Abstract
Simple Summary
Exosomes are nanometer-sized vesicles released by different cells that are important in the normal functioning of the body. In cancer, exosomes have been found to promote tumor growth and metastasis by carrying functional biomolecules and acting on different target sites in the body. Understanding the mechanism by which cancers modulate exosome secretion is crucial to studying cancer biology and developing new therapeutic approaches. Herein, we summarize the biological processes involved in the generation of exosomes, and the factors affecting the targeting and internalization of exosomes. By outlining the mechanisms regulating exosome dynamics, we link exosome secretion with characteristics of cancer cells and suggest methods for improving future research.
Abstract
Exosomes are mediators of intercellular communication in normal physiology and diseases. While many studies have emerged on the function of exosomal cargoes, questions remain regarding the origin of these exosomes. The packaging and secretion of exosomes in different contexts modify exosomal composition, which may in turn impact delivery, uptake and cargo function in recipient cells. A mechanistic understanding of exosome biology is therefore crucial to investigating exosomal function in complex biological systems and to the development of novel therapeutic approaches. Here, we outline the steps in exosome biogenesis, including endosome formation, MVB formation, cargo sorting and extracellular release, as well as exosome absorption, including targeting, interaction with recipient cells and the fate of internalized exosomes. In addition to providing a framework of exosome dynamics, we summarize current evidence on major pathways and regulatory mechanisms. We also highlight the various mechanisms observed in cancer and point out directions to improve study design in exosome biology. Further research is needed to illuminate the relationship between exosome biogenesis and function, which will aid the development of translational applications.
Keywords: exosome, extracellular vesicle, intercellular communication, biogenesis, targeting, regulation, cancer, endocytosis
1. Introduction
Extracellular vesicles (EVs) are a heterogeneous group of lipid bilayer-bound vesicles secreted by various cell types [1]. In recent years, many studies have emerged on the function of EVs in cancer and categorized them based on their morphology, cargo and donor cells [2,3]. Exosomes are a subset of EVs with a diameter of 30–150 nm and are known to regulate intercellular communication in normal physiology and diseases [4]. They carry cargoes, such as proteins, lipids, DNA and RNAs, including messenger RNA (mRNA) and noncoding RNA (ncRNA) [2], and mediate the exchange of functional contents between cells [5]. Studies have found that exosomes mediate various physiologic processes, such as neuronal communication, immune response and wound healing [6,7,8], while in cancer, exosomes are implicated in metastasis, immune evasion, therapy resistance and angiogenesis [3,9,10,11]. Notably, cancer cells utilize various mechanisms to regulate the amount and composition of exosomes to promote cancer development [12]. However, the unaccounted heterogeneity of secreted exosomes and the difficulty of harvesting EVs reproducibly reveal a bottleneck in our understanding of EV dynamics, both in vitro and especially in a biological system.
In ascending order of size, EVs are classified into exomeres, exosomes, ectosomes, apoptotic bodies and large oncosomes, of which exomeres and exosomes are classified into small EVs (sEVs), and the others are known as large EVs (lEVs) [13]. However, the observation that exomeres, which co-isolate with EVs, are non-membrane-bound particles has given rise to the term extracellular particles (EPs), used to classify the heterogeneous group of small, non-membrane-bound particles that includes exomeres [14]. While ectosomes, apoptotic bodies and large oncosomes are formed from the plasma membrane, exosomes are uniquely of endosomal origin and subject to numerous mechanisms of regulation and cargo loading [13].
In this review, we present the current knowledge on exosome biogenesis and relevant regulatory pathways. In addition, we provide a framework to understand exosome dynamics by highlighting mechanisms of exosome absorption. Finally, we suggest future directions of studying EV biogenesis to address research gaps and common experimental pitfalls.
2. Mechanisms of Exosome Biogenesis
The pathway of exosome biogenesis is the most well studied among the subsets of EVs. Given their functional importance and the translational applications focused on exosomes exclusively, a mechanistic understanding of exosome biogenesis and absorption is necessary to forming functional hypotheses.
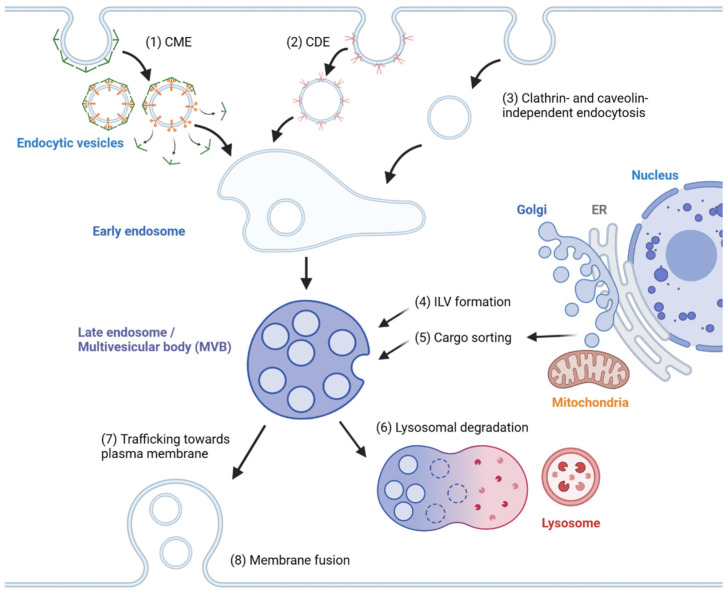
In general, exosome biogenesis is preceded by endocytosis, resulting in the formation of early endosomes. Several mechanisms cause the early endosome to incorporate cargo into intraluminal vesicles (ILVs), forming multivesicular bodies (MVBs) or late endosomes [15]. During maturation, MVBs may import cargoes from other organelles, including the trans-Golgi network (TGN), endoplasmic reticulum (ER) and other cytosolic compartments [16,17], further modifying the composition of ILVs. MVBs may fuse with lysosomes, resulting in degradation [2]. Alternatively, MVBs are transported to the plasma membrane, followed by membrane fusion, releasing ILVs as exosomes. The exosomes travel across extracellular space and reach a recipient cell, where several factors mediate the interaction between the exosome and the recipient cell [18]. Internalized exosomes are incorporated into early endosomes following the typical endosomal pathway and may be released again through recycling endosomes [19]. The overview of exosome biogenesis is depicted in Figure 1.
Figure 1.
Overview of exosome biogenesis. Endocytic vesicles are generated by (1) clathrin-mediated endocytosis (CME), (2) caveolin-dependent endocytosis (CDE) and (3) clathrin- and caveolin-independent endocytosis. After the fusion of endocytic vesicles to form early endosomes, (4) ILVs are generated by ESCRT-dependent and ESCRT-independent pathways, resulting in the formation of multivesicular bodies (MVBs). (5) Cargoes are sorted into ILVs from various organelles, including the trans-Golgi network (TGN), endoplasmic reticulum (ER) and mitochondria. (6) MVBs can then fuse with lysosomes and undergo lysosomal degradation. (7) Alternatively, MVBs are trafficked towards the plasma membrane and (8) undergo membrane fusion for the extracellular release of ILVs as exosomes.
To summarize, exosome biogenesis consists of endosome formation, MVB formation, cargo sorting and extracellular release, while exosome absorption consists of targeting to the recipient cell, internalization and re-release. Each step is regulated by molecular pathways and environmental factors, leading to changes in the amount, composition, and eventually the dynamics of exosomes in a biological system. Despite sharing these common steps, exosomes isolated from biofluids are still highly heterogeneous due to cell-type-specific regulation of biogenesis and absorption, as well as the intracellular heterogeneity of MVBs and ILVs, the mechanisms for which will be highlighted in this section.
2.1. Endosome Formation
Early endosomes are the precursors of MVBs. Although a crucial condition for subsequent steps, it has yet to be sufficiently characterized in the context of exosome biogenesis. There are multiple pathways of endocytosis, including clathrin-mediated endocytosis (CME), caveolin-dependent endocytosis (CDE) and clathrin- and caveolin-independent endocytosis [20,21]. The mechanism for CME has been reviewed in detail elsewhere [20]. In short, a FCH domain only (FCHO) initiation complex is formed on the plasma membrane, forming a clathrin-coated pit. These proteins, along with adaptor protein 2 (AP2) and cargo-specific adaptor proteins, mediate cargo selection, followed by clathrin coat assembly and dynamin-mediated vesicle scission. The clathrin coat is finally disassembled by the ATPase heat shock cognate 70 (HSC70) and auxilin [20]. Other pathways include CDE, the clathrin-independent carriers/GPI-AP-enriched early endosomal compartments (CLIC/GEEC) pathway and the ARF6-associated pathway. Though the detailed mechanism of these pathways are not well defined, the reorganization of the actin cytoskeleton appears to play a central role in endosome formation, especially in pathways independent of clathrin or caveolin coating [21].
2.2. MVB Formation and Cargo Encapsulation
The endosomal sorting complex required for transport (ESCRT)-dependent pathway has been well studied for its involvement in ILV generation. The ESCRT machinery comprises ESCRT-0, -I, -II and -III complexes and Vps4 [22]. ESCRT-0 binds to ubiquitinated cargoes via the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) subunit, while binding to phosphatidylinositol-3-phosphate (PI3P) on endosomal membranes, resulting in cargo enrichment on the limiting membrane of endosomes [22,23]. ESCRT-I is recruited to ESCRT-0 via the interaction of the ESCRT-I subunit, tumor susceptibility gene 101 (Tsg101) protein, with Hrs. This is followed by ESCRT-II binding to ESCRT-I. Together, they promote the inward budding of the endosomal membrane. Recruited by ESCRT-II, the ESCRT-III subunits Vps20, Snf7, Vps2 and Vps24 are sequentially assembled and promote the growth of the Snf7 polymer around the neck of the inward bud, driving further deformation, membrane fission and the formation of ILV [24]. The ESCRT-III subunit Did2 then replaces Vps24 and activates ATP hydrolysis by Vps4, resulting in the cleavage and disassembly of the ESCRT-III complex [24].
Numerous ESCRT-associated machinery cooperate with ESCRT in the formation of ILVs. Non-canonical ESCRT-dependent pathways, such as the Syndecan-Syntenin-Alix pathway and the his domain protein tyrosine phosphatase (HD-PTP) pathway also recruit ESCRT-III and VPS4 for the budding and scission of ILVs. The Syndecan–Syntenin–Alix pathway is initiated by the presence of the ubiquitous transmembrane protein Syndecan, recruiting Syntenin, which in turn binds Alix [25]. Proteins that are recognized by Syndecan, Syntenin or Alix are preferentially sorted into exosomes, such as fibroblast growth factor receptor (FGFR), lysyl-tRNA synthetase and tetraspanins, including CD9, CD63 and CD81 [25,26,27]. The phosphorylation of Syntenin by proteins and the cleavage by heparinase regulates the activity of the Alix-dependent pathway [28,29,30]. The HD-PTP pathway is initiated by the binding of HD-PTP to ESCRT-0, recruiting ESCRT-III and VPS4, thus re-entering the typical ESCRT-dependent pathway [31]. Components of the ESCRT pathway may interact with cargoes to promote their exosomal sorting; namely, the interaction of BAG6 and beta-catenin with Tsg101 and ESCRT-III, respectively, mediates their exosomal secretion [32,33].
