Abstract
Nitric oxide (NO) and carbon monoxide (CO) represent a pair of biologically active gases with an increasingly well-defined range of effects on circulating platelets. These gases interact with platelets and cells in the vessels and heart and exert fundamentally similar biological effects, albeit through different mechanisms and with some peculiarity. Within the cardiovascular system, for example, the gases are predominantly vasodilators and exert antiaggregatory effects, and are protective against damage in myocardial ischemia-reperfusion injury. Indeed, NO is an important vasodilator acting on vascular smooth muscle and is able to inhibit platelet activation. NO reacts with superoxide anion (O2(−•)) to form peroxynitrite (ONOO(−)), a nitrosating agent capable of inducing oxidative/nitrative signaling and stress both at cardiovascular, platelet, and plasma levels. CO reduces platelet reactivity, therefore it is an anticoagulant, but it also has some cardioprotective and procoagulant properties. This review article summarizes current knowledge on the platelets and roles of gas mediators (NO, and CO) in cardioprotection. In particular, we aim to examine the link and interactions between platelets, NO, and CO and cardioprotective pathways.
Keywords: platelets, preconditioning, remote conditioning, nitrosating agents, gasotransmitters
1. Introduction
Among cardiovascular diseases, myocardial infarction remains the leading cause of death and morbidity worldwide, representing 46% of all cardiovascular deaths in men and 38% in women [1]. The major challenge in facing myocardium loss after infarcting ischemia, is to reduce the so-called ischemia reperfusion injury (IRI) to limit ischemia/hypoxia-driven oxidative stress, inflammation, and intracellular Ca2+ overload and all forms of cell death [2,3]. IRI is due to a series of events in which several mechanisms play a pivotal role. These events and mechanisms include calcium overload, dissipation of mitochondrial membrane potential, endothelial dysfunction, platelet activation, microembolization, immune activation, autophagy, and, eventually, all forms of cell death [3]. In this regard, several therapeutic approaches to spare the ischemic myocardium have been proposed and different intracellular mechanisms are proven to be involved both in the pre- and post-ischemic phase and can, therefore, be targeted to achieve a more effective protected state of the myocardium. All of these mechanisms in some way involve nitric oxide (NO) and reactive oxygen species (ROS), which also lead to the formation of reactive nitrogen species (RNS) [4,5]. In determining reperfusion injury, the first minutes of reperfusion are really important. In particular, mitochondrial permeability transition pore (MPTP) opening and subsequent cell death by apoptosis and necrosis by rupture of the cell membrane of cardiomyocytes play a central role in this phase. In triggering the long-lasting opening of MPTPs, redox stress, mitochondrial Ca2+ overload, acute restoration of a physiological pH, and adenosine triphosphate (ATP) depletion are involved [6], followed by inflammatory response [7]. Molecular oxygen reduction induces ROS production during ischemia, but, especially, during early reperfusion, and it has been proposed that these ROS induce several lesions. In particular, ROS modulate the activity of enzymes, such as cytochrome and xanthine oxidase, cyclooxygenase, and caspases, leading to catecholamine oxidation and polymorphonuclear (PMN) activation [6]. However, it is important to remember that small amounts of ROS in the early stages of reperfusion are protective, as reported by several researchers [8,9,10]. Another important gas involved in platelet function and cardioprotection is carbon monoxide (CO).
The role played by gases and platelets in IRI is double and opposite; in fact, they appear to be involved in IRI [11,12,13] but also in the cardioprotection directly [14,15] or with production of cardioprotective molecule [16,17]. It is known that in the first phase of reperfusion, platelet activation occurs with consequent accumulation in the ischemic area [18]. Platelets participate in IRI by several mechanisms: aggregation and microthrombi formation, platelet–leukocyte aggregation, release of exosomes and vasoconstrictors, plasma membrane-derived vesicles (PMVs) and apoptotic body formation, and spinal afferent nerve activation [19]. In particular, activation and subsequent platelet–leukocyte interactions and platelet aggregation with the formation of microthrombi in small cardiac vessels and capillaries exert a key role in causing cardiac tissue damage [19]. Platelet activation and interactions with leukocytes and vascular endothelium are followed by the release of the platelet granules content and, thus, the development of an inflammatory response [13]. In this scenario the response of cardioprotective maneuvers is fundamental to reduce the IRI. In particular, gasotransmitters are emerging as cardioprotective factors interconnected to platelet function. In light of this, to the best of our knowledge, this review is the first to emphasize and examine the close relationships between gases, such as NO, derivatives and carbon monoxide (CO), and platelet function and interconnections with cardioprotection. These interconnections are also examined based on the results obtained from our original studies, e.g., [8,14].
2. Cardioprotection
Myocardial cell death due to IRI is one of the main causes of morbidity and mortality in the western world which can be reduced through cardioprotection protocols. Platelets and gasotransmitters play a role in cardioprotective scenarios. The first studies in the field of cardioprotection were by Maroko and colleagues in the early 1970s, subsequently the group of Murry, Reimer, and Jennings described the phenomenon of ischemic preconditioning (IP) in 1986 [20,21,22]. Another important step toward cardioprotection is the study by Vinten Johansen’s group in which intermittent interruption of coronary blood flow in the very early stage of reperfusion leads to cardioprotection [23]. This protection was referred to as postconditioning (PostC) and consists of short cycles of IR at the end of prolonged ischemia [23]. These cardioprotective protocols are able to significantly reduce the IRI by activating signaling pathways capable of modifying cardiac function with a reduction of infarct size and mechanical dysfunction.
All these protective protocols start from the release of ligands that induce the activation of the molecular pathway in the ischemic myocardium. This ligand–receptor interaction activates complex cascades involving several molecules, e.g., membrane G protein, growth factor receptors, signaling enzymes, such as NOS/cGMP-dependent protein kinase G (PKG), protein kinase C (PKC), ATP-sensing potassium channels (KATP), and ROS [24,25]. The end effector is the MPTP in which the protective cascade prevents pore formation, leading to protection [26,27]. The first pathway involved in cardioprotection has been referred to as reperfusion injury salvage kinase (RISK) [28].
In 2009, Lecour et al. reported another important protective signaling pathway, enhancement of survival activating factor (SAFE) [29]. Other kinases have been activated in this pathway compared to RISK, especially the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathway, which inhibits MPTP opening, thereby promoting cardiac survival [29,30]. Another important and fundamental pathway involved in cardioprotection, with relation to platelets, is represented by the NO/PKG pathway [31]. This pathway is activated by NO and natriuretic peptides (NPs; e.g., ANP (atrial NP), BNP (brain NP), and CNP (C-type NP)); in particular, NO can start this pathway by activating the soluble guanylate cyclase (sGC), while NPs activate the particulate GC (pGC). Both sGC and pGC, when activated, produce cGMP as a second messenger. Undoubtedly, cGMP exerts its physiological actions largely through targeting PKG. However, the effects brought about by cGMP can be significantly different depending on its subcellular localization [31,32]. PKG induces activation of a protein on the mitochondrial outer membrane (MOM), with consequentially opening of the mitochondrial KATP channel (mitoKATP) on the mitochondrial inner membrane, thus increasing production of mitochondrial ROS. Also in this pathway, the end effector is represented by the inhibition of MPTP and reduction in cell death [31,32].
Another important cardioprotective protocol is represented by remote ischemic conditioning (RIC), in which a non-target organ or tissue is exposed to short periods of IR for conditioning with a significative reduction of cardiac infarct size [33]. This protocol induces protection if applied before (remote ischemic preconditioning, RIPC), during (remote ischemic perconditioning, RiPerC), or after (remote ischemic postconditioning, RiPostC) the myocardial ischemic insult.
Several studies reported that the signal transfer of RIC was dependent by the humoral and neuronal pathways. In particular, preclinical experiments report the possibility of achieving humoral signal transfer, in which the cardioprotection of RIC is transferred with plasma or plasma-dialysate from conditioned donors to the heart isolated from a rodent, subjected to ex vivo ischemia/reperfusion (IR). It appears that RIC attenuates platelet activation in patients with coronary artery disease after treadmill exercise [34] or after coronary angiography [35]. Although RIC attenuates platelet activation, it is unclear whether or not RIC-induced cardioprotective signal transfer involves platelets or its derivatives, such as exosomes and PMVs [15,36].
