Abstract
Simple Summary
Prostate cancer is the most common malignancy (after skin cancer) in men, and also the most common cancer among cancer survivors in the US. We assessed the long-term burden of depression across three prostate cancer risk groups, and evaluated the association between regret and long-term depression. A large proportion of localized prostate cancer patients continued to experience long-term depression. The proportion with high depression increased over time for all risk groups. Higher regret at 24-month follow-up was significantly associated with high depression at 24-month follow-up, after adjusting for covariates. Patient-centered survivorship care strategies are needed to address depression and regret, and improve outcomes in prostate cancer care.
Abstract
Background: While psychological difficulties, such as depression, among prostate cancer patients are known, their longitudinal burden remains understudied. We assessed the burden of depression across low-, intermediate- and high-risk prostate cancer groups, and the association between regret and long-term depression. Methods: Secondary analysis of data from a multi-centered randomized controlled study among localized prostate cancer patients was carried out. Assessments were performed at baseline, and at 3-, 6-, 12- and 24-month follow-up. Depression was assessed using the Center for Epidemiologic Studies Depression (CES-D) scale. A CES-D score ≥ 16 indicates high depression. Regret was measured using the regret scale of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC). The proportion of patients with high depression was compared over time, for each risk category. Logistic regression was used to assess the association between regret, and long-term depression after adjusting for age, race, insurance, smoking status, marital status, income, education, employment, treatment, number of people in the household and study site. Results: The study had 743 localized prostate cancer patients. Median depression scores at 6, 12 and 24 months were significantly larger than the baseline median score, overall and for the three prostate cancer risk groups. The proportion of participants with high depression increased over time for all risk groups. Higher regret at 24-month follow-up was significantly associated with high depression at 24-month follow-up, after adjusting for covariates. Conclusions: A substantial proportion of localized prostate cancer patients continued to experience long-term depression. Patient-centered survivorship care strategies can help reduce depression and regret, and improve outcomes in prostate cancer care.
Keywords: localized prostate cancer, prostate cancer risk groups, depression, decision regret, longitudinal assessment
1. Introduction
Prostate cancer is the most common type of cancer (after skin cancer) in men in the United States. In 2023, there will be 288,300 new cases of prostate cancer and 34,700 prostate cancer-related death [1]. Currently, there are approximately 3,253,416 prostate cancer survivors in the United States [1]. Cancer diagnosis is a source of great distress, anxiety and depression for patients [2,3,4]. Estimates of the rate of depression accompanying prostate cancer diagnosis vary. In an analysis of Surveillance, Epidemiology and End Results (SEER) Medicare-linked data, 8.54% of newly diagnosed prostate cancer patients had a depression diagnosis [5]. This estimate is likely to be conservative, because Medicare claims data have been found to have poor sensitivity for clinical diagnosis of depression [6]. A meta-analysis showed that the prevalence of depression among prostate cancer patients ranged from 14 to 18% [7]. Studies have uniformly demonstrated the association between depression in prostate cancer and increase in the use of health services, poor quality of life and higher mortality [5,8,9,10,11,12]. One study showed that prostate cancer patients with depression who received androgen deprivation therapy experienced elevated periods of hospitalization [13]. Depression in patients with prostate cancer may be an unidentified factor in the variability in care, contributing to poorer long-term treatment outcomes [7].
Previous studies that examined the levels of depression among prostate cancer patients have been limited by small sample sizes, homogenous sample, reliance on claims data for depression diagnosis, or a cross-sectional design [7,8]. For example, few studies have employed longitudinal data after treatment [11,12]. Initial symptoms may change over time, so cross-sectional associations provide only a partial picture. Pre-treatment symptoms and traits are important to consider, since symptoms of depression may be related to what treatment option the patient selects [14,15]. Treatment decision regret may also contribute to the persisting longitudinal presence of depression [16]. Decision regret is the undesirable emotion of distress following a decision, and can manifest when the outcome of a decision is compared with the likely outcome of an unchosen alternative [17,18]. Thus, when a treatment decision is made in an uncertain or preference-sensitive situation, such as prostate cancer, it may lead to decision regret [9,17,19,20,21,22,23,24].
While studies have examined the longitudinal psychosocial consequences of curative treatments in men with prostate cancer [25], few have assessed the association between decision regret and depression among prostate cancer patients. In this paper, we present a secondary analysis using data from a randomized controlled trial of decision-making strategies for men with localized prostate cancer. Our objective is two-fold. First, we examine longitudinally, the proportion of those with high depression across three prostate cancer risk groups. Second, we assess the association between long-term depression and decision regret in our cohort of prostate cancer patients, for three different prostate cancer risk groups.
2. Materials and Methods
2.1. Study Design and Conduct
The overall study methodology has been described previously [26,27,28,29]. Briefly, in this multi-centered randomized controlled trial study, the intervention was a web-based, Patient Preferences for Prostate Cancer Care (PreProCare) instrument, that uses an adaptive choice based a conjoint (ACBC) analysis technique for preference assessment. The result of the PreProCare intervention was a list of five attributes that the participant valued most. All participants completed self-administered outcome assessments at baseline (prior to the intervention) and at the 3-, 6-, 12- and 24-month follow-up. Local institutional review boards approved the study.
