Abstract
Garlic (Allium sativum) has historically been associated with antioxidant, immunomodulatory, and microbiocidal properties, mainly due to its richness in thiosulfates and sulfur-containing phytoconstituents. Sepsis patients could benefit from these properties because it involves both inflammatory and refractory processes. We evaluated the effects of thiosulfinate-enriched Allium sativum extract (TASE) on the immune response to bacterial lipopolysaccharide (LPS) by monocytes from healthy volunteers (HVs) and patients with sepsis. We also explored the TASE effects in HIF-1α, described as the key transcription factor leading to endotoxin tolerance in sepsis monocytes through IRAK-M expression. Our results showed TASE reduced the LPS-triggered reactive oxygen species (ROS) production in monocytes from both patients with sepsis and HVs. Moreover, this extract significantly reduced tumor necrosis factor (TNF)-α, interleukin-1β, and interleukin-6 production in LPS-stimulated monocytes from HVs. However, TASE enhanced the inflammatory response in monocytes from patients with sepsis along with increased expression of human leukocyte antigen-DR. Curiously, these dual effects of TASE on immune response were also found when the HV cohort was divided into low- and high-LPS responders. Although TASE enhanced TNFα production in the LPS-low responders, it decreased the inflammatory response in the LPS-high responders. Furthermore, TASE decreased the HIF-1α pathway-associated genes IRAK-M, VEGFA and PD-L1 in sepsis cells, suggesting HIF-1α inhibition by TASE leads to higher cytokine production in these cells as a consequence of IRAK-M downregulation. The suppression of this pathway by TASE was confirmed in vitro with the prolyl hydroxylase inhibitor dimethyloxalylglycine. Our data revealed TASE’s dual effect on monocyte response according to status/phenotype and suggested the HIF-1α suppression as the possible underlying mechanism.
Keywords: thiosulfinates, garlic extract, allicin, inflammation, endotoxin tolerance, sepsis, Allium sativum, immunomodulation
1. Introduction
For more than 5000 years, garlic (Allium sativum) has traditionally been associated with bactericidal, antiviral, anti-inflammatory and antiparasitic properties [1]. Recently, garlic extract and its relative compounds have also shown anticancer effects, such as antiproliferative ability, cell death induction, and reduction of migration and metastasis [2,3,4,5]. One of the most interesting attributes of Allium sativum is its antioxidant capacity. This property has been explained by its richness in thiosulfates and sulfur-containing phytoconstituents [1], including allicin, alliin, S-allyl cysteine, vinyldithiins, and flavonoids such as quercetin [1]. Treatment with allicin has been shown to protect against the detrimental effects of nephropathy in a model of diabetes due to its antioxidant capacity and Nrf2 induction [6]. Moreover, this compound reduced reactive oxygen species (ROS) production in murine aortic endothelial cells that had undergone high glucose/hypoxia-induced injury [7].
Regarding the immunomodulatory properties of garlic, most studies have described its anti-inflammatory effects. Garlic has been shown to prevent inflammatory cytokine expression in LPS-activated human whole blood cells, adipocytes, and placental explants [8,9,10]. Some authors have reported that allicin had been shown in vitro to attenuate inflammation by tumor necrosis factor (TNF)-α in epithelial cells and during hypoxia–reoxygenation in cardiomyocytes [11,12]. Moreover, a randomized, double-blind, clinical trial of aged garlic extract supplementation revealed an inflammation reduction in adults with obesity [13]. However, other authors have argued that allicin exhibits an immunomodulatory role rather than overall anti-inflammatory effects, given that this compound had an enhanced proinflammatory response in acute malaria [14], increased TNF-α production in murine macrophages [15], and augmented interferon-γ release in monocyte cultures from patients with vaginitis [16].
In this regard, sepsis patients could benefit from the effects of garlic as this pathology is defined as a dysregulated inflammatory response against an infection. In a recent study, we found that thiosulfinate-enriched Allium sativum extract (TASE) used as an adjuvant to ceftriaxone treatment can improve weight and clinical signs (ocular secretions, piloerection) as well as reduce hepatic edema, vacuolization, and inflammation in a rat peritonitis model of sepsis [17]. Nevertheless, it should be noted animal models do not accurately reproduce the complexity of immune response observed in humans [18,19]. According to several authors, sepsis patients exhibit a first phase of systemic inflammation (SIRS) or “cytokine storm” followed by an immunosuppression phase known as compensatory anti-inflammatory response syndrome (CARS). CARS causes a protracted immunosuppression characterized by neutrophilia, increased regulatory T numbers, endotoxin tolerance phenotype in monocytes, high IL-10 and TGF-β levels, and high lymphocyte apoptosis [20,21,22].
Concerning the molecular mechanisms in sepsis, we and others have reported that monocytes from patients with sepsis demonstrated an endotoxin tolerance status in which they are unable to respond to subsequent bacterial stimuli such as lipopolysaccharide (LPS) [23,24,25,26]. This type of cell reprogramming is characterized by low proinflammatory cytokine production, human leukocyte antigen (HLA)-DR expression, increased phagocytosis activity, enhanced tissue remodeling, and impaired antigen presentation [23,24,25,26,27]. According to our previous data, hypoxia inducible factor-1α (HIF-1α) mediated the endotoxin tolerance phenotype by decreasing the cytokine production through the overexpression of IRAK-M pseudokinase and lowering antigen presentation via PD-L1 overexpression [23,24,25,26,27,28,29]. In this line, allicin and other garlic components have been shown to suppress the HIF-1α pathway in human cancer cells [3,4,30]. Nevertheless, the effects of these types of compounds in sepsis monocytes and their phenotype remain unknown.
Herein, we aimed to study the effects of a TASE on monocytes from healthy volunteers (HVs) and patients with sepsis recruited at hospital emergency department admission. In these cells, we analyzed ROS levels, the inflammatory response to the LPS challenge, and the HIF-1α pathway to assess the relevance of the endotoxin tolerance phenotype on the potential immunomodulatory properties of TASE.
