Abstract
Introduction
With availability of direct‐acting antivirals (DAA), most persons with inherited bleeding disorders are currently cured of hepatitis C virus (HCV) infection. The risk of liver‐related complications following HCV cure has not been reported for this population.
Aim
Reporting liver‐related complications during long‐term chronic HCV infection and following sustained virological response (SVR) in this population.
Methods
Retrospective follow‐up of a prospective single‐centre cohort of HCV antibody‐positive persons with inherited bleeding disorders. Primary endpoint was liver‐related complications [hepatocellular carcinoma (HCC), decompensated cirrhosis, bleeding gastroesophageal varices]. Liver‐related complications were reported separately during chronic HCV and following SVR, stratified for interferon‐based and DAA‐based SVR.
Results
In total 309/381 (81%) HCV antibody‐positive individuals developed chronic HCV infection. Median follow‐up was 44 years [interquartile range (IQR): 34–50]. Liver‐related complications occurred in 36/309 (12%) of individuals with chronic HCV infection after median 31 years of chronic infection. Of 199 individuals with SVR, 97 were cured with interferon‐based regimens and 102 with DAA after median infection durations of 29 and 45 years, respectively. At end of follow‐up, respectively, 21% and 42% had advanced fibrosis or cirrhosis. Post‐SVR, seven (4%) individuals had a liver‐related complication, mainly HCC (n = 4). Incidence of liver‐related complications per 100 patient‐years post‐SVR follow‐up was .2 for interferon‐cured and 1.0 for DAA‐cured individuals (p = .01).
Conclusion
Successful HCV treatment does not eliminate the risk of liver‐related complications in persons with inherited bleeding disorders. Due to higher baseline risk, incidence was higher after DAA than interferon‐based SVR. We advise continuing HCC surveillance post‐SVR in all with advanced fibrosis or cirrhosis.
Keywords: antiviral agents, cirrhosis, haemophilia, hepatocellular carcinoma, viral hepatitis
1. INTRODUCTION
Before 1992, nearly all persons with severe inherited bleeding disorders were infected with hepatitis C virus (HCV) through contaminated clotting factor products. 1 Among the 70%–80% of HCV‐infected individuals who developed chronic HCV infection, 1 , 2 liver‐related morbidity has been one of the most frequent causes of death. In contrast to the general HCV population, the onset of HCV infection can be reasonably estimated among people with inherited bleeding disorders as most individuals were infected at their first infusion with clotting factor concentrates. 3
Earlier studies from an international prospective cohort reported liver‐related complications in 13% of persons with chronic HCV infection after median 23 years infection duration. 3 , 4 These data originate from the era of previously used and less effective interferon‐based HCV therapy. Since then, treatment of HCV has improved greatly with the introduction of direct‐acting antivirals (DAA) that achieve sustained virological response (SVR) in >95% of persons. 5
Although successful HCV treatment is considered to substantially reduce the risk of liver‐related complications, this risk is not entirely eradicated. 6 , 7 In the general HCV population, liver‐related complications following SVR are more frequent after DAA‐induced SVR than following interferon‐induced SVR because in contrast to interferon, also patients with more advanced liver disease are eligible for DAA therapy. 6 , 8 To our knowledge, it has not been reported yet whether this applies to persons with inherited bleeding disorders. This issue is important to assess, as various HCV populations may differ markedly in prevalence of factors associated with unfavourable outcomes [e.g., human immunodeficiency virus (HIV) and hepatitis B virus (HBV) co‐infections, 9 alcohol use, overweight 10 and socioeconomic status 11 ]. Furthermore, some authors argue that repeated exposure to HCV via frequent administration of contaminated clotting factor products has resulted in increased intra‐individual HCV quasispecies diversity in this population, which could potentially influence disease course. 12 , 13 Finally, their almost universally long period of HCV infection makes them especially at risk for developing liver‐related complications.
Nine years after the previous publication, 4 almost all participants in our cohort are currently successfully treated for their HCV infection. Our aims were to describe liver‐related outcomes after median 35 years of chronic HCV infection and following successful HCV treatment in a large cohort of persons with inherited bleeding disorders. Additionally, we compared the incidence of liver‐related complications between DAA‐induced and interferon‐induced SVR.
2. METHODS
2.1. Design and participants
This study was a retrospective follow‐up of a prospective single‐centre cohort conducted at the Van Creveldkliniek Haemophilia Treatment Centre (Department of Benign Haematology, University Medical Centre Utrecht, The Netherlands). This centre provides care for approximately 50% of the Dutch haemophilia population. The study cohort was set up in 2005 and consisted of all individuals with inherited bleeding disorders ≥18 years who ever tested HCV antibody‐positive. 3 Data of HCV antibody‐positive individuals who joined the haemophilia treatment centre after 2005 were added. All persons with inherited bleeding disorders treated with plasma‐derived clotting factor products from large plasma pools were systematically screened for HCV infection since 1992. 1 Local haemophilia and liver‐related guidelines are reported in the Supplementary Data. The study was approved by the medical ethics committee of the University Medical Centre Utrecht.
2.2. Data collection
We collected age, body mass index (BMI), haemophilia‐related variables (type and severity of the bleeding disorder), alcohol use, HBV and HIV co‐infections, and date and cause of death. HCV‐related variables were date of infection, HCV RNA and genotype results, and HCV treatment history. Liver‐related variables were abdominal ultrasound results, Fibroscan (Echosens, Paris, France) measurements, liver‐related laboratory results, results of endoscopy of the upper gastrointestinal tract and liver‐related clinical complications.