2.2. Study Participants
2.2.1. Study Sites
The University of Pennsylvania (UPenn) was the primary and coordinating site (site 1). Site 2 was the Corporal Michael J. Crescenz Veterans Administration Medical Center (CMCVAMC) and Site 3 was Fox Chase Cancer Center/Temple University Hospital (FCCC). All study sites were located in Philadelphia, PA, USA. Based on sample size estimates, the total target accrual goal was 720 participants. The power calculations for specific aims assumed the availability of 720 participants who were eligible for randomization into one of the two intervention groups. We assumed a conservative intra-class correlation of 0.3, 4 follow-up measures per subject, and a two-sided level of significance of α = 0.05. The sample size was adjusted to accommodate a 10% missing or dropout rate by 24 months. We had 80% power to detect a 1.2-point difference in the prostate cancer index or SF-36 sub-scale [26].
2.2.2. Study Eligibility Criteria
The study eligibility criteria were: (1) newly diagnosed with localized prostate cancer (low risk: PSA ≤ 10 ng/mL, Gleason ≤ 6, and stage T1c–T2a; intermediate risk: PSA > 10–≤ 20 ng/mL, or Gleason 7, or stage T2b; and high risk: PSA > 20 ng/mL, or Gleason score 8–10, or stage T2c); (2) treatment naïve (as of study entry); (3) age ≥ 18 years; and (4) able to provide informed consent. The exclusion criteria were: (1) distant, metastatic or un-staged prostate cancer at diagnosis; (2) unable to communicate in English; and (3) treatment for prostate cancer already underway.
2.3. Recruitment and Randomization
Recruitment involved following steps: (1) obtaining consent from the patient’s urologist/physician for reviewing medical records; (2) determining eligibility via medical records; (3) screening to assess willingness to participate; and (4) obtaining informed consent and Health Insurance Portability and Accountability Act (HIPAA) permissions. The study biostatistician created randomization sequences for each site using a pseudo-random number generator with random blocking, varying in size from two to six. The treatment assignments were placed in sealed, opaque envelopes. Research coordinators opened the envelope and notified the participants of the group assignment. The study investigators were blinded to the group assignment.
2.4. The PreProCare Intervention
Participants in the intervention group completed the web-based, ACBC instrument, PreProCare, to assess their individual preferences. Briefly, this three-part tool was arranged as follows: a brief introduction to the instrument was provided in part one. In the second part, the participants ranked the attributes of various treatments (‘not important’ to ‘extremely important’). In the third part, choice scenarios, consisting of combinations of attributes, were presented based on the attribute ranking, and participants selected the combination that they most preferred. At end of the task, a graph and a list of the five attributes most preferred by the participant were generated. The participant had the option to have a printout of the output to share with his provider. On average, the PreProCare instrument required about 30 min to complete. Usual care group participants received standard care.
2.5. Assessments
Depression: Depression was measured at baseline and all follow-up time points using the Center for Epidemiologic Studies Depression (CES-D) scale. The CES-D is a 20-item, self-report scale used to identify depression in the general population. It covers major components of depression, with an emphasis on affective components: depressed mood; feelings of guilt and worthlessness; feelings of helplessness and hopelessness; psychomotor retardation; loss of appetite; and sleep disorders [30]. The score ranges from 0 to 60, and a higher score indicates higher depression level. A score of 16 or more is indicative of ‘high’ depression.
Regret: Regret was measured using the five-item regret subscale of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) [31,32,33]. The score ranges from 0 to 100, with a higher score indicating greater decisional regret.
Treatment: Data on primary treatment, such as active surveillance, open radical prostatectomy, robot-assisted radical prostatectomy and radiation therapy (intensity modulated radiation therapy, brachytherapy, or proton therapy) were obtained via medical charts and a self-report.
Other covariates: Self-reported data on age, income, race and ethnicity, education, marital status and employment were obtained at baseline. Data on height, weight, smoking status and health insurance were abstracted from electronic medical records. Data on Prostate Specific Antigen (PSA) levels, TNM stage, grade and histology, and the Gleason score were also abstracted from electronic medical records.
2.6. Statistical Analysis
First, we compared the socio-demographic and clinical variables between the three prostate cancer risk groups. We then longitudinally examined the proportion of patients with ‘high depression’ for the three prostate cancer risk groups. Finally, we conducted logistic regression to assess the association between high depression and decision regret at 24-month follow-up, after adjusting for covariates. The covariates were operationalized as follows: regret score, age and number of people in the household were continuous variables; treatment was surgery, radiation, or active surveillance (reference category); race was white, African American, or other (reference category); education was college and higher, or some college (reference category); marital status was married or other (reference category); employment status was employed (full/part time), or other (reference category); income was ≤USD 40,000, USD 40–75,000, or >USD 75,000 (reference category); smoking was never, or current/ever (reference category); health insurance was Medicare/Medicaid, or other (reference category); and the study site was UPenn or FCCC/CMCVAMC (reference category). We conducted separate analysis for each prostate cancer risk group.
3. Results
We recruited 743 localized prostate cancer patients for the study. Comparison of baseline characteristics across the three prostate cancer risk groups is presented in Table 1. Age, number of people in the household, race and ethnicity, insurance, smoking status, marital status, income, education and employment were comparable between the three prostate cancer risk groups. The distribution of treatment varied across the prostate cancer risk groups (p < 0.0001). The low-risk group had the highest proportion of those on active surveillance, compared to intermediate- and high-risk groups (53.9% vs. 18.2% and 11.6%, respectively). On the other hand, the high-risk group had the highest proportion of those treated with surgery, compared to the low- and intermediate-risk groups (69.8% vs. 29.1% and 57.9%, respectively).
Table 1.
