Abstract
Purpose of Review
Increased risk of type 2 diabetes mellitus (T2D) among individuals with overweight or obesity is well-established; however, questions remain about the temporal dynamics of weight change (gain or loss) on the natural course of T2D in this at-risk population. Existing epidemiologic evidence is limited to studies that discretely sample and assess excess weight and T2D risk at different ages with limited follow-up, yet changes in weight may have time-varying and possibly non-linear effects on T2D risk. Predicting the impact of weight change on the risk of T2D is key to informing primary prevention. We critically review the relationship between weight change, trajectory groups (i.e., distinct weight change patterns), and T2D risk among individuals with excess weight in recently published T2D prevention randomized controlled trials (RCTs) and longitudinal cohort studies.
Recent Findings
Overall, weight trajectory groups have been shown to differ by age of onset, sex, and patterns of insulin resistance or beta-cell function biomarkers. Lifestyle (diet and physical activity), pharmacological, and surgical interventions can modify an individual’s weight trajectory. Adolescence is a critical etiologically relevant window during which onset of excess weight may be associated with higher risk of T2D. Changes in insulin resistance and beta-cell function biomarkers are distinct but related correlates of weight trajectory groups that evolve contemporaneously over time. These multi-trajectory markers are differentially associated with T2D risk.
Summary
T2D risk may differ by the age of onset and duration of excess body weight, and the type of weight loss intervention. A better understanding of the changes in weight, insulin sensitivity, and beta-cell function as distinct but related correlates of T2D risk that evolve contemporaneously over time has important implications for designing and targeting primary prevention efforts.
Keywords: Weight change, Weight trajectory, Type 2 diabetes mellitus, Weight loss interventions
Introduction
Excess body weight defined by body mass index [BMI] as overweight or obesity is associated with an increased risk of type 2 diabetes mellitus (T2D) [1]. T2D is a heterogeneous and multifactorial chronic disease characterized by hyperglycemia resulting from the combination of peripheral tissue resistance to insulin action and inadequate insulin secretion (i.e., beta-cell dysfunction). Among susceptible individuals, excess weight effects on T2D may be mediated by insulin resistance and beta-cell malfunction. These mediation (indirect) effects may be stronger with adiposity [2]. The risk of T2D is elevated among individuals with increased abdominal visceral and subcutaneous fat depots, and intrahepatic triglyceride content independent of excess body weight [3–5]. Conversely, greater subcutaneous leg fat is positively correlated with lower T2D risk with or without excess weight [5–7].
While there is evidence from prospective cohort studies that the risk of T2D is higher among individuals with overweight (risk ratio [RR]: 2.99; 95% CI: 2.42–3.72) or obesity (RR: 7.19; 95% CI: 5.74–9.00) than that with normal weight [1], the clinical (i.e., differences between study participants) and methodological (i.e., differences in the measurement, timing, and definition of excess weight or T2D) diversities contributed to heterogeneity of the measures of association. The effects of misclassifying both excess weight and T2D on delineating causal relations can be unpredictable [8, 9]. Moreover, independent of excess weight, normal life course fluctuations in insulin sensitivity and resistance are expected during puberty [10], pregnancy [11], post menopause [12], and with older age [13]. Without appropriate control for these potential confounding factors, delineating the causal relation between weight changes and T2D risk in observational studies remains problematic. While weight gain and loss are associated with increased and decreased T2D risk, respectively, among individuals with excess weight and/or adiposity, it remains unclear whether the temporal dynamics of life course weight change have transient or sustained effects on the natural course of T2D.
Weight Loss Interventions and T2D Risk Among Individuals with Excess Weight
Lifestyle (diet and physical activity), pharmacological, and surgical interventions can be used to promote and maintain weight loss [14–16], and to reduce the risk of T2D as demonstrated in numerous large RCT studies [17–23]. Sustained weight loss for more than 12–36 months has been shown to improve insulin action (sensitivity) and improve glucose tolerance [24, 25].
There is moderate-to-strong evidence that specialized dietary interventions involving low-fat and/or low-carbohydrate diets [26], very-low-energy diets [27], Mediterranean diet [28], alternative-day fasting [29], and intermittent fasting [30] promote short-term weight loss. However, the success of any diet relies primarily on adherence, which tends to be poor in the long term [31]. While some of the evidence points to reduced T2D risk via mechanisms linked to increased glucose tolerance or insulin sensitivity, weight loss does not always translate to reduced T2D risk [26–28, 32]. Moreover, the evidence for any dietary intervention on glycemic outcomes independent of weight loss is weak and different mechanisms are postulated for how different diets impact insulin resistance or beta-cell dysfunction [26–28, 32]. When controlled for total daily energy intake, diets of different macronutrient compositions are associated with similar weight loss but with differential effects on the reduction of T2D risk factors. In the POUNDS LOST trial [33], diets with different compositions of fat, protein, and carbohydrates led to similar weight loss at 6 months and similar weight regain at 2 years. The reduction in fasting serum insulin levels was larger with the high-protein diet than that with the average-protein diet (10% vs 4%, p = 0.07) [33]. In the PREVention of diabetes through lifestyle intervention and population studies In Europe and around the World (PREVIEW) study, different weight maintenance diets, i.e., a high-protein (HP) and low glycemic index (GI) diet versus a moderate protein (MP) and moderate GI diet, combined with either high intensity (HI) or moderate intensity physical activity (PA) did not result in substantial differences for percent body weight change. On average, across diet groups, there were − 11% and − 5% body weight changes after an 8-week weight reduction and 3-year weight maintenance intervention period, respectively [17]. Although adherence to target diet intakes over the 3-year follow-up period varied among participants, this study showed lower T2D risk (<4%) for all intervention groups (diet, physical activity, or both) than was expected under scenarios of no intervention (10–16%) [17, 34]. However, after adjusting for differences in weight loss, men had larger reductions in metabolic syndrome Z-scores than women [34].
