Abstract
Depression is a major health problem for which most patients are not effectively treated. This underscores a need to identify new targets for the development of antidepressants with improved efficacy. Studies have shown that blockade of low-affinity/high-capacity transporters, such as organic cation transporters (OCTs) and the plasma membrane monoamine transporter (PMAT), with decynium-22 can produce antidepressant-like effects and inhibit serotonin clearance in brain when the serotonin transporter is pharmacologically or genetically compromised. In vitro studies show that OCTs/PMAT are also capable of norepinephrine transport, raising the possibility that decynium-22 might enhance the antidepressant-like effects of norepinephrine transporter inhibitors. Using in vivo electrochemistry, we show that local administration of decynium-22 into dentate gyrus of hippocampus enhanced the ability of the norepinephrine transporter blocker, desipramine, but not the dual norepinephrine/serotonin transporter blocker venlafaxine, to inhibit norepinephrine clearance. In parallel, systemic administration of decynium-22 (0.32 mg/kg) enhanced the antidepressant-like effects of desipramine (32 mg/kg), but not those of venlafaxine, in the tail suspension test, underscoring the heterogeneous response of mice to antidepressants, including those that share similar mechanisms of action. Systemic administration of normetanephrine, a potent blocker of OCT3, failed to potentiate the antidepressant-like effects of desipramine, suggesting that the actions of decynium-22 to augment the antidepressant-like effects of desipramine are likely mediated by another OCT isoform and/or PMAT. Taken together with existing literature, concurrent blockade of OCTs and/or PMAT merits further investigation as an adjunctive therapeutic for desipramine-like antidepressant drugs.
Keywords: Norepinephrine transporter, Serotonin transporter, Antidepressant, Decynium-22, Desipramine, Venlafaxine
1. Introduction
Drugs that block high-affinity/low-capacity monoamine transporters, such as norepinephrine and serotonin transporters, are among the most commonly prescribed treatments for depression. Blockade of these transporters increases extracellular norepinephrine and serotonin in brain regions important for mood regulation. This increase in extracellular monoamines is thought to trigger therapeutic downstream mechanisms responsible for antidepressant effects (Frazer, 1997; Daws et al., 2013). Unfortunately, these treatments often leave patients without full symptom relief (Kirsch et al., 2008; Sinyor et al., 2010), spurring investigations into alternative drug targets that enhance the therapeutic utility of commonly prescribed antidepressants.
Low-affinity/high-capacity “uptake 2” monoamine transporters are gaining attention as novel targets to treat psychiatric disorders (Schildkraut and Mooney, 2004; Rahman et al., 2008; Bacq et al., 2012; Daws et al., 2013; Horton et al., 2013; Krause-Heuer et al., 2017; Orrico-Sanchez et al., 2019). Such transporters include organic cation transporters (OCTs) and plasma membrane monoamine transporter (PMAT), which, in vitro, are capable of high efficiency serotonin uptake (Duan and Wang, 2010). In vivo experiments utilizing decynium-22, a potent blocker of OCTs/PMAT, show that local application of decynium-22 in hippocampus can inhibit serotonin clearance in serotonin transporter heterozygous (SERT+/−) and knockout (SERT−/−) mice (Baganz et al., 2008), and enhance the ability of fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), to inhibit serotonin clearance in wild-type mice (Horton et al., 2013). In parallel, systemic administration of decynium-22 produces antidepressant-like effects in the tail suspension test in SERT+/− and SERT−/− mice, and enhances the antidepressant-like effect of a sub-effective dose of fluvoxamine in wild-type mice (Baganz et al., 2008, 2010; Horton et al., 2013). Given decynium-22 does not have appreciable activity at serotonin, norepinephrine, or dopamine transporters (Fraser-Spears et al., 2019), these findings raise the possibility that blockers of decynium-22-sensitive transporters, collectively OCTs and PMAT, may be effective adjunct therapies administered with SSRIs in the treatment of depression.
OCT3 blockade is suggested to enhance neurochemical and antidepressant-like effects of norepinephrine transporter targeting antidepressants (Rahman et al., 2008). Normetanephrine, a metabolite of norepinephrine and potent OCT3 blocker (Martel et al., 1999; Schildkraut and Mooney, 2004; Graf et al., 2013), enhanced the antidepressant-like effects of the norepinephrine transporter blocker, desipramine, in mice (Rahman et al., 2008). Moreover, normetanephrine enhanced the increase in extracellular norepinephrine following venlafaxine, a serotonin and norepinephrine reuptake inhibitor (SNRI), in prefrontal cortex of rats (Rahman et al., 2008). These studies suggest that inhibitors of OCT3, and potentially other OCT isoforms and PMAT, may also be used to enhance the therapeutic benefit of blockers that act at norepinephrine and/or serotonin transporters, such as desipramine and venlafaxine.
To investigate this possibility, we hypothesized that decynium-22 enhances desipramine- and venlafaxine-induced inhibition of norepinephrine clearance from extracellular fluid in dentate gyrus of hippocampus, a brain region with dense norepinephrine transporter expression (Swanson et al., 1987), and implicated in mediating antidepressant effects of drugs (Campbell and MacQueen, 2004; Malykhin and Coupland, 2015). Moreover, we hypothesized that this enhanced inhibition of norepinephrine clearance would be paralleled by decynium-22 enhancing the antidepressant-like effects of these drugs.
2. Materials and methods
2.1. Animals
Naïve adult male C57BL/6 mice obtained from our in-house breeding colony were used for all experiments. Previously we found no sex differences in the antidepressant-like effect of desipramine or the SSRI escitalopram in the tail suspension test, therefore only male mice were used (Mitchell et al., 2013). Experiments were conducted in mice between 3 and 10 months of age. Animals were housed in a temperature-controlled (24 °C) vivarium maintained on a 12/12-hr light/dark cycle (lights on at 7:00 a.m.) in plastic cages (29 cm × 18 cm × 13 cm) containing 7090 Teklad sani-chip bedding (Envigo, East Millstone, NJ). Animals were given free access to water and Teklad LM-485 mouse/rat sterilizable diet 7012 chow (Envigo, East Millstone, NJ). Mice were weaned at P21 and housed in groups of no more than five same sex peers. All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal, Resources, Commission of Life Sciences, National Research Council, https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf), and with the approval of the Institutional Animal Care and Use Committee, The University of Texas Health San Antonio (approval number: 20090119).
2.2. High-speed chronoamperometry
In vivo high-speed chronoamperometry experiments were conducted in adult male mice using methods adapted from Daws and Toney (2007; Baganz et al., 2008; Daws et al., 2016; Horton et al., 2013). In vivo high-speed chronoamperometry affords real-time, msec to msec, recording of monoamine clearance, thereby providing a readout of transporter efficiency. Carbon fiber electrodes were fabricated as described in Williams et al. (2007; Daws et al., 2016) based on published methods (Gerhardt, 1995; Perez and Andrews, 2005). Briefly, a single carbon fiber (30 μm diameter) was sealed within fused silica tubing (Schott, North America). Nafion coating (5% solution; Sigma-Aldrich, St. Louis, MO) was applied to carbon fiber electrodes to prevent the passage of anions in extracellular fluid to the carbon fiber electrode surface (Daws and Toney, 2007). Sensitivity to norepinephrine and ascorbic acid (Sigma-Aldrich, St. Louis, MO) were evaluated. Only electrodes displaying a selectivity ratio for norepinephrine over ascorbic acid greater than 100:1 and a linear response (r2 ≥ 0.9) to norepinephrine were used.
