Abstract
Background: The effectiveness of anti-TNF or ustekinumab (UST) as a second-line biologic after vedolizumab (VDZ) failure has not yet been described. Aims and Methods: In this retrospective multicenter cohort study, We aim to investigate the effectiveness of anti-TNF and UST as second-line therapy in patients with Crohn’s disease (CD) who failed VDZ as a first-line treatment. The primary outcome was clinical response at week 16–22. Secondary outcomes included the rates of clinical remission, steroid-free clinical remission, CRP normalization, and adverse events. Results: Fifty-nine patients who failed on VDZ as a first-line treatment for CD were included; 52.8% patients received anti-TNF and 47.2% UST as a second-line therapy. In initial period (Week 16–22), the clinical response and remission rate was similar between both groups: 61.2% vs. 68%, p = 0.8 and 48.3% vs. 56%, p = 0.8 on anti-TNF and UST therapy, respectively. Furthermore, in the maintenance period the rate was similar: 75% vs. 82.3%, p = 0.8 and 62.5% vs. 70.5%, p = 0.8, respectively. Of the patients, 12 out of the 59 stopped the therapy, without a significant difference between the two groups (p = 0.6). Conclusion: Second-line biological therapy after VDZ failure therapy was effective in >60% of the patients with CD. No differences in effectiveness were detected between the use of anti-TNF and UST as a second line.
Keywords: Crohn’s disease, drug positioning, treatment response, treatment failure, ustekinumab, vedolizumab, anti-TNF
1. Introduction
Biological therapies have revolutionized the management of Crohn’s disease (CD). Monoclonal antibodies against tumor necrosis factor alpha (anti-TNF) have been the corner stone of CD therapy since start the century [1,2,3,4]. More recently two additional biologicals, with different mechanism of action, have been added to the CD armamentarium: vedolizumab (VDZ) (α4β7 integrin inhibitor) [5,6,7,8,9,10] and ustekinumab (UST) (anti-interleukin-12/23) [2,6,7,9,11,12,13,14,15,16]. Despite the good efficacy, loss of response remains a serious concern across all therapeutic agents; the annual risk of loss of response to anti-TNF approaches 20–37% [17,18]. A similar rate of loss of response was described also in VDZ and UST [15,16,17,18,19,20]. In the last few years VDZ has been used as first-line therapy in CD with similar effectiveness as anti-TNF and very good safety profile [9,21,22,23].
With the advent of more agents, there is a dire need to develop not only positioning but also sequencing strategies. Nonetheless, there is limited guidance on optimal choice of agents as second-line therapies because of the absence of head-to-head trials after VDZ failure [3,24,25,26]. Accordingly, the aim of this study was to assess comparative effectiveness in terms of the induction of clinical response and remission with respect to anti-TNF versus UST in CD patients who showed failure of VDZ therapy. In addition, we attempted to identify predictors for the induction and maintenance of remission.
2. Materials and Methods
This was a multicenter retrospective cohort study. We included adult patients with an established CD diagnosis who received VDZ as a first-line therapy and switched to a second-line therapy that was either anti-TNF or UST. Clinical, endoscopic and laboratory data were extracted from the patient’s files and electronic records.
Patients eligible for inclusion in the study had to satisfy the following criteria: adult patients (≥18 years) with a confirmed CD who received VDZ as a first-line therapy and were switched to either anti-TNF or UST for a second-line therapy. All patients must have active disease and had follow-up for a minimum of 16 weeks after starting anti-TNF or UST therapy, patients who failed the therapy prior to week 16 were also included. Patients with under 16 weeks of follow-up post induction, with ostomy or a change in diagnosis to ulcerative colitis or inflammatory bowel disease unclassified (IBD-U) were excluded.
Demographic and clinical information that was collected included: age, gender, smoking status, type of anti-TNF agent, date of diagnosis, disease duration, disease location and Behavioral (Montreal classification), presence of perianal disease, duration of treatment with VDZ, concomitant immunomodulator at initiation of second-line therapy, corticosteroid use, C-reactive protein (CRP), fecal calprotectin (FCP), Harvey–Bradshaw Index (HBI), requirement for subsequent hospitalization, surgery and adverse events.
The study was approved by the Sheba Medical Center ethics committee. The patients’ consent was waived.
2.1. Outcome Definitions
The primary outcome was defined as a clinical response (defined by a reduction of HBI ≥ 3) [27] at week 16. Clinical response was assessed at the following time-points: initial response (week 16–22) and maintenance response (week 52).
Main secondary outcomes included the following in the two time points after induction: clinical remission (HBI ≤ 4) [27]; steroid-free clinical remission; drug discontinuation; CRP normalization ((CRP serum concentration levels less than normal range as per the cut-off used in the corresponding institutions) in patients with elevated baseline CRP; FCP normalization (<250 µg/mg) in patients with elevated baseline FCP.
Clinical and demographic characteristics were compared between the patients who received anti-TNF and UST, in order to identify potential predictive factors for clinical response. An adverse event is defined as any adverse reaction that occurs after initiating treatment.