3. Some Aspects of Platelet Activation
Platelets are small anucleated cells derived from the fragmentation of megakaryocytes and represent a key component involved in the intricate process of hemostasis together with vascular endothelium, coagulation, and fibrinolysis. Platelets contain three types of granules (i.e., alpha, dense, and lysosomes) and the secretion of molecules stored in these granules in response to stimulus is able to modulate aggregation and thrombus formation [37]. Platelets may be activated by various compounds, including collagen, thromboxane A2 (TXA2), coagulation factors (thrombin), adenosine diphosphate (ADP), and serotonin, by their binding to receptors on the platelet surface. Alterations of platelet function can result in pathological consequences, such as arterial thrombosis or hemorrhage. Platelets are also known for their ability to influence immune response, tumor progression, and inflammation [38]. Specifically, during sepsis, platelet hyperactivation can exacerbate coagulation and inflammation by promoting endothelial dysfunction, neutrophil extracellular traps (NETs) formation, and generation of microthrombi [39,40]. Platelet responses include adhesion to adhesive molecules such as collagen, secretion of compounds from their granules leading to form a hemostatic plug or thrombus [40].
The process by which platelets form a plug is known as primary hemostasis, whereas secondary steps activate the procoagulant system. Platelet events involved in primary hemostasis mainly consist in adhesion, secretion, and aggregation [41]. When platelets are stimulated, hydrolysis of phosphoinositide and synthesis of eicosanoids represent two key interrelated signal transduction cascades leading to platelet activation, which will result in the elevation of intracellular calcium levels. When intraplatelet Ca2+ levels exceed a specific threshold, platelets undergo rapid shape change driven by the actin cytoskeleton and shift from the resting discoid to a flattened morphology with the extension of multiple filopodia and lamellipodia [42,43].
NO and Platelets
Platelet function is regulated by a dynamic equilibrium between agonists and inhibitory substances, including the well-documented gasotransmitter NO. NO inhibits platelets by preventing platelet adhesion, activation, aggregation, and disaggregating previously aggregated platelets [44]. The NO/cGMP/PKG pathway has been postulated as the main mechanism by which NO inhibits platelet function in vivo and in vitro [45] (Figure 1). NO acting on platelets mainly derives from endothelial cells (which represent the principal source of vascular NO) stimulated by different stimuli, including shear stress, vascular endothelial growth factor (VEGF), insulin, bradykinin, and other stimuli able to increase intracellular calcium concentration and activate eNOS. However, a large body of evidence reported platelets as sources of the two NOS isoforms, i.e., eNOS and inducible NOS (iNOS), albeit there is not unanimous agreement on both expression and enzymatic activity of NOS in platelets [46,47]. In other words, platelet ability to produce itself NO that acts to prevent also other circulating platelets has not been definitely clarified yet. Interestingly, it has been demonstrated that human platelets can be divided into two distinct populations, positive or negative, for the expression of functionally active eNOS [48], thus reflecting the heterogeneity of platelet population and megakaryocytes. Indeed, the questions about the potential NOS expression in distinct platelet populations and whether platelets themselves are able to produce NO remain still open and new experimental approaches are needed to solve them.
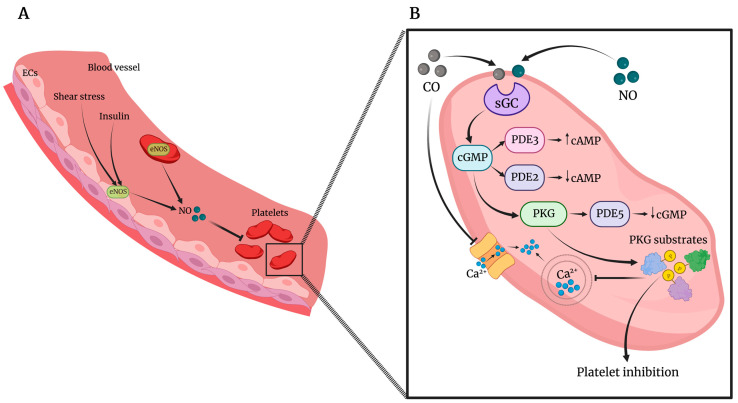
Figure 1.
(A). Nitric oxide (NO) in blood vessels is mainly synthetized by endothelial nitric oxide synthase (eNOS), expressed by endothelial cells (ECs), and it is enhanced by stimuli such as insulin and shear stress. Platelets could also express eNOS, representing, therefore, another source of NO in the blood stream. (B). In platelets, NO enhances guanosine 3’,5’-cyclic monophosphate (cGMP) synthesis through the activation of soluble guanylate cyclase (sGC). cGMP activates phosphodiesterase 3 (PDE3) and PDE2 activity, modulating platelet cyclic adenosine monophosphate (cAMP) levels. cGMP activates PKG, leading to the activation of PDE5 and reduction of cGMP levels, and is responsible for the phosphorylation of many substrates involved in mechanisms of inhibition of platelet activity, including intracellular Ca2+ release. Similarly, CO also stimulates the activation of sGC and inhibits Ca2+ entry.
NO regulates cGMP levels and the cGMP inhibitory effects predominantly depend on the PKG, the major effector of cGMP signaling in the cardiovascular system [49,50,51,52,53] (Figure 1).
Specifically, PKG interferes with platelet function by inhibiting many agonist-induced activation pathways, including the reorganization of cytoskeleton, secretion of platelet granules [45,54], the increase in intraplatelet calcium levels [55], and integrin activation [56]. Of note, besides the activation of sGC, NO as well as NO metabolites can influence platelet response by cGMP-independent mechanisms even if at higher concentrations than those needed for sGC activation [57,58]. Mechanisms of NOS activation in platelets as well as in other cells include increase in intracellular calcium levels, phosphorylation at Ser633, Ser1177, and Thr495, and the interaction with proteins such as caveolin, Hsp70, and Hsp90 [59,60].
Activation of platelet eNOS has been shown to be promoted also by β2-adrenoceptor [61], insulin [62], and acetylsalicylic acid [63] by mechanisms independent of calcium and dependent on NOS phosphorylation. Platelet NOS activity is increased by cAMP/PKA pathway activated by adenosine, forskolin, and, potentially, by every antiaggregating substance able to increase intraplatelet cAMP via receptor-dependent and -independent mechanisms [64] (Table 1).
Table 1.
The main stimuli and pathways involved in nitric oxide (NO) and carbon monoxide (CO)-induced effects on platelets. Abbreviations: eNOS endothelial nitric oxide synthase; iNOS inducible nitric oxide synthase; HO-1 heme oxygenase 1; cGMP cyclic guanosine monophosphate; HNO Nitroxyl; PKG protein kinase cGMP-dependent; IL-1β interleukin-1 β; PKC protein kinase C; ONOO− peroxynitrite.
| Gas | Stimuli | Production | Main Pathway | Effect(s) |
|---|---|---|---|---|
| NO | Shear stress, VEGF, insulin | Endothelial cells (eNOS) |
NO/cGMP/PKG | Vasodilation |
| [Ca]i increase, interaction protein (HSP70, HSP90, caveolin), insulin, β2 stimulation, acetylsalicylic acid, adenosine, and forskolin |
Platelets (eNOS) |
NO/cGMP/PKG | Reduction of adhesion, activation, and aggregation |
|
| Inflammation | Platelets iNOS |
NO/cGMP/PKG | Increased production of NO correlates with IL-1β |
|
| Conditioning ischemia | Cardiac cells (eNOS or iNOS) |
NO/cGMP/PKG S-nitrosylation |
Cardioprotection | |
| HNO | Conditioning ischemia | Cardiac cells (eNOS?) | PKCε translocation to the mitochondria |
Cardioprotection |
| ONOO− | Metabolic diseases | NO + O2− | nitration carbonylation and peroxidation |
Alteration of haemostatic functions |
| CO | Hemin and sodium arsenite | Platelet HO-1 |
cGMP/PKG | Reduction of aggregation and release of ADP and 5-HT |
| Conditioning ischemia | Cardiac cells HO-1 |
Opening of KATP channel and closure of the MPTP. |
Cardioprotection |
Other studies showed calcium-dependent platelet eNOS stimulation [65,66], as well as eNOS activation by Thr495 de-phosphorylation [67]. As mentioned, NO is an important negative regulator of signal transmission during blood platelet activation [68,69], then reduced NO synthesis or action are implicated in platelet hyperreactivity [70].
As mentioned, physiological hemostasis is the result of a dynamic state between pro- and anticoagulation processes, which can be influenced by gas mediators including NO, then any imbalance between the pro- and anti-coagulant processes may be responsible for bleeding or blood clots. An important cause of reduced protective action of NO on platelets is due to increased levels of ROS. Indeed, ROS represent a well-established second messenger for intraplatelet signal and both increased ROS synthesis and impaired ROS neutralization in platelets are deeply involved in the thrombotic process [71,72]. In particular, the rapid reaction of NO with superoxide anion (O2−) leads to peroxynitrite (ONOO−) generation [41,73], an oxidant agent able to activate or inhibit the hemostatic functions of platelets [74,75]. Targets of ONOO− action in platelets are lipids and proteins, resulting in nitration of tyrosine and carbonylation of many proteins [76,77,78] and lipid peroxidation that causes changes in the structure of platelet membrane and alterations of membrane receptors. In light of this, platelet redox imbalance found in some metabolic diseases, such as diabetes, dyslipidemia, and metabolic syndrome, may promote a prothrombotic state leading to occlusive arterial thrombi associated with myocardial infarction and stroke [72,79,80,81,82,83].