Additional data for the current follow‐up were collected until 31 January 2021. For individuals who moved to another haemophilia treatment centre since 2012, updated information on HCV status and occurrence of primary and secondary endpoints was requested from the other centre.
2.3. Outcomes and definitions
The primary endpoint was development of a first liver‐related complication, defined as the occurrence of decompensated cirrhosis, bleeding gastroesophageal varices or HCC. Decompensated cirrhosis was defined as cirrhosis with ascites, clinically diagnosed hepatic encephalopathy, hepatorenal syndrome or jaundice. Secondary endpoints were occurrence of individual liver‐related complications, liver‐related mortality and overall survival.
As defined previously, 3 , 4 the date of first exposure to large pool clotting factor products or cryoprecipitate was assumed to be the date of HCV infection. For the individuals in whom this date was unknown, the median date of HCV infection from the cohort was imputed (i.e., January 1970). For individuals born after this date, the median age at first treatment for persons with severe haemophilia in our centre was imputed. Median age at first treatment in our centre was 1 year during the 1970s and 1980s. 14 Presence of advanced fibrosis or cirrhosis was defined as any Fibroscan result ≥9.5 kPa, if diagnosed with radiologic imaging, or if there was a history of liver‐related complications as defined above. Severity of cirrhosis was classified using the Child–Pugh score. Additional definitions are presented in the Supplementary Data.
2.4. Statistical analysis
Descriptive data were presented as numbers (percentages) or median [with interquartile range (IQR) or range]. Characteristics were reported at the time of last clinical evaluation, regardless of HCV status, unless otherwise noted. Differences between groups were assessed for statistical significance using Fischer exact tests or Mann–Whitney U tests as appropriate.
Occurrence of liver‐related complications was reported with 95% confidence interval (CI) for the entire cohort since HCV infection and for three subgroups: during chronic HCV infection, following spontaneous HCV clearance and following successful HCV treatment. For the analysis of liver‐related complications during chronic HCV infection or following spontaneous HCV clearance, progression to clinical endpoints was compared using Kaplan–Meier analysis. Follow‐up started on the assumed date of HCV infection and ended at the moment of treatment‐induced SVR, occurrence of the first liver‐related complication, last clinical evaluation or death, whichever came first. For the analysis of overall survival, follow‐up ended at the last clinical evaluation or death, regardless of HCV status. Analysis of liver‐related complications was stratified for HIV/HCV co‐infected individuals, individuals with chronic HCV mono‐infection and those with spontaneous HCV clearance. Regarding overall survival, the group with spontaneous HCV clearance was additionally stratified for HIV status. Kaplan–Meier curves were truncated if the number of persons at risk in a subgroup was below 10. To address the potential issue of competing risk of mortality and liver transplantation, the cumulative incidence function was calculated including mortality and liver transplantation as competing risks to liver‐related complications in a sensitivity analysis.
To assess the association between overweight (BMI ≥25 kg/m2) and liver‐related complications during chronic HCV infection, Cox proportional hazards regression, adjusted for severe alcohol use and HIV infection, was performed in individuals with ≥35 years of chronic HCV infection without liver‐related complications.
Finally, liver‐related complications following SVR were reported as incidence per 100 patient‐years follow‐up with 95%CI. Follow‐up for the post‐SVR period started at cessation of the successful antiviral treatment and ended at the last clinical evaluation, liver‐related complication or death, whichever came first. This analysis was stratified for treatment type, that is, interferon‐based SVR and DAA‐based SVR. Differences in incidence rates between these treatment types were expressed as rate ratio (RR) with 95%CI. Data were analysed using R (version 3.6.1, Vienna, Austria).
3. RESULTS
In total 382 persons were included (Table 1; 93% haemophilia A or B). Of these, 309 (81%) developed chronic HCV infection (Figure 1). HCV transmission occurred before 1991. Fifty‐five (14%) individuals tested HIV antibody‐positive, all infected before June 1985. Median follow‐up after HCV infection was 44 years (IQR 34–50, in total 15,784 patient‐years). Median duration of chronic HCV infection was 35 years (IQR 27–43) and for those with successful antiviral therapy median follow‐up post‐SVR was 6 years (IQR 4–16).
TABLE 1.
Characteristics of HCV antibody‐positive persons with inherited bleeding disorders at their last clinical evaluation
| Spontaneous clearance | Ever diagnosed with chronic HCV | |
|---|---|---|
| Number | 73 | 309 a |
| Total number of follow‐up years | 2944 | 12,839 |
| Median follow‐up (years) | 41 (35–50) | 44 (34–50) |
| Male sex | 71 (97%) | 297 (96%) |
| Diagnosis | ||
| Haemophilia A | 54 (74%) | 250 (81%) |
| Haemophilia B | 11 (15%) | 42 (14%) |
| Von Willebrand disease | 6 (8%) | 8 (3%) |
| Other b | 2 (3%) | 9 (3%) |
| Severe bleeding disorder | 53 (73%) | 234 (76%) |
| Age at HCV infection (years) | 6 (1–22) | 8 (2–18) |
| Age at end of follow‐up (years) | 51 (40–63) | 52 (43–63) |
| HCV genotype | ||
| 1 c | 178 (58%) | |
| 2 | 29 (9%) | |
| 3 | 26 (8%) | |
| 4 | 7 (2%) | |
| 5 | 2 (1%) | |
| Unknown | 73 (100%) | 67 (22%) |
| HIV infection | 7 (10%) | 48 (16%) |
| HBV infection | ||
| HBsAg positive | 4 (6%) | 6 (2%) |
| HBsAg negative, anti‐HBc positive | 23 (32%) | 122 (40%) |
| History of severe alcohol use d | 5 (7%) | 37 (12%) |
| Body Mass Index (kg/m2) | 26 (24–28) | 25 (22–27) |
| At least advanced fibrosis e | 2 (3%) f | 110 (36%) |
| Child–Pugh A/B/C | 1/1/0 | 74/26/10 |
| Alanine transaminase (IU/L) | 22 (16–27) | 28 (18–52) |
| Aspartate transaminase (IU/L) | 24 (20–29) | 26 (18–43) |
| Platelet count (*109/L) | 236 (208–304) | 215 (156–262) |
| Bilirubin (μmol/L) | 10 (7–13) | 11 (8–16) |
| Albumin (g/L) | 42 (38–43) | 42 (38–45) |
| International normalised ratio | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) |
Data are number (percentage) or median (interquartile range) unless otherwise noted. Anti‐HBc, hepatitis B core antibodies; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
One individual spontaneously cleared HCV after >20 years of chronic infection.