2. Results
2.1. Thiosulfinate-Enriched Allium sativum Extract Reduces Lipopolysaccharide-Triggered Oxidative Stress in Monocytes from Both Patients with Sepsis and Healthy Volunteers
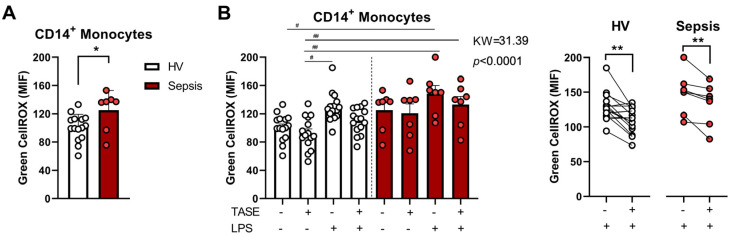
Due to the antioxidant ability of garlic, we tested its potential effects on the oxidative status of monocytes after an inflammatory insult. To do that, we isolated monocytes from both patients with sepsis and HVs (Table 1) and stimulated them with LPS and in combination with TASE. As Figure 1A illustrates, monocytes from patients with sepsis exhibited higher oxidative stress than HV monocytes. Curiously, despite the fact that TASE did not reduce the septic monocytes’ basal oxidative stress levels, it was able to reduce the increase in stress generated by an inflammatory insult in monocytes from both patients with sepsis and HVs (Figure 1B).
Table 1.
Baseline characteristics of sepsis patients and healthy controls at hospital admission.
| Controls (n = 15) | Sepsis (n = 7) | p-Value | |
|---|---|---|---|
| Age—years | 51.53 ± 5.21 | 56 ± 22.8 | 0.57 |
| Sex, male—n (%) | 9 (60) | 4 (57.1) | 0.99 |
| Comorbidities | |||
| Hypertension | 1 (6.7) | 2 (28.6) | 0.23 |
| Diabetes Mellitus | 1 (6.7) | 1 (14.3) | 0.99 |
| COPD | 1 (6.7) | 2 (28.6) | 0.23 |
| Current smoker | 2 (15.4) | 2 (28.6) | 0.56 |
| Temperature—°C | 36.5 ± 0.74 | 37.66 ± 1.6 | 0.03 * |
| HR—bpm | 84.64 ± 22.7 | 92.29 ± 18.6 | 0.44 |
| RR—bpm | 15.1 ± 2.5 | 19.14 ± 4.6 | 0.03 * |
| SBP—mm Hg | 124.7 ± 33.1 | 120 ± 43.87 | 0.78 |
| MBP—mm Hg | 86.4 ± 12.3 | 81.52 ± 24.6 | 0.54 |
| Leukocytes—103/µL | 6.94 ± 1.2 | 12.2 ± 5.83 | 0.01 * |
| Neutrophils—103/µL | 4.18 ± 1.33 | 10.25 ± 5.69 | 0.01 * |
| Lymphocytes—103/µL | 2.27 ± 0.66 | 1.18 ± 0.41 | <0.01 ** |
| Monocytes—103/µL | 0.48 ± 0.13 | 0.42 ± 0.23 | 0.25 |
| Platelets—103/µL | 236.8 ± 45.1 | 205.9 ± 88.3 | 0.72 |
| Creatinine—mg/dL | 0.91 ± 0.17 | 1.54 ± 1.37 | 0.08 |
| CRP—mg/L | 1.35 ± 0.9 | 136.4 ± 93.5 | <0.001 *** |
| Bilirubin—mg/dL | 0.73 ± 0.41 | 0.42 ± 0.18 | 0.1 |
| qSOFA | - | 0.81 ± 0.73 | - |
Data are expressed as mean ± SD and percentage (number inside parentheses). COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; HR, heart rate; MBP, mean blood pressure; qSOFA, Quick Sequential Organ Failure Assessment score; SBP, systolic blood pressure. *, p < 0.05; **, p < 0.01; ***, p < 0.001 in Chi-square or unpaired t tests.
Figure 1.
Thiosulfinate-enriched Allium sativum extract (TASE) effects on oxidative stress of monocytes from both HV and septic patients. (A) Monocytes from HV (n = 15) and septic patients (n = 7) were labeled with Green CellROX. Mean intensities of fluorescence (MIF) on gated CD14+ monocytes determined by flow cytometry are shown. *, p < 0.05 in unpaired t test. (B) Monocytes from HV (n = 15) and septic patients (n = 7) were stimulated with 10 ng/mL of LPS in the presence or not of 3 µg/mL of TASE for 16 h, then cells were labeled with Green CellROX. MIF on gated CD14+ monocytes determined by flow cytometry are shown. KW, Kruskal–Wallis statistic; #, p < 0.05; ##, p < 0.01 in Dunn’s post hoc test. *, p < 0.05; **, p < 0.01 in paired t test. White dots represent individual HV values and red dots individual sepsis values. Bars express mean ± SD. Left panels show individual paired analyses.
2.2. Thiosulfinate-Enriched Allium sativum Extract Exhibits Divergent Effects on Monocytes from Patients with Sepsis Compared with Healthy Volunteers
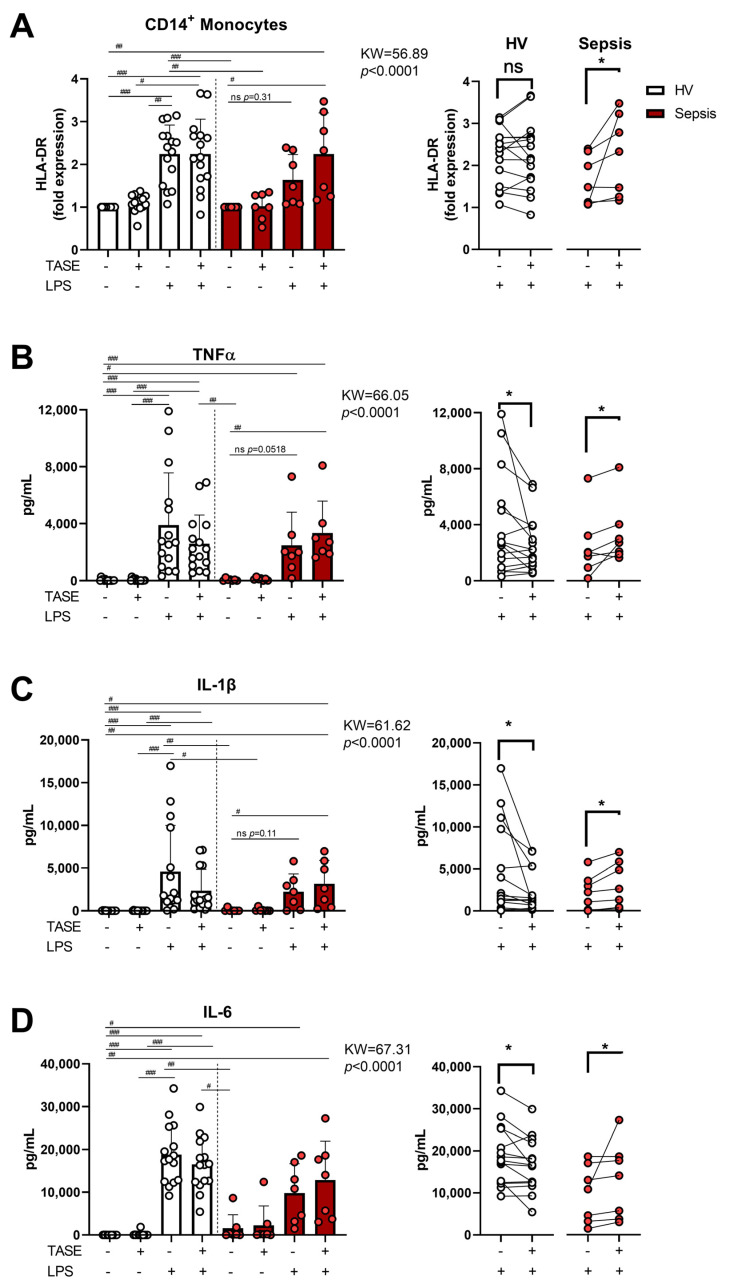