The recording assembly consisted of a Nafion-coated carbon-fiber electrode attached to a four-barreled micropipette (FHC, Bowdoin) such that their tips were separated by 200 μm. The barrels of the micropipette were filled with either norepinephrine (200 μM), decynium-22 (10 μM), desipramine (400 μM or 800 μM), venlafaxine (400 μM), a combination of decynium-22 and desipramine or venlafaxine, or phosphate-buffer saline (PBS) vehicle. The electrode assembly was lowered into the dentate gyrus of dorsal hippocampus (anteroposterior, −1.6 from bregma; mediolateral, +0.5 from midline; dorsoventral, −2.0 from dura) of an anesthetized mouse (Franklin and Paxinos, 1997). A mixture of alpha-chloralose (35 mg/kg) and urethane (350 mg/kg) given intraperitoneally (i.p.) at a volume of 0.25 ml was used to anesthetize mice in all experiments (Sigma-Aldrich, St. Louis, MO). Breathing was facilitated by a metal tube inserted into the trachea of anesthetized mice before mice were placed into a stereotaxic frame. Body temperature was maintained at 36–37 °C by a water circulated heating pad.
High-speed chronoamperometric recordings were made using a FAST-12 or FAST-16 system (Quanteon, Nicholasville, KY). Oxidation potentials consisted of 100-ms pulses of +0.55 V alternated with 900 ms intervals during which the resting potential was maintain at 0.0 V. The active electrode voltage was applied with respect to a sliver chloride reference electrode placed in the extracellular fluid of the superficial cortex. Oxidation and reduction currents were digitally integrated during the last 80 ms of each 100 ms voltage pulse.
Exogenous norepinephrine was applied into the dentate gyrus region of the hippocampus by pressure ejection. Once reproducible norepinephrine signals of ~0.75 μM were obtained, drug or vehicle was applied locally into the dentate gyrus. Norepinephrine was pressure ejected 7 min after drug or vehicle to allow sufficient time for drug to diffuse to the recording site and to limit electrode sensitivity degradation that can occur when utilizing norepinephrine transporter blockers for in vivo amperometry (Davidson et al., 2000). Norepinephrine was then applied at 5 min intervals until norepinephrine clearance time returned to pre-drug values. The T80 time course parameter, defined as the time for the signal to decline by 80% of the peak signal amplitude, and peak signal amplitude were analyzed.
An electrolytic lesion was made to mark the placement of electrode tip. Brains were removed, frozen, and stored at −80 °C for later histological analysis. At that time brains were thawed to −18 °C and sliced into 20-μm-thick sections and stained with thionin for histological verification of electrode location.
2.3. Tail suspension test
Tail suspension test experiments were carried out as described by Steru et al. (1985; for a review see Castagné et al., 2011). All experiments were conducted between 12:00 noon and 5:00 p.m., with a 1–2 h (h) acclimation period in the testing room before the first injection was administered. We first evaluated if decynium-22 could enhance the antidepressant-like effects of venlafaxine in the tail suspension test. In these experiments, all mice received either saline or decynium-22 at 0.1 or 0.32 mg/kg intraperitoneal (i.p.) 1 h before testing, followed 30 min later by an i.p. injection of either venlafaxine at 0.5, 1, 2, 4, or 8 mg/kg or saline (control condition). Drug concentrations for decynium-22 were based on previous studies showing that 0.1 and 0.32 mg/kg decynium-22 are behaviorally active doses in the tail suspension test when co-administered with the SSRI fluvoxamine (Horton et al., 2013).
In the second set of experiments, we investigated if decynium-22 (blocker of OCTs and PMAT) and normetanephrine, a potent blocker of OCT3, could enhance the antidepressant-like effect of desipramine. In these experiments, all mice received either saline, decynium-22 at 0.1 mg/kg or normetanephrine at 56 mg/kg i.p. 1 h before testing, followed 30 min later by an i.p. injection of desipramine at 0.32, 10, 32 mg/kg or saline (control condition). This drug administration protocol was selected in order to be consistent with our previously published procedure (Baganz et al., 2008; Horton et al., 2013; Mitchell et al., 2015 & 2017). The concentration of normetanephrine was chosen based on previous studies showing that 56 mg/kg normetanephrine is a behaviorally active dose in the tail suspension test when co-administered with desipramine (Rahman et al., 2008). In subsequent experiments, mice received decynium-22 at 0.32 mg/kg with 32 mg/kg desipramine. Neither decynium-22 at 0.1 or 0.32 mg/kg nor normetanephrine at 56 mg/kg alter locomotor behavior (data not shown, P > 0.05; Horton et al., 2013).
Before testing, an aluminum bar (2 × 0.3 × 10 cm) was fastened to the mouse tail using adhesive tape placed at a 90° angle to the long-itudinal axis of the tail, with 3–4 cm between the base of the tail and the bar. A hole in the bar, opposite the tail taped end, was then secured to a hook in the ceiling of a visually isolated box (40 × 40 × 40 cm). Mice were suspended by their tails for 6 min. The ventral surface and the front and hind limbs of the mouse were recorded using a digital video camera facing the suspension box. Immobility was defined as the absence of initiated movement, and included passive swaying. Total immobility was recorded in seconds (s) by observers blinded to the mouse treatment conditions. A mouse was excluded from the study if it climbed its tail and/or the aluminum bar for greater than or equal to 3 s. Mice were tested only once, and all mice were assigned randomly to treatment conditions.
We selected the tail suspension test, rather than (or in addition to) the forced swim test, for two reasons. First, using the same C57BL/6 mouse strain as in the present studies, we found the forced swim test to be relatively insensitive to detection of reduced time spent immobile after a range of desipramine doses (3.2–32 mg/kg), and insensitive to the same dose range of the SSRI, fluvoxamine (Koek et al., 2018). This contrasts to our findings using the tail suspension test where we detect robust antidepressant-like effects of desipramine and SSRIs (Baganz et al., 2008; Horton et al., 2013; Mitchell et al., 2015, 2016, 2017). Second, the tail suspension test was used in the studies of Rahman et al. (2008) who showed potentiation of the antidepressant-like effect of desipramine and venlafaxine by the uptake-2 blocker, normetanephrine.
2.4. Drugs
Decynium-22 [1,1′-Diethyl-2,2′cyanine iodide], venlafaxine [(+/−)-1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl cyclohexanol] hydrochloride, desipramine (desmethylimipramine) hydrochloride, and DL-normetanephrine (DL-α-[aminomethyl]-4-hydroxy-3-methoxybezenemethanol) hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in physiologic saline and injected i.p. at doses expressed as salt weight per kilogram body weight. The injection volume was 10 ml/kg.
It is worth emphasizing that at the very low doses of decynium-22 used in these studies, we are selectively targeting OCTs and PMAT. Decynium-22 has activity at the alpha-1 adrenoceptor, but at concentrations that greatly exceed those used here (Russ et al., 1996; and see Horton et al., 2013). Decynium-22 also has off-target effects that involve inhibition of dopaminergic and GABAergic synaptic transmission in the substantia nigra pars compacta and the spontaneous (pacemaker) activity of nigral neurons (Lloyd et al., 2019). However, the concentrations used in the Lloyd et al. study (25 μM) vastly exceed those used in our behavioral (0.1 and 0.32 mg/kg) and chronoamperometry (~0.05 μM) studies, and therefore are unlikely to play any role in the experimental outcomes described here. Note that although the barrel concentration of decynium-22 in these studies was 10 μM, there is an estimated 200-fold dilution in drug concentration before it reaches the recording electrode (Daws et al., 2016).
2.5. Data analysis
Data were analyzed by ANOVA and by multiple comparisons using Tukey’s test irrespective of the statistical significance of ANOVA outcomes, consistent with Hsu (1996; page 177) and Maxwell et al. (2018; page 260). Prism 6.0 (GraphPad, San Diego, CA) was used to perform statistical analysis.