bDeficiency factor II (n = 1), VII (n = 1), X (n = 4), XIII (n = 2), or haemophilia carrier (n = 2).
cSubtypes: 1a n = 53, 1a/b n = 5, 1b n = 74, 1c n = 1, unknown n = 45.
dAlcohol intake > 20 units/week.
eFibroscan ≥ 9.5 kPa or radiological, histological or clinical diagnosis.
fBoth were diagnosed with alcoholic cirrhosis.
FIGURE 1.
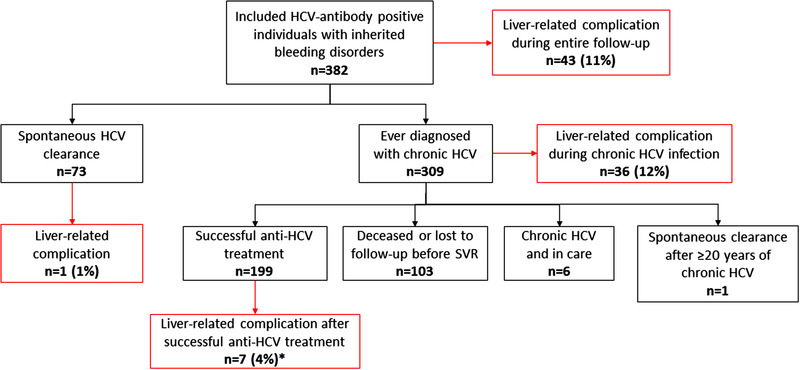
Flowchart.
Liver‐related complications were defined as the occurrence of decompensated cirrhosis, variceal bleeding or hepatocellular carcinoma. *One individual had a liver‐related complication both during chronic HCV infection and after successful HCV treatment. HCV, hepatitis C virus; SVR, sustained virological response
3.1. Liver‐related complications and overall survival since HCV infection
In the complete cohort, 43 (11%, 95%CI 8%–15%) of the 382 HCV antibody‐positive individuals developed a liver‐related complication during the entire follow‐up (Table 2). At the end of follow‐up, 97 (25%, 95%CI 21%–30%) individuals were deceased. Among the 309 individuals ever diagnosed with chronic HCV infection, liver‐related death was the most common cause of death (n = 23, 7%, 95%CI 5%–11%; Table 2). Overall survival was comparable between HIV‐negative persons with either chronic HCV infection or spontaneous HCV clearance (Figure 2). Between 1990 and 2000, 24 (50%, 95%CI 35%–65%) individuals with HIV/HCV co‐infection died, of whom 23 because of either acquired immune deficiency syndrome (AIDS, n = 16) or liver‐related complications (n = 7).
TABLE 2.
Liver‐related complications and mortality after median 44 years since HCV infection in HCV antibody‐positive persons with inherited bleeding disorders
| Complete cohort | Ever diagnosed with chronic HCV | |||
|---|---|---|---|---|
| Number | 382 | 309 | ||
| Spontaneous clearance | Ever diagnosed with chronic HCV | HIV/HCV | HCV mono | |
| Number | 73 | 309 | 48 | 261 |
| Data are reported as number (percentage, 95% confidence interval) | ||||
| Liver‐related complication | 1 a (1, 0–7) | 42 (14, 10–18) | 16 (33, 20–48) | 26 (10, 7–14) |
| Decompensated cirrhosis | 1 (1, 0–7) | 31 (10, 7–14) | 15 (31, 19–46) | 16 (6, 4–10) |
| Hepatocellular carcinoma | 1 (1, 0–7) | 17 (6, 3–9) | 2 (4, 1–14) | 15 (6, 3–9) |
| Gastroesophageal bleeding | 1 (1, 0–7) | 10 (3, 2–6) | 2 (4, 1–14) | 8 (3, 1–6) |
| Liver transplantation | 0 | 4 (1, 0–3) | 0 | 4 (2, 0–4) |
| All‐cause mortality | 14 (19, 10–30) | 83 (27, 22–32) | 29 (60, 45–74) | 54 (21, 16–26) |
| HIV/AIDS | 2 (3, 0–10) | 18 (6, 3–9) | 18 (38, 24–53) | 0 |
| Liver‐related | 1a (1, 0–7) | 23 (7, 5–11) | 10 (21, 10–35) | 13 (5, 3–8) |
| Non‐variceal haemorrhage | 1 (1, 0–7) | 11 (4, 2–6) | 0 | 11 (4, 2–7) |
| Malignancy b | 4 (5, 2–13) | 7 (2, 1–5) | 0 | 7 (3, 1–5) |
| Other | 4 (5, 2–13) | 16 (5, 3–8) | 0 | 16 (6, 4–10) |
| Unknown | 2 (3, 0–10) | 8 (3, 3–8) | 1 (2, 0–11) | 7 (3, 1–5) |
AIDS, acquired immune deficiency syndrome; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
aDiagnosed with alcoholic cirrhosis.
bMalignancies not related to either HIV or HCV.