Lifestyle-focused interventions have been shown to induce clinically significant weight loss over approximately 3–4 years [23, 35]. However, as highlighted in the Diabetes Prevention Study (DPS), the protective effects of intervention (versus control) on T2D risk dissipates with longer follow-up (i.e., 58% [23] and 33% [19] lower risk of T2D at 4 and 13 years, respectively). Similarly, the US-based multicenter Diabetes Prevention Program (DPP) study showed that an intensive lifestyle change program, which led to an average weight loss of approximately 6%, reduced the risk of developing T2D by 58% (95%CI: 48–66%) compared to placebo (standard lifestyle recommendations) after 2.8 years [35]. After 10 years, the intensive lifestyle group still had a lower incidence of T2D than the placebo group, but the between-group difference was smaller (34%; 95%CI: 24–42%) [36].
Weight loss achieved by pharmacotherapy interventions has been shown to consistently improve glycemic control in individuals with overweight/obesity with greater HbA1c reduction in those with T2D in numerous RCTs [37]. However, there have been fewer RCTs that examined the effect of pharmacotherapy in reducing the risk of T2D. In the SCALE obesity and prediabetes trial [22], 2254 adults with prediabetes and excess weight were randomly assigned to daily subcutaneous injections of liraglutide 3.0 mg (n = 1505) or placebo (n = 749). Liraglutide was associated with an average weight loss of 4.3% compared to placebo after 160 weeks. The time to onset of T2D was 2.7 times longer (p < 0.01) and the risk of T2D was 79% (hazard ratio [HR]: 0.21; 95% CI: 0.13–0.34) lower in the liraglutide group than those in the placebo group. In another trial [20], a total of 309 (out of 383) individuals with obesity, who lost ≥ 5% of their body weight initially achieved an average weight loss of 14.4 kg after 8-week treatment with a very-low-energy diet, were randomized to orlistat 120 mg or placebo. The average weight regain (4.6 vs 7.0 kg) and cumulative T2D incidence (5.2% vs 10.9%) were lower with orlistat vs placebo at 3-year follow-up. In the largest and longest RCT that examined antiobesity pharmacotherapy for prevention of T2D, a total of 3305 individuals with obesity were randomized to orlistat or placebo in addition to lifestyle counseling. At 4-year follow-up, orlistat was associated with a higher proportion of participants who lost ≥ 5% of their baseline body weight (52.8% vs 37.3%), greater average weight loss (5.8 vs 3.0 kg), and a lower cumulative incidence of T2D than placebo (6.2% vs 9.0%) corresponding to a risk reduction of 37.3% [38]. In the DPP study, weight loss with metformin explained 64% of its beneficial effect on T2D risk [39]. Overall, these pharmacotherapy interventions (e.g., liraglutide, orlistat) are hypothesized to function as a glucagon-like peptide-1 (GLP-1) receptor agonist, a hormone that regulates appetite, to induce gut microbiome dysbiosis. These antiobesity drugs modulate the composition of gut microbiota (i.e., decrease obesity-related but increase lean-related phenotypes) and modify metabolic signaling pathways that influence insulin sensitivity or resistance related to the risk of T2D.
Bariatric surgery, which induces large and sustained weight loss, has been shown to reduce T2D risk. In the Swedish Obesity Study (SOS) [21], 1658 individuals who underwent bariatric surgery and had an average weight loss of 31 kg after 1 year and 1771 controls who were matched for obesity and received usual care were compared at 15-year follow-up. Despite partial weight regain, the risk of T2D was 83% lower in the bariatric surgery group than that in the control group (HR: 0.17; 95% CI, 0.13–0.21). This protective effect differed depending on the presence (HR: 0.13; 95%CI: 0.09–0.18) or absence (HR: 0.25; 95%CI: 0.19–0.32) of impaired fasting glucose at baseline, but not baseline excess weight status.
