Abstract
Chronic pain can be a debilitating condition, leading to profound changes in nearly every aspect of life. However, the reliance on opioids such as oxycodone for pain management is thought to initiate dependence and addiction liability. The neurobiological intersection at which opioids relieve pain and possibly transition to addiction is poorly understood. Using RNA sequencing pathway analysis in rats with complete Freund’s adjuvant (CFA)-induced chronic inflammation, we found that the transcriptional signatures in the medial prefrontal cortex (mPFC; a brain region where pain and reward signals integrate) elicited by CFA in combination with oxycodone differed from those elicited by CFA or oxycodone alone. However, the expression of Egr3 was augmented in all animals receiving oxycodone. Furthermore, virus-mediated overexpression of EGR3 in the mPFC increased mechanical pain relief but not the affective aspect of pain in animals receiving oxycodone, whereas pharmacological inhibition of EGR3 via NFAT attenuated mechanical pain relief. Egr3 overexpression also increased the motivation to obtain oxycodone infusions in a progressive ratio test without altering the acquisition or maintenance of oxycodone self-administration. Taken together, these data suggest that EGR3 in the mPFC is at the intersection of nociceptive and addictive-like behaviors.
Keywords: Self-administration, Pain, Progressive ratio, Oxycodone, Motivation
Introduction
Chronic or persistent recurring pain has become an enormous global health crisis that incurs profound financial and societal burdens1,2. Pain encompasses unpleasant sensory and affective experiences, which often result in restricted activity, opioid dependence, anxiety, depression, and a poorer quality of life3,4. Cognitive and emotional appraisal are dysregulated in individuals with chronic pain, concomitant with a heightened negative assessment of pain. The alleviation of pain associated with aversive stimuli can therefore reinforce behaviors that promote the transition to addiction5. For example, opioids such as oxycodone are effective therapeutics for analgesia and pain management6 but can lead to maladaptive neuroplasticity in the mesocorticolimbic dopamine system, resulting in abuse liability7. The neurobiological basis of the relationship between antinociception and susceptibility to addiction is not well understood and warrants further examination.
The brain’s reward center, including the prefrontal cortex (PFC) in the mesocorticolimbic dopamine system8, is highly relevant in the context of the affective-motivational need for pain relief. The medial prefrontal cortex (mPFC) continually undergoes transcriptional and morphological plasticity, which underlies the enduring behavioral adaptations in response to chronic pain and opioid exposure9–13. Furthermore, neuronal ensembles in the mPFC encode learned associations of drug-paired contexts14, making it an important region where pain processing and reward mechanisms overlap.
The sensory component of pain is measured through mechanical and thermal thresholds for a painful stimulus whereas affective pain is the aversive, motivational, or emotional aspect of pain15–18.The sensory dimension identifies the physical characteristics of the noxious stimuli (mechanical stimuli) prompting withdrawal reflexes. On the contrary, the affective-motivational dimension of pain evaluates the emotional component of pain by linking unpleasantness with the noxious stimulus triggering defensive or coping behaviors19. Importantly, even if a painful stimulus can elicit similar degrees of pain sensation, they can engage different degrees of pain affect20. Additionally, the sensory processing of mechanical pain engages divergent neural substrates and pathways in comparison to the affective dimension of pain11. Thus, understanding these varied aspects of pain processing is critical for devising effective pharmacological and psychosocial therapeutic measures against nociception. The mPFC is a critical neural substrate that transforms sensory stimulus and encodes chronification of pain in addition to its association with affective pain processing due to its amygdalar connections11. Despite being ostensibly used to study nociception in rodent models, the neural mediators that transduce mechanical nociception remain stubbornly elusive21 and thus, warrant further investigation in implicated neural substrates.
Early growth response 3 (EGR3) is a master transcriptional regulator expressed in the mPFC and other brain regions. EGR3 typically exhibits an activity-dependent expression profile and is upregulated by cellular intermediates such as neuregulin1, calcineurin, N-methyl-d-aspartate receptors, and neurotrophins22–26. In turn, EGR3 modulates numerous downstream targets that regulate processes such as synaptic plasticity, axonal and dendritic extension, and receptor function25,27, all of which are involved in drug-induced neuroadaptations. EGR3 has therefore been implicated in various psychiatric disorders, and there is emerging evidence that it governs drug-induced maladaptive plasticity28–31. How EGR3 in the mPFC regulates the opioid-induced transcriptional programming common to pain processing is currently unknown.
Here, we demonstrate that oxycodone elicits unique transcriptional signatures in the mPFCs of rats with or without complete Freund’s adjuvant (CFA)-induced inflammatory pain. According to a top-down analysis of transcriptional profiling that identified gene alterations under various treatment conditions, we posit that EGR3 is a master transcriptional regulator underlying oxycodone-induced transcriptomic change. We also show that viral and pharmacological manipulation of EGR3 in the mPFC regulates behavioral plasticity pertaining to discrete nociceptive and reward domains.
Materials and Methods
Animals
Male Sprague-Dawley rats (Envigo Laboratories, Indianapolis, IN) weighing 250–275 g were used for all experiments. Animals were housed at 22–25 °C under a 12:12-h reverse light-dark cycle with access to food and water ad libitum. All behavioral testing was conducted during the dark cycle. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo.
Drugs
Oxycodone (generously gifted from the NIDA drug supply program) dissolved in 0.9% sterile saline was administered cumulatively at doses of 0.1, 0.32, 1.0, and 3.2 mg/kg body weight for Von Frey tests. Oxycodone was administered at a dose of 1 mg/kg for place escape avoidance paradigm and locomotor tests, whereas 0.15 mg/kg/infusion (inf) was used for self-administration. To ensure the appropriate dose was used for each animal injection, volumes were adjusted according to the rat’s body weight. For self-administration, oxycodone was delivered by syringe pumps, and injection volumes were adjusted daily according to body weight32.
Cyclosporin (CYSP) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in saline containing 2% dimethyl sulfoxide at a dose of 4 μg/μl, which has been shown to inhibit calcineurin activity. Control animals received 2% dimethyl sulfoxide dissolved in saline (vehicle). CFA was purchased from Thermo Fisher Scientific (Waltham, MA) and dissolved in paraffin oil.
Inflammatory pain induction
To induce inflammatory pain, 0.1 ml of CFA (containing killed Mycobacterium butyricum)33 dissolved in paraffin oil was injected subcutaneously into the rear portion of the plantar side of the right hind paw of each rat under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen at flow rate of 5 L min−1). Rats that served as controls for the inflammatory pain condition were injected with 0.1 mL of sterile saline.
Viral-mediated gene expression
Rats received bilateral intracranial injection of volume-to-volume mixture of AAV-Cre (serotype 2) and a double-floxed inverted open reading frame (DIO) Cre-dependent adeno-associated virus (AAV-serotype 2) vector to express EGR3 fused to EYFP (referred to as AAV-EGR3 in the future). EF1a-DIO vector backbone was obtained from Karl Deisseroth lab. EGR3 was cloned into the DIO framework in AAV serotype 2 (5.1×102 genomic particles per μL) by the vector core at UNC Chapel Hill and was provided by the lobo lab. Control rats received AAV-Cre-GFP (referred to as AAV-GFP). Injectors were set at 10° with coordinates described previously32,34: AP, +3.2 mm; ML, +1.3 mm; DV, −3.2 mm. Viruses were manually infused over 5 min (0.2 μL/min, total infusion volume of 1 μL), and the needles remained in place for 10 min to ensure diffusion of the AAV. Animals were allowed 14 days for maximal viral expression and recovery before testing. The viral overexpression of EGR3 was timed to mimic the potentiated EGR3 expression in the mPFC observed in rats administered with chronic oxycodone and to mimic the modulation of pain perception in both mechanical and affective pain paradigms. All rats with EGR3 overexpression received CFA for inflammatory pain induction and were assessed for mechanical pain, affective pain, and locomotor activity. For progressive ratio behavior tests (described below), rats underwent viral surgical procedures and received either Cre-inducible AAV-EGR3 or AAV-GFP in the mPFC 14 days prior to jugular catheterization (to ensure maximal expression) to assess active drug taking and motivation behaviors.