Demographics and clinical variables across prostate cancer risk groups.
| Low Risk (n = 254) | Intermediate Risk (n = 247) | High Risk (n = 225) | p-Value | ||
|---|---|---|---|---|---|
| Mean age, years (SD) | 63.9 (8.4) | 63.6 (7.4) | 63.6 (7.4) | 0.8193 | |
| Mean Number of people in the household (SD) | 2.3 (0.98) | 2.2 (0.96) | 2.5 (1.12) | 0.0698 | |
| Race/Ethnicity | White | 198 (79.8) | 194 (80.8) | 188 (87.0) | 0.3005 |
| African American | 40 (16.1) | 36 (15.0) | 23 (10.7) | ||
| Other | 10 (4.0) | 10 (4.2) | 5 (2.3) | ||
| Insurance | Medicare/Medicaid | 106 (42.9) | 83 (33.5) | 84 (38.8) | 0.1326 |
| Managed care | 109 (44.1) | 138 (56.3) | 109 (50.5) | ||
| Private | 32 (12.9) | 24 (9.8) | 23 (10.5) | ||
| Smoking status | No | 147 (58.1) | 145 (58.7) | 129 (58.4) | 0.4850 |
| Yes | 18 (7.1) | 13 (5.3) | 21 (9.5) | ||
| Prior history | 88 (34.8) | 89 (36.0) | 71 (32.1) | ||
| Marital status | Married | 203 (82.2) | 186 (77.2) | 186 (84.9) | 0.0942 |
| Other | 44 (17.8) | 55 (22.8) | 33 (15.1) | ||
| Income | ≤USD 40,000 | 41 (19.8) | 30 (14.7) | 28 (14.4) | 0.4627 |
| USD 40,000–75,000 | 46 (22.2) | 41 (20.1) | 41 (21.0) | ||
| >USD 75,000 | 120 (57.9) | 133 (65.2) | 126 (64.6) | ||
| Education | Some college | 84 (39.3) | 81 (38.6) | 80 (39.4) | 0.9828 |
| College and other | 130 (60.8) | 129 (61.4) | 123 (60.6) | ||
| Employment | Full/part time | 113 (52.8) | 126 (60.3) | 123 (60.3) | 0.1981 |
| Other | 101 (47.2) | 83 (39.7) | 81 (39.7) | ||
| Treatment Type | Active surveillance | 137 (53.9) | 45 (18.2) | 26 (11.6) | <0.0001 |
| Surgery | 74 (29.1) | 143 (57.9) | 157 (69.8) | ||
| Radiation | 19 (7.5) | 41 (16.6) | 32 (14.2) | ||
| Site | UPenn | 174 (68.5) | 195 (78.9) | 173 (77.6) | <0.0001 |
| VA | 18 (7.1) | 1 (0.4) | 3 (1.3) | ||
| FCCC | 62 (24.4) | 51 (20.6) | 49 (21.1) | ||
Note. Data are presented as a no. (%), unless otherwise indicated. Missing values: prostate cancer risk group (n = 17), age (n = 19), number of people in household (n = 98), race/ethnicity (n = 22), insurance (n = 18), smoking (n = 5), marital status (n = 19), income (n = 120), education (n = 99), employment (n = 99) and treatment type (n = 52). Abbreviations: SD, standard deviation; UPenn, University of Pennsylvania; CMCVAMC, Corporal Michael J. Crescenz Veterans Administration Medical Center; FCCC, Fox Chase Cancer Center.
The distribution of CES-D scores over time and by prostate cancer risk group is presented in Table 2. We used paired Wilcoxon signed rank test of the medians to test the difference in the medians (baseline and 3 months, baseline and 6 months, baseline and 12 months and baseline and 24 months). The results indicated that the medians at 6, 12 and 24 months were significantly different from baseline median values, overall and for the three prostate cancer risk groups. The mean regret score at 3 month was 9.4 (standard deviation (SD) 17.3), 8.1 (SD 16.6) at 6 month, 7.2 (SD 15.2) at 12 month and 7.7 (SD 15.7) at 24 month.
Table 2.
Distribution of CES-D scores over time, and by prostate cancer risk group.
| Baseline | 3 m | 6 m | 12 m | 24 m | |
|---|---|---|---|---|---|
| ALL | |||||
| Mean | 9.04 | 9.52 | 11.69 | 15.40 | 16.02 |
| SD | 8.12 | 8.21 | 7.99 | 5.55 | 4.91 |
| Median | 7.00 | 7.00 | 12.00 | 14.00 | 15.00 |
| Wilcoxon signed rank test of medians—compared to baseline median value | p-value 0.4219 | p-value < 0.0001 | p-value < 0.0001 | p-value < 0.0001 | |
| Low Risk | |||||
| Mean | 8.12 | 9.20 | 11.42 | 15.15 | 16.02 |
| SD | 7.73 | 8.67 | 7.57 | 4.82 | 4.79 |
| Median | 6.00 | 6.00 | 12.00 | 14.00 | 14.00 |
| Wilcoxon signed rank test of medians—compared to baseline median value | p-value 0.0811 | p-value < 0.0001 | p-value < 0.0001 | p-value < 0.0001 | |
| Intermediate Risk | |||||
| Mean | 9.60 | 10.37 | 11.93 | 15.87 | 16.06 |
| SD | 8.48 | 8.69 | 8.55 | 5.66 | 5.09 |
| Median | 7.00 | 9.00 | 13.00 | 14.00 | 15.00 |
| Wilcoxon signed rank test of medians—compared to baseline median value | p-value 0.8132 | p-value 0.0049 | p-value < 0.0001 | p-value < 0.0001 | |
| High Risk | |||||
| Mean | 9.30 | 9.09 | 11.59 | 15.15 | 15.96 |
| SD | 7.94 | 7.27 | 7.94 | 6.14 | 4.87 |
| Median | 7.00 | 7.00 | 12.00 | 15.00 | 15.00 |
| Wilcoxon signed rank test of medians—compared to baseline median value | p-value 0.8691 | p-value < 0.0001 | p-value < 0.0001 | p-value < 0.0001 | |
Abbreviations: SD, standard deviation.
