Abstract
Background:
The impact of comorbid disease states on the development of atrial and ventricular arrhythmias in patients with hypertrophic cardiomyopathy (HCM) remains unresolved.
Objective:
Evaluate the association of comorbidities linked to arrhythmias in other cardiovascular diseases (e.g., obesity, systemic hypertension, diabetes, obstructive sleep apnea, renal disorders, tobacco, and alcohol use) to atrial fibrillation (AF) and sudden cardiac death (SCD) events in a large cohort of HCM patients.
Methods:
A total of 2269 patients, 54 ± 15 years of age, 1392 males, were evaluated at the Tufts HCM Institute between 2004 and 2018 and followed for an average of 4 ± 3 years for new-onset clinical AF and SCD events (appropriate defibrillation for ventricular tachyarrhythmias, resuscitated cardiac arrest, or SCD).
Results:
One or more comorbidity was present in 75% of HCM patients, including 50% with ≥2 comorbidities, most commonly obesity (body mass index [BMI] ≥ 30 kg/m2) in 43%. New-onset atrial fibrillation developed in 11% of our cohort (2.6%/year). On univariate analysis, obesity was associated with a 1.7-fold increased risk for AF (p = .03) with 12% of obese patients developing AF (3.3%/year) as compared to 7% of patients with BMI < 25 kg/m2 (1.6%/year; p = .006). On multivariate analysis, age and LA transverse dimension emerged as the only variables predictive of AF. Comorbidities, including obesity, were not independently associated with AF development (p > .10 for each).
SCD events occurred in 3.3% of patients (0.8%/year) and neither obesity nor other comorbidities were associated with increased risk for SCD (p > .10 for each).
Conclusions:
In adult HCM patients comorbidities do not appear to impact AF or SCD risk. Therefore, for most patients with HCM, adverse disease related events of AF and SCD appear to be primarily driven by underlying left ventricular and atrial myopathy as opposed to comorbidities.
Keywords: atrial fibrillation, comorbidities, hypertrophic cardiomyopathy, obesity, sudden death
1 |. INTRODUCTION
Comorbid conditions are known to increase risk for morbidity and mortality in many cardiovascular diseases and are associated with development of atrial fibrillation (AF) and arrhythmic sudden cardiac death (SCD).1–7 Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease occurring in up to 1 in 200 individuals, with patients at risk for a number of adverse disease related events including AF and potentially lethal ventricular tachyarrhythmias.8–15 Occurrence of these arrhythmias has previously been ascribed primarily to the underlying structural abnormalities in HCM including left ventricular (LV) hypertrophy, LV outflow obstruction, microvascular ischemia, left atrial (LA) enlargement, and myocardial fibrosis.8,9 More recently, obesity and other comorbidities have been demonstrated to impact clinical course in HCM, primarily via their impact on outflow obstruction and progressive heart failure.15–21 However, whether such comorbidities affect development of clinically relevant arrhythmias in HCM remains unresolved. Therefore, we have taken the opportunity to evaluate the potential impact of obesity and other comorbidities on development of AF and SCD events in a large consecutive cohort of HCM patients.
2 |. METHODS
The database of the Tufts HCM Institute was interrogated and 2269 consecutive patients ≥18 years of age, evaluated between 2004 and 2018, were included. HCM diagnosis was based on echocardiographic and/or cardiac magnetic resonance imaging evidence of a hypertrophied and nondilated LV (wall thickness ≥15 mm) in the absence of another cardiac or systemic disease capable of producing a similar magnitude of hypertrophy at some time during their clinical course.9
Past medical history was assessed at initial clinical evaluation in our center for pre-existing comorbidities, medical conditions or diseases that were simultaneous present with HCM, based on medical records and patients’ self-reported history. Comorbidities were assessed at initial clinical evaluation in our center, and not updated to reflect newly diagnosed comorbidities over the follow-up period, to determine impact of comorbidities at index visit on the subsequent development of events over time. Comorbidities included a diagnosis of systemic hypertension (blood pressure >140/90 mmHg and/or treatment with antihypertensive medications), diabetes mellitus (fasting blood glucose >126 mg/dl, non-fasting blood glucose >200 mg/dl, and/or use of oral hypoglycemics/insulin), obstructive sleep apnea (diagnosed by polysomnography), chronic renal disease (eGFR <90 ml/min), thyroid disease (hyperthyroidism or hypothyroidism), pulmonary disease (e.g., asthma and chronic obstructive pulmonary disease verified by pulmonary function tests), and obesity.3,5–7,22 History was obtained for alcohol consumption, that is, ≥1 alcoholic beverage/month, with moderate or heavy consumption as ≥7 alcoholic beverages/week,23 as well as smoking history characterized as former, active, or never. Normal BMI was defined as 18.5 to <25 kg/m2 and obesity was defined as BMI ≥ 30 kg/m2.1,2
Clinical features of HCM were also assessed at initial evaluation and included traditional HCM-related sudden death risk markers: family history of sudden death due to HCM, massive LV hypertrophy (wall thickness ≥30 mm), unexplained recent syncope, nonsustained VT (NSVT) on ambulatory ECG monitoring, diffuse and extensive late gadolinium enhancement (LGE) (comprising about ≥15% of LV mass), LV apical aneurysm and endstage with systolic dysfunction (EF < 50%)9,10; as well as echocardiographic and cardiac MRI imaging findings: maximum LV wall thickness, ejection fraction, left atrial dimension, LVOT gradient, and LGE.9
Patients were prospectively followed for either SCD events (appropriate ICD interventions for ventricular arrhythmias, resuscitated cardiac arrest, or sudden death),9 or new-onset clinical AF.13 Patients with such events before initial clinical evaluation at our center were excluded from the analysis of specified end-points. Newly diagnosed AF was defined as ≥1 clinically overt episode documented by electrocardiogram (ECG) or telemetry, requiring medical attention and consideration for treatment13; brief and infrequent asymptomatic (silent) AF episodes identified fortuitously by interrogation of implantable defibrillator memory that did not result in altering treatment based on the judgement of the managing cardiologist were excluded from analysis, given the currently uncertain implications of thesebrief episodes of subclinical AF in HCM,9 as were AF episodes in the first 3 months after surgical myectomy.13 Most recent clinical status and survival status were obtained at routine follow-up in our center (usually on annual visits), or with hospital visit, telephone contact with patients, family members or information from referring physicians, as well as survival by social security death index.