Bilateral cannulation and microinjections
To pharmacologically inhibit EGR3, bilateral guide cannulae (C235GS, Plastics One) targeting the mPFC were placed with the following coordinates relative to bregma: AP, +2.9 mm; ML, ±0.6 mm; DV, −3 mm. Animals were then handled daily during the recovery phase to habituate them to the microinjection procedure. Animals were randomly assigned to receive either CSPA (4 μg/μL) or vehicle (2% dimethyl sulfoxide dissolved in saline); 0.5 μL per side was injected bilaterally into the mPFC for 1 min (rate of 0.5μL/min) and then allowed to diffuse for 10 min. Microinjections were repeated daily for 3 days to ensure detection of long-term transcriptional changes. Von Frey, place escape avoidance, and locomotor tests were performed 24 h after the last infusion.
Von Frey test
Weighted von Frey filaments (4, 6, 8, 10, 15, and 26 g; North Coast Medical, Morgan Hill, CA) were used to measure mechanical hyperalgesia35. Rats were placed in plastic chambers on a grid (IITC Life Science) that provided access to the bottoms of the rats’ hind paws. Von Frey filaments, in order of ascending weight, were applied perpendicular to the plantar surface of the hind paw from below the grid floor. A withdrawal of the paw from the applied force was considered a positive behavioral response. The mechanical threshold (percent maximal effect) was identified as the lowest force that evoked a positive behavioral response in at least two of three applications to the hind paw. Von Frey responses were measured prior to drug administration, 20 min after each drug dose was delivered, and immediately before the next drug treatment. The test continued until the percent maximal effect at the 26-g filament was reached. Forces greater than 26 g would physically lift an untreated paw and were not considered.
Place escape avoidance paradigm
Place escape avoidance was used to assess the affective dimension of pain because this paradigm takes into account the past pain felt, the context of pain, and anticipation of future unpleasantness—all of which are vital aspects of the affective-motivational dimension of pain36. Briefly, rats were placed on a mesh grid, for experimenter access, and a cage painted half black and half white was placed atop the grid, housing the rat. The right inflamed paw was poked when the rat was on the black side of the chamber, whereas the left paw was poked when the rat entered the white side. The aversive (black chamber) or non-aversive (white chamber) stimulus was administered every 15 s for 30 min to emulate “chronic pain” as previously described37. Percent time spent on the white side of the chamber was calculated (white side stimulus/total number of stimuli ´ 100) and used as an indicator of escape/avoidance learning. Oxycodone was administered 20 min before the testing session.
Locomotor activity
An infrared motion-sensor system (AccuScan Instruments, Inc., Columbus, OH) fitted outside transparent plastic cages with Versa Max animal activity software (Omnitech Electronics, Inc., Columbus, OH) was used to measure locomotor activity. Total distance traveled during the 1h test was used as a measure of overall locomotion38.
Jugular catheterization and patency testing
All rats were implanted with chronic indwelling jugular catheters, as previously described32. Briefly, rats were anesthetized using ketamine and xylazine (60 and 5 mg/kg, respectively, intraperitoneally), the right jugular vein was isolated, and the catheter was inserted. The other end of the catheter was fitted to a vascular access harness (Instech, PA). Rats were allowed 5 days to recover from the surgical procedure, and catheter patency was preserved by flushing them daily with 0.2 mL of enrofloxacin (4 mg mL−1) in heparinized saline (50 IU mL−1 in 0.9% sterile saline). One day prior to self-administration training, catheter patency was confirmed by loss of muscle tone and righting reflex following an intravenous infusion of ketamine hydrochloride (0.5 mg/kg in 0.05 mL). Only rats with patent catheters were used in behavioral studies.
Oxycodone self-administration and progressive ratio after EGR3 overexpression
Rats were trained to self-administer oxycodone (0.15 mg/kg/inf) for 3 h each day for 10 days in a soundproof operant chamber equipped with active and inactive nose pokes32. Responses in the active nose poke resulted in an infusion of oxycodone at fixed ratio 1 that was incrementally increased by 1 each day for 10 days to fixed ratio 10. Responses in the inactive nose poke resulted in no programmed consequences. Following training, the animals were allowed to self-administer oxycodone on a progressive ratio schedule of reinforcement for a maximal session duration of 6 h (day 11). The session for each animal ended if the animal failed to earn an infusion within the last 1 h. The schedule of delivery progressively increased after each infusion in a schedule of 1, 2, 3, 4, 6, 8, 12, 24, 32, 48, etc.32,39. After testing, the catheters were flushed with heparin-saline, and the animals were returned to the colony room.
RNA sequencing
Rats received either saline or CFA in their right hind paws and were administered saline or oxycodone (1 mg/kg, intraperitoneally) for 7 days. Thirty minutes after the last drug treatment, animals were killed and the mPFC tissues were collected with 2mm biopsy punches. RNA sequencing was performed as previously described32. Briefly, RNA libraries were generated using the Illumina TruSeq RNA library preparation kit, and sequencing was performed on the Illumina HiSeq 2500 system with a 50-cycle single-end flow cell. The RN5 (rat) genome was used for alignments using the TopHat (version 2.0.13) alignment algorithm and the UCSC refGene annotation set. For differential expression analysis, Cuffdiff with default parameters was utilized.
Quantitative PCR
mRNA from tissue punches (2mm punches obtained from the mPFC region) was isolated and purified using TRIzol (Ambion, Austin, TX) and the MicroElute total RNA kit (Omega Bio-tek Inc., Norcross, GA). RNA concentrations were measured on a NanoDrop, and 500 ng was reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Reaction mixtures were prepared with iQ SYBR Green Supermix (Bio-Rad Laboratories) and run on an iQ5 system (Bio-Rad Laboratories). Reactions were run in triplicates, and expression was quantified using a relative threshold cycle method with Gapdh as a housekeeping gene. The primer sequences are as follows: Gapdh, 5′-AACGACCCCTTCATTGAC-3′ (forward) and 5′-TCCACGACATACTCAGCA-3′ (reverse); Egr3, 5′-ATGGCTACAGAGAATGTGATGGA-3′ (forward) and 5′-TGGAAGGAGAGTCGAAAGCG-3′ (reverse).
Immunoblotting
2mm mPFC tissue punches of each rat were homogenized with 50 μL of RIPA buffer containing proteinase and phosphatase inhibitors. Protein concentrations were determined by Bradford protein assay. Immunoblotting was performed by loading 30 μg of protein per lane into 4–20% gradient Tris-SDS polyacrylamide gels. After electrophoresis, proteins were then transferred to nitrocellulose membranes and blocked with Rockland buffer. Membranes were incubated in primary antibody (mouse anti-Egr3, 1:300; Santa Cruz Biotechnology, Dallas, TX) diluted in Rockland blocking buffer overnight at 4 °C. Membranes were incubated with antibodies to GAPDH (1: 10,000; Cell Signaling Technology, Danvers, MA) for 1 h at room temperature (22–25 °C) to ensure equal protein loading. Membranes were then washed in Tris-buffered saline with 0.1% Tween-20 and incubated with appropriate IRDye secondary antibodies (1: 5,000; LI-COR, Inc., Lincoln, NE) diluted in Rockland blocking buffer at room temperature for 1 h. Membranes were imaged on a LI-COR system and quantified by densitometry analysis with ImageJ software (National Institutes of Health, Bethesda, MD). Expression in each lane was normalized to its loading control and treatment condition.
