Abstract
Nitroreductase (NTR) has the ability to activate nitro group-containing prodrugs and decompose explosives; thus, the evaluation of NTR activity is specifically important in pharmaceutical and environmental areas. Numerous studies have verified effective fluorescent methods to detect and image NTR activity; however, near-infrared (NIR) fluorescence probes for biological applications are lacking. Thus, in this study, we synthesized novel NIR probes (NIR-HCy-NO2 1–3) by introducing a nitro group to the hemicyanine skeleton to obtain fluorescence images of NTR activity. Additionally, this study was also designed to propose a different water solubility and investigate the catalytic efficiency of NTR. NIR-HCy-NO2 inherently exhibited a low fluorescence background due to the interference of intramolecular charge transfer (ICT) by the nitro group. The conversion from the nitro to amine group by NTR induced a change in the absorbance spectra and lead to the intense enhancement of the fluorescence spectra. When assessing the catalytic efficiency and the limit of detection (LOD), including NTR activity imaging, it was demonstrated that NIR-HCy-NO2 1 was superior to the other two probes. Moreover, we found that NIR-HCy-NO2 1 reacted with type I mitochondrial NTR in live cell imaging. Conclusively, NIR-HCy-NO2 demonstrated a great potential for application in various NTR-related fields, including NTR activity for cell imaging in vivo.
Keywords: nitroreductase, fluorescent probes, near-infrared, bioimaging, mitochondria
1. Introduction
Reductase is a kind of enzyme that chemically reduces a substrate. Nitroreductase (NTR) is a type of reductase and a type of flavoenzyme, which involves a nicotinamide adenine dinucleotide (phosphate) hydrate (NAD(P)H)-dependent reduction for nitro group-containing compounds, such as nitroaromatic and nitroheterocyclic molecules [1]. The determination of NTR activity is especially significant in vivo because most nitro group-containing compounds exhibit high cytotoxicity [2]. NTR has attracted great attention since it has started to be used as an activator of nitro group-containing prodrugs and a decomposer of explosives, such as trinitrotoluene (TNT) [3,4]. Therefore, the detection of NTR is highly significant in pharmaceutical and environmental areas. NTR is expressed in some bacteria and eukaryotic species, and it has been particularly utilized in therapeutic technologies for tumor-targeted delivery, such as cancer chemotherapy, also known as gene-directed enzyme prodrug therapy (GDEPT) [5,6,7,8,9].
NTR is classified into two different types: oxygen-insensitive type I NTR and oxygen-sensitive type II NTR. Type I NTR is mainly used as a nitroaromatic prodrug activator in GDEPT, and type II NTR is used for the selective imaging of hypoxic tumors because of its overexpression and the little interference that oxygen has under hypoxic conditions [10,11,12,13,14,15,16,17,18,19]. Further, previous studies on NTR activity fluorescence imaging have mostly focused on type II NTR [7,8,9] because fluorescence imaging for the activity of type I NTR is relatively insufficient. Previous reports have suggested the possibility of the mitochondrial existence of type I NTR based on the bacterial origin of mitochondria in fluorescence imaging [20,21,22]. As a prodrug activator, type I NTR can potentially present activity for nitroaromatic prodrugs when employing fluorescence imaging.
Functional fluorescent probes have the ability to identify and distinguish species of interest (SOI) in complex systems, such as intracellular or in vivo systems [23]. To utilize the advantages of functional fluorescent probes, various SOI have been applied to sensors and bioimaging, including for the detection and imaging of NTR. The probes for NTR detection are enzymatically activated by NTR, reducing the nitro group to the amine group, which induces a conversion from the electron-withdrawing group (EWG) to the electron-donating group (EDG) [2,24,25]. The enzymatic reduction induced by the activity of NTR results in a remarkable enhancement in the fluorescence spectra due to the intramolecular charge transfer (ICT) of the amine group, known as a strong EDG, utilizing the change in the functional group induced by NTR. However, most of the probes still suffer from the limitations of a high fluorescence background and a slow response, making the majority of probes useful only for the detection and imaging of NTR in cytoplasm. Moreover, the fluorescence probes proposed in previous studies were mostly viable in the visible range in order to assess the detection and imaging of NTR activities; however, fluorescence in the visible region is unfavorable for in vivo imaging due to the transmission and absorbance of biomolecules [26]. Therefore, near-infrared (NIR) probes are much more suitable for in vivo imaging due to their relatively low absorbance and good transmission in the NIR region.
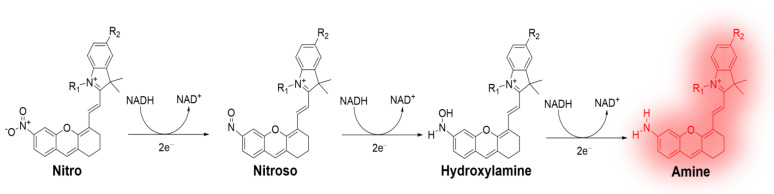
In this study, we describe the novel NIR fluorescence probe NIR-HCy-NO2 for the intracellular and in vivo imaging of NTR activity through the highly selective enzymatic reaction of NTR (Figure 1). We used a hemicyanine skeleton as a fluorogenic backbone, introducing a nitro group as a selective NTR-responsive moiety and fluorescence quencher. NIR-HCy-NO2 derivatives (NIR-HCy-NO2 2 and NIR-HCy-NO2 3) are also designed to increase water solubility, which was achieved by introducing a sulfonate (-SO3−) and quaternary ammonium group to the indolium part of NIR-HCy-NO2 [27,28]. NIR-HCy-NO2 showed a low fluorescence because of the interference of ICT by the nitro group and the reduction induced by NTR, which induced an enhancement in the fluorescence spectra due to the effect of ICT on the amine group. According to previous studies, indolium cation as a mitochondria tracker enables the verification of mitochondrial NTR [29]. This approach, which utilizes indolium cation in NIR-HCy-NO2, can be also applied to the mitochondrial imaging of NTR activity. Previously reported NTR probes have shown that the 4-nitrobenzene group, as an enzyme response moiety, should be introduced to the fluorophore backbone, and an additional elimination reaction is essential for the enhancement of the fluorescence [30,31]. In our newly synthesized NIR-HCy-NO2 derivatives, the nitro group, the enzymatically reactive part, was directly conjugated to the NIR fluorophore backbone in order to achieve a fast response. The hemicyanine skeleton-based NIR probe has been previously applied to live zebrafish larvae for in vivo imaging. However, larval zebrafish have limitations in the in vivo imaging model due to their nonmammalian status and relatively thin skin, which is highly penetrable by light. Thus, fast responsive NIR-emitted NTR sensors should be used in NTR activity-related practical applications, such as in live cell and animal imaging.
