Abstract
Simple Summary
Pediatric thyroid ultrasound-guided fine-needle aspiration includes the use of dedicated ultrasound guidance algorithms to improve the accuracy and increase the diagnostic yield of the procedure. The refinement of imaging criteria and risk stratification can potentially spare cytological assessment of benign nodules. Molecular testing can also be used to differentiate benign from malignant lesions and to guide their further management. The assistance of artificial intelligence tools will be helpful in this investigation.
Abstract
Thyroid fine-needle aspiration (FNA) is a commonly used diagnostic cytological procedure in pediatric patients for the evaluation of thyroid nodules, triaging them for the detection of thyroid cancer. In recent years, greater attention has been paid to thyroid FNA in this setting, including the use of updated ultrasound score algorithms to improve accuracy and yield, especially considering the theoretically higher risk of malignancy of these lesions compared with the adult population, as well as to minimize patient discomfort. Moreover, molecular genetic testing for thyroid disease is an expanding field of research that could aid in distinguishing benign from cancerous nodules and assist in determining their clinical management. Finally, artificial intelligence tools can help in this task by performing a comprehensive analysis of all the obtained data. These advancements have led to greater reliance on FNA as a first-line diagnostic tool for pediatric thyroid disease. This review article provides an overview of these recent developments and their impact on the diagnosis and management of thyroid nodules in children.
Keywords: thyroid nodule, pediatrics, fine-needle aspiration, ultrasound, molecular pathology
1. Introduction
In recent years, the overall incidence of thyroid malignancy has increased mainly due to significant improvements in high-resolution ultrasonography (US)-guided fine-needle aspiration (FNA), leading to earlier cancer detection [1]. US is a non-invasive imaging method that is suitable for thyroid evaluation and is well accepted by patients of all ages, whereas FNA is considered the best method for evaluating thyroid nodules to minimize unnecessary surgeries or establish the proper extent of surgery [2,3]. Histological assessment remains the reference standard for thyroid nodule diagnosis [4]. According to the Surveillance, Epidemiology, and End Results (SEER) database, thyroid cancer cases in individuals under 20 years old account for only 2.3% of all thyroid cancer diagnoses, but they still represent the second most common adolescent malignant neoplasms, sharing a relatively low mortality with their adult counterparts even in advanced forms (globally <2%), although the rarity of these conditions in children makes data collection challenging [5,6,7,8,9,10]. Currently, there is no consensus on the age upper cut-off for defining the pediatric group for thyroid diseases, with different options proposed by the World Health Organization (19 years old), the American Thyroid Association (18 years), and the American Academy of Pediatrics (21 years), with some studies suggesting 14 or 22 years old cut-off for a better prognostic value [10].
Substantial differences in the clinical, pathological and molecular aspects of pediatric thyroid cancers make their preoperative assessment more challenging. From a clinical standpoint, pediatric nodules are relatively less frequent (0.2–5% vs. 20–70%), but they have a greater risk of malignancy (mean ROM, 19–26% vs. 5–15%) and cancer prevalence (estimated from 1.6-fold to 2.5-fold higher), with the peak of incidence being among 15–19 years old [2,3,4,6,7,10,11,12,13,14]. Moreover, pediatric patients often present with occult diffuse infiltrative lesions and extrathyroidal extension (e.g., metastases in regional lymph nodes or pulmonary metastases), stressing the role of non-invasive methods (US + FNA) for their correct assessment [3,6]. They are more often associated with female sex (especially in the post-pubertal >14 years age group, probably due to different endocrine, metabolic and immune characteristics), Asian origins, radiation exposure and inherited syndromes (5–15% of cases) [7,9,10,15]. Correctly recognizing malignant nodules in children is essential because of their greater rate of complications related to surgical treatment, with potential long-term impact on growth and bone health, and for their better response to radioactive iodine therapy (RAI) [2,10]. For preoperative assessment, the commonly used US size cut-offs for risk stratification of thyroid nodules may not be adequate for the age thyroid volume; moreover, the molecular landscape of the most frequent histotype, papillary thyroid carcinoma (PTC), representing approximately 80–90% of cases, is characterized by lower rates of BRAF p.V600E mutation and higher rates of RET/PTC translocations, as compared to what is described in the adult counterpart, and 15–40% of PTC cases are of histological high-risk variants (e.g., tall cell, diffuse sclerosing and solid/trabecular variants) [3,7,9,10,14,15]. As in adults, the second and third most frequent histotypes are follicular thyroid carcinoma (FTC) and medullary thyroid carcinoma (MTC), respectively, while poorly differentiated thyroid carcinoma (PDTC) and anaplastic thyroid carcinoma (ATC) are particularly rare entities [10].
For these reasons, a more comprehensive understanding of the thyroid cancer background in pediatrics is mandatory to ensure their best management. This review will provide an overview of the current state of the field and the various new methods and technologies that are being used to improve the accuracy of thyroid FNA in this population.
2. Materials and Methods
We focus on the most recent literature to provide an update on the advancements in the thyroid nodules’ assessment in pediatrics, performing a literature search (date of search: 1 January 2023) in PubMed (Medline) for studies published from 1 January 2020 to 31 December 2022 using the following search terms: “thyroid FNA” and “pediatric(s)” or “children”, requiring the terms “pediatric(s)” or “children” to appear in the title. To further expand the information to articles published before the censoring period, a secondary search of the literature was manually conducted from the references of our primary search, including papers by the application of the same inclusion and exclusion criteria. Articles satisfying the following inclusion criteria were included in our review (regardless of the study design): [1] study was written in English; [2] the full article could be obtained. Articles satisfying the following exclusion criteria were excluded from our review: [1] study was written in a non-English language; [2] the full article was not available; [3] study was not related to thyroid FNA in pediatrics; [4] study was not published in a peer-reviewed journal. Literature review and data extraction were conducted independently by two reviewers (D.S. and F.P.). Doubts or disagreements regarding the inclusion or exclusion of manuscripts were resolved through discussion between the reviewers until a consensus was reached (search strategy summary in Table 1).
Table 1.