Data analysis
All statistical analyses were performed with Prism (GraphPad Software Inc., La Jolla, CA). Either a Student’s t test or two-way ANOVA was used to determine significance followed by Tukey’s post hoc analysis as appropriate. Significance was defined as a two-tailed P value of <0.05, and data are presented as means and standard errors.
Results
Oxycodone elicits unique transcriptional signatures in the mPFC
RNA sequencing was performed on mPFC tissues from rats with or without chronic inflammatory pain induced by CFA (Fig. 1a). A total of 145 genes were significantly modulated in the chronic inflammatory state. There was an upregulation of biological processes involved in the ensheathment of axons/neurons, myelination, glial cell development, and oligodendrocyte differentiation (Fig. 1b). Administration of oxycodone to animals with chronic inflammation significantly altered 326 genes, with an upregulation in the expression of genes involved in cellular communication and synaptic signaling and a downregulation in processes pertaining to myelination and neuron ensheathment (Fig. 1c). By contrast, administration of oxycodone by itself altered the expression of 132 genes, including those involved in responses to endogenous stimuli, metabolism, cell differentiation, and cellular development (Fig. 1d). The mRNA transcript profile for oxycodone administered to animals in a state of chronic pain revealed a general suppression of genes in processes related to cell differentiation, tissue development, the MAPK pathway, metabolism, and behavior (Fig. 1e). In total, 54 genes were uniquely altered between the oxycodone and in CFA with oxycodone treatments (Table 1).
Figure 1. RNA sequencing reveals discrete transcriptomics for altered biological processes based on nociceptive induction and treatments.
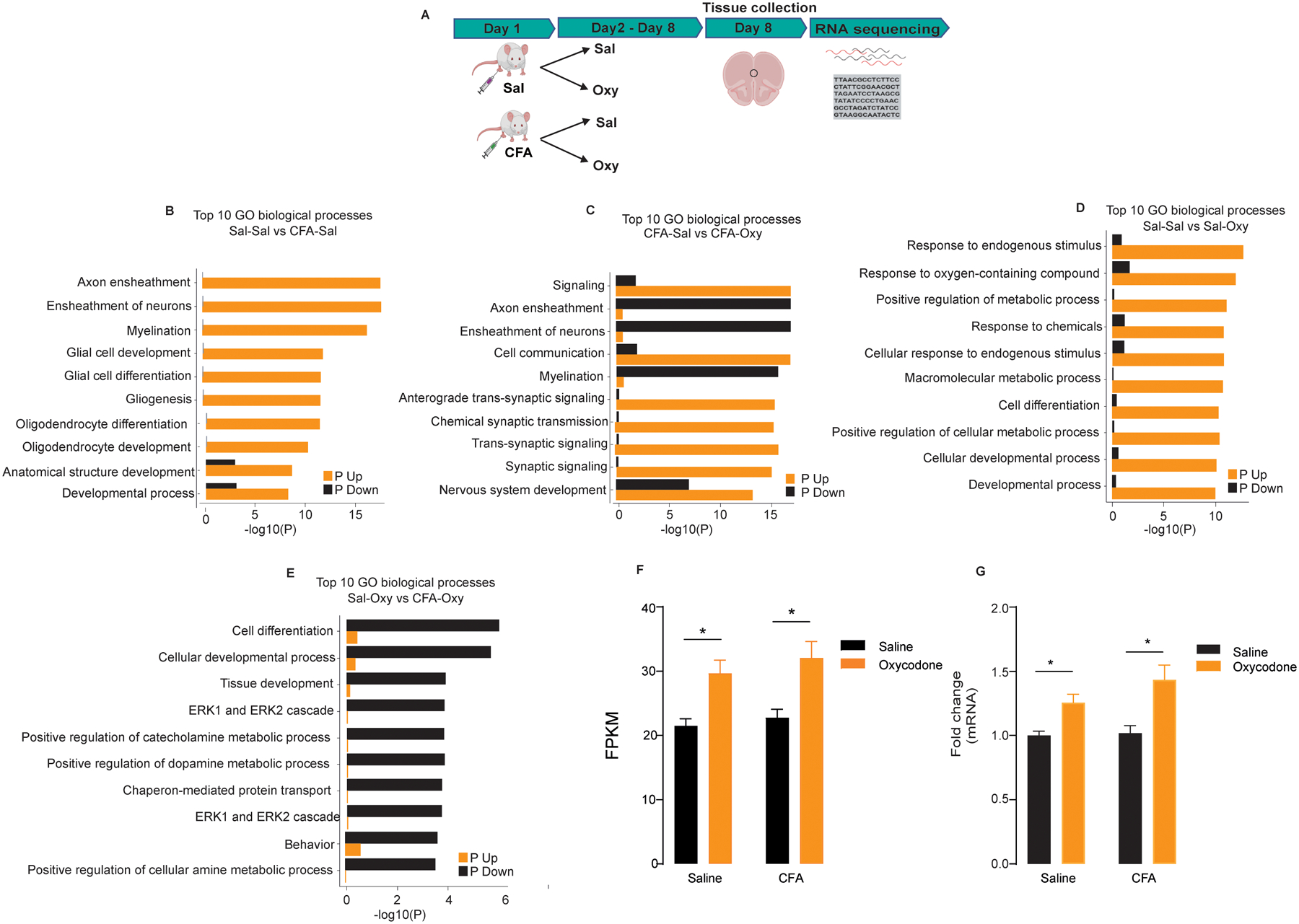
(a) Experimental timeline for RNA sequencing of the mPFC under various treatment conditions. (b) mRNA transcript profile comparing saline-treated rats that did not receive CFA to rats that received CFA. (c) mRNA transcript profile comparing CFA-treated rats that received either saline or oxycodone. (d) mRNA transcript profile comparing saline versus oxycodone treatments in rats that did not receive CFA. (e) mRNA transcript profile comparing oxycodone-treated rats that did not receive CFA to rats that received CFA. (f) Egr3 transcript changes expressed as fragments per kilobase of exon per million mapped fragments (FKPM) between CFA and saline rats that received either oxycodone or saline. (g) mRNA expression of Egr3 expressed in fold change between CFA and saline rats that received either oxycodone or saline. All data are presented as means ± SEMs with statistical significance (*) at P < 0.05. * represents significant differences between oxycodone groups of saline and CFA with their respective controls (saline + saline and saline + CFA)
Table 1:
List of uniquely altered genes between oxycodone and oxycodone with CFA treatment.