Figure 1.
Reduction mechanism of NIR-HCy-NO2 probe triggered by NTR.
Herein, the three derivatives of NIR-HCy-NO2 are applied to intracellular and in vivo mouse imaging, respectively. It was hypothesized that NIR-HCy-NO2 has high sensitivity and selectivity for NTR in the presence of NADH and that it would provide intracellular and in vivo NTR activity imaging.
2. Results
2.1. Design and Synthesis of NIR-HCy-NO2 1–3
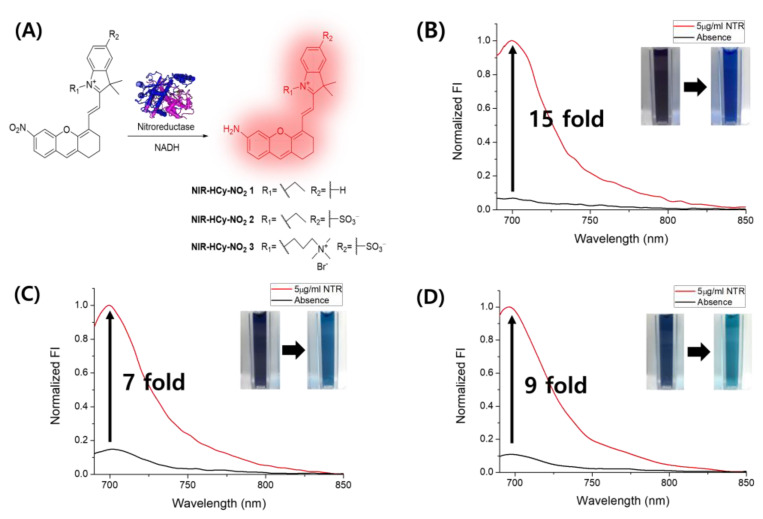
To design and synthesize NIR fluorophore for NTR detection, the hemicyanine skeleton was chosen as a fluorescence unit because of its long wavelength (longer than λ = 650 nm) and ability to minimize autofluorescence and biological damage [32,33]. First, the bottom-up approach was reported to synthesize the NIR-emitted hemicyanine structure [34], and the structural flexibility was one of the attractive points for the use of the bottom-up approach. Previously, we developed novel alkaline phosphatase (ALP)-targeted NIR fluorescent probes (NIR-Phos-1 and NIR-Phos-2) using the same synthesis approach, and the hydroxyl group, which introduced the hemicyanine skeleton, was used as an NIR fluorophore for the ALP activity bioimaging [35]. In this study, we designed NIR-HCy-NO2 derivatives, which are direct nitro group-modified hemicyanines. To achieve their synthesis, the nitro group, containing a tricyclic compound as a core intermediate, was combined with indolium derivatives which each have different functional groups (Scheme S1). NIR-HCy-NO2 2 and NIR-Hcy-NO2 3 had a better water solubility than NIR-Hcy-NO2 1 due to the introduction of sulfonate and quaternary ammonium to the indolium part in NIR-Hcy-NO2. As expected, the introduction of polar functional groups increased the water solubility of NIR-Hcy-NO2. NIR-Hcy-NO2 3 only reacted with NTR in PBS, and the NIR-Hcy-NO2 1 and NIR-Hcy-NO2 2 reactions were conducted in a cosolvent of PBS and ACN. However, NIR-HCy-NO2 1, the most nonpolar probe, responded with the highest enhancement of fluorescence by NTR in the developed probes (Figure 2).
Figure 2.
Nitroreductase (NTR)-mediated reduction and activation of NIR-HCy-NO2 (A) and fluorescence enhancement of NIR-HCy-NO2 in the presence of 5 µg/mL NTR and 50 µM NADH over 30 min (B–D). (B) NIR-HCy-NO2 1 in 1× PBS (pH 7.4, 20% (v/v) ACN), (C) NIR-HCy-NO2 2 in 1× PBS (pH 7.4, 5% (v/v) ACN), and (D) NIR-HCy-NO2 3 in 1× PBS (pH 7.4). The emission spectra were recorded using λex = 672 nm.
