Abstract
The 2018 outbreak of myxomatosis in the Iberian hare (Lepus granatensis) has been hypothesized to originate from a species jump of the rabbit‐associated myxoma virus (MYXV), after natural recombination with an unknown poxvirus. Iberian hares were long considered resistant to myxomatosis as no prior outbreaks were reported. To provide insights into the emergence of this recombinant virus (ha‐MYXV), we investigated serum samples from 451 Iberian hares collected over two time periods almost two decades apart, 1994–1999 and 2017–2019 for the presence of antibodies and MYXV‐DNA. First, we screened all serum samples using a rabbit commercial indirect ELISA (iELISA) and then tested a subset of these samples in parallel using indirect immunofluorescence test (IFT), competitive ELISA (cELISA) and qPCR targeting M000.5L/R gene conserved in MYXV and ha‐MYXV. The cut‐off of iELISA relative index 10 = 6.1 was selected from a semiparametric finite mixture analysis aiming to minimize the probability of false positive results. Overall, MYXV related‐antibodies were detected in 57 hares (12.6%) including 38 apparently healthy hares (n = 10, sampled in 1994–1999, none MYXV‐DNA positive, and n = 28 sampled in 2017–2019 of which four were also ha‐MYXV‐DNA positive) and 19 found‐dead and ha‐MYXV‐DNA‐positive sampled in 2018–2019. Interestingly, four seronegative hares sampled in 1997 were MYXV‐DNA positive by qPCR, the result being confirmed by sequencing of three of them. For the Iberian hares hunted or live trapped (both apparently health), seroprevalence was significantly higher in 2017–2019 (13.0%, CI95% 9.2–18.2%) than in 1994–1999 (5.4%, CI95% 3.0–9.6%) (p = .009). Within the second period, seroprevalence was significantly higher in 2019 compared to 2017 (24.7 vs 1.7% considering all the sample, p = .007), and lower during the winter than the autumn (p < .001). While our molecular and serological results show that Iberian hares have been in contact with MYXV or an antigenically similar virus at least since 1996, they also show an increase in seroprevalence in 2018–2019. The remote contact with MYXV may have occurred with strains that circulated in rabbits, or with unnoticed strains already circulating in Iberian hare populations. This work strongly suggests the infection of Iberian hares with MYXV or an antigenically related virus, at least 20 years before the severe virus outbreaks were registered in 2018.
Keywords: emerging disease, myxomatosis, myxoma virus, lagomorph diseases, hare diseases, ha‐MYXV, recombination, Lepus granatensis, Iberian hare, qPCR, ELISA, Iberian Peninsula
1. INTRODUCTION
Myxomatosis is caused by infection with myxoma virus (MYXV), belonging to genus Leporipoxvirus from subfamily Chordopoxvirinae and family Poxviridae. MYXV has a 163 kbp‐long dsDNA genome, which replicates in the cytoplasm of the infected cells (Murphy et al., 1995) and is transmitted mainly by biting arthropods or direct contact with infected animals (Mead‐Briggs & Vaughan, 1975). The main vectors belong to families Culicidae and Simuliidae and order Siphonaptera, while lice, ticks and mites play a less important role in transmission (Bertagnoli & Marchandeau, 2015).
In the European rabbit (Oryctolagus cuniculus), MYXV generally induces a severe, often fatal, generalized disease characterized by a swollen head, eyelids and ears, blepharoconjunctivitis with mucopurulent ocular and nasal discharge, cutaneous lesions and inflammation and oedema of the genitalia and perianal skin (Bertagnoli & Marchandeau, 2015; Kerr et al., 2015). Since its intentional introduction in Europe in 1952, myxomatosis became endemic in European rabbit populations (Bertagnoli & Marchandeau, 2015; Villafuerte et al., 2017a). According to the case fatality rate, average survival time and clinical signs induced in rabbits, the MYXV strains can be classified in I (higher) to V (lower) virulence grades (Kerr & Best, 1998).
Until recently, the European rabbit was the only Iberian lagomorph considered highly susceptible to infection by the MYXV. While sporadic cases of myxomatosis have been reported in brown hares Lepus europaeus (Jacotot et al., 1954; Magallon et al., 1953; Barlow et al., 2014), the endemic and declining Iberian hare (Lepus granatensis) (Carro & Soriguer, 2017) was considered resistant to MYXV infection, with no confirmed cases until 2018, when a large outbreak was reported in southwestern Spain (García‐Bocanegra et al., 2019). This outbreak recurred in subsequent years and has been spreading throughout the Iberian Peninsula (Duarte et al., 2021; García‐Bocanegra et al., 2020; MAPA, 2020).
A novel strain of MYXV was genotyped from dead Iberian hares during the 2018 outbreak (Carvalho et al., 2020; Dalton et al., 2019; Pinto et al., 2019). These studies demonstrated a natural recombinant virus (ha‐MYXV), which has additional genetic material from an unknown poxvirus (mainly a ∼2.8 kbp insertion disrupting m009L gene), hypothesized to have allowed the species jump event and/or increased virulence for Iberian hares (Dalton et al., 2019; Pinto et al., 2019). Although ha‐MYXV DNA was detected in a few wild and domestic rabbits (Abade dos Santos, 2020, 2020), the rarity of detection of ha‐MYXV in rabbits supports the apparent preferential circulation of MYXV and ha‐MYXV in rabbits and hares, respectively. The ha‐MYXV, either isolated from Iberian hare or European rabbit, proved to be pathogenic for the wild rabbit (Abade dos Santos et al., 2022b). The susceptibility of hares to MYXV classic strains infection has not been investigated, although it was shown in vitro that it cannot infect and replicate in hare cell cultures (Águeda‐Pinto et al., 2022). However, classic MYXV was recently associated with cases of co‐infection with ha‐MYXV in Iberian hare and wild rabbit, demonstrating that under certain circumstances, it can systemically infect the Iberian hare (Abade dos Santos et al., 2022a).
This study aims to provide insights into the emergence of myxomatosis in the Iberian hare, by assessing the presence of antibodies against MYXV antigens and MYXV‐DNA in specimens from Spain and Portugal, before and during the outbreaks reported in 2018.
To accomplish that, we performed a serological and molecular survey of myxoma or antigenically related viruses in samples collected in two different periods, over 20 years apart (1994–2019) to investigate the putative contact of Iberian hare populations with MYXV or MYXV‐like viruses in the past, and encompassing the ha‐MYXV emergence recorded in 2018. We started by screening all serum samples with a commercial rabbit indirect ELISA (iELISA), and then tested a subset of positive and negative samples with indirect immunofluorescence test (IFT), competitive ELISA (cELISA) and qPCR. Our aim was to explore uncovered clues about the past circulation of the virus in hares and the recent emergence of highly pathogenic ha‐MYXV in Iberian hares. Given that the origin of the ha‐MYXV is yet to be explained, several hypotheses are put forward in this manuscript, along with those considered in the past.
2. MATERIAL AND METHODS
2.1. Sample collection and serum preparation
Blood samples were collected from 451 Iberian hares, mostly from the central‐south‐western Iberian Peninsula, coinciding with regions that were covered by research studies in Portugal and Spain, and where the recent myxomatosis infections have been detected in this species. Iberian hares included in the study were either live captured with long nets (apparently healthy, n = 88), or post‐mortem, either hunted (apparently healthy, n = 313) or found dead in the field (presumably sick, n = 50). Live captures and hunting samples were considered in this study as random sampling, contrary to the animals found dead in the field, presumably sick. The number of samples from each sampling period was: 186 from 1994 to 1999 (random sampling only); and 265 from 2017 to 2019 (random sample n = 215, found dead, n = 50) (Table 1). The samples from 2018 and 2019 were collected after the emergence of myxomatosis (middle and late 2018 for Spain and Portugal, respectively). Hares were hunted according to the Spanish, Portuguese and European legislation. Thus, no animal was sacrificed for this study.
TABLE 1.
