Abstract
Colorectal cancer (CRC) is a leading cause of mortality worldwide. We conducted a genome-wide association study meta-analysis of 100,204 CRC cases and 154,587 controls of European and East Asian ancestry, identifying 205 independent risk associations, of which 50 were unreported. We performed integrative genomic, transcriptomic and methylomic analyses across large bowel mucosa and other tissues. Transcriptome- and methylome-wide association studies revealed an additional 53 risk associations. We identified 155 high confidence effector genes functionally linked to CRC risk, many of which had no previously established role in CRC. These have multiple different functions, and specifically indicate that variation in normal colorectal homeostasis, proliferation, cell adhesion, migration, immunity and microbial interactions determines CRC risk. Cross-tissue analyses indicated that over a third of effector genes most likely act outside the colonic mucosa. Our findings provide insights into colorectal oncogenesis, and highlight potential targets across tissues for new CRC treatment and chemoprevention strategies.
Editor summary:
A multi-ancestry genome-wide association study meta-analysis, combined with transcriptome- and methylome-wide association analyses identify risk loci associated with colorectal cancer. Credible effector genes and their target tissues are also highlighted, showing that over a third probably act outside the colonic mucosa.
INTRODUCTION
Colorectal cancer (CRC), which affects approximately 1.9 million people worldwide annually1, has a strong heritable basis2. Our understanding of CRC genetics has been informed by genome-wide association studies (GWAS), which have so far identified 150 statistically independent risk variants3,4. To provide a comprehensive description of CRC genetics, we brought together the great majority of GWAS performed to date. We complemented GWAS with transcriptome- and methylome-wide association analyses (TWAS and MWAS; Fig. 1). Through integration of these data, we investigated the genes and mechanisms underlying established and novel CRC risk loci. We identified credible effector genes and the tissues in which they act, informing our understanding of colorectal tumorigenesis.
Figure 1. Summary of the study data and analytical design, and the number of previously unreported CRC risk loci discovered.

The figure illustrates the information for the different analyses used: GWAS (green), TWAS (blue), MWAS (yellow) used to identify additional risk loci. These are later used to select credible effector genes annotated to functions and tissues.
RESULTS
Genetic architecture of colorectal cancer
We performed a meta-analysis of CRC GWAS data sets, comprising 100,204 CRC cases and 154,587 controls (73% European and 27% East Asian ancestry) (Supplementary Tables 1 & 2). We identified 205 associations, including 37 single-nucleotide polymorphisms (SNPs) at novel loci (sentinel risk SNPs > 1 megabase (Mb) from another significant SNP), 13 independent novel risk SNPs in conditional analysis (Table 1), and 155 previously reported SNPs or proxies Table 1, Supplementary Tables 3–4, Supplementary figures 1 & 2). There was limited heterogeneity ascribable to population effects (Supplementary Table 2, Supplementary figure 3), although four risk variants (rs12078075, rs57939401, rs151127921 and rs5751474) were monomorphic in East Asian participants (Table 1).
Table 1.
Previously unreported colorectal cancer risk associations identified by genome-wide association study analysis.
| SNP | Cytoband | Position (bp, GRCh37) | Risk/Alt Allele | RAF (EUR) | RAF (EAS) | OR (95% CI) |
P-value | I2 (%) | Closest gene (RefSeq) |
|---|---|---|---|---|---|---|---|---|---|
| rs34963268 * | 1p36.12 | 22,710,877 | G/C | 0.84 | 0.77 | 1.07 (1.05–1.09) | 6.28E-16 | 31 | ZBTB40 |
| rs5028523 | 1q24.3 | 172,864,224 | A/G | 0.53 | 0.05 | 1.04 (1.03–1.06) | 1.44E-08 | 0 | TNFSF18 |
| rs12137232 | 1q32.1 | 201,885,446 | G/T | 0.52 | 0.19 | 1.04 (1.03–1.05) | 7.71E-09 | 15 | LMOD1 |
| rs12078075 | 1q32.1 | 205,163,798 | G/A | 0.09 | 0 | 1.07 (1.05–1.10) | 1.94E-08 | 0 | DSTYK |
| rs2078095 | 1q43 | 240,408,346 | G/A | 0.28 | 0.23 | 1.04 (1.03–1.06) | 2.08E-08 | 0 | FMN2 |
| rs4668039 | 2q24.3 | 169,025,379 | G/A | 0.2 | 0.52 | 1.04 (1.03–1.06) | 3.32E-08 | 12 | STK39 |
| rs704417 | 3p14.1 | 64,252,424 | T/C | 0.51 | 0.89 | 1.05 (1.03–1.06) | 4.35E-10 | 0 | PRICKLE2 |
| rs7623129 * | 3p14.1 | 64,624,426 | C/T | 0.56 | 0.51 | 1.04 (1.02–1.05) | 1.51E-08 | 5 | ADAMTS9 |
| rs2388976 | 4q26 | 115,502,406 | A/G | 0.44 | 0.45 | 1.04 (1.02–1.05) | 1.75E-08 | 17 | UGT8 |
| rs10006803 | 4q31.3 | 151,501,208 | C/G | 0.5 | 0.45 | 1.04 (1.02–1.05) | 2.58E-08 | 0 | LRBA |
| rs1426947 | 4q34.1 | 175,420,523 | T/C | 0.42 | 0.66 | 1.04 (1.03–1.05) | 7.48E-10 | 0 | HPGD |
| rs3930345 | 5q14.3 | 82,881,255 | C/T | 0.8 | 0.75 | 1.05 (1.03–1.06) | 6.82E-09 | 10 | VCAN |
| rs472959 | 5q35.1 | 172,324,558 | A/G | 0.46 | 0.46 | 1.04 (1.03–1.05) | 4.71E-09 | 24 | ERGIC1 |
| rs1294437 | 6p25.1 | 6,749,789 | C/T | 0.65 | 0.23 | 1.04 (1.03–1.06) | 1.21E-08 | 0 | LY86 |
| rs9379084 * | 6p24.3 | 7,231,843 | G/A | 0.88 | 0.8 | 1.07 (1.05–1.09) | 1.79E-12 | 9 | RREB1 |
| rs209142 * | 6p22.1 | 28,862,617 | C/G | 0.39 | 0.52 | 1.04 (1.02–1.05) | 3.66E-08 | 20 | TRIM27 |
| rs57939401 | 6p21.1 | 45,572,071 | A/G | 0.1 | 0.13 | 1.07 (1.04–1.09) | 3.51E-10 | 0 | RUNX2 |
| rs6912214 * | 6p12.1 | 55,721,302 | T/C | 0.55 | 0.83 | 1.04 (1.03–1.05) | 1.55E-08 | 20 | BMP5 |
| rs145997965 * | 6q21 | 106,482,613 | C/T | 0.02 | 0 | 1.21 (1.13–1.29) | 1.26E-08 | 0 | PRDM1 |
| rs6911915 | 6q22.1 | 117,809,031 | C/T | 0.44 | 0.43 | 1.05 (1.03–1.06) | 3.99E-12 | 3 | DCBLD1 |
| rs151127921 | 6q23.2 | 133,993,925 | T/C | 0.02 | 0 | 1.17 (1.11–1.24) | 3.19E-08 | 24 | EYA4 |
| rs1182197 | 7p22.2 | 2,863,289 | A/C | 0.63 | 0.7 | 1.04 (1.03–1.05) | 5.32E-09 | 0 | GNA12 |
| rs12539962 | 7q11.23 | 73,167,259 | C/T | 0.72 | 0.63 | 1.04 (1.03–1.05) | 2.96E-08 | 27 | ABHD11 |
| rs2527927 | 7q22.1 | 99,477,426 | G/A | 0.55 | 0.71 | 1.04 (1.03–1.06) | 3.31E-10 | 2 | OR2AE1 |
| rs60911071 | 8p21.2 | 23,664,632 | G/C | 0.95 | 0.64 | 1.06 (1.04–1.09) | 2.24E-08 | 0 | STC1 |
| rs826732 | 8q12.1 | 59,742,639 | C/G | 0.5 | 0.59 | 1.04 (1.03–1.06) | 6.26E-10 | 7 | TOX |
| rs11557154 | 9p13.3 | 34,107,505 | T/C | 0.14 | 0.59 | 1.05 (1.04–1.07) | 6.02E-10 | 14 | DCAF12 |
| rs10978941 | 9q31.2 | 110,373,819 | C/T | 0.83 | 0.87 | 1.06 (1.04–1.08) | 2.29E-12 | 0 | KLF4 |
| rs7038489 * | 9q34.2 | 136,682,468 | C/T | 0.89 | 0.99 | 1.08 (1.05–1.1) | 1.1E-08 | 48 | VAV2 |
| rs11789898 | 9q34.2 | 136,925,663 | T/G | 0.18 | 0.08 | 1.05 (1.04–1.07) | 6.28E-09 | 36 | BRD3 |
| rs1775910 * | 10p12.1 | 29,096,942 | G/C | 0.25 | 0.32 | 1.04 (1.03–1.06) | 3.11E-08 | 17 | LOC100507605 |
| rs1773860 | 10p12.1 | 29,291,556 | T/C | 0.49 | 0.35 | 1.04 (1.03–1.05) | 3.49E-09 | 6 | LOC100507605 |
| rs10751097 | 11q13.3 | 69,938,433 | A/G | 0.4 | 0.31 | 1.05 (1.03–1.06) | 2.14E-12 | 0 | ANO1 |
| rs497916 | 11q23.3 | 118,758,089 | T/C | 0.28 | 0.17 | 1.04 (1.03–1.06) | 3.37E-08 | 0 | CXCR5 |
| rs7297628 | 12q14.2 | 64,404,555 | T/C | 0.54 | 0.75 | 1.04 (1.03–1.05) | 1.39E-08 | 30 | SRGAP1 |
| rs11178634 | 12q21.1 | 71,518,329 | G/T | 0.62 | 0.7 | 1.05 (1.03–1.06) | 1.36E-11 | 34 | TSPAN8 |
| rs7299936 * | 12q24.21 | 115,934,000 | A/G | 0.56 | 0.18 | 1.04 (1.02–1.05) | 3.73E-08 | 0 | MED13L |
| rs116964464 | 13q12.13 | 27,543,193 | T/C | 0.03 | 0.04 | 1.11 (1.07–1.15) | 4.83E-09 | 3 | USP12 |
| rs1078563 * | 13q34 | 110,352,851 | G/C | 0.33 | 0.28 | 1.04 (1.03–1.05) | 1.53E-08 | 0 | IRS2 |
| rs1497077 | 14q22.1 | 52,491,655 | C/T | 0.66 | 0.76 | 1.04 (1.03–1.06) | 3.64E-08 | 0 | NID2 |
| rs8031386 | 15q23 | 72,508,799 | A/C | 0.26 | 0.54 | 1.04 (1.03–1.06) | 4.50E-09 | 12 | PKM2 |
| rs11247566 * | 17p13.3 | 835,371 | G/A | 0.55 | 0.52 | 1.04 (1.02–1.05) | 2.92E-08 | 35 | NXN |