Once we established that TASE prevents an increase in the basal oxidative stress of LPS-challenged monocytes, we studied its effects in the inflammatory response. We analyzed the expression of human leukocyte antigen (HLA)-DR and the production of inflammatory cytokines in response to TASE, LPS, and their combination. Our data indicated that TASE did not exhibit a patent effect on the HLA-DR expression of HV monocytes. In contrast, HLA-DR expression was higher in LPS-challenged septic monocytes in the presence of TASE than in septic monocytes stimulated with LPS alone (Figure 2A). Thus, the effects of TASE on proinflammatory cytokine production after LPS stimulation was revealed to be the opposite in HV monocytes compared with septic monocytes. In addition, whereas TASE induced a decrease in TNF-α, IL-1β, and IL-6 production in LPS-stimulated HV monocytes, it provoked an increment of these cytokines in the septic monocytes (Figure 2B). Other immune markers deregulated in sepsis, such as CD16 or CD206, as well as the anti-inflammatory cytokine IL-10 and the cytokine CXCL-10, did not exhibit a clear pattern (Supplementary Figures S1 and S2).
Figure 2.
Effects of thiosulfinate-enriched Allium sativum extract (TASE) in LPS response of monocytes from both HV and septic patients. (A) Monocytes from HV (n = 15) and septic patients (n = 7) were stimulated with 10 ng/mL for 16 h in the presence or not of 3 µg/mL of TASE and labeled with cytometry antibodies. Fold induction of HLA-DR mean intensities of fluorescence against basal on gated CD14+ monocytes determined by flow cytometry are shown. (B–D) Monocytes from HV (n = 15) and septic patients (n = 7) were stimulated with 10 ng/mL of LPS for 16 h in combination or not with 3 µg/mL of GE and inflammatory cytokine levels of TNF-α (B), IL-1β (C) and IL-6 (D) were measured. Concentrations are shown. KW, Kruskal–Wallis statistic; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 in Dunn’ post hoc test. *, p < 0.05 in paired t test. White dots represent individual HV values and red dots individual sepsis values. Left panels show paired analysis of individual values and bars express mean ± SD.
2.3. Thiosulfinate-Enriched Allium sativum Extract Increases the Lipopolysaccharide Response of Monocytes with an Endotoxin-Tolerant Phenotype
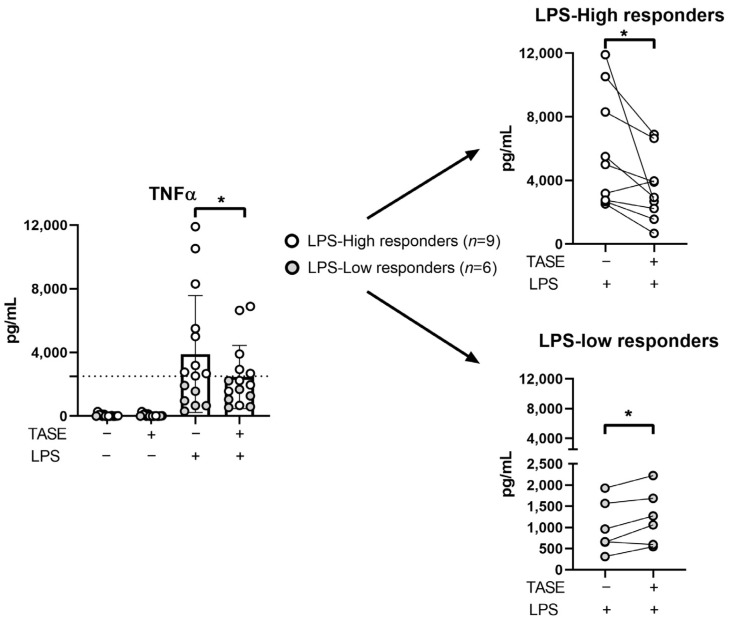
Given that TASE exhibited dual immunomodulatory properties in HV and septic monocytes, and sepsis is highly related to an endotoxin-tolerant phenotype in which monocytes poorly respond to LPS [23,24,25,26], we split the HV cohort into two groups: low- and high-LPS responders. The garlic extract induced a significantly high response to LPS in low-responder monocytes, whereas it reduced the response in high-responder monocytes (Figure 3), indicating that TASE improved the response to LPS in endotoxin-tolerant monocytes.
Figure 3.
Comparison of thiosulfinate-enriched Allium sativum extract (TASE) effects in LPS response of low- and high-responder monocytes. Monocytes from HV (n = 15) were stimulated with 10 ng/mL of LPS for 16 h in combination or not with 3 µg/mL of TASE. The HV cohort was split into two groups according to the LPS response of their monocytes (cut-off 2500 pg/mL of TNF-α). TNF-α levels in cell culture supernatant are shown. *, p < 0.05 in paired t test. White dots represent individual high responders values and grey dots individual low responders values. Left panels show paired analysis of individual values and bars express mean ± SD.
