Abstract
Multiple myeloma (MM) is an incurable disease characterized by the presence of malignant plasma cells in the bone marrow that secrete specific monoclonal immunoglobulins into the blood. Obesity has been associated with the risk of developing solid and hematological cancers, but its role as a risk factor for MM needs to be further explored. Here, we evaluated whether 32 genome-wide association study (GWAS)-identified variants for obesity were associated with the risk of MM in 4189 German subjects from the German Multiple Myeloma Group (GMMG) cohort (2121 MM cases and 2068 controls) and 1293 Spanish subjects (206 MM cases and 1087 controls). Results were then validated through meta-analysis with data from the UKBiobank (554 MM cases and 402,714 controls) and FinnGen cohorts (914 MM cases and 248,695 controls). Finally, we evaluated the correlation of these single nucleotide polymorphisms (SNPs) with cQTL data, serum inflammatory proteins, steroid hormones, and absolute numbers of blood-derived cell populations (n = 520). The meta-analysis of the four European cohorts showed no effect of obesity-related variants on the risk of developing MM. We only found a very modest association of the POC5rs2112347G and ADCY3rs11676272G alleles with MM risk that did not remain significant after correction for multiple testing (per-allele OR = 1.08, p = 0.0083 and per-allele OR = 1.06, p = 0.046). No correlation between these SNPs and functional data was found, which confirms that obesity-related variants do not influence MM risk.
Keywords: multiple myeloma, obesity, genetic variants, susceptibility
1. Introduction
Multiple myeloma (MM) is an incurable disease characterized by the presence of malignant plasma cells in the bone marrow that secrete specific monoclonal immunoglobulins (also called M-protein) into the blood and/or urine [1,2]. M protein levels have been traditionally used to diagnose the disease and to monitor residual disease using protein electrophoresis (PEL), immunofixation electrophoresis (IFE), free light chain nephelometry (FLC), and liquid chromatography–mass spectrometry (LC-MS) methods [3]. However, despite the substantial advances during the last decade in identifying specific biomarkers and even the biological mechanisms underlying MM onset, there are no consistent risk factors other than male gender, age, African American ethnicity, obesity, and positive family history of lymphatohematopoietic cancer (LHC) and monoclonal gammopathy of undetermined significance (MGUS). Among these factors, obesity is a potentially interesting modifiable factor as it has been associated with several solid and hematological cancers [4,5,6,7,8,9,10,11], as well as tumor development by its interaction between cancer stem cells and macrophages or the tumor environment [12,13]. Although the analysis of both laboratory and clinical data has suggested complex associations between obesity and MM, the underlying genetic factors remain elusive. Thus, the aim of this study was to evaluate whether 32 GWAS-identified polymorphisms for obesity could influence the risk of MM. We also assessed whether these SNPs could exert their effect on MM risk by modulating host immune responses through comprehensive functional analysis.
2. Results
Selected polymorphisms did not deviate from Hardy Weinberg Equilibrium (HWE) in the control population (p < 0.001). The analysis of the discovery population only showed that each copy of the MTCH2rs3817334T allele slightly decreased the risk of developing MM (OR = 0.89, p = 0.024). After the meta-analysis of all study cohorts, we could not replicate this finding but found a weak effect of the ADCY3rs11676272 and POC5rs2112347 SNPs on the risk of developing MM. However, these associations did not remain significant after multiple testing (OR = 1.06, p = 0.046 and OR = 1.08, p = 0.0083; Table 1). Association estimates did not substantially change after correction for body mass index (BMI).
Table 1.
Meta-analysis of the discovery cohort with GWAS data from the UKBiobank and FinnGen projects.
| Gene_SNP ID | Discovery Cohort (n = 5482) |
UKBiobank (n = 403,268) |
FinnGen (n = 249,609) |
Meta-Analysis (n = 658,359) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) ∂ | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | PHet | |
| ADCY3_rs11676272 | 1.04 (1.13–0.95) | 0.36 | 1.05 (0.93–1.17) | 0.44 | 1.09 (0.99–1.19) | 0.084 | 1.06 (1.00–1.11) | 0.046 | 0.47 0.36 |
