Abstract
Psoriatic arthritis (PsA) is a systemic inflammatory condition characterised by multiple clinical manifestations. Over the last decade, significant progress has been made in understanding the pathobiology of the disease. An expanded set of targeted therapies have emerged and have shown efficacy in PsA. Nevertheless, there is still a substantial subset of patients who experience no response or only a partial response to currently licensed therapies. The heterogeneous nature of the disease, together with a varying level of severity at presentation and disease activity during follow-up, brings tremendous challenges to devising management strategies. While there are certain pathophysiological similarities between PsA and rheumatoid arthritis (RA), it has become clear that there are discriminating features between these two conditions at the clinical, cellular, and molecular levels. However, there is a degree of overlap in the clinical approach when treating both PsA and RA, given that many biological and targeted therapies have proven efficacy for both pathologies. With an increasing understanding of the relevance of the IL-23/IL-17 axis in PsA, pharmacological agents blocking this pathway have provided promising possibilities for patients with PsA.
Keywords: psoriatic arthritis, targeted therapy, IL-17 pathway
1. Introduction
Psoriatic arthritis (PsA) is a heterogeneous, chronic inflammatory condition affecting one in five individuals with psoriasis and has been shown to affect about 0.1% of the population [1,2]. It is characterised by a diverse array of clinical features, which include peripheral arthritis, axial spondyloarthritis, dactylitis, enthesitis, uveitis, psoriasis, and nail disease [3]. PsA is recognised as part of the spectrum of psoriatic diseases [4]. It was once thought to be a relatively benign disease; however, it is now well recognised to have a functional burden similar to other inflammatory arthritic conditions such as rheumatoid arthritis (RA) and the axial spondyloarthritides (axSpA) [5].
PsA is progressive and destructive in a substantial subset of individuals. It has been confirmed that damage occurs early in the disease, with nearly 50% of patients showing evidence of radiographic damage at a median interval disease duration of 2 years [6]. Another cross-sectional study illustrated that a diagnostic delay of 6 months contributed to worse outcomes, including the development of peripheral joint erosions, arthritis mutilans, and poor quality of life [7].
Therefore, there is compelling evidence supporting attempts at early diagnosis and prompt institution of proven disease-modifying therapy in PsA. Despite this awareness, delay in diagnosis remains a major challenge in the care of those with PsA. A population-based survey showed an average diagnostic delay of 5 years from the commencement of musculoskeletal symptoms to a final diagnosis of PsA [8]. Numerous factors likely contribute to the challenge of early identification of PsA; these include the heterogeneity of presentation, often only a minimal acute phase response, and limited objective joint swelling compared with RA. Potentially most importantly, the lack of biomarkers to assist in ascertaining a diagnosis remains challenging [9].
The quest to find an optimal and effective therapy that will induce cure or remission has expanded the research agenda to examine new compounds targeting different pathways involved in the persistence of the signs and symptoms of PsA. Table 1 summarises the targeted therapies discussed in this review with their corresponding mode of action.
Table 1.
Targeted therapies for psoriatic arthritis.
| Therapy | Mechanism of Action |
|---|---|
| Infliximab | Chimeric monoclonal antibody against TNF-α |
| Etanercept | Soluble TNF receptor p75-immunoglobulin G1 fusion protein |
| Adalimumab | Fully human anti-TNF-α monoclonal antibody |
| Golimumab | Fully human immunoglobulin G1 kappa anti-TNF-α antibody |
| Certolizumab pegol |
Fab fragment of anti-TNF- α monoclonal antibody |
| Ustekinumab | Fully human immunoglobulin G1 kappa monoclonal antibody against the shared p40 subunit of IL-12 and IL-23 |
| Guselkumab | Fully human immunoglobulin G1 lambda monoclonal antibody against the p19 subunit of IL-23 |
| Risankizumab | Fully human immunoglobulin G1 lambda monoclonal antibody against the p19 subunit of IL-23 |
| Secukinumab | Fully human immunoglobulin G1 kappa monoclonal antibody against IL-17A |
| Ixekizumab | Humanised immunoglobulin G4 monoclonal antibody against IL-17A |
| Brodalumab | Fully human immunoglobulin G2 monoclonal antibody against IL-17RA |
| Bimekizumab | Humanised immunoglobulin G1 monoclonal antibody against IL-17A and IL-17F |
| Tofacitinib | Inhibitor of JAK1 and JAK3 |
| Upadacitinib | Selective inhibitor of JAK1 |
| Filgotinib | Selective inhibitor of JAK1 |
| Deucravacitinib | Selective TYK2 inhibitor |
| Abatacept | Selective T cell co-stimulator inhibitor |
2. Immunopathology of PsA, Genetics, and Environment
2.1. Immunopathology
The importance of T lymphocytes has been consistently shown in psoriasis and PsA [10,11]. The key role of T cells in the immunopathogenesis of PsA is illustrated by the presence of T cell response cytokines, namely interferon γ, IL-2, IL-4 TNFα, and interleukin (IL)-17A, and in both the synovial fluid and inflamed synovium [12]. Over the last decade, studies have highlighted key drivers of disease in PsA, and the focus has shifted significantly, from TNFα and Th1 response cytokines, to Th17 cells, IL-23, and IL-17 [13]. These targets have proven relevant in randomised controlled trials (RCT) and have been approved by international regulatory authorities.
When an antigen is presented to the initial T cell, IL-12 is upregulated. This results in the differentiation and proliferation of Th1 cells contributing to pro-inflammatory cytokines, including TNFα, being released [14]. On the other hand, IL-23 is thought to trigger Th17 cell differentiation [15]. This results in the release of IL-22 and IL-17, leading to the upregulation of TNFα [16].
Abundant B lymphocyte aggregates have been noted in the synovium of patients with PsA. However, it is unclear what role, if any, B-cells have in disease onset and perpetuation. Notably, PsA has not been linked with circulating autoantibodies [17]. In addition, monoclonal antibodies targeting CD20 expressed on B cells have only shown a modest benefit in treating PsA [18].
The synovial tissue in PsA shares similarities to those observed in other spondyloarthritis (SpA) but contrasts with the synovium in RA. Compared to RA, synovial macroscopic examination in PsA reveals a distinctly different vascular pattern with potentially important pathogenic implications [19]. The vascular patterns described in the joints in PsA have been reported to be comparable to those noted in the dermis of the skin and nailfold capillaries in psoriasis, thus suggesting a shared link at the tissue level [20].
In another model, McGonagle and colleagues propose that entheseal structures, which are intimately associated with the synovium, are the initial sites of musculoskeletal disease in PsA [21]. In this model, it is hypothesised that synovitis is triggered by the release of proinflammatory cytokines from the closely related enthesis, an anatomic region at the junction of tendon to bone. In a mouse model, IL-23 resulted in entheseal-predominant inflammatory arthritis comparable to what is seen in SpA with bone erosion and new bone formation [22].
2.2. Genetics
PsA has a highly heritable component, especially when compared with RA. The recurrence risk ratio in first-degree relatives compared with the general population is in excess of 27, which is considerably higher than the recurrence risk ratio for psoriasis or RA [23]. Susceptibility to PsA and psoriasis is associated with class I major histocompatibility complex (MHC) alleles. This is contrary to RA, which is linked to class II MHC alleles. Nuances in the specific associated genetic susceptibility to the ‘psoriatic spectrum’ exist. For example, a cross-sectional study demonstrated that HLA-C*06 confers susceptibility for psoriasis but not for PsA. Furthermore, HLA-B*08, B*27, B*38, and B*39 with particular subtypes of these alleles are associated with particular ‘sub-phenotypes’, with symmetric or asymmetric sacroiliitis, enthesitis, dactylitis, and synovitis [24]. Despite these well-characterised genetic associations with sub-phenotypes, no role for genotyping has been established in selecting treatment for individual patients.
2.3. Environment
The role of environmental factors as a trigger of PsA in genetically susceptible individuals is the subject of intense research. Environmental risk factors such as trauma (Koebner’s phenomenon), infections, smoking, and immunological triggers (such as rubella vaccination) have each been posited to be risk factors for the onset of PsA [25].
3. Unmet Needs in Therapeutics—The Challenges
New treatment paradigms have recently emerged with the recognition of novel therapies targeting specific cytokines and signalling pathways. Despite these expanding therapeutic targets, the overall improvement in response to therapies has been disappointing.
At least 40% of PsA patients exhibit no response or only a partial response. Clinical trials have failed to consistently show an American College of Rheumatology criteria for 20% improvement (ACR20) in more than 60% of patients [3,26]. Therefore, there is growing interest in developing and testing other therapeutic concepts to expand the repertoire of treatment modalities available to treat patients with PsA.
The heterogeneity of PsA challenges practicing rheumatologists in choosing the most suitable therapy for their patients. To address these challenges, international bodies including the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR) have issued recommendations to guide clinicians [27]. The recent GRAPPA recommendations, published in 2021, suggest addressing all active disease domains and PsA-relevant comorbidities in an individual patient and driving treatment decisions based on the domain with the highest level of activity. However, this approach can be insensitive for the many individuals who exhibit persistent disease in several domains [28].
Combining therapies with different modes of action is an appealing area of interest. This strategy has the theoretical potential to induce remission. One novel approach has been the development of a bispecific TNF-inhibitor (TNFi) and IL-17 inhibitor (targeted variable domain immunoglobulin), which acknowledges the proven efficacy of each cytokine in treating PsA. However, this was not superior in clinical trials to adalimumab in PsA or, indeed, RA. Nevertheless, there were no major safety signals in these relatively small studies, inhibiting two cytokines [29]. In a proof-of-principle clinical trial of this bispecific inhibitor in PsA in inadequate methotrexate responders, the ACR50/ACR70 response and the Psoriasis Area and Severity Index criteria for 75% improvement (PASI75)/PASI90 was statistically superior to adalimumab at multiple time intervals. These promising efficacy results were combined amidst comparable safety parameters to that of the TNFi comparator (adalimumab). However, further development and testing of the agent have not been pursued [30].
Numerous case reports have described improvement in disease manifestations using dual biologic combinations, including TNF-inhibitors (TNFi) and gulselkumab (IL-23 inhibition) or ustekinumab (IL 12-23 inhibition). Given the lack of blinding in these studies, the results must be interpreted cautiously because of the possibility of publication bias [31].
The AFFINITY (NCT05071664) multicenter double-blind RCT evaluating a combined strategy of golimumab and guselkumab (IL-23 inhibition) versus guselkumab monotherapy is currently enrolling participants. This study aims to answer important questions about the efficacy and safety of implementing dual cytokine inhibition in the setting of psoriatic arthritis [32].
With an increase in pathogenic understanding of the processes involved in the disease, treatments now include compounds targeting TNF, IL-12/23, IL-23, IL-17, JAK-STAT, and phosphodiesterase type 4 (PDE4). However, a recent systematic review revealed that minimal disease activity (MDA) is only attained in 17% of those prescribed conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) and in up to only 57% of those on biologic DMARD (bDMARD) [33].