2.4. Thiosulfinate-Enriched Allium sativum Extract Reduces HIF-1α Pathway Activation in Sepsis Monocytes
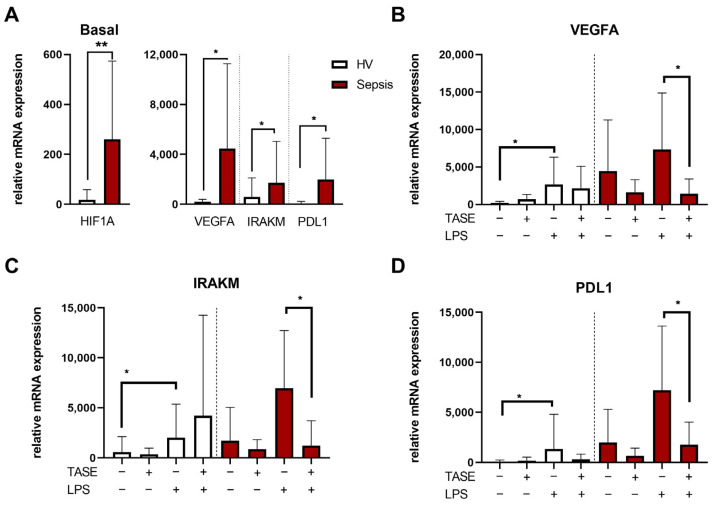
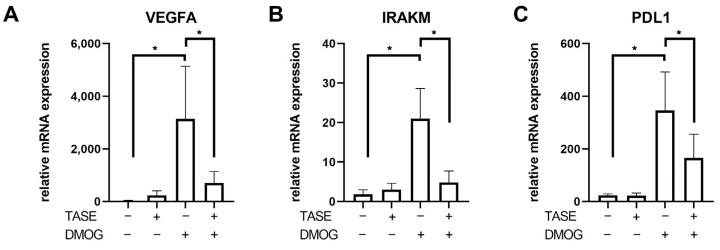
The HIF-1α pathway is one of the key mechanisms involved in the monocyte phenotype during sepsis; thus, we explored the effect of TASE on this pathway. First, HIF-1α activation was analyzed in monocytes from patients with sepsis and HVs. As expected, the septic monocytes exhibited a basal overexpression of the HIF1A gene and some of its target genes, including VEGFα, IRAKM, and PD-L1 compared with HVs (Figure 4A). It should be noted that no other basal differences between sepsis and healthy monocytes were found in membrane markers or cytokine levels, except for a slight decrease in CD33 in sepsis monocytes (Supplementary Figure S3). The expression of these HIF-1α-pathway related genes was shown to be reduced in septic monocytes treated with TASE, with a significant reduction of their expression in the LPS-challenged monocytes (Figure 4B–D). In contrast, in monocytes from HVs, TASE did not reveal a clear effect on the expression of these genes, either basally or in combination with LPS (Figure 4B–D). To confirm the involvement of TASE in the modulation of the HIF-1α pathway, we treated HV monocytes with DMOG, a pharmacological HIF-1α inductor. We found that TASE treatment reduced the DMOG-triggered overexpression of the HIF-1α-related genes (Figure 5).
Figure 4.
Expression of HIF-1α and related genes in monocytes from HV and septic patients. (A) Basal expressions of HIF1A, VEGFA, IRAKM, and PDL1 in monocytes from HV (n = 7) and septic patients (n = 7) by RT-qPCR are shown. *, p < 0.05; ** p < 0.01 in Mann–Whitney t test. (B–D) Monocytes from HV and septic patients were stimulated with 10 ng/mL of LPS for 16 h in combination or not with 3 µg/mL of TASE. Relative expressions of VEGFA (B), IRAKM (C), and PDL1 (D) are shown. *, p < 0.05 in paired t test. Bars represent mean ± SD.
Figure 5.
Expression of HIF-1α pathway related genes in monocytes treated with DMOG. Monocytes from HV (n = 5) were stimulated with 100 µM of DMOG for 16 h in combination or not with 3 µg/mL of TASE. Relative expressions of VEGFA (A), IRAKM (B), and PDL1 (C) are shown. *, p < 0.05 in paired t test. Bars represent mean ± SD.
3. Discussion
Allium sativum has traditionally been associated with beneficial effects in infectious processes due to its antimicrobial, anti-inflammatory, and antioxidant properties [6,17,31,32]. However, it has not yet been introduced in clinical settings. Perhaps its most interesting application could be as an adjuvant to certain treatments [2,17]. In this regard, we and others have evaluated the potential use of garlic and its associated compounds in animal models of endotoxemia or sepsis, thereby revealing its protective effects [17,33,34,35].
Sepsis can lead to an increase in ROS, especially in monocytes [36]. Herein, we found that TASE reduced the increase in oxidative stress caused by the LPS inflammatory insult in both septic and HV monocytes. These data are in accordance with other studies that found the Allium sativum derivatives S-allyl cysteine and alliin reduced nitric oxide generation in LPS-treated rats and intracellular ROS in LPS-stimulated THP1-derived macrophages, respectively [33,37].
In a previous study, using the same TASE as a adjuvant, we found TASE reduced inflammation in a rat peritonitis sepsis model [17]. We found different results when we evaluated the effects of TASE on the human monocyte response. Even though the extract decreased the LPS response of HV monocytes, it increased this response in monocytes from patients with sepsis. It should be noted, animal models mainly reproduce the SIRS phase of sepsis and not the complexity of the immune response observed in humans [18,19], which can be also characterized by CARS and immunosuppression [20,21,22]. A reasonable explanation for these divergent effects is that human septic monocytes are locked in an endotoxin-tolerant status, as we observed the same divergent effect of TASE when we split the HV cohort into high (non-tolerant) and low (tolerant) responders. This result suggests that a possible immunomodulatory effect of Allium sativum depends on the polarization of the monocytes at that precise moment. This fact should be considered when designing studies to achieve a precision-medicine approach in studies involving garlic or its derivative compounds.
In previous studies, we have described the HIF-1α pathway as the major transcription factor for endotoxin tolerance in monocytes by IRAK-M and PD-L1 upregulation, leading to low cytokine production and decreased antigen presentation, respectively [24,25,26,38]. IRAK-M is a pseudokinase widely known for its role in toll such as receptor signaling inhibition [28,29,38,39,40]. Here, we observed that TASE reduced the expression of both IRAK-M and PD-L1 in LPS-challenged monocytes from patients with sepsis. This result was reproduced in monocytes from HVs when the HIF-1α pathway was induced by the prolyl hydroxylase inhibitor DMOG. Some other studies have proposed regulation of this pathway by garlic-derived compounds and similar antioxidant phytochemicals [41,42]. Along these lines, allicin and diallyl trisulfide suppressed the HIF-1α pathway in cancer and other pathogenic contexts [3,4,7,13,30]. Thus, we propose the suppression of the HIF-1α pathway and the subsequent decrease in IRAK-M as the possible underlying cause of the differential/dual effects of TASE in monocytes.
In our study, septic monocytes were isolated from blood samples taken in the emergency department prior to any treatment. According to Santos et al., this is typically a moment in time with a higher endotoxin-tolerant phenotype and higher ROS levels in septic monocytes [36]. Our data indicate that, at this time, septic patients could benefit from the immunomodulatory properties of Allium sativum. Furthermore, a longitudinal study evaluating the antioxidant properties of TASE at various stages of sepsis and in combination with various treatments would be advisable to better define the treatment window and to optimize its use.