Weight Trajectory Groups and the Risk of T2D Among Individuals with Excess Weight
The characterization of weight change patterns (i.e., trajectory groups) with and without interventions is critical to understanding the long-term effects of weight change on T2D risk. The use of objective and reproducible methods to delineate the weight trajectory groups is vital if we are to understand the different multifactorial pathways of T2D development. Particularly, the role of correlates of weight trajectory groups that could promote or reduce the risk of T2D, such as diet, sedentary behavior, sleep, and aging. The best ways to delineate subtypes or subgroups of individuals with excess weight who share the same features of T2D disease natural history have been the subject of study for decades, and refinement is ongoing [40, 41]. Numerous and sometimes conflicting weight trajectory groups have been proposed without clarity of clinical relevance to T2D [42–49]. These weight trajectory groups have traditionally been based on body mass index (BMI) and weight measured in kilograms or pounds.
Latent class growth analysis (LCGA) [50, 51] or group-based trajectory modeling (GBTM) and growth mixture modeling (GMM) [52, 53] can be used to characterize weight trajectory groups beyond pre-post intervention weight assessments. Weight trajectory groups derived from these methods can be used to identify critical periods of weight change that may be etiologically relevant to T2D risk. These models can provide more nuanced insights into the temporal dynamics between excess weight and correlates of T2D risk. Both the LCGA and GMM models assume the existence of mutually exclusive and exhaustive groups of individuals that can be differentiated by the presence or absence of latent “unobserved” traits that follow distinct developmental pathways (i.e., weight change patterns).
LCGA is a finite mixture semi-parametric model [50, 51] whereby individual weight change patterns within a subgroup are assumed to be homogenous, weight change over time is modeled on a continuous scale (i.e., scaled growth), polynomial restrictions (e.g., quadratic and cubic functions) are placed on the shape of a subgroup (trajectory group), and the variance and covariance estimates for the growth factors (i.e., intercept and slope) within a trajectory group are assumed to be fixed to zero.
In contrast to LCGA, GMM is a finite mixture parametric model [52, 53] that allows for individual-level variation within subgroups that are characterized by different growth patterns (i.e., random effects – intercepts and slopes). Individuals are assigned to the “latent” trajectory group with the highest probability of describing the subject’s weight change pattern.
Previous studies [42–49] have shown that correlates of T2D risk may vary across different weight trajectory groups; however, to our knowledge, no published research has assessed the strength of evidence linking T2D risk to different weight trajectory groups. LGCA and GMM approaches have identified three [46–48] to seven [42] weight trajectory groups with different shapes defined by an increase or decrease in weight relative to excess weight status among individuals 13 + years of age.
Recently, using GMM, Luo et al. [42] identified seven weight trajectory groups defined by excess weight status with a stable, modest, or rapid increase in a cohort of middle-aged Australian women (Australian Longitudinal Study on Women’s Health [ALSWH], n = 12,302). Compared to the stable normal weight trajectory group, other trajectory groups (e.g., overweight or obesity at baseline with a modest or rapid increase during follow-up) had higher risk of T2D (HR range: 2.06 to 8.35) over a 16-year follow-up period. Older age of onset and shorter duration of excess weight were associated with lower risk of T2D. A separate analysis (n = 11,192) showed that even among adolescents and young adults (18–23 years) [43], although only six distinct trajectory groups were identified, older age at onset of excess weight was negatively associated with risk of T2D (HR: 0.87; 95% CI 0.79–0.96) and duration of excess weight was positively and linearly associated with T2D risk (p < 0.01). The cumulative incidence of T2D was much lower among adolescents and young adults than that among middle-aged women (1.5% [43] versus 11.2% [42], p < 0.01) during the 16-year study follow-up period.
In a younger cohort (n = 1387; 13–18-year olds) [44], Wu et al. showed that the risk of T2D was higher among participants with overweight (HR: 5.31; 95% CI: 2.16–13.08) and obesity (HR: 10.01; 95% CI: 3.70–27.11) during early childhood defined by tri-ponderal mass index (TMI) than that among participants who had normal weight. Similar risk was observed even when the obesity definition was determined by the BMI Z-score. Compared to a stable weight trajectory group, adolescents in the “rapid decrease,” “rapid increase and then stable,” and “persistent increase” weight trajectory groups had higher risk of T2D in early adulthood. A higher proportion of male than female participants were observed in the “rapid decrease,” “rapid increase then stable,” and “persistent increase” trajectory groups (p = 0.04). These trajectory groups also had higher baseline blood pressure (p < 0.01), cholesterol (p = 0.01), and triglyceride levels (p < 0.01) than the stable weight group. In contrast, these covariates and the risk of T2D were not different between BMI Z-score–defined trajectory groups. These findings suggest that TMI- (vs BMI-) defined weight trajectory groups during adolescence might provide better discrimination for T2D incidence. This further underscores the fact that the relationship between the body fat percentage and the mass divided by height squared is quite different in adolescent vs adult populations. In addition, findings lend credence to the hypothesis that during adolescence beta-cell dysfunction or insulin resistance due to excess weight could persist even after subsequent weight reduction. It is also possible that the pathophysiological mechanisms that predispose some adolescents to T2D may only be partially related to obesity [54]. Other causal mechanisms suspected involve genetic susceptibility, puberty-induced insulin sensitivity, poor diet, sedentariness, fetal exposure to maternal obesity, and gestational diabetes [54].
