Abstract
Background
Alpha‐gal syndrome (AGS) is an IgE‐mediated allergy to galactose‐alpha‐1,3‐galactose. Clinical presentation ranges from hives to anaphylaxis; episodes typically occur 2–6 h after exposure to alpha‐gal‐containing products. In the United States, lone star tick bites are associated with the development of AGS. To characterize features of AGS, we evaluated a cohort of patients presenting for care at the University of North Carolina, focusing on symptoms, severity, and identifying features unique to specific alpha‐gal‐containing product exposures.
Methods
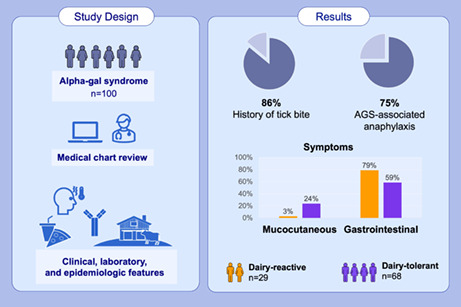
We performed a chart review and descriptive analysis of 100 randomly selected patients with AGS during 2010–2019.
Results
Median age at onset was 53 years, 56% were female, 95% reported White race, 86% reported a history of tick bite, and 75% met the criteria for anaphylaxis based on the involvement of ≥2 organ systems. Those reporting dairy reactions were significantly less likely to report isolated mucocutaneous symptoms (3% vs. 24%; ratio [95% CI]: 0.1 [0.1, 0.3]) than those who tolerated dairy, and were more likely to report gastrointestinal symptoms (79% vs. 59%; ratio [95% CI]: 1.3 [0.7, 2.6]), although this difference was not statistically significant. Dairy‐tolerant patients demonstrated higher alpha‐gal sIgE titers (as a percentage of total IgE) than dairy‐reactive patients (GM 4.1 [95% CI: 2.7, 6.1] vs. GM 2.5 [95% CI: 1.3, 4.8], respectively; ratio −1.6 [95% CI: −1.0, 3.9]).
Conclusion
While tick exposure is common in the southern United States, nearly all AGS patients reported a tick bite. Gastrointestinal symptoms were prominent among those reporting reactions to dairy. Anaphylaxis was common, underscoring the severity and need to raise awareness of AGS among patients and providers.
Keywords: alpha‐gal syndrome; anaphylaxis; galactose‐alpha‐1,3‐galactose; red meat allergy; tick bite
This report provides a comprehensive description of the epidemiology, clinical presentation, and laboratory testing trends among large cohort of patients diagnosed with alpha‐gal syndrome in the United States. Eighty six percent reported a history of tick bite, and 75% met the criteria for anaphylaxis based on the involvement of more than 2 organ systems. Patients reporting dairy reactions were significantly less likely to report isolated mucocutaneous symptoms (3% vs. 24%) than those who tolerated dairy and were more likely to report gastrointestinal symptoms (79% vs. 59%).Abbreviation: AGS, alpha‐gal syndrome
1. INTRODUCTION
Alpha‐gal syndrome (AGS) is an immunoglobulin E (IgE)‐mediated allergy to non‐primate mammalian meat products (e.g., ‘red meat’ such as beef, pork, or lamb) and other mammalian‐derived products including dairy. The condition was first recognized in 2007 among patients with pre‐existing serum IgE antibodies to galactose‐alpha‐1,3‐galactose (alpha‐gal) who experienced immediate‐onset life‐threatening hypersensitivity reactions following intravenous administration of the monoclonal antibody cetuximab, which contains numerous alpha‐gal moieties. 1 , 2
Unlike other food allergies, AGS is most often characterized by delayed onset (2–6 h) allergic reactions after ingestion of red meat, dairy, or other mammalian‐derived products. Symptom onset is more rapid with intramuscular or intravenous exposures. Clinical presentation is broad, ranging from urticaria and gastrointestinal distress to angioedema and life‐threatening anaphylaxis; other factors such as alcohol consumption and exercise can also potentiate the response. 3 , 4 A subset of patients report gastrointestinal (GI) symptoms (including abdominal pain, nausea, vomiting, and diarrhea) without mucocutaneous, respiratory, or cardiovascular symptoms. 5 , 6 Diagnosis is typically based on the history of allergic reactions upon exposure to alpha‐gal containing products coupled with elevated serum IgE antibodies to alpha‐gal (≥0.1 IU/ml). 3 , 4 , 7 , 8 Onset is most common during adulthood and management of AGS requires a multifaceted, patient‐centered approach, including prevention of tick bites, an avoidance diet, antihistamine use, and awareness of alpha‐gal presence in many food and non‐food products. 8 , 9
Cases of AGS have been reported worldwide. While risk factors are not fully known, the bite of various tick species has been associated with its development. 10 , 11 , 12 In the United States, AGS development is most often associated with the lone star tick (Amblyomma americanum). 13 Serum IgE specific to alpha‐gal (sIgE) antibody levels have been shown to increase following tick bites and may decrease in some patients who avoid tick bites. 10 , 13 , 14 Questions remain regarding natural progression of clinical AGS in relation to sIgE titers, disease management, and tick re‐exposures over time.