The ESCRT-independent pathway involves a combination of lipid raft components, which together regulate the budding and formation of ILVs [34,35]. The neutral sphingomyelinase 2 (nSMase2)-ceramide pathway involves the conversion of sphingomyelin to ceramide by nSMase2, which allows ceramide to self-associate into raft-like macrodomains, leading to a negative membrane curvature that favors inward budding [36]. Microtubule-associated protein 1 light chain 3 (LC3), a well-known marker of autophagy, is located on the limiting membrane of MVBs and recruits the FAN protein, which promotes nSMase2-mediated ceramide production [37,38].
Other lipid raft components, such as cholesterol, caveolin-1 and flotillins, have been linked to exosome secretion and the ILV sorting of cargoes [12,39,40,41]. Proteins that promote cholesterol transfer to MVBs, such as ORP1L and STARD3, positively regulate the formation of ILVs [39,42]. While the precise mechanism of how cholesterol mediates ILV formation is unknown, cholesterol is found to promote flotillin 2 secretion in oligodendroglial cells, and flotillin-1 knock-down inhibits caveolin-1 secretion in PC-3 cells [41,43]. Thus, it is attractive to hypothesize whether cholesterol, caveolin-1 and flotillins constitute an alternative pathway of ILV formation, and whether they are related to ESCRT or ESCRT-independent machinery. However, cholesterol can have different effects on exosome release depending on the cell type; therefore, mechanistic studies using consistent cell types are preferred before a pathway can be confidently established.
Tetraspanins are integral membrane proteins that contribute to protein scaffolding in tetraspanin-enriched microdomains, which mediate adhesion and signal transduction [44]. CD63 has been found to interact with Apolipoprotein E to promote ILV formation in melanocytes independently of ESCRT and ceramide [45]. CD63 has also been used to promote reporter cargo loading in engineered exosomes [46]. Tetraspanin-6 (TSPN6) promotes exosomal secretion in HEK293 cells by interacting with Syntenin, likely co-opting the Syntenin–Syndecan–Alix pathway [30,47], though conflicting evidence suggests that TSPN6 inhibits exosome release in MCF-7 cells due to directing cargoes towards lysosomal degradation [48]. As common exosome biomarkers, CD9 and CD81 are enriched on exosomes, but their mechanisms are not well-defined [49]. While CD9 interacts with and mediates the exosomal secretion of CD10 [50], specific CD81 ligands appear to be abundant on tetraspanin-enriched microdomains [51], suggesting CD81 involvement in cargo sorting. Generally, tetraspanins mediate cargo loading into ILVs via molecular interaction, but their role in the formation of ILV, or its relevance or necessity for the ESCRT and ESCRT-independent pathways, has yet to be clearly investigated.
2.3. Cargo Sorting to MVBs
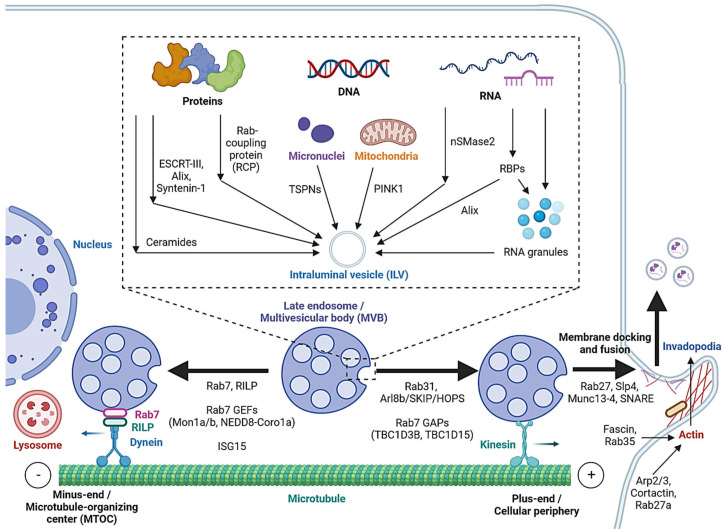
Cargo sorting modulates the composition of ILVs and eventually exosomes. Exosomal cargo include proteins, amino acids, lipids, metabolites, DNA and RNAs, including messenger RNA (mRNA), microRNA (miRNA), long non-coding RNA (lncRNA), circular RNA (circRNA) and PIWI-interacting RNA (piRNA) [2,52]. The state of the donor cell can impact the proteomic and lipidomic profiles of the exosomal cargo [53]. As mentioned, protein cargoes are sorted into ILVs by interacting with components of the ESCRT-dependent or ESCRT-independent pathways, such as ESCRT-III, Alix, Syntenin-1 and ceramides [25,26,27,32,33,53]. In addition, Hsp90 alpha has been found to sort into ILVs by interacting with Rab coupling protein (RCP) [54] (Figure 2).
Figure 2.
Cargo sorting mechanisms and the regulation of MVB trafficking and fusion with the cell membrane. Proteins are sorted into ILVs by associating with ESCRT-dependent and ESCRT-independent machinery and Rab coupling protein (RCP). Tetraspanins and PINK1 are involved in the loading of genomic DNA (gDNA) and mitochondrial DNA (mtDNA), respectively. RNA loading is regulated by nSMase2 and RNA-binding proteins (RBPs). Alix binds to the RBP Ago2 and promotes miRNA loading. RNA granules formed by liquid–liquid phase separation (LLPS) contain RNAs and RBPs and promote miRNA, lncRNA and circRNA loading. MVBs can be fated towards lysosomal degradation by translocation towards the microtubule minus-end or the perinuclear region. Alternatively, they are fated towards secretion by translocation towards the plus-end or the cellular periphery. Actin polymerization at invadopodia stabilizes MVB docking sites. MVB fusion is mediated by SNARE proteins, which are regulated by Rab27 and its effectors, Slp4 and Munc13-4.
While cell-free DNA (cfDNA) originates from various sources, a significant portion is localized on the surface or in the lumen of EVs [55]. Tetraspanins directly interact with cancer cell micronuclei, resulting in genomic DNA (gDNA) loading to exosomes [56]. Exosomal release also serves as a mechanism for removing harmful gDNA from the cytoplasm of healthy cells [57], whereas mitochondrial DNA (mtDNA) loading is initiated by mitochondrial damage, activating PINK1, which promotes the interaction of mitochondria and MVBs in an autophagy-independent manner [58].
Various mechanisms have been studied for RNA loading. nSMase2, which is involved in the ESCRT-independent pathway, has been reported to regulate the exosomal loading of miRNA in cancer cells [59,60]. Substance P (SP)/Neurokinin-1 Receptor (NK1R) signaling also increases miRNA expression and exosomal loading in colonocytes [61]. In addition, a number of RNA-binding proteins (RBPs), such as heterogeneous nuclear ribonucleoproteins (hnRNPs), Argonaute 2 (AGO2), Y-Box-Binding Protein-1 (YBX-1), Serine and Arginine-Rich Splicing Factor (SRSF1) and Major Vault Protein (MVP), bind to specific miRNAs motifs to facilitate their sorting to exosomes [5,62,63]. Meanwhile, hnRNPs also mediate the sorting of lncRNAs [64,65], and ESCRT-II and hnRNPA2B1 have been found to mediate the sorting of circRNAs [66,67]. Alix, well-known for its role in the ESCRT-dependent pathway, was also demonstrated to enrich miRNA in EVs via binding to Ago2 and miRNA [68].
In cells, biomolecular condensates, such as RNA granules, are formed by liquid–liquid phase separation (LLPS) [69]. Mechanistically, RNAs and RBPs contribute to RNA granule formation, which in turn contains components such as hnRNPA2 that facilitate miRNA, lncRNA and circRNA sorting into exosomes [69,70]. YBX-1 also forms LLPS-mediated condensates to promote miRNA loading into exosomes [71]. In addition, post-transcriptional modifications of miRNA sequences, in particular 3′ end adenylation and 3′ end uridylation, favor the cellular retention and exosomal release of miR-2909, respectively [72].
2.4. Extracellular Release
MVB translocation and fusion with the plasma membrane are required for the release of ILVs as exosomes. It primarily involves SNARE proteins that are well-known for their role in mediating membrane fusion events [73]. Various upstream mechanisms regulate the structure of SNAREs in different cell types that favor the fusion of MVBs with the plasma membrane, namely, the Fas/Fap-1/caveolin-1 cascade regulates the formation of SNARE containing SNAP25 and VAMP5 in mesenchymal stem cells [74], while the lncRNA HOTAIR regulates the formation of SNARE containing SNAP23 and VAMP3 in hepatocellular carcinoma [75].
As mentioned, MVBs can be fated towards the degradative pathway or the secretory pathway, based on their eventual fusion with lysosomes or with the cell membrane. Cytoskeleton filaments are crucial for MVB transport and docking. Actin reorganization, invadopodia formation and cortactin promote exosome secretion [76,77]. Cortactin binds to Arp2/3 and to actin filaments, leading to the stabilization of MVB docking sites and promote exosome secretion [77]. Coordinately, Rab27a promotes MVB docking by preventing Coronin1b localization to invadopodia, antagonization of cortactin and actin disassembly [77]. Rab35 binds and localizes the actin-bundling protein Fascin to actin, which facilitates actin cross-linking [78]. Accordingly, impeding Cortactin and Fascin-1 reduced EV secretion by regulating invadopodia formation [79]. Microtubules, on the other hand, play a role in the MVB fate by enabling movement towards their minus-ends or the plus-ends [80]. Trafficking towards the plus-end or the cellular periphery promotes membrane docking and the secretory fate of MVBs, whereas the minus-end is anchored in the perinuclear microtubule-organizing center (MTOC) and promotes the lysosomal degradative fate of MVBs.
Rab7 regulates MVB movement along cytoskeleton microtubules. Cargo movement towards the plus-end and minus-end of microtubules is mediated by kinesin and the dynein–dynactin complex, respectively [81]. Rab7 binds to its trafficking adaptor protein, Rab-interacting lysosomal protein (RILP), to recruit the dynein motor complex, thus promoting the transport of Rab7-positive vesicles towards the perinuclear region [82]. Cleavage of RILP due to inflammasome activation inhibits Rab7 binding to dynein motor complex, leading to kinesin-mediated movement of Rab7-positive vesicles to the cell periphery [83]. Thus, Rab7 appears to direct MVBs towards degradation, and its regulation alters the fate of MVBs. Rab31 recruits the GTPase-activating protein (GAP) TBC1D2B, which inhibits Rab7, resulting in increased MVB membrane fusion and exosome secretion [84]. Arl8b, also present on endosomal membranes, initiates the Arl8b/SKIP/HOPS cascade to recruit another GAP TBC1D15, which inhibits Rab7 [80]. As opposed to GAPs, the guanine nucleotide exchange factors (GEFs) Mon1a/b and NEDD8-Coro1a activate Rab7 by converting it to its GTP-bound state, resulting in lysosomal targeting and reduced EV secretion [85,86]. Additionally, induction of ISGylation via interferon-stimulating gene 15 (ISG15) overexpression was found to induce MVB protein degradation and colocalization with lysosomes [87].