Given the financial costs associated with bDMARDs and tsDMARDs therapy, another important challenge in treating PsA relates to the costs of available treatments. Notwithstanding the reduction in costs of targeted biological therapies in recent years, a more precise selection of initial treatments could be greatly improved if biomarkers, including genetic, cellular, and other molecular markers, were identified and validated in predicting treatment responses. This could conceivably enable patients to experience either minimal disease activity or remission at the earliest stage possible.
4. TNFα
TNF inhibitors (TNFi) were the first licensed biologics for the management of PsA and are now commonly utilised. There are five TNFi agents approved for use (adalimumab, etanercept, infliximab, certolizumab pegol, and golimumab). There is good evidence that TNFi can slow or prevent the radiographic progression in peripheral PsA as demonstrated by stable measures of structural damage such as radiological measurements of erosions, and joint space narrowing [34,35,36,37,38]. One of the disappointing aspects of the novel therapies has been the inability to retard a cardinal characteristic of PsA progression, new bone formation.
It is worth noting that the response to TNFi whether used as monotherapy or with concomitant csDMARD (usually methotrexate) does not differ greatly [39]. However, these studies were not appropriately powered to allow a sound conclusion on whether combination therapy provides additional benefits as opposed to TNFi or csDMARD monotherapy in the early stages of PsA.
The SEAM-PsA study evaluated the efficacy of methotrexate monotherapy, etanercept monotherapy or methotrexate in conjunction with etanercept in patients with a relatively short disease duration [39]. One very important finding of this trial was that methotrexate treatment on its own does provide benefits in the management of treatment-naïve patients, which, while an established therapy, has had very little data to date. However, the trial did not include a placebo control arm, making this finding difficult to confirm definitively. Another important finding from this study is that combination therapy confers very little additional benefit over etanercept monotherapy.
TNFi has been demonstrated to be a potent therapy for all disease phenotypes and is recommended by GRAPPA and EULAR for entheseal involvement and dactylitis [27,28]. However, all the key phase III RCTs assessing TNFi in PsA used different measures to evaluate enthesitis, thus rendering comparison challenging [34,35,36,37,38].
With a paucity of data regarding the effectiveness of TNF inhibition on axial disease in PsA, the recommendations stem from data for axSpA studies [40,41,42,43,44]. TNFi appears to have an impact on axial inflammation by reducing pain, decreasing markers of active disease such as C-reactive protein (CRP) and improving the lumbar spinal range of motion. However, it is not entirely clear whether improvement in these indices translates to a true impact on the radiographic progression in the axial skeleton, and, therefore, whether these agents can be considered truly disease-modifying, especially for axial disease [45].
As in RA, uncontrolled inflammation can lead to bone and cartilage destruction and erosion in patients with PsA. One fundamental difference to RA, referenced already, is that the bony architectural changes in PsA are characterised by the presence of both catabolic and anabolic bone changes [46]. These changes suggest the presence of increased resorption (resulting in bone erosions and osteolysis) coupled with enhanced bone formation, such as syndesmophytes and enthesophytes. In PsA, normal bone remodelling homeostasis and the crosstalk between osteoclasts and osteoblasts are disrupted. A number of cytokines and growth factors normally associated with osteoclasts and osteoblasts differentiation are dysregulated, with the result being the complex bone phenotype involving both erosive changes and new bone formation seen in PsA [47]. While TNFi retard the progression of bone erosion, they do not appear to prevent the phenomenon of new bone formation seen in PsA.
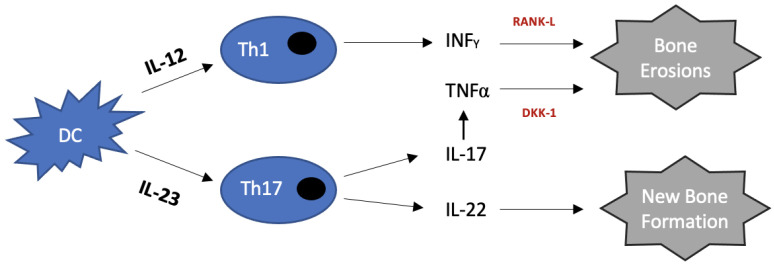
TNFα plays an important part in the formation of erosions as it induces osteoclast differentiation by triggering the expression of receptor activator nuclear factor-kB ligand in the synovium, which is a key player for osteoclast differentiation [48]. TNFα also stimulates the expression of Dickkopf-related protein 1 (Dkk-1) by synovial fibroblasts, which suppress osteoblast differentiation, further promoting the formation of erosions (Figure 1) [48,49]. Based on this knowledge, it is clear that inhibition of TNFα by the currently approved compounds (TNFi) does not prevent new bone formation in PsA. Indeed these findings imply that other cytokines, such as IL-22, may be at the centre of this excessive bone formation [50].
Figure 1.
Pathogenesis of psoriatic arthritis. (Adapted with permission from Mantravadi, S. et al., Tumor necrosis factor inhibitors in psoriatic arthritis; published by Expert Review of Clinical Pharmacology, 2017. Copyright 2017 by Taylor and Francis) Dendritic (DC) and T helper (Th17 and Th1) cells produce several cytokines. TNFα promotes osteoclastogenesis via RANK-L and inhibits osteoblastogenesis via Dkk-1. This ultimately results in bone erosion formation [49]. IL-22 is involved in new bone formation [50,51].
5. IL-12/23
Arguably the most important advance over the last two decades has been the recognition that the IL12/23 and IL-17 cytokine axis plays a major role in the biology of psoriatic disease. As a result, growing interest has emerged in developing therapeutic agents targeting this pathway directly via IL-17 inhibition or upstream via inhibition of IL-12 and 23.
Ustekinumab is a human monoclonal antibody targeting the p40 subunit shared by IL-12 and IL-23 [52]. It was first approved for the management of plaque psoriasis following two phase III trials (PHOENIX 1 and 2) [53,54].
Following the PHOENIX trials in psoriasis patients, the PSUMMIT 1 and PSUMMIT 2 Phase III trials were performed to assess ustekinumab in patients with PsA [55,56]. Based on the results of these two trials, ustekinumab received approval from both the Food and Drug Administration (FDA) and naïve European Medicines Agency (EMA) for the management of PsA, representing the first approval of a novel agent for PsA in a decade.
In PSUMMIT 1, 615 TNFi-naïve PsA patients were randomised 1:1:1 to receive a placebo vs. 45 mg vs. 90 mg of ustekinumab. ACR20/50/70 and PASI75 responses were evaluated at 24 weeks, with ACR20 as the primary outcome. Ustekinumab showed superiority to the placebo; in the 90 mg ustekinumab group, the ACR20/50/70 response was achieved in 49.5%, 27.9%, and 14.2%, whereas the placebo responses were 22.8%, 8.7%, and 2.4%, respectively. In PSUMMIT 1, ACR20 response rates were sustained at 52 weeks regardless of concomitant methotrexate use [55].
In contrast to PSUMMIT 1, PSUMMIT 2 included 58% of patients who had previous TNFi exposure but experienced an inadequate response. A clinical response to ustekinumab was shown regardless of prior TNFi exposure; however, the magnitude of the clinical response was lesser in the TNFi-inadequate responders in contrast to the naïve patients [56].
Most of the participants in the TNFi-experienced arm discontinued TNFi due to inadequate response, with most having been treated with at least two prior TNFi. It is possible that this specific group of patients had a more recalcitrant disease phenotype [57].
The IL23–Th17–IL–17 axis has been associated with the activation of osteoclastogenesis and thus promotes erosive changes [58]. Radiographic progression was evaluated in the PSUMMIT 1 and 2 trials using the PsA-modified Van Der Heijde-Sharp (vdH-S) scoring system, which scores erosions and joint space narrowing. At week 24, more participants in the combined ustekinumab-treated group demonstrated no progression compared to the control (91.7% vs. 83.8%, p = 0.005). A reduction in progression was also noted in patients originally receiving the placebo who transitioned over to the active arm. The proportion of patients with features of ‘arthritis mutilans’ (‘pencil-in-cup’ or gross osteolysis) also remained at a low level and stable [52]. It is worth noting that, once again, the scoring system employed to evaluate the X-rays of the hands and feet of the patients did not take full account of new bone formation [59]. Furthermore, the duration of the trial may have been too short to assess this aspect of disease modification meaningfully.
Although preliminary efficacy has been demonstrated in an open-label, proof-of-concept trial evaluating ustekinumab in axSpA [60], a large phase III study in axSpA was terminated as the study’s primary and secondary endpoints were not met, thereby raising important questions about the disease-modifying effect of ustekinumab in axial PsA.
6. IL-23
Responses in collagen-induced arthritis animal models have demonstrated that loosing the IL-23 gene (p19−/−) was protective, whereas a loss of the IL-12 gene (p35−/−) worsened arthritis. This led to efforts to inhibit exclusively IL-23 in humans with PsA, which have been shown to be efficacious [61].
IL-23 dysregulation plays a central role in enthesitis. IL-23 expression has been shown to induce enthesitis in mice along with several features which are also seen in humans, including periostitis, entheseal and periosteal new bone formation, and bone erosion. Later, a ‘secondary’ synovitis appears to develop, which has destructive properties similar to ‘arthritis mutilans’ as seen in PsA [22,62].
Guselkumab and risankizumab are monoclonal antibodies targeting the p19 subunit of IL-23, thus, have no biological influence on IL-12.
These therapies have demonstrated clear benefits in psoriasis treatment, with superiority, versus adalimumab and ustekinumab [63,64,65,66]. Guselkumab is the only anti-IL-23p19 currently approved by the EMA and the FDA for the treatment of PsA. Guselkumab 100 mg administered every 4 weeks (Q4W) versus every 8 weeks (Q8W) were studied in the placebo-controlled randomised DISCOVER-1 and DISCOVER-2 phase III trials in active PsA. In both trials, guselkumab administered at both dose frequencies significantly improved the manifestations of PsA compared to a placebo at 24 weeks [67,68]. Sustained improvements were also noted through 1 year [69,70].
In DISCOVER-1, previous exposure to one or two TNFi was allowed but was limited to ∼30% of the study cohort, while all participants in DISCOVER-2 were biologic-naïve. The primary endpoint (ACR20 at week 24) for DISCOVER-1 (N = 381) was experienced significantly by patients in the guselkumab group every Q4W (58%) and Q8W (52%) than in the placebo group (22%) [67]. DISCOVER-2 included a larger population of PsA patients (N = 739); again, significantly greater proportions of patients receiving guselkumab (at both dose regimens; Q4W and Q8W) reached an ACR20 response at week 24 compared to the placebo group [68]. The pooled safety data through 1 year of the two phase III DISCOVER trials did not identify any new safety signals, guselkumab at both doses was well tolerated by PsA patients, and the safety was consistent with that established in psoriasis patients who received treatment with guselkumab [71].
The efficacy of guselkumab in enthesitis resolution is worth mentioning. Among 1118 randomised patients in both DISCOVER trials who had ≥1 Leeds Enthesitis Index (LEI) site examined, 65% had enthesitis at baseline. Guselkumab Q4W and Q8W were superior to the placebo in enthesitis resolution at week 24 (45% and 50% vs. 29%). Enthesitis resolution rates continued to increase, and by week 52, 58% of patients randomised to guselkumab achieved resolution [72]. Nevertheless, direct comparisons with other available treatments in this domain make this finding difficult to interpret.