Search strategy summary.
| Items | Specifications |
|---|---|
| Date of Search | 1 January 2023 |
| Databases and other sources searched | PubMed (Medline) |
| Search terms used | Search terms: “thyroid FNA” and “pediatric(s)” or “children” |
| Timeframe | From 1 January 2020 to 31 December 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| (1) study was written in English language; | |
| (2) the full article could be obtained. | |
| Exclusion criteria: | |
| (1) study was written in non-English language; | |
| (2) the full article was not available; | |
| (3) study was not related to thyroid FNA in pediatrics; | |
| (4) study was not published in a peer-reviewed journal. | |
| Selection process | The literature review and the data extraction were conducted independently by two reviewers (D.S. and F.P.). |
| A secondary search of the literature was manually conducted from the references of our primary search included papers by the application of the same inclusion and exclusion criteria. | |
| Doubts or disagreements regarding the inclusion or exclusion of manuscripts were resolved through a discussion between the reviewers until a consensus was reached. |
3. Discussion
3.1. Ultrasound Assessment of Thyroid Nodules in Pediatrics
Worldwide, the most employed ultrasound Thyroid Imaging Reporting and Data Systems (TI-RADS) are the American College of Radiology (ACR) TI-RADS, European Thyroid Association (EU) TI-RADS and American Thyroid Association (ATA) guidelines, with known limits in identifying malignant tumors other than PTC [3,15,16,17]. ACR and EU TI-RADS have been successfully tested and implemented in adult practice, the former because of the simplicity and effectiveness of the scoring system, the latter for its pattern recognition approach, but both have not been well studied in children [18]. Furthermore, some authors blame all classification systems for their high missed malignancy rate (approximately 50%), supposedly due to the employment of non-optimal size cut-offs for FNA selection, resulting in a high rate of benign FNA (up to 80%), or either for the non-recognition of unique imaging pediatric characteristics, such as ectopic intrathyroidal thymic tissue (2–5%) resembling microcalcifications [2,3,6,7,18,19,20,21]. Currently, ATA guidance is the only published guideline for the management of thyroid nodules harboring a section explicitly dedicated to the pediatric population [15]. According to ATA guidelines, US-FNA in children is indicated for all solid or partly solid nodules greater than 1 cm in size regardless of their other echographic features (Figure 1A–E) [15]. US-FNA is also recommended for all nodules smaller than 1 cm harboring suspicious features or clinical risk factors, such as Hashimoto thyroiditis, which involves approximately 20–25% of malignant pediatric cases vs. <5% of benign nodules (Table 2) [2,6,15].
Figure 1.
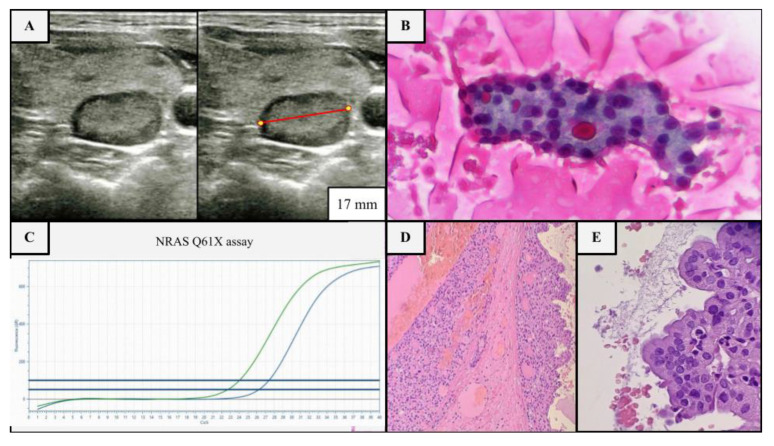
Example of US, cytological and molecular assessment of a thyroid nodule in a 17-year-old female patient with multinodular goiter without clinical risk factors. (A) Nodular thyroid lesion of the right lobe, 17 mm in diameter (red line), isoechoic, with a mixed composition and smooth margins, no microcalcifications, deserving FNA according to ATA guidelines (≥1 cm partly solid nodule), but not deserving it according to ACR TI-RADS (class 2) (B) FNA shows a blood-rich background with fluid colloid, rare foamy histiocytes and numerous groups of thyroid cells mainly arranged in sheets and microfollicular structures, sometimes with oxyphilic appearance (class IV sec. TBSRTC, Papanicolau stained smear, ×40). (C) Molecular test (real time PCR) was performed, since the indeterminate cytology, starting from the needle wash, revealing the NRAS p.Q61X mutation (blue line mutated allele, green line wild type allele), a low-risk mutation group, which confirms the clonality of the lesion and its likely low biological aggressiveness. (D,E) Accordingly, a hemi-thyroidectomy was performed, revealing a partly cystic nodule composed of oncocytic thyrocytes, with pushing borders and a thin fibrotic capsule without invasion or lymphovascular infiltration, leading to the final histological diagnosis of NRAS-mutant oncocytic follicular adenoma (H & E, 10× and 40×).
Table 2.
US suspicious features and clinical risk factors prompting FNA assessment of pediatric thyroid nodules less than 1 cm in size according to the ATA guidelines [2,15].
| US Suspicious Features | Clinical Risk Factors |
|---|---|
|
|
This differs from what is applied to the adult population by all the systems, with FNA performed only once the nodules are ≥1 cm in size and harbor suspicious US features (Table 3).
Table 3.