In conclusion, we have evaluated the antioxidant and immunomodulatory capacity of a TASE in the context of sepsis and endotoxin tolerance. Our data revealed a differential and dual effect of Allium sativum extract in monocytes according to their basal status. TASE had an anti-inflammatory role in non-tolerant monocytes, whereas it had a proinflammatory effect on tolerant ones. Our results indicated that HIF-1α pathway inhibition by TASE in tolerant monocytes appeared to be responsible for this divergent effect. Thus, we have identified a new mechanism to explain the possible immunomodulatory activity of garlic in the context of sepsis. Our data reinforce the idea of a possible therapeutic opportunity for garlic-derived compounds in sepsis, nevertheless further in vivo studies are required to demonstrate its possible clinical benefit.
4. Materials and Methods
4.1. Study Design and Participants
Patients who met the criteria for sepsis according to the Sepsis-3 definition [43] were recruited from the Emergency Department of La Paz University Hospital; the age- and sex-matched HVs were recruited from the Blood Donor Services of La Paz University Hospital. The clinical characteristics of the patients and HVs are shown in Table 1. Blood samples were collected at admission and prior to any treatment. The exclusion criteria were pregnancy, having received immunosuppressants at admission (i.e., chemotherapy or corticosteroids), and immunodeficiency (primary or acquired). The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the La Paz University Hospital Clinical Research Ethics Committee. An informed consent document was obtained from all the included patients and HVs.
4.2. Thiosulfinate-Enriched Allium sativum Extract Preparation
The thiosulfinate-enriched Allium sativum extract (TASE) was obtained following a patented protocol for the production of lyophilized garlic extract (WO 2008/102036 A1) that is commercially available under the brand name Aliben (Aliben Foods S.L., Ciudad Real, Spain). The thiosulfinate content was determined as described [2], showing 7.03 µg of allicin per mg of lyophilizate. The lyophilizate extract was dissolved in sterile saline (sodium chloride 0.9% from Fresenius Kabi, Barcelona, Spain) to a final stock concentration of 30 mg/mL (≈10 mM allicin). The solution was then filtered with 0.22 µm syringe (JetBiofil, Guangzhou, China). The composition of organic compounds in TASE is shown in Table 2.
Table 2.
Composition of organic compounds of lyophilized thiosulfinate-enriched Allium sativum extract (TASE) from purple garlic of Las Pedroñeras (Cuenca, Spain) used in this study.
| Compound | TASE (mg/g) |
|---|---|
| Total Polyphenols | 13.91 |
| Total Flavonoids | 3.22 |
| Diallyl thiosulfinate (allicin) | 7.03 |
| S-allyl-L-cysteine | 0.08 |
| Leucine | 0.586 |
| Isoleucine | 0.500 |
| Valine | 0.477 |
| Methionine | 0.316 |
| Cysteine | 0.811 |
| Phenylalanine | 0.556 |
| Tyrosine | 4.499 |
| Aspartic Acid | 0.901 |
| Glutamic Acid | 2.866 |
| Arginine | 4.090 |
| Lysine | 0.617 |
| Histidine | 0.891 |
| Threonine | 0.812 |
| Serine | 0.385 |
| Glycine | 0.215 |
| Alanine | 0.897 |
| Thiamine (B1) | 0.552 |
| Riboflavin (B2) | 0.002 |
| Niacin (B3) | 0.026 |
| Pantothenic acid (B5) | 1.556 |
| Biotin (B7) | 0.251 |
| Cobalamin (B12) | 0.898 |
| Ascorbic Acid (C) | 3.347 |
| Linoleic Acid (F) | 0.276 |
| Tocopherol (E) | 0.007 |
| Menadione (K3) | 0.007 |
4.3. Human Monocyte Isolation
Fresh blood from venipuncture was collected in K2-EDTA anticoagulant tubes (Vacuette, Greiner BioOne, San Sebastián de los Reyes, Spain) and submitted to Ficoll-Plus (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) gradient according to the manufacturer’s instructions. The layer of peripheral blood mononuclear cells (PBMCs) was washed twice with phosphate-buffered saline (PBS). The monocyte population was enriched by an adherence selectivity protocol [44,45]. Briefly, the percentage of monocytes in total PBMCs was measured by flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA), then 500,000 monocytes per well were cultured in Costar 6-well tissue culture-treated plates (Corning, Corning, NY, USA) in Roswell Park Memorial Institute (RPMI) medium (Gibco, Darmstadt, Germany) without serum for 1 h at 37 °C. The non-adherent cells (mainly lymphocytes) were then discarded, and the adhered cells were washed thrice and cultured with RPMI supplemented with 10% fetal bovine serum (Gibco) and 0.01% penicillin and streptomycin mix (Thermofisher, Waltham, MA, USA) at 37 °C in a humidified atmosphere with 5% CO2. Monocyte purity was tested by CD14 labeling and flow cytometry analysis (average 71% of CD14+ cells).
4.4. In Vitro Stimulation
The monocyte cultures from both patients and controls were stimulated with 3 µg/mL of filtered TASE and/or 10 ng/mL LPS from Escherichia coli O111:B4 (Merck KGaA, Darmstadt, Germany) for 16 h using the same volume of vehicle (saline) as a negative control. The supernatants were stored at −20 °C until inflammatory cytokine quantification. Cells were divided into 2 tubes for intracellular ROS measurement and flow cytometry characterization.
4.5. Cell Reactive Oxygen Species Measurement
To determine intracellular ROS levels, we employed CellROX Green Reagent (Life Technologies, Carlsbad, CA, USA), following the manufacturer’s instructions as previously described [46]. Briefly, 5 µM CellROX reagent was added to the cell cultures 30 min before harvesting, and the cells were co-stained with an anti-human CD14 allophycocyanin antibody (Immunostep, Salamanca, Spain). Samples were acquired with a FACSCalibur (BD Biosciences) and the data were analyzed with FlowJo software (BD Biosciences).
4.6. Flow Cytometry Characterization
Monocytes from cell cultures were labeled with a cocktail of Alexa Fluor 488 anti-human CD14 (BioLegend San Diego, CA, USA), BUV496 anti-human HLA-DR (BD Biosciences), BV 510 anti-human CD16 (BioLegend), BV711 anti-human CD206 (BioLegend), BV605 anti-human CD33 (BioLegend), and BUV395 anti-human CD162 (BD Biosciences). True-Stain Monocyte Blocker (BioLegend) reagent was added prior to the label protocol to block the nonspecific binding of some fluorochromes to monocytes. Cells were acquired on a Cytek Aurora Spectral Cytometer (Cytek Biosciences, Fremont, CA, USA) and the data were analyzed employing OMIQ (Dotmatics, Woburn, MA, USA).
