Abstract
Introduction
Late HIV diagnosis is associated with increased morbidity, mortality and risk of onward transmission. Increasing HIV early diagnosis is still a priority. In this observational study with historical control, we determined the impact of an opportunistic HIV screening strategy in the reduction of late diagnosis and missed opportunities for earlier diagnosis.
Methods
The screening programme was implemented in the emergency department (ED) of the Hospital de Cascais between September 2018 and September 2021. Eligible patients were aged 18–64 years, with no known HIV diagnosis or antibody testing performed in the previous year, and who required blood work for any reason. Out of the 252 153 emergency visits to the ED, we identified 43 153 (17.1%) patients eligible for HIV testing. Among the total population eligible for the screening, 38 357 (88.9%) patients were ultimately tested for HIV. Impact of the ED screening was determined by analysing late diagnosis in the ED and missed opportunities at different healthcare settings 3 years before and 3 years after the start of the ED screening.
Results
After 3 years of automated HIV ED testing, we found 69 newly diagnosed HIV cases (54% male, 39% Portuguese nationals, mean age 40.5 years). When comparing the characteristics of HIV diagnoses made in the ED, we observed a significant reduction in the number of people with late HIV diagnosis before and after implementation of the screening programme (78.4% vs. 39.1%, respectively; p = 0.0291). The mean number of missed opportunities for diagnosis also fell (2.6 vs. 1.5 annual encounters with the healthcare system per patient, p = 0.0997).
Conclusions
People living with HIV in Cascais and their providers miss several opportunities for earlier diagnosis. Opportunistic screening strategies in settings previously deemed to be unconventional, such as EDs, are feasible and effective in mitigating missed opportunities for timely HIV diagnosis.
Keywords: automated screening, early diagnosis, HIV, missed opportunities
INTRODUCTION
Despite multiple efforts to reduce the HIV epidemic over recent decades and the important therapeutic advances achieved over this time, HIV infection is still a public health challenge causing a large number of deaths worldwide [1]. Global efforts aim at tackling HIV/AIDS disease by stopping its transmission [2]. However, late diagnosis of HIV infection (CD4 T‐cell count ≤ 350 cells/μL at diagnosis) [3], thought to increase the likelihood of transmission, is still a major local, national and European problem [1].
Late HIV diagnosis is problematic for affected individuals, who experience increased morbidity and mortality, and because of missed opportunities by public health systems to interrupt transmission [4]. Official numbers from the Portuguese General Directorate for Health indicate that 2800 people living with HIV are unaware of their infection, with one in two receiving their diagnosis at a late stage [5]. A similar 50% rate of late diagnosis was reported in a large hospital in Lisbon by Miranda et al. [6], reporting higher rates in those of male gender, patients with heterosexual transmission, immigrants originating from sub‐Saharan Africa and patients aged between 31 and 55 years old. The results of Miranda et al. were also consistent with previous reports in other countries [7, 8, 9]. Considering the negative impact of late HIV detection in the prognosis of the patient, reaching and testing those at risk of infection remain a major challenge.
National and international guidelines alike recommend enhancing HIV screening and linkage to care (LTC) practices [10, 11, 12]. In its evidence‐based guidance on integrated HIV testing, the European Centre for Disease Prevention and Control urges countries to increase testing coverage and uptake towards achieving the United Nations' epidemic control goals for 2030 [11]. However, to date, HIV screening has typically followed traditional models, requiring dedicated staff and resources that are separate from routine clinical practice [13]. Instead of integrating screening into the regular provision of care for all eligible patients, conventional approaches often rely on a case‐by‐case decision, which may reinforce the deterring stigma associated with testing for these infections [14]. Besides, screening and testing awareness campaigns have had no impact in lowering late diagnosis numbers, and insufficient HIV testing in different healthcare settings seems to be generalized [15].
According to the Portuguese General Directorate for Health recommendations, people aged 18–64 should be offered HIV testing at least once in their lifetime, and more often depending on risk behaviour [10]. Despite these recommendations, implementation of HIV screening and LTC in formal healthcare organizations in Portugal is low and heterogeneous. Healthcare professionals and the health system itself have been identified as one of the major barriers to HIV testing, allegedly because of lack of time, lack of training, uneasiness discussing sexual health with patients or even fear of being perceived as discriminatory by patients [16].
To overcome this perceived barrier, we implemented a completely automated HIV screening programme in a general emergency department (ED) that is independent from physician request, thereby removing the ‘human factor’ from testing decisions. The aim of our study was to evaluate the impact of this opportunistic screening programme, specifically in terms of decreasing late HIV diagnosis and reducing missed opportunities for earlier diagnosis.
METHODS
Design and study procedure
>A systematic, opportunistic HIV and hepatitis C virus (HCV) screening programme [17] was prospectively implemented at the ED of Hospital de Cascais Dr. José de Almeida in Portugal, which receives 98 000 visits per year. In this paper, we report on HIV screening results only. Between 12 September 2018 and 11 September 2021, all patients presenting at the ED for any reason received an offer of universal HIV testing unless they met the following exclusion criteria (Figure 1): (a) age < 18 or > 64 years; (b) blood work not required; or (c) recorded HIV infection or HIV test within the previous year of presentation. In patients who met these requirements, the electronic health record (EHR) automatically generated a request for an HIV test by means of an algorithm that was implemented to select ED patients to be screened and excludes people already known to be HIV‐positive. At the time of the blood draw, the nursing staff informed the patient about the opportunity to test for HIV and the explicit possibility of opting out (‘As hospital policy, your laboratory tests will include an HIV or HCV test unless you decline. Do you have any questions?’). If a patient met the eligibility criteria but was unable to give verbal informed consent (altered state of consciousness, impaired cognitive capacities, language barriers), the decision to include the patient in the screening programme was based on medical opinion. Physicians could always independently order tests, based on clinical suspicion, during patient evaluation. In that case, the patient was not included for screening because the EHR automatically identified the physician's test order.