FIGURE 2.
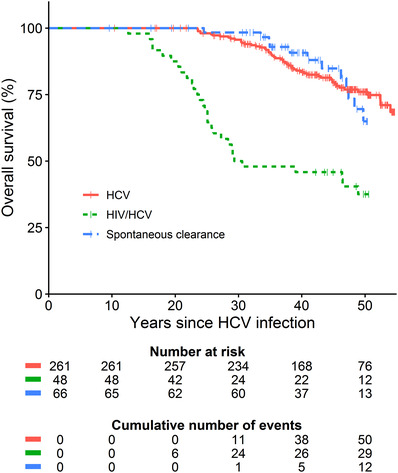
Overall survival stratified by infection status. HIV‐positive individuals with spontaneous HCV clearance were excluded from this analysis as the number at risk in this subgroup was below 10 at start of follow‐up (n = 7). HCV, hepatitis C virus; HIV, human immunodeficiency virus
3.2. Liver‐related complications during chronic HCV infection
After median 31 years (range 16–49) of chronic HCV infection, 36/309 (12%, 95%CI 8%–16%) individuals developed a liver‐related complication. Main liver‐related complications were ascites (n = 21, 7%, 95%CI 4%–10%), HCC (n = 14, 5%, 95%CI 3%–7%), variceal bleeding (n = 8, 3%, 95%CI 1%–5%) and hepatic encephalopathy (n = 6, 2%, 95%CI 1%–4%). Barcelona Clinic Liver Cancer (BCLC) stage at diagnosis of HCC was 0 (n = 1), A (n = 5), B (n = 2), C (n = 4) and D (n = 2). Liver‐related complications were more frequent in the HIV/HCV co‐infected group (29% vs. 8%, p < .001), mainly because of higher incidence of decompensated cirrhosis (29% vs. 5%, p < .001). In HIV/HCV co‐infected individuals, liver‐related complications started after 20 years of HCV infection (Figure 3). After the introduction of more effective and less hepatotoxic HIV therapy in 1996, the incidence of liver‐related complications decreased in HIV co‐infected persons. In those diagnosed with chronic HCV mono‐infection, liver‐related complications started after 30 years of chronic HCV infection, with increasing incidence after 40 years.
FIGURE 3.
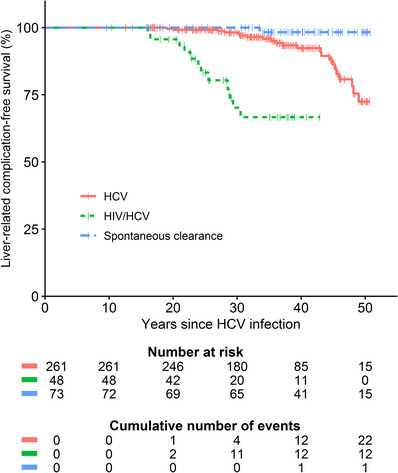
Liver‐related complication‐free survival stratified by infection status. Liver‐related complications were defined as the occurrence of decompensated cirrhosis, variceal bleeding or hepatocellular carcinoma. HCV, hepatitis C virus; HIV, human immunodeficiency virus
In the sub‐analysis including 150 individuals with ≥35 years of chronic HCV infection without prior liver‐related complication, 16 individuals developed a liver‐related complication during chronic HCV infection. No significant association was found between being overweight and the occurrence of liver‐complications (adjusted HR 1.1, 95%CI .7–5.8, p = .9). Among 226 individuals with chronic HCV who had an ultrasound examination, 46/226 (25%) had their most recent ultrasound examination indicating steatosis. Twelve (5%) of these individuals had a liver‐related complication before achieving SVR, which was not associated with an ultrasound indicating steatosis (HR adjusted for HIV and severe alcohol use: .4, 95%CI .1–3.3, p = .4).
As there were no cases of liver transplantation prior to development of a liver‐related complication, only mortality was included as competing risk in the sensitivity analysis (Supplementary Figure S1). In this analysis, the incidence of liver‐related complications was similar to the incidence in the regular survival analysis for all groups.
3.3. Antiviral therapy and SVR rates
Of the 309 persons diagnosed with chronic HCV infection, 223 (72%) were treated at least once with anti‐HCV therapy. SVR percentages for first treatment with an interferon‐based regimen were 40% (21/53) for interferon monotherapy, 43% (18/42) for interferon + ribavirin and 57% (55/97) for peg‐interferon + ribavirin. SVR‐rate was 68% (15/22) for first‐ or second‐generation DAA + peg‐interferon + ribavirin and 96% (86/90) for interferon‐free treatment based on second‐generation DAA.
In total 199/223 (89%) of ever‐treated individuals obtained SVR. Of the remaining individuals ever diagnosed with chronic HCV infection, 103 had either died (n = 75) or were lost to follow‐up before SVR (n = 28), one individual spontaneously cleared the virus after 20 years of chronic HCV infection and six individuals remained chronically HCV‐infected while in care in January 2021. At end of follow‐up, 63/199 (32%) successfully treated individuals had at least advanced fibrosis. Laboratory parameters of liver function (serum bilirubin, PT, albumin values) did not change from pre‐treatment to 4 years post‐SVR in those with at least advanced fibrosis before start of HCV therapy (results not shown).