Data are expressed as mean ± SD for continuous variables and proportions for categorical variables. Odds ratio was calculated for each variable for each clinical outcome. Comparison of Kaplan Meier curves was performed using log-rank testing. For all testing, a p value of .05 cutoff was used for determination of significance. Variables that were found to be significantly associated with an outcome on univariable logistic regression were included in multivariable logistic regression analysis for identification of independent predictors. This study was reviewed and approved by the Tufts Health Sciences Institutional Review Board, permitting use of patient medical information for research.
3 |. RESULTS
3.1 |. Study group
Of the 2269 patients, age at initial evaluation at Tufts HCM Institute was 54 ± 15 years (range = 18–92 years); 1392 (61%) were male, and patients were followed for an average of 4.0 ± 3.4 years for clinical outcome. 1702 (75%) had ≥1 medical comorbidity and 50% of patients had ≥2 comorbidities, most commonly obesity (43%), hyperlipidemia (39%) and/or hypertension (27%; Table 1). In addition to comorbid medical conditions, 10% of patients reported active smoking history and 6% at least moderate alcohol consumption. Prevalence of ≥1 comorbidities increased by age: 43% of patients <40 years, compared to 77% age 40–59 years, and 87% ≥60 years of age (p < .001).
TABLE 1.
Demographics and clinical characteristics of 2269 HCM patients
| Demographic | |
|---|---|
| Male (%) | 61% |
| Age at initial evaluation | 54 ± 15 |
| Max LV wall thickness (mm) | 19 ± 4 |
| LV wall thickness ≥30 mm | 4% |
| Ejection fraction | 64 ± 6 |
| Ejection fraction <50% | 4% |
| Left atrial dimension | 42 ± 7 |
| Left atrial dimension ≥45 mm | 32% |
| LVOT gradient ≥30 mmHg-rest or provocation, % | 62% |
| Family history of HCM | 22% |
| Family history-SD | 8% |
| Syncope | 10% |
| LV apical aneurysm | 3% |
| CMR performed | 1349 |
| LGE present | 57% |
| LGE, % | 6 ± 6% |
| Extensive LGE (≥15% LV mass) | 5% |
| NSVT on ambulatory monitoring | 13% |
| History of AF before initial visit | 20% |
| NYHA class III/IV at initial visit | 37% |
| Appropriate ICD intervention before initial visit | 1.5% |
| Out of hospital cardiac arrest before initial visit | 1.4% |
| BMI, kg/m2 | 30 ± 6 |
| <25 kg/m2 | 20% |
| 25–29 kg/m2 | 37% |
| ≥30 kg/m2 | 43% |
| Stage 1: BMI = 30–34 kg/m2 | 25% |
| Stage 2: BMI = 35–39 kg/m2 | 11% |
| Stage 3: BMI = ≥40 kg/m2 | 7% |
| History of hypertension | 27% |
| SBP, mmHg | 125 ± 17 |
| DBP, mmHg | 75 ± 10 |
| History of diabetes | 11% |
| Diet controlled | 21% |
| Oral medication | 61% |
| Insulin | 18% |
| History of CAD | 8% |
| History of hypothyroid | 9% |
| History of hyperlipidemia | 39% |
| History of chronic kidney disease | 3% |
| Stage 2: GFR = 60–90 | 20% |
| Stage 3: GFR = 30–59 | 56% |
| Stage 4: GFR = 15–29 | 11% |
| Stage 5: GFR <15 or on dialysis | 13% |
| History of obstructive sleep apnea | 12% |
| History of pulmonary disease | 10% |
| History of tobacco use | 36% |
| Active tobacco use | 10% |
| Former tobacco use | 26% |
| Alcohol use | 59% |
| Moderate or heavy alcohol use | 6% |
| Number of comorbidities* | 1.6 ± 1.4 |
| 0 | 25% |
| 1 | 25% |
| 2 | 24% |
| 3 | 15% |
| ≥4 | 10% |
| Cardiovascular events during follow-up | |
| Progressive HF symptoms (NYHA class I/II to III/IV) | |
| Septal myectomy | 32% |
| Alcohol septal ablation | 7% |
| Heart transplant | 1.6% |
| New onset AF | 8.7% |
| Appropriate ICD intervention VT/VF during follow up | 2.8% |
| Out of hospital cardiac arrest | 0.4% |
| Deaths | 150 (6.6%) |
| Non-HCM related | 126 (5.6%) |
| Age at death | 69 ± 14 |
| HCM related | 24 (1%) |
| Age at death | 56 ± 15 |
| Sudden death | 4 |
| Heart failure death | 13 |
| Postoperative death | 5 |
| Stroke related death | 2 |
Note: Values as mean ± SD, or number (% of subjects), when applicable.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CMR, cardiac magnetic resonance; DBP, diastolic blood pressure; HF, heart failure; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVOT, left ventricular outflow tract; SBP, systolic blood pressure; VT/VF, ventricular tachycardia/ventricular fibrillation.
Symbols: comorbidities included hypertension, hyperlipidemia, diabetes, coronary artery disease, obesity, obstructive sleep apnea, kidney, and pulmonary disease.