Sex and age classes descriptive statistics of the Iberian hares analyzed in this study. Number of hares analyzed in 1994–1999 and 2017–2019, by sex (F, female; M, male) and age classes (J, juveniles; A, adults). ND not determined
| Sampling methods | Total | 1994–1999 | 2017–2019 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | ND | J | A | ND | F | M | ND | J | A | ND | |||
| Live | Nets | 88 | 24 | 40 | 0 | 15 | 49 | 0 | 12 | 12 | 0 | 4 | 20 | 0 |
| Post‐mortem | Hunted | 313 | 3 | 4 | 115 | 0 | 7 | 115 | 107 | 80 | 4 | 16 | 173 | 2 |
| Found dead | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 22 | 8 | 5 | 37 | 8 | |
| Total | 451 | 27 | 44 | 115 | 15 | 56 | 115 | 139 | 114 | 12 | 25 | 230 | 10 | |
| Total | 1994 | 1996 | 1997 | 1998 | 1999 | 2017 | 2018 | 2019 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Live | Nets | 88 | 0 | 0 | 25 | 22 | 17 | 0 | 0 | 24 |
| Post‐mortem | Hunted | 313 | 30 | 22 | 37 | 0 | 33 | 60 | 90 | 41 |
| Found dead | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 20 | |
| Total | 451 | 30 | 22 | 62 | 22 | 50 | 60 | 120 | 85 |
Blood collection from live hares was obtained by venipuncture of the marginal ear vein or the external jugular vein (Abade dos Santos et al., 2019) and from dead animals by cardiac puncture. Blood was transferred into plain tubes, centrifuged for 10 min at 1500g at 4°C and the serum was preserved at −20°C until analysis. In the case of found‐dead hares, as blood was coagulated, the sample used was sera collected from the heart and large vessels, often haemolyzed.
2.2. Indirect ELISA
Antibodies specific for MYXV were detected by a commercial iELISA (Civtest® Cuni Mixomatosis, Hipra, Girona, Spain), following the manufacturer's instructions. The protein A‐horseradish peroxidase provided with the kit was used as conjugate to detect the IgG isotype. Briefly, serum samples were added to the plate (100 μl/well) at a dilution of 1:40 in dilution solution (according to manufacturer indication) and incubated for 20 min at 37°C. After three washes with 300 μl washing solution/well, protein A‐horseradish peroxidase conjugate was added (100 μl/well) and incubated for 20 min at 37°C. The plate was washed three times and 3,3′,5,5′‐tetramethylbenzidine was added (100 μl/well) as liquid substrate and incubated for 10 min at room temperature. The reaction was stopped with 100 μl/well of H2SO4 and the optical density (OD) was measured in a spectrophotometer at 450 nm. Positive and negative controls (rabbit sera, as provided in the kit) and samples were tested in duplicate in each plate. Results were expressed as relative index 10 (RI10) using the formula:
2.3. iELISA cut‐off estimation
As the iELISA was applied to a different species, for which it was not originally validated, a preliminary evaluation to establish the appropriate cut‐off was needed. Particularly, we aimed to maximize the diagnostic specificity to be able to detect antibodies to MYXV, or an antigenically related virus, minimizing the proportion of false positives. The cut‐off was estimated by finite mixture models (Swart et al., 2021). Finite mixture models allow to characterize the distributions of the subgroups (seropositive and seronegative in this study) within bimodal datasets (Benaglia et al., 2009), thus being an alternative tool to estimate the cut‐off of serological tests in the absence of reference tests (Migchelsen et al., 2017; Peel et al., 2014; Swart et al., 2021). Finite mixture models were implemented using the expectation‐maximization algorithm for mixtures of univariate semiparametric distributions, from the package ‘mixtools’ (Benaglia et al., 2009) in R. Sera were also analyzed using the cut‐off of the test defined by the manufacture for the detection of anti‐MYXV rabbit IgGs (IR10 ≥ 2.0 positive; IR10 < 2.0 negative).
2.4. Comparison with other serological methods
Other serological tests were used to confirm and validate a subset of positive and negative iELISA results, increasing the security of the results presented. These included (i) the cELISA developed in the Instituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna “Bruno Ubertini” (International Reference Laboratory for the myxomatosis) which reagents and technique are available from the OIE International Reference Laboratory (OIE, 2020), (ii) the titration of the serum MYXV antibodies using serial dilutions in the commercial iELISA (CIVTEST) and (iii) an indirect IFT developed in the National Reference Laboratory (INIAV, I.P) (Abade dos Santos et al., 2022b). The serum samples were tested in a randomized manner, having also in consideration the reduced volume of serum available. The protocol for the sera titration by iELISA was the same described above, using antibody diluter to prepare the different dilutions. The dilutions used were 1:40 (the dilution recommended by the manufacturer), 1:200, 1:400, 1:800 and 1:1600. Titre was estimated as the last dilution with IR10 above the cut‐off (using two replicates).
The protocol for the IFT was performed according to the following procedures. Rabbit kidney cells (RK13 cell line, CCL‐37) were seeded in 96‐wells cell culture microplates, cultured with minimum essential medium Eagle (MEM medium) and 5% of foetal bovine serum (FBS) (Sigma–Aldrich, St. Louis, Missouri, USA). At 70% cell confluence, the medium was removed and cells were infected at a multiplicity of infection of 0.1, with ha‐MYXV diluted with MEM. After 1‐h adsorption with regular manual agitation at 37°C, MEM medium with 2% of FBS was added to the cells. When cytopathogenic effect (CPE) was evident (after 3–5 dpi), the MYXV‐infected RK13 cells were fixed using cold methanol 70%–acetone 30%–water (v/v) for 10 min. The fixator was removed and the cell layer was washed two times (5 min each) with the PBS‐Tween washing buffer .05%. Blocking was carried out with incubation with 5% non‐fat dry milk prepared in PBS‐Tween .05% for 15 min. After two washes with PBS‐Tween 0.05%, 50 μl of each sera dilution, diluted in PBS pH 7.2, was added to each well and incubated at room temperature for 1 h with agitation. Negative (PBS) and positive controls (control serum) were always included in the test. After two washes (5 min each) with PBS‐Tween 0.05% under agitation, the secondary antibody (anti‐rabbit IgG (whole molecule), produced in goat and conjugated with FITC (Sigma–Aldrich), diluted in PBS pH 7.2, was incubated for 1 h, at room temperature, with agitation. After two final wash steps (5 min each) with PBS‐Tween 0.05% with slow agitation, the cells were observed in an inverted fluorescence microscope. The CPE is characterized by rounding of the cells at the foci, with an increase in cell multiplication (forming cell clusters), followed by detachment. Titre was estimated as the last dilution with positive and specific fluorescence signal (two replicates) using twofold serial dilutions.
2.5. Necropsy
Necropsies were carried out at the Nacional Institute of Agrarian and Veterinary Research, Portugal, the Department of Animal Health, Universidad de Córdoba and the Instituto de Investigación en Recursos Cinegéticos (UCLM & CSIC), Spain.
2.6. Molecular diagnosis
Sera samples from hares collected in Portugal were analyzed according to Duarte et al. (2014). The diploid targeted gene M000.5L/R is present in the MYXV and MYXV‐like genomes, but not in other leporipoxviruses, such as Shope Fibroma Virus. Sera were diluted at 1:3 in PBS. Total DNA was extracted from 200 μl diluted serum samples, using the MagAttract 96 cador Pathogen Kit in a BioSprint 96 nucleic acid extractor (Qiagen, Hilden, Germany), according to the manufacturer's protocol. Contamination of the field samples during the nucleic acid extraction procedure was minimized by extracting the serum samples separately (old from new). No positive reference samples (strong positives) were extracted in the same plates along with the serum samples being analyzed. Contamination of the PCR reagents was ruled out by using several negative controls and a recombinant plasmid of a known sequence as positive control.
For the detection of DNA of both the classical MYXV strains and the novel ha‐MYXV isolate, a conserved region of the M071L or M000.5L/R gene was amplified by PCR or real‐time PCR, respectively, as previously described (Cavadini et al., 2010; Duarte et al., 2014). Using TaKaRa LA Taq DNA polymerase (TaKaRa, Japan), a specific ha‐MYXV PCR was carried out with primers directed to M009L gene (Dalton et al., 2019). Alternatively, the qPCR multiplex method that differentiates MYXV and ha‐MYXV strains was used (Abade dos Santos et al., 2021).
2.7. Statistical analysis
Generalized linear models (GLMs) were used to assess the effect of the independent variables sex, age, type of sampling (random versus found dead), season and year on the iELISA results. The iELISA results considered were positive/negative; thus, a binomial GLM with logit link was used. The reference classes of the independent factors were set as ‘females’, ‘adults’, ‘randomly collected samples’, season ‘autumn’ and year ‘2017’. Collinearity was checked by estimating the Variance Inflation Factor with a threshold of 3. A two‐tailed 2‐sample test for equality of proportions without continuity correction was used to compare seroprevalence between periods. Significance was set at .05.
