Abstract
Simple Summary
Endometrial (EC) and cervical cancers (CC) do not respond well to chemotherapy and alternative therapeutic options are lacking. Recently, EC and CC patients’ outcome were improved thanks to immunotherapy-based treatments leading to their approval in the management of these diseases. However, not all patients respond to such treatments, especially in relapsed settings. In the present review, we focused on the immunological features of both EC and CC and presented the upcoming clinical trials targeting the immune system alone or in combination that will allow to improve patient’s care. We also raise some questions that need to be solved in this field and presented the expected progresses.
Abstract
Endometrial cancer (EC) is the seventh most common tumor in women, and prognosis of recurrent and metastatic disease is poor. Cervical cancer (CC) represents the fifth most common gynecological cancer. While ECs are more common in developed countries, the incidence of CC has decreased due to the recent implementation of large screening and vaccination programs. Until very recently, patients with advanced or unresectable EC or CC had very limited treatment options and were receiving in first line setting platinum/taxane-based chemotherapy (CT). Significant progress in the treatment of gynecological cancers has occurred in the last few years, with the use of innovative targeted therapies and immunotherapy. However, targeting the immune system in patients with gynecological tumors remains challenging and is not always successful. In ovarian cancer, several immunotherapy treatment regimens have been investigated (as monotherapy and combination therapy in first and subsequent lines of treatment) and showed poor responses. Therefore, we specifically focused our review on EC and CC for their specific immune-related features and therapeutic results demonstrated with immunotherapy. We report recent and current immunotherapy-based clinical trials and provide a review of emerging data that are likely to impact immunotherapy development based on increased biomarkers’ identification to monitor response and overcome resistance.
Keywords: cervical cancers, endometrial cancers, gynecological cancers, immunotherapy, tumor microenvironment
1. Introduction
Gynecological cancers include ovarian, cervical, endometrial, vaginal, and vulvar cancers that all present specific clinical and biological features. Up to 1,398,601 women were diagnosed with gynecological cancers worldwide in 2020 and over 671,875 related deaths were reported [1] (Table 1). The present review will focus on uterine-associated tumors, i.e., endometrial cancers (EC) (upper part, uterine corpus) and cervical cancers (CC) (lower part, uterine cervix), which showed less pronounced responses to chemotherapy (CT) than ovarian cancers, underlining the need for further therapies including immunotherapy-based treatment, in patients with EC and CC. The use of immunotherapy and in particular immune checkpoint inhibitors (ICIs) drastically changed the management and outcomes of patients, with the ability to boost endogenous immunity against unique tumor antigens, resulting in long-lasting responses in some selected patients. In patients with ovarian cancer, only a limited benefit was achieved, as compared with other tumors [2], and is partly explained because of high tumor heterogeneity and predominant immunosuppressive tumor microenvironment (TME). Several trials are currently investigating the use of ICIs alone or in combination, with high expectations in innovative therapies.
Table 1.
Worldwide incidence and mortality rates of gynecological cancers in 2020.
| Worldwide New Cases [1] | Worldwide Deaths [1] | Worldwide Female Cancer Rank [1] | 5-Year Survival Rates (United States) | |
|---|---|---|---|---|
| Cervical Cancers | 604,127 | 341,831 | 5th | ~66% in the whole population [3] Low stage: 92% and 58% if lymph nodes are invaded [4] |
| Endometrial Cancers | 417,367 | 97,370 | 7th | Early stages: >80% (95% for stage I) [3,5] Recurrent/advanced disease: 20-25% [5] |
| Ovarian Cancers | 313,959 | 207,252 | 9th | ~49.7% [3] |
| Vaginal Cancers | 17,908 | 7,995 | >10th | ~49% in the whole population with a variation between 35–78% [6,7] Early stage: ~85% [7] |
| Vulvar Cancers | 45,240 | 17,427 | >10th | ~70.3% [3] |
However, results with immunotherapy in patients with EC and CC are still disappointing, especially in the second line setting. Therefore, we reviewed recent achievements and suggested implementations in line with the currently expected progress.
1.1. Endometrial Cancers
EC ranks seventh among the most common diagnosed gynecological cancer worldwide and is the fourth most common female cancer in Europe with an incidence of 16.4–20.2/100,000 [1]. Up to 80% of ECs are diagnosed owing to associated postmenopausal bleeding and are mainly locally advanced/confined to the uterus. The mortality rate remains low at 2.7–3.7/100,000 [1,8]. The 5-year overall survival (OS) in patients with stage I EC is over 95% and remains over 50% in EC with invaded lymph nodes but reduced to only 20–25% in patients with advanced/recurrent/metastatic disease [5] (Table 1). Furthermore, EC patients at high and at high–intermediate risk are more prone to relapse with 16–35% relapsing patients compared with patients at intermediate and at low risk relapsing in 9% of the cases [9] (Table 2 and Table 3). Several risk factors have been associated with an increased likelihood of developing EC and a poorer OS including obesity, type 2 diabetes, prior breast or ovarian cancer, and advanced age (Figure 1) [10,11,12,13,14].
Table 2.
Endometrial cancer risk groups (Oaknin et al., 2022 [5]). The table can be used in stage III-IVA with complete resection without residual disease and does not apply to stage III-IVA with residual disease or for stage IV. dMMR, deficient mismatch repair; EC, endometrial cancer; G1-G3, grade 1-3; IHC, immunohistochemistry; LVSI, lymphovascular space invasion; MSI-H, microsatellite instability high/hypermutated; NSMP, no specific molecular profile; p53-abn, p53-abnormal; POLEmut, polymerase ε-ultramutated.
| Risk Group | Description |
|---|---|
| Low risk | Stage IA (G1-G2) with endometrioid type (dMMR or MSI-H and NSMP) and no or focal LVSI Stage I–III POLEmut EC |
| Intermediate risk | Stage IA G3 with endometrioid type (dMMR and NSMP) and no or focal LVSI Stage IA non-endometrioid type (serous, clear-cell, undifferentiated carcinoma, carcinosarcoma, mixed) and/or p53-abn cancers without myometrial invasion and no or focal LVSI Stage IB (G1-G2) with endometrioid type (dMMR and NSMP) and no or focal LVSI Stage II G1 endometrioid type (dMMR and NSMP) and no or focal LVSI |
| High-intermediate risk | Stage I endometrioid type (dMMR and NSMP) any grade and any depth of invasion with substantial LVSI Stage IB G3 with endometrioid type (dMMR and NSMP) regardless of LVSI Stage II G1 endometrioid type (dMMR and NSMP) with substantial LVSI Stage II G2-G3 endometrioid type (dMMR and NSMP) |
| High risk | All stages and all histologies with p53-abn and myometrial invasion All stages with serous or undifferentiated carcinoma including carcinosarcoma with myometrial invasion All stage III and IVA with no residual tumor, regardless of histology and regardless of molecular subtype |
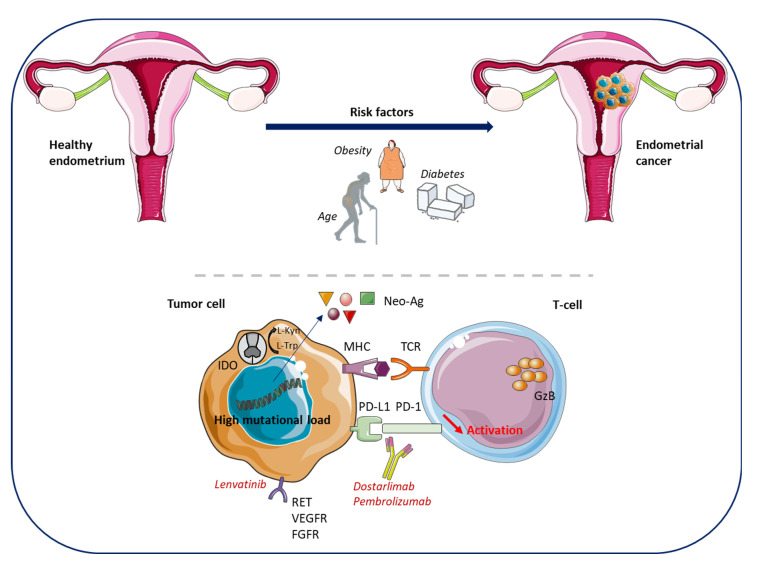
Figure 1.
Endometrial cancer (EC)—development, immune environment and approved treatments. Risk factors linked to the development of EC include obesity, type 2 diabetes, prior breast or ovarian cancer, and age. The POLEmut and the MSI-H subtypes of EC have a high mutational load resulting in tumor-specific neo-antigen production, favoring cytotoxic T cell infiltration within tumors. EC cells are known to express IDO that converts L-Trp in L-Kyn, driving regulatory T cells’ differentiation and inhibiting CD8pos and NK cells. EC cells express high levels of RET, VEGFR, and FGFR that can be targeted with Lenvatinib. EC cells also express PD-L1 and the use of Dostarlimab or Pembrolizumab, permitting the reactivation of the immune system and the anti-tumor response. Ag, antigens; EC, endometrial cancer; FGFR, fibroblast growth factor receptor; GzB, granzyme B; IDO, indoleamine 2,3-dioxygenase; Kyn, kynurenine; MSI-H, microsatellite instability hypermutated; POLEmut, polymerase ε mutation; RET, rearranged during transfection (tyrosine kinase receptor); Trp, tryptophan; VEGFR, vascular endothelial growth factor. The figure was drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ (accessed on 2 September 2022)).
In the first-line setting, treatment regimen is based on hysterectomy, with or without adjuvant radiotherapy (RT), or taxane-platinum CT, or hormonotherapy. In advanced or recurrent EC not eligible for tumor resection, no established standard of care in the second-line setting currently exists. CT in this patient population has limited success, and its use particularly faces constraints related to fertility preservation.
The clinical trials Keynote-158 and -146, and more specifically the phase III Keynote-775 trials, prompted the Food and Drug Administration (FDA), in 2019, to approve the combination of the anti-PD-1 pembrolizumab (Keytruda®) with the tyrosine kinase inhibitor lenvatinib (Lenvima) in patients with progressive EC after prior systemic therapy and patients not eligible for RT or curative surgery not identified with tumors with microsatellite instability hypermutated (MSI-H) or with deficient mismatch repair (dMMR) (Figure 1) [15,16,17,18]. The European Medicines Agency (EMA) approved, in 2021, the combined therapy in patients with progressive EC during or following CT and patients not eligible for curative surgery or RT. The GARNET trial investigating dostarlimab (Jemperli) in patients not responding to CT led the FDA and the EMA to approve dostarlimab as a monotherapy in 2021 in patients with MSI-H/dMMR tumors (Figure 1) [19,20,21], and further progress in the management of patients with EC is expected with innovative treatments. However, the prognosis of these patients with advanced/metastatic, progressive, or relapsing EC after at least one prior systemic treatment is poor. Hence, the development of new innovative treatments and better identification of the patients who are more likely to respond to dedicated treatments should be encouraged.
Table 3.
FIGO staging for endometrial cancers (Koskas et al., 2021 [22]). FIGO, International Federation of Gynecology and Obstetrics grading; M, metastasis; N, lymph nodes; T, tumor; x, any number.
| FIGO Stage | TNM Category | Description |
|---|---|---|
| I | T1N0M0 | Tumor confined to the uterus, including endocervical glandular involvement |
| IA | T1aN0M0 | Tumor confined to the endometrium or invades <50% of the myometrial wall |
| IB | T1bN0M0 | Invasion ≥ 50% of the myometrium |
| II | T2N0M0 | Tumor invades cervical stroma, but does not extend beyond the uterus |
| III | T3N0-1M0 | Local and/or regional spread of the tumor |
| IIIA | T3aN0M0 | Tumor involves serosa or adnexa (direct extension or metastasis) |
| IIIB | T3bN0M0 | Tumor involves vagina or parametrial (direct extension or metastasis) |
| IIIC | T1-3N1M0 | Tumor with pelvic and/or para-aortic lymph node metastasis |
| IIIC1 | T1-3N1M0 | Tumor with positive pelvic nodes |
| IIIC2 | T1-3N1M0 | Tumor with positive para-aortic nodes with or without positive pelvic lymph nodes |
| IV | Tumor invades bladder and/or bowel mucosa and/or involves distant organs | |
| IVA | T4NxM0 | Tumor invades bladder mucosa and/or bowel mucosa |
| IVB | TxNxM1 | Distant metastasis, including intra-abdominal metastasis and/or inguinal nodes |
1.2. Cervical Cancers
Cervical cancer (CC) is the fifth most frequent and a fatal cancer in women and ranks the ninth most common cancer diagnosed worldwide [1] (Table 1). In Europe, CC is the fourth most common female cancers with an incidence rate of 7.0–14.5/100,000 cases, and the mortality rate is low at 2.0–6.1/100,000 cases [1]. However, the incidence rate greatly varies according to socioeconomic status and a higher incidence (18.8% vs. 11.3%) and mortality (12.4% vs. 5.2%) is reported in low/medium economically developed countries [1,23]. Currently, thanks to advances in the understanding of the aetiopathogenesis of CC and its natural history, these tumors are considered to be a sexually transmitted disease, associated with chronic infection by various genotypes of the human papillomavirus (HPV) (Figure 2). Prior infection with HPV has been reported as a major risk factor for CC development, with 96% of the CC identified in HPV-positive patients [24,25]. More than 150 HPV genotypes have been identified, with type 16 and 18 HPV classified as the highest risk. The implementation of preventive HPV vaccination programs and large screening strategies has contributed to reduce CC incidence and mortality in developed countries [23,25]. Other risk factors for CC development include sexually transmittable co-infections, such as Human Immunodeficiency Virus (HIV), Chlamydia trachomatis, and genital Herpes Simplex Virus (HSV), but also tobacco, early age of sexual debut, multiple sexual partners, a high number of childbirth, long-term use of oral contraceptives, and age (Figure 2) [1,26,27,28]. Interestingly, seropositivity to HSV type 2, but not type 1, was shown to be associated with CC in a cohort of 8814 women [29]. The prevalence of HPV infection is relatively high; however, infection is generally fought off by the immune system. Infected individuals rarely develop chronic infections. The carcinogenic mechanism is mainly related to an increased expression of the E6 and E7 viral genes. The related proteins, E6 and E7, inactivate p53 and the retinoblastoma (Rb) gene, respectively, which are central in cell cycle regulation [30]. Their inactivation leads to genomic instability which translates into an appearance of dysplasia with a peak of incidence maximum at 10 years after viral exposition, and neoplastic progression may occur 15 to 20 years later. Cell-mediated immunity via antigen-presenting cells (APCs) is responsible for the activation of the immune response against infections and contribute to the clearance or persistence of the infectious process [31]. However, several ways of evading the immune system exist such as the increased PD-L1 expression, and the downregulation of the major histocompatibility complex (MHC) on the surface of APCs induced by the interaction with the viral protein E5 (Figure 2) [32]. Therefore, this interaction between the virus and the immune system supports the rationale for immunotherapy and therapeutic vaccines in CC.
