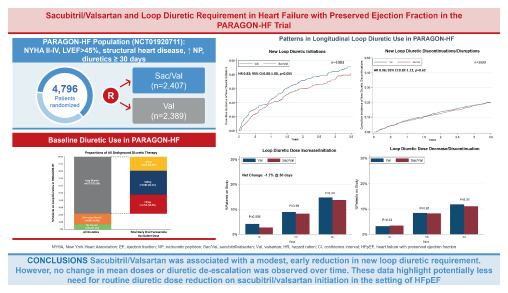
ABSTRACT
Aims
As sacubitril/valsartan may potentiate early natriuresis, expert consensus documents recommend diuretic dose reduction on first initiation. However, there are limited data on the effects of sacubitril/valsartan on the background of varying diuretic regimens or on diuretic requirements over time in heart failure (HF) with preserved ejection fraction (HFpEF).
Methods and results
In this post hoc analysis of PARAGON‐HF, of the 4796 patients, background diuretic therapy was distributed as follows: 341 (7%) on no diuretic, 698 (15%) on non‐loop diuretic, and 3757 (78%) were on loop diuretics (1255, 1589, and 913 were on <40, 40 and >40 mg furosemide equivalent doses, respectively). The primary composite outcome of total HF hospitalizations and cardiovascular death was analysed using semiparametric proportional rates methods. The cumulative incidence of the primary composite outcome (first events) was lowest in patients on no diuretic and highest in those on >40 mg of loop diuretic (p < 0.001). The effects of sacubitril/valsartan (vs. valsartan) on the primary composite outcome (recurrent events) did not significantly vary by baseline diuretic use (p interaction = 0.65). Treatment effects on safety outcomes were similar across diuretic categories. Sacubitril/valsartan reduced new loop diuretic initiations over the course of the trial (hazard ratio 0.83; 95% confidence interval 0.68–1.00, p = 0.055), with similar mean loop diuretic dose and rates of diuretic discontinuation between treatment groups in follow‐up. Patients randomized to sacubitril/valsartan experienced a slight early reduction in diuretic initiation or dose escalation at 30 days after initiation (net reduction 1.7%, p = 0.02), but these differences were not sustained beyond this timepoint.
Conclusions
Patients with HFpEF on higher baseline diuretic doses were at heightened risk of HF events, but similarly benefited from sacubitril/valsartan with a consistent safety profile across a range of diuretic doses. Initiation of sacubitril/valsartan was associated with modestly lower new loop diuretic requirement in follow‐up.
Keywords: Heart failure with preserved ejection fraction, Sacubitril/valsartan, Diuretics
Sacubitril/valsartan and loop diuretic requirement in heart failure with preserved ejection fraction in the PARAGON‐HF trial.
Introduction
Clinical congestion represents a hallmark feature of heart failure (HF) with preserved ejection fraction (HFpEF) and its management, typically with loop diuretics, is guideline‐recommended as a key component of comprehensive care. Higher doses of loop diuretics however have been associated with markers of neurohormonal excess and progression of kidney disease; as such, loop diuretic sparing strategies are welcome in clinical practice. Neprilysin inhibition results in increases in circulating natriuretic peptides which in turn promote diuresis and natriuresis. 1 In patients with HF with reduced ejection fraction (HFrEF), sacubitril/valsartan was associated with reduced loop diuretic requirement compared with treatment with enalapril. 2 Expert consensus guidelines now suggest consideration of reduced loop diuretic requirement upon initiation of sacubitril/valsartan; however, limited data exist on the effect of sacubitril/valsartan on longitudinal diuretic use specifically in patients with HFpEF. 3 This may be particularly important in HFpEF given a tendency to greater preload dependence compared to patients with HFrEF. 4
The PARAGON‐HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction) trial demonstrated a modest, but statistically non‐significant reduction in the primary composite outcome of total HF hospitalizations and cardiovascular death with sacubitril/valsartan versus valsartan. 5 Secondary analyses have suggested the potential for accentuated clinical benefit of sacubitril/valsartan in proximity to HF hospitalization. 6 Some have even suggested that initiation of sacubitril/valsartan may be preferentially considered in patients with a more congested HF phenotype. 7 , 8
In this secondary analysis of the PARAGON‐HF trial, we sought to assess the efficacy and safety of sacubitril/valsartan relative to valsartan according to background diuretic therapy and to examine the effect of sacubitril/valsartan on longitudinal patterns of diuretic use.