2.5.1. High-speed chronoamperometry
The time to clear 80% of the peak norepinephrine signal (T80) in seconds, and norepinephrine signal amplitude were measured before and after drug application. Pre- and post-values were analyzed using paired t-tests (Table 1). The post-drug T80 and signal amplitude were then expressed as a percent change from baseline (pre-drug). Each parameter was analyzed using a two-factor ANOVA (desipramine or venlafaxine alone vs with decynium-22) followed by Tukey’s post hoc multiple comparisons test. Data are expressed as mean ± S.E.M. P < 0.05 was considered statistically significant.
Table 1.
Summary of pre- and post-norepinephrine signal amplitudes and time course (T80) for norepinephrine clearance following pressure-ejection of norepinephrine and treatments into dentate gyrus.
| Vehicle | Desipramine (54 pmol) | Venlafaxine (54 pmol) | Desipramine (108 pmol) | Vehicle | Desipramine (54 pmol) | Venlafaxine (54 pmol) | Desipramine (108 pmol) | ||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Vehicle | Vehicle | Vehicle | Decynium-22 | Decynium-22 | Decynium-22 | Decynium-22 | ||
| Ampl (μM) | Pre | 0.8 ± 0.06 | 0.7 ± 0.05 | 0.7 ± 0.06 | 0.8 ± 0.31 | 0.7 ± 0.03 | 0.7 ± 0.06 | 0.8 ± 0.11 | 0.8 ± 0.18 |
| Post | 0.8 ± 0.11 | 0.7 ± 0.09 | 0.6 ± 0.09 | 0.8 ± 0.14 | 0.6 ± 0.05 | 0.8 ± 0.11 | 0.9 ± 0.15 | 0.7 ± 0.21 | |
| T80 (s) | Pre | 110 ± 7.2 | 106 ± 8.5 | 102 ± 11.4 | 65 ± 13.9 | 116 ± 10.3 | 111 ± 15.8 | 100 ± 10.9 | 96 ± 29.9 |
| Post | 111 ± 9.9 | 118 ± 9.9 | 105 ± 12.3 | 142 ± 28.4a | 119 ± 12.8 | 143 ± 17.1a | 117 ± 13.1 | 322 ± 107b |
Pre- and post-drug values for peak signal amplitude, and clearance time after local application of vehicle (PBS), desipramine (54 and 108 pmol), decynium-22 (1.4 pmol), venlafaxine (54 pmol), or combination of decynium-22 given with desipramine or venlafaxine.
P < 0.05 represents post-drug values being significantly different from pre-drug values (paired t-test).
P = 0.06. Mean ± S.E.M.; n = 6–13 group.
2.5.2. Tail suspension test
In the first set of tail suspension test experiments, we evaluated the effect of 0.1 and 0.32 mg/kg decynium-22 on the antidepressant-like actions of venlafaxine. Dose-response data were analyzed using a 2-factor ANOVA (venlafaxine, decynium-22) followed by Tukey’s post hoc multiple comparisons test (Fig. 2A). Additionally, a one-factor ANOVA followed by Tukey’s post hoc multiple comparisons test was used to investigate if 0.1 or 0.32 mg/kg decynium-22 altered immobility time in the tail suspension test (Fig. 2B).
Fig. 2.
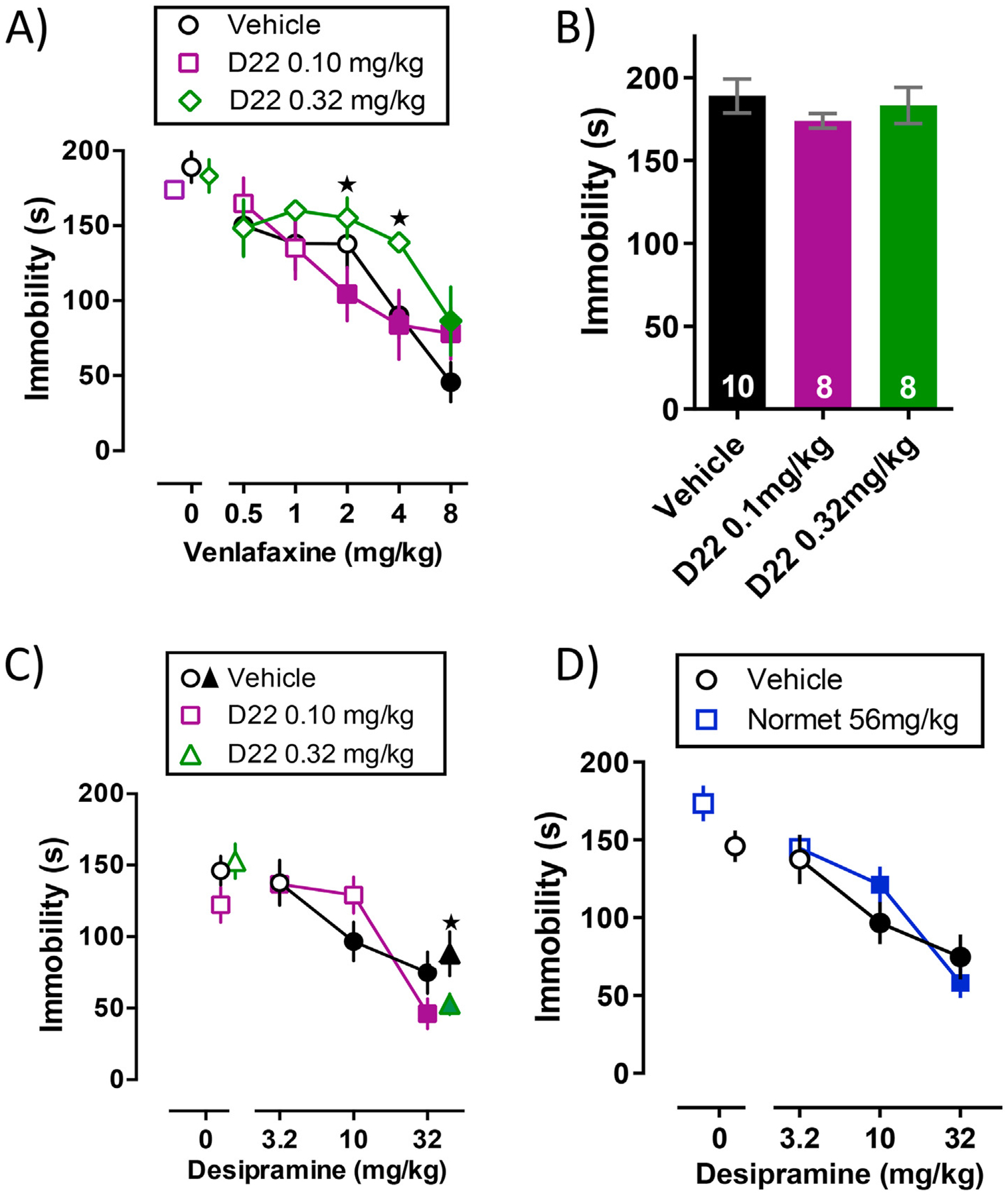
Antidepressant-like effects of desipramine, but not venlafaxine, were enhanced by co-administration of decynium-22. (A) Immobility time (s) in the tail suspension test after acutely administered venlafaxine in combination with 0.1 or 0.32 mg/kg decynium-22 (D22) or saline. Solid symbols represent a significant difference from saline with Tukey’s post hoc multiple comparisons test after a two-factor ANOVA (venlafaxine, decynium-22). *P < 0.05 represents 0.1 mg/kg decynium-22 significantly different from 0.32 mg/kg decynium-22; Tukey’s post hoc test for multiple comparisons. (B) Immobility time (s) in the tail suspension test after acute administration of vehicle, 0.1 or 0.32 mg/kg decynium-22. Decynium-22 does not affect immobility time when administered alone. (C) Immobility time (s) in the tail suspension test after acutely administered desipramine in combination with 0.1 mg/kg decynium-22 or saline. Solid circles and squares represent a significant difference from saline with Tukey’s post hoc multiple comparisons test after a two-factor ANOVA (desipramine, decynium-22). In separate experiments (results represented by triangles), immobility time in the tail suspension test was examined after acutely administered desipramine (32 mg/kg) in combination with 0.32 mg/kg decynium-22. *P < 0.05 represents 0.32 mg/kg decynium + 32 mg/kg desipramine significantly different from saline + 32 mg/kg desipramine, as determined by student’s t-test. (D) There is no difference in immobility time between mice given normetanephrine (normet) or vehicle in conjunction with desipramine. Data are mean ± S.E.M.; n = 8–12 per group.