ACR TI-RADS, EU-TIRADS and ATA guidelines FNA flowcharts for the adult population compared to ATA recommendations for pediatrics [15,16,17].
| Adults | Pediatrics | |||||
|---|---|---|---|---|---|---|
| ACR Classes | Final Indication ACR | EU Classes | Final Indication EU | ATA Classes | Final Indication ATA | ATA |
| ACR1 | No FNA | EU1 | No FNA | ATA1 | No FNA | No FNA if <1 cm and no US or clinical risk factors |
| ACR2 | No FNA | EU2 | No FNA | ATA2 | FNA or follow if ≥2 cm | |
| ACR3 | FNA if ≥2.5 cm | EU3 | FNA if >2 cm | ATA3 | FNA if ≥1.5 cm | FNA if <1 cm and US or clinical risk factors |
| Follow if ≥1.5 cm | ||||||
| ACR4 | FNA if ≥1.5 cm | EU4 | FNA if >1.5 cm | ATA4 | FNA if ≥1 cm | FNA to any nodule ≥1 cm solid/partly solid |
| Follow if ≥1 cm | ||||||
| ACR5 | FNA if ≥1 cm | EU5 | FNA if >1 cm | ATA5 | FNA if ≥1 cm | |
| Follow if ≥0.5 cm | Follow if ≤1 cm | |||||
In this direction, Dunya et al. investigated 77 pediatric FNA, half of which had suspicious or malignant cytology findings. Using ATA guidelines rather than the ACR TI-RADS system, they observed a better sensitivity but a worse positive predictive value [7]. Indeed, the ATA system tends to produce a higher number of false-positive results than the ACR system, even if both demonstrated only modest overall diagnostic performance [12]. A slightly superior impact was noted for ACR TI-RADS in the setting of indeterminate class III Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) nodules, due to its ability to appropriately select those nodules that require surgery [22]. According to the ATA, hyperfunctioning lesions are usually resected surgically and do not necessarily require FNA [7,15]. Finally, in the setting of autoimmune thyroiditis, US should be performed, especially in children with other clinical risk factors, considering that PTCs arisen in this context harbor a favorable outcome despite their more frequent multifocal pattern of growth (Table 2) [2]. At the same time, there is no evidence suggesting increased aggressiveness for malignancies arising in genetic cancer syndromes, so prophylactic thyroidectomy is no longer recommended, and US surveillance every 1–3 years is indicated [2]. Nonetheless, prophylactic surgery remains recommended in children harboring RET mutations (MEN syndromes) and at risk of medullary thyroid carcinoma (MTC) onset, with the timing of surgery determined by the known progression risk of every specific mutated codon [2]. However, the US-based surveillance of nodule growth did not prove reliable in assessing the malignant nature of children’s lesions, given the particularly slow growth of many thyroid cancers [1,14].
3.2. Fine-Needle Aspiration (FNA) of Thyroid Nodules in Pediatrics
The classification of thyroid FNA samples using The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) is recommended for children by ATA guidance, which is characterized by more aggressive criteria for FNA than in adults [6,23]. Furthermore, all the systems suffer from a lack of well-defined FNA risk of malignancy (ROM) for pediatric nodules, especially in the indeterminate categories (TBSRTC classes III–IV), although the ultrasound features predictive of malignancy coincide with those of adults [7,14,20]. However, the implementation of Rapid On-Site Evaluation (ROSE) has proven effective in reducing the non-diagnostic rate in the pediatric cohort, on average, halving it [20,24]. Based on the generally literature-recognized higher overall ROM in children, ATA guidelines suggest that all FNA positive and indeterminate nodules should undergo surgical excision and comprehensive cervical lymph nodes US examination (Table 4 and Table 5) [4,15].
Table 4.
Thyroid FNA risk of malignancy (ROM) in adults according to current TBSRTC (The Bethesda System for Reporting Thyroid Cytopathology), SIAPEC (Società Italiana di Anatomia Patologica e di Citopatologia diagnostica) and BTA (British Thyroid Association) reporting thyroid cytology classifications [23,25,26].
| Adults | |||||
|---|---|---|---|---|---|
| TBSRTC | ROM (%) |
SIAPEC | ROM (%) |
BTA | ROM (%) |
| I | 1–4 | TIR1-TIR1c | nd | THY1-THY1c | 4 |
| II | <3 | TIR2 | <3 | THY2-THY2c | 1.4 |
| III | 5–15 | TIR3A | <10 | THY3a | 17 |
| IV | 15–30 | TIR3B | 15–30 | THY3b | up to 40 |
| V | 60–75 | TIR4 | 60–80 | THY4 | up to 68 |
| VI | 97–99 | TIR5 | >95 | THY5 | up to 100 |
Table 5.
Thyroid FNA ROM according to the main pediatric studies using TBSRTC categories [23].
| Pediatrics | ||||||
|---|---|---|---|---|---|---|
| Studies | TBSRTC Categories (%) | |||||
| I | II | III | IV | V | VI | |
| Amirazodi et al. [27] | 0 | 16 | 67 | - | 71 | 100 |
| Buryk et al. [28] | 0 | 10 | 0 | 50 | 86 | 100 |
| Cherella et al. [29] | 11 | 0.7 | 44 | 71 | 73 | 97 |
| Heider et al. [30] | - | - | 36 | 20 | 100 | - |
| Jia et al. [31] | 0 | 0.8 | 15.6 | 54.5 | 100 | 100 |
| Kardelen et al. [32] | 0 | 25 | 100 | 75 | 85.7 | 100 |
| Lale et al. [33] | 17 | 0 | 50 | 47 | 100 | 100 |
| Monaco et al. [34] | 0 | 7 | 28 | 58 | 100 | 100 |
| Norlen et al. [35] | 0 | 0 | 22 | 100 | 100 | 100 |
| Pantola et al. [36] | 0 | 0 | 8.3 | 10 | 100 | 100 |
| Partyka et al. [37] | 0 | 1.5 | 21 | 57.1 | 100 | 100 |
| Rossi et al. [38] | 0 | 0 | 11.8 | 81.8 | 100 | 100 |
| Wang et al. [39] | 0 | 7 | 20 | 25 | 100 | 100 |
| Suh et al. [40] | 22 | 3.2 | 75 | 50 | 100 | 100 |
| Tuli et al. [21] | 0 | 0 | 0 | 77.8 | 100 | 100 |
| Vuong et al. [41] | 30 | 50 | 66.6 | 36.4 | 100 | 99.3 |
| Average | 5.3 | 10.4 | 38.5 | 50.6 | 95.3 | 99.8 |
The assessment of ROM in a single cytological category and its comparison with its adult counterpart has been extensively explored in recent years, with mixed results. Higher ROM for pediatric patients has been described in the setting of Bethesda classes III, IV and V by Dunya et al., and a study on 340 pediatric FNA confirmed this finding, with greater TBSRTC expected ROM in classes ≥III, suggesting that FNA should be performed for all nodules with ACR TI-RADS score ≥4, independently of their size [7,31]. Single reports even suggest differential ROM depending on the side of the gland affected by nodular disease (e.g., right vs. left lobe) [21]. In contrast, an extensive meta-analysis involving 3687 pediatric and 145,066 adult patients with thyroid nodules showed a comparable frequency and ROM for benign (class II) and indeterminate (classes III and IV) categories, with a relatively higher rate of diagnostic resection for indeterminate classes in the pediatric population, suggesting the risk of overtreatment based on comparable ROM with the adult counterpart [41]. However, possible selection biases can explain this underestimation of ROM in pediatric patients, accounting for a potential increase in the relative risk from 1.6-fold to 2.5-fold compared to adults [12]. A more detailed analysis investigated the relationship between cytological atypia (e.g., nuclear or architectural only, nuclear and architectural at the same time, oncocytic atypia) and the final ROM of class III, revealing a more prominent impact of nuclear features on the final ROM, irrespective of the coexistence of architectural atypia [42]. Moreover, this study further stressed the possible role of repeat biopsy in indeterminate cases of nuclear-only atypia, suggesting a more conservative approach than the ATA guidelines [42]. Finally, regardless of the generally higher ROM, the overall comparable rate of indeterminate FNA diagnoses in children and adults should rule out the possibility that pathologists may fear overdiagnosis in young patients, without falling back on the comfortable security of Bethesda classes III-IV categories, prompting just US follow-up or FNA repetition.