While presence of alpha‐gal sIgE is an established diagnostic criterion, levels do not correlate directly with symptoms or severity. 8 , 15 Current diagnostic assays utilize a reference value of ≥0.1 IU/ml to indicate a positive test; however, approximately 2% of patients will not have an alpha‐gal sIgE level that crosses the limit of detection of the assays. 8 Additionally, asymptomatic alpha‐gal sensitization can also occur, as not all patients who test positive for alpha‐gal sIgE will exhibit symptoms or experience reactions to mammalian products, suggesting additional host factors associated with clinical illness. For example, the sensitization rate (positive to alpha‐gal sIgE) was found to be 22% among a cohort of patients undergoing endoscopy without a history of AGS in North Carolina, 16 while an asymptomatic cohort in Tennessee showed a sensitization rate of 20.8%. 1 Additionally, a study conducted in Germany to characterize the prevalence of alpha‐gal sIgE positivity among forest service employees and hunters found that 35% were sensitized to alpha‐gal, but only 8.6% reported clinical symptoms of AGS. 17 While Mabelane et al reported an alpha‐gal sIgE up to 2.0 IU/ml may have predictive value, 18 a more recent study conducted in an allergy clinic in Germany found that an sIgE titer of at least 0.54 IU/ml was predictive of AGS, 19 but neither cut‐off has been validated in other settings. Thus, alpha‐gal sIgE is a helpful diagnostic tool, but cannot be relied upon solely for a diagnosis.
In specific geographic regions including the southeastern United States, increasing evidence suggests that AGS contributes substantially to food allergy incidence and is an important cause of anaphylaxis. 20 , 21 Nonetheless, most reports on AGS have been limited to relatively small, time‐limited observational studies and case series. To better characterize the clinical and laboratory features of AGS, we evaluated a large cohort of patients presenting for evaluation to the University of North Carolina (UNC) Allergy and Immunology Clinic (Chapel Hill, North Carolina), focusing on differences in symptoms, severity of disease, and identification of features unique to specific alpha‐gal‐containing product exposures. This study provides information on exposure, clinical manifestations, and the natural progression of AGS. These data can inform prevention and management strategies and guide future work aimed at understanding this newly identified unique syndrome.
2. METHODS
2.1. Study design and patient identification
We performed a retrospective medical chart review and descriptive analysis of randomly selected patients with AGS who sought care at UNC during 2010–2019. Study participants included patients of all ages with symptoms consistent with allergic reaction to mammalian products and evidence of sensitization to alpha‐gal, defined as ≥0.1 IU of alpha‐gal sIgE per milliliter of serum (IU/ml). A random number generator was used to select 100 patients among the 446 patients who visited the UNC Clinic for AGS during this period. The Centers for Disease Control and Prevention (CDC) Human Research Protection Office and UNC Office of Human Research Ethics both determined the study exempt from further review under 45 CFD 46.101(b); approval from an institutional review board was not required and no patients were contacted.
2.2. Data collection
Data were abstracted from electronic and paper medical charts using a standardized abstraction form and entered into a Research Electronic Data Capture (REDCap) database. 22 , 23 Data collected included demographic information, patient medical history (including diagnoses of Lyme disease, Rocky Mountain spotted fever, southern tick‐associated rash illness [STARI], ehrlichiosis, anaplasmosis or babesiosis), allergy history before onset of AGS, and reported history of any tick or chigger bites (including timing and characteristics of any exposures or bites). Detailed data regarding onset and severity of AGS reactions, including whether any reaction resulted in an anaphylactic episode and if emergency care was received, how many reactions were experienced prior to diagnosis, and the time‐of‐day reactions typically occurred were collected. Data on exposure to specific alpha‐gal‐containing products and associated self‐reported symptoms were also collected. In addition, data from referring providers and specialists were included, which significantly expanded the amount of information available for chart abstraction and allowed for details not typically available for a retrospective chart review.
We collected available laboratory data from the medical chart, including all reported measurements of total and alpha‐gal sIgE antibody levels. Serum IgE antibody levels specific to other mammalian animal products (i.e., beef, pork, lamb/mutton, and cow's milk) were also abstracted as well as data for other allergens, including skin‐test and serum‐based diagnostics. We collected data on exposure to and allergic history of inhalants (i.e., dust mite, cockroach, tree, grass or weeds, mold, cat or dog dander), food (i.e. peanuts, tree nuts, shrimp, wheat), and venomous insects (fire ants, honey bee, yellow jacket, wasp, yellow‐faced hornet, or white‐faced hornet). For total IgE, a positive cut‐off was not applied, as values vary by age and sex. In some instances, the ratio of alpha‐gal sIgE to total IgE has been found to be clinically significant, especially among non‐atopic patients who might have low total IgE. 7 , 18 We therefore also report the ratios of alpha‐gal sIgE to total IgE when both tests were available.
2.3. Anaphylaxis as outcome measure of severity
To assess severity of AGS episodes, we abstracted all self‐reported and physician‐documented signs and symptoms, which we categorized based on the organ system involved: mucocutaneous (e.g., hives/urticaria, itching, redness of palms or soles, lip swelling, flushing, throat swelling, rash, tongue swelling, congestion, rhinorrhea, itchy throat); respiratory (e.g., shortness of breath, chest tightness, wheezing, respiratory distress or apnea, cough); cardiovascular (e.g., dizziness, syncope, hypotension, weakness or numbness, chest pain, tachycardia or palpitations); and gastrointestinal (e.g., abdominal pain or cramping, nausea, vomiting, diarrhea, constipation, heartburn, indigestion, difficulty swallowing). Headache and muscle/joint pain or swelling were classified as ‘other’. Anaphylaxis was defined as any reaction involving ≥2 organ systems. 24 Patient data were reviewed independently by two medical providers to confirm final anaphylactic designation.
2.4. Characterization of patients experiencing AGS‐associated reactions to dairy products
Avoidance of red meat is a mainstay of AGS management; however, for a subset of patients, avoidance of other mammalian derivatives including bovine‐derived milk or dairy products may also be necessary to prevent additional reactions. 8 , 25 We therefore identified patients experiencing AGS reactions associated with ingestion of dairy products (dairy product‐reactive patients) and compared their clinical presentation and laboratory characteristics to patients who did not report signs or symptoms of AGS associated with dairy products (dairy product‐tolerant patients). Symptoms with dairy consumption can be due to etiologies other than AGS, including lactose intolerance, cow's milk allergy and lactase deficiency; therefore we report patients as dairy‐reactive only if (i) chart documentation stated that symptom onset was at least 2 h after dairy consumption [guidelines suggest the standard measurement interval to assess malabsorption and intolerance is 30 min], 26 (ii) documentation stated dairy‐related symptoms were new in onset with AGS diagnosis, and (Iii) dairy‐related gastrointestinal symptoms occurred in conjunction with non‐GI signs/symptoms (e.g., urticaria, angioedema, hypotension). Using these criteria, 3 charts were excluded from symptom analysis portion of the results.