Half-maximal effective dose (ED50) and maximal effect (Emax) for venlafaxine alone or in combination with 0.1 or 0.32 mg/kg decynium-22 were derived from data in Fig. 2A and are summarized in Table 2. ED50 values were calculated using methods detailed in Koek et al.(2009; Mitchell et al., 2017). F ratio tests in GraphPad Prism were used to compare the linear portion of log dose-response curves with respect to their slopes and intercepts. For example, a non-significant F ratio for slopes and a significant F ratio for intercepts show that dose-response curves are parallel but occupy different positions on the dose axis. Emax was defined as the greatest observed percent change of immobility from vehicle control. Emax values were analyzed with a one-factor ANOVA followed by Tukey’s post hoc multiple comparisons test (Table 2).
Table 2.
The potency (ED50) and maximal anti-immobility effects (Emax) for venlafaxine when administered alone or with decynium-22 in the tail suspension test.
| Vehicle | Decynium-22 (0.1 mg/kg) | Decynium-22 (0.32 mg/kg) | |
|---|---|---|---|
| Venlafaxine | |||
| ED50 (mg/kg) | 3.6 | 5.0 | 9.2a |
| Emax (% control) | 76 ± 7 | 55 ± 10 | 53 ± 12 |
Mean ± S.E.M.
P < 0.05 different from venlafaxine/vehicle group (F ratio test); n = 8–12.
In the second set of tail suspension test experiments, the effects of 0.1 mg/kg decynium-22 and 56 mg/kg normetanephrine on desipramine-induced antidepressant-like effects in the tail suspension test were evaluated in parallel with experiments evaluating the antidepressant-like effect of desipramine alone (Mitchell et al., 2015). Dose-response data were analyzed by 2-factor ANOVAs (desipramine, decynium-22; desipramine, normetanephrine). ED50 and Emax values for the antidepressant-like effects of desipramine alone (originally published in Mitchell et al., 2015) and co-administered with 0.1 mg/kg decynium-22 or co-administered with 56 mg/kg normetanephrine are summarized in Table 3. Student’s t-test was used to evaluate the difference in Emax values for desipramine alone and desipramine co-administered with 0.1 mg/kg decynium-22 or 56 mg/kg normetanephrine (Table 3).
Table 3.
The ED50 and Emax values for desipramine to reduce immobility time in the tail suspension test when administered alone or with the “uptake-2 blockers” decynium-22 or normetanephrine.
| Vehicle | Decynium-22 (0.1 mg/kg) | Normetanephrine (56 mg/kg) | |
|---|---|---|---|
| Desipramine | |||
| ED50 (mg/kg) | 23.0/22.7a | 28.1 | 16.5 |
| Emax (% control) | 48 ± 8/49 ± 10a | 62 ± 9 | 67 ± 6 |
Mean ± S.E.M.
Values for P90/P300 wild-type mice originally published in Mitchell et al. (2015). n = 8–10.
In a follow up tail suspension test study, student’s t-test was used to test the hypothesis that 0.32 mg/kg decynium-22 enhances the antidepressant-like effect of 32 mg/kg desipramine (Fig. 2C).
All data are expressed as mean ± S.E.M., except ED50 values, which are expressed as the mean. P < 0.05 was considered statistically significant.
3. Results
3.1. Intrahippocampal co-administration of decynium-22 with desipramine, but not venlafaxine, enhances inhibition of norepinephrine clearance from extracellular fluid of dentate gyrus
To test the hypothesis that decynium-22 enhances the inhibiting effect of norepinephrine transporter blockers on norepinephrine clearance, we used in vivo chronoamperometry according to well established protocols (Daws and Toney, 2007; Baganz et al., 2008, 2010; Horton et al., 2013; Daws et al., 2016). Clearance of locally applied norepinephrine from extracellular fluid in the dentate gyrus of anesthetized mice was compared before and after application of venlafaxine, desipramine, decynium-22, PBS, or decynium-22 in combination with venlafaxine or desipramine or PBS (Fig. 1A) to the dentate gyrus of hippocampus. In our initial studies (Experiment 1), 54 pmol of venlafaxine or desipramine was administered. This pmol amount typically does not cause significant inhibition of norepinephrine clearance, and was selected in order to determine if decynium-22 could bolster the actions of subthreshold amounts of desipramine and venlafaxine to inhibit norepinephrine clearance. Given that decynium-22 does not have activity at norepinephrine transporter (serotonin or dopamine transporters) (Fraser-Spears et al., 2019) any action of decynium-22 to enhance the ability of desipramine or venlafaxine to inhibit norepinephrine clearance would not be due to decynium-22 occupying spare norepinephrine transporter or serotonin/norepinephrine transporters, respectively. In subsequent experiments (Experiment 2), we increased the dose of desipramine to 108 pmol, an amount that robustly inhibits norepinephrine clearance. Unfortunately, higher concentrations of venlafaxine could not be tested using chronoamperometry due to interference with the recording properties of the carbon fiber electrode. The amount of decynium-22 applied was 1.4 pmol, which is the maximum that can be delivered without interfering with properties of the recording electrode (Baganz et al., 2008). Norepinephrine was pressure ejected into dentate gyrus to obtain reproducible signals of ~0.75 μM, then drug or vehicle was locally ejected into dentate gyrus followed 5 min later by ejection of norepinephrine.
Fig. 1.