Statistical analysis was performed in R (R Core Team, 2017). Maps were produced in QGIS 2.6.1 Brighton software (QGIS Development Team, 2011).
3. RESULTS
3.1. Calculation of iELISA cut‐off value
Finite mixture modelling estimated the seronegative population as having a normal distribution of RI10 with mean = .830 and standard deviation = 1.900 and the positive populations with mean = 11.359 and standard deviation = 1.902. The cut‐off RI10 = 6.1 was chosen to assure a probability of belonging to the seropositive population >90% (Figure 1). This criterion reduced the percentage of positives from 61.36% (54 out of 88) to 32.9% (29 out of 88) with regards to RI10 = 2.0, recommended by the manufacturer for rabbits’ sera.
FIGURE 1.
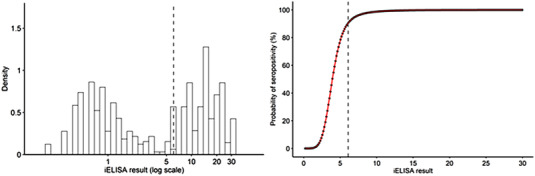
(Left) Density histogram of the log‐transformed indirect ELISA results and probability of belonging to the seropositive population. (Right) Selected cut‐off IR10 = 6.1 as a dashed gray line
3.2. Seroprevalence according to the iELISA results
The results obtained with the iELISA (RI10 = 6.1) for each year are shown in Table 2 and Figures 1 and 2a.
TABLE 2.
Summary of the clinical, molecular and serological (iELISA) results in Iberian hares by year of sampling. Serological status presented as seropositive (Ab+) or seronegative (Ab−) for anti‐MYXV antibodies
| Laboratory test results | 1994 | 1996 | 1997 | 1998 | 1999 | 1994–1999 | 2017 | 2018 | 2019 | 2017‐2019 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab+ | Ab− | Ab+ | Ab− | Ab+ | Ab− | Ab+ | Ab− | Ab+ | Ab‐ | Ab+ | Ab− | Ab+ | Ab− | Ab+ | Ab− | Ab+ | Ab‐ | Ab+ | Ab‐ | |
| Macroscopic lesions present | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 2 | 5 | 2 |
| MYXV positive qPCR | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 16 | 19 | 7 | 10 | 23 | 29 |
| MYXV negative qPCR | 0 | 30 | 1 | 21 | 2 | 57 | 2 | 20 | 5 | 44 | 10 | 172 | 1 | 59 | 0 | 54 | 11 | 14 | 12 | 127 |
| Not tested by qPCR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 22 | 2 | 38 | 7 | 60 |
| Ab positive/total sample | 0/30 (0%) | 1/22 (4.5%) | 2/62 (3.2%) | 2/22 (9.1%) | 5/50 (10.0%) | 10/186 (5.4%) | 1/60 (1.7%) | 25/120 (20.8%) | 21/85 (24.7%) | 47/265 (17.7%) | ||||||||||
| Ab positive/randomly collected samples | 0/30 (0%) | 1/22 (4.5%) | 2/62 (3.2%) | 2/22 (9.1%) | 5/50 (10.0%) | 10/186 (5.4%) | 1/60 (1.7%) | 13/90 (14.4%) | 14/65 (21.5%) | 28/215 (13.0%) | ||||||||||
FIGURE 2.
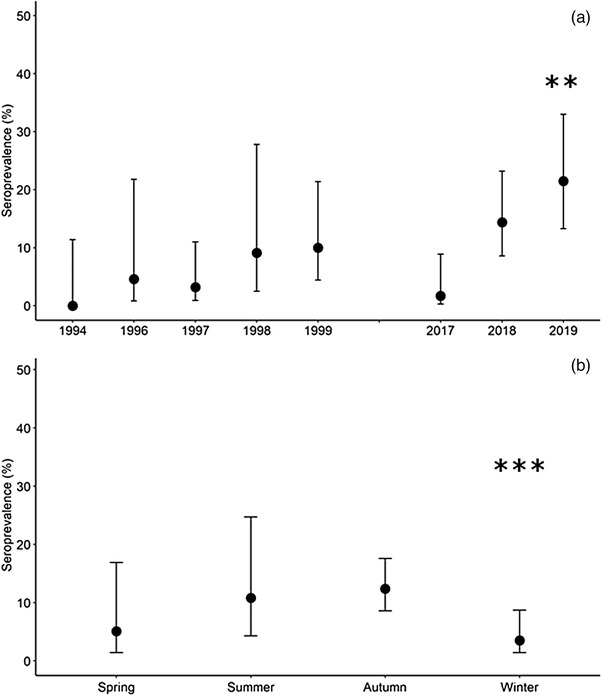
Seroprevalence in Iberian hares by year (a) and season (b). Seroprevalence includes only results from hares randomly sampled (hunted or live captured), showing the proportion of the sample with antibodies recognizing myxoma virus antigens with 95% confidence interval. Statistically significant differences highlighted (**p < .01, ***p < .001).
According to these results, the antibodies recognising MYXV antigens were detected in 57 Iberian hares. Interestingly, 5.4% (CI95% 3.0–9.6%, 10 out of 186) of the samples randomly collected between 1994 and 1999 were classified as seropositive (Table 2). In 2017, 1.7% of sera (CI95% 0.3–8.9%, one out of 60) were seropositive, while in 2018–2019, the apparent seropositivity was 17.4% (CI95% 12.2–24.2%, 27 out of 155). The apparent seropositivity in the found‐dead hares’ subset was 38.0% (CI95% 25.9–51.9%, 19 out of 50), more than double that in the randomly collected sera subset.
Seropositive samples were detected in two out of the five (40%) administrative units included in the study in 1994–1999 as well as in 11 of 20 units included in 2017–2019 (Figure 3).
FIGURE 3.
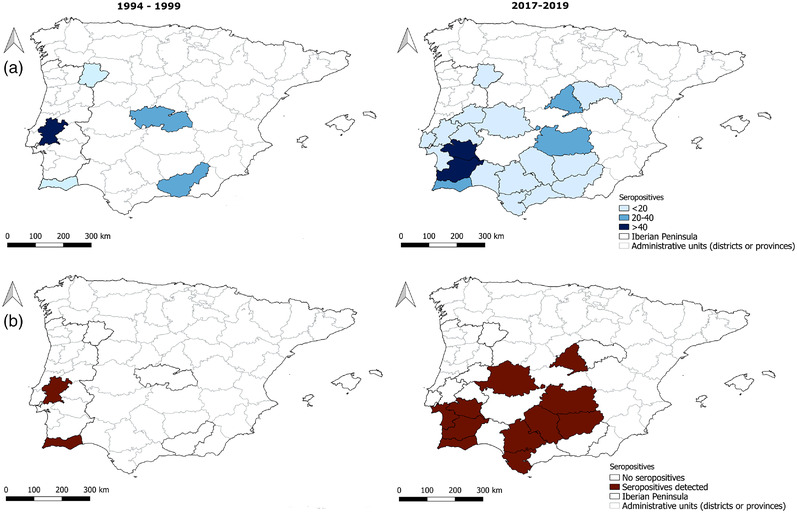
Sample size and seropositivity by administrative units in 1994–1999 and 2017–2019. (a) Number of Iberian hares sampled; (b) administrative units with seropositive Iberian hares, considering the whole sample. Administrative units are districts in Portugal and provinces in Spain.
For the Iberian hares randomly sampled (hunted or live trapped), seroprevalence based on the iELISA was significantly higher in the year 2019 (β = 2.906, p = .007) compared with the remaining years and was lower during the winter (β = −2.155, p < .001), compared with autumn (Table 3 and Figure 2b). No significant differences were found between sex and age classes.
TABLE 3.
Summary of the results of the binomial generalized linear model of seroprevalence. Reference classes were set as females, adults, season autumn and year 2017
| Variable | β | Standard error (β) | p Value |
|---|---|---|---|
| Intercept | −3.388 | 1.049 | .001 |
| Sex | |||
| Males | .335 | 0.398 | .400 |
| Age | |||
| Juveniles | −1.163 | 0.791 | .141 |
| Season | |||
| Winter | −2.155 | 0.590 | <.001 |
| Spring | −1.336 | 0.919 | .146 |
| Summer | .497 | 0.805 | .537 |
| Year of sampling | |||
| 1994 | −13.452 | 1,071.6 | .990 |
| 1996 | .220 | 1.448 | .879 |
| 1997 | −0.278 | 1.290 | .829 |
| 1998 | 0.811 | 1.382 | .558 |
| 1999 | 1.852 | 1.233 | .133 |
| 2018 | 2.024 | 1.073 | .059 |
| 2019 | 2.906 | 1.083 | .007 |
As demonstrated in Figure 3, seroprevalence was significantly higher in 2017–2019 (13.0%, CI95% 9.2–18.2%) than in 1994–1999 (5.4%, CI95% 3.0–9.6%) (p = .009). In Figure 4, in addition to the variation in seropositivity over the years, the evolution of the antibody titre can be noted.