| ADPGK_rs7164727 | 1.01 (1.10–0.91) | 0.86 | 0.98 (0.87–1.10) | 0.71 | 1.07 (0.97–1.18) | 0.20 | 0.98 (0.92–1.04) | 0.63 | |
| AKAP6_rs17522122 | 0.94 (1.06–0.84) | 0.31 | 1.08 (0.96–1.21) | 0.22 | 1.02 (0.93–1.12) | 0.66 | 1.01 (0.95–1.08) | 0.70 | 0.22 0.97 |
| BDNF_rs6265 | 1.00 (1.36–0.74) | 1.00 | 0.97 (0.83–1.12) | 0.65 | 0.97 (0.86–1.10) | 0.63 | 0.97 (0.88–1.07) | 0.51 | |
| DNASE1_rs1053874 | 1.08 (1.20–0.96) | 0.19 | 1.04 (0.92–1.18) | 0.52 | 0.91 (0.82–1.00) | 0.056 | 1.00 (0.93–1.06) | 0.88 | 0.12 0.73 |
| FAIM2_rs7138803 | 0.99 (1.08–0.91) | 0.85 | 1.01 (0.90–1.13) | 0.90 | 0.95 (0.86–1.04) | 0.28 | 0.98 (0.93–1.04) | 0.48 | |
| FLT3_rs1933437 | 0.94 (1.04–0.83) | 0.27 | 0.98 (0.85–1.09) | 0.69 | 0.91 (0.81–1.01) | 0.090 | 0.94 (0.87–1.00) | 0.054 | 0.77 0.34 0.008 |
| FTO_rs1421085 | 0.95 (1.04–0.85) | 0.33 | 1.10 (0.97–1.24) | 0.14 | 1.01 (0.93–1.11) | 0.77 | 1.01 (0.95–1.06) | 0.78 | |
| FTO_rs7190492 | 1.06 (1.16–0.98) | 0.15 | 0.93 (0.79–1.05) | 0.27 | 1.01 (0.92–1.10) | 0.79 | 1.02 (0.96–1.08) | 0.54 | |
| GNPDA2_rs10938397 | 1.05 (1.14–0.96) | 0.26 | 1.01 (0.90–1.12) | 0.93 | 1.01 (0.93–1.10) | 0.80 | 1.03 (0.97–1.08) | 0.37 | 0.62 0.69 |
| GPRC5B_rs12444979 | 1.13 (1.62–0.79) | 0.51 | 0.96 (0.82–1.13) | 0.65 | 1.03 (0.90–1.17) | 0.70 | 1.01 (0.91–1.12) | 0.85 | |
| ITH4_rs4687657 | 1.04 (1.15–0.94) | 0.48 | 0.97 (0.85–1.10) | 0.61 | 1.07 (0.96–1.18) | 0.22 | 1.03 (0.97–1.10) | 0.33 | 0.72 0.93 |
| KCTD15_rs11084753 | 1.04 (1.17–0.92) | 0.53 | 0.99 (0.87–1.10) | 0.91 | 0.99 (0.89–1.08) | 0.84 | 1.00 (0.94–1.07) | 0.88 | |
| LMOD1_rs2820312 | 0.99 (1.09–0.91) | 0.89 | 0.96 (0.85–1.08) | 0.49 | 0.96 (0.87–1.06) | 0.40 | 0.97 (0.92–1.03) | 0.36 | 0.94 0.61 0.99 |
| LOC400652_rs17782313 | 1.06 (1.15–0.96) | 0.23 | 1.01 (0.89–1.15) | 0.88 | 1.03 (0.92–1.16) | 0.57 | 1.04 (0.97–1.10) | 0.24 | |
| MAF_rs1424233 | 0.99 (1.07–0.92) | 0.83 | 1.01 (0.89–1.11) | 0.89 | 1.00 (0.91–1.08) | 0.99 | 1.00 (0.95–1.05) | 0.95 | |
| MC4R_rs17700633 | 1.04 (1.15–0.94) | 0.43 | 0.99 (0.88–1.12) | 0.92 | 0.91 (0.82–1.02) | 0.096 | 0.98 (0.92–1.05) | 0.60 | 0.37 0.92 0.43 |
| MST1R_rs2230590 | 0.95 (1.04–0.85) | 0.26 | 1.00 (0.88–1.14) | 0.98 | 0.97 (0.89–1.07) | 0.57 | 0.97 (0.91–1.03) | 0.30 | |
| MTCH2_rs3817334 | 0.89 (0.99–0.81) | 0.024 | 0.98 (0.88–1.10) | 0.78 | 0.99 (0.91–1.08) | 0.91 | 0.95 (0.90–1.01) | 0.12 | |
| NEGR1_rs2815752 | 0.95 (1.04–0.86) | 0.32 | 1.03 (0.91–1.14) | 0.63 | 1.05 (0.96–1.14) | 0.29 | 1.01 (0.95–1.06) | 0.83 | 0.16 |
| NPC1_rs1805081 | 1.16 (1.35–0.91) | 0.19 | 0.93 (0.83–1.05) | 0.24 | 1.02 (0.93–1.11) | 0.71 | 1.00 (0.93–1.07) | 0.96 | 0.20 0.88 |
| NT5C2_rs11191580 | 0.98 (1.14–0.79) | 0.81 | 0.97 (0.78–1.19) | 0.77 | 1.03 (0.88–1.21) | 0.73 | 1.00 (0.89–1.09) | 0.94 | |
| PCSK1_rs6235 | 1.01 (1.11–0.91) | 0.92 | 1.10 (0.98–1.21) | 0.099 | 1.05 (0.95–1.14) | 0.35 | 1.05 (0.98–1.12) | 0.16 | 0.36 |
| POC5_rs2112347 | 1.09 (1.17–1.00) | 0.055 | 1.07 (0.95–1.21) | 0.28 | 1.07 (0.98–1.18) | 0.13 | 1.08 (1.02–1.13) | 0.0083 | 0.96 |
| SEC16B_rs543874 | 1.03 (1.14–0.90) | 0.63 | 0.93 (0.81–1.08) | 0.35 | 1.04 (0.92–1.16) | 0.56 | 1.01 (0.93–1.08) | 0.87 | 0.67 |
| SH2B1_rs7359397 | 0.94 (1.06–0.84) | 0.33 | 0.97 (0.86–1.09) | 0.63 | 1.12 (1.02–1.23) | 0.017 | 1.03 (0.96–1.09) | 0.41 |
0.043 0.19 |
| STK33_rs10769908 | 1.00 (1.10–0.88) | 0.96 | 0.91 (0.77–1.03) | 0.14 | 1.06 (0.97–1.15) | 0.17 | 1.00 (0.94–1.06) | 0.91 | |
| TFAP2B_rs2206277 | 1.03 (1.16–0.91) | 0.65 | 1.13 (0.96–1.32) | 0.14 | 1.00 (0.90–1.10) | 0.97 | 1.04 (0.96–1.11) | 0.34 | 0.17 |
| TMEM18_rs6548238 | 0.94 (1.06–0.83) | 0.30 | 1.05 (0.89–1.18) | 0.53 | 0.99 (0.86–1.10) | 0.84 | 0.98 (0.91–1.06) | 0.65 | 0.73 |
| TRAF3_rs10133111 | 1.05 (1.19–0.92) | 0.46 | 1.00 (0.87–1.16) | 0.97 | 1.10 (0.99–1.23) | 0.080 | 1.06 (0.99–1.15) | 0.11 | 0.68 |
| UHRF1BP1_rs11755393 | 0.99 (1.14–0.82) | 0.90 | 1.02 (0.90–1.14) | 0.79 | 0.97 (0.88–1.07) | 0.53 | 0.99 (0.91–1.06) | 0.72 | 0.95 |
| ZZZ3_rs17381664 | 0.97 (1.06–0.87) | 0.51 | 0.95 (0.84–1.07) | 0.39 | – | – | 0.96 (0.88–1.03) | 0.29 | 0.91 |
Abbreviatures: SNP, single-nucleotide polymorphism; OR, odds ratio; CI, confidence interval. A fixed effect model was assumed for the meta-analysis of all cohorts. ∂ Association estimates were adjusted for age and sex and were calculated according to log-additive model of inheritance. p ≤ 0.05 in bold.