FIGURE 1.
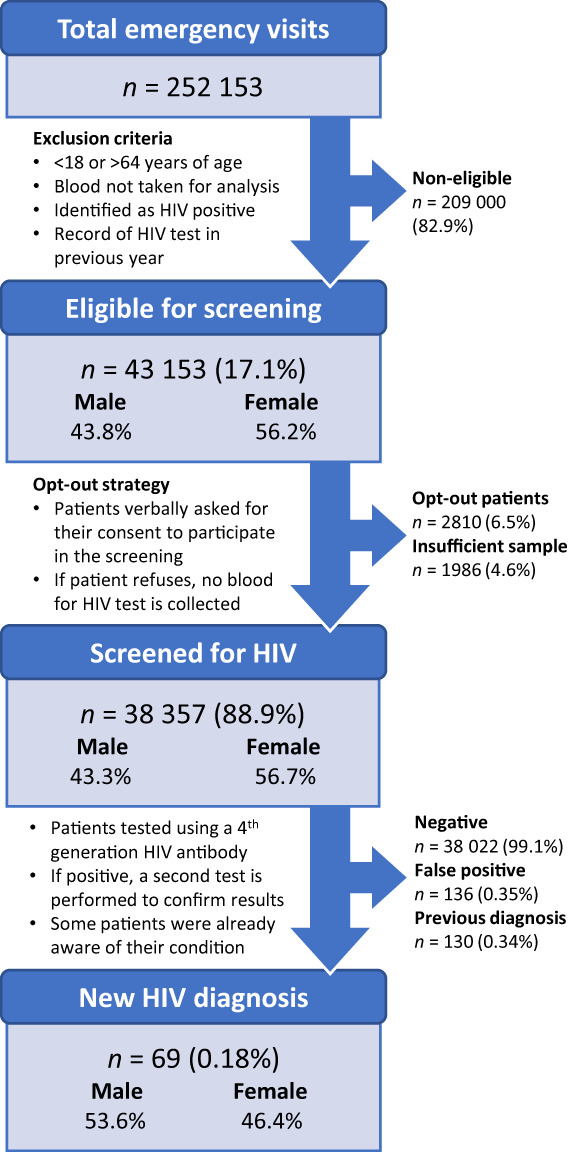
Flowchart depicting the number of patients who met study inclusion criteria for HIV screening and the results obtained in the test
Healthcare workers in the ED received specific training for this project. LTC, defined as confirmation of a first medical appointment after diagnosis, was guaranteed by a specialized nurse. The LTC team received a daily email with the reactive results from the previous day, to ensure automatic follow‐up of all cases. All patients with a reactive first‐line HIV test were contacted by the LTC team and called to the hospital to have this result confirmed, independently of positivity range. If the confirmatory HIV test came back negative, the case was defined as a false‐positive. The HIV 1/2 differentiation test was performed on the same sample. The Ethics Committee of the Hospital of Cascais approved the conduct of this project.
Description of the TEST model
We employed the TEST model to promote changes in our care system and to extend screening and LTC [17]. TEST is based on four main pillars, namely testing and linkage integrated into the normal clinical circuit, using existing infrastructure and personnel to generate efficiencies (T); EHR modifications to automate patient eligibility assessment and laboratory request orders (E); systemic policy change (S); and training, continuous quality improvement and feedback (T).
Immunoassay test for screening
Serum samples were analysed using the ADVIA Centaur® Immunoassay System (Siemens Healthineers, Erlangen, Germany) employing sandwich‐type chemiluminescent immunoassays for the simultaneous qualitative detection of HIV p24 antigen and HIV‐1 and ‐2 antibodies. Reactive HIV antibody tests were confirmed by repeating the fourth‐generation HIV antibody test and by using the HIV‐1/HIV‐2 differentiation test, as applicable. Patient samples with anti‐HIV‐1/2 antibodies underwent a second test on the same sample by Standard Diagnostics, Inc. (SD HIV‐1/2 3.0) to confirm and differentiate HIV‐1/2 antibodies.
Comparison with a historical control group
We analysed the database of the Hospital de Cascais Dr. José de Almeida to characterize new diagnoses and the number of missed opportunities for diagnosis, comparing the group of patients identified in the screening with a historical control group. Patients were eligible to enter the control group if they met the following criteria: (a) HIV diagnosis at the ED between 12 September 2015 and 11 September 2018; (b) aged between 18 and 64 years; and (c) absence of recorded HIV infection or HIV test in the EHR. HIV tests for this group of patients were ordered based solely on clinical presenting conditions and doctor's suspicion. Late HIV diagnosis was defined as CD4 count ≤ 350 cells/μL [3].
Previous healthcare encounters at any national public healthcare facility in Portugal were retrospectively investigated in patients from the screening programme and the control group, using the Portuguese National Health Service centralized database – private and foreign healthcare system records are not available to us. Missed opportunities were defined as the failure of a previously untested patient to obtain HIV testing despite attending one or more visits in healthcare facilities in the 12 months prior to diagnosis.