In contrast to the findings by Luo [43] and Wu [44] et al., a cohort study by Tirosh et al. (n = 37,674) [45] showed that as adolescents transition into adulthood, increasing BMI-defined weight trajectory groups are equivalent to an annual rate of rise in BMI of about 0.3 BMI units per year at 17 years of age, corresponding to a total of 15 kg, or 4 BMI units with a 13-year follow-up. However, after adjustment for time-varying BMI assessments during adulthood, elevated BMI in adolescence was not associated with higher risk of T2D. This suggests that T2D risk may be a function of increased BMI closer to the time of T2D diagnosis than BMI at earlier assessments during adolescence [45].
In a cohort of individuals with and without excess weight at baseline, Lv et al. [46] identified three GMM-based BMI-defined trajectory groups (low-increasing [n = 5136], medium-increasing [n = 1914], and high-increasing [n = 239]) in a Chinese cohort of 20–50 years old individuals who were followed for about 11 ± 5 years. Compared to the low-increasing group, belonging to the medium-increasing and high-increasing group was associated with a higher risk of T2D (HR: 1.40; 95% CI: 1.00–1.97 and 2.45; 95% CI: 1.32–4.56, respectively). Higher baseline and long-term BMI levels were associated with increased risk of T2D. Overall, trajectory group-specific slopes increased gradually from 0.64 to 0.82 (BMI units) during 20–30 years, then decreased from 0.81 to 0.13 during 31–41 years, and finally increased negatively to − 0.68 until 50 years. These results suggest that increased velocity of weight change is positively correlated with T2D risk, independent of baseline BMI levels. However, these findings should be interpreted cautiously because of the potential for unmeasured confounding effects (e.g., sleep apnea). Moreover, findings may not be generalizable to non-Asian populations.
Using GMM, applied to a British cohort (N = 6705) of individuals without T2D between the ages 35 to 55 years, Vistisen et al. [47] identified three weight trajectory groups among incident cases of T2D (n = 645; 9.6%) defined by BMI during a median follow-up of 14 years: stable overweight, progressive weight gainers, and persistently obese. The stable overweight group (n = 604; 94%) experienced slightly worsening of beta-cell function and insulin sensitivity 5 years prior to a T2D diagnosis. The progressive weight gainers (n = 15) exhibited a pattern of consistent weight gain before a T2D diagnosis that increased linearly with blood pressure and exponentially with insulin resistance a few years before a T2D diagnosis. The persistently obese (n = 26) experienced an initial beta-cell compensation followed by loss of beta-cell function, whereas insulin sensitivity was relatively stable. Female participants were more likely to belong to the persistently obese group than in the stable overweight or T2D-free group (p < 0.02). The stable overweight and progressive weight gain groups had a higher prevalence of family history than the T2D-free group (p < 0.04). Consistent with Tirosh et al. [45], the majority of individuals diagnosed with T2D did not have substantial weight gain prior to a T2D diagnosis (stable overweight group). However, T2D incidence was preceded by different patterns of excess weight and insulin resistance; these findings underscore the heterogeneity of pathophysiological pathways associated with T2D [55].
With a 14-year follow-up, Nano et al. [48] found a 9% cumulative incidence of T2D in a cohort of 6223 individuals who were 55 + years old. Three BMI-defined distinct trajectory groups were identified, including a progressive overweight (n = 481; 85.1%), progressive weight loss (n = 59; 10.4%), and persistently high BMI group (n = 25; 4.4%). The persistently high BMI group was characterized by a concave up increasing function of insulin resistance and a rapid decrease of beta-cell function. This group also had an increase in T2D incidence from 6 to 19% during an 8-year follow-up period. The progressive overweight group had a smaller increase in T2D risk from 2 to 14%, had fluctuations in insulin resistance, and marked beta-cell dysfunction. Similar to Vistisen et al.’s study [47], most incident T2D cases had excess weight at baseline and continued to gain weight during follow-up. T2D incidence was not preceded by a recent weight gain but rather preceding sustained weight gain. These findings highlight the challenges in both predicting and targeting T2D prevention interventions since many at-risk individuals with overweight or normal weight [56] might be missed when using existing screening strategies. Strategies with higher predictive properties are needed to better discriminate between high- and low-risk weight trajectory groups based not only on BMI change but at least one other biomarker of insulin resistance or beta-cell function.
Using LCGA, Yacamán-Méndez et al. [49] categorized 7203 Danish participants ages 7–55 years from the Stockholm Diabetes Prevention Program cohort into five trajectory groups: stable normal weight, stable overweight trajectory, lean increasing weight, overweight from early adulthood, and overweight from late adulthood trajectory. Compared to the stable normal group, a higher fraction of T2D risk was attributed to other trajectory groups among women (population attributable fraction [PAF] 47.40%; 95% CI 38.06–55.34%) and men (PAF: 42.91%; 95% CI 31.47–52.45%). The cumulative incidence of T2D was 65% higher with onset of excess weight during early than late adulthood (RR: 1.65; 95%CI: 1.40, 1.95).
