Abstract
Simple Summary
Bacillus of Calmette-Guérin (BCG) is the gold standard as per adjuvant intravesical treatment for intermediate and high-risk non-muscle invasive bladder cancer (NMIBC). Nevertheless, drug-related toxicity, compliance, and a shortage of BCG availability make the completion of the planned treatment schedule challenging in many patients, thus possibly impacting survival outcomes. No one specific BCG strain out of the several available ones worldwide has so far demonstrated its superiority profile in prolonging time to recurrence and progression. In our systematic review and network meta-analysis, we compared to most widely adopted BCG strains and demonstrated that BCG strain Tice, RIVM, and Tokyo 172 could display potential enhanced benefits, thus possibly supporting the use of such strains for future BCG trials in NMIBCs.
Abstract
Background: In an era of Bacillus of Calmette-Guérin (BCG) shortages, the comparative efficacy from different adjuvant intravesical BCG strains in non-muscle invasive bladder cancer (NMIBC) has not been clearly elucidated. We aim to compare, through a systematic review and meta-analysis, the cumulative BC recurrence rates and the best efficacy profile of worldwide available BCG strains over the last forty years. Methods: PubMed, Scopus, Web of Science, Embase, and Cochrane databases were searched from 1982 up to 2022. A meta-analysis of pooled BC recurrence rates was stratified for studies with ≤3-y vs. >3-y recurrence-free survival (RFS) endpoints and the strain of BCG. Sensitivity analysis, sub-group analysis, and meta-regression were implemented to investigate the contribution of moderators to heterogeneity. A random-effect network meta-analysis was performed to compare BCG strains on a multi-treatment level. Results: In total, n = 62 series with n = 15,412 patients in n = 100 study arms and n = 10 different BCG strains were reviewed. BCG Tokyo 172 exhibited the lowest pooled BC recurrence rate among studies with ≤3-y RFS (0.22 (95%CI 0.16–0.28). No clinically relevant difference was noted among strains at >3-y RFS outcomes. Sub-group and meta-regression analyses highlighted the influence of NMIBC risk-group classification and previous intravesical treated categories. Out of the n = 11 studies with n = 7 BCG strains included in the network, BCG RIVM, Tice, and Tokyo 172 presented with the best-predicted probability for efficacy, yet no single strain was significantly superior to another in preventing BC recurrence risk. Conclusion: We did not identify a BCG stain providing a clinically significant lower BC recurrence rate. While these findings might discourage investment in future head-to-head randomized comparison, we were, however, able to highlight some potential enhanced benefits from the genetically different BCG RIVM, Tice, and Tokyo 172. This evidence would support the use of such strains for future BCG trials in NMIBCs.
Keywords: bladder cancer, non-muscle invasive bladder cancer, recurrence rate, BCG immunotherapy, BCG strain, network meta-analysis
1. Introduction
The standard of care for intermediate and high-risk non-muscle invasive bladder cancer (NMIBC) status post trans-urethral resection of bladder tumor (TURBT) with or without secondary resection (Re-TUR) is represented by an adjuvant induction course of intravesical Bacillus of Calmette-Guérin (BCG) followed by an adequate maintenance schedule for at least one year [1]. Additionally, Randomized Controlled Trial (RCT) evidence has suggested that the three years of the maintenance schedule originally described by Lamm et al. (i.e., a total of n = 27 intravesical instillations within 3 years) was able to provide an additional reduction of recurrence in high-risk cases, but there was no difference in progression and cancer survival when the data was examined over the initial one-year period [2]. Of note, this was not similarly true for intermediate risk tumors [3]. Nevertheless, drug-related toxicity, compliance, and a shortage of BCG availability make the completion of the planned treatment schedule challenging for many patients. In particular, BCG production and global distribution remain particularly fraught. The currently available strains adopted for NMIBC are indeed the result of years of increasing genotypic diversity from the original mycobacterium bovis seed released in 1921 by Calmette and Guérin as per the tuberculosis vaccine [4,5]. The subsequent end of production of the BCG Connaught strain in 2012 resulted in a global shortage of BCG, for which European Association of Urology (EAU) Guidelines developed a statement by the NMIBC Panel exploring the possible available options including reduced maintenance schedule and frequency or alternative treatments such as device-assisted chemotherapy instillations (microwave-induced hyperthermia [RITE] or electromotive drug administration [EMDA]) [6]. Unfortunately, in most cases, these options are experimental or less effective while significantly more expensive, highlighting the issues of BCG shortages [7,8].
According to the literature, there is evidence that genetically distinct BCG strains have been associated with differences in immune responses, providing possible differences in recurrence rate outcomes [4]. Despite this evidence, the currently available EAU Guidelines do not offer any conclusive indications as to individual BCG strains and their impact on anti-tumor efficacy.
One relatively recent meta-analysis that compared different BCG strains was published in 2017 by Boehm et al. [9]. Although the tremendous effort endured by the authors, the outcomes presented were mainly made with intravesical chemotherapy as the common comparator for the expression of the effect size estimators as well on BCG naïve population and with no clear distinction on the different available time-dependent recurrence rate endpoints commonly adopted by the NMIBC series. Additionally, in the last 5 years, there has been renewed interest in retrospective cohort studies which assess this unresolved topic, making an updated analysis on the topic especially germane.
Therefore, our aim was to provide an up-to-date review of the literature on the topic of BCG strain comparison by conducting a cumulative meta-analysis of event rates for bladder cancer recurrence assessed at different recurrence-free survival (RFS) endpoints. Accordingly, we developed a systematic review and meta-analysis comparing the effectiveness of different treatments of the most commonly adopted BCG strains, with varying doses and schedules accounting for the most known and validated NMIBC survival confounders. We also conducted a network meta-analysis, including those studies that adopted different BCG strains on a multi-treatment level, to explore the compared efficacy of one specific strain over the other. Furthermore, we aimed to be as inclusive as possible in our inclusion criteria to assess and balance the relative effect of different BCG schedules, dosages, and a variety of clinical scenarios.
2. Methods
This systematic review and network meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. A research question was established based on the Patient-Index test-Comparator-Outcome-Study design (PICOS) criteria as the following: is there any superiority in terms of efficacy profile for preventing bladder cancer recurrence rate amongst different available BCG strains?
Furthermore, our goal was to compare current evidence within all the available retrospective/prospective and/or single-/multicenter cohort studies applying different BCG strains in the adjuvant setting of patients treated by therapeutic and/or sampling trans-urethral resection (TUR) followed or not followed by secondary resection (Re-TUR). In particular, we determined the pooled cumulative recurrence rate and a network multi-treatment comparison meta-analysis among all the available BCG strains utilized worldwide over the last forty years. Finally, our review has been revised and approved by PROSPERO with the following reference number: CRD42022380372.
2.1. Evidence Acquisition
We performed a systematic review of the literature in PubMed, Scopus, Web of Science, Embase, and Cochrane from 1982 to November 2022 to identify studies that examined the implementation of intravesical BCG immunotherapy for the adjuvant treatment of NMIBC and evaluated the pooled event rate trends and network indirect/direct comparison between the available series. The reference lists of the included studies were also screened for relevant articles. Both original prospective and retrospective cohort studies were included and critically evaluated (Level of Evidence: II and III-a), as well as randomized controlled trials (RCTs) focused on different BCG outcomes with comparisons on schedule, dose, and concomitant additional therapies where the type of strain administered was specified. Case reports, abstracts, and meeting reports were excluded from the analysis. Search terms included, but were not limited to: bladder cancer, AND non-muscle invasive bladder cancer AND adjuvant BCG immunotherapy or intravesical BCG implementation AND BCG strain or sub-strain AND efficacy profile AND recurrence rate AND; secondary fields: non-muscle invasive bladder cancer and recurrence rate; BCG strain and efficacy profile; BCG intravesical immunotherapy and schedule; BCG intravesical immunotherapy and dose. A comprehensive list of primary and secondary fields of search criteria has been presented in Supplementary Materials Table S1.
2.2. Selection of the Studies and Criteria of Inclusion
Entry into the analysis was restricted to data from original articles that examined patients with primary and/or recurrent NMIBC diagnoses, classified as intermediate- and/or high-risk according to the European Organization for Research and Treatment of Cancer (EORTC) and/or EAU groups and that reported the rate of BC recurrences over the course of variable follow-up time after the administration of intravesical BCG immunotherapy. In addition, only those studies which included data to reconstruct the number of the recurrence events assigned per each different BCG strain and that was structured on a single or multiple comparison arm level were considered suitable for further consideration. In the multi-comparison experiences, studies were considered eligible if at least one of the involved study arms declared the strain of BCG adopted regardless of the schedule, dosage, and BC history of the participants.
Articles were excluded if they met one or more of the following criteria: inadequate information for data extraction or quality assessment; inclusion of study population consisting of <10 patients; presented outcomes which dealt with other topics (e.g., BCG non for BC immunotherapy, BCG, and other outcomes rather than recurrence such as BC progression and/or side effects).
Five authors (FDG, VA, EB, CMS, EDB) independently screened the titles and abstracts of all articles using predefined inclusion criteria. The full-text articles were examined independently by the five (FDG, VA, EB, CMS, EDB) to determine whether or not they met inclusion criteria. Final inclusion was determined by the consensus of all investigators. Selected articles were then critically analyzed. The following data were extracted from the included studies by using a standardized form: the origin of study (institution and period of enrollment), size of the study population, period of time prospectively/retrospectively covered (i.e., Recurrence-free survival [RFS] endpoints, mean/median follow-up time), previous BC history (only primary, only recurrent, mixed primary/recurrent NMIBCs), previous intravesical therapy (i.e., previous intravesical chemotherapy [CHT] and/or BCG naïve or already BCG treated), EAU risk group (only intermediate, only high-risk, mixed intermediate/high-risk), schedule of BCG (only induction, induction plus maintenance), BCG dose (i.e., full, half, one-third). Finally, baseline clinical and demographic patient information and tumor features (e.g., mean/median age, range of patients, gender representation, percentage of smoking, etc.) were annotated.
2.3. Assessment of Quality for Studies Included and Statistical Analysis
To assess the risk of bias (RoB), all included experiences were independently reviewed using the “Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies” provided by the National Health Institute (NIH) [11]. Biases screened included selection bias, information bias, and measurement bias or confounding bias (including cointerventions, differences at baseline in patient characteristics, etc.). Studies were rated as poor quality, fair or good, with higher RoB leading to poor quality (“−”) ratings and low RoB leading to good quality (“+”) ratings. Publication bias was tested both by visual assessment of the Deeks’ funnel plot and calculation of the p-value using the Deeks’ asymmetry test [12]. The ‘Trim and Fill’ method was implemented to explore the possible nature of studies “missed” in the review [13]. Statistical analyses, along with reporting and interpretation of the results, were conducted according to the previously described methodology [14,15,16,17] and consisted of two analytical steps.
First, a conventional meta-analysis of the pooled event rate (i.e., BC recurrence rate) and 95% confidence intervals (CIs) was performed using random effect according to DerSimonian–Laird method [18]. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual trials one at a time and recalculating the pooled estimates for the remaining studies (leave-one-out meta-analysis). Evaluation for the presence of heterogeneity was done using [12]: (I) Cochran’s Q-test with p < 0.05 signifying heterogeneity; (II) Higgins I2 test with inconsistency index (I2) = 0–40%, heterogeneity might not be important; 30–60%, moderate heterogeneity; 50–90%, substantial heterogeneity; and 75–100%, considerable heterogeneity. Our results are graphically displayed as forest plots on a per-single study arm level, with pooled results indicating the overall BC recurrence rate across each series implementing different types of BCG strains. Subgroup analyses were performed looking at differences in categorical confounders (e.g., EAU risk group, BC history, study design, BCG schedule, BCG dosage, prev. intravesical CHT, etc.). Meta-regression analyses were performed using available continuous variables retrieved from the studies. Pooled weighted estimates were plotted against the following available quantitative variables: mean/median age of the patients, the total number of patients study arm patients, range of study time screened (months retrospectively or prospectively imputed), the relative percentage gender distribution as well as smoking and BCG-specific study characteristics.