3.2 |. Obesity
Obesity was present in 43% of patients, including 25% with BMI 30–34 kg/m2, 11% with BMI 35–39 kg/m2, and 7% with BMI ≥ 40 kg/m2; only 20% of patients had normal BMI. BMI did not increase with age, with no difference in BMI evident in patients <40 years as compared to ≥60 years of age (29 ± 7 vs. 29 ± 6 kg/m2, p = .46). Compared to patients with normal BMI, obese patients also had a greater burden of other comorbidities (80% of obese patients with ≥1 other comorbidities vs. 53% of patients with normal BMI, p < .001).
3.3 |. Atrial fibrillation
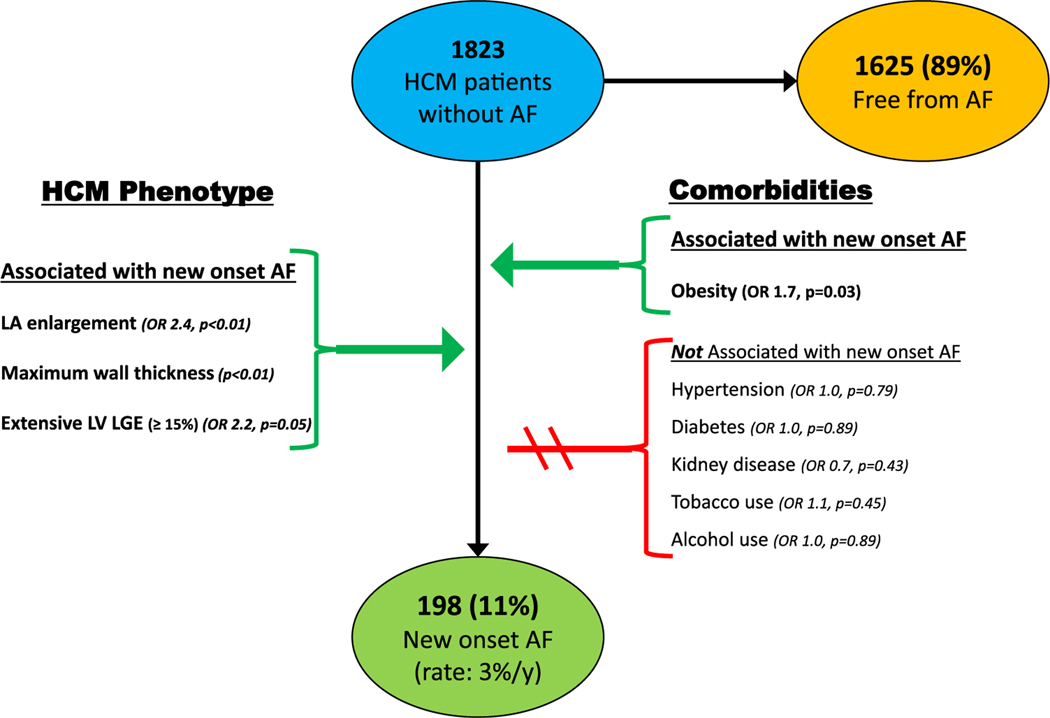
Of the 1823 patients without prior AF history, 198 (11%) developed new-onset AF over follow-up (2.6%/year) at age 56 ± 14, 3.2 ± 3 years from initial clinical evaluation.
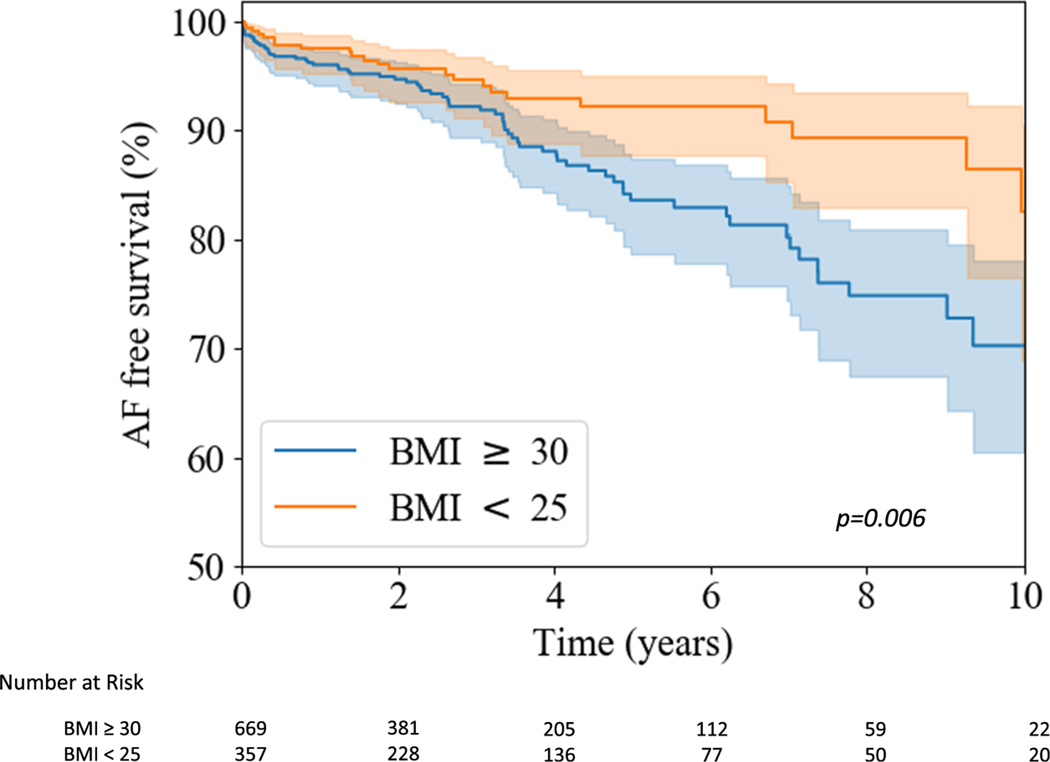
On univariate analysis, obesity was associated with a 1.7-fold increase in risk for AF development compared to patients with normal BMI (95% CI: 1.0, 2.7, p = .03). Overall, 12% of obese patients developed AF (rate of 3.3%/year), as compared to just 7% with normal BMI (rate of 1.6%/year; p = .006) (Figure 1).