| Genes | P-Value | FDR |
|---|---|---|
| Chchd10 | 7.81E-18 | 1.01E-13 |
| Tbcb | 8.57E-14 | 5.57E-10 |
| Sepw1 | 5.66E-13 | 2.45E-09 |
| Cetn3 | 3.07E-12 | 9.97E-09 |
| Echs1 | 2.01E-10 | 5.22E-07 |
| Clip3 | 2.87E-10 | 6.22E-07 |
| Scn4b | 3.36E-10 | 6.25E-07 |
| Sulf1 | 4.05E-10 | 6.59E-07 |
| Apold1 | 6.85E-09 | 9.89E-06 |
| Myh11 | 2.50E-08 | 3.24E-05 |
| Sccpdh | 2.96E-08 | 3.50E-05 |
| Mx2 | 4.77E-08 | 4.91E-05 |
| Mif | 4.91E-08 | 4.91E-05 |
| Acta2 | 6.31E-08 | 5.86E-05 |
| Cyr61 | 6.88E-08 | 5.96E-05 |
| Hapln4 | 2.53E-07 | 0.000205 |
| Cdhr1 | 3.05E-07 | 0.000233 |
| Grcc10 | 4.00E-07 | 0.000289 |
| Col11a2 | 9.59E-07 | 0.000656 |
| Chid1 | 2.20E-06 | 0.001432 |
| Vwa5b2 | 3.30E-06 | 0.001989 |
| Fcho1 | 3.37E-06 | 0.001989 |
| Col27a1 | 6.02E-06 | 0.003386 |
| Hsp90aa1 | 6.25E-06 | 0.003386 |
| Ucma | 1.23E-05 | 0.006393 |
| Stac2 | 1.28E-05 | 0.006393 |
| Foxp2 | 1.48E-05 | 0.006987 |
| Vwa3a | 1.52E-05 | 0.006987 |
| Brd2 | 1.56E-05 | 0.006987 |
| Syt2 | 1.77E-05 | 0.00769 |
| Miat | 1.88E-05 | 0.007878 |
| Btg2 | 2.10E-05 | 0.00855 |
| Tagln | 2.30E-05 | 0.009071 |
| Hprt1 | 2.42E-05 | 0.00926 |
| Scn1b | 2.62E-05 | 0.00975 |
| LOC690918 | 3.50E-05 | 0.012643 |
| Ube2b | 3.61E-05 | 0.01268 |
| Ptgds | 4.00E-05 | 0.013698 |
| Rn5-8s | 4.74E-05 | 0.015446 |
| Atf3 | 4.85E-05 | 0.015446 |
| Ndufa1 | 4.87E-05 | 0.015446 |
| Chrna5 | 6.03E-05 | 0.018652 |
| RT1-M5 | 7.82E-05 | 0.023659 |
| RGD1562161 | 8.35E-05 | 0.024275 |
| RGD1566401 | 8.40E-05 | 0.024275 |
| Kcnv2 | 0.000118 | 0.033238 |
| Nnat | 0.000128 | 0.035342 |
| Nxph3 | 0.000159 | 0.043066 |
| Kcnk4 | 0.000172 | 0.0456 |
| Anxa6 | 0.000176 | 0.0458 |
| Tyrp1 | 0.000186 | 0.047388 |
| Npy | 0.000193 | 0.047388 |
| Aldoart2 | 0.000194 | 0.047388 |
| Cdh1 | 0.000197 | 0.047388 |
Oxycodone upregulates Egr3 in the mPFC
Notably, oxycodone increased Egr3 expression irrespective of the inflammatory state. Oxycodone administration to animals that received CFA or saline increased in the amount of Egr3 expressed as FKPM (two-way ANOVA: pain effect, F(1,32) = 0.9979, P > 0.05; drug effect, F(1,32) = 22.01, P < 0.001; interaction, F(1,32) = 0.08879, P > 0.05, n = 9/group) and fold change (two-way ANOVA: pain effect, F(1,32) = 1.626, P > 0.05; drug effect, F(1,32) = 18.81, P < 0.002; interaction, F(1,32) = 1.070, P > 0.05, n = 9/group) (Fig. 1f, g).
EGR3 overexpression in the mPFC potentiates the effect of opioids on mechanical pain without altering affective pain
To examine how EGR3 affects oxycodone-induced antinociception, we performed a Von Frey test for mechanical analgesia in animals with EGR3 overexpression in the mPFC (Fig. 2a, b). There was a significant difference in the mechanical threshold at the 1.0-mg/kg dose, suggesting that EGR3 potentiates mechanical pain relief (two-way ANOVA: drug effect, F(3,40) = 20.78, P < 0.0001; virus effect, F(1,40) = 5.470, P < 0.05; interaction, F(3,56) = 1.159, P > 0.05; n = 6/group) (Fig. 2c). No significant differences were observed between EGR3-overexpressing (AAV-EGR3-AAV) and control (AAV-GFP) rats for mechanical threshold at the 0.1-, 0.32-, and 3.2-mg/kg doses. To assess affective responses to pain, animals were evaluated in the place escape avoidance paradigm; a locomotor test was used as a behavioral control. EGR3 overexpression did not alter the preference for the white side (non-painful stimulus-paired side) of the place escape avoidance chamber (t10 = 1.540, P > 0.05; n = 6/group) or overall locomotion (t10 = 0.8506, P > 0.05; n = 6/group) (Fig. S1a, b)
Figure 2. EGR3 in the mPFC regulates mechanical nociception.
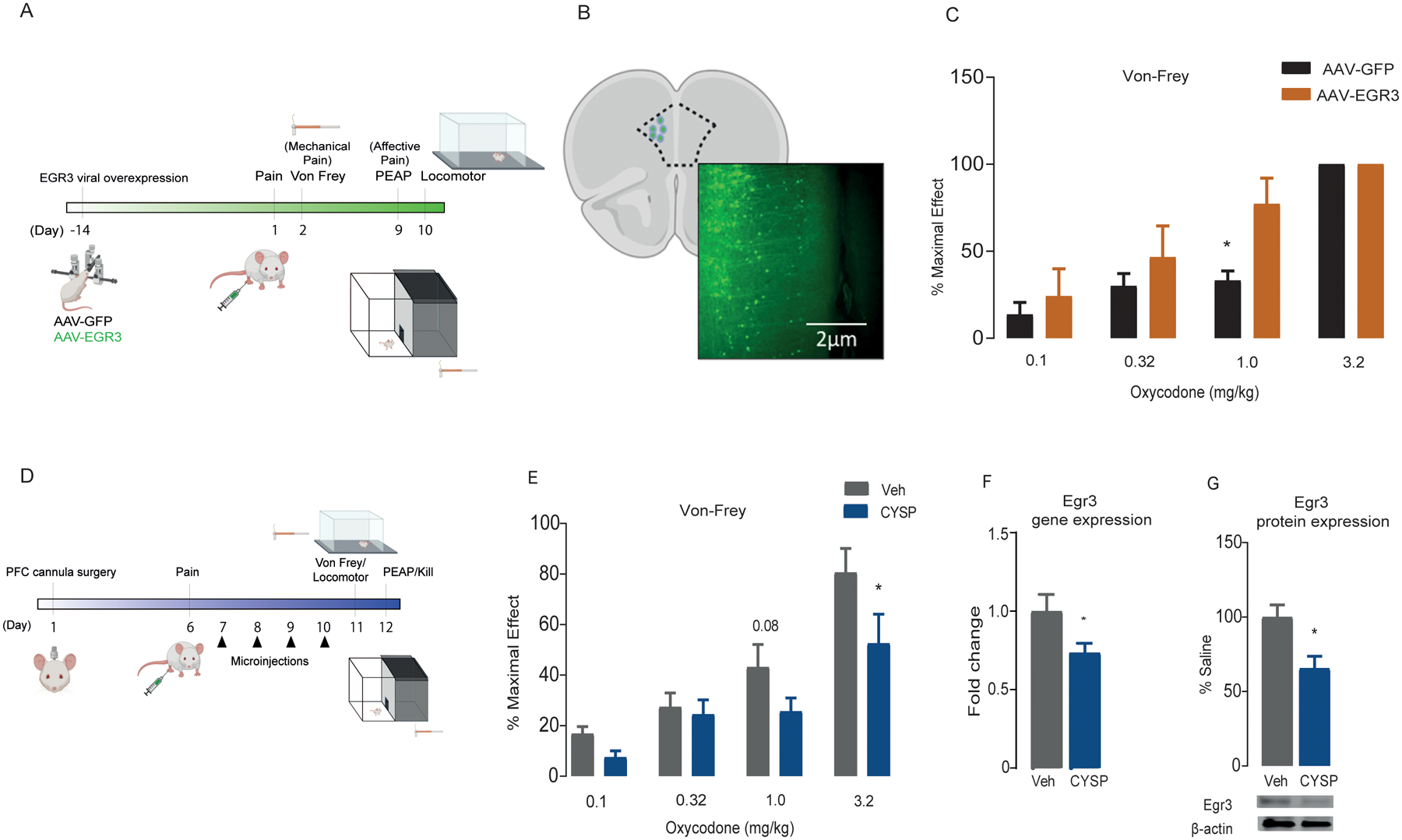
(a) Experimental timeline for EGR3 overexpression in the mPFC followed by nociceptive and locomotor behavior tests. (b) Anatomical placement of viral infection with AAV-cre in combination with AAV-DIO-EGR3 (referred to as AAV-EGR3) and representative image (10x) of AAV infection in the mPFC. (c) Percent maximal effect determined through Von Frey filament test for mechanical hyperalgesia at increasing doses of oxycodone in rats that were microinjected in the mPFC with AAV-GFP or AAV-EGR3. * represents significant differences between AAV-GFP and AAV-EGR3 in % maximal effect at 1 mg/kg dose (d) Experimental timeline for bilateral CYSP injections into the mPFC followed by nociceptive and locomotor behavior tests. (e) Percent maximal effect determined through Von Frey filament test for mechanical hyperalgesia at increasing doses of oxycodone in vehicle- and CYSP-microinjected rats. * represents significant differences between vehicle and CYSP in % maximal effect at 3.2 mg/kg dose EGR3 gene (f) and protein (g) expression in the mPFC in vehicle and CYSP-microinjected rats. * represents significant differences between vehicle and CYSP in EGR3 expression. All data are presented as means ± SEMs with statistical significance (*) at P < 0.05.