Second, a random-effect network meta-analysis within a Bayesian framework [19] was performed to evaluate the relative efficacy of the available studies comparing different intravesical BCG strains on a multi-treatment level. Odds ratios (ORs) with 95% CI of each contrast between strains on the outcome of BC recurrence were displayed. The surface under the cumulative ranking curve (SUCRA) [20] was used to estimate the ranking probabilities for the different BCG stains in order to obtain a treatment hierarchy for the outcome of BC recurrence. An absolute measure of fit residual deviance was considered to formally check the model’s overall fit [21]. The presence of inconsistency in network meta-analysis was evaluated by a loop-specific approach [22]. Inconsistency with 95% CIs between direct and indirect analysis for the comparison of each outcome was calculated to assess the presence of inconsistency in each loop. Inconsistency was defined as a disagreement between direct and indirect evidence with a 95% CI excluding 0.
Calculations were accomplished using the ‘meta’ and ‘network setup’ packages on Stata version 17.1 (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Search Results
The initial search yielded n = 282 articles (PubMed: 258; Cochrane: 17; and Embase: 7). n = 57 were excluded as they contained overlapping data or were duplicates appearing in multiple databases. Of the remaining n = 220, n = 25 were further excluded because they did not examine BCG therapy as per adjuvant intravesical use in NMIBCs (n = 23), did not report information regarding the type of BCG strain adopted (n = 27) or were review papers, editorials, or abstracts (n = 43). Full-text articles were then reevaluated and critically analyzed for the remaining n = 125 journal references. Within this in-depth review, a further n = 63 did not meet the inclusion criteria. The remaining n = 62 studies were included in the quantitative analysis (Supplementary Materials Figure S1). No study was considered to be seriously flawed, and performance bias was overall low, with some attrition bias due to incomplete outcome data across all the studies. Individual RoB, as well as visual assessment of the Deeks’ funnel plots, are illustrated in Supplementary Materials Table S2 and Supplementary Material Figure S2, respectively.
3.2. Characteristics of The Populations, Study Design, and Location
Sixty-two studies [2,3,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] were included in the systematic review with a total of n = 15,412 patients who received adjuvant intravesical BCG following therapeutic and/or sampling TUR ± Re-TUR with EAU intermediate/high-risk NMIBCs. The number of single study arms was n = 100 [2,3,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82], including a total of n = 10 [2,3,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] different BCG strains adopted. A comprehensive list of study characteristics is presented in Table 1. The study period was from 1982 to 2022, including both male and female subjects with mean age comprised of 53 to 77 who had been followed for a median time of 43 months (range, 12–108 months). The vast majority of the studies (n = 34, [24,25,26,27,28,29,34,35,36,37,38,39,40,41,42,48,50,51,53,55,56,60,62,63,65,68,69,71,75,76,77,79,80,81]) were single-arm with no direct comparison across other strains. On the contrary, the remaining n = 27 [2,3,23,30,31,32,33,43,44,45,47,49,52,54,57,58,59,61,64,66,67,70,72,73,74,78,82] were dual or multi-treatment comparison analysis. The cumulative sample size for each study arm was directly associated with the study design with n = 79 [2,3,23,24,25,26,27,28,29,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,60,62,64,65,67,68,69,70,71,72,73,74,75,76,79,80,82] prospective cohort analysis including from a minimum to a maximum of n = 9 and n = 816 patients respectively. Additionally, out of the prospective series, n = 12 were head-to-head randomized controlled comparisons yet not primarily focused on strain comparison but rather assessing differences in BCG maintenance schedule (n = 7, [2,44,45,47,52,64,82]), dose (n = 4, [3,23,54,74]) or additional related adjuvant therapies (n = 1), [72]). Retrospective series were in total n = 21 [30,31,32,33,59,61,63,66,77,78,81], accounting for a consistently larger median sample (from n = 27 to n = 1142) and investigating all the multi-treatments BCG comparisons (n = 5, [30,32,33,59,78]). Finally, the systematic review included worldwide experiences, with the vast majority of the studies deriving from Europe (n = 20), followed by Japan (n = 12) and the USA (n = 8), while in several cases (n = 12) there a multicenter design was implemented with no primary institution contribution detectable (Table 1).
Table 1.
Clinical, demographic, and BCG-related features of the studies and patients enrolled in the systematic review and meta-analysis. MIBC: muscle-invasive bladder cancer; EAU: European Association of Urology; BCG: Bacillus Calmette–Guérin; yr: Year; mo: Months.
| Study Author | Year | Study Design | Sample Size (n) | Median Age (yr) |
Male Gender (%) |
EAU Risk Group |
Previous BC History | BCG Strain |
BCG Schedule | BCG Dose | Previous BCG | Follow-Up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agrawal [23] | 2007 | Prospective | 128 | 64.5 | 71.8 | Only Intermediate | Primary /Recurrent |
Danish 1331 | Induction & Maintenance | Full Dose vs. one-third Dose | Naïve | 36 |
| Ali-el-dein [24] | 1999 | Prospective | 58 | 58.5 | 72.4 | Intermediate/ High-risk |
Primary /Recurrent |
Pasteur | Induction & Maintenance | Full Dose | Naïve | 30 |
| Arend [25] | 2016 | Prospective | 190 | 67.4 | 84.2 | Intermediate/ High-risk |
Primary /Recurrent |
Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 24 |
| Bilen [26] | 2000 | Prospective | 21 | 53 | 95.2 | Only High-risk | Primary /Recurrent |
Connaught | Induction only | Full Dose | Naïve | 18 |
| Brosman [27] | 1982 | Prospective | 39 | 63.4 | 73.7 | Intermediate/ High-risk |
Recurrent only | Tice | Induction & Maintenance | Full Dose | Recurrent | 24 |
| Cai [28] | 2008 | Prospective | 81 | 69.8 | 86.4 | Only High-risk | Recurrent only | Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 15 |
| Cheng [29] | 2005 | Prospective | 102 | 70.1 | 71.5 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance | Full Dose | Naïve | 23 |
| D’Andrea [30] | 2020 | Retrospective | 660 | 63 | 89.7 | Intermediate/ High-risk |
Primary /Recurrent |
Moreau/Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 41 |
| Dai Koguchi [31] | 2020 | Retrospective | 78 | 76 | 82.5 | Intermediate/ High-risk |
Primary /Recurrent |
Tokyo 172 | Induction & Maintenance/ Induction only | Half-dose | Naïve | 35 |
| Del Giudice [32] | 2021 | Retrospective | 422 | 67 | 67.7 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught/RIVM/Tice | Induction & Maintenance | Full Dose | Naïve | 73 |
| Del Giudice [33] | 2022 | Retrospective | 852 | 68 | 74.4 | Only High-risk |
Primary only | Tice/RIVM | Induction & Maintenance | Full Dose | Naïve | 53 |
| Dereijke [34] | 2005 | Prospective | 84 | - | 94 | Only High-risk |
Primary /Recurrent |
Connaught | Induction only | Full Dose | Naïve & Recurrent | 60 |
| Di Lorenzo [35] | 2010 | Prospective | 40 | 71.4 | 55 | Only High-risk |
Recurrent only | Connaught | Induction & Maintenance | Full Dose | Recurrent | 16 |
| Di Stasi [36] | 2006 | Prospective | 105 | 67 | 81.9 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance | Full Dose | Naïve | 88 |
| Farah [37] | 2014 | Prospective | 60 | 61.7 | 83.3 | Intermediate/ High-risk |
Primary /Recurrent |
RIVM | Induction & Maintenance | Full Dose | Naïve | 48 |
| Friedrich [38] | 2007 | Prospective | 163 | 67 | 80.4 | Intermediate/ High-risk |
Primary /Recurrent |
RIVM | Induction only | Full Dose | Naïve | 36 |
| Gontero [39] | 2013 | Prospective | 120 | 67.5 | - | Only Intermediate | Primary only | Connaught | Induction & Maintenance | One-third Dose | Naïve | 12 |
| Gruenwald [40] | 1997 | Prospective | 40 | 68.5 | 87.5 | Only High-risk |
Primary /Recurrent |
Pasteur | Induction only | Full Dose | Naïve | 28 |
| Hemdan [41] | 2014 | Prospective | 126 | - | 79.2 | Intermediate. /High-risk |
Primary only | Tice | Induction & Maintenance | Full Dose | Naïve | 60 |
| Herr [42] | 2012 | Prospective | 156 | 68 | 67.5 | Only High-risk |
Primary /Recurrent |
Connaught | Induction only | Full Dose | Naïve | 24 |
| Herr [43] | 2011 | Prospective | 816 | 64 | 73 | Only High-risk |
Primary /Recurrent |
Connaught | Induction only | Full Dose | Naïve & Recurrent | 24 |
| Herr [44] | 2007 | Prospective | 805 | 65 | 76 | Only High-risk |
Recurrent only | Connaught | Induction & Maintenance /Induction only |
Full Dose | Naïve & Recurrent | 24 |
| Hinotsu [45] | 2011 | Prospective | 83 | - | 95.2 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance /Induction only |
Full Dose | Naïve & Recurrent | 24 |
| Hinotsu [46] | 2006 | Prospective | 40 | 64.3 | 78 | Intermediate/ High-risk |
Primary /Recurrent |
Tokyo 172 | Induction only | Full Dose | Naïve | 36 |
| Hudson [47] | 1987 | Prospective | 42 | 67 | 71.4 | Only Intermediate | Primary only | Pasteur | Induction & Maintenance /Induction only |
Full Dose | Naïve | 17 |
| Ibrahiem [48] | 1988 | Prospective | 17 | 55 | 82.3 | Intermediate/ High-risk |
Recurrent only | Montreal Armand Frappier | Induction & Maintenance | Full Dose | Naïve & Recurrent | 28 |
| Inamoto [49] | 2013 | Prospective | 38 | 72.5 | 85 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught/Tokyo 172 | Induction & Maintenance | Full Dose /Half Dose |
Naïve & Recurrent | 12 |
| Jarvien [50] | 2009 | Prospective | 44 | 68 | 77.2 | Intermediate/ High-risk |
Recurrent only | Pasteur | Induction & Maintenance | Full Dose | Naïve | 60 |
| Kamat [51] | 1994 | Prospective | 95 | 54.1 | 86 | Intermediate/ High-risk |
Primary /Recurrent |
Danish 1331 | Induction Only | Full Dose | Naïve | 60 |
| Koga [52] | 2010 | Prospective | 51 | 74 | 79 | Intermediate/ High-risk |
Primary /Recurrent |
Tokyo 172 | Induction & Maintenance /Induction only |
Full Dose | Naïve | 29 |
| Lamm [2] | 2000 | Prospective | 384 | 67 | 90.1 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance /Induction only |
Full Dose | Naïve & Recurrent | 84 |
| Lamm [53] | 1995 | Prospective | 469 | 66.5 | 84 | Intermediate/ High-risk |
Primary /Recurrent |
Tice | Induction & Maintenance | One-third Dose | Naïve | 30 |