The COSMOS trial, which exclusively included TNFi-inadequate responders, demonstrated the superiority of guselkumab Q8W (ACR20 response of 44.4% at week 24) compared to a placebo (ACR20 response of 19.8% at 24 weeks), thus suggesting that guselkumab is an appropriate therapy for PsA patients who are intolerant to or lack response to TNFi [73].
The question of radiographic progression was evaluated only in the DISCOVER-2 trial, and progression was significantly lower in the guselkumab Q4W group than in the placebo group at 24 weeks. As referenced earlier, this is one major challenge in clinical trials where scores of radiographic progression are assessed over a relatively short time period.
The long-term efficacy analysis of patients treated with guselkumab through 2 years as part of the DISCOVER-2 was recently published. Mean changes in the PsA-modified vdH-S score from weeks 52 to week 100 indicated that the low rates of progression seen in guselkumab-treated patients at earlier time points continued through week 100 [74].
The efficacy of guselkumab regarding retarding new bone formation was not assessed, and only long-term follow-up will determine whether IL-23 inhibition can delay or prevent new bone formation.
As in the case of ustekinumab, a phase II proof-of-principle study of risankizumab in ankylosing spondylitis failed to meet the primary endpoint [75]. One posited theory is that IL-23 is involved in the initiation of disease but not in the ‘perpetuation’ of axial inflammation in spondyloarthropathy [76,77]. It is unclear how and if this would apply to axial PsA. Interestingly, a post hoc analysis of DISCOVER-1 and DISCOVER-2 reported that patients with active PsA and imaging-proven sacroiliitis who received guselkumab (regardless of the dose regimen utilised) had greater mean improvements in measures of axial disease activity scores than the patients treated with a placebo [78]. Based on these results, the STAR study, a phase IV RCT of guselkumab in biologic-naïve patients with active axial PsA, is currently recruiting participants. It will be able to determine if guselkumab truly has a disease-modifying effect on the axial skeleton in PsA (NCT04929210).
7. IL17
IL-17 is an inflammatory cytokine produced by Th17 T cells and other cells, and its presence has been demonstrated in plaque psoriasis and enthesitis [79].
The IL-17 superfamily comprises six ligands (IL-17A to IL-17F) which can bind to five subtypes of receptors (IL-17RA to IL-17RE). IL-17A is the best characterised ligand of the IL-17 family. It can exist as a homodimer or in a heterodimer with IL-17F and signals through the IL-17RA and IL-17RC receptor complexes. Once bound to a receptor, IL-17A upregulates inflammatory gene expression [80,81].
While not present in significant concentrations in RA joint fluid, IL-17A-producing conventional CD8 + T cells have been demonstrated in the joint fluid of patients with active PsA, and the titers correlate with disease activity [82,83]. Il-17A has been implicated as an amplifier of the inflammatory response in the entheses, including other cytokines released by resident mesenchymal cells [22,84,85].
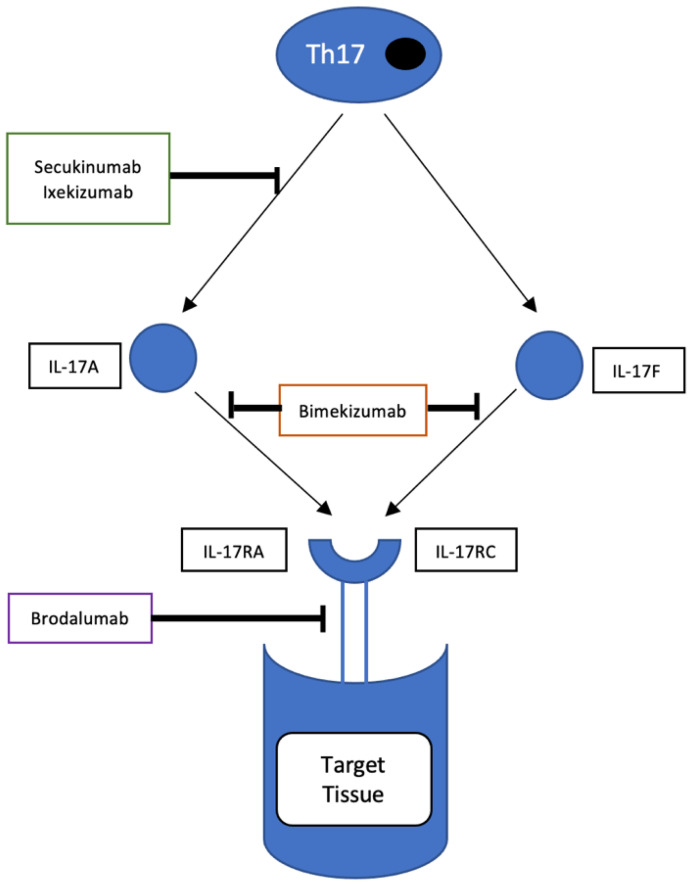
With basic science highlighting the importance of the Th17 pathway in PsA, a number of new therapies targeting this pathway have been developed. The mechanism of action of IL-17 inhibitors is shown in Figure 2. Secukinumab is a fully human anti-IL-17A monoclonal antibody approved for the treatment of PsA, psoriasis, and ankylosing spondylitis following the favorable results of several large RCTs [86,87,88,89,90,91].
Figure 2.
Mechanism of action of IL-17 inhibitors. Secukinumab and ixekizumab bind to IL-17A and inhibit its interaction with the IL-17 receptor. Bimekizumab binds to Il-17A and IL-17F and inhibits the activation of the IL-17RA/RC complex. Brodalumab binds to IL-17RA and blocks the actions of IL-17A,E,F.
The results from phase III studies, FUTURE 1 and FUTURE 2, showed that secukinumab results in rapid and significant improvements in the manifestations of PsA, sustained for up to 3 years of treatment. Clinical benefits were noted in biologic-naïve patients and those with prior TNFi treatment [88,89,92,93,94,95,96]. Perhaps not surprisingly, responses were better in those without prior exposure to biologic DMARDs. Only the TNFi non-responders who received the 300 mg dose had a statistically significant benefit compared to the placebo [97]. FUTURE 1 included intravenous loading followed by a subcutaneous regimen and did not assess a dose higher than 150 mg. FUTURE 2 utilised a subcutaneous loading and maintenance dosing of 300 mg, 150 mg, and 75 mg.
FUTURE 1 demonstrated a 50.5% and 50.0% ACR20 response at 24 weeks to secukinumab at 75 mg and 150 mg, respectively, and FUTURE 2 demonstrated a 29.3%, 51.0%, and 54.0% ACR20 response for doses at 75, 150, and 300 mg, respectively, versus 15.3% for the placebo [88,89]. Regulatory bodies subsequently approved the 300 mg and 150 mg dosing regimens for use in PsA.
The data from FUTURE 1 showed that secukinumab significantly inhibited structural damage through week 24 with benefits maintained to 2 years [96]; however, FUTURE 2 did not examine radiographic progression. This efficacy in radiographic disease progression was confirmed in FUTURE 5, which evaluated the effects of secukinumab 300 mg and 150 dose regimens on structural damage through 52 weeks. This study showed that both doses, with or without subcutaneous loading, provided sustained low rates of progression compared to the placebo [98]. IL-17A has been implicated in promoting osteoblast differentiation and thus may stimulate new bone formation [99,100]. A small open-label study of secukinumab demonstrated no worsening of catabolic and anabolic bone changes in PsA patients. This study utilised MRI and power doppler ultrasound to assess structural damage as opposed to conventional radiographs used in most clinical trials. These are promising data showing that secukinumab may have an impact on blocking new bone formation; however, the lack of a placebo control makes it challenging to interpret these results [101].
The safety data from Future 1 and 2 were similar to those in psoriasis. Importantly, there were no new cases of tuberculosis. Most adverse events were due to upper respiratory tract infections, which were only marginally increased in incidence in the secukinumab-treated group; these occurrences did not appear to be dose related. Candida infections were more prevalent in the secukinumab-treated arm [89,96].
Ixekizumab is a high-affinity monoclonal antibody that has specificity for IL-17A. Ixekizumab demonstrated its superiority over a placebo in the SPIRIT-P1 and SPIRIT-P2 phase III trials in patients with active PsA and is approved for use in PsA, psoriasis, and asSpA [102,103].
Secukinumab and ixekizumab have both demonstrated efficacy in outcome measures of axial inflammation and are therapeutic options for patients with axial PsA [104,105].
There has been a welcome increase in head-to-head randomised control trials in PsA in recent publications. The efficacy and safety of ixekizumab were compared with adalimumab; ixekizumab was superior in achieving concurrent improvement of joint and skin disease in PsA patients who experienced an inadequate response to csDMARDs [106]. McInnes et al. evaluated the efficacy and safety of secukinumab with adalimumab; secukinumab was at least as efficacious as the TNFi on musculoskeletal endpoints but exceeded on skin outcomes [107]. These two important studies have challenged the historic precedent for selecting TNFi as the initial biological agent for PsA patients and have highlighted potential alternatives with differing mechanisms of action.
Another agent targeting the IL-17 pathway is brodalumab, a fully monoclonal antibody with a unique mode of action. Brodalumab binds with high affinity to the IL-17 receptor subunit A (IL-17RA), resulting in the blockade of the action of multiple proinflammatory cytokines of the IL-17 superfamily beyond that of IL-17A alone. This agent is approved for the treatment of psoriasis by the FDA and EMA and is approved only in Japan for PsA. The findings from the phase II AMVISION-1 and AMVISION-2 trials suggest that brodalumab is a promising therapy for PsA. Approximately 30% of patients included in these two trials had prior treatment with biologics. Significantly more patients treated with brodalumab (at both doses of 140 mg and 210 mg at week 0 and 1 and every 2 weeks) reached an ACR20 response at 16 weeks compared to placebo (45.8% and 47.9% for the 140 mg and 210 mg doses, respectively, versus 20.9% in the placebo-treated group, p < 0.0001). These results were sustained at week 24. ACR50/70 and PASI75/90/100 responses were achieved by significantly greater proportions of patients who received brodalumab and this group also experienced significantly higher proportions of dactylitis and enthesitis resolution versus the placebo group. The adverse event rates in the brodalumab groups were similar to the placebo [108]. This may represent another treatment option for patients with PsA.
IL-17F has the highest structural homology with IL-17A (close to 50%), with overlapping biology with IL-17A. However, the role it plays in immune-mediated inflammatory diseases remains less clear. IL-17A and IL-17F bind to the same complex of IL-17RA and IL-17RC [109]. IL-17F has similar actions to IL-17A but has less potent properties since it has less affinity for the IL-17RA/RC complex. However, levels of IL-17F have been shown to be higher in psoriatic skin tissue and serum (30-fold higher on average) than levels of IL-17A [110]. Studies have established that neutralising both IL-17 ligands (IL-17A and IL-17F) more potently suppresses in-vitro cytokine responses and neutrophil chemotaxis than targeting either ligand alone [111]. This biological basis has informed the development of dual and bispecific IL-17A and IL-17F inhibitors, and the clinical data thus far have been encouraging, especially for bimekizumab. Data from the phase 2b BE ACTIVE study showed that Bimekizumab, a monoclonal IgG1 antibody which selectively inhibits IL-17F and IL-17A, accounted for rapid improvements in skin and joint endpoints in PsA patients [112].