2.2. Statistical Methods
The primary and secondary end points were calculated and compared for both the anti-TNF and UST patient groups. All variables were reported as mean ± standard deviation (SD) or proportions. Between-group comparisons were performed using unpaired t tests, Chi-square, Fisher’s exact or Wilcoxon rank testing, as appropriate. A survival analysis curve with COX regression was constructed for analysis of time to treatment discontinuation. The model was adjusted for age at diagnosis, disease behavior, presence of perianal disease, and steroid use at baseline. P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics software version 22. The data-extraction sheets contained only anonymized data.
3. Results
3.1. Baseline Characteristics
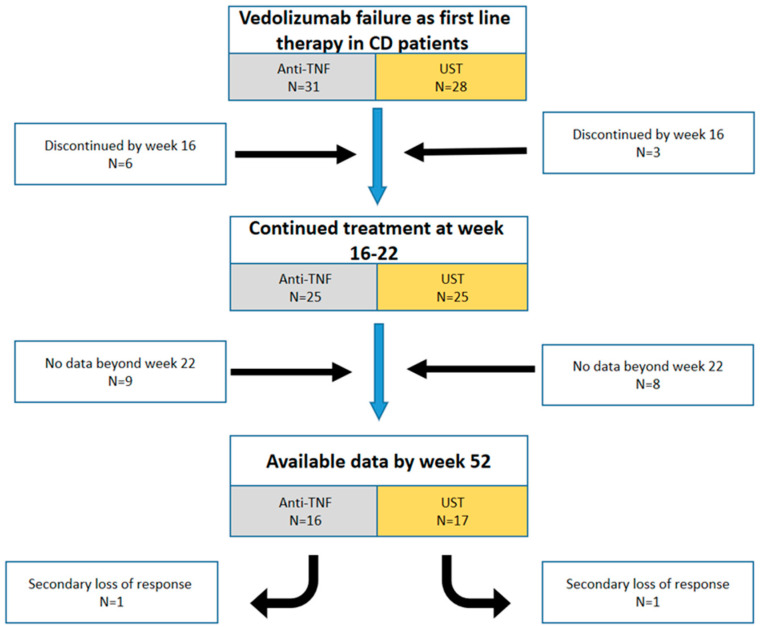
Fifty-nine patients from eleven centers in six different countries (six Europe, five Israel) were included in the study. The clinical and demographic characteristics of the patients are detailed in Table 1. All patients received VDZ as a first-line biological therapy and experienced therapy failure. The median time of VDZ treatment was 13.5 months (interquartile range (IQR) 5–18). The main reason for the discontinuation of VDZ was a lack of response (clinically, endoscopic and biomarker non- response (47.6%, 32.4% and 6.8%, respectively)). The rest discontinued the treatment because of: adverse event (3.3%), active extraintestinal manifestation (3.3%), surgery (3.3%) and active perianal disease (3.3%). Active disease was the indication for the second-line therapy initiation. A total of 31 out of the 59 patients were switched to anti-TNF (61.3% infliximab, 35.5% adalimumab, 3.2% certolizumab), and the remaining 28 patients were switched to UST (52.8% vs. 47.2%, respectively (p = 0.7)). There were no significant differences between the two groups as detailed in Table 1. Figure 1 describes the patient flow during the study.
Table 1.
Patients’ demographic and clinical characteristics.
| Overall | UST | Anti-TNF | p-Value | |
|---|---|---|---|---|
| Patient number, N (%) | 59 | 28 (47.5) | 31 (52.5) | 0.7 |
| Demographics | ||||
| Mean (SD) age, years | 52 (19) | 58 (17.5) | 46 (19) | 0.9 |
| Gender [male], n(%) | 25 (42.4) | 12 (42.8) | 13 (41.9) | 0.9 |
| Gender [female], n(%) | 34 (57.6) | 16 (57.1) | 18 (58) | 0.9 |
| Smoking status | ||||
| Current, n (%) | 9 (15.2) | 5 (17.8) | 4 (12.9) | 0.6 |
| Former, n (%) | 13 (22) | 8 (28.5) | 5 (16.1) | 0.3 |
| Never smoked, n (%) | 37 (62.8) | 15 (53.5) | 22 (70.9) | 0.5 |
| Clinical characteristics | ||||
| Disease duration, years (IQR) | 35 (23–56.5) | 43 (25–60) | 36 (21–48) | 0.4 |
| Disease location | ||||
| Ileal | 19 (32.4) | 11 (39.2) | 8 (25.8) | 0.4 |
| Colonic | 18 (30.5) | 9 (32.1) | 9 (29) | 0.8 |
| Ileocolonic | 21 (35.5) | 7 (25) | 14 (45.1) | 0.2 |
| Upper-GI | 1 (1.6) | 1 (3.5) | 0 (0) | 0.4 |
| Behavior | ||||
| Non-stricturing, non-penetrating | 39 (66.2) | 20 (71.4) | 19 (61.2) | 0.7 |
| Stricturing | 16 (27.1) | 7 (25) | 9 (29) | 0.7 |
| Penetrating | 4 (6.7) | 1 (3.5) | 3 (9.6) | 0.3 |
| Perianal disease | 5 (8.4) | 3 (10.7) | 2 (6.4) | 0.5 |
| History of surgery | 17 (28.8) | 8 (28.5) | 9 (29) | 0.9 |
| Mean vedolizumab therapy duration, months (IQR) | 12 (5–17) | 14.3 (6–18.7) | 10 (4–14) | 0.1 |
| Disease activity at treatment onset | ||||
| HBI median, (IQR) | 8 (6–9) | 9 (7–10.7) | 7 (6–9) | 0.6 |
| Elevated CRP, n (%) | 36 (66.6) | 16 (59.2) | 20 (74) | 0.7 |
| Elevated FCP, n (%) | 26 (68.4) | 12 (66.5) | 14 (70) | 0.9 |
| Concomitant corticosteroid | 23 (38.9) | 13 (46.4) | 10 (32.2) | 0.6 |
| Concomitant immunomodulators | 6 (10.1) | 1 (3.5) | 5 (16.1) | 0.1 |
Abbreviations: n, number; UST, ustekinumab; anti-TNF, anti-tumor necrosis factor; IQR, interquartile range; Upper-GI, upper gastrointestinal; HBI, Harvey–Bradshaw Index, CRP, C-reactive protein; FCP, Fecal Calprotectin; SD, Standard deviation.
