Abstract
Compared to neurotypical peers, autistic adolescents show greater cognitive inflexibility (CI) which manifests at the behavioral and cognitive level and potentially increases vulnerability for the development of internalizing (INT) and externalizing (EXT) symptoms. This systematic review and meta‐analysis explored the association between CI and INT/EXT in autistic adolescents. PubMed, EMBASE, MEDLINE, PsycINFO and Web of Science databases were searched to identify relevant studies until April 2022 (PROSPERO protocol: CRD42021277294). Systematic review included 21 studies (n = 1608) of CI and INT, and 15 studies (n = 1115) of CI and EXT. A pooled effect size using Pearson's correlation between CI and INT/EXT was calculated and the moderating effects of age, sex, IQ and study quality were investigated using meta‐regressions. Sensitivity analyses were completed to investigate the impact of measure variance for CI and co‐occurring ADHD on the overall effects. Greater CI is associated with increased INT (nine studies; n = 833; r = 0.39 (moderate effect), 95% confidence interval [0.32, 0.46]) and EXT (six studies; n = 295; r = 0.48 (large effect), 95% confidence interval [0.38, 0.58]). Results withheld when only using parental reports of CI and excluding autistic adolescents with co‐occurring ADHD. Increased CI may be a transdiagnostic vulnerability factor that can increase autistic adolescents' rigid or perseverative patterns of unhelpful cognition and behaviors and reduce their ability to access psychological interventions. Addressing CI may improve autistic children and adolescents' engagement with psychological therapy for co‐occurring mental health difficulties.
Keywords: autism spectrum disorder, cognitive inflexibility, cognitive flexibility, externalizing, internalizing, meta‐analysis, systematic review
Lay Summary
This systematic review and meta‐analysis explored the relationship between cognitive inflexibility (CI) and symptoms of anxiety, depression and behavioral difficulties in autistic children and adolescents. CI refers to increased rigidity and perseveration in thinking and behavior and was found to be associated with increased mental health symptoms in autistic adolescents. Addressing and targeting individual differences in CI may improve autistic children and adolescents' engagement with psychological therapy for co‐occurring mental health difficulties.
INTRODUCTION
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterized by social communication difficulties and restricted and repetitive behaviors and sensory anomalies (American Psychiatric Association, 2013) that affects 1 in 54 children (Centers for Disease Control and Prevention, 2019). In both population derived sample estimates and meta‐analysis that have examined psychiatric co‐occurring conditions amongst autistic individuals, 70% of autistic1 children and adolescents have at least one co‐occurring condition (Simonoff et al., 2008), between 20% and 41% experience internalizing conditions including anxiety and mood disorders, and between 12% and 30% experience externalizing conditions such as oppositional defiant and conduct disorder (Lai et al., 2019; Simonoff et al., 2008).
Given that co‐occurring psychiatric conditions negatively impact the quality of life for autistic children and adolescents (van Steensel et al., 2012), identifying possible vulnerability factors can inform clinical assessment, formulation and intervention. Recent systematic reviews have highlighted that individual differences in executive function (EF) amongst autistic individuals may pose a significant risk factor for the development and maintenance of psychopathology (Demetriou et al., 2018; Uddin, 2021). The unitary (i.e., different components within EF may correlate with each other to suggest a common underlying process) and diversity (i.e., different EF processes also show separability when assessed using performance‐based vs rater‐report measures, and may load onto different latent constructs) (Friedman & Miyake, 2017) highlights that it may be possible to adopt a dimensional approach to better understand the unique impact of individual EF processes above and beyond the common EF factor contributing to the behavioral differences observed across autistic individuals (Demetriou et al., 2018). Furthermore, the degree of heterogeneity in performance across different EF domains is more significant in young people from neurodiverse backgrounds compared to their neurotypical peers. Reasons accounting for widespread heterogeneity may be related to a number of factors including method of EF assessment, age range of participants, and level of individual functioning, further suggesting a common EF factor may not be able to inform different subtypes of EF difficulties amongst autistic young people (Demetriou et al., 2019).
Adopting a dimensional approach by focusing on a single executive function domain can also support the establishment and critical evaluation of evidence‐base to explore whether the identified construct may be suitable for intervention as an explicit treatment target. Such knowledge is crucial for supporting clinicians to make informed decisions when adapting clinical interventions to treat psychopathology for autistic children and young people (Demetriou et al., 2018; Kenworthy et al., 2014; Morris & Mansell, 2018; Uddin, 2021). One important executive function domain under recent scrutiny in autism research is cognitive flexibility, especially when considered from a developmental perspective across adolescence (Uddin, 2021). Cognitive flexibility enables one to develop a well‐organized response in an efficient manner and act in a goal‐directed way, and increased cognitive flexibility is associated with being better able to adapt to novel situations and generalize problem‐solving skills across a variety of settings (Kenworthy et al., 2014). For autistic young people, cognitive flexibility plays an important buffering role against increasing development demands during adolescence from biological (changes in hormones, neural reorganization in the adolescent brain), psychological (increased peer‐sensitivity including reward and rejection), and social (changes in peer relationships and increasing independence from family) perspectives (Uddin, 2021). One recent study found that different aspects of cognitive and social flexibility reported by parents accounted for individual differences in social adaptive functioning and communication skills in autistic youths aged 7–17 years, such that greater flexibility supported the ability for young people to function independently when transitioning to young adulthood (Bertollo et al., 2020), and is a protective factor against maladjustment through puberty.
Reduced cognitive flexibility, or cognitive inflexibility (CI), can also be a risk factor in development for autistic young people (Uddin, 2021). Compared to adolescents with ADHD and neurotypical peers, autistic adolescents and their parents report greater CI and reduced emotional control and reduced self‐monitoring (Kenworthy et al., 2022). Parent‐report of CI in autistic children and adolescents (aged 5–18 years) directly predicted externalizing symptoms and indirectly predicted internalizing symptoms via intolerance of uncertainty (Ozsivadjian et al., 2021). Another recent study using a range of neuropsychological tasks to measure CI demonstrated associations with internalizing symptoms across both adolescence and early adulthood, with inflexibility accounting for the stability of symptoms across timepoints (Hollocks et al., 2022). This suggests that CI may be one mechanism through which emotional difficulties are maintained longitudinally.
The definition of CI and its assessment shows variance across empirical literature (Ionescu, 2012). At the behavioral level, cognitive flexibility has been assessed by observing one's ability to switch between different sets of rules and instructions (or set‐shifting), finding alternative solutions, and even multitasking (Cragg & Chevalier, 2012; Geurts et al., 2009). At the conceptual level, flexibility is less clearly defined, and has been related to cognitive control that falls under executive function, shifting between and generating alternative strategies when problem solving in light of conflicting evidence (Bennett & Müller, 2010; Garcia‐Garcia et al., 2010), engaging in adaptive behaviors in a goal‐oriented manner based on environmental changes (Deák, 2003), and even divergent thinking and creativity (Cretenet & Dru, 2009; Dietrich & Kanso, 2010). Cognitive mechanisms interact with environmental factors such as task demands, contextual cues, and sensorimotor aspects, and continues to mature over one's lifetime as cognitive flexibility (Ionescu, 2012). Given the complexity in the definition of cognitive flexibility and the number of cognitive, sensorimotor, and environmental factors that need to be considered during its assessment, empirical research has used a wide range of experimental tasks, neurocognitive tasks, and self‐ and observer questionnaire reports to capture cognitive flexibility at the behavioral and cognitive level across contexts (Ionescu, 2012). Examining differences in cognitive flexibility therefore also requires consideration and comparison across different assessment methods, given that different experimental and neurocognitive tasks and questionnaires may draw on different mechanisms underlying cognitive flexibility in different contexts.
Previous systematic and literature reviews on the topic of CI have evaluated the psychometric properties of standardized measures, including their discriminability (Leung & Zakzanis, 2014) and ecological validity when completed by autistic individuals (Geurts et al., 2009). No review to date has explored how CI may be associated with internalizing and externalizing symptoms in autistic children and adolescents. The current systematic review and meta‐analysis has two objectives:
Aim 1: What is the relationship between CI and internalizing symptoms (INT; e.g., anxiety and mood symptoms/disorders?) in autistic children and adolescents?
Aim 2: What is the relationship between CI and externalizing symptoms (EXT; e.g., aggression, rule‐breaking) in autistic children and adolescents?
Exploratory Aim: To explore whether any significant relationships observed in Aim 1 and/or 2 may be moderated by participants' mean age, gender (proportion of male participants), mean full‐scale IQ, study quality, and modality of assessment.
We hope that a close examination of the empirical literature can aid clinical practice through generating hypotheses about the potential benefits of directly targeting CI to boost therapeutic engagement and outcomes in this clinical population when working with psychiatric co‐occurring conditions.
METHODS
Search strategy
This review followed the PRISMA 2020 Checklist (Page et al., 2021), see Prospero (CRD42021277294) for study protocol. Peer‐reviewed journal articles published in English until April 11, 2022 were retrieved from PubMed, EMBASE, MEDLINE, PsycINFO and Web of Science. The earliest relevant article identified using the search terms was published in 1964. Synonyms of the following key words were used in identifying relevant articles across each database: autism, children/adolescent, CI, INT (Aim 1) and EXT (Aim 2) (Appendix A for full search strategy). Search terms were kept broad to explore which internalizing and externalizing conditions have been researched in relation to CI in adolescents with ASD. Literature only using ADHD as an outcome measure were excluded given the changes in classification and the predominant construct overlap between ADHD and neurodevelopmental conditions (Rietz et al., 2021). After collating results using EndNote library, duplicates were first removed before screening titles, abstracts and full‐text articles based on the inclusion/exclusion criteria. Reference lists of included studies were screened to identify relevant articles.
Study selection
The inclusion/exclusion criteria described followed the participant, exposure, comparison, outcome (Table 1) outlined by conducting systematic reviews and meta‐analyses of observational studies of etiology (Dekkers et al., 2019). Both cross‐sectional and longitudinal quantitative studies published in English and in peer‐reviewed journals were included in the review. Qualitative studies, systematic review/meta‐analyses, opinion articles, gray literature and non‐English publications were excluded.
TABLE 1.
Summary of inclusion and exclusion criteria as per Participant Exposure Comparison Outcome
| Inclusion criteria | Exclusion criteria |
|---|---|
| Participant | |
|
|
| Exposure | |
|
|
| Comparison | |
|
|
| Outcome | |
|
|
Quality appraisal
Quality appraisal was completed by using The Standard Quality Assessment Criteria for Evaluating Primary Research papers from a Variety of Fields (Kmet et al., 2004) (Appendix B for description). The cut‐off for inclusion ranges from being liberal (0.55) to conservative (0.75), with the current study adopting a moderately conservative threshold of 0.60 for study inclusion (Kmet et al., 2004). All studies were assessed independently by two assessors, who met to discuss and review any discrepancies in scoring, with final discussion outcomes being reflected by the quality appraisal scores provided in Tables 3 and 4. The interclass correlation coefficient between the two assessors showed moderate agreement (κ = 0.73) with a 95% confidence interval of (0.64–0.81).
TABLE 3.