Methodologic and Conceptual Issues Associated with Attributing T2D Risk to Changes in Weight
Random assignment of individuals to levels of an independent variable of interest (i.e., intervention) is critically important to determine the causal effects [57, 58]. Herein, we have been concerned with the impact of weight loss on the subsequent risk of T2D among persons with excess weight (Fig. 1). More precisely, we have been focusing on the causal effects of the weight loss per se, as opposed to the interventions (i.e., lifestyle, surgery, and pharmacotherapy) which produce the weight loss. Yet, as has been noted elsewhere [59], we can only truly assess the causal effects of intervention assignment with confidence via randomization. Many of the factors that plausibly mediate or serve as mechanisms of the effects of intervention assignment, such as various changes in anatomy, physiology, and behavior, cannot be assigned to individuals, but only observed [60]. We are therefore left in the precarious position of trying to draw causal inferences from data in which the postulated causal factors (i.e., weight loss) have not been randomized. This creates many challenges.
Fig. 1.
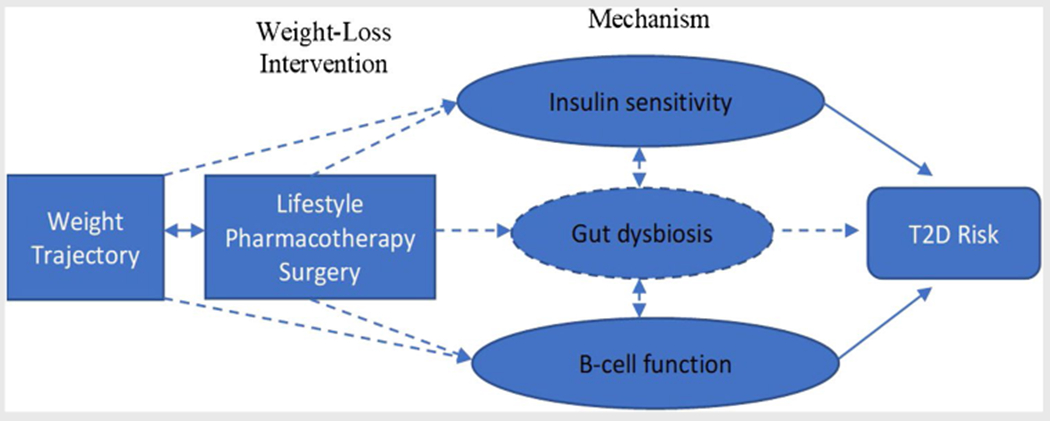
Current evidence on the relationship between weight change and risk of T2D. Some evidence (solid lines); inconclusive evidence (dashed lines)
Specifically, in weight loss interventions, several variables including changes in metabolism, hormones, behavior, and environment plausibly mediate the effects of interventions on subsequent risk of T2D. Moreover, separate examination of the temporal and dynamic changes in these mediating variables may be needed. For example, some studies have noted the importance of separating out the effects of negative energy balance producing weight loss from the effects of finally landing at and then potentially maintaining a lower body mass [61].
Disentangling these causal effects is further complicated by the fact that none of the mediating (intermediary) variables is likely to be measured with perfect accuracy. Therefore, measurement error can distort associations and, in turn, limit the ability to correctly identify causal effects [62]. More challenging still is that the degree of measurement error may be larger in some groups than in others [63] or for some variables than for others [64] leading to biased estimation of causal effects when both modeled in multi-variable analysis or in competing non-nested analysis [65]. Further still, the fact that the relations among putative mediating variables and their upstream causes and downstream effects may be nonlinear and the pattern of nonlinearity may differ across these variables [66] can lead to potentially erroneous conclusions if only a single form of relationship is modeled and that form better fits the actual relationship for one postulated mediator than for another. For example (Fig. 1), if one mediator (e.g., insulin sensitivity) had a linear relationship with an outcome (e.g., T2D) and another mediator (e.g., beta-cell function) had a markedly nonlinear relationship with the outcome, but only linear mediation was modeled, the former postulated mediator might be thought to have an important causal effect and the latter not, even if this was not the case.
Yet further complexities involve the fact that there are likely multiple mechanisms through which weight loss interventions affect risk of T2D and other important health outcomes. For example, there is some evidence suggesting that the reduction of body fat per se or at least some depots of body fat (especially visceral or omental body fat) may reduce risk of T2D (or alleviate some health problems), at least in some rodent models [67]. Yet, in humans, while data are far more limited, current interventional data (though not always randomized data) are inconsistent regarding this hypothesized effect of visceral fat reduction [68]. Alternatively, some evidence in humans suggests that the composition of the diet, particularly a lower carbohydrate diet, may lead to important reductions in the risk of T2D [69]. In contrast, carefully controlled studies in mice and rats suggest effects of diet composition, including the amount of sugar in the diet, that are at best equivocal and inconsistent in terms of their effects on lifespan, at worst largely null, and may depend on whether the diets are restricted in energy, isocaloric, or ad libitum [70–72]. This suggests the importance of experimental control and of looking at aspects of dietary changes. Findings from rodent studies also suggest that reduction in energy intake, regardless of the composition of the diet, results in a reduced mortality rate, and data from non-human primate studies suggest inconsistent effects on mortality rate, but consistent beneficial effects of caloric restriction on reduced T2D risk, independent of marked differences in diet composition [73–75].