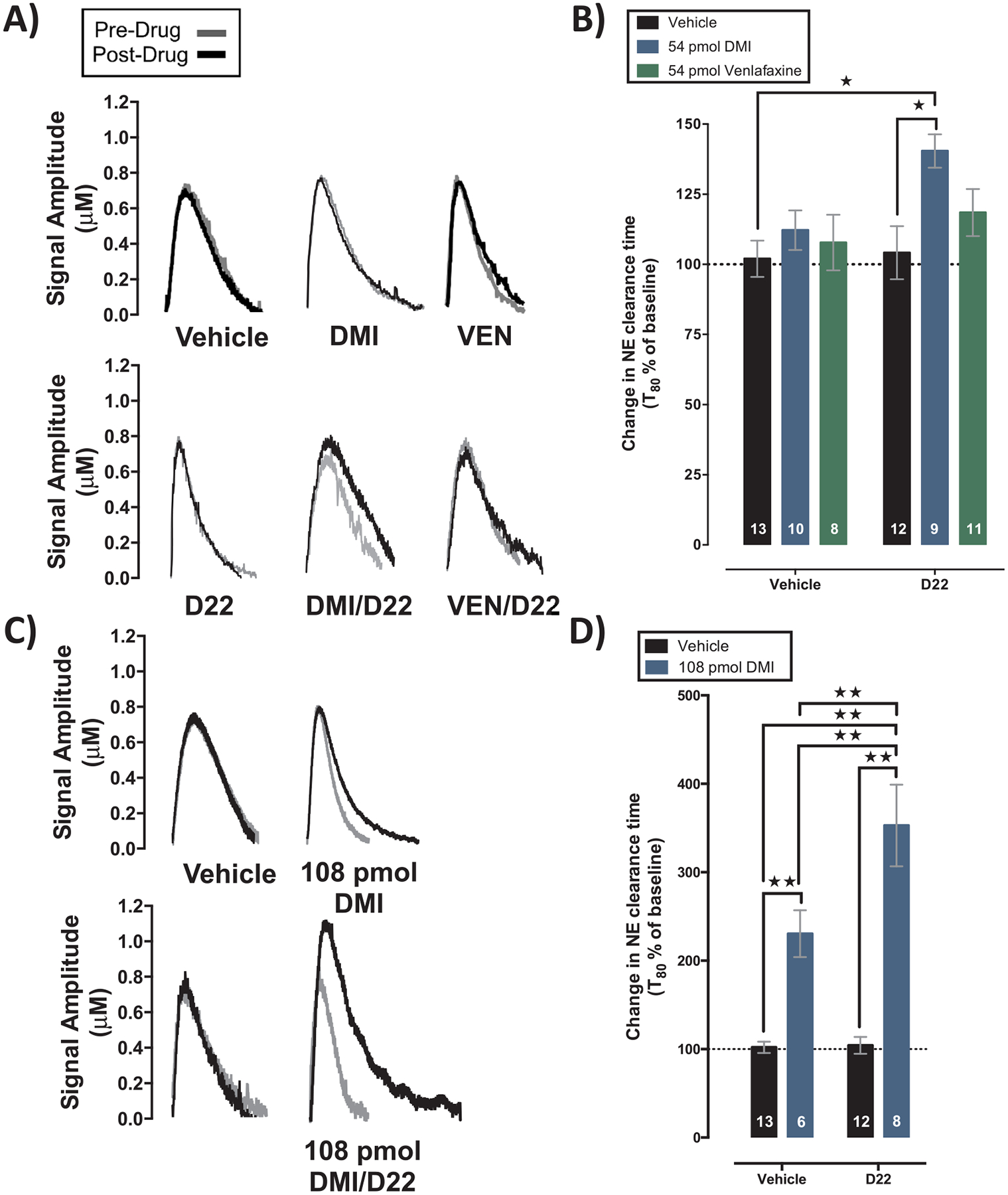
Decynium-22 enhances the ability of desipramine to inhibit norepinephrine clearance in dentate gyrus. (A and C) Representative oxidation currents produced by pressure-ejecting norepinephrine into dentate gyrus before (grey) and 7 min after (black) local application of vehicle (PBS), desipramine (DMI; 54 pmol and 108 pmol), venlafaxine (VEN; 54 pmol), decynium-22 (D22; 1.4 pmol), or decynium-22 given with desipramine or venflafaxine. Traces are superimposed for ease of comparison. (B and D) Change in norepinephrine clearance time (T80). Tukey’s multiple comparison tests following 2-way ANOVA show that (B) 54 pmol desipramine given with decynium-22 significantly inhibits norepinephrine clearance and (D) 108 pmol desipramine given with decynium-22 significantly inhibits norepinephrine clearance compared to vehicle, decynium-22, and 108 pmol desipramine; 108 pmol desipramine also significantly inhibits norepinephrine clearance compared to vehicle and decynium-22. *P < 0.05. **P < 0.01. Data are mean ± S.E.M.; n = 6–13 as depicted in the bars for each treatment group. Note the different scales on y-axis of B and D.
Experiment 1: Representative traces for vehicle, decynium-22 (1.4 pmol), desipramine (54 pmol), venlafaxine (54 pmol), and decynium-22/desipramine, decynium-22/venlafaxine cocktails are shown in Fig. 1A. Two-way ANOVA of the percent change in T80 clearance time from pre-drug values revealed a main effect of treatment to increase norepinephrine clearance time [F(2,57) = 11.97, P = 0.0185], with a main effect of decynium-22 to augment this effect [F(1,57) = 5.926, P = 0.044], and no statistically significant interaction [F (2,57) = 3.819, P = 0.264] (Fig. 1B). Post-hoc analysis showed that neither decynium-22 (P = 1.00), venlafaxine (P = 0.99), nor 54 pmol desipramine (P = 0.94) administered alone significantly increased T80 clearance time compared to vehicle control. Co-administration of decynium-22 with venlafaxine also failed to impact T80 clearance time relative to vehicle control (P = 0.64). In contrast, co-administration of decynium-22 with 54 pmol desipramine significantly increased T80 clearance time compared to vehicle control group (P = 0.015), and decynium-22 alone (P = 0.029), and approached statistical significance compared to venlafaxine alone (P = 0.12), and 54 pmol desipramine alone (P = 0.19). None of the treatments significantly influenced signal amplitude (see Table 1). Average signal amplitude and T80 values for all groups (inclusive of Experiment 2) before treatment were 0.74 ± 0.03 μM, and 109 ± 5 s respectively.
Experiment 2: Representative traces for vehicle, decynium-22 (1.4 pmol), desipramine (108 pmol), and decynium-22/desipramine cocktails are shown in Fig. 1C. Two-way ANOVA of the percent change in T80 clearance time from pre-drug values revealed a main effect of 108 pmol desipramine to increase norepinephrine clearance time [F (1,35) = 56.21, P < 0.0001], a main effect of decynium-22 to augment this effect [F(1,35) = 6.127, P = 0.009], and a significant interaction [F(1,35) = 5.708, P = 0.012] (Fig. 1D). Post-hoc analysis showed that administration of 108 pmol desipramine significantly increased T80 clearance time compared to vehicle control (P = 0.0025) and decynium-22 administered alone (P = 0.0034). Co-administration of decynium-22 with 108 pmol desipramine significantly increased T80 clearance time compared to vehicle control (P < 0.0001), decynium-22 administered alone (P < 0.0001), and 108 pmol desipramine administered alone (P = 0.0098). None of the treatments significantly increased signal amplitude (see Table 1). Average signal amplitude and T80 values for all groups before treatment were 0.74 ± 0.03 μM, and 102 ± 8 s respectively.
Thus, the key finding from these studies is that pmol amounts of desipramine, which alone did not inhibit norepinephrine clearance, do inhibit the time for norepinephrine to be cleared from extracellular space when given in combination with decynium-22. Similarly, the effect of desipramine given in pmol amounts that robustly inhibit norepinephrine clearance was potentiated by decynium-22. These findings are similar to our data showing that decynium-22 enhances the ability of the SSRI, fluvoxamine, to inhibit serotonin clearance from the CA3 region of hippocampus, and where we also found that decynium-22 enhances the ability of fluvoxamine to produce antidepressant-like effects (Horton et al., 2013). Assuming antidepressant-like behavior parallels inhibition of norepinephrine clearance in the hippocampus, our data suggest that decynium-22 should also enhance the antidepressant-like effects of desipramine, and perhaps venlafaxine. We tested this hypothesis by conducting dose-response analysis of the effects of decynium-22 co-administered with venlafaxine or desipramine on immobility time in the tail suspension test.