Summary of studies examining the relationship between cognitive inflexibility (CI) in children and adolescents with ASD and internalizing symptoms (n = 21)
| Author (year); country | ASD diagnosis (criteria; measure) | N (male) | Age (years; M, SD); IQ (M, SD) | Cognitive flexibility (CF) measure | Internalizing (INT) symptom measure | Main findings | Quality score |
|---|---|---|---|---|---|---|---|
| Carter Leno et al. (2022); Canada | DSM‐IV‐TR; ADOS; ADI‐R |
ASD: 242 (204) Typical shifting: 144 Atypical shifting: 98 |
Age: T1: 3.46 T5: 7.77 T6: 8.73 T7: 9.71 T8: 10.77 FSIQ (T6): Typical shifting: 86.55 (18.96) Atypical shifting: 82.70 (19.21) |
BRIEF‐Shift (parent) | CBCL–internalizing (teacher) |
Confounding variables controlled for: family income and autism symptom severity Cognitive Inflexibility and INT: Atypical Shifting vs. Typical Shifting: Greater CI significantly moderated the relationship between family‐stressful life events (F‐SLE) and future internalizing problems only in the group with atypical shifting abilities |
0.79 |
| Dieckhaus et al. (2021); USA |
DSM‐5; ASD: ADOS‐2; ADHD: MINI‐Kid. |
ASD: 35 (35) ADHD: 83 (63) |
Age: ASD: 9.85 (0.88) ADHD: 9.56 (0.87) FSIQ: ASD: 101.63 (13.88) ADHD: 97.54 (15.02) |
BRIEF‐Shift (Parent) | CBCL – Anxiety (Parent) |
Confounding variables controlled for: gender and ethnoracial identity CI: 54% of ASD and 46% of ADHD group showed clinically elevated scores on Shift subscale (greater CI) INT: 51% of ASD and 36% of ADHD group showed clinically elevated anxiety problems on CBCL CI and INT: In both ASD and ADHD groups – greater CI associated with greater anxiety scores (ASD: Spearman's rho = 0.61, p < 0.001; ADHD: Spearman's rho = 0.60, p < 0.001) |
0.82 |
| Hollocks et al. (2022); UK | ICD‐10; ADOS‐2, ADI‐R | ASD: 81 (74) |
Age: Wave 2: 15.4 (0.45) Wave 3: 23.2 (0.79) FSIQ (Wave 2): 83.5 (17.8) |
WASI–Block Design Opposite Words Trail Making WCST (Performance‐based) |
SDQ–emotional problems (parent–wave 2) BAI (parent–wave 3) BDI (parent–wave 3) |
Confounding variables controlled for: verbal IQ, restricted and repetitive behaviors CI and INT (Age 16): CI significantly associated with increased emotional problems CI and INT (Age 23): greater CI at age 16 predicted greater anxiety and depression at age 23. CI partially mediated the relationship between anxiety, depression and emotional problems between age 16 and 23 |
0.83 |
| * Ozsivadjian et al. (2021); UK | DSM‐5; DAWBA | ASD: 95 (71) |
Age: 11 (3.2) FSIQ: (n = 59) 98.5 (2.3) |
FS‐R (Parent) |
RCADS ‐ Total (Parent) SDQ–emotional problems (parent) |
Confounding variable controlled for: autism symptom severity CI and INT: CI positively associated with RCADS total (r = 0.39) and SDQ‐E (r = 0.34). CI did not significantly predict internalizing symptoms. CI significantly predicted higher intolerance of uncertainty (β =0.73, SE = 0.09; p ≤ 0.01) |
0.91 |
| Uljarevic et al. (2022); Australia |
DSM‐5; ADI‐R; ADOS‐2. |
PTEN‐ASD: 38 (30) Macro‐ASD: 25 (21) PTEN no ASD: 23 (15) |
Age: PTEN‐ASD: 8.93 (4.75) Macro‐ASD: 11.99 (5.15) PTEN‐no ASD: 8.94 (4.85) FSIQ: PTEN‐ASD: 66.32 (13.71) Macro‐ASD: 74.30 (24.50) PTEN‐no ASD: 99.14 (17.40) |
BRIEF–shift (parent) | CBCL 1.5–5/6–18–anxiety (parent) |
Confounding variable controlled for: FSIQ CIs: Macro‐ASD group > PTEN‐no ASD group. CI and INT: Over the whole sample: there is a significant positive correlation between CI and anxiety (r = 0.53, p < 0.01). |
0.86 |
| *Crawley et al. (2020); UK | ADI‐R; ADOS. |
ASD: 321 (232) NT: 251 (171) |
Age: ASD: 16.67 (5.92) NT: 16.93 (6.02) FSIQ: ASD: 103.6 (15.28) NT: 108.95 (12.82) |
Probabilistic reversal learning (PRL) (Performance‐based) |
BAI (parent for children; self for adolescents); BYI‐II–anxiety (parent for children; self for adolescents) |
Confounding variable controlled for: IQ, restricted and repetitive behavior CI: ASD < NT on task accuracy; ASD > NT on number of perseverative errors (greater CI) CI and INT: in ASD children, perseverative errors positively correlated with anxiety (r = 0.34) |
1 |
| * Sesso et al. (2020); Italy |
DSM‐5; K‐SADS‐PL; ADI‐R; ADOS. |
ADHD: 64 (56) ADHD + ASD: 19 (18) ADHD + ODD/CD: 43 (39) ADHD + ASD + ODD/CD: 25 (24) |
Age: ADHD: 10.02 (2.49) ADHD + ASD: 9.58 (2.69) ADHD + ODD/CD: 9.37 (2.95) ADHD + ASD + ODD/CD: 8.4 (2.24) FSIQ: ADHD: 93 (14.98) ADHD + ASD: 92.69 (17) ADHD + ODD/CD: 96.86 (16.05) ADHD + ASD + ODD/CD: 98.94 (18.06) |
BRIEF‐2 shift (parent) | CBCL 6–18‐internalizing problems (parent) |
Confounding variable controlled for: none CI: no significant between‐group differences CI and INT: Items from the Shift (BRIEF) subscale and internalizing symptoms (CBCL) loaded onto the same principal component factor. For ASD group, there was a positive correlation between CI and internalizing problems ( r = 0 .51, p = 0.04) |
0.91 |
| Trimarco et al. (2020); Italy | DSM‐5; ADOS‐2 |
ASD: 21 (4) PKU: 15 (8) Control: 14 (6) |
Age: ASD: 9.83 (1.95) PKU: 10.26 (2.26) NT: 10.20 (1.99) FSIQ: ASD: 94.33 (18.94) PKU: 95.47 (12.50) |
NEPSY‐II: Switching, Response Set, Animal Sorting, Design Fluency (Performance‐based) | CBCL 6–18 internalizing problems (parent) |
Confounding variable controlled for: none CI: ASD < NT group on design fluency and response set. No differences on switching tasks across PKU, ASD and NT groups INT: ASD > NT and PKU groups |
0.73 |
| Dajani et al. (2016); USA | ASD: ADOS‐G; ADOS‐2; ADI‐R; ADHD: DICA‐IV; CPRS‐R:L. |
ASD: 24 (18) ADHD: 31 (22) NT: 44 (31) |
Age: ASD: 10.30 (1.44) ADHD: 9.74 (1.24) NT: 10.47 (1.03) FSIQ: ASD: 102.48 (12.3) ADHD: 109.68 (12.64) NT: 119.66 (13.21) |
BRIEF–shift (parent) | CBCL 6–18 internalizing problems (parent) |
Confounding variable controlled for: head motion CI: weaker left SPL to right SPL connectivity is related to greater CI and worse emotional control in children |
0.82 |
| Berenguer et al. (2018); Spain |
DSM‐5; ASD: SCQ; ADI‐R ADHD: SDQ |
ASD: 30 (27) ADHD: 35 (32) ASD + ADHD: 22 (21) NT: 37 (23) |
Age: ASD: 8.39 (1.3) ADHD: 9.14 (1.4) ASD + ADHD: 8.86 (1.3) NT: 8.54 (1.2) FSIQ: ASD: 100.37 (12.4) ADHD: 99.03 (9.8) ASD + ADHD: 102.86 (13.0) NT: 102.11 (8.9) |
BRIEF–BRI (Teacher) | SDQ emotional problems (parents) |
Confounding variables controlled for: sex, vocabulary and educational level of parents CI: ASD + ADHD > ASD, ADHD > NT INT: ASD, ADHD, ASD + ADHD > NT |
0.86 |
| *Gardiner et al. (2018); Canada |
DSM‐IV‐TR; ADI‐R; ADOS. |
ASD: 59 (51) NT: 67 (33) |
Age: ASD: 10.07 (2.09) NT: 9.44 (1.73) FSIQ: ASD: 107.47 (13.25) NT: 111.37 (12.78) |
BRIEF–shift (parent) | BASC‐2–internalizing behaviors (parent) |
Confounding variable controlled for: IQ CI: ASD > NT INT: ASD > NT on depression symptoms CI and INT: No significant association between CI and anxiety; For the ASD group ‐ shift (β = 0.35, p = 0.02) and emotional control (β = 0.37, p = 0.03) scales emerged as unique significant contributors towards depression symptom severity. Greater CI also was associated with greater internalizing symptoms (r = 0.54) |
0.91 |
| * Vogan et al. (2018); Canada |
ADOS/ ADOS‐2 |
ASD: 39 (34) NT: 34 (20) |
Age: ASD: 10.6 (1.8) NT: 11.2 (2.1) FSIQ: ASD: 103.3 (14.7) NT: 115.4 (11.7) |
BRIEF–BRI (parent) | CBCL–anxious/depressed (parent) |
Confounding variable controlled for: age CI and INT: ASD group–Behavioral Regulation Index (BRI) from BRIEF showed significant correlation with anxiety/depression symptom severity ( r = 0.45 , p < 0.01) rated 2 years later. Regression analyses showed that more BRI problems at T1 predicted later symptoms of anxiety/depression (p < 0.01) at T2 (18% of variance) |
0.82 |
| *Lieb et al. (2017); USA | DSM‐IV‐TR | ASD: 127 (103) | Age: 13.95 (1.6) | BRIEF–shift (parent) |
CBCL–depression (parent) YSR‐depression (self) |
Confounding variables controlled for: age, gender, mode of participation CI and INT: CI positively associated with CBCL‐D ( r = 0.46 , p < 0.01) and YSR‐D (r = 0.34, p < 0.01) |
0.86 |
| Dajani et al. (2016); USA | ASD: ADOS‐G; ADOS‐2; ADI‐R; ADHD: DICA‐IV; CPRS‐R:L. |
ASD: 30 (23) ADHD: 93 (72) ASD + ADHD: 66 (55) NT: 128 (98) |
Age: ASD: 9.76 (1.36) ADHD: 9.79 (1.21) ASD + ADHD: 10.45 (1.40) NT: 10.03 (1.18) FSIQ: ASD: 106.10 (14.88) ADHD: 107.31 (11.67) ASD + ADHD: 99.99 (15.98) NT: 115.76 (12.23) |
BRIEF (Parent) NEPSY‐II Statue subtest WISC‐IV Backward Digit Span (Performance‐based) |
CBCL 6–18–Anxiety/Depression (Parent) |
Confounding variable controlled for: diagnosis CI: ASD primarily in the “impaired” class for executive function (78%) (including 47% of children with ASD only, and 92% of children with both ASD and ADHD), with 20% in the “average” class. CI and INT: Socioemotional problems (i.e., including highest level of anxiety and depression) based on EF profile: “impaired” EF > “average” EF > “above average” EF |
0.86 |
| * Andersen et al. (2015); Norway | K‐SADS‐PL |
ASD: 34 (28) NT: 45 (29) |
Age: ASD: 11.6 (2.0) NT: 11.4 (1.5) FSIQ: ASD: 99.9 (17.4) NT: 104.5 (13.1) |
CW4 (Performance‐based) | CBCL–Internalizing Problems (Parent) |
Confounding variable controlled for: age CI: ASD > NT group, both showed similar rates of improvement over time. INT: ASD > NT on depression symptoms. ASD group showed improvement over time. CI and INT: Neither group showed any significant correlation between changes in flexibility and changes in depression. At baseline, greater internalizing symptoms was associated with greater CI ( r = 0.47 ). |
0.77 |
| Lawson et al. (2015); USA |
DSM‐IV‐TR; ASD: ADI‐R; ADOS; ADHD: ADHD Rating Scale‐IV |
ASD: 70 (63) ADHD: 55 (39) |
Age: ASD: 10.07 (1.77) ADHD: 8.93 (2.69) FSIQ: ASD: 107.01 (19) ADHD: 111.53 (16.85) |
BRIEF–Shift (Parent) | CBCL–Anxiety/Depression (Parent) |
Confounding variables controlled for: age, gender CI: ASD > ADHD group. CI and INT: Across the whole sample, CI is positively associated with Anxious/Depressed (r = 0.39, p < 0.001) scale. Greater CI is also associated with higher anxious/depressed symptoms in the ASD group (B = 0.288, p < 0.001) |
0.91 |
| * Hollocks et al. (2014); UK |
ICD‐10; ADI‐R; ADOS; SCQ. |
ASD: 90 (82) |
Age: 15.5. (0.47) FSIQ: 84.5 (17.2) |
Card Sorting Task ‐ adapted from WCST (Performance‐based) | SDQ ‐ Emotional Symptoms (Parent) |
Confounding variable controlled for: age CI and INT: Poorer card sorting task performance was associated with greater anxiety (r = −0.24 , p < 0.05) and greater depression (r = −0.23, p < 0.05) |
0.91 |
| Tachibana et al. (2013); Japan | DSM‐IV‐TR |
ASD: 11 (8) [Intervention group: 6 (4); Control group: 5 (4)] |
Age: ASD: 9.24 (0.82) [Intervention: 8.93 (0.71); Control: 9.62 (0.84)] FSIQ: ASD: 93.36 (13.20) [Intervention: 92.67 (15.66); Control: 94.20 (11.30)] |
WCST (Performance‐based) | CBCL–Anxiety/Depression (Parent) |
Confounding variable controlled for: none CI: intervention group showed significant improvement in number of “perseverative errors” and “categories achieved” on WCST compared to control group INT: Intervention group showed significant improvement on depression/anxiety symptom severity compared to controls |
0.67 |
| Teunisse et al. (2012); The Netherlands | DSM‐IV | ASD: 20 (20) |
Age: 13.7 (1) FSIQ: 105.5 (13) |
WCST‐S, CANTAB ID/ED (Performance‐based); BFRS‐R (Parent); BRIEF–shift (parent) |
CBCL/4–18 total problems (parent) |
Confounding variable controlled for: none CI: CANTAB ID/ED and WCST‐S are positively associated (r = 0.46, p < 0.05). Both parent‐based flexibility rating scales are positively associated with each other (r = 0.65, p < 0.01) CI and Total Problems: Both parent‐based flexibility scales (BFRS‐R, r = 0.51, p < 0.05; BRIEF Shift Score, r = 0.54, p < 0.05) significantly correlated with total problem score on CBCL. Neuropsychological tests did not significantly correlate with CBCL |
0.64 |
| * Yerys et al. (2009); USA |
DSM‐IV; ADI/ADI‐R; ADOS; ADHD: Inattentive Type on the DSM‐IV ADHD parent rating scale |
ASD: 28 (20) ASD + ADHD: 21 (18) NT: 21 (13) |
Age: ASD: 9.7 (2.12) ASD + ADHD: 9.65 (1.62) NT:10.3 (1.76) FSIQ: ASD: 117.39 (18.68) ASD + ADHD: 111.24 (13.56) NT: 116.24 (11.53) |
BRIEF‐Shift (Parent) | BASC–internalizing problems (parent) |
Confounding variable controlled for: none CI: ASD + ADHD > ASD and NT, ASD group > NT INT: ASD and ASD + ADHD groups > NT group CI and INT: ASD and ASD + ADHD groups combined ‐ CI positively associated with internalizing symptoms (r = 0.46) |
0.82 |
| Happé et al. (2006); UK | DSM‐IV |
ASD: 32 (32) ADHD: 30 (30) NT: 32 (32) |
Age: ASD: 10.9 (2.4) ADHD: 11.6 (1.7) NT: 11.2 (2.0) FSIQ: ASD: 99.7 (18.7) ADHD: 99.1 (17.7) NT: 106.8 (13.4) |
Verbal Fluency; Design Fluency; CANTAB ID/ED (Performance‐based) |
SDQ Emotional Problems (Parent) |
Confounding variables controlled for: age and FSIQ CI: ASD group: age positively associated with performance on Categories and Design fluency, and ID/ED CI and INT: Within the ADHD group, partialling out age revealed a significant correlation between Flexibility and SDQ Emotional symptoms (r = −0.56, p = 0.001). There were no associations between SDQ scores and flexibility in ASD or NT group |
0.82 |
Note: *Indicates studies included in meta‐analysis. Statistics in italics and bold are correlations used for meta‐analysis.