| rs1791373 | 18p11.31 | 3,616,779 | T/A | 0.43 | 0.14 | 1.04 (1.03–1.06) | 1.13E-08 | 0 | DLGAP1 |
| rs10409772 | 19p13.3 | 5,840,926 | A/C | 0.09 | 0.29 | 1.07 (1.05–1.09) | 1.33E-10 | 6 | FUT6 |
| rs9983528 | 21q22.3 | 47,772,439 | A/G | 0.13 | 0.24 | 1.07 (1.05–1.09) | 5.10E-13 | 0 | PCNT |
| rs4616575 | 22q12.1 | 29,406,076 | T/G | 0.52 | 0.56 | 1.04 (1.03–1.05) | 1.49E-10 | 0 | ZNRF3 |
| rs130651 | 22q13.1 | 39,644,273 | G/A | 0.33 | 0.08 | 1.05 (1.03–1.07) | 2.92E-10 | 46 | PDGFB |
| rs5751474 | 22q13.2 | 43,689,542 | A/G | 0.79 | 0 | 1.05 (1.03–1.07) | 1.80E-08 | 52 | SCUBE1 |
| rs34256596 * | 22q13.2 | 43,778,431 | A/G | 0.26 | 0.4 | 1.05 (1.03–1.06) | 5.86E-09 | 0 | MPPED1 |
| rs9330814 * | 22q13.31 | 46,364,191 | T/C | 0.33 | 0.68 | 1.05 (1.03–1.07) | 1.28E-09 | 33 | WNT7B |
P-values calculated from a fixed-effects meta-analysis
conditional SNP association, with P-values and ORs derived from analysis conditional on known risk loci within 1Mb; RAF, risk allele frequency; EUR, European ancestry population; EAS, East Asian ancestry population; OR, odds ratio; I2, fraction of variance attributable to between study heterogeneity; bp, base pairs. Association statistics for European and East Asian populations are detailed in Supplementary Table 3.
Using linkage-disequilibrium (LD) score regression (LD hub), we estimated the heritability of CRC attributable to all common genetic variants to be similar in Europeans (h2 0.11, s.d. 0.008) and East Asians (h2 0.09, s.d. 0.006), which translates to 73% of familial CRC risk. Restricting estimates to the 205 GWAS-significant SNPs explained 19.7% of this familial risk. We evaluated the performance of a polygenic risk score (PRS) based on these SNPs in two cohorts independent of the GWAS discovery samples7,8. For Europeans and East Asians, individuals in the top PRS decile exhibited odds ratios of 2.22 (95%CI: 1.92–2.57; P = 1.80 × 10−26) and 1.96 (95%CI: 1.64–2.34; P = 8.9 × 10−14) compared to the remaining individuals. Corresponding areas under the receiver operating characteristic curve (AUC) were 0.62 (95%CI: 0.60–0.63) and 0.60 (95%CI: 0.59–0.62).
Discovery of risk loci by TWAS and MWAS
TWAS was performed by implementing the PredictDB pipeline using mRNA expression data from 1,107 colorectal mucosa samples as reference (709 in house, 368 GTEx transverse colon) 9,10. In addition to associations identified by GWAS or those previously reported by TWAS (PYGL and TRIM4 11,12), we identified 15 novel associations at Bonferroni-corrected significance (PBonferroni, Table 2, Supplementary Tables 5 & 6, Supplementary figure 4). We extended the main TWAS to a transcript isoform-wide association study (TIsWAS), both to ascertain whether specific transcripts could account for TWAS associations and to identify previously unreported risk associations (Supplementary Tables 7 & 8). For a third of TWAS genes, a significant association with CRC risk was found for a single mRNA isoform (Supplementary Table 7). The TIsWAS also identified eight loci associated with CRC risk (Table 3). To improve power for discovery, and because some CRC risk SNPs may not exert their effects in colorectal mucosa, we also conducted a cross-tissue TWAS using our in-house RNA sequencing (RNAseq) data and the full GTEx and Depression Genes and Networks (DGN) project data (49 tissues)13. We identified a further 23 risk associations (Table 4, Supplementary Tables 9–13).
Table 2.
Colorectal cancer risk associations identified by a colorectal mucosa-specific transcriptome-wide association study.
| # | ENSEMBL identifier | Gene | Chr | Start (bp, GRCh37) | End (bp, GRCh37) | P S-MultiXcan | Mean z score | Effect size | n models | n indep | Top GWAS SNP at <1Mb | SNP position | P GWAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ENSG00000171621 | SPSB1 | 1 | 9,352,939 | 9,429,591 | 2.96E-06 | 4.569 | 0.077 | 3 | 1 | rs2075971 | 9,407,104 | 1.96E-07 |
| 2 | ENSG00000142632 | ARHGEF19 | 1 | 16,524,712 | 16,539,104 | 2.32E-06 | −4.610 | −0.046 | 7 | 1 | rs2132851 | 16,537,752 | 7.20E-07 |
| ENSG00000237276 | ANO7P1 | 1 | 16,542,404 | 16,554,522 | 1.27E-06 | −4.801 | −0.054 | 3 | 1 | rs2132851 | 16,537,752 | 7.20E-07 | |
| 3* | ENSG00000237190 | CDKN2AIPNL | 5 | 133,737,778 | 133,747,589 | 1.37E-09 | 1.665 | 0.045 | 3 | 3 | rs647161 | 134,499,092 | 8.53E-18 |
| 4 | ENSG00000260653 | RP11–114G11.5 | 7 | 57,404,172 | 57,419,535 | 1.37E-06 | −4.829 | −0.494 | 1 | 1 | rs4242307 | 57,477,102 | 2.28E-03 |
| 5 | ENSG00000204175 | GPRIN2 | 10 | 46,994,087 | 47,005,643 | 3.38E-14 | −7.582 | −1.709 | 1 | 1 | rs10906949 | 47,698,776 | 1.58E-04 |
| 6 | ENSG00000180210 | F2 | 11 | 46,740,730 | 46,761,056 | 2.80E-07 | 5.136 | 0.257 | 1 | 1 | rs7109707 | 46,818,814 | 5.30E-07 |
| ENSG00000123444 | KBTBD4 | 11 | 47,595,014 | 47,600,561 | 5.48E-07 | 5.008 | 0.053 | 1 | 1 | rs7109707 | 46,818,814 | 5.30E-07 | |
| 7 | ENSG00000213445 | SIPA1 | 11 | 65,405,568 | 65,418,401 | 2.81E-06 | −3.033 | −0.046 | 2 | 2 | rs570760 | 65,833,631 | 2.88E-07 |
| 8 | ENSG00000166106 | ADAMTS15 | 11 | 130,318,869 | 130,346,532 | 3.86E-06 | 4.515 | 0.125 | 2 | 2 | rs7936386 | 130,462,505 | 9.18E-08 |
| 9 | ENSG00000174106 | LEMD3 | 12 | 65,563,351 | 65,642,107 | 2.15E-06 | 3.040 | 0.076 | 3 | 3 | rs59829994 | 65,560,831 | 1.39E-07 |
| 10* | ENSG00000234608 | MAPKAPK5-AS1 | 12 | 112,277,588 | 112,280,706 | 6.15E-14 | 3.544 | 0.050 | 6 | 6 | rs653178 | 112,007,756 | 2.51E-24 |
| 11 | ENSG00000167173 | C15orf39 | 15 | 75,487,984 | 75,504,510 | 2.14E-07 | 4.036 | 0.100 | 3 | 2 | rs17338413 | 75,474,936 | 2.15E-07 |
| ENSG00000260274 | RP11–817O13.8 | 15 | 75,660,496 | 75,661,925 | 2.93E-06 | 3.090 | 0.096 | 2 | 2 | rs17338413 | 75,474,936 | 2.15E-07 | |
| 12 | ENSG00000166822 | TMEM170A | 16 | 75,476,952 | 75,499,395 | 1.05E-06 | −3.464 | −0.041 | 7 | 4 | rs4888408 | 75,432,824 | 9.14E-07 |
| 13 | ENSG00000131748 | STARD3 | 17 | 37,793,318 | 37,819,737 | 8.11E-07 | 4.933 | 0.143 | 1 | 1 | rs2313171 | 37,833,842 | 2.77E-07 |
| ENSG00000161395 | PGAP3 | 17 | 37,827,375 | 37,853,050 | 9.59E-07 | 4.777 | 0.043 | 7 | 1 | rs2313171 | 37,833,842 | 2.77E-07 | |
| ENSG00000141736 | ERBB2 | 17 | 37,844,361 | 37,886,606 | 2.96E-06 | 2.679 | 0.032 | 3 | 3 | rs2313171 | 37,833,842 | 2.77E-07 | |
| 14 | ENSG00000152217 | SETBP1 | 18 | 42,260,138 | 42,648,475 | 3.11E-07 | 4.339 | 0.093 | 2 | 2 | rs12958322 | 42,309,786 | 2.60E-07 |
| 15 | ENSG00000267100 | ILF3-AS1 | 19 | 10,762,538 | 10,764,520 | 2.70E-07 | 4.689 | 0.079 | 2 | 2 | rs10408721 | 10,758,319 | 5.71E-08 |
SMultiXcan uses a two-sided F-test to quantify the significance of the joint fit of the linear regression of the phenotype on predicted expression from multiple tissue models jointly. All associations shown were transcriptome-wide significant after Bonferroni correction for 12,017 genes with an S-MultiXcan model (i.e. P = 0.05/12,017 = 4.16 × 10−6 for the PS-MultiXcan). Genes with boundaries less than 1Mb apart were considered to be in the same cluster. This resulted in 13 CRC associations, for which all TWAS-significant genes were > 1 Mb away from and independent of any GWAS-significant SNP (PGWAS < 5 × 10−8) As expected SNPs close to genome-wide significance were found in all cases. Two further gene associations (*) were < 1Mb from a GWAS-significant SNP, but in analysis conditional on the SNP showed a minimally changed association (Supplementary Table 6) and remained significant at P = 4.16 × 10−6. # indicates the number of novel TWAS loci. z score and effect size are calculated as the mean across S-PrediXcan models from the TWAS reference data sets. n models shows the number of reference data sets for which the S-PrediXcan elastic nets produced genetically-predicted expression models, with the n indep showing the number of those models that were statistically independent. The SNP with the lowest CRC GWAS P-value within 1Mb of the gene is also shown.