Methods
Study design
The PARAGON‐HF trial study design and results have been previously reported. 8 In brief, PARAGON‐HF was a double‐blind, randomized, controlled trial comparing sacubitril/valsartan versus valsartan in patients ≥50 years of age with symptomatic HF (New York Heart Association class II–IV), left ventricular ejection fraction ≥45%, evidence of structural heart disease, elevated natriuretic peptides, and necessity for diuretics for at least 30 days assessed at the time of screening. Participants were allowed to be screened, but not randomized, during hospitalization for HF. Exclusion criteria included symptomatic hypotension or systolic blood pressure <100 mmHg at screening, estimated glomerular filtration rate <30 ml/min/1.73 m2, or serum potassium >5.2 mmol/L at screening. The ethics committee at each study site approved the study protocol and study participants provided written informed consent.
Clinical endpoints
The primary endpoint was the composite of total (first and recurrent) HF hospitalizations and cardiovascular death. Safety outcomes included the occurrence of hypotension (systolic blood pressure <100 mmHg), elevations in serum creatinine (≥2.0, ≥2.5 , and ≥3.0 mg/dl), and drug discontinuation.
Statistical analysis
All patients with diuretic dose information were included in this analysis. Patients with missing loop diuretic doses were not considered in this analyses. In longitudinal analyses, patients were removed at the time point where data were missing and included again at a subsequent time point where data were available. Use of mineralocorticoid receptor antagonist (MRA) alone was considered in the ‘no diuretic’ category. Total daily furosemide dose equivalents were calculated for all patients on a loop diuretic. Bumetanide 1 mg, torsemide 20 mg, azosemide 60 mg, and ethacrynic acid 100 mg were considered equivalent to 40 mg of intravenous furosemide and 80 mg of oral furosemide. A small proportion of patients (n = 330) were on more than one diuretic therapy (most frequently loop diuretic and thiazide or thiazide‐like diuretic). In these cases, patients were analysed as part of the group of the most potent diuretic in the combination (i.e. categorized as part of the ‘loop diuretic’ subgroup if treated with both a loop and non‐loop diuretic).
Baseline patient characteristics were compared according to diuretic use across the following categories: no diuretic, non‐loop diuretic and furosemide dose equivalents of >40, =40 and <40 mg. Data are reported as mean ± standard deviation, median (interquartile range) for skewed distributions, and frequency (percentage) for categorical variables. Student's t‐tests, Pearson chi‐square tests, and ANOVA tests were used where appropriate.
Treatment effects of sacubitril/valsartan versus valsartan, across diuretic categories, on the primary composite outcome as well as total HF hospitalizations were analysed using semiparametric proportional rates methods of Lin et al. 9 Time to first events analyses were performed using Cox regression for first primary outcomes, its components, and the renal composite outcome. Cumulative incidence of first events was displayed using Kaplan–Meier curves. Treatment effects on safety outcomes were also analysed with Cox regression.
Temporal changes in mean loop diuretic dose over the course of the trial were evaluated using a repeated measures mixed‐effect model. Treatment, time, and the interaction between assigned treatment and time were included as fixed effects. The percentage of patients experiencing a loop diuretic dose decrease or increase was calculated at 30, 180 and 365 days post randomization and compared between treatment groups using regression analysis. Time to initiation among baseline non‐users of loop diuretics and discontinuation or disruption among baseline users were analysed using Cox regression models and displayed with Kaplan–Meier curves. Loop diuretic disruption was defined as any interruption in diuretic use for ≥30 days. No adjustments were made for multiple testing.
All analyses were conducted using STATA 17 (Stata Corp., College Station, TX, USA). A p‐value of <0.05 was considered statistically significant.
Results
Baseline diuretic use in PARAGON‐HF
Of the 4796 patients, background diuretic therapy was distributed as follows: 7.1% (n = 341) were on no diuretic, 14.5% (n = 698) on non‐loop diuretic, and 78.3% (n = 3757) on loop diuretic (Figure 1 ). Of those on loop diuretic, 33.4% (n = 1255), 42.2% (n = 1589), and 24.3% (n = 913) were on <40, 40 and >40 mg furosemide equivalent doses, respectively (Figure 1 ). The most frequently prescribed total daily oral furosemide diuretic dose was 40 mg, followed by 20 and 80 mg (Figure 1 ). A small proportion of patients (n = 330) were on more than one diuretic therapy with the most common combination being a loop diuretic and thiazide or thiazide‐like diuretic.