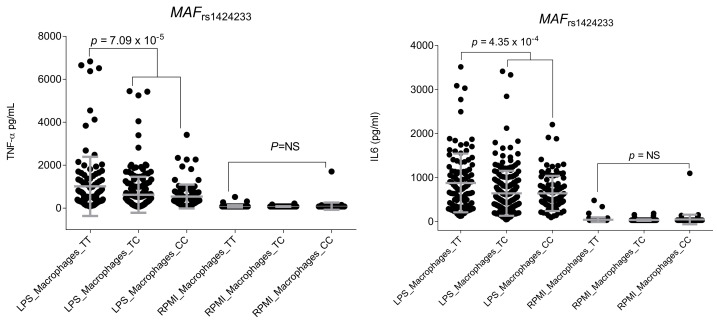
Functional experiments did not suggest any functional effect of the ADCY3rs11676272 and POC5rs2112347 SNPs on the modulation of host immune responses, which suggested that, if any, the functional effect of these SNPs on MM risk was not mediated through the modulation of immune responses. Intriguingly, we found a novel and statistically significant association of the MAFrs1424233 SNP with levels of TNFα after the stimulation of human macrophages with LPS (p = 7.09 × 10−5; Figure 1).
Figure 1.
Correlation of the MAFrs1424233 SNP with TNFα and IL6 levels after stimulation of macrophages with LPS (n = 406).
In addition, although it did not remain significant after correction for multiple testing, we found a correlation between this SNP and IL6 levels after stimulation of macrophages with LPS (p = 4.35 × 10−4; Figure 1), which might explain, at least in part, the link between obesity and inflammation. No significant correlation between the rest of the obesity-related SNPs and cQTL data, serum steroid hormone levels, serum inflammatory proteins, or absolute number of blood-derived cell populations was detected.
3. Discussion
This two-stage case–control association study showed that there is no significant association between GWAS-identified variants for obesity and MM risk. These findings were in agreement with a recent study that, using a Mendelian randomization strategy, demonstrated that SNPs associated with BMI, hip and waist circumference, waist-to-hip ratio, and childhood obesity were not involved in the modulation of MM risk [14]. In line with these negative findings, we neither found a positive correlation between obesity-related SNPs and cQTL data, serum inflammatory protein levels, steroid hormone levels, nor absolute numbers of blood-derived cell populations in the Human Functional Genomics Project (HFGP) cohort, which reinforced the hypothesis suggesting no effect of GWAS-identified SNPs for obesity in modulating MM risk. Interestingly, although it had no effect on MM risk, we found a potentially interesting correlation between the MAFrs1424233 polymorphism and TNFα and IL6 levels after stimulation of macrophages with LPS that confirmed the role of the MAF locus in modulating macrophage-mediated immune responses, a well-known phenomenon. MAF, which is expressed selectively in macrophages, positively regulates IL10 production in these cells after stimulation with LPS [15]. Similarly, it has been reported that MAF induces cytokine production [16] and promotes IL10-mediated anti-inflammatory responses through inhibition of the inflammasome [17]. Finally, another recent study showed that MAF, in addition to participating in the control of IL10 production and lipogenesis, is a negative regulator of IL2, which modulates Th1, Th2 and Th17 immune responses in a context-specific manner [18]. Considering that activated macrophages have the ability to initially produce proinflammatory cytokines (TNF, IL1β, IL6, and IL12) and, subsequently, induce the production of IL10 in response to LPS, it seems conceivable to suggest that genetic markers within the MAF locus might, at least in part, account for the link between obesity, lipogenesis and inflammation.
4. Methods and Materials
4.1. Study Participants
This two-stage case–control association study included 658.359 subjects from four European cohorts. The discovery cohort consisted of 2121 MM cases and 2068 healthy controls recruited from four German clinical trials (GMMG-HD3/ISRCTN064413384, GMMG-HD4/ISRCTN64455289, GMMG-HD5/ISRCTN05745813, and GMMG-HD6/NCT02495922) [19] and a Spanish cohort that consisted of 1293 subjects (206 MM and 1087 healthy controls) recruited at two Spanish medical institutions (Virgen de las Nieves University Hospital, Granada, Spain, and Morales Meseguer Hospital, Murcia, Spain). Demographic and clinical details of the GMMG cohort have been previously published [19], and data regarding the Spanish cohort are included in Supplementary Table S1. The analysis of the discovery cohort was followed by meta-analysis with independent GWAS on 403,268 subjects from the UKBiobank (554 MM cases and 402,714 healthy controls; UKBiobank TOPMed-imputed) and 249,609 from the FinnGen cohort (914 MM cases and 248,695 healthy controls; Risteys7). Details on these GWAS data have been previously reported [20,21]. All MM patients were diagnosed according to the International Myeloma Working Group (IMWG) criteria [2,22,23]. The study was approved by the ethical committee of participant institutions, and all participants gave written informed consent to participate in the study.