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder; ADI‐(R), Autism Diagnostic Interview (Revised); ADOS, Autism Diagnostic Observation Schedule; APSD, Antisocial process Screening Device; ASD, Autism Spectrum Disorder; BASC, Behavior Assessment System for Children; BFRS‐R, Behavior Flexibility Rating Scale‐Revised; BRIEF‐(S), Behavior Rating Inventory of Executive Function (Shift subscale); CANTAB ID/ED, Cambridge Neuropsychological Test Automated Battery Intra/Extra dimensional set shift; CBCL, Child Behavior Checklist; CD, Conduct Disorder; CF, Cognitive Flexibility; CI, Cognitive inflexibility; CPRS, Conner's Parent Rating Scale; CU, Callous Unemotional; CW4, Color Word Interference task–condition 4; DAWBA, Development and Wellbeing Assessment; DICA, IV, Diagnostic Interview for Children and Adolescents IV; DSM, Diagnostic Statistical Manual; EXT, Externalizing; FS‐R, The Flexibility Scale‐Revised; FSIQ, Full Scale IQ; ICD, International Classification of Disease; K‐SADS‐PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School‐Age Children–Present and Lifetime version; NEPSY‐II, A Developmental NEuroPSYchological assessment; NT, Neurotypical; ODD, Oppositional Defiant Disorder; PACS, Parental Account of Childhood Symptoms; PKU, Phenylketonuria; RBS‐R, Repetitive Behavior Scale–Revised; RCADS, Revised Child Anxiety and Depression Scale; SDQ, Strength and Difficulties Questionnaire; WCST, Wisconsin Card Sorting Task; WISC, Wechsler Intelligence Scale for Children.
TABLE 4.
Summary of studies examining the relationship between cognitive inflexibility (CI) in children and adolescents with ASD and externalizing symptoms (n = 15)
| Author (year); country | ASD diagnosis (criteria; measure) | N (male) | Age (years; M, SD); IQ (M, SD) | Cognitive flexibility (CF) measure | Externalizing symptom (EXT) measure | Main findings | Quality score |
|---|---|---|---|---|---|---|---|
| Carter Leno et al. (2022); Canada | DSM‐IV‐TR; ADOS; ADI‐R |
ASD: 242 (204) Typical shifting: 144 Atypical shifting: 98 |
Age: T1: 3.46 T5: 7.77 T6: 8.73 T7: 9.71 T8: 10.77 FSIQ (T6): Typical shifting: 86.55 (18.96) Atypical shifting: 82.70 (19.21) |
BRIEF‐shift (parent) | CBCL–externalizing (teacher) |
Confounding variables controlled for: family income and autism symptom severity. Cognitive inflexibility and EXT: Atypical shifting vs. Typical shifting: Atypical shifting vs. typical shifting: greater CI moderated (though did not reach statistical significance) the relationship between family‐stressful life events (F‐SLE) and future externalizing problems only in the group with atypical shifting abilities |
0.79 |
| Hollocks et al. (2022); UK | ICD‐10; ADOS‐2, ADI‐R | ASD: 81 (74) |
Age: Wave 2: 15.4 (0.45) Wave 3: 23.2 (0.79) FSIQ (Wave 2): 83.5 (17.8) |
WASI–block design Opposite Words Trail making WCST (performance‐based) |
SDQ–conduct problems (parent, wave 2 and 3) |
Confounding variables controlled for: verbal IQ, restricted and repetitive behaviors CI and EXT (Age 16): CI showed moderate (though nonsignificant) association with increased EXT CI and EXT (Age 23): greater CI at age 16 predicted greater EXT at age 23 |
0.83 |
| * Ozsivadjian et al. (2021); UK | DSM‐5; DAWBA | ASD: 95 (71) |
Age: 11 (3.2) FSIQ: (n = 59) 98.5 (2.3) |
FS‐R (Parent) |
RCADS–total score (parent) SDQ–conduct problems (Parent) |
Confounding variable controlled for: autism symptom severity CI and EXT: CI positively associated with RCADS total (r = 0.39); and SDQ‐B ( r = 0.51 ). CI significantly predicted higher externalizing symptoms (β = 0.57, SE = 0.13; p ≤ 0.01). |
0.91 |
| * Sesso et al. (2020); Italy |
DSM‐5; K‐SADS‐PL; ADI‐R; ADOS |
ADHD: 64 (56) ADHD+ASD: 19 (18) ADHD + ODD/CD: 43 (39) ADHD + ASD + ODD/CD: 25 (24) |
Age: ADHD: 10.02 (2.49) ADHD+ASD: 9.58 (2.69) ADHD + ODD/CD: 9.37 (2.95) ADHD+ASD + ODD/CD: 8.4 (2.24) FSIQ: ADHD: 93 (14.98) ADHD+ASD: 92.69 (17) ADHD + ODD/CD: 96.86 (16.05) ADHD+ASD + ODD/CD: 98.94 (18.06) |
BRIEF‐2 Shift (Parent) | CBCL 6–18 Externalizing Problems (Parent) |
Confounding variable controlled for: none CI: no significant between‐group differences in CF. EXT: ADHD + ASD + ODD/CD > ADHD+ASD on externalizing problems CI and EXT: For ASD group, there was no significant correlation between CI and externalizing problems ( r = 0 .32, p = 0.22) |
0.91 |
| Trimarco et al. (2020); Italy |
DSM‐5; ADOS‐2 |
ASD: 21 (4) PKU: 15 (8) Control: 14 (6) |
Age: ASD: 9.83 (1.95) PKU: 10.26 (2.26) Control: 10.20 (1.99) FSIQ: ASD: 94.33 (18.94) PKU: 95.47 (12.50) |
NEPSY‐II: switching, response set, animal sorting, design fluency (performance‐based) |
CBCL 6–18 externalizing problems (parent) |
Confounding variable controlled for: none CI: ASD < PKU/Control groups on design fluency and response set. No group differences on switching tasks EXT: ASD > NT on externalizing problems |
0.73 |
| Berenguer et al. (2018); Spain |
DSM‐5; SDQ; SCQ; ADI‐R. |
ASD: 30 (27) ADHD: 35 (32) ASD + ADHD: 22 (21) NT: 37 (23) |
Age: ASD: 8.39 (1.3) ADHD: 9.14 (1.4) ASD + ADHD: 8.86 (1.3) NT: 8.54 (1.2) FSIQ: ASD: 100.37 (12.4) ADHD: 99.03 (9.8) ASD + ADHD: 102.86 (13.0) NT: 102.11 (8.9) |
BRIEF–BRI (teacher) | SDQ–behavioral Problems (Parent) |
Confounding variables controlled for: sex, vocabulary and educational level of parents CI: ASD + ADHD > ASD or ADHD > NTs groups EXT: ASD + ADHD, ADHD > ASD > NT on externalizing problems |
0.86 |
| *Gardiner et al. (2018); Canada |
DSM‐IV‐TR; ADI‐R; ADOS |
ASD: 59 (51) NT: 67 (33) |
Age: ASD: 10.07 (2.09) NT: 9.44 (1.73) FSIQ: ASD: 107.47 (13.25) NT: 111.37 (12.78) |
BRIEF‐shift (parent) | BASC‐2–externalizing behavior (parent) |
Confounding variable controlled for: IQ CI: ASD > NT CI and EXT: Greater CI significantly associated with greater externalizing symptoms (r = 0.59) |
0.91 |
| Maddox et al. (2018); USA |
DSM‐IV‐TR; ADOS‐2 |
ASD: 182 (172) |
Age: 9.32 (2.25) IQ: 104.26 (18.67) |
BRIEF–shift (parent); RBS‐R sameness (parent) |
BASC‐2 aggression (parent) |
Confounding variables controlled for: age, IQ, recruitment site CI and EXT: Greater CI significantly associated with more challenging behaviors when measured using RBS‐R sameness scale (B = 0.171, p < 0.05), but not BRIEF Shift scale (B = 0.085, p > 0.05) |
0.82 |
| * Vogan et al. (2018); Canada | ADOS/ADOS‐2. |
ASD: 39 (34) NT: 34 (20) |
Age: ASD: 10.6 (1.8) NT: 11.2 (2.1) FSIQ: ASD: 103.3 (14.7) NT: 115.4 (11.7) |
BRIEF–BRI (parent) | CBCL–aggressiveness (parent) |
Confounding variable controlled for: age CI and EXT: In ASD group, BRI (BRIEF) showed significant correlation with CBCL Aggressiveness scale ( r = 0.61 , p < 0.001) rated 2 years later. Regression analyses showed that more executive function difficulties at T1 predicted later aggressive behavior. |
0.82 |
| Dajani et al. (2016); USA | ASD: ADOS‐G; ADOS‐2; ADI‐R; ADHD: DICA‐IV; CPRS‐R:L. |
ASD: 30 (23) ADHD: 93 (72) ASD + ADHD: 66 (55) NT: 128 (98) |
Age: ASD: 9.76 (1.36) ADHD: 9.79 (1.21) ASD + ADHD: 10.45 (1.40) NT: 10.03 (1.18) FSIQ: ASD: 106.10 (14.88) ADHD: 107.31 (11.67) ASD + ADHD: 99.99 (15.98) NT: 115.76 (12.23) |
BRIEF (parent); NEPSY‐II: Statue subtest; WISC‐IV: Backward Digit Span (Performance‐based) |
CBCL 6–18–Aggression (parent) |
Confounding variable controlled for: Diagnosis CI difficulties: ASD primarily in the “impaired” class for executive function (78%) (including 47% of children with ASD only, and 92% of children with both ASD and ADHD), with 20% in the “average” class CI and EXT: Socioemotional problems (i.e., including highest level of aggression) based on EF profile: “impaired” EF > “average” EF > “above average” EF |
0.86 |
| * Andersen et al. (2015); Norway | K‐SADS‐PL |
ASD: 34 (28) NT: 45 (29) |
Age: ASD: 11.6 (2.0) NT: 11.4 (1.5) FSIQ: ASD: 99.9 (17.4) NT: 104.5 (13.1) |
CW4 (Performance‐based) | CBCL–externalizing problems (parent) |
Confounding variable controlled for: age CI: ASD > NT group, and both groups showed similar rates of improvement in flexibility over time CI and EXT: At baseline, greater externalizing symptoms did not show significant association with greater CI ( r = 0.24 ) |
0.77 |
| Lawson et al. (2015); USA |
DSM‐IV‐TR; ASD: ADI‐R; ADOS; ADHD: ADHD Rating Scale‐IV. |
ASD: 70 (63) ADHD: 55 (39) |
Age: ASD: 10.07 (1.77) ADHD: 8.93 (2.69) FSIQ: ASD: 107.01 (19) ADHD: 111.53 (16.85) |
BRIEF–shift (parent) | CBCL–aggression (parent) |
Confounding variables controlled for: age, gender CI: ASD diagnosis associated with greater CI CI and EXT: Across the whole sample, CI was positively associated with CBCL aggressive behavior (r = 0.30, p = 0.001) scale. For ASD group, greater CI was associated with higher EXT (B = 0.23, p < 0.001). |
0.91 |
| Teunisse et al. (2012); The Netherlands | DSM‐IV | ASD: 20 (20) |
Age: 13.7 (1) FSIQ: 105.5 (13) |
WCST‐S; CANTAB ID/ED (Performance‐based); BFRS‐R (Parent); BRIEF‐shift (parent) |
CBCL/4–18 Total Problems (Parent) |
Confounding variable controlled for: none CI: There is a positive correlation between performance on CANTAB ID/ED and WCST‐S (r = 0.46, p < 0.05) and between both parent‐based flexibility rating scales (r = 0.65, p < 0.01). CI and Total Problems: Both parent‐based flexibility scales (BFRS‐R, r = 0.51, p < 0.05; BRIEF Shift Score, r = 0.54, p < 0.05) significantly correlated with total problem score on CBCL. Neuropsychological tests did not significantly correlate with CBCL. |
0.64 |
| * Yerys et al. (2009); USA |
DSM‐IV; ADI/ADI‐R; ADOS; ADHD: Inattentive Type on the DSM‐IV ADHD parent rating scale |
ASD: 28 (20) ASD + ADHD: 21 (18) NT: 21 (13) |
Age: ASD: 9.7 (2.12) ASD + ADHD: 9.65 (1.62) NT:10.3 (1.76) FSIQ: ASD: 117.39 (18.68) ASD + ADHD: 111.24 (13.56) NT: 116.24 (11.53) |
BRIEF–shift (parent) | BASC–externalizing problems (parent) |
Confounding variable controlled for: none CI: ASD + ADHD > ASD > NT groups. EXT: ASD and ASD + ADHD > NT CI and EXT: ASD and ASD + ADHD groups combined–CI positively associated with externalizing symptoms (r = 0.38) |
0.82 |
| Rogers et al. (2006); UK | DSM‐IV |
ASD low CU: 18 (18) ASD high CU: 10 (10) |
Age: ASD Low CU: 14.51 (2.34) ASD High CU: 14.60 (2.58) FSIQ: ASD Low CU: 92.1 (22.2) ASD High CU: 85.0 (13.5) |
CANTAB ID/ED (Performance‐based) | APSD (Teacher) |
Confounding variable controlled for: none CI: Both CU‐high and CU‐low children performed poorly on ID/ED task, no group differences on errors. |
0.68 |
Note: *Indicates studies included in meta‐analysis. Statistics in italics and bold are correlations used for meta‐analysis.