Given that insulin has been shown to inhibit platelet function also through a rapid increase in NO-mediated cGMP and cAMP [84] and platelets are targets of insulin action, in conditions of insulin-resistance such as central obesity, type 2 diabetes, hypertension, the inhibitory actions of insulin on platelets are impaired [85]. Specifically, platelets from insulin-resistant subjects show multi-step defects of NO/cGMP/PKG [86]. Since a crucial feature to initiate signaling events leading to platelet activation is the increase in intracellular Ca2+ [87] and cGMP exerts its effects on platelets mainly through a reduction of intracellular Ca2+ [54], these findings indicate the presence in insulin-resistance of alterations in Ca2+ fluxes handling. However, lifestyle interventions aimed at reducing body weight can restore platelet sensitivity to NO/cGMP/PKG accompanied by an improvement of insulin resistance and a decrease in inflammation [88,89]. Platelets isolated from obese subjects have been shown to exhibit reduced NO-dependent antiplatelet activity, both of endogenous and NO donor origin. The reason for this seems to be due to the lower insulin sensitivity typical of these subjects. From a molecular point of view, this suggests lower GC activation. In these conditions, NO deficiency is compensated by the use of antioxidant substances such as N-acetyl-L-cysteine (NAC) or antioxidant enzymes such as superoxide dismutase (SOD), which inactivates the extracellular superoxide anion [90]. Recent studies have shown that both NO donors and cGMP analogs are able to increase the antiplatelet activity of drugs ligating the P2Y12 receptor [53].
On the other hand, platelet NO production has also been recognized as a novel mechanism of platelet activation. As known, the disproportionate host inflammatory response to pathogens during sepsis may be due to the effects of a cytokine storm characterized by a huge production and action of inflammatory cytokines [91,92]. Some of these inflammatory factors may have a role in the activation of the inducible isoform of NO-synthase in platelets. For example, platelets from dengue patients show increased iNOS expression and NO production. In this clinical setting, platelet NO production correlates with the inflammatory cytokine IL-1β and severity of disease, data also confirmed by in vitro IL-1β stimulation which reproduced platelet response in vivo [93].
4. Role of NO in Cardioprotection and Platelets
The signal transduction pathways involved in cardioprotection include an important contribution both from redox signaling due to the production of ROS but also from NO itself and its derivatives, such as nitroxyl (HNO) [94,95].
Following the production of NO, important modifications of proteins by S-nitrosylation were detected. The role of NO in cardioprotection has been extensively studied. The intervention of platelets in the cardioprotective field is given by the production of molecules able to exert their protective effect by directly or indirectly inducing the production of NO [96,97].
An important molecule of platelet origin, platelet activating factor phosphoglyceride (PAF), is able to act as an autocrine/paracrine mediator on various experimental models, including cardiomyocytes and isolated hearts. While at high concentrations (1–10 nmol/L) it shows direct and indirect negative effects on the heart, at low concentrations (pM), PAF demonstrates a protective effect, similar to ischemic preconditioning [12,16]. The cardioprotective action of PAF is due to the activation of the RISK pathway, particularly with PKC/protein kinase B (Akt)/NOS involvement [16,98]. The cardioprotective action exerted by low doses of PAF also depends on NO-mediated S-nitrosylation of proteins involved in calcium transport, such as L-type Ca2+ channels; thus, reducing Ca2+ overload during myocardial ischemia–reperfusion [98]. Platelets allow the release of sphingosine-1-phosphate (S1P) produced from membrane sphingosine through the action of sphingosine kinase [17].
The protective action of S1P takes place directly through the S1P1, S1P2, and S1P3 receptors present in cardiomyocytes, with consequent activation of the RISK and enhancement of survival activating factor (SAFE) protective pathways [36]. S1P itself is an important activator of endothelial NO synthase (eNOS) at the platelet level [99]. It is well known that purinergic type 2 receptor subtypes (ADP-binding P2Y1, purinergic Y-type 12 (P2Y12), and ATP-binding P2X1) are involved in both aggregation and platelet shape change. Recently, it has been observed that P2Y12 inhibitors, including prasugrel [100], cangrelor [101], and ticagrelor [102], also display cardioprotective effects [103]. Currently, the potential cardioprotective mechanism of the action of P2Y12 inhibitors is not fully understood. Their cardioprotective action could be due to phosphorylation of sphingosine, activation of PI3k/Akt signaling pathways, and/or blockade of the ENT1 transporter, the adenosine transported, resulting in increased tissue levels of adenosine, thereby reducing cardiac damage, by mechanisms other than those attributed to inhibition of the NLRP3 complex [104,105,106,107]. Recent studies have shown that in patients aged 70 years and older at high risk of bleeding, clopidogrel is a favorable alternative to ticagrelor and prasugrel, as it results in fewer bleeding events without an increase in the combined endpoint of death from all causes, myocardial infarction, stroke, and hemorrhage [103,108].
5. The Role of CO in Cardioprotection and Platelet
Differently from NO, only a limited number of studies have been carried out on CO effects on platelets and CO and cardioprotection.
5.1. CO and Cardioprotection
The cardioprotective action of CO is mediated by the opening of KATP and consequent inhibition of the opening of the MPTP. It has been observed that CO donors (CORM) [109] demonstrate a protective effect, reporting a certain influence of sex on cardioprotection. In fact, as known from the literature, sex determines the expression of specific genes and proteins involved in protection of mitochondrial and myocardial function, such as Akt [109].
The protective action exerted by CO at low doses allows the maintenance of mitochondrial function, in fact, it has been observed that in these conditions it is able to maintain the mitochondrial membrane potential stable, which is altered in ischemia/reperfusion injury [97].
It has also been reported that CO is able to modulate the synthesis of mitochondrial ROS, the activity of some hemoproteins, including cytochrome c, and inflammation (inflammasome-dependent) [110,111]. Although mitochondrial ROS are involved in cardioprotection [8,9,10,112] and mitochondrial ROS induced by NO are cardioprotective [113], it is not known if ROS generated by exogenous CO are cardioprotective.
5.2. CO and Platelets
Although early studies showed stimulating CO effects on platelet aggregation, more recent studies have indicated a CO ability to inhibit platelet aggregation and release of ADP and serotonin from their granules [114,115] (Table 1).
CO and NO share some chemical and biological properties [116]. Indeed, exogenously added CO inhibits platelet aggregation mainly by elevating intracellular levels of cGMP [116]. Gaseous CO shows antiaggregating properties with similarity with NO in increasing sGC activity as result of direct binding of CO to the iron present in the heme moiety of sGC, even if CO is 30–100 times less potent than NO [115,117]. There is agreement on the concept that the inhibitory actions of CO on platelets are relatively low in comparison to those exerted by the endothelium-released agents NO and prostacyclin [118]. Actually, only high concentrations of gaseous CO (100%) seem to reduce platelet aggregation via sGC activation [117].
Platelets are the target but also the source of CO given that platelets express heme oxygenase 1 (HO-1) and are involved in multiple steps of heme and bilirubin metabolism [119]. In the presence of HO-1 activators, such as hemin- and sodium arsenite, platelet agonist-induced aggregations are reduced and margination and rolling are prevented, these effects are abolished by the HO-1 inhibitor zinc protoporphyrin IX (ZnPP-IX) and reproduced by CO [120,121]. Even if under basal condition, HO-1 does not significantly influence platelet-dependent clot formation in vivo, in the presence of increased HO-1 production, platelet-dependent thrombus formation is suppressed [122]. These findings induced the authors to suggest that the enhanced HO-1 expression may be a mechanism able to reduce platelet activation under prothrombotic states. A study using HO-1 knockout mice found normal platelet number, bleeding time, and platelet aggregating characteristics, but accelerated thrombosis, at least partially, due to platelet activation, which was rescued by inhaled CO [123] or CO-donor [124].
The unexpected physiological roles of CO in the cardiovascular system have justified the development of CO-releasing compounds [125] and the evaluation of their effects also on platelet function. Actually, CO-donor compounds have been shown to effectively inhibit human platelets without involving the activation of sGC [114] even if NO- and CO-mediated effects on platelets seem to be interlinked given that the inhibition of sGC increases the inhibitory CO effects.