4.2. SNP Selection and Genotyping
Genetic variants were selected on the basis of previously published research (Table 2) [24]. Genotyping of selected SNPs was carried out at GENYO (www.genyo.es; Granada, Spain) using KASPar assays (LGC Genomics, Hoddesdon, UK) according to previously reported protocols [25]. For internal quality control, ~5% of samples were randomly selected and included as duplicates. Concordance between the original and the duplicate samples for the selected SNPs was ≥99.0%. The call rate was higher than 90% with the exception of the HIVEP1rs2228213 SNP, which was removed from further analysis.
Table 2.
Selected obesity-related SNPs.
| Gene Name | dbSNP rs# | Effect Allele | Context | References |
|---|---|---|---|---|
| ADCY3 | rs11676272 | G | missense_variant | [26,27,28,29,30,31] |
| AKAP6|NPAS3 | rs17522122 | G | 3_prime_UTR_variant | [32,33,34,35,36] |
| ADPGK |ADPGK-AS | rs7164727 | T | downstream_gene_variant | [32,34,35,37] |
| BDNF|BDNF-AS | rs6265 | A | missense_variant | [29,33,35,38,39,40,41] |
| DNASE1 | rs1053874 | A | missense_variant | [37,42] |
| FAIM2|BCDIN3D | rs7138803 | A | intergenic_variant | [27,29,32,33,34,35,36,37,38,43,44,45,46] |
| FLT3 | rs1933437 | C | missense_variant | [35] |
| FTO | rs1421085 | C | intron_variant | [38] |
| FTO | rs7190492 | A | intron_variant | [40] |
| GNPDA2 | rs10938397 | G | intergenic_variant | [27,29,32,33,34,35,36,37,38,43,44,45,47,48,49,50,51,52] |
| GPRC5B|GPR139|PDILT | rs12444979 | T | intergenic_variant | [44] |
| HIVEP1 | rs2228213 | A | missense_variant | [33,34,35,38] |
| ITH4 | rs4687657 | T | missense_variant | [37] |
| KCTD15 | rs11084753 | A | intergenic_variant | [51] |
| LMOD1 | rs2820312 | A | missense_variant | [34,37,42,51] |
| LOC400652|LOC342784 | rs17782313 | C | intergenic_variant | [32,51,53,54] |
| MAF | rs1424233 | T | regulatory_region_variant | [54] |
| MC4R | rs17700633 | A | n/s | [53] |
| MST1R | rs2230590 | C | missense_variant | [36,37,42] |
| MTCH2 | rs3817334 | T | intron_variant | [32,34,35,36,37,38,44,45] |
| NEGR1|LOC105378797 | rs2815752 | C | intron_variant | [44,51] |
| NPC1|SLC35F4 | rs1805081 | C | missense_variant | [54] |
| NT5C2 | rs11191580 | C | intron_variant | [36] |
| PCSK1 | rs6235 | C | missense_variant | [33] |
| POC5|FLJ35779 | rs2112347 | G | intergenic_variant | [29,32,33,34,35,36,37,38,43,44,45,48,50] |
| SEC16B | rs543874 | G | upstream_gene_variant | [26,27,29,32,33,34,35,36,37,38,44,45,47,49,50,55,56,57] |
| SH2B1 | rs7359397 | T | intron_variant | [44] |
| STK33 | rs10769908 | C | intron_variant | [51] |
| TFAP2B | rs2206277 | A | intron_variant | [29,33,36,38,43,58] |
| TMEM18 | rs6548238 | T | TF_binding_site_variant | [51] |
| TRAF3 | rs10133111 | A | 3_prime_UTR_variant | [36,37] |
| UHRF1BP1 | rs11755393 | G | missense_variant | [37,42] |
| ZZZ3 | rs17381664 | C | intron_variant | [32,35,36,43] |
Abbreviature: SNP, single nucleotide polymorphism.
4.3. Statistical Analysis and Meta-Analysis
The HWE test was performed in the control group (alive subjects) by a standard observed-expected chi-square (χ2) test. Logistic regression analyses adjusted for age and gender were used to assess the effects of the genetic polymorphisms on MM risk in the discovery populations using a log-additive model. A gender-stratified association analysis adjusted for age was also performed to detect the gender-specific effects of selected SNPs on MM risk. All analyses were conducted using STATA (version 20.0). Subsequently, in order to validate the most interesting associations, a meta-analysis of the discovery populations with GWAS data of the UKBiobank and FinnGen cohorts was conducted using METAL. The I2 statistic was used to assess statistical heterogeneity between cohorts. The pooled OR was computed using a fixed-effect model. The Bonferroni method was used to account for multiple testing, and a p-value of 0.0016 (0.05/31 SNPs) was set as the study-wide significance threshold.
