Abstract
Simple Summary
Colorectal cancer (CRC) screening is one of the most effective measures to prevent CRC resulting in a decrease in CRC mortality. Mortality reduction (MR) from CRC screening was estimated based on large-scale randomized control trials (RCTs) as well as in model studies, as there is a wide range on CRC-specific MR and a lack of estimates of all-cause MR. We found that biennial FIT, gFOBT, single/5-yearly FS, and 10-yearly colonoscopy screenings reduced CRC-specific mortality significantly, and 10-yearly colonoscopy is the most effective with a mortality reduction of 73%. The effectiveness of screening increases at younger screening initiation ages and higher adherences. Our findings also suggest that adherence is an important factor in CRC-specific mortality and is an explanation for discrepancy in thus far published pooled estimates.
Abstract
(1) Background: The aim of this study was to pool and compare all-cause and colorectal cancer (CRC) specific mortality reduction of CRC screening in randomized control trials (RCTs) and simulation models, and to determine factors that influence screening effectiveness. (2) Methods: PubMed, Embase, Web of Science and Cochrane library were searched for eligible studies. Multi-use simulation models or RCTs that compared the mortality of CRC screening with no screening in general population were included. CRC-specific and all-cause mortality rate ratios and 95% confidence intervals were calculated by a bivariate random model. (3) Results: 10 RCTs and 47 model studies were retrieved. The pooled CRC-specific mortality rate ratios in RCTs were 0.88 (0.80, 0.96) and 0.76 (0.68, 0.84) for guaiac-based fecal occult blood tests (gFOBT) and single flexible sigmoidoscopy (FS) screening, respectively. For the model studies, the rate ratios were 0.45 (0.39, 0.51) for biennial fecal immunochemical tests (FIT), 0.31 (0.28, 0.34) for biennial gFOBT, 0.61 (0.53, 0.72) for single FS, 0.27 (0.21, 0.35) for 10-yearly colonoscopy, and 0.35 (0.29, 0.42) for 5-yearly FS. The CRC-specific mortality reduction of gFOBT increased with higher adherence in both studies (RCT: 0.78 (0.68, 0.89) vs. 0.92 (0.87, 0.98), model: 0.30 (0.28, 0.33) vs. 0.92 (0.51, 1.63)). Model studies showed a 0.62–1.1% all-cause mortality reduction with single FS screening. (4) Conclusions: Based on RCTs and model studies, biennial FIT/gFOBT, single and 5-yearly FS, and 10-yearly colonoscopy screening significantly reduces CRC-specific mortality. The model estimates are much higher than in RCTs, because the simulated biennial gFOBT assumes higher adherence. The effectiveness of screening increases at younger screening initiation ages and higher adherences.
Keywords: colorectal cancer, screening, mortality reduction, simulation models, randomized control trials, meta-analysis
1. Introduction
The incidence and mortality of colorectal cancer (CRC) accounts for approximately 10% of all cancers worldwide, with an estimated 1.93 million new cases diagnosed and 0.94 million deaths in 2020 [1,2]. The 5-year CRC survival in 2014 was over 60% in high-income countries, and less than 50% in South American and Asian countries [3,4]. The majority of CRC arises from precursor lesions in the classic pathway with the most common lesions being adenomas and serrated pathways with polypus serrated lesions [4,5]. Usually, it takes 10–15 years for these precursor lesions to progress to CRC [6,7]. CRC screening is one of the most effective measures to prevent CRC resulting in decrease in CRC mortality [4,8]. As such, CRC screening is recommended by the World Health Organization (WHO) and has been implemented in several countries [4,9]. Biannual FIT for people under 75 years of age is the most common screening scenario in countries where population-based CRC screening have been implemented [4].
The mortality reduction (MR), life years gained (LYG) and quality-adjusted life year (QALY) gained from CRC screening were evaluated in large-scaled randomized control trials (RCTs) and in model studies. Five RCTs with guaiac-based fecal occult blood tests (gFOBT) in 765,685 participants and four RCTs with flexible sigmoidoscopy (FS) in 458,022 participants on CRC screening have been reported in the CRC handbook of the International Agency for Research on Cancer [4]. In addition, several models have been widely used to evaluate CRC screening scenarios efficiently and economically [10,11,12,13,14].
Published RCTs and simulation models present a varying range on CRC-specific MR. Four systematic reviews and meta-analyses combined the results of RCTs with gFOBT and FS in intention-to-screen analysis [15,16,17,18]. These reviews included studies that reported relative risks for CRC-specific mortality, ranging from 0.78 to 0.91 using gFOBT and from 0.33 to 0.78 using FS [15,16,17,18]. In the pooled analyses, gFOBT screening leads to a MR of 17% with 95% confidence intervals (95% CI) of 8–25% [17], and that FS results in a MR of 28% (95% CI: 20–35%) [15,16]. The model studies showed 24–79%, 8–84%, 25–56%, 16–94%, and 55–81% CRC-specific MRs on biennial fecal immunochemical tests (FIT), biennial gFOBT, single FS, 10-yearly colonoscopy, and 5-yearly FS screening, respectively [12,13,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Adherence rates, adenoma detection rates and dwelling time were used in several model studies in the sensitivity analyses [10,13,19,23,40,41,44].
In conclusion, there is uncertainty over CRC-specific MRs and lack of estimates of all-cause MR in the general population due to CRC screening, and the model studies tend to give higher estimates than RCTs for disease-specific mortalities. Therefore, in this systematic review and meta-analysis, we aim to synthesize and compare the effectiveness of different CRC screening interventions in the general population on all-cause and CRC-specific MR compared with no screening in RCTs and simulation models. In addition, we aim to evaluate the factors that influence screening effectiveness to determine how CRC screening could be improved.
2. Materials and Methods
We registered a predefined protocol of this study in the International Prospective Registry of Systematic Reviews (PROSPERO registration number: CRD42021270887). This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA), 2020 statement [45].
2.1. Data Sources and Search Strategies
We conducted a systematic literature search in PubMed, Embase, Web of Science and Cochrane library for published RCT studies (1 January 2006 to 31 July 2022) and model studies (1 January 2016 to 31 July 2022) with the following keywords: (“randomized controlled trials”) for RCTs and (“computer simulation” or “models” or “modelling” or “Markov chain”) for simulation models, (“mortality” or “cost-benefit analysis” or “effectiveness” or “life-year”), (“early detection of cancer” or “mass screening” or “fecal immunochemical test” or “fecal occult blood test“ or “colonoscopy” or “sigmoidoscopy”), and (“colorectal cancer” or “bowel cancer” or “colon cancer” or “rectum cancer”). The keywords retrieving RCTs were used for the Cochrane library since this database only includes trial studies. Detailed search strategies for all databases are shown in Supplementary Materials Table S1.
2.2. Study Selection and Data Extraction
Two reviewers (SZ, JS) independently screened the potentially relevant studies based on eligibility criteria and extracted data from included studies. A study was eligible for inclusion if the following criteria were met: (1) multi-use simulation model or the latest publication of RCT compared commonly used CRC screening scenarios with no screening in general population; (2) original study published in English with outcomes on survival, death number, and CRC-specific or all-cause MR by CRC screening. Studies published as conference abstract, editorial, review, research protocol, study design, or implementation report of screening program without original data were excluded. For a detailed overview of the inclusion and exclusion criteria see Supplementary Materials Box S1.
Study information, country/population, screening scenario, adherence to screening, screening time, and follow-up time were extracted for both study types. Screening program, number of participants and person-years of observation in screening and control group, all-cause/CRC death number, and compliance-adjusted outcomes were obtained for RCTs; model used, and all-cause/CRC mortality in screening and no screening scenario for model studies.
2.3. Quality Assessment for RCTs and Simulation Models
For the RCT studies, we applied the revised Cochrane risk-of-bias tool for randomized trials (RoB2) [46]. There are five domains in this tool, including randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of reported results. We classified the risk of bias on each domain and study as “low”, “high”, or “some concerns”.
For the simulation models, we selected the study with the most complete description to evaluate model quality. A qualitative assessment framework included modelling approach, model parameters, transparency of data sources/assumptions, and external validation to assess the overall risk of bias (Supplementary Materials Box S2) [47]. When there were two or more items that did not provide a clear description, high risk of bias was assessed; otherwise, low risk. The two reviewers resolved disagreements on study selection, data extraction, and assessments by discussion and further review, or arbitrage by a third author (GdB).
2.4. Data Synthesis and Analysis
Data were synthesized and analyzed separately according to screening interventions (gFOBT or FS) in RCT studies. The primary outcomes were all-cause and CRC-specific MR, as measured by calculating the mortality rate ratio between screening and no screening scenarios. We used the original data in a pooled analysis of an intention to screen analysis, which included all individuals as randomized. Because of various follow-up times, observed person-years were extracted to calculate mortality density. RCT studies providing compliance-adjusted results were included in the compliance-adjusted analysis. Rate ratio, hazard ratio, and relative risk were assumed to be similar in the case of large sample sizes and were pooled directly. Regarding the model studies, we summarized data by screening scenarios and synthesized the commonly used global and Dutch scenarios.