Figure 1.
Chart of the patients’ flow during the study period. Abbreviations: CD, Crohn’s disease; anti-TNF, anti-Tumor Necrosis Factor; UST, ustekinumab.
3.2. Treatment Outcomes
3.2.1. Initial Response
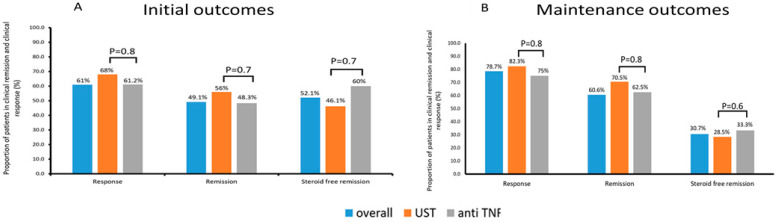
An initial response (week 16–22) was achieved in 61% of the patients (19/31 (61.2%) and 17/25 (68%) on anti-TNF and UST therapy, respectively (p = 0.8)). Rates of clinical remission were similar between both groups (15/31 (48.3%) and 14/25 (56%), (p = 0.7). Systemic corticosteroids were discontinued in 16/23 (69.5%) patients who were on corticosteroids at the treatment onset. Twelve out of 23 (52.1%) patient achieved corticosteroid-free remission (6/10 (60%) and 6/13 (46.1%), (p = 0.7) (Figure 2).
Figure 2.
Outcomes in the initial (A) and maintenance period (B). The proportion of patients achieving clinical remission (HBI ≤ 4), clinical response (delta HBI ≥ 3) and steroid-free remission during the initial period (week 16–22) (A), and the proportion of patients achieving clinical remission, clinical response and steroid free remission during the maintenance period (week 52) (B); HBI: Harvey–Bradshaw index; UST: ustekinumab; anti-TNF: anti-tumor necrosis factor.
3.2.2. Maintenance of Response
Follow up results in the maintenance period (median 53 weeks [IQR 48–58]) were available for 33/59 patients (16 (48.5%) and 17 (51.5%), respectively). A further 26 patients were excluded from the analysis of this time period (for 17 patients no data were available and 9 discontinued the therapy by week 16 (Figure 1).
The overall response rate was 78.7% (12/16 (75%) and 14/17 (82.3%) on anti-TNF and UST therapy, respectively (p = 0.8)). Clinical remission was achieved by 22/33(60.6%) patients (10/16 (62.5%) and 12/17 (70.5%), (p = 0.8), respectively). Of the 23 patients who were on corticosteroids at the treatment onset, 13 had available follow up results. The corticosteroid discontinuation rate was 58.3% in patients who were on corticosteroids at treatment onset. Corticosteroid-free remission was achieved by 4/13 (30.7%) patients (2/6 (33.3%) and 2/7 (28.5%), (p = 0.9)) (Figure 2).
3.2.3. Changes in CRP and FCP
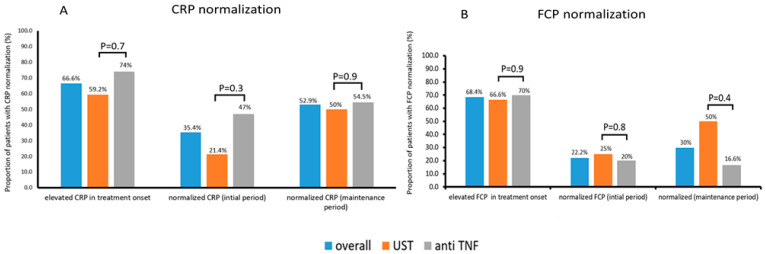
CRP levels were available in 54 out of the 59 patients and were elevated in 36 (66.6%) of them at treatment onset (20/27 (74%) and 16/27 (59.2%), on anti-TNF and UST therapy, respectively (p = 0.7)). CRP normalized in 11/31 (35.4%) patients (8/17 (47%) and 3/14 (21.4%), (p = 0.3)) with elevated baseline and available follow-up (median 17 weeks (IQR 15–22)) CRP levels in the initial response period. In the maintenance period, CRP values were available for 17 patients with a median follow up of 55 weeks (IQR 49.5–55), and they normalized in 9/17 (52.9%) of those with an elevated CRP at baseline (6/11 (54.5%) and 3/6 (50%), (p = 0.9) (Figure 3).