| Martinez-Pieniro [54] | 2002 | Prospective | 500 | - | 89.3 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance | Full Dose /one-third Dose |
Naïve | 69 |
| Martinez-Pieniro [55] | 1990 | Prospective | 67 | 65 | 82 | Intermediate/ High-risk |
Primary /Recurrent |
Pasteur | Induction only | Full Dose | Naïve | 36 |
| Marttila [56] | 2016 | Prospective | 115 | 69.5 | 74 | Only Intermediate | Primary /Recurrent |
Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 90 |
| Melekos [57] | 1996 | Prospective | 46 | 65.4 | 89.1 | Intermediate/ High risk |
Primary /Recurrent |
Pasteur/Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 35 |
| Mukherjee [58] | 1992 | Prospective | 21 | - | - | Intermediate/ High-risk |
Primary /Recurrent |
Glaxo /Pasteur |
Induction only | Full Dose | Naïve & Recurrent | 12 |
| Nowak [59] | 2021 | Retrospective | 590 | 71.1 | 85.3 | Only High Risk | Primary only | Moreau/ RIVM/Tice |
Induction & Maintenance | Full Dose | Naïve & Recurrent | 40 |
| Oddens [3] | 2012 | Prospective | 1355 | 69 | 80.7 | Intermediate/ High-risk |
Primary /Recurrent |
Tice | Induction & Maintenance | Full Dose/ one-third Dose |
Naïve | 84 |
| Ojea [60] | 2007 | Prospective | 430 | - | 88 | Only Intermediate | Primary /Recurrent |
Connaught | Induction & Maintenance | One-third Dose | Naïve & Recurrent | 57 |
| Okamura [61] | 2011 | Retrospective | 75 | 68 | 88.8 | Intermediate/ High-risk |
Primary /Recurrent |
Tokyo 172 | Induction & Maintenance/ Induction only |
Full Dose | Naïve | 66 |
| Oosterlinck [62] | 2011 | Prospective | 48 | 70 | 81.3 | Intermediate/ High-risk |
Primary only | Tice | Induction & Maintenance | Full Dose | Naïve | 56 |
| Ourfali [63] | 2019 | Retrospective | 402 | - | - | Only High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance | Full Dose | Naïve | 24 |
| Palou [64] | 2001 | Prospective | 126 | 63 | 95 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance /Induction only |
Full Dose | Naïve | 78 |
| Peyromaure [81] | 2003 | Retrospective | 57 | 65.4 | - | Intermediate/ High-risk |
Primary /Recurrent |
Connaught | Induction & Maintenance | Full Dose | Naïve & Recurrent | 48 |
| Porena [65] | 2010 | Prospective | 64 | 68.7 | 87.5 | Only High-risk | Primary only | Tice | Induction & Maintenance | Full Dose | Naïve | 44 |
| Prasanna [66] | 2017 | Retrospective | 103 | 77 | 83 | Intermediate/ High-risk |
Primary /Recurrent |
Tice | Induction & Maintenance | Full Dose | Naïve & Recurrent | 15 |
| Rentsch [67] | 2014 | Prospective | 131 | 72 | 83.3 | Only High risk | Primary /Recurrent |
Connaught/ Tice |
Induction only | Full Dose | Naïve & Recurrent | 51.4 |
| Rintala [68] | 1991 | Prospective | 51 | 68 | 76.4 | Intermediate/ High-risk |
Primary /Recurrent |
Pasteur | Induction only | Full Dose | Naïve | 28 |
| Sekine [69] | 2001 | Prospective | 42 | 72 | 80.9 | Only High-risk |
Primary /Recurrent |
Tokyo 172 | Induction only | Full Dose | Naïve | 47 |
| Sengiku [70] | 2013 | Prospective | 129 | 70.7 | 76.1 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught/ Tokyo 172 |
Induction only | Full Dose | Naïve | 28 |
| Shinka [71] | 1997 | Prospective | 141 | - | 83 | Intermediate/ High-risk |
Primary only | Tokyo 172 | Induction only | Full Dose | Naïve | 60 |
| Shinka [72] | 1989 | Prospective | 56 | 67.8 | - | Only High-risk |
Recurrent only | Tokyo 172 | Induction only | Full Dose | Naïve | 20 |
| Sood [73] | 2020 | Prospective | 104 | 58 | 94.1 | Intermediate/ High-risk |
Primary only | Sii Onco BCG | Induction & Maintenance | Full Dose | Naïve | 36 |
| Steinberg [74] | 2016 | Prospective | 398 | 70 | 78.4 | Intermediate/ High-risk |
Primary /Recurrent |
Connaught/ Tice |
Induction & Maintenance | Full Dose /one-third Dose |
Naïve & Recurrent | 24 |
| Sylvester [75] | 2010 | Prospective | 281 | 66 | NR | Intermediate/ High-risk |
Primary /Recurrent |
Tice | Induction only | Full Dose | Naïve | 108 |
| Takashi [76] | 1998 | Prospective | 84 | 65.3 | 75 | Only High-risk |
Primary /Recurrent |
Tokyo 172 | Induction only | Full Dose | Naïve | 56 |
| Takashi [77] | 1997 | Retrospective | 30 | 65.6 | 83.3 | Only High-risk |
Primary /Recurrent |
Tokyo 172 | Induction only | Full Dose | Naïve | 60 |
| Witjes [78] | 2016 | Retrospective | 2099 | - | 82.8 | Intermediate/ High risk |
Primary /Recurrent |
Connaught/ Tice |
Induction & Maintenance | Full Dose | Naïve & Recurrent | 62.4 |
| Witjes [79] | 1999 | Prospective | 55 | - | 85.7 | Only High-risk |
Primary only | Tice | Induction & Maintenance | Full Dose | Naïve | 12.3 |
| Yalcinkaya [80] | 1997 | Prospective | 80 | 55.2 | 55 | Intermediate/ High risk |
Primary /Recurrent |
Connaught | Induction only | Full Dose | Naïve | 33 |
| Yoo [82] | 2012 | Prospective | 126 | 61.7 | 81.5 | Intermediate/ High risk |
Primary /Recurrent |
Tice | Induction & Maintenance /Induction only |
Full Dose | Naïve | 24 |
3.3. BCG-Specific Characteristics: Stains, Schedule, and Dose
The type of BCG schedule administration retrieved accounted for study arms assessing induction only (n = 39 [2,26,31,34,38,40,42,43,44,45,46,47,51,52,55,58,61,64,67,68,69,70,71,72,75,76,77,80,82]) and induction followed by any type of maintenance schedule (n = 61 [2,3,23,24,25,27,28,29,30,31,32,33,35,36,37,39,41,44,45,47,48,49,50,52,53,54,56,57,59,60,61,62,63,64,65,66,70,71,72,73,73,74,74,75,76,77,78,81,82]).
Similarly, the majority of the single study arm based their comparison on a full BCG dose (n = 87 [2,3,23,24,25,26,27,28,29,30,32,34,35,36,37,38,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,56,57,58,59,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,79,80,81,82]) followed by half-dose n = 2 [31,49] with only n = 9 [3,48,53,54,60,74] experiences that fractioned the BCG administration to one-third of the original dose.
BCG Connaught was the most represented strain adopted, with a total sample of the patient included in this group accounting for n = 6960 patients. Overall, n = 23 [2,26,29,32,34,35,36,39,42,43,44,45,49,54,60,63,64,67,70,74,78,80,81] studies and n = 33 single-arm comparisons were based on its utilization. Moreover, before the suspension of its production happened in 2012, this strain was also the most used from the 2000s onwards. This strain was also significantly implemented in prospective longitudinal cohort studies with only n = 4 [33,64,79,82] retrospective series maintaining a good balance regarding the type of schedule and dose administered.
BCG Tice was the second most represented strain adopted, accounting for a total of n = 5326 patients. Overall, n = 21 [3,25,27,28,30,32,33,41,53,56,57,59,62,65,66,67,74,75,78,79,82] studies and n = 27 single-arm comparisons were based on its utilization. This strain was mostly studied in prospective longitudinal cohort studies with only n = 6 [30,32,33,59,66,78] retrospective series with a predominance of induction plus maintenance schedule and full dose administered.
For the BCG Tokyo 172 strain, there were a total of n = 726 patients, with all the studies deriving from Japan. Overall, n = 11 [31,46,49,52,61,69,70,71,72,76,77] studies and n = 16 single-arm comparisons were analyzed. This strain was also significantly utilized in prospective longitudinal cohort studies with only n = 5 [31,61,77] retrospective series maintaining a good balance regarding the type of schedule, with a full dose mostly administered.
The Pasteur strain accounted for a total of n = 8 [24,47,48,50,55,57,58,68] studies. All the studies included in this subgroup were prospective, considering a full-dose BCG treatment, with a good balance regarding the type of schedule and only full dose adopted.
Regarding the remaining series and related BCG stains, there were n = 11 [23,30,32,33,37,38,48,51,58,59,73] studies and n = 15 [23,30,32,33,37,38,48,51,58,59,73] single-arm comparisons for a total of further n = 2021 patients. Six strains were mentioned in the studies, specifically RIVM (n = 5 [32,33,37,38,59]), Danish 1331 (n = 3 [23,51]), Moreau (n = 2 [30,59]), Glaxo (n = 2 [58]), Montreal Armand Frappier (n = 1 [48]) and Sii Onco BCG (n = 2 [73]). These strains were mostly studied in prospective longitudinal cohort studies with only n = 4 [30,32,33,59] retrospective series.
3.3.1. BC Recurrence Rate Meta-Analysis
From the n = 62 studies analyzed [2,3,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82], the cumulative BC recurrence rate within the n = 100 single-arm assessed varied from 0.08 (95%CI −0.06–0.24) to 0.88 (95%CI 0.57–1.19) irrespective of any confounders, sub-group, or BCG-specific covariates. As expected, at this preliminary assessment, there was substantial heterogeneity across every single study arm with I2 82.67%, Q (101): 583.26, p < 0.001. Publication bias was initially assessed by Galbright and Funnel plot (Supplementary Materials Figure S2A). The inspection of both plots suggested that there was no small-study effect, with the smaller studies tending to have higher recurrence rate estimates, suggesting the absence of publication bias (Egger test, p = 0.19). Additionally, the “Trim and Fill” method suggested that only n = 2 studies would have needed to be included to remove residual asymmetry from the funnel plot. The main contribution to single-arm variability was identified by both demographic cohort imbalances and clinical BC characteristics and, most importantly, by BCG-specific confounders such as strains, administration schedule, dosage, as well as RFS time points, and study design. A detailed list of sub-group analyses is presented in (Supplementary Materials Figure S3A,B). The total number of BCG strains assessed was n = 10. Nevertheless, given the observed wide variability in the influence of those BCG strains studies with no more than n = 150 patients per study arm on pooled estimates, BCG Danish 1331 (pts. n = 130), Glaxo (pts. n = 24), Montreal Armand Frappier (pts. n = 17), and Sii-onco (pts. n = 87) were excluded from the cumulative recurrence rate meta-analysis. Additionally, since the well knows the influence of the time-dependent effect on BC recurrences by predefined RFS cut-off within each study, the results had been reported stratified by ≤3-y and >3-y RFS timepoints, respectively. Such criterion is derived, on the one hand, from the common literature-based RFS endpoints for NMIBCs usually set at 3-y and 5-y and, on the other hand, from the observation of non-clinically relevant variability observed within these predefined ranks across the studies enclosed.