Due to differences in the population, trial procedures, model assumptions, and interventions of the included RCTs and simulation models, the expected effect value of these studies were not identical. To ensure that all effects were represented in the pooled effects, and not overly influenced by one study, a bivariate random model was applied to estimate pooled all-cause or CRC-specific mortality rate ratio with 95% CI. The no screening group/scenario was the reference. We then qualitatively synthesized all-cause MRs in model studies.
Meta regression was applied before subgroup analyses to explore possible sources of heterogeneity (I2 ≥ 50%) when there were more than 10 studies/scenarios. Our subgroup analyses followed if heterogeneity existed or when we observed differences in factors affecting screening effectiveness among the pooled studies. We classified subgroups by risk of bias, adherence, screening initiation age, population, model, or screening scenario and assessed publication bias by funnel plots or Egger’s test. A two-sided p < 0.05 was considered statistically significant. Statistical analysis was performed using meta package in R 3.6.1 and Review Manager (Version 5.4.1, The Cochrane Collaboration, 2020, London, UK).
3. Results
3.1. Literature Search
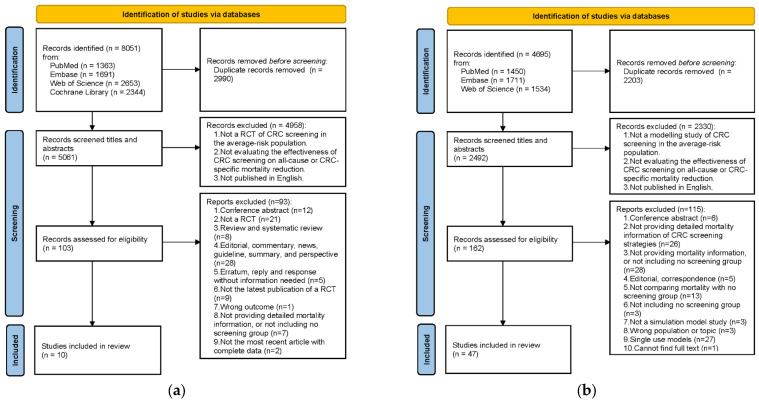
The literature search identified 8051 studies for RCTs and 4695 studies for model studies. A total of 103 RCTs and 162 model studies remained after removing duplicates and reviewing titles and abstracts. We excluded 93 RCTs and 115 model studies following the exclusion criteria. Thus, 10 RCTs and 47 model studies were eligible for inclusion (Figure 1).
Figure 1.
Flowchart of the selection process. (a) Studies on RCTs; (b) Studies on simulation model studies.
3.2. RCTs
3.2.1. Quality Assessment
Concerning deviations from intended interventions, five RCTs [48,49,50,51,52] showed some concerns on risk of bias because blind methods were not used, and the other five studies [53,54,55,56,57] were evaluated as high risk because of the lack of blind methods and an appropriate analysis to estimate effect of adhering to intervention. The study of Thiis-Evensen et al. was deemed to have some concerns on randomization process due to small sample size [57]. Overall, five studies were considered as low risk, four studies as some concerns, and one study as high risk of bias (Supplementary Materials Figure S1).
3.2.2. Study Characteristics
Five RCTs used gFOBT and the other five RCTs used FS as CRC screening interventions (Table 1). There were four European trials and one US trial with gFOBT screening [49,51,52,54,56]. The total number of participants ranged from 46,551 in the US trial to 360,492 in the Finland study. Adherence rates varied from 57.0% to 90.0%. The RCTs with FS screening consisted of four European trials and one US trial [48,50,53,55,57]. Among these trials, the total number of participants ranged from 799 to 170,034. The adherence rates ranged from 57.8% to 86.6%.
Table 1.
Characteristics of randomized controlled trials.
| Study | Program | Intervention | Age | Screening Years | Follow-Up Time (Years) | Total No. of Participants | Total No. of Screening Group | Adherence Rate in Screening Group (%) | Total No. of Control Group | Results of Compliance-Adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| A Shaukat US, 2013 [52] |
Minnesota Colon Cancer Control Study | Annual or biennial gFOBT | 50–80 | 1976–1992 | 30.0 | 46,551 | 31,157 | 90.0 | 15,394 | All-cause and CRC-specific |
| A Shaukat Finland, 2021 [51] |
Funen Fecal Occult Blood Trial | Biennial gFOBT | 45–75 | 1985–2002 | 30.0 | 61,933 | 30,966 | 66.8 | 30,964 | All-cause and CRC-specific |
| E Lindholm Sweden, 2008 [54] |
Goteborg FOBT Trial | 2–3 times gFOBT | 60–64 | 1982–1995 | Mean 15.5/8.7 a | 68,308 | 34,144 | 70.0 | 34,164 | N/A |
| J H Scholefield UK, 2012 [49] |
Nottingham Trial | Biennial gFOBT | 45–74 | 1981–1991 | Median 19.5 | 151,975 | 76,056 | 57.0 | 75,919 | CRC-specific |
| J Pitkäniemi Finland, 2015 [56] |
Finnish FOBT Screening Programme | Biennial gFOBT | 60–69 | 2004–2012 | Median 4.5 | 360,492 | 180,210 | 68.8 | 180,282 | N/A |
| E Thiis-Evensen Norway, 2013 [57] |
Telemark Polyp Study | Single FS | 50–59 | 1983–1996 | 26.0 | 799 | 400 | 81.0 | 399 | N/A |
| C Senore Italy, 2022 [50] |
SCORE Trial | Single FS | 55–64 | 1995–1999 | Median 18.8 | 34,272 | 17,136 | 57.8 | 17,136 | All-cause and CRC-specific |
| Ø Holme Norway, 2018 [53] |
NORCCAP | Single FS with or without FOBT | 50–64 | 1999–2001 | Median 14.8 | 98,678 | 20,552 | 63.1 | 78,126 | N/A |
| P F Pinsky US, 2019 [55] |
PLCO Cancer Screening | FS at baseline and at year 3 or 5 | 55–74 | 1993–2001 | Median 17.0 | 154,887 | 77,443 | 86.6 | 77,444 | N/A |
| W Atkin UK, 2017 [48] |
UKFSST | Single FS | 55–64 | 1994–1999 | Median 17.0 | 170,034 | 57,098 | 71.1 | 112,936 | All-cause and CRC-specific |
a 15.5 years from the first invitation, 8.6 years from the last screening occasion. N/A: Not available. The study did not provide the result.
3.2.3. Synthesis Results of CRC-Specific and All-Cause MR
In the intention-to-screen analyses, the pooled estimates of all-cause and CRC-specific mortality rate ratios of gFOBT trials were 1.00 (95% CI: 0.99–1.01, p = 0.65) and 0.88 (95% CI: 0.80–0.96, p = 0.005), and of FS trials 1.02 (95% CI: 0.97–1.06, p = 0.46) and 0.76 (95% CI: 0.68–0.84, p < 0.001), respectively. There were heterogeneities among the gFOBT studies on the CRC-specific mortality rate ratio (I2 = 59%, p = 0.04) and among the FS studies on the all-cause mortality rate ratio (I2 = 88%, p < 0.001) (Table 2). The funnel plots do not suggest relevant publication bias (Supplementary Materials Figure S2).
Table 2.
Overview of randomized controlled trials on impact of CRC screening: results on all-cause and CRC-specific mortality (Intention-to-screen analyses a).
| Studies | Screening Group | Control Group | All-Cause Mortality Rate Ratio (95 CI%) b |
CRC-Specific Mortality Rate Ratio (95 CI%) b |
||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Deaths | CRC-Specific Deaths | Total Person-Years of Observation | All-Cause Deaths | CRC-Specific Deaths | Total Person-Years of Observation | |||
| FOBT | ||||||||
| A Shaukat 2013 [52] | 22,076 | 437 | 951,047 | 10,944 | 295 | 469,897 | 1.00 (0.97, 1.02) | 0.73 (0.63, 0.85) |
| A Shaukat 2021 [51] | 22,474 | 786 | 605,023 | 22,535 | 851 | 603,953 | 1.00 (0.98, 1.01) | 0.92 (0.84, 1.02) |
| E Lindholm 2008 [54] | 10,591 | 252 | 471,072 | 10,432 | 300 | 471,980 | 1.02 (0.99, 1.04) | 0.84 (0.71, 1.00) |
| J H Scholefield 2012 [49] | 40,681 | 1176 | 1,296,712 | 40,550 | 1300 | 1,296,614 | 1.00 (0.99, 1.02) | 0.90 (0.84, 0.98) |
| J Pitkäniemi 2015 [56] | 8000 | 170 | 805,480 | 7963 | 164 | 805,693 | 1.00 (0.97, 1.04) | 1.04 (0.84, 1.28) |
| Meta-analysis: | ||||||||
| No. of studies | 5 | 5 | ||||||
| Pooled estimate | 1.00 (0.99, 1.01) | 0.88 (0.80, 0.96) | ||||||
| Test for overall effect: P | 0.65 | 0.005 | ||||||
| Heterogeneity: I2 (%)/Tau2/P | 0/0.00/0.74 | 59/0.01/0.04 | ||||||
| FS | ||||||||
| E Thiis-Evensen 2013 [57] | 188 | 1 | 8441 | 151 | 7 | 8997 | 1.33 (1.07, 1.64) | 0.15 (0.02, 1.24) |
| C Senore 2022 [50] | 3062 | 122 | 296,730 | 3155 | 157 | 295,013 | 0.96 (0.92, 1.01) | 0.77 (0.61, 0.98) |
| P F Pinsky 2019 [55] | 22,562 | 416 | 1,234,900 | 22,652 | 546 | 1,222,450 | 0.99 (0.97, 1.00) | 0.75 (0.66, 0.86) |
| W Atkin 2017 [48] | 13,279 | 353 | 902,198 | 26,409 | 996 | 1,780,738 | 0.99 (0.97, 1.01) | 0.70 (0.62, 0.79) |
| Ø Holme 2018 [53] | 3809 | 122 | 291,075 | 13,433 | 530 | 1,114,581 | 1.09 (1.05, 1.13) | 0.88 (0.72, 1.07) |
| Meta-analysis: | ||||||||
| No. of studies | 5 | 5 | ||||||
| Pooled estimate | 1.02 (0.97, 1.06) | 0.76 (0.68, 0.84) | ||||||
| Test for overall effect: P | 0.46 | <0.001 | ||||||
| Heterogeneity: I2 (%)/Tau2/P | 88/0.00/<0.001 | 35/0.00/0.19 | ||||||
a Intention-to-screen analyses: including all individuals as randomized. Not be adjusted by compliance. b Control group was used as the reference.