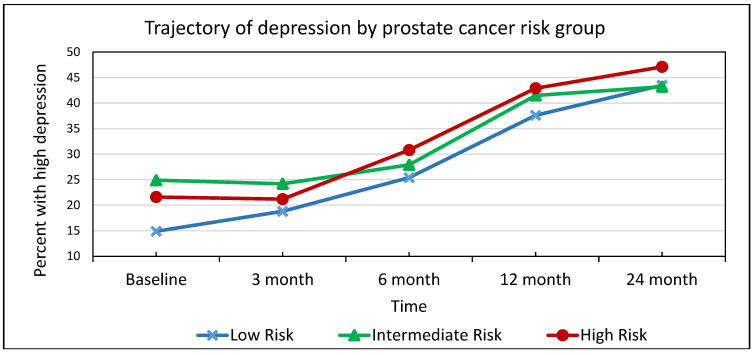
As seen in Figure 1, the proportion of patients with high depression increased between baseline and 24 months for risk groups. For the low-risk group, the proportion with high depression was 14.9% baseline and 43.5% at 24-month. These proportions were 24.9% and 43.2%, respectively, for the intermediate-risk group; and 21.6% and 47.1%, respectively, for the high-risk group. The increase in the proportion of patients with high depression over time was statistically significant for all three prostate cancer risk groups (0.05 level.)
Figure 1.
Trajectory of high depression by prostate cancer risk group *. * The increase in the proportion of patients with high depression over time was statistically significant for all three prostate cancer risk groups (0.05 level).
In Table 3, we present the results of the multivariable logistic regression for association between 24-month depression and regret, by prostate cancer risk group. We observed that for all prostate cancer risk groups, regret was significantly associated with the risk of high depression. For one unit increase in the 24-month regret score, there was a 4 percent increase in the risk of high depression at 24-month follow-up, on average, after adjusting for age, race, insurance status, smoking status, marital status, income, education, employment, treatment, study site and the number of people in the household.
Table 3.
Multivariable logistic regression for association between 24-month depression and regret, by prostate cancer risk group.
| Dependent Variable: High Depression at 24 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Risk | Intermediate Risk | High Risk | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Regret score | 1.06 | 1.01, 1.11 | 0.0111 | 1.04 | 1.01, 1.08 | 0.0188 | 1.04 | 1.01, 1.07 | 0.0209 |
| Age | 0.98 | 0.89, 1.06 | 0.5402 | 1.03 | 0.96, 1.10 | 0.4154 | 0.96 | 0.89, 1.04 | 0.3199 |
| Number of people in household | 1.44 | 0.86, 2.42 | 0.1647 | 1.02 | 0.64, 1.65 | 0.9116 | 1.17 | 0.75, 1.83 | 0.4780 |
| Smoking | |||||||||
| Never | 0.22 | 0.10, 0.59 | 0.0031 | 0.51 | 0.22, 1.17 | 0.1136 | 1.06 | 0.48, 2.23 | 0.8900 |
| Current/ever (ref) | - | - | - | - | - | - | - | - | - |
| Insurance | |||||||||
| Medicare/Medicaid | 0.37 | 0.12, 1.17 | 0.0903 | 0.93 | 0.36, 2.37 | 0.8723 | 1.74 | 0.69, 4.39 | 0.2385 |
| Other (ref) | - | - | - | - | - | - | - | - | - |
| Treatment | |||||||||
| Surgery | 0.71 | 0.25, 2.05 | 0.5303 | 0.67 | 0.24, 1.87 | 0.4492 | 1.03 | 0.30, 3.61 | 0.9613 |
| Radiation | 0.39 | 0.07, 2.26 | 0.2956 | 0.44 | 0.12, 1.55 | 0.1998 | 2.47 | 0.53, 11.58 | 0.2497 |
| Active surveillance (ref) | - | - | - | - | - | - | - | - | - |
| Race | |||||||||
| White | 1.82 | 0.10, 35.70 | 0.6922 | 1.61 | 0.21, 12.24 | 0.6480 | 1.52 | 0.07, 32.60 | 0.7882 |
| African American | 2.37 | 0.07, 78.90 | 0.6304 | 3.34 | 0.29, 38.30 | 0.3322 | 3.26 | 0.12, 91.60 | 0.4875 |
| Other (reference) | - | - | - | - | - | - | - | - | - |
| Education | |||||||||
| College and more | 0.88 | 0.29, 2.59 | 0.8105 | 1.74 | 0.69, 4.36 | 0.2408 | 1.83 | 0.72, 4.65 | 0.2023 |
| Some college (ref) | - | - | - | - | - | - | - | - | - |
| Marital status | |||||||||
| Married | 0.45 | 0.09, 2.23 | 0.3305 | 0.67 | 0.21, 2.11 | 0.4957 | 0.97 | 0.24, 3.94 | 0.9678 |
| Other (ref) | - | - | - | - | - | - | - | - | - |
| Employment status | |||||||||
| Employed | 1.67 | 0.49, 5.69 | 0.4154 | 0.75 | 0.31, 1.85 | 0.5363 | 1.19 | 0.49, 2.92 | 0.6943 |
| Other (ref) | - | - | - | - | - | - | - | - | - |
| Income | |||||||||
| ≤USD 40,000 | 1.97 | 0.37, 10.55 | 0.4287 | 3.80 | 0.85, 17.72 | 0.4249 | 0.86 | 0.19, 3.76 | 0.8419 |
| USD 40–75,000 | 4.39 | 1.29, 14.98 | 0.0179 | 0.65 | 0.22, 1.88 | 0.0803 | 2.74 | 0.96, 7.75 | 0.0579 |
| >USD 75,000 (ref) | - | - | - | - | - | - | - | - | - |
| Study Site | |||||||||
| UPenn | 1.78 | 0.59, 5.34 | 0.2975 | 0.55 | 0.22, 1.36 | 0.1930 | 1.06 | 0.48, 2.34 | 0.5935 |
| FCCC/VA (ref) | - | - | - | - | - | - | - | - | - |
Abbreviations: UPenn, University of Pennsylvania; CMCVAMC, Corporal Michael J. Crescenz Veterans Administration Medical Center; FCCC, Fox Chase Cancer Center. OR: odds ratio. CI: confidence interval.