FIGURE 4.
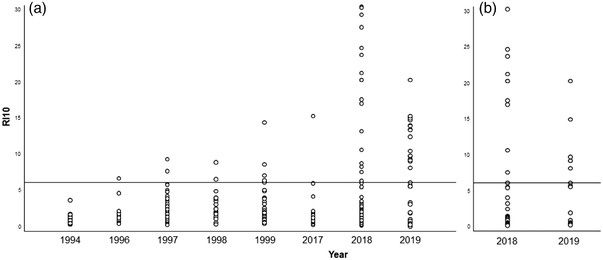
Dot graph with the RI10 values from serum samples collected in different years. The left graph (a) refers to data from serum samples collected from hunted or live trapped hares. The right graph (b) refers to data from found‐dead hares’ serum samples. The horizontal line point out the IR value of IR 6.1 indicates the adjusted ELISA cut‐off value.
3.3. Comparison with other serological methods
A subset of samples (88 out of 451, 19.5%) was submitted to one or two additional serological tests to confirm the iELISA results using the IR10 = 6.1 (Table 4).
TABLE 4.
Summary of results obtained in cross‐validation tests
| Serological positive evidence | ||||
|---|---|---|---|---|
| N. pos iELISA/N. tested | N. pos cELISA/N. iELISA‐pos | N. pos IFT/N. iELISA‐pos | N. pos (cELISA, IFT and iELISA)/N. sera tested by the 3 methods | |
| Old sera | 12/26 (46.2%) | 2/8 (25%) | 8/8 (100%) | 2/8 (25.0%) |
| Recent sera | 23/62 (37.1%) | 13/13 (100%) | 10/10 (100%) | 10/28 (35.7%) |
| Total | 35/88 (39.8%) | 15/21 (71.4%) | 18/18 (100%) | 12/36 (33.3%) |
| Serological negative evidence | ||||
|---|---|---|---|---|
| N. neg iELISA/N. tested | N. neg cELISA/N. iELISA‐neg | N. neg IFT/N. iELISA‐neg | N. neg (cELISA & IFT& iELISA)/N. sera tested by the 3 methods | |
| Old sera | 14/26 (53.8%) | 14/14 (100.0%) | 3/4 (75%) | 3/8 (37.5%) |
| Recent sera | 39/62 (62.9%) | 20/28 (71.4%) | 17/26 (65.4%) | 9/28 (32.1%) |
| Total | 53/88 (60.2%) | 34/40 (85%) | 20/30 (66.7%) | 12/36 (33.3%) |
Doubtful results were excluded from the analysis.
Of a 21 iELISA‐positive samples subgroup, 15 also tested positive in the cELISA; of the 40 iELISA‐negative samples subgroup, six had positive cELISA results. Eighteen out of the 28 IFT‐positive samples were also positive in the iELISA. All the 20 IFT‐negative samples had also iELISA‐negative results. Twelve serum samples, two of which from the 1990s, were simultaneously positive in iELISA, cELISA and IFT.
Within an iELISA‐positive sera subgroup (n = 21), the number of cELISA positives was lower (two out of eight, 25%) in the group of old sera than in the group of recent sera (13 out of 13, 100%). All IFT‐positive results were in agreement with the iELISA results (Table 4). Seventeen sera from the cELISA titres ranging between 10 and 1280 were also tested by IFT, the majority (n = 9) showing equivalent titres and most remaining with small deviation.
In 73% of sera from 1994 to 1999, there was a concordance between iELISA result and the other methods, when performed. Moreover, 75% of recent sera tested by at least one method other than the iELISA were also concordant.
A total of 33 sera with RI10 values lower than 2.0 were negative in the iELISA, either in the dilution recommended by the manufacturer (one out of 40), as well as in higher dilutions (one out of 200, one out of 400, one out of 800 and one out of 1600), indicating that there was no inhibition phenomenon. From the 27 sera with iELISA RI10 values ranging between 2.0 and 6.1 (therefore considered negative in this study), six were tittered by iELISA, showing OD values inversely proportional to the dilution factor and proportional to the RI10 value. From a total of 35 sera with RI10 values higher than 6.1, 18 were titrated with the iELISA showing titres between 40 and >1600.
For example, one serum from the 1990s (Panc 86, Figures 5c and d) showed marking intracytoplasmic inclusion bodies around the initial focus of MYXV in RK13 cell infected layers (cells forming clusters of dead cells). The effect of antibody dilution and the lack of labelling in mock cells validated the assay. Similar results were obtained with recent sera exemplified by serum LCAP‐002 (Figures 5a and b). The cytoplasmic staining pattern of the IFT reveals the intracytoplasmic multiplication of virus.
FIGURE 5.
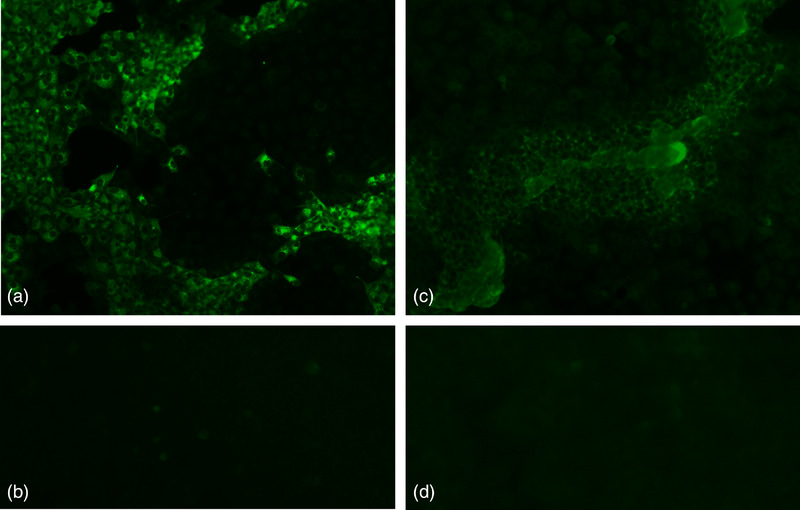
Immunofluorescence test. (a) Hare serum LCAP‐002 (2019), diluted at 1/40, 100×. (b) Mock‐infected cells incubated with the serum LCAP‐002, diluted at 1/40, 100×. (c) Hare serum Panc 86, diluted 1/80, 100× and (D) mock‐infected cells incubated with the serum Panc 86, diluted at 1/40, 100×.
3.4. Molecular diagnosis
MYXV‐DNA was detected by qPCR in serum from three asymptomatic and seronegative samples collected in 1997 in Benavente, Santarém, Portugal, with Cq values ranging from 30.6 to 35.4 (Table 5). These qPCR results were confirmed by cloning and sequencing part of gene M000.5 R/L (Table 5). BLAST analysis of these 80 nt‐long sequences revealed 100% of similarity with the ha‐MYXV strains (MK340973 and MK836424) and with a strain isolated in 2017 in Germany (KP723386) from a rabbit. These sequences differ from the positive control ruling out contaminations.
TABLE 5.
Serological and molecular information of the ancient sera MYXV‐DNA positive
| Serum ID | Sex, Age | Collection date | RI10 | Cq a | Sequenced | Access number |
|---|---|---|---|---|---|---|
| Panc 2 | F, Adult | June 1997 | 3.8 | 32.1 | Yes | OK905447 |
| Panc 6 | F, Adult | June 1997 | 3.8 | 30.6 | Yes | OK905449 |
| Panc 20 | M, Adult | August 1997 | 0.4 | 35.4 | Yes | OK905448 |
M000.5L/R qPCR.
In the samples from 2018 to 2019, MYXV‐DNA was detected in 67.6% of the tested seropositive hares and 29.9% of the tested seronegative hares (Table 2).