Pharmacological inhibition of EGR3 in the mPFC attenuates the effect of opioids on mechanical pain
To test whether EGR3 bidirectionally affects oxycodone-induced antinociception, we pharmacologically inhibited NFAT, an upstream regulator of EGR340, by bilaterally injecting CYSP, a calcineurin/NFAT inhibitor, into the mPFC (Fig. 2d). CYSP prevents NFAT from translocating to the nucleus and activating EGR3. Intra-mPFC inhibition of EGR3, through NFAT, attenuated the relief of mechanical hyperalgesia elicited by 3.2 mg/kg oxycodone (two-way ANOVA: drug effect, F(3,56) = 21.07, P < 0.0001; virus effect, F(1,56) = 8.216, P < 0.05; interaction, F(3,56) = 1.159, P > 0.05; n = 8/group) (Fig. 2e). CYSP did not affect the preference for the white side of the place escape avoidance chamber (t(14) = 0.6079, P > 0.05) or overall locomotion (t(14) = 1.1085, P > 0.05) (Fig. S1c, d), suggesting that suppression of EGR3 activity does not influence affective pain. The downregulation of EGR3 via NFAT inhibition was confirmed by quantitative PCR (t(11) = 2.057, P = 0.03) and Western blotting (t(11) = 2.969, P = 0.01) (Fig. 2f, g)
EGR3 overexpression increases the rewarding effects of oxycodone
To determine if EGR3 alters the rewarding effects of opioids, we employed a volitional model of oxycodone self-administration (Fig. 3a). Virus-mediated overexpression of EGR3 in the mPFC did not alter the acquisition of oxycodone self-administration, according to the mean number of infusions of 0.15 mg/kg oxycodone (F(9, 180) = 0.5802, P > 0.05; n = 10/group) (Fig. 2b). However, EGR3 overexpression in the mPFC increased the total number of infusions earned (t(18) = 2.164, P = 0.04) and the breakpoint (t(18) = 2.24, P = 0.03) during a 6-h progressive ratio test (Fig. 3c, d), indicating that EGR3 regulates the motivational domain of reward processing.
Figure 3. EGR3 overexpression in the mPFC increase the rewarding effects of oxycodone.
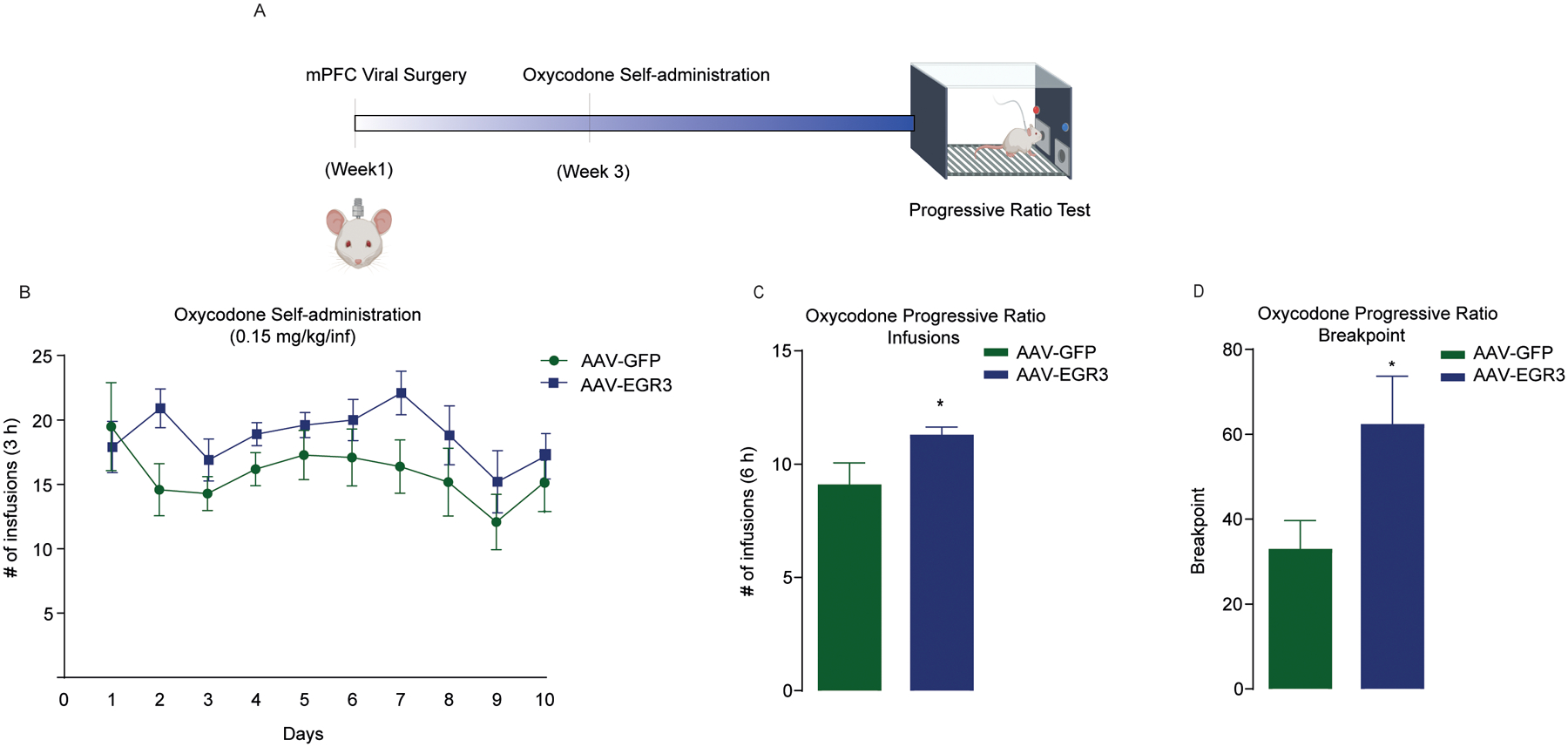
(a) Experimental timeline for EGR3 overexpression in the mPFC followed by oxycodone self-administration and progressive ratio behavior. Numbers of infusions (b, c) and breakpoints (d) in a 6-h progressive ratio test in rats microinjected with AAV-GFP or AAV-EGR3. * represents significant differences between AAV-GFP and AAV-EGR3 in number of infusion and breakpoint. All data are presented as means ± SEMs with statistical significance (*) at P < 0.05.