FIGURE 1.
Development of atrial fibrillation in obese versus nonobese patients. Kaplan–Meier curve comparing survival free from new onset atrial fibrillation in obese patients and patients with normal body mass index. BMI, body mass index
Other comorbidities were not significantly associated with AF development in HCM (Figure 2). Specifically systemic hypertension (OR = 1.0, p = .79), diabetes (OR = 1.0, p = .89), chronic kidney disease (OR = 0.7, p = .43), obstructive sleep apnea (OR = 0.8, p = .33), pulmonary disease (OR = 1.5, p = .10), tobacco (OR = 1.1, p = .45) and alcohol (OR = 1.0, p = .89) were unassociated with AF development (Table 2).
FIGURE 2.
Risk factors for atrial fibrillation in HCM. Univariate association of clinical factors and comorbidities on development of new onset atrial fibrillation in 1823 HCM patients. AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular
TABLE 2.
Demographics and clinical characteristics of 1823 HCM patients with and without new onset clinical atrial fibrillation over follow-up
| Demographic | HCM without AF (n = 1625) | New onset AF (n = 198) | Odds ratio/p value |
|---|---|---|---|
| Male (%) | 60% | 61% | 1.0 (0.7, 1.4); p = .94 |
| Age | 52 ± 15 | 56 ± 14 | p < .001 |
| Max LV wall thickness (mm) | 19 ± 4 | 20 ± 4 | p = .001 |
| Ejection fraction | 64 ± 5 | 64 ± 7 | p = 1.0 |
| Ejection fraction <50% | 1.8% | 5% | 2.5 (1.2, 5.4); p = .02 |
| Left atrial dimension | 40 ± 6 | 44 ± 7 | p <. 001 |
| Left atrial dimension ≥45 mm | 24% | 43% | 2.4 (1.8, 3.2); p < .0001 |
| LVOT gradient ≥30 mmHg-rest or provocation, % | 63% | 67% | 1.2 (0.9, 1.7); p = .24 |
| % LGE by CMR | 5.6 ± 5 | 7.4 ± 8 | p = .03 |
| % LGE ≥ 15% | 4% | 9% | 2.2 (1.0,4.8); p = .05 |
| Family history-SD | 8% | 13% | 1.7 (1.1, 2.6); p = .02 |
| Syncope | 9% | 14% | 1.6 (1.0, 2.4); p = .04 |
| NSVT on ambulatory monitoring | 8% | 12% | 1.4 (0.9, 2.3); p = .15 |
| NYHA class at initial visit | |||
| I | 36% | 25% | 0.6 (0.4, 0.8); p = .002 |
| II | 29% | 35% | 1.3 (1.0, 1.8); p = .08 |
| III/IV | 35% | 40% | 1.2 (0.9, 1.7); p = .15 |
| BMI, kg/m2 | 30 ± 6 | 31 ± 6 | p = .04 |
| <25 kg/m2 | 22% | 15% | 0.6 (0.4, 1.0); p = .05 |
| 25–29 kg/m2 | 37% | 37% | 1.4 (0.9, 2.3); p = .17 |
| ≥30 kg/m2 | 41% | 48% | 1.7 (1.0, 2.7); p = .03 |
| History of Hypertension | 28% | 27% | 1.0 (0.7, 1.3); p = .79 |
| SBP, mmHg | 126 ± 17 | 124 ± 16 |
p =.11 |
| DBP, mmHg | 75 ± 10 | 73 ± 9 | p = .007 |
| History of diabetes | 11% | 11% | 1.0 (0.6, 1.7); 0.89 |
| History of CAD | 8% | 10% | 1.3 (0.8, 2.1); p = .3 |
| History of hypothyroid | 9% | 10% | 1.1 (0.7, 1.9); p = .64 |
| History of hyperlipidemia | 40% | 42% | 1.1 (0.8, 1.5); p = .56 |
| History of chronic kidney disease | 3% | 2% | 0.7 (0.2, 1.9); 0.43 |
| History of obstructive sleep apnea | 11% | 9% | 0.8 (0.5, 1.3); p = .33 |
| History of pulmonary disease | 9% | 13% | 1.5 (0.1, 2.4); p = .10 |
| History of tobacco use | 36% | 39% | 1.1 (0.8, 1.6); p = .45 |
| History of alcohol use | 60% | 60% | 1.0 (0.7, 1.4); p = .89 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CMR, cardiac magnetic resonance; DBP, diastolic blood pressure; LGE, late gadolinium enhancement; LV, left ventricular; LVOT, left ventricular outflow tract; SBP, systolic blood pressure.
When examining clinical features in HCM patients with and without AF development, those with new-onset AF had increased LA transverse dimension (44 ± 7 vs. 40 ± 6, p < .001), maximum wall thickness (20 ± 4 vs. 19 ± 4, p = .001), as well as extensive LV LGE (9% vs. 4%, p = .05) but did not differ with respect to other clinical features including LV ejection fraction, LVOT obstruction, or baseline New York Heart Association functional class (Table 2).
On multivariate analysis, only age and LA transverse dimension were associated with AF development, while notably obesity did not reach statistical significance (OR = 1.3 [95% CI = 0.95, 1.79], p = .10; Table S1).