The results of two phase III clinical trials of bimekizumab for PsA have been very recently reported. The BE OPTIMAL trial included only biologic naïve-patients allocated to one of three groups: bimekizumab, a placebo, or adalimumab (the reference group). However, the study was not powered to allow statistical comparisons between adalimumab and bimekizumab. Here, an ACR50 response at 16 weeks was the selected primary endpoint. The study achieved its primary endpoint, with a significantly larger proportion of patients experiencing an ACR50 at 16 weeks in the bimekizumab-treated group (44%) versus the placebo group (10%). Active treatment also inhibited radiographic progression in patients in both the overall population (a subgroup with elevated CRP) and in those with at least one bone erosion at baseline [113].
The BE COMPLETE study included patients with prior TNFi use and included two groups—a bimekizumab-treated group and a placebo-treated group. Bimekizumab was superior to the placebo in achieving an ACR50 response at 16 weeks (43% versus 7%, respectively). Moreover, bimekizumab demonstrated rapid improvements in clinical manifestations of PsA, with separation from the placebo as early as week 4 [114].
The results from these trials highlight another possible agent for PsA. However, some important questions remain, especially regarding the efficacy of bimekizumab in the resolution of dactylitis and enthesitis. BE COMPLETE did not include these domains in the secondary efficacy analyses due to low numbers of patients with dactylitis and enthesitis, resulting in a lack of statistical power to assess those endpoints adequately. As such, these domains were analysed by pooling data from the two trials, and the results were reported by BE OPTIMAL. Bimekizumab only performed modestly better than the placebo for these disease domains. Complete enthesitis resolution was seen in 50% of those treated with bimekizumab compared to 35% in the placebo group at week 16, p = 0.0083 and complete dactylitis resolution was observed in 76% in the bimekizumab group compared to 51% in the placebo group, p = 0.0022 (an odds ratio of 1.9 for enthesitis resolution; an odds ratio of 3.4 for dactylitis resolution). Disappointingly, dual blockade therapy appears to have similar efficacy in both biologic-naïve patients and those previously treated with TNFi. In fact, the ACR50 response was nearly the same in BE COMPLETE and BE OPTIMAL. While bimekizumab seems to be a new effective, safe therapy for PsA, the true advantage of combined inhibition of IL-17A and IL-17F versus IL-17A alone remains to be demonstrated. Bimekizumab was superior to other therapeutic targets when efficacy was compared in direct head-to-head trials in psoriasis, with higher rates of fungal infections than adalimumab and secukinumab [115,116,117]. It is likely that direct comparative studies of bimekizumab versus other therapies for PsA will provide a better understanding of the future role of this compound in the PsA treatment algorithm.
8. Janus Kinase (JAK) Inhibitors
The JAK family of non-receptor, intracellular tyrosine kinases comprises four members: JAK1-3 and tyrosine kinase (TYK) 2. They represent a family of intracellular signal transducers with downstream regulation of activators of transcription (STAT). The JAK-STAT signalling pathway can be activated by many important proinflammatory cytokines involved in the pathogenesis of PsA, such as those related to the IL-12/23 and the IL-17 axes [118,119,120]. Consequently, targeting the JAK-STAT pathway via small molecules, JAK inhibitors (JAKi), has a biological basis and has been the subject of intense research in PsA.
Tofacitinib, an oral JAKi which inhibits signalling through JAK3 and JAK1, is approved for use in PsA. The approval of tofacitinib for PsA was based on two phase III RCTs: OPAL Broaden, which included patients naïve to TNFi and OPAL Beyond, which included patients who had an inadequate response to previous TNFi use. [121,122] OPAL Broaden included adalimumab as an active comparator. In the TNFi-naïve patients, the efficacy of tofacitinib with respect to joint and skin responses was (disappointingly) similar to that of adalimumab (ACR20 response at 3 months was achieved in 50% of the 5 mg-tofacitinib group, 60% of the 10 mg-tofacitinib group, and 52% of the adalimumab group versus 33% in the placebo-treated group) [121]. Nevertheless, and importantly, significant improvements were also seen in the patients who had prior inadequate responses to TNFi [122].
The biology of the JAK-STAT signalling pathway is complex but it is clearly an important modulator in normal cell survival and growth [123]. Consequently, concerns have been articulated about the true selectivity of the small molecular inhibitors, and the safety of their use regarding malignancy risks and serious infections. Four cases of herpes zoster infections were reported in OPAL Broaden and three in OPAL Beyond in patients treated with tofacitinib. While these data confirm a statistically significant increased risk of herpes zoster infection, the increased clinical risk is poorly understood. An open-label long-term extension study, analysed at 3 years, which included patients from the two phase III tofacitinib PsA RCTs did not reveal any new safety signals [124]. However, further long-term safety data will be required, especially with respect to the risk of neoplasms. Furthermore, as a result of the ORAL surveillance study in RA, higher rates of major cardiovascular events and malignancies in tofacitinib-treated patients compared to TNFi-treated patients appear to have been confirmed [125].
Clinical trials assessing the efficacy and safety of various JAKi in PsA have exploded in recent years. Upadacinitib, an oral JAK1 selective inhibitor, was evaluated in a phase III trial that included patients who had an inadequate response to csDMARD [126] and those who had had inadequate responses to TNFi. [127] Upadacitinib was superior to the placebo in these trials and was non-inferior to adalimumab in SELECT-PSA1. Rates of herpes zoster were increased compared to TNFi but were not more serious than infections in those not treated with JAKi [126]. Long-term safety data are awaited regarding upadacinitib, although it is to be noted that it has been approved across the highest number of indications in its class.
Filgotinib is an oral selective JAK1 inhibitor. It showed efficacy over a placebo in a phase II clinical trial, which included patients with PsA who had an inadequate response to csDMARD [128].
TYK2 is an intracellular kinase member of the JAK family and signals through the JAK–STAT pathway. It mediates downstream signalling by numerous cytokines, including IL-23, which are targets in PsA and psoriasis. Unlike the other members of the JAK family (JAK1/2/3), the TYK2 signalling pathways are restricted to select immune pathways [129,130]. Deucravacitinib is a novel oral selective TYK2 inhibitor which binds to the regulatory domain of TYK2, thus rendering the enzyme inactive. Deucravacitinib showed promising results in a phase II trial which included patients who had an inadequate response to csDMARD, and two phase III trials are currently enrolling to further assess the results in PsA patients [131,132,133].
The second generation of JAKi (filgotinib and upadacitinib) were initially developed to increase the selectivity of inhibition while maintaining or improving efficacy. It is hoped that selective inhibition will lead to superior safety endpoints compared to non-selective JAKi, though this has not yet been confirmed in head-to-head studies.
9. Other Targets
Menon et al. reported that IL-17-producing T cells (mainly CD8 + T cells) are more abundant in the synovial fluid of those with PsA than RA. The authors also demonstrated that raised synovial fluid IL-17 + CD4-negative T cells correlated with elevated acute phase reactants, features of active power doppler ultrasound synovitis and erosive disease [82]. Abatacept, a selective T cell co-stimulation modulator fused to the extracellular domain of human cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), was studied as a potential pharmacologic intervention in PsA. However, when assessed in a RCT, abatacept only demonstrated a modest response with marginally better efficacy in patients naïve to TNFi compared to patients who had prior exposure to TNFi [134]. Abatacept is approved for use in PsA, but given its relatively low efficacy, the EULAR recommendations suggest that its use should be considered only after other bDMARD classes have failed [27].
There are other cellular and molecular targets approved for the treatment of RA and that are of potential interest in PsA which are not currently approved for the management of PsA. These include anti-B-cell, anti-IL-1, and anti-IL-6 targeted therapies. Notwithstanding the previous demonstration of B-cell aggregates [17] and the upregulation of pro-inflammatory cytokines (including IL-6) in the synovial tissue in active PsA, inhibition of these targets has led to disappointing results [18,135].
The relative lack of efficacy of these agents suggests that the presence of certain cytokines and cells at the site of inflammation does not necessarily indicate their relevance as a therapeutic target in the treatment of the disease.
10. Future Direction and Remaining Questions
The therapies available for treating PsA have evolved markedly over the past decade. Despite the important developments in drug discovery, a considerable research agenda and unanswered questions remain. (Table 2 summarises the key clinical trials evaluating the targeted therapies in PsA)
Table 2.
Clinical trials in psoriatic arthritis.