Rab GTPases also control the MVB distribution, membrane docking and SNARE-mediated membrane fusion. Slp4 and Munc13-4 are downstream effectors of Rab27 that bind to and promote the formation of SNARE proteins to mediate the fusion of MVBs with the plasma membrane [88,89,90]. GEFs that regulate Rab27a/b, such as the DENN domain-containing protein Rab-3GEP (MADD) and FAM45A, promote endosomal maturation [91,92], and the stabilization of Rab27a by KIBRA also contributes to exosome secretion [93]. Rab27b regulates the trafficking of MVBs towards the plasma membrane by its effector Slac2b [88]. Rab11 and Rab35 enhance exosomal secretion in leukemia and oligodendrocytes, respectively [94,95]. Calcium ions (Ca2+) is required for the Munc13-4- and Rab11-mediated pathways of exosome secretion [96,97].
In summary, cytoskeleton components, such as actin filaments and microtubules, are crucial in MVB trafficking, docking and release. These processes are regulated by Rabs, which are in turn controlled by their GEFs and GAPs. Further study of Rabs and their effectors may help uncover viable mechanisms that modulate the volume and composition of secreted exosomes.
3. Mechanisms of Exosome Absorption
3.1. Exosome Transport and Targeting to Recipient Cells
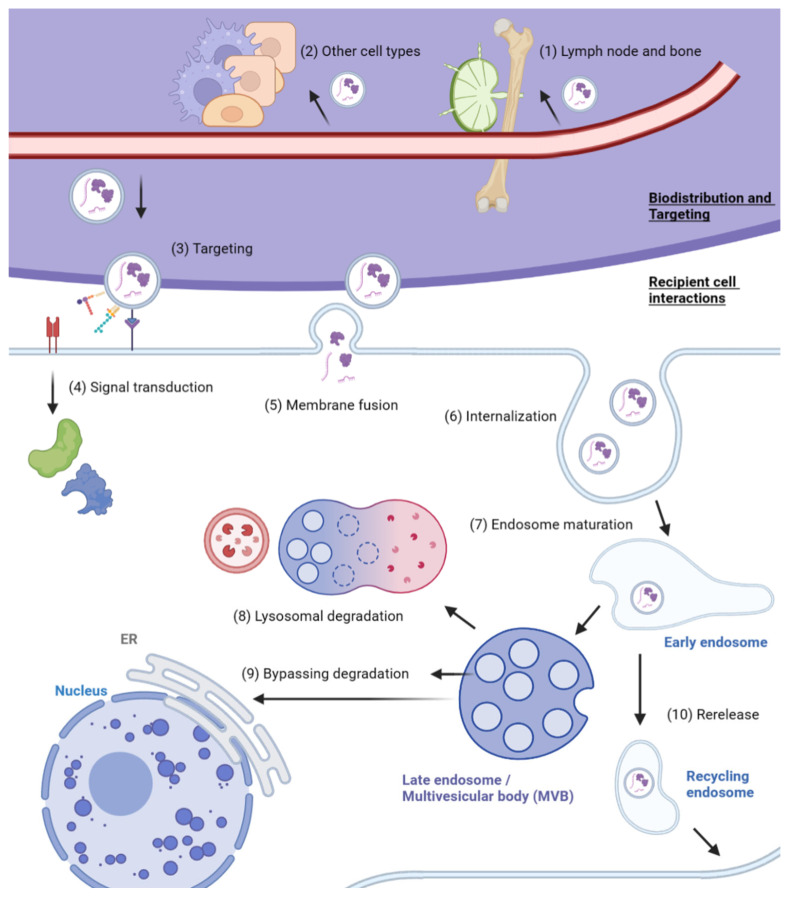
After exosomes are released extracellularly, they are distributed systematically to facilitate local and distant intercellular communication. Exosomes have been found in numerous biofluids, including blood, serum, saliva, synovial fluid and breast milk [98]. The transport of exosomes is highly relevant to estimating their tissue-specific effector functions, and the progress on studying the biodistribution of exogenously administered exosomes has been reviewed in detail elsewhere [99]. In general, smaller EVs are less prone to retention in lymph nodes and bone tissue [14], and the intravenous injection of unmodified tumor-derived exosomes resulted in poor tumor accumulation of exosomes compared to intratumoral injection [100]. The route of administration, in addition to molecular targeting and donor cell type, are important considerations to modulate exosome biodistribution [101]. The overview of exosome uptake is depicted in Figure 3.
Figure 3.
Overview of exosome biodistribution and uptake. The biodistribution of secreted exosomes is affected by (1) retention in lymph nodes and bone tissue and (2) competition from other cell types that vary in the rate of exosome uptake. Exosomes can be (3) targeted to recipient cells by receptor–ligand interaction, the presence of complex lipids on the exosome membrane, and recognition moieties from the donor cell type. Exosomes exert their functions in three ways—(4) activating signal transduction by receptor–ligand binding, (5) membrane fusion to release contents and (6) internalization via endocytic pathways. (7) Endosomes containing internalized exosomes eventually mature into multivesicular bodies (MVBs) and (8) undergo lysosomal degradation together with the exosomes. (9) Exosomal cargo may bypass degradation by passive diffusion, activation by acidification, RNA transfer to endoplasmic reticulum (ER) or release into the cytosol by exosome fusion with the endosomal membrane. (10) Internalized exosomes can also be re-released from early endosomes into the extracellular space via recycling endosomes.
While all cells undergo non-selective exosome uptake, the difference in the exosome uptake capability of different cell types appears to be the main factor accounting for the organ-specific accumulation of tumor-derived exosomes [102]. On the other hand, several mechanisms also affect the delivery of exosomes to target tissues, including the specific interaction between exosomal surface molecules and cell surface receptors [103,104,105], complex lipids on the exosome surface [106,107] and recognition moieties favoring the same donor and recipient cell type [3,108,109]. This derives from the observation that cancer cell-derived exosomes preferentially fuse with their cell of origin compared to other cell types [110], and enhance the delivery of a chemotherapeutic agent to the donor cell type [111]. Specifically, the pattern of integrins and tetraspanins determines exosome targeting. ITGαvβ5 mediates liver tropism, and ITGα6β4 and ITGα6β1 mediate lung tropism, while ITGα4β7 mediates exosome distribution to the small intestine [112,113]. Notably, blockage of ITGαvβ5 and ITGα6β4 binding to fibronectin and laminin receptors, respectively, reduced their associated organ-specific exosome uptake and organotropic metastases [112]. As for tetraspanins, exosomes displaying the TSPAN8-alpha4 integrin complex are preferentially taken up by pancreatic cells [105], and CD63+ exosomes released by neuroblastoma specifically target neuronal dendrites, as opposed to CD63- exosomes, which target neurons and also glial cells [114]. Other exosomal proteins, such as integrin-associated protein (CD47), CD55 and CD59 facilitate immune evasion, leading to extended time in the circulation [115,116].
3.2. Interaction with Recipient Cells and Internalization
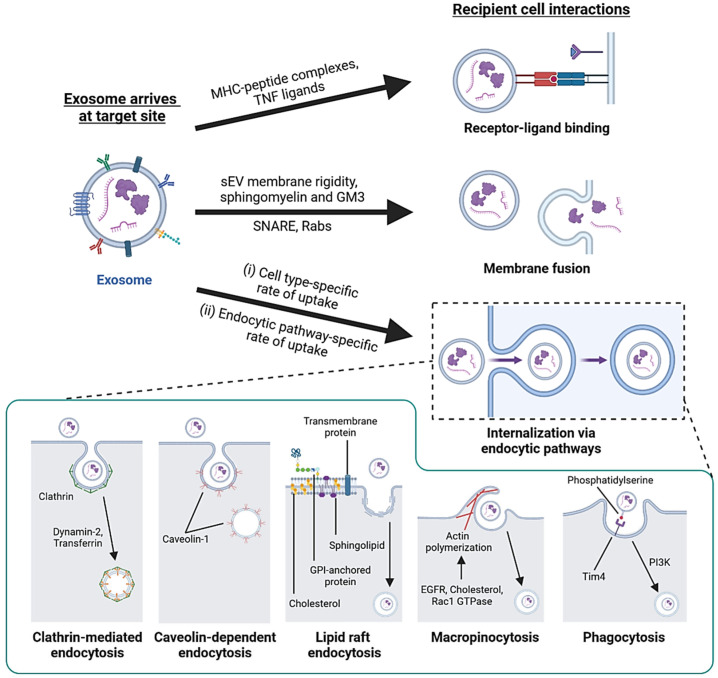
After transportation to recipient cells, exosomes can function in three ways—internalization, membrane fusion or receptor–ligand binding (Figure 4). Internalization is a temperature-sensitive process where cells rapidly uptake exosomes via endocytic pathways that subject exosomes to various internalized fates [117]. To a lesser extent, exosomes fuse with the plasma membrane and directly release their contents into the cytosol. Additionally, ligands on the exosome surface can bind with cell surface receptors to trigger signaling cascades in target cells [118]. Exosomes interact with cell surface receptors by carrying molecules, such as MHC–peptide complexes and tumor necrosis factor (TNF) ligands, which usually function as signal transduction in the immune response and induction of apoptosis in cancer cells [118,119]. Exosome fusion with the plasma membrane involves the formation of a hemi-fusion stalk between the lipid bilayers, followed by stalk expansion and opening of the fusion pore [120,121]. SNAREs and Rab proteins are involved in membrane fusion [122]. Moreover, membrane fusion is promoted for exosomes secreted by tumor cells in acidic pH conditions due to the increased exosomal membrane rigidity and the high content of sphingomyelin and ganglioside GM3 lipids that mediate membrane fusion [107].
Figure 4.
Interactions of exosomes with recipient cells. Exosomes carrying MHC–peptide complexes or TNF ligands undergo receptor–ligand interaction with recipient cells to activate immune cells or trigger apoptosis in cancer cells. Exosomes that have high membrane rigidity and enrichment in sphingomyelin and GM3 have increased fusion efficiency. SNARE and Rab proteins are required for membrane fusion. Most exosomes are taken up by internalization. Different cell types differ in overall exosome uptake efficiency, and each cell may use more than one endocytic pathway for exosome uptake in parallel. Endocytic pathways, such as clathrin-mediated endocytosis (CME), caveolin-dependent endocytosis (CDE), lipid raft endocytosis, macropinocytosis and phagocytosis, contribute to exosome uptake.