4. Discussion
We observed that among newly diagnosed prostate cancer patients undergoing treatment or those on active surveillance: (1) median depression scores at 6, 12 and 24 months were significantly higher than the baseline median score, overall and for the three prostate cancer risk groups; (2) the proportion with high depression increased over time (between baseline and 24-month follow-up), for all prostate cancer risk groups; and (3) higher regret was significantly associated with high depression at the 24-month follow-up, after adjusting for age, race, insurance status, smoking status, marital status, income, education, employment, treatment, study site and the number of people in the household.
Among patients living with other types of cancers, such as breast cancer [34,35,36,37], head and neck cancer [38,39,40], and esophageal cancer [41,42], depression and anxiety were found to be the most common problems, and were associated with impaired outcomes [40,43,44,45]. The research focusing on the association between depression and regret among cancer patients is limited. Among breast cancer patients undergoing reconstructive procedures, increased depression and lower satisfaction with information were associated with an increased likelihood of experiencing regret [46]. One study of adolescent and young adult cancer patients showed an association between regret and negative psychological outcomes, including anxiety and depression [47].
Our results make an important contribution to the limited existing literature on the assessment of the association between depression and treatment decision regret. As most men treated for localized prostate cancer can expect to live many years after diagnosis, the treatment-related morbidities are often experienced over an extended period of time, leading to treatment regret that can have long-term negative consequences on patients’ quality of life and depression [21,48]. Androgen deprivation therapy was shown to be associated with an increased risk of diagnosis of depression [49]. With increased survivorship and burden of disease, it is important to consider the physical and mental health of prostate cancer survivors. Previous studies found that the rates of depressive symptoms among prostate cancer survivors ranged from 16 to 30% [5,7,8,25,50].
Research has demonstrated that a large proportion of patients with prostate cancer experience some regret after treatment, and this regret tends to increase over time [21,22,23,24,51,52]. Diefenbach and Mohamed (2009) found that prostate cancer survivors who were regretful about their treatment choice(s) had a lower quality of life compared to those who were not regretful [53]. Previous studies have also found that men who had a passive role in treatment decision-making had higher treatment regret than those who assumed an active or collaborative role, and that among those who participated in medical decision-making, 94% did not experience treatment regret [54]. Our results are in concordance with research that reported an association between treatment decision regret and depression [24,25]. While the evidence for the impact of treatment regret on depression among prostate cancer survivors is evolving, its contribution can be important. Davison and Goldenberg (2003) found that emotional functioning was significantly better at follow-up among prostate cancer patients who participated in their treatment decision than those who did not [55]. In a longitudinal study of patients undergoing prostatectomy, shared decision-making was protective against regret, and patients with higher depression were more likely to have regret [24]. This suggests that treatment regret can have implications for depression.
We would like to note the strength and limitations of our study. The strength of our study is that this is a secondary analysis of one of the largest multicenter randomized controlled study that assessed self-reported regret and depression, using valid instruments over a 24-month follow-up period. Some of the limitations of our study are as follows: our study sites were urban academic institutions. Between baseline and 24 months, there were missing data due to lost to follow-up, as well as due to missing data. Most of the participants were married, college graduates and almost two-thirds had an annual household income of USD 75,000 or higher. Our results showed that among low-risk group participants, smoking status and income was associated with depression. Thus, future studies in patients with diverse characteristics and in different clinical settings are needed to enhance the generalizability of our findings.
5. Conclusions
Our study has several important clinical implications. In this secondary analysis of a large randomized controlled study, we assessed the burden of depression, and the association between decision regret and depression in localized prostate cancer patients. We observed that a substantial proportion of localized prostate cancer patients continued to experience long-term depression, irrespective of their prostate cancer risk group status. In addition, a positive association between decision regret and depression was noted, albeit the size of the effect was small. Studies, including our prior research, have demonstrated that depression in prostate cancer patients is associated with impaired outcomes of care. Thus, effective survivorship care strategies for prostate cancer must address depression and regret. Patient-centered strategies that facilitate patient participation in decision-making can help lower decision regret. Screening and surveillance for depression must be incorporated into clinical care of prostate cancer. Similarly, coordinated strategies are necessary for improving depression treatment uptake and retention in depression care.
Acknowledgments
We thank the patient and provider members of the study advisory group. We also thank all study participants.