4.7. Inflammatory Cytokine Quantification
The concentration of interleukin (IL)-1β, TNF-α, CCL2 (MCP-1), IL-6, IL-10, CXCL8 (IL-8), and CXCL10 (IP-10) were quantified in monocyte supernatant by a LEGENDplex HU Essential Immune Response Panel, following the manufacturer’s protocol. Samples were acquired in a FACSCalibur (BD Biosciences), and the data were analyzed with the LEGENDplex Data Analysis Software Suite (Qognit, Inc. San Carlos, CA, USA).
4.8. Dimethyloxalylglycine In Vitro Model
To simulate the overactivation of the HIF-1α pathway observed in patients with sepsis, dimethyloxalylglycine (DMOG), a well-known pharmacological inductor of the HIF-1α pathway, was used as previously described [24]. Monocytes were treated with 100 μM DMOG in the presence or not of 3 µg/mL TASE for 16 h. The relative expression of HIF-1α-dependent genes was quantified by real-time quantitative PCR (RT-qPCR).
4.9. Relative mRNA Expression Quantification
Total mRNA from monocyte cultures was isolated with the Monarch Total RNA Miniprep Kit (New England Biolabs, Ipswich, MA, USA). The complementary DNA was obtained by reverse transcription of 0.5 μg total RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster, CA, USA). The gene expression levels were analyzed by RT-qPCR (Applied Biosystems 7300).
4.10. Statistical Analysis
For sepsis vs. control comparisons of quantitative variables, distribution normality was checked by Shapiro–Wilk test in order to define the parametric (t test) and non-parametric (Mann–Whitney) analysis. Kruskal–Wallis with Dunn’s post hoc test was used for multiple group comparisons. Paired t test was performed to compare the various in vitro stimulation conditions. For qualitative variables, Fisher exact test was used. p-values of <0.05 were considered significant at a 95% confidence interval. Statistical analyses were performed with IBM SPSS 23 (Armonk, NY, USA) and GraphPad Prism 8 (San Diego, CA, USA) software. The sample size (n) and the statistical test in each analysis are shown in the figure legends. All experiments were performed in at least five biological replicates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076234/s1.
Author Contributions
Conceptualization, F.J.R.-C., J.A.-O., E.L.-C. and J.M.P.-O.; data curation, J.F.R.; formal analysis, F.J.R.-C., J.A.-O. and R.L.-R.; investigation, F.J.R.-C., V.T.-A., R.L.-R. and R.d.C.; methodology, J.M.P.-O., V.T.-A., R.L.-R., M.B.-G., C.R.-J. and J.A.-O.; resources, F.J.R.-C., E.L.-C., J.M.P.-O., N.B.-R., L.A.G. and J.F.R.; writing—original draft, F.J.R.-C., E.L.-C., J.A.-O. and J.M.P.-O.; writing—review and editing, F.J.R.-C., E.L.-C., J.A.-O., N.B.-R. and J.M.P.-O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All patients and controls were treated according to recommended criteria of confidentiality. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the local Ethics Committee (La Paz University Hospital, Madrid, PI-3761).
Informed Consent Statement
All included patients and healthy volunteers fulfill an informed consent.
Data Availability Statement
Datasheets can be provided by corresponding authors under reasonable request.
Conflicts of Interest
J.F.R. and L.A.G. are part of the authors of the registered brand Aliben© (European Trademark number 10543429) which entitles the lyophilized Allium sativum extract to be employed in this study (patent WO 2008/102036 A1). Method for obtaining a freeze-dried, stable extract from plants of the Allium genus. J.M.P.-O., L.A.G., J.F.R., and F.J.R.-C. are co-contributors of a national registered patent (Spanish Trademark number ES2675282A1), which employs the lyophilized Allium sativum extract, its use for the manufacture of a medicinal product for the treatment of diseases, and its obtaining procedure.
Funding Statement
This work was partially supported by the Comunidad de Madrid under grant P2022/BMD-7212 to R.d.C. and Spanish Ministry of Health-Carlos III Health Institute (ISCIII) under grants CB06/02/0053 to R.d.C. and CD21/00059 to J.A.-O.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.El-Saber Batiha G., Magdy Beshbishy A., G Wasef L., Elewa Y.H.A., A Al-Sagan A., Abd El-Hack M.E., Taha A.E., M Abd-Elhakim Y., Prasad Devkota H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Ortiz J.M., Galan-Moya E.M., de la Cruz-Morcillo M.A., Rodriguez J.F., Gracia I., Garcia M.T., Redondo-Calvo F.J. Cost Effective Use of a Thiosulfinate-Enriched Allium Sativum Extract in Combination with Chemotherapy in Colon Cancer. Int. J. Mol. Sci. 2020;21:2766. doi: 10.3390/ijms21082766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei Z., Shan Y., Tao L., Liu Y., Zhu Z., Liu Z., Wu Y., Chen W., Wang A., Lu Y. Diallyl Trisulfides, a Natural Histone Deacetylase Inhibitor, Attenuate HIF-1α Synthesis, and Decreases Breast Cancer Metastasis. Mol. Carcinog. 2017;56:2317–2331. doi: 10.1002/mc.22686. [DOI] [PubMed] [Google Scholar]
- 4.Pandey N., Tyagi G., Kaur P., Pradhan S., Rajam M.V., Srivastava T. Allicin Overcomes Hypoxia Mediated Cisplatin Resistance in Lung Cancer Cells through ROS Mediated Cell Death Pathway and by Suppressing Hypoxia Inducible Factors. Cell. Physiol. Biochem. 2020;54:748–766. doi: 10.33594/000000253. [DOI] [PubMed] [Google Scholar]
- 5.Borlinghaus J., Albrecht F., Gruhlke M.C.H., Nwachukwu I.D., Slusarenko A.J. Allicin: Chemistry and Biological Properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arellano-Buendía A.S., Castañeda-Lara L.G., Loredo-Mendoza M.L., García-Arroyo F.E., Rojas-Morales P., Argüello-García R., Juárez-Rojas J.G., Tapia E., Pedraza-Chaverri J., Sánchez-Lozada L.G., et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants. 2020;9:1134. doi: 10.3390/antiox9111134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin K.-H., Wei Y.-M., Liu C.-H., Liu J.-S., Huang I.-C., Viswanadha V.P., Huang C.-Y., Kuo W.-W. Diallyl Trisulfide Suppresses High-Glucose-Induced Cardiomyocyte Apoptosis by Targeting Reactive Oxygen Species-Mediated Hypoxia-Inducible Factor-1α/Insulin-like Growth Factor Binding Protein 3 Activation. J. Agric. Food Chem. 2021;69:11696–11708. doi: 10.1021/acs.jafc.1c02384. [DOI] [PubMed] [Google Scholar]