3.4 |. Sudden cardiac death
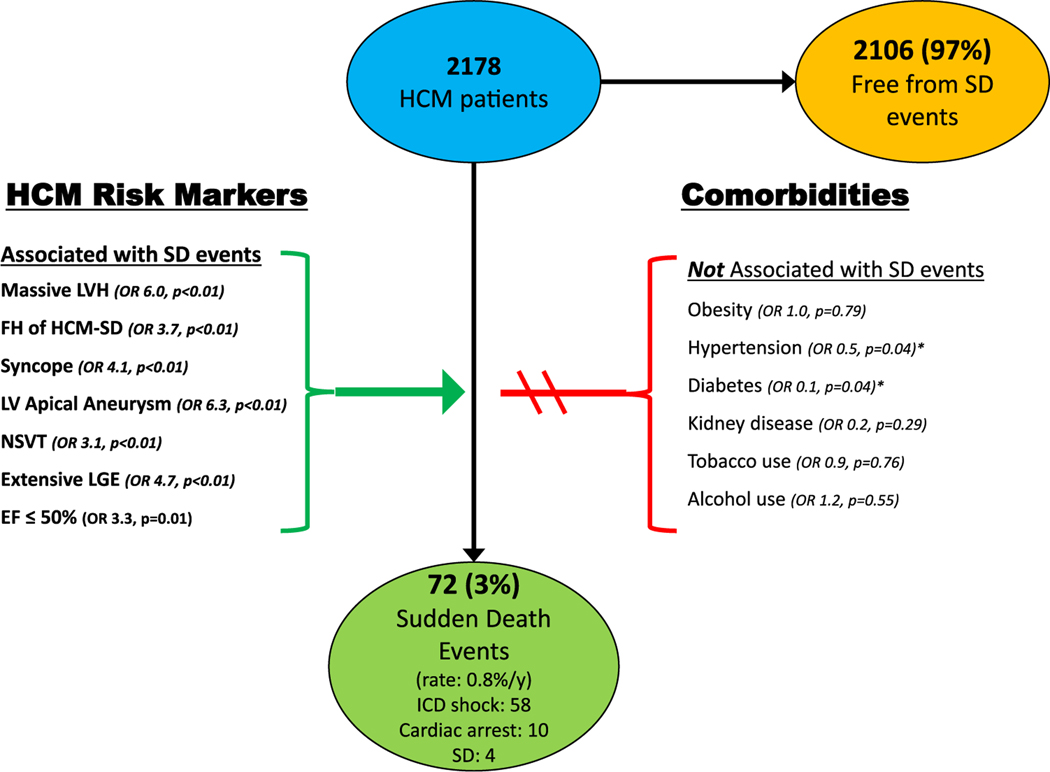
Of the 2178 patients with no prior history of sustained ventricular tachyarrhythmias, 72 (3%) experienced a SCD event during the follow-up period (0.8%/year), including 58 with appropriate ICD therapy for VT/VF, 10 with out of hospital cardiac arrest, and 4 with sudden death.
Comorbidities, including obesity, were not associated with an increase in risk for SCD events (Figure 3). In contrast, HCM patients with SCD events were younger (45 ± 15 vs. 54 ± 15, p < .001) and healthier overall, with less frequent systemic hypertension (OR = 0.51, p = .04), diabetes (OR = 0.05, p = .04), and hyperlipidemia (OR = 0.59, p = .05) compared to patients without events. Neither the proportion of obese patients (36% vs. 43%, p = .20) nor BMI (29 ± 7 vs. 30 ± 6, p = .20) differed between patients with and without SCD events. Other comorbidities were also not associated with SCD events including chronic kidney disease (OR = 0.2, p = .29), obstructive sleep apnea (OR = 0.4, p = .10), pulmonary disease (OR = 0.4, p = .11), tobacco (OR = 0.9, p = .76) or alcohol use (OR = 1.2, p = .55; Table 3).
FIGURE 3.
Risk factors for sudden death events in HCM. Univariate association of clinical factors and comorbidities on sudden cardiac death events in 2178 HCM patients. *Patients with SCD events were statistically less likely to have hypertension and diabetes. EF, ejection fraction; FH, family history; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricular; NSVT, nonsustained ventricular tachycardia; SD, sudden death
TABLE 3.
Demographics and clinical characteristics of 2178 HCM patients with and without sudden death events over follow-up
| Demographic | HCM without SD events (n = 2106) | SD events (n = 72) | Odds ratio/p-value |
|---|---|---|---|
| Male (%) | 61% | 69% | 1.5 (0.9, 2.4); p = .15 |
| Age | 54 ± 15 | 45 ± 15 | p < .001 |
| Max LV wall thickness (mm) | 19 ± 4 | 22 ± 6 | p < .001 |
| LV wall thickness ≥30 mm | 4% | 19% | 6.0 (3.0–11.3); p < .0001 |
| Ejection fraction | 64 ± 6 | 63 ± 7 | p = .17 |
| Ejection fraction <50% | 3% | 7% | 3.3 (1.3, 8.7); p = .01 |
| Left atrial dimension | 42 ± 7 | 42 ± 7 | p = 1.0 |
| LVOT gradient ≥30 mmHg-rest or provocation, % | 62% | 49% | 0.6 (0.4, 0.9); p = .02 |
| Family history-SD | 8% | 24% | 3.7 (2.1, 6.5); p < .001 |
| Syncope | 9% | 29% | 4.1 (2.4, 7.0); p < .001 |
| LV apical aneurysm | 2% | 11% | 6.3 (2.8, 14.0); p < .001 |
| Extensive LGE (≥15% LV mass) | 4% | 17% | 4.7 (1.7, 12.9); p = .003 |
| NSVT on ambulatory monitoring | 13% | 32% | 3.1 (1.8, 5.1); p < .001 |
| ≥1 sudden death risk marker | 24% | 89% | 25.6 (12.2, 53.8); p < .001 |
| History of AF before initial visit | 17% | 19% | 1.2 (0.6, 2.1); p = .6 |
| NYHA class III/IV at initial visit | 37% | 24% | 0.5 (0.3, 0.9); p = .02 |
| BMI, kg/m2 | 30 ± 6 | 29 ± 7 | p = .2 |
| <25 kg/m2 | 20% | 26% | 1.4 (0.8, 2.6); p = .25 |
| 25–29 kg/m2 | 37% | 38% | 0.8 (0.4, 1.5); p = .45 |
| ≥30 kg/m2 | 43% | 36% | 0.6 (0.3, 1.3); p = .20 |
| History of Hypertension | 27% | 16% | 0.5 (0.3, 0.9); p = .04 |
| SBP, mmHg | 126 ± 17 | 123 ± 14 | p = .14 |
| DBP, mmHg | 75 ± 10 | 72 ± 9 | p = .01 |
| History of diabetes | 11% | 0% | 0.05 (0, 0.9); p = .04 |
| History of CAD | 8% | 14% | 1.8 (0.9, 3.5); p = .10 |
| History of hypothyroid | 9% | 4% | 0.4 (0.1, 1.4); p = .17 |
| History of hyperlipidemia | 39% | 28% | 0.6 (0.3, 1.0); p = .05 |
| History of chronic kidney disease | 3% | 0% | 0.2 (1.01, 3.6); p = .29 |
| History of obstructive sleep apnea | 12% | 6% | 0.4 (0.2, 1.2); p = .10 |
| History of pulmonary disease | 10% | 4% | 0.4 (0.1, 1.2); p = .11 |
| History of tobacco use | 36% | 34% | 0.9 (0.5, 1.6); p = .76 |
| History of alcohol use | 59% | 63% | 1.2 (0.7, 2.1); p = .55 |
| Appropriate ICD intervention VT/VF | – | 80% | – |
| Out of hospital cardiac arrest | – | 14% | – |
| Sudden death | – | 6% | – |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CMR, cardiac magnetic resonance; DBP, diastolic blood pressure; ICD, Implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVOT, left ventricular outflow tract; SBP, systolic blood pressure; VT/VF, ventricular tachycardia/ventricular fibrillation
When examining HCM clinical features in HCM patients with and without events, those with SCD events had more traditional SCD risk markers, including massive LV hypertrophy (OR = 6.0, p < .001), family history of HCM-related SCD (OR = 3.7, p < .001), unexplained syncope (OR = 4.1, p < .001), LV apical aneurysm (OR = 6.3, p < .001), NSVT (OR = 3.1, p < .001), extensive LGE (OR = 4.7, p = .003), and endstage with systolic dysfunction (EF < 50%; OR 3.3, p = .01), (Figure 3 and Table 3).8–10 On multivariate analysis, massive LV hypertrophy, family history of HCM related SCD, unexplained syncope and NSVT were positive independent predictors for SCD events (p < .05; Table S2). Notably, there was no difference in other morphologic features including LA dimension, LV ejection fraction, or AF history as compared to patients without sudden death.
4 |. DISCUSSION