3.3. Molecular Testing in Pediatrics
Thyroid molecular genetic testing offers the possibility of obtaining additional information to assist in treatment decisions, particularly for indeterminate nodules at FNA, helping to guide the choice of surgery or observation, even if ATA still does not recommend such analyses on cytological pediatric specimens [2]. To date, only Next-Generation Sequencing (NGS) tests have been validated in children, although large comprehensive studies are lacking, while gene expression classifier tools have not yet been extensively tested and validated [2,8]. Some studies have suggested that microRNA (miRNA) pathways in children are similar to those in adults and that the combination of NGS analysis with miRNA expression raised the diagnostic accuracy in some cases [43]. Children have a high detection rate (approximately 80–90%) of pathogenic variants in differentiated thyroid cancers (DTC) and are more likely to have gene rearrangements rather than point mutations, among which DICER1 variants are more common than in adults (Table 6) [8,44,45]. In particular, about 30% of pediatric cancers harbor BRAF/RAS mutations, while gene fusions are present in up to 60% of cases, mainly in younger patients with PTC, usually involving RET or NTRK1/3, as also ALK, BRAF and PPARG [8]. Overall, similar survival rates have been observed in tumors with point mutations and rearrangements [8]. As in adults, more aggressive cancer behavior is expected in cases harboring p.V600E or other BRAF point mutations or BRAF-like fusions (e.g., RET/PTC1, NTRK3/ETV6, ALK rearrangements), although these correlations remain unconfirmed in children [46]. Among the genetic alterations encountered in thyroid cancers of adults, TERT promoter (pTERT) mutations, particularly p.C288T and p.C250T, have shown an incidence of 5–25% in some series. This data is confirmed by The Cancer Genome Atlas cohort with 9.4% of these cancers harboring pTERT mutations, none of them being pediatric. The relatively low frequency of pTERT mutations in children (<5%) has been confirmed by different studies that failed to demonstrate such alterations in different series [47,48,49]. Exceptions are represented by sporadic reports demonstrating isolated cases with p.C288T mutation in PTC or even rarer studies showing slightly higher prevalence (27%), always in restricted cohorts [43,50]. As per pTERT mutations, the presence of highly aggressive mutations in TP53 are quite rare in pediatric population [8]. An indeterminate FNA cytological result combined with a less aggressive molecular variant, such as RAS, DICER1, PTEN, TSHR mutations or PAX8-PPARG rearrangements, may also prompt a conservative surgical approach in children too [2]. The pathogenic role of some of these low-risk mutations is not fully understood, and the association of copy number variation (CNV) is suspected to be necessary for malignant transformation [51]. According to Baran et al., low- and high-risk mutations account for approximately 50% and 30% of malignant pediatric nodules, respectively, while the remaining cases harbor relatively uncommon variants (APC, BLM, PPM1D mutations, FGFR1 rearrangements) which require further investigation [46]. In addition, they observed a correlation between the Bethesda category and the presence of high-risk genetic alterations, opening the possibility of creating an effective cytological and molecular combined algorithm of risk classification, as previously performed with ultrasound and FNA [1,19,46]. DICER1 mutations seem to correlate with the macrofollicular variant of FTC, in which have been found in up to 50% of cases, and seem to be involved in the progression from FTC to PDTC [10]. PTC with solid pattern of growth seems to be correlated with NTRK fusions instead [10]. Interestingly, in pediatric population has been found a higher prevalence of STRN-ALK rearrangements (7% vs. 3%, respectively), both in FTC and PTC, in classic papillary and in diffuse sclerosing variants [10]. Molecular analysis is also pivotal for the diagnosis and management of medullary thyroid carcinoma (MTC), a rare type of thyroid cancer (3–5% of cases) that originates from parafollicular C cells. MTC is associated with activating mutations in the RET proto-oncogene, usually in exon 10 or 11, and different RET mutations correlate variably with disease progression and response to therapy. Patients with germline mutations in RET have a higher risk of developing MTC and may require prophylactic thyroidectomy; therefore, pediatric MTC prompts the evaluation of syndromic association. Molecular assessment is useful also for the application of targeted therapies that specifically inhibit the RET signaling pathway in advanced or metastatic MTC, such as vandetanib and cabozantinib [10].
Table 6.