- 8.Quintero-Fabián S., Ortuño-Sahagún D., Vázquez-Carrera M., López-Roa R.I. Alliin, a Garlic (Allium sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes. Mediat. Inflamm. 2013;2013:e381815. doi: 10.1155/2013/381815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris A., Thornton C.E., Xu B., Hennessy A. Garlic Increases IL-10 and Inhibits TNFalpha and IL-6 Production in Endotoxin-Stimulated Human Placental Explants. Placenta. 2005;26:828–834. doi: 10.1016/j.placenta.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Keiss H.-P., Dirsch V.M., Hartung T., Haffner T., Trueman L., Auger J., Kahane R., Vollmar A.M. Garlic (Allium sativum L.) Modulates Cytokine Expression in Lipopolysaccharide-Activated Human Blood Thereby Inhibiting NF-KappaB Activity. J. Nutr. 2003;133:2171–2175. doi: 10.1093/jn/133.7.2171. [DOI] [PubMed] [Google Scholar]
- 11.Lang A., Lahav M., Sakhnini E., Barshack I., Fidder H.H., Avidan B., Bardan E., Hershkoviz R., Bar-Meir S., Chowers Y. Allicin Inhibits Spontaneous and TNF-Alpha Induced Secretion of Proinflammatory Cytokines and Chemokines from Intestinal Epithelial Cells. Clin. Nutr. 2004;23:1199–1208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Deng X., Yang P., Gao T., Liu M., Li X. Allicin Attenuates Myocardial Apoptosis, Inflammation and Mitochondrial Injury during Hypoxia-Reoxygenation: An in Vitro Study. BMC Cardiovasc. Disord. 2021;21:200. doi: 10.1186/s12872-021-01918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C., Mathews A.E., Rodrigues C., Eudy B.J., Rowe C.A., O’Donoughue A., Percival S.S. Aged Garlic Extract Supplementation Modifies Inflammation and Immunity of Adults with Obesity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin. Nutr. ESPEN. 2018;24:148–155. doi: 10.1016/j.clnesp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y., Zhu X., Wang Q., Jiang Y., Shang H., Cui L., Cao Y. Allicin Enhances Host Pro-Inflammatory Immune Responses and Protects against Acute Murine Malaria Infection. Malar. J. 2012;11:268. doi: 10.1186/1475-2875-11-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang N.S., Moon E.Y., Cho C.G., Pyo S. Immunomodulating Effect of Garlic Component, Allicin, on Murine Peritoneal Macrophages. Nutr. Res. 2001;21:617–626. doi: 10.1016/S0271-5317(01)00269-X. [DOI] [Google Scholar]
- 16.Islam N., Iqbal J., Hasan N., Fatima Z., Thakur H., Hasan N., Mahdi A.A., Khan T., Bano F., Ansari I.A. Allicin from Garlic Suppresses TNF-α and Augments IFN-γ Expressions in Monocyte Cultures from Patients with Vaginitis. Nat. Prec. 2008 doi: 10.1038/npre.2008.1560.1. [DOI] [Google Scholar]
- 17.Redondo-Calvo F.J., Bejarano-Ramírez N., Baladrón V., Montenegro O., Gómez L.A., Velasco R., Villasanti N., Illescas S., Franco-Sereno M.T., Gracia I., et al. Black Garlic and Thiosulfinate-Enriched Extracts as Adjuvants to Ceftriaxone Treatment in a Rat Peritonitis Model of Sepsis. Biomedicines. 2022;10:3095. doi: 10.3390/biomedicines10123095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroder K., Irvine K.M., Taylor M.S., Bokil N.J., Le Cao K.-A., Masterman K.-A., Labzin L.I., Semple C.A., Kapetanovic R., Fairbairn L., et al. Conservation and Divergence in Toll-like Receptor 4-Regulated Gene Expression in Primary Human versus Mouse Macrophages. Proc. Natl. Acad. Sci. USA. 2012;109:E944–E953. doi: 10.1073/pnas.1110156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L., et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss R.S., Monneret G., Payen D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss R.S., Coopersmith C.M., McDunn J.E., Ferguson T.A. The Sepsis Seesaw: Tilting toward Immunosuppression. Nat. Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao C., Yu M., Chai Y. Pathological Alteration and Therapeutic Implications of Sepsis-Induced Immune Cell Apoptosis. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-2015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavaillon J.-M., Adib-Conquy M. Bench-to-Bedside Review: Endotoxin Tolerance as a Model of Leukocyte Reprogramming in Sepsis. Crit. Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avendaño-Ortiz J., Maroun-Eid C., Martín-Quirós A., Lozano-Rodríguez R., Llanos-González E., Toledano V., Gómez-Campelo P., Montalbán-Hernández K., Carballo-Cardona C., Aguirre L.A., et al. Oxygen Saturation on Admission Is a Predictive Biomarker for PD-L1 Expression on Circulating Monocytes and Impaired Immune Response in Patients With Sepsis. Front. Immunol. 2018;9:2008. doi: 10.3389/fimmu.2018.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avendaño-Ortiz J., Maroun-Eid C., Martín-Quirós A., Toledano V., Cubillos-Zapata C., Gómez-Campelo P., Varela-Serrano A., Casas-Martin J., Llanos-González E., Alvarez E., et al. PD-L1 Overexpression During Endotoxin Tolerance Impairs the Adaptive Immune Response in Septic Patients via HIF1α. J. Infect. Dis. 2018;217:393–404. doi: 10.1093/infdis/jix279. [DOI] [PubMed] [Google Scholar]