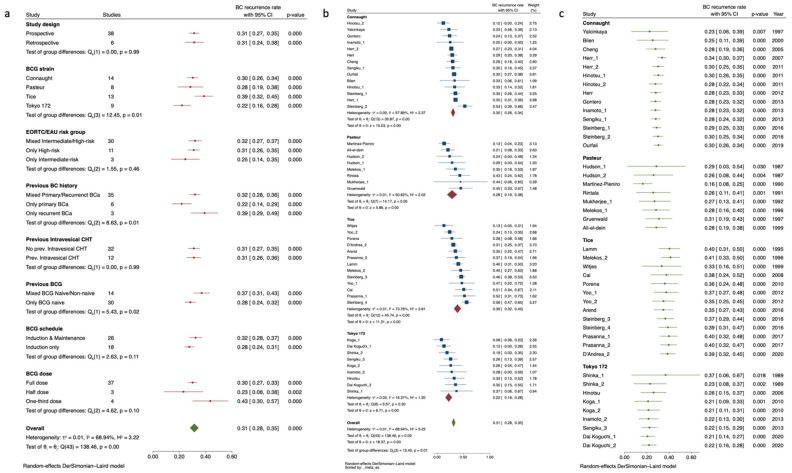
3.3.2. BC Recurrence Rate by ≤3-y RFS Endpoints and BCG Strain
N = 2 BCG strains were evaluated by only n = 2 study arms [23,73], as well as the study of Di Lorenzo et al. [35] was the sole significantly affecting the heterogeneity statistic, and these were therefore removed from the analysis. Thus, the sub-sample of 1 up to 3 y RFS included n = 33 studies [31,39,40,42,43,44,45,46,47,49,52,53,55,57,58,63,65,66,68,70,71,74,79,80,82] assessing n = 4 BCG strains (i.e., Connaught, Pasteur, Tice, and Tokyo 172) and n = 5123 patients accounting for a cumulative recurrence rate of 0.31 (95%CI 0.28–0.35). This data clustering led to a significant reduction in the overall in-studies heterogeneity, now accounting for I2 68.94%, Q (95): 138.46, p = 0.001. At this time, an inspection of the funnel plot suggested that there was no small-study effect, with the smaller studies tending to have higher recurrence rate estimates, suggesting an absence of publication bias (Egger test, p = 0.67). The “Trim and Fill” method suggested that no “missing” studies needed to be included to remove any asymmetry from the funnel plot (Supplementary Materials Figure S2B). Residual inconsistency was found to be attributable to previous BC history, previous BCG treatment, and BCG strains adopted, as detailed in the list of sub-group analysis Figure 1a. On meta-regression analysis, we found no significant nor clinical correlation among all quantitative moderators assessed in the study except from the increasing relative percentages of smokers across the studies reporting the information (Coeff: 0.01, SE: 0.004, p = 0.006) and the percentages of previously BCG treated patients (Coeff: 0.002, SE: 0.0009, p = 0.004) as depicted in Supplementary Materials Figure S4A.
Figure 1.
Sub-groups analysis for BC recurrence rate by studies with ≤3-y RFS endpoints exploring heterogeneity according to categorical confounders (e.g., study, BCG, and NMIBC characteristics) (a). Forrest plot depicting BC recurrence rate by studies with ≤3-y RFS endpoints grouped by BCG strains and sorted by increasing effect size (b). Forrest-plot for cumulative meta-analysis by studies with ≤3-y RFS endpoints grouped according to BCG strain and sorted by year of publication (c). BC: Bladder Cancer; BCG: Bacillus Calmette–Guérin; Bladder Cancer; CHT: Chemotherapy; EAU: European Association of Urology.
A close focus on BCG strains across the ≤3-y RFS studies is shown in Figure 1b as an incremental rate by BC recurrence rate and in Figure 1c as a cumulative meta-analysis by publication year. BCG Tokyo 172 showed the lowest recurrence rate with a pooled estimate of 0.22 (95%CI 0.16–0.28) and a reduction of the observed cumulative percentage of recurrence events over the years. The pooled rate of BC recurrence was slightly higher and overlapping for both BCG Connaught and Pasteur (i.e., 0.30, 95%CI 0.26–0.34 and 0.28, 95%CI 0.19–0.38, respectively) while pooled rate observed for BCG Tice was 0.39, 95%CI 0.32–0.45). Interestingly, except from BCG Tokyo 172, we registered a slight but consistent increase in the recurrence rate by cumulative meta-analysis sorted by publication year, possibly indicating a trend toward reduction in efficacy over the years of the administration of such strains.
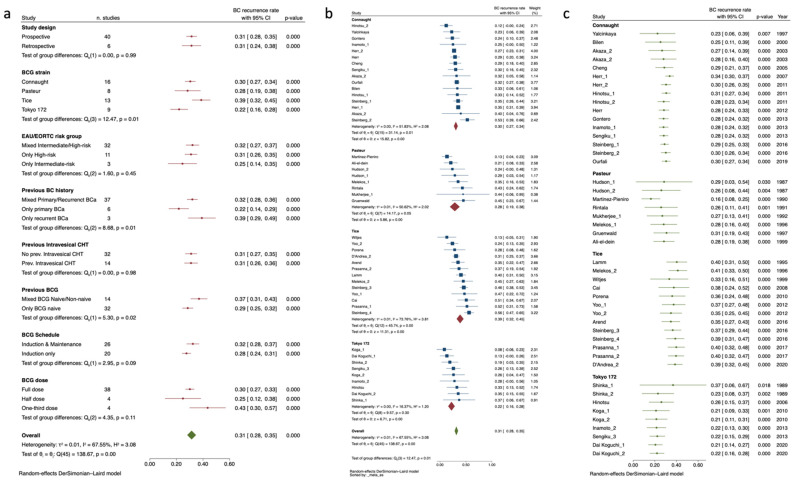
3.3.3. BC Recurrence Rate for >3-y RFS Endpoint and BCG Strain
Due to the significantly reduced sample size, the studies of Mukherjee et [58] and Jarvien et al. [50] were significantly affecting the heterogeneity statistic and were removed from the analysis. Moreover, BCG Moreau was only represented by the study arm of Nowak et al. [59] and thus was excluded from the analysis. Finally, one of the study arms from Herr et al. [44], which accounted for only recurrent BC patients, was removed as per sensitivity analysis. Thus, the updated sub-group of studies reporting more than 3-y RFS timepoints accounted for n = 26 studies assessing in total n = 4 BCG strains (i.e., Connaught, RIVM, Tice, and Tokyo 172) with a cumulative sample size of n = 9207 patients distributed in n = 40 study arms and showing a pooled recurrence rate of 0.40 (95%CI 0.36–0.43). The overall measured study arm heterogeneity resulted, however substantial, with I2 85.93%, Q (95): 277.1, p < 0.001. The rest of the within-study variability for recurrence rate was mainly due to already intravesical CHT-treated patients (0.36, 95%CI 0.31–0.41 vs. 0.48, 95%CI 0.44–0.58) with no further influence from the previously mentioned sub-group category, not even BCG strains. Detailed sub-groups analysis is presented in Figure 2a. Inspection of the funnel plot revealed no small-study effect, with the smaller studies tending to have higher recurrence rate estimates, suggesting an absence of publication bias (Egger test, p = 0.99). Nevertheless, the “Trim and Fill” method suggested that n = 6 “missing” studies would have needed to remove the visual asymmetry from the funnel plot, thus justifying part of the observed heterogeneity (Supplementary Materials Figure S2c). Additionally, the residual heterogeneity was further explored by meta-regression analysis (Supplementary Materials Figure S4B) and once again identified primarily the total sample groups distribution and the relative percentage of previous intravesical CHT administration with direct incremental correlation with the effects sizes (Coeff: 0.0001, SE: 0.000052, p = 0.001, and Coeff: 0.0048, SE: 0.0022, p = 0.032 respectively). Interestingly, male and female gender revealed an inversed relationship with the effect size (Coeff: −0.0075, SE: 0.0024, p = 0.002, and Coeff: 0.004, SE: 0.0014, p = 0.006), which is in line with sex-driven differences in NMIBC recurrence rate outcomes over long term follow-up. Finally, as expected, increasing percentages of subjects who had previously undergone BCG were significantly associated with an increased rate of recurrences over time (Coeff: 0.0032, SE: 0.0009, p = 0.001).
Figure 2.
Sub-groups analysis for BC recurrence rate by studies with >3-y RFS endpoints exploring heterogeneity according to categorical confounders (e.g., study, BCG, and NMIBC characteristics) (a). Forrest plot depicting BC recurrence rate by studies with >3-y RFS endpoints grouped by BCG strains and sorted by increasing effect size (b). Forrest-plot for cumulative meta-analysis by studies with >3-y RFS endpoints grouped according to BCG strain and sorted by year of publication (c). BC: Bladder Cancer; BCG: Bacillus Calmette–Guérin; Bladder Cancer; CHT: Chemotherapy; EAU: European Association of Urology.
At long-term RFS endpoints, there were no clinical nor significant differences in the effect sizes for the n = 4 BCG strains analyzed (Figure 2b). Similar to the previous analysis, BCG Tice showed a slight yet non-significant higher reported recurrence rate when compared with BCG Connaught, RIVM, and Tokyo 172. This last strain also presented here the lowest pooled recurrence rate (0.35, 95%CI 0.21–0.48) yet the highest inter-strain study heterogeneity I2 78.61%, Q (95): 28.05, p < 0.001. Finally, each BCG strain assessed throughout cumulative meta-analysis by year of publication demonstrated a constant and significant trend towards a reduction in efficacy, confirming the same trajectory observed at shorter RFS endpoints (Figure 2c).
3.3.4. Evidence Structure of Network Meta-Analysis for BCG Strain Comparison
In total, n = 11 [30,32,33,49,57,58,59,67,70,74,78] studies accounting for n = 7 different BCG strains assessed across n = 24 study arms met the inclusion criteria and were reviewed for the network mixed-treatment meta-analysis of BC recurrence. The range of study time was wide, accounting for a total of n = 5549 patients treated with intravesical BCG from 1992 to 2022. Of note, the study from Del Giudice et al. [32] and Nowak et al. [59] were the sole experiences directly comparing three strains at once, while the rest was focused on dual comparison within two strains. The network map on multiple comparisons is shown in Supplementary Materials Figure S5. Most of the studies focused on the comparison with BCG Tice. This strain had the highest number of interactions being adopted by n = 8 studies within a range of 26 years, followed by BCG Connaught (n = 6 interactions), BCG RIVM (n = 3 interactions), and BCG Moreau, Pasteur, Tokyo 172 (n = 2 interactions) with BCG Glaxo being adopted only in n = 1 comparison. Half of the studies were conducted in a retrospective design with a consistently larger total sample size reviewed, varying from a minimum of n = 112 patients to a maximum of n = 1142 in the treatment arms of Del Giudice et al. [32] and Witjes et al. [78] respectively. As expected, this was inversely represented when the design was prospective with per single study arm ranging between n = 9 and n = 325 in the study of Mukherjee [58] and Steinberg [74], respectively. The vast majority of the arms from the studies included in the network meta-analysis were homogeneous in the assessment of patients presenting with mixed EAU intermediated/high-risk NMIBCs (n = 17 [30,32,49,57,58,70,74,78]), that received a course of induction followed by maintenance schedule (n = 18 [30,32,33,49,57,59,74,78]), and with the administration of a full BCG dose (n = 23 [30,32,33,49,57,58,59,67,70,74,78]) throughout predefined timepoints for RFS set at 5-y (n = 16 [32,33,34,58,59,67,70,78]). Finally, there was more heterogeneity in the distribution of intravesically naïve arms both for previous BCG and intravesical CHT, respectively (n = 13 [32,33,49,59,67,70,74]).