Molecular signatures of thyroid cancer in pediatric and adult; SNV: single nucleotide variation; pTERT: TERT promoter [10].
| Mutation Type | Molecular Landscape | Pediatric | Adult |
|---|---|---|---|
| PTC | |||
| SNV | BRAF V600E | <70% | 30–90% |
| pTERT | <5% | 5–25% | |
| DICER1 | 5–10% | <5% | |
| HRAS, KRAS, NRAS | <10% | <35% | |
| Fusion | RET | 20–80% | 5–35% |
| PAX8-PPARG | <5% | <5% | |
| NTRK | <20% | <5% | |
| BRAF | <20% | <3% | |
| ALK | <10% | <3% | |
| FTC | |||
| SNV | HRAS, KRAS, NRAS | <50% | 10–60% |
| Fusion | DICER1 | <25% | 1% |
| PTEN | <25% | <1% | |
| PAX8-PPARG | <20% | 35–50% | |
In summary, molecular analysis of thyroid nodules, especially thyroid FNA specimens, is a valuable tool in the management of thyroid lesions, providing important diagnostic and prognostic information that can guide clinical decision-making and improve patient outcomes.
3.4. Future Perspectives
Artificial intelligence (AI) may play a significant role in the analysis of ultrasound and other imaging data (radiomics). Indeed, complex algorithms can automate the process of interpreting images, allowing for faster and more accurate diagnoses even in this field, as well as for the prediction of molecular alterations (Figure 2) [52].
Figure 2.
Putative future combined approach for correct identification of malignant thyroid nodules.
According to a recent meta-analysis, computer-assisted diagnosis has proven to have reliable diagnostic performance, comparable to that of radiologists [53]. A machine learning approach to identify pediatric thyroid nodules requiring surgical intervention demonstrated good performance, but additional data on larger cohorts are needed before their application in clinical practice [54]. These tools can also be trained to autonomously identify and classify cells based on their morphological features and so to detect cancer cells. A study used a combination of clinical data, microscopic characteristics obtained from FNA and imaging features to triage indeterminate and follicular lesions into high- or low-risk categories, reaching a diagnostic accuracy comparable to that of TI-RADS [55]. Currently, in clinical practice, there are no reliable methods for analyzing cells directly from FNA specimens owing to the complex and multi-layered nature of these samples, as well as the lack of standardized algorithms [52]. Even if the benefits and possible challenges of an AI approach are currently well known, the same is still not true for their pediatric counterparts, in which AI and machine learning still represents an emerging field, and the development of useful candidate algorithms to be applied on histology/cytology for computer-aided diagnosis are mainly limited by the relative rarity of these tumors and their intrinsic structural heterogeneity [56]. Another approach may be the application of Matrix-Assisted Laser Desorption/Ionization—Mass Spectrometry imaging (MALDI-MSI) technology to investigate the spatial distribution of biomolecules (proteins, lipids and metabolites) directly in situ, potentially capable of performing a triage of pediatric thyroid nodules [57]. This technology has been previously used to investigate proteomic data of adult indeterminate nodules, NIFTPs and Hashimoto thyroiditis starting from FNA specimens [58,59,60,61].
4. Conclusions
It is important to prioritize the development of treatment guidelines and risk stratification tools to accurately identify pediatric patients who would benefit from surgical intervention. In the future, the potential combination of all clinical, radiological, cytological and molecular data merged with the assistance of artificial intelligence will certainly improve thyroid nodule management and surveillance, and will aid in the understanding of the key biomolecular pathways involved in thyroid oncogenic processes. In this direction, multicentric efforts and collaborations can further help in increasing the number of case series describing uncommon clinical entities and molecular alterations in this group of patients, enhancing our capabilities of developing robust and reliable AI tools for their assessment.
Author Contributions
Conceptualization, D.S., V.L. and F.P.; methodology, D.S. and F.P.; investigation, D.S. and F.P.; data curation, D.S. and F.P.; writing—original draft preparation, D.S., S.C., A.I.P., D.L., A.I.P., A.G., M.G., V.L., A.C. and F.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Regione Lombardia, regional law no. 9/2020, resolution no. 3776/2020 and Ricerca Finalizzata GR-2019-12368592.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Seminati D., Mane E., Ceola S., Casati G., Putignano P., Garancini M., Gatti A., Leni D., Pincelli A.I., Fusco N., et al. An Indeterminate for Malignancy FNA Report Does Not Increase the Surgical Risk of Incidental Thyroid Carcinoma. Cancers. 2022;14:5427. doi: 10.3390/cancers14215427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldfarb M., Dinauer C. Differences in the Management of Thyroid Nodules in Children and Adolescents as Compared to Adults. Curr. Opin. Endocrinol. Diabetes Obes. 2022;29:466–473. doi: 10.1097/MED.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 3.Scappaticcio L., Maiorino M.I., Iorio S., Docimo G., Longo M., Grandone A., Luongo C., Cozzolino I., Piccardo A., Trimboli P., et al. Exploring the Performance of Ultrasound Risk Stratification Systems in Thyroid Nodules of Pediatric Patients. Cancers. 