Discussion
Opioids are currently amongst the most commonly prescriped drugs for pain relief and analgesia6,41. However, because they modulate the mesolimbic dopamine system, repeated use of opioids can lead to dependence and addiction42,43. The idea that chronic pain can induce affective-emotional behaviors implicates pain in reward processing, learning, and goal-oriented functions. Many studies have examined alterations in gene expression caused by chronic pain44–46 or drugs of abuse47,48, but potential transcriptomic changes from exposure to oxycodone in a state of chronic inflammatory pain is not well characterized. Here, we elucidate a comprehensive understanding of the distinct transcriptomic signatures unique to oxycodone exposure in the presence or absence of chronic inflammatory pain, revealing the transcriptional regulation at the intersection of oxycodone exposure and oxycodone exposure during pain.
The expression of genes involved in oligodendrocyte differentiation and myelination was elevated by inflammation-induced nociception, highlighting the important role of oligodendrocytes in the nociceptive state. The differentiation and activity of oligodendrocyte precursor cells is increased by neural activity and experiences49, whereas dysregulated function of these cells is associated with decreased motor activity50–52, depression-like behavior53, and neuroinflammatory responses54,55. Prior studies showed that opioids such as methadone and buprenorphine increase the expression of myelin-related genes in early development56,57. Furthermore, oligodendrocyte precursor cell differentiation and transcripts for myelination increase with heroin self-administration32. Conversely, there was a decrease in genes related to myelination and glial cell differentiation in rats with CFA-induced chronic inflammation such that the expression of genes upregulated by chronic pain was reversed by oxycodone, showing that the opioid can counter the effects of the nociceptive state at the transcriptional level. Oxycodone administered to animals with chronic inflammation also upregulated genes associated with cell-to-cell communication and synaptic signaling. However, exposure to oxycodone in the absence of inflammation upregulated genes in processes that play vital roles in synaptic plasticity58, memory formation59, glutamatergic receptor regulation60, signal transduction, cell differentiation, and responses to environmental stress61, representing a transcriptomic profile that may be unique to oxycodone only exposure62,63.
Preclinical and clinical evidence demonstrate that stressful stimuli, including pain, activate EGR proteins64–67. Immediate early genes such as Egr3 underlie gene-environment interactions and mediate neuronal activity underlying higher-order processes such as learning, cognition, and reward mechanisms31. EGR3 has also been implicated in modulating drug-induced maladaptive plasticity that is influenced by sex and cell-type specificity29,30. However, it is not clear how EGRs regulate the mechanical or emotional aspects of pain transmission or how such regulation intersects reward and motivation. We focused on EGR3 because it influences immunomodulation, synaptic plasticity, cellular differentiation, and glial cell dynamics, which are modified by oxycodone treatment, making it a suitable candidate for investigation underlying nociception and reward modalities. We found that overexpression of EGR3 in the mPFC increased the mechanical pain threshold, whereas inhibition of EGR3 decreased it. This is consistent with the dual role of mPFC in pain processing, both exerting top-down control and receiving nociceptive inputs68–71. For example, neuromodulation of this region attenuates mechanical pain72, and mPFC projection neurons regulate pain threshold73.
The mPFC region is also heavily involved in encoding the value of reward and action-outcome associations74,75 through its modulatory effects on inhibitory control, a phenomenon that is disrupted in pain processing and drug addiction11,76. Overexpression of EGR3 in the mPFC increased the motivation to self-administer oxycodone in a progressive ratio schedule of delivery, which is a classic method to evaluate the reinforcing effects of drugs77 and aids in parsing out the motivational aspect of addiction. A potential explanation for this finding is the regulation of glutamatergic and GABAergic synaptic plasticity by EGR3 (by regulating the transcription of receptor subunits)78,79, which could fine-tune the neurotransmission to and from the mPFC11. Furthermore, BDNF, an ubiquitous neurotrophic factor that is known to be altered by exposure to drugs of abuse and pain80,81, regulates NFAT82 and EGR324 and could be an underlying mechanism of action observed in this study. However, EGR3 also influences other neuroadaptations such as myelination and mitochondrial transcriptomics that are essential to drug-induced plasticity32,83,84.
It is important to consider that the experiments were conducted with mPFC region that includes both prelimbic (PrL) and infralimbic (IL) cortexes. Both PrL and IL have been reported to have distinct functionalities underlying addiction and pain processing85–91. Thus, it could be possible that Egr3 changes observed here reflect the net change in both the regions or changes in one region over the other. Additionally, it is noteworthy that inhibition of NFAT pathway through CYSP can have widespread effects through alteration of calcium dynamics92 and immune signaling93 mechanisms that might have contributed to the behavioral effects.
In summary, this study identifies EGR3 as a modulator of opioid-related plasticity underlying both reward and nociceptive processing. We propose the NFAT-EGR3 pathway in the mPFC as a cellular mediator in regulating mechanical nociception and motivation. Future studies will aim to better unravel the distinct and/or common downstream targets of EGR3 through which such functional consequences are achieved. Because sex differences are thought to play a vital role in behavioral responding influenced by EGR330, future studies should also evaluate the effects in female rats. Our study also indicates that the transcriptomics of other immediate early genes in mesolimbic dopamine substrates should be examined to understand the plasticity events driving maladaptive behaviors.
Supplementary Material
Figure S1. EGR3 in the mPFC does not regulate affective nociception or locomotor behaviors. Percent time spent in the white side of a place escape avoidance chamber (a) and total locomotion (b) of AAV-GFP- and AAV-EGR3-microinjected rats. Percent time spent in the white side of a place escape avoidance chamber (c) and total locomotion (d) of vehicle- and CYSP-microinjected rats. All data are presented as means ± SEMs.
Acknowledgements
This work was supported by the National Institute on Drug Abuse (NIDA; R01DA037257, S1-R01DA037257, and R21DA044486 to D.M.D.), National Institute of Neurological Disorders and Stroke (NINDS; F99NS108543 to J.A.S.), National Institute of General Medical Sciences (NIGMS; R25GM09545902 to The State University of New York at Buffalo). The NIDA Drug Supply Program generously gifted the oxycodone used in these studies. We thank Karen Dietz for copy editing the manuscript. We also thank Jacob Converse and Mason Hochstetler for their support in conducting experiments.
Footnotes
Competing interests
The authors declare no competing interests.