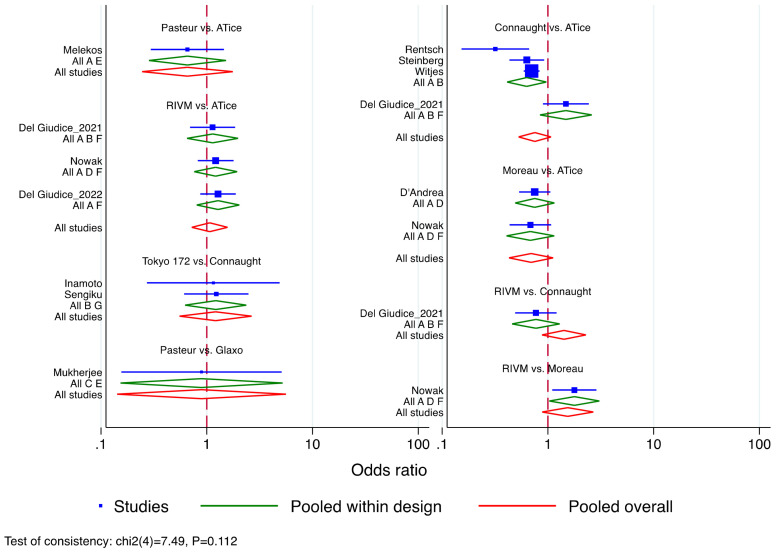
3.3.5. Network Meta-Analysis for Risk of BC Recurrence among Intravesical BCG Strains
Given its wider adoption over the range of study time, BCG Tice was considered for predefined reference (Supplementary Materials Table S3). All the mixed comparisons were non-significant in the efficacy profile for the risk of BC recurrence. However, all the strains provided a trend towards significance in line with reported variability across literature assessing the BCG strain’s efficacy. These findings were therefore implemented to explore the probabilities that each treatment was the best under the consistency model as depicted in the rankogram of the different BCG stains in Supplementary Materials Figure S6. RIVM had a 27.2% cumulative probability of being the best BCG strain as well as the 30.6% and 21.8% of being the second and third best, respectively. Overall, this was followed by BCG Tice (12.5%, 31.7%, and 32.4%) and BCG Tokyo 172 (21.9%, 15.7%, and 14.7%). These assumptions were further confirmed by testing for the inconsistency of the displayed model (p = 0.11). The individual study results, grouped by treatment contrast and design, are displayed in Figure 3. All the assessed comparisons crossed the reference line yet with some interesting insight. BCG Connaught resulted superior to Tice, but this was true only in those treatment contrast where the BCG induction was not followed by any maintenance schedule [67]. The opposite was indeed found when an adequate BCG schedule was implemented [32,74,78]. Finally, in the investigation of the contrast between RIVM vs. Tice and Moreau vs. RIVM and Tice, there were overlapping results in the first case and a tendency favoring Moreau over the last two strains. These results were, in conclusion, validated by further exploring inconsistency by side-splitting of each node of the contrast comparison. The findings once again supported the consistent results aforementioned.
Figure 3.
Forrest plot for bladder cancer (BC) risk of recurrence showing the individual study results grouped by treatment contrast and design.
4. Discussion
Adjuvant BCG immunotherapy for prolonging RFS in intermediate/high-risk NMIBC patients has corroborated on multiple occasions its superiority when compared to endoscopic resection alone and/or adjuvant intravesical chemotherapy [83,84]. This was confirmed by individual-patient data meta-analysis [9,85] and RCT evidence comparing BCG with Epirubicin and interferon, MMC, or Epirubicin alone [50,86]. Moreover, the way BCG should be administered in terms of schedule and dose has also reached a universal uniform agreement. Recently, the phase III non-inferiority NIMBUS trial [87] has indeed failed in demonstrating reduced BCG frequency and dose to provide similar RFS in high-risk patients. This was clear with reduced schedule leading after 12 months of median follow-up to more than double the relative percent of recurrence rate, thus stopping the trial to avoid harm in the reduced frequency arm. Interestingly, the rationale from the NIMBUS trial was based on in-vivo animal trials showing robust immuno-react effects with less than the conventional number of BCG instillations. Despite not reaching the pre-established endpoints, the NIMBUS trial can be considered a comprehensive example of the logistic complexity related to the worldwide BCG supply chain yet delivering a critical message about the beneficial and life-saving effect of this medication in the arduous path of NMIBC patients. The trial was sample size re-adjusted during the course of the enrollment while it was struggling to increase the number of Institutions and patients due to the lack of BCG availability across European countries.
The concept of “adequate BCG exposure”, just lately introduced in the EAU Guidelines, may be interpreted as a proposed alternative for the necessity of facing the world crisis in BCG production. While the original Lamm protocol [2] consisting of a 3-year schedule is indeed the most efficient in preventing recurrence from the high-risk category, this seems nowadays challenge to apply in routine clinical practice both from a patient (due to toxicity and compliance to treatment profile) and industrial chain supply perspective.
The chance to test different BCG strains for exploring more efficient BCG properties derived from variable immunogenicity and reactogenicity in the prevention of recurrence and/or progression is, therefore, particularly appealing since no clear evidence has so far demonstrated the superiority of one strain over the other. Of note, this lack of high-quality evidence has induced the American Food and Drug Administration (FDA) to not expand the manufacturing indication towards other than non-American BCG stains, even during the hardest BCG shortage period.
The hypothesis for existing variable BCG strains efficacy is, however, concrete if we do account for the historical developments and worldwide distribution of the oral and intradermal TB vaccine. The wide variability in BCG strain genetic profiling is indeed conferred by serial laboratories passages which have led to a continuous modification of the freeze-dried seed lots nowadays cultured by different manufacturing companies across the world. Subsequent deletions of the region of difference 1 (RD1) responsible for the protein secretion system ESX-1 have provided different BCG sub-strains, which are nowadays implemented in clinical practice [4]. The interplay between these genetic differences and immuno-reactivity for clinical outcomes was tested in an RCT setting. The experience from Rentsch et al. [66] was able to highlight the longer RFS of BCG RIVM and Connaught, respectively, when compared to BCG Tice. Similar was observed in the largest European cohort study on T1 G3 NMIBCs from Witjes et al. [77], which identified BCG Connaught superiority in preventing recurrence. After adjusting for adequate confounders usually associated with recurrence in NMIBCs, the authors found a significantly longer time to first recurrence on Connaught as compared with Tice (HR, 1.48; 95%CI: 1.20–1.82). The common denominator of these experiences highlighting such different efficacy profiles was represented by the implementation of an induction-only schedule. The results were indeed the opposite when the sole sub-group of patients who had undergone maintenance was reviewed. In the study of Witjes [77], BCG Tice revealed a long-acting potential exhibiting improved efficacy outcomes in the long-term maintenance setting opposing a decrease in the immune response over time demonstrated by BCG Connaught.
In our study, we did not find any consistent differences in the relative efficacy of the BCG strains adopted both at per cumulative event rate level stratified by RFS timepoints and at the network metanalysis of the direct and indirect treatment comparisons. This was also true when comparing our outcomes to those previously published in other series [88,89,90,91]. However, at a closer analysis of our results, BCG Tokyo 172 was the sole exhibiting a relatively lower percentage of pooled BC recurrence (i.e., 0.22, 95%CI 0.16–0.27) but only in the 1 to 3 years RFS time points, yet this not translated in any BCG contrasts superiority at network assessment (Figure 3). Of note, when interpreting results from studies assessing outcomes at longer RFS endpoints (i.e., >3-y RFS), we noticed a slight but constant decrease in the recurrence rate per strain when a cumulative meta-analysis by publication year was applied. While we would be cautious in deeming conclusive conjectures, this could be interpreted as a tendency for lower efficacy of BCG over the course of follow-up, testifying an indirect loss of efficacy potentially related to lowered immunoreactivity. On the other hand, this observed phenomenon could be related to the variation in the NMIBC grading system over time (i.e., 1973 vs. 2004/2016) so that patients enrolled in the different series might not always be overlapping in terms of risk stratification. The additional explanation includes the possibility of intradermal BCG exposure prior to intravesical instillations as per the tuberculosis vaccine to which a large subset of the population in Europe had been exposed in the past four decades.
In addition, in the network meta-analysis, the rank of probable best strains for lowering recurrence was mainly identified in BCG RIVM and Tice. This is in line with what was recently documented by Del Giudice et al. [31] in a large comparative retrospective series of Connaught, RIVM, and Tice. These last two BCG strains exhibited indeed longer RFS, which was also related to a possible better tolerability profile leading to a longer course of maintenance and a total number of instillations delivered. An interesting point of debate was furthermore underlined in a subsequent multicenter experience by the same groups of authors focused on the direct comparison of the sole RIVM and Tice among high-risk NMIBCs [32,92]. What emerged at the sub-group analysis level was that Tice was able to exhibit longer RFS only in those cases where a strict adhesion to EAU guidelines recommendations was followed with regard to routinely performing secondary resection.
Our study is not devoid of limitations. First and most importantly, we would readily admit the existence of substantial single-study variability both within patients population and tumor characteristics as well as per single-strain adopted by each of the different study designs included. While indeed, NMIBC recommendations guidelines for clinical trial development [93] insist on the necessity to avoid hyper-categorization of the different confounders and cohort features which otherwise would be too far from clinical practice reproducibility, this aspect, however, significantly influenced the pooled estimates from meta-analytic calculations across a wide range of study assessed. However, we deeply attempted to reduce the single study and arm heterogeneity by constantly repeating sub-group analysis and meta-regression for the most valuable confounders, including schedule, dosage, previous BC history, and naïve vs. non-naïve intravesical patients. This led to a significant reduction in the interpretation of the displayed results to the sole BCG strains, which have been more consistently reported in the literature, while the less utilized could have been only mentioned yet not cumulatively compared. Moreover, clinical practice and Guidelines have widely changed over the range of the study period, and for this reason, imperative indications such as Re-TUR in high-risk cases could not have been captured. Additionally, our analysis was mainly focused on the outcome of BC recurrence within different BCG strains, and we were not able to cumulatively represent the same analyses on progression and cancer-specific survival due to a lack of an adequate similar number of series assessing the outcomes. However, this could have been misleading since several additional factors, such as the influence of upfront cystectomy in highest-risk patients as well as alternative strategies for BCG unresponsive patients, could have been missed. Finally, we did not report any cumulative estimates on the well-known BCG-related adverse effects and toxicity events across the arms screened nor in the single BCG strains assessment. While this was out of the scope of the current report, we would readily recognize the possible effect of such a confounder of patients censored among the studies due to side effects related to drop-out.