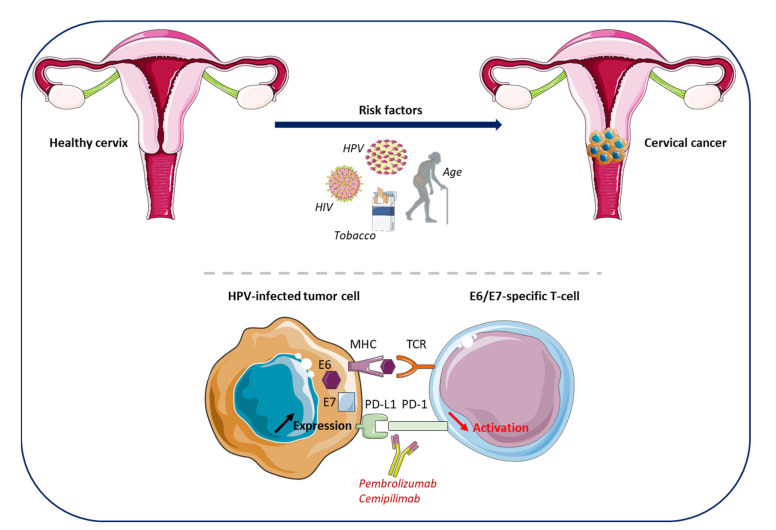
Figure 2.
Cervical cancer (CC)—development, immune environment and approved treatments. HPV represents the major risk factor for CC development. Other risk factors include sexually transmittable co-infections such as HIV, Chlamydia trachomatis or genital HSV, tobacco, early age of sexual debut, multiple sexual partners, a high number of childbirths, long-term use of oral contraceptives, and age. HPV infection is highly prevalent but is eradicated in most cases by the immune system. However, a small percentage of women will develop chronic infections, resulting in the development of cancer cells that evade the immune system and, thus, leading to tumor development. One mechanism of HPV-infected cells to overcome the immune response is an increase of the PD-L1 expression at their cell surface, resulting in the inhibition of the T-lymphocyte activation through the interaction with PD-1. This inhibition can be reversed using anti-PD-1 antibodies (Pembrolizumab, Cemipilimab). CC, cervical cancer; HIV, Human Immunodeficiency Virus; HPV, Human Papilloma Virus; HSV, Herpes Simplex Virus; PD-1, programmed cell death protein 1. The figure was drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ (accessed on 2 September 2022)).
Prognostic factors for CC include the stage of the tumor, lymph node status, and histology [33] and disparities are found according to race/ethnicity [34]. Cervical adenocarcinoma and adenosquamous carcinoma, accounting for 20% and 4% of all CCs, respectively, are generally associated with poorer prognosis in patients with locally advanced stages (defined according to the International Federation of Gynecology and Obstetrics grading (FIGO) II/III (Table 4)) when compared with the major representative class of squamous cell carcinoma, which accounts for 70% of CCs. Importantly, in patients having received concurrent chemoradiotherapy (CRT), the worst survival rate is reported in patients with cervical adenocarcinoma compared with adenosquamous patients (HR 1.14, 95%CI 1.03–1.27). Considering lymph node involvement, the 5-year survival rate in stages IB–IIA CC with and without lymph node involvement are, respectively, 51–78% and 88–95% (Table 1) [4,35]. More advanced stages showed more reduced survival rates.
Table 4.
FIGO staging for cervical cancers (Cibula et al., 2018 [36]). FIGO, International Federation of Gynecology and Obstetrics grading; T, tumor; x, any number. * Pelvic sidewall is defined as the muscle, fascia, neurovascular structures, and skeletal portions of the bony pelvis.
| FIGO Stage | T Category | Description |
|---|---|---|
| Tx | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| I | T1 | Cervical carcinoma confined to the uterus (extension to corpus should be disregarded) |
| IA | T1a | Invasive carcinoma diagnosed only by microscope. Stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less; vascular space involvement, venous or lymphatic, does not affect classification |
| IA1 | T1a1 | Measured stromal invasion of 3.0 mm or less in depth and 7.0 mm or less in horizontal spread |
| IA2 | T1a2 | Measured stromal invasion of more than 3.0 mm and not more than 5.0 mm, with a horizontal spread of 7.0 mm or less |
| IB | T1b | Clinically visible lesion confined to the cervix or microscopic lesion greater than IA2 (T1a2). Includes all macroscopically visible lesions, even those with superficial invasion |
| IB1 | T1b1 | Clinically visible lesion 4.0 cm or less in greatest dimension |
| IB2 | T1b2 | Clinically visible lesion more than 4.0 cm or less in greatest dimension |
| II | T2 | Cervical carcinoma invading beyond the uterus but not to the pelvic wall or to lower third of the vagina |
| IIA | T2a | Tumor without parametrial invasion |
| IIA1 | T2a1 | Clinically visible lesion 4.0 cm or less in greatest dimension |
| IIA2 | T2a2 | Clinically visible lesion more than 4.0 cm in greatest dimension |
| IIB | T2b | Tumor with parametrial invasion |
| III | T3 | Tumor extending to the pelvic sidewall * and/or involving the lower third of the vagina and/or causing hydronephrosis or non-functioning kidney |
| IIIA | T3a | Tumor involving the lower third of the vagina but not extending to the pelvic wall |
| IIIB | T3b | Tumor extending to the pelvic wall and/or causing hydronephrosis or nonfunctioning kidney |
| IVA | T4 | Tumor invading the mucosa of the bladder or rectum and/or extending beyond the true pelvis (bullous edema is not sufficient to classify a tumor as T4) |
| IVB | Tumor invading distant organs |
The treatment of CC patients varies according to the extent of the tumor. In early stages, conization or hysterectomy are recommended [36]. Adjuvant RT or CRT is considered in the presence of combined risk factors after primary radical surgery when lymph nodes are invaded and positive surgical margins are detected. In locally advanced tumors, the combination of CRT with cisplatin or carboplatin remains the standard of care in patients with a poorer health status [37]. Interestingly, in advanced stages, the phase III trial GOG-204 did not show differences in patients treated with cisplatin combined with paclitaxel, topotecan, gemcitabine, or vinorelbine [38]. Considering the high overall response rate (ORR) (29.1%), median progression-free survival (PFS) (5.8 months), median OS (12.8 months), and a favorable toxicity profile, the combination of cisplatin with paclitaxel is the preferred option in the first-line setting in patients with advanced/metastatic CC not eligible for surgery or RT. The addition of bevacizumab to standard CT is recommended for patients with a good performance status and recurrent, persistent, or metastatic CC [36,39,40,41]. Nevertheless, their outcomes remain poor with a 5-year recurrence rate between 28 to 73.6% for stages IIB-IVB, and treatment options are very limited [42]. Moreover, several side effects are reported with CT, especially in elderly patients, and no alternative therapy after two lines of CT is available.
Some recent advances in immunotherapy, resulting from the trials Keynote-826 and EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9, led to the approval of two new drugs: pembrolizumab and the anti-PD-1 cemipilimab (Libtayo) (Figure 2) [43,44]. The FDA approved pembrolizumab in the first-line alone or in association with CT, with or without bevacizumab, in PD-L1 positive CC with persistent, recurrent, or first-line metastatic disease and who had never received CT [45]. Both the FDA and EMA approved cemipilimab in patients with persistent, recurrent, or metastatic CC regardless of their PD-L1 status, and in case of disease progression during or after platinum-based CT [46]. Other trials are underway to further investigate new therapeutic strategies based on the recently demonstrated sensitivity of CC to immunotherapy and to identify biomarkers predictive for a treatment response.
2. The Immune Microenvironment of Endometrial Cancers and Immunotherapy-Based Clinical Trials
The TME includes immune cells, stromal cells, blood cells, and extracellular matrix, playing an active role in tumor development [47]. TME composition differs between tumor types. Innate and adaptive mechanisms are continuously involved in eliminating cancer cells. Therapeutic agents, ICIs or other molecules, can induce and potentiate immunosurveillance through increased tumor antigen (Ag) presentation and T-cell activity, or target the TME through the inhibition of immune mechanisms that promote tumor growth. In several cancer types, immunotherapy has improved patients’ survival [48,49,50,51]. With the exception of platinum-sensitivity, gynecological cancers do not substantially benefit from usual CT regimens. Despite the emergence of new therapies, both incidence and mortality are increasing in patients with EC. As recent advances in the management of EC disease arose from immunotherapy, new therapeutic strategies targeting the immune system need to be identified to improve patients’ survival and better adapt treatment according to tumor phenotypes. To this end, better identification of biomarkers to monitor treatment response is required. We provide an overview of the immune microenvironment in EC and present the main trials investigating immunotherapy and their respective objectives.
2.1. Immune Environment of Endometrial Cancers
Endometrial tissue contains numerous leukocytes varying in number and phenotype throughout the menstrual cycle [52]. Leukocytes are more abundant before menstruation, probably in relation to the immune protection required during endometrial disruption. Therefore, tumor immune response may be specifically enhanced in EC cells. A high expression of immune checkpoints (ICP) and their ligands have been reported in ECs, but expression levels vary according to studies and patient cohorts, tumor grade, histology, or mutation status. EC can be classified into four distinct molecular subtypes linked to clinicopathological features [5]: (i) pathogenic polymerase ε (POLE) variants (POLEmut) [53], (ii) dMMR or MSI-H tumors, (iii) p53-mutant (p53-mut), and (iv) no specific molecular profile (NSMP) (Table 2). About 30% of ECs are MSI-H [54]. Therefore, EC represents the most frequent cancer with this dMMR status.
The POLEmut and the MSI-H subtypes show a high mutational load and are thus associated with a higher immunogenic profile, with more tumor-specific neo-Ag resulting in CD4pos and CD8pos tumor infiltration. Several studies have showed that these subtypes are characterized by an increase of PD-1pos, PD-L1pos and CD8pos T cells, activated CD8pos T cells (GranzymeBpos), and overexpress genes involved in their cytotoxic functions (T-bet, Eomes, interferon γ (IFN-γ), perforin) and exhaustion (LAG-3, Tim-3, TIGIT) [55,56,57,58]. Regardless of EC molecular subtype, the number of intratumoral CD8pos T cells correlates with a better OS and CD8pos/FOXP3pos ratio with disease-free survival [59,60]. Interestingly, Tumeh et al., suggested that tumor regression following anti-PD1 therapy requires pre-existing CD8pos T cells [61]. However, the presence of FOXP3pos regulatory T cells (Tregs) impacts the prognosis of patients but not OS [62]. In line with the approval of pembrolizumab for all EC and dostarlimab for MSI-H EC, the MSI-H status may be used as a biomarker of response to ICIs [63]. A recent meta-analysis showed that PD-L1 expression is significantly associated with advanced tumor stages and that PD-L1 overexpression in immune cells, but not tumor cells, correlates with poorer survival, suggesting its use as a biomarker to stratify patients eligible for anti-PD1/L1 therapy [64]. Therefore, other therapeutic options and biomarkers need to be provided in patients with tumors not sensitive to anti-PD-1 treatment or MSS.
The immune modulatory molecule indoleamine 2,3-dioxygenase 1 (IDO1) involved in EC, converts L-tryptophan into the immunosuppressive metabolite L-kynurenine, that can promote Tregs cell differentiation, induce tolerogenic dendritic cells (DC), and myeloid-derived suppressor cells (MDSC) (Figure 2) [65]. High levels of IDO1 in EC are associated with disease progression and impaired clinical outcome [66]. IDO (1 and/or 2) expression in EC correlates with the number of nodal metastases, low number of CD8pos T cells and natural killer (NK) cells within the tumor, and decreased PFS [66,67,68]. In 2018, it was shown that the majority of EC cells that express IDO also express PD-L1 [69]. IDO is mostly expressed in MSI-H EC but also in a subset of proficient MMR (pMMR) patients, highlighting that anti-IDO therapy may be an option for non MSI-H EC patients.
Lymphocyte activation gene-3 (LAG-3), also known as CD223, is an ICP participating in the immune escape of tumors. LAG-3 is expressed by activated T cells, NK cells, B cells, and DC and binds to the MHC class II (MHC-II) or to the galectin-3 (Gal-3) expressed by tumor cells. The binding of LAG-3 and its ligands increase intratumoral T cells, the inhibition of CD4pos T cell activation and CD8pos T cell cytotoxic functions, and tumor cell evasion of apoptosis [70,71,72]. In EC, around 30% of the tumor cells and 25% of the immune cells express this ICP with a prevalent expression in POLEmut and dMMR tumors [71,72]. Interestingly, LAG-3 expression is correlated with Gal-3 in EC, predominantly in dMMR EC tumors [71]. The blockade of LAG-3, therefore, represents an interesting strategy in EC, but a better characterization of the population more likely to benefit from this therapeutic approach needs to be clearly defined. In addition, the inhibition of the CD8pos T cells’ antitumor response differs from LAG-3 and PD-1 related responses, suggesting that a combined inhibitory strategy could enhance antitumor immune responses in EC. In line with this hypothesis, the RELATIVITY-047 phase II/III clinical trial demonstrated a benefit of anti-LAG-3 combined with anti-PD-1 in melanoma patients, allowing the FDA’s approval of the combined treatment with relatlimab and nivolumab [73].
Altogether, these findings laid the groundwork to further investigate immunotherapy in EC. Therefore, several trials have been carried out to evaluate the efficacy of ICIs, alone or combined to other targeted therapeutics, but detailed mechanisms of action still need to be precised and a better identification of eligible patients needs to be performed.
2.2. Immunotherapy in Endometrial Cancers: Recent and Ongoing Trials (Table 5)
Recent investigations with immunotherapy, especially anti-PD1/L1, have been conducted in patients with EC, and response is associated with higher rates of tumor-infiltrating lymphocytes (TILs) in patients with MSI-H EC. Herein, we will present recently completed and ongoing trials investigating immunotherapy alone or in combination in EC. Other studies investigating targeted therapies such as the cyclin-dependent PI3K, WEE1, and HER2 inhibitors have been performed; however, these approaches are not presented here.
Table 5.