2.2. Optical Properties of NIR-HCy-NO2 1–3
To confirm a change in the optical properties following the enzymatic reduction induced by NTR, we examined the differences in the absorbance spectra in the absence or presence of NTR. In the absorbance spectra, NIR-HCy-NO2 1–3 absorbed at λabs = 552, 594, and 600 nm in the absence of NTR, and the absorbance peak increased at λabs = 604, 662, and 658 nm in the presence of NTR and NADH (Figure S30) [36]. To explain the relationship between the change in the absorbance spectra and the enhancement in the fluorescence spectra, the reduction of NIR-HCy-NO2 1–3 by NTR was carried out under the same conditions, and the fluorescence signals of all NIR-HCy-NO2 1–3 were enhanced 15-, 7-, and 9-fold, respectively, compared to the absence of NTR (Figure 2B–D). Additionally, following the reduction induced by NTR, the color of the NIR-HCy-NO2 1 solution changed from violet to blue, NIR-HCy-NO2 2 changed from navy blue to blue, and NIR-HCy-NO2 3 changed from navy blue to emerald. These color changes in NIR-HCy-NO2 1–3 were caused by the bathochromic effect (i.e., redshift), as confirmed in the absorbance spectra. Additionally, the red light-absorbed fluorophores were generally observed as a blue-green color in the solution; a similar trend was observed in NIR-HCy-NO2 1–3. High-resolution mass spectrometry (HR-MS) was used to show that the bathochromic effect was caused by the change from the nitro group to the amine group; NIR-HCy-NO2 1–3 were converted to hydroxylamine (NIR-HCy-NHOH) as the intermediate and were then reduced to amine (NIR-HCy-NH2) as the final product. The hydroxylamine intermediate m/z values of NIR-HCy-NO2 1–3 were calculated at 413.2224 ([NIR-HCy-NHOH 1]+), 515.1611 ([NIR-HCy-NHOH 2 + Na]+), and 293.6209 ([NIR-HCy-NHOH 3 + Na]2+) and observed to be 413.2231, 515.1613, and 293.6211, respectively. The amine product m/z values of NIR-HCy-NO2 1–3 were also calculated at 397.2274 ([NIR-Hcy-NH2 1]+), 499.1662 ([NIR-Hcy-NH2 2 + Na]+), and 285.6235 ([NIR-Hcy-NH2 3 + Na]2+) and were found to be 397.2226, 499.1636, and 285.6252, respectively. Additionally, the azoxy form of NIR-HCy-NO2 1 was found at 403.2112 ([NIR-HCy 1-N=NO-NIR-HCy 1]2+ calculated m/z = 403.2092). NIR-HCy-NO2 1–3 were reduced, which was in line with the expected process (Figure 1) and confirmed in the abovementioned results (Figures S31–S33). In previous studies, NIR-HCy-NHOH 1–3 were reduced via four-electron transfer, and NIR-HCy-NH2 1–3 were reduced through two-electron transfer from hydroxylamine intermediates. Specifically, the formation of azoxy compounds was induced by the reaction between hydroxylamine and nitroso intermediates to produce more stable azoxy compounds than the intermediate forms. This can be explained indirectly through the azoxy formation of NIR-HCy-NO2 1, in which nitroso intermediates were produced during the NTR reduction process. Specifically, the bathochromic effect and fluorescence enhancement occurred when the nitro group (a strong EWG) was reduced to an amine group (a strong EDG) by NTR. As a result, the fluorescence emissions of NIR-HCy-NO2 1–3 themselves were very weak, which is consistent with their nonemissive characteristic; this was due to the quenching effect of the six-nitro substitution on the hemicyanine skeleton. However, reductive NIR-HCy-NO2 1–3 showed fluorescent properties due to the removal of ICT interference by the nitro group (Figure 2B–D) [37,38].
To confirm the exact excitation and emission wavelengths of reduced NIR-HCy-NO2 1–3 by NTR, the emission spectra were recorded using the same excitation wavelength (λex = 672 nm) (Figure S34). All three probes emitted at longer wavelengths, in order from longest to shortest, of 3, 2, and 1 in the NIR region. Additionally, NIR-HCy-NO2 1–3 exhibited a Stokes shift of more than 20 nm, similar to the Stokes shift of other NIR dyes. Additionally, to cross-check the fluorescence-emitted form, fully reduced forms (HCy-NH2 1–3) were obtained using the chemical reduction method (Figures S35–S37). The spectra of HCy-NH2 1–3 were almost the same as the NTR reduction result (Figures S34 and S38), and the conversion from a strong EWG to a strong EDG was quite important to emit fluorescence due to the ICT effect. Moreover, kinetically, the reduction reaction of NIR-HCy-NO2 1–3 samples by NTR was dependent on time and was saturated in less than 20 min (Figure S39). Specifically, the reductive reaction of NIR-HCy-NO2 1 and NIR-HCy-NO2 3 was saturated within 10 min (Figure S39A,C).
2.3. Selectivity Study
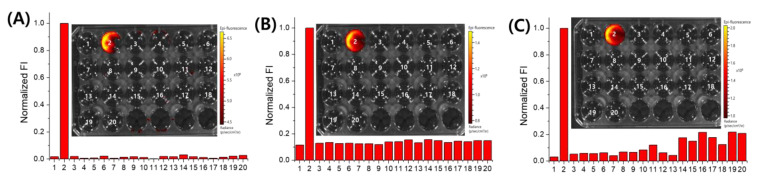
After confirming the reaction condition and optical properties of NIR-HCy-NO2 1–3, including the reaction time, excitation, and emission wavelength, we evaluated the selectivity of NIR-HCy-NO2 1–3 for NTR over other various types of biological and chemical species. The fluorometric change in NIR-HCy-NO2 1–3 by NTR and other analytes was measured. Metal cations (Na+, K+, Mg2+, Ca2+, and Hg2+), halogen anions (Br− and I−), amino acids (L-cysteine, DL-homocysteine, L-phenylalanine, and glycine), and proteins (BSA, ALP, GOx, thrombin, AchE, lysozyme, and trypsin) were used as the analytes. The fluorometric response of NIR-HCy-NO2 1–3 by NTR was much higher than the other analytes (Figure 3). Thus, NIR-HCy-NO2 1–3 reacted selectively with NTR, and, specifically, NIR-HCy-NO2 1 showed the highest fluorescence response compared to the others.
Figure 3.
Fluorescence enhancement of NIR-HCy-NO2 in the presence of alkali, alkaline earth metals, heavy metals, amino acids, and proteins. (A) NIR-HCy-NO2 1 in 1× PBS (pH 7.4, 20% (v/v) ACN), (B) NIR-HCy-NO2 2 in 1× PBS (pH 7.4, 5% (v/v) ACN), and (C) NIR-HCy-NO2 3 in 1× PBS (pH 7.4); reaction condition: 5 µM NIR-HCy-NO2 1–3 at 37 °C for 30 min; 1, NIR-HCy-NO2; 2, 5 µg/mL NTR + 50 µM NADH; 3, 50 mM NaCl; 4, 50 mM KCl; 5, 50 mM MgCl2; 6, 50 mM CaCl2; 7, 50 mM HgCl2; 8, 50 mM KBr; 9, 50 mM KI; 10, 1 mM L-cysteine; 11, 1 mM DL-homocysteine; 12, 1 mM L-phenylalanine; 13, 1 mM glycine; 14, 1 mg/mL BSA; 15, 1 U/mL GOx; 16, 1 U/mL thrombin; 17, 1 U/mL ALP; 18, 1 U/mL AchE; 19, 0.1 mg/mL lysozyme; 20, 0.1 mg/mL trypsin.