5. Conclusions
Our results do not support any clear advantage of one specific BCG strain over another. The evidence from our results is in line with previous meta-analyses, yet it updates the current state of the art by exploring time-dependent endpoints of BC recurrence rates and extrapolating the comparisons from the trials exhibiting different BCG strains as the common denominator. These findings make the decision to invest in future head-to-head RCTs on BCG strain comparison challenging while possibly diminishing the interest in the manufacturing supply chain to drive the production among strains. However, according to our pooled estimates and network results, future research could be oriented toward BCG Tokyo 172, RIVM, and Tice, which showed possible insights into efficacy profiling. This could be of particular interest, especially now that international NMIBC recommendations from various Guidelines have emphasized the importance of adequate risk-group assessment, secondary resection, as well as frequency, and dose of adjuvant BCG immunotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15071937/s1, Table S1. Comprehensive list of primary and secondary search criteria fields. NMIBC: Non-Muscle Invasive Bladder Cancer; BCG: Bacillus Calmette–Guérin; CHT: Chemotherapy; TURB: Tran-surethral resection of the bladder. Table S2. Risk assessment of individual study enrolled according to the “Quality assessment tool for observational Cohort and Cross-Sectional Studies” released by the National Health Institute (NIH). NA: Not applicable. Table S3. Multi-treatment level comparison (OR, 95%CI) for the risk of BC recurrence among the n = 7 available BCG stains assessed in the network meta-analysis (BCG Tice considered as the ref-erence standard). BCG: Bacillus Calmette–Guérin; OR: odd ratio; CI: Confidence Interval; SE: standard error. Figure S1. PRISMA flow diagram. NMIBC: Non-Muscle Invasive Bladder Cancer; BCG: Bacillus Calmette–Guérin. Figure S2. Publication bias for recurrence rate across the total studies included expressed by Gal-bright plot (I), Funnel plot with regression-based Egger-test for small-study effects (II) and Funnel plot after study’s imputation with the “Trim and Fill” method (III) (A). Publication bias for BC recurrence rate by studies with ≤3-yr RFS endpoints assessed throughout Galbright plot (I), Funnel with regression-based Egger test for small-study effects (II), Funnel plot after the “Trim and Fill” method (III) (B). Publication bias for BC recurrence rate by studies with >3-yr RFS endpoints as-sessed throughout Galbright plot (I), Funnel with regression-based Egger test for small-study effects (II), Funnel plot after study’s imputation with the “Trim and Fill” method (III) (C). RFS: Recur-rence-free survival; CI: Confidence Interval. Figure S3. Forrest plot depicting BC recurrence rate sorted by BCG strains for all the studies enrolled in the systematic review and meta-analysis (A). Sub-groups analysis exploring heterogeneity ac-cording to BC recurrence rate stratified by categorical confounders (e.g., study, BCG and NMIBC characteristics) (B). Forrest-plot for cumulative meta-analysis sorted by publication year and strat-ified according to BCG strain (C). BC: Bladder Cancer; BCG: Bacillus Calmette–Guérin; NMIBC: Non-Muscle Invasive Bladder Cancer; CHT: Chemotherapy; EAU: European Association of Urology. Figure S4. Meta-regression analysis for studies with ≤3-yr RFS endpoint modeling BC recurrence rate according to available continuous demographic, clinic, and BCG-related variables (A). Me-ta-regression analysis for studies with >3-yr RFS endpoint modeling BC recurrence rate according to available continuous demographic, clinic and BCG-related variables (B) BC: Bladder Cancer; BCG: Bacillus Calmette–Guérin; CHT: Chemotherapy; RFS: Recurrence-free survival. Figure S5. Network map for multiple-treatment comparison out of the n = 7 BCG strains included in the analysis. Figure S6. The surface under the cumulative ranking curve (SUCRA) stratified by each BCG strains (A) and its related cumulative probability for being the best vs. the worst BCG treatment strain (B). BCG: Bacillus Calmette–Guérin.
Author Contributions
Conceptualization, F.D.G., V.A., B.I.C., A.S. and E.D.B.; methodology, F.D.G., A.S., E.B., C.M.S., D.C., D.D., B.P., W.K., R.A., G.M.B., T.S., B.I.C., R.P. and O.S.T.; software, P.V.; A.M., C.M.S., L.A., A.G., V.D., B.P., M.L.E., B.M. and Ł.N.; validation, P.V., F.C., M.M., L.S.M., K.M., S.B., E.L., F.S., R.A. and M.F.; formal analysis, F.D.G., V.A., E.B., C.M.S., G.M.B., T.S., A.M., A.G., A.C., S.A. and E.L.; investigation, S.S., G.M., W.K., K.M., O.S.T., S.C., F.C., B.I.C., S.B., M.L.E., P.V. and A.C.; resources, S.S., R.A., D.D., S.C., E.D.B., F.C. and M.L.E.; data curation, F.D.G., T.S., V.A., E.B., C.M.S., D.C., V.D., B.M., Ł.N., P.V., M.M., E.L., G.M., L.A., F.S., K.H.T., M.F., O.S.T. and M.L.E.; writing—original draft preparation S.S., M.M., E.D.B., A.M., A.C., S.A., A.G., F.S., K.H.T., R.P., S.C., O.S.T., F.C., D.C., V.D. and S.A.; writing—review and editing, F.D.G., S.A., V.A., E.B., C.M.S., G.M.B., S.S., G.M., D.D., K.H.T., B.M., Ł.N., L.S.M. and B.P.; visualization, V.D., D.C., L.S.M., L.A., M.M., F.S., A.M., E.L., K.M., W.K., K.H.T., R.P., M.F., S.B., T.S., B.M. and Ł.N.; supervision, F.D.G., A.S., S.A., D.D., A.G., L.A., G.M., A.C., R.A., F.S.,B.P., W.K., K.M., R.P., M.F., G.M.B., S.C., S.B. and L.S.M.; project administration, A.S., B.I.C. and E.D.B.; funding acquisition: NA; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Conflicts of Interest
Authors have no conflict of interest to disclose.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Escrig J.L.D., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ) Eur. Urol. 2021;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Lamm D.L., Blumenstein B.A., Crissman J.D., Montie J.E., Gottesman J.E., Lowe B.A., Sarosdy M.F., Bohl R.D., Grossman H.B., Beck T.M., et al. Maintenance bacillus calmette-guerin immunotherapy for recurrent ta, t1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study. J. Urol. 2000;163:1124–1129. doi: 10.1016/S0022-5347(05)67707-5. [DOI] [PubMed] [Google Scholar]
- 3.Oddens J., Brausi M., Sylvester R., Bono A., van de Beek C., van Andel G., Gontero P., Hoeltl W., Turkeri L., Marreaud S., et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guérin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. Eur. Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Gan C., Mostafid H., Khan M.S., Lewis D.J. BCG immunotherapy for bladder cancer—The effects of substrain differences. Nat. Rev. Urol. 2013;10:580–588. doi: 10.1038/nrurol.2015.114. Erratum in Nat. Rev. Urol. 2015, 12, 360. [DOI] [PubMed] [Google Scholar]
- 5.Ritz N., Hanekom W.A., Robins-Browne R., Britton W.J., Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 2008;32:821–841. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M., Burger M., Compérat E., Palou Redorta J., van Rhijn B., Rouprêt M., Shariat S., Sylvester R., Zigeuner R., Gontero P., et al. Statement Concerning the Shortage of BCG Vaccine from the EAU Guidelines Panel on Non-Muscle-IInvasive Bladder Cancer. [(accessed on 13 January 2023)]. Available online: https://d56bochluxqnz.cloudfront.net/documents/guideline-appendices/non-muscle-invasive-bladder-cancer/Updated-statement-concerning-the-shortage-of-BCG-vaccine-NMIBC-Panel-2018.pdf.
- 7.Angulo J.C., Álvarez-Ossorio J.L., Domínguez-Escrig J.L., Moyano J.L., Sousa A., Fernández J.M., Gómez-Veiga F., Unda M., Carballido J., Carrero V., et al. Hyperthermic Mitomycin C in Intermediate-risk Non–muscle-invasive Bladder Cancer: Results of the HIVEC-1 Trial. Eur. Urol. Oncol. 2022;6:58–66. doi: 10.1016/j.euo.2022.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Carrion D., Pradere B., Soria F., del Giudice F., Moschini M. Editorial commentary on: “Reduced vs full-dose BCG therapy in bladder cancer: A systematic review and meta-analysis”. Actas Urol. Esp. 2022;47:S2173–S5786. doi: 10.1016/j.acuroe.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Boehm B.E., Cornell J.E., Wang H., Mukherjee N., Oppenheimer J.S., Svatek R.S. Efficacy of bacillus Calmette-Guérin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J. Urol. 2017;198:503–510. doi: 10.1016/j.juro.2017.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. [(accessed on 23 February 2022)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 12.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Duval S., Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z., Liu H., Wang Y., Zhang C., Xu T. Determining optimal maintenance schedules for adjuvant intravesical bacillus Calmette–Guerin immunotherapy in non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Curr. Med. Res. Opin. 2017;33:1379–1387. doi: 10.1080/03007995.2017.1326889. [DOI] [PubMed] [Google Scholar]
- 15.Busetto G., Del Giudice F., De Berardinis E., Sperduti I., Ferro M., Maggi M., Gross M., Sciarra A., Eisenberg M. A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J. Androl. 2020;22:360–367. doi: 10.4103/aja.aja_101_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Del Giudice F., Pecoraro M., Vargas H.A., Cipollari S., De Berardinis E., Bicchetti M., Chung B.I., Catalano C., Narumi Y., Catto J.W.F., et al. Systematic Review and Meta-Analysis of Vesical Imaging-Reporting and Data System (VI-RADS) Inter-Observer Reliability: An Added Value for Muscle Invasive Bladder Cancer Detection. Cancers. 2020;12:2994. doi: 10.3390/cancers12102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Giudice F., Kasman A.M., De Berardinis E., Busetto G.M., Belladelli F., Eisenberg M.L. Association between male infertility and male-specific malignancies: Systematic review and meta-analysis of population-based retrospective cohort studies. Fertil. Steril. 2020;114:984–996. doi: 10.1016/j.fertnstert.2020.04.042. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Lu G., Ades A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 20.Salanti G., Ades A.E., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalter D.J., Best N.G., Carlin B.P., Van Der Linde A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2002;64:583–639. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- 22.Song F., Altman D.G., Glenny A.-M., Deeks J.J. Validity of indirect comparison for estimating efficacy of competing interventions: Empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal M.S., Agrawal M., Bansal S., Agarwal M., Lavania P., Goyal J. The Safety and Efficacy of Different Doses of Bacillus Calmette Guérin in Superficial Bladder Transitional Cell Carcinoma. Urology. 2007;70:1075–1078. doi: 10.1016/j.urology.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Ali-El-Dein B., Nabeeh A., Ismail E.-H., Ghoneim M.A. Sequential bacillus calmette-guerin and epirubicin versus bacillus calmette-guerin alone for superficial bladder tumors: A randomized prospective study. J. Urol. 1999;162:339–342. doi: 10.1016/S0022-5347(05)68555-2. [DOI] [PubMed] [Google Scholar]