Ongoing clinical trials testing immunotherapies alone or in combination in endometrial cancer. DC, dendritic cells; EC, endometrial cancer; FGFR, fibroblast growth factor receptor; FRα, folate receptor α; HER2, Human Epidermal Receptor-2; IDO1, Indoleamine 2,3-dioxygenase; MDSC, myeloid-derived suppressor cells; ORR, overall response rate; OS, overall survival; PARPi, Poly(ADP-ribose) polymerase inhibitor; PFS, progression-free survival; TAM, tumor-associated macrophages.
| Clinical Trial | Phase | Condition or Disease | Number of Patients | Drugs Combination | Mechanism of Action | Primary Endpoint (Time Frame) | Status |
|---|---|---|---|---|---|---|---|
| First-line treatment | |||||||
| DOMENICA (NCT05201547) [74] | III | dMMR (IIIC2/IV or first recurrent EC without curative treatment by RT, CT or surgery) | 142 | Dostarlimab vs. Chemotherapy | Anti-PD-1 (dostarlimab) | PFS (5 years) | Recruiting |
| AtTEND (NCT03603184) [75] |
III | EC with residual disease after surgery or inoperable FIGO III–IV | 550 | Atezolizumab or placebo + taxane platinum-based CT | Anti-PD-L1 (atezolizumab) | OS and PFS (2 years) | Active, not recruiting |
| RUBY (NCT03981796) [76] |
III | FIGO III-IV or first recurrent EC | 785 | Dostarlimab (or placebo) + taxane platinum-based CT followed by dostarlimab or niraparib (or placebo) | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Investigator assessed PFS and OS (6 years) |
Active, not recruiting |
| EnGOT-en9 (NCT03884101) [77] | III | First-line treatment of FIGO stage III, IVA, IVB or recurrent EC | 875 | Pembrolizumab+ lenvatinib vs. taxane platinum-based CT |
Anti-PD-1 (pembrolizumab) and protein kinase inhibitor (lenvatinib) | PFS (31 months) and OS (45 months) | Active, not recruiting |
| Advanced and recurrent EC | |||||||
| NRG-GY018 (NCT03914612) [78] |
III | FIGO stage III, IVA, IVB or recurrent EC | 810 | Pembrolizumab or placebo + taxane platinum-based CT | Anti-PD-1 (pembrolizumab) | PFS (5 years) | Active, not recruiting |
| GYNET (NCT04652076) [79] | I/II | Pretreated advanced EC | 240 | Netrin-1 mAbs (NP137) + Carboplatin Plus Paclitaxel and/or Pembrolizumab | Anti-PD-1 (pembrolizumab) and anti-netrin-1 (NP137) | Dose limiting toxicity occurrence, ORR (12 weeks up to 2 years) |
Recruiting |
| NCT04885413 [80] | II | Recurrent/advanced EC | 37 | Niraparib + sintilimab | PARPi (niraparib) and anti-PD-1 (sintilimab) | ORR (6 months) | Recruiting |
| NCT02912572 [81] | II | Recurrent or persistent EC MSI-H and/or POLEmut, MSS | 105 | Avelumab or Avelumab and Talazoparib or Avelumab and Axitinib | Anti-PD-L1 (avelumab) and anti-HER2 (talazoparib) and tyrosine kinase inhibitor (axitinib) | Activity of avelumab + talazoparib in EC, activity of avelumab + axitinib in MSS patients assessed by frequency of patients with PFS > 6 months or with objective tumor response (2 years) | Recruiting |
| NCT05036681 [82] | II | Metastatic EC not amenable to surgery or RT MSS | 30 | Futibatinib and Pembrolizumab | FGFR1-4 (futibatinib) and anti-PD-1 (pembrolizumab) | ORR, safety, and tolerability (1 year) | Recruiting |
| POD1UM-204 (NCT04463771) [83] | II | Advanced or metastatic EC with CT progression | 300 | Retifanlimab +/− epacadostat or pemigatinib or anti-LAG3 and anti-TIM3 | Anti-PD-1 (Retifanlimab) and anti-IDO1 (epacadostat) | ORR, PFS (2.5 years) | Recruiting |
| NCT05156268 [84] | II | Persistent/recurrent EC (including CS) | 25 | Pembrolizumab + olaparib | Anti-PD-1 (pembrolizumab) and PARPi (olaparib) | ORR (24 weeks) | Recruiting |
| NCT03526432 [85] | II | Pretreated advanced, recurrent, or persistent EC | 55 | Atezolizumab + bevacizumab | Anti-PD-L1 (atezolizumab) and anti-angiogenic (bevacizumab) | ORR (3 years) | Active, not recruiting |
| NCT03016338 [86] | II | Pretreated advanced/recurrent EC | 51 | Dostarlimab + niraparib | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Clinical benefit rate (16 weeks) | Active, not recruiting |
| DOMEC (NCT03951415) [87] | II | Recurrent or persistent EC | 55 | Olaparib + Durvalumab | Anti-PD-L1 (durvalumab) and PARPi (olaparib) | PFS (6 months) | Active, not recruiting |
| DUO-E (NCT04269200) [88] | III | After first-line treatment of advanced/recurrent EC | 699 | Taxan platinum-based CT and durvalumab followed by placebo vs. durvalumab vs. durvalumab and olaparib | Anti-PD-L1 (durvalumab) and PARPi (olaparib) | PFS (4 years) | Recruiting |
| Recurrent/relapsed/advanced EC dMMR and/or MSI-H only | |||||||
| CAN-RESPOND (NCT05550558) [89] | II | Recurrent sporadic dMMR EC | 43 | Camrelizumab + anlotinib | Anti-PD-1 (camrelizumab) and multitarget tyrosine kinase inhibitor (anlotinib) | ORR (24 months) | Not yet recruiting |
| NCT05419817 [90] | II | Recurrent EC and other solid tumors dMMR post PD1 exposure | 30 | Pembrolizumab + Sitravatinib | Anti-PD-1 (pembrolizumab) and receptor tyrosine kinases inhibitor (Sitravatinib) | ORR (12 weeks) | Recruiting |
| NCT05112601 [91] | II | Recurrent dMMR | 12 | Ipilimumab + Nivolumab vs. nivolumab | Anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) | PFS (5 years) | Recruiting |
| Recurrent/relapsed/advanced EC MSS only | |||||||
| IMGN853 (NCT03835819) [92] | II | Advanced or recurrent serous EC, MSS, FRαpos | 35 | Mirvetuximab Soravtansine (IMGN853) and Pembrolizumab | Anti-FRα (Mirvetuximab soravtansine) and anti-PD-1 (pembrolizumab) | ORR, PFS (6 months) | Recruiting |
2.2.1. Anti-PD1/PD-L1
The rationale for the use of antibodies blocking PD-1 or PD-L1 is supported by results from several trials that have tested their efficacy to improve treatment response, or better identify specific drugs likely to benefit dedicated patients, based on efficacy and tolerance. The phase II study JapicCTI-163212 enrolled 64 patients with EC, CC, and soft-tissue sarcoma and tested the clinical efficacy of the anti-PD-1 nivolumab [93]. Patients with EC presented an ORR of 22.7%, a median PFS of 3.4 months, and a 12-month OS of 48.5%. No differences in PD-L1 expression were observed in EC patients. EC patients with the MSI-H phenotype all achieved partial responses. This study, therefore, suggests that MSI-H status may be used as a predictive factor to select patients more likely to respond to nivolumab. Whether the anti-PD-1 nivolumab can be used such as pembrolizumab to treat a patient with EC still needs to be evidenced.
In pretreated patients, the anti-PD-L1 atezolizumab showed an ORR of 13% in a phase I study (NCT01375842) [94]. A phase II study showed that the PD-L1 inhibitor avelumab exhibited promising activity in dMMR EC patients, regardless of PD-L1 level, highlighting that molecular characterization is more useful than PD-L1 expression analysis for patient selection [95]. The phase II trial PHAEDRA (ANZGOG1601) enrolled 71 patients with advanced EC, including 36 with dMMR and 35 with pMMR [96]. In this study, the anti-PD-L1 durvalumab showed promising activity and manageable safety in dMMR patients regardless of prior lines of CT.
The phase III trial RUBY (NCT03981796) recently reported, for the first time, a significant clinical benefit with dostarlimab combined with standard-of-care CT in patients with FIGO III-IV or first recurrent EC patients [76,97]. Moreover, a favorable trend in survival was observed in all patient regardless of molecular subtype (dMMR/MSI-H and pMMR/MSS).
Several studies are currently investigating ICI in monotherapy. First, the phase III trial DOMENICA (NCT05201547) aims to assess PFS with dostarlimab vs. CT in 142 dMMR/MSI-H patients with FIGO stages IIIC2/IV in first-line advanced or first recurrence settings [74]. Secondly, the trial NRG-GY018 (NCT03914612) investigates the efficacy of pembrolizumab alone, in comparison to taxane platinum-based CT in 810 patients who have not previously received first-line CT [78]. In addition, the phase III trial AtTEND (NCT03603184) [75] has planned to enroll, in the first-line setting, 550 EC patients with residual diseases after surgery or inoperable FIGO III-IV stages and to allocate them to receive atezolizumab versus a placebo combined with platinum-based doublet and to assess whether atezolizumab combined with CT is beneficial to patients.
2.2.2. ICI and Anti-Angiogenic/Tyrosine Kinase Inhibitors
Lenvatininb is a kinase inhibitor with anti-angiogenic features already approved in the treatment of EC patients, along with pembrolizumab. Several trials have investigated the combination of these inhibitors with ICI. The phase II trial NCT03367741 showed that the combination of nivolumab and cabozantinib is beneficial to patients with recurrent ECs, treated with 3 or more prior regimens [98]. The inhibitor of multiple tyrosine kinases cabozantinib targeting the Met/Hepatocyte Growth Factor Receptor (HGFR), the AXL receptor tyrosine kinase, and the Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) showed a median PFS of 5.3 months with the combination therapy vs. 1.9 months with cabozantinib alone. Among patients treated with immunotherapy, non-progressive patients had proportions of activated tissue-resident γδ T cells significantly higher than progressive patients. The combination of these inhibitors with immunotherapy may be beneficial to patients having received prior treatments. A phase II trial (NCT02912572) is currently evaluating the combination of avelumab with the multikinase inhibitor of VEGFR-1, -2, and -3 and the Platelet Derived Growth Factor Receptor (PDGRF) axitinib in EC patients with a recurrent or persistent disease. The phase III trial EnGOT-en9/LEAP-001 (NCT03884101) has planned to enroll 875 patients with FIGO stage III, IVA, IVB, or recurrent EC and will evaluate PFS and OS in first-line treatment with pembrolizumab and lenvatinib vs. taxane platinum-based CT [77]. Interestingly, the phase II trial CAN-RESPOND aims to evaluate the ORR in 43 patients with a relapsing dMMR EC treated with anti-PD-1 camrelizumab combined with the multitarget tyrosine kinase inhibitor anlotinib [89]. In the patients having received prior anti-PD-1 therapy, the phase II trial (NCT05419817) will assess the ORR after the administration of pembrolizumab along with the tyrosine kinase inhibitor sitravatinib [90].
2.2.3. ICI and Polymerase Inhibitors
The outcomes and safety of the combination of avelumab with the polyadenosine diphosphate-ribose polymerase (PARP) inhibitor (PARPi) talazoparib were assessed in a phase II trial that enrolled 35 recurrent pMMR EC patients [81]. Four patients had partial responses and eight were still progression-free at 6 months. While homologous recombination deficiency (HRD) was associated with clinical benefits and better PFS, tumor mutation burden, TILs, and PD-1 status were not identified as predictive factors. Post-hoc analyses showed that patients treated with this combination, who were at a progression-free interval (PFI) at 6 months or longer had clinical benefits. Avelumab with talazoparib can be further considered for recurrent pMMR patients with EC.
The phase II trial (NCT02912572) is currently recruiting patients to evaluate the efficacy of the combinations (i) avelumab and talazoparib or (ii) avelumab with axitinib in recurrent/persistent EC or MSS-recurrent/persistent EC patients, respectively. The trial assessed the rate of patients with a PFS above 6 months or with objective tumor response in a time frame of 2 years. This trial will also evaluate the duration of OS, PFS, and immune-related objective response, giving insights into the rationale of using this combination in MSS patients in need for new treatment options and better identifying and characterizing patients in failure with this regimen.
The phase III trial RUBY (NCT03981796), which has already reported clear benefits for EC treated with dostarlimab and taxane platinum-based CT, will evaluate maintenance with dostarlimab or niraparib [76]. Another ongoing phase III trial is DUO-E (NCT04269200), which will evaluate, on 699 patients with advanced/recurrent EC, the PFS after the combination of durvalumab with olaparib in the first-line regimen [88]. The phase II trial DOMEC (NCT03951415) has already recruited 55 patients with recurrent/persistent EC treated with olaparib and durvalumab [87]. Besides PFS, secondary endpoints include the evaluations of ORR, OS, and the use of tumor biopsies for the quantification of predictive biomarkers including dMMR/POLE status, HRD status, and several immune factors (CD3, CD4, CD8, CD103, PD-1, CD161, LAG3, CTLA-4, NKG2A, FOXP3, NK, and PD-L1) on myeloid and tumor cells, and describe myeloid infiltration (CD68, CD14, CD33, and CD163). Other trials currently evaluating the combination of PARPi with immunotherapy are listed in Table 5.
2.2.4. ICI and Other Targeted Therapies
The innovative phase I/II trial GYNET (NCT04652076) [79] has planned to recruit over 240 patients with CC and EC. The aim is to evaluate the efficacy of restoring apoptosis in tumor cells, using the first-in-class humanized monoclonal antibody that blocks Netrin-1 (NP137) alone or combined with an anti-PD-1 [99]. Netrin-1 is a secreted protein involved in axon guidance, bone remodeling, and metabolic homeostasis. Used as a tumor biomarker, Netrin-1 is highly expressed in cancers, and especially in aggressive cancers. Netrin-1 expression was assumed to favor tumorigenesis, while promoting epithelial to mesenchymal transition (EMT) [100,101].