2.4. Quantitative Analysis
The quantitative analysis of NIR-HCy-NO2 1–3 was performed with various concentrations of NTR under the physiological condition (PBS (pH 7.4) in the presence of 50 μM of NADH at 37 °C). The fluorescence signals of NIR-HCy-NO2 1–3 increased the NTR concentration dependently. The trend line R-square (R2) values of NIR-HCy-NO2 1–3 were 0.9888, 0.9969, and 0.9964, respectively, and all three probes showed good linearity over the concentration range of 0.125–5 μg/mL of NTR (Figure S40). The detection limits of NIR-HCy-NO2 1–3 were 8, 114, and 181 ng/mL NTR, respectively. The sensitivity was the opposite trend to the probe polarity. Thus, the probe polarity was quite an important factor in deciding the detection limit.
2.5. Michaelis–Menten Kinetics
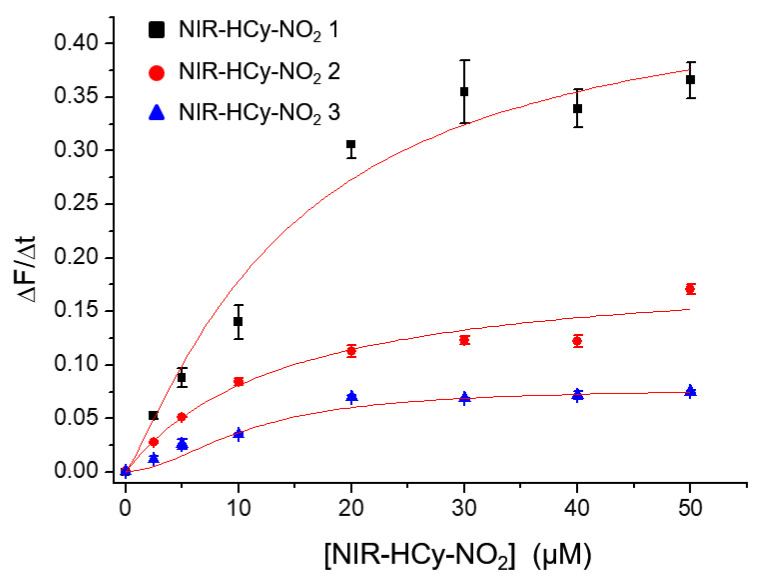
To elucidate the enzyme–substrate interaction between NTR and NIR-HCy-NO2 1–3, Michaelis–Menten kinetics were conducted. In Figure 4, the initial reaction rates of the enzymatic reduction were dependent on the concentration of NIR-HCy-NO2 1–3 (0–40 μM), and the reduction induced by NTR was saturated above 40 μM of NIR-HCy-NO2 1–3. Consequently, the kinetic curves (Figure 4) followed the Michaelis—Menten equation. In the kinetics results, the Michaelis constant (Km) and the catalytic rate constant (kcat) of NTR for NIR-HCy-NO2 1–3 were obtained.
Figure 4.
Michaelis–Menten curve of NIR-HCy-NO2 1–3 reacted with 5 µg/mL NTR and 50 µM NADH at 37 °C. NIR-HCy-NO2 1 reacted in 1× PBS (pH 7.4, 20% (v/v) ACN), NIR-HCy-NO2 2 in 1× PBS (pH 7.4, 5% (v/v) ACN), and NIR-HCy-NO2 3 in 1× PBS (pH 7.4).
As shown in Table S1, the catalytic efficiency (kcat/Km) increased in the order of NIR-HCy-NO2 1–3, and it was indicated that NIR-HCy-NO2 1 was reduced more effectively by NTR compared to NIR-HCy-NO2 2 and NIR-HCy-NO2 3. Additionally, the Km value of NTR for all three probes was lower than the kinetic values obtained in previous NTR probe studies under similar conditions. Thus, NIR-HCy-NO2 1–3 had a better affinity to NTR compared to the other probes reported in previous studies (Table S2). While NIR-HCy-NO2 1 was superior to nitrofurazone in terms of its catalytic efficiency, NIR-HCy-NO2 2 and NIR-HCy-NO2 3 were lower. The above results suggest that NIR-HCy-NO2 1 is a more suitable substrate for NTR kinetics analysis than the other two probes. It was also suggested that the charged function groups (sulfonate and quaternary ammonium) in indolium interrupt the reduction induced by NTR.
2.6. NTR Activity Imaging in Live Cells
We expanded the biological application of NIR-HCy-NO2 1–3 to NTR activity imaging in live cells. The A549 cell line was selected for confocal fluorescence images. First, the cytotoxicity tests of NIR-HCy-NO2 1–3 were performed to determine the probes’ nontoxic concentration level. All the probes were nontoxic at high concentrations (≥20 μM) for cell imaging after 1 h, suggesting that NIR-HCy-NO2 1–3 were biocompatible (Figure S41) for live cell NTR activity imaging.
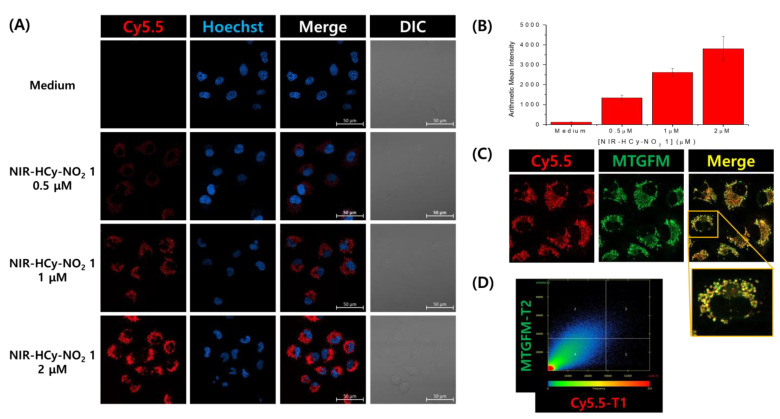
At a low concentration (<5 μM), NIR-HCy-NO2 1 induced sufficient fluorescence signals for imaging after 10 min (Figure 5A). However, the fluorescence signals of NIR-HCy-NO2 2 and NIR-HCy-NO2 3 were not detected at 40 μM after 30 min of treatment (Figure S42A). The arithmetic mean intensity of NIR-HCy-NO2 1 increased in a concentration-dependent manner; the arithmetic mean intensity of each concentration of NIR-HCy-NO2 1 increased 10.1-, 19.8-, and 28.8-fold, respectively, compared to the untreated group (Figure 5B). However, the arithmetic mean intensities of NIR-HCy-NO2 2 and NIR-HCy-NO2 3 increased up to about 6.2- and 2.7-fold, respectively (Figure S42B), which was too small a fold change considering their treatment concentrations. In the Michaelis–Menten kinetics results, the catalytic efficiencies of NIR-HCy-NO2 2 and NIR-HCy-NO2 3 were relatively lower than NIR-HCy-NO2 1, and the trend was the same as live cell imaging (Figure 4, Figure 5 and Figure S42 and Table S2). Additionally, NIR-HCy-NO2 1 was localized in mitochondria, which was overlapped with a MitoTracker (Figure 5C). The scatter plot of the two channels (MitoTracker (MTGFM) and NIR-HCy-NO2 1 (Cy5.5)) showed a linear form and tendencies to synchronize with a Mender’s colocalization coefficient of 0.9748 (Figure 5D).