4.4. Cell Isolation, Differentiation, and Cytokine Quantitative Trait Loci (cQTL) in Relation to the GWAS-Identified Variants for Obesity
With the aim of determining whether those SNPs associated with obesity at the GWAS level had a role in modulating immune responses, we performed in vitro stimulation experiments and measured cytokine production (interferon (IFN) γ, interleukin (IL) 1Ra, IL1β, IL6, IL8, IL10, TNFα, IL17, and IL22) after stimulation of peripheral blood mononuclear cells (PBMCs), whole blood, or monocyte-derived macrophages (MDMs) from 408 healthy subjects of the 500 functional genomic (500FG) cohort from the HFGP with lipopolysaccharide (LPS; 1 or 100 ng/mL), phytohemagglutinin (PHA; 10 μg/mL), Pam3Cys (10 μg/mL), and CpG (ODN M362; 100 ng/mL) as an experimental model for cytokine production capacity. Details on PBMC isolation, macrophage differentiation and stimulation assays have been reported elsewhere [59,60]. The HFGP study was approved by the Arnhem-Nijmegen Ethical Committee (no. 42561.091.12), and biological specimens were collected after informed consent was obtained. After log transformation, cytokine levels were correlated with the SNPs of interest using a linear regression model with age and sex as co-factors in R (http://www.r-project.org/, accessed on 7 November 2022). A significance threshold of 0.000179 (0.05/31SNPs/9cytokines) was used for the cytokine quantitative trait loci (cQTL) analysis.
4.5. Correlation between GWAS-Identified Polymorphisms and Cell Counts of 91 Blood-Derived Immune Cell Populations and Serum/Plasmatic Proteomic Profile
We also investigated whether the selected polymorphisms had an impact on blood cell counts by analyzing a set of 91 manually annotated immune cell populations and genotype data from the 500FG cohort that consisted of 408 healthy subjects (Supplementary Table S2). Cell populations were measured by 10-color flow cytometry (Navios flow cytometer, Beckman Coulter) after blood sampling (2–3 h), and cell count analysis was performed using the Kaluza software (Beckman Coulter, v.1.3). In order to reduce inter-experimental noise and increase statistical power, cell count analysis was performed by calculating parental and grandparental percentages, which were defined as the percentage of a certain cell type within the cell-populations one or two levels higher in the hierarchical definitions of cell sub-populations [61]. Detailed laboratory protocols for cell isolation, reagents, gating and flow cytometry analysis have been reported elsewhere [62], and the accession number for the raw flow cytometry data and analysed data files are available upon request to the authors (http://hfgp.bbmri.nl). A proteomic analysis was also performed in serum and plasma samples from the 500FG cohort. Circulating proteins were measured using the commercially available Olink Inflammation panel (Olink, Sweden), which resulted in the measurement of 103 different biomarkers (Supplementary Table S3). Protein levels were expressed on a log2-scale as normalized protein expression values and normalized using bridging samples to correct for batch variation. Considering the number of proteins (n = 103) and cell populations (n = 91) tested, p-values of 0.000016 and 0.000017 were, respectively, set as the significant thresholds for the proteomic and cell-level variation analyses.
4.6. Correlation between Obesity-Related SNPs and Serum Steroid Hormones
Finally, we also measured serum levels of seven steroid hormones (androstenedione, cortisol, 11-deoxy-cortisol, 17-hydroxy progesterone, progesterone, testosterone, and 25 hydroxy vitamin D3) in 280 healthy subjects from the 500FG cohort that did not undergo hormone replacement therapy or take oral contraceptives. After log transformation, the correlation between steroid hormone levels and obesity SNPs was evaluated by linear regression analysis adjusted for age and sex. The significance threshold was set to 0.00023 (0.05/31 SNPs/7 hormones). Complete protocol details of steroid hormone measurements have been reported elsewhere [59].
5. Conclusions
In summary, our findings suggest that GWAS-identified variants for obesity do not influence the risk of MM and that the MAF locus might play a role in modulating the inflammatory alterations and lipogenesis, leading to the development of obesity.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076029/s1.
Author Contributions
Conception and design: K.H., A.F., J.S.; Development of methodology: J.S.; Acquisition of data: M.d.P.G., D.C., A.M., S.C., A.J., F.C., M.G.N., Y.L., K.H., A.F., J.S.; Functional data: M.G.N., R.T.H., Y.L.; Analysis and interpretation of data: J.M.S.-M., A.J.C.-S., R.T.H., J.S.; Writing, review, and/or revision of the manuscript: J.M.S.-M., A.J.C.-S., A.F., J.S.; Administrative, technical, or material support: J.M.S.-M., R.T.H., D.C., F.C., A.J., K.H., A.F.; Study supervision: A.F., J.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Boards of all institutions participating in patient recruitment.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Genetic data from the Spanish cohort are available at the GENYO data infrastructure (https://ftp.genyo.es/, accessed on 20 March 2023). These data are available upon reasonable request. Data from the German population are available from four German clinical trials (GMMG-HD3/ISRCTN064413384, GMMG-HD4/ISRCTN64455289, GMMG-HD5/ISRCTN05745813 and GMMG-HD6/NCT02495922). Functional data used in this study are available at the BBMRI-NL data infrastructure (https:// hfgp.bbmri.nl/, accessed on 2 July 2021), where they have been meticulously catalogued and archived using the MOLGENIS open-source platform for scientific data. This allows flexible data querying and downloading, including sufficiently rich metadata and interfaces for machine processing (R statistics, REST API) using FAIR principles to optimize Findability, Accessibility, Interoperability, and Reusability. These datasets are not publicly available because they contain information that could compromise the research participants’ privacy.
Conflicts of Interest
The authors declare that no conflict of interest exists in relation to the work described.