| Study | Comparison | Population | TNFi-IR (%) |
MTX Use (%) |
Primary Outcome | Primary Endpoint (Weeks) | ACR20 (%) |
|---|---|---|---|---|---|---|---|
| TNF-α inhibitors | |||||||
| Antoni et al. (IMPACT 2) (N = 200) [36] |
IFX vs. PBO | csDMARD-IR or NSAID-IR | 0 | 46 | ACR20 | 14 | 58 (IFX) vs. 11 (PBO) * |
| Mease et al. (N = 205) [35] |
ETN vs. PBO | NSAID-IR | 0 | 41 | ACR20 | 12 | 59 (ETN) vs. 15 (PBO) * |
| Mease et al. (ADEPT) (N = 313) [34] |
ADA vs. PBO | NSAID-IR | 0 | 50.5 | ACR20 | 12 | 58(ADA) vs. 14 (PBO) * |
| Kavanaugh et al. (GO-REVEAL) (N = 405) [37] |
GOL (50 mg, 100 mg) vs. PBO | NSAID-IR | 0 | 48 | ACR20 | 24 | 48 (combined) vs. 9 (PBO) * |
| Mease et al. (RAPID-PsA) (N = 409) [38] |
CTZ (200 mg Q2W, 400 mg Q4W) vs. PBO | csDMARD-IR | NR 38.9 prior TNFi | 64 | ACR20 | 12 | 58 (Q2W) 51.9 (Q4W) vs. 24.3 (PBO) * |
| IL-12, 23p40 inhibitor | |||||||
| McInnes et al. (PSUMMIT 1) (N = 615) [55] | UST (45 mg, 90 mg) vs. PBO | csDMARD-IR | 0 | 48 | ACR20 | 24 | 42.4 (45 mg) 49.5 (90 mg) 22.8 (PBO) * |
| Ritchlin et al. (PSUMMIT 2) (N = 312) [56] | UST (45 mg, 90 mg) vs. PBO | Mixed csDMARD-IR/TNFi-IR | 57.7 | 50 | ACR20 | 24 | 43.8 (combined) 20.2 (PBO) * |
| Il-23p19 inhibitors | |||||||
| Deodhar et al. (DISCOVER-1) (N = 381) [67] | GKM (Q4W, Q8W) vs. PBO | Mixed csDMARD-IR/Apremilast-IR/TNFi-IR | 12 | 55 | ACR20 | 24 | 59 (Q4W) 52 (Q8W) 22 (PBO) * |
| Mease et al. (DISCOVER-2) (N = 739) [68] | GKM (Q4W, Q8W) vs. PBO | csDMARD-IR | 0 | 60 | ACR20 | 24 | 64 (Q4W) 64 (Q8W) 33 (PBO) * |
| Coates et al. (COSMOS) (N = 285) [73] | GKM vs. PBO | TNFi-IR | 84 | 55 | ACR20 | 24 | 44.4 (GKM) vs. 19.8 (PBO) * |
| Kristensen et al. (KEEPsAKE-1) (N = 964) [141] |
RKM vs. PBO | csDMARD-IR | 0 | 65.2 | ACR20 | 24 | 57.3 (RKM) vs. 33.5 (PBO) * |
| Östör et al. (KEEPsAKE-2) (N = 443) [142] | RKM vs. PBO | Mixed csDMARD-IR/Bio-IR | 46 | 47.1 | ACR20 | 24 | 51.3 (RKM) vs. 26.5 (PBO) * |
| Il-17A inhibitors | |||||||
| Mease et al. (FUTURE 1) (N = 606) [88] | SEC (150 mg, 75 mg) vs. PBO | Mixed csDMARD-IR/TNFi-IR | 29.4 | 61 | ACR20 | 24 | 50 (150 mg) 50.5 (75 mg) 17.3 (PBO) |
| McInnes et al. (FUTURE 2) (N = 397) [89] |
SEC (300 mg, 150 mg, 75 mg) vs. PBO | Mixed csDMARD-IR/TNFi-IR | 35 | 47 | ACR20 | 24 | 54 (300 mg) 51 (150 mg) 29 (75 mg) 15 (PBO) * |
| McInnes et al. (EXCEED) (N = 853) [107] |
SEC vs. ADA | csDMARD-IR | 0 | 85 | ACR20 | 52 | 67 (SEC) 62 (ADA) |
| Mease et al. (SPIRIT-P1) (N = 417) [103] |
IXE (Q2W, Q4W) vs. PBO vs. ADA (reference arm) | csDMARD-IR | 0 | 54.2 | ACR20 | 24 | 62.1 (Q2W) 57.9 (Q4W) 30.2 (PBO) * 57.4 (ADA) |
| Nash et al. (SPIRIT-P2) (N = 363) [102] |
IXE (Q2W, Q4W) vs. PBO | TNFi-IR | 91 | 41 | ACR20 | 24 | 48 (Q2W) 53 (Q4W) 20 (PBO) * |
| IL-17RA inhibitor | |||||||
| Mease et al. (AMVISION-1 and AMVISION-2) (N = 962) [108] |
Brodalumab (140 mg, 210 mg) vs. PBO | Mixed csDMARD-IR/Bio-IR | NR | NR | ACR20 | 16 | 45.8 (140 mg) 47.9 (210 mg) 20.9 (PBO) * |
| IL-17A/F inhibitor | |||||||
| McInnes et al. (BE-OPTIMAL) (N = 852) [113] |
BKM vs. PBO vs. ADA (reference arm) | csDMARD-IR | 0 | 58 | ACR50 (44% BKZ vs. 10% PBO) * |
16 | 62.2 (BKZ) vs. 24 (PBO) * 68.6 (ADA) |
| Merola et al. (BE-COMPLETE) (N = 400) [114] | BKM vs. PBO | TNFi-IR | 88 | 43 | ACR50 (43% BKZ vs. 7% PBO) * |
16 | 67 (BKZ) vs. 15.8 (PBO) * |
| JAK inhibitors | |||||||
| Mease et al. (OPAL Broaden) (N = 422) [121] |
TOFA (5 mg BD, 10 mg BD) vs. PBO vs. ADA (reference arm) | csDMARD-IR | 0 | 84 | ACR20 | 12 | 50 (5 mg) vs. 61 (10 mg) vs. 33 (PBO) * 52% (ADA) |
| Gladman et al. (OPAL Beyond) (N = 394) [122] |
TOFA (5 mg BD, 10 mg BD) vs. PBO | TNFi-IR | 100 | 73 | ACR20 | 12 | 50 (5 mg) vs. 47 (10 mg) vs. 24 (PBO) * |
| McInnes et al. (SELECT-PsA 1) (N = 1704) [126] | UPA (15 mg, 30 mg) vs. PBO vs. ADA (reference arm) | csDMARD-IR | 0 | 84 | ACR20 | 12 | 70.6 (15 mg) vs. 78.5 (30 mg) vs. 36.2 (PBO) * 65 (ADA) |
| Mease et al. (SELECT-PSA 2) (N = 641) [127] |
UPA (15 mg, 30 mg) vs. PBO | Bio-IR | NR 92% Bio-IR |
37 | ACR20 | 12 | 56.9 (15 mg) vs. 63.8 (30 mg) vs. 24.1 (PBO) * |
| Mease et al. (EQUATOR) (N = 131) [128] |
Filgotinib vs. PBO | csDMARD-IR | 0 | 55 | ACR20 | 16 | 80 (filgotinib) vs. 33 (PBO) * |
| Other targets | |||||||
| Mease et al. (N = 203) [133] |
Deucravacitinib (6 mg, 12 mg) vs. PBO | Mixed csDMARD-IR/TNFi-IR | NR 15.8% prior TNFi | 54.7 | ACR20 | 16 | 52.9 (6 mg) vs. 62.7 (12 mg) vs. 31.8 (PBO) * |
| Mease et al. (ASTRAEA) (N = 424) [134] |
ABA vs. PBO | Mixed csDMARD-IR/TNFi-IR | 61 | 60 | ACR20 | 24 | 39.4 (ABA) vs. 22.3 (PBO) * |
* Statistically significant result; MTX, methotrexate; TNFi-IR, tumour necrosis factor inhibitor inadequate response; csDMARD-IR, conventional synthetic disease modifying anti-rheumatic drug inadequate response; Bio-IR, biologic inadequate response; NSAID-IR, non-steroidal anti-inflammatory drug inadequate response; IFX, infliximab; PBO, placebo; ETN, etanercept; ADA, adalimumab; GOL, golimumab; CTZ, certolizumab pegol; UST, ustekinumab; GKM, guselkumab; RKM, rizankisumab; SEC, secukinumab; IXE, ixekizumab; BKM, bimekizumab; TOFA, tofacitinib; UPA, upadacitinib; ABA, abatacept.
The need to individualise treatment in this heterogeneous patient group is of paramount importance. In patients with refractory disease, there is often a need to change from one bDMARD to another, and in general, drug survival has been shown to be remarkably short [136,137,138,139]. In addition to the challenge of drug survival, there is no informed strategy for selecting the optimal treatment for an individual patient based on the biology of their disease, as identified by biomarkers. Currently, the most active domain of an individual’s disease (e.g., enthesitis, nail disease, etc.) is used to select a specific target. Furthermore, patients can experience differential responses for their different psoriatic disease manifestations, an example being that therapies targeting the IL-17 pathway can result in dramatic psoriasis responses while improvement in peripheral arthritis may not be as impressive.
Immunophenotyping based upon T-helper cell subsets in the peripheral blood of PsA patients has been put forward as a treatment selection strategy. In a proof-of-principle study, using stratifying of PsA based on a peripheral lymphocyte subtype analysis to inform treatment, there was a signal for improved outcomes [140]. This study represents an exciting potential avenue into precision medicine in PsA with the opportunity to improve our patients’ outcomes. Whether this biological classification and stratification of individual patients should be carried out based on peripheral blood immunophenotyping or synovial tissue profiling and analysis is an important question that remains unanswered.
Most clinical trials assessing novel agents in PsA focus heavily on the efficacy of the therapies to treat peripheral arthritis. As a result, the efficacy relating to the management of other domains (e.g., enthesitis and dactylitis) is generally assessed as secondary endpoints in these trials. This results in challenges for physicians when selecting a therapy to treat these disease domains.
In addition to the lack of biomarkers to inform treatment selection, there is also a paucity of biomarkers to assess disease activity. Acute phase reactants are non-specific and are not always elevated in active disease and are therefore unreliable biological indicators to assess therapeutic responses and disease activity.
If the goal of treatment has not been achieved, whether switching to another immunomodulator or strategies involving the combination or ‘sequential’ use of targeted therapies with different mechanisms of action is another area of research.
11. Conclusions
TNFi was, for a long-time, established as the most effective treatment for all the various disease manifestations of PsA. However, a substantial number of patients do not respond adequately to TNFi. The plethora of new therapies that have emerged from the understanding of the role of the IL-23/IL-17 axis in the pathogenesis of PsA has offered more options for patients with PsA, but at a population level, they have not shown overall improved outcomes when compared with TNFi. Solely long-term data will determine whether these therapies can retard or halt the process of new bone formation, which causes a great deal of pain, deformity, and disability.
Studies are ongoing, and they are hoped to discover further relevant targets, broaden the selection of treatments, and ultimately help optimise patient care. However, for the moment, prevention or cure does not appear to be on the horizon.