- 25.Arends T.J., Nativ O., Maffezzini M., de Cobelli O., Canepa G., Verweij F., Moskovitz B., van der Heijden A.G., Witjes J.A. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guérin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non–Muscle-invasive Bladder Cancer. Eur. Urol. 2016;69:1046–1052. doi: 10.1016/j.eururo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Bilen C.Y., Özen H., Aki F.T., Aygün C., Ekici S., Kendi S. Clinical experience with BCG alone versus BCG plus epirubicin. Int. J. Urol. 2000;7:206–209. doi: 10.1046/j.1442-2042.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 27.Brosman S.A., Brosman S.A. Experience with Bacillus Calmette-Guerin in Patients with Superficial Bladder Carcinoma. J. Urol. 1982;128:27–30. doi: 10.1016/S0022-5347(17)52736-6. [DOI] [PubMed] [Google Scholar]
- 28.Cai T., Nesi G., Tinacci G., Zini E., Mondaini N., Boddi V., Mazzoli S., Bartoletti R. Can Early Single Dose Instillation of Epirubicin Improve Bacillus Calmette-Guerin Efficacy in Patients With Nonmuscle Invasive High Risk Bladder Cancer? Results From a Prospective, Randomized, Double-Blind Controlled Study. J. Urol. 2008;180:110–115. doi: 10.1016/j.juro.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Cheng C.W., Chan S.F.P., Chan L.W., Chan C.K., Ng C.F., Cheung H.Y., Chan S.Y.E., Wong W.S., Lai F.M.-M., To K.F., et al. Twelve-year follow up of a randomized prospective trial comparing bacillus Calmette-Guerin and epirubicin as adjuvant therapy in superficial bladder cancer. Int. J. Urol. 2005;12:449–455. doi: 10.1111/j.1442-2042.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 30.D’Andrea D., Soria F., Abufaraj M., Pones M., Gontero P., Machado A.T., Waksman R., Enikeev D.V., Glybochko P.V., Adonias S.P., et al. Comparative Effectiveness of Intravesical BCG-Tice and BCG-Moreau in Patients with Non–muscle-invasive Bladder Cancer. Clin. Genitourin. Cancer. 2019;18:20–25.e2. doi: 10.1016/j.clgc.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Koguchi D., Matsumoto K., Hirayama T., Moroo S., Kobayashi M., Katsumata H., Ikeda M., Iwamura M. Impact of maintenance therapy using a half dose of the bacillus Calmette–Guérin Tokyo strain on recurrence of intermediate and high-risk nonmuscle invasive bladder cancer: A retrospective single-center study. BMC Urol. 2020;20:194. doi: 10.1186/s12894-020-00766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Giudice F., Busetto G.M., Gross M.S., Maggi M., Sciarra A., Salciccia S., Ferro M., Sperduti I., Flammia S., Canale V., et al. Efficacy of three BCG strains (Connaught, TICE and RIVM) with or without secondary resection (re-TUR) for intermediate/high-risk non-muscle-invasive bladder cancers: Results from a retrospective single-institution cohort analysis. J. Cancer Res. Clin. Oncol. 2021;147:3073–3080. doi: 10.1007/s00432-021-03571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Giudice F., Flammia R.S., Chung B.I., Moschini M., Pradere B., Mari A., Soria F., Albisinni S., Krajewski W., Szydełko T., et al. Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis. Cancers. 2022;14:887. doi: 10.3390/cancers14040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Reijke T.M., Kurth K.H., Sylvester R.J., Hall R.R., Brausi M., Van De Beek K., Landsoght K.E.J., Carpentier P., European Organization for the Research and Treatment of Cancer-Genito-Urinary Group Bacillus calmette-guerin versus epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: Results of a european organization for the research and treatment of cancer—Genito-urinary group phase iii trial (30906) J. Urol. 2005;173:405–409. doi: 10.1097/01.ju.0000150425.09317.67. [DOI] [PubMed] [Google Scholar]
- 35.Di Lorenzo G., Perdonà S., Damiano R., Faiella A., Cantiello F., Pignata S., Ascierto P., Simeone E., De Sio M., Autorino R. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer. Cancer. 2010;116:1893–1900. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 36.Di Stasi S.M., Giannantoni A., Giurioli A., Valenti M., Zampa G., Storti L., Attisani F., De Carolis A., Capelli G., Vespasiani G., et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: A randomised controlled trial. Lancet Oncol. 2006;7:43–51. doi: 10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 37.Farah N.B., Ghanem R., Amr M. Treatment efficacy and tolerability of intravesical Bacillus Calmette-Guerin (BCG)—RIVM strain: Induction and maintenance protocol in high grade and recurrent low grade non-muscle invasive bladder cancer (NMIBC) BMC Urol. 2014;14:11. doi: 10.1186/1471-2490-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrich M.G., Pichlmeier U., Schwaibold H., Conrad S., Huland H. Long-Term Intravesical Adjuvant Chemotherapy Further Reduces Recurrence Rate Compared with Short-Term Intravesical Chemotherapy and Short-Term Therapy with Bacillus Calmette-Guérin (BCG) in Patients with Non–Muscle-Invasive Bladder Carcinoma. Eur. Urol. 2007;52:1123–1130. doi: 10.1016/j.eururo.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 39.Gontero P., Oderda M., Mehnert A., Gurioli A., Marson F., Lucca I., Rink M., Schmid M., Kluth L.A., Pappagallo G., et al. The Impact of Intravesical Gemcitabine and 1/3 Dose Bacillus Calmette-Guérin Instillation Therapy on the Quality of Life in Patients with Nonmuscle Invasive Bladder Cancer: Results of a Prospective, Randomized, Phase II Trial. J. Urol. 2013;190:857–862. doi: 10.1016/j.juro.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 40.Gruenwald I.E., Stein A., Rashcovitsky R., Shifroni G., Lurie A. A 12 versus 6-week course of bacillus Calmette-Guerin prophylaxis for the treatment of high risk superficial bladder cancer. J. Urol. 1997;157:487–491. doi: 10.1016/S0022-5347(01)65183-8. [DOI] [PubMed] [Google Scholar]
- 41.Hemdan T., Johansson R., Jahnson S., Hellström P., Tasdemir I., Malmström P.-U., Members of the Urothelial Cancer Group of the Nordic Association of Urology 5-Year Outcome of a Randomized Prospective Study Comparing bacillus Calmette-Guérin with Epirubicin and Interferon-α2b in Patients with T1 Bladder Cancer. J. Urol. 2014;191:1244–1249. doi: 10.1016/j.juro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Herr H.W. Intravesical Bacillus Calmette-Guérin Outcomes in Patients With Bladder Cancer and Asymptomatic Bacteriuria. J. Urol. 2012;187:435–437. doi: 10.1016/j.juro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Herr H.W., Dalbagni G., Donat S.M. Bacillus Calmette-Guérin Without Maintenance Therapy for High-Risk Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2011;60:32–36. doi: 10.1016/j.eururo.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 44.Herr H.W. Age and Outcome of Superficial Bladder Cancer Treated with Bacille Calmette-Guérin Therapy. Urology. 2007;70:65–68. doi: 10.1016/j.urology.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Hinotsu S., Akaza H., Naito S., Ozono S., Sumiyoshi Y., Noguchi S., Yamaguchi A., Nagamori S., Terai A., Nasu Y., et al. Maintenance therapy with bacillus Calmette-Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int. 2010;108:187–195. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 46.Hinotsu S., Akaza H., Isaka S., Kanetake H., Kubota Y., Kuroda M., Shinohara N., Shinka T., Tachibana M., Naito S., et al. Sustained prophylactic effect of intravesical bacille Calmette-Guérin for superficial bladder cancer: A smoothed hazard analysis in a randomized prospective study. Urology. 2006;67:545–549. doi: 10.1016/j.urology.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 47.Hudson M.A., Ratliff T.L., Gillen D.P., Haaff E.O., Dresner S.M., Catalona W.J. Single Course Versus Maintenance Bacillus Calmette-Guerin Therapy for Superficial Bladder Tumors: A Prospective, Randomized Trial. J. Urol. 1987;138:295–298. doi: 10.1016/S0022-5347(17)43125-9. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahiem E.-H.I., Ghoneim M.A., Nigam V., Brailovsky C., Elhilali M.M. Prophylactic Maltose Tetrapalmitate and Bacillus Calmette-Guerin Immunotherapy of Recurrent Superficial Bladder Tumors: Preliminary Report. J. Urol. 1988;140:498–500. doi: 10.1016/S0022-5347(17)41701-0. [DOI] [PubMed] [Google Scholar]
- 49.Inamoto T., Ubai T., Nishida T., Fujisue Y., Katsuoka Y., Azuma H. Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guérin prophylaxis in nonmuscle invasive bladder cancer: Results of a randomized prospective comparison. Urol. Ann. 2013;5:7–12. doi: 10.4103/0974-7796.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Järvinen R., Kaasinen E., Sankila A., Rintala E., FinnBladder Group Long-term Efficacy of Maintenance Bacillus Calmette-Guérin versus Maintenance Mitomycin C Instillation Therapy in Frequently Recurrent TaT1 Tumours without Carcinoma In Situ: A Subgroup Analysis of the Prospective, Randomised FinnBladder I Study with a 20-Year Follow-up. Eur. Urol. 2009;56:260–265. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Kamat M., Kulkarni J., Tongaonkar H., Dalal A. Intravesical Bacillus Calmette-Guerin For Superficial Bladder Cancer: Experience With Danish 1331 Strain. J. Urol. 1994;152:1424–1428. doi: 10.1016/S0022-5347(17)32436-9. [DOI] [PubMed] [Google Scholar]
- 52.Koga H., Ozono S., Tsushima T., Tomita K., Horiguchi Y., Usami M., Hirao Y., Akaza H., Naito S. BCG Tokyo Strain Study Group Maintenance intravesical bacillus Calmette-Guérin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: Randomized controlled trial by the BCG Tokyo Strain Study Group. Int. J. Urol. 2010;17:759–766. doi: 10.1111/j.1442-2042.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 53.Lamm D.L., Blumenstein B.A., Crawford E.D., Crissman J.D., Lowe B.A., Smith J.A., Sarosdy M.F., Schellhammer P.F., Sagalowsky A.I., Messing E.M., et al. Randomized intergroup comparison of bacillus calmette-guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urol. Oncol. Semin. Orig. Investig. 1995;1:119–126. doi: 10.1016/1078-1439(95)00041-F. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Piñeiro J., Flores N., Isorna S., Solsona E., Sebastián J., Pertusa C., Rioja L., Vela R., Camacho J., Nogueira J., et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int. 2002;89:671–680. doi: 10.1046/j.1464-410X.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 55.Piñeiro J.A.M., León J.J., Piñeiro L.M., Fiter L., Mosteiro J.A., Navarro J., Matres M.J.G., Cárcamo P. Bacillus Calmette-Guerin Versus Doxorubicin Versus Thiotepa: A Randomized Prospective Study in 202 Patients with Superficial Bladder Cancer. J. Urol. 1990;143:502–506. doi: 10.1016/S0022-5347(17)40002-4. [DOI] [PubMed] [Google Scholar]
- 56.Marttila T., Järvinen R., Liukkonen T., Rintala E., Boström P., Seppänen M., Tammela T., Hellström P., Aaltomaa S., Leskinen M., et al. Intravesical Bacillus Calmette-Guérin Versus Combination of Epirubicin and Interferon-α2a in Reducing Recurrence of Non–Muscle-invasive Bladder Carcinoma: FinnBladder-6 Study. Eur. Urol. 2016;70:341–347. doi: 10.1016/j.eururo.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Melekos M.D., Zarakovitis I., Dandinis K., Fokaefs E., Chionis H., Dauaher H., Barbalias G. BCG versus epirubicin in the prophylaxis of multiple superficial bladder tumours: Results of a prospective randomized study using modified treatment schemes. Int. Urol. Nephrol. 1996;28:499–509. doi: 10.1007/BF02550957. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee A., Persad R., Smith P.J.B. Intravesical BCG Treatment for Superficial Bladder Cancer: Long-term Results using Two Different Strains of BCG. BJU Int. 1992;69:147–150. doi: 10.1111/j.1464-410X.1992.tb15486.x. [DOI] [PubMed] [Google Scholar]