Funding Statement
This work was supported by grants from the Instituto de Salud Carlos III (Madrid, Spain; PI17/02256 and PI20/01845), from the Consejería de Salud y Familia de la Junta de Andalucía (PY20/01282) and from the Dietmar Hopp Foundation and the German Ministry of Education and Science (BMBF: CLIOMMICS (01ZX1309)).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Palumbo A., Anderson K. Multiple myeloma. N. Engl. J. Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020;95:548–567. doi: 10.1002/ajh.25791. [DOI] [PubMed] [Google Scholar]
- 3.Barnidge D.R., Tschumper R.C., Theis J.D., Snyder M.R., Jelinek D.F., Katzmann J.A., Dispenzieri A., Murray D.L. Monitoring M-proteins in patients with multiple myeloma using heavy-chain variable region clonotypic peptides and LC-MS/MS. J. Proteome Res. 2014;13:1905–1910. doi: 10.1021/pr5000544. [DOI] [PubMed] [Google Scholar]
- 4.Basen-Engquist K., Chang M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Maskarinec G., Erber E., Gill J., Cozen W., Kolonel L.N. Overweight and obesity at different times in life as risk factors for non-Hodgkin’s lymphoma: The multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2008;17:196–203. doi: 10.1158/1055-9965.EPI-07-0716. [DOI] [PubMed] [Google Scholar]
- 7.Chiu B.C., Soni L., Gapstur S.M., Fought A.J., Evens A.M., Weisenburger D.D. Obesity and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2007;18:677–685. doi: 10.1007/s10552-007-9013-9. [DOI] [PubMed] [Google Scholar]
- 8.Larsson S.C., Wolk A. Obesity and risk of non-Hodgkin’s lymphoma: A meta-analysis. Int. J. Cancer. 2007;121:1564–1570. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 9.Skibola C.F. Obesity, diet and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2007;16:392–395. doi: 10.1158/1055-9965.EPI-06-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtman M.A. Obesity and the risk for a hematological malignancy: Leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083–1101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strom S.S., Yamamura Y., Kantarijian H.M., Cortes-Franco J.E. Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol. Biomark. Prev. 2009;18:1501–1506. doi: 10.1158/1055-9965.EPI-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillers-Ziemer L.E., McMahon R.Q., Hietpas M., Paderta G., LeBeau J., McCready J., Arendt L.M. Obesity Promotes Cooperation of Cancer Stem-Like Cells and Macrophages to Enhance Mammary Tumor Angiogenesis. Cancers. 2020;12:502. doi: 10.3390/cancers12020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter A., Kreis N.N., Hoock S.C., Solbach C., Louwen F., Yuan J. Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells, Obesity and the Tumor Microenvironment of Breast Cancer. Cancers. 2022;14:3908. doi: 10.3390/cancers14163908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Went M., Sud A., Law P.J., Johnson D.C., Weinhold N., Forsti A., van Duin M., Mitchell J.S., Chen B., Kuiper R., et al. Assessing the effect of obesity-related traits on multiple myeloma using a Mendelian randomisation approach. Blood Cancer J. 2017;7:e573. doi: 10.1038/bcj.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao S., Liu J., Song L., Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J. Immunol. 2005;174:3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trabanelli S., Ercolano G., Wyss T., Gomez-Cadena A., Falquet M., Cropp D., Imbratta C., Leblond M.M., Salvestrini V., Curti A., et al. c-Maf enforces cytokine production and promotes memory-like responses in mouse and human type 2 innate lymphoid cells. EMBO J. 2022;41:e109300. doi: 10.15252/embj.2021109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T.H., Yang K., Kim M., Kim H.S., Kang J.L. Apoptosis inhibitor of macrophage (AIM) contributes to IL-10-induced anti-inflammatory response through inhibition of inflammasome activation. Cell Death Dis. 2021;12:19. doi: 10.1038/s41419-020-03332-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrysova L., Alvarez-Martinez M., Luisier R., Cox L.S., Sodenkamp J., Hosking C., Perez-Mazliah D., Whicher C., Kannan Y., Potempa K., et al. c-Maf controls immune responses by regulating disease-specific gene networks and repressing IL-2 in CD4(+) T cells. Nat. Immunol. 2018;19:497–507. doi: 10.1038/s41590-018-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinhold N., Meissner T., Johnson D.C., Seckinger A., Moreaux J., Forsti A., Chen B., Nickel J., Chubb D., Rawstron A.C., et al. The 7p15.3 (rs4487645) association for multiple myeloma shows strong allele-specific regulation of the MYC-interacting gene CDCA7L in malignant plasma cells. Haematologica. 2015;100:e110–e113. doi: 10.3324/haematol.2014.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagliano Taliun S.A., VandeHaar P., Boughton A.P., Welch R.P., Taliun D., Schmidt E.M., Zhou W., Nielsen J.B., Willer C.J., Lee S., et al. Exploring and visualizing large-scale genetic associations by using PheWeb. Nat. Genet. 2020;52:550–552. doi: 10.1038/s41588-020-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Maldonado J.M., Collado R., Cabrera-Serrano A.J., Ter Horst R., Galvez-Montosa F., Robles-Fernandez I., Arenas-Rodriguez V., Cano-Gutierrez B., Bakker O., Bravo-Fernandez M.I., et al. Type 2 Diabetes-Related Variants Influence the Risk of Developing Prostate Cancer: A Population-Based Case-Control Study and Meta-Analysis. Cancers. 2022;14:2376. doi: 10.3390/cancers14102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003;121:749–757. doi: 10.1046/j.1365-2141.2003.04355.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 24.Sainz J., Frank B., da Silva Filho M.I., Hoffmeister M., Rudolph A., Butterbach K., Chang-Claude J., Brenner H., Hemminki K., Forsti A. GWAS-identified common variants for obesity are not associated with the risk of developing colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2014;23:1125–1128. doi: 10.1158/1055-9965.EPI-13-1354. [DOI] [PubMed] [Google Scholar]