4. DISCUSSION
This study provides strong molecular and serological evidence for the exposure of Iberian hare populations to MYXV, or other viruses antigenically similar to MYXV (MYXV like viruses), over two decades before the occurrence of the first reported outbreak of myxomatosis in Iberian hares in mid‐2018 (Carvalho et al., 2020; Dalton et al., 2019; García‐Bocanegra et al., 2019; Pinto et al., 2019). These results bring new insights into the emergence of a new natural recombinant, and highly pathogenic, ha‐MYXV strain. Together with LeHV‐5 (Abade dos Santos et al., 2020) and other pathogens such as Taenia pisiformis or Micipsella iberica (Abade dos Santos et al., 2022 a), ha‐MYXV threatens this iconic species (Lepus granatensis) geographically restricted to the Iberian Peninsula (Duarte et al., 2020). To assess the earlier contact of Iberian hares with MYXV or viruses antigenically similar to MYXV, we used an integrated diagnosis strategy, combining serological and molecular analyses.
As the iELISA assay was applied to a distinct lagomorph species for which it was not originally validated, a preliminary evaluation to estimate its diagnostic performance was carried out. Particularly, it was crucial to ensure the high diagnostic specificity of the method to support the claim that antibodies against MYXV, or a related virus, were already present in sera decades before ha‐MYXV strain was recognized in the Iberian hare as a pathogen. Since the previous contact of Iberian hares with MYXV or MYXV‐like virus could not be ruled out initially, it was not possible to assume any sera as negative or positive reference. This precluded using conventional approaches to evaluate the diagnostic performance of the iELISA. Although rabbits and hares belong to the same taxonomic family Leporidae, the relative affinity of protein A towards Iberian hare's IgG has not been estimated and compared with the affinity towards rabbit's IgG. There is a significant difference in the affinity of protein A towards IgG from different species (Biolabs, 2020). Since a dissimilar affinity to the Iberian hare IgG could affect the performance and outcome of the iELISA, it was necessary to ensure that hares testing positive had been truly exposed to the MYXV or an MYXV‐like virus. We thus selected an extremely high cut‐off to maximize the positive predictive value of the iELISA, at the expense of potentially missing weak positive sera. Therefore, the overall seroprevalence determined by iELISA is probably underestimated.
The cut‐off was selected by finite mixture models, which are independent of a reference test or reference samples (Swart et al., 2021). Finite mixture models allow characterizing the distributions of the seropositive and seronegative subgroups within bimodal datasets (Benaglia et al., 2009), thus being an alternative tool to estimate the cut‐off of serological tests in the absence of reference tests (Peel et al., 2014) and materials. These models have been increasingly used to evaluate the performance of diagnostic tests in the absence of reference tests in humans (Ades et al., 2017; Baughman et al., 2006; Migchelsen et al., 2017; Seck et al., 2019), livestock (Charlier et al., 2016; Deng et al., 2016; Nielsen et al., 2007) and wildlife (Peel et al., 2014). Alternative optimizations of the diagnostic performance of iELISA, namely lower cut‐off values which would achieve higher sensitivity, were not considered due to the absolute need to maximize the diagnostic specificity, having in mind the purpose of this study.
Given the large scale of the current outbreak of myxomatosis in Iberian hares, it is not surprising that the seroprevalence in randomly collected samples was significantly higher in 2018–2019 when compared with 1994–1999 and 2017 (Figure 2), the periods before the first reported outbreak. The GLM identified 2019 as the year having significantly higher seroprevalence (21.5%, CI95% 13.3–33.0%) (Figure 2). Interestingly, samples from 1999 showed non‐significant statistically elevated values of seroprevalence (10.0%, CI95% 4.4–21.4%), not associated with any known reported outbreak of mortality. This lack of reporting could be due to deficient sanitary surveillance in the Iberian hare during the 1990s (Leighton et al., 1995). Sanitary surveillance of wild lagomorphs in Europe, and particularly in the Iberian Peninsula, has been improving in the last decades, despite being far from being an optimized system, similar to what happens in general with wildlife diseases (Cardoso et al., 2022). The different profiles of RI10 in the samples from the 2018–2019 epizootic and 1994–1999 (Figures 3 and 4) could be due to the long‐term storage of the latter (some of them for over 25 years). Despite care to avoid freeze–thaw cycles, sera were stored at −20°C, so that some loss of antibodies might have occurred (Boadella & Gortázar, 2011). The serological results obtained after the emergence of myxomatosis in the Iberian hare in 2018, comprising the sera with the higher titres, are not surprising. It is, however, interesting to note that there is great variability in the titre of antibodies obtained. Samples collected in 2018 from sick or recovered animals (for which there was not considerable time for the decay of the antibody titre), reflected the different courses of infection in the Iberian hare, similarly to what is seen in rabbits.
To validate the results obtained in the iELISA with sera from the 1990s, which were unprecedented, we carried out a comparison with two additional methods. Only a subset of the samples was tested due to the reduced volume of blood available from many animals. The results achieved with different methods were not always concordant, as was the case of seven out of 21 iELISA‐positive samples that were negative in the cELISA. Six of these samples date back to the 1990s, again suggesting that long‐term preservation may have affected the sample quality with an adverse effect on test results. The quality of serum samples may have been more critical to the cELISA, a monoclonal antibody‐based competitive test, than to the iELISA or IFT, where polyclonal antibodies are detected. A total of 18 iELISA‐positive samples were submitted to IFT, all testing positively. The presence of fluorescence in the cytoplasm of infected cells due to the formation of well‐defined intracytoplasmic inclusion bodies corroborated the iELISA results (Figure 5). Eleven sera were positive by IFT and/or cELISA, but negative by iELISA suggesting that the iELISA may have failed detection using a RI10 of 6.1. It was also possible to verify that all IFT/cELISA‐positive samples that were negative in iELISA had an RI10 of 2.0–6.1. Overall, our results highlight the lower sensitivity of the iELISA at the selected cut‐off (RI10 = 6.1), which was expected since it is intended to maximize the specificity of the test. Two samples from the 1990s were positive in all the diagnostic tests used, supporting the infection of Iberian hares with MYXV, or an antigenically similar virus, decades before the current outbreak.
Despite being recognized that some hare species other than L. granatensis are susceptible to myxomatosis (Barlow et al., 2014; Jacotot et al., 1954; Magallon & Bazin, 1953), the scarcity of scientific evidence regarding the putative infection of Iberian hare by MYXV gives room to different explanations. Notwithstanding co‐existing with the European rabbits where annual myxomatosis outbreaks occur in the Iberian Peninsula, the Iberian hares appear to be refractory to infection by the MYXV strains (Fenner & Fantini, 1999; Wibbelt & Frolich, 2005). However, until now no serological evidence had been provided to support or exclude this hypothesis.
Our results report, for the first time, the presence of antibodies recognising MYXV or MYXV‐like antigens and the detection of viral DNA in Iberian hare populations since at least 1996, supporting the theory of early infections by MYXV, or a virus antigenically similar to MYXV, in this host species, possibly in unapparent forms of disease that justify the lack of published reports. This hypothesis is further reinforced by the detection of MYXV‐specific DNA (gene M000.5R/L) that does not differentiate from ha‐MYXV. Underreporting of clinical signs in hares (excluding those due to the lack of active prospection) may indicate resistance to strains circulating at the time (Figure 6, Hypothesis A), or the circulation of low virulence strains
FIGURE 6.
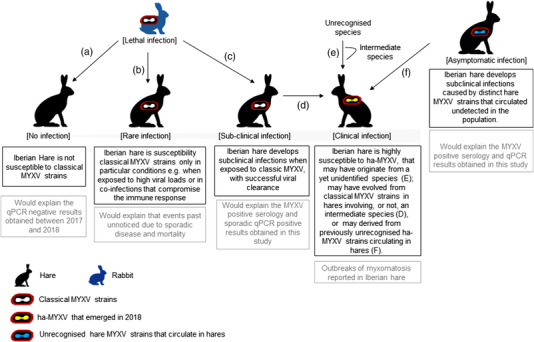
Hypotheses for Iberian hare susceptibility to MYXV and MYXV‐related viruses. (a) Hares are not susceptible to infection by MYXV strains. (b) Hares are susceptible to MYXV if exposed to high infectious doses or if immunodepressed. (c) Hares are infected by MYXV, but develop subclinical disease. (d) ha‐MYXV emerged in hares infected with rabbit strains, by a recombination event that increased virulence of the virus towards hares. (e) ha‐MYXV emerged in hares after a species jump event, involving or not, an intermediate host. (f) ha‐MYXV emerged in hares from a low virulent strain that has circulated unnoticed in the population
In infected domestic rabbits, IgM can be detected 5–6 days post‐infection and during the following 30–40 days, whereas IgG peaks at days 20–30 and can be detected up to 2 years (OIE Terrestrial Manual, 2018). The seropositivity between 1996 and 1999 (3.2–10.0%) suggests that, in these years, the sampled Iberian hare populations were in contact with MYXV or an antigenically related virus, which may have resulted from a large epidemic outbreak in the wild rabbit. A study from Spain reported a peak in myxomatosis prevalence in rabbit farms in 1997 (Rosell et al., 2019).