The pooled estimates of rate ratios in the compliance-adjusted analysis showed that gFOBT screening reduced all-cause mortality by 1% (0.99, 95% CI: 0.97–1.00, p = 0.01), CRC–specific mortality by 21% (0.79, 95% CI: 0.68–0.91, p = 0.001), and FS screening reduced CRC–specific mortality by 41% (0.59, 95% CI: 0.51–0.70, p < 0.001). However, there was no significant reduction in all-cause mortality of FS screening compared with no screening (0.97, 95% CI: 0.91–1.03, p = 0.26) (Supplementary Materials Figure S3).
3.2.4. Subgroup Analysis
Subgroup analysis by adherence showed that there was a significant difference between the ≥70% and <70% adherence groups, and CRC-specific MR by FOBT increased with higher adherence (0.78 (0.68, 0.89) vs. 0.92 (0.87, 0.98), Psub = 0.03). For all-cause MR by FS, subgroups of initiation age and risk of bias did not eliminate heterogeneity among studies in intention-to-screen analyses (initiation age: Psub = 0.08; risk of bias: Psub = 0.19) (Supplementary Materials Table S2). In compliance-adjusted analyses, annual gFOBT had a larger significant reduction in CRC-specific mortality than biennial gFOBT (0.65 (0.52, 0.81) vs. 0.84 (0.74, 0.94), Psub = 0.045) (Supplementary Materials Table S3).
3.3. Simulation Models
3.3.1. Study Characteristics and Quality Assessment
Nine simulation models were identified, including three Cancer Intervention and Surveillance Modeling Network (CISNET) CRC models (CRC Simulated Population Model for Incidence and Natural History (CRC-SPIN) [14,24,30,31,42], Simulation Model of Colorectal Cancer (SimCRC) [14,24,30,31,36,42] and MIcrosimulation SCreening ANalysis-Colon (MISCAN-Colon) [14,20,21,22,24,25,29,30,31,35,36,37,38,39,42,58,59,60,61,62,63,64,65,66,67]), adenoma and serrated pathway to colorectal cancer (ASCCA) [12,19,26,27,28,43], Microsimulation-based colon modelling open-source tool (CMOST) [23,41], Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model (CRC-AIM) [40,44,68,69], Decision analytic Markov cohort model [32,70,71,72], Multistate Markov model [73,74], and Policy1-Bowel [13,33,34,75]. Six models were assessed as low risk of bias, and three models as high risk (Supplementary Materials Table S4).
Five main scenarios were extracted from included studies. In total, 15 studies assessed the effectiveness of biennial FIT screening from 55–75 years [12,19,22,26,27,28,30,31,36,40,43,59,60,62,63], 4 studies biennial gFOBT screening from 45–80 years [25,31,36,40], 6 studies single FS screening from 50–75 years [20,21,24,25,29,42], 7 studies 10-yearly colonoscopy screening from 55–75 years [22,30,31,36,40,43,62], and 4 studies 5-yearly FS screening from 55–75 years [30,31,36,40] (Table 3 and Supplementary Materials Tables S5–S8). These studies used ASCCA, CRC-AIM, SimCRC, CRC-SPIN, or MISCAN-Colon models, and applied the US, Australian, and European population.
Table 3.
Characteristics of simulation models on biennial FIT screening from the age of 55 to 75.
| Model | Study No. | Study | Simulate Population | Screening Age | Simulation Period/Follow-Up Time (Years) | Adherence Rate in Screening Group (%) | CRC Mortality of No Screening Group | CRC Mortality of Screening Group |
|---|---|---|---|---|---|---|---|---|
| ASCCA | 1 | Bronzwaer, M. E. S. (2018) [19] | Dutch | 55–75 | 2014–2044 | 73% (FIT)/92% (FIT-positive CS) | 42.3/100,000 | 20.4/100,000 |
| 2 | Greuter, M. J. (2016) [26] | Dutch | 55–75 | 2014–2044 (30 years) | 63% (FIT)/82% (FIT-positive CS) | 44.0/100,000 | 21.0/100,000 | |
| 3 | Greuter, M. J. (2016) [27] | Dutch | 55–75 | Lifetime (11 rounds) | 63% (FIT)/96% (FIT-positive CS) | 28.3/1000 | 12.2/1000 | |
| 4 | Greuter, M. J. (2016) [28] | Dutch | 55–75 | 2014–2044 (30 years) | 63% (FIT)/96% (FIT-positive CS) | 44.0/100,000 | 23.3/100,000 | |
| 5 | Greuter, M. J. E. (2017) [12] | Dutch | 55–75 | Lifetime | 72.6% (FIT)/92% (FIT-positive and Surveillance CS) | 28.2/1000 | 13.5/1000 | |
| 6 | Vleugels, J. L. A. (2017) [43] | Dutch | 55–75 | Lifetime | 73% (FIT)/92% (FIT-positive CS) | 28.2/1000 | 14.0/1000 | |
| CRC-AIM | 7 | Piscitello, A. (2020) [40] | US | 55–75 | Lifetime | 100% | 31.7/1000 | 12.0/1000 |
| SimCRC | 8 | Knudsen, A. B. (2016) [31] | US | 55–75 | Lifetime | 100% | 28.0/1000 | 9.0/1000 |
| 9 | Knudsen, A. B. (2021) [30] | US | 55–75 | Lifetime | 100% | 34.0/1000 | 12.0/1000 | |
| 10 | Meester, R. G. S. (2018) [36] | US White Female | 55–75 | Lifetime | 100% | 25.7/1000 | 8.7/1000 | |
| US Black Female | 55–75 | Lifetime | 100% | 30.0/1000 | 12.0/1000 | |||
| US White Male | 55–75 | Lifetime | 100% | 31.0/1000 | 10.0/1000 | |||
| US Black Male | 55–75 | Lifetime | 100% | 27.0/1000 | 10.0/1000 | |||
| CRC-SPIN | 8 | Knudsen, A. B. (2016) [31] | US | 55–75 | Lifetime | 100% | 27.0/1000 | 10.0/1000 |
| 9 | Knudsen, A. B. (2021) [30] | US | 55–75 | Lifetime | 100% | 32.0/1000 | 12.0/1000 | |
| MISCAN-Colon | 8 | Knudsen, A. B. (2016) [31] | US | 55–75 | Lifetime | 100% | 28.0/1000 | 11.0/1000 |
| 9 | Knudsen, A. B. (2021) [30] | US | 55–75 | Lifetime | 100% | 34.0/1000 | 15.0/1000 | |
| 10 | Meester, R. G. S. (2018) [36] | US White Female | 55–75 | Lifetime | 100% | 21.9/1000 | 9.9/1000 | |
| US Black Female | 55–75 | Lifetime | 100% | 28.4/1000 | 12.4/1000 | |||
| US White Male | 55–75 | Lifetime | 100% | 27.2/1000 | 11.2/1000 | |||
| US Black Male | 55–75 | Lifetime | 100% | 29.6/1000 | 12.6/1000 | |||
| 11 | Cenin, D. R. (2020) [22] | Australian | 54–74 | Lifetime 40–100 | 100% | 29.0/1000 | 16.0/1000 | |