Figure 3.
Biomarker normalization. The proportion of patients with CRP normalization (A) (CRP serum concentration levels less than normal range as per cut-off used in the corresponding institutions) and FCP normalization (B) (FCP < 250 µg/g); CRP, C-reactive protein; FCP, Fecal Calprotectin; UST, ustekinumab; anti-TNF, anti-tumor necrosis factor.
FCP levels were available in 38 out of the 59 patients and were elevated in 26 (68.4%) at treatment onset (14/20 (70%) and 15/22 (66.6%), on anti-TNF and UST therapy, respectively (p = 0.9)). FCP normalized in 4/18 (22.2%) patients (2/10 (20%) and 2/8 (25%), (p = 0.8)) with elevated baseline and available follow-up (median 16 weeks (IQR 14–20.5)) FCP levels in the initial response period. FCP values at the maintenance period were available for 10 patients, and they normalized with a median follow-up of 52 weeks (IQR 49–55) in 3/10 (30%) of those with an elevated FCP at baseline (1/6 (16.6%) and 2/4 (50%), (p = 0.4) (Figure 3).
3.3. Discontinuation of Therapy
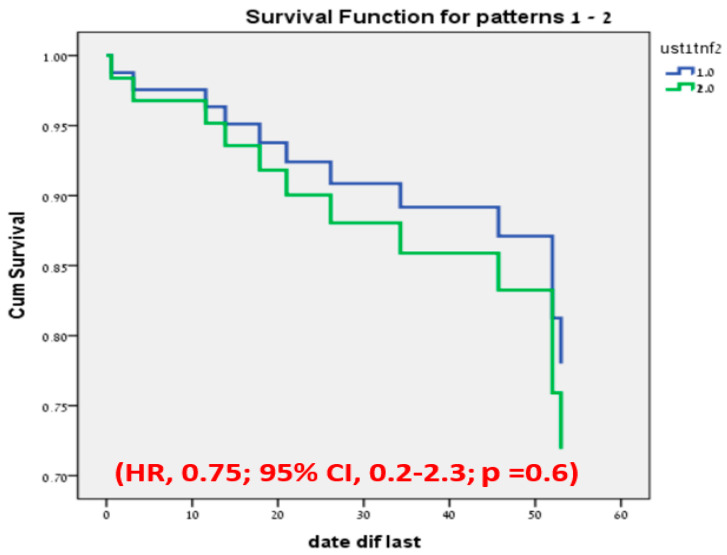
The overall treatment discontinuation was observed in 12 patients, with a median follow-up duration of 23.5 (IQR 6.5–50.5) weeks. A total of 7 patients out of 31 (22.5%) on anti-TNF with a median time of 21 weeks (IQR 14–46) and 4/28 (14.2%) on UST therapy with a median time of 34 weeks (6.5–52.5) discontinued treatment without any significant difference between the groups (p = 0.6) (Figure 4).
Figure 4.
Cox regression test of treatment discontinuation-free analysis; UST1, ustekinumab; TNF2, anti-tumor necrosis factor; HR, hazard ratio; CI, confidence interval; p, p-value.
The main reason for the discontinuation of both treatment groups was a lack of response (anti-TNF: 5/7 (71.4%), UST:3/4 (75%). The rest discontinued the treatment because of: patient decision (n = 1), surgery (n = 1), and immunogenicity (n = 1). During the follow-up period, 28% of the patients required dose escalation (32% vs. 21.4% on anti-TNF and UST therapy, respectively; (p = 0.4)) without any significant change in the discontinuation rate in the booth subgroups (30% vs. 16.6%, respectively; p = 0.6).
3.4. Safety
The adverse events that were documented during the entire follow-up period are listed in Table 2. Overall, 7 (10%) patients reported adverse events (4 (10.8%) patients on anti-TNF and 3 (9%) on UST). Adverse events were a cofactor for therapy discontinuation in 4 patients as is described in Table 2. Five patients out of the 70 (7.1%) required surgical intervention (2 (5.8%) and 3 (10.3%) on anti-TNF and UST therapy, respectively, (p = 0.5)). Ten patients (14.2%) were hospitalized at least once (5/37 (14.7%) and 5/33 (17.2%), respectively, (p = 0.8).
Table 2.