Most exosomes are taken up through internalization [121,123], utilizing endocytic pathways, such as CME, lipid raft endocytosis, macropinocytosis and other mechanisms. As mentioned in Section 2.1, CME involves the formation of clathrin-coated pit with AP2 adaptor proteins, which deforms and favors inward membrane budding and dynamin-mediated scission, followed by uncoating to form endocytic vesicles that fuse with and deposit contents into endosomes [20,124]. Inhibition of AP2 or dynamin-2 function has been reported to reduce exosome uptake [117,125]. Lipid raft endocytosis is a possible route of exosome internalization. These rafts are enriched in sphingomyelin, cholesterol and flotillins, which facilitate clathrin- and caveolin-independent endocytosis [126,127]. Inhibiting the biosynthesis of sphingolipids or the intracellular transport of cholesterol results in reduced exosome uptake [128,129]. Macropinocytosis involves the formation of membrane invaginations by lamellipodia, which then pinch off to form macropinosomes [121]. Exosome uptake by macropinocytosis is not sensitive to exosomal composition due to the lack of direct contact between the cell and internalized material [130]. The mechanism is regulated by Rac1 GTPase, Actin and cholesterol [130]. Growth factors also promote micropinocytosis, such as that induced by EGFR signaling in cancer cells expressing Ras [131,132].
Exosome uptake by CDE is possible, but conflicting evidence exists. Reduction in caveolin-1 by genetic knockdown leads to reduced exosome uptake [133], while genetic knockout increases exosome uptake in fibroblasts [134]. Exosome uptake by phagocytosis is primarily utilized by immune cells and explains the higher rate of exosome uptake in macrophages [125,135]. Cargoes internalized in phagosomes are thought to be directed towards lysosomal degradation [136]. Phosphatidylinositol-3-kinase (PI3K) is required for forming phagosomes, and its pharmacological inhibition reduces exosome uptake in phagocytic cells [125,137]. Phosphatidylserine (PS) enriched on exosomes is recognized by the TIM4 receptor on macrophages and facilitates phagocytosis [125]. Consequently, the masking of PS on exosomes by annexin-V treatment reduces the exosomal uptake by immune cells [7,138].
3.3. Fate of Internalized Exosomes
Following internalization, endosomes containing exosomes mature into MVBs, eventually undergoing lysosomal degradation in the target cell [139]. However, some cargoes bypass degradation by passive diffusion across the exosomal membrane [140], or if the exosomal cargo function is activated by acidification, as in the case of transforming growth factor (TGF) beta-1 [141]. In addition, the interaction of ER with endosomes allows the transfer of exosomal RNAs into the ER and facilitates the translation of exosomal RNA [142]. Cargoes may also be released into the cytosol by membrane fusion between exosomes and endosome [123], which can be enhanced by molecules, such as the exosomal surface lipid lysobisphosphatidic acid (LBPA) [143].
Recycling endosomes, which typically serve to return endocytosed plasma membranes to the cell surface, can facilitate the re-release of exosomes back to the extracellular space, allowing the penetration of exosomes across multiple cell layers [144,145]. The exosome biogenesis and absorption discussed are summarized in Table 1 and Table 2.
Table 1.
Steps in exosome biogenesis and associated pathways.
| Steps | Pathways | Mechanisms | References |
|---|---|---|---|
| Exosome biogenesis | |||
| Endosome formation | Clathrin-mediated endocytosis | FCHO, AP2, adaptor proteins, Dynamin, HSC70, auxilin | [20] |
| Caveolin-dependent endocytosis | Caveolin-1 | [21] | |
| Clathrin- and caveolin-independent endocytosis | CLIC/GEEC; ARF6; Actin | [20,21] | |
| MVB formation | ESCRT-dependent pathway | ESCRT-0/I/II/III, VPS4 | [22,23,24] |
| Non-canonical ESCRT-dependent pathways | Syndecan-Syntenin-Alix, heparinase; HD-PTP | [25,28,29,30,31] | |
| ESCRT-independent pathway | LC3, nSMase2, ceramide; cholesterol, caveolin-1, flotillins; TSPNs (CD63, TSPN6, CD9, CD81) |
[34,35,36,37,38] [39,40,41,42,43] [45,46,47,48,49,50,51] |
|
| Cargo sorting | Protein-sorting pathways | ESCRT or ESCRT-independent pathways; Rab coupling protein | [25,26,27,32,33,53,54] |
| DNA-sorting pathways | TSPN interaction with micronuclei; PINK1 | [56,58] | |
| RNA-sorting pathways | nSMase2, SP/NK1R, RBPs, ESCRT-II, Alix; LLPS, 3′ end adenylation or uridylation |
[5,59,60,61,62,63,64,65,66,67,68] [69,70,71,72] |
|
| Extracellular release | SNARE-mediated membrane fusion | Fas/Fap-1/caveolin-1, lncRNA HOTAIR | [74,75] |
| Actin reorganization | Cortactin, Arp2/3, Rab27a, Coronin1b; Rab25, Fascin | [76,77,78,79] | |
| Microtubule-mediated transport | Rab7, RILP; Rab31, Arl8b/SKIP/HOPS, Rab7 GAPs (TBC1D2B, TBC1D15), Rab7 GEFs (Mon1a/b, NEDD8-Coro1a); ISG15 | [80,81,82] [83,84,85,86] [87] |
|
| Rab-regulated trafficking and fusion | Rab27a/b, Rab27 GEFs (MADD, FAM45A), KIBRA, Slp4, Munc13-4; Rab11; Rab35, Ca2+ | [88,89,90,91,92,93] [94,95,96,97] |
|
Abbreviations: TSPN, tetraspanin; SP/NK1R, substance P/neurokinin-1 receptor; RBP, RNA-binding proteins; LLPS, liquid–liquid phase separation; lncRNA, long non-coding RNA; GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor.
Table 2.
Steps in exosome absorption and associated factors.
| Steps | Factors/Processes | Mechanisms | References |
|---|---|---|---|
| Exosome absorption | |||
| Exosome biodistribution and organ-specific accumulation | Retention in lymph nodes and bone | Exosome size | [14] |
| Route of administration | Intratumoral, intravenous, etc. | [99,100,101] | |
| Cell type-specific rate of exosome uptake | Cell type-dependent factors | [102] | |
| Targeting to recipient cell | Receptor–ligand interaction | RVG, SDF-1; integrins (αvβ5, α6β4, α6β1, α4β7), TSPNs (TSPAN8, CD63), CD47 | [103,104,105] [112,113,114,115,116] |
| Lipid targeting | Phosphatidylethanolamine, sphingomyelin | [106,107] | |
| Cell-type-specific exosome uptake | Recognition moieties from the donor cell type | [108,109,110,111] | |
| Recipient cell interactions | Signal transduction via receptors | MHC-peptide complex, TNF receptors | [118,119] |
| Membrane fusion | Rabs, SNARE; membrane rigidity and lipid content | [107,122] | |
| Clathrin-mediated endocytosis | Dynamin-2; transferrin | [117,124,125] | |
| Lipid raft endocytosis | Cholesterol, sphingolipid, flotillin | [126,127,128,129] | |
| Macropinocytosis | Rac1 GTPase, actin, cholesterol, EGFR | [130,131,132] | |
| Caveolin-dependent endocytosis | Caveolin-1 | [133,134] | |
| Phagocytosis | PI3K, phosphatidylserine | [125,135,136,137,138] | |
| Internalized exosomes | Lysosomal degradation | Trafficking to lysosomes (e.g., Rab7) | [80,139] |
| Bypassing lysosomal degradation | Passive diffusion; cargo activation by acidification; RNA transfer to ER; fusion with endosomal membrane (e.g., LBPA) | [108,140,141,142,143] | |
| Re-release | Sorting to recycling endosomes | [144,145] | |
Abbreviations: RVG, rabies viral glycoprotein; TSPN, tetraspanin; ER, endoplasmic reticulum; LBPA, lysobisphosphatidic acid.
4. Relevance of Exosome Secretion and Uptake in Cancer and Cancer Therapy
4.1. Regulation of Exosome Secretion in Cancer
Exosomes promote cancer development by priming premetastatic niches, evading immune surveillance and developing resistance to therapy [3,9,10,11]. To this effect, various mechanisms are utilized by cancer cells to regulate the amount and composition of exosomes.
Exosomal biogenesis is directly affected by the aberrant expression of exosome biogenesis machinery. For the formation of MVBs, the proteins Hrs, Hsp90 and Syntenin act as oncogenes to promote exosome release and promote cell invasion in cancer cells [76,146,147]. Notably, Vps4A and HD-PTP may act as tumor suppressors, either by promoting the exosomal secretion or the lysosomal degradation of oncogenic proteins, resulting in reduced cell proliferation and metastasis in donor cells [31,33]. Dysregulation of molecular signaling pathways in cancer have also been found to modulate exosome release, such as the Ras/Raf/MEK/ERK [148], Ca2+ [97], glutamine [149], p53 [150], mTOR [151], STAT3 [152] and GPCR signaling pathways [153].
In addition, miRNAs can negatively regulate the expression of biogenesis machinery [154], while lncRNAs can regulate exosome release by regulating gene expression [75,155] or promoting the colocalization of SNARE components [75]. Post-transcriptional modifications of biogenesis machinery, such as phosphorylation [28], GlcNac modification [156], mono-ubiquitination [157] and SUMOylation [65], are also mechanisms of modulating exosome biogenesis in cancer cells. Extracellular environment and stresses can also affect exosome secretion. The tumor microenvironment (TME) is observed to regulate exosome secretion by hypoxia- and acidity-related regulation of cellular signaling [107,158]. Lactate released by cancer cells in the TME also recruits tumor-associated macrophages (TAMs), which secrete exosomes with tumorigenic cargo [159].
4.2. The Role of Exosome Targeting in Cancer and Drug Delivery
Cancer-specific alterations to exosome biogenesis are relevant with respect to how they modulate exosome function. Tumor-derived exosomes produced in the low-pH tumor microenvironment have higher membrane rigidity and increased sphingomyelin content [107]. They are more readily taken up by tumor cells via membrane fusion, which promotes the paracrine diffusion of malignancy [107]. Tumor cells also preferentially uptake exosomes from donor cells of the same type due to conservation of cellular signatures [108], contributing to the horizontal transfer of cargo between tumor cells.
In addition to the variety of tumorigenic and metabolic effects induced in tumor cells, exosomes also mediate the crosstalk of tumor cells, the extracellular matrix, stromal cells and immune cells [160]. Exosomes regulate the macrophage polarization between M1 and M2 phenotypes, and can induce the transformation towards M2 macrophages that promote tumor growth [161]. Tumor-derived exosomes also act on T cells, B cells and natural killer (NK) cells, activating immune responses while contributing to immune evasion [160].
The study of exosome targeting and uptake is especially relevant to their use as drug delivery vehicles. To generate exosomes that display desired surface molecules, genetic engineering or chemical engineering approaches have been employed. Genetic engineering refers to the incorporation of ligand-expressing plasmids into donor cells, while chemical engineering involves chemical conjugation or electroporation of collected exosomes [62,162]. Genetic engineering is more favorable towards exosome integrity, as it utilizes the natural biogenesis pathways, whereas direct modification methods may negatively impact the stability of the membrane structure, as well as the endogenous and exogenous targeting molecules, which hinders the targeting effectiveness of the produced exosomes.