3.4. Liver‐related complications following successful HCV treatment
Total follow‐up after SVR was 1626 patient‐years (median 16, IQR 14–22) for the interferon‐cured group (n = 97) and 385 years (median 4, IQR 3–5) for the DAA‐cured group (n = 102). Infection duration was significantly longer among DAA‐cured individuals (45 vs. 29 years, p < .001; Table 3) and advanced fibrosis or cirrhosis at the end of follow‐up was significantly more common in the DAA‐cured group than in the interferon‐cured group (42% vs. 21%, p = .001). Seven (4%, 95%CI 1.6–7.2) persons developed nine liver‐related complications following SVR, of whom three individuals were interferon‐cured and four DAA‐cured (Table 4). These events were HCC (n = 4), ascites (n = 3) and variceal bleeding (n = 2). BCLC stage of post‐SVR HCC was A (n = 2), C (n = 1) and D (n = 1). Median time between end of successful treatment and first liver‐related event was 25 months (range 11–157). Only one of these individuals had a liver‐related complication before SVR, with recurrent HCC following successful HCV treatment.
TABLE 3.
Characteristics of successfully treated individuals, stratified for therapy type
| Interferon‐cured | DAA‐cured | |
|---|---|---|
| Number | 97 | 102 |
| Age (median, IQR) (years) | ||
| At start of treatment | 37 (28–44) | 49 (41–60) |
| At end of follow‐up | 54 (47–64) | 52 (45–63) |
| HCV infection duration (years) | 29 (23–34) | 45 (38–48) |
| Advanced fibrosis or cirrhosis a | 20 (21%) | 43 (42%) |
| Child–Pugh A/B/C | 18/2/0 | 42/1/0 |
| HCV genotype | ||
| 1 | 36 (37%) | 90 (88%) |
| 2 | 21 (22%) | 2 (2%) |
| 3 | 17 (18%) | 6 (6%) |
| 4 | 1 (1%) | 3 (3%) |
| 5 | 1 (1%) | 0 |
| Unknown | 21 (21%) | 1 (1%) |
| HIV co‐infection | 9 (9%) | 12 (12%) |
| No prior (Peg)‐interferon treatment | 85 (88%) | 61 (60%) |
| History of severe alcohol use b | 9 (9%) | 10 (10%) |
| Body mass index (median, IQR) (kg/m2) | 26 (23–28) | 25 (22–28) |
| Platelet count (median, IQR) | ||
| Prior to successful treatment | 221 (181–271) | 206 (165–254) |
| Two to four year post‐SVR | 234 (195–289) | 228 (185–270) |
| APRI ≥1.0 | ||
| Prior to successful treatment | 20/91 (22%) | 27/102 (27%) |
| Two to four years post‐SVR | 3/80 (4%) | 4/37 (11%) |
| FIB‐4 ≥3.25 | ||
| Prior to successful treatment | 2/91 (2%) | 14/102 (14%) |
| Two to four years post‐SVR | 1/80 (1%) | 4/37 (11%) |
Data are reported as number (percentage) unless otherwise noted. Characteristics reported at the most recent clinical visit, unless otherwise noted. APRI, AST to Platelet Ratio Index; DAA, direct‐acting antivirals; FIB‐4, Fibrosis‐4 Score; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; SVR, sustained virological response.
†Defined as a Fibroscan result ≥9.5 kPa or radiological, histological or clinical diagnosis.
‡Defined as an alcohol intake > 20 units per week.
TABLE 4.
Liver‐related complications following successful HCV treatment
| Interferon‐cured | DAA‐cured | |
|---|---|---|
| Number | 97 | 102 |
| Follow‐up since SVR (years) | ||
| Median, IQR | 16 (14–22) | 4 (3–5) |
| Group total | 1626 | 385 |
| Liver‐related complication after SVR a | 3 (3%) | 4 (4%) b |
| Per 100 patient‐years | .2 (95% CI .05–.5) | 1.0 (95% CI .3–2.5) |
| Per 100 patient‐years (only F3/F4 c ) | 1.0 (95% CI .2–2.7) | 2.2 (95% CI .7–5.2) |
| Hepatocellular carcinoma after SVR | 1 (1%) | 3 (3%) b |
| Per 100 patient‐years | .1 (95% CI .003–.3) | .8 (95% CI .2–2.1) |
| Per 100 patient‐years (only F3/F4 c ) | .3 (95% CI .02–1.6) | 1.6 (95% CI .4–4.4) |
| Liver‐related death after SVR | 0 | 1 (1%) |
| Per 100 patient‐years | 0 | .3 (95% CI .01–1.3) |
| Per 100 patient‐years (only F3/F4 c ) | 0 | .5 (95% CI .03–2.7) |
| All‐cause mortality after SVR | 4 (4%) | 4 (4%) |
| Per 100 patient‐years | .2 (95% CI .8–5.9) | 1.0 (95% CI .3–2.5) |
Data are reported as number (percentage) or incidence (95% confidence interval) unless otherwise noted. CI, confidence interval; DAA, direct‐acting antivirals; HCV, hepatitis C virus; IQR, interquartile range; SVR, sustained virological response.
aDefined as hepatocellular carcinoma, decompensated cirrhosis or variceal bleeding.
bOne individual had a liver‐related event prior to SVR (hepatocellular carcinoma), and a recurrent hepatocellular carcinoma following SVR.
cAdvanced fibrosis or cirrhosis. Defined as a Fibroscan result ≥9.5 kPa or radiological, histological or clinical diagnosis.