Adverse events.
| Patients Number | Drug | Adverse Events | Stopped Therapy | Required Hospitalization |
|---|---|---|---|---|
| Patient 1 | UST | Rash in the extremities and itchy | Yes | No |
| Patient 2 | Anti-TNF | Allergic reaction to anti TNF | No | No |
| Patient 3 | Anti-TNF | Fatigue, arthralgia, myalgia | No | No |
| Patient 4 | Anti-TNF | Worsening in ITP | Yes | Yes |
| Patient 5 | UST | Uneasiness, loss of strength | Yes | No |
| Patient 6 | Anti-TNF | Pancreatitis | No | Yes |
| Patient 7 | UST | Lung cancer | Yes | Yes |
Abbreviations: UST, ustekinumab; anti-TNF, anti-tumor necrosis factor; ITP, Immune Thrombocytopenia.
4. Discussion
Our study is a comparative real-world study in CD that attempts to address an important evidence gap: to compare the effectiveness and safety of anti-TNF agents and UST as a second-line biologic after VDZ failure. Our retrospective data from 59 CD patients demonstrated that second-line anti-TNF-treated patients had similar rates of clinical effectiveness and adverse event to second-line UST cohort in both induction and maintenance period (52 weeks’ post-treatment initiation). These results support the effectiveness and safety of anti-TNF and UST as a second-line biologic treatment after VDZ failure.
VDZ is efficacious in both TNF-naïve and TNF-failure populations [5,7,8,9,10]. Despite the adequate efficacy about 39–42% of the patients will stop the therapy mainly due to loss of response and adverse events [28,29,30]. The choice of drug positioning is based upon clinicians’ experience, costs and drug availability in the region. Moreover, data regarding efficacy of anti-TNF and UST therapy after VDZ failure as a first-line therapy are very limited.
There is only one study that evaluated the efficacy and safety of anti-TNF as a second-line biologic after VDZ failure in pediatric IBD patients. In particular, Dolinger, M. et al. [31] included 21 pediatric patients with mostly colonic disease (19/21). The study included only six CD patients with numerically lower remission rates than in UC/IBD-U (50% vs. 80% p = 0.27). Three out of the six patients discontinued anti-TNF therapy after a median duration of 15 (7–24) weeks. No serious adverse events, hospitalizations or serious infections attributable to anti-TNF therapy were described in the study. In our study, the overall clinical remission was achieved in 49.1% after the induction period without significantly difference between anti-TNF and UST. The remission rate remained high in the maintenance period (60.6%) and steroid-free clinical remission was achieved in a greater proportion of patients (52.1%).
Earlier studies have shown the effectiveness of UST in CD patients refractory to anti-TNFs. However, these studies do not describe the effectiveness of UST as second-line biologic after VDZ failure as a first-line therapy [32,33,34,35].
In our study 20.3% (12/59) of the patients discontinued the therapy, while the majority continued the therapy with an acceptable response (78.7%%) and remission rates (60.6%). Effectiveness of anti-TNF and UST as a second-line biologic after VDZ failure suggest that the effectiveness may not be compromised by prior VDZ exposure, as shown in previous studies [36,37,38].
Only four adverse events were as cofactors for therapy discontinuation (one anti-TNF, three UST), adding further to the literature supporting anti-TNF and UST safety profiles [39,40].
Important limitations of the study need to be acknowledged. The major limitation of this study is the retrospective multicenter nature real world (RWE) evidence of the data collection, with the potential biases including recall bias; missing some laboratory data, including drug levels and anti-drug antibodies; heterogeneity in the scheduled visit dates and reporting bias of side effects which patients may not have reported or may not have been recorded. These limitations are not unique to our study and are similar to most multicenter RWE series. The second limitation is the lack of data pertaining to the response of extra intestinal manifestations and perianal disease. Further limitations include the relatively modest number of patients, that may be secondary to the limited indication of VDZ as a first-line therapy in many countries. Due to this low number of patients, we were unable to calculate the predictors of clinical response. The study cohort was narrow in terms of age (51.8 years old (IQR 32–70)) and may not well reflect the responses of all patients. There is no representation for the young under the age of 30. The time definition was also challenging to implement due to different follow-up and treatment approaches in the different centers. Thirty-seven percent of the cohort had available endoscopic follow-up examinations, with a time lag between the endoscopies and a median of 44 weeks (IQR 21–57.5).
Despite the aforementioned limitations, our study is the largest to data addressing the effectiveness of second-line biologic after VDZ failure. Further research would be required to identify the most effective treatment regimen for Crohn’s disease patients failing VDZ as first-line biological therapy.
In conclusion, second line therapy after VDZ failure therapy was effective in more than 60% of the patients with CD. No differences in effectiveness were detected between the use of anti-TNF and UST as a second line.