Generally, genetic vectors encoding for a fusion product between an exosomal membrane component and a targeting moiety have been used to modify exosomes from donor cells. For example, lysosomal-associated membrane protein 2b (Lamp2b) fusion with αv integrin-specific iRGD peptide has been used to target breast cancer cells [163], and Lamp2b fusion with neuron-specific rabies viral glycoprotein (RVG) peptide targets acetylcholine receptors in the brain [103]. Platelet-derived growth factor receptor (PDGFR) on the exosomal surface has also been fused with antibodies targeting CD3 and EGFR to produce synthetic multivalent antibody-retargeted exosomes (SMART-Exos) which simultaneously target T cells and breast cancer cells [164]. In addition, glycosylphosphatidylinositol (GPI) anchor peptides, inherently enriched on the exosomal membrane, have been fused with anti-EGFR nanobodies to facilitate targeting to cancer cells [165,166].
4.3. Cancer Exosomes as a Therapeutic Target
Cancer-specific dysregulation of exosome biogenesis has given rise to the pharmacological inhibition of exosome machinery as a potential avenue of treatment. To inhibit exosome biogenesis and release, pathways, such as nSMase2 [167], Syntenin [168] and Ras signaling [148,169], have been targeted. GW4869 is a well-studied nSMase2 inhibitor that inhibits the ESCRT-independent nSMase2-ceramide pathway [167]. It sensitizes anti-programmed death-ligand 1 (PD-L1) therapy by inhibiting exosomal secretion of PD-L1 by cancers [170], and also reduces glioma progression by promoting the cellular retention of tumor-suppressing miRNA [171]. While GW4869 inhibits exosome release, it stimulates multivesicle release in breast adenocarcinoma cells [172], suggesting its nonspecific action may hinder the inhibition of overall EV release when considering its potential for clinical translation. Manumycin A is an inhibitor of Ras farnesyltransferase (FTase), thereby inhibiting Ras/Raf/ERK1/2 signaling required for the ESCRT-dependent pathway and reduced EV release in prostate cancer cell lines [148].
Besides exosome biogenesis, exosome transport can also be inhibited via various approaches. HER2-positive EVs induce the formation of premetastatic niches and promote resistance to breast cancer therapy [173]. A hemofiltration system utilizing customizable affinity matrices comprising exosome-binding antibodies, lectins and aptamers was proposed to remove HER2-positive cancer-derived EVs [173]. In humans, regular hemodialysis was confirmed to reduce circulating EVs via adsorption to the dialysis membrane, though this reduction is not specific to the EV origin [174]. Alternatively, antibodies can be used to interfere with exosome-mediated disease progression. Treatment with anti-CD9 and anti-CD63 antibodies increased the macrophage internalization of EVs in vitro and decreased the lung and lymph node metastasis of breast cancer in mice [175]. However, this approach is not specific to tumor-derived EVs and may interfere with normal exosome physiology. In the future, antibodies against tumor-specific exosomal markers may reduce off-target effects, and their viability should be explored. The combination of exosome-depleting adjuncts with chemotherapy agents may increase the efficacy, reduce metastasis and minimize chemoresistance.
Finally, EV uptake inhibitors have been explored to reduce cancer-derived EV uptake by both cancer and non-cancer cells, with the aim of reducing EV-mediated chemoresistance, proliferation and metastasis [176]. Dynasore is a non-competitive inhibitor of dynamin1, dynamin2 and dynamin-related protein 1 (Drp1), which are required for production of clathrin-coated endocytic vesicles during CME [177]. Dynasore is commonly used to inhibit CME [178] and reduces the uptake of exosomes in vitro [179]. However, dynasore has non-specific effects by regulating actin bundling [178], which may indicate its potential role in exosome release, though this is poorly characterized. Ikarugamycin (IKA) specifically inhibits CME and not other endocytic pathways, and it acts by targeting transferrin receptors involved in CDE [180]. Its inhibitory effect is potent, fast-acting and reversible, and thus, it is suitable for research use; however, its specificity and toxicity have not been studied sufficiently to assess its viability for clinical translation. Heparin sulfate proteoglycans (HSPGs), such as Syndecan, are internalizing coreceptors that modulate cancer-derived exosome uptake [181]. Heparin is a clinically used anticoagulant that competitively inhibits HSPGs on cells and was found to a cause dose-dependent and charge density-dependent reduction in exosome uptake [182]. Alternatively, healthy pancreatic tissue surrounding pancreatic cancer releases the lectin Reg3β, which binds to exosomal surface glycoproteins and reduces the uptake of cancer-derived exosomes in vivo [183]. Functionally, Reg3β reduces EV-mediated cancer cell migration and macrophage phenotypic switching in vitro [183], suggesting its potential role in reducing cancer-derived EV uptake by both cancer and non-cancer cells.
To select drugs for clinical translation, the solubility and potency of inhibitors should be adequately tested [167]. Currently, there are difficulties in clinical translation in terms of drug efficacy, drug specificity and gaps in mechanistic knowledge. While some drugs target only one protein in EV biogenesis, other drugs may have multiple targets, such as Manumycin A, which targets both Ras farnesyltransferase and nSMase2. Dynasore may target both EV release and EV uptake via different mechanisms. While inhibitors that target multiple pathways may be more efficacious, their downstream effects are more difficult to control precisely. In addition, various pathways of EV release and EV uptake act in parallel, with each pathway contributing to a portion of EV flow. As such, a single EV inhibitor may not be sufficient to inhibit EV release or uptake completely. GW4869 and manumycin A used together produced significantly better EV reduction than manumycin A alone [148], suggesting that multiple pathway inhibitors are needed to completely block EV release. On the other hand, this may support the notion of designing specific therapies that inhibit disease-specific EV flow with significantly less alteration of the normal physiological roles of EVs.
However, the observation that promoting MVB formation can be either oncogenic (via the activation of Hrs and Syntenin) or tumor suppressing (via the activation of Vps4A and HD-PTP) in a molecule-specific and cell-type-specific manner is a major barrier to translational application. This confusion reflects an insufficient understanding of the mechanism of action of the therapeutic compounds or of the biological pathway itself. In addition, despite the identification of key machinery in exosome biogenesis, conflicting evidence remains regarding the upstream regulators of the mechanisms [37,38]. Therefore, the identification of novel effectors and further understanding of exosome mechanisms in pathway-specific and cell-type-specific contexts will aid the identification of new therapeutic approaches.
5. Challenges and Perspectives for Designing Studies on Exosome Biology
In the recent decade, the heterogeneity of exosomes has been brought to light. As a result, exosomes have been further divided into small exosomes (Exo-S, 30–90 nm) and large exosomes (Exo-L, 90–150 nm) [184]. Even within the same MVB, subpopulations of ILVs can be produced via different mechanisms. This gives rise to exosomal subpopulations with distinct biological and physical properties [185]. The basic differentiation of exosomes from other EVs relies on the isolation and characterization methods advised by ISEV [186], including nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM) and western blotting. Eventually, techniques may be required to further differentiate between the physically and compositionally distinct exosomal subpopulations, such as flow cytometry and proteomic techniques [1]. These methods, however, suffer from the lack of a standardized technique and the lack of consistent and specific markers for EV subpopulations [1].
Proteins should be tested for their involvement with major biogenesis pathways in order to gain further understanding of the interaction or independence of exosome biogenesis mechanisms. For example, the use of specific pathway inhibitors has been used to block endocytic pathways for the study of exosome uptake in vitro [121].
Given the multiple downstream functions of exosomal machinery, gain-of-function or loss-of-function studies for studying exosome secretion may alter other cellular processes besides the pathway of interest [2,18], which may indirectly impact exosome secretion, potentially yielding false-positive results. Experimental reproducibility is also negatively impacted without proper standardization and documentation of culture conditions, exosome collection protocols and pre-analytical variables [1,187], the effects of which have yet to be quantified scientifically.
Reliable methods are required for studying exosome uptake. One method is the loading of EVs with luciferin substrate, followed by the administration to luciferase-expressing cells and the measurement of bioluminescence, which studies the transfer of exosomal contents into cytosol [121,137]. To visualize the interaction between exosomes and cells, studies may also stain exosomes with fluorescent lipophilic membrane-binding dyes, which can be subsequently measured by fluorescence microscopy or flow cytometry [121]. However, these methods merely track exosomes and their surface lipids and cannot be used to visualize the fate of intracellular cargoes themselves.
While exosome bioengineering provides a promising future for targeted drug delivery, several barriers stand in the way of its translation. Genetic modification of donor cells applies only to targeting molecules and fusion products that can be encoded within a genetic vector. The feasibility of modifying exosomes from patient-specific donor cells via a genetic approach is also uncharacterized [188]. Furthermore, targeting moieties intended for exosomal incorporation can be degraded within donor cells, though this can be overcome via the addition of glycosylation motifs to stabilize the targeting peptide [189]. On the other hand, chemical engineering overcomes several of these limitations, at the cost of requiring strict reaction conditions that ensure the stability of the exosome membranes, intrinsic targeting molecules and extrinsic targeting molecules [188].
6. Conclusions
Given the complex networks of protein interactions, individual proteins may exert more than one effect on the exosomes produced. As mentioned, some proteins have dual roles by simultaneously promoting and inhibiting different mechanisms in EV biogenesis. Moreover, the function of a protein in exosome biogenesis can be cell-type-specific. Homologs from the same protein family are responsible for loading different cargos into ILVs. Therefore, observations from a particular cell type, protein and conditions should be well-documented and cautiously interpreted when applied in other contexts. Currently, despite being aware of the heterogeneity of exosomes and MVBs, it is unknown which mechanisms can regulate such heterogeneity and the extent to which these mechanisms are cell-type-specific.
Recent studies in the regulation of exosome release have enabled the framework of exosome biogenesis and absorption to be identified; thus, we have attempted to unite current evidence and point out common elements in exosome biology in this review. However, cell-type-specific pathways and interactions between mechanisms remain ambiguous. Delineating these mechanisms will be crucial to the conceptualization and development of novel, specific and effective therapeutic approaches. However, exosome isolation methods and the analysis of the exosome composition are unstandardized. Establishing reliable methods in characterizing exosome subpopulations is needed for overcoming the current bottleneck.
Numerous translational opportunities have been proposed for exosomes and their biogenesis. Exosomes have been suggested as biomarkers for the diagnosis and evaluation of cancer progression, but heterogeneity has been an obstacle for the analysis of EV composition and obstructing the detection of disease-specific exosomes. Additionally, exosomes can act as drug delivery vectors for cancer therapy. Exosome engineering approaches can incorporate targeting moieties that improve organ-specific targeting. However, the low yield and compositional heterogeneity is an obstacle to harvesting and controlling the side effects of engineered EVs. Further study in exosome biology and bioengineering is needed to optimize the specific targeting and efficient manufacturing of exosomes for translational medicine.