2021;13:5304. doi: 10.3390/cancers13215304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis G.L., Waguespack S.G., Bauer A.J., Angelos P. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Pediatric Thyroid. Thyroid. 2015;25:716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies L., Welch H.G. Increasing Incidence of Thyroid Cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 6.Daniels K.E., Shaffer A.D., Garbin S., Squires J.H., Vaughan K.G., Viswanathan P., Witchel S.F., Mollen K.P., Yip L., Monaco S.E., et al. Validity of the American College of Radiology Thyroid Imaging Reporting and Data System in Children. Laryngoscope. 2022 doi: 10.1002/lary.30425. early view . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunya G., Dance L., Grimmer J.F. Comparing ATA Guidelines vs TI-RADS for Evaluation of Pediatric Thyroid Lesions. Int. J. Pediatr. Otorhinolaryngol. 2022;164:111411. doi: 10.1016/j.ijporl.2022.111411. [DOI] [PubMed] [Google Scholar]
- 8.Gallant J.-N., Chen S.-C., Ortega C.A., Rohde S.L., Belcher R.H., Netterville J.L., Baregamian N., Wang H., Liang J., Ye F., et al. Evaluation of the Molecular Landscape of Pediatric Thyroid Nodules and Use of a Multigene Genomic Classifier in Children. JAMA Oncol. 2022;8:1323–1327. doi: 10.1001/jamaoncol.2022.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krane J.F. Improving Risk Assessment in Indeterminate Pediatric Thyroid FNA Biopsies. Cancer Cytopathol. 2022;130:326–327. doi: 10.1002/cncy.22543. [DOI] [PubMed] [Google Scholar]
- 10.Guleria P., Srinivasan R., Rana C., Agarwal S. Molecular Landscape of Pediatric Thyroid Cancer: A Review. Diagnostics. 2022;12:3136. doi: 10.3390/diagnostics12123136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A., Ly S., Castroneves L.A., Frates M.C., Benson C.B., Feldman H.A., Wassner A.J., Smith J.R., Marqusee E., Alexander E.K., et al. A Standardized Assessment of Thyroid Nodules in Children Confirms Higher Cancer Prevalence than in Adults. J. Clin. Endocrinol. Metab. 2013;98:3238–3245. doi: 10.1210/jc.2013-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherella C.E., Cibas E.S., Wassner A.J. Re: “The Use of the Bethesda System for Reporting Thyroid Cytopathology in Pediatric Thyroid Nodules: A Meta-Analysis” by Vuong et al. Thyroid. 2021;31:1441. doi: 10.1089/thy.2021.0116. [DOI] [PubMed] [Google Scholar]
- 13.Kim P.H., Yoon H.M., Hwang J., Lee J.S., Jung A.Y., Cho Y.A., Baek J.H. Diagnostic Performance of Adult-Based ATA and ACR-TIRADS Ultrasound Risk Stratification Systems in Pediatric Thyroid Nodules: A Systematic Review and Meta-Analysis. Eur. Radiol. 2021;31:7450–7463. doi: 10.1007/s00330-021-07908-8. [DOI] [PubMed] [Google Scholar]
- 14.Cimbek E.A., Polat R., Sönmez B., Beyhun N.E., Dinç H., Saruhan H., Karagüzel G. Clinical, Sonographical, and Pathological Findings of Pediatric Thyroid Nodules. Eur. J. Pediatr. 2021;180:2823–2829. doi: 10.1007/s00431-021-04032-z. [DOI] [PubMed] [Google Scholar]
- 15.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tessler F.N., Middleton W.D., Grant E.G., Hoang J.K., Berland L.L., Teefey S.A., Cronan J.J., Beland M.D., Desser T.S., Frates M.C., et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 17.Russ G., Bonnema S.J., Erdogan M.F., Durante C., Ngu R., Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seminati D., Capitoli G., Leni D., Fior D., Vacirca F., Di Bella C., Galimberti S., L’Imperio V., Pagni F. Use of Diagnostic Criteria from ACR and EU-TIRADS Systems to Improve the Performance of Cytology in Thyroid Nodule Triage. Cancers. 2021;13:5439. doi: 10.3390/cancers13215439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leni D., Seminati D., Fior D., Vacirca F., Capitoli G., Cazzaniga L., Di Bella C., L’Imperio V., Galimberti S., Pagni F. Diagnostic Performances of the ACR-TIRADS System in Thyroid Nodules Triage: A Prospective Single Center Study. Cancers. 2021;13:2230. doi: 10.3390/cancers13092230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W., Phillips S.A., Newbury R.O., Naheedy J.H., Newfield R.S. Diagnostic Utility of Fine Needle Aspiration Cytology in Pediatric Thyroid Nodules Based on Bethesda Classification. J. Pediatr. Endocrinol. Metab. 2021;34:449–455. doi: 10.1515/jpem-2020-0645. [DOI] [PubMed] [Google Scholar]
- 21.Tuli G., Munarin J., Agosto E., Matarazzo P., Quaglino F., Mormile A., de Sanctis L. Predictive Factors of Malignancy in Pediatric Patients with Thyroid Nodules and Performance of the Italian Classification (SIAPEC 2014) in the Outcome of the Cytological FNA Categories. Endocrine. 2021;74:365–374. doi: 10.1007/s12020-021-02784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora S., Khoury J., Trout A.T., Chuang J. Improving Malignancy Prediction in AUS/FLUS Pediatric Thyroid Nodules with the Aid of Ultrasound. Horm. Res. Paediatr. 2020;93:239–244. doi: 10.1159/000509118. [DOI] [PubMed] [Google Scholar]
- 23.Ali S.Z., Cibas E.S. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 24.Muri R., Trippel M., Borner U., Weidner S., Trepp R. The Impact of Rapid On-Site Evaluation on the Quality and Diagnostic Value of Thyroid Nodule Fine-Needle Aspirations. Thyroid. 2022;32:667–674. doi: 10.1089/thy.2021.0551. [DOI] [PubMed] [Google Scholar]
- 25.Nardi F., Basolo F., Crescenzi A., Fadda G., Frasoldati A., Orlandi F., Palombini L., Papini E., Zini M., Pontecorvi A., et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014;37:593–599. doi: 10.1007/s40618-014-0062-0. [DOI] [PubMed] [Google Scholar]