Table 3.
Colorectal cancer risk associations identified by a colorectal mucosa-specific transcript isoform-wide association study (TIsWAS).
| # | ENSEMBL identifier | Gene | Chr | Start (bp, GRCh37) | End (bp, GRCh37) | P S-MultiXcan | Mean z score | Effect size | n models | n indep | Top GWAS SNP at <1Mb | SNP location | P GWAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ENST00000609196 | ACP6 | 1 | 147,101,453 | 147,131,116 | 6.43E-11 | −1.264 | −0.048 | 4 | 3 | rs1541187 | 147,051,493 | 1.44E-04 |
| ENST00000493129 | ACP6 | 1 | 147,127,341 | 147,142,574 | 1.65E-23 | −5.781 | −0.482 | 2 | 2 | rs1541187 | 147,051,493 | 1.44E-04 | |
| 2 | ENST00000273153 | CSRNP1 | 3 | 39,183,346 | 39,195,066 | 9.99E-07 | 4.891 | 0.099 | 1 | 1 | rs4676609 | 39,214,256 | 4.63E-06 |
| 3 | ENST00000274695 | CDKAL1 | 6 | 20,534,688 | 21,232,635 | 1.29E-06 | −4.841 | −0.046 | 1 | 1 | rs9295474 | 20,652,717 | 7.61E-08 |
| 4 | ENST00000481601 | CCDC183 | 9 | 139,694,767 | 139,702,192 | 9.60E-07 | −4.490 | −0.048 | 2 | 2 | rs2811736 | 139,651,954 | 3.12E-05 |
| ENST00000464157 | ABCA2 | 9 | 139,902,688 | 139,903,240 | 7.39E-07 | −4.951 | −0.235 | 1 | 1 | rs2811736 | 139,651,954 | 3.12E-05 | |
| 5 * | ENST00000543000 | PLEKHG6 | 12 | 6,426,733 | 6,427,529 | 3.30E-09 | 6.003 | 0.076 | 3 | 2 | rs10849433 | 6,406,904 | 6.73E-17 |
| 6 | ENST00000448790 | TOX4 | 14 | 21,945,335 | 21,967,315 | 1.22E-07 | 5.290 | 0.498 | 1 | 1 | rs3811252 | 22,855,779 | 2.11E-05 |
| 7 | ENST00000478981 | BNIP2 | 15 | 59,955,092 | 59,961,148 | 9.91E-07 | −4.893 | −0.326 | 1 | 1 | rs7182962 | 59,945,783 | 6.04E-08 |
| 8 | ENST00000310144 | PSMC5 | 17 | 61,904,543 | 61,909,379 | 4.18E-10 | 6.247 | 0.553 | 1 | 1 | rs12449782 | 61,576,249 | 2.18E-05 |
As per Table 2, SMultiXcan uses a two-sided F-test to quantify the significance of the joint fit of the linear regression of the phenotype on predicted expression from multiple tissue models jointly. All associations shown were transcriptome-wide significant after Bonferroni correction for 27,941 transcripts with an S-MultiXcan model (i.e. P = 0.05/27,941 = 1.79 × 10−6 for the PS-MultiXcan). Novel associations were called when >1Mb from both a GWAS-significant SNP and a TWAS locus. As expected, all these loci showed evidence of a risk association in the full TWAS (FDR < 0.05, P < 2.86 × 10−3). Transcripts with boundaries < 1 Mb apart were considered to be in the same cluster. This resulted in seven CRC associations. One further association (*) was identified based on conditional TIsWAS analysis (Supplementary Table 8). Other annotations are as per Table 2.
Table 4.
Colorectal cancer risk associations identified by cross-tissue transcriptome-wide association study.
| # | Gene | Chr | Start (bp, GRCh37) | End (bp, GRCh37) | P S-MultiXcan | Tissue | Mean z score | Effect size | n models | n indep | Top GWAS SNP at <1Mb | SNP location | P GWAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RPL5 | 1 | 93,297,540 | 93,307,481 | 2.27E-07 | All | −1.160 | −0.167 | 2 | 2 | rs7530780 | 93,130,268 | 4.18E-05 |
| 2 | LINGO4 | 1 | 151,772,740 | 151,778,546 | 2.73E-08 | All | 1.666 | 0.034 | 27 | 6 | rs9826 | 151,778,899 | 3.81E-06 |
| 3 | FAM98A | 2 | 33,808,725 | 33,824,429 | 2.98E-06 | Immune | 4.672 | 0.166 | 1 | 1 | rs1448561 | 33,854,344 | 5.92E-07 |
| 4 | FBLN7 | 2 | 112,895,962 | 112,945,793 | 1.28E-06 | All | −0.711 | −0.023 | 28 | 10 | rs7580507 | 112,879,209 | 2.71E-07 |
| 5 | ARHGEF4 | 2 | 131,671,559 | 131,804,836 | 2.33E-08 | All | −0.243 | −0.026 | 14 | 8 | rs73960398 | 131,795,345 | 4.86E-06 |
| 6 | GBE1 | 3 | 81,538,850 | 81,811,312 | 1.95E-12 | All | −0.557 | −0.032 | 8 | 7 | rs554330436 | 81.039,172 | 1.69E-04 |
| 7 | DIRC2 | 3 | 122,513,642 | 122,599,986 | 1.25E-06 | All | 0.812 | 0.003 | 16 | 13 | rs6774610 | 122,521,477 | 6.85E-07 |
| 8 | GAB1 | 4 | 144,258,304 | 144,395,721 | 1.11E-07 | All | 1.756 | 0.040 | 10 | 6 | rs72726477 | 143,517,452 | 2.91E-05 |
| 9 | FBXO38 | 5 | 147,763,498 | 147,822,399 | 2.11E-06 | Mesenchymal | 4.677 | 0.287 | 2 | 2 | rs35548425 | 147,816,153 | 1,80E-07 |
| 10 | EPB41L2 | 6 | 131,160,487 | 131,384,462 | 2.70E-11 | Gastrointestinal | −1.720 | −0.018 | 8 | 6 | rs12662663 | 131,398,523 | 6.71E-08 |
| EPB41L2 | 6 | 131,160,487 | 131,384,462 | 2.96E-09 | All | −0.108 | 0.024 | 24 | 11 | rs12662663 | 131,398,523 | 6.71E-08 | |
| 11 | CDK6 | 7 | 92,234,235 | 92,465,908 | 8.00E-14 | All | 0.281 | 0.037 | 8 | 6 | rs143120528 | 92,258,733 | 2.49E-07 |
| 12 | PSMD13 | 11 | 236,546 | 252,984 | 3.89E-06 | Mesenchymal | 1.737 | 0.113 | 3 | 2 | rs7394572 | 432,436 | 4.88E-06 |
| IFITM1 | 11 | 313,506 | 314,456 | 6.73E-07 | All | −0.090 | −0.071 | 33 | 18 | rs7394572 | 432,436 | 4.88E-06 | |
| 13 | RHOG | 11 | 3,848,208 | 3,862,213 | 1.58E-06 | Gastrointestinal | −1.862 | −0.232 | 2 | 2 | rs10835185 | 3,862,343 | 5.97E-08 |
| RHOG | 11 | 3,848,208 | 3,862,213 | 8.27E-07 | Mesenchymal | −4.929 | −0.476 | 1 | 1 | rs10835185 | 3,862,343 | 5.97E-08 | |
| OR51E2 | 11 | 4,701,401 | 4,719,084 | 7.44E-06 | Colon Sigmoid | 4.480 | 0.336 | 1 | 1 | rs10835185 | 3,862,343 | 5.97E-08 | |
| 14 | ME3 | 11 | 86,152,150 | 86,383,678 | 2.62E-06 | Gastrointestinal | −0.215 | −0.125 | 5 | 5 | rs74402426 | 86,161,656 | 1.89E-05 |
| 15 | TAGLN | 11 | 117,070,037 | 117,075,052 | 5.80E-09 | All | −2.118 | −0.111 | 14 | 9 | rs1035237 | 116,727,850 | 5.43E-08 |
| 15 | PCSK7 | 11 | 117,075,499 | 117,103,241 | 2.67E-06 | Mesenchymal | 3.281 | 0.311 | 2 | 2 | rs1035237 | 116,727,850 | 5.43E-08 |
| 16 | CLIP1 | 12 | 122,755,979 | 122,907,179 | 7.61E-08 | All | 0.664 | 0.026 | 6 | 5 | rs1716169 | 123,716,930 | 1.58E-06 |
| 17 | ATP2C2 | 16 | 84,402,133 | 84,497,793 | 4.44E-07 | Gastrointestinal | 1.903 | 0.021 | 7 | 5 | rs7187803 | 84,501,660 | 1.07E-05 |
| ATP2C2 | 16 | 84,402,133 | 84,497,793 | 2.89E-07 | All | 0.754 | 0.010 | 23 | 14 | rs7187803 | 84,501,660 | 1.07E-05 | |
| 18 | CBFA2T3 | 16 | 88,941,266 | 89,043,612 | 1.11E-06 | Mesenchymal | 4.871 | 0.253 | 1 | 1 | rs502258 | 88,968,547 | 9.90E-06 |
| 19 | LLGL1 | 17 | 18,128,901 | 18,148,149 | 3.05E-06 | Immune | −4.667 | −0.469 | 1 | 1 | rs6502570 | 17,183,255 | 2.63E-06 |