- 25.Rios-Tamayo R., Lupianez C.B., Campa D., Hielscher T., Weinhold N., Martinez-Lopez J., Jerez A., Landi S., Jamroziak K., Dumontet C., et al. A common variant within the HNF1B gene is associated with overall survival of multiple myeloma patients: Results from the IMMEnSE consortium and meta-analysis. Oncotarget. 2016;7:59029–59048. doi: 10.18632/oncotarget.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J., Nishimura K.K., Fernandez-Rhodes L., Haessler J., Bien S., Graff M., Lim U., Lu Y., Gross M., Fornage M., et al. Trans-ethnic analysis of metabochip data identifies two new loci associated with BMI. Int. J. Obes. 2018;42:384–390. doi: 10.1038/ijo.2017.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felix J.F., Bradfield J.P., Monnereau C., van der Valk R.J., Stergiakouli E., Chesi A., Gaillard R., Feenstra B., Thiering E., Kreiner-Moller E., et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 2016;25:389–403. doi: 10.1093/hmg/ddv472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stergiakouli E., Gaillard R., Tavare J.M., Balthasar N., Loos R.J., Taal H.R., Evans D.M., Rivadeneira F., St Pourcain B., Uitterlinden A.G., et al. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 2014;22:2252–2259. doi: 10.1002/oby.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shungin D., Winkler T.W., Croteau-Chonka D.C., Ferreira T., Locke A.E., Magi R., Strawbridge R.J., Pers T.H., Fischer K., Justice A.E., et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helgeland O., Vaudel M., Sole-Navais P., Flatley C., Juodakis J., Bacelis J., Koloen I.L., Knudsen G.P., Johansson B.B., Magnus P., et al. Characterization of the genetic architecture of infant and early childhood body mass index. Nat. Metab. 2022;4:344–358. doi: 10.1038/s42255-022-00549-1. [DOI] [PubMed] [Google Scholar]
- 31.Warrington N.M., Howe L.D., Paternoster L., Kaakinen M., Herrala S., Huikari V., Wu Y.Y., Kemp J.P., Timpson N.J., St Pourcain B., et al. A genome-wide association study of body mass index across early life and childhood. Int. J. Epidemiol. 2015;44:700–712. doi: 10.1093/ije/dyv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graff M., Scott R.A., Justice A.E., Young K.L., Feitosa M.F., Barata L., Winkler T.W., Chu A.Y., Mahajan A., Hadley D., et al. Genome-wide physical activity interactions in adiposity—A meta-analysis of 200,452 adults. PLoS Genet. 2017;13:e1006528. doi: 10.1371/journal.pgen.1006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulit S.L., Stoneman C., Morris A.P., Wood A.R., Glastonbury C.A., Tyrrell J., Yengo L., Ferreira T., Marouli E., Ji Y., et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019;28:166–174. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann T.J., Choquet H., Yin J., Banda Y., Kvale M.N., Glymour M., Schaefer C., Risch N., Jorgenson E. A Large Multiethnic Genome-Wide Association Study of Adult Body Mass Index Identifies Novel Loci. Genetics. 2018;210:499–515. doi: 10.1534/genetics.118.301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler T.W., Justice A.E., Graff M., Barata L., Feitosa M.F., Chu S., Czajkowski J., Esko T., Fall T., Kilpelainen T.O., et al. The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study. PLoS Genet. 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice A.E., Winkler T.W., Feitosa M.F., Graff M., Fisher V.A., Young K., Barata L., Deng X., Czajkowski J., Hadley D., et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 2017;8:14977. doi: 10.1038/ncomms14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama M., Okada Y., Kanai M., Takahashi A., Momozawa Y., Ikeda M., Iwata N., Ikegawa S., Hirata M., Matsuda K., et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 2017;49:1458–1467. doi: 10.1038/ng.3951. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson T., Rask-Andersen M., Pan G., Hoglund J., Wadelius C., Ek W.E., Johansson A. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat. Med. 2019;25:1390–1395. doi: 10.1038/s41591-019-0563-7. [DOI] [PubMed] [Google Scholar]
- 40.Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A., Styrkarsdottir U., Gretarsdottir S., Thorlacius S., Jonsdottir I., et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z., Guo Y., Shi H., Liu C.L., Panganiban R.A., Chung W., O’Connor L.J., Himes B.E., Gazal S., Hasegawa K., et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 2020;145:537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turcot V., Lu Y., Highland H.M., Schurmann C., Justice A.E., Fine R.S., Bradfield J.P., Esko T., Giri A., Graff M., et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 2018;50:26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berndt S.I., Gustafsson S., Magi R., Ganna A., Wheeler E., Feitosa M.F., Justice A.E., Monda K.L., Croteau-Chonka D.C., Day F.R., et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013;45:501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Lango Allen H., Lindgren C.M., Luan J., Magi R., et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood A.R., Tyrrell J., Beaumont R., Jones S.E., Tuke M.A., Ruth K.S., Consortium G., Yaghootkar H., Freathy R.M., Murray A., et al. Variants in the FTO and CDKAL1 loci have recessive effects on risk of obesity and type 2 diabetes, respectively. Diabetologia. 2016;59:1214–1221. doi: 10.1007/s00125-016-3908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heard-Costa N.L., Zillikens M.C., Monda K.L., Johansson A., Harris T.B., Fu M., Haritunians T., Feitosa M.F., Aspelund T., Eiriksdottir G., et al. NRXN3 is a novel locus for waist circumference: A genome-wide association study from the CHARGE Consortium. PLoS Genet. 