- 26.Cross P., Chandra A., Giles T., Liverpool R., Johnson S., Kocjan G., Poller D., Stephenson T. Guidance on the Reporting of Thyroid Cytology Specimens. January 2016. The Royal College of Pathologists; London, UK: 2016. [Google Scholar]
- 27.Amirazodi E., Propst E.J., Chung C.T., Parra D.A., Wasserman J.D. Pediatric Thyroid FNA Biopsy: Outcomes and Impact on Management over 24 Years at a Tertiary Care Center. Cancer Cytopathol. 2016;124:801–810. doi: 10.1002/cncy.21750. [DOI] [PubMed] [Google Scholar]
- 28.Buryk M.A., Simons J.P., Picarsic J., Monaco S.E., Ozolek J.A., Joyce J., Gurtunca N., Nikiforov Y.E., Feldman Witchel S. Can Malignant Thyroid Nodules Be Distinguished from Benign Thyroid Nodules in Children and Adolescents by Clinical Characteristics? A Review of 89 Pediatric Patients with Thyroid Nodules. Thyroid. 2015;25:392–400. doi: 10.1089/thy.2014.0312. [DOI] [PubMed] [Google Scholar]
- 29.Cherella C.E., Angell T.E., Richman D.M., Frates M.C., Benson C.B., Moore F.D., Barletta J.A., Hollowell M., Smith J.R., Alexander E.K., et al. Differences in Thyroid Nodule Cytology and Malignancy Risk between Children and Adults. Thyroid. 2019;29:1097–1104. doi: 10.1089/thy.2018.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heider A., Arnold S., Lew M., Pang J., Rabah R., Bruch S., Thomas I., Menon R., Cantley R., Davenport R., et al. Malignant Risk of Indeterminate Pediatric Thyroid nodules—An Institutional Experience. Diagn. Cytopathol. 2019;47:993–998. doi: 10.1002/dc.24266. [DOI] [PubMed] [Google Scholar]
- 31.Jia M.R., Baran J.A., Bauer A.J., Isaza A., Surrey L.F., Bhatti T., McGrath C., Jalaly J., Mostoufi-Moab S., Adzick N.S., et al. Utility of Fine-Needle Aspirations to Diagnose Pediatric Thyroid Nodules. Horm. Res. Paediatr. 2021;94:263–274. doi: 10.1159/000519307. [DOI] [PubMed] [Google Scholar]
- 32.Kardelen Al A.D., Yılmaz C., Poyrazoglu S., Tunca F., Bayramoglu Z., Bas F., Bundak R., Gilse Senyurek Y., Ozluk Y., Yegen G., et al. The Role Of Thyroid Fine-Needle Aspiration Cytology In The Treatment And Follow-Up Of Thyroid Nodules In The Pediatric Population. Acta Endocrinol. 2019;15:333–341. doi: 10.4183/aeb.2019.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lale S.A., Morgenstern N.N., Chiara S., Wasserman P. Fine Needle Aspiration of Thyroid Nodules in the Pediatric Population: A 12-Year Cyto-Histological Correlation Experience at North Shore-Long Island Jewish Health System. Diagn. Cytopathol. 2015;43:598–604. doi: 10.1002/dc.23265. [DOI] [PubMed] [Google Scholar]
- 34.Monaco S.E., Pantanowitz L., Khalbuss W.E., Benkovich V.A., Ozolek J., Nikiforova M.N., Simons J.P., Nikiforov Y.E. Cytomorphological and Molecular Genetic Findings in Pediatric Thyroid Fine-Needle Aspiration. Cancer Cytopathol. 2012;120:342–350. doi: 10.1002/cncy.21199. [DOI] [PubMed] [Google Scholar]
- 35.Norlén O., Charlton A., Sarkis L.M., Henwood T., Shun A., Gill A.J., Delbridge L. Risk of Malignancy for Each Bethesda Class in Pediatric Thyroid Nodules. J. Pediatr. Surg. 2015;50:1147–1149. doi: 10.1016/j.jpedsurg.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 36.Pantola C., Kala S., Khan L., Pantola S., Singh M., Verma S. Cytological Diagnosis of Pediatric Thyroid Nodule in Perspective of the Bethesda System for Reporting Thyroid Cytopathology. J. Cytol. 2016;33:220–223. doi: 10.4103/0970-9371.190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partyka K.L., Huang E.C., Cramer H.M., Chen S., Wu H.H. Histologic and Clinical Follow-up of Thyroid Fine-Needle Aspirates in Pediatric Patients. Cancer Cytopathol. 2016;124:467–471. doi: 10.1002/cncy.21713. [DOI] [PubMed] [Google Scholar]
- 38.Rossi E.D., Straccia P., Martini M., Revelli L., Lombardi C.P., Pontecorvi A., Fadda G. The Role of Thyroid Fine-Needle Aspiration Cytology in the Pediatric Population: An Institutional Experience. Cancer Cytopathol. 2014;122:359–367. doi: 10.1002/cncy.21400. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Mehrad M., Ely K.A., Liang J., Solórzano C.C., Neblett W.W., 3rd, Coogan A.C., Weiss V.L. Incidence and Malignancy Rates of Indeterminate Pediatric Thyroid Nodules. Cancer Cytopathol. 2019;127:231–239. doi: 10.1002/cncy.22104. [DOI] [PubMed] [Google Scholar]
- 40.Suh J., Choi H.S., Kwon A., Chae H.W., Kim H.-S. Adolescents with Thyroid Nodules: Retrospective Analysis of Factors Predicting Malignancy. Eur. J. Pediatr. 2020;179:317–325. doi: 10.1007/s00431-019-03507-4. [DOI] [PubMed] [Google Scholar]
- 41.Vuong H.G., Chung D.G.B., Ngo L.M., Bui T.Q., Hassell L., Jung C.K., Kakudo K., Bychkov A. The Use of the Bethesda System for Reporting Thyroid Cytopathology in Pediatric Thyroid Nodules: A Meta-Analysis. Thyroid. 2021;31:1203–1211. doi: 10.1089/thy.2020.0702. [DOI] [PubMed] [Google Scholar]
- 42.Cherella C.E., Hollowell M.L., Smith J.R., Zendejas B., Modi B.P., Cibas E.S., Wassner A.J. Subtype of Atypia on Cytology and Risk of Malignancy in Pediatric Thyroid Nodules. Cancer Cytopathol. 2022;130:330–335. doi: 10.1002/cncy.22544. [DOI] [PubMed] [Google Scholar]
- 43.Franco A.T., Labourier E., Ablordeppey K.K., Surrey L.F., Mostoufi-Moab S., Isaza A., Adzick N.S., Kazahaya K., Kumar G., Bauer A.J. miRNA Expression Can Classify Pediatric Thyroid Lesions and Increases the Diagnostic Yield of Mutation Testing. Pediatr. Blood Cancer. 2020;67:e28276. doi: 10.1002/pbc.28276. [DOI] [PubMed] [Google Scholar]