Similarly, the timing of food may matter. It may be that periodic bouts of undernutrition, even if, on average, energy intake is not altered over integrated periods of time, may result in periodic losses and regains of weight that appear salubrious and lifespan enhancing in rodent models [76]. Some human physiology studies make similar predictions about the value of periodically draining aspects of bodily energy reserves [77], despite the fact that observational epidemiologic data show deleterious associations between weight variability and longevity in humans [78]. Whether these differences are an indication of different causal systems at play in rodents versus humans, confounding in human studies despite the very best attempts to reduce it (as indeed seems quite likely from an animal study in which observational and randomized interventional estimates of causal effects can be compared), [79] or other factors is still unknown.
Summary
Overall, when weight loss interventions are targeted at at-risk populations, heterogeneous and nonlinear patterns of weight change are often observed but are analyzed as pre-post intervention weight change. These nonlinear trends are in part due to weight changes that reflect time periods that define switching between a weight loss and weight maintenance intervention phase. Identifying homogeneous groups of individuals that follow similar weight trajectory groups with and without intervention exposure may provide important insights for understanding interruptions to the natural course of T2D among individuals with excess weight.
Weight trajectory groups irrespective of interventions have been shown to differ by age of onset, sex, and risk of T2D based upon patterns of insulin resistance or beta-cell function biomarkers. Excess weight that persists from childhood into adulthood is associated with higher risk of T2D. Adolescence is a critical etiologically relevant window during which onset of excess weight may be associated with higher risk of T2D especially among women. Among women, the post-menopausal period is associated with changes in body composition that could negatively impact insulin sensitivity and beta-cell function. Preventing or delaying the onset of excess weight in early adulthood and reducing the duration of an excess weight status may substantially lower the risk of T2D. Compared to pre-post intervention characterization of weight change and T2D risk, weight trajectory groups provide an intuitive population-level generalization of the temporal relationship between weight change and risk of T2D. However, there is inconsistent nomenclature of trajectory shapes and substantial heterogeneity in measures of association that summarize the relationship between weight trajectory groups and T2D risk. Additionally, individual-level variations within trajectory groups cannot be ruled out; therefore, a cautious interpretation of trajectory group effects is warranted. A better understanding of not just the developmental course of excess weight trajectory groups but also insulin resistance, gut dysbiosis, and beta-cell function is needed (Fig. 1). These distinct but related antecedents of T2D risk may evolve contemporaneously over time with important implications for the design and targeting of primary prevention efforts. Clever and creative study designs that help us disentangle these questions, deal with cross-species generalization, separate causation from correlation, and deal with the various statistical and methodological challenges raised represent an important and exciting opportunity for the near future.
Conflict of Interest
Arthur H. Owora reports grants to his institution from Eli Lilly & Co., Inc, Merck & Co., Inc, Soleno Therapeutics, Inc., and the National Institutes of Health, outside the submitted work. Dr. Allison reports personal fees from Alkermes, Inc.; personal fees from Amin, Talati, Wasserman (Glanbia); other from Big Sky Health, Inc.; personal fees from Biofortis Innovation Services/Merieux NutriSciences; grants and personal fees from California Walnut Commission; personal fees from Clark Hill PLC; other from International Life Sciences Institute of North America (ILSI); personal fees from Kaleido Biosciences and from Law Offices of Ronald Marron; personal fees from Medpace/Gelesis; personal fees from Nestec/Nestle; personal fees from Novo Nordisk Fonden; personal fees from Sports Research Corporation; personal fees from Tomasik, Kostin, & Kasserman; other from Northarvest Bean Grower’s Association; grants from Alliance for Potato Research and Education; other from Almond Board; grants from American Egg Board; grants and personal fees from Arnold Ventures; grants from Dairy Management, Inc.; grants from Eli Lilly and Co.; grants from Herbalife International; grants from Mars, Incorporated; grants and other from Mondelez; grants from National Cattlemen’s Beef Association; grants and other from Peanut Institute; grants from Reckitt Bencksier Group PLC; grants from Soleno Therapeutics; grants from USDA; and grants from National Institutes of Health, outside the submitted work.
Xuan Zhang reports grants to his institution from Eli Lilly & Co., Inc, and National Institutes of Health, outside the submitted work.
Nana A. Gletsu-Miller reports grants to her institution from Eli Lilly & Co., Inc, and National Institutes of Health, outside the submitted work. Kishore M. Gadde reports grants to his institution from AstraZeneca, BioKier, Indiana University Foundation, and National Institutes of Health, outside the submitted work.
Footnotes
Human and Animal Rights This article is a review of recent published work on the topic of weight trajectories and the risk of type 2 diabetes. It reports previous publications based on human and animal trials.