Comorbid medical conditions are important contributors to the development of arrhythmias in non-HCM cardiac conditions,1–7 and while obesity and other comorbidities in HCM have been linked to heart failure progression,15–21 their impact on arrhythmias remains unknown. Thereby we prospectively examined the influence of comorbidities on the risk for development of AF and SCD in over 2200 HCM patients followed at our center.
We found no evidence that obesity or other comorbidities impact risk for SCD in HCM. Indeed, HCM patients with SCD events were younger, had similar BMI, and were less likely to have diabetes, hypertension, or hyperlipidemia than HCM patients without such events. These findings are in sharp contrast to the general cardiovascular population without HCM in which these comorbidities are associated with a substantial increase in SCD risk.5–7,24 The absence of a relationship between SCD and comorbidities supports the hypothesis that malignant ventricular tachyarrhythmias in HCM are predominantly secondary to the unstable ventricular myocardial substrate including disorganized myocyte architecture, microvascular disease, and interstitial and replacement fibrosis.8–11 Notably, SCD is particularly uncommon in older HCM patients with a higher burden of comorbidities,8,25,26 substantiating our conclusion that comorbidities do not increase risk for ventricular tachyarrhythmias in HCM.
On univariate analysis obesity was associated with the risk for development of AF in HCM. In our cohort, obese patients were nearly twofold more likely to develop AF than HCM patients with normal BMI. This relationship between obesity and AF risk in HCM is nearly identical to the reported risk of obesity on AF in the general cardiovascular population without HCM.1–4 Our findings are also consistent with prior retrospective studies demonstrating that obese HCM patients had higher prevalence of AF and larger LA size.16,17,27 In contrast, on multivariate analysis, when controlling for other disease features including LA size, obesity did not reach statistical significance. This likely reflects the more direct impact that LA size has on AF development in HCM, while the effect of obesity is likely more indirect, contributing in part to LA enlargement and diastolic dysfunction. However, the overall relationship between obesity and AF suggests a more substantial role of obesity on clinical course in HCM than previously regarded, particularly since obesity has also been associated with both outflow obstruction and progressive heart failure.15–19
While AF is clearly linked to hypertension, tobacco and heavy alcohol use, diabetes, and obstructive sleep apnea in general cardiovascular populations,1–4 we found no evidence that these variables increased risk of new onset AF in HCM. The absence of substantial impact of comorbidities on disease progression is likely due to the strong influence of primary HCM disease features, including an associated underlying atrial myopathy, on AF development.13,28–31 Thereby comorbidities do not have the potential to impact the natural history of HCM to the same degree as in the general cardiovascular population. For example, LA structural remodeling occurs in HCM secondary to elevated LA filling pressures from LV diastolic dysfunction, from mitral regurgitation resulting from LV outflow obstruction, as well as from an undefined intrinsic atrial myopathy, which likely serve as primary driver for AF development.28–31
In contrast, the ability for obesity to potentially impact AF and heart failure in HCM supports attention on weight loss to potentially mitigate arrhythmia risk, and reinforces the need for targeted lifestyle modification strategies in obese HCM patients.15–7,32,33 This is particularly relevant given the high prevalence of obesity in HCM—over 40% of patients with BMI ≥ 30 found in the present and other HCM cohorts, which is substantially higher than in the general US population16–19—and highlights the critical need for focus on healthy lifestyle for overall health status in HCM patients.32
4.1 |. Limitations
Comorbidities in our study were defined based on medical history at time of initial visit to our HCM center. For example, we did not prospectively perform sleep studies on asymptomatic patients. Therefore, we cannot exclude a role for obstructive sleep apnea in AF development in HCM.34–36 In addition, the present analysis was designed to evaluate newly diagnosed symptomatic AF and we did not include subclinical (asymptomatic) AF episodes usually recognized fortuitously by the interrogation of implantable devices, given the currently uncertain implications of these usually brief episodes of subclinical AF in HCM.37,38 This decision also avoids selection bias in AF detection, since subclinical AF is detected predominantly in patients judged at high-risk for arrhythmia-related sudden death by interrogation of dual-chamber ICDs.37 In contrast, given the potential of subclinical AF to lead to adverse clinical events, further research is needed on the impact of subclinical AF in HCM.