Figure 1.
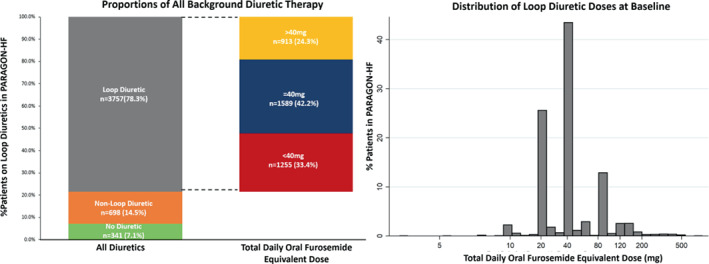
Baseline diuretic use in PARAGON‐HF.
Patient characteristics
There were several notable differences in patient characteristics across diuretic categories (online supplementary Table Appendix S1 ). Patients receiving the highest doses of diuretic (>40 mg) at baseline tended to have worse kidney function, higher body mass index, lower left ventricular ejection fraction, higher systolic blood pressure, and were more often treated with MRA.
Efficacy and safety outcomes according to baseline diuretic use
Overall, the cumulative incidence of the primary composite and its components as well as the renal composite outcome were lowest in patients on no diuretic or non‐loop diuretic and highest in those receiving furosemide dose equivalent of >40 mg (all p < 0.001; online supplementary Figure Appendix S1 ).
Efficacy of sacubitril/valsartan compared to valsartan with regard to the primary composite outcome did not significantly vary by baseline diuretic use (p interaction = 0.65): no diuretic (rate ratio [RR] 0.68; 95% confidence interval [CI] 0.39–1.19), <40 mg (RR 0.93; 95% CI 0.70–1.24), 40 mg (RR 0.87; 95% CI 0.68–1.11), >40 mg (RR 0.84; 95% CI 0.65–1.09), non‐loop diuretic (RR 1.20; 95% CI 0.74–1.96). Treatment effects of sacubitril/valsartan versus valsartan were consistent regardless of background diuretic use for total HF hospitalizations (p interaction = 0.72) and first HF hospitalization or cardiovascular death (p interaction = 0.52) (Table 1 ). Treatment effects remained similarly consistent across a range of loop diuretic dose (online supplementary Table S2 ).
Table 1.
Treatment effect on clinical outcomes according to background diuretic therapy
| No diuretic (n = 341) | <40 mg (n = 1255) | 40 mg (n = 1589) | >40 mg (n = 913) | Non‐loop diuretic (n = 698) | p interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | ||
| Total HFH + CV death | |||||||||||
| Event rate per 100 py | 9.3 (6.2–14.7) | 6.6 (4.5–10.0) | 11.3 (9.3–13.9) | 10.9 (8.8–13.4) | 14 (11.8–16.7) | 12.4 (10.5–14.8) | 29.8 (24.7–36.2) | 24.4 (20.3–29.5) | 5.0 (3.5–7.3) | 6.0 (4.4–8.6) | |
| Rate ratio (95% CI) | 0.68 (0.39–1.19) | 0.93 (0.70–1.24) | 0.87 (0.68–1.11) | 0.84 (0.65–1.09) | 1.20 (0.74–1.96) | 0.65 | |||||
| Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | Val | Sac/Val | ||
| Total HFH | |||||||||||
| Event rate per 100 py | 6.3 (3.9–10.6) | 4.4 (2.7–7.8) | 9.1 (7.3–11.4) | 8.1 (6.4–10.4) | 10.3 (8.4–12.7) | 9.3 (7.6–11.4) | 25.5 (20.7–31.7) | 20.3 (16.6–25.1) | 3.3 (2.1–5.6) | 4.2 (3.0–6.2) | |
| Rate ratio (95% CI) | 0.68 (0.35–1.32) | 0.87 (0.63–1.20) | 0.88 (0.66–1.18) | 0.82 (0.62–1.10) | 1.27 (0.70–2.32) | 0.72 | |||||
| First HFH + CV death | |||||||||||