CO is able to reduce the calcium signal elicited by platelet agonists by a direct effect on calcium entry [126], thus, confirming a role for HO activity in modulating platelet response. Different mechanisms could explain CO effect on intraplatelet calcium levels. On the one hand, CO can induce a cGMP-mediated decrease in calcium release from intracellular stores or an acceleration in the rate of its back-sequestration. On the other hand, CO shows the ability to directly inhibit the pathway involved in calcium entry [126]. The direct role of CO on capacitative calcium entry may be responsible for its antiaggregatory action.
Other cGMP-independent mechanisms by which CO-donors can inhibit platelet function include the CO ability to interfere with glycoprotein-mediated HS1 phosphorylation, a signaling molecule involved downstream of glycoprotein activation. In particular, it has been shown that during lipopolysaccharide (LPS)-induced platelet activation, the signal transmitted between membrane glycoproteins and HS1 is suppressed by CO-releasing molecules [127,128].
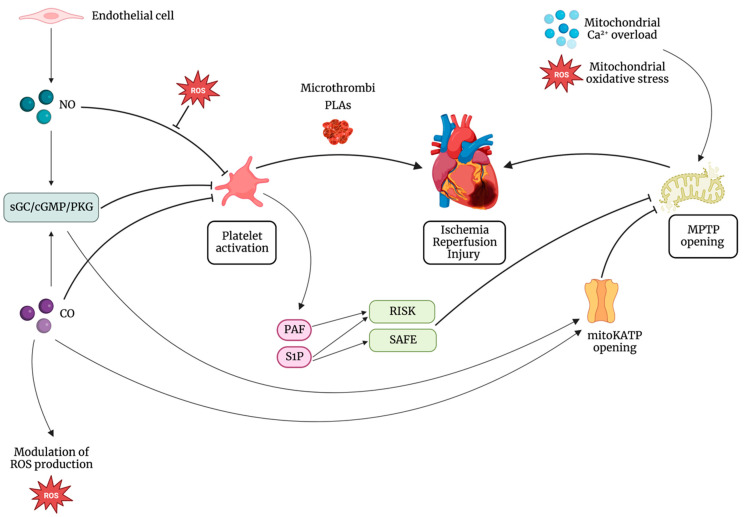
The effect of CO on platelets may be also due to its effect on the cytochrome P450 enzymes with subsequent prevention of generation of arachidonic acid, a powerful proaggregating agent [129,130]. Figure 2 summarizes platelet–gases interactions in the cardioprotective scenario.
Figure 2.
Mitochondrial permeability transition pore (MPTP) opening is the main end effector in ischemia reperfusion injury (IRI). On one side, platelets contribute to IRI mainly by microthrombi formation and platelet-leukocyte aggregates (PLAs). On the other side, molecules of platelet origin, such as platelet activating factor phosphoglyceride (PAF) and sphingosine-1-phosphate (S1P), lead to the activation of cardioprotective pathways, such as the reperfusion injury salvage kinase (RISK) pathway and the survivor activating factor enhancement (SAFE) pathway, all targeting inhibition of MPTP opening. Besides, the interaction between platelets and gasotransmitters has a central role in cardioprotection. Nitric oxide (NO) activates the soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP)/cGMP-dependent protein kinase (PKG) pathway, leading to the inhibition of platelet adhesion, activation, and aggregation. NO can inhibit platelet response also by cGMP-independent mechanisms. NO inhibitory effect on platelets is reduced in the presence of increased levels of reactive oxygen species (ROS). Also, PKG leads to the opening of the mitochondrial ATP-dependent K+ channel (mitoKATP) and subsequent inhibition of MPTP. Carbon monoxide (CO) exerts its cardioprotective action triggering the opening of mitoKATP with consequent inhibition of the opening of MPTP and modulating mitochondrial ROS production. Furthermore, it can inhibit platelet activation both by cGMP-dependent and cGMP-independent mechanisms.
6. Conclusions
After years of intensive basic research, in vivo studies and ongoing clinical trials have provided evidence of the potential clinical relevance of the application of exogenous gasotransmitters and modulation of their endogenous production. However, large-scale clinical application is finding it difficult to be implemented, although the importance of gasotransmitter interaction has been suggested as a potential therapeutic strategy.
Gasotransmitters are among the molecules that have been shown to play a central role in triggering ischemic preconditioning [94,131,132] and mediating the effects of postconditioning [133,134,135]. Complex signaling pathways act synergistically in providing cardioprotection, and gasotransmitters are no exception. Several substrates, for example, undergo post-transcriptional modulation through nitrosylation of specific residues, and complex interactions between gasses have been demonstrated in several experimental models. It seems clear that most cardioprotective signaling pathways share an involvement of mitochondria, and several mitochondrial components have been shown to be selectively targeted by gases [136,137]. We have seen that two gasotransmitters, namely NO and CO, affect both platelets and cardioprotective pathways (Figure 2). Other gases, such as ROS, RNS, and H2S, are important in this field. Future studies may investigate whether the role of gases on platelets is necessary for cardioprotection.
Acknowledgments
The authors of the present manuscript are supported by grants from the Department of Clinical and Biological Sciences of Turin University (PAGP_RILO_22; PENC_RILO_22 and RUSI_RILO_22) to P.P., C.P. and I.R., and by Fondo di Beneficenza Intesa San Paolo (PAGP_RIC_COMP_21_01) to P.P.
Abbreviations
| ADP | adenosine diphosphate |
| ANP | atrial NP |
| ATP | adenosine triphosphate |
| BNP | brain NP |
| CNP | C-type NP |
| CO | carbon monoxide |
| CORM | CO donors |
| eNOS | endothelial NOS |
| HNO | nitroxyl |
| HO-1 | heme oxygenase 1 |
| iNOS | inducible NOS |
| IP | ischemic preconditioning |
| IR | ischemia reperfusion |
| IRI | ischemia reperfusion injury |
| JAK | Janus kinase |
| KATP | ATP-sensing potassium channels |
| LPS | lipopolysaccharide |
| mitoKATP | mitochondrial KATP channel |
| MOM | mitochondrial outer membrane |
| MPTP | mitochondrial permeability transition pore |
| NAC | N-acetyl-L-cysteine |
| NETs | neutrophil extracellular traps |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NPs | natriuretic peptides |
| O2− | superoxide anion |
| ONOO− | peroxynitrite |
| P2Y12 | purinergic Y-type 12 |
| PAF | platelet activating factor phosphoglyceride |
| pGC | particulate GC |
| PKC | protein kinase C |
| PKG | cGMP-dependent protein kinase |
| PMN | polymorphonuclear |
| PMVs | plasma membrane-derived vesicles |
| PostC | Postconditioning |
| RIC | remote ischemic conditioning |
| RIPC | remote ischemic preconditioning |
| RiPerC | remote ischemic perconditioning |
| RiPostC | remote ischemic postconditioning |
| RISK | reperfusion injury salvage kinase |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| SAFE | enhancement of survival activating factor |
| sGC | soluble guanylate cyclase |
| SOD | superoxide dismutase |
| STAT3 | signal transducer and activator of transcription 3 |
| TXA2 | thromboxane A2 |
| VEGF | vascular endothelial growth factor |
| ZnPP-IX | zinc protoporphyrin IX |
Author Contributions
P.P., C.P. and I.R. conceived and wrote the article; C.B. and E.M. revised the manuscript and conceived figures and table. P.P. and C.P. share the senior investigation authorship for this manuscript. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Yellon D.M., Hausenloy D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Davidson S.M., Adameová A., Barile L., Cabrera-Fuentes H.A., Lazou A., Pagliaro P., Stensløkken K.-O., Garcia-Dorado D. EU-Cardioprotection Cost Action (CA16225) Mitochondrial and Mitochondrial-Independent Pathways of Myocardial Cell Death during Ischaemia and Reperfusion Injury. J. Cell. Mol. Med. 2020;24:3795–3806. doi: 10.1111/jcmm.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J., Druhan L.J., Zweier J.L. Reactive Oxygen and Nitrogen Species Regulate Inducible Nitric Oxide Synthase Function Shifting the Balance of Nitric Oxide and Superoxide Production. Arch. Biochem. Biophys. 2010;494:130–137. doi: 10.1016/j.abb.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hałucha K., Rak-Pasikowska A., Bil-Lula I. Protective Role of Platelets in Myocardial Infarction and Ischemia/Reperfusion Injury. Cardiol. Res. Pract. 2021;2021:5545416. doi: 10.1155/2021/5545416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valikeserlis I., Athanasiou A.-A., Stakos D. Cellular Mechanisms and Pathways in Myocardial Reperfusion Injury. Coron. Artery Dis. 2021;32:567–577. doi: 10.1097/MCA.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez B., Heusch G., Ovize M., Van de Werf F. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Penna C., Rastaldo R., Mancardi D., Raimondo S., Cappello S., Gattullo D., Losano G., Pagliaro P. Post-Conditioning Induced Cardioprotection Requires Signaling through a Redox-Sensitive Mechanism, Mitochondrial ATP-Sensitive K+ Channel and Protein Kinase C Activation. Basic Res. Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 9.Downey J.M., Cohen M.V. A Really Radical Observation. Basic Res. Cardiol. 2006;101:190–191. doi: 10.1007/s00395-006-0586-3. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi Y.M., Yokoyama T., Horikawa Y., Roth D.M., Patel H.H. Reactive Oxygen Species Trigger Ischemic and Pharmacological Postconditioning: In Vivo and In Vitro Characterization. Life Sci. 2007;81:1223–1227. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslov L.N., Popov S.V., Mukhomedzyanov A.V., Naryzhnaya N.V., Voronkov N.S., Ryabov V.V., Boshchenko A.A., Khaliulin I., Prasad N.R., Fu F., et al. Reperfusion Cardiac Injury: Receptors and the Signaling Mechanisms. Curr. Cardiol. Rev. 2022;18:63–79. doi: 10.2174/1573403X18666220413121730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penna C., Bassino E., Alloatti G. Platelet Activating Factor: The Good and the Bad in the Ischemic/Reperfused Heart. Exp. Biol. Med. 2011;236:390–401. doi: 10.1258/ebm.2011.010316. [DOI] [PubMed] [Google Scholar]
- 13.Schanze N., Bode C., Duerschmied D. Platelet Contributions to Myocardial Ischemia/Reperfusion Injury. Front. Immunol. 2019;10:1260. doi: 10.3389/fimmu.2019.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo I., Femminò S., Barale C., Tullio F., Geuna S., Cavalot F., Pagliaro P., Penna C. Cardioprotective Properties of Human Platelets Are Lost in Uncontrolled Diabetes Mellitus: A Study in Isolated Rat Hearts. Front. Physiol. 2018;9:875. doi: 10.3389/fphys.2018.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieder H.R., Tsoumani M., Andreadou I., Schrör K., Heusch G., Kleinbongard P. Platelet-Mediated Transfer of Cardioprotection by Remote Ischemic Conditioning and Its Abrogation by Aspirin But Not by Ticagrelor. Cardiovasc. Drugs Ther. 2022 doi: 10.1007/s10557-022-07345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penna C., Alloatti G., Cappello S., Gattullo D., Berta G., Mognetti B., Losano G., Pagliaro P. Platelet-Activating Factor Induces Cardioprotection in Isolated Rat Heart Akin to Ischemic Preconditioning: Role of Phosphoinositide 3-Kinase and Protein Kinase C Activation. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2512–H2520. doi: 10.1152/ajpheart.00599.2004. [DOI] [PubMed] [Google Scholar]
- 17.Vito C.D., Hadi L.A., Navone S.E., Marfia G., Campanella R., Mancuso M.E., Riboni L. Platelet-Derived Sphingosine-1-Phosphate and Inflammation: From Basic Mechanisms to Clinical Implications. Platelets. 2016;27:393–401. doi: 10.3109/09537104.2016.1144179. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler M., Alt K., Paterson B.M., Kanellakis P., Bobik A., Donnelly P.S., Hagemeyer C.E., Peter K. Highly Sensitive Detection of Minimal Cardiac Ischemia Using Positron Emission Tomography Imaging of Activated Platelets. Sci. Rep. 2016;6:38161. doi: 10.1038/srep38161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler M., Wang X., Peter K. Platelets in Cardiac Ischaemia/Reperfusion Injury: A Promising Therapeutic Target. Cardiovasc. Res. 2019;115:1178–1188. doi: 10.1093/cvr/cvz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maroko P.R., Libby P., Bloor C.M., Sobel B.E., Braunwald E. Reduction by Hyaluronidase of Myocardial Necrosis Following Coronary Artery Occlusion. Circulation. 1972;46:430–437. doi: 10.1161/01.CIR.46.3.430. [DOI] [PubMed] [Google Scholar]
- 21.Maroko P.R., Libby P., Sobel B.E., Bloor C.M., Sybers H.D., Shell W.E., Covell J.W., Braunwald E. Effect of Glucose-Insulin-Potassium Infusion on Myocardial Infarction Following Experimental Coronary Artery Occlusion. Circulation. 1972;45:1160–1175. doi: 10.1161/01.CIR.45.6.1160. [DOI] [PubMed] [Google Scholar]
- 22.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z.-Q., Corvera J.S., Halkos M.E., Kerendi F., Wang N.-P., Guyton R.A., Vinten-Johansen J. Inhibition of Myocardial Injury by Ischemic Postconditioning during Reperfusion: Comparison with Ischemic Preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 24.Tullio F., Angotti C., Perrelli M.-G., Penna C., Pagliaro P. Redox Balance and Cardioprotection. Basic Res. Cardiol. 2013;108:392. doi: 10.1007/s00395-013-0392-7. [DOI] [PubMed] [Google Scholar]
- 25.Heusch G. Myocardial Ischaemia-Reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 26.Penna C., Perrelli M.-G., Pagliaro P. Mitochondrial Pathways, Permeability Transition Pore, and Redox Signaling in Cardioprotection: Therapeutic Implications. Antioxid. Redox Signal. 2013;18:556–599. doi: 10.1089/ars.2011.4459. [DOI] [PubMed] [Google Scholar]
- 27.Boengler K., Heusch G., Schulz R. Mitochondria in Postconditioning. Antioxid. Redox Signal. 2011;14:863–880. doi: 10.1089/ars.2010.3309. [DOI] [PubMed] [Google Scholar]
- 28.Rossello X., Yellon D.M. The RISK Pathway and Beyond. Basic Res. Cardiol. 2018;113:2. doi: 10.1007/s00395-017-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecour S. Activation of the Protective Survivor Activating Factor Enhancement (SAFE) Pathway against Reperfusion Injury: Does It Go beyond the RISK Pathway? J. Mol. Cell. Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Hadebe N., Cour M., Lecour S. The SAFE pathway for cardioprotection: Is this a promising target? Basic Res. Cardiol. 2018;113:9. doi: 10.1007/s00395-018-0670-5. [DOI] [PubMed] [Google Scholar]
- 31.Park M., Sandner P., Krieg T. cGMP at the centre of attention: Emerging strategies for activating the cardioprotective PKG pathway. Basic Res. Cardiol. 2018;113:24. doi: 10.1007/s00395-018-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa A.D., Pierre S.V., Cohen M.V., Downey J.M., Garlid K.D. cGMP signalling in pre- and post-conditioning: The role of mitochondria. Cardiovasc. Res. 2007;77:344–352. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 33.Le Page S., Bejan-Angoulvant T., Angoulvant D., Prunier F. Remote ischemic conditioning and cardioprotection: A systematic review and meta-analysis of randomized clinical trials. Basic Res. Cardiol. 2015;110:11. doi: 10.1007/s00395-015-0467-8. [DOI] [PubMed] [Google Scholar]
- 34.Battipaglia I., Scalone G., Milo M., Di Franco A., Lanza G.A., Crea F. Upper arm intermittent ischaemia reduces exercise-related increase of platelet reactivity in patients with obstructive coronary artery disease. Heart. 2011;97:1298–1303. doi: 10.1136/hrt.2011.226415. [DOI] [PubMed] [Google Scholar]
- 35.Lanza G.A., Cesarano M., De Vita A., Villano A., Milo M., Russo G., Crea F. Effect of Remote Ischemic Preconditioning on Coronary Procedure-Related Impairment of Vascular Dilator Function. J. Am. Coll. Cardiol. 2016;68:2490–2492. doi: 10.1016/j.jacc.2016.08.071. [DOI] [PubMed] [Google Scholar]
- 36.Davidson S.M., Andreadou I., Barile L., Birnbaum Y., Cabrera-Fuentes H.A., Cohen M.V., Downey J.M., Girao H., Pagliaro P., Penna C., et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019;115:1156–1166. doi: 10.1093/cvr/cvy314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z., Delaney M.K., O’Brien K.A., Du X. Signaling During Platelet Adhesion and Activation. Arter. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semple J.W., Italiano J.E., Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 39.Barale C., Melchionda E., Morotti A., Russo I. Prothrombotic Phenotype in COVID-19: Focus on Platelets. Int. J. Mol. Sci. 2021;22:13638. doi: 10.3390/ijms222413638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veer C.V., Van Der Poll T., De Stoppelaar S.F. The role of platelets in sepsis. Thromb. Haemost. 2014;112:666–677. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 41.Lufrano M., Balazy M. Interactions of peroxynitrite and other nitrating substances with human platelets: The role of glutathione and peroxynitrite permeability. Biochem. Pharmacol. 2002;65:515–523. doi: 10.1016/S0006-2952(02)01584-8. [DOI] [PubMed] [Google Scholar]