| 20 | PSMC3IP | 17 | 40,725,329 | 40,729,849 | 2.21E-06 | All | 1.575 | 0.108 | 11 | 9 | rs12949918 | 40,526,273 | 1.39E-06 |
| BECN1 | 17 | 40,963,673 | 40,985,158 | 1.14E-06 | Immune | 4.824 | 0.547 | 2 | 2 | rs12949918 | 40,526,273 | 1.39E-06 | |
| 21 | SMAD4 | 18 | 48,554,764 | 48,611,415 | 2.75E-06 | Mesenchymal | 4.750 | 0.653 | 2 | 2 | rs12958467 | 48,481,751 | 4.69E-07 |
| 22 | ATP8B1 | 18 | 55,313,658 | 55,470,547 | 2.54E-06 | Immune | −4.704 | −0.203 | 1 | 1 | rs8097764 | 55,317,896 | 1.49E-07 |
| 23 | LIF | 22 | 30,636,528 | 30,640,922 | 4.96E-06 | Colon Sigmoid | −4.566 | −0.201 | 1 | 1 | rs12484740 | 30,606,927 | 4.97E-06 |
SMultiXcan uses a two-sided F-test to quantify the significance of the joint fit of the linear regression of the phenotype on predicted expression from multiple tissue models jointly. TWAS tests were performed separately for the following tissue categories: “Colon_sigmoid”: GTEx (n=318 samples; PBonferroni = 8.12 × 10−6 for the PS-PrediXcan); “Immune”: DGN + GTEx Cells_EBV-transformed_lymphocytes + GTEx Whole_Blood + GTEx_Spleen (n=1,966 samples; PBonferroni = 3.34 × 10−6 for the PS-MultiXcan); “Mesenchymal”: GTEx Adipose_Subcutaneous + GTEx Adipose_Visceral_Omentum + GTEx Cells_Cultured_fibroblasts (n=1,533 samples; PBonferroni = 3.96 × 10−6 for the PS-MultiXcan); “Gastrointestinal”: the 6 in-house colorectal mucosa datasets + GTEx Pancreas + GTEx Liver + GTEx Stomach + GTEx Terminal_Ileum + GTEx Oesophageal_Mucosa + GTEx Colon_Transverse (n=2,615 samples; PBonferroni = 3.34 × 10−6 for the PS-MultiXcan); “All”: the 6 in-house colorectal mucosa datasets + all GTEx 49 tissues + DGN (n=16,832 samples; PBonferroni = 2.31 × 10−6 for the PS-MultiXcan). Other annotations are as per Table 2.
To complement the TWAS, identify further CRC risk loci and gain mechanistic insights, we extended the PredictDB pipeline to perform MWAS based on quantitative methylation data from histologically normal colorectal mucosa (Supplementary Methods). We found significant associations between CRC risk and methylation of individual CpGs at 69 loci (Supplementary Tables 14 & 15). This included seven novel independent risk loci (Table 5). Risk SNPs may influence CRC risk through changes in the CpG methylation status of regulatory elements leading to changes in gene expression. We therefore explored the relationship between gene expression, CpG methylation and CRC risk in colorectal mucosa for 6,722 genes with both TWAS and MWAS predictions. There was a strong tendency for genes to be represented in both TWAS and MWAS (P < 10−7, Fisher’s exact test). Subsequently, we conditioned TWAS associations on the top MWAS-significant CpG within 1Mb, finding that 67/91 (75%) genes did not retain a significant TWAS association (PBonferroni > 5.50 × 10−4; Supplementary Table 16). Our data are consistent with a model in which many CRC risk SNPs act through changes in DNA methylation, although formal causality analysis could not be performed to exclude reverse causation or possible confounders.
Table 5.
Colorectal cancer risk associations identified by methylome-wide association study.
| # | CpG | Annotated Gene | Chr | Probe location (bp, GRCh37) | Probe annotation | P S-MultiXcan | Mean z score | Effect size | n models | n indep | Top GWAS SNP at <1Mb | SNP location | P GWAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | cg01716680 | GJA4 | 1 | 35,259,750 | S Shore | 3.41E-07 | −5.099 | −0.164 | 1 | 1 | rs57975061 | 34,890,238 | 2.42E-06 |
| 2 | cg15917621 | NRBP1 | 2 | 27,650,478 | N Shore | 1.61E-07 | −3.301 | −0.094 | 2 | 2 | rs4665972 | 27,598,097 | 1.58E-07 |
| 3 | cg02609692 | LMX1B | 9 | 129,389,125 | Island | 4.24E-07 | 5.058 | 0.112 | 1 | 1 | rs4075850 | 130,169,301 | 1.76E-06 |
| 4* | cg12931523 | TTLL13 | 15 | 90,793,004 | S Shore | 7.74E-09 | 4.511 | 0.067 | 3 | 3 | rs71407320 | 91,185,291 | 3.61E-08 |
| cg05239308 | TTLL13 | 15 | 90,793,057 | S Shore | 1.54E-07 | 5.364 | 0.114 | 3 | 2 | rs71407320 | 91,185,291 | 3.61E-08 | |
| cg27018984 | TTLL13 | 15 | 90,796,558 | S Shelf | 3.64E-09 | −5.900 | −0.089 | 1 | 1 | rs71407320 | 91,185,291 | 3.61E-08 | |
| 5 | cg02086790 | AXIN1 | 16 | 375,327 | Island | 2.75E-07 | 2.471 | 0.042 | 3 | 3 | rs9921222 | 375,782 | 7.10E-07 |
| 6* | cg09894072 | PLA2G15 | 16 | 68,279,487 | Island | 2.26E-07 | 5.176 | 0.096 | 1 | 1 | rs9939049 | 68,812,301 | 1.95E-12 |
| 7 | cg15135657 | LOC100631378 | 19 | 38,346,511 | S Shore | 1.55E-07 | −2.170 | −0.032 | 2 | 2 | rs55876653 | 39,146,780 | 2.10E-06 |
SMultiXcan uses a two-sided F-test to quantify the significance of the joint fit of the linear regression of the phenotype on predicted expression from multiple tissue models jointly. All associations shown were methylome-wide significant after Bonferroni correction for 88,888 CpGs with an S-PrediXcan model (P = 0.05/88,888 = 5.62 × 10−7 for the PS-MultiXcan). Pairs of CpGs or strings of adjacent CpGs within 1Mb of one another were considered to lie within the same cluster. Five CRC associations were found for which all CpGs were > 1 Mb away from GWAS-significant SNP (PGWAS < 5 × 10−8), although near a SNP close to genome-wide significance. Two further associations for 4 CpGs (*) were identified based on conditional MWAS analysis (Supplementary Table 15). Novel CpG hits were all independent of each other and of GWAS SNPs and TWAS genes. Other annotations are as per Table 2.
Effector genes and biological pathways of CRC oncogenesis
A major, largely unfulfilled aim of cancer GWAS is to identify genes and functional mechanisms that may ultimately be clinically useful targets, for example in chemoprevention. The large GWAS and TWAS datasets in this study address this aim by enabling a detailed functional analysis of the molecular mechanisms contributing to CRC risk. Since TWAS approaches do not identify causal genes directly, we used our data to compile a set of 155 credible effector genes from the independent associations identified through GWAS, TWAS, TIsWAS and MWAS (details in Supplementary Table 17 and Supplementary Methods).