| Event rate per 100 py | 6.4 (4.5–9.3) | 6.0 (4.2–8.7) | 7.6 (6.4–9.0) | 7.3 (6.3–8.8) | 8.7 (7.5–10.1) | 8.4 (7.3–9.7) | 16.8 (14.5–19.4) | 13.9 (11.9–16.3) | 4.0 (2.9–5.5) | 4.2 (3.1–5.7) | |
| Hazard ratio (95% CI) | 0.92 (0.55–1.5) | 0.93 (0.73–1.19) | 0.95 (0.77–1.17) | 0.84 (0.68–1.04) | 1.06 (0.68–1.65) | 0.52 | |||||
| CV death | |||||||||||
| Event rate per 100 py | 3.0 (1.8–5.1) | 2.1 (1.2–3.8) | 2.2 (1.6–3.0) | 2.7 (2.1–3.6) | 3.7 (30–4.6) | 3.2 (2.5–4.0) | 4.3 (3.3–5.6) | 4.1 (3.1–5.3) | 1.7 (1.0–2.7) | 1.8 (1.1–2.8) | |
| Hazard ratio (95% CI) | 0.69 (0.31–1.52) | 1.20 (0.79–1.81) | 0.84 (0.61–1.16) | 0.95 (0.65–1.38) | 1.06 (0.54–2.07) | 0.52 | |||||
| Discontinuation due to SAE | |||||||||||
| Event rate per 100 py | 4.5 (2.8–7.1) | 3.2 (2.0–5.4) | 4.4 (3.5–5.5) | 5.2 (4.2–6.4) | 5.8 (4.9–7.0) | 4.9 (4.0–6.0) | 8.7 (7.1–10.6) | 7.1 (5.7–8.8) | 3.4 (2.3–4.8) | 4.5 (3.4–6.1) | |
| Hazard ratio (95% CI) | 0.71 (0.35–1.42) | 1.14 (0.83–1.56) | 0.84 (0.64–1.10) | 0.81 (0.60–1.08) | 1.35 (0.84–2.17) | 0.43 | |||||
CI, confidence interval; CV, cardiovascular; HFH, heart failure hospitalization; py, patient‐years; Sac/Val, sacubitril/valsartan; SAE, serious adverse event; Val, valsartan.
Treatment effects on most safety outcomes were similar across diuretic categories (online supplementary Table S3 ). Interaction testing did not detect significant heterogeneity by diuretic use group. There was however a signal for greater hypotension with sacubitril/valsartan when considering patients on any loop diuretic dose as an aggregate (p interaction = 0.01). A similar risk of discontinuation due to serious adverse events was observed with sacubitril/valsartan versus valsartan irrespective of baseline diuretic use.
Changes in diuretic use over time
The mean furosemide equivalent dose was 50 ± 49 mg in the valsartan‐treated group and 51 ± 51 mg in the sacubitril/valsartan‐treated group at baseline. The mean furosemide equivalent dose did not differ between study arms during trial follow‐up (p = 0.53) (Figure 2 ). Overall, 1116 patients were identified as diuretic non‐users and 3680 as diuretic users, at baseline. Among baseline diuretic non‐users, treatment with sacubitril/valsartan resulted in a modest reduction in new diuretic initiations that did not meet statistical significance (hazard ratio [HR] 0.83; 95% CI 0.68–1.00, p = 0.055; Figure 3A ). Conversely, there was no significant difference on new diuretic discontinuations or treatment disruptions in baseline diuretic users over the course of the trial (HR 0.96; 95% CI 0.82–1.12, p = 0.62; Figure 3B ). When net change in diuretic dose was analysed cross‐sectionally, patients randomized to sacubitril/valsartan experienced a slight early reduction in diuretic use at 30 days after initiation (net reduction 1.7%, p = 0.02) but no net differences in diuretic use were observed later in follow‐up (Figure 4 ).
Figure 2.