Author Contributions
Conceptualization, S.C. and R.J.; data curation, S.C. and R.J.; formal analysis, S.C. and R.J.; funding acquisition, R.J.; investigation, S.C. and R.J.; methodology, S.C., R.J. and K.H.M.; project administration, R.J.; resources, T.G., D.K.N., N.V., K.V.A., A.J.W., S.B.M. and R.J.; software, S.C., K.H.M. and R.J.; supervision, R.J.; validation, S.C. and R.J.; visualization, S.C. and R.J.; writing—original draft, S.C. and R.J.; writing—review and editing, S.C., J.J.G., T.G., K.H.M., D.K.N., N.V., K.V.A., A.J.W., S.B.M. and R.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University of Pennsylvania (protocol code 818201, July 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data were collected as part of a large randomized controlled study and contain sensitive information. As such, the data cannot be shared.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
Patient-Centered Outcomes Research Institute—CE-12-11-4973. Trials Registration: Clinicaltrials.gov-NCT02032550.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Smith H.R. Depression in cancer patients: Pathogenesis, implications and treatment (Review) Oncol. Lett. 2015;9:1509–1514. doi: 10.3892/ol.2015.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Panjwani A., Li M. Recent trends in the management of depression in persons with cancer. Curr. Opin. Psychiatr. 2021;34:448–459. doi: 10.1097/YCO.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 5.Jayadevappa R., Malkowicz S.B., Chhatre S., Gallo J.J. The burden of depression in prostate cancer. Psychooncology. 2012;21:1338–1345. doi: 10.1002/pon.2032. [DOI] [PubMed] [Google Scholar]
- 6.Townsend L., Walkup J.T., Crystal S., Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol. Drug Saf. 2012;21:163–173. doi: 10.1002/pds.2310. [DOI] [PubMed] [Google Scholar]
- 7.Watts S., Leydon G., Birch B., Prescott P., Lai L., Eardley S., Lewith G. Depression and anxiety in prostate cancer: A systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4:e003901. doi: 10.1136/bmjopen-2013-003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fervaha G., Izard J.P., Tripp D.A., Rajan S., Leong D.P., Siemens D.R. Depression and prostate cancer: A focused review for the clinician. Urol. Oncol. 2019;37:282–288. doi: 10.1016/j.urolonc.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Fervaha G., Izard J.P., Tripp D.A., Aghel N., Shayegan B., Klotz L., Niazi T., Fradet V., Taussky D., Lavallée L.T., et al. Psychological morbidity associated with prostate cancer: Rates and predictors of depression in the RADICAL PC study. Can. Urol. Assoc. J. 2021;15:181–186. doi: 10.5489/cuaj.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquini M., Biondi M. Depression in cancer patients: A critical review. Clin. Pract. Epidemiol. Ment. Health. 2007;3:2. doi: 10.1186/1745-0179-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Zhang Y., Wang Y. A 5-year follow-up assessment of anxiety and depression in postoperative prostate cancer patients: Longitudinal progression and prognostic value. Psychol. Health Med. 2023;28:529–539. doi: 10.1080/13548506.2022.2115083. [DOI] [PubMed] [Google Scholar]
- 12.Hu S., Li L., Wu X., Liu Z., Fu A. Post-surgery anxiety and depression in prostate cancer patients: Prevalence, longitudinal progression, and their correlations with survival profiles during a 3-year follow-up. Ir. J. Med. Sci. 2021;190:1363–1372. doi: 10.1007/s11845-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirl W.F., Siegel G.I., Goode M.J., Smith M.R. Depression in men receiving androgen deprivation therapy for prostate cancer: A pilot study. Psychooncology. 2002;11:518–523. doi: 10.1002/pon.592. [DOI] [PubMed] [Google Scholar]
- 14.Latini D.M., Hart S.L., Knight S.J., Cowan J.E., Ross P.L., DuChane J., Carroll P.R. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J. Urol. 2007;178:826–832. doi: 10.1016/j.juro.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 15.van den Bergh R.C.N., Essink-Bot M.-L., MSc M.J.R., Wolters T., Schröder F.H., Bangma C.H., MSc E.W.S. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115:3868–3878. doi: 10.1002/cncr.24446. [DOI] [PubMed] [Google Scholar]
- 16.Wallis C.J.D., Zhao Z., Huang L.-C., Penson D.F., Koyama T., Kaplan S.H., Greenfield S., Luckenbaugh A.N., Klaassen Z., Conwill R., et al. Association of Treatment Modality, Functional Outcomes, and Baseline Characteristics With Treatment-Related Regret Among Men With Localized Prostate Cancer. JAMA Oncol. 2022;8:50–59. doi: 10.1001/jamaoncol.2021.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becerra Pérez M.M., Menear M., Brehaut J.C., Légaré F. Extent and predictors of decision regret about health care decisions: A systematic review. Med. Decis. Mak. 2016;36:777–790. doi: 10.1177/0272989X16636113. [DOI] [PubMed] [Google Scholar]
- 18.Connolly T., Reb J. Regret aversion in reason-based choice. Theory Decis. 2011;73:35–51. doi: 10.1007/s11238-011-9269-0. [DOI] [Google Scholar]