Author Contributions
Conceptualization, A.A. (Ahmad Albshesh) and U.K.; methodology, A.A. (Ahmad Albshesh) and U.K.; software, A.A. (Ahmad Albshesh); validation, A.A. (Ahmad Albshesh) and U.K.; formal analysis, A.A. (Ahmad Albshesh); investigation, A.A. (Ahmad Albshesh); resources and data curation, A.A. (Ahmad Albshesh), L.B., T.S.F., M.T., S.R.V., K.T., D.P., E.V.S., E.Z., D.D., X.R., A.B.-G.S., A.A. (Alessandro Armuzzi), T.L., N.M., H.Y. and S.B.-H.; writing—original draft preparation, A.A. (Ahmad Albshesh); writing—review and editing, L.B., T.S.F., M.T., S.R.V., K.T., D.P., E.V.S., E.Z., D.D., X.R., A.B.-G.S., A.A. (Alessandro Armuzzi), T.L., N.M., H.Y. and S.B.-H.; visualization, A.A. (Ahmad Albshesh); supervision, U.K.; project administration, A.A. (Ahmad Albshesh). All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
This retrospective study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee of Sheba Medical Center. The patients’ consent was waived.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Ahmad Albshesh—received speaking fees from Takeda; Stephan Vavricka—has received consulting fees, speakers honorary and unrestricted research grants from Abbott, Alfasigma, Amgen, Arenapharm, Falk Pharma GmbH, Ferring Pharmaceuticals, Gilead, iQuone, Janssen, MSD, Permamed, Pfizer Inc, Sanofi-Aventis, Takeda, Tillotts, UCB, and Vifor; Eran Zittan—received research support and consulting fees from Janssen, AbbVie, Takeda, Neopharm, Celgene and Pfizer; David Drobne—has served as a speaker, a consultant and an advisory board member for MSD, Abbvie, Takeda, Pfizer, Janssen, and Krka; Alessandro Armuzzi—consulting, advisory board and/or lecture fees and/or research support: AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celltrion, Eli Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Novartis, Pfizer, Protagonist-Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda; Triana lobaton—Financial support for research from Abbvie, Viatris, MSD, Mundipharma, Biogen, Janssen, Pfizer and Takeda; Speaker fees from Ferring, MSD, Abbvie, Janssen, Amgen, Fresenius Kabi, Galapagos, Viatrisand Takeda; Consultancy fee from Janssen, Galapagos, Amgen, Bristol Myers Squibb Fresenius Kabiand Takeda; Nitsan Maharshak—has received speaking and/or consulting fees from Pfizer, Takeda, Janssen, Ferring, BiomX, BMS, Nestle and grant support from Takeda, Janssen, Abbott, Abbvie, Pfizer, BMS, Nestle, Trobix; Henit Yanai: reports institutional research grants from Pfizer and the ISF; consulting fees from Abbvie, Janssen, Pfizer, and Takeda; honoraria for lectures from Abbvie, Janssen, Pfizer, and Takeda; participation in a Data Safety Monitoring Board or Advisory Board from Abbvie, Pfizer, and Takeda; Shomron Ben-Horin—consulting and advisory board fees and/or research support—Abbvie, MSD, Janssen, Takeda, and CellTrion; Uri Kopylov—research support- Jannsen Medtronic Takeda, advisory and speaker fees- Abbvie BMS Janssen Medtronic Novartis Pfizer Takeda. All other authors have no conflicts of interest to report.
Funding Statement
This research received no funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dreesen E., Gils A. Letter: Overcoming secondary loss of response to infliximab-it is not the drug, it is how you use it! Authors’ reply. Aliment. Pharmacol. Ther. 2018;48:1029–1030. doi: 10.1111/apt.14974. [DOI] [PubMed] [Google Scholar]
- 2.Kopylov U., Seidman E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2016;9:513–526. doi: 10.1177/1756283X16638833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stidham R.W., Lee T.C.H., Higgins P.D.R., Deshpande A.R., Sussman D.A., Singal A.G., Elmunzer B.J., Saini S.D., Vijan S., Waljee A.K. Systematic review with network meta-analysis: The efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment. Pharmacol. Ther. 2014;39:1349–1362. doi: 10.1111/apt.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preda C., Fulger L.E., Gheorghe L., Gheorghe C., Goldis A., Trifan A., Tantau M., Tantau A., Negreanu L., Manuc M., et al. Infliximab and Adalimumab in Crohn’s disease: Real-life data from a national cohort study. Curr. Health Sci. J. 2016;42:115–124. doi: 10.12865/CHSJ.42.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stidham R.W., Lee T.C.H., Higgins P.D.R., Deshpande A.R., Sussman D.A., Singal A.G., Waljee A.K. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Vermeire S., Loftus E.V., Jr., Colombel J.-F., Feagan B.G., Sandborn W.J., Sands B.E., Danese S., D’Haens G.R., Kaser A., Panaccione R., et al. Long-term Efficacy of Vedolizumab for Crohn’s Disease. J. Crohns Colitis. 2017;11:412–424. doi: 10.1093/ecco-jcc/jjw176. [DOI] [PubMed] [Google Scholar]