2.5. Longitudinal analyses
Patients with >1 alpha‐gal sIgE test result, clinical, and exposure information for follow‐up visits were identified and included in the longitudinal analysis. We describe changes in AGS reactions, fluctuations in total IgE and alpha‐gal sIgE results, and interval tick exposure over time.
2.6. Statistical analyses
Descriptive statistics were calculated for each variable. Summaries of continuous variables are expressed as medians (first quartile – third quartile [Q1–Q3]) or as geometric means (GMs) and corresponding 95% CIs, and summaries of categorical variables are expressed as proportions. Ratios (95% CIs) were used to compare proportions. Differences in means (95% Student CIs) and medians (95% bootstrap CIs) were used to compare continuous variables. The GMs (95% CIs) of alpha‐gal sIgE and separately of total IgE were computed and their ratio (95% CIs) was used to compare according to dairy tolerance. Log10‐transformed alpha‐gal sIgE results for all tests were also compared across dairy tolerance groups using a linear mixed model with a random intercept to account for anticipated correlation among samples from the same patient. The difference (95% CI) in alpha‐gal sIgE as a percent of total IgE for each of the first, highest, and average positive observed values were computed to compare dairy tolerance groups. Pearson's correlation coefficient (95% CI) was used to estimate correlation between paired maximum alpha‐gal sIgE and corresponding total IgE values. Data management, analyses, and visualizations were performed using SAS v9.4 (SAS Institute, https://www.sas.com) and R v4.0.3 software (https://r‐project.org).
3. RESULTS
3.1. Demographic, clinical, and exposure characteristics
All 100 study patients presented to UNC during March 2010–March 2019. Most patients were female (n = 66, 66%) and White race (n = 95, 95%; Table 1). The median age for all patients was 53 years (Q1–Q3: 42–60); seven patients were ≤19 years of age. The most frequently reported pre‐existing medical conditions were heartburn or reflux (n = 37, 37%) and hypertension (n = 31, 31%). Non‐food allergies were more frequently reported than food allergies (n = 68, 68% vs. n = 17, 17%, respectively); the most common pre‐existing allergies were to medical supplies (n = 51, 51%), and environmental or seasonal allergies (n = 39, 39%). Eighty‐six patients (86%) reported history of any tick or chigger bites, and diagnosis of previous tickborne illness was reported by 16 (16%), including Lyme disease (n = 6, 6%) Rocky Mountain spotted fever (n = 8, 8%), and STARI (n = 2, 2%); no patients (0%) reported previous illness due to ehrlichiosis, anaplasmosis, or babesiosis (Table 1).
TABLE 1.
Demographic, clinical, and tick exposure characteristics of patients with alpha‐gal syndrome, University of North Carolina Allergy Clinic, 2010–2019
|
All patients N = 100 |
95% CI | ||
|---|---|---|---|
| N | % | ||
| Demographics | |||
| Age at onset, years | |||
| Median (Q1–Q3) | 53 (42–60) | ||
| 0–19 | 7 | 7.0 | 3.4, 13.7 |
| 20–39 | 12 | 12.0 | 7.0, 19.8 |
| 40–59 | 48 | 48.0 | 38.5, 57.7 |
| 60+ | 29 | 29.0 | 21.0, 38.5 |
| Sex, female | 66 | 66.0 | 56.3, 74.5 |
| Race | |||
| White | 95 | 95.0 | 88.8, 97.8 |
| Non‐White | 5 | 5.0 | 2.2, 11.1 |
| Hispanic ethnicity | 1 | 1.0 | 0, 5.4 |
| Clinical characteristics | |||
| Weight at presentation, median (Q1‐Q3), kg | 75.4 (62.0–96.2) | ||
| Medical history a | |||
| Heartburn/reflux | 37 | 37.0 | 28.2, 46.8 |
| Hypertension | 31 | 31.0 | 22.8, 40.6 |
| Recurrent sinus infections | 22 | 22.0 | 15.0, 31.1 |
| Asthma | 22 | 22.0 | 15.0, 31.1 |
| Previous tickborne illness b | 16 | 16.0 | 10.1, 24.4 |
| Diabetes type II | 8 | 8.0 | 4.1, 15.0 |
| Coronary artery disease | 7 | 7.0 | 3.4, 13.7 |
| Emphysema/COPD | 6 | 6.0 | 2.8, 12.5 |
| Eczema | 6 | 6.0 | 2.8, 12.5 |
| Allergy history | |||
| Any food allergy c | 17 | 17.0 | 10.9, 25.5 |
| Any nuts | 8 | 8.0 | 4.1, 15.0 |
| Shellfish | 6 | 6.0 | 2.8, 12.5 |
| Eggs | 4 | 4.0 | 1.6, 9.8 |
| Any non‐food allergy | 68 | 68.0 | 58.3, 76.3 |
| Pharmaceuticals d | 51 | 51.0 | 41.3, 60.6 |
| Environmental/seasonal | 39 | 39.0 | 30.0, 48.8 |
| Stinging insects | 18 | 18.0 | 11.7, 26.7 |
| Animals | 6 | 6.0 | 2.8, 12.5 |
| History of tick bite | 86 | 86.0 | 77.9, 91.5 |
Note: Data in table are reported as n, % unless otherwise indicated. Categories may not sum to 100% if data were unknown, or if categories were not mutually exclusive.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; kg, kilograms; Q1, Q3, first and third quartiles, respectively.