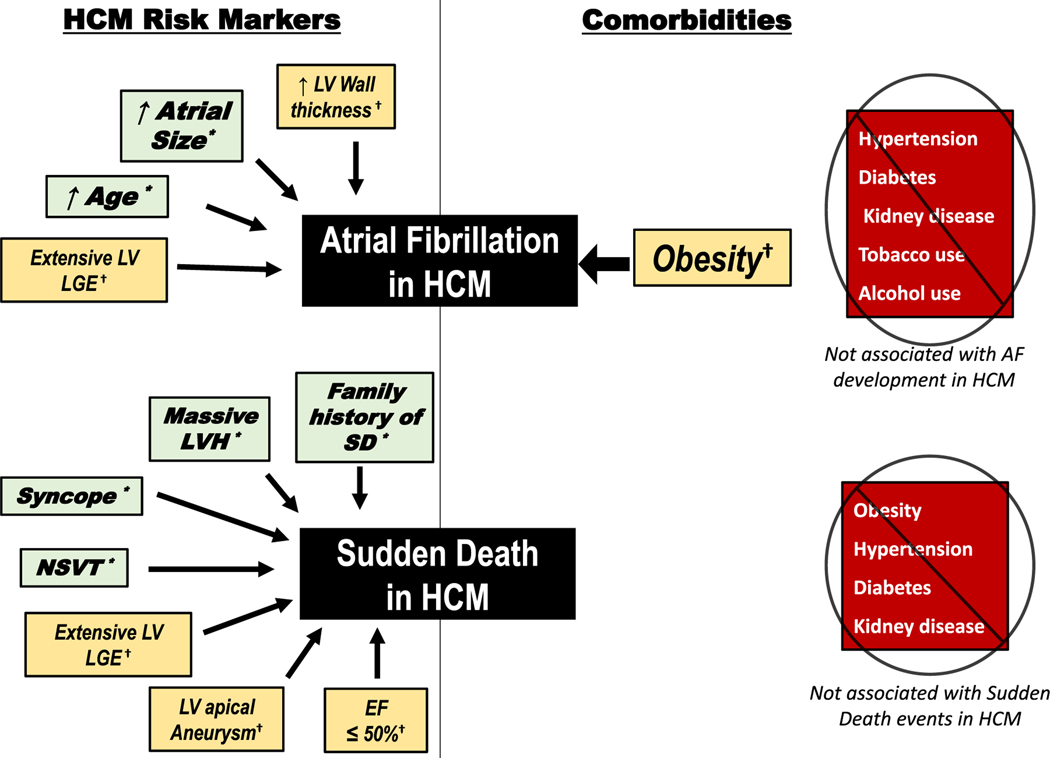
In conclusion, unlike other cardiovascular conditions, most comorbidities do not appear to have a major impact on arrhythmia development in HCM (Figure 4). SCD is unrelated to comorbidities including obesity, and most comorbidities are not linked to AF development. Therefore, for most patients with HCM, AF and SCD appear to be primarily driven by the underlying left ventricular and atrial myopathy as opposed to comorbidities.
FIGURE 4.
Impact of clinical factors and comorbidities on atrial fibrillation and sudden cardiac death in hypertrophic cardiomyopathy. HCM clinical variables associated with atrial fibrillation development (top) and sudden death events (bottom) in a large cohort of 2269 HCM patients (left). While obesity is associated with development of atrial fibrillation on univariate analysis, other comorbidities are not associated with atrial fibrillation development (top right), and obesity was not independently associated with AF development on multivariate analysis. Neither obesity or other comorbidities are associated with sudden death events (including appropriate ICD interventions for ventricular tachyarrhythmias, out of hospital cardiac events, or sudden death) (bottom right). *Associated with events on both univariate and multivariate analysis. †Associated with events on univariate but not on multivariate analysis. EF, ejection fraction; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricular; NSVT, nonsustained ventricular tachycardia; SD, sudden death
Supplementary Material
Acknowledgments
Dr. Martin Maron is a steering committee member for Cytokinetics and a consultant for Imbria Pharmaceuticals; Dr. Rowin has a research grant from Pfizer.