| Australian | 54–74 | Lifetime 40–100 | Realistic adherence | 29.0/1000 | 22.0/1000 | |||
| 12 | Gini, A. (2021) [62] | Dutch | 55–75 | 2018–2050 | 71.3% | 26.5/1000 | 19.5/1000 | |
| 13 | Cenin, D. (2022) [60] | Chinese | 55–75 | Lifetime | 100% | 11.0/1000 | 5.0/1000 | |
| 14 | Buskermolen, M. (2022) [59] | Dutch | 55–75 | Lifetime | 72.4% | 37.3/1000 | 10.1/1000 | |
| 15 | Heinävaara, S. (2022) [63] | Finnish | 55–74 | Lifetime | 100% | 23.0/1000 | 14.0/1000 |
3.3.2. Synthesis Results of CRC-Specific and All-Cause MR
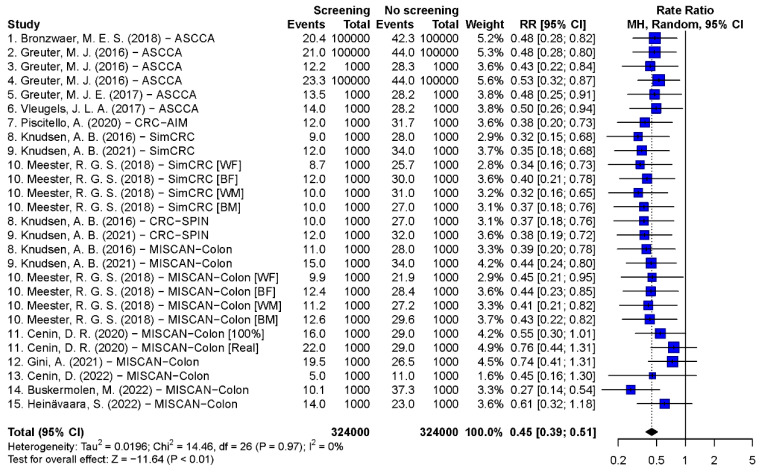
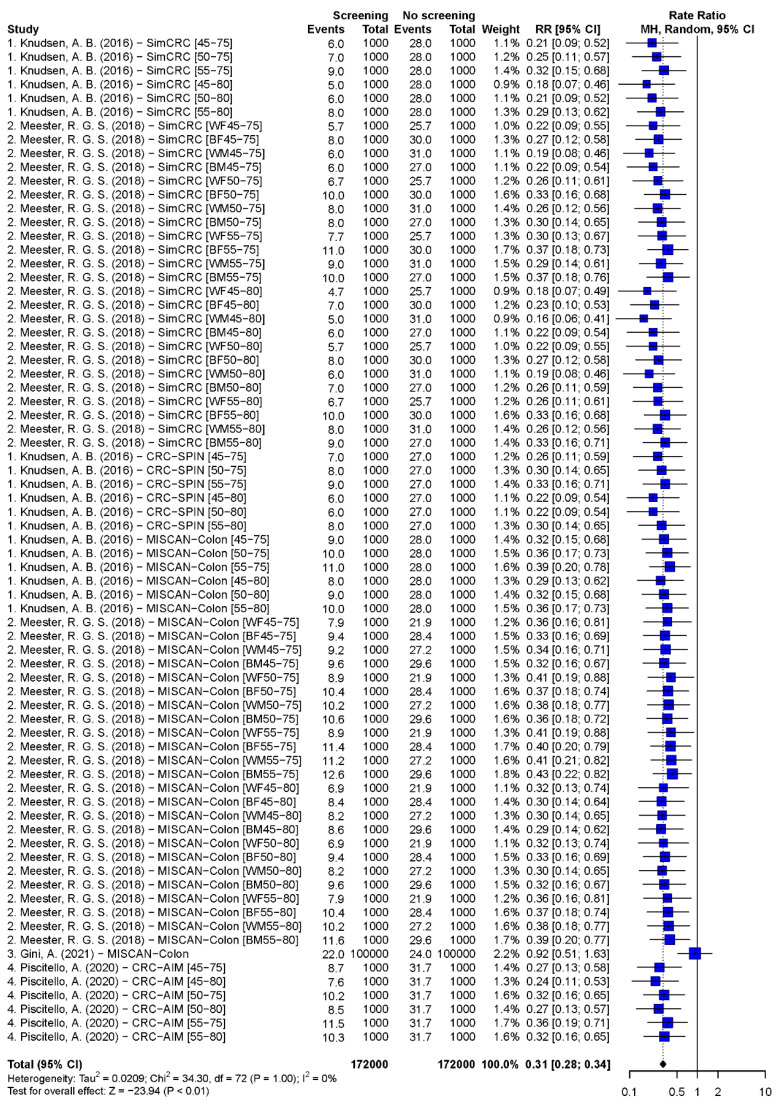
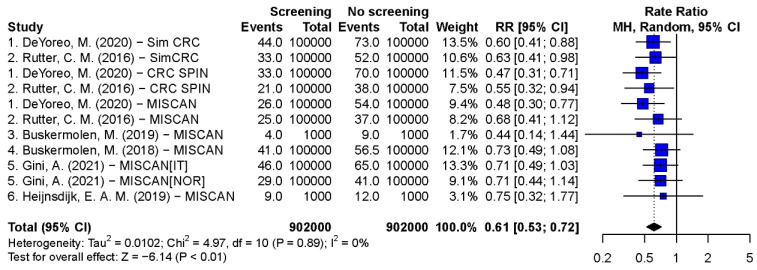
Pooled estimates of CRC-specific mortality rate ratios of scenarios with 55–75 years biennial FIT, 45–80 years biennial gFOBT, 50–75 years single FS, 55–75 years 10-yearly colonoscopy, and 55–75 years 5-yearly FS were 0.45 (95% CI: 0.39–0.51, p < 0.001), 0.31 (95% CI: 0.28–0.34, p < 0.001), 0.61 (95% CI: 0.53–0.72, p < 0.001), 0.27 (95% CI: 0.21–0.35, p < 0.001), and 0.35 (95% CI: 0.29–0.42, p < 0.001), respectively (Figure 2, Figure 3, Figure 4 and Supplementary Materials Figures S4 and S5). The Egger’s tests indicated that there was a publication bias in model studies on CRC-specific MR except for single FS scenario (Supplementary Materials Table S9). The results of screenings from 50 to 75 and 45 to 75 years are shown in Supplementary Materials Figures S6–S8. The pooled rate ratios of 45–75 years biennial FIT, 10-yearly colonoscopy, and 5-yearly FS were 0.38 (95% CI: 0.32–0.46, p < 0.001), 0.17 (95% CI: 0.13–0.24, p < 0.001), and 0.27 (95% CI: 0.22–0.33, p < 0.001), respectively.
Figure 2.
Forest plots of the CRC-specific mortality rate ratio on biennial FIT screening from the age of 55 to 75 [12,19,22,26,27,28,30,31,36,40,43,59,60,62,63].
Figure 3.
Forest plots of the CRC-specific mortality rate ratio on biennial gFOBT screening from the age of 45 to 80 [25,31,36,40].
Figure 4.
Forest plots of the CRC-specific mortality rate ratio on single FS screening from the age of 50 to 75 [20,21,24,25,29,42].
The all-cause MR was presented in two studies. In the one from Norway, the results of the 3% CRC risk population were selected [20,76]. A total of 1.1–1.4% all-cause MR was shown with perfect adherence annual/biennial FIT and single FS/colonoscopy from 50 to 79 years [20]. In the Dutch study, single FS reduced 0.62% of all-cause mortality with 73% adherence [29] (Supplementary Materials Table S14).
3.3.3. Subgroup Analysis
There were heterogeneities among studies with 55–75 years 10-yearly colonoscopy on CRC-specific MR (I2 = 55%, p < 0.001), and meta regression showed that adherence was the main source for heterogeneity (Supplementary Materials Figure S4 and Table S10). The pooled estimate of 100% adherence subgroup was 0.21 (95% CI: 0.17–0.27), and of realistic adherence subgroup was 0.70 (95% CI: 0.50–0.97) (Psub < 0.001). The results of biennial gFOBT by adherence also indicated a significant difference between 100% and 69% adherence groups (0.30 (0.28, 0.33) vs. 0.92 (0.51, 1.63), Psub < 0.001). For single FS, the rate ratio of UK population was lower than that of non-UK population (0.56 (0.48,0.66) vs. 0.70 (0.61,0.80), Psub = 0.004) (Supplementary Materials Tables S11–S13).
3.3.4. The Comparison of Model Studies and RCTs
For CRC-specific MR, pooled estimates of RCTs showed a 12% (4–20%) and 24% (16–32%) reduction in the intention-to-screen analyses, and 21% (9–31%) and 41% (30–49%) reduction in the compliance–adjusted analysis in gFOBT and single FS scenarios, respectively. In model studies, biennial gFOBT presented 69% (66–72%) and single FS 39% (28–47%) reduction. In total, 1% of all–cause MR was found in RCTs with gFOBT when adjusted compliance, and 0.62–1.1% reductions were shown in model studies with single FS.
4. Discussion
By systematic selection of RCTs and multiple-use simulation models on all-cause and CRC-specific MR of CRC screening, 10 RCTs and 47 model studies, including 9 simulation models, were retrieved. Our pooled results show that biennial FIT, gFOBT, single/5-yearly FS, and 10-yearly colonoscopy screenings reduced CRC-specific mortality significantly, and 10-yearly colonoscopy is the most effective with a mortality reduction of 73%. Approximately 1% all-cause MR was presented in FIT, gFOBT, and FS scenarios with high adherence or adjusted compliance. Adherence is a crucial factor on the effectiveness of CRC screening, and higher adherence leads to more significant MR. In model studies, younger screening initiation ages were associated with higher MR than older ages.
Although RCTs should be considered as golden standard for evaluation of benefits and cost-effectiveness of screening strategies, trials request large amounts of time and medical resources, and not all potential scenarios can be evaluated. Simulation models eliminate these disadvantages and can assess multiple scenarios [47]. However, our main finding was that the pooled MRs in model studies tended to be higher than in RCTs. An explanation for this overestimation might be uncertainties in model parameters and in assumptions on CRC progression, and most model studies assume ideal parameters and lack external validation [47]. Another possible explanation might be that model studies applied a perfect adherence with lifetime follow-up, which resulted in more than realistic MR [13,77]. Many individuals in screening ages are not screened properly in the real world [77,78].