Main outcomes and measures
Variables considered in this study were: sex; age, categorized into four strata (18–29, 30–39, 40–49 and 50–64 years); and country of origin, categorized into three groups (Portuguese, non‐Portuguese or unknown). The main outcomes reported were the number of HIV screenings performed, positive HIV tests, late HIV diagnoses and the number of missed opportunities.
Statistical analysis
All data points were analysed descriptively and the resulting statistics are reported as percentages or mean ± SD. We performed an unpaired Student's t‐test to contrast the mean value of late HIV diagnoses, CD4 count and missed opportunities in the screening group compared with the historical control. This comparison was bilateral and assumed confidence intervals of 95%. Statistical analysis was performed using GraphPad Prism 9 (v. 9.3.1).
RESULTS
Population screened and demographic characteristics
Between 12 September 2018 and 11 September 2021, there were 252 153 emergency visits to the general ED at Hospital de Cascais (Figure 1). The EHR identified 43 153 (17.1%) patients eligible for HIV testing (see eligibility criteria in the Methods section). Of these, 2810 (6.5%) declined to participate in the screening. Among the total population of people eligible for the screening, 38 357 (88.9%) patients were ultimately tested for HIV. The demographic characteristics of these patients are reported in Table 1. A significant proportion of screened patients, 21 779/38 357 (56.8%), were between 40 and 64 years of age. Approximately two‐thirds were of Portuguese origin and one‐third were from other countries.
TABLE 1.
Demographic characteristics of patients tested and diagnosed with HIV per year of emergency department (ED) screening
| Parameter | First year a | Second year b | Third year c | Total d | 3 years pre‐screening e | p‐value* |
|---|---|---|---|---|---|---|
| Total emergency visits (n) | 100 501 | 79 625 | 72 027 | 252 153 | 282 751 | ND |
| People screened for HIV (n) | 15 242 | 12 090 | 11 025 | 38 357 | NA | NA |
| Sex [n (%)] | ||||||
| Male | 7130 (43) | 6061 (44) | 5706 (45) | 18 897 (43.8) | NA | NA |
| Female | 9731 (57) | 7607 (56) | 6915 (55) | 24 253 (56.2) | NA | NA |
| Age range (years) [n (%)] | ||||||
| 18–29 | 3684 (24.1) | 2835 (23.5) | 2344 (21.3) | 8863 (23.1) | NA | NA |
| 30–39 | 3118 (20.5) | 2431 (20.1) | 2166 (19.6) | 7715 (20.1) | NA | NA |
| 40–49 | 3570 (23.4) | 2894 (23.9) | 2708 (24.6) | 9172 (23.9) | NA | NA |
| 50–64 | 4870 (31.9) | 3930 (32.5) | 3808 (34.5) | 12 607 (32.9) | NA | NA |
| Country of origin [n (%)] | ||||||
| Portugal | 10 469 (68.7) | 7919 (65.5) | 7189 (65.2) | 25 577 (66.7) | NA | NA |
| Other than Portugal | 4647 (30.5) | 4069 (33.7) | 3743 (34) | 12 459 (32.5) | NA | NA |
| Unknown (%) | 126 (0.8) | 102 (0.8) | 93 (0.8) | 321 (0.8) | NA | NA |
| New HIV diagnosis (n) | 36 | 17 | 16 | 69 | 37 | ND |
| Sex [n (%)] | ||||||
| Male | 21 (58.3) | 9 (52.9) | 7 (43.7) | 37 (53.6) | 26 (70.3) | ND |
| Female | 15 (41.7) | 8 (47.1) | 9 (56.3) | 32 (46.4) | 11 (29.7) | ND |
| Age range (years) [n (%)] | ||||||
| 18–29 | 10 (27.8) | 3 (17.7) | 2 (12.5) | 15 (21.7) | 6 (16.2) | ND |
| 30–39 | 8 (22.2) | 4 (23.5) | 3 (18.8) | 15 (21.7) | 7 (18.9) | ND |
| 40–49 | 5 (13.9) | 8 (47) | 9 (56.3) | 22 (31.9) | 12 (32.4) | ND |
| 50–64 | 13 (36.1) | 2 (11.8) | 2 (12.4) | 17 (24.6) | 12 (32.4) | ND |
| Country of origin [n (%)] | ||||||
| Portugal | 12 (33.3) | 7 (41.2) | 8 (50) | 27 (39.1) | 16 (43.2) | ND |
| Other than Portugal | 24 (66.7) | 10 (58.8) | 8 (50) | 42 (60.9) | 21 (56.8) | ND |
| Mode of transmission [n (%)] | ||||||
| HTS | 27 (75) | 11 (64.7) | 14 (87.5) | 52 (75.4) | 28 (75.7) | ND |
| MSM | 9 (25) | 5 (29.4) | 2 (12.5) | 16 (23.2) | 8 (21.6) | ND |
| IVDU | 0 | 1 (5.9) | 0 | 1 (1.4) | 1 (2.7) | ND |
| CD4 count (cells/μL) (mean ± SD) | 417 ± 247 | 407 ± 233 | 385 ± 299 | 411 ± 253 | 211 ± 150 | <0.0001 |
| Late diagnosis f [n (%)] | ||||||
| Yes (CD4 ≤ 350 cells/μL) | 12 (33.3) | 6 (35.3) | 9 (56.2) | 27 (39.1) | 29 (78.4) | 0.0291 |
| No (CD4 > 350 cells/μL) | 21 (58.3) | 9 (52.9) | 7 (43.8) | 37 (53.6) | 8 (21.6) | ND |
| Unknown | 3 (8.3) | 2 (11.8) | 0 | 5 (7.2) | 0 | ND |
| Patients with at least one healthcare encounter [n (%)] | 13 (36.1) | 10 (58.8) | 5 (31.3) | 28 (40.6) | 25 (67.6) | 0.0829 |
| Number of missed opportunities per patient (mean ± SD) | 1.4 ± 2.9 | 2.1 ± 3.9 | 0.9 ± 1.9 | 1.5 ± 3.0 | 2.6 ± 3.6 | 0.0997 |
Note: Bold values highlight screening and historical control values.