Data availability
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- 1.Goldberg DS & McGee SJ Pain as a global public health priority. BMC Public Health [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlhamer J et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67, 1001–1006, doi: 10.15585/mmwr.mm6736a2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage SR, Kirsh KL & Passik SD Challenges in using opioids to treat pain in persons with substance use disorders. Addict Sci Clin Pract 4, 4–25, doi: 10.1151/ascp08424 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price DD Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772, doi: 10.1126/science.288.5472.1769 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Navratilova E & Porreca F Reward and motivation in pain and pain relief. Nat Neurosci 17, 1304–1312, doi: 10.1038/nn.3811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips JK, Ford MA & Bonnie RJ Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. . (2017). [PubMed]
- 7.Kosten TR & George TP The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1, 13–20, doi: 10.1151/spp021113 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanchoo SJ, Swann AC & Dafny N Descending glutamatergic pathways of PFC are involved in acute and chronic action of methylphenidate. Brain Res 1301, 68–79, doi: 10.1016/j.brainres.2009.08.095 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz AE, Yau HJ, Centeno MV, Apkarian AV & Martina M Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A 106, 2423–2428, doi: 10.1073/pnas.0809897106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Bao C, Gao J, Gu Y & Dong X-W Pain modulates neural responses to reward in the medial prefrontal cortex. Hum Brain Mapp 41, 1372–1381, doi: 10.1002/hbm.24882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong W-Y, Stohler CS & Herr DR Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol 56, 1137–1166, doi: 10.1007/s12035-018-1130-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X-B, Liang B & Gao Y-J The increase of intrinsic excitability of layer V pyramidal cells in the prelimbic medial prefrontal cortex of adult mice after peripheral inflammation. Neuroscience Letters 611, 40–45, doi: 10.1016/j.neulet.2015.11.030 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Liu SS et al. Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci 39, 4162–4178, doi: 10.1523/jneurosci.0274-19.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossert JM et al. Role of Projections from Ventral Medial Prefrontal Cortex to Nucleus Accumbens Shell in Context-Induced Reinstatement of Heroin Seeking. The Journal of Neuroscience 32, 4982, doi: 10.1523/JNEUROSCI.0005-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs PN Beyond Reflexive Measures to Examine Higher Order Pain Processing in Rats. Pain Research and Management 5, 828517, doi: 10.1155/2000/828517 (2000). [DOI] [Google Scholar]
- 16.Johansen Joshua P, Fields Howard L & Manning Barton H The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences 98, 8077–8082, doi: 10.1073/pnas.141218998 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaBuda CJ & Fuchs PN A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163, 490–494, doi: 10.1006/exnr.2000.7395 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Casey K Sensory motivational and central control determinants of pain a new conceptual model. (1968).
- 19.Lumley MA et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 67, 942–968, doi: 10.1002/jclp.20816 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNabb CT, Uhelski ML & Fuchs PN A direct comparison of affective pain processing underlying two traditional pain modalities in rodents. Neuroscience Letters 507, 57–61, doi: 10.1016/j.neulet.2011.11.051 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Peirs C & Seal RP Neural circuits for pain: Recent advances and current views. Science 354, 578–584, doi: 10.1126/science.aaf8933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldredge LC et al. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development 135, 2949–2957, doi: 10.1242/dev.023960 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hippenmeyer S et al. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 36, 1035–1049, doi: 10.1016/s0896-6273(02)01101-7 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR & Russek SJ Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem 281, 29431–29435, doi: 10.1074/jbc.C600167200 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Yamada K et al. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc Natl Acad Sci U S A 104, 2815–2820, doi: 10.1073/pnas.0610765104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamagata K et al. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn Mem 1, 140–152 (1994). [PubMed] [Google Scholar]
- 27.Herndon CA, Ankenbruck N, Lester B, Bailey J & Fromm L Neuregulin1 signaling targets SRF and CREB and activates the muscle spindle-specific gene Egr3 through a composite SRF-CREB-binding site. Exp Cell Res 319, 718–730, doi: 10.1016/j.yexcr.2013.01.001 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Pfaffenseller B et al. Differential expression of transcriptional regulatory units in the prefrontal cortex of patients with bipolar disorder: potential role of early growth response gene 3. Transl Psychiat 6, e805–e805, doi: 10.1038/tp.2016.78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra R et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci 35, 7927–7937, doi: 10.1523/jneurosci.0548-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engeln M et al. Sex-Specific Role for Egr3 in Nucleus Accumbens D2-Medium Spiny Neurons Following Long-Term Abstinence From Cocaine Self-administration. Biol Psychiatry 87, 992–1000, doi: 10.1016/j.biopsych.2019.10.019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers KT et al. The Immediate Early Gene Egr3 Is Required for Hippocampal Induction of Bdnf by Electroconvulsive Stimulation. Front Behav Neurosci 12, 92–92, doi: 10.3389/fnbeh.2018.00092 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin JA et al. A Novel Role for Oligodendrocyte Precursor Cells (OPCs) and Sox10 in Mediating Cellular and Behavioral Responses to Heroin. Neuropsychopharmacology 43, 1385–1394, doi: 10.1038/npp.2017.303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren K & Dubner R Inflammatory Models of Pain and Hyperalgesia. ILAR Journal 40, 111–118, doi: 10.1093/ilar.40.3.111 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G & Watson C The Rat Brain in Stereotaxic Coordinates. Academic Press, New York, 400. 6th Edition (2005). [Google Scholar]
- 35.Deuis JR, Dvorakova LS & Vetter I Methods Used to Evaluate Pain Behaviors in Rodents. Frontiers in Molecular Neuroscience 10, doi: 10.3389/fnmol.2017.00284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs PN & McNabb CT The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integr Neurosci 11, 61–72, doi: 10.1142/s0219635212500045 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Bree D et al. Characterization of the Affective Component of Acute Postoperative Pain Associated with a Novel Rat Model of Inguinal Hernia Repair Pain. CNS Neurosci Ther 22, 146–153, doi: 10.1111/cns.12483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner CT et al. Ubiquitin-proteasomal regulation of chromatin remodeler INO80 in the nucleus accumbens mediates persistent cocaine craving. Sci Adv 5, eaay0351, doi: 10.1126/sciadv.aay0351 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gancarz AM, Kausch MA, Lloyd DR & Richards JB Between-session progressive ratio performance in rats responding for cocaine and water reinforcers. Psychopharmacology (Berl) 222, 215–223, doi: 10.1007/s00213-012-2637-9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengarajan J et al. Sequential Involvement of NFAT and Egr Transcription Factors in FasL Regulation. Immunity 12, 293–300, doi: 10.1016/S1074-7613(00)80182-X (2000). [DOI] [PubMed] [Google Scholar]
- 41.Zjawiony JK et al. Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogs. Frontiers in Psychiatry 10, doi: 10.3389/fpsyt.2019.00157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fields HL & Margolis EB Understanding opioid reward. Trends in neurosciences 38, 217–225, doi: 10.1016/j.tins.2015.01.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Y et al. Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci 17, 254–261, doi: 10.1038/nn.3622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guedon J-MG et al. Current gene therapy using viral vectors for chronic pain. Mol Pain 11, 27–27, doi: 10.1186/s12990-015-0018-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience 114, 529–546, doi: 10.1016/s0306-4522(02)00341-x (2002). [DOI] [PubMed] [Google Scholar]