Footnotes
Disclosure
Other authors: No disclosures.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. [DOI] [PubMed] [Google Scholar]
- 2.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. [DOI] [PubMed] [Google Scholar]
- 3.Miller JD, Aronis KN, Chrispin J, et al. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol. 2015;66: 2899–2906. [DOI] [PubMed] [Google Scholar]
- 4.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors. The atherosclerosis risk in communities (ARIC) Study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayaraman R, Reinier K, Nair S, et al. Risk factors of sudden cardiac death in the young: multiple-year community-wide assessment. Circulation. 2018;137:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finocchiaro G, Papadakis M, Dhutia H, et al. Obesity and sudden cardiac death in the young: clinical and pathological insights from a large national registry. Eur. J Prev Cardiol. 2018;25:395–401. [DOI] [PubMed] [Google Scholar]
- 7.Ha FJ, Han HC, Sanders P, et al. Sudden cardiac death in the young: incidence, trends, and risk factors in a nationwide study. Circ Cardiovasc Qual Outcomes. 2020;13:e006470. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. [DOI] [PubMed] [Google Scholar]
- 9.Ommen SR, Mital S, Burke MA, et al. 2020. AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol, 2020(76):e159–e240. [DOI] [PubMed] [Google Scholar]
- 10.Maron MS, Rowin EJ, Wessler BS, et al. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissler-Snir A, Adler A, Williams L, Gruner C, Rakowski H. Prevention of sudden death in hypertrophic cardiomyopathy: bridging the gaps in knowledge. Eur Heart J. 2017;38:1728–1737. [DOI] [PubMed] [Google Scholar]
- 12.Christiaans I, Birnie E, Bonsel GJ, et al. Manifest disease, risk factors for sudden cardiac death, and cardiac events in a large nationwide cohort of predictively tested hypertrophic cardiomyopathy mutation carriers: determining the best cardiological screening strategy. Eur Heart J. 2011;32:1161–1170. [DOI] [PubMed] [Google Scholar]
- 13.Rowin EJ, Hausvater A, Link MS, et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136:2420–2436. [DOI] [PubMed] [Google Scholar]
- 14.Hodges K, Rivas CG, Aguilera J, et al. Surgical management of left ventricular outflow tract obstruction in a specialized hypertrophic obstructive cardiomyopathy center. J Thorac Cardiovasc Surg. 2019; 157:2289–2299. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Schaff HV, Nishimura RA, et al. Impact of body mass index on outcome of septal myectomy for obstructive hypertrophic cardiomyopathy. Ann Thorac Surg. 2021. S0003-4975(21)00563-4 [DOI] [PubMed] [Google Scholar]
- 16.Olivotto I, Maron BJ, Tomberli B, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62:449–457. [DOI] [PubMed] [Google Scholar]
- 17.Fumagalli C, Maurizi N, Day SM, et al. Association of obesity with adverse long-term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2020;Jan 1 5(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen CM, Ball CA, Hebl VB, et al. Effect of body mass index on exercise capacity in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;121:100–106. [DOI] [PubMed] [Google Scholar]
- 19.Canepa M, Sorensen LL, Pozios I, et al. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Cui H, Ji K, et al. Impact of type 2 diabetes mellitus on mid-term mortality for hypertrophic cardiomyopathy patients who underwent septal myectomy. Cardiovasc Diabetol. 2020;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserstrum Y, Barriales-Villa R, Fernández-Fernández X, et al. The impact of diabetes mellitus on the clinical phenotype of hypertrophic cardiomyopathy. Eur Heart J. 2019;40:1671–1677. [DOI] [PubMed] [Google Scholar]
- 22.Singleton MJ, Imtiaz-Ahmad M, Kamel H, et al. Association of atrial fibrillation without cardiovascular comorbidities and stroke risk: from the REGARDS study. J Am Heart Assoc. 2020;9:e016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth A, Teo KK, Rangarajan S, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386:1945–1954. [DOI] [PubMed] [Google Scholar]
- 24.Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of sudden cardiac death: a systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2018;28:543–556. [DOI] [PubMed] [Google Scholar]
- 25.Alashi A, Smedira NG, Popovic ZB, et al. Characteristics and outcomes of elderly patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2021;10:e018527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maron BJ, Rowin EJ, Casey SA, et al. Risk stratification and outcome of patients with hypertrophic cardiomyopathy >=60 years of age. Circulation. 2013;127:585–593. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Yu M, Cui J, Liu S, Yuan J, Qiao S. Impact of body mass index on left atrial dimension in HOCM patients. Open Med (Wars). 2021; 16:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowin EJ, Sridharan A. Thinking outside the heart to treat atrial fibrillation in hypertrophic cardiomyopathy. J Am Heart Assoc. 2020; 9:e016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SP, Ashley EA, Homburger J, et al. Incident atrial fibrillation is associated with MYH7 sarcomeric gene variation in hypertrophic cardiomyopathy. Circ Heart Fail. 2018;11:e005191. [DOI] [PubMed] [Google Scholar]
- 30.Sivalokanathan S, Zghaib T, Greenland GV, et al. Hypertrophic Cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol. 2019;5:364–375. [DOI] [PubMed] [Google Scholar]
- 31.Papavassiliu T, Germans T, Flüchter S, et al. CMR findings in patients with hypertrophic cardiomyopathy and atrial fibrillation. J Cardiovasc Magn Reson. 2009;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reineck E, Rolston B, Bragg-Gresham JL, et al. Physical activity and other health behaviors in adults with hypertrophic cardiomyopathy. Am J Cardiol. 2013;111:1034–1039. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan R, Lau DH, Brooks AG, et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol. 2021;7:630–641 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 34.Konecny T, Brady PA, Orban M, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597–1602. [DOI] [PubMed] [Google Scholar]
- 35.Pedrosa RP, Drager LF, Genta PR, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 36.Eleid MF, Konecny T, Orban M, et al. High prevalence of abnormal nocturnal oximetry in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:1805–1809. [DOI] [PubMed] [Google Scholar]
- 37.Rowin EJ, Orfanos A, Esta NA, et al. Occurrence and natural history of clinically silent episodes of atrial fibrillation in hypertrophic cardiomyopathy. Am J Cardiol. 2017;119:1862–1865. [DOI] [PubMed] [Google Scholar]
- 38.Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507–1516. (S0140-6736(21)01698-6 Online ahead of print). doi: 10.1016/S0140-6736(21)01698-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.