Our pooled estimates of RCTs showed CRC-specific MR of 12% in gFOBT and 24% in single FS screenings compared to control group. Previous systematic review and meta-analyses reported 12% and 18% MR with gFOBT screening, respectively [79,80]. Others reported 26–28% CRC-specific MR with single FS screening [15,16,80]. The compliance-adjusted analysis showed that FS screening decreased CRC-specific mortality by 41%, which is in accordance with Brenner et al. [15]. Regarding all-cause MR, prior study indicated that single FS and gFOBT screening had little or no reduction in all-cause mortality compared [80]. However, gFOBT screening slightly reduced all-cause mortality by 1% in our results, possibly because only screening participants in intervention group were included, which amplified the effect of screening.
All included scenarios decreased CRC-specific mortality significantly in model studies. Several reviews of model studies concluded that all CRC screening strategies were more effective than no screening [81,82]. In addition, in our pooled estimates, CRC-specific MR of 10-yearly colonoscopy was the highest among all scenarios, while MR of 10-yearly colonoscopy with realistic adherence was not dominant. In general, the adherence of FIT or gFOBT is higher than that of colonoscopy [83]. Thus, although colonoscopy is convinced to have a strong capability in CRC and adenoma screening, the conclusion that the dominance of colonoscopy scenario is not absolute in reality considering the adherence [83,84]. This is consistent with the result of Zhong et al. [83]. Another interesting finding was that biennial gFOBT showed higher MR than biennial FIT. However, gFOBT has lower sensitivity and specificity for CRC than FIT, which results in lower MR than other fecal-based scenarios [31,85,86]. This may be explained by the wider screening age range used with gFOBT, and that all except one study used perfect adherence and lifetime follow-up.
Another finding was that adherence and screening initiation age are crucial factors on effectiveness of CRC screening. Most models included aimed to compare the cost-effectiveness of screening scenarios under optimal conditions. For that, a 100% compliances was assumed. However, in daily screening practice, only part of the invited population will attend CRC screening, which will reduce the screening efficiency [87]. Reported estimates for CRC screening adherence are over 60% in high-income countries in Europe, and generally less than 40% in Eastern European countries [88]. Therefore, the use of the real adherence estimates in simulation models will show more realistic values for the evaluation of screening scenarios. Additionally, the WHO stated that a high adherence is the critical factor for a successful screening program implementation [88,89]. Prior studies indicated that several measures contribute to the improvement in adherence, including telephone contact with a navigator, narrative invitation letters, and an approach in which the awareness of CRC and of purpose of CRC screening is strengthened by using an enhanced procedural informational brochure [90,91,92,93]. For screening initiation age, a prior study also revealed that the effectiveness of CRC screening was influenced [73]. American Cancer Society recommends that starting CRC screening at age 45 instead of 50 leads to more favorable cost-effectiveness [31,73]. Our finding also suggested that younger screening initiation ages are correlated with higher CRC-specific MR. Because we did not consider screening costs, we can, however, not conclude early initiation ages are dominant scenarios.
An explanation for the publication bias in model studies is that screening techniques are sensitive to early cancers and precursor lesions, which could be detected and treated at early stage [4,8]. The majority of results are positive due to early diagnosis, which does not introduce bias into our results.
Strengths and Limitations
This study combined results reported in the latest English publication of CRC screening RCTs worldwide, which is the best representative of CRC screening effectiveness. This is also the first study that pooled the benefits of CRC screening in model studies and compared the outcomes with RCTs. Additionally, this study reviewed the effects of CRC screening on all-cause MR. There are some limitations in our study. First, populations in both RCT and model studies were only from Europe, the United States and Australia, so generalization of findings to other parts of the world should be carried out with caution. Second, this study did not include cost, detection rate, and false positive rates, which need to be considered when evaluating optimal screening scenarios and should therefore be added to future research. Third, the assumptions and parameters of the simulation models differed, which leads to a variability in results. There were relatively more publications on CRC-SPIN, SimCRC, and MISCAN-Colon, which are the three main models recommended by CISNET. Thus, the effects of these three models might have a greater impact on the pooled estimates compared to the other models. This resulted inevitably in a quasi-publication bias. Fourth, this study focused on commonly used scenarios. Further systematic reviews and meta-analyses focusing on scenarios with other ages, intervals, and novel screening techniques are necessary to expand the scope of screening effectiveness assessment. Fifth, only perfect adherence and widely used screening ages were considered in the model studies. Screening interval, age of screening initiation, and adherence, which might influence screening effectiveness, were included in scenario construction or sensitivity analyses in some model studies. However, there are no studies that explored MR as a function of different screening scenarios and adherence.
5. Conclusions
Our systematic review and meta-analysis provides a summary of the latest RCT and model studies of CRC screening on all-cause and CRC-specific MR. Commonly adopted global and Dutch screening scenarios could decrease CRC-specific mortality significantly, and 10-yearly colonoscopy screening is likely to be the most effective. Compliance-adjusted outcome with gFOBT in RCTs showed 1% of all-cause MR, and 0.62–1.1% reductions were shown in model studies with single FS screenings. Our findings suggest that adherence is an important factor in CRC-specific mortality and is an explanation for discrepancy in pooled estimates. Therefore, increased CRC screening adherence improves screening effectiveness. In model studies real-life adherence data should be used, and external validation should be performed for realistic outcomes. Lower screening initiation ages reduces CRC mortality.
Acknowledgments
The authors acknowledge the information specialist (Sjoukje van der Werf) of Central Medical Library (CMB) in University Medical Centre Groningen for helping develop the search strategies of this review. The author, Senshuang Zheng, received support from Chinese scholarship council (CSC) for her research. The council had no role in the study design, data analysis, interpretation, or reporting of the results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15071948/s1. References [13,20,21,22,23,24,25,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,47,48,49,50,51,52,53,54,55,56,57,58,60,62,63,64,70,74,75,94,95], Table S1: Literature search strategy; Box S1: Selection criteria for articles; Box S2: Qualitative assessment framework on model characteristics; Table S2: Subgroup analysis of RCTs (Intention-to-treat analyses); Table S3: Subgroup analysis of RCTs on CRC-specific mortality reduction by FOBT (Compliance-adjusted analysis); Table S4: Quality assessment of included models; Table S5: Characteristics of simulation models on biennial gFOBT screening from the age of 45 to 80; Table S6: Characteristics of simulation models on single FS screening from the age of 50 to 75; Table S7: Characteristics of simulation models on 10-yearly colonoscopy screening from the age of 55 to 75; Table S8: Characteristics of simulation models on 5-yearly flexible sigmoidoscopy (FS) screening from the age of 55 to 75; Table S9: Egger’s Test for publication bias of simulation modelling studies on CRC-specific mortality reduction; Table S10: Meta regression of simulation models on CRC-specific mortality reduction by 10-yearly colonoscopy screening from the age of 55 to 75; Table S11: Subgroup analysis of simulation models on CRC-specific mortality reduction by 10-yearly colonoscopy screening from the age of 55 to 75; Table S12: Subgroup analysis of simulation models on CRC-specific mortality reduction by biennial gFOBT screening from the age of 45 to 80; Table S13: Subgroup analysis of simulation models on CRC-specific mortality reduction by single FS screening from the age of 50 to 75; Table S14: Characteristics of simulation models on all-cause mortality reduction of CRC screening; Figure S1: Quality assessment of included RCTs articles; Figure S2: Funnel plots for assessing publication bias (Intention-to-treat analyses). a. All-cause mortality rate ratio on FOBT screening programs; b. CRC-specific mortality rate ratio on FOBT screening programs; c. All-cause mortality rate ratio on FS screening programs; and d. CRC-specific mortality rate ratio on FS screening programs; Figure S3: Forest plots of the compliance-adjusted analysis. a. All-cause mortality rate ratio on FOBT screening programs; b. CRC-specific mortality rate ratio on FOBT screening programs; c. All-cause mortality rate ratio on FS screening programs; d. CRC-specific mortality rate ratio on FS screening programs; Figure S4: Forest plots of the CRC-specific mortality rate ratio on 10-yearly colonoscopy screening from the age of 55 to 75; Figure S5: Forest plots of the CRC-specific mortality rate ratio on 5-yearly FS screening from the age of 55 to 75; Figure S6. Forest plots of the CRC-specific mortality rate ratio on biennial FIT screening. a. from the age of 50 to 75; b. from the age of 45 to 75; Figure S7: Forest plots of the CRC-specific mortality rate ratio on 10-yearly colonoscopy screening. a. from the age of 50 to 75; b. from the age of 45 to 75; Figure S8: Forest plots of the CRC-specific mortality rate ratio on 5-yearly FS screening. a. from the age of 50 to 75; and b. from the age of 45 to 75.