Incidence rates of liver‐related complications following SVR were significantly higher in the DAA‐cured group than in the interferon‐cured group (RR 5.6, 95%CI 1.2–30.2, p = .01). For individuals with at least advanced fibrosis, the difference between interferon and DAA was less pronounced with incidence rates of 1.0 and 2.2 per 100 patient‐years in the interferon and DAA group, respectively (RR 2.2, 95%CI .5–11.9, p = .28). Four persons were diagnosed with HCC post‐SVR, of which three were de novo HCCs. HCC incidence rates per 100 patient‐years post‐SVR follow‐up in persons with at least advanced fibrosis were .3 for the interferon‐cured group and 1.6 for the DAA‐cured group (RR 4.6, 95%CI .5–131.4, p = .12). One individual with HCC following DAA‐based HCV eradication had a liver‐related death.
4. DISCUSSION
Following the introduction of highly effective DAA, virtually all persons with inherited bleeding disorders in our centre are now successfully treated for their HCV infection. Although successful HCV treatment substantially reduces the risk of liver‐related complications, a residual risk remains. In our cohort, seven (4%, 95%CI 1.6–7.2) of the 199 successfully treated individuals had a liver‐related complication during median six years post‐SVR follow‐up.
Our findings on the post‐SVR clinical course of DAA‐cured individuals with inherited bleeding disorders are in line with data from the European and American general HCV populations. This includes that the most frequent liver‐related event following SVR in was HCC 15 , 16 and that post‐SVR HCC incidence rates tended to be higher after DAA‐based than interferon‐based cure. 6 , 8 Importantly, interferon‐based therapies were used years before the introduction of DAA therapy, with potential risk of confounding by age, infection duration and year of treatment. Indeed, both groups differed in characteristics such as age, infection duration and year of HCV cure. Of note, studies in the general HCV population with proper adjustment for differences in baseline characteristics demonstrated that SVR with DAA and interferon‐based regimens result in similar HCC risk reduction. 6 , 8 Nonetheless, it appears that individuals treated with DAA have an inherently higher baseline HCC risk due to a higher prevalence of severe liver disease and longer infection duration. Hence, post‐SVR HCC is expected to be more frequent in the DAA era.
The post‐SVR HCC incidence of DAA‐cured individuals with at least advanced fibrosis observed in our study was above the threshold for cost‐effective post‐SVR HCC surveillance of 1.3% per year reported by a recent analysis from Canada. 17 Validated prognostic tools to identify patients with cirrhosis having a sufficiently low risk to omit HCC surveillance are not yet available. 18 , 19 Furthermore, non‐invasive tools for the assessment of liver fibrosis are insufficiently accurate in detecting fibrosis regression following SVR. 18 , 20 , 21 Thus, in our opinion, continued bi‐annual HCC surveillance with ultrasound ± alpha fetoprotein post‐SVR for individuals with bleeding disorders and HCV‐induced advanced fibrosis or cirrhosis is justified, in accordance with current HCV and HCC guidelines for the general HCV population. 19 , 22 , 23
Being overweight after 35 years of chronic HCV infection was not significantly associated with increased occurrence of liver‐related complications during chronic HCV infection. Although BMI is associated with hepatic steatosis in HCV‐infected individuals, 24 there is inconsistency in literature regarding the association between being overweight and accelerated fibrosis progression in HCV patients. 10 , 25 A recent analysis of a large database on hospital inpatient discharge data in the USA indicated that non‐alcoholic steatohepatitis (NASH) diagnosis based on ICD‐10 codes was strongly associated with HCC among people with haemophilia. 26 For individuals without advanced fibrosis or cirrhosis, additional HCC risk factors such as NASH, HBV infection and severe alcohol use can justify continuing liver fibrosis assessments following successful HCV treatment. Robust data on epidemiology and clinical outcomes of NASH in people with inherited bleeding disorders are still lacking, and are therefore an important topic for future research.
In the previous analysis of our cohort in 2012, the majority of included persons still had chronic HCV infection 4 and after a median duration of 33 years, 9% had developed a liver‐related complication. For the current analysis, median HCV infection duration was extended to 35 years and complications during chronic HCV infection had increased to 12%. Although median infection duration was not extended much due to successful antiviral therapy, incidence of liver‐related complications during chronic HCV infection had increased further. Since DAAs were allowed in The Netherlands for individuals with at least advanced fibrosis in November 2014, five patients were diagnosed with de novo HCC. In the seven years before DAA, seven cases were identified, indicating that prevalence did not decrease sharply following introduction of DAA. Physicians must therefore remain aware of the risk of liver‐related complications, even after 35 years of complication‐free chronic HCV infection and after SVR. Taken together, this underlines the importance of the arrival of DAAs and that HCV micro‐elimination is paramount in this population with uniform long HCV infection duration.