References
- 1.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309–19. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. [DOI] [PubMed] [Google Scholar]
- 3.Bragg F, Tang K, Guo Y, et al. Associations of general and central adiposity with incident diabetes in Chinese men and women. Diabetes Care. 2018;41(3):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 5.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels. Diabetes Care. 2004;27(2):372. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer D, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Subcutaneous thigh fat area is unrelated to risk of type 2 diabetes in a prospective study of Japanese Americans. Diabetologia. 2011;54(11):2795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston EH. Lower body subcutaneous fat accumulation and diabetes mellitus risk. Surg Obes Relat Dis. 2006;2(3):362–8. [DOI] [PubMed] [Google Scholar]
- 8.Owora AH. Commentary: diagnostic validity and clinical utility of HbA1c tests for type 2 diabetes mellitus. Curr Diabetes Rev. 2018;14(2):196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32(Suppl 3):S56–59. [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Jacobs DR, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162(4):1008–14. [DOI] [PubMed] [Google Scholar]
- 12.Lindheim SR, Buchanan TA, Duffy DM, et al. Original articlescomparison of estimates of insulin sensitivity in pre- and postmenopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. J Soc Gynecol Investig. 1994;1(2):150–4. [DOI] [PubMed] [Google Scholar]
- 13.Shimokata H, Muller DC, Fleg JL, Sorkin J, Ziemba AW, Andres R. Age as independent determinant of glucose tolerance. Diabetes. 1991;40(1):44–51. [DOI] [PubMed] [Google Scholar]
- 14.Uusitupa M, Khan TA, Viguiliouk E, et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11(11):2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–42. [DOI] [PubMed] [Google Scholar]
- 16.Merlotti C, Morabito A, Ceriani V, Pontiroli AE. Prevention of type 2 diabetes in obese at-risk subjects: a systematic review and meta-analysis. Acta Diabetol. 2014;51(5):853–63. [DOI] [PubMed] [Google Scholar]
- 17.Raben A, Vestentoft PS, Brand-Miller J, et al. The PREVIEW intervention study: results from a 3-year randomized 2 x 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes Obes Metab. 2021;23(2):324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer VL, Vangeepuram N, Fei K, et al. Outcomes of a weight loss intervention to prevent diabetes among low-income residents of East Harlem. New York Health Educ Behav. 2019;46(6):1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindström J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56(2):284–93. [DOI] [PubMed] [Google Scholar]
- 20.Richelsen B, Tonstad S, Rössner S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care. 2007;30(1):27–32. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409. [DOI] [PubMed] [Google Scholar]
- 23.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 24.Clamp LD, Hume DJ, Lambert EV, Kroff J. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr Diabetes. 2017;7(6):e282–e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115(3):466–79. [DOI] [PubMed] [Google Scholar]
- 27.Parretti HM, Jebb SA, Johns DJ, Lewis AL, Christian-Brown AM, Aveyard P. Clinical effectiveness of very-low-energy diets in the management of weight loss: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2016;17(3):225–34. [DOI] [PubMed] [Google Scholar]
- 28.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9(1):1–12. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y, Cai T, Zhou Z, et al. Health effects of alternate-day fasting in adults: a systematic review and meta-analysis. Frontiers in Nutrition. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patikorn C, Roubal K, Veettil SK, et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. 2021;4(12):e2139558–e2139558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. journal of the american college of cardiology. 2018;71(1):69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164(4):302–11. [DOI] [PubMed] [Google Scholar]
- 33.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen P, Meinert Larsen T, Westerterp-Plantenga M, et al. Men and women respond differently to rapid weight loss: metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes Metab. 2018;20(12):2840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diabetes Prevention Program Research G, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet (London, England). 2009;374(9702):1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadde KM, Atkins KD. The limits and challenges of antiobesity pharmacotherapy. Expert Opin Pharmacother. 2020;21(11):1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–61. [DOI] [PubMed] [Google Scholar]
- 39.Lachin JM, Christophi CA, Edelstein SL, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes. 2007;56(4):1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allison DB, Heshka S. Toward an empirically derived typology of obese persons: derivation in a nonclinical sample. Int J Eat Disord. 1993;13(1):93–108. [DOI] [PubMed] [Google Scholar]
- 41.Heo M, Faith MS, Mott JW, Gorman BS, Redden DT, Allison DB. Hierarchical linear models for the development of growth curves: an example with body mass index in overweight/obese adults. Stat Med. 2003;22(11):1911–42. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Hodge A, Hendryx M, Byles JE. BMI trajectory and subsequent risk of type 2 diabetes among middle-aged women. Nutr Metab Cardiovasc Dis. 2021;31(4):1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Hodge A, Hendryx M, Byles JE. Age of obesity onset, cumulative obesity exposure over early adulthood and risk of type 2 diabetes. Diabetologia. 2020;63(3):519–27. [DOI] [PubMed] [Google Scholar]