Author Contributions
Conceptualization, S.S., C.O. and D.V.; methodology, S.S. and C.O.; resources S.S.; writing—original draft preparation, S.S., C.O. and D.V.; writing—review and editing, S.S., C.O. and D.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alinaghi F., Calov M., Kristensen L.E., Gladman D.D., Coates L.C., Jullien D., Gottlieb A.B., Gisondi P., Wu J.J., Thyssen J.P., et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019;80:251–265.e219. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Scotti L., Franchi M., Marchesoni A., Corrao G. Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;48:28–34. doi: 10.1016/j.semarthrit.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Veale D.J., Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391:2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 4.Qi F., Tan Y., Yao A., Yang X., He Y. Psoriasis to Psoriatic Arthritis: The Application of Proteomics Technologies. Front. Med. 2021;8:681172. doi: 10.3389/fmed.2021.681172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchlin C.T., Colbert R.A., Gladman D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 6.Kane D., Stafford L., Bresnihan B., FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology. 2003;42:1460–1468. doi: 10.1093/rheumatology/keg384. [DOI] [PubMed] [Google Scholar]
- 7.Haroon M., Gallagher P., FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015;74:1045. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh A., Helliwell P., Ritchlin C.T. Psoriatic Arthritis and Burden of Disease: Patient Perspectives from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Rheumatol. Ther. 2016;3:91–102. doi: 10.1007/s40744-016-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng B.C.K., Jadon D.R. Unmet needs in psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2021;35:101693. doi: 10.1016/j.berh.2021.101693. [DOI] [PubMed] [Google Scholar]
- 10.Veale D.J., Barnes L., Rogers S., FitzGerald O. Immunohistochemical markers for arthritis in psoriasis. Ann. Rheum. Dis. 1994;53:450. doi: 10.1136/ard.53.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veale D., Yanni G., Rogers S., Barnes L., Bresnihan B., Fitzgerald O. Reduced synovial membrane macrophage numbers, elam-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. 1993;36:893–900. doi: 10.1002/art.1780360705. [DOI] [PubMed] [Google Scholar]
- 12.Van Kuijk A.W.R., Reinders-Blankert P., Smeets T.J.M., Dijkmans B.A.C., Tak P.P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann. Rheum. Dis. 2006;65:1551. doi: 10.1136/ard.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeremenko N., Paramarta J.E., Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr. Opin. Rheumatol. 2014;26:361–370. doi: 10.1097/BOR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 14.Coates L.C., FitzGerald O., Helliwell P.S., Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin. Arthritis Rheum. 2016;46:291–304. doi: 10.1016/j.semarthrit.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 15.De Vlam K., Gottlieb A.B., Mease P.J. Current concepts in psoriatic arthritis: Pathogenesis and management. Acta Derm. Venereol. 2014;94:627–634. doi: 10.2340/00015555-1833. [DOI] [PubMed] [Google Scholar]
- 16.Lories R.J., McInnes I.B. Primed for inflammation: Enthesis-resident T cells. Nat. Med. 2012;18:1018–1019. doi: 10.1038/nm.2854. [DOI] [PubMed] [Google Scholar]
- 17.Veale D.J., Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open. 2015;1:e000025. doi: 10.1136/rmdopen-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mease P., Kavanaugh A., Genovese M., Ritchlin C., Rosengren S., Quistberg A. Rituximab in psoriatic arthritis provides modest clinical improvement and reduces expression of inflammatory biomarkers in skin lesions. Arthritis Rheum. 2010;62:S818. [Google Scholar]
- 19.Reece R.J., Canete J.D., Parsons W.J., Emery P., Veale D.J. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999;42:1481–1484. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Veale D.J., Ritchlin C., FitzGerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2005;64:ii26. doi: 10.1136/ard.2004.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGonagle D., Gibbon W., Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137–1140. doi: 10.1016/S0140-6736(97)12004-9. [DOI] [PubMed] [Google Scholar]
- 22.Sherlock J.P., Joyce-Shaikh B., Turner S.P., Chao C.-C., Sathe M., Grein J., Gorman D.M., Bowman E.P., McClanahan T.K., Yearley J.H., et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+ CD4− CD8− entheseal resident T cells. Nat. Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 23.Chandran V., Schentag C.T., Brockbank J.E., Pellett F.J., Shanmugarajah S., Toloza S.M.A., Rahman P., Gladman D.D. Familial aggregation of psoriatic arthritis. Ann. Rheum. Dis. 2009;68:664. doi: 10.1136/ard.2008.089367. [DOI] [PubMed] [Google Scholar]
- 24.Haroon M., Winchester R., Giles J.T., Heffernan E., FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann. Rheum. Dis. 2016;75:155. doi: 10.1136/annrheumdis-2014-205461. [DOI] [PubMed] [Google Scholar]
- 25.Pattison E., Harrison B.J., Griffiths C.E.M., Silman A.J., Bruce I.N. Environmental risk factors for the development of psoriatic arthritis: Results from a case–control study. Ann. Rheum. Dis. 2008;67:672. doi: 10.1136/ard.2007.073932. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bosch F., Coates L. Clinical management of psoriatic arthritis. Lancet. 2018;391:2285–2294. doi: 10.1016/S0140-6736(18)30949-8. [DOI] [PubMed] [Google Scholar]
- 27.Gossec L., Baraliakos X., Kerschbaumer A., de Wit M., McInnes I., Dougados M., Primdahl J., McGonagle D.G., Aletaha D., Balanescu A., et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020;79:700. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coates L.C., Soriano E.R., Corp N., Bertheussen H., Callis Duffin K., Campanholo C.B., Chau J., Eder L., Fernández-Ávila D.G., FitzGerald O., et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022;18:465–479. doi: 10.1038/s41584-022-00798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese M.C., Weinblatt M.E., Mease P.J., Aelion J.A., Peloso P.M., Chen K., Li Y., Liu J., Othman A.A., Khatri A., et al. Dual inhibition of tumour necrosis factor and interleukin-17A with ABT-122: Open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology. 2018;57:1972–1981. doi: 10.1093/rheumatology/key173. [DOI] [PubMed] [Google Scholar]
- 30.Mease P.J., Genovese M.C., Weinblatt M.E., Peloso P.M., Chen K., Othman A.A., Li Y., Mansikka H.T., Khatri A., Wishart N., et al. Phase II Study of ABT-122, a Tumor Necrosis Factor- and Interleukin-17A-Targeted Dual Variable Domain Immunoglobulin, in Patients With Psoriatic Arthritis With an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2018;70:1778–1789. doi: 10.1002/art.40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibodeaux Q., Ly K., Reddy V., Smith M.P., Liao W. Dual biologic therapy for recalcitrant psoriasis and psoriatic arthritis. JAAD Case Rep. 2019;5:928–930. doi: 10.1016/j.jdcr.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Research J., Development L. A Study of Guselkumab and Golimumab Combination Therapy in Participants with Active Psoriatic Arthritis. [(accessed on 13 December 2022)];2021 Available online: https://ClinicalTrials.gov/show/NCT05071664.
- 33.Zardin-Moraes M., da Silva A.L.F.A., Saldanha C., Kohem C.L., Coates L.C., Henrique L.R., Palominos P.E., Chakr R.M.d.S. Prevalence of Psoriatic Arthritis Patients Achieving Minimal Disease Activity in Real-world Studies and Randomized Clinical Trials: Systematic Review with Metaanalysis. J. Rheumatol. 2020;47:839. doi: 10.3899/jrheum.190677. [DOI] [PubMed] [Google Scholar]
- 34.Mease P.J., Gladman D.D., Ritchlin C.T., Ruderman E.M., Steinfeld S.D., Choy E.H., Sharp J.T., Ory P.A., Perdok R.J., Weinberg M.A. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 35.Mease P.J., Kivitz A.J., Burch F.X., Siegel E.L., Cohen S.B., Ory P., Salonen D., Rubenstein J., Sharp J.T., Tsuji W. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 36.Antoni C., Krueger G.G., de Vlam K., Birbara C., Beutler A., Guzzo C., Zhou B., Dooley L.T., Kavanaugh A. Infliximab improves signs and symptoms of psoriatic arthritis: Results of the IMPACT 2 trial. Ann. Rheum. Dis. 2005;64:1150–1157. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavanaugh A., McInnes I., Mease P., Krueger G.G., Gladman D., Gomez-Reino J., Papp K., Zrubek J., Mudivarthy S., Mack M. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four–week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–986. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 38.Mease P.J., Fleischmann R., Deodhar A.A., Wollenhaupt J., Khraishi M., Kielar D., Woltering F., Stach C., Hoepken B., Arledge T., et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann. Rheum. Dis. 2014;73:48. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mease P.J., Gladman D.D., Collier D.H., Ritchlin C.T., Helliwell P.S., Liu L., Kricorian G., Chung J.B. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial. Arthritis Rheumatol. 2019;71:1112–1124. doi: 10.1002/art.40851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun J., van der Horst-Bruinsma I.E., Huang F., Burgos-Vargas R., Vlahos B., Koenig A.S., Freundlich B. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double-blind trial. Arthritis Rheum. 2011;63:1543–1551. doi: 10.1002/art.30223. [DOI] [PubMed] [Google Scholar]
- 41.Van der Heijde D., Kivitz A., Schiff M.H., Sieper J., Dijkmans B.A., Braun J., Dougados M., Reveille J.D., Wong R.L., Kupper H. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 42.Inman R.D., Davis J.C., Jr., Heijde D.V.D., Diekman L., Sieper J., Kim S.I., Mack M., Han J., Visvanathan S., Xu Z. Efficacy and safety of golimumab in patients with ankylosing spondylitis: Results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–3412. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 43.Van der Heijde D., Dijkmans B., Geusens P., Sieper J., DeWoody K., Williamson P., Braun J. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–591. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 44.Landewé R., Braun J., Deodhar A., Dougados M., Maksymowych W., Mease P., Reveille J., Rudwaleit M., Van Der Heijde D., Stach C. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orr C., Veale D.J. Therapeutic targets in psoriatic arthritis. Int. J. Clin. Rheumatol. 2015;10:489–499. doi: 10.2217/ijr.15.52. [DOI] [Google Scholar]
- 46.Schett G. Bone Formation in Psoriatic Arthritis: A Report from the GRAPPA 2013 Annual Meeting. J. Rheumatol. 2014;41:1218. doi: 10.3899/jrheum.140173. [DOI] [PubMed] [Google Scholar]
- 47.Paine A., Ritchlin C. Bone remodeling in psoriasis and psoriatic arthritis: An update. Curr. Opin. Rheumatol. 2016;28:66–75. doi: 10.1097/BOR.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 48.Ritchlin C.T., Haas-Smith S.A., Li P., Hicks D.G., Schwarz E.M. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Investig. 2003;111:821–831. doi: 10.1172/JCI200316069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schett G., Gravallese E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lories R.J., Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum. Dis. Clin. 2012;38:555–567. doi: 10.1016/j.rdc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Mantravadi S., Ogdie A., Kraft W.K. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev. Clin. Pharmacol. 2017;10:899–910. doi: 10.1080/17512433.2017.1329009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnsson H.J., McInnes I.B. Interleukin-12 and interleukin-23 inhibition in psoriatic arthritis. Clin. Exp. Rheumatol. 2015;33:S115–S118. [PubMed] [Google Scholar]
