The molecular pathology underlying Parkinson's disease (PD) is characterized by α‐synuclein (a‐syn) misfolding and aggregation, 1 beginning with the formation of a‐syn oligomers. A‐syn oligomers are responsible for exerting neurotoxicity 2 and for driving the propagation of pathology through intercellular transmission and seeded aggregation. 3 In recent years, the mechanism of a‐syn aggregation has been compared with that of prion proteins and recent studies have used real‐time quaking induced conversion (RT‐QuIC) or protein misfolding cyclic amplification (PMCA) to detect a‐syn oligomers in cerebrospinal fluid, 4 submandibular glands, 5 skin biopsies, 6 or nasal mucosa. 7
Here, we exploited the prion‐like properties of a‐syn aggregates to detect the presence of seeding‐competent a‐syn oligomers in saliva, an easily accessible biofluid in which increased concentration of a‐syn aggregates have been detected in PD patients.8, 9 We have applied RT‐QuIC to a cohort of 37 de novo PD patients and 23 sex‐ and age‐matched healthy subjects (HS), and we have analyzed and compared different kinetic parameters including: lag‐phase; rate of change; area under the curve (AUC); Thioflavin‐T maximum value (Vmax); and percent increase of Thioflavin‐T fluorescence from baseline. Detailed methods are provided in Supplementary Appendix S1.
Salivary samples derived from PD patients exhibit a greater seeding‐capacity in the a‐syn RT‐QuIC assay compared to salivary samples from HS (Fig. 1A,B, and Bi). Of 37 PD salivary samples, 31 (86%) reached the fluorescence threshold and were, therefore, deemed RT‐QuIC positive, with the remaining 6 (14%) being RT‐QuIC negative. Among the 23 HS samples, 5 (22%) were deemed RT‐QuIC positive and the remaining 18 (78%) RT‐QuIC negative. The average lag‐phase was significantly shorter in PD samples than in HS samples, whereas the other kinetic parameters were all significantly higher in PD samples relative to HS (Supplementary Fig. S1 and Supplementary eTable S1). To evaluate the capability of the RT‐QuIC kinetic parameters to distinguish between PD and HS subjects, we used principal components analysis (PCA) and we found a segregation of PD patient and HS across the PC1 axis (Supplementary Fig. S2 ). Therefore, to evaluate the diagnostic potential of our RT‐QuIC assay we extracted the PC1 Eigenvalue—that represents a composite value of all RT‐QuIC parameters submitted to PCA—for each subject and performed receiver operating characteristic (ROC) analysis. We found that salivary RT‐QuIC possessed good diagnostic accuracy, with sensitivity of 83.78% (95% confidence interval [CI], 68.86–92.35), specificity of 82.61% (95% CI, 62.86–93.02), and a likelihood ratio of 4.818 (Fig. 1C). When we immunodepleted saliva of both PD patients and HS from a‐syn, we detected an increased lag‐phase and a significantly decreased Thioflavin‐T‐Vmax in PD patients, whereas we did not detect any difference in HS (Supplementary Fig. S3), therefore, supporting the specificity of the assay for the a‐syn aggregates.
FIG 1.
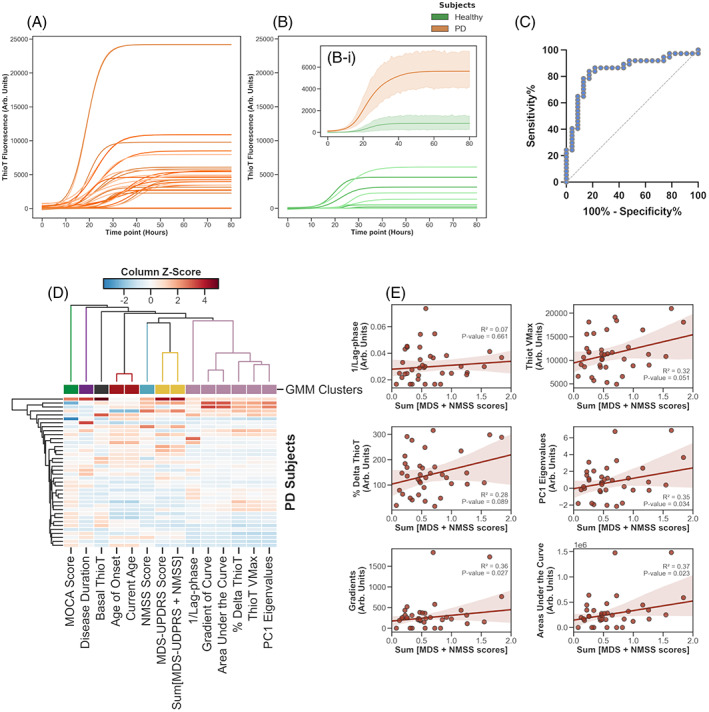
Logistic growth curves fitted to the raw kinetic curve data obtained from RT‐QuIC reactions seeded with the saliva of 37 PD patients (A) (orange lines) and 23 healthy subjects (B) (green lines). The average kinetic curves for the PD and HS subject groups are shown in (Bi); error bands represent standard deviation from the mean. Row data used to generate growth curves have been corrected for individual baseline values to eliminate the background. ThT fluorescence threshold normalized for baseline has been fixed at 2990.5 relative fluorescence units (RFU). (C) ROC analysis of PC1 Eigenvalues for all subjects: sensitivity of 83.78% (95% CI, 68.86–92.35), specificity of 82.61% (95% CI, 62.86–93.02), and a likelihood ratio of 4.818 and AUC = 0.8437. (D) A heatmap of PD subject data, which identified 7 nominal clusters, with the RT‐QuIC parameters forming one cluster (light purple) and the clinical parameters (yellow and turquoise) forming other two closely associated clusters. Agglomerative hierarchical clustering demonstrates that the RT‐QuIC parameters cluster (light purple) is hierarchically closer to the clinical scores clusters (yellow and turquoise), and more distant to other clusters: age/age of onset (red), disease duration (purple) and MoCA score (green), as well as basal Thio‐T (dark‐grey). (E ) Linear regression performed between RT‐QuIC kinetic parameters and the summed MDS‐UPDRS and NMSS scores. Pearson's correlation test found statistically significant correlations with three RT‐QuIC parameters: PC1 Eigenvalues (P = 0.034, r 2 = 0.35); gradients of fitted curves (P = 0.027, r 2 = 0.36); and areas under fitted curves (P = 0.023, r 2 = 0.37). RT‐QuIC, real‐time quaking induced conversion; PD, Parkinson's disease; ThT, Thioflavin‐T; ROC, receiver operating characteristic; CI, confidence interval; AUC, area under the curve; MoCA, Montreal Cognitive Assessment; MDS‐UPDRS, The Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale; NMSS, The Non Motor Symptoms Score.