- 7.Sands B.E., Sandborn W.J., Van Assche G., Lukas M., Xu J., James A., Lasch K. Vedolizumab as induction and maintenance therapy for crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm. Bowel Dis. 2017;23:97–106. doi: 10.1097/MIB.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 8.Sands B.E., Sandborn W.J., Van Assche G., Lukas M., Xu J., James A., Lasch K. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 9.Kopylov U., Avni-Biron I., Ron Y., Koslowsky B., Waterman M., Daher S., Ungar B., Schwartz D., Zittan E., Openhaim M., et al. Effectiveness and safety of vedolizumab for maintenance treatment in inflammatory bowel disease-The Israeli real world experience. Dig. Liver Dis. 2019;51:68–74. doi: 10.1016/j.dld.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Macaluso F.S., Fries W., Renna S., Viola A., Muscianisi M., Cappello M., Sicilian Network for Inflammatory Bowel Disease (SN-IBD) Effectiveness and safety of vedolizumab in biologically naïve patients: A real-world multi-centre study. United Eur. Gastroenterol. J. 2020;8:1045–1055. doi: 10.1177/2050640620948802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubesch A., Rueter L., Farrag K., Krause T., Stienecker K., Hausmann J., Blumenstein I. Short and Long-Term Effectiveness of Ustekinumab in Patients with Crohn’s Disease: Real-World Data from a German IBD Cohort. J. Clin. Med. 2019;8:2140. doi: 10.3390/jcm8122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liefferinckx C., Verstockt B., Gils A., Noman M., Van Kemseke C., Macken E., Belgian Inflammatory Bowel Disease Research and Development Group [BIRD Group] Long-term Clinical Effectiveness of Ustekinumab in Patients with Crohn’s Disease Who Failed Biologic Therapies: A National Cohort Study. J. Crohns Colitis. 2019;13:1401–1409. doi: 10.1093/ecco-jcc/jjz080. [DOI] [PubMed] [Google Scholar]
- 13.Feagan B.G., Sandborn W.J., Gasink C., Jacobstein D., Lang Y., Friedman J.R., Blank M.A., Johanns J., Gao L.-L., Miao Y., et al. Ustekinumab as induction and maintenance therapy for crohn’s disease. N. Engl. J. Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 14.Dreesen E., Van Stappen T., Ballet V., Peeters M., Compernolle G., Tops S., Van Steen K., Van Assche G., Ferrante M., Vermeire S., et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment. Pharmacol. Ther. 2018;47:346–355. doi: 10.1111/apt.14452. [DOI] [PubMed] [Google Scholar]
- 15.Amiot A., Grimaud J.-C., Peyrin-Biroulet L., Filippi J., Pariente B., Roblin X., Buisson A., Stefanescu C., Trang-Poisson C., Altwegg R., et al. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016;14:1593–1601.e2. doi: 10.1016/j.cgh.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Shmidt E., Kochhar G., Hartke J., Chilukuri P., Meserve J., Chaudrey K., Koliani-Pace J.L., Hirten R., Faleck D., Barocas M., et al. Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24:2461–2467. doi: 10.1093/ibd/izy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisbert J.P., Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: A review. Am. J. Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 18.Billioud V., Sandborn W.J., Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: A systematic review. Am. J. Gastroenterol. 2011;106:674–684. doi: 10.1038/ajg.2011.60. [DOI] [PubMed] [Google Scholar]
- 19.Dalal R.S., Njie C., Marcus J., Gupta S., Allegretti J.R. Predictors of ustekinumab failure in crohn’s disease after dose intensification. Inflamm. Bowel Dis. 2021;27:1294–1301. doi: 10.1093/ibd/izaa282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyrin-Biroulet L., Danese S., Argollo M., Pouillon L., Peppas S., Gonzalez-Lorenzo M., Lytras T., Bonovas S. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients with Crohn’s Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019;17:838–846.e2. doi: 10.1016/j.cgh.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Attauabi M., Höglund C., Fassov J., Pedersen K.B., Hansen H.B., Wildt S., Burisch J. Vedolizumab as first-line biological therapy in elderly patients and those with contraindications for anti-TNF therapy: A real-world, nationwide cohort of patients with inflammatory bowel diseases. Scand. J. Gastroenterol. 2021;56:1040–1048. doi: 10.1080/00365521.2021.1946588. [DOI] [PubMed] [Google Scholar]
- 22.Baumgart D.C., Bokemeyer B., Drabik A., Stallmach A., Schreiber S. Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice--a nationwide consecutive German cohort study. Aliment. Pharmacol. Ther. 2016;43:1090–1102. doi: 10.1111/apt.13594. [DOI] [PubMed] [Google Scholar]
- 23.Kopylov U., Verstockt B., Biedermann L., Sebastian S., Pugliese D., Sonnenberg E., Steinhagen P.R., Arebi N., Ron Y., Kucharzik T., et al. Effectiveness and Safety of Vedolizumab in Anti-TNF-Naïve Patients with Inflammatory Bowel Disease-A Multicenter Retrospective European Study. Inflamm. Bowel Dis. 2018;24:2442–2451. doi: 10.1093/ibd/izy155. [DOI] [PubMed] [Google Scholar]