The following assessed conditions were not reported by any patients: chronic bronchitis, fibrotic/restrictive lung disease, blood clots, health failure, stroke, congenital heart disease, inflammatory bowel disease (Crohn's disease and ulcerative colitis), HIV/AIDS or other immune deficiency, autoimmune disease, or type 1 diabetes.
Tickborne illnesses of interest included Lyme disease (N = 6), Rocky Mountain spotted fever (N = 8), STARI (N = 2), ehrlichiosis (N = 0), anaplasmosis (N = 0), and babesiosis (N = 0).
The following food allergies not listed in table include pre‐existing cow's milk allergy (N = 3), wheat (N = 3), soy (N = 2), mango (N = 1), and pork‐cat syndrome (N = 1).
Includes latex (N = 4), iodine (N = 5), and medications (N = 46).
3.2. Characteristics of AGS reactions
Just more than half of patients (n = 53, 53%) experienced onset of AGS during 2015–2018 (Table 2). When month of AGS onset was known (n = 50), summer was most frequently reported (June, July, August; n = 28, 56%). Among patients with known month and year of both AGS onset and tick bite preceding AGS onset (n = 16), the median time from tick bite to onset was 2 months (Q1–Q3: 1–4 months). Among the 39 patients reporting the time of day of their reactions, most occurred in the evening (6 PM–12 AM; n = 16, 41%) or overnight (12 AM–6 AM; n = 31, 80%; results are not mutually exclusive). Nearly half of patients required emergency care for an AGS reaction (n = 41, 41%) or were prescribed an epinephrine auto‐injector (n = 40, 40%; Table 2).
TABLE 2.
Characteristics of reactions among patients with alpha‐gal syndrome, University of North Carolina Allergy Clinic, 2010–2019
|
All patients N = 100 |
95% CI | ||
|---|---|---|---|
| N | % | ||
| Onset and diagnosis | |||
| Onset year | |||
| Before 2000 | 4 | 4.0 | 1.6, 9.8 |
| 2000–2004 | 2 | 2.0 | 0.1, 7.0 |
| 2005–2009 | 9 | 9.0 | 4.8, 16.2 |
| 2010–2014 | 29 | 29.0 | 21.0, 38.5 |
| 2015–2018 | 53 | 53.0 | 43.3, 62.5 |
| Onset season a | |||
| Winter | 5 | 5.0 | 2.2, 11.1 |
| Spring | 8 | 8.0 | 4.1, 15.0 |
| Summer | 28 | 28.0 | 20.1, 37.5 |
| Fall | 10 | 10.0 | 5.5, 17.4 |
| Unknown/not reported | 50 | 50.0 | 40.4, 59.6 |
| Time from tick bite to onset, median (Q1–Q3), months b | 2 (1–4) | ||
| Clinical characteristics and outcomes of reactions | |||
| Reaction time of day | |||
| Morning (6 AM–12 PM) | 4 | 4.0 | 1.6, 9.8 |
| Afternoon (12 PM–6 PM) | 4 | 4.0 | 1.6, 9.8 |
| Evening (6 PM–12 AM) | 16 | 16.0 | 10.1, 24.4 |
| Overnight (12 AM–6 AM) | 31 | 31.0 | 22.9, 40.6 |
| Unknown/not reported | 61 | 61.0 | 51.2, 70.0 |
| Organ system involvement c | |||
| Mucocutaneous | 87 | 87.0 | 79.0, 92.2 |
| Gastrointestinal | 66 | 66.0 | 56.3, 74.5 |
| Cardiovascular | 31 | 31.0 | 22.9, 40.6 |
| Respiratory | 24 | 24.0 | 16.7, 33.2 |
| Other | 6 | 6.0 | 2.8, 12.5 |
| Anaphylaxis as defined by >1 organ system involved | 75 | 75.0 | 65.7, 82.5 |
| Self‐reported anaphylaxis | 21 | 21.0 | 14.2, 30.0 |
| Prescribed epinephrine auto‐injector d | 40 | 40.0 | 30.9, 49.8 |
| Emergency care visit | 41 | 41.0 | 31.9, 50.8 |
| Exposures associated with reactions | |||
| Food products | |||
| Unspecified red meat | 56 | 56.0 | 46.2, 65.3 |
| Beef | 47 | 47.0 | 37.5, 56.7 |
| Pork | 32 | 32.0 | 23.7, 41.7 |
| Dairy | 32 | 32.0 | 23.7, 41.7 |
| Gelatin/glycerin | 6 | 6.0 | 2.8, 12.5 |
| Lamb/mutton | 5 | 5.0 | 2.2, 11.1 |
| Other (including fumes) | 5 | 5.0 | 2.2, 11.1 |
| Non‐food products | |||
| Vaccines e | 1 | 1.0 | 0, 5.4 |
| Monoclonal antibodies f | 2 | 2.0 | 0.1, 7.0 |
Note: Data in table are reporting as n, % unless otherwise indicated. Categories might not sum to 100% if data points were unknown, or if categories were not mutually exclusive.
Abbreviations: CI, confidence interval; Q1, Q3, first and third quartiles, respectively.
Seasons defined as winter (December, January, February), spring (March, April, May), summer (June, July, August), fall (September, October, November).
N = 15 patients reported month and year of tick bite and alpha‐gal syndrome onset.
Symptoms grouped by organ system are show in Figure 1.
For any reason either before or after onset of alpha‐gal syndrome.
N = 1 patient reported a reaction associated with Varicella vaccine; other vaccines assessed without associated reactions included Herpes Zoster, MMR, Rabies, Yellow Fever, oral Typhoid, and Influenza.
N = 2 patients reported reactions associated with monoclonal antibodies (Xolair, Abatacept [Orencia]). Other pharmaceuticals assessed without associated reactions included xenotransplantation and certain medications (cetuximab, heparin, pancreatic enzymes, crotalidae antivenom, and equine antivenom).