Author Contributions
Conceptualization, G.H.d.B., M.J.W.G. and S.Z.; methodology, G.H.d.B. and S.Z.; software and validation, S.Z.; investigation, data curation, formal analysis, and writing—original draft preparation, S.Z. and J.J.A.S.; writing—review and editing, G.H.d.B., M.J.W.G., G.K.-U. and W.L.; visualization, G.H.d.B., S.Z., M.J.W.G. and G.K.-U.; supervision, G.H.d.B., M.J.W.G. and W.L.; project administration, G.H.d.B. and M.J.W.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.International Agency for Research on Cancer GLOBOCAN 2020. [(accessed on 10 February 2023)]. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0.
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C.J., Esteve J., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer . IARC Handbooks of Cancer Prevention. Volume 17. International Agency for Research on Cancer; Lyon, France: 2019. Colorectal cancer screening; p. 300. [Google Scholar]
- 5.Greuter M.J., Xu X.M., Lew J.B., Dekker E., Kuipers E.J., Canfell K., Meijer G.A., Coupe V.M. Modeling the Adenoma and Serrated pathway to Colorectal CAncer (ASCCA) Risk Anal. 2014;34:889–910. doi: 10.1111/risa.12137. [DOI] [PubMed] [Google Scholar]
- 6.Han Y.D., Oh T.J., Chung T.H., Jang H.W., Kim Y.N., An S., Kim N.K. Early detection of colorectal cancer based on presence of methylated syndecan-2 (SDC2) in stool DNA. Clin. Epigenetics. 2019;11:51. doi: 10.1186/s13148-019-0642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society Can Colorectal Polyps and Cancer Be Found Early? [(accessed on 10 February 2023)]. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/detection.html.
- 8.Bevan R., Rutter M.D. Colorectal Cancer Screening-Who, How, and When? Clin. Endosc. 2018;51:37–49. doi: 10.5946/ce.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Cancer Control: Module 3: Early Detection. WHO Press; Geneva, Switzerland: 2007. p. 50. [PubMed] [Google Scholar]
- 10.Fisher D.A., Saoud L., Hassmiller Lich K., Fendrick A.M., Ozbay A.B., Borah B.J., Matney M., Parton M., Limburg P.J. Impact of screening and follow-up colonoscopy adenoma sensitivity on colorectal cancer screening outcomes in the CRC-AIM microsimulation model. Cancer Med. 2021;10:2855–2864. doi: 10.1002/cam4.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goede S.L., Rabeneck L., van Ballegooijen M., Zauber A.G., Paszat L.F., Hoch J.S., Yong J.H., Kroep S., Tinmouth J., Lansdorp-Vogelaar I. Harms, benefits and costs of fecal immunochemical testing versus guaiac fecal occult blood testing for colorectal cancer screening. PLoS ONE. 2017;12:e0172864. doi: 10.1371/journal.pone.0172864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greuter M.J.E., de Klerk C.M., Meijer G.A., Dekker E., Coupe V.M.H. Screening for Colorectal Cancer with Fecal Immunochemical Testing with and without Postpolypectomy Surveillance Colonoscopy: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2017;167:544–554. doi: 10.7326/M16-2891. [DOI] [PubMed] [Google Scholar]
- 13.Lew J.B., St John D.J.B., Macrae F.A., Emery J.D., Ee H.C., Jenkins M.A., He E., Grogan P., Caruana M., Greuter M.J.E., et al. Benefits, Harms, and Cost-Effectiveness of Potential Age Extensions to the National Bowel Cancer Screening Program in Australia. Cancer Epidemiol. Biomark. Prev. 2018;27:1450–1461. doi: 10.1158/1055-9965.EPI-18-0128. [DOI] [PubMed] [Google Scholar]
- 14.Naber S.K., Knudsen A.B., Zauber A.G., Rutter C.M., Fischer S.E., Pabiniak C.J., Soto B., Kuntz K.M., Lansdorp-Vogelaar I. Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PLoS ONE. 2019;14:e0220234. doi: 10.1371/journal.pone.0220234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner H., Stock C., Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmunzer B.J., Hayward R.A., Schoenfeld P.S., Saini S.D., Deshpande A., Waljee A.K. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmunzer B.J., Singal A.G., Sussman J.B., Deshpande A.R., Sussman D.A., Conte M.L., Dwamena B.A., Rogers M.A., Schoenfeld P.S., Inadomi J.M., et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: A network meta-analysis. Gastrointest. Endosc. 2015;81:700–709.e3. doi: 10.1016/j.gie.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., Cheng Z., Ma Y., He C., Lu Y., Zhao Y., Chang X., Zhang Y., Bai Y., Cheng N. Effectiveness of Screening Modalities in Colorectal Cancer: A Network Meta-Analysis. Clin. Color. Cancer. 2017;16:252–263. doi: 10.1016/j.clcc.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Bronzwaer M.E.S., Greuter M.J.E., Bleijenberg A.G.C., JEG I.J., Dekker E., Coupe V.M.H. Impact of differences in adenoma and proximal serrated polyp detection rate on the long-term effectiveness of FIT-based colorectal cancer screening. BMC Cancer. 2018;18:465. doi: 10.1186/s12885-018-4375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buskermolen M., Cenin D.R., Helsingen L.M., Guyatt G., Vandvik P.O., Haug U., Bretthauer M., Lansdorp-Vogelaar I. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A microsimulation modelling study. BMJ. 2019;367:l5383. doi: 10.1136/bmj.l5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buskermolen M., Gini A., Naber S.K., Toes-Zoutendijk E., de Koning H.J., Lansdorp-Vogelaar I. Modeling in Colorectal Cancer Screening: Assessing External and Predictive Validity of MISCAN-Colon Microsimulation Model Using NORCCAP Trial Results. Med. Decis. Mak. 2018;38:917–929. doi: 10.1177/0272989X18806497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cenin D.R., Naber S.K., de Weerdt A.C., Jenkins M.A., Preen D.B., Ee H.C., O’Leary P.C., Lansdorp-Vogelaar I. Cost-Effectiveness of Personalized Screening for Colorectal Cancer Based on Polygenic Risk and Family History. Cancer Epidemiol. Biomark. Prev. 2020;29:10–21. doi: 10.1158/1055-9965.EPI-18-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deibel A., Deng L., Cheng C.Y., Schlander M., Ran T., Lang B., Krupka N., Beerenwinkel N., Rogler G., Wiest R., et al. Evaluating key characteristics of ideal colorectal cancer screening modalities: The microsimulation approach. Gastrointest. Endosc. 2021;94:379–390.e7. doi: 10.1016/j.gie.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 24.DeYoreo M., Lansdorp-Vogelaar I., Knudsen A.B., Kuntz K.M., Zauber A.G., Rutter C.M. Validation of Colorectal Cancer Models on Long-term Outcomes from a Randomized Controlled Trial. Med. Decis. Mak. 2020;40:1034–1040. doi: 10.1177/0272989X20961095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gini A., Buskermolen M., Senore C., Anttila A., Novak Mlakar D., Veerus P., Csanadi M., Jansen E.E.L., Zielonke N., Heinavaara S., et al. Development and Validation of Three Regional Microsimulation Models for Predicting Colorectal Cancer Screening Benefits in Europe. MDM Policy Pract. 2021;6:2381468320984974. doi: 10.1177/2381468320984974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greuter M.J., Berkhof J., Canfell K., Lew J.B., Dekker E., Coupe V.M. Resilience of a FIT screening programme against screening fatigue: A modelling study. BMC Public Health. 2016;16:1009. doi: 10.1186/s12889-016-3667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greuter M.J., Berkhof J., Fijneman R.J., Demirel E., Lew J.B., Meijer G.A., Stoker J., Coupe V.M. The potential of imaging techniques as a screening tool for colorectal cancer: A cost-effectiveness analysis. Br. J. Radiol. 2016;89:20150910. doi: 10.1259/bjr.20150910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greuter M.J., Demirel E., Lew J.B., Berkhof J., Xu X.M., Canfell K., Dekker E., Meijer G.A., Coupe V.M. Long-Term Impact of the Dutch Colorectal Cancer Screening Program on Cancer Incidence and Mortality-Model-Based Exploration of the Serrated Pathway. Cancer Epidemiol. Biomark. Prev. 2016;25:135–144. doi: 10.1158/1055-9965.EPI-15-0592. [DOI] [PubMed] [Google Scholar]
- 29.Heijnsdijk E.A.M., Csanadi M., Gini A., Ten Haaf K., Bendes R., Anttila A., Senore C., de Koning H.J. All-cause mortality versus cancer-specific mortality as outcome in cancer screening trials: A review and modeling study. Cancer Med. 2019;8:6127–6138. doi: 10.1002/cam4.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen A.B., Rutter C.M., Peterse E.F.P., Lietz A.P., Seguin C.L., Meester R.G.S., Perdue L.A., Lin J.S., Siegel R.L., Doria-Rose V.P., et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325:1998–2011. doi: 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knudsen A.B., Zauber A.G., Rutter C.M., Naber S.K., Doria-Rose V.P., Pabiniak C., Johanson C., Fischer S.E., Lansdorp-Vogelaar I., Kuntz K.M. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016;315:2595–2609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladabaum U., Mannalithara A., Meester R.G.S., Gupta S., Schoen R.E. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology. 2019;157:137–148. doi: 10.1053/j.gastro.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lew J.B., St John D.J.B., Macrae F.A., Emery J.D., Ee H.C., Jenkins M.A., He E., Grogan P., Caruana M., Sarfati D., et al. Evaluation of the benefits, harms and cost-effectiveness of potential alternatives to iFOBT testing for colorectal cancer screening in Australia. Int. J. Cancer. 2018;143:269–282. doi: 10.1002/ijc.31314. [DOI] [PubMed] [Google Scholar]