We identified molecular pathways enriched in effector genes using Enrichr (https://maayanlab.cloud/Enrichr/) (Supplementary Table 18). This analysis was complemented with DEPICT based on the GWAS SNPs (https://data.broadinstitute.org/mpg/depict/) (Supplementary Table 19). CRC effectors were principally enriched in genes regulating TGF-β/BMP, Wnt WNT and Hippo pathways. A number of the credible effector genes that map to these pathways have no established role in CRC, including the intestinal stem cell regulator ZNRF314, the TGF repressor LEMD315, and the EMT regulator RREB116.
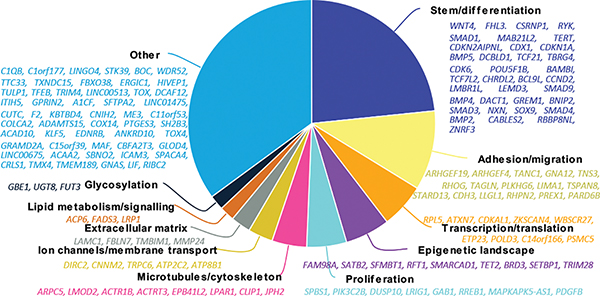
To complement the pathway analysis, we performed gene-level functional annotation based on the principal cellular function of each effector gene as reported in the literature (Figure 2, Supplementary Table 20). Thirty-six genes (mostly Wnt and BMP family members) were annotated to colorectal homeostasis (i.e. cellular stemness/differentiation). Intriguingly, 16 genes (including ARHGEF19, ARHGEF4, GNA12, RHOG, TAGLN, TSPAN8, STARD13 and LLGL1) were linked to cell migration through RhoA/ROCK signaling. We found eight genes (SPSB1, PIK3C2B, DUSP1, LRIG1, GAB1, RREB1, MAPKAPK5-AS1 and PDGFB) to act within the Ras/Raf growth factor signaling pathway. In addition to the previously reported association at FUT2, the novel fucosyltransferase effector genes FUT3 and FUT6 supported a relationship between the gut microbiome and CRC risk17. Inflammation is important in CRC18, and the TWAS association at the FADS gene cluster and PTGES3, specifically highlighted the role of prostaglandin metabolism in CRC risk. Finally, our data also indicated several effector genes with roles in ion transport and cytoskeletal components (Fig. 2, Supplementary Table 20).
Figure 2. Effector genes for CRC risk and the cellular processes in which they act.
Pie chart describing the proportion and list of effector genes allocated to each process.
Although our pathway analysis and functional annotation indicated that the colorectum was the likely target tissue of many effector genes (Supplementary Tables 19 & 20), some genes were associated with principal roles in other tissue types, for example neuronal cells (LINGO4, TULP1 and CNIH2) and leukocytes (TOX, TOX4 and MAF, plus many candidate genes within the MHC region) (Supplementary Table 20). We therefore performed a systematic analysis of effector gene tissue specificity, based on the premise that TWAS associations tend to be present in tissues in which a gene functionally affects CRC risk. Cross-tissue analysis showed that all but one effector gene exhibited a TWAS association (FDRTWAS < 0.05) in at least one tissue and 52 (34%) genes showed an association in multiple tissues (Supplementary figure 5). For 26 (17%) genes, associations were confined to the colorectal mucosa (PTWAS Bonferroni-significant in mucosa, PTWAS > FDR elsewhere). In contrast, 67 genes (43%) showed no evidence of a TWAS association in colorectal mucosa (FDRTWAS > 0.05). Notably, 12 (8%) gene associations were present only in immune cells (Supplementary figure 5, Supplementary Table 11) and four (3%) were restricted to mesenchymal cells (Supplementary figure 5, Supplementary Table 12).
Linking colorectal cancer risk to other traits
To gain insight into the role of potentially modifiable risk factors in CRC genetics, we performed cross-trait LD score regression analyses19 using publicly available GWAS summary statistics for 171 phenotypes. Twelve genetic correlations remained significant (two-sided Z-test, Bonferroni-corrected P < 2.93 × 10−4). Notably, positive associations with CRC risk (Supplementary Table 21) included insulin resistance (raised fasting insulin and glucose), smoking, and obesity (body mass index - BMI, waist-to-hip ratio - WHR, waist circumference), traits that have previously been reported in observational epidemiological studies to be associated with CRC risk3,20,21. These associations not only highlight shared biology, but also suggest that public health interventions to reduce cardiometabolic disease will additionally lower CRC burden.
DISCUSSION
We report a comprehensive genetic analysis of CRC risk in the general population. To identify the most credible effector genes for each risk variant, we performed detailed annotation using tissue-specific gene expression and other relevant data types. Our study is twice as large as previous CRC GWAS, and also includes participants of both European and East Asian ancestries, demonstrating that most loci are shared across these ancestral groups. This increased power for GWAS, coupled with complementary analyses, including TWAS and MWAS, identified 103 previously unreported risk associations and identified 155 effector genes. These data substantially expand our existing knowledge regarding the impact of common genetic variation on the heritable risk of CRC.
The availability of large, multi-omic data sets has allowed us to assign the most likely target/effector genes of GWAS and TWAS associations (Fig. 3), and confidence in these assignments will increase as additional functional data are reported in the literature. It is clear that pathways (e.g., Wnt, BMP, Hippo) involved in normal intestinal homeostasis play important roles in CRC risk, suggesting that modulation of normal mucosal dynamics has the potential to prevent colorectal neoplasia. The gut flora is intimately involved in normal bowel homeostasis, and effector genes are likely to be involved in microbial interactions. By contrast, Ras pathway activity is thought to be more important during repair or tumorigenesis, and the Ras effector genes we have found may act after tumor initiation. Our finding of multiple risk genes involved in cell adhesion and migration naturally suggests roles in malignant progression, although effects earlier in tumorigenesis also remain plausible. Similarly, immune pathway effector genes could, in principle, have their effects on normal cell function or at any stage of tumorigenesis, from mediating day-to-day microbial interactions to killing of cells in early neoplastic transformation or established tumors.
Figure 3. Representation of effector genes and their putative actions in the colorectum.
Diagram representing the processes that the combined GWAS, TWAS and MWAS analyses have unveiled as relevant to CRC risk. Exemplar effector genes from cellular processes and pathways (in capitals) are chosen to depict each category.
Cross-tissue analyses indicated that the colorectal mucosa was the most likely site of action of many effector genes, but some genes are more likely to act in different tissue types. For example, it is highly likely that genes such as HIVEP1, LIF, SH2B3, TOX and TOX4 (and probably genes in the MHC region) influence the development of CRC through immune cell variation, and that EDNRB influences risk through effects on blood vessels. An unexpected finding was that several credible effector genes have primary roles in neurogenesis, raising the intriguing possibility that the enteric nervous system is involved in CRC risk.
While germline genetics has guided the development of drugs to prevent cardiovascular disease (e.g. statins and PCSK9 inhibitors), such a paradigm has yet to be realized for cancer. Since almost all CRCs develop from colonic polyps, and up to 40% of the screened population will be diagnosed with one or more polyps, CRC is particularly well-suited to evaluate novel chemopreventive agents. Our findings highlight candidate targets for chemoprevention, such as gut microbiota, prostaglandin metabolism, and signaling through the Wnt, BMP and Hippo pathways. Specific potential targets in the near term include CDK6, which is targeted by drugs in clinical use for cancer therapy, such as palbociclib and ribociclib. Similarly, Wnt pathway activity can be targeted indirectly using porcupine inhibitors (e.g. LGK974, ETC159, CGX-1321 and RXC004) that prevent Wnt ligand palmitoylation22, although future approaches may more specifically target effector genes such as WNT4 and ZNRF3. Hence, adapted forms of these drugs or modified dosing regimens could be repurposed for chemoprevention, possibly initially for high-risk groups, such as those with in the top PRS percentiles or Lynch Syndrome cases. Based on our data, we speculate that in the longer term, targeted approaches based on demethylation of specific CpG sites from MWAS could be effective means of prevention with minimal toxicity.
The identification of additional risk associations has the potential to provide further biological insights into CRC. However, cohort numbers required in European and East Asian populations to identify additional risk SNPs through GWAS are likely to be prohibitive. Indeed, to identify SNPs explaining 80% of the heritable risk of CRC risk loci, thus providing comprehensive biological insights, will require sample sizes in excess of 500,000 cases and at least that number of controls (Supplementary figure 6). This is far higher than a previous estimate23, which was based on a small subset of the GWAS included herein. Extending GWAS to African and other populations may detect further risk SNPs, including population specific ones. Complementary approaches such as TWAS and MWAS are demonstrably useful for the discovery of further risk loci, especially if, and when, reference data sets from multiple populations are made available.
Overall, our findings demonstrate the power of multi-omics to provide new insights into the biological basis of CRC, including both the identification of candidate effector genes and support for previously unsuspected functional mechanisms. Importantly, several of the genes and pathways we have identified are potential targets for CRC treatment or chemoprevention.
Methods
The research presented in this study complies with all relevant ethical regulations, and has been approved by the South Central Ethics Committee (UK) (reference number 17/SC/0079).