In certain conditions, such as immunosuppression induced by pathogens, hares may eventually become more susceptible to rabbit MYXV (Figure 6, Hypothesis B). Another possibility is that MYXV was responsible for spillover events or subclinical infections unnoticed in Iberian hares (Figure 6, Hypothesis C). Also, the recombination event that generated ha‐MYXV may have involved an intermediate host (Figure 6, Hypothesis D), similarly to many emerging viruses (e.g. SARS‐CoV or MERS‐CoV). In addition to a putative recent species jumps from rabbit to hare, our results support the possibility of one or more MYXV strains already circulating unnoticed in the hare populations (Figure 6, Hypotheses E and F). Increased virulence of this strain/strains would lead to recent outbreaks, as previously suggested (Dalton et al., 2019; Pinto et al., 2019). The possibility that ha‐MYXV originated from a distinct hare MYXV that was already circulating unnoticed in wild hare populations for decades under subclinical forms of the disease, would explain myxomatosis never being reported before in Iberian hares (Figure 6, Hypothesis F). Finally, myxoma‐like viruses, as the Hare Fibroma Virus or other non‐identified poxviruses, may also be considered a potential cause of the antibody observed response in the periods before the reported ha‐MYXV outbreak.
The higher seroprevalence in our sample during the summer could suggest seasonal transmission like that of MYXV in European rabbits, possibly also mediated by arthropod vectors (García‐Bocanegra et al., 2010). The long‐term contact of the European rabbits with MYXV strains, endemic in Europe for more than 60 years, resulted in a relatively high seroprevalence in wild populations, mainly in adults, and a co‐adaptation between the virus and the host (Alves et al., 2019; Duarte et al., 2018; Villafuerte et al., 2017b). The Iberian hare is more solitary than the gregarious European rabbit, attaining lower densities (Gortazar et al., 2007), which could hinder MYXV transmission (Villafuerte et al., 2017b). In addition, the reproductive characteristics of hares, with less and smaller litters compared with the European rabbit (Alves et al., 2002; Gonçalves et al., 2002), introduce a lower number of susceptible juveniles to the population, which may lead to greater containment of outbreaks due to the existence of fewer susceptible animals. The detection of viral MYXV‐DNA by qPCR in four serum samples from 1997, collected in Benavente, Portugal, demonstrates that Iberian hares have been infected by such virus decades before the known outbreaks. These virological results obtained from sera samples probably underestimate the real prevalence of infection, as the serum is not an appropriate sample for MYXV‐DNA detection. While molecular tests detect viral DNA, which is assumed to mean infection, the serological tests reveal the presence of specific antibodies, which last longer than viremia, as assessed in European rabbits (Kerr, 2012). The mismatch between the timeframes of viral positivity (earlier and shorter) and antibody positivity (later and long‐lasting) may therefore explain our data.
However, regarding the 1990's samples, no other alternative biological material was available. The amplification of longer viral DNA fragments from qPCR‐positive sera was not accomplished, probably due to DNA degradation. Sequencing of the small amplicon did not allow the identification of the MYXV strain.
In conclusion, despite the origin of the ha‐MYXV still being unclear, our study shows that virus antigenically similar to MYXV sporadically infected Iberian hares at least over two decades ago and raises the hypothesis of a recent increase in virulence of a MYXV strain, or an antigenically similar strain already circulating in hares, that could have caused the ongoing outbreak. Other hypotheses to explain the results include, among others, the occurrence of continued MYXV spillover events for Iberian hare and undetected subclinical disease (Figure 5). Our results also highlight the need to continue and improve sanitary surveillance of this neglected game species.
AUTHOR CONTRIBUTIONS
Conception and design: F. A. A. S., N. S., M. D. D. and P. C. A. Acquisition of data: F. A. A. S., N. S., C. L. C., M. M. H., C. G., I. G. B. and L. C.). Analysis and interpretation of data: F. A. A. S., N. S., L. C., M. D. D. and P. C. A. Contributed equally: M. D. and P. C. A.
CONFLICT OF INTEREST
The authors declare no competing interests.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as no animal was killed for this study.
ACKNOWLEDGEMENTS
This work was financed by the Fundo Florestal Permanente, Government of Portugal, (Project +Coelho 2, ref. 2019014300001, as part of the Action Plan for the Control of Rabbit Viral Haemorrhagic Disease (Dispatch no. 4757/2017 of 31 May) and by the Spanish Ministry of Science and Innovation (PID2019‐111080RB‐C21). Fundação para a Ciência e Tecnologia (FCT) funded Nuno Santos (grant SFRH/BPD/116596/2016) and Fábio A. Abade dos Santos (SFRH/BD/137067/2018). The Centre for Interdisciplinary Research in Animal Health (CIISA) from the Faculty of Veterinary Medicine (University of Lisbon) also contributed to this study (CIISA‐UIDP/CVT/00276/2020, LA/P/0059/2020 – AL4AnimalS). MMH was funded by Junta de Comunidades de Castilla‐La Mancha and the European Regional Development Fund (SBPLY/17/180501/000514). We would like to thank the Epidemiological Surveillance Program in Wildlife of the Government of Andalusia for providing valuable samples for this study.
Abade dos Santos, F. A. , Santos, N. , Carvalho, C. L. , Martinez‐Haro, M. , Gortázar, C. , García‐Bocanegra, I. , Capucci, L. , Duarte, M. , & Alves, P. C. (2022). Retrospective serological and molecular survey of myxoma or antigenically related virus in the Iberian hare, Lepus granatensis . Transboundary and Emerging Diseases, 69, 3637–3650. 10.1111/tbed.14734
Contributor Information
Fábio A. Abade dos Santos, Email: fabio.abade@iniav.pt.