- 44.Bauer A.J. Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol. Metab. Clin. N. Am. 2017;46:389–403. doi: 10.1016/j.ecl.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Mollen K.P., Shaffer A.D., Yip L., Monaco S.E., Huyett P., Viswanathan P., Witchel S.F., Duvvuri U., Simons J.P. Unique Molecular Signatures Are Associated with Aggressive Histology in Pediatric Differentiated Thyroid Cancer. Thyroid. 2022;32:236–244. doi: 10.1089/thy.2021.0317. [DOI] [PubMed] [Google Scholar]
- 46.Baran J.A., Halada S., Bauer A.J., Ricarte-Filho J.C., Isaza A., Surrey L.F., McGrath C., Bhatti T., Jalaly J., Mostoufi-Moab S., et al. Indeterminate Thyroid Fine-Needle Aspirations in Pediatrics: Exploring Clinicopathologic Features and Utility of Molecular Profiling. Horm. Res. Paediatr. 2022;95:430–441. doi: 10.1159/000526116. [DOI] [PubMed] [Google Scholar]
- 47.Cancer Genome Atlas Research Network Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oishi N., Kondo T., Nakazawa T., Mochizuki K., Inoue T., Kasai K., Tahara I., Yabuta T., Hirokawa M., Miyauchi A., et al. Frequent BRAF and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan. Endocr. Pathol. 2017;28:103–111. doi: 10.1007/s12022-017-9470-y. [DOI] [PubMed] [Google Scholar]
- 49.Chakraborty D., Shakya S., Ballal S., Agarwal S., Bal C. BRAFV600E and TERT Promoter Mutations in Paediatric and Young Adult Papillary Thyroid Cancer and Clinicopathological Correlation. J. Pediatr. Endocrinol. Metab. 2020;33:1465–1474. doi: 10.1515/jpem-2020-0174. [DOI] [PubMed] [Google Scholar]
- 50.Geng J., Liu Y., Guo Y., Wang H., Tai J., Jin Y., Zhang J., Yu Y., Wang S., Song Y., et al. Correlation between TERT C228T and Clinic-Pathological Features in Pediatric Papillary Thyroid Carcinoma. Sci. China Life Sci. 2019;62:1563–1571. doi: 10.1007/s11427-018-9546-5. [DOI] [PubMed] [Google Scholar]
- 51.Chernock R.D., Rivera B., Borrelli N., Hill D.A., Fahiminiya S., Shah T., Chong A.-S., Aqil B., Mehrad M., Giordano T.J., et al. Poorly Differentiated Thyroid Carcinoma of Childhood and Adolescence: A Distinct Entity Characterized by DICER1 Mutations. Mod. Pathol. 2020;33:1264–1274. doi: 10.1038/s41379-020-0458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorrenti S., Dolcetti V., Radzina M., Bellini M.I., Frezza F., Munir K., Grani G., Durante C., D’Andrea V., David E., et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers. 2022;14:3357. doi: 10.3390/cancers14143357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L., Gao J., Wang Q., Yin J., Yu P., Bai B., Pei R., Chen D., Yang G., Wang S., et al. Computer-Aided Diagnosis Systems in Diagnosing Malignant Thyroid Nodules on Ultrasonography: A Systematic Review and Meta-Analysis. Eur. Thyroid J. 2020;9:186–193. doi: 10.1159/000504390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radebe L., van der Kaay D.C.M., Wasserman J.D., Goldenberg A. Predicting Malignancy in Pediatric Thyroid Nodules: Early Experience with Machine Learning for Clinical Decision Support. J. Clin. Endocrinol. Metab. 2021;106:e5236–e5246. doi: 10.1210/clinem/dgab435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wildman-Tobriner B., Taghi-Zadeh E., Mazurowski M.A. Artificial Intelligence (AI) Tools for Thyroid Nodules on Ultrasound, From the AJR Special Series on AI Applications. Am. J. Roentgenol. 2022;219:547–554. doi: 10.2214/AJR.22.27430. [DOI] [PubMed] [Google Scholar]
- 56.van der Kamp A., van der Kamp A., Waterlander T.J., de Bel T., van der Laak J., van den Heuvel-Eibrink M.M., Mavinkurve-Groothuis A.M.C., de Krijger R.R. Artificial Intelligence in Pediatric Pathology: The Extinction of a Medical Profession or the Key to a Bright Future? Pediatr. Dev. Pathol. 2022;25:380–387. doi: 10.1177/10935266211059809. [DOI] [PubMed] [Google Scholar]
- 57.Capitoli G., Piga I., Galimberti S., Leni D., Pincelli A.I., Garancini M., Clerici F., Mahajneh A., Brambilla V., Smith A., et al. MALDI-MSI as a Complementary Diagnostic Tool in Cytopathology: A Pilot Study for the Characterization of Thyroid Nodules. Cancers. 2019;11:1377. doi: 10.3390/cancers11091377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piga I., Capitoli G., Clerici F., Galimberti S., Magni F., Pagni F. Toxic Chemical and Biological Agents: Detection, Diagnosis and Health Concerns. Springer; Dordrecht, The Netherlands: 2020. Recent Advances in MALDI Mass Spectrometry Imaging: A Cutting-Edge Tool for the Diagnosis of Thyroid Nodules; pp. 253–254. [Google Scholar]
- 59.Pagni F., L’Imperio V., Bono F., Garancini M., Roversi G., De Sio G., Galli M., Smith A.J., Chinello C., Magni F. Proteome Analysis in Thyroid Pathology. Expert Rev. Proteom. 2015;12:375–390. doi: 10.1586/14789450.2015.1062369. [DOI] [PubMed] [Google Scholar]
- 60.Smith A., Galli M., Piga I., Denti V., Stella M., Chinello C., Fusco N., Leni D., Manzoni M., Roversi G., et al. Molecular Signatures of Medullary Thyroid Carcinoma by Matrix-Assisted Laser Desorption/ionisation Mass Spectrometry Imaging. J. Proteom. 2019;191:114–123. doi: 10.1016/j.jprot.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Mainini V., Pagni F., Garancini M., Giardini V., De Sio G., Cusi C., Arosio C., Roversi G., Chinello C., Caria P., et al. An Alternative Approach in Endocrine Pathology Research: MALDI-IMS in Papillary Thyroid Carcinoma. Endocr. Pathol. 2013;24:250–253. doi: 10.1007/s12022-013-9273-8. [DOI] [PubMed] [Google Scholar]