- 34.Lew J.B., St John D.J.B., Xu X.M., Greuter M.J.E., Caruana M., Cenin D.R., He E., Saville M., Grogan P., Coupe V.M.H., et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: A modelling study. Lancet Public Health. 2017;2:e331–e340. doi: 10.1016/S2468-2667(17)30105-6. [DOI] [PubMed] [Google Scholar]
- 35.Meester R.G.S., Doubeni C.A., Zauber A.G., van Ballegooijen M., Corley D.A., Lansdorp-Vogelaar I. Impact of adenoma detection on the benefit of faecal testing vs. colonoscopy for colorectal cancer. Int. J. Cancer. 2017;141:2359–2367. doi: 10.1002/ijc.30933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meester R.G.S., Peterse E.F.P., Knudsen A.B., de Weerdt A.C., Chen J.C., Lietz A.P., Dwyer A., Ahnen D.J., Siegel R.L., Smith R.A., et al. Optimizing colorectal cancer screening by race and sex: Microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2974–2985. doi: 10.1002/cncr.31542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meulen M.P.V., Kapidzic A., Leerdam M.E.V., van der Steen A., Kuipers E.J., Spaander M.C.W., de Koning H.J., Hol L., Lansdorp-Vogelaar I. Do Men and Women Need to Be Screened Differently with Fecal Immunochemical Testing? A Cost-Effectiveness Analysis. Cancer Epidemiol. Biomark. Prev. 2017;26:1328–1336. doi: 10.1158/1055-9965.EPI-16-0786. [DOI] [PubMed] [Google Scholar]
- 38.Peterse E.F.P., Meester R.G.S., de Jonge L., Omidvari A.H., Alarid-Escudero F., Knudsen A.B., Zauber A.G., Lansdorp-Vogelaar I. Comparing the Cost-Effectiveness of Innovative Colorectal Cancer Screening Tests. J. Natl. Cancer Inst. 2021;113:154–161. doi: 10.1093/jnci/djaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterse E.F.P., Meester R.G.S., Siegel R.L., Chen J.C., Dwyer A., Ahnen D.J., Smith R.A., Zauber A.G., Lansdorp-Vogelaar I. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2964–2973. doi: 10.1002/cncr.31543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piscitello A., Saoud L., Fendrick A.M., Borah B.J., Hassmiller Lich K., Matney M., Ozbay A.B., Parton M., Limburg P.J. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLoS ONE. 2020;15:e0244431. doi: 10.1371/journal.pone.0244431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakash M.K., Lang B., Heinrich H., Valli P.V., Bauerfeind P., Sonnenberg A., Beerenwinkel N., Misselwitz B. CMOST: An open-source framework for the microsimulation of colorectal cancer screening strategies. BMC Med. Inform. Decis. Mak. 2017;17:80. doi: 10.1186/s12911-017-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutter C.M., Knudsen A.B., Marsh T.L., Doria-Rose V.P., Johnson E., Pabiniak C., Kuntz K.M., van Ballegooijen M., Zauber A.G., Lansdorp-Vogelaar I. Validation of Models Used to Inform Colorectal Cancer Screening Guidelines: Accuracy and Implications. Med. Decis. Mak. 2016;36:604–614. doi: 10.1177/0272989X15622642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vleugels J.L.A., Greuter M.J.E., Hazewinkel Y., Coupe V.M.H., Dekker E. Implementation of an optical diagnosis strategy saves costs and does not impair clinical outcomes of a fecal immunochemical test-based colorectal cancer screening program. Endosc. Int. Open. 2017;5:E1197–E1207. doi: 10.1055/s-0043-113565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher D.A., Karlitz J.J., Jeyakumar S., Smith N., Limburg P., Lieberman D., Fendrick A.M. Real-world cost-effectiveness of stool-based colorectal cancer screening in a Medicare population. J. Med. Econ. 2021;24:654–664. doi: 10.1080/13696998.2021.1922240. [DOI] [PubMed] [Google Scholar]
- 45.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 47.Koleva-Kolarova R.G., Zhan Z., Greuter M.J., Feenstra T.L., De Bock G.H. Simulation models in population breast cancer screening: A systematic review. Breast. 2015;24:354–363. doi: 10.1016/j.breast.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Atkin W., Wooldrage K., Parkin D.M., Kralj-Hans I., MacRae E., Shah U., Duffy S., Cross A.J. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: The UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389:1299–1311. doi: 10.1016/S0140-6736(17)30396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholefield J.H., Moss S.M., Mangham C.M., Whynes D.K., Hardcastle J.D. Nottingham trial of faecal occult blood testing for colorectal cancer: A 20-year follow-up. Gut. 2012;61:1036–1040. doi: 10.1136/gutjnl-2011-300774. [DOI] [PubMed] [Google Scholar]
- 50.Senore C., Riggi E., Armaroli P., Bonelli L., Sciallero S., Zappa M., Arrigoni A., Casella C., Crosta C., Falcini F., et al. Long-Term Follow-up of the Italian Flexible Sigmoidoscopy Screening Trial. Ann. Intern. Med. 2022;175:36–45. doi: 10.7326/M21-0977. [DOI] [PubMed] [Google Scholar]
- 51.Shaukat A., Kaalby L., Baatrup G., Kronborg O., Duval S., Shyne M., Mandel J.S., Church T.R. Effects of Screening Compliance on Long-term Reductions in All-Cause and Colorectal Cancer Mortality. Clin. Gastroenterol. Hepatol. 2021;19:967–975.e2. doi: 10.1016/j.cgh.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Shaukat A., Mongin S.J., Geisser M.S., Lederle F.A., Bond J.H., Mandel J.S., Church T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 53.Holme O., Loberg M., Kalager M., Bretthauer M., Hernan M.A., Aas E., Eide T.J., Skovlund E., Lekven J., Schneede J., et al. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann. Intern. Med. 2018;168:775–782. doi: 10.7326/M17-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindholm E., Brevinge H., Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br. J. Surg. 2008;95:1029–1036. doi: 10.1002/bjs.6136. [DOI] [PubMed] [Google Scholar]
- 55.Pinsky P.F., Miller E.A., Zhu C.S., Prorok P.C. Overall mortality in men and women in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J. Med. Screen. 2019;26:127–134. doi: 10.1177/0969141319839097. [DOI] [PubMed] [Google Scholar]
- 56.Pitkaniemi J., Seppa K., Hakama M., Malminiemi O., Palva T., Vuoristo M.S., Jarvinen H., Paimela H., Pikkarainen P., Anttila A., et al. Effectiveness of screening for colorectal cancer with a faecal occult-blood test, in Finland. BMJ Open Gastroenterol. 2015;2:e000034. doi: 10.1136/bmjgast-2015-000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thiis-Evensen E., Kalager M., Bretthauer M., Hoff G. Long-term effectiveness of endoscopic screening on incidence and mortality of colorectal cancer: A randomized trial. United Eur. Gastroenterol. J. 2013;1:162–168. doi: 10.1177/2050640613483290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babela R., Orsagh A., Ricova J., Lansdorp-Vogelaar I., Csanadi M., De Koning H., Reckova M. Cost-effectiveness of colorectal cancer screening in Slovakia. Eur. J. Cancer Prev. 2021;31:415–421. doi: 10.1097/CEJ.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 59.Buskermolen M., Naber S.K., Toes-Zoutendijk E., van der Meulen M.P., van Grevenstein W.M.U., van Leerdam M.E., Spaander M.C.W., Lansdorp-Vogelaar I. Impact of surgical versus endoscopic management of complex nonmalignant polyps in a colorectal cancer screening program. Endoscopy. 2022;54:871–880. doi: 10.1055/a-1726-9144. [DOI] [PubMed] [Google Scholar]
- 60.Cenin D., Li P., Wang J., de Jonge L., Yan B., Tao S., Lansdorp-Vogelaar I. Optimising colorectal cancer screening in Shanghai, China: A modelling study. BMJ Open. 2022;12:e048156. doi: 10.1136/bmjopen-2020-048156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Csanadi M., Gini A., Koning H., Szeles G., Pitter J.G., Oroszi B., Pataki P., Fadgyas-Freyler P., Korponai G., Voko Z., et al. Modeling costs and benefits of the organized colorectal cancer screening programme and its potential future improvements in Hungary. J. Med. Screen. 2021;28:268–276. doi: 10.1177/0969141320968598. [DOI] [PubMed] [Google Scholar]
- 62.Gini A., van Ravesteyn N.T., Jansen E.E.L., Heijnsdijk E.A.M., Senore C., Anttila A., Novak Mlakar D., Veerus P., Csanadi M., Zielonke N., et al. The EU-TOPIA evaluation tool: An online modelling-based tool for informing breast, cervical, and colorectal cancer screening decisions in Europe. Prev. Med. Rep. 2021;22:101392. doi: 10.1016/j.pmedr.2021.101392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinavaara S., Gini A., Sarkeala T., Anttila A., de Koning H., Lansdorp-Vogelaar I. Optimizing screening with faecal immunochemical test for both sexes—Cost-effectiveness analysis from Finland. Prev. Med. 2022;157:106990. doi: 10.1016/j.ypmed.2022.106990. [DOI] [PubMed] [Google Scholar]