Self‐reported anaphylaxis was documented for 21 patients, while 75 met criteria for anaphylaxis based on reporting involvement of ≥2 organ systems; one patient who self‐reported anaphylaxis did not meet criteria when organ system involvement was reviewed (Table 2). Mucocutaneous signs and symptoms (n = 87, 87%) were the most common, with hives/urticaria (n = 68, 68%) being the most frequently reported overall, followed by gastrointestinal symptoms (n = 66, 66%) including abdominal pain or cramping (n = 66, 66%) and nausea or vomiting (n = 34, 34%). Respiratory (n = 24, 24%) or cardiovascular symptoms were less frequently reported (n = 31, 31%; Table 2; Figure S1).
Food exposures associated with AGS reactions were more frequently reported than non‐food exposures (Table 2). Unspecified red meat was the most frequently reported food exposure (n = 48, 48%) associated with a reaction; when the mammalian meat product or derivative associated with a reaction was specified, beef was the most frequently reported (n = 47, 47%), followed by pork (n = 32, 32%). Reactions to non‐food products including gelatin/glycerin (n = 6, 6%), vaccines (n = 1, 1%) and monoclonal antibodies (n = 2, 2%) were uncommon.
3.3. Dairy product tolerance
Thirty two patients reported AGS symptoms associated with dairy product exposure (Table 2), 29 of whom met criteria for dairy‐related symptoms attributed to AGS. These patients reported gastrointestinal symptoms more often compared to 67 dairy product‐tolerant patients (n = 23, 79% vs. n = 40, 59%, respectively; Figure 1 [purple bars]). Gastrointestinal symptoms were reported with any mammal‐food and/or product exposure in the dairy‐reactive group. However, isolated mucocutaneous symptoms (Figure 1, orange bars) were more common among dairy product‐tolerant patients (n = 16, 24% vs. n = 1, 3%, respectively).
FIGURE 1.
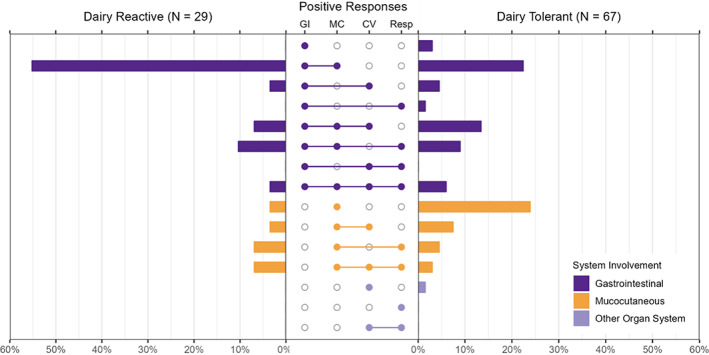
Comparison of organ system involvement of allergic reactions stratified by dairy product tolerance among patients with alpha‐gal syndrome, University of North Carolina Allergy Clinic, 2010–2019. Horizontal bars represent the proportion of patients reporting symptoms grouped by organ system, stratified by those who reported AGS reactions to dairy products (e.g., dairy product‐reactive; n = 29) on the left vs. those who did not report reactions to dairy products (e.g., dairy product‐tolerant, n = 67) on the right; one patient (dairy product‐tolerant) only reported symptoms classified as ‘other’ and is not displayed in the figure. Combinations of body system involvement are represented by filled‐in circles in middle panel; single body system involvement indicate only symptoms classified as such were reported (e.g., only GI, only MC, only CV, only resp.). Abbreviations: CV, cardiovascular; GI, gastrointestinal; MC, mucocutaneous; Resp, respiratory
3.4. Laboratory characteristics
Two hundred fifty two alpha‐gal sIgE test results were available for the 100 patients; 247 were positive (Table 3). The GM (95% CI) of the first positive alpha‐gal sIgE test among all patients was 5.3 IU/ml (95% CI: 3.9, 7.2 IU/ml). The GMs of all positive values were similar between patients who react to dairy compared with patients with dairy tolerance (4.9 IU/ml [95% CI: 2.8, 8.7] vs. 3.2 [95% CI: 1.9, 5.4]). Additionally, paired total IgE and alpha‐gal sIgE values were available for 22 patients who reacted to dairy products, and 54 patients who were tolerant of dairy products (Figure 2). A positive relationship was identified between alpha‐gal sIgE and total IgE among dairy product‐tolerant patients (r = 0.4 [95% CI: 0.2, 0.6]) and among dairy product‐reactive patients (r = 0.2 [95% CI: −0.2, 0.6]) (Figure 2 left and center panels). The average ratio of maximum alpha‐gal sIgE to its corresponding total IgE was measured as lower among dairy‐reactive patients (GM [95% CI]: 2.5 [1.3, 4.8]), compared with dairy‐tolerant patients (GM [95% CI]: 4.1 [2.7, 6.1]; Figure 2, right panel). Results for other allergy testing, including IgE antibodies to several different mammalian extracts, are shown in Table S1.
TABLE 3.