Author Contributions
Conceptualization, N.C.H.L. and J.W.P.Y.; writing—original draft preparation, N.C.H.L.; writing—review and editing, N.C.H.L. and J.W.P.Y.; supervision, J.W.P.Y.; funding acquisition, J.W.P.Y. Figures were created with BioRender.com (accessed on 26 March 2023). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work was funded by the Research Grants Council General Research Fund (Project No. 17105322), the National Natural Science Foundation of China General Program (Project No. 81872340 and 82072626), and the University Research Committee Seed Fund for Basic Research (Project No. 202111159009) of the University of Hong Kong.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezaie J., Ahmadi M., Ravanbakhsh R., Mojarad B., Mahbubfam S., Shaban S.A., Shadi K., Berenjabad N.J., Etemadi T. Tumor-derived extracellular vesicles: The metastatic organotropism drivers. Life Sci. 2022;289:120216. doi: 10.1016/j.lfs.2021.120216. [DOI] [PubMed] [Google Scholar]
- 4.Crescitelli R., Lässer C., Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021;16:1548–1580. doi: 10.1038/s41596-020-00466-1. [DOI] [PubMed] [Google Scholar]
- 5.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Men Y., Yelick J., Jin S., Tian Y., Chiang M.S.R., Higashimori H., Brown E., Jarvis R., Yang Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019;10:4136. doi: 10.1038/s41467-019-11534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolte-’t Hoen E.N., Buschow S.I., Anderton S.M., Stoorvogel W., Wauben M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B., Wang M., Gong A., Zhang X., Wu X., Zhu Y., Shi H., Wu L., Zhu W., Qian H., et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 9.Osaki M., Okada F. Exosomes and Their Role in Cancer Progression. Yonago Acta Med. 2019;62:182–190. doi: 10.33160/yam.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. Exosome-mediated metabolic reprogramming: The emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct. Target. Ther. 2020;5:242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Q.F., Li W.J., Hu K.S., Gao J., Zhai W.L., Yang J.H., Zhang S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer. 2022;21:207. doi: 10.1186/s12943-022-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang T., Atukorala I., Mathivanan S. Biogenesis of Extracellular Vesicles. Subcell. Biochem. 2021;97:19–43. doi: 10.1007/978-3-030-67171-6_2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., Mark M.T., Molina H., Martin A.B., Bojmar L., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 16.Kwon S.H., Oh S., Nacke M., Mostov K.E., Lipschutz J.H. Adaptor Protein CD2AP and L-type Lectin LMAN2 Regulate Exosome Cargo Protein Trafficking through the Golgi Complex. J. Biol. Chem. 2016;291:25462–25475. doi: 10.1074/jbc.M116.729202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Mattia T., Tomasetto C., Alpy F. Faraway, so close! Functions of Endoplasmic reticulum–Endosome contacts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158490. doi: 10.1016/j.bbalip.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien K., Ughetto S., Mahjoum S., Nair A.V., Breakefield X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022;39:110651. doi: 10.1016/j.celrep.2022.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon H.T., Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 21.Mayor S., Parton R.G., Donaldson J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 2014;6:16758. doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt O., Teis D. The ESCRT machinery. Curr. Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vietri M., Radulovic M., Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 24.Pfitzner A.K., Mercier V., Jiang X., Moser von Filseck J., Baum B., Šarić A., Roux A. An ESCRT-III Polymerization Sequence Drives Membrane Deformation and Fission. Cell. 2020;182:1140–1155.e1118. doi: 10.1016/j.cell.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.B., Kim H.R., Park M.C., Cho S., Goughnour P.C., Han D., Yoon I., Kim Y., Kang T., Song E., et al. Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. J. Cell Biol. 2017;216:2201–2216. doi: 10.1083/jcb.201605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larios J., Mercier V., Roux A., Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020;219:4113. doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Li Y., Liu P., Gong D., Zhou H., Li W., Zhang H., Zheng W., Xu J., Cheng H., et al. Phosphatase Shp2 regulates biogenesis of small extracellular vesicles by dephosphorylating Syntenin. J. Extracell. Vesicles. 2021;10:e12078. doi: 10.1002/jev2.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyenne V., Labouesse M., Goetz J.G. The Small GTPase Ral orchestrates MVB biogenesis and exosome secretion. Small GTPases. 2018;9:445–451. doi: 10.1080/21541248.2016.1251378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roucourt B., Meeussen S., Bao J., Zimmermann P., David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazan J.M., Desrochers G., Martin C.E., Jeong H., Kharitidi D., Apaja P.M., Roldan A., St Denis N., Gingras A.C., Lukacs G.L., et al. Endofin is required for HD-PTP and ESCRT-0 interdependent endosomal sorting of ubiquitinated transmembrane cargoes. iScience. 2021;24:103274. doi: 10.1016/j.isci.2021.103274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuldner M., Dörsam B., Shatnyeva O., Reiners K.S., Kubarenko A., Hansen H.P., Finkernagel F., Roth K., Theurich S., Nist A., et al. Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53. Theranostics. 2019;9:6047–6062. doi: 10.7150/thno.36378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Q., Lv L., Wei J., Lei X., Lin H., Li G., Cao J., Xie J., Yang W., Wu S., et al. Vps4A mediates the localization and exosome release of β-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019;457:47–59. doi: 10.1016/j.canlet.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 34.Skotland T., Hessvik N.P., Sandvig K., Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid. Res. 2019;60:9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson G. Isolation of Lipid Rafts (Detergent-Resistant Microdomains) and Comparison to Extracellular Vesicles (Exosomes) Methods Mol. Biol. 2021;2187:99–112. doi: 10.1007/978-1-0716-0814-2_6. [DOI] [PubMed] [Google Scholar]
- 36.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 37.Leidal A.M., Huang H.H., Marsh T., Solvik T., Zhang D., Ye J., Kai F., Goldsmith J., Liu J.Y., Huang Y.H., et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam-Klages S., Adam D., Wiegmann K., Struve S., Kolanus W., Schneider-Mergener J., Krönke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/S0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 39.Eden E.R., Sanchez-Heras E., Tsapara A., Sobota A., Levine T.P., Futter C.E. Annexin A1 Tethers Membrane Contact Sites that Mediate ER to Endosome Cholesterol Transport. Dev. Cell. 2016;37:473–483. doi: 10.1016/j.devcel.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albacete-Albacete L., Navarro-Lérida I., López J.A., Martín-Padura I., Astudillo A.M., Ferrarini A., Van-Der-Heyden M., Balsinde J., Orend G., Vázquez J., et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J. Cell Biol. 2020;219:6178. doi: 10.1083/jcb.202006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phuyal S., Hessvik N.P., Skotland T., Sandvig K., Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm L.P., Wendling C., Védie B., Kobayashi T., Chenard M.P., Tomasetto C., Drin G., Alpy F. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 2017;36:1412–1433. doi: 10.15252/embj.201695917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strauss K., Goebel C., Runz H., Möbius W., Weiss S., Feussner I., Simons M., Schneider A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 2010;285:26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kummer D., Steinbacher T., Schwietzer M.F., Thölmann S., Ebnet K. Tetraspanins: Integrating cell surface receptors to functional microdomains in homeostasis and disease. Med. Microbiol. Immunol. 2020;209:397–405. doi: 10.1007/s00430-020-00673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., Marks M.S., Rubinstein E., Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bebelman M.P., Bun P., Huveneers S., van Niel G., Pegtel D.M., Verweij F.J. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020;15:102–121. doi: 10.1038/s41596-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 47.Guix F.X., Sannerud R., Berditchevski F., Arranz A.M., Horré K., Snellinx A., Thathiah A., Saido T., Saito T., Rajesh S., et al. Tetraspanin 6: A pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol. Neurodegener. 2017;12:25. doi: 10.1186/s13024-017-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghossoub R., Chéry M., Audebert S., Leblanc R., Egea-Jimenez A.L., Lembo F., Mammar S., Le Dez F., Camoin L., Borg J.P., et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA. 2020;117:5913–5922. doi: 10.1073/pnas.1922447117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escola J.M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 50.Mazurov D., Barbashova L., Filatov A. Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. FEBS J. 2013;280:1200–1213. doi: 10.1111/febs.12110. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Hernandez D., Gutiérrez-Vázquez C., Jorge I., López-Martín S., Ursa A., Sánchez-Madrid F., Vázquez J., Yáñez-Mó M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira J.V., da Rosa Soares A., Ramalho J., Máximo Carvalho C., Cardoso M.H., Pintado P., Carvalho A.S., Beck H.C., Matthiesen R., Zuzarte M., et al. LAMP2A regulates the loading of proteins into exosomes. Sci. Adv. 2022;8:eabm1140. doi: 10.1126/sciadv.abm1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S., Wang C., Ma B., Xu M., Xu S., Liu J., Tian Y., Fu Y., Luo Y. Mutant p53 Drives Cancer Metastasis via RCP-Mediated Hsp90α Secretion. Cell Rep. 2020;32:107879. doi: 10.1016/j.celrep.2020.107879. [DOI] [PubMed] [Google Scholar]
- 55.Grabuschnig S., Bronkhorst A.J., Holdenrieder S., Rosales Rodriguez I., Schliep K.P., Schwendenwein D., Ungerer V., Sensen C.W. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int. J. Mol. Sci. 2020;21:8062. doi: 10.3390/ijms21218062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yokoi A., Villar-Prados A., Oliphint P.A., Zhang J., Song X., De Hoff P., Morey R., Liu J., Roszik J., Clise-Dwyer K., et al. Mechanisms of nuclear content loading to exosomes. Sci. Adv. 2019;5:eaax8849. doi: 10.1126/sciadv.aax8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi A., Okada R., Nagao K., Kawamata Y., Hanyu A., Yoshimoto S., Takasugi M., Watanabe S., Kanemaki M.T., Obuse C., et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabas N., Palmer S., Mitchell L., Ismail S., Gohlke A., Riley J.S., Tait S.W.G., Gammage P., Soares L.L., Macpherson I.R., et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 2021;220:6049. doi: 10.1083/jcb.202006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., Lin Y., Xiang P., Tang Y., Hu X., et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018;46:1659–1670. doi: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakirtzi K., Man Law I.K., Fang K., Iliopoulos D., Pothoulakis C. MiR-21 in Substance P-induced exosomes promotes cell proliferation and migration in human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;317:G802–G810. doi: 10.1152/ajpgi.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie S., Zhang Q., Jiang L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes. 2022;12:498. doi: 10.3390/membranes12050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabbiano F., Corsi J., Gurrieri E., Trevisan C., Notarangelo M., D’Agostino V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles. 2020;10:e12043. doi: 10.1002/jev2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C., Luo Y., He W., Zhao Y., Kong Y., Liu H., Zhong G., Li Y., Li J., Huang J., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020;130:404–421. doi: 10.1172/JCI130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C., Zheng H., Luo Y., Kong Y., An M., Li Y., He W., Gao B., Zhao Y., Huang H., et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J. Clin. Investig. 2021;131:146431. doi: 10.1172/JCI146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C., Yu H., Han F., Lai X., Ye K., Lei S., Mai M., Lai M., Zhang H. Tumor-suppressive circRHOBTB3 is excreted out of cells via exosome to sustain colorectal cancer cell fitness. Mol. Cancer. 2022;21:46. doi: 10.1186/s12943-022-01511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Z., Zhao R., Li B., Qi Y., Qiu W., Guo Q., Zhang S., Zhao S., Xu H., Li M., et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol. Cancer. 2022;21:16. doi: 10.1186/s12943-021-01485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iavello A., Frech V.S., Gai C., Deregibus M.C., Quesenberry P.J., Camussi G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int. J. Mol. Med. 2016;37:958–966. doi: 10.3892/ijmm.2016.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roden C., Gladfelter A.S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2021;22:183–195. doi: 10.1038/s41580-020-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan V.H., Dignon G.L., Zerze G.H., Chabata C.V., Silva R., Conicella A.E., Amaya J., Burke K.A., Mittal J., Fawzi N.L. Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol. Cell. 2018;69:465–479.e467. doi: 10.1016/j.molcel.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X.M., Ma L., Schekman R. Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. eLife. 2021;10:71982. doi: 10.7554/eLife.71982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wani S., Kaul D. Cancer cells govern miR-2909 exosomal recruitment through its 3’-end post-transcriptional modification. Cell Biochem. Funct. 2018;36:106–111. doi: 10.1002/cbf.3323. [DOI] [PubMed] [Google Scholar]