Nuno Santos, Email: nuno.santos@cibio.up.pt.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. cd_value_code=text
REFERENCES
- Abade dos Santos, F. , Carvalho, C. , Monteiro, M. , Carvalho, P. , Mendonça, P. , Peleteiro, M. C. , & Duarte, M. D. (2020). Recombinant myxoma virus infection associated with high mortality in rabbit farming (Oryctolagus cuniculus). Transboundary and Emerging. Diseases, 1–6. 10.1111/tbed.13899 [DOI] [PubMed] [Google Scholar]
- Abade dos Santos, F. , Carvalho, C. , Pinto, A. , Rai, R. , Monteiro, M. , Carvalho, P. , Mendonça, P. , Peleteiro, M. C. , Parra, F. , & Duarte, M. D. (2020). Detection of recombinant hare myxoma virus in wild rabbits (Oryctolagus cuniculus algirus). Viruses, 12, 1–12. 10.3390/v12101127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abade dos Santos, F. A. , Duarte, M. D. , Carvalho, C. L. , Monteiro, M. , Carvalho, P. , Mendonça, P. , Valente, P. C. L. G. , Sheikhnejad, H. , Waap, H. , & Gomes, J. (2022a). Genetic and morphological identification of filarial worm from Iberian hare in Portugal. Scientific Reports, 12, 9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abade dos Santos, F. A. , Carvalho, C. L. , Parra, F. , Dalton, K. P. , Peleteiro, M. C. , & Duarte, M. D. (2021). A quadruplex qPCR for detection and differentiation of classic and natural recombinant myxoma virus strains of leporids. International Journal of Molecular Science, 22(21), 12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abade dos Santos, F. A. , Monteiro, M. , Pinto, A. , Carvalho, C. L. , Peleteiro, M. C. , Carvalho, P. , Mendonça, P. , Carvalho, T. , & Duarte, M. D. (2020). First description of a herpesvirus infection in genus Lepus. PloS One, 15, e0231795–e0231795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abade dos Santos, F. A. , Carvalho, C. L. , Valente, P. C. L. G. , Armés, H. , Reemers, S. S. , Peleteiro, M. C. , Calonge Sanz, I. , Dalton, K. P. , Parra, F. , & Duarte, M. D. (2022b). Evaluation of commercial myxomatosis vaccines against recombinant myxoma virus (ha‐MYXV) in Iberian Hare and wild rabbit. Vaccines (Basel), 10, 356. 10.3390/vaccines10030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abade dos Santos, F. A. , Dalton, K. P. , Casero, M. , Álvarez, Á. L. , Parra, F. , Carvalho, C. L. , & Duarte, M. D. (2022). Co‐infection by classic MYXV and ha‐MYXV in Iberian hare (Lepus granatensis) and European wild rabbit (Oryctolagus cuniculus algirus). Transboundary and Emerging Diseases, 69(4), 1684–1690. [DOI] [PubMed] [Google Scholar]
- Ades, A. E. , Price, M. J. , Kounali, D. , Akande, V. A. , Wills, G. S. , Mcclure, M. O. , Muir, P. , & Horner, P. J. (2017). Original contribution proportion of tubal factor infertility due to chlamydia: Finite mixture modeling of serum antibody titers. American Journal of Epidemiology, 185, 124–134. [DOI] [PubMed] [Google Scholar]
- Águeda‐Pinto, A. , Kraberger, S. , Everts, A. , Gutierrez‐Jensen, A. , Glenn, H. L. , Dalton, K. P. , Podadera, A. , Parra, F. , Martinez‐Haro, M. , Viñuelas, J. A. , Varsani, A. , McFadden, G. , Rahman, M. M. , & Esteves, P. J. (2022). Identification of a novel myxoma virus C7‐like host range factor that enabled a species leap from rabbits to hares. MBio, 13, e0346121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, J. M. , Carneiro, M. , Cheng, J. Y. , de Matos, A. L. , Rahman, M. M. , Loog, L. , Campos, P. F. , Wales, N. , Eriksson, A. , Manica, A. , Strive, T. , Graham, S. C. , Afonso, S. , Bell, D. J. , Belmont, L. , Day, J. P. , Fuller, S. J. , Marchandeau, S. , Palmer, W. J. , … Jiggins, F. M. (2019). Parallel adaptation of rabbit populations to myxoma virus. Science, 363, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, P. C. , Gonçalves, H. , Santos, M. , & Rocha, A. (2002). Reproductive biology of the Iberian hare, Lepus granatensis, in Portugal. Mammalian Biology, 67, 358–371. [Google Scholar]
- Barlow, A. , Lawrence, K. , Everest, D. , Dastjerdi, A. , Finnegan, C. , & Steinbach, F. (2014). Confirmation of myxomatosis in a European brown hare in Great Britain. Veterinary Record, 175, 75–76. [DOI] [PubMed] [Google Scholar]
- Baughman, A. L. , Bisgard, K. M. , Lynn, F. , & Meade, B. D. (2006). Mixture model analysis for establishing a diagnostic cut‐off point for pertussis antibody levels. Statistics Medicine, 25(17), 2994–3010. [DOI] [PubMed] [Google Scholar]
- Benaglia, T. , Chauveau, D. , Hunter, D. , & Young, D. (2009). Mixtools: An R package for analyzing finite mixture models. Journal of Statistical Software, 32, 1–23. [Google Scholar]
- Bertagnoli, S. , & Marchandeau, S. (2015). The myxoma virus. Rev. Sci. Tech. Off. Int. Epiz., 34, 549–556. [PubMed] [Google Scholar]
- Biolabs (2020). Affinity of protein A/G for IgG types from different species [Online] Available at https://www.neb.com/tools‐and‐resources/selection‐charts/affinity‐of‐protein‐ag‐for‐igg‐types‐from‐different‐species (accessed May 1, 2020)
- Boadella, M. , & Gortázar, C. (2011). Effect of haemolysis and repeated freeze‐thawing cycles on wild boar serum antibody testing by ELISA. BMC Research Notes, 4, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, B. , García‐Bocanegra, I. , Acevedo, P. , Cáceres, G. , Alves, P. C. , & Gortázar, C. (2022). Stepping up from wildlife disease surveillance to integrated wildlife monitoring in Europe. Research in Veterinary Science, 144, 149–156. [DOI] [PubMed] [Google Scholar]
- Carro, F. , & Soriguer, R. C. (2017). Long‐term patterns in Iberian hare population dynamics in a protected area (Doñana National Park) in the SW Iberian Peninsula: effects of wheather conditions and plant cover. Integrative Zoology, 12(1), 49–60. [DOI] [PubMed] [Google Scholar]
- Carvalho, F. A. , Abade Dos Santos, M. , Monteiro, Carvalho, P. , Mendonça, P. , & Duarte, M. D. (2020). First cases of myxomatosis in Iberian hares (Lepus granatensis) in Portugal. Veterinary Record Case Reports, 8, 1–5. [Google Scholar]
- Cavadini, P. , Botti, G. , Barbieri, I. , Lavazza, A. , & Capucci, L. (2010). Molecular characterization of SG33 and Borghi vaccines used against myxomatosis. Vaccine, 28, 5414–5420. [DOI] [PubMed] [Google Scholar]
- Charlier, J. , Ghebretinsae, A. , Meyns, T. , Czaplicki, G. , Vercruysse, J. , & Claerebout, E. (2016). Veterinary parasitology antibodies against Dictyocaulus viviparus major sperm protein in bulk tank milk : Association with clinical appearance, herd management and milk production. Veterinary Parasitology, 232, 36–42. [DOI] [PubMed] [Google Scholar]
- Dalton, K. P. , Martín, J. M. , Nicieza, I. , Podadera, A. , Llano, D. , Casais, R. , Gimenez, S. , Badiola, I. , Agüero, M. , Duran, M. , Buitrago, D. , Romero, L. J. , García, E. , & Parra, F. (2019). Myxoma virus jumps species to the Iberian hare. Transboundary and Emerging Diseases, 66(6), 2218–2226. [DOI] [PubMed] [Google Scholar]
- Deng, H. , Dam‐deisz, C. , Luttikholt, S. , Maas, M. , Nielen, M. , Swart, A. , Vellema, P. , Van Der Giessen, J. , & Opsteegh, M. (2016). Risk factors related to Toxoplasma gondii seroprevalence in indoor‐housed Dutch dairy goats. Preventive Veterinary Medicine, 124, 45–51. [DOI] [PubMed] [Google Scholar]
- Duarte, M. D. , Barros, S. C. , Henriques, A. M. , Fagulha, M. T. , Ramos, F. , Luís, T. , & Fevereiro, M. (2014). Development and validation of a real time PCR for the detection of myxoma virus based on the diploid gene M000. 5L/R. Journal of Virological Methods, 196, 219–224. [DOI] [PubMed] [Google Scholar]
- Duarte, M. D. , Carvalho, C. , Abade dos Santos, F. A. , Monteiro, J. , Monteiro, M. , Carvalho, P. , Mendonça, P. , Tavares Santos, P. , & Melo, P. (2020). The health and future of the six hare species in Europe: A closer look at the Iberian Hare., In: Lagomorphs. IntechOpen (in press).
- Duarte, M. D. , Carvalho, C. L. , Abade, F. A. , dos Santos, J. , Gomes, P. C. , Alves, P. J. , Esteves, J. , Abrantes, A. M. , Lopes, M. P. , Serronha, A. , Santos, N. , Santos, P. T. , Vaz, Y. , Carvalho, J. , Pinto, F. C. , & Amaro, J. (2021). +Coelho 2: Desenvolvimento e implementação de medidas práticas impulsionadoras da recuperação dos leporídeos silvestres em Portugal. +Coelho 2 GT, Lisbon.
- Duarte, M. D. , Carvalho, C. L. , Abade, F. A. , dos Santos, J. , Gomes, P. C. , Alves, P. J. , Esteves, J. , Abrantes, A. M. , Lopes, M. P. , Serronha, A. , Santos, N. , Santos, P. T. , Vaz, Y. , Carvalho, J. , Pinto, F. C. , Amaro, J. , & Cunha, M. V. (2018). +Coelho: Avaliação Ecossanitária das Populações Naturais de Coelho Bravo Visando o Controlo da Doença Hemorrágica Viral.