- 26.Shalova I.N., Lim J.Y., Chittezhath M., Zinkernagel A.S., Beasley F., Hernández-Jiménez E., Toledano V., Cubillos-Zapata C., Rapisarda A., Chen J., et al. Human Monocytes Undergo Functional Re-Programming during Sepsis Mediated by Hypoxia-Inducible Factor-1α. Immunity. 2015;42:484–498. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Biswas S.K., Lopez-Collazo E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Maldifassi M.C., Atienza G., Arnalich F., López-Collazo E., Cedillo J.L., Martín-Sánchez C., Bordas A., Renart J., Montiel C. A New IRAK-M-Mediated Mechanism Implicated in the Anti-Inflammatory Effect of Nicotine via A7 Nicotinic Receptors in Human Macrophages. PLoS ONE. 2014;9:e108397. doi: 10.1371/journal.pone.0108397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Fresno C., Soler-Rangel L., Soares-Schanoski A., Gómez-Piña V., González-León M.C., Gómez-García L., Mendoza-Barberá E., Rodríguez-Rojas A., García F., Fuentes-Prior P., et al. Inflammatory Responses Associated with Acute Coronary Syndrome Up-Regulate IRAK-M and Induce Endotoxin Tolerance in Circulating Monocytes. J. Endotoxin Res. 2007;13:39–52. doi: 10.1177/0968051907078623. [DOI] [PubMed] [Google Scholar]
- 30.Song B., Shu Y., Cui T., Fu P. Allicin Inhibits Human Renal Clear Cell Carcinoma Progression via Suppressing HIF Pathway. Int. J. Clin. Exp. Med. 2015;8:20573–20580. [PMC free article] [PubMed] [Google Scholar]
- 31.Mousa A.M., Soliman K.E., Alhumaydhi F., Almatroudi A., Rugaie O.A., Allemailem K.S., Alrumaihi F., Khan A., Rezk M.Y., Aljasir M., et al. Garlic Extract Alleviates Trastuzumab-Induced Hepatotoxicity in Rats Through Its Antioxidant, Anti-Inflammatory, and Antihyperlipidemic Effects. JIR. 2021;14:6305–6316. doi: 10.2147/JIR.S339092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arreola R., Quintero-Fabián S., López-Roa R.I., Flores-Gutiérrez E.O., Reyes-Grajeda J.P., Carrera-Quintanar L., Ortuño-Sahagún D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015;2015:e401630. doi: 10.1155/2015/401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayraktar O., Tekin N., Aydın O., Akyuz F., Musmul A., Burukoglu D. Effects of S-Allyl Cysteine on Lung and Liver Tissue in a Rat Model of Lipopolysaccharide-Induced Sepsis. Naunyn-Schmiedebergs Arch. Pharmacol. 2015;388:327–335. doi: 10.1007/s00210-014-1076-z. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.K., Park Y.J., Ko M.J., Wang Z., Lee H.Y., Choi Y.W., Bae Y.-S. A Novel Natural Compound from Garlic (Allium sativum L.) with Therapeutic Effects against Experimental Polymicrobial Sepsis. Biochem. Biophys. Res. Commun. 2015;464:774–779. doi: 10.1016/j.bbrc.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Redondo-Calvo F.J., Montenegro O., Padilla-Valverde D., Villarejo P., Baladrón V., Bejarano-Ramírez N., Galán R., Gómez L.A., Villasanti N., Illescas S., et al. Thiosulfinate-Enriched Allium Sativum Extract as an Adjunct to Antibiotic Treatment of Sepsis in a Rat Peritonitis Model. Appl. Sci. 2021;11:4760. doi: 10.3390/app11114760. [DOI] [Google Scholar]
- 36.Santos S.S., Carmo A.M., Brunialti M.K.C., Machado F.R., Azevedo L.C., Assunção M., Trevelin S.C., Cunha F.Q., Salomao R. Modulation of Monocytes in Septic Patients: Preserved Phagocytic Activity, Increased ROS and NO Generation, and Decreased Production of Inflammatory Cytokines. Intensive Care Med. Exp. 2016;4:5. doi: 10.1186/s40635-016-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M., Lu J., Yang S., Chen Y., Yu J., Guan S. Alliin Alleviates LPS-Induced Pyroptosis via Promoting Mitophagy in THP-1 Macrophages and Mice. Food Chem. Toxicol. 2022;160:112811. doi: 10.1016/j.fct.2022.112811. [DOI] [PubMed] [Google Scholar]
- 38.Escoll P., del Fresno C., García L., Vallés G., Lendínez M.J., Arnalich F., López-Collazo E. Rapid Up-Regulation of IRAK-M Expression Following a Second Endotoxin Challenge in Human Monocytes and in Monocytes Isolated from Septic Patients. Biochem. Biophys. Res. Commun. 2003;311:465–472. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi K., Hernandez L.D., Galán J.E., Janeway C.A., Medzhitov R., Flavell R.A. IRAK-M Is a Negative Regulator of Toll-like Receptor Signaling. Cell. 2002;110:191–202. doi: 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 40.van ’t Veer C., van den Pangaart P.S., van Zoelen M.A.D., de Kruif M., Birjmohun R.S., Stroes E.S., de Vos A.F., van der Poll T. Induction of IRAK-M Is Associated with Lipopolysaccharide Tolerance in a Human Endotoxemia Model. J. Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 41.Aminova L.R., Siddiq A., Ratan R.R. Antioxidants, HIF Prolyl Hydroxylase Inhibitors or Short Interfering RNAs to BNIP3 or PUMA, Can Prevent Prodeath Effects of the Transcriptional Activator, HIF-1α, in a Mouse Hippocampal Neuronal Line. Antioxid. Redox Signal. 2008;10:1989–1998. doi: 10.1089/ars.2008.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun B.D., Son S.W., Choi S.Y., Kuh H.J., Oh T.-J., Park J.K. Anti-Cancer Activity of Phytochemicals Targeting Hypoxia-Inducible Factor-1 Alpha. Int. J. Mol. Sci. 2021;22:9819. doi: 10.3390/ijms22189819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cubillos-Zapata C., Hernández-Jiménez E., Toledano V., Esteban-Burgos L., Fernández-Ruíz I., Gómez-Piña V., Del Fresno C., Siliceo M., Prieto-Chinchiña P., Pérez de Diego R., et al. NFκB2/P100 Is a Key Factor for Endotoxin Tolerance in Human Monocytes: A Demonstration Using Primary Human Monocytes from Patients with Sepsis. J. Immunol. 2014;193:4195–4202. doi: 10.4049/jimmunol.1400721. [DOI] [PubMed] [Google Scholar]
- 45.del Fresno C., García-Rio F., Gómez-Piña V., Soares-Schanoski A., Fernández-Ruíz I., Jurado T., Kajiji T., Shu C., Marín E., Gutierrez del Arroyo A., et al. Potent Phagocytic Activity with Impaired Antigen Presentation Identifying Lipopolysaccharide-Tolerant Human Monocytes: Demonstration in Isolated Monocytes from Cystic Fibrosis Patients. J. Immunol. 2009;182:6494–6507. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- 46.Lin C.-C., Chiang T.-H., Sun Y.-Y., Lin M.-S. Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients. 2019;11:700. doi: 10.3390/nu11030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasheets can be provided by corresponding authors under reasonable request.