Figure 5.
Confocal fluorescence images and arithmetic mean intensity graph of living A549 cells stained with various concentrations of NIR-HCy-NO2 1 (A,B). Colocalization of NIR-HCy-NO2 1 and MTGFM in living A549 cells (C,D). (A) Fluorescence and bright images of living A549 cells stained with various concentrations of NIR-HCy-NO2 1. (B) The arithmetic mean intensity dependent on the treated concentration of NIR-HCy-NO2 1. (C) Fluorescence images of NIR-HCy-NO2 1 and MTGFM and a merged image. (D) Intensity scatter plot of NIR-HCy-NO2 1 and MTGFM. The cell experiments were performed under normoxia conditions.
Interestingly, the NIR-HCy-NO2 1 signal was oxygen concentration-independent in the live cell (Figure S43). In a previous study, a Cy7-based fluorescence probe detected mitochondrial NTR in an A549 cell line under normoxia, and it was identified as type I NTR, which is oxygen-independent [20]. In this study, NIR-HCy-NO2 1 was also able to detect type I mitochondrial NTR under the same conditions and cell line. Moreover, the fluorescence signals of NIR-HCy-NO2 1 were similar under normoxia and hypoxia, and this suggested that NIR-HCy-NO2 1 was mainly reduced by type I mitochondrial NTR (Figure S43B). To verify the relationship between the signal enhancement and reductase, dicoumarol was pretreated as the reductase inhibitor, and the signal in the treated group decreased by up to 40% compared to the untreated group (Figure S44). As with previous results, NIR-HCy-NO2 1 is more suitable for NTR activity imaging than NIR-HCy-NO2 2 and NIR-HCy-NO2 3.
2.7. In Vivo Imaging
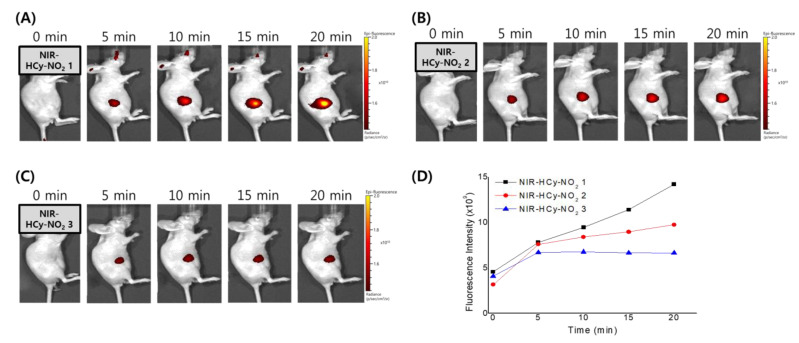
We next applied NIR-HCy-NO2 to in vivo fluorescence imaging in a xenograft model using an IVIS Spectrum system. Prior to imaging, we first determined the adequate concentration of each probe for injection. As shown in Figure S45, the fluorescent background signals from prereacted NIR-HCy-NO2 1–3 were not observed from 5–50 µM in PBS. Based on their cytotoxicity results, 20 µM was decided on for NIR-HCy-NO2 1–3, considering a sufficiently strong fluorescence signal for in vivo imaging. Then, nude mice bearing A549 xenograft tumors were intratumorally injected with each probe and monitored in a treatment time–course manner. In Figure 6D, the fluorescence signal of all three NIR-HCy-NO2 initially enhanced after the injection, and NIR-HCy-NO2 1 showed the strongest fluorescence signal among them. The fluorescence of NIR-HCy-NO2 1 was not saturated until 20 min post-injection, while that of NIR-HCy-NO2 2 and NIR-HCy-NO2 3 were saturated at 10 and 5 min, respectively. Thus, NIR-HCy-NO2 1 showed the best performance for in vivo tumor imaging among all NIR-HCy-NO2 probes due to the strong signal and the continuous reaction with the reductase (Figure 6D).
Figure 6.
In vivo imaging of tumors with NIR-HCy-NO2 in xenograft models. BALB/c nude mice bearing A549 tumors were injected intratumorally with 100 µL of PBS containing 20 µM of NIR-HCy-NO2 1 (A), NIR-HCy-NO2 2 (B), and NIR-HCy-NO2 3 (C). Fluorescence images were acquired at different time points after the injection of each probe (D).
3. Discussion
In summary, we succeeded in the design and synthesis of novel NIR off–on probes NIR-HCy-NO2 1–3 for NTR activity imaging. The water solubility of NIR-HCy-NO2 derivatives was different depending on the functional group introduced to NIR-HCy-NO2, and all NIR-HCy-NO2 1–3 reacted selectively with NTR. The fluorescence intensity and absorbance spectra of NIR-HCy-NO2 1–3 changed due to the reduction reaction induced by NTR, and the LODs of NIR-HCy-NO2 1–3 were under 200 ng/mL of NTR. Among them, the LOD of NIR-HCy-NO2 1 was the lowest with 8 ng/mL of NTR, and the catalytic efficiency was the highest at 0.22 ± 0.03 μM−1·s−1. In intracellular NTR activity imaging, the performance of NIR-HCy-NO2 1 was overwhelmingly good, and the signal was reduced with a dicoumarol treatment known as the reductase inhibitor. Additionally, the NIR-HCy-NO2 1 response was oxygen-independent, and it was considered that NIR-HCy-NO2 1 should be used to detect and image type I mitochondrial NTR as the potential target in live cells. NIR-HCy-NO2 1 showed a strong fluorescence intensity and sustained reactivity in vivo. In conclusion, NIR-HCy-NO2 1 showed a good performance among the three derivatives, and similar trends were observed in the Michaelis–Menten kinetics and intracellular and in vivo NTR activity imaging. In further studies, NIR-HCy-NO2 has the potential to be applied to various fields related to NTR, and it is able to be used for intracellular and in vivo NTR activity imaging.