Galactose‐alpha‐1,3, galactose‐specific IgE and total IgE test results among patients with alpha‐gal syndrome stratified by dairy tolerance, University of North Carolina Allergy Clinic, 2010–2019
|
All patients N = 97 |
Dairy tolerant N = 68 |
Dairy reactive N = 29 |
Comparison (95% CI) |
|
|---|---|---|---|---|
| Alpha‐gal sIgE | ||||
| Total number of tests performed | 252 | 173 | 79 | |
| Mean (SD) tests per patient | 2.6 (1.7) | 2.5 (1.8) | 2.6 (1.6) | 0 (−0.8, 0.7) |
| Median (Q1–Q3) | 2.0 (1–3) | 2.0 (1–3) | 2.0 (1–3) | 0 (−1.0, 0.5) |
| Patients with ≥1 negative test, n (%) | 5 (5.2) | 2 (2.9) | 3 (10.3) | −7.4 (−15.2, 0) |
| Results, GM (95% CI), kU/L | ||||
| First positive values | 5.3 (3.9, 7.2) | 5.3 (3.7, 7.6) | 5.3 (2.8, 10.1) | 1.0 (0.5, 2.1) |
| Highest positive values | 6.3 (4.6, 8.5) | 6.5 (4.5, 9.2) | 5.8 (3.0, 11.2) | 1.1 (0.5, 2.4) |
| All positive values | 3.6 (2.7, 4.9) | 3.2 (1.9, 5.4) | 4.9 (2.8, 8.7) | 0.7 (0.3, 1.4) |
| Total IgE | ||||
| Total number of tests performed | 148 | 99 | 49 | |
| Mean (SD) tests per patient | 1.9 (1.3) | 1.8 (1.4) | 2.1 (1.2) | −0.3 (−1.0, 0.3) |
| Median (Q1–Q3) | 1 (1–2) | 1 (1–2) | 2 (1–3) | −1.0 (−2.0, 0) |
| Results, GM (95% CI), kU/L | ||||
| All values | 118.8 (95.7, 147.5) | 114.9 (78.4, 168.4) | 129.1 (83.0, 200.8) | 0.9 (0.5, 1.6) |
| Alpha‐gal sIgE as a percentage of total IgE | ||||
| First positive values | 4.1 (2.8, 5.8) | 4.5 (2.9, 6.9) | 3.1 (1.6, 6.0) | 1.4 (−1.9, 4.2) |
| Highest positive values | 4.7 (3.2, 6.8) | 3.5 (2.2, 5.7) | 8.5 (4.7, 14.9) | −5.0 (−11.5, −0.6) |
| Average positive values | 3.6 (2.5, 5.0) | 4.1 (2.7, 6.1) | 2.5 (1.3, 4.8) | 1.6 (−1.0, 3.9) |
Abbreviations: Alpha‐gal sIgE, alpha‐gal‐specific immunoglobulin E; CI, confidence interval; GM, geometric mean; Q1, Q3, first and third quartile, respectively; SD, standard deviation.
FIGURE 2.
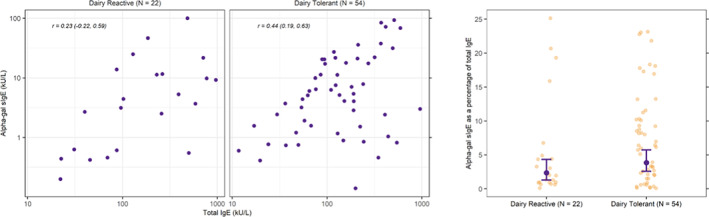
Relationship between galactose‐alpha‐1,3, galactose specific IgE and total IgE stratified by dairy tolerance among patients with alpha‐gal syndrome, University of North Carolina Allergy Clinic, 2010–2019. Dairy product‐tolerant visualizations (center and right panels) represent 54 paired total IgE and alpha‐gal sIgE tests among 54 patients who did not report AGS‐associated reactions to dairy products. Dairy product‐reactive visualizations (left and center panels represent 22 paired total IgE and alpha‐gal sIgE tests among 22 patients who reported AGS‐associated reactions to dairy products. Paired test results with a patient's highest recorded alpha‐gal sIgE value were selected for those with multiple pairs of tests. Right panel shows the distribution of alpha‐gal sIgE as a percentage of total IgE (scatter plots) with geometric mean and 95% confidence intervals depicted with black dot and lines; dairy‐reactive: GM (95% CI): 2.5 (1.3, 4.8)); non‐dairy: GM (95% CI): 4.1 (2.7, 6.1); difference (95% CI): 1.6 (−1.0, 3.9). Abbreviations: Alpha‐gal sIgE, alpha‐gal‐specific immunoglobulin E; CI, confidence interval; GM, geometric mean
3.5. Longitudinal analyses
Thirteen patients had at least five alpha‐gal sIgE results, spanning approximately 8 years (5.2 years on average; Figure 3). There was an overall downward trend of alpha‐gal sIgE levels over time, but only one patient (patient K) had a documented negative result (<0.1 IU/ml) approximately 5.1 years after the first test. Alpha‐gal sIgE test results are shown for all 100 patients in Figure S2.
FIGURE 3.
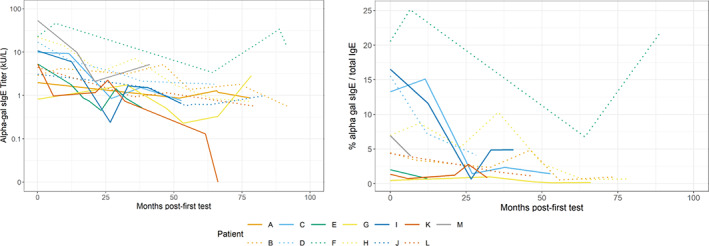
Galactose‐alpha‐1,3, galactose specific IgE results by month since first test among patients with Alpha‐gal Syndrome, University of North Carolina Allergy Clinic, 2010–2019. Alpha‐gal sIgE results are shown by month post‐first test 13 patients with at least five tests. Month 0 is the referent time point, indicating the initial test in chart. One patient (patient K) demonstrated a negative result, which is displayed as an sIgE value of 0. Note that while colors have been repeated, this represents no association between these patients; line types differentiate results from different patients.