- 64.Naber S.K., Almadi M.A., Guyatt G., Xie F., Lansdorp-Vogelaar I. Cost-effectiveness analysis of colorectal cancer screening in a low incidence country: The case of Saudi Arabia. Saudi J. Gastroenterol. 2021;27:208–216. doi: 10.4103/sjg.sjg_526_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naber S.K., Kundu S., Kuntz K.M., Dotson W.D., Williams M.S., Zauber A.G., Calonge N., Zallen D.T., Ganiats T.G., Webber E.M., et al. Cost-Effectiveness of Risk-Stratified Colorectal Cancer Screening Based on Polygenic Risk: Current Status and Future Potential. JNCI Cancer Spectr. 2020;4:pkz086. doi: 10.1093/jncics/pkz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Meulen M.P., Lansdorp-Vogelaar I., Goede S.L., Kuipers E.J., Dekker E., Stoker J., van Ballegooijen M. Colorectal Cancer: Cost-effectiveness of Colonoscopy versus CT Colonography Screening with Participation Rates and Costs. Radiology. 2018;287:901–911. doi: 10.1148/radiol.2017162359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., de Jonge L., Cenin D.R., Li P., Tao S., Yang C., Yan B., Lansdorp-Vogelaar I. Cost-effectiveness analysis of colorectal cancer screening in Shanghai, China: A modelling study. Prev. Med. Rep. 2022;29:101891. doi: 10.1016/j.pmedr.2022.101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher D.A., Saoud L., Finney Rutten L.J., Ozbay A.B., Brooks D., Limburg P.J. Lowering the colorectal cancer screening age improves predicted outcomes in a microsimulation model. Curr. Med. Res. Opin. 2021;37:1005–1010. doi: 10.1080/03007995.2021.1908244. [DOI] [PubMed] [Google Scholar]
- 69.Karlitz J.J., Fendrick A.M., Bhatt J., Coronado G.D., Jeyakumar S., Smith N.J., Plescia M., Brooks D., Limburg P., Lieberman D. Cost-Effectiveness of Outreach Strategies for Stool-Based Colorectal Cancer Screening in a Medicaid Population. Popul. Health Manag. 2022;25:343–351. doi: 10.1089/pop.2021.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladabaum U., Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016;151:427–439.e6. doi: 10.1053/j.gastro.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Ladabaum U., Mannalithara A., Brill J.V., Levin Z., Bundorf K.M. Contrasting Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening Under Commercial Insurance vs. Medicare. Am. J. Gastroenterol. 2018;113:1836–1847. doi: 10.1038/s41395-018-0106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladabaum U., Mannalithara A., Mitani A., Desai M. Clinical and Economic Impact of Tailoring Screening to Predicted Colorectal Cancer Risk: A Decision Analytic Modeling Study. Cancer Epidemiol. Biomark. Prev. 2020;29:318–328. doi: 10.1158/1055-9965.EPI-19-0949. [DOI] [PubMed] [Google Scholar]
- 73.Chen C., Stock C., Hoffmeister M., Brenner H. Optimal age for screening colonoscopy: A modeling study. Gastrointest. Endosc. 2019;89:1017–1025.e12. doi: 10.1016/j.gie.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 74.Heisser T., Hoffmeister M., Brenner H. Model based evaluation of long-term efficacy of existing and alternative colorectal cancer screening offers: A case study for Germany. Int. J. Cancer. 2022;150:1471–1480. doi: 10.1002/ijc.33894. [DOI] [PubMed] [Google Scholar]
- 75.Lew J.B., Feletto E., Worthington J., Roder D., Canuto K., Miller C., D’Onise K., Canfell K. The potential for tailored screening to reduce bowel cancer mortality for Aboriginal and Torres Strait Islander peoples in Australia: Modelling study. J. Cancer Policy. 2022;32:100325. doi: 10.1016/j.jcpo.2022.100325. [DOI] [PubMed] [Google Scholar]
- 76.Helsingen L.M., Vandvik P.O., Jodal H.C., Agoritsas T., Lytvyn L., Anderson J.C., Auer R., Murphy S.B., Almadi M.A., Corley D.A., et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ. 2019;367:l5515. doi: 10.1136/bmj.l5515. [DOI] [PubMed] [Google Scholar]
- 77.Powell K., Prasad V. Colorectal cancer screening at a younger age: Pitfalls in the model-based recommendation of the USPSTF. BMJ Evid. Based Med. 2022;27:206–208. doi: 10.1136/bmjebm-2021-111793. [DOI] [PubMed] [Google Scholar]
- 78.Choi K.S., Lee H.Y., Jun J.K., Shin A., Park E.C. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J. Gastroenterol. Hepatol. 2012;27:1070–1077. doi: 10.1111/j.1440-1746.2011.06944.x. [DOI] [PubMed] [Google Scholar]
- 79.Fitzpatrick-Lewis D., Ali M.U., Warren R., Kenny M., Sherifali D., Raina P. Screening for Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Color. Cancer. 2016;15:298–313. doi: 10.1016/j.clcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Jodal H.C., Helsingen L.M., Anderson J.C., Lytvyn L., Vandvik P.O., Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: A systematic review and network meta-analysis. BMJ Open. 2019;9:e032773. doi: 10.1136/bmjopen-2019-032773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel S.S., Kilgore M.L. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control. 2015;22:248–258. doi: 10.1177/107327481502200219. [DOI] [PubMed] [Google Scholar]
- 82.Ran T., Cheng C.Y., Misselwitz B., Brenner H., Ubels J., Schlander M. Cost-Effectiveness of Colorectal Cancer Screening Strategies-A Systematic Review. Clin. Gastroenterol. Hepatol. 2019;17:1969–1981.e15. doi: 10.1016/j.cgh.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Zhong G.C., Sun W.P., Wan L., Hu J.J., Hao F.B. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: A systematic review and meta-analysis. Gastrointest. Endosc. 2020;91:684–697.e15. doi: 10.1016/j.gie.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 84.Zauber A.G., Winawer S.J., O’Brien M.J., Lansdorp-Vogelaar I., van Ballegooijen M., Hankey B.F., Shi W., Bond J.H., Schapiro M., Panish J.F., et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin J.S., Piper M.A., Perdue L.A., Rutter C., Webber E.M., O’Connor E., Smith N., Whitlock E.P. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US); Rockville, MD, USA: 2016. [PubMed] [Google Scholar]
- 86.Whitlock E.P., Lin J.S., Liles E., Beil T.L., Fu R. Screening for colorectal cancer: A targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 87.Wu W., Huang J., Yang Y., Gu K., Luu H.N., Tan S., Yang C., Fu J., Bao P., Ying T., et al. Adherence to colonoscopy in cascade screening of colorectal cancer: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022;37:620–631. doi: 10.1111/jgh.15762. [DOI] [PubMed] [Google Scholar]
- 88.Ponti A., Anttila A., Ronco G., Senore C., Basu P., Segnan N., Tomatis M. Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening. European Commission; Brussels, Belgium: 2017. Against Cancer; p. 333. [Google Scholar]
- 89.World Health Organization . Comprehensive Cervical Cancer Control: A Guide to Essential Practice. 2nd ed. WHO Press; Geneva, Switzerland: 2014. p. 364. [PubMed] [Google Scholar]
- 90.Jandorf L., Braschi C., Ernstoff E., Wong C.R., Thelemaque L., Winkel G., Thompson H.S., Redd W.H., Itzkowitz S.H. Culturally targeted patient navigation for increasing african americans’ adherence to screening colonoscopy: A randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2013;22:1577–1587. doi: 10.1158/1055-9965.EPI-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen J.D., King A.J., Carcioppolo N., Krakow M., Samadder N.J., Morgan S. Comparing tailored and narrative worksite interventions at increasing colonoscopy adherence in adults 50–75: A randomized controlled trial. Soc. Sci. Med. 2014;104:31–40. doi: 10.1016/j.socscimed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Ling B.S., Schoen R.E., Trauth J.M., Wahed A.S., Eury T., Simak D.M., Solano F.X., Weissfeld J.L. Physicians encouraging colorectal screening: A randomized controlled trial of enhanced office and patient management on compliance with colorectal cancer screening. Arch. Intern. Med. 2009;169:47–55. doi: 10.1001/archinternmed.2008.519. [DOI] [PubMed] [Google Scholar]
- 93.Myers R.E., Sifri R., Daskalakis C., DiCarlo M., Geethakumari P.R., Cocroft J., Minnick C., Brisbon N., Vernon S.W. Increasing colon cancer screening in primary care among African Americans. J. Natl. Cancer Inst. 2014;106:dju344. doi: 10.1093/jnci/dju344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carter J.L., Coletti R.J., Harris R.P. Quantifying and monitoring overdiagnosis in cancer screening: A systematic review of methods. BMJ. 2015;350:g7773. doi: 10.1136/bmj.g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eddy D.M., Hollingworth W., Caro J.J., Tsevat J., McDonald K.M., Wong J.B., ISPOR-SMDM Modeling Good Research Practices Task Force Model transparency and validation: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med. Decis. Mak. 2012;32:733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or Supplementary Materials.