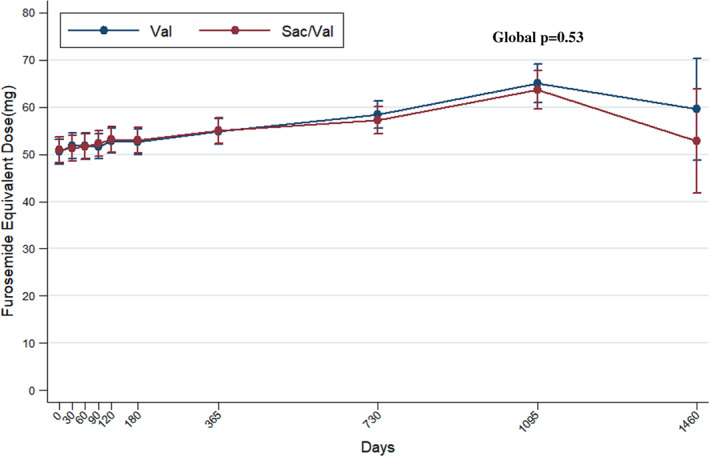
Mean loop diuretic dose over time according to treatment assignment. Sac/Val, sacubitril/valsartan; Val, valsartan.
Figure 3.
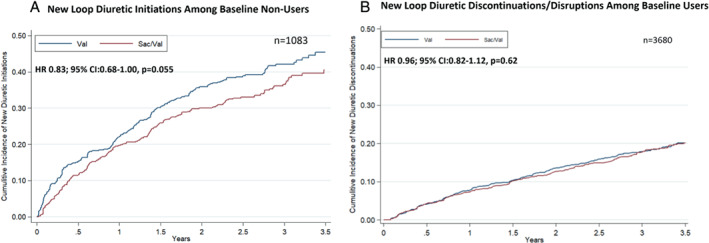
Treatment effect on loop diuretic utilization in study follow‐up. Kaplan–Meier curves displaying (A) the time to loop diuretic initiation among patients not on loop diuretic at baseline and (B) time to discontinuation or disruption among patients being treated with loop diuretic at baseline. Disruption was defined as any interruption in diuretic use of ≥30 days. CI, confidence interval; HR, hazard ratio; Sac/Val, sacubitril/valsartan; Val, valsartan.
Figure 4.
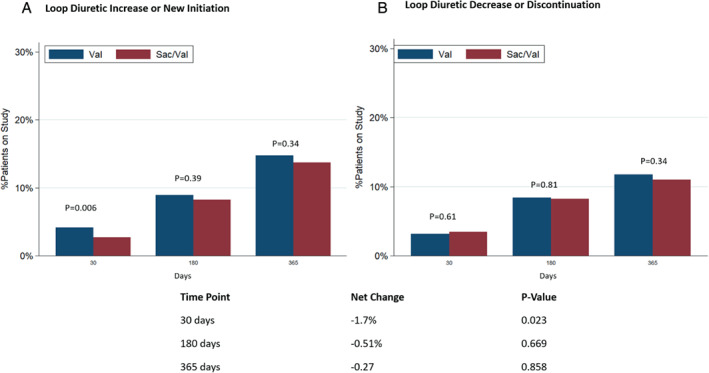
Changes in diuretic use at 30, 180 and 365 days according to treatment assignment. (A) Loop diuretic increase or new initiation among patients not on loop diuretic at baseline. (B) Loop diuretic dose decrease or discontinuation among patients taking loop diuretic at baseline. Sac/Val, sacubitril/valsartan; Val, valsartan.
Discussion
In this post hoc analysis of the PARAGON‐HF trial, we found that sacubitril/valsartan compared to valsartan exhibited consistent benefits and safety profile across a range of diuretic use and dosing, and that treatment with sacubitril/valsartan was associated with an early modest reduction in loop diuretic dose requirement. However, mean loop diuretic dose and rates of discontinuation remained similar in follow‐up. This suggests that the early borderline reduction in loop diuretic dose requirement associated with sacubitril/valsartan in patients with HFpEF may reflect another facet of prevention of worsening HF (Graphical Abstract).