Abbreviations: IVDU, intravenous drug use; HTS, heterosexual sex; MSM, men who have sex with men; NA, not applicable; ND, not determined; SD, standard deviation.
Statistical comparison between screening and historical control group.
From 12 September 2018 to 11 September 2019.
From 12 September 2019 to 11 September 2020.
From 12 September 2020 to 11 September 2021.
From 12 September 2018 to 11 September 2021.
From 12 September 2015 to 11 September 2018.
As determined by the CD4 count (cells/μL).
Outcome of the HIV ED screening programme
We obtained 335 reactive HIV antibody tests (0.87% of the samples screened). Of these, 199 patients were confirmed to be infected with HIV (0.52% of the total population screened), 130 (65.3%) of whom were already aware of their HIV condition (not reported during the evaluation or included in their health records). Among the 69 new HIV diagnoses found during the 3‐year screening period, more than half (56.5%) were identified in people between 40 and 64 years of age (Table 1). The mean age was 40.5 ± 11.3 years. Of all new HIV diagnoses, 42/69 (60.9%) were obtained in people whose country of origin was not Portugal; 26/42 (61.9%) of these were of African origin. All patients receiving a new HIV diagnosis were successfully linked to care.
Regarding the mode of HIV acquisition, 52/69 (75.4%) patients reported heterosexual sex, 16/69 (23.2%) reported they were men who have sex with men, and there was one case of HIV infection through intravenous drug use identified in the screening programme (1.4%). LTC was achieved in 100% of cases, with 26/69 (37.7%) patients being referred to other hospitals because their home address was outside the Hospital de Cascais area of influence. Finally, 5/69 (7.2%) cases were HIV‐2 infection.
New HIV diagnoses before and after the implementation of screening
Between 12 September 2015 and 11 September 2018, 37 patients were diagnosed with an HIV infection at our ED unit (Table 1). Of these, 26/37 (70.3%) were male and 24/37 (64.8%) were aged > 40 years. The country of origin was Portugal in 16/37 (43.2%) cases. In 2018, the new screening programme identified nine patients between 12 September and 31 December, accounting for 21.4% of all 42 cases identified that year in our hospital. In 2019, all 36 new HIV cases identified at the ED were discovered through the screening programme, followed by 17/20 in 2020 and 16/21 in 2021.
Late diagnosis and missed opportunities of HIV diagnosis
The mean CD4 count at diagnosis for new patients diagnosed at the ED in the 3 years prior the screening (12 September 2015 to 11 September 2018) was 211 ± 150 cells/μL. During this period, 29/37 (78.4%) HIV‐diagnosed patients were defined as late presenters (Figure 2a). We observed an increasing trend over the 3 years: 7/11 (63.6%), 11/14 (78.6%) and 11/12 (91.7%) during September 2015 to September 2016, September 2016 to September 2017, and September 2017 to September 2018, respectively. After implementing the screening (September 2018 to September 2021), the percentage of late presenters dropped to 27/69 (39.1%; p = 0.0291). Late diagnosis rates were considerably lower than in any of the 3 years prior to the screening: 12/36 (33.3%) in the first year, 6/17 (35.3%) in the second year and 9/16 (56.2%) in the third year (Table 1). The mean CD4 count at diagnosis during this period increased by 94% (Figure 2b), reaching 411 ± 253 cells/μL (p < 0.0001) and remaining well above the 350 cells/μL late diagnosis threshold during the 3 years (Table 1).
FIGURE 2.
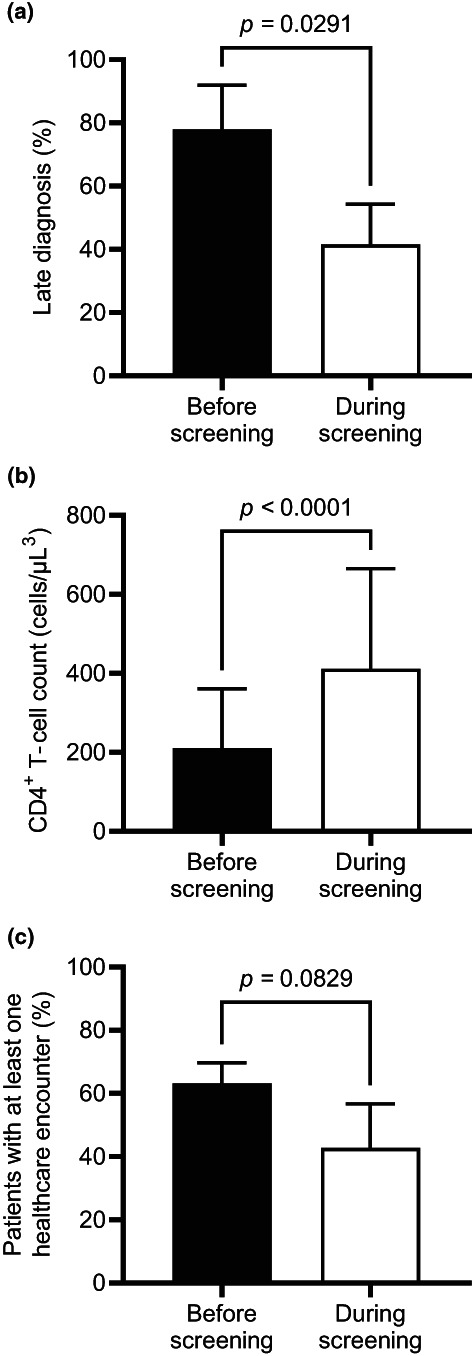
Impact of screening on late diagnosis cases, CD4 T‐cell count and missed opportunities for diagnosis, grouped in the periods before (12 September 2015 to 11 September 2018) and after (12 September 2018 to 11 September 2021) the implementation of the screening. Graphs depict mean ± SD. Statistical analysis was performed using an unpaired Student's t‐tests. (a) Percentage of patients presenting with late HIV diagnosis. (b) Patient CD4 T‐cell count at diagnosis. (c) Percentage of people living with HIV with at least one healthcare encounter in the year before diagnosis
Upon implementing the screening programme, we observed a 20.5% drop in the percentage of patients with previously missed opportunities for diagnosis compared with the historical control group (Figure 2c). The mean number of total missed opportunities for diagnosis compared with the previous 3 years fell from 2.6 ± 3.6 to 1.5 ± 3.0 annual encounters with the healthcare system per patient (p = 0.0997).