- 44.Wu YF, Fan HY, Chen YC, Kuo KL, Chien KL. Adolescent tri-ponderal mass index growth trajectories and incident diabetes mellitus in early adulthood. J Clin Endocrinol Metab. 2021;106(8):e2919–27. [DOI] [PubMed] [Google Scholar]
- 45.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv J, Fan B, Wei M, et al. Trajectories of early to mid-life adulthood BMI and incident diabetes: the China Health and Nutrition Survey. BMJ Open Diabetes Res Care. 2020;8(1): e000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vistisen D, Witte DR, Tabák AG, et al. Patterns of obesity development before the diagnosis of type 2 diabetes: the Whitehall II cohort study. PLoS Med. 2014;11(2):e1001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nano J, Dhana K, Asllanaj E, et al. Trajectories of BMI before diagnosis of type 2 diabetes: the Rotterdam Study. Obesity (Silver Spring). 2020;28(6):1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yacamán-Méndez D, Trolle-Lagerros Y, Zhou M, et al. Life-course trajectories of weight and their impact on the incidence of type 2 diabetes. Sci Rep. 2021;11(1):12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139–57. [DOI] [PubMed] [Google Scholar]
- 51.Nagin D, Nagin D. Group-based modeling of development. Cambridge, UNITED STATES: Harvard University Press; 2005. [Google Scholar]
- 52.Muthén BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29(1):81–117. [Google Scholar]
- 53.Muth´en B. Chapter 19 Latent variable analysis. Growth mixture modeling and related techniques for longitudinal data. In Kaplan D ed The Sage handbook of quantitative methodology for the social sciences Thousand Oakes: Sage Publications., Inc.; 2004:345–368. [Google Scholar]
- 54.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353:113–37. [DOI] [PubMed] [Google Scholar]
- 55.Chen ME, Chandramouli AG, Considine RV, Hannon TS, Mather KJ. Comparison of β-cell function between overweight/obese adults and adolescents across the spectrum of glycemia. Diabetes Care. 2018;41(2):318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klitgaard HB, Kilbak JH, Nozawa EA, Seidel AV, Magkos F. Physiological and lifestyle traits of metabolic dysfunction in the absence of obesity. Curr Diab Rep. 2020;20(6):17. [DOI] [PubMed] [Google Scholar]
- 57.Owora AH, Dawson J, Gadbury G, et al. Randomisation can do many things – but it cannot “fail.” Significance. 2022;19(1):20–3. [Google Scholar]
- 58.Rubin DB. Practical implications of modes of statistical inference for causal effects and the critical role of the assignment mechanism. Biometrics. 1991;47(4):1213–34. [PubMed] [Google Scholar]
- 59.Yanovski SZ, Bain RP, Williamson DF. Report of a National Institutes of Health-Centers for Disease Control and Prevention workshop on the feasibility of conducting a randomized clinical trial to estimate the long-term health effects of intentional weight loss in obese persons. Am J Clin Nutr. 1999;69(3):366–72. [DOI] [PubMed] [Google Scholar]
- 60.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 61.Müller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5(4):413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fritz MS, Kenny DA, MacKinnon DP. The combined effects of measurement error and omitting confounders in the single-mediator model. Multivariate Behav Res. 2016;51(5):681–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aaby D, Siddique J. Effects of differential measurement error in self-reported diet in longitudinal lifestyle intervention studies. Int J Behav Nutr Phys Act. 2021;18(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murrah WM. Compound bias due to measurement error when comparing regression coefficients. Educ Psychol Meas. 2020;80(3):548–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCracken MW. Parameter estimation and tests of equal forecast accuracy between non-nested models. Int J Forecast. 2004;20(3):503–14. [Google Scholar]
- 66.Valeri L, Lin X, VanderWeele TJ. Mediation analysis when a continuous mediator is measured with error and the outcome follows a generalized linear model. Stat Med. 2014;33(28):4875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7(3):438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein S. Is visceral fat responsible for the metabolic abnormalities associated with obesity?: implications of omentectomy. Diabetes Care. 2010;33(7):1693–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly T, Unwin D, Finucane F. Low-carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. Int j environ res public health. 2020;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell SJ, Bernier M, Mattison JA, et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019;29(1):221–228.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasselli JR, Weindruch R, Heymsfield SB, et al. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes Res. 2005;13(4):693–702. [DOI] [PubMed] [Google Scholar]
- 72.Le Couteur DG, Solon-Biet S, Cogger VC, et al. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci. 2016;73(6):1237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattison JA, Colman RJ, Beasley TM, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith DL Jr, Yang Y, Nagy TR, et al. Weight cycling increases longevity compared with sustained obesity in mice. Obesity (Silver Spring). 2018;26(11):1733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci USA. 2014;111(47):16647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou H, Yin P, Liu L, et al. Body-weight fluctuation was associated with increased risk for cardiovascular disease, all-cause and cardiovascular mortality: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019;10:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ejima K, Li P, Smith DL Jr, et al. Observational research rigour alone does not justify causal inference. Eur J Clin Invest. 2016;46(12):985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