- 73.Yoon T.Y., Munson M. SNARE complex assembly and disassembly. Curr. Biol. 2018;28:R397–R401. doi: 10.1016/j.cub.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Kou X., Xu X., Chen C., Sanmillan M.L., Cai T., Zhou Y., Giraudo C., Le A., Shi S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018;10:8524. doi: 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L., Peng X., Li Y., Zhang X., Ma Y., Wu C., Fan Q., Wei S., Li H., Liu J. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol. Cancer. 2019;18:78. doi: 10.1186/s12943-019-0990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N., Tyska M.J., Weaver A.M. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinha S., Hoshino D., Hong N.H., Kirkbride K.C., Grega-Larson N.E., Seiki M., Tyska M.J., Weaver A.M. Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell Biol. 2016;214:197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marat A.L., Ioannou M.S., McPherson P.S. Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton. Mol. Biol. Cell. 2012;23:163–175. doi: 10.1091/mbc.e11-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beghein E., Devriese D., Van Hoey E., Gettemans J. Cortactin and fascin-1 regulate extracellular vesicle release by controlling endosomal trafficking or invadopodia formation and function. Sci. Rep. 2018;8:15606. doi: 10.1038/s41598-018-33868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jongsma M.L., Bakker J., Cabukusta B., Liv N., van Elsland D., Fermie J., Akkermans J.L., Kuijl C., van der Zanden S.Y., Janssen L., et al. SKIP-HOPS recruits TBC1D15 for a Rab7-to-Arl8b identity switch to control late endosome transport. EMBO J. 2020;39:e102301. doi: 10.15252/embj.2019102301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Granger E., McNee G., Allan V., Woodman P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin. Cell Dev. Biol. 2014;31:20–29. doi: 10.1016/j.semcdb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001;11:1680–1685. doi: 10.1016/S0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 83.Wozniak A.L., Adams A., King K.E., Dunn W., Christenson L.K., Hung W.T., Weinman S.A. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 2020;219:12074. doi: 10.1083/jcb.201912074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei D., Zhan W., Gao Y., Huang L., Gong R., Wang W., Zhang R., Wu Y., Gao S., Kang T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31:157–177. doi: 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinchen J.M., Ravichandran K.S. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464:778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fei X., Li Z., Yang D., Kong X., Lu X., Shen Y., Li X., Xie S., Wang J., Zhao Y., et al. Neddylation of Coro1a determines the fate of multivesicular bodies and biogenesis of extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12153. doi: 10.1002/jev2.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villarroya-Beltri C., Baixauli F., Mittelbrunn M., Fernández-Delgado I., Torralba D., Moreno-Gonzalo O., Baldanta S., Enrich C., Guerra S., Sánchez-Madrid F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016;7:13588. doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 89.Alnaas A.A., Watson-Siriboe A., Tran S., Negussie M., Henderson J.A., Osterberg J.R., Chon N.L., Harrott B.M., Oviedo J., Lyakhova T., et al. Multivalent lipid targeting by the calcium-independent C2A domain of synaptotagmin-like protein 4/granuphilin. J. Biol. Chem. 2021;296:100159. doi: 10.1074/jbc.RA120.014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He J., Johnson J.L., Monfregola J., Ramadass M., Pestonjamasp K., Napolitano G., Zhang J., Catz S.D. Munc13-4 interacts with syntaxin 7 and regulates late endosomal maturation, endosomal signaling, and TLR9-initiated cellular responses. Mol. Biol. Cell. 2016;27:572–587. doi: 10.1091/mbc.e15-05-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Zhang K., Qi L., Hu Q., Shen Z., Liu B., Deng J., Zhang C., Zhang Y. DENN domain-containing protein FAM45A regulates the homeostasis of late/multivesicular endosomes. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:916–929. doi: 10.1016/j.bbamcr.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Imai A., Ishida M., Fukuda M., Nashida T., Shimomura H. MADD/DENN/Rab3GEP functions as a guanine nucleotide exchange factor for Rab27 during granule exocytosis of rat parotid acinar cells. Arch. Biochem. Biophys. 2013;536:31–37. doi: 10.1016/j.abb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Song L., Tang S., Han X., Jiang Z., Dong L., Liu C., Liang X., Dong J., Qiu C., Wang Y., et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 2019;10:1639. doi: 10.1038/s41467-019-09720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Savina A., Vidal M., Colombo M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 95.Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M.A., Bakhti M., Grønborg M., Möbius W., Rhee J., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Messenger S.W., Woo S.S., Sun Z., Martin T.F.J. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J. Cell Biol. 2018;217:2877–2890. doi: 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 98.Qin J., Xu Q. Functions and application of exosomes. Acta Pol. Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 99.Morishita M., Takahashi Y., Nishikawa M., Takakura Y. Pharmacokinetics of Exosomes—An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J. Pharm. Sci. 2017;106:2265–2269. doi: 10.1016/j.xphs.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 100.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiklander O.P., Nordin J.Z., O’Loughlin A., Gustafsson Y., Corso G., Mäger I., Vader P., Lee Y., Sork H., Seow Y., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horibe S., Tanahashi T., Kawauchi S., Murakami Y., Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18:47. doi: 10.1186/s12885-017-3958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 104.Vinas J.L., Spence M., Gutsol A., Knoll W., Burger D., Zimpelmann J., Allan D.S., Burns K.D. Receptor-Ligand Interaction Mediates Targeting of Endothelial Colony Forming Cell-derived Exosomes to the Kidney after Ischemic Injury. Sci. Rep. 2018;8:16320. doi: 10.1038/s41598-018-34557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rana S., Yue S., Stadel D., Zoller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Toda Y., Takata K., Nakagawa Y., Kawakami H., Fujioka S., Kobayashi K., Hattori Y., Kitamura Y., Akaji K., Ashihara E. Effective internalization of U251-MG-secreted exosomes into cancer cells and characterization of their lipid components. Biochem. Biophys. Res. Commun. 2015;456:768–773. doi: 10.1016/j.bbrc.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 107.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sancho-Albero M., Navascués N., Mendoza G., Sebastián V., Arruebo M., Martín-Duque P., Santamaría J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019;17:16. doi: 10.1186/s12951-018-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hazan-Halevy I., Rosenblum D., Weinstein S., Bairey O., Raanani P., Peer D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015;364:59–69. doi: 10.1016/j.canlet.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiao L., Hu S., Huang K., Su T., Li Z., Vandergriff A., Cores J., Dinh P.U., Allen T., Shen D., et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10:3474–3487. doi: 10.7150/thno.39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saari H., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 112.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park E.J., Prajuabjinda O., Soe Z.Y., Darkwah S., Appiah M.G., Kawamoto E., Momose F., Shiku H., Shimaoka M. Exosomal regulation of lymphocyte homing to the gut. Blood. Adv. 2019;3:24877. doi: 10.1182/bloodadvances.2018024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laulagnier K., Javalet C., Hemming F.J., Chivet M., Lachenal G., Blot B., Chatellard C., Sadoul R. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol. Life Sci. 2018;75:757–773. doi: 10.1007/s00018-017-2664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Logtenberg M.E.W., Scheeren F.A., Schumacher T.N. The CD47-SIRPα Immune Checkpoint. Immunity. 2020;52:742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clayton A., Harris C.L., Court J., Mason M.D., Morgan B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 117.Escrevente C., Keller S., Altevogt P., Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Munich S., Sobo-Vujanovic A., Buchser W.J., Beer-Stolz D., Vujanovic N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tkach M., Kowal J., Zucchetti A.E., Enserink L., Jouve M., Lankar D., Saitakis M., Martin-Jaular L., Théry C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012–3028. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prada I., Meldolesi J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016;17:1296. doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joshi B.S., de Beer M.A., Giepmans B.N.G., Zuhorn I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano. 2020;14:4444–4455. doi: 10.1021/acsnano.9b10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mettlen M., Chen P.H., Srinivasan S., Danuser G., Schmid S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018;87:871–896. doi: 10.1146/annurev-biochem-062917-012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng D., Zhao W.L., Ye Y.Y., Bai X.C., Liu R.Q., Chang L.F., Zhou Q., Sui S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 126.Teissier E., Pécheur E.I. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur. Biophys. J. 2007;36:887–899. doi: 10.1007/s00249-007-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Glebov O.O., Bright N.A., Nichols B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 128.Koumangoye R.B., Sakwe A.M., Goodwin J.S., Patel T., Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Izquierdo-Useros N., Naranjo-Gómez M., Archer J., Hatch S.C., Erkizia I., Blanco J., Borràs F.E., Puertas M.C., Connor J.H., Fernández-Figueras M.T., et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerr M.C., Teasdale R.D. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 131.Lim J.P., Gleeson P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 132.Nakase I., Kobayashi N.B., Takatani-Nakase T., Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 2015;5:10300. doi: 10.1038/srep10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nanbo A., Kawanishi E., Yoshida R., Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 2013;87:10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Svensson K.J., Christianson H.C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L.M., Mörgelin M., Belting M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rudt S., Müller R.H. In vitro phagocytosis assay of nano- and microparticles by chemiluminescence. III. Uptake of differently sized surface-modified particles, and its correlation to particle properties and in vivo distribution. Eur. J. Pharm. Sci. 1993;1:31–39. doi: 10.1016/0928-0987(93)90015-3. [DOI] [Google Scholar]