DISCUSSION
In this study, we analysed the results of an HIV opportunistic screening programme, after 3 years' implementation at the ED of the Hospital de Cascais. We found a significant decrease in late HIV diagnosis and a reduction in the number of missed opportunities for earlier diagnosis since starting the screening intervention. To our knowledge, this is the first report of a systematic, clinician‐independent, opportunistic screening programme for HIV infection in an ED in Portugal.
Electronic health record‐based opportunistic HIV screenings in EDs allow for the identification of new HIV cases among people who may underestimate their exposure risk or lack access to traditional HIV testing services [19, 20, 21]. They are also useful for detecting and controlling possible local disease outbreaks [22]. The US Centers for Disease Control and Prevention has recommended opportunistic HIV screenings in EDs since 2006 [12]. The total number of emergency visits during the second and third years of the study was lower due to the COVID‐19 pandemic (see later for an additional explanation), but we typically receive close to 100 000 patients per year at our ED. Systematic HIV testing in this medical setting provides a unique opportunity to reach a wide population of patients who do not consider themselves at risk or have other reasons for not seeking patient‐initiated testing, potentially allowing for early diagnosis [23]. We used an automatic EHR‐initiated method, which has been associated with better implementation of testing recommendations, overcoming physicians' decision to order a test, and avoiding stigmatization on grounds of sexual orientation and gender [24]. In fact, patients generally agree to be tested when offered an HIV test [25]. Only 6.5% of eligible patients in our study opted out of screening. Although nursing staff received repeat training on consent procedures, it is plausible that some patients may not have been informed about testing. We mitigated this risk during intake interviews of patients with reactive tests by confirming that test information had originally been provided. Simultaneously, our declination rate is in line with results from other projects deploying similar strategies, where acceptability was also > 90% [26, 27].
After the programme was implemented in 12 September 2018, cases detected at ED screening accounted for 40.5% of all patients presenting for care at the HIV unit of the Hospital de Cascais. Moreover, the total number of new HIV diagnoses in our ED increased from 37 in the historical control period to 77 (+108%) during the screening period, despite a reduction of ~20–30% in overall emergency visits during the COVID‐19 pandemic years, with the consequent reduction in patients screened and the number of positive cases detected. Indeed, the number of new HIV diagnoses fell by more than 50% in the second and third years compared with the first year. Other groups have also acknowledged this challenge while addressing the number of new HIV‐positive cases detected in ED screenings [29, 30]. All these observations strengthen the idea that screening opportunistically yields more diagnosis and better outcomes of earlier diagnosis than risk‐based testing alone.
The analysis of the country of origin of the individuals tested in the screening revealed possible socioeconomic implications affecting HIV risk in Portugal. Even though about two‐thirds of the patients screened were of Portuguese origin, over 60% of HIV cases were confirmed among people whose country of origin was not Portugal. Notably, 7.2% of infections were of HIV‐2, a finding associated with the historical presence of Portugal in African countries, namely Guinea‐Bissau, which has a high prevalence of HIV‐2 [30, 31]. Previous studies have reported a higher burden of missed opportunities for HIV diagnosis among migrant populations in Europe, particularly those of African origin [32, 33]. These individuals generally present important socioeconomic vulnerabilities which prevent proper access to healthcare services and create a lower degree of HIV infection awareness [34, 35]. The results of our screening initiative prompt us to speculate that similar structural factors related to poor living conditions are in play in Portugal, as ED is often the primary entry point of migrant populations into the healthcare system.
People with early HIV diagnosis can benefit from antiretroviral treatment at a stage before clinical manifestations develop, avoiding serious comorbidities and dramatically improving their quality of life [36]. Late HIV diagnosis results in worse treatment outcomes and higher costs for healthcare systems [6, 37]. This is particularly important for patients aged > 50 because they are at higher risk of developing serious comorbidities and are frequently underdiagnosed [38, 39]. Early detection of HIV infection is also crucial to limit the viral spread attributable to people unaware of their condition and would contribute to halting the HIV epidemic [40, 41]. However, most newly diagnosed HIV patients in Portugal are late presenters [42]. Previous studies have shown that patients with HIV have numerous encounters with the healthcare system prior to the detection of their condition, representing missed opportunities for diagnosis [18, 43, 44]. Our non‐targeted, opportunistic HIV screening was successful in decreasing both the incidence of late HIV presentation and the missed opportunities for diagnosis. As such, we advocate the general implementation of screening and LTC practices in any healthcare organization using EHRs, as they have been shown to be successful in multiple settings [23].