Diuretic therapy is central to the management of patients with HF regardless of left ventricular ejection fraction; however, studies characterizing the use and impact on outcomes in patients with HFpEF are limited. In this study, 78% of patients were on loop diuretic therapy at randomization, in keeping with the requirement in PARAGON‐HF to be treated with at least intermittent diuretic therapy at screening. The mean and median furosemide equivalent doses were 50 and 40 mg, respectively, with the most frequently prescribed total daily furosemide equivalent dose being 40 mg. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) Americas, 62% of patients were on loop diuretic at baseline with a median baseline furosemide equivalent dose of 40 mg. 10
As expected, patients receiving the highest doses of diuretic experienced the highest risk of clinical outcomes including the primary composite outcome (and its components) and the renal composite outcome. This is consistent with other studies demonstrating a similar dose‐dependent association between higher loop diuretic dose and worse clinical outcomes in both HFpEF 10 , 11 and HFrEF. 12 , 13 Whether this association is related to baseline HF severity and adverse patient risk profile, degree of congestion, or direct deleterious effects of high‐dose loop diuretics is uncertain. 14
Based on the postulated diuretic sparing effects, consensus statements have offered preferential consideration of sacubitril/valsartan in congested HF phenotypes which has been extrapolated to treatment algorithms in HFpEF. Indeed, the potential for sacubitril/valsartan to counteract residual congestion was thought to partly explain recent observations demonstrating the greatest absolute and relative benefits of sacubitril/valsartan in patients most recently hospitalized for HF. 5 In this study, although clinical risk profiles varied by diuretic dose, the clinical benefits of sacubitril/valsartan versus valsartan remained consistent irrespective of background diuretic therapy.
A similar safety profile of sacubitril/valsartan relative to valsartan was also observed across a wide range of diuretic doses. As expected, hypotension occurred more frequently in sacubitril/valsartan‐treated patients. While interaction testing did not reveal significant differences across groups, there was a signal for increased hypotension when any dose of loop diuretic was considered in aggregate. In clinical practice, diuretic‐naïve patients who are newly being started on sacubitril/valsartan should be monitored for hypotension, and appropriate measures should be undertaken to mitigate these potential risks. Event rates were too small to evaluate treatment effect on the renal composite outcome; however, notably, important rises in serum creatinine did not differ across the range of diuretic therapy.
Understanding the diuretic sparing effects of HF medical therapy is of particular interest given the potential need for diuretic dose reduction to mitigate the risk for over diuresis, hypotension, and worsening renal function. Consensus guidelines suggest diuretic dose reduction on sacubitril/valsartan initiation on the basis of sustained net reduction in loop diuretic dose observed in the HFrEF population. 2 , 15 , 16 In contrast, in the present study of well characterized HFpEF patients, mean loop diuretic dose remained similar between treatment groups in the absence of significant impact on loop diuretic dose decrease or discontinuations over the course of follow‐up. These differences in sacubitril/valsartan diuretic effect in patients with HFrEF versus HFpEF are observed despite similar burden of metrics of congestion as indicated by similar mean baseline loop diuretic doses in PARADIGM‐HF (48 mg for sacubitril/valsartan and 50 mg for enalapril) and PARAGON‐HF (51 mg for sacubitril/valsartan and 50 mg for valsartan).
The discrepancy in influence of sacubitril/valsartan on diuretic dose between patients with HFpEF versus HFrEF may lie in reasons for diuretic dose titration. Downstream diuretic dose adjustments are frequently made to mitigate the deleterious effects of diuretics including hypotension, even in the face of persistent congestion. Patients with HFpEF are known to have a blood pressure profile that is significantly higher in comparison to patients with HFrEF. 2 Indeed, the baseline systolic blood pressure among randomized patients in PARAGON‐HF 1 was approximately 10 mmHg higher than patients in PARADIGM‐HF. 3 The difference in baseline blood pressure in these two HF phenotypes may partially explain the observed variability in diuretic dose adjustment.
On the other hand, sacubitril/valsartan resulted in a borderline significant 17% reduction in new loop diuretic initiations and a small early net reduction in overall loop diuretic requirement. Worsening HF is common in patients with HFpEF, and may occur even in those in ambulatory care, and often managed with escalation of loop diuretics. 17 The borderline reduction in new loop diuretic use may represent another facet of prevention of worsening HF with sacubitril/valsartan.
Several limitations of this analysis are worth noting. First, the analyses of the efficacy and safety of sacubitril/valsartan by background diuretic therapy were not pre‐specified and thus should be considered hypothesis generating. Second, sample sizes within some evaluated subgroups were small. Third, diuretic dose information was not available for all patients at all time‐points. Fourth, diuretic utilization was compared between treatment groups at pre‐determined study visits and therefore may not be granular enough to account for changes that may have occurred between study visits. Lastly, the specific rationale by patients or clinicians for diuretic dose decrease or discontinuation was not available. It is possible that alterations in diuretic regimen may have been in response to other clinical changes and thus may not necessarily reflect reduced congestion in all cases.