- 42.Bearer E., Prakash J., Li Z. Actin dynamics in platelets. Int. Rev. Cytol. 2002;217:137–182. doi: 10.1016/s0074-7696(02)17014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begonja A.J., Pluthero F.G., Suphamungmee W., Giannini S., Christensen H., Leung R., Lo R.W., Nakamura F., Lehman W., Plomann M., et al. FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets. Blood. 2015;126:80–88. doi: 10.1182/blood-2014-07-587600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moncada S., Higgs E.A. Nitric Oxide and the Vascular Endothelium. In: Moncada S., Higgs A., editors. The Vascular Endothelium I. Handbook of Experimental Pharmacology. Volume 176/I. Springer; Berlin/Heidelberg, Germany: 2006. [DOI] [PubMed] [Google Scholar]
- 45.Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J. Thromb. Haemost. 2012;10:167–176. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- 46.Gambaryan S., Tsikas D. A review and discussion of platelet nitric oxide and nitric oxide synthase: Do blood platelets produce nitric oxide from l-arginine or nitrite? Amino Acids. 2015;47:1779–1793. doi: 10.1007/s00726-015-1986-1. [DOI] [PubMed] [Google Scholar]
- 47.Böhmer A., Gambaryan S., Tsikas D. Human blood platelets lack nitric oxide synthase activity. Platelets. 2014;26:583–588. doi: 10.3109/09537104.2014.974024. [DOI] [PubMed] [Google Scholar]
- 48.Radziwon-Balicka A., Lesyk G., Back V., Fong T., Loredo-Calderon E.L., Dong B., El-Sikhry H., A El-Sherbeni A., El-Kadi A., Ogg S., et al. Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation. Cardiovasc. Res. 2017;113:1719–1731. doi: 10.1093/cvr/cvx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preedy M.E.J., Baliga R.S., Hobbs A.J. Multiplicity of Nitric Oxide and Natriuretic Peptide Signaling in Heart Failure. J. Cardiovasc. Pharmacol. 2020;75:370–384. doi: 10.1097/FJC.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 50.Blanton R.M. cGMP Signaling and Modulation in Heart Failure. J. Cardiovasc. Pharmacol. 2020;75:385–398. doi: 10.1097/FJC.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dang T.A., Schunkert H., Kessler T. cGMP Signaling in Cardiovascular Diseases: Linking Genotype and Phenotype. J. Cardiovasc. Pharmacol. 2020;75:516–525. doi: 10.1097/FJC.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 52.Dunkerly-Eyring B., Kass D.A. Myocardial Phosphodiesterases and Their Role in cGMP Regulation: Linking Genotype and Phenotype. J. Cardiovasc. Pharmacol. 2020;75:483–493. doi: 10.1097/FJC.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degjoni A., Campolo F., Stefanini L., Venneri M.A. The NO/cGMP/PKG pathway in platelets: The therapeutic potential of PDE5 inhibitors in platelet disorders. J. Thromb. Haemost. 2022;20:2465–2474. doi: 10.1111/jth.15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walter U., Gambaryan S. cGMP: Generators, Effectors and Therapeutic Implications. Springer; Berlin/Heidelberg, Germany: 2009. cGMP and cGMP-Dependent Protein Kinase in Platelets and Blood Cells; pp. 533–548. [DOI] [PubMed] [Google Scholar]
- 55.Geiger J., Nolte C., Walter U. Regulation of calcium mobilization and entry in human platelets by endothelium-derived factors. Am. J. Physiol. 1994;267:C236–C244. doi: 10.1152/ajpcell.1994.267.1.C236. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian H., Zahedi R.P., Sickmann A., Walter U., Gambaryan S. Phosphorylation of CalDAG-GEFI by protein kinase A prevents Rap1b activation. J. Thromb. Haemost. 2013;11:1574–1582. doi: 10.1111/jth.12271. [DOI] [PubMed] [Google Scholar]
- 57.Tsikas D., Ikic M., Tewes K.S., Raida M., Frölich J.C. Inhibition of platelet aggregation by S-nitroso-cysteine via cGMP-independent mechanisms: Evidence of inhibition of thromboxane A2 synthesis in human blood platelets. FEBS Lett. 1999;442:162–166. doi: 10.1016/S0014-5793(98)01633-0. [DOI] [PubMed] [Google Scholar]
- 58.Kobsar A., Simonis S., Klinker E., Koessler A., Kuhn S., Boeck M., Koessler J. Specific inhibitory effects of the NO donor MAHMA/NONOate on human platelets. Eur. J. Pharmacol. 2014;735:169–176. doi: 10.1016/j.ejphar.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 59.Butt E., Bernhardt M., Smolenski A., Kotsonis P., Fröhlich L.G., Sickmann A., Meyer H.E., Lohmann S.M., Schmidt H.H.H.W. Endothelial Nitric-oxide Synthase (Type III) Is Activated and Becomes Calcium Independent upon Phosphorylation by Cyclic Nucleotide-dependent Protein Kinases. J. Biol. Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- 60.Boo Y.C., Sorescu G., Boyd N., Shiojima I., Walsh K., Du J., Jo H. Shear Stress Stimulates Phosphorylation of Endothelial Nitric-oxide Synthase at Ser1179 by Akt-independent Mechanisms: Role of Protein Kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 61.Queen L.R., Xu B., Horinouchi K., Fisher I., Ferro A. β2 -Adrenoceptors Activate Nitric Oxide Synthase in Human Platelets. Circ. Res. 2000;87:39–44. doi: 10.1161/01.RES.87.1.39. [DOI] [PubMed] [Google Scholar]
- 62.Schulz C., Fichtlscherer B., Kemp B.E., Fisslthaler B., Busse R., Fleming I. AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thromb. Haemost. 2003;90:863–871. doi: 10.1160/TH03-04-0228. [DOI] [PubMed] [Google Scholar]
- 63.O’Kane P., Xie L., Liu Z., Queen L., Jackson G., Ji Y., Ferro A. Aspirin acetylates nitric oxide synthase type 3 in platelets thereby increasing its activity. Cardiovasc. Res. 2009;83:123–130. doi: 10.1093/cvr/cvp120. [DOI] [PubMed] [Google Scholar]
- 64.Russo I., Doronzo G., Mattiello L., De Salve A., Trovati M., Anfossi G. The activity of constitutive nitric oxide synthase is increased by the pathway cAMP/cAMP-activated protein kinase in human platelets. New insights into the antiaggregating effects of cAMP-elevating agents. Thromb. Res. 2004;114:265–273. doi: 10.1016/j.thromres.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 65.Radomski M., Palmer R., Moncada S. Characterization of the l-arginine: Nitric oxide pathway in human platelets. Br. J. Pharmacol. 1990;101:325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radomski M.W., Palmer R.M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel B., Sharifi M., Milward A.D., Oberprieler N.G., Gibbins J.M., Parkin S., Naseem K.M. Platelet nitric oxide synthase is activated by tyrosine dephosphorylation: Possible role for SHP-1 phosphatase. J. Thromb. Haemost. 2006;4:2423–2432. doi: 10.1111/j.1538-7836.2006.02160.x. [DOI] [PubMed] [Google Scholar]
- 68.Mehta J.L., Chen L.Y., Kone B.C., Mehta P., Turner P. Identification of constitutive and inducible forms of nitric oxide synthase in human platelets. J. Lab. Clin. Med. 1995;125:370–377. [PubMed] [Google Scholar]
- 69.Radomski M.W., Zakar T., Salas E. Methods in Enzymology. Volume 269. Academic Press; Cambridge, MA, USA: 1996. [9] Nitric oxide in platelets; pp. 88–107. [DOI] [PubMed] [Google Scholar]
- 70.Loscalzo J., Jin R.C. Vascular nitric oxide: Formation and function. J. Blood Med. 2010;1:147–162. doi: 10.2147/JBM.S7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D., Knight A.R., Taylor E.L., Oettrich J., Ruskovska T., et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morotti A., Barale C., Melchionda E., Russo I. Platelet Redox Imbalance in Hypercholesterolemia: A Big Problem for a Small Cell. Int. J. Mol. Sci. 2022;23:11446. doi: 10.3390/ijms231911446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krötz F., Sohn H.-Y., Pohl U. Reactive Oxygen Species: Players in the Platelet Game. Arter. Thromb. Vasc. Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 74.A Moro M., Darley-Usmar V.M., A Goodwin D., Read N.G., Zamora-Pino R., Feelisch M., Radomski M.W., Moncada S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc. Natl. Acad. Sci. USA. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mondoro T.H., Shafer B.C., Vostal J.G. Peroxynitrite-Induced Tyrosine Nitration and Phosphorylation in Human Platelets. Free Radic. Biol. Med. 1997;22:1055–1063. doi: 10.1016/S0891-5849(96)00510-2. [DOI] [PubMed] [Google Scholar]