- 19.Skyring T.A., Mansfield K.J., Mullan J.R. Factors Affecting Satisfaction with the Decision-Making Process and Decision Regret for Men with a New Diagnosis of Prostate Cancer. Am. J. Men’s Health. 2021;15:15579883211026812. doi: 10.1177/15579883211026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilding S., Downing A., Selby P., Cross W., Wright P., Watson E.K., Wagland R., Kind P., Donnelly D., Hounsome L., et al. Decision regret in men living with and beyond nonmetastatic prostate cancer in the United Kingdom: A population-based patient-reported outcome study. Psycho-Oncology. 2020;29:886–893. doi: 10.1002/pon.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz L.M., Cullen J., Kim D.J., Elsamanoudi S., Hudak J., Rn M.C., Travis J., Kuo H., Rice K.R., Porter C.R., et al. Longitudinal Regret After Treatment for Low- and Intermediate-Risk Prostate Cancer. Cancer. 2017;123:4252–4258. doi: 10.1002/cncr.30841. [DOI] [PubMed] [Google Scholar]
- 22.Bradley C., Ilie G., MacDonald C., Massoeurs L., Vo J.D.C.-T., Rutledge R.D.H. Treatment Regret, Mental and Physical Health Indicators of Psychosocial Well-Being among Prostate Cancer Survivors. Curr. Oncol. 2021;28:3900–3917. doi: 10.3390/curroncol28050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry D.L., Hong F., Blonquist T.M., Halpenny B., Xiong N., Filson C.P., Master V.A., Sanda M.G., Chang P., Chien G.W., et al. Decision regret, adverse outcomes and treatment choice in men with localized prostate cancer: Results from a multi-site randomized trial. Urol. Oncol. 2021;39:493.e9–493.e15. doi: 10.1016/j.urolonc.2020.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meissner V.H., Simson B.W., Dinkel A., Schiele S., Ankerst D.P., Lunger L., Gschwend J.E., Herkommer K. Treatment decision regret in long-term survivors after radical prostatectomy: A longitudinal study. BJU Int. 2022 doi: 10.1111/bju.15955. [DOI] [PubMed] [Google Scholar]
- 25.Erim D.O., Bensen J.T., Mohler J.L., DrPH E.T.H.F., Song L., Farnan L., Delacroix S.E., Peters E.S., Erim T.N., Chen R.C., et al. Prevalance and Predictors of probable depression in prostate cancer survivors. Cancer. 2019;125:3418–3427. doi: 10.1002/cncr.32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayadevappa R., Chhatre S., Gallo J.J., Wittink M., Morales K.H., Malkowicz S.B., Lee D., Guzzo T., Caruso A., Van Arsdalen K., et al. Treatment preference and patient centered prostate cancer care: Design and rationale. Contemp. Clin. Trials. 2015;45:296–301. doi: 10.1016/j.cct.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Jayadevappa R., Chhatre S., Gallo J.J., Wittink M., Morales K.H., Lee D.I., Guzzo T.J., Vapiwala N., Wong Y.-N., Newman D.K., et al. Patient-Centered Preference Assessment to Improve Satisfaction with Care Among Patients With Localized Prostate Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2019;37:964–973. doi: 10.1200/JCO.18.01091. [DOI] [PubMed] [Google Scholar]
- 28.Chhatre S., Jefferson A., Cook R., Meeker C.R., Kim J.H., Hartz K.M., Wong Y.-N., Caruso A., Newman D.K., Morales K.H., et al. Patient-centered recruitment and retention for a randomized controlled study. Trials. 2018;19:205–214. doi: 10.1186/s13063-018-2578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayadevappa R., Chhatre S., Gallo J.J., Malkowicz S.B., Schwartz J.S., Wittink M.N. Patient-Centered Approach to Develop the Patient’s Preferences for Prostate Cancer Care (PreProCare) Tool. MDM Policy Pract. 2019;4:1–13. doi: 10.1177/2381468319855375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff L.S. The CES-D Scale: A self report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 31.Clark J.A., Bokhour B.G., Inui T.S., Silliman R.A., Talcott J.A. Measuring patients’ perceptions of the outcomes of treatment for early prostate cancer. Med. Care. 2003;41:923–936. doi: 10.1097/00005650-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Clark J.A., Inui T.S., Silliman R.A., Bokhour B.G., Krasnow S.H., Robinson R.A., Spaulding M., Talcott J.A. Patients’ perceptions of quality of life after treatment for early prostate cancer. J. Clin. Oncol. 2003;21:3777–3784. doi: 10.1200/JCO.2003.02.115. [DOI] [PubMed] [Google Scholar]
- 33.Roth A., Nelson C.J., Rosenfeld B., Warshowski A., O’shea N., Scher H., Holland J.C., Slovin S., Curley-Smart T., Reynolds T., et al. Assessing anxiety in men with prostate cancer: Further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX–PC) Psychosomatics. 2006;47:340–347. doi: 10.1176/appi.psy.47.4.340. [DOI] [PubMed] [Google Scholar]
- 34.Theresa P.Y., Shawnna C., Richard E.T., Ryan F., Avinoam N., Harish L., Charles Y.J. Distress, Depression, and the Effect of ZIP Code in Pancreaticobiliary Cancer Patients and Their Significant Others. J. Am. Coll. Surg. 2023;236:339–349. doi: 10.1097/XCS.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 35.Fann J.R., Thomas-Rich A.M., Katon W.J., Cowley D., Pepping M., McGregor B.A., Gralow J. Major depression after breast cancer: A review of epidemiology and treatment. Gen. Hosp. Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel D., Riba M.B. Managing anxiety and depression during treatment. Breast. 2015;21:97–103. doi: 10.1111/tbj.12355. [DOI] [PubMed] [Google Scholar]