- Fenner, F. , & Fantini, B. (1999). History of myxomatosis: An experiment in evolution. CABI., In: Biological Control of Vertebrate Pests. New York, USA. [Google Scholar]
- Garcia‐Bocanegra, I. , Astorga, R. , Napp, S. , Casal, J. , Huerta, B. , Borge, C. , & Arenas, A. (2010). Myxomatosis in wild rabbit: Design of control programs in Mediterranean ecosystems. Preventive Veterinary Medicine, 93, 42–50, 10.1016/j.prevetmed.2009.09.013 [DOI] [PubMed] [Google Scholar]
- García‐Bocanegra, I. , Camacho‐Sillero, L. , Caballero‐Gómez, J. , Aguero, M. , Gómez‐Guillamón, F. , Ruiz‐Casas, J. , Díaz‐Cao, J. , García, E. , Ruano, M. , & Haza, R. (2020). Monitoring of emerging myxoma virus epidemics in Iberian hares. Transboundary and Emerging Diseases, 68(3), 1275–1282. [DOI] [PubMed] [Google Scholar]
- García‐Bocanegra, I. , Camacho‐Sillero, L. , Risalde, M. A. , Dalton, K. , Caballero‐Gómez, J. , Aguero, M. , Zorrila, I. , & Gómez‐Guillamón, F. (2019). First outbreak of myxomatosis in Iberian hares (Lepus granatensis). Transboundary and Emerging Diseases, 66(6), 2204–2208. [DOI] [PubMed] [Google Scholar]
- Gonçalves, H. , Alves, P. C. , & Rocha, A. (2002). Seasonal variation in the reproductive activity of the wild rabbit (Oryctolagus cuniculus algirus) in a Mediterranean ecosystem. Wildlife Research, 29, 165–173. [Google Scholar]
- Gortazar, C. , Millán, J. , Acevedo, P. , Escudero, M. A. , Marco, J. , & de Luco, D. F. (2007). A large‐scale survey of brown hare Lepus Europaeus and Iberian Hare L. granatensis populations at the limit of their ranges. Wildlife Biology, 13, 244–250. [Google Scholar]
- Jacotot, H. , Vallee, A. , & Virat, B. (1954). Sur un cas de myxomatose chez le lievre. Annales de l'Institut Pasteur, 86, 105–107. [PubMed] [Google Scholar]
- Kerr, P. J. (2012). Myxomatosis in Australia and Europe: A model for emerging infectious diseases. Antiviral Research, 93, 387–415. [DOI] [PubMed] [Google Scholar]
- Kerr, P. J. , & Best, S. M. (1998). Myxoma virus in rabbits Myxoma virus and myxomatosis biological control for the Myxomatosis in rabbits in Australia: Development of resistance associated with. Revue Scientifique et Techniqiue, 7, 256–268. [DOI] [PubMed] [Google Scholar]
- Kerr, P. J. , Liu, J. , Cattadori, I. , Ghedin, E. , Read, A. F. , & Holmes, E. C. (2015). Myxoma virus and the leporipoxviruses: An evolutionary paradigm. Viruses, 7, 1020–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton, F. A. , Artois, M. , Capucci, L. , Gavier‐Widén, D. , & Morisse, J. P. (1995). Antibody response to rabbit viral haemorrhgic disease virus in red foxes (Vulpes vulpes) consuming livers of infected rabbits (Oryctolagus cuniculus). Journal of Wildlife Diseases, 31, 541–544. [DOI] [PubMed] [Google Scholar]
- Magallon, P. , & Bazin, J. (1953). La myxomatose du lievre. Bulletin de l'Office International des Épizooties, 39, 765–769. [Google Scholar]
- MAPA (2020). Ministerio de agricultura, pesca y alimentación.
- Mead‐Briggs, A. R. , & Vaughan, J. A. (1975). The differential transmissibility of myxoma virus strains of differing virulence grades by the rabbit flea Spilopsyllus cuniculi (Dale)*. The Journal of Hygiene, 75, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migchelsen, S. J. , Martin, D. L. , Southisombath, K. , Turyaguma, P. , Heggen, A. , Rubangakene, P. P. , Joof, H. , Makalo, P. , Cooley, G. , Gwyn, S. , Solomon, A. W. , Holland, J. , Courtright, P. , Willis, R. , Alexander, N. D. E. , David, C. , Mabey, W. , & Roberts, C. (2017). Defining seropositivity thresholds for use in trachoma elimination studies. PLoS Neglected Tropical Diseases, 16(8), e0010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, F. A. , Fauquet, C. M. , Bishop, D. H. L. , Ghabrial, S. A. , Jarvis, A. W. , Martelli, G. P. , Mayo, M. A. , & Summers, M. (1995). Virus taxonomy: Classification and nomenclature of Viruses. Springer Verlag, Vienna, Austria: Sixth report of the International Committee for the Taxonomy of Viruses. Archives of Virology, Suppl. 10.
- Nielsen, S. S. , Toft, N. , Jørgensen, E. , & Bibby, B. M. (2007). Bayesian mixture models for within‐herd prevalence estimates of bovine paratuberculosis based on a continuous ELISA response. Preventive Veterinary Medicine, 81, 290–305. [DOI] [PubMed] [Google Scholar]
- OIE (2020). Myxomatosis [Online] Available at https://www.oie.int/en/animal‐health‐in‐the‐world/animal‐diseases/Myxomatosis/(accessed May 26, 2020)
- OIE Terrestrial Manual (2018). Myxomatosis, 1371–1388. In: Lagomorpha.
- Peel, A. J. , Pulliam, J. R. C. , Luis, A. D. , Plowright, R. K. , O'Shea, T. J. , Hayman, D. T. S. , Wood, J. L. N. , Webb, C. T. , & Restif, O. (2014). The effect of seasonal birth pulses on pathogen persistence in wild mammal populations. Proceedings of the Royal Society B: Biological, 281, 20132962–20132962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, A. , De Matos, A. L. , Abrantes, M. , Kraberger, S. , Risalde, M. A. , Gort, C. , Mcfadden, G. , Varsani, A. , & Esteves, P. J. (2019). Genetic characterization of a recombinant myxoma virus in the Iberian Hare (Lepus granatensis). Viruses, 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing. https://www.R‐project.org/
- Rosell, J. M. , De Fuente, L. F. , Parra, F. , Dalton, K. P. , Badiola, J. I. , Ana, P. , Rozas, D. , Badiola, J. J. , Fern, D. , Casal, J. , Casas, J. , Garriga, R. , & Fern, M. (2019). Myxomatosis and rabbit haemorrhagic disease: A 30‐year study of the occurrence on commercial farms in Spain. Animals (Basel), 9(10), 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, A. F. A. , Carvalho, C. L. , Peleteiro, M. C. , Gabriel, S. , Patrício, R. , Abade, F. A. , Carvalho, C. L. , Peleteiro, M. C. , Gabriel, S. I. , Patrício, R. , Carvalho, J. , Cunha, M. V , & Duarte, M. D. (2019). Blood collection from the external jugular vein of Oryctolagus cuniculus algirus sedated with midazolam : Live sampling of a subspecies at risk Blood collection from the external jugular vein of Oryctolagus cuniculus algirus sedated with midazolam: Live. Wildlife Biology, 1, 1–10. 10.2981/wlb.00588 [DOI] [Google Scholar]
- Seck, M. C. , Badiane, A. S. , Thwing, J. , Moss, D. , Fall, F. B. , Gomis, J. F. , Deme, A. B. , Diongue, K. , Sy, M. , Mbaye, A. , Ndiaye, T. , Gaye, A. , Ndiaye, Y. D. , & Diallo, M. A. (2019). Serological data shows low levels of chikungunya exposure in senegalese nomadic pastoralists. Pathogens, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart, A. , Maas, M. , Vries, A. , Cuperus, T. , & Opsteegh, M. (2021). Bayesian binary mixture models as a flexible alternative to cut‐off analysis of ELISA results, a. Viruses, 13, 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte, R. , Castro, F. , Ramírez, E. , Cotilla, I. , Parra, F. , Delibes‐Mateos, M. , Recuerda, P. , & Rouco, C. (2017a). Large‐scale assessment of myxomatosis prevalence in European wild rabbits (Oryctolagus cuniculus) 60 years after first outbreak in Spain. Research In Veterinary. Science, 114, 281–286. [DOI] [PubMed] [Google Scholar]
- Villafuerte, R. , Castro, F. , Ramírez, E. , Cotilla, I. , Parra, F. , Delibes‐Mateos, M. , Recuerda, P. , & Rouco, C. (2017b). Large‐scale assessment of myxomatosis prevalence in European wild rabbits (Oryctolagus cuniculus) 60 years after first outbreak in Spain. Research in Veterinary Science, 114, 281–286. [DOI] [PubMed] [Google Scholar]
- Wibbelt, G. , & Frolich, K. (2005). Infectious diseases in European brown hare (lepus europaeus). Wildlife Biology in Practice, 1, 10.2461/wbp.2005.1.11 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. cd_value_code=text