4. DISCUSSION
In 2020, the United States Department of Health and Human Services Tickborne Diseases Working Group issued a report to Congress emphasizing the importance of AGS and need for better understanding of the disease that impacts quality of life. 27 This is one of the largest objective chart reviews of patients with AGS in the United States to date and helps better define this syndrome to allow for early identification and high‐quality treatment. While tick bites are common in the southern United States, nearly all patients (86%) reported a history of tick or chigger bites, consistent with their association to AGS development. Notably, ‘chigger’ bites can be confused with bites from larval ticks, particularly those of lone star ticks where numerous bites in a single exposure are common. Most patients reported onset in the summer when tick activity is highest, with a 2‐month average latency between bite and onset. We also examined the clinical and laboratory features of AGS in relation to severity and found that most patients with AGS met criteria for anaphylaxis, underscoring the severity and the continued need to raise awareness of this syndrome. Additionally, patients who experienced signs or symptoms of AGS reactions associated with consumption of dairy products demonstrated a prominence of gastrointestinal symptoms compared to those who did not report reactions to dairy products, despite similarities in alpha‐gal sIgE titers. Taken together, these unique clinical features better inform both the diagnosis and management of patients presenting with AGS.
The relative paucity of cardiovascular and respiratory symptoms in AGS patients represents a clinical deviation from other food allergies. In a recent update of our understanding of shellfish allergy, one of the most common food allergies in adults, approximately 53% of patients reported respiratory symptoms and 33% reported cardiovascular symptoms. 28 Instead, our study found that AGS is characterized by mucocutaneous and gastrointestinal manifestations without significant respiratory or cardiovascular symptoms, which is consistent with recent studies showing that AGS can cause gastrointestinal distress (e.g., episodic abdominal pain, nausea, and diarrhea) in the absence of symptoms commonly seen in allergic reactions to food products, including anaphylaxis. 18 , 29 Cardiovascular and respiratory symptoms were almost exclusively reported by those who objectively qualified as having a more severe outcome (anaphylaxis). 30 Gastrointestinal symptoms were also commonly reported, which is notable as patients and providers may overlook abdominal pain and cramping as a feature of an allergic reaction, underappreciating the systemic response to alpha‐gal. While we did note an increase in the number of patients presenting to care with onset in more recent years, increased education among patients and clinicians around both AGS and anaphylaxis is nonetheless important to help detect this condition early and guide appropriate management.
Tolerance of dairy products is an important branch‐point in the clinical management of a patient with AGS as it impacts the need to restrict the diet more fully to avoid continued symptoms. We further described the difference among these two groups; patients who reported reactions to dairy products most frequently reported experiencing both gastrointestinal and mucocutaneous symptoms. And those who did not report reactions to dairy products were more likely to report cardiovascular or respiratory symptoms, though they were still uncommon. Possible explanations for the number of patients reporting gastrointestinal symptoms, includes local referring patterns, the reputation of the UNC clinic and patients, more severely affected seeking additional management guidance. Laboratory results were not predictive of reactivity to dairy products. This supports other literature that the quantitative value, while helpful, is not the only predictor of disease severity and outcome, and might not depend on the specific alpha‐gal exposure. However, those who were diary product‐reactive had a lower ratio of alpha‐gal sIgE to total IgE. Whereas a positive sIgE to alpha‐gal is relevant for diagnosis, the lack of a direct correlation suggests that clinical AGS is a complex association between sensitization and other host factors. Furthermore, since patients most commonly react to ingested alpha‐gal, the number of alpha‐gal sIgE reactive B cells, and other alpha‐gal reactive immunoglobulins (IgG, IgM, IgA) that bind and block antigen in the gut, likely have a role in the phenotype of this condition. Other factors, such as alphagalactosidase and the composition of gut flora, may also contribute to absorption and subsequent reactions. When treating patients with AGS, clinicians should obtain a detailed dietary history, specifically noting whether symptoms occur with exposure to dairy products, as this will impact avoidance diet recommendations.
This study is limited due to the retrospective nature of the design and subject to the availability of data in the chart. Additionally, since patients presented to a specialty allergy clinic often after having been seen by another provider, data (particularly laboratory results) were not always complete, and some patients had multiple visits and laboratory results without defined intervals, which may be biased by perception of symptoms or access to care and thus overrepresented in our analysis. Recall bias should also be considered, particularly for historical information related to tick bites and tick exposure. As such, details on tick bites, including timing of the tick bite relative to onset or presentation were limited. Moreover, the percentage of charts reporting a diagnosis of RMSF (8%) is high and, given antigen cross‐reactivity with Rickettsia amblyommatis, may not reflect actual RMSF due to Rickettsia rickettsii. Despite these limitations, this study includes the most robust analysis to date of patient‐specific longitudinal alpha‐gal sIgE data. Based on the 13 patients included in Figure 3, alpha‐gal sIgE generally decreased over time but only resolved in one patient, possibly due to re‐exposure to ticks or that alpha‐gal sIgE may decrease slowly in some persons.
In summary, this report demonstrates that AGS is both a severe allergy, with nearly 75% of patients meeting criteria for anaphylaxis, and also distinct from other food allergies in its symptom profile. With tick bites common across most of the United States and other regions of the world, a substantial number of people are at risk for developing this potentially fatal disease. Yet, many providers are not aware of the condition, and patients may suffer for years before an accurate diagnosis. Understanding the predominance of gastrointestinal and mucocutaneous symptoms will allow providers to be better adept to recognize this condition.
AUTHOR CONTRIBUTIONS
AMB, DCB, CLA, CBB, LRP, GJK, SPC, and PAA designed the study. CLA and SPC acquired the data. BJB, AMB, DCB, and ESJ analyzed the data. AMB, SCP, and PAA drafted the manuscript. SCP and PAA provided supervision and oversight for the study. All authors revised the manuscript for critically important intellectual content.
FUNDING INFORMATION
UNC was funded under 19IPA1908943 from CDC.
CONFLICT OF INTEREST
Scott P. Commins is on the speaker's bureau of Genentech; Alison M. Binder, Dena Cherry‐Brown, Brad J. Biggerstaff, Emma S. Jones, Claire L. Amelio, Charles B. Beard, Lyle R. Petersen, Gilbert J. Kersh, and Paige A. Armstrong report no conflicts of interest.