The phase II trial POD1UM-204 (NCT04463771) is an umbrella study investigating the anti-PD-1 retifanlimab in patients with advanced or metastatic EC who progressed during or after taxane platinum-based CT [83]. Monotherapy will be evaluated in patients with PD-L1neg, MSI-H, or dMMR/POLEmut EC and retifanlimab combined with an anti-FGFR2 and will be investigated in patients with mutated ECs or combined with an anti-IDO1 molecule in MSS and PD-L1pos EC patients. The role of IDO-1 in EC was already mentioned. Fibroblast growth factor receptor (FGFR) plays a role in angiogenesis, and FGFR2 mutations found in around 15% of EC leads to FGFR overexpression and favors the proliferation of tumor cells and metastasis [102,103]. Shorter disease-free survival and OS are observed in these patients, especially at an early stage, owing to the lack of treatment options. Therefore, the trial POD1UM-204 will support the management of EC patients through a better identification of EC patients that are potential responders to anti-FGFR2 therapy and will determine if patients with a non MSI-H EC benefit from the inhibition of IDO-1. In MSI-H patients progressive after anti-PD-L1 treatment, the POD1UM-204 trial has proposed an additional treatment with anti-TIM3/LAG-3. OS and PFS will be assessed in all groups of patients. Another phase II (NCT05036681) study will aim at testing the inhibition of FGFR along with anti-PD-1 in metastatic EC patients with an MSS profile who are not candidates for surgery [82].
Other ICI combinations will be evaluated in a subset of EC patients with recurrent dMMR who will receive nivolumab with or without ipilimumab (NCT05112601, phase II trial) [91].
Folate receptors (FR) contribute to cell proliferation via the transportation of folate vitamins across cell membranes. The major subunit FR-α is highly expressed in gynecological cancers. The FR-α expression in EC is significantly higher than in endometrial hyperplasia or normal endometrium [104]. The phase II IMGN853 trial (NCT03835819) will evaluate the ORR and PFS in patients with advanced/recurrent MSS EC and who are FR-α positive, following the administration of the antibody-drug conjugate (ADC) mirvetuximab soravtansine (IMGN853), along with anti-PD-1 [92].
3. The Immune Microenvironment of Cervical Cancers and Immunotherapy-Based Clinical Trials
3.1. Immune Environment of Cervical Cancers
Almost all the patients with CC are HPV-positive [24]. The most frequent HPV detected in CC samples is HPV16 and HPV18, encoding for E6 and E7 proteins known as tumor drivers by suppressing p53 and Rb genes, respectively, and both participating in immune evasion through an increased PD-L1 expression and induction of an immune tolerant environment [105,106,107]. In addition, inhibition of p53 and Rb preventing normal cell-cycle control and apoptosis leads to the proliferation of HPV-infected damaged cells [30]. HPV infection drives a specific immune response with the proliferation of E6- and E7-specific T cells. However, in CC patients, this immune response is impaired and highlights the need to boost the immune system by ICI combined or not with other targeted therapies.
In 2007, Piersma et al., evaluated the CD8pos/FOXP3pos ratio in the CC tissue and blood of patients before a hysterectomy [108]. The authors showed a ratio in patients with localized tumors higher than in metastatic tumors, suggesting its use as a prognostic factor in CC patients. Immunohistochemistry (IHC) analysis in CC tissues—but not in blood samples—from treatment-naïve patients showed that CD8pos/FOXP3pos combined with the presence of type 1 macrophages is a significant independent favorable prognostic factor [109,110]. Moreover, a high ratio was associated with more favorable clinical outcomes in patients with CC after neoadjuvant CT [111]. The 5-year survival rate was reported as significantly lower in CC patients presenting a high Treg tumor-infiltration [112].
Another series of 21 patients with CC showed that 11 tumors responding to CT showed an inflammatory status higher than non-responding tumors (N = 10) [113]. Additionally, patients in response presented a higher CD8pos lymphocyte tumor-infiltrate rate, and higher PD-1, -L1, and -L2 levels as well as higher mutation rates in PIK3CA and KDR/VEGFR. Another study, conducted on 20 patients with CC, reported that tumor samples had decreased CD3pos, CD4pos, Tregs, and CD8pos T cells during the first week of CRT [114]. After 3 to 5 weeks of treatment, CD4pos and CD8pos T cells presented an activated phenotype. In corresponding blood samples, very few activated T cells were observed.
The presence of the tumor-associated antigens E6 and E7 proteins, the high mutational load, and/or the presence of immune infiltrate, provides new promising approaches to identify predictive favorable factors for a response with immunotherapy. Some studies reported that prior CT may change TME in patients with CC through increased infiltration of several immune-related cells, favoring TME sensitivity to ICI [115]. Targeting the immune system gives rise to new opportunities to improving outcomes in patients with CC.
3.2. Immunotherapy in Cervical Cancers: Recent and Ongoing Trials (Table 6)
The standard of care relies on different factors, including tumor extension, and multidisciplinary approaches with RT, CT, and/or surgical resection are usually required. Immunotherapy has drastically changed the treatment of many cancers, and its critical role is also expected for the future management in patients with CC.
Table 6.
Ongoing clinical trials testing immunotherapies alone or in combination in cervical cancer. CC, cervical cancer; CT, chemotherapy; CRT, chemoradiotherapy; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
| Clinical Trial | Phase | Condition or Disease | Number of Patients | Drugs Combination | Mechanism of Tion | Primary Endpoint (Time Frame) | Status |
|---|---|---|---|---|---|---|---|
| First-line treatment | |||||||
| NCT05311566 [116] | II | FIGO IB2-IIIB | 92 | Camrelizumab plus concurrent CRT | Anti-PD-1 (camrelizumab) | OS (3 years) | Recruiting |
| NCT04974944 [117] | II | FIGO IVB, Recurrent or Persistent | 172 | Camrelizumab and apatinib vs. paclitaxel and cisplatin and carboplatin and bevacizumab | Anti-PD-1 (camrelizumab) and anti-VEGFR2 (apatinib) | PFS (36 months) | Recruiting |
| NCT05511623 [118] | II | FIGO IIIC2 | 112 | Tislelizumab and concurrent CRT | Anti-PD-1 (tislelizumab) | PFS and side effects (3 years) | Not yet recruiting |
| FERMATA (NCT03912415) [119] |
III | FIGO IVB | 316 | BCD-100 + CT +/− bevacizumab | Anti-PD-1 (BCD-100) | OS (3 years) | Recruiting |
| BEATcc (NCT03556839) [120] |
III | FIGO IVB | 404 | Atezolizumab + CT+ bevacizumab | Anti-PD-L1 (atezolizumab) | PFS and OS (48 months) | Active, not recruiting |
| NCT04982237 [121] | III | FIGO IVB | 440 | Cadonilimab + CT +/− bevacizumab | Anti-PD-1/CTLA-4 bispecific antibody (Cadonilimab, AK104) | PFS and OS (2 years) | Recruiting |
| NCT04973904 [122] | II | Recurrent, Refractory and Metastatic CC | 35 | Toripalimab + paclitaxel + cisplatin + bevacizumab | Anti-PD-1 (toripalimab) | ORR (1 year) | Not yet recruiting |
| Recurrent/persistent/metastatic CC | |||||||
| ITTACC (NCT05614453) [123] | II | After Platinum-Based CT | 57 | Sitravatinib Tislelizumab |
Tyrosine kinase inhibitor (sitravatinib) and anti-PD-1 (tislelizumab) | ORR (2 years) | Not yet recruiting |
| NCT03108495 [124] | II | Recurrent, metastatic, or persistent CC | 189 | LN-145 and LN-145 + Pembrolizumab | Autologous tumor infiltrating lymphocytes (TIL) infusion (LN-145) followed by IL-2 | ORR and AE (60 months) | Recruiting |
| NCT04646005 [125] | II | Recurrent/Metastatic HPV16 CC | 105 | Cemiplimab + ISA101b vaccine | Anti-PD-1 (cemipilimab) | ORR (36 months) | Recruiting |
| NCT05247619 [126] | II | Recurrent, metastatic, or persistent CC | 49 | Tislelizumab/Bevacizumab/Paclitaxel/Cisplatin/Carboplatin | Anti-PD-1 (tislelizumab) | Median PFS (24 months) | Recruiting |
| NCT05033132 [127] | II | Advanced CC | 177 | Balstilimab or balstilimab + Zalifrelimab | Anti-PD-L1 (balstilimab) and anti-CTLA-4 (zalifrelimab) | ORR (36 months) | Recruiting |
| ALARICE (NCT03826589) [128] | NA | Persistent or recurrent CC after platinum-based CT |
23 | Avelumab and Axitinib | Anti-PD-L1 (avelumab) and tyrosine kinase inhibitor (axitinib) | ORR (2years) | Recruiting |
| NCT03808857 [129] | II | Recurrent or metastatic, PD-L1 positive who failed in platinum-based CT | 80 | GB226 | Anti-PD-1 inhibitor (GB226) | ORR (2 years) | Recruiting |
| STAR (NCT04068753) [130] | II | Recurrent or progressive CC | 66 | Dostarlimab and niraparib | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Proportion of patients with response to treatment (1 year) | Recruiting |
| NCT05446883 [131] | III | First-Line treatment of persistent, recurrent or metastatic CC | 498 | QL1706 and placebo and paclitaxel and cisplatin/carboplatin with or without bevacizumab | Anti-PD-1 and –CTLA-4 (QL1706) | PFS and OS (2 years) | Recruiting |
| ATOMICC (NCT03833479) [132] | II | Maintenance therapy for patients with high-risk locally advanced CC | 132 | TSR-042 | Anti-PD-1 (TSR-042) | PFS (30 months) | Recruiting |
| ATEZOLACC (NCT03612791) [133] | II | Locally advanced CC | 189 | Atezolizumab and RCT | Anti-PD-L1 (atezolizumab) | PFS (24 months) | Recruiting |
3.2.1. PD-1 and PD-L1 Inhibitors
The phase II trial NRG-GY002 (NCT02257528) evaluated the anti-PD-1 nivolumab in 25 patients with persistent or recurrent CC after one prior systemic CT [134]. Although the median OS was 14.5 months, the benefit from nivolumab as a monotherapy was not demonstrated; however, an acceptable safety profile was reported. Among 88% of the patients tested for PD-L1 expression, 77.3% expressed PD-L1 (combined positive score (CPS) cut-off of >1%) but no significant correlation between tumor response and PD-L1 expression was identified. The Checkmate 358 trial enrolled 19 patients with CC, including patients with vaginal/vulvar cancer to receive nivolumab [135]. With a median follow-up of 19.2 months, CC patients reached a median OS of 21.9 (95%CI 15.1-not reached (NR)) months, and the ORR was 26.3% (95%CI 9.1–51.2). The duration of response (DOR) was not reached, and CC patients treated with nivolumab reported a stabilized health status and health-related quality of life. Further trials investigating nivolumab are still required in patients with CC.
Recently, the PD-1 inhibitor balstilimab was investigated in a phase II study in 161 patients with recurrent and/or metastatic CC after one prior line of platinum-based CT [136]. The ORR was 15% (95%CI 10–21.8%) in the overall population, and 20% and 7.9% in PD-L1pos and PD-L1neg patients with CC, respectively. Response to balstilimab was reported as independent of PD-L1 expression and histology, suggesting a potential benefit in a larger population of CC patients, compared with pembrolizumab exclusively recommended in PD-L1pos CC.
The phase III trial CALLA evaluated the combination of CT with durvalumab compared to CT alone in CC patients with FIGO stages IB2 to IVA, naïve of treatment, regardless of lymph node status [137]. Unfortunately, in March 2022, the trial showed no benefit from this combination [138]. However, the GOG-3047 trial will investigate pembrolizumab with CT and RT in a similar cohort of patients [78], and will determine whether CC patients naïve of treatment, may benefit from anti-PD-1 combined with CT. In addition, the phase II trial ATEZOLACC will investigate CRT with or without atezolizumab in patients with locally advanced CC [133]. Finally, the ongoing trial NRG-GY017 is currently evaluating the combination of CT with ICI [139]. With 40 patients enrolled, the study aims to assess the administration of atezolizumab 3 weeks before and/or with CRT in CC patients with positive lymph nodes and FIGO IB2-IVA. The phase II trial ATOMICC will evaluate the role of anti-PD-1 in CC patients with a high-risk type of tumor in maintenance therapy (NCT03833479) [132].
3.2.2. CTLA-4 Inhibitors
The phase II study (NCT01693783) enrolled 42 patients with advanced CC to investigate ipilimumab in the second-line setting [140]. The ORR was 8.3%, and median PFS and median OS were 2.5 and 8.5 months, respectively. The intratumoral expression of CD3, CD4, CD8, FOXP3, IDO, and PD-L1 in pre- and post-treatment samples will be evaluated. More recently, the phase I trial GOG-8829 tested the addition of ipilimumab after standard definitive CRT in 21 CC patients with positive pelvic lymph nodes [141]. The OS and PFS were, respectively, 90% and 81% at one year. In peripheral blood, rates of PD-1posCD4pos and CD8pos T cells after CRT increased, and respective levels were maintained after ipilimumab administration. This study suggests that using immunotherapy after CRT might be a promising and well tolerated approach. Further studies are needed to see if this expression correlates with the tumor and is used as a predictive factor of treatment response.
3.2.3. ICI and Tyrosine Kinase Inhibitors
The multicenter open-label single-arm study CLAP, testing the combination of camrelizumab with the VEGFR-2 tyrosine kinase inhibitor apatinib in patients with advanced CC [142], enrolled 44 patients to receive 200 mg camrelizumab every 2 weeks and 250 mg apatinib once a day and showed an ORR of 55.6% (95%CI 40–70.4%) with 23 partial responses and 2 complete responses. This combination was considered as promising in advanced CC patients, even after prior lines of anti-angiogenic molecules or two or more lines of CT. Larger trials are needed to further investigate these encouraging results as listed in Table 6. In 2023, the tumor tissue collection from the CLAP trial further explored tumor-infiltrating lymphocytes before and after treatment [143]. Multiplex-immunofluorescence showed that CD8pos T cell density in invasive margins in responder patients was higher than in non-responders and was associated with prolonged PFS. In addition, the higher ratio of macrophages (CD68pos) to CD8pos T cells observed in non-responder patients was associated with poorer PFS. No significant results were observed with FOXP3pos regulatory T cells. This observation suggests that the localization of tumor-infiltrating CD8pos T cells might be a biomarker of treatment response.