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder; ADI‐(R), Autism Diagnostic Interview (Revised); ADOS, Autism Diagnostic Observation Schedule; APSD, Antisocial Process Screening Device; ASD, Autism Spectrum Disorder; BASC, Behavior Assessment System for Children; BFRS‐R, Behavior Flexibility Rating Scale‐Revised; BRIEF‐(S), Behavior Rating Inventory of Executive Function (Shift subscale); CANTAB ID/ED, Cambridge Neuropsychological Test Automated Battery Intra/Extra dimensional set shift; CBCL, Child Behavior Checklist; CD, Conduct Disorder; CF, Cognitive Flexibility; CI, Cognitive inflexibility; CPRS, Conner's Parent Rating Scale; CU, Callous Unemotional; CW4, Color Word Interference task–condition 4; DAWBA, Development and Wellbeing Assessment; DICA, IV, Diagnostic Interview for Children and Adolescents IV; DSM, Diagnostic Statistical Manual; EXT, Externalizing; FS‐R, The Flexibility Scale‐Revised; FSIQ, Full Scale IQ; ICD, International Classification of Disease; K‐SADS‐PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School‐Age Children–Present and Lifetime version; NEPSY‐II, A Developmental NEuroPSYchological assessment; NT, Neurotypical; ODD, Oppositional Defiant Disorder; PACS, Parental Account of Childhood Symptoms; PKU, Phenylketonuria; RBS‐R, Repetitive Behavior Scale–Revised; RCADS, Revised Child Anxiety and Depression Scale; SDQ, Strength and Difficulties Questionnaire; WCST, Wisconsin Card Sorting Task; WISC, Wechsler Intelligence Scale for Children.
Data extraction
Table 3 (INT) and Table 4 (EXT) show information extracted from studies included in the systematic review: (1) author, year and country of publication, (2) ASD diagnosis criteria and measure, (3) sample size and gender, (4) mean and standard deviation of age and full scale IQ (where available), (5) CI measure, (6) INT or EXT measure, (7) main findings of CI, INT/EXT, and the association between CI and INT/EXT, (8) quality appraisal score.
Data analysis
For each meta‐analysis, Pearson's correlation coefficient (r) was chosen as a commonly reported effect size measure in observational studies. The first/last authors of studies that did not report Pearson's correlation (n = 19) were contacted via email on two occasions to request the relevant association. Six authors could not be reached or no longer had access to the raw dataset, and four authors responded with the relevant correlation coefficients that were included in the respective meta‐analyses, and nine authors did not respond. When two or more symptom measures are used, specific scales for INT or EXT are used rather than total problem score.
Meta‐analyses were conducted using RStudio (Core Team, 2019) and the metafor package in R (Viechtbauer, 2019). Due to possible variations in study outcomes because of differences in participant characteristics such as age, gender, IQ, and so forth, a random‐effects meta‐analysis model was used. The effect size for each study was first converted to Fisher's Z, which was subsequently converted back to a summary correlation. To interpret the magnitude of effect sizes, Cohen's guidelines (Cohen, 1988) for small (r = 0.10), moderate (r = 0.30) and large (r = 0.50) effects were applied. To assess the degree of heterogeneity across studies, Cochran's Q test and the Higgin's and Thompson's I2 tests were used. Heterogeneity is indicated by either a statistically significant result from Q test (p < 0.05), or higher I2 value (75% = substantial heterogeneity, 50% = moderate heterogeneity, 25% = low heterogeneity) (Higgins et al., 2003). Funnel plots were generated to inspect possible asymmetry that may indicate risk of publication bias, as indicated by a significant Egger's test statistic (p < 0.05) (Egger et al., 1997). Several study characteristics were explored using independent meta‐regressions as potential moderators: (1) mean age, (2) gender (proportion of male participants), (3) mean FSIQ, (4) study quality. Finally, to explore whether the overall effect sizes from each meta‐analysis are influenced by (1) CI measurement; (2) co‐occurring ADHD‐diagnosis, separate post hoc sensitivity analyses were completed for studies using parent report measures of CI only, and for studies where adolescents did not have a reported co‐occurring ADHD diagnosis.
RESULTS
Search results
The PRISMA diagram (Figure 1) summarizes the literature search process (Moher et al., 2009). The first author performed the initial literature search across all databases on 3rd September 2021 and an updated literature search on 11th April 2022, removed study duplicates, and completed title, abstract and full‐text screening. A second coder independently screened ~10% of abstracts (n = 83; Kappa coefficient = 0.96), and ~ 10% of full‐text articles (n = 27; Kappa coefficient = 0.96) with high inter‐rater reliability. The 24 articles were selected for quality assessment. The 21 studies measured CI and INT (Aim 1), including nine Pearson's correlations for meta‐analysis. The 15 studies measured CI and EXT (Aim 2), including six Pearson's correlation coefficients for meta‐analysis.
FIGURE 1.
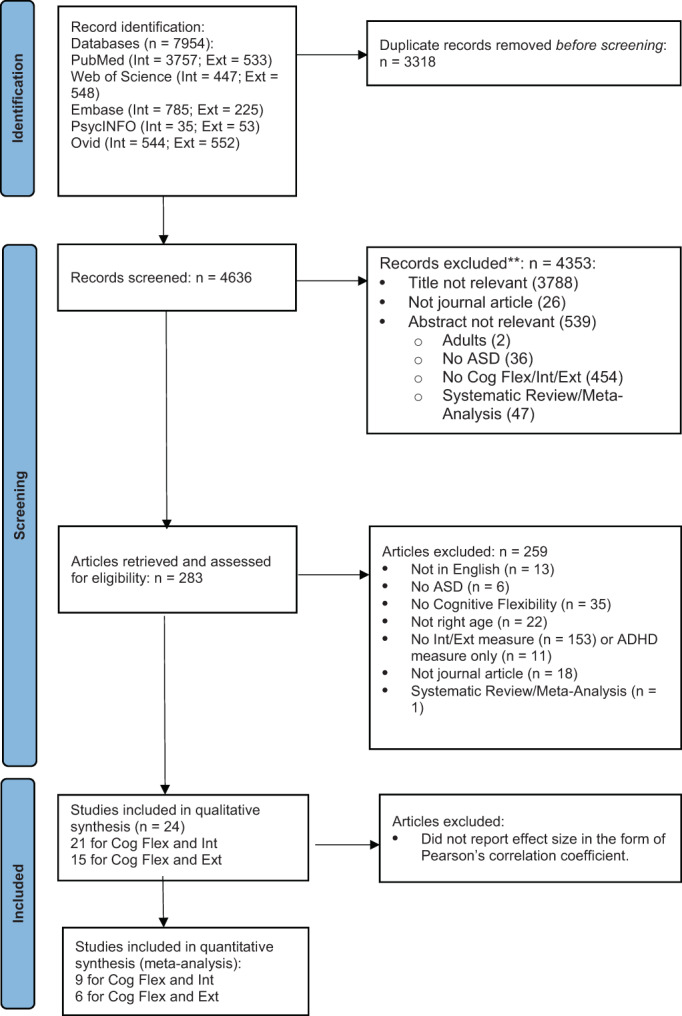
PRISMA diagram
Study characteristics
Tables 2 summarizes the characteristics for the 24 included studies. Of the 21 studies included for Aim 1, six studies reported family socioeconomic status (SES), three included largely low to middle income families (Carter Leno et al., 2022; Dieckhaus et al., 2021; Yerys et al., 2009), and three used either parental (Berenguer et al., 2018) or maternal education (Andersen et al., 2015; Gardiner & Iarocci, 2018) as an estimate of family SES (on average achieved secondary education completion). Of the 15 studies included for Aim 2, five studies reported SES, two included families from low to middle SES (Carter Leno et al., 2022; Yerys et al., 2009), and three included families where mothers or parents completed secondary school education on average (Andersen et al., 2015; Berenguer et al., 2018; Gardiner & Iarocci, 2018).
TABLE 2.
Study characteristics of included 24 full‐text articles
| Aim 1 (21 studies)–CI and INT (n = 1608) | Aim 2 (15 studies)–CI and EXT (n = 1115) | |||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| Sample size | 76.57 (75.48) | 11–321 | 74.33 (62.06) | 20–242 |
| % male | 82.51 (17.06) | 19–100 | 83.60 (19.46) | 19–100 |
| Age (years) | 11.14 (2.45) | 7.77–16.67 | 10.75 (2.19) | 7.77–15.4 |
| FSIQ | (20 studies) | (15 studies) | ||
| 97.68 (10.37) | 69.49–114.75 | 99.51 (8.61) | 83.5–114.75 | |
| Ethnicity | (% ‐ six studies) | (% ‐ three studies) | ||
| Caucasian | 69.44 (16.61) | 42.86–86.61 | 72.98 (7.73) | 65.31–80.77 |
| Mixed/other ethnicity | 23.07 (12.87) | 8.66–42.86 | 21.84 (5.63) | 15.93–27.14 |
| Black | 6.27 (5.76) | 1.59–14.29 | 7.1 (1.5) | 6.04–8.16 |
| Asian | 2.72 (3.78) | 0–7.94 | 2.86 (1.72) | 1.65–4.08 |
| Study quality | 0.83 (0.08) | 0.64–1 | 0.82 (0.08) | 0.64–0.91 |
| Recruitment | (n = studies) | (n = studies) | ||
| Clinical sites (including hospitals/university clinic) | 9 | 7 | ||
| Community settings | 7 | 5 | ||
| School | 1 | 1 | ||
| Longitudinal datasets | 4 | 2 | ||
| Comorbidities | (n = participants; six studies) | (n = participants; four studies) | ||
| ADHD | 153 | 153 | ||
| ODD/CD | 25 | 25 | ||
| PTEN mutation | 38 | — | ||
| Macroencephaly | 25 | — | ||
| CI measure | (n = studies) | (n = studies) | ||
| Parent report | 13 | 10 | ||
| Teacher report | 1 | 1 | ||
| Neurocognitive/task measure | 9 | 6 | ||
Abbreviations: ADHD, attention deficit hyperactivity disorder; CD, conduct disorder; CI, cognitive inflexibility; EXT, externalizing; FSIQ, full scale IQ; INT, internalizing; ODD, oppositional defiant disorder.
Measurement of CI
Across the 24 studies included in this systematic review, 15 studies used a parent report measure to examine CI in children and adolescents with ASD. The 13 of those 15 studies used the shift scale or behavioral regulation index of the Behavior Rating Inventory of Executive Function (BRIEF; Gioia et al., 2000), one study used The Flexibility Scale‐Revised (FS‐R; Strang et al., 2017), and one study also used the Sameness subscale from the Repetitive Behavior Scale‐Revised (Maddox et al., 2018). Only one study used the teacher report version of the BRIEF (Berenguer et al., 2018). Using parent and teacher reports, autistic children and adolescents with co‐occurring ADHD were found to have greater CI compared to adolescents with ASD only (Berenguer et al., 2018; Yerys et al., 2009), who in turn had great CI compared to adolescents with ADHD only (Dieckhaus et al., 2021; Lawson et al., 2015), with neurotypical adolescents being rated with lowest CI (Andersen et al., 2015; Berenguer et al., 2018; Gardiner & Iarocci, 2018; Yerys et al., 2009). Only one study found there to be no significant differences in parent‐rated CI when comparing adolescents with ASD and ADHD to adolescents with ADHD only, with ADHD and Oppositional Defiant Disorder/Conduct Disorder (ODD/CD), or with ASD, ADHD and ODD/CD (Sesso et al., 2020). Parents also reported that autistic adolescents with microencephaly experienced greater CI compared to adolescents with PTEN mutation and without ASD, but did not differ from autistic adolescents with PTEN mutation, suggesting that CI may be uniquely associated with ASD above and beyond the effect of PTEN mutation (Uljarević et al., 2022).