Data availability
Summary level data for the full set of Asian and European GWAS are available through GWAS catalog (accession number GCST90129505). For individual-level data, CCFR, CORECT, CORSA_2 and GECCO are deposited in dbGaP (phs001415.v1.p1, phs001315.v1.p1, phs001078.v1.p1, phs001903.v1.p1, phs001856.v1.p1 and phs001045.v1.p1). NSCCG and COIN are available in the European Genome-phenome Archive under accession numbers EGAS00001005412 (NSCCG), EGAS00001005421 (COIN). UK Biobank data are available through http://www.ukbiobank.ac.uk/ and Finnish data through THL Biobank. Access to individual-level data for the remaining studies is controlled through oversight committees. CCFR 1 and CCFR 2 data can be requested by submitting an application for collaboration to the CCFR (forms, instructions and contact information can be located at (www.coloncfr/collaboration.org). Applications for individual level data from the QUASAR2 and SCOT clinical trials will be assessed by the Translational Research Steering Committees that oversee those studies. Individual level data from the CORGI (UK1) study will be made available subject to standard institutional agreements. Application forms for these three studies, and for Scotland Phase 1, Scotland Phase 2, SOCCS, DACHS4 and Croatia, will be provided by emailing a request to access.crc.gwas.data@outlook.com. For access to CORSA_1, please contact gecco@fredhutch.org. For Generation Scotland (GS) access is through the GS Access Committee (GSAC) (access@generationscotland.org). Applications for The Lothian Birth Cohort data should be made through https://www.ed.ac.uk/lothian-birth-cohorts/data-access-collaboration. For details of the application process for Aichi1, Aichi2, BBJ, Guanzhou1, HCES, HCES2, Korea and Shanghai cohorts, please go to https://swhs-smhs.app.vumc.org/ or contact Dr. Zheng at wei.zheng@vanderbilt.edu.
CRC-relevant epigenome data were obtained from the NCBI Gene Expression Omnibus (GEO) database under accession number GSE77737 and GSE36401.
Genetically predicted models of gene expression and methylation have been deposited in the Zenodo repository (https://zenodo.org/deposit/6472285).
Code availability
All bioinformatics and statistical analysis tools used in this study are open source, details of which are available in the Methods section and in the Reporting Summary. No custom code was used to process or analyse data. Details on URLs used can be found in the Supplementary Note.
Statistics and reproducibility
No statistical method was used to predetermine sample size. The experiments were not randomized. Data exclusion from each analysis is explained below in the corresponding sections. Informed consent was obtained for all participants in the study. A description of the different datasets and cohorts used is included in the Supplementary Note.
Criteria for declaring new CRC risk associations
Multi-omic studies present inherent difficulties for deciding on what constitutes a novel GWAS, TWAS or MWAS association. To declare statistically significant associations, for GWAS we have used the established threshold of P = 5 × 10−8. We applied this to both loci >1Mbp from a previously known SNP and analyses conditioned on the most significant SNP within 1Mb region. For TWAS or MWAS we also followed convention and used a Bonferroni correction P = 0.05/N, where N is the number of gene models successfully derived from the reference tissue. Furthermore, for TIsWAS and cross-tissue TWAS, we used Bonferroni-corrected P-value thresholds for significance in each of the reference tissue data sets separately, owing to the overlap in between tissue groups and the fact that many eQTLs are present across tissues. A further common practice, is that a new association should be located >1Mb from another association (from this study or previously reported), whether a genome-wide significant GWAS SNP, a TWAS gene or an MWAS CpG. However, use of the 1Mb distance convention introduces a further problem in that, whilst the location of a GWAS SNP and MWAS CpG can be defined precisely, the location of a gene cannot. We therefore defined a gene’s boundaries by the canonical transcript and novel associations must lie 1Mb from both those boundaries. Since TWAS and MWAS associations can affect multiple nearby genes or CpGs (e.g. owing to co-regulation or LD between eQTLs or mQTLs), we have conservatively assigned each TWAS and MWAS association to a single locus (defined as a group of genes or CpGs that are significantly associated with CRC risk and lie < 1Mb apart). Locus boundaries must be > 1Mb from another association to be declared an independent risk association.
We have also performed conditional analyses across GWAS, TWAS and MWAS. This is standard practice in GWAS (see below) 24, whereby nearby SNPs with no or limited correlation can be independently associated with CRC risk. Conditioning TWAS, TIsWAS and MWAS on GWAS using sMIST also allowed us to identify risk associations that were independent of the GWAS associations within 1Mb, based on a Pconditional that (i) remained Bonferroni-significant at the unconditional analysis threshold, and (ii) was within one order of magnitude as Punconditional. A much larger number of TWAS and MWAS associations fulfilled only criterion (i) after conditioning on a GWAS association within 1Mb (Supplementary Table 6, 8 and 15). Whilst we could not exclude the possibility that some of these associations resulted from additional SNPs independent of a nearby GWAS SNP for example, we conservatively did not declare these as novel risk associations.
GWAS data analysis
Meta-analysis:
Within each of the 31 analytical units, we conducted logistic regression under a log-additive model to examine the association between allelic dosage for each genetic variant and the risk of CRC, adjusted for unit-specific covariates. Meta-analysis under a fixed-effects inverse-variance weighted model was performed using META v1.725. Variants in the meta-analysis only included those with an imputation quality score (info/R2) > 0.4, MAF > 0.005, and seen in at least 15 analytical units. The I2 statistic was calculated to quantify between study heterogeneity and variants with I2 > 65% were excluded. A total of 8,782,440 variants were taken forward in the meta-analysis. Meta-analysis of risk estimates was conducted under an inverse variance weighted, fixed-effects model3. None of the analytical units showed strong evidence of genomic inflation (λ ranged from 0.95 to 1.28), and the λ value for the meta-analysis was 1.30 (λ1000 = 1.01) Supplementary figure 3). To account for any -ancestral differences between analytical units, we implemented MR-MEGA v0.1.526, including 10 principal components (PCs) in the analysis. To measure the probability of associations being false positives, the Bayesian False-Discovery Probability (BFDP)3 was calculated based on a plausible odds ratio (OR) of 1.2 (based on the 95th percentile of the meta-analysis OR values) and a prior probability of association of 10−5.
Definition of known and novel GWAS SNP risk associations:
We identified all previously reported CRC associations at P < 5 × 10−8 by referencing the NHGRI-EBI Catalog of human GWAS and by searching PubMed (performed June 2021)3. Additional articles were ascertained through references cited in primary publications (Supplementary Table 4). Where multiple studies reported associations in the same region (r2 > 0.1 and within 500kb-1Mb of the index SNP), we considered all variants with genome-wide significant associations. Given the improved power and coverage of our study over previous works, we identified the most strongly associated variant at each known signal and used lead variants for further analyses, rather than the previously reported index variants (Supplementary Table 3). A genome-wide significant risk variant was considered novel if >1Mb from a known risk variant.
GWAS conditional analysis:
To identify independent association signals at the discovered CRC risk associations, we performed conditional analyses using GCTA-COJO24 on the meta-analysis summary statistics. Analyses were performed separately for European and East Asian ancestry populations, to account for LD structure differences. The conditioned data were meta-analyzed together as described above, and associations with Pconditional < 5 × 10−8 were considered novel secondary associations. As reference for LD estimation, we made use of genotyping data from 6,684 unrelated samples of East Asian ancestry, and 4,284 samples from combined UK10K and European samples in 1000 Genomes.
Heritability analysis
We used the LDSC regression package with default parameters as implemented in LD Hub27 to estimate the SNP heritability from the GWAS meta-analysis summary statistics data3. SNPs were filtered to HapMap3 SNPS with 1000 Genomes EUR MAF above 5%. SNPs with imputation info score < 0.9, MAF < 0.01 and within the major histocompatibility complex (MHC) region (i.e. SNPs between 26Mb and 34Mb on chromosome six were excluded. Precalculated LD scores files computed using 1000 Genome European data were used.
The contribution of risk SNPs to the familial risk of CRC was calculated as , where λ0 is the familial risk to first-degree relatives of CRC cases, assumed to be 2.228, and λk is the familial relative risk associated with SNP k, calculated as , where pk is the risk allele frequency for SNP k, qk = 1−pk, and rk is the estimated per-allele OR from the meta-analysis3,29.
Pleiotropy analysis
We explored cross-trait pleiotropic effects using the LDSC regression package with default parameters30 as implemented in LD Hub. The summary statistics for 252 phenotypes were extracted from LD Hub. For comparability of results across the traits we limited our analysis to the CRC GWAS of European ancestry. After excluding GWAS performed on non-European cohorts, traits where the LD Hub output came with the following warning messages: “Caution: using this data may yield results outside bounds due to relative low Z score of the SNP heritability of the trait” and “Caution: using this data may yield less robust results due to minor departure of the LD structure”, as well as highly correlated traits, 171 phenotypes were included in the analysis. The departure of the LD structure means departure from the assumption of equal LD structure between two datasets, e.g due to differences in population structure between the study populations. SNPs from the MHC (chr6 26M~34M) region were removed for all traits prior to analysis.
Sample size prediction
To estimate the sample size required to detect a given proportion of the GWAS heritability, we made use of GENESIS software (GENetic Effect-Size distribution Inference from Summary-level data)31, which implements a likelihood-based approach to model the effect-size distribution in conjunction with LD information, using the three-component model (mixture of two normal distributions). The percentage of GWAS heritability explained for a projected sample size was based on power calculations for the discovery of genome-wide significant SNPs3. The genetic variance explained was calculated as the proportion of total GWAS heritability explained by SNPs reaching genome-wide significance at a given sample size.