3.2. Blockade of decynium-22-sensitive transporters enhances the antidepressant-like effects of desipramine, but not venlafaxine, in the tail suspension test
As expected, systemic administration of venlafaxine dose-dependently reduced immobility time in the tail suspension test [F(5, 128) = 18.56, P < 0.0001] (Fig. 2A). In contrast to experiments with SSRIs, decynium-22 had either no effect (0.1 mg/kg decynium-22 dose) on the ability of venlafaxine to reduce immobility time or dampened (0.32 mg/kg decynium-22 dose) venlafaxine’s antidepressant-like effect [F(2, 128) = 3.49, P = 0.03]. There was no statistically significant interaction between the antidepressant-like effects of venlafaxine alone or with co-administration of decynium-22 [F(10, 128) = 1.16, P = 0.32]. Between treatment comparisons showed greater immobility in the tail suspension test when mice were treated with a combination of 0.32 mg/kg decynium-22 and either 2 or 4 mg/kg venlafaxine in comparison with mice treated with a combination of 0.1 mg/kg decynium-22 and either 2 or 4 mg/kg venlafaxine (P = 0.05, P = 0.03). The lowest effective dose was 4.0 mg/kg venlafaxine for saline treated mice, 2.0 mg/kg venlafaxine for mice treated with 0.1 mg/kg decynium-22, and 8 mg/kg venlafaxine for mice treated with 0.32 mg/kg decynium-22. Published (Horton et al., 2013) and preliminary data suggest decynium-22 doses < 0.32 mg/kg do not inhibit overall mobility. In the present study, immobility in mice treated with 0.1 and 0.32 mg/kg decynium-22 were compared with saline treated mice to confirm decynium-22 alone does not increase immobility time in the tail suspension test (Fig. 2B, without venlafaxine treatment condition). One-way ANOVA showed decynium-22 did not impact immobility in comparison with saline treated mice [F(2, 23) = 0.68, P = 0.52]. Tukey’s multiple comparisons test indicated that 0.1 mg/kg decynium-22 did not significantly change locomotor activity compared to saline (P = 0.49) and 0.32 mg/kg decynium-22 (P = 0.78). Similarly, 0.32 mg/kg decynium-22 did not statistically change locomotor activity compared to saline (P = 0.89). Therefore, the increased immobility time in the tail suspension test is likely due to the combined effects of decynium-22 (0.32 mg/kg) with venlafaxine.
ED50 and Emax values for venlafaxine to reduce immobility time in the tail suspension test with and without co-administration of decynium-22 are summarized in Table 2. The ED50 of venlafaxine to reduce immobility time in the tail suspension test was significantly increased by co-administration of decynium-22 [F(2,116) = 3.53, P = 0.03]. Among treatment comparisons showed co-administration of 0.32 mg/kg decynium-22, but not 0.1 mg/kg decynium-22, significantly reduced the potency of venlafaxine to reduce immobility in the tail suspension test (P < 0.01, P = 0.36; Table 2). Emax values for venlafaxine to reduce immobility time in the tail suspension test were unaltered by co-administration of decynium-22 at 0.1 or 0.32 mg/kg [F(2, 21) = 1.62, P = 0.22].
The antidepressant-like effects of venlafaxine were not enhanced by co-administration of 0.1 or 0.32 mg/kg decynium-22; however, studies have shown that the antidepressant-like effects of desipramine can be potentiated by 56 mg/kg of the OCT3 blocker normetanephrine (Rahman et al., 2008). We therefore examined whether 0.1 mg/kg decynium-22 or 56 mg/kg normetanephrine enhanced the antidepressant-like effects of 3.2, 10, and 32 mg/kg desipramine in the tail suspension test. Systemic administration of desipramine dose dependently decreased immobility time in the tail suspension test [F(3, 79) = 14.91, P < 0.0001]. There was no statistically significant interaction between antidepressant-like effects of desipramine alone or with co-administration of 0.1 mg/kg decynium-22 [F(3, 79) = 2.33, P = 0.0804]. We tested if 0.1 mg/kg decynium-22 or 56 mg/kg normetanephrine could enhance the antidepressant-like effects of 3.2, 10, and 32 mg/kg desipramine in the tail suspension test by comparing ED50 and Emax values with those from mice treated with desipramine alone (Table 3 and Fig. 2C and 2D) [desipramine/vehicle data originally published in Mitchell et al. (2015) with permission, and carried out concurrently with these studies]. ED50 values were unaltered by co-administration of 0.1 mg/kg decynium-22 [F(1,51) = 0.29, P = 0.59] or 56 mg/kg normetanephrine [F(1,56) = 1.37, P = 0.25] in comparison to desipramine alone. Similarly, Emax values were not altered by co-administration of 0.1 mg/kg decynium-22 (P = 0.32) or 56 mg/kg normetanephrine (P = 0.17) compared with desipramine.
In an effort to compare the effects of decynium-22 on venlafaxine and desipramine at the same doses of decynium-22, we examined 32 mg/kg desipramine in combination with 0.32 mg/kg decynium-22 in a separate cohort of mice. We found that immobility time following either decynium-22 (0.32 mg/kg) or desipramine injection was in line with our first experiments (for decynium-22, compare Fig. 2B and 2C), adding to the reproducibility of these data (triangle symbols in Fig. 2C). However, unlike 0.1 mg/kg decynium-22 in combination with 32 mg/kg desipramine, 0.32 mg/kg decynium-22 in combination with 32 mg/kg desipramine significantly reduced immobility time compared to mice treated with 32 mg/kg desipramine and saline alone [t (17) = 2.134, P = 0.04]. These findings support the notion that blockade of OCTs/PMAT can enhance the antidepressant-like effects of at least some drugs.
4. Discussion
Major findings from the current study are that decynium-22 enhances the ability of desipramine to inhibit norepinephrine clearance in dentate gyrus (at either a subthreshold pmol amount of desipramine, or an amount that robustly inhibits norepinephrine clearance) (Fig. 1). This result maps onto decynium-22 (0.32 mg/kg) enhancing the antidepressant-like effect of desipramine (32 mg/kg) in the tail suspension test (Fig. 2C). In contrast, co-administration of decynium-22 with a subthreshold concentration of venlafaxine into dentate gyrus did not significantly inhibit norepinephrine clearance, nor did systemically administered 0.1 mg/kg decynium-22 potentiate the antidepressant-like effects of venlafaxine, while 0.32 mg/kg decynium-22 reduced the potency of venlafaxine to produce antidepressant-like effects in the tail suspension test (Fig. 2A and Table 2). Together with our earlier findings that decynium-22 augments the ability of fluvoxamine to inhibit serotonin clearance and produce antidepressant-like effects (Baganz et al., 2008; Horton et al., 2013), these results suggest that concurrent blockade of OCTs and/or PMAT is a potential means to increase therapeutic efficacy of at least some antidepressant drugs.
Clinically there is a 2–6 week refractory period before onset of therapeutic effects following treatment with tricyclic antidepressants, as well as other monoamine transporter blockers including SNRIs (Katz et al., 2004; Gartlehner et al., 2008; Martiny et al., 2012). While the reason for this delay is not yet fully understood, it has been hypothesized that the increase in extracellular norepinephrine following treatment with tricyclic antidepressants is undermined by uptake-2 transporters, which clear norepinephrine away from the extracellular space before a therapeutic concentration of norepinephrine is reached (Iversen, 1965; Hendley et al., 1970; Koepsell, 2004; Schildkraut and Mooney, 2004). Levels of normetanephrine, a potent OCT3 blocker and metabolite of norepinephrine, have been shown to accumulate with tricyclic antidepressant treatment; however, this process can take 2–6 weeks (Mooney et al., 2008). Once normetanephrine levels are high enough to block OCT3 then norepinephrine levels can rise to sustain a therapeutic concentration (Schildkraut and Mooney, 2004). Hypothetically, normetanephrine could enhance the acute antidepressant effects of norepinephrine transporter blockers if the two were co-administered. Consistent with this idea, Rahman et al. (2008) showed that normetanephrine potentiated the venlafaxine-induced increase of extracellular norepinephrine in frontal cortex of rats and enhanced the antidepressant-like effect of 10 mg/kg desipramine in the tail suspension test in mice. Our neurochemical data showing that decynium-22 enhanced the ability of desipramine to inhibit norepinephrine clearance in dentate gyrus (Fig. I.B and Table 1), and enhanced the antidepressant-like effects of desipramine (32 mg/kg) (Fig. 2C) are consistent with their findings. In contrast, under the present conditions decynium-22 failed to augment the ability of venlafaxine to inhibit norepinephrine clearance in dentate gyrus (Fig. 1B and Table 1). This may seem at odds with findings of Rahman and co-workers, where normetanephrine robustly potentiated venlafaxine-induced increases in extracellular norepinephrine in frontal cortex; however, there are several factors potentially contributing to this apparent discrepancy, including route of administration (intra hippocampal vs intraperitoneal), brain region (dentate gyrus vs frontal cortex), species (mouse vs rat), assay (chronoamperometry vs microdialysis) and time of measurement (min vs h). Thus, it cannot be ruled out that under different conditions, we might detect enhancement of venlafaxine-induced inhibition of norepinephrine clearance. For example, it is possible that a higher concentration of venlafaxine, given in concert with decynium-22, may reveal enhanced inhibition of norepinephrine clearance in dentate gyrus (as trends are evident, Fig. 1B). Unfortunately, higher concentrations of venlafaxine and decynium-22 could not be tested using chronoamperometry due to interference with the recording properties of the carbon fiber electrode. Desipramine is a more potent blocker of norepinephrine transporter than venlafaxine (Tatsumi et al., 1997), which may explain why we were able to detect potentiation of desipramine-induced inhibition of norepinephrine clearance by decynium-22, but not that of venlafaxine.