- 46.Descalzi G et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal 10, doi: 10.1126/scisignal.aaj1549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robison AJ & Nestler EJ Transcriptional and epigenetic mechanisms of addiction. Nature reviews. Neuroscience 12, 623–637, doi: 10.1038/nrn3111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Chen SR, Chen H & Pan HL RE1-silencing transcription factor controls the acute-to-chronic neuropathic pain transition and Chrm2 receptor gene expression in primary sensory neurons. J Biol Chem 293, 19078–19091, doi: 10.1074/jbc.RA118.005846 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson EM et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304, doi: 10.1126/science.1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenzie IA et al. Motor skill learning requires active central myelination. Science 346, 318–322, doi: 10.1126/science.1254960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao L et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19, 1210–1217, doi: 10.1038/nn.4351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang SH et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16, 571–579, doi: 10.1038/nn.3357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birey F et al. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron 88, 941–956, doi: 10.1016/j.neuron.2015.10.046 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lytle JM, Chittajallu R, Wrathall JR & Gallo V NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia 57, 270–285, doi: 10.1002/glia.20755 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Castaneda A & Gaultier A Adult oligodendrocyte progenitor cells - Multifaceted regulators of the CNS in health and disease. Brain Behav Immun 57, 1–7, doi: 10.1016/j.bbi.2016.01.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE & Sato-Bigbee C The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 36, 409–421, doi: 10.1159/000365074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE & Sato-Bigbee C Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 56, 1017–1027, doi: 10.1002/glia.20675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korb E & Finkbeiner S Arc in synaptic plasticity: from gene to behavior. Trends Neurosci 34, 591–598, doi: 10.1016/j.tins.2011.08.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bramham CR et al. The Arc of synaptic memory. Exp Brain Res 200, 125–140, doi: 10.1007/s00221-009-1959-2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkerson JR et al. A role for dendritic mGluR5-mediated local translation of Arc/Arg3.1 in MEF2-dependent synapse elimination. Cell Rep 7, 1589–1600, doi: 10.1016/j.celrep.2014.04.035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu YX et al. DUSP1 is controlled by p53 during the cellular response to oxidative stress. Mol Cancer Res 6, 624–633, doi: 10.1158/1541-7786.Mcr-07-2019 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Nestler EJ, Barrot M & Self DW DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A 98, 11042–11046, doi: 10.1073/pnas.191352698 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nestler EJ Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10, 136–143, doi: 10.9758/cpn.2012.10.3.136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko SW et al. Selective contribution of Egr1 (zif/268) to persistent inflammatory pain. J Pain 6, 12–20, doi: 10.1016/j.jpain.2004.10.001 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Olsson T, Mohammed AH, Donaldson LF, Henriksson BG & Seckl JR Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res 23, 349–353, doi: 10.1016/0169-328x(94)90246-1 (1994). [DOI] [PubMed] [Google Scholar]
- 66.Kerr JR et al. Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Infect Dis 197, 1171–1184, doi: 10.1086/533453 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Dolan S, Hastie P, Crossan C & Nolan AM Co-induction of cyclooxygenase-2 [correction of cyclooxyenase-2] and early growth response gene (Egr-1) in spinal cord in a clinical model of persistent inflammation and hyperalgesia. Mol Pain 7, 91–91, doi: 10.1186/1744-8069-7-91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kummer KK, Mitrić M, Kalpachidou T & Kress M The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. International journal of molecular sciences 21, 3440, doi: 10.3390/ijms21103440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dale J et al. Scaling Up Cortical Control Inhibits Pain. Cell Rep 23, 1301–1313, doi: 10.1016/j.celrep.2018.03.139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji G & Neugebauer V CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur J Neurosci 39, 455–466, doi: 10.1111/ejn.12432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee M et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 35, 5247–5259, doi: 10.1523/jneurosci.3494-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou H et al. A novel neuromodulation strategy to enhance the prefrontal control to treat pain. Mol Pain 15, 1744806919845739, doi: 10.1177/1744806919845739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin J-B et al. dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. The Journal of Clinical Investigation 130, 6555–6570, doi: 10.1172/JCI127607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baldo BA Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci 39, 366–377, doi: 10.1016/j.tins.2016.03.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gourley SL, Lee AS, Howell JL, Pittenger C & Taylor JR Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci 32, 1726–1734, doi: 10.1111/j.1460-9568.2010.07438.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volkow ND, Fowler JS & Wang G-J The addicted human brain: insights from imaging studies. The Journal of clinical investigation 111, 1444–1451, doi: 10.1172/JCI18533 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stafford D, LeSage MG & Glowa JR Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 139, 169–184, doi: 10.1007/s002130050702 (1998). [DOI] [PubMed] [Google Scholar]
- 78.Li L et al. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci 35, 76–88, doi: 10.1016/j.mcn.2007.02.004 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts DS et al. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A 102, 11894–11899, doi: 10.1073/pnas.0501434102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu B, Liu Y, Li N, Zhang J & Zhang X Oxycodone regulates incision-induced activation of neurotrophic factors and receptors in an acute post-surgery pain rat model. J Pain Res 11, 2663–2674, doi: 10.2147/JPR.S180396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schoenbaum G, Stalnaker TA & Shaham Y A role for BDNF in cocaine reward and relapse. Nat Neurosci 10, 935–936, doi: 10.1038/nn0807-935 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Groth RD & Mermelstein PG Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 8125–8134, doi: 10.1523/JNEUROSCI.23-22-08125.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones EA et al. Interactions of Sox10 and Egr2 in myelin gene regulation. Neuron Glia Biol 3, 377–387, doi: 10.1017/S1740925X08000173 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cole SL et al. Cocaine-induced neuron subtype mitochondrial dynamics through Egr3 transcriptional regulation. Molecular Brain 14, 101, doi: 10.1186/s13041-021-00800-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubio FJ et al. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci 49, 165–178, doi: 10.1111/ejn.14203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palombo P et al. Inactivation of the Prelimbic Cortex Impairs the Context-Induced Reinstatement of Ethanol Seeking. Front Pharmacol 8, 725–725, doi: 10.3389/fphar.2017.00725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peters J, LaLumiere RT & Kalivas PW Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 6046–6053, doi: 10.1523/JNEUROSCI.1045-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang N et al. Role of Glutamatergic Projections from the Ventral CA1 to Infralimbic Cortex in Context-Induced Reinstatement of Heroin Seeking. Neuropsychopharmacology 43, 1373–1384, doi: 10.1038/npp.2017.279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z et al. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Reports 12, 752–759, doi: 10.1016/j.celrep.2015.07.001 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Fan X-C et al. Hypersensitivity of Prelimbic Cortex Neurons Contributes to Aggravated Nociceptive Responses in Rats With Experience of Chronic Inflammatory Pain. Frontiers in Molecular Neuroscience 11, doi: 10.3389/fnmol.2018.00085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma L et al. Spontaneous Pain Disrupts Ventral Hippocampal CA1-Infralimbic Cortex Connectivity and Modulates Pain Progression in Rats with Peripheral Inflammation. Cell Reports 29, 1579–1593.e1576, doi: 10.1016/j.celrep.2019.10.002 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Kaminska B, Figiel I, Pyrzynska B, Czajkowski R & Mosieniak G Treatment of hippocampal neurons with cyclosporin A results in calcium overload and apoptosis which are independent on NMDA receptor activation. British journal of pharmacology 133, 997–1004, doi: 10.1038/sj.bjp.0704177 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rojanathammanee L, Floden AM, Manocha GD & Combs CK Attenuation of microglial activation in a mouse model of Alzheimer’s disease via NFAT inhibition. Journal of Neuroinflammation 12, 42, doi: 10.1186/s12974-015-0255-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. EGR3 in the mPFC does not regulate affective nociception or locomotor behaviors. Percent time spent in the white side of a place escape avoidance chamber (a) and total locomotion (b) of AAV-GFP- and AAV-EGR3-microinjected rats. Percent time spent in the white side of a place escape avoidance chamber (c) and total locomotion (d) of vehicle- and CYSP-microinjected rats. All data are presented as means ± SEMs.
Data Availability Statement
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.