TWAS analysis
Gene expression models for the six in-house expression datasets were generated using the PredictDB v7 pipeline for a total of 1,077 participants9,10. Elastic net model building with 10-fold cross-validation was performed independently for each dataset. The elastic net models for GTEx v8 Colon Transverse were obtained from the PredictDB data repository (http://predictdb.org/) and had been generated using the same pipeline. Models were computed using HapMap2 SNPs ±1Mb from each gene, together with covariate factors estimated using PEER32, clinical covariates when appropriate (age, sex and, where appropriate, case-control status, type of polyp and anatomic location in the colorectum), and three PCs from the individual dataset’s SNP genotype data. Transcriptome-wide association tests were then performed for each dataset with the S-PrediXcan feature using summary statistics from the GWAS meta-analysis. We used individual level GWAS data from GECCO (n=8,725) to derive the LD reference covariance matrix. S-MultiXcan analysis was then undertaken across datasets. Significant associations were declared using Bonferroni correction (0.05/number of gene models from S-MultiXcan). As recommended33, an additional filter of a TWAS association statistic, PS-PrediXcan ≤ 10−4, in at least one individual reference data set was implemented to minimize potential errors due to LD mismatches. Genes localizing to the HLA/MHC region (chr6:28,477,797–33,448,354bp) were excluded.
Transcript-based TWAS analyses (TIsWAS) were likewise performed by using transcript-level data from the SOCCS, BarcUVa-Seq and GTEx Colon Transverse datasets.
Additional TWAS analyses were similarly performed using the non-colonic mucosa tissue data available from GTEx. These correspond to S-PrediXCan elastic net models from 48 additional GTEx tissues with eQTL data and the DGN whole blood cohort. Five tissue groupings were tested: “Sigmoid colon”, corresponding to muscle and other sub-epithelial tissues; “Immune”, comprising DGN + GTEx Cells_EBV-transformed_lymphocytes + GTEx Whole_Blood + GTEx_Spleen (n=1,966 samples); “Mesenchymal”, comprising GTEx Adipose_Subcutaneous + GTEx Adipose_Visceral_Omentum + GTEx Cells_Cultured_fibroblasts (n=1,533 samples); “Gastrointestinal”, comprising six in-house datasets + GTEx Pancreas + GTEx Liver + GTEx Stomach + GTEx Terminal_Ileum + GTEx Oesophageal_Mucosa + GTEx Colon_Transverse; n=2,615 samples); and “All”, comprising the six in-house datasets + all 49 GTEx tissues + DGN (n=16,832 samples).
The predictive performance of the models for TWAS and TisWAS across the datasets was similar. For the TWAS models the number of genes successfully predicted with R2 > 0.01 (equivalent of R>0.1) varied between 3308 for the BarcUVa data set and 5092 for SOCCS rectum, while GTEx Colon Transverse models were available for 6295 genes. The mean CV-based prediction R2 for all genes varied between 0.09 (25–75th percentile 0.04–0.12) for BarcUVa to 0.19 for INTERMPHEN (0.07–0.24), compared with 0.12 (0.04–0.16) for GTEx Colon Transverse model. The numbers were slightly higher when comparing the overlapping 736 genes only. The in-house TisWAS models were constructed for a lesser number of transcripts (n=4632 for BarcUVa dataset and n=11262 for SOCCS rectum dataset) compared to GTEx Colon Transverse (n=15500), owing to greater read depth and larger sample size for GTEx. The mean R2 for all genes varied from 0.07 (0.03–0.09) for BarcUVa to 0.16 for SOCCS colon (0.07–0.21). GTEx Colon Transverse had mean R2 0.10 (0.03–0.12).
MWAS analysis
Methylation beta values were calculated based on the manufacturer’s standard, ranging from 0 to 1. Quality control and data normalization were performed in R using the ChAMP software pipeline for the EPIC and 450K arrays34. Briefly, we filtered out failed probes with detection P > 0.02 in >5% of samples, probes with <3 reads in >5% of samples per probe and all non-CpG probes. Samples with failed probes >0.1 were also excluded from downstream analyses. We discarded all probes with SNPs within 10bp of the interrogated CpG (from 1,000 Genomes Project, CEU population)35, and probes that ambiguously mapped to multiple locations in the human genome with up to two mismatches33. We only considered probes mapping to autosomes and those overlapping between the EPIC and the 450K arrays. Normalization was achieved using the Beta MIxture Quantile (BMIQ) method. Per probe methylation models were created using the PredictDB pipeline on the normalized methylation matrix and the genotypes as per TWAS eQTL analysis. To optimize power, we restricted our analysis to 263,341–238,443 (for the 450K array) and 377,678 (for the EPIC array) probes annotated to Islands, Shores and Shelves, and discarded “Open Sea” regions. Further analysis was performed as per the TWAS. CpGs were annotated to a known GWAS signal if within 1Mb of a genome-wide significant GWAS risk SNP and otherwise considered novel. For the MWAS models the number of CpG probes successfully predicted with R2 > 0.01 (equivalent of R>0.1) varied from 24325 for INTERMPHEN rectum to 30385 for COLONOMICS. The mean CV-based prediction R2 for all genes varied from 0.14 (25th-7th percentile 0.07–0.16) for INTERMPHEN proximal dataset to 0.19 for SOCCS (0.07–0.25).
Conditional analysis using sMiST for TWAS and MWAS findings
S-MultiXcan is a powerful method for assessing predicted gene expression across multiple tissues and samples, but cannot readily undertake conditional analysis to determine independence of a TWAS or MWAS association from other GWAS, TWAS or MWAS associations. We therefore used the summary statistics-based Mixed effects Score Test (sMiST)36 method to perform conditional analysis of TWAS, TIsWAS and MWAS data adjusting for GWAS risk SNPs. sMiST can assess the total effect, including both predicted molecular features (gene expression or methylation) and the residual direct effects of SNPs that are not explained by predicted molecular features, on CRC risk. To be consistent with S-MultiXcan, we only assessed the association of predicted molecular features. We first confirmed that there was a strong correlation between the sMiST and S-MultiXcan results, with minimal discordance (Supplementary figure 4). In view of this, we used sMiST to perform conditional TWAS and MWAS analysis for each of the significantly associated genes or CpGs respectively, conditioning on the lead GWAS-significant SNP (if present) within 1Mb (Supplementary Tables 6, 8 & 15). We also conditioned TWAS on TWAS, TIsWAS on TIsWAS and MWAS on MWAS. We also conducted TWAS conditioned on MWAS analyses for the genes for which both significant genetically predicted expression and methylation models were produced by the PredictDB pipeline. Where multiple CpGs were annotated to the same gene, we selected the association with the lowest MWAS P-value. We determined the number of genes associated (at Bonferroni-corrected P = 0.05/6,722 = 7.44 × 10−6) with CRC risk in both TWAS and MWAS (n=43), TWAS-only (n=54), MWAS-only (n=91) or neither (n=6,534).”
Effector gene identification
To identify the most credible target or “effector” genes at each CRC risk locus, a pragmatic approach was utilized. After excluding the MHC region, pseudogenes and transcripts of uncertain significance (generally RPNNNN or ACNNN), the following hierarchical inclusion criteria were used.
For significant (Bonferroni-corrected PTWAS < 0.05) TWAS genes at a locus, the gene most strongly associated with CRC risk in any tissue, as long as its PTWAS was at least an order of magnitude lower than any other gene at the locus. (N=112)
For loci included under (1), additional genes that remained significant (FDR < 0.05) in conditional TWAS-TWAS analysis including the lead gene. (N=9)
At GWAS loci not included under (1), the most significant (FDR < 0.05) TWAS gene, as long as its PTWAS was at least an order of magnitude lower than any other gene at the locus. (N=17)
TIsWAS analysis consistent with the approach used for TWAS as described in (1–3) above. (N=16)
Genes harboring missense or truncating variants in LD (r2 > 0.9) with sentinel GWAS SNPs. (N=1)
A set of 155 genes was identified, which corresponds to about two thirds of the CRC risk loci from GWAS, TWAS and MWAS (Supplementary Table 17).
The area under the receiver operating characteristics curve (AUC)
We calculated the confounder adjusted AUC of PRS in discriminating individuals with and without CRC by using the propensity score weighting to account for potentially different distribution of confounders between cases and controls37. We adjusted for age, sex, and four PCs as confounders. We obtained the 95% confidence intervals (CI) by bootstrapping and a total of 500 bootstrap samples were generated. We calculated adjusted AUCs using the R package ROCt.
Supplementary Material
Funding and acknowledgements
At the Institute of Cancer Research, this work was supported by Cancer Research UK (C1298/A25514 - RSH). Additional support was provided by the National Cancer Research Network. In Edinburgh, the work was supported by Programme Grant funding from Cancer Research UK (C348/A12076 to MGD, C6199/A16459 to IT), EU ERC Advanced Grant EVOCAN, and the infrastructure and staffing of the Edinburgh CRUK Cancer Research Centre. CFR was supported by a Marie Sklodowska-Curie Intra-European Fellowship Action (IEF-301077) for the INTERMPHEN project and received considerable help from many staff in the Department of Endoscopy at the John Radcliffe Hospital in Oxford. Support from the European Union [FP7/207–2013, grant 258236], FP7 collaborative project SYSCOL, and COST Actions EuColonGene and TransColonCan are also acknowledged [BM1206 and CA17118] (IT). We are grateful to many colleagues within UK Clinical Genetics Departments (for CORGI) and to many collaborators who participated in the VICTOR, QUASAR2 and SCOT trials. We also thank colleagues from the UK National Cancer Research Network (for NSCCG). IT acknowledges funding from Cancer Research UK Programme Grant C6199/A27327.