4. Materials and Methods
4.1. Characterization of Optical Properties
The optical property measurements were performed in 1× phosphate buffered saline (PBS) (10 mM, pH 7.4) containing acetonitrile (ACN). NIR-HCy-NO2 1 was measured in 1× PBS (pH 7.4, 20% (v/v) ACN), NIR-HCy-NO2 2 was measured in 1× PBS (pH7.4, 5% (v/v) ACN), and NIR-HCy-NO2 3 was measured in 1× PBS (pH 7.4) without ACN, respectively. All of the optical analyses were carried out using 5 μM of NIR-HCy-NO2 1–3, and NADH was added to all three probe solutions until the amount reached 50 μM. The mixture was incubated at 37 °C for 30 min. The UV-visible spectra were measured using a spectrophotometer (DU800, Beckman Coulter, Brea, CA, USA), and the fluorescence was measured using a fluorescence spectrometer (FS-2, SCINCO, Seoul, Republic of Korea) and an imaging reader (CYTATION5, BioTek, Winooski, VT, USA), respectively. The fluorescence spectra were recorded in the range from 685 to 850 nm, with λex = 672 nm from a xenon lamp.
4.2. Mass Analysis of Reduced NIR-HCy-NO2 1–3
To prepare the reaction mixture for mass analysis, 500 μM of NIR-HCy-NO2 1–3 were reduced by 1 μM of NTR with 500 μM of NADH in PBS (10 mM, pH 7.4) at room temperature for 3 min. To quench the enzymatic reaction, β-mercaptoethanol was added to the reaction mixture until the concentration was 2%. The product mass was measured using a high-resolution mass spectrometer (micrOTOF-QII, Bruker Daltonik, Bremen, Germany) in the electrospray ionization (ESI) mode.
4.3. Cell Culture
Non-small cell lung cancer adenocarcinoma A549 cell lines were obtained from the Bioevaluation Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin (P/S). The cells were cultured at 37 °C under 5% CO2.
4.4. Cytotoxicity Assay
The cytotoxicity assay was carried out using the methylene blue staining method (Methylene blue, Sigma-Aldrich, St. Louis, MO, USA). A549 cells were seeded into 96-well cell culture plates at 2 × 104/well and preincubated in DMEM (10% (v/v) FBS and 1% (v/v) P/S). After the medium in the wells was removed, NIR-HCy-NO2 1–3 (100 μL/well) at concentrations of 0–40 μM were added to the wells of the treatment group, respectively. The cells were incubated for 1 h at 37 °C under 5% CO2 and then fixed for over 1 h by adding 10% formalin solution (50 μL/well). After each well attached to fixed cells was washed with PBS (pH 7.4), the fixed cells were stained with 2% methylene blue working solution (50% (v/v) methanol) for 1 h. The stained cells were washed strongly with distilled water and dried sufficiently at room temperature. The cells were lysed using 0.5% hydrogen chloride, and an imaging reader (CYTATION5, BioTek, Winooski, VT, USA) was used to measure the OD600 (absorbance value) of each well. Cell viability was calculated using the following formula: cell viability (%T) = At/Ac × 100 (%), where At denotes the absorbance value of the treated group, and Ac denotes the absorbance value of the untreated group.
4.5. Confocal Fluorescence Imaging in Living Cells
A549 cells (4 × 104/well) were plated on µ-Slide 4 Well (ibidi, Gräfelfing, Germany) and were allowed to adhere for 24 h. All staining procedures were carried out under the normoxia condition. The cells were incubated in serum-free DMEM at 37 °C with NIR-HCy-NO2 1 (0.5, 1, and 2 μM) for 10 min, or NIR-HCy-NO2 2 and NIR-HCy-NO2 3 (40 µM) for 30 min. Then, the cells were washed with DPBS (0.4 mL × 2 times) and were further incubated with 5 μg/mL of Hoechst 33,342 in a serum-free DMEM at 37 °C for 5 min. After washing with DPBS (0.4 mL × 3 times), fluorescence imaging was performed with an LSM 800 confocal fluorescence microscope (ZEISS, Jena, Germany) at a 40× water immersion objective lens. The fluorescence signal of the cells incubated with Hoechst 33,342 and NIR-HCy-NO2 1–3 was collected at 400–600 nm using a semiconductor laser at 405 nm as an excitation resource of Hoechst 33,342 and at 645–700 nm using a semiconductor laser at 640 nm as an excitation resource of NIR-HCy-NO2 1–3, respectively.
For the comparison of the fluorescence images between normoxia and hypoxia, the hypoxic culture was carried out for 12 h at 37 °C under 5% CO2 and 2% O2. The staining procedure of NIR-HCy-NO2 1 and Hoechst 33,352 and the condition of confocal fluorescence imaging were performed in the same way as previously outlined.
A549 cells were used for the colocalization imaging, and the confocal imaging for colocalization was carried out in the same culture and using the NIR-HCy-NO2 1 staining condition with fluorescence confocal imaging. Then, the cells were treated with 0.5 μM of MitoTacker® Green FM (MTGFM) for 30 min and were washed with DPBS (0.4 mL × 3 times). Fluorescence imaging was carried out in the same conditions outlined for the previous experiment; the fluorescence signal was collected at 400–650 nm using a semiconductor laser at 490 nm as an excitation resource of MTGFM.
4.6. Reductase Inhibition Test in Live Cells
Dicoumarol (Sigma-Aldrich) was used for reductase inhibition. A549 cells were pretreated for 4 h with dicoumarol (500 µM), and then NIR-HCy-NO2 1 (10 µM) was added for 20 min. The fluorescence intensity in A549 cells was measured using a Synergy H1 multimode plate reader (BioTek instruments, Winooski, VT, USA).