2009;5:e1000539. doi: 10.1371/journal.pgen.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Rhodes L., Graff M., Buchanan V.L., Justice A.E., Highland H.M., Guo X., Zhu W., Chen H.H., Young K.L., Adhikari K., et al. Ancestral diversity improves discovery and fine-mapping of genetic loci for anthropometric traits-The Hispanic/Latino Anthropometry Consortium. HGG Adv. 2022;3:100099. doi: 10.1016/j.xhgg.2022.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin S., Cule M., Basty N., Tyrrell J., Beaumont R.N., Wood A.R., Frayling T.M., Sorokin E., Whitcher B., Liu Y., et al. Genetic Evidence for Different Adiposity Phenotypes and Their Opposing Influences on Ectopic Fat and Risk of Cardiometabolic Disease. Diabetes. 2021;70:1843–1856. doi: 10.2337/db21-0129. [DOI] [PubMed] [Google Scholar]
- 49.Ng M.C.Y., Graff M., Lu Y., Justice A.E., Mudgal P., Liu C.T., Young K., Yanek L.R., Feitosa M.F., Wojczynski M.K., et al. Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet. 2017;13:e1006719. doi: 10.1371/journal.pgen.1006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakaue S., Kanai M., Tanigawa Y., Karjalainen J., Kurki M., Koshiba S., Narita A., Konuma T., Yamamoto K., Akiyama M., et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021;53:1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PubMed] [Google Scholar]
- 51.Willer C.J., Speliotes E.K., Loos R.J., Li S., Lindgren C.M., Heid I.M., Berndt S.I., Elliott A.L., Jackson A.U., Lamina C., et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couto Alves A., De Silva N.M.G., Karhunen V., Sovio U., Das S., Taal H.R., Warrington N.M., Lewin A.M., Kaakinen M., Cousminer D.L., et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 2019;5:eaaw3095. doi: 10.1126/sciadv.aaw3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loos R.J., Lindgren C.M., Li S., Wheeler E., Zhao J.H., Prokopenko I., Inouye M., Freathy R.M., Attwood A.P., Beckmann J.S., et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyre D., Delplanque J., Chevre J.C., Lecoeur C., Lobbens S., Gallina S., Durand E., Vatin V., Degraeve F., Proenca C., et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat. Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 55.Graff M., Ngwa J.S., Workalemahu T., Homuth G., Schipf S., Teumer A., Volzke H., Wallaschofski H., Abecasis G.R., Edward L., et al. Genome-wide analysis of BMI in adolescents and young adults reveals additional insight into the effects of genetic loci over the life course. Hum. Mol. Genet. 2013;22:3597–3607. doi: 10.1093/hmg/ddt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y., Day F.R., Gustafsson S., Buchkovich M.L., Na J., Bataille V., Cousminer D.L., Dastani Z., Drong A.W., Esko T., et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 2016;7:10495. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monda K.L., Chen G.K., Taylor K.C., Palmer C., Edwards T.L., Lange L.A., Ng M.C., Adeyemo A.A., Allison M.A., Bielak L.F., et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat. Genet. 2013;45:690–696. doi: 10.1038/ng.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradfield J.P., Vogelezang S., Felix J.F., Chesi A., Helgeland O., Horikoshi M., Karhunen V., Lowry E., Cousminer D.L., Ahluwalia T.S., et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 2019;28:3327–3338. doi: 10.1093/hmg/ddz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ter Horst R., Jaeger M., Smeekens S.P., Oosting M., Swertz M.A., Li Y., Kumar V., Diavatopoulos D.A., Jansen A.F.M., Lemmers H., et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell. 2016;167:1111–1124.e1113. doi: 10.1016/j.cell.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Oosting M., Smeekens S.P., Jaeger M., Aguirre-Gamboa R., Le K.T.T., Deelen P., Ricano-Ponce I., Schoffelen T., Jansen A.F.M., et al. A Functional Genomics Approach to Understand Variation in Cytokine Production in Humans. Cell. 2016;167:1099–1110.e1014. doi: 10.1016/j.cell.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Orru V., Steri M., Sole G., Sidore C., Virdis F., Dei M., Lai S., Zoledziewska M., Busonero F., Mulas A., et al. Genetic variants regulating immune cell levels in health and disease. Cell. 2013;155:242–256. doi: 10.1016/j.cell.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aguirre-Gamboa R., Joosten I., Urbano P.C.M., van der Molen R.G., van Rijssen E., van Cranenbroek B., Oosting M., Smeekens S., Jaeger M., Zorro M., et al. Differential Effects of Environmental and Genetic Factors on T and B Cell Immune Traits. Cell Rep. 2016;17:2474–2487. doi: 10.1016/j.celrep.2016.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genetic data from the Spanish cohort are available at the GENYO data infrastructure (https://ftp.genyo.es/, accessed on 20 March 2023). These data are available upon reasonable request. Data from the German population are available from four German clinical trials (GMMG-HD3/ISRCTN064413384, GMMG-HD4/ISRCTN64455289, GMMG-HD5/ISRCTN05745813 and GMMG-HD6/NCT02495922). Functional data used in this study are available at the BBMRI-NL data infrastructure (https:// hfgp.bbmri.nl/, accessed on 2 July 2021), where they have been meticulously catalogued and archived using the MOLGENIS open-source platform for scientific data. This allows flexible data querying and downloading, including sufficiently rich metadata and interfaces for machine processing (R statistics, REST API) using FAIR principles to optimize Findability, Accessibility, Interoperability, and Reusability. These datasets are not publicly available because they contain information that could compromise the research participants’ privacy.