- 136.Gordon S. Phagocytosis: An Immunobiologic Process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 137.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z., et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuyama K., Sun H., Mitsutake S., Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen C., Zong S., Wang Z., Lu J., Zhu D., Zhang Y., Zhang R., Cui Y. Visualization and intracellular dynamic tracking of exosomes and exosomal miRNAs using single molecule localization microscopy. Nanoscale. 2018;10:5154–5162. doi: 10.1039/C7NR08800K. [DOI] [PubMed] [Google Scholar]
- 140.Tian T., Zhu Y.L., Hu F.H., Wang Y.Y., Huang N.P., Xiao Z.D. Dynamics of exosome internalization and trafficking. J. Cell Physiol. 2013;228:1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 141.Shelke G.V., Yin Y., Jang S.C., Lässer C., Wennmalm S., Hoffmann H.J., Li L., Gho Y.S., Nilsson J.A., Lötvall J. Endosomal signalling via exosome surface TGFβ-1. J. Extracell. Vesicles. 2019;8:1650458. doi: 10.1080/20013078.2019.1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heusermann W., Hean J., Trojer D., Steib E., von Bueren S., Graff-Meyer A., Genoud C., Martin K., Pizzato N., Voshol J., et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 2016;213:173–184. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., Sun H., Tanowitz M., Liang X.H., Crooke S.T. Intra-endosomal trafficking mediated by lysobisphosphatidic acid contributes to intracellular release of phosphorothioate-modified antisense oligonucleotides. Nucleic Acids Res. 2017;45:5309–5322. doi: 10.1093/nar/gkx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lakhal S., Wood M.J. Exosome nanotechnology: An emerging paradigm shift in drug delivery: Exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 146.Zhong L., Liao D., Li J., Liu W., Wang J., Zeng C., Wang X., Cao Z., Zhang R., Li M., et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct. Target. Ther. 2021;6:59. doi: 10.1038/s41392-020-00414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imjeti N.S., Menck K., Egea-Jimenez A.L., Lecointre C., Lembo F., Bouguenina H., Badache A., Ghossoub R., David G., Roche S., et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA. 2017;114:12495–12500. doi: 10.1073/pnas.1713433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Datta A., Kim H., Lal M., McGee L., Johnson A., Moustafa A.A., Jones J.C., Mondal D., Ferrer M., Abdel-Mageed A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017;408:73–81. doi: 10.1016/j.canlet.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang F., Oudaert I., Tu C., Maes A., Van der Vreken A., Vlummens P., De Bruyne E., De Veirman K., Wang Y., Fan R., et al. System Xc(-) inhibition blocks bone marrow-multiple myeloma exosomal crosstalk, thereby countering bortezomib resistance. Cancer Lett. 2022;535:215649. doi: 10.1016/j.canlet.2022.215649. [DOI] [PubMed] [Google Scholar]
- 150.Cooks T., Pateras I.S., Jenkins L.M., Patel K.M., Robles A.I., Morris J., Forshew T., Appella E., Gorgoulis V.G., Harris C.C. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mitani F., Lin J., Sakamoto T., Uehara R., Hikita T., Yoshida T., Setiawan A., Arai M., Oneyama C. Asteltoxin inhibits extracellular vesicle production through AMPK/mTOR-mediated activation of lysosome function. Sci. Rep. 2022;12:6674. doi: 10.1038/s41598-022-10692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dorayappan K.D.P., Wanner R., Wallbillich J.J., Saini U., Zingarelli R., Suarez A.A., Cohn D.E., Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verweij F.J., Bebelman M.P., Jimenez C.R., Garcia-Vallejo J.J., Janssen H., Neefjes J., Knol J.C., de Goeij-de Haas R., Piersma S.R., Baglio S.R., et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 2018;217:1129–1142. doi: 10.1083/jcb.201703206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y.D., Zhuang X.P., Cai D.L., Cao C., Gu Q.S., Liu X.N., Zheng B.B., Guan B.J., Yu L., Li J.K., et al. Let-7a regulates EV secretion and mitochondrial oxidative phosphorylation by targeting SNAP23 in colorectal cancer. J. Exp. Clin. Cancer Res. 2021;40:31. doi: 10.1186/s13046-020-01813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang F.W., Cao C.H., Han K., Zhao Y.X., Cai M.Y., Xiang Z.C., Zhang J.X., Chen J.W., Zhong L.P., Huang Y., et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J. Clin. Investig. 2019;129:727–743. doi: 10.1172/JCI122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan Z., Cao L., Wu Y., Wang B., Song Z., Yang J., Cheng L., Yang X., Zhou X., Dai Z., et al. Bisecting GlcNAc modification diminishes the pro-metastatic functions of small extracellular vesicles from breast cancer cells. J. Extracell. Vesicles. 2020;10:e12005. doi: 10.1002/jev2.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Giovannone A.J., Reales E., Bhattaram P., Fraile-Ramos A., Weimbs T. Monoubiquitination of syntaxin 3 leads to retrieval from the basolateral plasma membrane and facilitates cargo recruitment to exosomes. Mol. Biol. Cell. 2017;28:2843–2853. doi: 10.1091/mbc.e17-07-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun H., Meng Q., Shi C., Yang H., Li X., Wu S., Familiari G., Relucenti M., Aschner M., Wang X., et al. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatology. 2021;74:2633–2651. doi: 10.1002/hep.32009. [DOI] [PubMed] [Google Scholar]
- 159.Yang K., Fan M., Wang X., Xu J., Wang Y., Tu F., Gill P.S., Ha T., Liu L., Williams D.L., et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29:133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou Y., Zhang Y., Gong H., Luo S., Cui Y. The Role of Exosomes and Their Applications in Cancer. Int. J. Mol. Sci. 2021;22:2204. doi: 10.3390/ijms222212204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baig M.S., Roy A., Rajpoot S., Liu D., Savai R., Banerjee S., Kawada M., Faisal S.M., Saluja R., Saqib U., et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020;69:435–451. doi: 10.1007/s00011-020-01318-0. [DOI] [PubMed] [Google Scholar]
- 162.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 164.Cheng Q., Shi X., Han M., Smbatyan G., Lenz H.J., Zhang Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018;140:16413–16417. doi: 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kooijmans S.A., Aleza C.G., Roffler S.R., van Solinge W.W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Gassart A., Geminard C., Fevrier B., Raposo G., Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 167.Tallon C., Hollinger K.R., Pal A., Bell B.J., Rais R., Tsukamoto T., Witwer K.W., Haughey N.J., Slusher B.S. Nipping disease in the bud: nSMase2 inhibitors as therapeutics in extracellular vesicle-mediated diseases. Drug Discov. Today. 2021;26:1656–1668. doi: 10.1016/j.drudis.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leblanc R., Kashyap R., Barral K., Egea-Jimenez A.L., Kovalskyy D., Feracci M., Garcia M., Derviaux C., Betzi S., Ghossoub R., et al. Pharmacological inhibition of syntenin PDZ2 domain impairs breast cancer cell activities and exosome loading with syndecan and EpCAM cargo. J. Extracell. Vesicles. 2020;10:e12039. doi: 10.1002/jev2.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Datta A., Kim H., McGee L., Johnson A.E., Talwar S., Marugan J., Southall N., Hu X., Lal M., Mondal D., et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci. Rep. 2018;8:8161. doi: 10.1038/s41598-018-26411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang G., Xie L., Li B., Sang W., Yan J., Li J., Tian H., Li W., Zhang Z., Tian Y., et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat. Commun. 2021;12:5733. doi: 10.1038/s41467-021-25990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu X., Liu Y., Li Y., Chen H., Zhang Y., Liu J., Deng S., Zheng Y., Sun X., Wang J., et al. Selective exosome exclusion of miR-375 by glioma cells promotes glioma progression by activating the CTGF-EGFR pathway. J. Exp. Clin. Cancer Res. 2021;40:16. doi: 10.1186/s13046-020-01810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Menck K., Sönmezer C., Worst T.S., Schulz M., Dihazi G.H., Streit F., Erdmann G., Kling S., Boutros M., Binder C., et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles. 2017;6:1378056. doi: 10.1080/20013078.2017.1378056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marleau A.M., Chen C.S., Joyce J.A., Tullis R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ruzicka M., Xiao F., Abujrad H., Al-Rewashdy Y., Tang V.A., Langlois M.A., Sorisky A., Ooi T.C., Burger D. Effect of hemodialysis on extracellular vesicles and circulating submicron particles. BMC Nephrol. 2019;20:294. doi: 10.1186/s12882-019-1459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nishida-Aoki N., Tominaga N., Takeshita F., Sonoda H., Yoshioka Y., Ochiya T. Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis. Mol. Ther. 2017;25:181–191. doi: 10.1016/j.ymthe.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hayatudin R., Fong Z., Ming L.C., Goh B.H., Lee W.L., Kifli N. Overcoming Chemoresistance via Extracellular Vesicle Inhibition. Front. Mol. Biosci. 2021;8:629874. doi: 10.3389/fmolb.2021.629874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kirchhausen T., Macia E., Pelish H.E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzym. 2008;438:77–93. doi: 10.1016/s0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dutta D., Donaldson J.G. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell Logist. 2012;2:203–208. doi: 10.4161/cl.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chiba M., Kubota S., Sato K., Monzen S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci. Rep. 2018;8:11972. doi: 10.1038/s41598-018-30446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elkin S.R., Oswald N.W., Reed D.K., Mettlen M., MacMillan J.B., Schmid S.L. Ikarugamycin: A Natural Product Inhibitor of Clathrin-Mediated Endocytosis. Traffic. 2016;17:1139–1149. doi: 10.1111/tra.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sento S., Sasabe E., Yamamoto T. Application of a Persistent Heparin Treatment Inhibits the Malignant Potential of Oral Squamous Carcinoma Cells Induced by Tumor Cell-Derived Exosomes. PLoS ONE. 2016;11:e0148454. doi: 10.1371/journal.pone.0148454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.P., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bonjoch L., Gironella M., Iovanna J.L., Closa D. REG3β modifies cell tumor function by impairing extracellular vesicle uptake. Sci. Rep. 2017;7:3143. doi: 10.1038/s41598-017-03244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019;14:1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Willms E., Johansson H.J., Mäger I., Lee Y., Blomberg K.E.M., Sadik M., Alaarg A., Smith C.I.E., Lehtiö J., El Andaloussi S., et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chiang C.Y., Chen C. Toward characterizing extracellular vesicles at a single-particle level. J. Biomed. Sci. 2019;26:9. doi: 10.1186/s12929-019-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frolova L., Li I.T.S. Targeting Capabilities of Native and Bioengineered Extracellular Vesicles for Drug Delivery. Bioengineering. 2022;9:496. doi: 10.3390/bioengineering9100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hung M.E., Leonard J.N. Stabilization of exosome-targeting peptides via engineered glycosylation. J. Biol. Chem. 2015;290:8166–8172. doi: 10.1074/jbc.M114.621383. [DOI] [PMC free article] [PubMed] [Google Scholar]