In summary, sacubitril/valsartan exerts consistent benefits across a range of diuretic categories and doses with a similar safety profile. Treatment with sacubitril/valsartan did not influence the mean loop diuretic dose or rate of diuretic discontinuation in follow‐up, but did result in a modest, borderline significant reduction in new loop diuretic initiation. Taken together, this early reduction in new diuretic requirement underscores the clinical benefits of sacubitril/valsartan in preventing worsening HF. In contrast to HFrEF, however, routine diuretic dose reduction on sacubitril/valsartan initiation in HFpEF may be less warranted.
Funding
PARAGON‐HF was funded by Novartis Pharmaceuticals Corporation.
Conflict of interest: S.C. is supported by the Canadian Child's Scholarship. B.L.C. has been a consultant for Amgen, AO Biome, Biogen, Boehringer Ingelheim, Corvia, Gilead, Myokardia, Cardurion, and Novartis. K.J. has received support form the National Institute of Health (Training Grant 5‐T32 HL007604). A.S.D. has received research grant support from AstraZeneca, Alnylam, and Novartis; and has received consulting fees and/or honoraria from Abbott, AstraZeneca, Alnylam, Boehringer Ingelheim, Boston Scientific, Biofourmis, Corvidia, DalCor Pharma, Novartis, Relypsa, and Regeneron. M.L. is an employee of Novartis. J.J.V.M. has received grants and his employer paid by AstraZeneca, Theracos, and GlaxoSmithKline during the conduct of the study and grants and his employer being paid by Novartis, Amgen, Bristol‐Myers Squibb, Bayer, Abb‐vie, Dal‐Cor, Kidney Research UK, and Cardurion and grants from British Heart Foundation. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Puretech Health. M.V. has received research grants from and/or serves on advisory boards for American Regent, Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, has participated in speaking engagements supported by AstraZeneca, Roche Diagnostics, and Novartis, and participates in clinical trial committees for studies sponsored Occlutech, Impulse Dynamics, Galmed, Bayer AG, and Novartis. All other authors have nothing to disclose.
Supporting information
Appendix S1. Supporting information.
References
- 1. Pascual‐Figal D, Bayés‐Genis A, Beltrán‐Troncoso P, Caravaca‐Pérez P, Conde‐Martel A, Crespo‐Leiro MG, et al. Sacubitril‐valsartan, clinical benefits and related mechanisms of action in heart failure with reduced ejection fraction. A review. Front Cardiovasc Med. 2021;8:754499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail. 2019;21:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al.; PARAGON‐HF Investigators and Committees. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- 5. Vaduganathan M, Claggett BL, Desai AS, Anker SD, Perrone SV, Janssens S, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. 2020;75:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–77. [DOI] [PubMed] [Google Scholar]
- 7. Rosano GMC, Moura B, Metra M, Böhm M, Bauersachs J, Ben Gal T, et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23:872–81. [DOI] [PubMed] [Google Scholar]
- 8. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail. 2017;5:471–82. [DOI] [PubMed] [Google Scholar]
- 9. Lin DY, Wi LJ, Yang I, Ying Z. Semiparametric regression of the mean and rate functions for recurrent events. J R Stat Soc Ser B Stat Methodol. 2000;62:711–30. [Google Scholar]
- 10. Kalogeropoulos AP, Thankachen J, Butler J, Fang JC. Diuretic and renal effects of spironolactone and heart failure hospitalizations: a TOPCAT Americas analysis. Eur J Heart Fail. 2020;22:1600–10. [DOI] [PubMed] [Google Scholar]
- 11. Abdel‐Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long‐term outcomes in elderly patients with heart failure after hospitalization. Am Heart J. 2010;160:264–271.e1. [DOI] [PubMed] [Google Scholar]
- 12. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–64. [DOI] [PubMed] [Google Scholar]
- 13. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20:738–47. [DOI] [PubMed] [Google Scholar]
- 15. Pharithi RB, Ferre‐Vallverdu M, Maisel AS, O'Connell E, Walshe M, Sweeney C, et al. Sacubitril‐valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail. 2020;7:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, et al. Real‐world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21:588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nanayakkara S, Patel HC, Kaye DM. Hospitalisation in patients with heart failure with preserved ejection fraction. Clin Med Insights Cardiol. 2018;12:1179546817751609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