To our knowledge, our study is among the first to report data on incidence of liver‐related complications following SVR in persons with inherited bleeding disorders. These data originate from a centre that provides care for a large part of the Dutch haemophilia population, with very long and consistent follow‐up of individuals ever infected with HCV. Nonetheless, several limitations apply to our study. The survival curve of liver‐related complications during chronic HCV infection might overestimate the incidence rate at the end of follow‐up, as censoring due to successful treatment was not completely random because of patients with more advanced liver disease having a lower chance of achieving SVR with interferon‐based treatment. Also, the exact date of HCV seroconversion was unknown since HCV testing was not available at the time of HCV transmission. As previously described, 3 , 4 the date of first exposure to large pool clotting factor products or cryoprecipitate was used, which was frequently followed by elevated transaminases indicating non‐A, non‐B hepatitis. 27 , 28
Furthermore, our definition of at least advanced liver fibrosis if any Fibroscan result was ≥9.5 kPa might have falsely classified some individuals as having at least advanced fibrosis, because several factors, such as inflammation and a non‐fasting state, can lead to overestimation of fibrosis. Therefore, the incidence of post‐SVR liver‐related complications in individuals definitively having at least advanced fibrosis will have been higher. In contrast, regression or normalisation of Fibroscan values post‐SVR can be seen despite biopsy showing persistent cirrhosis. 20 , 21 Thus, we chose this definition to reduce the chance of missing individuals with at least advanced fibrosis post‐SVR. Liver biopsies were not performed for staging of extent of fibrosis in in our cohort of people with inherited bleeding disorders, related to the assumed risk of post‐procedural bleeding and early availability of Fibroscan measurements. Furthermore, NASH data were not systematically collected, as controlled attenuation parameter measurements during Fibroscan have only recently become available in our centre. Also, individuals who deceased before 1992 were not retrospectively tested for HCV antibodies. Therefore, some early mortality in HCV‐infected persons from our centre might have been missed. However, this effect was likely small, as liver‐related mortality was uncommon among people with haemophilia in these days. 29 Furthermore, post‐SVR follow‐up of DAA‐cured individuals was still relatively limited, precluding definitive conclusions on liver‐related complications during long‐term post‐DAA follow‐up. Finally, in comparison to the previous follow‐up studies, 3 , 4 the cohort in the current analysis was smaller as two centres from the UK (included in our previous report) were not able to participate in the current work due to Brexit and Covid‐19 unforeseen circumstances. Due to the smaller cohort and the limited number of post‐SVR liver‐related complications, we were unable to reliably assess predictors of post‐SVR liver‐related complications.
5. CONCLUSION
Nearly all HCV‐infected persons with inherited bleeding disorders in our centre have achieved HCV clearance. Nonetheless, our data show that a residual risk of liver‐related complications remains following SVR. Presumably due to higher baseline risk, incidence of liver‐related complications following DAA is higher than following interferon‐based treatment. Therefore, we strongly advise continuing bi‐annual HCC surveillance in all persons with advanced fibrosis or cirrhosis prior to successful DAA treatment.
AUTHOR CONTRIBUTIONS
Concept and design: Cas J. Isfordink, Karel J. van Erpecum, Kathelijn Fischer, Joop E. Arends, Evelien P. Mauser‐Bunschoten. Data collection: Cas J. Isfordink. Data analysis: Cas J. Isfordink, Kathelijn Fischer. Cas J. Isfordink wrote a first draft of the manuscript. All authors contributed to interpretation of the data and critically reviewed the manuscript, read and approved the final version of the manuscript.
CONFLICT OF INTEREST
C.J. Isfordink has received research funding from Gilead, not related to this study. K.J. van Erpecum participated in advisory boards of Gilead, Janssen‐Cilag, BMS, Abbvie, and MSD and received research grants from Gilead, Janssen‐Cilag and the Dutch Cancer Society (KWF Kankerbestrijding). The Van Creveldkliniek has received speaker's fees from Bayer, Baxter/Shire, SOBI/Biogen, CSL Behring and NovoNordisk; consultancy fees from Bayer, Biogen, CSL‐Behring, Freeline, NovoNordisk, Roche and SOBI; and research support from Bayer, Baxter/Shire, Novo Nordisk, Pfizer and Biogen for the work done by K. Fischer. P.R. van der Valk had received an research grant from Baxalta. L.F.D. van Vulpen has received research grants from CSL Behring and Griffols and is consultant for Sobi and Tremeau (funds to the institute). R.E.G. Schutgens has received research support from Bayer, Baxalta, CSL Behring, Novo Nordisk, Pfizer, Sobi, and Sanquin. J.E. Arends reports fees paid to the institution from Gilead, Janssen‐Cilag, Abbvie and MSD for advisory membership, all outside the submitted work. All other authors report no conflict of interest.
Supporting information
supporting Information
supporting Information
ACKNOWLEDGMENTS
No funding was received in order to prepare this manuscript.