Regardless of neurochemical results, striking was our finding that in spite of rigorous dose-response analysis, we were unable to detect enhancing effects of decynium-22 on the antidepressant-like effects of venlafaxine (Fig. 2A, Tables 2 and 3). Rahman et al. (2008) did not examine the ability of normetanephrine to augment the antidepressant-like effects of venlafaxine, so it is difficult to generalize. However, our findings underscore the heterogeneous response of mice to antidepressants, including those that share similar mechanisms of actions.
The inability of decynium-22 to augment the antidepressant-like effect of venlafaxine was unexpected given that venlafaxine also blocks the serotonin transporter and we have previously shown marked effects of decynium-22 to enhance the antidepressant-like effects of the SSRI fluvoxamine (Horton et al., 2013). Preliminary data have shown that decynium-22 can reduce locomotor behavior, possibly through ɑ1-adrenergic antagonism, which would increase immobile behavior in the tail suspension test and may appear as a reduction in venlafaxine potency (Horton et al., 2013). However, this is unlikely because neither 0.1 nor 0.32 mg/kg decynium-22 alone increased immobility time in the tail suspension test (Fig. 2B). Venlafaxine has a higher affinity for the serotonin transporter compared to the norepinephrine transporter, while desipramine is relatively selective for the norepinephrine transporter (Owens et al., 1997). Therefore, outcomes may be a result of potency differences at the two transporters or a more complex interplay between norepinephrine and serotonin systems in producing antidepressant-like effects than previously thought.
In separate experiments, we examine the effect of normetanephrine (56 mg/kg) on the antidepressant-like effect of desipramine (3.2, 10 and 32 mg/kg) in the tail suspension test. Unlike Rahman et al. (2008), we found no evidence for normetanephrine to enhance the antidepressant-like effect of desipramine; however, variation in tail suspension test protocols used should be noted. Rahman et al. (2008) used Swiss Webster mice, a strain gauge to measure immobility, and tail suspension began 60 min (min) after desipramine administration. Experiments in the current study used C57BL/6 mice, an observer blinded to treatment scored immobility from a recording, and tail suspension began 30 min after desipramine administration. Factors such as these are known to influence results obtained using the tail suspension test and may explain the discrepancies between our data and that reported by Rahman and co-workers (2008; Cryan et al., 2005). Nonetheless, our data do not support a role for concurrent OCT3 blockade with normetanephrine enhancing the antidepressant-like response to desipramine. Notwithstanding, the possibility remains that drugs such as normetanephrine and decynium-22 may shorten the latency to therapeutic benefit (Schildkraut and Mooney, 2004). To this end, studies utilizing behavioral paradigms sensitive to chronic antidepressant administration, such as novelty induced hypophagia (Dulawa and Hen, 2005), will be important avenues for future investigation.
5. Conclusions
The OCT/PMAT blocker decynium-22 enhanced the ability of desipramine to inhibit norepinephrine clearance in dentate gyrus and produce antidepressant-like effects in the tail suspension test. Normetanephrine, a potent OCT3 blocker, failed to augment the antidepressant-like effects of desipramine, suggesting that the ability of decynium-22 to do so is likely mediated by other OCT isoforms (OCT1 or OCT2) or PMAT. However, given reports of normetanephrine enhancing the antidepressant-like effect of desipramine under different conditions (Rahman et al., 2008), a role for OCT3 cannot be ruled out. Interestingly, decynium-22 did not enhance the ability of venlafaxine to inhibit norepinephrine clearance or produce antidepressant-like effects, underscoring the heterogeneous responses of mice to antidepressants that share common mechanisms of action. Taken together with existing literature (Schildkraut and Mooney, 2004; Baganz et al., 2008; Rahman et al., 2008; Bacq et al., 2012; Daws et al., 2013; Horton et al., 2013; Krause-Heuer et al., 2017; Orrico-Sanchez et al., 2019), concurrent blockade of OCTs and/or PMAT remains a promising line of investigation for enhancing the therapeutic effects of at least some antidepressant drugs. Ultimately, future neurochemical and behavioral experiments will help identify which monoamine transporters and drug combinations should be targeted to produce the greatest therapeutic effects.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Ms. Melissa Vitela and Ms. Myrna Herrera-Rosales. Some data from this manuscript are reprinted in a thesis titled “Mechanisms contributing to lack of antidepressant efficacy in juveniles and adolescents: Discovering novel targets for improved therapeutics” (Publication date 6/2016). Reprint request for thesis will be by Nathan Mitchell, nathan.c.mitchell@gmail.com, 7703 Floyd Curl Drive, San Antonio, TX, 78229-3900, USA.
Funding
This work was supported by the National Institutes of Health R01 MH093320 to LCD and WK and R01 MH106978 to LCD. MAB was supported in part by a training grant [T32 NS082145, PI David Morilak].