- 76.Bruckdorfer K. The nitration of proteins in platelets. Comptes Rendus L’académie Sci. Ser. III Sci. Vie. 2001;324:611–615. doi: 10.1016/S0764-4469(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 77.Sabetkar M., Low S.Y., Naseem K.M., Bruckdorfer K. The nitration of proteins in platelets: Significance in platelet function1,2. Free Radic. Biol. Med. 2002;33:728–736. doi: 10.1016/S0891-5849(02)00890-0. [DOI] [PubMed] [Google Scholar]
- 78.Olas B., Nowak P., Ponczek M., Wachowicz B. Resveratrol, a natural phenolic compound may reduce carbonylation proteins induced by peroxynitrite in blood platelets. Gen. Physiol. Biophys. 2006;25:215–222. [PubMed] [Google Scholar]
- 79.Kabbani S.S., Watkins M.W., Ashikaga T., Terrien E.F., Holoch P.A., Sobel B.E., Schneider D.J. Platelet Reactivity Characterized Prospectively: A Determinant of Outcome 90 Days after Percutaneous Coronary Intervention. Circulation. 2001;104:181–186. doi: 10.1161/01.CIR.104.2.181. [DOI] [PubMed] [Google Scholar]
- 80.Trip M.D., Cats V.M., van Capelle F.J., Vreeken J. Platelet Hyperreactivity and Prognosis in Survivors of Myocardial Infarction. N. Engl. J. Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 81.Vanschoonbeek K., Feijge M.A.H., Keuren J.F.W., Hemker H.C., Lodder J.J., Hamulyák K., Van Pampus E.C.M., Heemskerk J.W.M. Thrombin-induced hyperactivity of platelets of young stroke patients: Involvement of thrombin receptors in the subject-dependent variability in Ca2+ signal generation. Thromb. Haemost. 2002;88:931–937. doi: 10.1055/s-0037-1613336. [DOI] [PubMed] [Google Scholar]
- 82.Barale C., Cavalot F., Frascaroli C., Bonomo K., Morotti A., Guerrasio A., Russo I. Association between High On-Aspirin Platelet Reactivity and Reduced Superoxide Dismutase Activity in Patients Affected by Type 2 Diabetes Mellitus or Primary Hypercholesterolemia. Int. J. Mol. Sci. 2020;21:4983. doi: 10.3390/ijms21144983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barale C., Russo I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020;21:623. doi: 10.3390/ijms21020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trovati M., Anfossi G., Massucco P., Mattiello L., Costamagna C., Piretto V., Mularoni E., Cavalot F., Bosia A., Ghigo D. Insulin Stimulates Nitric Oxide Synthesis in Human Platelets and, Through Nitric Oxide, Increases Platelet Concentrations of Both Guanosine-3′, 5′-Cyclic Monophosphate and Adenosine-3′, 5′-Cyclic Monophosphate. Diabetes. 1997;46:742–749. doi: 10.2337/diab.46.5.742. [DOI] [PubMed] [Google Scholar]
- 85.Anfossi G., Russo I., Trovati M. Platelet dysfunction in central obesity. Nutr. Metab. Cardiovasc. Dis. 2009;19:440–449. doi: 10.1016/j.numecd.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Russo I., Del Mese P., Doronzo G., De Salve A., Secchi M., Trovati M., Anfossi G. Platelet Resistance to the Antiaggregatory Cyclic Nucleotides in Central Obesity Involves Reduced Phosphorylation of Vasodilator-Stimulated Phosphoprotein. Clin. Chem. 2007;53:1053–1060. doi: 10.1373/clinchem.2006.076208. [DOI] [PubMed] [Google Scholar]
- 87.Mammadova-Bach E., Nagy M., Heemskerk J.W., Nieswandt B., Braun A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium. 2018;77:39–48. doi: 10.1016/j.ceca.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Russo I., Traversa M., Bonomo K., De Salve A., Mattiello L., Del Mese P., Doronzo G., Cavalot F., Trovati M., Anfossi G. In Central Obesity, Weight Loss Restores Platelet Sensitivity to Nitric Oxide and Prostacyclin. Obesity. 2010;18:788–797. doi: 10.1038/oby.2009.302. [DOI] [PubMed] [Google Scholar]
- 89.Russo I., Penna C., Musso T., Popara J., Alloatti G., Cavalot F., Pagliaro P. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc. Diabetol. 2017;16:71. doi: 10.1186/s12933-017-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anfossi G., Russo I., Massucco P., Mattiello L., Trovati M. Platelet resistance to the antiaggregating effect of N-acetyl-l-cysteine in obese, insulin-resistant subjects. Thromb. Res. 2003;110:39–46. doi: 10.1016/S0049-3848(03)00284-6. [DOI] [PubMed] [Google Scholar]
- 91.Kinasewitz G.T., Yan S.B., Basson B., Comp P., A Russell J., Cariou A., Um S.L., Utterback B., Laterre P.-F., Dhainaut J.-F. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit. Care. 2004;8:R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinheiro M.B.M., Rozini S.V., Quirino-Teixeira A.C., Barbosa-Lima G., Lopes J.F., Sacramento C.Q., Bozza F.A., Bozza P.T., Hottz E.D. Dengue induces iNOS expression and nitric oxide synthesis in platelets through IL-1R. Front. Immunol. 2022;13:1029213. doi: 10.3389/fimmu.2022.1029213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mancardi D., Pagliaro P., Ridnour L.A., Tocchetti C.G., Miranda K., Juhaszova M., Sollott S.J., Wink D.A., Paolocci N. HNO Protects the Myocardium against Reperfusion Injury, Inhibiting the mPTP Opening via PKCε Activation. Antioxidants. 2022;11:382. doi: 10.3390/antiox11020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pagliaro P., Mancardi D., Rastaldo R., Penna C., Gattullo D., Miranda K.M., Feelisch M., A Wink D., A Kass D., Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/S0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 96.Penna C., Angotti C., Pagliaro P. Protein S-nitrosylation in preconditioning and postconditioning. Exp. Biol. Med. 2014;239:647–662. doi: 10.1177/1535370214522935. [DOI] [PubMed] [Google Scholar]
- 97.Shemarova I., Nesterov V., Emelyanova L., Korotkov S. Mitochondrial mechanisms by which gasotransmitters (H2S, NO and CO) protect cardiovascular system against hypoxia. Front. Biosci. 2021;13:105–130. doi: 10.52586/s556. [DOI] [PubMed] [Google Scholar]
- 98.Leary P.J., Rajasekaran S., Morrison R.R., Tuomanen E.I., Chin T.K., Hofmann P.A. A cardioprotective role for platelet-activating factor through NOS-dependent S-nitrosylation. Am. J. Physiol. Circ. Physiol. 2008;294:H2775–H2784. doi: 10.1152/ajpheart.00269.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Igarashi J., Michel T. S1P and eNOS regulation. Biochim. Biophys. Acta. 2008;1781:489–495. doi: 10.1016/j.bbalip.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Yokota T., Higuma T., Endo T., Nishizaki F., Hanada K., Yokoyama H., Yamada M., Okumura K., Tomita H. Prasugrel versus clopidogrel for residual thrombus burden in patients with ST-segment elevation myocardial infarction: An Optical Coherence Tomog-raphy Study. Coron. Artery Dis. 2018;29:663–669. doi: 10.1097/MCA.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 101.Pandit A., Aryal M.R., Pandit A.A., Jalota L., Hakim F.A., Mookadam F., Lee H.R., Tleyjeh I.M. Cangrelor versus clopidogrel in percutaneous coronary intervention: A systematic review and meta-analysis. Eurointervention. 2014;9:1350–1358. doi: 10.4244/EIJV9I11A226. [DOI] [PubMed] [Google Scholar]
- 102.Ye Y., Birnbaum G.D., Perez-Polo J.R., Nanhwan M.K., Nylander S., Birnbaum Y. Ticagrelor Protects the Heart Against Reperfusion Injury and Improves Remodeling After Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2015;35:1805–1814. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 103.Zhuang Y., Yu M.-L., Lu S.-F. Purinergic signaling in myocardial ischemia–reperfusion injury. Purinergic Signal. 2023;19:229–243. doi: 10.1007/s11302-022-09856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Penna C., Aragno M., Cento A.S., Femminò S., Russo I., Bello F.D., Chiazza F., Collotta D., Alves G.F., Bertinaria M., et al. Ticagrelor Conditioning Effects Are Not Additive to Cardioprotection Induced by Direct NLRP3 Inflammasome Inhibition: Role of RISK, NLRP3, and Redox Cascades. Oxidative Med. Cell. Longev. 2020;2020:9219825. doi: 10.1155/2020/9219825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bell R.M., Sivaraman V., Kunuthur S.P., Cohen M.V., Downey J.M., Yellon D.M. Cardioprotective Properties of the Platelet P2Y12 Receptor Inhibitor, Cangrelor: Protective in Diabetics and Reliant Upon the Presence of Blood. Cardiovasc. Drugs Ther. 2015;29:415–418. doi: 10.1007/s10557-015-6609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dost T. Cardioprotective properties of the platelet P2Y12 receptor inhibitor prasugrel on cardiac ischemia/reperfusion injury. Pharmacol. Rep. 2020;72:672–679. doi: 10.1007/s43440-019-00046-5. [DOI] [PubMed] [Google Scholar]