The work at Vanderbilt University Medical Center was supported by U.S. NIH grants R01CA188214, R37CA070867, UM1CA182910, R01CA124558, R01CA158473, and R01CA148667, as well as Anne Potter Wilson Chair funds from the Vanderbilt University School of Medicine (WZ). Sample preparation and genotyping assays at Vanderbilt University were conducted at the Survey and Biospecimen Shared Resources and Vanderbilt Microarray Shared Resource, supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485). Statistical analyses were performed on servers maintained by the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University (Nashville, TN).
GECCO: Genetics and Epidemiology of Colorectal Cancer Consortium: National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (U01 CA164930, U01 CA137088, R01 CA059045, R01201407, R01CA206279). Genotyping services were provided by the Center for Inherited Disease Research (CIDR) contract number HHSN268201200008I. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA015704. Scientific Computing Infrastructure at Fred Hutch funded by ORIP grant S10OD028685 (UP). Colorectal Cancer Transdisciplinary (CORECT) Study: The CORECT Study was supported by the National Cancer Institute, National Institutes of Health (NCI/NIH), U.S. Department of Health and Human Services (grant numbers U19 CA148107, R01 CA81488, P30 CA014089, R01 CA197350; P01 CA196569; R01 CA201407) and National Institutes of Environmental Health Sciences, National Institutes of Health (grant number T32 ES013678).
The Colon CFR participant recruitment and collection of data and biospecimens used in this study were supported by the NCI, NIH (grant number U01 CA167551). OFCCR was supported through funding allocated to the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783). The content of this manuscript does not necessarily reflect the views or policies of the NCI or any of the collaborating centers in the Colon Cancer Family Registry (CCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government, any cancer registry, or the CCFR.
Footnotes
Competing interests
AC is consultant to Bayer Pharma AG, Boehringer Ingelheim, and Pfizer Inc. for work unrelated to this manuscript; AS is an employee at Insitro, incl. consulting fees from BMS; HH is SAB for Invitae Genetics, Promega, and Genome Medical. Stock/Stock options for Genome Medical and GI OnDemand; JK is a consultant for Guardant Health; NP is a collaborator for Thrive and Exact, PGDx, CAGE, NeoPhore, Vidium, ManaTbio, and receives royalties for licensed technologies according to JHU rules; RKP collaborates with Eli Lilly, AbbVie, Allergan, Verily, and Alimentiv, which include consulting fees (outside of the submitted work); SAB has financial interest in Adaptive Biotechnologies; SBG is co-founder, Brogent International LLC; TSM receives research and honoraria from Merck Serono; ZKS’s immediate family member serves as a consultant in Ophthalmology for Alcon, Adverum, Gyroscope Therapeutics Limited, Neurogene, and RegenexBio (outside the submitted work). VM has research projects and owns stocks of Aniling. The remaining authors declare no competing interests.
References
- 1.Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Jiao S, Peters U, Berndt S, et al. Estimating the heritability of colorectal cancer. Hum Mol Genet. 2014;23(14):3898–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law PJ, Timofeeva M, Fernandez-Rozadilla C, et al. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun. 2019;10(1):2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Zhang F, Hu H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–487. [DOI] [PubMed] [Google Scholar]
- 6.Speed D, Holmes J, Balding DJ. Evaluating and improving heritability models using summary statistics. Nat Genet. 2020;52(4):458–462. [DOI] [PubMed] [Google Scholar]
- 7.Kvale MN, Hesselson S, Hoffmann TJ, et al. Genotyping Informatics and Quality Control for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics. 2015;200(4):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Burnett T, Kono S, et al. Trans-ethnic genome-wide association study of colorectal cancer identifies a new susceptibility locus in VTI1A. Nat Commun. 2014;5:4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamazon ER, Wheeler HE, Shah KP, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47(9):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbeira AN, Pividori M, Zheng J, Wheeler HE, Nicolae DL, Im HK. Integrating predicted transcriptome from multiple tissues improves association detection. PLoS Genet. 2019;15(1):e1007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bien SA, Su YR, Conti DV, et al. Genetic variant predictors of gene expression provide new insight into risk of colorectal cancer. Hum Genet. 2019;138(4):307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Lin W, Wen W, et al. Identifying Novel Susceptibility Genes for Colorectal Cancer Risk From a Transcriptome-Wide Association Study of 125,478 Subjects. Gastroenterology. 2021;160(4):1164–1178 e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battle A, Mostafavi S, Zhu X, et al. Characterizing the genetic basis of transcriptome diversity through RNA-sequencing of 922 individuals. Genome Res. 2014;24(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. [DOI] [PubMed] [Google Scholar]
- 15.Hirano Y, Iwase Y, Ishii K, Kumeta M, Horigome T, Takeyasu K. Cell cycle-dependent phosphorylation of MAN1. Biochemistry. 2009;48(7):1636–1643. [DOI] [PubMed] [Google Scholar]
- 16.Fattet L, Yang J. RREB1 Integrates TGF-beta and RAS Signals to Drive EMT. Dev Cell. 2020;52(3):259–260. [DOI] [PubMed] [Google Scholar]
- 17.Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuomisto AE, Makinen MJ, Vayrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Erzurumluoglu AM, Elsworth BL, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33(2):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson-Stuttard J, Papadimitriou N, Markozannes G, et al. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110(50):20224–20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YD, Hurson AN, Zhang H, et al. Assessment of polygenic architecture and risk prediction based on common variants across fourteen cancers. Nat Commun. 2020;11(1):3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-onlyreferences
- 24.Yang J, Ferreira T, Morris AP, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369–375, S361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magi R, Suleimanov YV, Clarke GM, et al. SCOPA and META-SCOPA: software for the analysis and aggregation of genome-wide association studies of multiple correlated phenotypes. BMC Bioinformatics. 2017;18(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speed D, Balding DJ. SumHer better estimates the SNP heritability of complex traits from summary statistics. Nat Genet. 2019;51(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992–3003. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Qi G, Park JH, Chatterjee N. Estimation of complex effect-size distributions using summary-level statistics from genome-wide association studies across 32 complex traits. Nat Genet. 2018;50(9):1318–1326. [DOI] [PubMed] [Google Scholar]
- 32.Stegle O, Parts L, Piipari M, Winn J, Durbin R . Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat Protoc. 2012;7(3):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbeira AN, Pividori M, Zheng J, Wheeler HE, Nicolae DL, Im HK. Integrating predicted transcriptome from multiple tissues improves association detection. PLoS Genet. 2019;15(1):e1007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Morris TJ, Webster AP, Yang Z, Beck S, Andrew F, Teschendorff AE (2017). “ChAMP: updated methylation analysis pipeline for Illumina BeadChips.” Bioinformatics, btx513. doi: 10.1093/bioinformatics/btx513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W, Laird PW, Shen H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017;45(4):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong X, Su YR, Barfield R, et al. A general framework for functionally informed set-based analysis: Application to a large-scale colorectal cancer study. PLoS Genet. 2020;16(8):e1008947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Borgne F, Combescure C, Gillaizeau F, et al. Standardized and weighted time-dependent receiver operating characteristic curves to evaluate the intrinsic prognostic capacities of a marker by taking into account confounding factors. Statistical Methods in Medical Research. 2018;27(11):3397–3410. doi: 10.1177/0962280217702416] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary level data for the full set of Asian and European GWAS are available through GWAS catalog (accession number GCST90129505). For individual-level data, CCFR, CORECT, CORSA_2 and GECCO are deposited in dbGaP (phs001415.v1.p1, phs001315.v1.p1, phs001078.v1.p1, phs001903.v1.p1, phs001856.v1.p1 and phs001045.v1.p1). NSCCG and COIN are available in the European Genome-phenome Archive under accession numbers EGAS00001005412 (NSCCG), EGAS00001005421 (COIN). UK Biobank data are available through http://www.ukbiobank.ac.uk/ and Finnish data through THL Biobank. Access to individual-level data for the remaining studies is controlled through oversight committees. CCFR 1 and CCFR 2 data can be requested by submitting an application for collaboration to the CCFR (forms, instructions and contact information can be located at (www.coloncfr/collaboration.org). Applications for individual level data from the QUASAR2 and SCOT clinical trials will be assessed by the Translational Research Steering Committees that oversee those studies. Individual level data from the CORGI (UK1) study will be made available subject to standard institutional agreements. Application forms for these three studies, and for Scotland Phase 1, Scotland Phase 2, SOCCS, DACHS4 and Croatia, will be provided by emailing a request to access.crc.gwas.data@outlook.com. For access to CORSA_1, please contact gecco@fredhutch.org. For Generation Scotland (GS) access is through the GS Access Committee (GSAC) (access@generationscotland.org). Applications for The Lothian Birth Cohort data should be made through https://www.ed.ac.uk/lothian-birth-cohorts/data-access-collaboration. For details of the application process for Aichi1, Aichi2, BBJ, Guanzhou1, HCES, HCES2, Korea and Shanghai cohorts, please go to https://swhs-smhs.app.vumc.org/ or contact Dr. Zheng at wei.zheng@vanderbilt.edu.
CRC-relevant epigenome data were obtained from the NCBI Gene Expression Omnibus (GEO) database under accession number GSE77737 and GSE36401.
Genetically predicted models of gene expression and methylation have been deposited in the Zenodo repository (https://zenodo.org/deposit/6472285).