- 37.Puigpinós-Riera R., Graells-Sans A., Serral G., Continente X., Bargalló X., Domènech M., Espinosa-Bravo M., Grau J., Macià F., Manzanera R., et al. Anxiety and depression in women with breast cancer: Social and clinical determinants and influence of the social network and social support (DAMA cohort) Cancer Epidemiol. 2018;55:123–129. doi: 10.1016/j.canep.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Duffy S.A., Ronis D.L., Valenstein M., Fowler K.E., Lambert M.T., Bishop C., Terrell J.E. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 39.Fan C.-Y., Chao H.-L., Lin C.-S., Huang W.-Y., Chen C.-M., Lin K.-T., Lin C.-L., Kao C.-H. Risk of depressive disorder among patients with head and neck cancer: A nationwide population-based study. Head Neck. 2017;40:312–323. doi: 10.1002/hed.24961. [DOI] [PubMed] [Google Scholar]
- 40.Huang R.-W., Chang K.-P., Marchi F., Loh C.Y.Y., Lin Y.-J., Chang C.-J., Kao H.-K. The impact of depression on survival of head and neck cancer patients: A population-based cohort study. Front. Oncol. 2022;12:871915. doi: 10.3389/fonc.2022.871915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellstadius Y., Lagergren P., Lagergren J., Johar A., Hultman C.M., Wikman A. Aspects of emotional functioning following oesophageal cancer surgery in a population-based cohort study. Psychooncology. 2015;24:47–53. doi: 10.1002/pon.3583. [DOI] [PubMed] [Google Scholar]
- 42.Oh T.K., Song I.-A., Park H.Y., Hwang J.-W. Pre-existing and new-onset depression among patients undergoing esophageal cancer surgery: A nationwide cohort study in South Korea. Esophagus. 2023;20:55–62. doi: 10.1007/s10388-022-00947-0. [DOI] [PubMed] [Google Scholar]
- 43.Kent E.E., Ambs A., Mitchell S.A., Clauser S.B., Smith A.W., Hays R.D. HRQOL surveillance efforts revealed poor health outcomes among many older adults and specifically among survivors of multiple myeloma and pancreatic cancer. Cancer. 2015;21:758–765. doi: 10.1002/cncr.29119. [DOI] [Google Scholar]
- 44.Kam D., Salib A., Gorgy G., Patel T.D., Carniol E.T., Eloy J.A., Baredes S., Park R.C.W. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2015;141:1075–1081. doi: 10.1001/jamaoto.2015.2480. [DOI] [PubMed] [Google Scholar]
- 45.Chiou W.Y., Lee M.S., Ho H.C., Hung S.K., Lin H.Y., Su Y.C., Lee C.C. Prognosticators and the relationship of depression and quality of life in head and neck cancer. Indian J. Cancer. 2013;50:14–20. doi: 10.4103/0019-509X.112279. [DOI] [PubMed] [Google Scholar]
- 46.Sheehan J., Sherman K.A., Lam T., Boyages J. Association of information satisfaction, psychological distress and monitoring coping style with post-decision regret following breast reconstruction. Psychooncology. 2007;16:342–351. doi: 10.1002/pon.1067. [DOI] [PubMed] [Google Scholar]
- 47.Mack J.W., Fasciano K.M., Block S.D. Adolescent and Young Adult Cancer Patients’ Experiences With Treatment Decision-making. Pediatrics. 2019;143:e20182800. doi: 10.1542/peds.2018-2800. [DOI] [PubMed] [Google Scholar]
- 48.Albkri A., Girier D., Mestre A., Costa P., Droupy S., Chevrot A. Urinary Incontinence, Patient Satisfaction, and Decisional Regret after Prostate Cancer Treatment: A French National Study. Urol. Int. 2018;100:50–56. doi: 10.1159/000484616. [DOI] [PubMed] [Google Scholar]
- 49.Dinh K.T., Reznor G., Muralidhar V., Mahal B., Nezolosky M.D., Choueiri T.K., Hoffman K.E., Hu J.C., Sweeney C.J., Trinh Q.-D., et al. Association of Androgen Deprivation Therapy With Depression in Localized Prostate Cancer. J. Clin. Oncol. 2016;34:1905–1912. doi: 10.1200/JCO.2015.64.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan L.-H., Lin M.-H., Pang S.-T., Wang J., Shih W.-M., Shih W.-M. Improvement of Urinary Incontinence, Life Impact, and Depression and Anxiety With Modified Pelvic Floor Muscle Training After Radical Prostatectomy. Am. J. Men’s Health. 2019;13:1557988319851618. doi: 10.1177/1557988319851618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Tol-Geerdink J.J., Leer J.W.H., Wijburg C.J., van Oort I.M., Vergunst H., van Lin E.J., Witjes J.A., Stalmeier P.F.M. Does a decision aid for prostate cancer affect different aspects of decisional regret, assessed with new regret scales? A randomized, controlled trial. Health Expect. 2016;19:459–470. doi: 10.1111/hex.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahal B.A., Chen M.-H., Bennett C.L., Kattan M.W., Sartor O., Stein K., D’Amico A.V., Nguyen P.L. The association between race and treatment regret among men with recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:38–42. doi: 10.1038/pcan.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diefenbach M.A., Mohamed N.E. Regret of treatment decision and its association with disease-specific quality of life following prostate cancer treatment. Cancer Investig. 2007;25:449–457. doi: 10.1080/07357900701359460. [DOI] [PubMed] [Google Scholar]
- 54.Davison B.J., So A.I., Goldenberg S.L. Quality of life, sexual function and decisional regret at 1 year after surgical treatment for localized prostate cancer. BJU Int. 2007;100:780–785. doi: 10.1111/j.1464-410X.2007.07043.x. [DOI] [PubMed] [Google Scholar]
- 55.Davison B.J., Goldenberg S.L. Decisional regret and quality of life after participating in medical decision-making for early-stage prostate cancer. BJU Int. 2003;91:14–17. doi: 10.1046/j.1464-410X.2003.04005.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data were collected as part of a large randomized controlled study and contain sensitive information. As such, the data cannot be shared.