3.2.4. ICI Combination
As reported in patients with other tumors, the combination of two immunotherapy drugs also appears to be a promising strategy in patients with CC. The phase I/II Checkmate-358, testing the combination of nivolumab and ipilimumab, enrolled 19 patients with recurrent or metastatic and virus-associated CC (including 5 patients with vulvar/vaginal cancer) and allocated them by randomization to a 24-month treatment or until progression, or unacceptable toxicity [144]. The primary endpoint, ORR, was higher in the combination arm, especially in patients who had not received any previous treatment. Responses to treatment were independent of PD-L1 status; however, significant toxicity was reported even if manageable. Another study evaluated the combination of balstilimab and the anti-CTLA-4 zalifrelimab in 155 women with recurrent and/or metastatic CC who relapsed after prior platinum-based CT [145]. The primary end-point ORR was 25.6% (95%CI 18.8–33.9) in the overall population and included 10 complete responses. The ORR was 32.8% in patients with PD-L1pos CC and 9.1% in patients with PD-L1neg CC, respectively. This promising combination showed durable clinical activity with a favorable tolerability. The ongoing phase II pilot study COLIBRI (NCT04256213) enrolled 40 patients to assess the CD8pos/FOXP3pos ratio in CC patients treated with nivolumab combined with ipilimumab before RCT [146], reporting variations in CD8pos/FOXP3pos ratio during treatment and validating its use as a biomarker for treatment response.
The humanized antibody tiragolumab targeting T-cell immunoreceptor with Ig and ITIM domains, TIGIT is an immune checkpoint inhibitor that blocks the binding of TIGIT to its ligand CD155, thereby enhancing T-cell and NK-cell anti-tumor activity. Tiragolumab is currently being investigated in the phase II trial SKYSCRAPER-04 (NCT04300647), comparing atezolizumab with or without tiragolumab in patients with metastatic and/or recurrent PD-L1pos CC [147]. The humanized antibody relatlimab targeting the immune checkpoint lymphocyte-activation gene 3 (LAG-3) is being evaluated in combination with nivolumab in a phase I/II trial in patients with CC among other tumors (NCT01968109) [148].
3.2.5. ICI, Anti-Angiogenics and Other Targets
Besides CT, other combinations involving anti-angiogenic molecules are being evaluated. The addition of bevacizumab to atezolizumab did not improve ORR in patients with advanced CC (NCT02921269) [149]. The study did not meet its primary endpoint, with none of the ten patients enrolled and having received bevacizumab 15 mg/kg and atezolizumab 1200mg q3w achieved an objective response. Ongoing trials are evaluating bevacizumab added to CT, pembrolizumab, and tisotumab vedotin, in the phase I/II trial (NCT03786081) in recurrent patients with FIGO IVB CC [150]. The ongoing phase III trial NCT05446883 aims to assess the 2-year PFS and OS following the administration of the novel dual-targeting anti-PD-1 and anti-CTLA4 ICI QL1706 combined with CT and added or not to bevacizumab in the first-line treatment of persistent/recurrent/metastatic CC patients [131].
3.2.6. Therapeutic Vaccines
The use of prophylactic HPV vaccines constitutes an important advance in the recent management of patients with CC and vaccination programs, recognized in their implementation and efficacy. Nevertheless, therapeutic vaccines are still in development. The E6 and E7 oncoproteins were expected to be used as promising targets and several novel vaccines were subsequently developed [151]. The fusion protein-based vaccine TVGV1 involving HPV16 E7 showed promising results in a mouse model, improving survival and increasing the production of HPV16 E7-specific CD8pos T cells [152].
An ongoing study aims to evaluate, in the adjuvant setting, the safety and efficacy of the fusion protein vaccine involving both E6 and E7 proteins as well as the capsid HPV16 viral protein L2, involved in HPV assembly and the infectious process (TA-CIN) (NCT02405221) [153]. Another promising strategy is the combination of vaccines with immunotherapy approaches. The ongoing phase II trial VolATIL (NCT03946358) aims to evaluate the combination of UCPVax vaccine and atezolizumab in patients with locally advanced or metastatic HPV-related tumors (anal, head, neck, cervical, and vulvar cancer) [154]. CT may also contribute to enhance the efficacy of therapeutic vaccines [155]. The results of the phase I/II CervISA study [156] are, in turn, eagerly awaited regarding the safety and immune modulation effects of the HPV16 long peptide E6/E7 therapeutic vaccine (ISA101/ISA101b) in combination with carboplatin and paclitaxel with or without bevacizumab in women with recurrent or advanced HPV16-positive CC. Greater knowledge on the TME and potential synergistic effect of this new strategy with other immunomodulatory treatments need to be explored.
4. Perspectives
Further research is required to improve outcomes in the first-line treatment in patients with CC, currently treated with platinum-doublet CT as a standard of care. Moreover, new investigation tools are necessary and, especially, reliable biomarkers to monitor responses and better select the patients who would most benefit from immunotherapy need to be explored. Furthermore, an immunotherapy-based combination with other approaches may be a promising strategy to improve OS in these tumors, according to specific patient profiles.
Based on the recent results from the RUBY trial, further improvement in the management of relapsing dMMR EC patients is currently highly expected. Results from the ongoing trial DOMENICA, dostarlimab vs. CT in EC patients with first-line advanced or recurrent diseases are awaited. Another issue to be solved would be to better evaluate the time to relapse after last treatment, and appropriately adjust next treatments’ ICI might be an option, according to the results provided from the combination of anti-LAG-3 and anti-PD-1 leading to significant benefits in recurrent/metastatic melanoma patients. One would assume that if the LAG-3 alone or combined with the PD-1 or PD-L1 inhibitor improves the response to treatment in EC patients, then it would result in better survival. We still wonder what would be the best therapeutic option in pMMR/MSS patients. The RUBY trial showed interesting results with anti-PD-1 in the first-line setting patients. Other ongoing trials will provide elements to answer and include (i) anti-FGFR in patients with MSS metastatic EC (NCT05036681), (ii) anti-FR-α in patients with MSS advanced/recurrent EC (IMGN853), (iii) avelumab and talazoparib or axitinib in patients with MSS-recurrent/persistent EC (NCT02912572), (iv) pembrolizumab with lenvatinib in patients with pMMR (LEAP-001) [157].
Other combinations using therapeutic vaccines, but also engineered T cell transfer, with CT or RT, are also promising therapeutic approaches. In that respect, a phase I/II study (NCT05194735) evaluates the administration of autologous T-cells engineered, expressing a TCR reactive to neo-Ag in patients with relapsed/refractory solid tumors, including EC [158]. Another phase I trial has planned to enroll patients with several CD70 positive solid tumor types, including advanced/metastatic CC, and evaluate the safety and tolerability of CD70-targeted chimeric antigen receptor (CAR)-T cells (NCT05518253) [159]. Hematologic cancers greatly benefit from the development of CAR technology, and this technique is now being evaluated in patients with gynecological cancers. In parallel, bispecific antibodies targeting two immune checkpoints, such as PD-1 or CTLA-4, are currently under development, and will be tested in clinical trials enrolling patients with different cancers, including EC and CC (NCT03517488) [160,161]. Another innovative treatment strategy is the use of the bifunctional ICI, M7824, comprising the extracellular domain of the human TGF-βRII linked to the C-terminus of the human anti-PD-L1 [162]. In vivo studies showed that anti-PD-L1 reduced TGF-β signaling in the TME, decreased tumor growth, and promoted CD8pos T cells’ and NK cells’ activation. The phase I/II trial (NCT04432597) tested the HPV Vaccine PRGN-2009 alone or combined with M7824 in 76 patients with HPV-positive cancers, including CC [163].
Altogether, these trials will help in the decision of the first-line treatment in patients with EC according to their MMR status, or after first recurrent disease without prior RT, CT, or surgery curative treatment. Such innovative treatment developments would benefit patients not responding to conventional therapies.
5. Conclusions
Until a few years ago, only limited treatment options were available in patients with advanced endometrial and cervical tumors. The increased understanding of the disease led to the emergence of new targets and therapeutic approaches. The most significant advances have been reported in the field of immunotherapy, highlighted by four pioneering studies. The basket trial Keynote-158 resulted in the approval by the FDA of pembrolizumab as a second-line treatment in cervical and endometrial cancers with PD-L1 and dMMR/MSI-H status, respectively. In patients with CC, the EMPOWER-CERVICAL-1 study is the first trial in over a decade showing survival benefits with the innovative cemiplimab, compared to the standard CT in the second-line setting, regardless of PD-L1 expression. In patients with EC, two studies marked a significant milestone leading both to treatment approval by the FDA and EMA in second-line treatment. In the GARNET trial, dostarlimab achieved a significant improvement in ORR and DOR in patients with MSI tumors. In patients with MSS tumors, the combination of pembrolizumab and lenvatinib was shown to improve DOR, PFS, and OS. Overall, all of these studies confirmed that these treatments had an acceptable toxicity profile and confirmed the already-known toxicities of immune checkpoint inhibitors.
Despite recent significant advances, tremendous progress is still expected to improve the management of EC and CC patients with an enhanced identification of predictive biomarkers for response, in both tumor and blood, to better determine the most efficient therapy and the most adequate schedule for administration.
Acknowledgments
The authors thank Sophie Darnis (Centre Léon Bérard) for helpful comments and valuable help for medical editorial assistance.
Author Contributions
Conceptualization, A.L., A.M.G.-L. and I.R.-C.; methodology, A.L. and A.M.G.-L.; writing—original draft preparation, A.L., A.M.G.-L. and I.R.-C.; writing—review and editing, A.L., A.M.G.-L., R.O., U.H. and I.R.-C.; supervision, A.L., A.M.G.-L. and I.R.-C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
A.L., A.M.G.-L., R.O. and U.H. declare no conflict of interest. I.R.-C. declares the following disclosures: Abbvie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; honoraria (institution) from GSK, MSD, Roche and BMS; advisory/consulting fees from Abbvie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; research grant/funding (self) from MSD, Roche and BMS; research grant/funding (institution) from MSD, Roche, BMS, Novartis, Astra Zeneca and Merck Sereno; and travel support from Roche and AstraZeneca and GSK.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Maiorano B.A., Maiorano M.F.P., Lorusso D., Maiello E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art and Future Perspectives. Cancers. 2021;13:4438. doi: 10.3390/cancers13174438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results Program SEER. [(accessed on 7 January 2023)];2022 Available online: https://seer.cancer.gov/index.html.
- 4.Kim S.M., Choi H.S., Byun J.S. Overall 5-Year Survival Rate and Prognostic Factors in Patients with Stage IB and IIA Cervical Cancer Treated by Radical Hysterectomy and Pelvic Lymph Node Dissection. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2000;10:305–312. doi: 10.1046/j.1525-1438.2000.010004305.x. [DOI] [PubMed] [Google Scholar]
- 5.Oaknin A., Bosse T.J., Creutzberg C.L., Giornelli G., Harter P., Joly F., Lorusso D., Marth C., Makker V., Mirza M.R., et al. Endometrial Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022;33:860–877. doi: 10.1016/j.annonc.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Survival Rates for Vaginal Cancer. [(accessed on 7 January 2023)]. Available online: https://www.cancer.org/cancer/vaginal-cancer/detection-diagnosis-staging/survival-rates.html.
- 7.Adams T.S., Rogers L.J., Cuello M.A. Cancer of the Vagina: 2021 Update. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2021;155((Suppl. 1)):19–27. doi: 10.1002/ijgo.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morice P., Leary A., Creutzberg C., Abu-Rustum N., Darai E. Endometrial Cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 9.Bendifallah S., Ouldamer L., Lavoue V., Canlorbe G., Raimond E., Coutant C., Graesslin O., Touboul C., Collinet P., Daraï E., et al. Patterns of Recurrence and Outcomes in Surgically Treated Women with Endometrial Cancer According to ESMO-ESGO-ESTRO Consensus Conference Risk Groups: Results from the FRANCOGYN Study Group. Gynecol. Oncol. 2017;144:107–112. doi: 10.1016/j.ygyno.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Friberg E., Orsini N., Mantzoros C.S., Wolk A. Diabetes Mellitus and Risk of Endometrial Cancer: A Meta-Analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 11.Secord A.A., Hasselblad V., Von Gruenigen V.E., Gehrig P.A., Modesitt S.C., Bae-Jump V., Havrilesky L.J. Body Mass Index and Mortality in Endometrial Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2016;140:184–190. doi: 10.1016/j.ygyno.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McVicker L., Cardwell C.R., Edge L., McCluggage W.G., Quinn D., Wylie J., McMenamin Ú.C. Survival Outcomes in Endometrial Cancer Patients According to Diabetes: A Systematic Review and Meta-Analysis. BMC Cancer. 2022;22:427. doi: 10.1186/s12885-022-09510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J.-Y., Kuo S.-J., Liaw Y.-P., Avital I., Stojadinovic A., Man Y., Mannion C., Wang J., Chou M.-C., Tsai H.-D., et al. Endometrial Cancer Incidence in Breast Cancer Patients Correlating with Age and Duration of Tamoxifen Use: A Population Based Study. J. Cancer. 2014;5:151–155. doi: 10.7150/jca.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Jiang W., Mao K., An Y., Su F., Kim B.Y.S., Liu Q., Jacobs L.K. Elevated Risks of Subsequent Endometrial Cancer Development among Breast Cancer Survivors with Different Hormone Receptor Status: A SEER Analysis. Breast Cancer Res. Treat. 2015;150:439–445. doi: 10.1007/s10549-015-3315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S., Balasubramaniam S., Zhang W., Zhang L., Sridhara R., Spillman D., Mathai J.P., Scott B., Golding S.J., Coory M., et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin. Cancer Res. 2020;26:5062–5067. doi: 10.1158/1078-0432.CCR-19-3979. [DOI] [PubMed] [Google Scholar]
- 16.Makker V., Colombo N., Casado Herráez A., Santin A.D., Colomba E., Miller D.S., Fujiwara K., Pignata S., Baron-Hay S., Ray-Coquard I., et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PubMed] [Google Scholar]
- 17.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.-P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenvatinib Plus Pembrolizumab in Patients with Either Treatment-Naive or Previously Treated Metastatic Renal Cell Carcinoma (Study 111/KEYNOTE-146): A Phase 1b/2 Study–The Lancet Oncology. [(accessed on 7 January 2023)]. Available online: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(21)00241-2/fulltext. [DOI] [PMC free article] [PubMed]
- 19.Oaknin A., Tinker A.V., Gilbert L., Samouëlian V., Mathews C., Brown J., Barretina-Ginesta M.-P., Moreno V., Gravina A., Abdeddaim C., et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020;6:1766. doi: 10.1001/jamaoncol.2020.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration . FDA Grants Accelerated Approval to Dostarlimab-Gxly for DMMR Endometrial Cancer. FDA; Silver Spring, MD, USA: 2021. [Google Scholar]
- 21.EMA . Jemperli. European Medicines Agency; Amsterdam, The Netherlands: 2021. [(accessed on 7 January 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli. [Google Scholar]
- 22.Koskas M., Amant F., Mirza M.R., Creutzberg C.L. Cancer of the Corpus Uteri: 2021 Update. Int. J. Gynecol. Obstet. 2021;155:45–60. doi: 10.1002/ijgo.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen P.A., Jhingran A., Oaknin A., Denny L. Cervical Cancer. Lancet Lond. Engl. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz N., Bosch F.X., de Sanjosé S., Herrero R., Castellsagué X., Shah K.V., Snijders P.J.F., Meijer C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 25.Bosch F.X., Lorincz A., Muñoz N., Meijer C.J.L.M., Shah K.V. The Causal Relation between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelzle D., Tanaka L.F., Lee K.K., Ibrahim Khalil A., Baussano I., Shah A.S.V., McAllister D.A., Gottlieb S.L., Klug S.J., Winkler A.S., et al. Estimates of the Global Burden of Cervical Cancer Associated with HIV. Lancet Glob. Health. 2021;9:e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca-Moutinho J.A. Smoking and Cervical Cancer. ISRN Obstet. Gynecol. 2011;2011:847684. doi: 10.5402/2011/847684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cervical Cancer–Risk Factors Cancer.Net. [(accessed on 5 January 2023)]. Available online: https://www.cancer.net/cancer-types/cervical-cancer/risk-factors.