Ten studies used a task‐based measure to examine CI in adolescents with ASD, including the NEuroPSYchological Assessment (NEPSY‐II; Trimarco et al., 2020), a probabilistic reversal learning paradigm (Crawley et al., 2020), Block Design2 (Hollocks et al., 2022), the Opposite Words task (Hollocks et al., 2022), Trail Making (Hollocks et al., 2022), Color Word Interference Task (CW‐4; Andersen et al., 2015), Wisconsin Card Sorting Task (WCST; Hollocks et al., 2014, 2022; Tachibana et al., 2013; Teunisse et al., 2012), and the Cambridge Neuropsychological Test Automated battery Intra/Extra dimensional set shift task (CANTAB ID/ED; Happé et al., 2006; Rogers et al., 2006; Teunisse et al., 2012). Compared to neurotypical peers, adolescents with ASD showed reduced task accuracy and greater perseverative errors (Crawley et al., 2020), and poorer performance on fluency based tasks involving generation of novel responses (Trimarco et al., 2020) or tasks requiring inhibiting interference from incorrect responses (Andersen et al., 2015). On switching tasks which assesses a range of executive functions including using environmental stimuli to modulate one's behavior in a goal‐directed manner and inhibiting interfering stimuli, one study found that adolescents with ASD performed similarly to neurotypical adolescents (Trimarco et al., 2020). Another found that performance on switching task improved by achieving a greater number of categories with fewer perseverative errors on the WCST after adolescents with ASD read aloud for 30 min five times a day for 5 weeks (Tachibana et al., 2013).
CI and INT
Table 3 shows a summary of results from the 21 studies that explored the association between INT and CI. Overall, many studies found that the parent/teacher reported CI significantly correlated with greater symptoms of anxiety (Dieckhaus et al., 2021; Lawson et al., 2015; Uljarević et al., 2022; Vogan et al., 2018), depression (Gardiner & Iarocci, 2018; Lawson et al., 2015; Lieb & Bohnert, 2017) and general emotional problems (Hollocks et al., 2022) in adolescents with ASD. Sesso et al. (2020) found that items from the shift subscale of BRIEF and internalizing subscale of CBCL loaded onto the same factor in a group of autistic adolescents, suggesting construct overlap in the two measurements. Ozsivadjian et al. (2021) also found that parent rated CI measured by FS‐R was not directly associated with INT, but rather was directly associated with greater intolerance of uncertainty, which in turn increased level of parent reported anxiety symptoms in adolescents with autism. Similarly, studies using neurocognitive assessment or experimental tasks to assess CI in adolescents with ASD also found that greater CI was associated with greater behavioral difficulties (Teunisse et al., 2012) including INT (Andersen et al., 2015), anxiety and depression (Crawley et al., 2020; Hollocks et al., 2014), and socioemotional problems (Dajani et al., 2016).
Two studies used a longitudinal study design and explored CI as a mediator of changes in INT severity over adolescence (Hollocks et al., 2022), and as a moderator between family stressful life events (F‐SLE) and future INT during childhood (Carter Leno et al., 2022). Greater CI at age 16 was found to be a predictor of greater anxiety and depression at age 23 amongst autistic adolescents, and also partially mediated changes in symptom severity of anxiety, depression and emotional problems between the ages of 16 and 23 (Hollocks et al., 2022). Amongst autistic children, CI only moderated the relationship between F‐SLE and future INT between the ages of 7 and 11 amongst those with atypical shifting abilities measured at age 8 as reported by parents, and not those with typical shifting abilities (Carter Leno et al., 2022).
Meta‐analyses of CI and INT
The meta‐analysis examining the association between CI and INT ranged from 0.24 and 0.54 across a total of nine studies (n = 833 children and adolescents with ASD) in five countries (Figure 2). Two studies included adolescents with both ASD and ADHD (n = 40) (Sesso et al., 2020; Yerys et al., 2009). A forest plot of the reported correlation coefficient between CI and INT estimates with 95% confidence interval for all the included studies is shown in Figure 2. The meta‐analysis showed a significant, moderate effect size, r = 0.39, p < 0.001, 95% CI [0.32, 0.46], indicating that higher CI was associated with higher levels of INT. Heterogeneity was low: Q(8) = 7.93, p = 0.44, I 2 = 13.17%. There was a nonsignificant moderator effect of participants' age (Q[1] = 3.38, p = 0.07), proportion of autistic male participants (Q[1] = 0.23, p = 0.63), mean FSIQ (Q[1] = 2.51, p = 0.11), and study quality (Q[1] = 2.51, p = 0.11). Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.13) nor Rank Correlation Test (p = 0.61) suggested evidence for publication bias. Post hoc sensitivity analyses (Appendix C [a]) found that a significant moderate effect size was maintained with only studies using parent‐report measures of CI (six studies; r = 0.48, p < 0.001, 95% CI [0.36, 0.52]), with only studies using performance‐based measures of CI (three studies; r = 0.34, p < 0.001, 95% CI [0.25, 0.44]), and when excluding studies with autistic adolescents and co‐occurring ADHD (seven studies; r = 0.38, p < 0.001, 95% CI [0.31, 0.45]).
FIGURE 2.
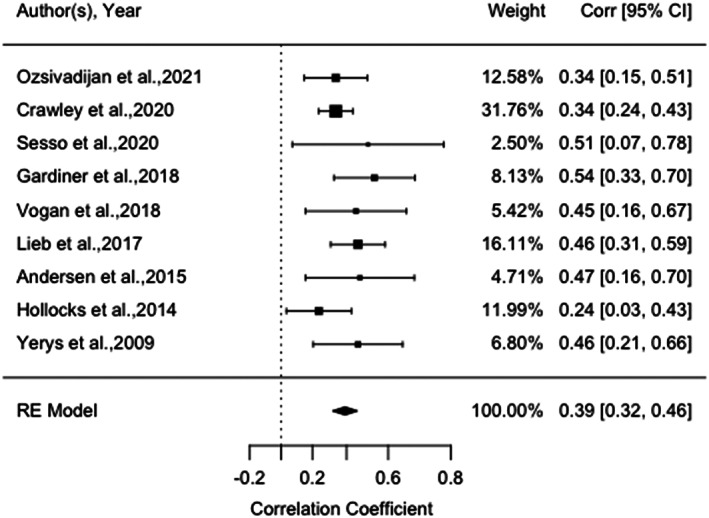
Forest plot of correlation between measures of cognitive flexibility and internalizing behaviors amongst autistic children and adolescents, and 95% confidence interval for random effects (RE) model
CI and EXT
Table 4 shows a summary of results from the 15 studies that explored the association between EXT and CI. The majority of studies used the BRIEF‐Shift scale parent measure of CI and found that greater CI in adolescents with ASD was associated with greater EXT (Gardiner & Iarocci, 2018; Lawson et al., 2015; Ozsivadjian et al., 2021; Vogan et al., 2018; Yerys et al., 2009). However, one study found increased EXT only correlated with greater CI as measured by the RBS‐R Sameness scale, but not by BRIEF‐Shift scale (Maddox et al., 2018). Only one study which included a sample of adolescents with ASD and ADHD found no association between CI and EXT (Sesso et al., 2020). Results from studies using neurocognitive assessment measures and cognitive tasks showed more mixed findings. One study which used a combination of CI measures from the NEPSY‐II and WISC‐IV showed that adolescents with ASD were more likely to show impaired executive function compared to adolescents with ADHD or neurotypical peers, and greater executive function impairment was associated with higher socioemotional difficulties including aggression (Dajani et al., 2016). In contrast, one study which used the CANTAB ID/ED found CI was not associated with levels of callous‐unemotional traits that may contribute towards greater EXT (Rogers et al., 2006), and another which used the color‐word interference task also found that CI was not significantly associated with EXT (Andersen et al., 2015). Another study which used a range of tasks (block design, trail making, opposite words task and WCST) also found that CI showed a moderate (nonsignificant) association with increased behavioral problems amongst autistic adolescents (Hollocks et al., 2022).
Two studies used a longitudinal study design and explored CI as a mediator of changes in EXT severity over adolescence (Hollocks et al., 2022), and as a moderator between family stressful life events (F‐SLE) and future EXT during childhood (Carter Leno et al., 2022). Greater CI at age 16 was found to be a predictor of greater behavioral problems at age 23 amongst autistic adolescents (Hollocks et al., 2022). Amongst autistic children, although CI did not significantly moderate the relationship between F‐SLE and future EXT between the ages of 7 and 11, a near‐significant trend was observed amongst those with atypical shifting abilities measured at age 8 as reported by parents compared to those with typical shifting abilities (Carter Leno et al., 2022).
Meta‐analyses of CI and EXT
The meta‐analysis examining the association between CI and EXT ranged from 0.24 and 0.61 across a total of six studies (n = 295 children and adolescents with ASD) in five countries (Figure 3). Five of the six studies used a parent report measure to assess CI in adolescents with ASD. A forest plot of the reported correlation coefficient between CI and EXT estimates with 95% confidence intervals for all the included studies are shown in Figure 3. The meta‐analysis showed a significant, large effect size, r = 0.48, p < 0.001, 95% CI [0.38, 0.58], indicating that higher CI was associated with higher levels of EXT. Heterogeneity was low: Q(5) = 6.40, p = 0.27, I2 = 14.63%. There was a nonsignificant moderator effect of participants' age (Q[1] = 0.08, p = 0.78), proportion of autistic male participants (Q[1] = 0.03, p = 0.87), mean FSIQ (Q[1] = 0.06, p = 0.80), and study quality (Q[1] = 0.06, p = 0.80). Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.27) nor Rank Correlation Test (p = 0.47) suggested evidence for publication bias. Post hoc sensitivity analyses (Appendix C [b]) showed a significant large effect size was maintained with only studies using parent‐report measures of CI (five studies; r = 0.51, p < 0.001, 95% CI [0.41, 0.60]), and when excluding studies with autistic adolescents and co‐occurring ADHD (four studies; r = 0.52, p < 0.001, 95% CI [0.40, 0.62]).
FIGURE 3.
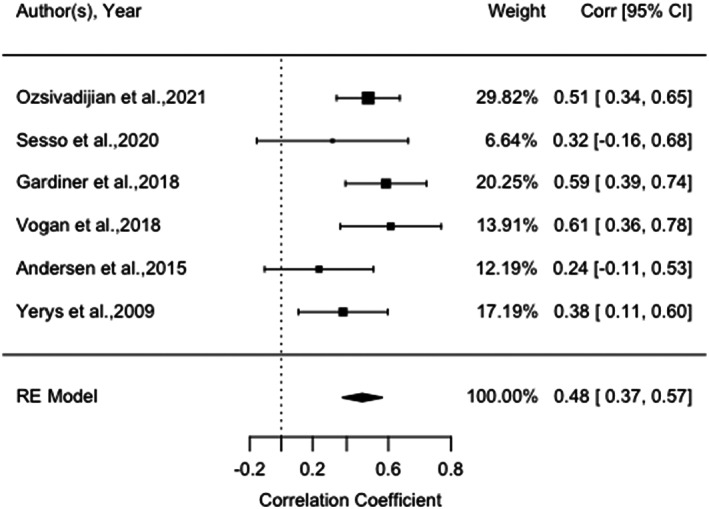
Forest plot of correlation between measures of cognitive inflexibility and externalizing behaviors amongst autistic children and adolescents, and 95% confidence interval for random effects (RE) model
DISCUSSION
CI, internalizing, and externalizing symptoms
The current systematic review and meta‐analysis found a significant and moderate to large effect size between CI and greater internalizing and externalizing symptoms in adolescents with ASD. Findings are robust given the low degree of heterogeneity across studies included in the meta‐analyses, and results withstood sensitivity analysis when only including parent‐report of CI or performance‐based measures of CI (for internalizing symptoms only) and excluding autistic adolescents with co‐occurring ADHD diagnosis. CI may be a transdiagnostic factor that can increase one's vulnerability to experiencing rigid or perseverative patterns of unhelpful cognition (e.g., rumination) and behaviors (e.g., avoidance, reduced activity, aggression) (Hollocks et al., 2022), resulting in maladaptive emotion regulation strategies that are less effective in the moment (Cai et al., 2018).
The current study found that the effect size of the association between CI and internalizing symptoms was greater when CI was measured using parent‐report measures (r = 0.48) compared to performance‐based task measures (r = 0.34). It is important to note that a major caveat is that only three studies used a performance‐based task measure and therefore the generalisability of this finding may be somewhat limited. However, this finding is significant when considering literature has highlighted issues around convergence of measurement between more ecologically valid reporter‐based measures (e.g., BRIEF) that assess how CI may affect daily functioning activities, compared to performance‐based measures of CI that assess more specific cognitive constructs in a lab‐based setting (e.g., WCST) (Uddin, 2021).