Two unexpected results of our study deserve further consideration. First, we observed that 130 patients of the screening were already aware of their HIV condition. As their disease background was not flagged in the EHR (probably because they were followed at another hospital and only attended our ED for an emergency visit), these patients were not automatically excluded from eligibility workflows. Nevertheless, it is intriguing that they did not opt out of screening to avoid extra testing. We speculate that this relates to confidentiality issues (i.e. having to disclose their condition to the nurse, perhaps in front of or close to other people). Second, the number of false‐positive results that we obtained seemed high: 136 false positives (> 40% of all reactive tests). This outcome amounts to a test specificity of 99.64%, which is in line with the 99.56–99.84% reported by the manufacturer for the assay we used [45], and above the WHO recommended threshold of 98% specificity for HIV tests [46]. Local prevalence influences how many false‐positive results are expected relative to true‐positive results: in low‐prevalence settings, a higher proportion of all positive tests are normally expected to be false‐positive [47].
Our study has some limitations. First, an inherent limitation of a monocentric study is that we must limit our conclusions to the area of influence of our hospital, which may not be representative of the general population. Second, we did not have access to the full medical record of patients analysed in the ‘missed opportunities for diagnosis’ section. Therefore, we cannot distinguish healthcare encounters where the patient presented with symptoms that could have been associated with HIV (e.g. oral candidiasis or herpes zoster) from encounters for other reasons not associated with this condition. Therefore, even though we applied the same parameters to study missed opportunities in patients identified before and during the screening, our data can only be taken as an approximation. Finally, the number of diagnoses and healthcare encounters during the COVID‐19 pandemic could have been negatively influenced by difficulties in accessing healthcare during lockdowns. Due to shifting healthcare restrictions, lockdowns and travel bans worldwide, a large number of migrant populations might not have been able to reach our ED. The impact of the pandemic reducing the number of HIV diagnoses has also been reported by other groups in multiple countries [48, 49, 50, 51, 52, 53]. Regarding late diagnosis and missed opportunities, data from 12 September 2018 to 11 September 2019 are unaffected by the COVID‐19 pandemic and still show a reduction in late diagnosis, mean number of previous healthcare encounters and percentage of patients with at least one healthcare encounter, all of which hints favourably at the benefit of our intervention. Given the impact of the COVID‐19 pandemic on worldwide detection of HIV in general, we can speculate about an increase in the late diagnosis rate in the coming years, but this has not been reported so far [48, 49, 50, 51, 52, 53]. Despite these limitations, our study demonstrated a robust methodology to generate essential HIV diagnoses and epidemiology data.
CONCLUSION
The implementation of an automated, opportunistic HIV screening in routine medical care effectively improved HIV diagnosis among individuals not previously aware of their condition, decreasing the incidence of late HIV presentation and reducing missed opportunities for diagnosis. We advocate the wider implementation of screening and LTC practices in all healthcare organizations using EHRs to reduce the spread and morbimortality associated with late HIV diagnosis and to improve LTC among people previously unaware of their condition.
AUTHOR CONTRIBUTIONS
IV‐P, AG, CE, MG and DM contributed to the conception and design of the work; IV‐P, AG, CE, MG and VC were responsible for data acquisition; IV‐P, AG, CE, and DM contributed to the analysis and interpretation of data and drafted the manuscript; all authors reviewed the manuscript critically for important intellectual content and provided final approval of the version to be published.
FUNDING INFORMATION
We acknowledge funding from Gilead Sciences' FOCUS program to support HIV and viral hepatitis screening and linkage to the first medical appointment after diagnosis. FOCUS funding does not support activities beyond the first medical appointment and is agnostic regarding how organizations handle subsequent patient care and treatment.
CONFLICT OF INTEREST
DM owns stock in and is an employee of Gilead Sciences. AC is an employee of Gilead Sciences. The remaining authors declare no conflicts of interest concerning the research, authorship and publication of this article. Data collection and management were conducted independently, with additional oversight by independent data monitoring agencies.
ACKNOWLEDGEMENTS
The authors thank the entire ED of Hospital de Cascais for their contribution to the FOCUS programme. We acknowledge the support of Dr Stela Lopes and Dr Joana Selada from Synlab Laboratory at the Hospital de Cascais. We also thank Anchel González Barriga and Vanessa Marfil Vives of Medical Statistics Consulting, Spain, for providing editorial support, in the form of medical writing and assembling tables based on authors' detailed directions, collating author comments, copyediting, fact‐checking and referencing.
Vaz‐Pinto I, Gorgulho A, Esteves C, et al. Increasing HIV early diagnosis by implementing an automated screening strategy in emergency departments. HIV Med. 2022;23(11):1153‐1162. doi: 10.1111/hiv.13431
Inês Vaz‐Pinto and Ana Gorgulho contributed equally to this work.