4.7. Fluorescence Imaging in Xenograft Mice
The in vivo imaging of the probes was determined using a xenograft mouse model. All animal experimental protocols were approved by the bioethics committee of the KRIBB. Six-week-old female nude mice were subcutaneously inoculated with A549 cells (5 × 106 cells) in the right flank. After two weeks, the mice were anesthetized with 2% isoflurane, and NIR-HCy-NO2 1–3 diluted in 100 µL of 1× PBS were injected into the tumor. The in vivo imaging was analyzed using the IVIS Lumina II luminescence imaging system (Caliper Life Science, Alameda, CA, USA) and Living Image software (Caliper Life Science).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076074/s1. References [39,40] are cited in Supplementary Materials.
Author Contributions
Conceptualization, S.H.L., C.S.P. and C.-S.L.; investigation, S.H.L., K.K.L., T.-H.H. and H.S.B.; validation, S.H.L. and C.-S.L.; writing—original draft preparation, S.H.L.; writing—review and editing, K.K.L., C.-S.L. and H.S.B.; project administration, C.-S.L.; funding acquisition, H.S.B. and C.-S.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animals were cared for in accordance with the guidelines provided by the Korea Research Institute of Bioscience and Biotechnology (KRIBB), and all experiments using mice were approved by KRIBB-IACUC (approval number: KRIBB-AEC-19084).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was supported by the National Research Council of Science & Technology (NST) grant by MSIT (No. CAP20013-000), the Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by MSIT (No. 2021-0-00725, Development of Monitoring Intelligence System Technology based on Multimodal Sensing for Monitoring the Risk of Infectious Diseases in Confined Spaces), and the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5472221 and KGM4542323). Finally, the authors acknowledge funding from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) and the Korea Smart Farm R&D Foundation (KosFarm) through the Smart Farm Innovation Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) and the Ministry of Science and ICT (MSIT), Rural Development Administration (RDA) (421042-04). The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Koder R.L., Miller A.-F. Steady-state kinetic mechanism, stereospecificity, substrate and inhibitor specificity of Enterobacter cloacae nitroreductase. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1998;1387:395–405. doi: 10.1016/S0167-4838(98)00151-4. [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira I.M., Bonatto D., Henriques J.A.P. Nitroreductases: Enzymes with environmental, biotechnological and clinical importance. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010;2:1008–1019. [Google Scholar]
- 3.Bryant C., DeLuca M. Purification and characterization of an oxygen-insensitive NAD (P) H nitroreductase from Enterobacter cloacae. J. Biol. Chem. 1991;266:4119–4125. doi: 10.1016/S0021-9258(20)64294-6. [DOI] [PubMed] [Google Scholar]
- 4.Hannink N., Rosser S.J., French C.E., Basran A., Murray J.A., Nicklin S., Bruce N.C. Phytodetoxification of TNT by transgenic plants expressing a bacterial nitroreductase. Nat. Biotechnol. 2001;19:1168–1172. doi: 10.1038/nbt1201-1168. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Hu L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 2009;29:29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 6.Bhaumik S., Sekar T., Depuy J., Klimash J., Paulmurugan R. Noninvasive optical imaging of nitroreductase gene-directed enzyme prodrug therapy system in living animals. Gene Ther. 2012;19:295–302. doi: 10.1038/gt.2011.101. [DOI] [PubMed] [Google Scholar]
- 7.McCormick N.G., Feeherry F.E., Levinson H.S. Microbial transformation of 2, 4, 6-trinitrotoluene and other nitroaromatic compounds. Appl. Environ. Microbiol. 1976;31:949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda Y., Ozaki M. Reduction of nitro and azo compounds by NADPH-cytochrome P-450 reductase-cytochrome c heme peptide system. Biol. Pharm. Bull. 1993;16:112–115. doi: 10.1248/bpb.16.112. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira I.M., Henriques J.A.P., Bonatto D. In silico identification of a new group of specific bacterial and fungal nitroreductases-like proteins. Biochem. Biophys. Res. Commun. 2007;355:919–925. doi: 10.1016/j.bbrc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Williams E.M., Little R.F., Mowday A.M., Rich M.H., Chan-Hyams J.V., Copp J.N., Smaill J.B., Patterson A.V., Ackerley D.F. Nitroreductase gene-directed enzyme prodrug therapy: Insights and advances toward clinical utility. Biochem. J. 2015;471:131–153. doi: 10.1042/BJ20150650. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Sun Y., Li J., Su Q., Yuan W., Dai Y., Han C., Wang Q., Feng W., Li F. Ultrasensitive near-infrared fluorescence-enhanced probe for in vivo nitroreductase imaging. J. Am. Chem. Soc. 2015;137:6407–6416. doi: 10.1021/jacs.5b04097. [DOI] [PubMed] [Google Scholar]
- 12.Huang B., Chen W., Kuang Y.-Q., Liu W., Liu X.-J., Tang L.-J., Jiang J.-H. A novel off–on fluorescent probe for sensitive imaging of mitochondria-specific nitroreductase activity in living tumor cells. Org. Biomol. Chem. 2017;15:4383–4389. doi: 10.1039/C7OB00781G. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Sun S., Li Q., Yue Y., Li Y., Shao S. A rapid response “turn-on” fluorescent probe for nitroreductase detection and its application in hypoxic tumor cell imaging. Analyst. 2015;140:574–581. doi: 10.1039/C4AN01934B. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Li X., Gao X., Zhang Y., Shi W., Ma H. Nitroreductase detection and hypoxic tumor cell imaging by a designed sensitive and selective fluorescent probe, 7-[(5-nitrofuran-2-yl) methoxy]-3 H-phenoxazin-3-one. Anal. Chem. 2013;85:3926–3932. doi: 10.1021/ac400750r. [DOI] [PubMed] [Google Scholar]