The convergence of effect sizes in the current meta‐analysis is significant to suggest that there is some shared unitary construct underlying CI, as the association between internalizing symptoms and CI remains when accounting for measurement differences. The stronger association with parent‐rated measures may be a combination of shared method variance, and that behavioral implications of CI can be more easily observed across different settings in daily lives by parents/carers. The latter is particularly important when considering how individual differences in cognitive flexibility may be either a risk factor or protective factor in the context of biopsychosocial changes during adolescence, and therefore the impact of CI on daily adaptive functioning and behavior in relation to psychopathology is more important for clinicians to assess and incorporate into formulation and treatment when working with autistic young people.
Although the current meta‐analysis did not explicitly examine the reciprocal impact of co‐occurring internalizing/externalizing symptoms on autistic adolescents' CI, it is possible that increased symptomatology can negatively impact autistic adolescents' flexible problem solving ability as reflected by frequent “stuck‐in‐set perseveration” errors during cognitive flexibility tasks (Crawley et al., 2020; Tachibana et al., 2013). For example, rumination over negative thoughts in depression can perpetuate over time, resulting in greater inactive and less flexible ways of thinking, rather than actively engaging with the environment and problem solving (Kashdan, 2010). Over time, pervasive negative cognitive style can also reduce behavioral flexibility and result in more rigid coping behaviors, further affecting one's emotional and social functioning (Kashdan, 2010). Individuals with heightened anxiety may also engage in experiential avoidance to reduce psychological distress, and deploy more rigid patterns of behavioral responses and experience persistent worries regardless of situational context (Borkovec, 1994).
However, the direction of causation between CI and behavioral symptoms remains ambiguous, as only three studies employed a longitudinal research design to provide insight from a developmental perspective (Andersen et al., 2015; Carter Leno et al., 2022; Hollocks et al., 2022). This is especially important as one meta‐analysis exploring changes in CI from childhood (<12 years) to adulthood (>18 years) found that adolescence (between 12–18 years) marked a period of significant heterogeneity for CI measured across studies (Demetriou et al., 2018). One study found that increased rigidity in thinking and rumination may be a predisposing and perpetuating factor that results in prolonged experience of distress from family stressful life events for autistic children aged 7–11 years, increasing their vulnerability to developing and maintaining internalizing symptoms across childhood (Carter Leno et al., 2022). However, it is unclear whether greater CI may have a direct effect on the development of externalizing symptoms before puberty (Carter Leno et al., 2022).
During adolescence, although improvements in CI were noted amongst children and adolescents with ASD aged 9–16 years, performance was still poorer compared to their neurotypical peers, and adolescents with ASD maintained greater levels of depression symptoms (Andersen et al., 2015). The relatively protracted maturation of cognitive flexibility for adolescents with ASD compared to neurotypical peers might mean less adaptable ways of coping with the challenges that arise during adolescence, and increase one's vulnerability to developing internalizing symptoms later in adulthood (Andersen et al., 2015).
When transitioning from adolescence to young adulthood, Hollocks et al. (2022) found that CI measured at the age of 16 continued to be associated with symptoms of anxiety and depression and at the age of 23, suggesting that it is an important cognitive mechanism that may influence the development and maintenance of internalizing symptoms over time. The same study also found that when controlling for restricted and repetitive behaviors (RRBs), CI measured at age 16 was significantly associated with externalizing symptoms at the ages of 16 and 23, suggesting that the continued impact of CI on emotion regulation is maintained across adolescent development, independent of RRBs considered to be core to ASD symptomatology.
The overlap between emotion regulation difficulties and CI in autism has been supported by neuroimaging studies where reduced connectivity between frontal and limbic regions of the brain may be associated with ineffective top‐down emotion regulation in response to negative emotions (Samson et al., 2015). Reduced top‐down emotion regulation may be especially evident during adolescence where the development of frontal lobes and executive functions matures at a slower rate compared to limbic brain regions for emotion processing (Blakemore & Robbins, 2012). Autistic adolescents may be even more vulnerable compared to neurotypical peers to feel overwhelmed by difficult emotions when unable to switch between maladaptive and adaptive emotion regulation strategies due to greater CI.
Measurement of CI
Most studies in the current review relied on parent‐report to assess CI, especially the shift scale of BRIEF. Both parent measures and cognitive tasks largely indicate greater CI amongst adolescents with ASD compared to neurotypical peers or peers with other neurodevelopmental conditions, though greater variation in performance were noted when using task‐performance based ratings. This may be due to experimental and neurocognitive tasks requiring a range of cognitive processes beyond cognitive flexibility to be employed for successful performance, and therefore it is difficult to unpick the extent to which CI may have contributed towards performance variance across individuals, without controlling for cognitive processes other than CI (Geurts et al., 2009).
Only one study explored the concordance between parent report of CI and adolescents' performance on neurocognitive tasks (Teunisse et al., 2012). Shared method variance was observed within parental measures and performance measures, though not between these measures of CI. Compared to task‐based measures, parental report of CI showed lower specificity as they also positively correlated with general behavioral problems, IQ and ASD symptomatology. The “Halo Effect”3 on the association between CI and behavioral measures rated by parents may be due to questions about executive function often including a component of emotional control (e.g., items on shift subscale of BRIEF uses words such as “resists,” “becomes upset,” “is disturbed by”). Parents reporting CI may take into consideration internalizing and externalizing symptoms and result in greater construct overlap. Therefore, it is important to be cautious when interpreting the positive associations identified in this meta‐analysis which is largely based on parent measures of CI.
Limitations
The current systematic review/meta‐analysis has several limitations. First, the majority of studies relied on parent reports of CI and emotional/behavioral difficulties, and therefore may result in inflated correlation across the measures due to shared methods variance (Podsakoff et al., 2003; Yorke et al., 2018). One recent study found parents perceived the magnitude of CI to be much greater compared to adolescents' self‐reports, and parents focused on observable behaviors at home/community compared to adolescents reporting on their inner experiences across multiple contexts including school (Kenworthy et al., 2022). Future studies should aim to assess CI by drawing on a range of perspectives including parents, teachers, self‐report, and objective assessment (e.g., cognitive assessment). Furthermore, the few studies that used task‐based measures showed greater individual variances in autistic adolescents' CI compared to parent reports, which may suggest greater heterogeneity in construct specificity across different tasks. Future studies may wish to use multiple tasks to extrapolate a latent construct of CI that may be more directly comparable across different studies.
Second, generalisability of findings is limited as study samples mostly failed to include autistic adolescents with intellectual disability. It is unclear for studies that did not report co‐occurring conditions amongst autistic adolescents whether this was not assessed/recorded or whether no co‐occurring conditions were found within the sample, the latter being unlikely given the high rates of psychiatric co‐occurring conditions found in this population (Simonoff et al., 2008). It may be possible that between‐subject differences in CI may be attributed to unreported co‐occurring conditions (such as ADHD) rather than ASD per se. Future studies can therefore benefit from more robustly assessing and explicitly reporting co‐occurring conditions in autistic adolescents.
Finally, the current study samples were largely boys. Sex differences in CI in autism have remained largely unexplored, with only one study including autistic children and adolescents aged 7–14 years suggesting that girls had poorer performance in WCST with greater perseverative errors and completing fewer categories compared to boys (Memari et al., 2013). Future studies can include more autistic females to further explore whether there are sex‐based differences in CI observed in autism, in relation to internalizing and externalizing symptoms over the course of development.
Clinical implications
The current meta‐analysis explored the association between CI and internalizing and externalizing symptoms in autistic children and adolescents, with the hope to highlight how this domain may be a possible treatment target that will enhance therapeutic outcomes when explicitly addressed in clinical interventions for psychopathology when working with this clinical group. Our findings suggest that CI does have associations with internalizing and externalizing symptoms in autistic children and adolescents, and evidence does support that clinicians should assess for and incorporate individual differences in CI into person‐centered formulation, and adapt clinical interventions to either explicitly target CI, or account for how CI may interfere with treatment efficacy and reception perceived by the young person. Accounting for individual differences in CI is especially important given many evidence‐based psychological treatments for mental health problems aim to bring about cognitive and behavioral change and thus are reliant on flexibility in both cognition and behavior.
As cognitive flexibility can support individuals to flexibly adapt to different situational demands (Kashdan, 2010), clinicians should more consistently evaluate individual differences in CI to guide assessment and personalization of treatment approach when working with autistic adolescents. Current adaptations to evidence‐based treatment for autistic adolescents with mental health conditions often focus on changing the format of communication and session structure, such as by having more frequent sessions and adopting more visual aids to make session material more concrete (Rodgers & South, 2021). However, such adaptations do not directly address constructs such as CI (Scarpa et al., 2021), which might affect engagement and response to therapeutic approaches that aim to increase awareness of alternative patterns of thinking and behavior (e.g., Cognitive Behavioral Therapy) (Rodgers & South, 2021), and reduce intervention effectiveness.
One approach that explicitly targets CI and executive functions such as planning and organization is called “Unstuck and On Target!” (Cannon et al., 2011), developed for educators to deliver in classroom settings for autistic students aged 8–11 years without intellectual disability, to support students in learning and utilizing their skills to increase flexibility in real‐life (Kenworthy et al., 2014). To increase children's perceived sense of control over flexible decision making in a nonthreatening way, the use of gamified digital platforms that have clear visual cues may help children more easily access, engage with, and adhere to new intervention approaches (Blackwell et al., 2021). Supporting autistic adolescents to internalize flexible thinking can shape their resilience and potentially buffer against adversity, such as family stressful life events, and support them to navigate more complex situations by better balancing self‐regulation and goal‐oriented behaviors (Scarpa et al., 2021).
CONFLICT OF INTEREST
Dr. Charman has served as a paid consultant to F. Hooffmann‐La Roche Ltd. And Servier; and has received royalties from Sage Publications and Guildford Publications. Dr. Russell has received royalties from Jessica Kingsley (Hachette) Publications. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
The current study was exempted from institutional review board review because it was based on reanalysis of published data.
ACKNOWLEDGMENTS
The authors thank all of the researchers who participated in the studies involved in the current article.
APPENDIX A.
Full Electronic Database Search Terms and history of preliminary scoping search results.
Planned search terms (See Appendix A for more information on preliminary scoping searches):
Main search terms include the following search constructs used for both aims:
Autism: ((Autis*) OR (Asperg*) OR (ASD) OR (ASC) OR (PDD)) AND
Children/adolescent: ((adolescen*) OR (young person) OR (young people) OR (youth*) OR (child*) OR (infant*) OR (toddler*)) AND
Cognitive flexibility †: ((cognitive flexib*) OR (cognitive inflexib*) OR (cognitive rigid*) OR (rigid*) OR (mental flexib*) OR (set shift*) OR (WCST) OR (Wisconsin Card Sorting Task) OR (Trail Making) OR (Brixton) OR (Haptic illusion) OR (Catbat) OR (Delis‐Kaplan Executive Function System) OR (Behavior Rating Inventory*) OR (Cognitive Flexibility Scale*)).
Aim 1 ‐ Internalizing symptoms: (Anxiety) OR (internali*) OR (OCD) OR (intrus*) OR (mood) OR (depress*) OR (affect*) OR (suicid*) OR (self‐harm*) OR (somati*) OR (PTSD) OR (Trauma*) OR (Phobia).
Aim 2 ‐ Externalizing symptoms: (aggress*) OR (antisocial*) OR (externali*) OR (delinquen*) OR (disrupt*) OR (conduct*) OR (anger*) OR (defiant) OR (hyperactiv*) OR (challenging behav*) OR (ADHD) OR (ODD) OR (oppositional*).
Preliminary scoping search results:
Main search terms: A preliminary scoping search using the main search terms on PubMed on July 12, 2021 generated 4093 results. Many of the search terms for cognitive flexibility were extracted from a published systematic review exploring cognitive flexibility in patients with Anorexia Nervosa (Miles et al., 2020).
Main ssarch terms and Aim 1: A preliminary scoping search using the main search terms and search terms unique to Aim 1 in PubMed on July 12, 2021 generated 1012 results.
Main search terms and Aim 2: A preliminary scoping search using the main search terms and search terms unique to Aim 2 in PubMed on July 12, 2021 generated 1097 results.
†Summary of main measures of cognitive flexibility as reported in (Miles et al., 2020).
Neurocognitive assessment measures and cognitive tasks
Wisconsin Cart Sorting Task (WCST).
Trail Making Test (TMT).
Berg's Card Sorting Task.
Brixton Spatial Anticipation Test.
CANTAB Intra‐and Extra‐Dimensional Task (ID/ED).
CatBat.
Controlled Oral Word Association Test.
Delis‐Kaplan Executive Function System (D‐KEFS) – in particular the color‐word interference task, TMT and verbal fluency task.
Haptic Illusions Task.
Hayling Sentence Completion Task.
-
2
Self‐report measures
Cognitive Flexibility Scale (CFS).
Shift subscale of Behavior Rating Inventory of Executive Functioning.
Detail and Flexibility questionnaire.
APPENDIX B.