Footnotes
CRediT authorship contribution statement
Melodi A. Bowman: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Nathan C. Mitchell: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization. W. Anthony Owens: Investigation. Rebecca E. Horton: Investigation. Wouter Koek: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Supervision. Lynette C. Daws: Conceptualization, Methodology, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, Louis F, Vialou V, Martres M-P, Chevarin C, Hamon M, Giros B, Gautron S, 2012. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol. Psychiatr 17, 926–939. [DOI] [PubMed] [Google Scholar]
- Baganz N, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC, 2008. Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc. Natl. Acad. Sci. U.S.A 105, 18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz N, Horton RE, Martin KP, Holmes A, Daws LC, 2010. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: Organic cation transporter 3, the smoking gun. Journal of Neuroscience 30 (45), 15185–15195. 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, MacQueen G, 2004. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci 29 (6), 417–426. [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD, 2011. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci Chapter 8, Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A, 2005. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev 29, 571–625. [DOI] [PubMed] [Google Scholar]
- Davidson C, Ellinwood EH, Douglas SB, Lee TH, 2000. Effect of cocaine, nomifensine, GBR 12909, and WIN 35428 on carbon fiber microelectrode sensitivity for voltammetric recording of dopamine. J. Neurosci. Methods 101, 75–83. [DOI] [PubMed] [Google Scholar]
- Daws LC, Koek W, Mitchell NC, 2013. Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders. ACS Chem. Neurosci 4, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Owens WA, Toney GM, 2016. High-speed chronoamperometry to measure biogenic amine release and uptake in vivo. In: Sitte, Harald, Bonisch, Heinz(Eds.), Neurotransmitter Transporters – Investigative Methods. Humana Press, pp. 53–81. [Google Scholar]
- Daws LC, Toney GM, 2007. In: Borland LM (Ed.), High-speed Chronoamperometry to Study Kinetics and Mechanism for Neuroscience (Michael AC. Taylor and Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Duan H, Wang J, 2010. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Therapeut 335, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R, 2005. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev 29, 771–783. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 1997. The Mouse Brain in Stereotaxic Coordinates. Academic Press, San Diego, California. [Google Scholar]
- Fraser-Spears R, Krause-Heuer AM, Basiouny M, Mayer FP, Manishimwe R, Wyatt NA, et al. , 2019. Comparative analysis of novel decynium-22 analogs to inhibit transport by the low-affinity, high-capacity biogenic amine transporters, organic cation transporters 2 and 3, and plasma membrane monoamine transporter. Eur. J. Pharmacol 842, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, 1997. Pharmacology of antidepressants. J. Clin. Psychopharmacol 17, 1–18 [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN, 2008. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann. Intern. Med 149, 734–750. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, 1995. Neuromethods: Voltammetry Methods in Brain Systems. In: In: Boulton AA, Baker GB, Adams RN (Eds.), Rapid Chronocoulometric Measurements of Norepinephrine Overflow and Clearance in CNS Tissues, vol. 27. Humana, Totowa, NJ, pp. 117–151. [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robbles MA, Vranijkovic O, Wheeler DS, Mantsch JR, Gasser PJ, 2013. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci 33, 11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendley ED, Taylor KM, Snyder SH, 1970. 3H-normetanephrine uptake in rat brain slices. Relationship to extraneuronal accumulation of norepinephrine. Eur. J. Pharmacol 12, 167–179. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Ownes WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC, 2013. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J. Neurosci 33, 10534–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, 1996. Multiple comparisons. In: Theory and Methods. Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- Iversen LL, 1965. The uptake of adrenaline by the rat isolated heart. Br. J. Pharmacol. Chemother 24, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A, 2004. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology 29, 566–579. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT, 2008. Initial severity and antidepressant benefits: meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP, 2009. Behavioral effects of γ-hydro-xybutyrate, its precursor γ-butyrolactone, and GABAB receptor agonists: time course and differential antagonism by the GABAB receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J. Pharmacol. Exp. Therapeut 330, 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Sandoval TL, Daws LC, 2018. Effects of the antidepressants desipramine and fluvoxamine on latency to immobility and duration of immobility in the forced swim test in adult male C57BL/6J mice. Behav. Pharmacol 29, 453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, 2004. Polyspecific organic cation transporters: their function and interactions with drugs. Trends Pharmacol. Sci 25, 375–381. [DOI] [PubMed] [Google Scholar]
- Krause-Heuer AM, Fraser-Spears R, Dobrowolski JC, Ashford ME, Wyatt NA, Roberts MP, Gould GG, Cheah WC, Ng CKL, Bhadbhade M, Zhang B, Greguric I, Wheate NJ, Kumar N, Koek W, Callaghan PD, Daws LC, Fraser BH, 2017. Evaluation of the antidepressant therapeutic potential of isocyanine and pseudoisocyanine analogues of the organic cation decynium-22. Eur. J. Med. Chem 137, 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JT, Martini A, McDouall A, Sood R, Freestone PS, Mercuri NB, Lipski J, 2019. Electrophysiological characterization of novel effects of the uptake-2 blocker decynium-22 (D-22) on dopaminergic neurons in the substantia nigra pars compacta. Neuroscience 396, 154–165. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Coupland NJ, 2015. Hippocampal neuroplasticity in major depressive disorder. Neuroscience 309, 200–213. [DOI] [PubMed] [Google Scholar]
- Martel F, Ribeiro L, Calhau C, Azevedo I, 1999. Comparison between uptake2 and rOCT1: effects of catecholamines, metanephrines and corticosterone. Naunyn-Schmiedeberg’s Arch. Pharmacol 359, 303–309. [DOI] [PubMed] [Google Scholar]
- Martiny K, Lunde M, Bech P, Plenge P, 2012. A short-term double-blind randomized controlled pilot trial with active or placebo pindolol in patients treated with venlafaxine for major depression. Nord. J. Psychiatr 66, 147–154. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD, Kelley K, 2018. Designing experiments and analyzing data. In: A Model Comparison Perspective, third ed. Routledge, New York. [Google Scholar]
- Mitchell NC, Gould GG, Smolik CM, Koek W, Daws LC, 2013. Antidepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: relationship with hippocampal serotonin and norepinephrine transporter expression and function. Front. Pharmacol 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Koek W, Daws LC, 2015. Antidepressant-like effects and basal immobility depend on age and serotonin transporter genotype. Gene Brain Behav. 14, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Owens WA, Vitela M, Gould G, Koek W, Daws LC, 2016. Ontogeny of SERT expression and antidepressant-like response to escitalopram in wild-type and SERT mutant mice. J. Pharmacol. Exp. Therapeut 358, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Bowman MA, Gould GG, Koek W, Daws LC, 2017. Ontogeny of norepinephrine transporter expression and antidepressant-like response to desipramine in wild-type and serotonin transporter mutant mice. J. Pharmacol. Exp. Therapeut 360, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney JJ, Samson JA, Hennen J, Pappalardo K, McHale N, Alpert J, Koutsos M, Schildkraut JJ, 2008. Enhanced norepinephrine output during long-term desipramine treatment: a possible role for the extraneuronal monoamine transporter (SLC22A3). J. Psychiatr. Res 42, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrico-Sanchez A, Chausset-Boissarie L, Alves de Sousa R, Coutens B, Rezai Amin S, Vialou V, Louis F, Hessani A, Dansette PM, Zornoza T, Gruszczynski C, Giros B, Guiard BP, Acher F, Pietrancosta N, Gautron S, 2019. Antidepressant efficacy of a selective organic cation transporter blocker in a mouse model of depression. Mol. Psychiatr 10.1038/s41380-019-0548-4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB, 1997. Neurotransmitter receptor and transporter binding profile of antidepressant and their metabolites. J. Pharmacol. Exp. Therapeut 283, 1305–1322. [PubMed] [Google Scholar]
- Perez XA, Andrews AM, 2005. Chronoamperometry to determine differential reductions in uptake in brain synaptosomes from serotonin transporter knockout mice. Anal. Chem 77, 818–826. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Ring RH, Young K, Platt B, Lin Q, Schechter LE, Rosenzweig-Lipson S, Beyer CE, 2008. Inhibition of uptake 2 (or extraneuronal monoamine transporter) by normetanephrine potentiates the neurochemical effects of venlafaxine. Brain Res. 1203, 68–78. [DOI] [PubMed] [Google Scholar]
- Russ H, Friedgen B, Königs B, Schumacher C, Graefe KH, Schömig E, 1996. Pharmacokinetic and α1-adrenoceptor antagonistic properties of two cyanine-type inhibitors of extraneuronal monoamine transport. Naunyn-Schmiedeberg’s Arch. Pharmacol 354, 268–274. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ, Mooney JJ, 2004. Toward a rapidly acting antidepressant: the normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. Am. J. Psychiatr 161, 909–911. [DOI] [PubMed] [Google Scholar]
- Sinyor M, Schaffer A, Levitt A, 2010. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can. J. Psychiatr 55, 126–135. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P, 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85, 367–370. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C, Bjorklund A, et al. , 1987. The limbic system. I. The septohippocampal system. Handbook of Chemical Neuroanatomy, vol. 5: Integrated Systems of the CNS, Part I. 5. Elsevier, Amsterdam, pp. 160–165. [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E, 1997. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol 340, 249–258. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A, 2007. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 5, e274. [DOI] [PMC free article] [PubMed] [Google Scholar]