We finally aimed to correlate RT‐QuIC kinetic parameters with the different clinical scores of PD patients. Z‐scores for all clinical and RT‐QuIC kinetic parameters were calculated and plotted on a heatmap. Performing agglomerative hierarchical clustering we identified that the clusters with the three principal clinical scores: The Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS), The The Non Motor Symptoms Score (NMSS), and the sum MDS‐UPDRS/NMSS were more closely associated to the cluster enriched by RT‐QuIC kinetic parameters (Fig. 1D). Interestingly, performing least squares regression we detected a significant positive correlation between summed MDS‐UPDRS part III and NMSS and some individual RT‐QuIC kinetic parameters (Fig. 1E), indicating that increased disease severity is significantly associated with a greater response in the salivary RT‐QuIC assay. A detailed discussion of the data is reported in Supplementary Appendix S1.
Financial Disclosures of All Authors (For The Preceding 12 Months)
All authors have no financial disclosures to report.
Conflict of Interest
All authors have no financial disclosures to report.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
1A‐B: G.V., A.S., A.B., M.G.S.
1 C: G.V., M.W., L.C., M.I.D.B., D.B., M.G.S.
2A‐C: G.V., M.M., M.G.S.
3A: G.V., M.M., M.I.D.B., D.B.
3B: A.S., G.F., AB, MGS.
Supporting information
Appendix S1 Supporting Information
Appendix S2 Supporting Information
Acknowledgments
We thank Dr. Aviva Tolkovsky for continuous helpful and constructive comments about the manuscript and the methodological approach.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Funding agencies: The work has been supported by a research training fellowship of the European Academy of Neurology (EAN); a research fellowship for young neurologist of the International Movement Disorders Society, a short‐term research fellowship of the European Molecular Biology Organization (EMBO) and a research grant from the Addenbrooke's Charitable Trust.
Contributor Information
Giorgio Vivacqua, Email: g.vivacqua@unicampus.it.
MariaGrazia Spillantini, Email: mgs11@cam.ac.uk.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α‐Synuclein in Lewy bodies. Nature 1997;388(6645):839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 2. Rockenstein E, Nuber S, Overk CR, et al. Accumulation of oligomer‐prone α‐synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 2014;137(5):1496–1513. 10.1093/brain/awu057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luk KC, Kehm V, Carroll J, et al. Pathological α‐synuclein transmission initiates Parkinson‐like neurodegeneration in nontransgenic mice. Science 2012;338(6109):949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shahnawaz M, Tokuda T, Waragai M, et al. Development of a biochemical diagnosis of Parkinson's disease by detection of α‐Synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74(2):163–172. 10.1001/jamaneurol.2016.4547 [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Becker K, Donadio V, et al. Skin α‐Synuclein aggregation seeding activity as a novel biomarker for Parkinson's disease. JAMA Neurol Published online September 2020;78(1):30‐40. 10.1001/jamaneurol.2020.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manne S, Kondru N, Jin H, et al. α‐Synuclein real‐time quaking‐induced conversion in the submandibular glands of Parkinson's disease patients. Mov Disord 2020;35(2):268–278. 10.1002/mds.27907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefani A, Iranzo A, Holzknecht E, et al. Alpha‐synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 2021;144(4):1118–1126. 10.1093/brain/awab005 [DOI] [PubMed] [Google Scholar]
- 8. Vivacqua G, Latorre A, Suppa A, et al. Abnormal salivary total and oligomeric alpha‐synuclein in parkinson's disease. PLoS One 2016;11(3):e0151156. 10.1371/journal.pone.0151156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivacqua G, Suppa A, Mancinelli R, et al. Salivary alpha‐synuclein in the diagnosis of Parkinson's disease and progressive Supranuclear palsy. Parkinsonism Relat Disord 2019;63:143–148. 10.1016/j.parkreldis.2019.02.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Appendix S2 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