Description of the Quality Appraisal Tool (Kmet et al., 2004):
This 14‐item tool has a detailed scoring protocol for examining (1) description of study objectives, (2) appropriateness of study design for addressing research question, (3) method of participant selection, (4) quality of participant information reported, (5) random allocation to treatment group (if applicable), (6) intervention blinding of investigators (if applicable), (7) intervention blinding of participants (if applicable), (8) description of outcome variables, (9) appropriateness of sample size, (10) appropriateness of statistical analysis, (11) estimate of variance for main results, (12) control for confounding variables, (13) sufficient detail in reporting of results, (14) whether results support conclusions drawn. Each item is rated on a scale of yes (2 points), partial (1 point), no (0 point) and not applicable (N/A). The summary score (between 0 and 1) is calculated in three steps: (1) calculate the total sum score = (number of “yes” *2 points) + (number of “partials” *1 point), (2) calculate the total possible sum = 28–(total number of “N/A" * 2 points); (3) create summary score (range 0–1) = total sum/total possible sum. This tools has been successfully used in the past for systematic reviews examining quantitative research in older adults with autism (Tse et al., 2021).
APPENDIX C.
Sensitivity analyses for cognitive inflexibility and internalizing symptoms.
To explore whether the effect size observed above between internalizing symptoms and cognitive flexibility remains when accounting for differences in method of measurement (i.e., parent report vs. task‐based measure), a post hoc sensitivity analysis was completed including only studies that used a parent report measure of cognitive flexibility (n = 6). The sensitivity analysis showed a significant, moderate effect size, r = 0.48, p < 0.001, 95% CI [0.36, 0.52], indicating that higher cognitive inflexibility was associated with higher levels of internalizing symptoms. There was no substantial degree of heterogeneity, Q(5) = 2.48, p = 0.78, I 2 = 0%. Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.56) nor Rank Correlation Test (p = 1.00) suggested evidence for publication bias.
A separate post hoc sensitivity analysis was completed including only studies that used performance‐based measures of cognitive flexibility (n = 3). The sensitivity analysis showed a significant, moderate effect size, r = 0.34, p < 0.001, 95% CI [0.25, 0.44], indicating that higher cognitive inflexibility was associated with higher levels of internalizing symptoms. There was no substantial degree of heterogeneity, Q(2) = 1.74, p = 0.41, I 2 = 0.01%. Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.66) nor Rank Correlation Test (p = 1.00) suggested evidence for publication bias.
To explore the extent to which the effect size observed between internalizing symptoms and cognitive flexibility is affected by co‐occurring ADHD, a post hoc sensitivity analysis was completed by excluding the two studies with young people with ASD and ADHD (Sesso et al., 2020; Yerys et al., 2009), leaving a total of seven studies in this analysis. The sensitivity analysis showed a significant, moderate effect size, r = 0.38, p < 0.001, 95% CI [0.31, 0.45], indicating that higher cognitive inflexibility was associated with higher levels of internalizing symptoms. There was no substantial degree of heterogeneity, Q(6) = 7.09, p = 0.31, I 2 = 16.99%. Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.26) nor Rank Correlation Test (p = 0.56) suggested evidence for publication bias.
-
b
Sensitivity analyses for cognitive inflexibility and externalizing symptoms.
To explore whether the effect size observed above between externalizing symptoms and cognitive flexibility remains when accounting for differences in method of measurement (i.e., parent report vs. task‐based measure), a post hoc sensitivity analysis was completed including only studies that used a parent report measure of cognitive flexibility (n = 5). The sensitivity analysis showed a significant, a significant, large effect size, r = 0.51, p < 0.001, 95% CI [0.41, 0.60], indicating that higher cognitive inflexibility was associated with higher levels of externalizing symptoms. There was no substantial degree of heterogeneity, Q(4) = 3.58, p = 0.47, I2 = 0%). Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.54) nor Rank Correlation Test (p = 0.82) suggested evidence for publication bias. Given only one study used behavioral task to measure cognitive flexibility, a sensitivity analysis could not be conducted.
To explore the whether the effect size observed between externalizing symptoms and cognitive flexibility remains when accounting for co‐occurring ADHD, a post hoc sensitivity analysis was completed by excluding the two studies with young people with ASD and ADHD (Sesso et al., 2020; Yerys et al., 2009), leaving a total of four studies in the analysis. The sensitivity analysis showed a significant, large effect size, r = 0.52, p < 0.001, 95% CI [0.40, 0.62], indicating that higher cognitive inflexibility was associated with higher levels of externalizing symptoms. There was no substantial degree of heterogeneity, Q(3) = 4.63, p = 0.20, I2 = 17.96%. Funnel plot did not show significant study asymmetry, and neither Egger's regression test (p = 0.56) nor Rank Correlation Test (p = 0.75) suggested evidence for publication bias.
Lei, J. , Charman, T. , Leigh, E. , Russell, A. , Mohamed, Z. , & Hollocks, M. J. (2022). Examining the relationship between cognitive inflexibility and internalizing and externalizing symptoms in autistic children and adolescents: A systematic review and meta‐analysis. Autism Research, 15(12), 2265–2295. 10.1002/aur.2826
Footnotes
This study uses both identity‐first and person‐first language when referring to autism, as studies in recent years have shown that the semantic choice of language when referring to autism is often debated without a general consensus being reached (Bury et al., 2020; Kenny et al., 2016; Vivanti, 2020).
Block design is included as a proxy for cognitive flexibility as it is a task that requires non‐verbal problem solving and loads significantly onto the latent construct measuring cognitive inflexibility, such as following through a well‐organized response in an efficient, flexible, and goal‐directed manner. Block design has previously been used as a clinical outcome measure of the latent construct of cognitive inflexibility in a clinical trial on “Unstuck and on Target” – an intervention aimed to target cognitive inflexibility in autistic children by Kenworthy et al. (2014).
The Halo Effect refers to the concept that a reporter rating on someone else's behavior may fail to distinguish between distinct and independent aspects of the behaviors observed, resulting in inflated inflation of correlation between the different types of behaviors observed (Saal et al., 1980).
Contributor Information
Jiedi Lei, Email: jiedi.lei@kcl.ac.uk.
Matthew J. Hollocks, Email: matthew.hollocks@kcl.ac.uk.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). American Psychiatric. [Google Scholar]
- Andersen, P. N. , Skogli, E. W. , Hovik, K. T. , Egeland, J. , & Øie, M. (2015). Associations among symptoms of autism, symptoms of depression and executive functions in children with high‐functioning autism: A 2 year follow‐up study. Journal of Autism and Developmental Disorders, 45(8), 2497–2507. 10.1007/s10803-015-2415-8 [DOI] [PubMed] [Google Scholar]
- Bennett, J. , & Müller, U. (2010). The development of flexibility and abstraction in preschool children. Merrill‐Palmer Quarterly, 56(4), 455–473. [Google Scholar]
- Berenguer, C. , Roselló, B. , Colomer, C. , Baixauli, I. , & Miranda, A. (2018). Children with autism and attention deficit hyperactivity disorder. Relationships between symptoms and executive function, theory of mind, and behavioral problems. Research in Developmental Disabilities, 83, 260–269. 10.1016/j.ridd.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Bertollo, J. R. , Strang, J. F. , Anthony, L. G. , Kenworthy, L. , Wallace, G. L. , & Yerys, B. E. (2020). Adaptive behavior in youth with autism Spectrum disorder: The role of flexibility. Journal of Autism and Developmental Disorders, 50(1), 42–50. 10.1007/s10803-019-04220-9 [DOI] [PubMed] [Google Scholar]
- Blackwell, S. , Zylberberg, A. , Scerif, G. , Miller, S. , & Woodcock, K. A. (2021). Understanding the psycho‐social context for a new early intervention for resistance to change that aims to strike a beneficial balance between structure and flexibility. BMC Psychiatry, 21(1), 621. 10.1186/s12888-021-03519-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, S.‐J. , & Robbins, T. W. (2012). Decision‐making in the adolescent brain. Nature Neuroscience, 15(9), 1184–1191. 10.1038/nn.3177 [DOI] [PubMed] [Google Scholar]
- Borkovec, T. D. (1994). The nature, functions, and origins of worry. In Davey G. & Tallis F. (Eds.), Worrying: Perspectives on theory, assessment, and treatment (pp. 5–33). Wiley & Sons. [Google Scholar]
- Bury, S. M. , Jellett, R. , Spoor, J. R. , & Hedley, D. (2020). “It defines who I am” or “It's something I have”: What language do [autistic] Australian adults [on the autism Spectrum] prefer? Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04425-3 [DOI] [PubMed] [Google Scholar]
- Cai, R. Y. , Richdale, A. L. , Uljarević, M. , Dissanayake, C. , & Samson, A. C. (2018). Emotion regulation in autism spectrum disorder: Where we are and where we need to go. Autism Research, 11(7), 962–978. 10.1002/aur.1968 [DOI] [PubMed] [Google Scholar]
- Cannon, L. , Kenworthy, L. , Alexander, K. C. , Werner, M. A. , & Anthony, L. (2011). Unstuck and on target! An executive function curriculum to improve flexibility for children with autism Spectrum disorders. Brookes Publishing Company. [Google Scholar]
- Carter Leno, V. , Wright, N. , Pickles, A. , Bedford, R. , Zaidman‐Zait, A. , Kerns, C. , Mirenda, P. , Zwaigenbaum, L. , Duku, E. , Bennett, T. , Georgiades, S. , Smith, I. , Vaillancourt, T. , Szatmari, P. , & Elsabbagh, M. (2022). Exposure to family stressful life events in autistic children: Longitudinal associations with mental health and the moderating role of cognitive flexibility. Autism, 26, 1656–1667. 10.1177/13623613211061932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2019). Data and statistics on autism Spectrum disorder|CDC. Centers for Disease Control and Prevention. U.S. Department of Health and Human Services. https://www.cdc.gov/ncbddd/autism/data.html [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Routledge. [Google Scholar]
- Core Team R . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Cragg, L. , & Chevalier, N. (2012). The processes underlying flexibility in childhood. Quarterly Journal of Experimental Psychology, 65(2), 209–232. 10.1080/17470210903204618 [DOI] [PubMed] [Google Scholar]
- Crawley, D. , Zhang, L. , Jones, E. J. H. , Ahmad, J. , Oakley, B. , Cáceres, A. S. J. , Charman, T. , Buitelaar, J. K. , Murphy, D. G. M. , Chatham, C. , Ouden, H. d. , Loth, E. , & Group, the E.‐A. L . (2020). Modeling flexible behavior in childhood to adulthood shows age‐dependent learning mechanisms and less optimal learning in autism in each age group. PLoS Biology, 18(10), e3000908. 10.1371/journal.pbio.3000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretenet, J. , & Dru, V. (2009). Influence of peripheral and motivational cues on rigid‐flexible functioning: Perceptual, behavioral, and cognitive aspects. Journal of Experimental Psychology, 138(2), 201–217. 10.1037/a0015379 [DOI] [PubMed] [Google Scholar]
- Dajani, D. R. , Llabre, M. M. , Nebel, M. B. , Mostofsky, S. H. , & Uddin, L. Q. (2016). Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Scientific Reports, 6, 36566. 10.1038/srep36566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák, G. O. (2003). The development of cognitive flexibility and language abilities. In advances in child development and behavior (Vol. 31, pp. 271–327). Academic Press. [DOI] [PubMed] [Google Scholar]
- Dekkers, O. M. , Vandenbroucke, J. P. , Cevallos, M. , Renehan, A. G. , Altman, D. G. , & Egger, M. (2019). COSMOS‐E: Guidance on conducting systematic reviews and meta‐analyses of observational studies of etiology. PLoS Medicine, 16(2), e1002742. 10.1371/journal.pmed.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou, E. A. , DeMayo, M. M. , & Guastella, A. J. (2019). Executive function in autism Spectrum disorder: History, theoretical models, empirical findings, and potential as an Endophenotype. Frontiers in Psychiatry, 10. 10.3389/fpsyt.2019.00753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou, E. A. , Lampit, A. , Quintana, D. S. , Naismith, S. L. , Song, Y. J. C. , Pye, J. E. , Hickie, I. , & Guastella, A. J. (2018). Autism spectrum disorders: A meta‐analysis of executive function. Molecular Psychiatry, 23(5), 1198–1204. 10.1038/mp.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckhaus, M. F. S. , Hardy, K. K. , Gutermuth Anthony, L. , Verbalis, A. , Kenworthy, L. , & Pugliese, C. E. (2021). Anxiety relates to classroom executive function problems in students with ASD, but not ADHD. Research in Autism Spectrum Disorders, 82, 101739. 10.1016/j.rasd.2021.101739 [DOI] [Google Scholar]
- Dietrich, A. , & Kanso, R. (2010). A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin, 136(5), 822–848. 10.1037/a0019749 [DOI] [PubMed] [Google Scholar]