- 15.Karan S., Cho M.Y., Lee H., Lee H., Park H.S., Sundararajan M., Sessler J.M., Hong K.S. Near-infrared fluorescent probe activated by nitroreductase for in vitro and in vivo hypoxic tumor detection. J. Med. Chem. 2021;64:2971–2981. doi: 10.1021/acs.jmedchem.0c02162. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q., Wang S., Li D., Yuan J., Xu J., Shao S. A mitochondria-targeting nitroreductase fluorescent probe with large Stokes shift and long-wavelength emission for imaging hypoxic status in tumor cells. Anal. Chim. Acta. 2020;1103:202–211. doi: 10.1016/j.aca.2019.12.063. [DOI] [PubMed] [Google Scholar]
- 17.Liu F., Zhang H., Li K., Xie Y., Li Z. A Novel NIR Fluorescent Probe for Highly Selective Detection of Nitroreductase and Hypoxic-Tumor-Cell Imaging. Molecules. 2021;26:4425. doi: 10.3390/molecules26154425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon S.A., Chun J., Kang C., Lee M.H. Self-Calibrating Bipartite Fluorescent Sensor for Nitroreductase Activity and Its Application to Cancer and Hypoxic Cells. ACS Appl. Bio Mater. 2021;4:2052–2057. doi: 10.1021/acsabm.0c01085. [DOI] [PubMed] [Google Scholar]
- 19.Wan S., Vohs T., Steenwinkel T.E., White W.R., Lara-Ramirez A., Luck R.L., Werner T., Tanasova M., Liu H. Near-Infrared Fluorescent Probes with Amine-Incorporated Xanthene Platforms for the Detection of Hypoxia. ACS Appl. Bio Mater. 2022;5:4294–4300. doi: 10.1021/acsabm.2c00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevalier A., Zhang Y., Khdour O.M., Kaye J.B., Hecht S.M. Mitochondrial nitroreductase activity enables selective imaging and therapeutic targeting. J. Am. Chem. Soc. 2016;138:12009–12012. doi: 10.1021/jacs.6b06229. [DOI] [PubMed] [Google Scholar]
- 21.Gray M.W., Burger G., Lang B.F. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann J.M. Converting bacteria to organelles: Evolution of mitochondrial protein sorting. Trends Microbiol. 2003;11:74–79. doi: 10.1016/S0966-842X(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 23.Chan J., Dodani S.C., Chang C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012;4:973–984. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Race P.R., Lovering A.L., Green R.M., Ossor A., White S.A., Searle P.F., Wrighton C.J., Hyde E.I. Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone: Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem. 2005;280:13256–13264. doi: 10.1074/jbc.M409652200. [DOI] [PubMed] [Google Scholar]
- 25.Pitsawong W., Hoben J.P., Miller A.-F. Understanding the broad substrate repertoire of nitroreductase based on its kinetic mechanism. J. Biol. Chem. 2014;289:15203–15214. doi: 10.1074/jbc.M113.547117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H., Luby-Phelps K., Mickey B.E., Habib A.A., Mason R.P., Zhao D. Dynamic near-infrared optical imaging of 2-deoxyglucose uptake by intracranial glioma of athymic mice. PLoS ONE. 2009;4:e8051. doi: 10.1371/journal.pone.0008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki E., Kojima H., Nishimatsu H., Urano Y., Kikuchi K., Hirata Y., Nagano T. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J. Am. Chem. Soc. 2005;127:3684–3685. doi: 10.1021/ja042967z. [DOI] [PubMed] [Google Scholar]
- 28.Xu X., Cao Y.-C., Liu J.A., Lin Y. Quaternary Ammonium Polyamidoamine Dendrimer Modified Quantum Dots as Fluorescent Probes for p-Fluorophenoxyacetic Acid Detection in Aqueous Solution. J. Fluoresc. 2017;27:2195–2200. doi: 10.1007/s10895-017-2160-9. [DOI] [PubMed] [Google Scholar]
- 29.Xiong X., Song F., Chen G., Sun W., Wang J., Gao P., Zhang Y., Qiao B., Li W., Sun S. Construction of Long-Wavelength Fluorescein Analogues and Their Application as Fluorescent Probes. Chem. Eur. J. 2013;19:6538–6545. doi: 10.1002/chem.201300418. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., He X., Wang Z., Yang R., Shi W., Ma H. In vivo imaging and detection of nitroreductase in zebrafish by a new near-infrared fluorescence off–on probe. Biosens. Bioelectron. 2015;63:112–116. doi: 10.1016/j.bios.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Zhu D., Xue L., Li G., Jiang H. A highly sensitive near-infrared ratiometric fluorescent probe for detecting nitroreductase and cellular imaging. Sens. Actuators B Chem. 2016;222:419–424. doi: 10.1016/j.snb.2015.08.093. [DOI] [Google Scholar]
- 32.Yang J., Choi J., Bang D., Kim E., Lim E.K., Park H., Suh J.S., Lee K., Yoo K.H., Kim E.K. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells. Angew. Chem. Int. Ed. 2011;123:461–464. doi: 10.1002/ange.201005075. [DOI] [PubMed] [Google Scholar]
- 33.Pansare V.J., Hejazi S., Faenza W.J., Prud’homme R.K. Review of long-wavelength optical and NIR imaging materials: Contrast agents, fluorophores, and multifunctional nano carriers. Chem. Mater. 2012;24:812–827. doi: 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard J.-A. De novo synthesis of phenolic dihydroxanthene near-infrared emitting fluorophores. Org. Biomol. Chem. 2015;13:8169–8172. doi: 10.1039/C5OB01223F. [DOI] [PubMed] [Google Scholar]
- 35.Park C.S., Ha T.H., Kim M., Raja N., Yun H.-S., Sung M.J., Kwon O.S., Yoon H., Lee C.-S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018;105:151–158. doi: 10.1016/j.bios.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Gürses A., Açıkyıldız M., Güneş K., Gürses M.S. Dyes and Pigments. Springer; Berlin/Heidelberg, Germany: 2016. Dyes and pigments: Their structure and properties; pp. 13–29. [Google Scholar]
- 37.Misra R., Bhttacharyya S. Intramolecular Charge Transfer: Theory and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2018. [Google Scholar]
- 38.Hao M., Chi W., Wang C., Xu Z., Li Z., Liu X. Molecular origins of photoinduced backward intramolecular charge transfer. J. Phys. Chem. C. 2020;124:16820–16826. doi: 10.1021/acs.jpcc.0c04218. [DOI] [Google Scholar]
- 39.Kubin R.F., Fletcher A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982;27:455–462. doi: 10.1016/0022-2313(82)90045-X. [DOI] [Google Scholar]
- 40.Choi H.S., Nasr K., Alyabyev S., Feith D., Lee J.H., Kim S.H., Ashitate Y., Hyun H., Patonay G., Strekowski L., et al. Synthesis and In Vivo Fate of Zwitterionic Near-Infrared Fluorophores. Angew. Chem. Int. Ed. 2011;50:6258–6263. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.