Funding information Gilead Sciences
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. UNAIDS . UNAIDS 2020 Global report2020 May 19th 2022. https://www.unaids.org/sites/default/files/media_asset/2020_aids‐data‐book_en.pdf
- 2. UNAIDS . 90‐90‐90, an ambitious treatment target to help end the AIDS epidemic. In: HIV/AIDS JUNPo , ed. UNAIDS; 2014. [Google Scholar]
- 3. Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61‐64. [DOI] [PubMed] [Google Scholar]
- 4. Mugavero MJ, Castellano C, Edelman D, Hicks C. Late diagnosis of HIV infection: the role of age and sex. Am J Med. 2007;120(4):370‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA) e a Direção‐Geral da Saúde (DGS) . Infeção VIH e SIDA em Portugal ‐ 2020. In: Jorge INdSdDR, ed. Lisbon; 2020.
- 6. Miranda MNS, Pingarilho M, Pimentel V, et al. Determinants of HIV‐1 late presentation in patients followed in Europe. Pathogens. 2021;10(7):835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwuji CC, Churchill D, Gilleece Y, Weiss HA, Fisher M. Older HIV‐infected individuals present late and have a higher mortality: Brighton, UK Cohort Study. BMC Public Health. 2013;13:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Op de Coul EL, van Sighem A, Brinkman K, et al. Factors associated with presenting late or with advanced HIV disease in The Netherlands, 1996‐2014: results from a national observational cohort. BMJ Open. 2016;6(1):e009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith RD, Delpech VC, Brown AE, Rice BD. HIV transmission and high rates of late diagnoses among adults aged 50 years and over. Aids. 2010;24(13):2109‐2115. [DOI] [PubMed] [Google Scholar]
- 10. Direçao‐Geral da Saúde (DGS) . Diagnóstico e Rastreio Laboratorial da Infeção pelo Vírus da Imunodeficiência Humana (VIH); 2014. 19th May 2022. https://www.dgs.pt/directrizes‐da‐dgs/normas‐e‐circulares‐normativas/norma‐n‐0582011‐de‐28122011‐jpg.aspx
- 11. European Centre for Disease Prevention and Control (ECDC) . Public health guidance on HIV, hepatitis B and C testing in the EU/EEA–an integrated approach. European Centre for Disease Prevention and Control (ECDC). 2018;1:1‐4. [Google Scholar]
- 12. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health‐care settings. MMWR Recomm Rep. 2006;55(RR‐14):1‐17. quiz CE1‐4. [PubMed] [Google Scholar]
- 13. Sullivan PS, Lyons MS, Czarnogorski M, Branson BM. Routine screening for HIV infection in medical care settings: a decade of Progress and next opportunities. Public Health Rep. 2016;131(Suppl 1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control (ECDC) . HIV Testing: Increasing Uptake and Effectiveness in the European Union; 2010. 19th May 2022. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/101129_GUI_HIV_testing.pdf
- 15. Deblonde J, De Koker P, Hamers FF, Fontaine J, Luchters S, Temmerman M. Barriers to HIV testing in Europe: a systematic review. Eur J Public Health. 2010;20(4):422‐432. [DOI] [PubMed] [Google Scholar]
- 16. Weber M, Barrett A, Finnerty F, Richardson D. Identifying barriers to HIV testing in hospital admissions to improve HIV testing. J Public Health (Oxf). 2019;41(2):e216. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez TH, Sullivan PS, Rothman RE, et al. A novel approach to realizing routine HIV screening and enhancing linkage to Care in the United States: protocol of the FOCUS program and early results. JMIR Res Protoc. 2014;3(3):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanditha NGA, St‐Jean M, Tafessu H, et al. Missed opportunities for earlier diagnosis of HIV in British Columbia, Canada: a retrospective cohort study. PLoS One. 2019;14(3):e0214012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Escudero DJ, Bahamon M, Panakos P, Hercz D, Seage GR 3rd, Merchant RC. How to best conduct universal HIV screening in emergency departments is far from settled. J Am Coll Emerg Physicians Open. 2021;2(1):e12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyons MS, Lindsell CJ, Ruffner AH, et al. Randomized comparison of universal and targeted HIV screening in the emergency department. J Acquir Immune Defic Syndr. 2013;64(3):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Rossi N, Dattner N, Cavassini M, Peters S, Hugli O, Darling KEA. Patient and doctor perspectives on HIV screening in the emergency department: a prospective cross‐sectional study. PLoS One. 2017;12(7):e0180389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faryar KA, Ancona RM, Reau Z, et al. HIV detection by an emergency department HIV screening program during a regional outbreak among people who inject drugs. PLoS One. 2021;16(5):e0251756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avery AK, Toro MD, Einstadter D. Decreasing missed opportunities for HIV testing in primary care through enhanced utilization of the electronic medical record. Clin Res. 2012;Suppl 4:doi: 10.4172/2155-6113.S4-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckwith CG, Flanigan TP, del Rio C, et al. It is time to implement routine, not risk‐based. HIV Testing Clin Infect Dis. 2005;40(7):1037‐1040. [DOI] [PubMed] [Google Scholar]
- 25. Baumann KE, Hemmige V, Kallen MA, Street RL, Giordano TP, Arya M. Whether patients want it or not, physician recommendations will convince them to accept HIV testing. J Int Assoc Provid AIDS Care. 2018;17:2325957417752258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman AE, Sattin RW, Miller KM, Dias JK, Wilde JA. Acceptance of rapid HIV screening in a southeastern emergency department. Acad Emerg Med. 2009;16(11):1156‐1164. [DOI] [PubMed] [Google Scholar]