- 29.Li S., Wen X. Seropositivity to Herpes Simplex Virus Type 2, but Not Type 1 Is Associated with Cervical Cancer: NHANES (1999–2014) BMC Cancer. 2017;17:726. doi: 10.1186/s12885-017-3734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yim E.-K., Park J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res. Treat. 2005;37:319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyer B.A., Zamarin D., Eskandar R.N., Mayadev J.M. Role of Immunotherapy in the Management of Locally Advanced and Recurrent/Metastatic Cervical Cancer. J. Natl. Compr. Cancer Netw. JNCCN. 2019;17:91–97. doi: 10.6004/jnccn.2018.7108. [DOI] [PubMed] [Google Scholar]
- 32.Otter S.J., Chatterjee J., Stewart A.J., Michael A. The Role of Biomarkers for the Prediction of Response to Checkpoint Immunotherapy and the Rationale for the Use of Checkpoint Immunotherapy in Cervical Cancer. Clin. Oncol. R. Coll. Radiol. G. B. 2019;31:834–843. doi: 10.1016/j.clon.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Meng Y., Chu T., Lin S., Wu P., Zhi W., Peng T., Ding W., Luo D., Wu P. Clinicopathological Characteristics and Prognosis of Cervical Cancer with Different Histological Types: A Population-Based Cohort Study. Gynecol. Oncol. 2021;163:545–551. doi: 10.1016/j.ygyno.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Watson M., Saraiya M., Benard V., Coughlin S.S., Flowers L., Cokkinides V., Schwenn M., Huang Y., Giuliano A. Burden of Cervical Cancer in the United States, 1998-2003. Cancer. 2008;113((Suppl. 10)):2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 35.Cervical Cancer Prognosis and Survival Rates–NCI. [(accessed on 5 January 2023)]; Available online: https://www.cancer.gov/types/cervical/survival.
- 36.Cibula D., Pötter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Meder C.H., Köhler C., Landoni F., Lax S., Lindegaard J.C., et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer. 2018;28:919–936. doi: 10.1097/IGC.0000000000001216. [DOI] [Google Scholar]
- 37.Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A., Clarke-Pearson D.L., Insalaco S. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 38.Monk B.J., Sill M.W., McMeekin D.S., Cohn D.E., Ramondetta L.M., Boardman C.H., Benda J., Cella D. Phase III Trial of Four Cisplatin-Containing Doublet Combinations in Stage IVB, Recurrent, or Persistent Cervical Carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tewari K.S., Sill M.W., Long H.J., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. N. Engl. J. Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitagawa R., Katsumata N., Shibata T., Kamura T., Kasamatsu T., Nakanishi T., Nishimura S., Ushijima K., Takano M., Satoh T., et al. Paclitaxel Plus Carboplatin versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:2129–2135. doi: 10.1200/JCO.2014.58.4391. [DOI] [PubMed] [Google Scholar]
- 41.Redondo A., Colombo N., McCormack M., Dreosti L., Nogueira-Rodrigues A., Scambia G., Lorusso D., Joly F., Schenker M., Ruff P., et al. Primary Results from CECILIA, a Global Single-Arm Phase II Study Evaluating Bevacizumab, Carboplatin and Paclitaxel for Advanced Cervical Cancer. Gynecol. Oncol. 2020;159:142–149. doi: 10.1016/j.ygyno.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Santin A.D., Sill M.W., McMeekin D.S., Leitao M.M., Brown J., Sutton G.P., Van Le L., Griffin P., Boardman C.H. Phase II Trial of Cetuximab in the Treatment of Persistent or Recurrent Squamous or Non-Squamous Cell Carcinoma of the Cervix: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011;122:495–500. doi: 10.1016/j.ygyno.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colombo N., Dubot C., Lorusso D., Caceres M.V., Hasegawa K., Shapira-Frommer R., Tewari K.S., Salman P., Hoyos Usta E., Yañez E., et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 44.Tewari K.S., Monk B.J., Vergote I., Miller A., de Melo A.C., Kim H.-S., Kim Y.M., Lisyanskaya A., Samouëlian V., Lorusso D., et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022;386:544–555. doi: 10.1056/NEJMoa2112187. [DOI] [PubMed] [Google Scholar]
- 45.US Food and Drug Administration . FDA Approves Pembrolizumab Combination for the First-Line Treatment of Cervical Cancer. FDA; Silver Spring, MD, USA: 2022. [Google Scholar]
- 46.EMA . Libtayo: Pending EC Decision. European Medicines Agency; Amsterdam, The Netherlands: 2022. [(accessed on 7 January 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/libtayo-0. [Google Scholar]
- 47.Anderson N.M., Simon M.C. The Tumor Microenvironment. Curr. Biol. CB. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.André T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., Smith D., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 49.Emens L.A., Ascierto P.A., Darcy P.K., Demaria S., Eggermont A.M.M., Redmond W.L., Seliger B., Marincola F.M. Cancer Immunotherapy: Opportunities and Challenges in the Rapidly Evolving Clinical Landscape. Eur. J. Cancer. 2017;81:116–129. doi: 10.1016/j.ejca.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 50.Low J.L., Walsh R.J., Ang Y., Chan G., Soo R.A. The Evolving Immuno-Oncology Landscape in Advanced Lung Cancer: First-Line Treatment of Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2019;11:175883591987036. doi: 10.1177/1758835919870360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown L.C., Desai K., Zhang T., Ornstein M.C. The Immunotherapy Landscape in Renal Cell Carcinoma. BioDrugs. 2020;34:733–748. doi: 10.1007/s40259-020-00449-4. [DOI] [PubMed] [Google Scholar]
- 52.Vanderstraeten A., Tuyaerts S., Amant F. The Immune System in the Normal Endometrium and Implications for Endometrial Cancer Development. J. Reprod. Immunol. 2015;109:7–16. doi: 10.1016/j.jri.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 53.León-Castillo A., Britton H., McConechy M.K., McAlpine J.N., Nout R., Kommoss S., Brucker S.Y., Carlson J.W., Epstein E., Rau T.T., et al. Interpretation of Somatic POLE Mutations in Endometrial Carcinoma. J. Pathol. 2020;250:323–335. doi: 10.1002/path.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonneville R., Krook M.A., Kautto E.A., Miya J., Wing M.R., Chen H.-Z., Reeser J.W., Yu L., Roychowdhury S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017;2017:PO.17.00073.. doi: 10.1200/PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pakish J.B., Zhang Q., Jazaeri A.A., Celestino J., Kwan S.Y., Mok S., Yates M., Lu K.H. Endometrial Cancer Subtypes and Immunotherapy: Is the Immune Microenvironment Different in Microsatellite Instable Endometrial Cancer? Gynecol. Oncol. 2016;141:6. doi: 10.1016/j.ygyno.2016.04.041. [DOI] [Google Scholar]
- 56.Pakish J.B., Zhang Q., Chen Z., Liang H., Chisholm G.B., Yuan Y., Mok S.C., Broaddus R.R., Lu K.H., Yates M.S. Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: Hereditary or Sporadic Origin Matters. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:4473–4481. doi: 10.1158/1078-0432.CCR-16-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howitt B.E., Shukla S.A., Sholl L.M., Ritterhouse L.L., Watkins J.C., Rodig S., Stover E., Strickland K.C., D’Andrea A.D., Wu C.J., et al. Association of Polymerase E-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 58.van Gool I.C., Eggink F.A., Freeman-Mills L., Stelloo E., Marchi E., de Bruyn M., Palles C., Nout R.A., de Kroon C.D., Osse E.M., et al. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kondratiev S., Sabo E., Yakirevich E., Lavie O., Resnick M.B. Intratumoral CD8+ T Lymphocytes as a Prognostic Factor of Survival in Endometrial Carcinoma. Clin. Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 60.de Jong R.A., Leffers N., Boezen H.M., ten Hoor K.A., van der Zee A.G.J., Hollema H., Nijman H.W. Presence of Tumor-Infiltrating Lymphocytes Is an Independent Prognostic Factor in Type I and II Endometrial Cancer. Gynecol. Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J.M., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kübler K., Ayub T.H., Weber S.K., Zivanovic O., Abramian A., Keyver-Paik M.-D., Mallmann M.R., Kaiser C., Serçe N.B., Kuhn W., et al. Prognostic Significance of Tumor-Associated Macrophages in Endometrial Adenocarcinoma. Gynecol. Oncol. 2014;135:176–183. doi: 10.1016/j.ygyno.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Halla K. Emerging Treatment Options for Advanced or Recurrent Endometrial Cancer. J. Adv. Pract. Oncol. 2022;13:45–59. doi: 10.6004/jadpro.2022.13.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamat @ Yusof M.N., Chew K.T., Kampan N., Abd Aziz N.H., Md Zin R.R., Tan G.C., Shafiee M.N. PD-L1 Expression in Endometrial Cancer and Its Association with Clinicopathological Features: A Systematic Review and Meta-Analysis. Cancers. 2022;14:3911. doi: 10.3390/cancers14163911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munn D.H., Mellor A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation and Tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ino K., Yoshida N., Kajiyama H., Shibata K., Yamamoto E., Kidokoro K., Takahashi N., Terauchi M., Nawa A., Nomura S., et al. Indoleamine 2,3-Dioxygenase Is a Novel Prognostic Indicator for Endometrial Cancer. Br. J. Cancer. 2006;95:1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Jong R.A., Kema I.P., Boerma A., Boezen H.M., der Want J.J.L.v., Gooden M.J.M., Hollema H., Nijman H.W. Prognostic Role of Indoleamine 2,3-Dioxygenase in Endometrial Carcinoma. Gynecol. Oncol. 2012;126:474–480. doi: 10.1016/j.ygyno.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 68.Ino K., Yamamoto E., Shibata K., Kajiyama H., Yoshida N., Terauchi M., Nawa A., Nagasaka T., Takikawa O., Kikkawa F. Inverse Correlation between Tumoral Indoleamine 2,3-Dioxygenase Expression and Tumor-Infiltrating Lymphocytes in Endometrial Cancer: Its Association with Disease Progression and Survival. Clin. Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 69.Mills A., Zadeh S., Sloan E., Chinn Z., Modesitt S.C., Ring K.L. Indoleamine 2,3-Dioxygenase in Endometrial Cancer: A Targetable Mechanism of Immune Resistance in Mismatch Repair-Deficient and Intact Endometrial Carcinomas. Mod. Pathol. 2018;31:1282–1290. doi: 10.1038/s41379-018-0039-1. [DOI] [PubMed] [Google Scholar]
- 70.Kozłowski M., Borzyszkowska D., Cymbaluk-Płoska A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines. 2022;10:2826. doi: 10.3390/biomedicines10112826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedman L.A., Ring K.L., Mills A.M. LAG-3 and GAL-3 in Endometrial Carcinoma: Emerging Candidates for Immunotherapy. Int. J. Gynecol. Pathol. 2020;39:203. doi: 10.1097/PGP.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y., Yang R., Xu C., Zhang Y., Deng M., Wu D., Tang F., Liu X., Han Y., Zhan Y., et al. Analysis of the Immune Checkpoint Lymphocyte Activation Gene-3 (LAG-3) in Endometrial Cancer: An Emerging Target for Immunotherapy. Pathol. Res. Pract. 2022;236:153990. doi: 10.1016/j.prp.2022.153990. [DOI] [PubMed] [Google Scholar]
- 73.Tawbi H.A., Schadendorf D., Lipson E.J., Ascierto P.A., Matamala L., Castillo Gutiérrez E., Rutkowski P., Gogas H.J., Lao C.D., De Menezes J.J., et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ARCAGY/GINECO GROUP Randomized Phase III Trial in MMR Deficient Endometrial Cancer Patients Comparing Chemotherapy Alone versus Dostarlimab in First Line Advanced/Metastatic Setting; Clinical Trial Registration NCT05201547; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05201547.
- 75.Mario Negri Institute for Pharmacological Research Phase III Double-Blind Randomized Placebo Controlled Trial of Atezolizumab in Combination With Paclitaxel and Carboplatin in Women With Advanced/Recurrent Endometrial Cancer; Clinical Trial Registration NCT03603184; clinicaltrials.gov. [(accessed on 29 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03603184.
- 76.Tesaro, Inc A Phase 3, Randomized, Double-Blind, Multicenter Study of Dostarlimab (TSR-042) Plus Carboplatin-Paclitaxel versus Placebo Plus Carboplatin-Paclitaxel in Patients with Recurrent or Primary Advanced Endometrial Cancer (RUBY); Clinical Trial Registration NCT03981796; clinicaltrials.gov. [(accessed on 29 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03981796.
- 77.Merck Sharp & Dohme LLC A Phase 3 Randomized, Open-Label, Study of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) versus Chemotherapy for First-Line Treatment of Advanced or Recurrent Endometrial Carcinoma (LEAP-001); Clinical Trial Registration NCT03884101; clinicaltrials.gov. [(accessed on 29 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03884101.
- 78.National Cancer Institute (NCI) A Phase III Randomized, Placebo-Controlled Study of Pembrolizumab (MK-3475, NSC #776864) in Addition to Paclitaxel and Carboplatin for Measurable Stage III or IVA, Stage IVB or Recurrent Endometrial Cancer; Clinical Trial Registration NCT03914612; clinicaltrials.gov. [(accessed on 29 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03914612.