- Egger, M. , Smith, G. D. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, N. P. , & Miyake, A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia, M. , Barceló, F. , Clemente, I. C. , & Escera, C. (2010). The role of the dopamine transporter DAT1 genotype on the neural correlates of cognitive flexibility. The European Journal of Neuroscience, 31(4), 754–760. 10.1111/j.1460-9568.2010.07102.x [DOI] [PubMed] [Google Scholar]
- Gardiner, E. , & Iarocci, G. (2018). Everyday executive function predicts adaptive and internalizing behavior among children with and without autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 11(2), 284–295. 10.1002/aur.1877 [DOI] [PubMed] [Google Scholar]
- Geurts, H. M. , Corbett, B. , & Solomon, M. (2009). The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences, 13(2), 74–82. 10.1016/j.tics.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia, G. A. , Isquith, P. K. , Guy, S. C. , & Kenworthy, L. (2000). Test review behavior rating inventory of executive function. Child Neuropsychology, 6(3), 235–238. 10.1076/chin.6.3.235.3152 [DOI] [PubMed] [Google Scholar]
- Happé, F. , Booth, R. , Charlton, R. , & Hughes, C. (2006). Executive function deficits in autism spectrum disorders and attention‐deficit/hyperactivity disorder: Examining profiles across domains and ages. Brain and Cognition, 61(1), 25–39. 10.1016/j.bandc.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks, M. J. , Charman, T. , Baird, G. , Lord, C. , Pickles, A. , & Simonoff, E. (2022). Exploring the impact of adolescent cognitive inflexibility on emotional and behavioural problems experienced by autistic adults. Autism, 26(5), 1229–1241. 10.1177/13623613211046160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks, M. J. , Jones, C. R. G. , Pickles, A. , Baird, G. , Happé, F. , Charman, T. , & Simonoff, E. (2014). The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 7(2), 216–228. 10.1002/aur.1361 [DOI] [PubMed] [Google Scholar]
- Ionescu, T. (2012). Exploring the nature of cognitive flexibility. New Ideas in Psychology, 30(2), 190–200. 10.1016/j.newideapsych.2011.11.001 [DOI] [Google Scholar]
- Kashdan, T. B. (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(7), 865–878. 10.1016/j.cpr.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, L. , Hattersley, C. , Molins, B. , Buckley, C. , Povey, C. , & Pellicano, E. (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Kenworthy, L. , Anthony, L. G. , Naiman, D. Q. , Cannon, L. , Wills, M. C. , Luong‐Tran, C. , Werner, M. A. , Alexander, K. C. , Strang, J. , Bal, E. , Sokoloff, J. L. , & Wallace, G. L. (2014). Randomized controlled effectiveness trial of executive function invention for children on the autism spectrum. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(4), 374–383. 10.1111/jcpp.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy, L. , Verbalis, A. , Bascom, J. , daVanport, S. , Strang, J. F. , Pugliese, C. , Freeman, A. , Jeppsen, C. , Armour, A. C. , Jost, G. , Hardy, K. , & Wallace, G. L. (2022). Adding the missing voice: How self‐report of autistic youth self‐report on an executive functioning rating scale compares to parent report and that of youth with attention deficit hyperactivity disorder or neurotypical development. Autism, 26(2), 422–433. 10.1177/13623613211029117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmet, L. , Lee, R. C. , Cook, L. S. , Lorenzetti, D. , Godlovitch, G. , & Einsiedel, E. (2004). Systematic review of the social. In Ethical, and legal dimensions of genetic cancer risk assessment technologies. Alberta Heritage Foundatoin for Medical Research. [Google Scholar]
- Lai, M.‐C. , Kassee, C. , Besney, R. , Bonato, S. , Hull, L. , Mandy, W. , Szatmari, P. , & Ameis, S. H. (2019). Prevalence of co‐occurring mental health diagnoses in the autism population: A systematic review and meta‐analysis. The Lancet Psychiatry, 6(10), 819–829. 10.1016/S2215-0366(19)30289-5 [DOI] [PubMed] [Google Scholar]
- Lawson, R. A. , Papadakis, A. A. , Higginson, C. I. , Barnett, J. E. , Wills, M. C. , Strang, J. F. , Wallace, G. L. , & Kenworthy, L. (2015). Everyday executive function impairments predict comorbid psychopathology in autism spectrum and attention deficit hyperactivity disorders. Neuropsychology, 29(3), 445–453. 10.1037/neu0000145 [DOI] [PubMed] [Google Scholar]
- Leung, R. C. , & Zakzanis, K. K. (2014). Brief report: Cognitive flexibility in autism Spectrum disorders: A quantitative review. Journal of Autism and Developmental Disorders, 44(10), 2628–2645. 10.1007/s10803-014-2136-4 [DOI] [PubMed] [Google Scholar]
- Lieb, R. W. , & Bohnert, A. M. (2017). Relations between executive functions, social impairment, and friendship quality on adjustment among high functioning youth with autism Spectrum disorder. Journal of Autism and Developmental Disorders, 47(9), 2861–2872. 10.1007/s10803-017-3205-2 [DOI] [PubMed] [Google Scholar]
- Maddox, B. B. , Cleary, P. , Kuschner, E. S. , Miller, J. S. , Armour, A. C. , Guy, L. , Kenworthy, L. , Schultz, R. T. , & Yerys, B. E. (2018). Lagging skills contribute to challenging behaviors in children with autism spectrum disorder without intellectual disability. Autism: The International Journal of Research and Practice, 22(8), 898–906. 10.1177/1362361317712651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari, A. H. , Ziaee, V. , Shayestehfar, M. , Ghanouni, P. , Mansournia, M. A. , & Moshayedi, P. (2013). Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Research in Developmental Disabilities, 34(10), 3218–3225. 10.1016/j.ridd.2013.06.033 [DOI] [PubMed] [Google Scholar]
- Miles, S. , Gnatt, I. , Phillipou, A. , & Nedeljkovic, M. (2020). Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review. Clinical Psychology Review, 81, 101905. 10.1016/j.cpr.2020.101905 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ, 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, L. , & Mansell, W. (2018). A systematic review of the relationship between rigidity/flexibility and transdiagnostic cognitive and behavioral processes that maintain psychopathology. Journal of Experimental Psychopathology, 9(3), 2043808718779431. 10.1177/2043808718779431 [DOI] [Google Scholar]
- Ozsivadjian, A. , Hollocks, M. J. , Magiati, I. , Happé, F. , Baird, G. , & Absoud, M. (2021). Is cognitive inflexibility a missing link? The role of cognitive inflexibility, alexithymia and intolerance of uncertainty in externalising and internalising behaviours in young people with autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(6), 715–724. 10.1111/jcpp.13295 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsakoff, P. M. , MacKenzie, S. B. , Lee, J.‐Y. , & Podsakoff, N. P. (2003). Common method biases in behavioral research: A critical review of the literature and recommended remedies. The Journal of Applied Psychology, 88(5), 879–903. 10.1037/0021-9010.88.5.879 [DOI] [PubMed] [Google Scholar]
- Rietz, E. D. , Pettersson, E. , Brikell, I. , Ghirardi, L. , Chen, Q. , Hartman, C. , Lichtenstein, P. , Larsson, H. , & Kuja‐Halkola, R. (2021). Overlap between attention‐deficit hyperactivity disorder and neurodevelopmental, externalising and internalising disorders: Separating unique from general psychopathology effects. The British Journal of Psychiatry, 218(1), 35–42. 10.1192/bjp.2020.152 [DOI] [PubMed] [Google Scholar]
- Rodgers, J. , & South, M. (2021). Commentary: Thinking flexibly about mental health and autism ‐ a commentary on Ozsivadjian et al. (2020). Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(6), 725–727. 10.1111/jcpp.13340 [DOI] [PubMed] [Google Scholar]
- Rogers, J. , Viding, E. , Blair, R. J. , Frith, U. , & Happé, F. (2006). Autism spectrum disorder and psychopathy: Shared cognitive underpinnings or double hit? Psychological Medicine, 36(12), 1789–1798. 10.1017/S0033291706008853 [DOI] [PubMed] [Google Scholar]
- Saal, F. E. , Downey, R. G. , & Lahey, M. A. (1980). Rating the ratings: Assessing the psychometric quality of rating data. Psychological Bulletin, 88(2), 413–428. 10.1037/0033-2909.88.2.413 [DOI] [Google Scholar]
- Samson, A. C. , Hardan, A. Y. , Lee, I. A. , Phillips, J. M. , & Gross, J. J. (2015). Maladaptive behavior in autism Spectrum disorder: The role of emotion experience and emotion regulation. Journal of Autism and Developmental Disorders, 45(11), 3424–3432. 10.1007/s10803-015-2388-7 [DOI] [PubMed] [Google Scholar]
- Scarpa, A. , Swain, D. M. , Factor, R. S. , Dahiya, A. V. , & Bertollo, J. R. (2021). Enhancing flexibility: A biosocial model for resilience to adversity in youth with autism. SAGE Open, 11(3), 21582440211037996. 10.1177/21582440211037997 [DOI] [Google Scholar]
- Sesso, G. , Cristofani, C. , Berloffa, S. , Cristofani, P. , Fantozzi, P. , Inguaggiato, E. , Narzisi, A. , Pfanner, C. , Ricci, F. , Tacchi, A. , Valente, E. , Viglione, V. , Milone, A. , & Masi, G. (2020). Autism Spectrum disorder and disruptive behavior disorders comorbidities delineate clinical phenotypes in attention‐deficit hyperactivity disorder: Novel insights from the assessment of psychopathological and neuropsychological profiles. Journal of Clinical Medicine, 9(12), E3839. 10.3390/jcm9123839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff, E. , Pickles, A. , Charman, T. , Chandler, S. , Loucas, T. , & Baird, G. (2008). Psychiatric disorders in children with autism Spectrum disorders: Prevalence, comorbidity, and associated factors in a population‐derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Strang, J. F. , Anthony, L. G. , Yerys, B. E. , Hardy, K. K. , Wallace, G. L. , Armour, A. C. , Dudley, K. , & Kenworthy, L. (2017). The flexibility scale: Development and preliminary validation of a cognitive flexibility measure in children with autism Spectrum disorders. Journal of Autism and Developmental Disorders, 47(8), 2502–2518. 10.1007/s10803-017-3152-y [DOI] [PubMed] [Google Scholar]
- Tachibana, Y. , Hwang, Y. , Abe, Y. , Goto, S. , Sugai, K. , & Kawashima, R. (2013). Reading aloud improves executive function of children with autism spectrum disorder: A pilot randomized controlled trial. International Journal on Disability and Human Development, 12(1), 91–101. 10.1515/ijdhd-2012-0128 [DOI] [Google Scholar]
- Teunisse, J.‐P. , Roelofs, R. L. , Verhoeven, E. W. M. , Cuppen, L. , Mol, J. , & Berger, H. J. C. (2012). Flexibility in children with autism spectrum disorders (ASD): Inconsistency between neuropsychological tests and parent‐based rating scales. Journal of Clinical and Experimental Neuropsychology, 34(7), 714–723. 10.1080/13803395.2012.670209 [DOI] [PubMed] [Google Scholar]
- Trimarco, B. , Manti, F. , Nardecchia, F. , Melogno, S. , Testa, M. , Meledandri, G. , Carducci, C. , Penge, R. , & Leuzzi, V. (2020). Executive functioning, adaptive skills, emotional and behavioral profile: A comparison between autism spectrum disorder and phenylketonuria. Molecular Genetics and Metabolism Reports, 23, 100577. 10.1016/j.ymgmr.2020.100577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, V. W. S., Lei, J., Crabtree, J., Mandy, W., & Stott, J. (2021). Characteristics of Older Autistic Adults: a Systematic Review of Literature. Review Journal of Autism and Developmental Disorders, 9(2), 184–207. 10.1007/s40489-021-00238-x [DOI]
- Uddin, L. Q. (2021). Brain mechanisms supporting flexible cognition and behavior in adolescents with autism Spectrum disorder. Biological Psychiatry, 89(2), 172–183. 10.1016/j.biopsych.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarević, M. , Frazier, T. W. , Rached, G. , Busch, R. M. , Klaas, P. , Srivastava, S. , Martinez‐Agosto, J. A. , Sahin, M. , Eng, C. , Hardan, A. Y. , & Developmental Synaptopathies Consortium . (2022). Brief report: Role of parent‐reported executive functioning and anxiety in insistence on sameness in individuals with germline PTEN mutations. Journal of Autism and Developmental Disorders, 52(1), 414–422. 10.1007/s10803-021-04881-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel, F. J. A. , Bögels, S. M. , & Dirksen, C. D. (2012). Anxiety and quality of life: Clinically anxious children with and without autism spectrum disorders compared. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 41(6), 731–738. 10.1080/15374416.2012.698725 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2019). Meta‐analysis package for R. CRAN.
- Vivanti, G. (2020). Ask the editor: What is the Most appropriate way to talk about individuals with a diagnosis of autism? Journal of Autism and Developmental Disorders, 50(2), 691–693. 10.1007/s10803-019-04280-x [DOI] [PubMed] [Google Scholar]
- Vogan, V. M. , Leung, R. C. , Safar, K. , Martinussen, R. , Smith, M. L. , & Taylor, M. J. (2018). Longitudinal examination of everyday executive functioning in children with ASD: Relations with social, emotional, and behavioral functioning over time. Frontiers in Psychology, 9. 10.3389/fpsyg.2018.01774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys, B. E. , Wallace, G. L. , Sokoloff, J. L. , Shook, D. A. , James, J. D. , & Kenworthy, L. (2009). Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 2(6), 322–333. 10.1002/aur.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorke, I. , White, P. , Weston, A. , Rafla, M. , Charman, T. , & Simonoff, E. (2018). The association between emotional and behavioral problems in children with autism Spectrum disorder and psychological distress in their parents: A systematic review and meta‐analysis. Journal of Autism and Developmental Disorders, 48(10), 3393–3415. 10.1007/s10803-018-3605-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