- 107.Aungraheeta R., Conibear A., Butler M., Kelly E., Nylander S., Mumford A., Mundell S.J. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016;128:2717–2728. doi: 10.1182/blood-2016-03-707844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gimbel M., Qaderdan K., Willemsen L., Hermanides R., Bergmeijer T., de Vrey E., Heestermans T., Gin M.T.J., Waalewijn R., Hofma S., et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–1381. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- 109.Knauert M., Vangala S., Haslip M., Lee P.J. Therapeutic Applications of Carbon Monoxide. Oxidative Med. Cell. Longev. 2013;2013:360815. doi: 10.1155/2013/360815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryter S.W. Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders. Antioxidants. 2020;9:1153. doi: 10.3390/antiox9111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M., Soares M. Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Di Lisa F., Canton M., Carpi A., Kaludercic N., Menabò R., Menazza S., Semenzato M., Valls-Lacalle L., Barba I., Miró-Casas E., et al. Mitochondrial Injury and Protection in Ischemic Pre- and Postconditioning. Antioxid. Redox Signal. 2011;14:881–891. doi: 10.1089/ars.2010.3375. [DOI] [PubMed] [Google Scholar]
- 113.Xu Z., Ji X., Boysen P.G. Exogenous nitric oxide generates ROS and induces cardioprotection: Involvement of PKG, mitochondrial KATP channels, and ERK. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1433–H1440. doi: 10.1152/ajpheart.00882.2003. [DOI] [PubMed] [Google Scholar]
- 114.Chlopicki S., Olszanecki R., Marcinkiewicz E., Lomnicka M., Motterlini R. Carbon monoxide released by CORM-3 inhibits human platelets by a mechanism independent of soluble guanylate cyclase. Cardiovasc. Res. 2006;71:393–401. doi: 10.1016/j.cardiores.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 115.Wu L., Wang R. Carbon Monoxide: Endogenous Production, Physiological Functions, and Pharmacological Applications. Pharmacol. Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 116.Marks G.S., Brien J.F., Nakatsu K., McLaughlin B.E. Does carbon monoxide have a physiological function? Trends Pharmacol. Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- 117.Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol. Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 118.Brüne B., Schmidt K.-U., Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. JBIC J. Biol. Inorg. Chem. 1990;192:683–688. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 119.Nowell S.A., Leakey J.E., Warren J.F., Lang N.P., Frame L.T. Identification of Enzymes Responsible for the Metabolism of Heme in Human Platelets. J. Biol. Chem. 1998;273:33342–33346. doi: 10.1074/jbc.273.50.33342. [DOI] [PubMed] [Google Scholar]
- 120.Morisaki H., Katayama T., Kotake Y., Ito M., Tamatani T., Sakamoto S., Ishimura Y., Takeda J., Suematsu M. Roles of Carbon Monoxide in Leukocyte and Platelet Dynamics in Rat Mesenteric during Sevoflurane Anesthesia. Anesthesiology. 2001;95:192–199. doi: 10.1097/00000542-200107000-00030. [DOI] [PubMed] [Google Scholar]
- 121.Morisaki H., Katayama T., Kotake Y., Ito M., Handa M., Ikeda Y., Takeda J., Suematsu M. Carbon Monoxide Modulates Endotoxin-induced Microvascular Leukocyte Adhesion through Platelet-dependent Mechanisms. Anesthesiology. 2002;97:701–709. doi: 10.1097/00000542-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 122.Peng L., Mundada L., Stomel J.M., Liu J.J., Sun J., Yet S.-F., Fay W.P. Induction of Heme Oxygenase-1 Expression Inhibits Platelet-Dependent Thrombosis. Antioxid. Redox Signal. 2004;6:729–735. doi: 10.1089/1523086041361677. [DOI] [PubMed] [Google Scholar]
- 123.True A.L., Olive M., Boehm M., San H., Westrick R.J., Raghavachari N., Xu X., Lynn E.G., Sack M.N., Munson P.J., et al. Heme Oxygenase-1 Deficiency Accelerates Formation of Arterial Thrombosis Through Oxidative Damage to the Endothelium, Which Is Rescued by Inhaled Carbon Monoxide. Circ. Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 124.Chen B., Guo L., Fan C., Bolisetty S., Joseph R., Wright M.M., Agarwal A., George J.F. Carbon Monoxide Rescues Heme Oxygenase-1-Deficient Mice from Arterial Thrombosis in Allogeneic Aortic Transplantation. Am. J. Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Motterlini R., Mann B., Johnson T., Clark J., Foresti R., Green C. Bioactivity and Pharmacological Actions of Carbon Monoxide-Releasing Molecules. Curr. Pharm. Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- 126.A Gende O. Carbon monoxide inhibits capacitative calcium entry in human platelets. Thromb. Res. 2004;114:113–119. doi: 10.1016/j.thromres.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 127.Liu D., Liang F., Wang X., Cao J., Qin W., Sun B. Suppressive Effect of CORM-2 on LPS-Induced Platelet Activation by Glycoprotein Mediated HS1 Phosphorylation Interference. PLoS ONE. 2013;8:e83112. doi: 10.1371/journal.pone.0083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nielsen V.G., Garza J.I. Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul. Fibrinolysis. 2014;25:801–805. doi: 10.1097/MBC.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 129.Vostal J.G., Fratantoni J.C. Econazole inhibits thapsigargin-induced platelet calcium influx by mechanisms other than cytochrome P-450 inhibition. Biochem. J. 1993;295:525–529. doi: 10.1042/bj2950525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Truss N.J., Warner T.D. Gasotransmitters and platelets. Pharmacol. Ther. 2011;132:196–203. doi: 10.1016/j.pharmthera.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 131.Zhu X.-Y., Yan X.-H., Chen S.-J. H(2)S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng li xue bao Acta Physiol. Sin. 2008;60:221–227. [PubMed] [Google Scholar]
- 132.Ananthakrishnan R., Li Q., O’Shea K.M., Quadri N., Wang L., Abuchowski A., Schmidt A.M., Ramasamy R. Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif. Cells Nanomed. Biotechnol. 2013;41:428–436. doi: 10.3109/21691401.2012.762370. [DOI] [PubMed] [Google Scholar]
- 133.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2014;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shao J., Miao C., Geng Z., Gu M., Wu Y., Li Q. Effect of eNOS on Ischemic Postconditioning-Induced Autophagy against Ischemia/Reperfusion Injury in Mice. BioMed Res. Int. 2019;2019:5201014. doi: 10.1155/2019/5201014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J., Cai X., Zhang Q., Li X., Li S., Ma J., Zhu W., Liu X., Wei M., Tu W., et al. Hydrogen sulfide restores sevoflurane postconditioning mediated cardioprotection in diabetic rats: Role of SIRT1/Nrf2 signaling-modulated mitochondrial dysfunction and oxidative stress: Nitric Oxide, Protein Kinases, and Mitochondria. J. Cell. Physiol. 2020;236:5052–5068. doi: 10.1002/jcp.30214. [DOI] [PubMed] [Google Scholar]
- 136.Heusch G., Boengler K., Schulz R. Cardioprotection: Nitric Oxide, Protein Kinases, and Mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 137.Pagliaro P., Moro F., Tullio F., Perrelli M.-G., Penna C. Cardioprotective Pathways During Reperfusion: Focus on Redox Signaling and Other Modalities of Cell Signaling. Antioxid. Redox Signal. 2011;14:833–850. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.