- 53.Leonardi C.L., Kimball A.B., Papp K.A., Yeilding N., Guzzo C., Wang Y., Li S., Dooley L.T., Gordon K.B. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 54.Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N., Guzzo C., Hsu M.C., Wang Y., Li S., et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 55.McInnes I.B., Kavanaugh A., Gottlieb A.B., Puig L., Rahman P., Ritchlin C., Brodmerkel C., Li S., Wang Y., Mendelsohn A.M., et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 56.Ritchlin C., Rahman P., Kavanaugh A., McInnes I.B., Puig L., Li S., Wang Y., Shen Y.-K., Doyle M.K., Mendelsohn A.M., et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 2014;73:990. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orr C., Veale D.J. Is there a need for new agents with novel mechanisms of action in psoriatic arthritis? Ann. Rheum. Dis. 2014;73:951. doi: 10.1136/annrheumdis-2013-204934. [DOI] [PubMed] [Google Scholar]
- 58.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., Tanaka S., Kodama T., Akira S., Iwakura Y., et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van der Heijde D., Sharp J., Wassenberg S., Gladman D.D. Psoriatic arthritis imaging: A review of scoring methods. Ann. Rheum. Dis. 2005;64:ii61. doi: 10.1136/ard.2004.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poddubnyy D., Hermann K.-G.A., Callhoff J., Listing J., Sieper J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: Results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS) Ann. Rheum. Dis. 2014;73:817–823. doi: 10.1136/annrheumdis-2013-204248. [DOI] [PubMed] [Google Scholar]
- 61.Murphy C.A., Langrish C.L., Chen Y., Blumenschein W., McClanahan T., Kastelein R.A., Sedgwick J.D., Cua D.J. Divergent pro-and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherlock J.P., Cua D.J. Interleukin-23 in perspective. Rheumatology. 2021;60:iv1–iv3. doi: 10.1093/rheumatology/keab461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papp K.A., Blauvelt A., Bukhalo M., Gooderham M., Krueger J.G., Lacour J.-P., Menter A., Philipp S., Sofen H., Tyring S. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2017;376:1551–1560. doi: 10.1056/NEJMoa1607017. [DOI] [PubMed] [Google Scholar]
- 64.Blauvelt A., Papp K.A., Griffiths C.E., Randazzo B., Wasfi Y., Shen Y.-K., Li S., Kimball A.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo-and active comparator–controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017;76:405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 65.Reich K., Armstrong A.W., Foley P., Song M., Wasfi Y., Randazzo B., Li S., Shen Y.-K., Gordon K.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo-and active comparator–controlled voyage 2 trial. J. Am. Acad. Dermatol. 2017;76:418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 66.Langley R., Tsai T.F., Flavin S., Song M., Randazzo B., Wasfi Y., Jiang J., Li S., Puig L. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: Results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2018;178:114–123. doi: 10.1111/bjd.15750. [DOI] [PubMed] [Google Scholar]
- 67.Deodhar A., Helliwell P.S., Boehncke W.-H., Kollmeier A.P., Hsia E.C., Subramanian R.A., Xu X.L., Sheng S., Agarwal P., Zhou B. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–1125. doi: 10.1016/S0140-6736(20)30265-8. [DOI] [PubMed] [Google Scholar]
- 68.Mease P.J., Rahman P., Gottlieb A.B., Kollmeier A.P., Hsia E.C., Xu X.L., Sheng S., Agarwal P., Zhou B., Zhuang Y. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–1136. doi: 10.1016/S0140-6736(20)30263-4. [DOI] [PubMed] [Google Scholar]
- 69.McInnes I.B., Rahman P., Gottlieb A.B., Hsia E.C., Kollmeier A.P., Chakravarty S.D., Xu X.L., Subramanian R.A., Agarwal P., Sheng S. Efficacy and safety of guselkumab, an interleukin-23p19–specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73:604–616. doi: 10.1002/art.41553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritchlin C.T., Helliwell P.S., Boehncke W.-H., Soriano E.R., Hsia E.C., Kollmeier A.P., Chakravarty S.D., Zazzetti F., Subramanian R.A., Xu X.L. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open. 2021;7:e001457. doi: 10.1136/rmdopen-2020-001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman P., Ritchlin C.T., Helliwell P.S., Boehncke W.-H., Mease P.J., Gottlieb A.B., Kafka S., Kollmeier A.P., Hsia E.C., Xu X.L., et al. Pooled Safety Results Through 1 Year of 2 Phase III Trials of Guselkumab in Patients With Psoriatic Arthritis. J. Rheumatol. 2021;48:1815–1823. doi: 10.3899/jrheum.201532. [DOI] [PubMed] [Google Scholar]
- 72.McGonagle D., McInnes I.B., Deodhar A., Schett G., Shawi M., Kafka S., Karyekar C.S., Kollmeier A.P., Hsia E.C., Xu X.L., et al. Resolution of enthesitis by guselkumab and relationships to disease burden: 1-year results of two phase 3 psoriatic arthritis studies. Rheumatology. 2021;60:5337–5350. doi: 10.1093/rheumatology/keab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coates L.C., Gossec L., Theander E., Bergmans P., Neuhold M., Karyekar C.S., Shawi M., Noël W., Schett G., McInnes I.B. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: Results through one year of a phase IIIb, randomised, controlled study (COSMOS) Ann. Rheum. Dis. 2022;81:359. doi: 10.1136/annrheumdis-2021-220991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McInnes I.B., Rahman P., Gottlieb A.B., Hsia E.C., Kollmeier A.P., Xu X.L., Jiang Y., Sheng S., Shawi M., Chakravarty S.D., et al. Long-Term Efficacy and Safety of Guselkumab, a Monoclonal Antibody Specific to the p19 Subunit of Interleukin-23, Through Two Years: Results From a Phase III, Randomized, Double-Blind, Placebo-Controlled Study Conducted in Biologic-Naive Patients With Active Psoriatic Arthritis. Arthritis Rheumatol. 2022;74:475–485. doi: 10.1002/art.42010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baeten D., Østergaard M., Wei J.C.-C., Sieper J., Järvinen P., Tam L.-S., Salvarani C., Kim T.-H., Solinger A., Datsenko Y., et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018;77:1295. doi: 10.1136/annrheumdis-2018-213328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Tok M.N., Na S., Lao C.R., Alvi M., Pots D., Van de Sande M.G., Taurog J.D., Sedgwick J.D., Baeten D.L., Van Duivenvoorde L.M. The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin-23 signaling. Front. Immunol. 2018;9:1550. doi: 10.3389/fimmu.2018.01550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGonagle D., Watad A., Sharif K., Bridgewood C. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front. Immunol. 2021;12:614255. doi: 10.3389/fimmu.2021.614255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mease P.J., Helliwell P.S., Gladman D.D., Poddubnyy D., Baraliakos X., Chakravarty S.D., Kollmeier A.P., Hsia E.C., Xu X.L., Sheng S., et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: A post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021;3:e715–e723. doi: 10.1016/S2665-9913(21)00105-3. [DOI] [PubMed] [Google Scholar]
- 79.Huynh D., Kavanaugh A. Psoriatic arthritis: Current therapy and future approaches. Rheumatology. 2015;54:20–28. doi: 10.1093/rheumatology/keu237. [DOI] [PubMed] [Google Scholar]
- 80.Gaffen S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amatya N., Garg A.V., Gaffen S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017;38:310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon B., Gullick N.J., Walter G.J., Rajasekhar M., Garrood T., Evans H.G., Taams L.S., Kirkham B.W. Interleukin-17+ CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014;66:1272–1281. doi: 10.1002/art.38376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jandus C., Bioley G., Rivals J.P., Dudler J., Speiser D., Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 84.Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–528. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J.J., Ruddy M.J., Wong G.C., Sfintescu C., Baker P.J., Smith J.B., Evans R.T., Gaffen S.L. An essential role for IL-17 in preventing pathogen-initiated bone destruction: Recruitment of neutrophils to inflamed bone requires IL-17 receptor–dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thaçi D., Blauvelt A., Reich K., Tsai T.F., Vanaclocha F., Kingo K., Ziv M., Pinter A., Hugot S., You R., et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J. Am. Acad. Derm. 2015;73:400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 87.Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K., Puig L., Nakagawa H., Spelman L., Sigurgeirsson B. Secukinumab in plaque psoriasis—Results of two phase 3 trials. N. Engl. J. Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 88.Mease P.J., McInnes I.B., Kirkham B., Kavanaugh A., Rahman P., Van Der Heijde D., Landewé R., Nash P., Pricop L., Yuan J. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 2015;373:1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 89.McInnes I.B., Mease P.J., Kirkham B., Kavanaugh A., Ritchlin C.T., Rahman P., Van der Heijde D., Landewé R., Conaghan P.G., Gottlieb A.B. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 90.Mease P., van der Heijde D., Landewé R., Mpofu S., Rahman P., Tahir H., Singhal A., Boettcher E., Navarra S., Meiser K. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann. Rheum. Dis. 2018;77:890–897. doi: 10.1136/annrheumdis-2017-212687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baeten D., Sieper J., Braun J., Baraliakos X., Dougados M., Emery P., Deodhar A., Porter B., Martin R., Andersson M. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 92.Kavanaugh A., McInnes I.B., Mease P.J., Hall S., Chinoy H., Kivitz A.J., Wang Z., Mpofu S. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: Results from the randomized placebo-controlled FUTURE 2 study. J. Rheumatol. 2016;43:1713–1717. doi: 10.3899/jrheum.160275. [DOI] [PubMed] [Google Scholar]
- 93.McInnes I.B., Mease P.J., Ritchlin C.T., Rahman P., Gottlieb A.B., Kirkham B., Kajekar R., Delicha E.-M., Pricop L., Mpofu S. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology. 2017;56:1993–2003. doi: 10.1093/rheumatology/kex301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hueber W., Patel D.D., Dryja T., Wright A.M., Koroleva I., Bruin G., Antoni C., Draelos Z., Gold M.H., Group P.S. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 95.Mease P., Kavanaugh A., Reimold A. Sustained improvements in the signs and syptoms of active psoriatic arthritis through 3 years: Efficacy and safety results from a phase 3 trial [abstract] 2016 ACR; Proceedings of the ARHP Annual Meeting; Washington, DC, USA. 11–16 November 2016. [Google Scholar]
- 96.Kavanaugh A., Mease P.J., Reimold A.M., Tahir H., Rech J., Hall S., Geusens P., Wang Z., Pricop L., Mpofu S. Secukinumab for long-term treatment of psoriatic arthritis: A two-year followup from a phase iii, randomized, double-blind placebo-controlled study. Arthritis Care Res. 2017;69:347–355. doi: 10.1002/acr.23111. [DOI] [PubMed] [Google Scholar]
- 97.Kavanaugh A., McInnes I.B., Hall S., Chinoy H., Kivitz A., Kandala S., Patekar M., Mpofu S. THU0411 Secukinumab Efficacy in Anti-TNF-Naive and Anti-TNF-IR Patients with Psoriatic Arthritis: Results of a Phase 3 Multicenter, Double-Blind, Placebo-Controlled Study (Future 2) Ann. Rheum. Dis. 2015;74:345. doi: 10.1136/annrheumdis-2015-eular.2178. [DOI] [Google Scholar]