- 79.NETRIS Pharma A Randomized, Multicenter, Open Label, Phase I/II Study to Evaluate the Safety, Clinical and Biological Activity of a Humanized Monoclonal Antibody Targeting Netrin-1 (NP137) in Combination With Carboplatin Plus Paclitaxel and/or Pembrolizumab in Patients With Locally Advanced/Metastatic Endometrial Carcinoma or Cervix Carcinoma Progressing/Relapsing after at Least One Prior Systemic Chemotherapy.; Clinical Trial Registration NCT04652076; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04652076.
- 80.Li J. An Open-Label, Single Arm, Phase II Trial of Niraparib in Combination with Anti-PD1 Antibody in Recurrent/Advanced Stage Endometrial Cancer Patients; Clinical Trial Registration NCT04885413; clinicaltrials.gov. [(accessed on 28 December 2022)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT04885413.
- 81.Konstantinopoulos P.A., Gockley A.A., Xiong N., Krasner C., Horowitz N., Campos S., Wright A.A., Liu J.F., Shea M., Yeku O., et al. Evaluation of Treatment with Talazoparib and Avelumab in Patients with Recurrent Mismatch Repair Proficient Endometrial Cancer. JAMA Oncol. 2022;8:1317–1322. doi: 10.1001/jamaoncol.2022.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.M.D. Anderson Cancer Center A Phase II Study of Futibatinib and Pembrolizumab in Metastatic Microsatellite Stable Endometrial Carcinoma; Clinical Trial Registration NCT05036681; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05036681.
- 83.Incyte Corporation An Umbrella Study of INCMGA00012 Alone and in Combination with Other Therapies in Participants with Advanced or Metastatic Endometrial Cancer Who Have Progressed on or after Platinum-Based Chemotherapy (POD1UM-204); Clinical Trial Registration NCT04463771; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04463771.
- 84.Memorial Sloan Kettering Cancer Center A Phase II Trial sof Pembrolizumab Plus Olaparib for the Treatment of Patients with Persistent/Recurrent Endometrial Cancers; Clinical Trial Registration NCT05156268; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05156268.
- 85.University of Oklahoma A Phase II, Single Arm Study of Atezolizumab + Bevacizumab in Women with Advanced, Recurrent or Persistent Endometrial Cancer; Clinical Trial Registration NCT03526432; clinicaltrials.gov. [(accessed on 29 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03526432.
- 86.University Health Network, Toronto A Phase II, Open Label Study of the Poly(ADP-Ribose) Polymerase Inhibitor Niraparib in Monotherapy or in Combination With Anti-PD1 Inhibitor TSR-042 in Recurrent Endometrial Cancer; Clinical Trial Registration NCT03016338; clinicaltrials.gov. [(accessed on 29 December 2022)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT03016338.
- 87.Kroep J.R. Durvalumab and Olaparib in Metastatic or Recurrent Endometrial Cancer; Clinical Trial Registration NCT03951415; clinicaltrials.gov. [(accessed on 28 December 2022)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT03951415.
- 88.AstraZeneca A Randomised, Multicentre, Double-Blind, Placebo-Controlled, Phase III Study of First-Line Carboplatin and Paclitaxel in Combination with Durvalumab, Followed by Maintenance Durvalumab with or without Olaparib in Patients with Newly Diagnosed Advanced or Recurrent Endometrial Cancer (DUO-E); Clinical Trial Registration NCT04269200; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04269200.
- 89.Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University Camrelizumab Plus Anlotinib in Patients with Recurrent Sporadic Mismatch Repair Deficient Endometrial Cancer (CAN-RESPOND): A Single-Arm, Multicentre, Phase 2 Study; Clinical Trial Registration NCT05550558; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05550558.
- 90.Mahdi H. Pembrolizumab with Sitravatinib in Recurrent Endometrial Cancer and Other Solid Tumors with Deficient Mismatch Repair System Post PD1 Exposure: Phase II Trial; Clinical Trial Registration NCT05419817; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05419817.
- 91.National Cancer Institute (NCI) A Randomized Phase II Trial of Nivolumab and Ipilimumab Compared to Nivolumab Monotherapy in Patients with Deficient Mismatch Repair System Recurrent Endometrial Carcinoma; Clinical Trial Registration NCT05112601; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05112601.
- 92.Konstantinopoulo P. A Phase 2, Two-Stage, Study of Mirvetuximab Soravtansine (IMGN853) in Combination with Pembrolizumab in Patients with Microsatellite Stable (MSS) Recurrent or Persistent Endometrial Cancer (EC); Clinical Trial Registration NCT03835819; clinicaltrials.gov. [(accessed on 28 December 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03835819.
- 93.Katsumata N., Tamura K., Hasegawa K., Matsumoto K., Mukai H., Takahashi S., Nomura H., Minami H. Efficacy and Safety of Nivolumab in Patients with Uterine Cervical Cancer, Uterine Corpus Cancer, or Soft-Tissue Sarcoma. Ann. Oncol. 2018;29:vii60. doi: 10.1093/annonc/mdy374.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J.F., Gordon M., Veneris J., Braiteh F., Balmanoukian A., Eder J.P., Oaknin A., Hamilton E., Wang Y., Sarkar I., et al. Safety, Clinical Activity and Biomarker Assessments of Atezolizumab from a Phase I Study in Advanced/Recurrent Ovarian and Uterine Cancers. Gynecol. Oncol. 2019;154:314–322. doi: 10.1016/j.ygyno.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 95.Konstantinopoulos P.A., Luo W., Liu J.F., Gulhan D.C., Krasner C., Ishizuka J.J., Gockley A.A., Buss M., Growdon W.B., Crowe H., et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019;37:2786–2794. doi: 10.1200/JCO.19.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antill Y., Kok P.S., Stockler M.R., Robledo K., Yip S., Parry M., Smith D., Spurdle A., Barnes E., Friedlander M.L., et al. Updated Results of Activity of Durvalumab in Advanced Endometrial Cancer (AEC) According to Mismatch Repair (MMR) Status: The Phase II PHAEDRA Trial (ANZGOG1601) Ann. Oncol. 2019;30:ix192. doi: 10.1093/annonc/mdz446.011. [DOI] [Google Scholar]
- 97.Jemperli (Dostarlimab) RUBY Phase III Trial Met Its Primary Endpoint in a Planned Interim Analysis in Patients with Primary Advanced or Recurrent Endometrial Cancer|GSK. [(accessed on 1 February 2023)]. Available online: https://www.gsk.com/en-gb/media/press-releases/jemperli-dostarlimab-ruby-phase-iii-trial-met-its-primary-endpoint-in-a-planned-interim-analysis-in-patients-with-primary-advanced-or-recurrent-endometrial-cancer/
- 98.Lheureux S., Matei D.E., Konstantinopoulos P.A., Wang B.X., Gadalla R., Block M.S., Jewell A., Gaillard S.L., McHale M., McCourt C., et al. Translational Randomized Phase II Trial of Cabozantinib in Combination with Nivolumab in Advanced, Recurrent, or Metastatic Endometrial Cancer. J. Immunother. Cancer. 2022;10:e004233. doi: 10.1136/jitc-2021-004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grandin M., Meier M., Delcros J.G., Nikodemus D., Reuten R., Patel T.R., Goldschneider D., Orriss G., Krahn N., Boussouar A., et al. Structural Decoding of the Netrin-1/UNC5 Interaction and Its Therapeutical Implications in Cancers. Cancer Cell. 2016;29:173–185. doi: 10.1016/j.ccell.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Bruikman C.S., Zhang H., Kemper A.M., van Gils J.M. Netrin Family: Role for Protein Isoforms in Cancer. J. Nucleic Acids. 2019;2019:3947123. doi: 10.1155/2019/3947123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun Y., Manceau A., Frydman L., Cappuccio L., Neves D., Basso V., Wang H., Fombonne J., Maisse C., Mehlen P., et al. Δ40p53 Isoform Up-Regulates Netrin-1/UNC5B Expression and Potentiates Netrin-1 pro-Oncogenic Activity. Proc. Natl. Acad. Sci. USA. 2021;118:e2103319118. doi: 10.1073/pnas.2103319118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adamczyk-Gruszka O., Horecka-Lewitowicz A., Gruszka J., Wawszczak-Kasza M., Strzelecka A., Lewitowicz P. FGFR-2 and Epithelial–Mesenchymal Transition in Endometrial Cancer. J. Clin. Med. 2022;11:5416. doi: 10.3390/jcm11185416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winterhoff B., Konecny G.E. Targeting Fibroblast Growth Factor Pathways in Endometrial Cancer. Curr. Probl. Cancer. 2017;41:37–47. doi: 10.1016/j.currproblcancer.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 104.Senol S., Ceyran A.B., Aydin A., Zemheri E., Ozkanli S., Kösemetin D., Sehitoglu I., Akalin I. Folate Receptor α Expression and Significance in Endometrioid Endometrium Carcinoma and Endometrial Hyperplasia. Int. J. Clin. Exp. Pathol. 2015;8:5633–5641. [PMC free article] [PubMed] [Google Scholar]
- 105.Allouch S., Malki A., Allouch A., Gupta I., Vranic S., Al Moustafa A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020;10:914. doi: 10.3389/fonc.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Münger K., Howley P.M. Human Papillomavirus Immortalization and Transformation Functions. Virus Res. 2002;89:213–228. doi: 10.1016/S0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 107.Tindle R.W. Immune Evasion in Human Papillomavirus-Associated Cervical Cancer. Nat. Rev. Cancer. 2002;2:59–64. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 108.Piersma S.J., Jordanova E.S., van Poelgeest M.I.E., Kwappenberg K.M.C., van der Hulst J.M., Drijfhout J.W., Melief C.J.M., Kenter G.G., Fleuren G.J., Offringa R., et al. High Number of Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes Is Associated with the Absence of Lymph Node Metastases in Patients with Large Early-Stage Cervical Cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 109.De Vos van Steenwijk P.J., Ramwadhdoebe T.H., Goedemans R., Doorduijn E.M., van Ham J.J., Gorter A., van Hall T., Kuijjer M.L., van Poelgeest M.I.E., van der Burg S.H., et al. Tumor-Infiltrating CD14-Positive Myeloid Cells and CD8-Positive T-Cells Prolong Survival in Patients with Cervical Carcinoma: Clinical Benefit of CD14+ Cells in Cervical Cancer. Int. J. Cancer. 2013;133:2884–2894. doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 110.Jordanova E.S., Gorter A., Ayachi O., Prins F., Durrant L.G., Kenter G.G., van der Burg S.H., Fleuren G.J. Human Leukocyte Antigen Class I, MHC Class I Chain-Related Molecule A, and CD8+/Regulatory T-Cell Ratio: Which Variable Determines Survival of Cervical Cancer Patients? Clin. Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 111.Liang Y., Lü W., Zhang X., Lü B. Tumor-Infiltrating CD8+ and FOXP3+ Lymphocytes before and after Neoadjuvant Chemotherapy in Cervical Cancer. Diagn. Pathol. 2018;13:93. doi: 10.1186/s13000-018-0770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shah W., Yan X., Jing L., Zhou Y., Chen H., Wang Y. A Reversed CD4/CD8 Ratio of Tumor-Infiltrating Lymphocytes and a High Percentage of CD4+FOXP3+ Regulatory T Cells Are Significantly Associated with Clinical Outcome in Squamous Cell Carcinoma of the Cervix. Cell. Mol. Immunol. 2011;8:59–66. doi: 10.1038/cmi.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martins P.R., Machado C.M.T., Coxir S.A., de Oliveira A.J., Moreira T.B., Campos L.S., Alcântara R., de Paula S.O.C., de Oliveira Salles P.G., Gollob K.J., et al. Cervical Cancer Patients That Respond to Chemoradiation Therapy Display an Intense Tumor Infiltrating Immune Profile before Treatment. Exp. Mol. Pathol. 2019;111:104314. doi: 10.1016/j.yexmp.2019.104314. [DOI] [PubMed] [Google Scholar]
- 114.Dorta-Estremera S., Colbert L.E., Nookala S.S., Yanamandra A.V., Yang G., Delgado A., Mikkelson M., Eifel P., Jhingran A., Lilie L.L., et al. Kinetics of Intratumoral Immune Cell Activation During Chemoradiation for Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:593–600. doi: 10.1016/j.ijrobp.2018.06.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Yu M., Jing Y., Cheng J., Zhang C., Cheng L., Lu H., Cai M.-C., Wu J., Wang W., et al. Baseline Immunity and Impact of Chemotherapy on Immune Microenvironment in Cervical Cancer. Br. J. Cancer. 2021;124:414–424. doi: 10.1038/s41416-020-01123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L. Effectiveness and Safety of Camrelizumab Combined with Concurrent Chemoradiotherapy for FIGO IB2-IIIB Cervical Cancer: A Single-Center, Single-Arm, Open-Phase II Clinical Study; Clinical Trial Registration NCT05311566; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05311566.
- 117.Huang X. An Open-Label, Phase 2 Randomized Trial of Camrelizumab (SHR1210) Plus Apatinib versus Paclitaxel and Cisplatin/Carboplatin Plus Bevacizumab as a First-Line Therapy in Patients with Stage IVB, Recurrent, or Persistent Cervical Cancer; Clinical Trial Registration NCT04974944; clinicaltrials.gov. [(accessed on 3 January 2023)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT04974944.
- 118.First Affiliated Hospital of Guangxi Medical University Efficacy and Safety of Tislelizumab Combined with Concurrent Chemoradiotherapy as First-Line Treatment for Stage IIIC2 Cervical Cancer; Clinical Trial Registration NCT05511623; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05511623.
- 119.Biocad An International Randomized Double-Blind Clinical Trial of BCD-100 Plus Platinum-Based Chemotherapy with and without Bevacizumab versus Placebo Plus Platinum-Based Chemotherapy with and without Bevacizumab as First-Line Treatment of Subjects with Advanced Cervical Cancer; Clinical Trial Registration NCT03912415; clinicaltrials.gov. [(accessed on 3 January 2023)];2020 Available online: https://clinicaltrials.gov/ct2/show/NCT03912415.
- 120.Grupo Español de Investigación en Cáncer de Ovario A Randomized Phase III Trial of Platinum Chemotherapy Plus Paclitaxel with Bevacizumab and Atezolizumab versus Platinum Chemotherapy Plus Paclitaxel and Bevacizumab in Metastatic (Stage IVB), Persistent, or Recurrent Carcinoma of the Cervix; Clinical Trial Registration NCT03556839; clinicaltrials.gov. [(accessed on 31 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03556839.
- 121.Akeso A Randomized, Double-Blind, Placebo-Controlled Phase III Study to Evaluate AK104 Plus Platinum-Containing Chemotherapy with or without Bevacizumab as First-Line Treatment for Persistent, Recurrent, or Metastatic Cervical Cancer; Clinical Trial Registration NCT04982237; clinicaltrials.gov. [(accessed on 31 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04982237.