- 24.Hazlewood G.S., Rezaie A., Borman M., Panaccione R., Ghosh S., Seow C.H., Kaplan G.G. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: A network meta-analysis. Gastroenterology. 2015;148:344–354.e5. doi: 10.1053/j.gastro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Singh S. Network meta-analysis to inform positioning of biologics in patients with Crohn’s disease: Promise and perils. Best Pract. Res. Clin. Gastroenterol. 2019;38–39:101614. doi: 10.1016/j.bpg.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Singh S., Fumery M., Sandborn W.J., Murad M.H. Systematic review and network meta-analysis: First- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment. Pharmacol. Ther. 2018;48:394–409. doi: 10.1111/apt.14852. [DOI] [PubMed] [Google Scholar]
- 27.Vermeire S., Schreiber S., Sandborn W.J., Dubois C., Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 2010;8:357–363. doi: 10.1016/j.cgh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson C., Marsal J., Bergemalm D., Vigren L., Björk J., Eberhardson M., Halfvarson J. Long-term effectiveness of vedolizumab in inflammatory bowel disease: A national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG) Scand. J. Gastroenterol. 2017;52:722–729. doi: 10.1080/00365521.2017.1304987. [DOI] [PubMed] [Google Scholar]
- 29.Engel T., Ungar B., Yung D.E., Ben-Horin S., Eliakim R., Kopylov U. Vedolizumab in IBD-Lessons from Real-world Experience; A Systematic Review and Pooled Analysis. J. Crohns Colitis. 2018;12:245–257. doi: 10.1093/ecco-jcc/jjx143. [DOI] [PubMed] [Google Scholar]
- 30.Allegretti J.R., Barnes E.L., Stevens B., Storm M., Ananthakrishnan A., Yajnik V., Korzenik J. Predictors of Clinical Response and Remission at 1 Year Among a Multicenter Cohort of Patients with Inflammatory Bowel Disease Treated with Vedolizumab. Dig. Dis. Sci. 2017;62:1590–1596. doi: 10.1007/s10620-017-4549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolinger M., Rolfes P., Phan B., Pan S., Dubinsky M. P082 anti-tnf efficacy after primary vedolizumab failure in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2020;26((Suppl. S1)):S71–S72. doi: 10.1093/ibd/zaa010.180. [DOI] [Google Scholar]
- 32.Albshesh A., Taylor J., Savarino E.V., Truyens M., Armuzzi A., Ribaldone D.G., Kopylov U. Effectiveness of Third-Class Biologic Treatment in Crohn’s Disease: A Multi-Center Retrospective Cohort Study. J. Clin. Med. 2021;10:2914. doi: 10.3390/jcm10132914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alric H., Amiot A., Kirchgesner J., Tréton X., Allez M., Bouhnik Y., Beaugerie L., Carbonnel F., Meyer A. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment. Pharmacol. Ther. 2020;51:948–957. doi: 10.1111/apt.15706. [DOI] [PubMed] [Google Scholar]
- 34.Taxonera C., Rodríguez C., Bertoletti F., Menchén L., Arribas J., Sierra M., Arias L., Martínez-Montiel P., Juan A., Iglesias E., et al. Clinical Outcomes of Golimumab as First, Second or Third Anti-TNF Agent in Patients with Moderate-to-Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2017;23:1394–1402. doi: 10.1097/MIB.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 35.Iborra M., Beltrán B., Clotet A.F., Gutiérrez A., Antolín B., Huguet J., De Francisco R., Merino O., Carpio D., García-López S., et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2019;50:278–288. doi: 10.1111/apt.15371. [DOI] [PubMed] [Google Scholar]
- 36.Dussias N., Rizzello F., Calabrese C., Passino A.S., Melotti L., Scaioli E., Gionchetti P. Effec-tiveness of ustekinumab after vedolizumab failure in patients with anti-TNF-refractory Crohn’s disease. J. Crohns Colitis. 2021;15:S528. doi: 10.1093/ecco-jcc/jjab076.689. [DOI] [Google Scholar]
- 37.Ritter T.E., Fourment C., Kuten S.A., Hardin T.C., Van Anglen L.J. 728°Second-Line Biologic Therapy After Vedolizumab. Am. J. Gastroenterol. 2019;114:S429–S430. doi: 10.14309/01.ajg.0000592448.04563.6a. [DOI] [Google Scholar]
- 38.Ritter T.E., Fourment C., Okoro T.C., Hardin T.C., Van Anglen L.J. Failure of Vedolizumab as First-Line Biologic Does Not Decrease Response Rates of Second-Line Therapy: 681. Off. J. Am. Coll. Gastroenterol. ACG. 2018;113:S382–S383. doi: 10.14309/00000434-201810001-00681. [DOI] [Google Scholar]
- 39.Adegbola S.O., Sahnan K., Warusavitarne J., Hart A., Tozer P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018;19:2244. doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engel T., Yung D.E., Ma C., Pariente B., Wils P., Eliakim R., Ungar B., Ben-Horin S., Kopylov U. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig. Liver Dis. 2019;51:1232–1240. doi: 10.1016/j.dld.2019.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.