- 98.Van der Heijde D., Mease P.J., Landewé R.B.M., Rahman P., Tahir H., Singhal A., Boettcher E., Navarra S., Zhu X., Ligozio G., et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology. 2020;59:1325–1334. doi: 10.1093/rheumatology/kez420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Croes M., Öner F.C., van Neerven D., Sabir E., Kruyt M.C., Blokhuis T.J., Dhert W.J., Alblas J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Jo S., Wang S.E., Lee Y.L., Kang S., Lee B., Han J., Sung I.-H., Park Y.-S., Bae S.-C., Kim T.-H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 2018;20:115. doi: 10.1186/s13075-018-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kampylafka E., d’Oliveira I., Linz C., Lerchen V., Stemmler F., Simon D., Englbrecht M., Sticherling M., Rech J., Kleyer A. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: Results from the prospective PSARTROS study. Arthritis Res. Ther. 2018;20:153. doi: 10.1186/s13075-018-1653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nash P., Kirkham B., Okada M., Rahman P., Combe B., Burmester G.-R., Adams D.H., Kerr L., Lee C., Shuler C.L., et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: Results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317–2327. doi: 10.1016/S0140-6736(17)31429-0. [DOI] [PubMed] [Google Scholar]
- 103.Mease P.J., van der Heijde D., Ritchlin C.T., Okada M., Cuchacovich R.S., Shuler C.L., Lin C.Y., Braun D.K., Lee C.H., Gladman D.D. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baraliakos X., Gossec L., Pournara E., Jeka S., Mera-Varela A., Angelo S., Schulz B., Rissler M., Nagar K., Perella C., et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: Results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann. Rheum. Dis. 2021;80:582. doi: 10.1136/annrheumdis-2020-218808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deodhar A., Gladman D.D., Bolce R., Sandoval D., Park S.Y., Liu Leage S., Nash P., Poddubnyy D. POS1045 Ixekizumab efficacy on spinal pain, disease activity and quality of life in patients with psoriatic arthritis presenting with symptoms suggestive of axial involvement. Ann. Rheum. Dis. 2021;80:798. doi: 10.1136/annrheumdis-2021-eular.1570. [DOI] [Google Scholar]
- 106.Mease P.J., Smolen J.S., Behrens F., Nash P., Leage S.L., Li L., Tahir H., Gooderham M., Krishnan E., Liu-Seifert H. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann. Rheum. Dis. 2020;79:123–131. doi: 10.1136/annrheumdis-2019-215386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McInnes I.B., Behrens F., Mease P.J., Kavanaugh A., Ritchlin C., Nash P., Masmitja J.G., Goupille P., Korotaeva T., Gottlieb A.B. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395:1496–1505. doi: 10.1016/S0140-6736(20)30564-X. [DOI] [PubMed] [Google Scholar]
- 108.Mease P.J., Helliwell P.S., Hjuler K.F., Raymond K., McInnes I. Brodalumab in psoriatic arthritis: Results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 2021;80:185. doi: 10.1136/annrheumdis-2019-216835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitra A., Raychaudhuri S., Raychaudhuri S.P. IL-17 and IL-17R: An auspicious therapeutic target for psoriatic disease. Actas Dermo Sifiliogr. 2014;105:21–33. doi: 10.1016/S0001-7310(14)70015-8. [DOI] [PubMed] [Google Scholar]
- 110.Kolbinger F., Loesche C., Valentin M.-A., Jiang X., Cheng Y., Jarvis P., Peters T., Calonder C., Bruin G., Polus F. β-Defensin 2 is a responsive biomarker of IL-17A–driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017;139:923–932.e928. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 111.Glatt S., Baeten D., Baker T., Griffiths M., Ionescu L., Lawson A.D., Maroof A., Oliver R., Popa S., Strimenopoulou F. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: Evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann. Rheum. Dis. 2018;77:523–532. doi: 10.1136/annrheumdis-2017-212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ritchlin C.T., Kavanaugh A., Merola J.F., Schett G., Scher J.U., Warren R.B., Gottlieb A.B., Assudani D., Bedford-Rice K., Coarse J. Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395:427–440. doi: 10.1016/S0140-6736(19)33161-7. [DOI] [PubMed] [Google Scholar]
- 113.McInnes I.B., Asahina A., Coates L.C., Landewé R., Merola J.F., Ritchlin C.T., Tanaka Y., Gossec L., Gottlieb A.B., Warren R.B., et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: A randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL) Lancet. 2022;401:25–37. doi: 10.1016/S0140-6736(22)02302-9. [DOI] [PubMed] [Google Scholar]
- 114.Merola J.F., Landewé R., McInnes I.B., Mease P.J., Ritchlin C.T., Tanaka Y., Asahina A., Behrens F., Gladman D.D., Gossec L., et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: A randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE) Lancet. 2023;401:38–48. doi: 10.1016/S0140-6736(22)02303-0. [DOI] [PubMed] [Google Scholar]
- 115.Reich K., Warren R.B., Lebwohl M., Gooderham M., Strober B., Langley R.G., Paul C., De Cuyper D., Vanvoorden V., Madden C., et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N. Engl. J. Med. 2021;385:142–152. doi: 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 116.Warren R.B., Blauvelt A., Bagel J., Papp K.A., Yamauchi P., Armstrong A., Langley R.G., Vanvoorden V., De Cuyper D., Cioffi C., et al. Bimekizumab versus Adalimumab in Plaque Psoriasis. N. Engl. J. Med. 2021;385:130–141. doi: 10.1056/NEJMoa2102388. [DOI] [PubMed] [Google Scholar]
- 117.Reich K., Papp K.A., Blauvelt A., Langley R.G., Armstrong A., Warren R.B., Gordon K.B., Merola J.F., Okubo Y., Madden C., et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487–498. doi: 10.1016/S0140-6736(21)00125-2. [DOI] [PubMed] [Google Scholar]
- 118.Veale D.J., McGonagle D., McInnes I.B., Krueger J.G., Ritchlin C.T., Elewaut D., Kanik K.S., Hendrikx T., Berstein G., Hodge J., et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology. 2019;58:197–205. doi: 10.1093/rheumatology/key070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamaoka K., Saharinen P., Pesu M., Holt V.E., 3rd, Silvennoinen O., O’Shea J.J. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fragoulis G.E., Siebert S., McInnes I.B. Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases. Annu. Rev. Med. 2016;67:337–353. doi: 10.1146/annurev-med-051914-021944. [DOI] [PubMed] [Google Scholar]
- 121.Mease P., Hall S., FitzGerald O., van der Heijde D., Merola J.F., Avila-Zapata F., Cieślak D., Graham D., Wang C., Menon S. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 122.Gladman D., Rigby W., Azevedo V.F., Behrens F., Blanco R., Kaszuba A., Kudlacz E., Wang C., Menon S., Hendrikx T. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 123.Liu K.D., Gaffen S.L., Goldsmith M.A. JAK/STAT signaling by cytokine receptors. Curr. Opin. Immunol. 1998;10:271–278. doi: 10.1016/S0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- 124.Nash P., Coates L.C., Fleishaker D., Kivitz A.J., Mease P.J., Gladman D.D., FitzGerald O., Wang C., Wu J., Hsu M.-A., et al. Safety and efficacy of tofacitinib up to 48 months in patients with active psoriatic arthritis: Final analysis of the OPAL Balance long-term extension study. Lancet Rheumatol. 2021;3:e270–e283. doi: 10.1016/S2665-9913(21)00010-2. [DOI] [PubMed] [Google Scholar]
- 125.Ytterberg S.R., Bhatt D.L., Mikuls T.R., Koch G.G., Fleischmann R., Rivas J.L., Germino R., Menon S., Sun Y., Wang C., et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 126.McInnes I.B., Anderson J.K., Magrey M., Merola J.F., Liu Y., Kishimoto M., Jeka S., Pacheco-Tena C., Wang X., Chen L., et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021;384:1227–1239. doi: 10.1056/NEJMoa2022516. [DOI] [PubMed] [Google Scholar]
- 127.Mease P.J., Lertratanakul A., Anderson J.K., Papp K., Van den Bosch F., Tsuji S., Dokoupilova E., Keiserman M., Wang X., Zhong S., et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021;80:312. doi: 10.1136/annrheumdis-2020-218870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mease P., Coates L.C., Helliwell P.S., Stanislavchuk M., Rychlewska-Hanczewska A., Dudek A., Abi-Saab W., Tasset C., Meuleners L., Harrison P., et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): Results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:2367–2377. doi: 10.1016/S0140-6736(18)32483-8. [DOI] [PubMed] [Google Scholar]
- 129.Nogueira M., Puig L., Torres T. JAK inhibitors for treatment of psoriasis: Focus on selective TYK2 inhibitors. Drugs. 2020;80:341–352. doi: 10.1007/s40265-020-01261-8. [DOI] [PubMed] [Google Scholar]
- 130.Burke J.R., Cheng L., Gillooly K.M., Strnad J., Zupa-Fernandez A., Catlett I.M., Zhang Y., Heimrich E.M., McIntyre K.W., Cunningham M.D. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci. Transl. Med. 2019;11:eaaw1736. doi: 10.1126/scitranslmed.aaw1736. [DOI] [PubMed] [Google Scholar]
- 131.A Study to Determine the Efficacy and Safety of Deucravacitinib Compared with Placebo in Participants with Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-Modifying Anti-Rheumatic Drugs. [(accessed on 19 December 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04908202.
- 132.A Study to Determine the Efficacy and Safety of Deucravacitinib Compared with Placebo in Participants with Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-Rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment. [(accessed on 19 December 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04908189.
- 133.Mease P.J., Deodhar A.A., van der Heijde D., Behrens F., Kivitz A.J., Neal J., Kim J., Singhal S., Nowak M., Banerjee S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 2022;81:815. doi: 10.1136/annrheumdis-2021-221664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mease P.J., Gottlieb A.B., van der Heijde D., FitzGerald O., Johnsen A., Nys M., Banerjee S., Gladman D.D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 2017;76:1550. doi: 10.1136/annrheumdis-2016-210724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mease P.J., Gottlieb A.B., Berman A., Drescher E., Xing J., Wong R., Banerjee S. The Efficacy and Safety of Clazakizumab, an Anti–Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults With Active Psoriatic Arthritis. Arthritis Rheumatol. 2016;68:2163–2173. doi: 10.1002/art.39700. [DOI] [PubMed] [Google Scholar]
- 136.Egeberg A., Rosenø N.A.L., Aagaard D., Lørup E.H., Nielsen M.-L., Nymand L., Kristensen L.E., Thyssen J.P., Thomsen S.F., Cordtz R.L., et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—A nationwide cohort study from the DANBIO and DERMBIO registries. Semin. Arthritis Rheum. 2022;53:151979. doi: 10.1016/j.semarthrit.2022.151979. [DOI] [PubMed] [Google Scholar]
- 137.Murray K., Turk M., Alammari Y., Young F., Gallagher P., Saber T., Fearon U., Veale D.J. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res. Ther. 2021;23:25. doi: 10.1186/s13075-020-02380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lindström U., Di Giuseppe D., Delcoigne B., Glintborg B., Möller B., Ciurea A., Pombo-Suarez M., Sanchez-Piedra C., Eklund K., Relas H., et al. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis. Data from the EuroSpA collaboration. Ann. Rheum. Dis. 2021;80:1410. doi: 10.1136/annrheumdis-2021-220097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pina Vegas L., Penso L., Claudepierre P., Sbidian E. Long-term Persistence of First-line Biologics for Patients With Psoriasis and Psoriatic Arthritis in the French Health Insurance Database. JAMA Dermatol. 2022;158:513–522. doi: 10.1001/jamadermatol.2022.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miyagawa I., Nakayamada S., Nakano K., Kubo S., Iwata S., Miyazaki Y., Yoshikawa M., Yoshinari H., Tanaka Y. Precision medicine using different biological DMARDs based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatology. 2019;58:336–344. doi: 10.1093/rheumatology/key069. [DOI] [PubMed] [Google Scholar]
- 141.Kristensen L.E., Keiserman M., Papp K., McCasland L., White D., Lu W., Wang Z., Soliman A.M., Eldred A., Barcomb L., et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann. Rheum. Dis. 2022;81:225–231. doi: 10.1136/annrheumdis-2021-221019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Östör A., Van den Bosch F., Papp K., Asnal C., Blanco R., Aelion J., Alperovich G., Lu W., Wang Z., Soliman A.M., et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann. Rheum. Dis. 2022;81:351. doi: 10.1136/annrheumdis-2021-221048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.