- 27. Hoxhaj S, Davila JA, Modi P, et al. Using nonrapid HIV technology for routine, opt‐out HIV screening in a high‐volume urban emergency department. Ann Emerg Med. 2011;58(1 Suppl 1):S79‐S84. [DOI] [PubMed] [Google Scholar]
- 28. Lara‐Paez G, Zuazo M, Blumenthal J, Coyne CJ, Hoenigl M. HIV and hepatitis C virus screening in the emergency department and linkage to care during COVID‐19: challenges and solutions. J Acquir Immune Defic Syndr. 2021;88(2):e14‐e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanford KA, Friedman EE, Schmitt J, et al. Routine screening for HIV in an Urban emergency department during the COVID‐19 pandemic. AIDS Behav. 2020;24(10):2757‐2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ceccarelli G, Giovanetti M, Sagnelli C, et al. Human immunodeficiency virus type 2: the neglected threat. Pathogens. 2021;10(11):1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valadas E, França L, Sousa S, Antunes F. 20 years of HIV‐2 infection in Portugal: trends and changes in epidemiology. Clin Infect Dis. 2009;48(8):1166‐1167. [DOI] [PubMed] [Google Scholar]
- 32. Dias S, Gama A, Tavares AM, et al. Are opportunities being missed? Burden of HIV, STI and TB, and unawareness of HIV among African migrants. Int J Environ Res Public Health. 2019;16(15):2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fakoya I, Álvarez‐Del Arco D, Monge S, et al. HIV testing history and access to treatment among migrants living with HIV in Europe. J Int AIDS Soc. 2018;21(Suppl 4):e25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. German D, Latkin CA. Social stability and health: exploring multidimensional social disadvantage. J Urban Health. 2012;89(1):19‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brantley ML, Kerrigan D, German D, Lim S, Sherman SG. Identifying patterns of social and economic hardship among structurally vulnerable women: a latent class analysis of HIV/STI risk. AIDS Behav. 2017;21(10):3047‐3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc. 2017;20(1):21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prabhu S, Harwell JI, Kumarasamy N. Advanced HIV: diagnosis, treatment, and prevention. Lancet HIV. 2019;6(8):e540‐e551. [DOI] [PubMed] [Google Scholar]
- 38. Guaraldi G, Zona S, Menozzi M, et al. Late presentation increases risk and costs of non‐infectious comorbidities in people with HIV: an Italian cost impact study. AIDS Res Ther. 2017;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Youssef E, Cooper V, Delpech V, Davies K, Wright J. Barriers and facilitators to HIV testing in people age 50 and above: a systematic review. Clin Med (Lond). 2017;17(6):508‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378(9787):256‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bellan SE, Dushoff J, Galvani AP, Meyers LA. Reassessment of HIV‐1 acute phase infectivity: accounting for heterogeneity and study design with simulated cohorts. PLoS Med. 2015;12(3):e1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miranda AC, Miranda M, Pingarilho M, et al. Determinants of HIV‐1 late presentation in a cohort of Portuguese HIV‐1 patients. AIDS Res Hum Retroviruses. 2021;37(11):846‐851. [DOI] [PubMed] [Google Scholar]
- 43. Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19(4):349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gargallo‐Bernad C, Sangrós‐González FJ, Arazo‐Garcés P, et al. Missed opportunities in the diagnosis of human immunodeficiency virus infection in the region of Aragon. Late diagnosis importance. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(2):100‐108. [DOI] [PubMed] [Google Scholar]
- 45. Food and Drug Administration . Assay for the detection of HIV p24 antigen and antibodies to human immunodeficiency virus type 1, including group O (HIV‐1 + “O”) and/or type 2 (HIV‐2); 2016. Accessed 2 June 2022. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package‐Insert‐‐‐ADVIA‐Centaur‐HIV‐Ag‐Ab‐Combo‐%28CHIV%29‐Assay.pdf
- 46. WHO . WHO Recommendations to Assure HIV Testing Quality; 2015. Accessed 2 June 2022. https://apps.who.int/iris/bitstream/handle/10665/179521/WHO_HIV_2015.15_eng.pdf
- 47. Centers for Disease Control and Prevention (CDC) . Factsheet about False‐Positive HIV Test Results; 2018. Accessed 2 June 2022. https://www.cdc.gov/hiv/pdf/testing/cdc-hiv-factsheet-false-positive-test-results.pdf
- 48. Mazzitelli M, Ciccullo A, Baldin G, et al. Has COVID‐19 changed the approach to HIV diagnosis? A multicentric Italian experience. Medicine (Baltimore). 2021;100(41):e27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eckardt P, Niu J, Montalvo S. Emergency room “opt‐out” HIV testing pre‐ and during COVID‐19 pandemic in a large community health system. J Int Assoc Provid AIDS Care. 2021;20:23259582211041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quiros‐Roldan E, Izzo I, Carriero C, et al. Decrease in new diagnosis of HIV/AIDS in the two years period 2019‐2020: impact of COVID‐19 pandemic. J Public Health Res. 2021;11(1):2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Romero‐Hernández B, Martínez‐García L, Rodríguez‐Dominguez M, et al. The negative impact of COVID‐19 in HCV, HIV, and HPV surveillance programs during the different pandemic waves. Front Public Health. 2022;10:880435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Darcis G, Vaira D, Moutschen M. Impact of coronavirus pandemic and containment measures on HIV diagnosis. Epidemiol Infect. 2020;148:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rick F, Odoke W, van den Hombergh J, Benzaken AS, Avelino‐Silva VI. Impact of coronavirus disease (COVID‐19) on HIV testing and care provision across four continents. HIV Med. 2022;23(2):169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