- 59.Nowak Ł., Krajewski W., Moschini M., Chorbińska J., Poletajew S., Tukiendorf A., Muilwijk T., Joniau S., Tafuri A., Antonelli A., et al. Assessment of the oncological outcomes of three different bacillus Calmette–Guérin strains in patients with high-grade T1 non-muscle-invasive bladder cancer. Arab. J. Urol. 2021;19:78–85. doi: 10.1080/2090598X.2021.1874628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ojea A., Nogueira J.L., Solsona E., Flores N., Gómez J.M.F., Molina J.R., Chantada V., Camacho J.E., Piñeiro L.M., Rodríguez R.H., et al. A Multicentre, Randomised Prospective Trial Comparing Three Intravesical Adjuvant Therapies for Intermediate-Risk Superficial Bladder Cancer: Low-Dose Bacillus Calmette-Guerin (27 mg) versus Very Low-Dose Bacillus Calmette-Guerin (13.5 mg) versus Mitomycin C. Eur. Urol. 2007;52:1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 61.Okamura T., Akita H., Ando R., Ikegami Y., Naiki T., Kawai N., Tozawa K., Kohri K. Single monthly bacillus Calmette-Guérin intravesical instillation is effective maintenance therapy to prevent recurrence in Japanese patients with non-muscle-invasive bladder cancer. Int. J. Clin. Oncol. 2011;17:477–481. doi: 10.1007/s10147-011-0314-3. [DOI] [PubMed] [Google Scholar]
- 62.Oosterlinck W., Kirkali Z., Sylvester R., da Silva F.C., Busch C., Algaba F., Collette S., Bono A. Sequential Intravesical Chemoimmunotherapy with Mitomycin C and Bacillus Calmette-Guérin and with Bacillus Calmette-Guérin Alone in Patients with Carcinoma in Situ of the Urinary Bladder: Results of an EORTC Genito-Urinary Group Randomized Phase 2 Trial (30993) Eur. Urol. 2011;59:438–446. doi: 10.1016/j.eururo.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 63.Ourfali S., Ohannessian R., Fassi-Fehri H., Pages A., Badet L., Colombel M. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guérin Connaught Strain for Bladder Cancer Patients. Eur. Urol. Focus. 2019;7:111–116. doi: 10.1016/j.euf.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Palou J., Laguna P., Millán-Rodríguez F., Hall R.R., Salvador-Bayarri J., Vicente-Rodríguez J. Control group and maintenance treatment with bacillus Calmette-Guerin for carcinoma in situ and/or high grade bladder tumors. J. Urol. 2001;165:1488. doi: 10.1016/S0022-5347(05)66333-1. [DOI] [PubMed] [Google Scholar]
- 65.Porena M., Del Zingaro M., Lazzeri M., Mearini L., Giannantoni A., Bini V., Costantini E. Bacillus Calmette-Guérin versus Gemcitabine for Intravesical Therapy in High-Risk Superficial Bladder Cancer: A Randomised Prospective Study. Urol. Int. 2010;84:23–27. doi: 10.1159/000273461. [DOI] [PubMed] [Google Scholar]
- 66.Prasanna T., Craft P., Balasingam G., Haxhimolla H., Pranavan G. Intravesical Gemcitabine versus Intravesical Bacillus Calmette–Guérin for the Treatment of Non-Muscle Invasive Bladder Cancer: An Evaluation of Efficacy and Toxicity. Front. Oncol. 2017;7:260. doi: 10.3389/fonc.2017.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rentsch C.A., Birkhäuser F.D., Biot C., Gsponer J.R., Bisiaux A., Wetterauer C., Lagranderie M., Marchal G., Orgeur M., Bouchier C., et al. Bacillus Calmette-Guérin Strain Differences Have an Impact on Clinical Outcome in Bladder Cancer Immunotherapy. Eur. Urol. 2014;66:677–688. doi: 10.1016/j.eururo.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 68.Rintala E., Jauhiainen K., Alfthan O., Hansson E., Juusela H., Kanerva K., Korhonen H., Permi J., Sotarauta M., Vaalasti T., et al. Intravesical Chemotherapy (Mitomycin C) versus Immunotherapy (Bacillus Calmette-Guérin) in Superficial Bladder Cancer. Eur. Urol. 1991;20:19–25. doi: 10.1159/000471653. [DOI] [PubMed] [Google Scholar]
- 69.Sekine H., Ohya K., Kojima S.-I., Igarashi K., Fukui I. Equivalent efficacy of mitomycin C plus doxorubicin instillation to bacillus Calmette-Guerin therapy for carcinoma in situ of the bladder. Int. J. Urol. 2001;8:483–486. doi: 10.1046/j.1442-2042.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 70.Sengiku A., Ito M., Miyazaki Y., Sawazaki H., Takahashi T., Ogura K. A Prospective Comparative Study of Intravesical Bacillus Calmette-Guérin Therapy with the Tokyo or Connaught Strain for Nonmuscle Invasive Bladder Cancer. J. Urol. 2013;190:50–54. doi: 10.1016/j.juro.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 71.Shinka T., Matsumoto M., Ogura H., Hirano A., Ohkawa T. Recurrence of Primary Superficial Bladder Cancer Treated with Prophylactic Intravesical Tokyo 172 Bacillus Calmette-Guerin: A Long-Term Follow-up. Int. J. Urol. 1997;4:139–143. doi: 10.1111/j.1442-2042.1997.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 72.Shinka T., Hirano A., Uekado Y., Kyoku I., Aoshi H., Ohkawa T. Intravesical Bacillus Calmette-Guérin Treatment for Superficial Bladder Tumours. BJU Int. 1989;63:610–615. doi: 10.1111/j.1464-410X.1989.tb05255.x. [DOI] [PubMed] [Google Scholar]
- 73.Sood R., Sharma H., Sharma B., Parekh S., Pujari P., Shewale S. A prospective comparative study to assess the efficacy and tolerability of 2 different doses of intravesical bacillus Calmette-Guerin (BCG) in patients with non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2020;38:433–439. doi: 10.1016/j.urolonc.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Steinberg R.L., Brooks N.A., Thomas L.J., Mott S.L., O’Donnell M.A. Bacillus Calmette-Guerin strain may not effect recurrence-free survival when used intravesically with interferon-alpha2b for non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2017;35:201–207. doi: 10.1016/j.urolonc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Sylvester R.J., Brausi M.A., Kirkels W.J., Hoeltl W., Da Silva F.C., Powell P.H., Prescott S., Kirkali Z., van de Beek C., Gorlia T., et al. Long-Term Efficacy Results of EORTC Genito-Urinary Group Randomized Phase 3 Study 30911 Comparing Intravesical Instillations of Epirubicin, Bacillus Calmette-Guérin, and Bacillus Calmette-Guérin plus Isoniazid in Patients with Intermediate- and High-Risk Stage Ta T1 Urothelial Carcinoma of the Bladder. Eur. Urol. 2010;57:766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takashi M., Katsuno S., Yuba H., Ohshima S., Wakai K., Ohno Y. Possible factors affecting response to intravesical bacillus Calmette-Guérin (Tokyo 172 Strain) therapy for carcinoma in situ of the bladder: A multivariate analysis. Int. Urol. Nephrol. 1998;30:713–722. doi: 10.1007/BF02564859. [DOI] [PubMed] [Google Scholar]
- 77.Takashi M., Shimoji T., Murase T., Sakata T., Sobajima T., Suzuki Y. Intravesical bacillus Calmette-Guérin (Tokyo 172 strain) therapy for carcinoma in situ of the bladder. Int. Urol. Nephrol. 1997;29:557–563. doi: 10.1007/BF02552201. [DOI] [PubMed] [Google Scholar]
- 78.Witjes J.A., Dalbagni G., Karnes R.J., Shariat S., Joniau S., Palou J., Serretta V., Larré S., di Stasi S., Colombo R., et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2099 patients with T1G3 non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016;34:484.e19–484.e25. doi: 10.1016/j.urolonc.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witjes W.P., König M., Boeminghaus F.P., Hall R.R., Schulman C.C., Zurlo M., Fittipaldo A., Riggi M., Debruyne F.M. Results of a European Comparative Randomized Study Comparing Oral Bropirimine versus Intravesical BCG Treatment in BCG-Naive Patients with Carcinoma in situ of the Urinary Bladder. Eur. Urol. 1999;36:576–581. doi: 10.1159/000020051. [DOI] [PubMed] [Google Scholar]
- 80.Yalçinkaya F., Kamiş L., Özteke O., Günlüsoy B., Yiĝitbasi O., Ünal S. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. Int. Urol. Nephrol. 1998;30:41–44. doi: 10.1007/BF02550276. [DOI] [PubMed] [Google Scholar]
- 81.Peyromaure M., Guérin F., Amsellem-Ouazana D., Saighi D., Debre B., Zerbib M. Intravesical Bacillus Calmette-Guerin Therapy for Stage T1 Grade 3 Transitional Cell Carcinoma of the Bladder: Recurrence, Progression and Survival in a Study of 57 Patients. J. Urol. 2003;169:2110–2112. doi: 10.1097/01.ju.0000066840.42991.4a. [DOI] [PubMed] [Google Scholar]
- 82.Yoo K.H., Lim T.J., Chang S.-G. Monthly intravesical bacillus Calmette-Guérin maintenance therapy for non-muscle-invasive bladder cancer: 10-year experience in a single institute. Exp. Ther. Med. 2011;3:221–225. doi: 10.3892/etm.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shelley M., Kynaston H.G., Court J.B., Wilt T.J., Coles B., Burgon K., Mason M.D. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 84.Shelley M., Wilt T., Court J., Coles B., Kynaston H., Mason M. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004;93:485–490. doi: 10.1111/j.1464-410X.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- 85.Malmström P.-U., Sylvester R.J., Crawford D.E., Friedrich M., Krege S., Rintala E., Solsona E., Di Stasi S.M., Witjes J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 86.Duchek M., Johansson R., Jahnson S., Mestad O., Hellström P., Hellsten S., Malmström P.-U., Members of the Urothelial Cancer Group of the Nordic Association of Urology Bacillus Calmette-Guérin Is Superior to a Combination of Epirubicin and Interferon-α2b in the Intravesical Treatment of Patients with Stage T1 Urinary Bladder Cancer. A Prospective, Randomized, Nordic Study. Eur. Urol. 2010;57:25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 87.Grimm M.-O., van der Heijden A.G., Colombel M., Muilwijk T., Martínez-Piñeiro L., Babjuk M.M., Türkeri L.N., Palou J., Patel A., Bjartell A.S., et al. Treatment of High-grade Non–muscle-invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations Versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial “NIMBUS”. Eur. Urol. 2020;78:690–698. doi: 10.1016/j.eururo.2020.04.066. [DOI] [PubMed] [Google Scholar]
- 88.Jiang S., Redelman-Sidi G. BCG in Bladder Cancer Immunotherapy. Cancers. 2022;14:3073. doi: 10.3390/cancers14133073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moon Y.J., Cho K.S., Jeong J.Y., Chung D.Y., Kang D.H., Jung H.D., Lee J.Y. Effects of intravesical BCG maintenance therapy duration on recurrence rate in high-risk non-muscle invasive bladder cancer (NMIBC): Systematic review and network meta-analysis according to EAU COVID-19 recommendations. PLoS ONE. 2022;17:e0273733. doi: 10.1371/journal.pone.0273733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Panebianco V., Pecoraro M., Del Giudice F., Takeuchi M., Muglia V.F., Messina E., Cipollari S., Giannarini G., Catalano C., Narumi Y. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J. Magn. Reson. Imaging. 2020;55:23–36. doi: 10.1002/jmri.27361. [DOI] [PubMed] [Google Scholar]
- 91.Panebianco V., Del Giudice F., Leonardo C., Sciarra A., Catalano C., Catto J.W. VI-RADS Scoring Criteria for Alternative Risk-adapted Strategies in the Management of Bladder Cancer During the COVID-19 Pandemic. Eur. Urol. 2020;78:e18–e20. doi: 10.1016/j.eururo.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicolazzo C., Busetto G.M., Del Giudice F., Sperduti I., Giannarelli D., Gradilone A., Gazzaniga P., de Berardinis E., Raimondi C. The long-term prognostic value of survivin expressing circulating tumor cells in patients with high-risk non-muscle invasive bladder cancer (NMIBC) J. Cancer Res. Clin. Oncol. 2017;143:1971–1976. doi: 10.1007/s00432-017-2449-8. [DOI] [PubMed] [Google Scholar]
- 93.Kamat A.M., Sylvester R.J., Böhle A., Palou J., Lamm D.L., Brausi M., Soloway M., Persad R., Buckley R., Colombel M., et al. Definitions, End Points, and Clinical Trial Designs for Non–Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J. Clin. Oncol. 2016;34:1935–1944. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.