- 122.Peking Union Medical College Hospital A Single-Arm, Multicenter, Phase II Study to Investigate Efficacy and Safety of Toripalimab Combined with Chemotherapy and Bevacizumab as First-Line Treatment in Patients with Recurrent, Refractory and Metastatic Cervical Cancer; Clinical Trial Registration NCT04973904; clinicaltrials.gov. [(accessed on 3 January 2023)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT04973904.
- 123.Australia New Zealand Gynaecological Oncology Group A Phase II Trial of Tislelizumab in Combination with Sitravatinib for Recurrent/Metastatic Cervical Cancer after Platinum-Based Chemotherapy; Clinical Trial Registration NCT05614453; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05614453.
- 124.Iovance Biotherapeutics, Inc A Phase 2, Multicenter Study to Evaluate the Efficacy and Safety Using Autologous Tumor Infiltrating Lymphocytes (LN-145) in Patients with Recurrent, Metastatic or Persistent Cervical Carcinoma; Clinical Trial Registration NCT03108495; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03108495.
- 125.Regeneron Pharmaceuticals A Phase 2 Study of Cemiplimab, an Anti-PD-1 Monoclonal Antibody, and ISA101b Vaccine in Patients with Recurrent/Metastatic HPV16 Cervical Cancer Who Have Experienced Disease Progression after First Line Chemotherapy; Clinical Trial Registration NCT04646005; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04646005.
- 126.Zhu J. A Clinical Study to Explore the Efficacy and Safety of Tislelizumab in Combination with Bevacizumab and Chemotherapy in Patients with Persistent, Recurrent, or Metastatic Cervical Cancer; Clinical Trial Registration NCT05247619; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05247619.
- 127.Agenus Inc An Open-Label, Multicenter Phase II Study Evaluating Balstilimab Alone or Balstilimab in Combination with Zalifrelimab in Patients with Advanced Cervical Cancer; Clinical Trial Registration NCT05033132; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05033132.
- 128.Tse D.K.-Y. Avelumab with Axitinib in Persistent or Recurrent Cervical Cancer after Platinum-Based Chemotherapy–A Proof-of-Concept Study (ALARICE Study); Clinical Trial Registration NCT03826589; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03826589.
- 129.Genor Biopharma Co., Ltd. Phase II Clinical Study to Evaluate the Efficacy and Safety of GB226 in Treatment of Recurrent or Metastatic Cervical Cancer Patients with PD-L1 Positive Who Failed in Platinum-Based Chemotherapy; Clinical Trial Registration NCT03808857; clinicaltrials.gov. [(accessed on 3 January 2023)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT03808857.
- 130.University of Oklahoma Phase II Trial of Niraparib in Combination with Dostarlimab in Patients with Recurrent or Progressive Cervix Cancer; Clinical Trial Registration NCT04068753; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04068753.
- 131.Qilu Pharmaceutical Co., Ltd. A Randomized, Double-Blind, Placebo-Controlled Phase III Study to Evaluate QL1706 Plus Paclitaxel-Cisplatin/Carboplatin with or without Bevacizumab for the First-Line Treatment of Persistent, Recurrent or Metastatic Cervical Cancer; Clinical Trial Registration NCT05446883; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05446883.
- 132.Grupo Español de Investigación en Cáncer de Ovario A Randomized, Open Label, Phase II Trial of Anti-PD1, TSR-042, as Maintenance Therapy for Patients with High-Risk Locally Advanced Cervical Cancer after Chemo-Radiation; Clinical Trial Registration NCT03833479; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03833479.
- 133.Roussy G., Cancer Campus, Grand Paris Randomized Phase II Trial Assessing the Inhibitor of Programmed Cell Death Ligand 1 (PD-L1) Immune Checkpoint Atezolizumab in Locally Advanced Cervical Cancer; Clinical Trial Registration NCT03612791; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03612791.
- 134.Santin A.D., Deng W., Frumovitz M., Buza N., Bellone S., Huh W., Khleif S., Lankes H.A., Ratner E.S., O’Cearbhaill R.E., et al. Phase II Evaluation of Nivolumab in the Treatment of Persistent or Recurrent Cervical Cancer ( NCT02257528/NRG-GY002) Gynecol. Oncol. 2020;157:161–166. doi: 10.1016/j.ygyno.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naumann R.W., Hollebecque A., Meyer T., Devlin M.-J., Oaknin A., Kerger J., López-Picazo J.M., Machiels J.-P., Delord J.-P., Evans T.R.J., et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019;37:2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Malley D.M., Oaknin A., Monk B.J., Selle F., Rojas C., Gladieff L., Berton D., Leary A., Moore K.N., Estevez-Diz M.D.P., et al. Phase II Study of the Safety and Efficacy of the Anti-PD-1 Antibody Balstilimab in Patients with Recurrent and/or Metastatic Cervical Cancer. Gynecol. Oncol. 2021;163:274–280. doi: 10.1016/j.ygyno.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 137.Mayadev J., Nunes A.T., Li M., Marcovitz M., Lanasa M.C., Monk B.J. CALLA: Efficacy and Safety of Concurrent and Adjuvant Durvalumab with Chemoradiotherapy versus Chemoradiotherapy Alone in Women with Locally Advanced Cervical Cancer: A Phase III, Randomized, Double-Blind, Multicenter Study. Int. J. Gynecol. Cancer. 2020;30:1065–1070. doi: 10.1136/ijgc-2019-001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Update on CALLA Phase III trial of Concurrent Use of IMFINZI and Chemoradiotherapy in Locally Advanced Cervical Cancer. [(accessed on 1 February 2023)]. Available online: https://www.astrazeneca.com/media-centre/press-releases/2022/update-on-calla-phase-iii-trial-for-imfinzi.html.
- 139.National Cancer Institute (NCI) Anti PD-L1 (Atezolizumab) as an Immune Primer and Concurrently with Extended Field Chemoradiotherapy for Node Positive Locally Advanced Cervical Cancer; Clinical Trial Registration NCT03738228; clinicaltrials.gov. [(accessed on 1 February 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03738228.
- 140.Lheureux S., Butler M.O., Clarke B., Cristea M.C., Martin L.P., Tonkin K.S., Fleming G.F., Tinker A., Hirte H.W., Tsoref D., et al. A Phase I/II Study of Ipilimumab in Women with Metastatic or Recurrent Cervical Carcinoma: A Study of the Princess Margaret and Chicago N01 Consortia. J. Clin. Oncol. 2015;33((Suppl. 15)):3061. doi: 10.1200/jco.2015.33.15_suppl.3061. [DOI] [Google Scholar]
- 141.Mayadev J.S., Enserro D., Lin Y.G., Da Silva D.M., Lankes H.A., Aghajanian C., Ghamande S., Moore K.N., Kennedy V.A., Fracasso P.M., et al. Sequential Ipilimumab after Chemoradiotherapy in Curative-Intent Treatment of Patients with Node-Positive Cervical Cancer. JAMA Oncol. 2020;6:92–99. doi: 10.1001/jamaoncol.2019.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lan C., Shen J., Wang Y., Li J., Liu Z., He M., Cao X., Ling J., Huang J., Zheng M., et al. Camrelizumab Plus Apatinib in Patients with Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:4095–4106. doi: 10.1200/JCO.20.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y., Lai Y., Peng H., Yan S., Liu Z., Tong C., Huang X. Multiparametric Immune Profiling of Advanced Cervical Cancer to Predict Response to Programmed Death-1 Inhibitor Combination Therapy: An Exploratory Study of the CLAP Trial. Clin. Transl. Oncol. 2022;25:256–268. doi: 10.1007/s12094-022-02945-1. [DOI] [PubMed] [Google Scholar]
- 144.Bristol-Myers Squibb Non-Comparative, Open-Label, Multiple Cohort, Phase 1/2 Study of Nivolumab Monotherapy and Nivolumab Combination Therapy in Subjects with Virus-Positive and Virus-Negative Solid Tumors; Clinical Trial Registration NCT02488759; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT02488759.
- 145.O’Malley D.M., Neffa M., Monk B.J., Melkadze T., Huang M., Kryzhanivska A., Bulat I., Meniawy T.M., Bagameri A., Wang E.W., et al. Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022;40:762–771. doi: 10.1200/JCO.21.02067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.ARCAGY/GINECO GROUP A Multicenter, Pilot Study Evaluating Immune Impact and Safety of Nivolumab in Combination with Ipilimumab (Immune Combination) before Initial RT-CT Treatment for Cervix Cancer.: The French GINECO–COLIBRI Study; Clinical Trial Registration NCT04256213; clinicaltrials.gov. [(accessed on 19 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04256213.
- 147.Hoffmann-La Roche A Phase II, Safety, and Efficacy Study of Tiragolumab Plus Atezolizumab and Atezolizumab Monotherapy in Patients with Metastatic and/or Recurrent PD-L1-Positive Cervical Cancer; Clinical Trial Registration NCT04300647; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04300647.
- 148.Bristol-Myers Squibb A Phase I/2a Dose Escalation and Cohort Expansion Study of the Safety, Tolerability, and Efficacy of Anti-LAG-3 Monoclonal Antibody (BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Advanced Solid Tumors; Clinical Trial Registration NCT01968109; clinicaltrials.gov. [(accessed on 5 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT01968109.
- 149.Friedman C.F., Snyder Charen A., Zhou Q., Carducci M.A., Buckley De Meritens A., Corr B.R., Fu S., Hollmann T.J., Iasonos A., Konner J.A., et al. Phase II Study of Atezolizumab in Combination with Bevacizumab in Patients with Advanced Cervical Cancer. J. Immunother. Cancer. 2020;8:e001126. doi: 10.1136/jitc-2020-001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seagen Inc A Phase 1b/2 Open-Label Trial of Tisotumab Vedotin (HuMax®-TF-ADC) Monotherapy and in Combination with Other Agents in Subjects with Recurrent or Stage IVB Cervical Cancer; Clinical Trial Registration NCT03786081; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03786081.
- 151.Wang R., Pan W., Jin L., Huang W., Li Y., Wu D., Gao C., Ma D., Liao S. Human Papillomavirus Vaccine against Cervical Cancer: Opportunity and Challenge. Cancer Lett. 2020;471:88–102. doi: 10.1016/j.canlet.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 152.Da Silva D.M., Skeate J.G., Chavez-Juan E., Lühen K.P., Wu J.-M., Wu C.-M., Kast W.M., Hwang K. Therapeutic Efficacy of a Human Papillomavirus Type 16 E7 Bacterial Exotoxin Fusion Protein Adjuvanted with CpG or GPI-0100 in a Preclinical Mouse Model for HPV-Associated Disease. Vaccine. 2019;37:2915–2924. doi: 10.1016/j.vaccine.2019.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins A Pilot Clinical Trial Assessing the Safety and Feasibility of Intramuscular Administration of the TA-CIN Vaccine as Adjuvant Therapy for Patients with History of HPV16 Associated Cervical Cancer; Clinical Trial Registration NCT02405221; clinicaltrials.gov. [(accessed on 5 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT02405221.
- 154.Centre Hospitalier Universitaire de Besancon A Phase II Study Evaluating the Interest to Combine UCPVax a CD4 TH1-Inducer Cancer Vaccine and Atezolizumab for the Treatment of Human PapillomaVirus Positive Cancers; Clinical Trial Registration NCT03946358; clinicaltrials.gov. [(accessed on 5 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03946358.
- 155.Hancock G., Hellner K., Dorrell L. Therapeutic HPV Vaccines. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:59–72. doi: 10.1016/j.bpobgyn.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 156.ISA Pharmaceuticals A Multicenter, Open Label Phase I/II Study to Determine the Safety and Immune Modulating Effects of the Therapeutic Human Papilloma Virus 16 (HPV16) E6/E7 Long Peptides Vaccine (ISA101/ISA101b) Immunotherapy in Combination with Standard of Care Therapy (Carboplatin and Paclitaxel with or without Bevacizumab) in Women with HPV16 Positive Advanced or Recurrent Cervical Cancer Who Have No Curative Treatment Options; Clinical Trial Registration NCT02128126; clinicaltrials.gov. [(accessed on 5 January 2023)];2019 Available online: https://clinicaltrials.gov/ct2/show/NCT02128126.
- 157.Marth C., Tarnawski R., Tyulyandina A., Pignata S., Gilbert L., Kaen D., Rubio M.J., Frentzas S., Beiner M., Magallanes-Maciel M., et al. Phase 3, Randomized, Open-Label Study of Pembrolizumab plus Lenvatinib versus Chemotherapy for First-Line Treatment of Advanced or Recurrent Endometrial Cancer: ENGOT-En9/LEAP-001. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2022;32:93–100. doi: 10.1136/ijgc-2021-003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alaunos Therapeutics Phase I/II Study of Autologous T Cells Engineered Using the Sleeping Beauty System to Express T-Cell Receptors (TCRs) Reactive against Cancer-Specific Mutations in Subjects with Solid Tumors; Clinical Trial Registration NCT05194735; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05194735.
- 159.Fang W. A Phase I Clinical Study of CD70-Targeting CAR-T Therapy in the Treatment of CD70-Positive Advanced/Metastatic Solid Tumors; Clinical Trial Registration NCT05518253; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05518253.
- 160.Xencor, Inc A Phase 2 Study of XmAb20717 in Patients with Selected Gynecological Malignancies and High-Risk Metastatic Castration-Resistant Prostate Cancer; Clinical Trial Registration NCT05032040; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05032040.
- 161.Xencor to Present Data from the Phase 1 Study of XmAb®20717 and Three Research Programs at the SITC Annual Meeting | Xencor, Inc. [(accessed on 5 January 2023)]. Available online: https://investors.xencor.com/news-releases/news-release-details/xencor-present-data-phase-1-study-xmabr20717-and-three-research.
- 162.Knudson K.M., Hicks K.C., Luo X., Chen J.-Q., Schlom J., Gameiro S.R. M7824, a Novel Bifunctional Anti-PD-L1/TGFβ Trap Fusion Protein, Promotes Anti-Tumor Efficacy as Monotherapy and in Combination with Vaccine. Oncoimmunology. 2018;7:e1426519. doi: 10.1080/2162402X.2018.1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.National Cancer Institute (NCI) Phase I/II Trial of HPV Vaccine PRGN-2009 Alone or in Combination with Anti-PD-L1/TGF-Beta Trap (M7824) in Subjects with HPV Positive Cancers; Clinical Trial Registration NCT04432597; clinicaltrials.gov. [(accessed on 3 January 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04432597.