Supporting information
Table S1
Figure S1
Figure S2
ACKNOWLEDGMENTS
Naomi Drexler (CDC) and Johanna Salzer (CDC) for thoughtful review of this manuscript.
Binder AM, Cherry‐Brown D, Biggerstaff BJ, et al. Clinical and laboratory features of patients diagnosed with alpha‐gal syndrome—2010–2019. Allergy. 2023;78:477‐487. doi: 10.1111/all.15539
Scott P. Commins and Paige A. Armstrong contributed equally.
REFERENCES
- 1. Chung CH, Mirakhur B, Chan E, et al. Cetuximab‐induced anaphylaxis and IgE specific for galactose‐alpha‐1,3‐galactose. N Engl J Med. 2008;358(11):1109‐1117. doi: 10.1056/NEJMoa074943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N‐linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix‐assisted laser desorption/ionization hybrid quadrupole‐quadrupole time‐of‐flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364(1):8‐18. doi: 10.1016/j.ab.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 3. Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose‐α‐1,3‐galactose. J Allergy Clin Immunol. 2009;123(2):426‐433.e2. doi: 10.1016/j.jaci.2008.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Commins SP, Jerath MR, Cox K, Erickson LD, Platts‐Mills T. Delayed anaphylaxis to alpha‐gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65(1):16‐20. doi: 10.1016/j.alit.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the α‐Gal Syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348‐2358.e4. doi: 10.1016/j.jaip.2019.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iweala OI, Choudhary SK, Commins SP. Food Allergy. Curr Gastroenterol Rep. 2018;20(5):17. doi: 10.1007/s11894-018-0624-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platts‐Mills TAE, Li R c, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the α‐Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15‐23.e1. doi: 10.1016/j.jaip.2019.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Commins SP. Diagnosis & management of alpha‐gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667‐677. doi: 10.1080/1744666X.2020.1782745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renz H, Allen KJ, Sicherer SH, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098. doi: 10.1038/nrdp.2017.98 [DOI] [PubMed] [Google Scholar]
- 10. Levin M, Apostolovic D, Biedermann T, et al. Galactose α‐1,3‐galactose phenotypes: Lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598‐602. doi: 10.1016/j.anai.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Nunen S. Tick‐induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac Allergy. 2015;5(1):3‐16. doi: 10.5415/apallergy.2015.5.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crispell G, Commins SP, Archer‐Hartman SA, et al. Discovery of alpha‐gal‐containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. doi: 10.3389/fimmu.2019.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose‐α‐1,3‐galactose. J Allergy Clin Immunol. 2011;127(5):1286‐1293.e6. doi: 10.1016/j.jaci.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim MS, Straesser MD, Keshavarz B, et al. IgE to galactose‐α‐1,3‐galactose wanes over time in patients who avoid tick bites. J Allergy Clin Immunol Pract. 2020;8(1):364‐367.e2. doi: 10.1016/j.jaip.2019.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose‐alpha‐1,3‐galactose. J Allergy Clin Immunol. 2014;134(1):108‐115.e11. doi: 10.1016/j.jaci.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose‐alpha‐1,3‐galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esophagus. 2016;29(6):558‐562. doi: 10.1111/dote.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha‐gal in forest service employees and hunters. Allergy. 2017;72(10):1540‐1547. doi: 10.1111/all.13156 [DOI] [PubMed] [Google Scholar]
- 18. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha‐gal IgE levels and alpha‐gal IgE: Total IgE ratio and oral food challenge‐proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841‐849. doi: 10.1111/pai.12969 [DOI] [PubMed] [Google Scholar]
- 19. Fischer J, Huynh HN, Hebsaker J, Forchhammer S, Yazdi AS. Prevalence and impact of type I sensitization to alpha‐gal in patients consulting an allergy unit. Int Arch Allergy Immunol. 2019;181:119‐127. doi: 10.1159/000503966 [DOI] [PubMed] [Google Scholar]
- 20. Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018;121(5):594‐597. doi: 10.1016/j.anai.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 21. Binder AM, Commins SP, Altrich ML, et al. Diagnostic testing for galactose‐alpha‐1,3‐galactose, United States, 2010 to 2018. Ann Allergy Asthma Immunol. 2021;126(4):411‐416.e1. S1081120620312746. doi: 10.1016/j.anai.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Academy of Allergy, Asthma, and Immunology . Conditions and Treatments, Anaphylaxis. https://www.aaaai.org/conditions‐treatments/allergies/anaphylaxis
- 25. Commins SP. Invited commentary: alpha‐gal allergy: tip of the iceberg to a pivotal immune response. Curr Allergy Asthma Rep. 2016;16(9):61. doi: 10.1007/s11882-016-0641-6 [DOI] [PubMed] [Google Scholar]
- 26. Keller J, Hammer HF, Afolabi PR, et al. European guideline on indications, performance and clinical impact of 13C‐breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC. United European Gastroenterol J. 2021;9(5):598‐625. doi: 10.1002/ueg2.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tick‐Borne Disease Working Group . Report to Congress; 2020. . https://www.hhs.gov/ash/advisory‐committees/tickbornedisease/reports/index.html
- 28. Wang HT, Warren CM, Gupta RS, Davis CM. Prevalence and characteristics of shellfish allergy in the pediatric population of the United States. J Allergy Clin Immunol Pract. 2020;8(4):1359‐1370.e2. doi: 10.1016/j.jaip.2019.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croglio MP, Commins SP, McGill SK. Isolated gastrointestinal alpha‐gal meat allergy is a cause for gastrointestinal distress without anaphylaxis. Gastroenterology. 2021;29:2178‐2180.e1. doi: 10.1053/j.gastro.2021.01.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampson HA, Muñoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report‐‐Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391‐397. doi: 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1
Figure S2