Isfordink CJ, van Erpecum KJ, Fischer K, et al. Liver‐related complications before and after successful treatment of chronic hepatitis C virus infection in people with inherited bleeding disorders. Haemophilia. 2023;29:106–114. 10.1111/hae.14668
DATA AVAILABILITY STATEMENT
A reasonable request for the data that support the findings of this study can be made at the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Mauser‐Bunschoten EP, Roosendaal G, van den Berg HM, et al. Hepatitis C infection and viremia in Dutch Hemophilia patients. J Med Virol. 1995;45(3):241‐246. [DOI] [PubMed] [Google Scholar]
- 2. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 3. Posthouwer D, Makris M, Yee TT, et al. Progression to end‐stage liver disease in patients with inherited bleeding disorders and hepatitis C: an international, multicenter cohort study. Blood. 2007;109(9):3667‐3671. [DOI] [PubMed] [Google Scholar]
- 4. Fransen van de Putte DE, Makris M, Fischer K, et al. Long‐term follow‐up of hepatitis C infection in a large cohort of patients with inherited bleeding disorders. J Hepatol. 2014;60(1):39‐45. [DOI] [PubMed] [Google Scholar]
- 5. Stedman CAM, Hyland RH, Ding X, Pang PS, McHutchison JG, Gane EJ. Once daily ledipasvir/sofosbuvir fixed‐dose combination with ribavirin in patients with inherited bleeding disorders and hepatitis C genotype 1 infection. Haemophilia. 2016;22(2):214‐217. [DOI] [PubMed] [Google Scholar]
- 6. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moon AM, Green PK, Rockey DC, Berry K, Ioannou GN. Hepatitis C eradication with direct‐acting anti‐virals reduces the risk of variceal bleeding. Aliment Pharmacol Ther. 2020;51(3):364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646‐654. [DOI] [PubMed] [Google Scholar]
- 9. Isfordink CJ, van Erpecum KJ, van der Valk M, Mauser‐Bunschoten EP, Makris M. Viral hepatitis in haemophilia: historical perspective and current management. Br J Haematol. 2021;195(2):174‐185. [DOI] [PubMed] [Google Scholar]
- 10. Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C‐related fibrosis progression. Am J Gastroenterol. 2002;97(9):2408‐2414. [DOI] [PubMed] [Google Scholar]
- 11. Omland LH, Osler M, Jepsen P, et al. Socioeconomic status in HCV infected patients – risk and prognosis. Clin Epidemiol. 2013;5(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. J Infect Dis. 1999;179(5):1062‐1069. [DOI] [PubMed] [Google Scholar]
- 13. Shire NJ, Horn PS, Rouster SD, et al. HCV kinetics, quasispecies, and clearance in treated HCV‐infected and HCV/HIV‐1‐coinfected patients with hemophilia. Hepatology. 2006;44(5):1146‐1157. [DOI] [PubMed] [Google Scholar]
- 14. Fischer K, Van Der Bom JG, Mauser‐Bunschoten EP, et al. Changes in treatment strategies for severe haemophilia over the last 3 decades: effects on clotting factor consumption and arthropathy. Haemophilia. 2001;7(5):446‐452. [DOI] [PubMed] [Google Scholar]
- 15. Pons M, Rodríguez‐Tajes S, Esteban JI, et al. Non‐invasive prediction of liver‐related events in patients with HCV‐associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72(3):472‐480. [DOI] [PubMed] [Google Scholar]
- 16. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet. 2019;393(10179):1453‐1464. [DOI] [PubMed] [Google Scholar]
- 17. Farhang Zangneh H, Wong WWL, Sander B, et al. Cost Effectiveness of Hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis C virus infection and advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17(9):1840‐1849. [DOI] [PubMed] [Google Scholar]
- 18. Berzigotti A, Tsochatzis E, Boursier J, et al. EASL Clinical Practice Guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659‐689. [DOI] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C – final update of the series. J Hepatol. 2020;73(5):1170‐1218. [DOI] [PubMed] [Google Scholar]
- 20. Sultanik P, Kramer L, Soudan D, et al. The relationship between liver stiffness measurement and outcome in patients with chronic hepatitis C and cirrhosis: a retrospective longitudinal hospital study. Aliment Pharmacol Ther. 2016;44(5):505‐513. [DOI] [PubMed] [Google Scholar]
- 21. D'Ambrosio R, Aghemo A, Fraquelli M, et al. The diagnostic accuracy of Fibroscan® for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59(2):251‐256. [DOI] [PubMed] [Google Scholar]
- 22. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723‐750. [DOI] [PubMed] [Google Scholar]
- 23. Isfordink CJ, Maan R, de Man RA, van Erpecum KJ, van der Meer AJ. Should we continue surveillance for hepatocellular carcinoma and gastroesophageal varices in patients with cirrhosis and cured HCV infection? Eur J Intern Med. 2021;94:6‐14. [DOI] [PubMed] [Google Scholar]
- 24. Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta‐analysis of individual patient data. Gastroenterology. 2006;130(6):1636‐1642. [DOI] [PubMed] [Google Scholar]
- 25. Rüeger S, Bochud PY, Dufour JF, et al. Impact of common risk factors of fibrosis progression in chronic hepatitis C. Gut. 2015;64(10):1605‐1615. [DOI] [PubMed] [Google Scholar]
- 26. Yang X, Jeong K, Yabes JG, Ragni MV. Prevalence and risk factor for hepatocellular carcinoma in individuals with hemophilia in the era of direct‐acting antiviral agents: a national inpatient sample study. Haemophilia. 2022;28(5):769‐775. [DOI] [PubMed] [Google Scholar]
- 27. Kernoff PBA, Lee CA, Karayiannis P, Thomas HC. High risk of non‐A non‐B hepatitis after a first exposure to volunteer or commercial clotting factor concentrates: effects of prophylactic immune serum globulin. Br J Haematol. 1985;60(3):469‐479. [DOI] [PubMed] [Google Scholar]
- 28. Fletcher M, Trowell J, Craske J, Pavier K, Rizza C. Non‐A non‐B hepatitis after transfusion of factor VIII in infrequently treated patients. Br Med J. 1983;287(6407):1754‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Triemstra M, Rosendaal FR, Smit C, Van Der Ploeg HM, Briët E. Mortality in patients with hemophilia: changes in a Dutch population from 1986 to 1992 and 1973 to 1986. Ann Intern Med. 1995;123(11):823‐827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supporting Information
supporting Information
Data Availability Statement
A reasonable request for the data that support the findings of this study can be made at the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


