Abstract
According to statistics and future predictions, meat consumption will increase in the coming years. Considering both the environmental impact of intensive livestock farming and the importance of protecting animal welfare, the necessity of finding alternative strategies to satisfy the growing meat demand is compelling. Biotechnologies are responding to this demand by developing new strategies for producing meat in vitro. The manufacturing of cultured meat has faced criticism concerning, above all, the practical issues of culturing together different cell types typical of meat that are partly responsible for meat’s organoleptic characteristics. Indeed, the existence of a cross talk between adipose and muscle cells has critical effects on the outcome of the co-culture, leading to a general inhibition of myogenesis in favor of adipogenic differentiation. This review aims to clarify the main mechanisms and the key molecules involved in this cross talk and provide an overview of the most recent and successful meat culture 3D strategies for overcoming this challenge, focusing on the approaches based on farm-animal-derived cells.
Keywords: in vitro meat, cells co-culture, muscle and adipose cell cross talk, scaffold, hydrogel, farm-animal-derived cells
1. Introduction
Meat and meat-derived products are the most consumed food and they are essential in a balanced diet to provide proteins, essential amino acids, vitamins, iron, zinc and fatty acids [1].
According to the Chatham House Report, today, 50% of the world’s habitable land is occupied by cropping and animal farming [2], while the livestock amounts to approximately 65% of all mammals on Earth [3].
This represents a serious problem since livestock and the food industry are involved in critical environmental issues; according to FAO, livestock is responsible for producing 7.1 gigatonnes of CO2-equivalent per year, which is 14.5% of the total emission of anthropogenic greenhouse gases [4].
As reported by the Global Energy Review–2022, the production of CO2 from the most impacting activities, that are combustion and industrial processes, is about 36.3 gigatonnes [5].
Another critical point is the use of water in the animal-derived food industry, which is the primary agricultural cause of water wastage and pollution, significantly contributing to world water withdrawals and the discharge of toxic compounds such as drug residues and agrochemicals into the groundwater [6].
Additionally, as agriculture and livestock are steadily growing, an increase in both agricultural land use and the related levels of greenhouse gases seems inevitable. This would make farming indirectly responsible for the loss of biodiversity since greenhouse gases play a role in climate change and rising temperatures, the leading causes of wild animals either abandoning their natural habitats or dying [2].
Finally, finding an alternative to animal-derived food consumption could solve several ethical issues related to animal welfare [7].
Considering all the drawbacks linked to livestock and farming activities, different strategies to answer the growing demand for meat have been proposed, from plant-based alternatives to insect flour, low-impact farming, and cultured meat [8].
In vitro meat represents one of the most promising, but at the same time challenging, alternatives to meat. This technique is based on growing cells in specific conditions to obtain a meat-like structure, endowed with meat sensory characteristics. The first attempt at culturing meat was performed in 1971 by Russel Ross, who used pig smooth muscle-derived cells to obtain fibers [9]. However, it was only in 2013 that the team of Professor Mark Post from the University of Maastricht developed the first cultured hamburger [10], opening new perspectives and challenges and paving the way toward modern approaches to in vitro meat culturing. Innovations in cell culturing methods have been applied to this field with good results, especially with the introduction of 3D supports that made obtaining a meat-looking product even more realistic [10,11,12,13]. Despite some of the difficulties involved in co-culturing those cell types that are naturally typical of meat, recent strides have led to promising results, propelling in vitro meat culturing towards new perspectives and development possibilities [1,13,14,15,16].
This review aims to provide an up-to-date overview of novel approaches in this field, examining the critical, technical aspects related to the necessity of obtaining a more realistic in vitro product. This study seeks to examine (i) the cross talk between adipose and muscle cells and (ii) the novel biotechnological approaches to meat culturing, with a specific emphasis on achievements in using 3D supports and large farm-animal-derived cells and their potential in the pursuit of a realistic in vitro meat.
2. The Cross Talk between Adipose and Muscle Cells
2.1. In Vitro Meat—The Challenge
Muscles are constituted by different tissues identified in meat, including contractile muscle fibers, connective tissue, blood vessels, lymph capillaries, motor endplate, and adipose tissue [17,18,19,20,21,22]. Among these, muscle fiber and connective tissue, including intramuscular fat, are the main ones responsible for meat’s organoleptic characteristics of tenderness and juiciness, together with water [23,24]. These characteristics are evaluated by consumers and determine the quality of meat together with the meat color [18,20,25]. Hence, it has been and still is difficult to recreate those characteristics in an in vitro condition of meat production [1]. One of the main challenges in this process is the co-culturing of different cell types.
Co-culturing is a technique that allows cultivating together different cell types, either directly or indirectly [26]. Briefly, in the direct system, different cell types are cultured spatially together in the same dish, while in the indirect system, different cell populations are cultured separately, for example, using Transwell inserts or specific chambers, and they only communicate via secretory factors [27]. These systems are already widely used for different purposes, such as in studies on the cross talk between different tissues, for example, between endothelial and smooth muscle cells or between microglia and neuronal cells [28,29], and in research on drug delivery [30,31]. However, their application in in vitro meat reproduction is only beginning to find its way.
Ideally, to properly reproduce meat in an in vitro condition, it would be necessary to co-culture muscle cells, adipocytes, nerve, and blood cells [1]. However, current in vitro meat production relies mainly on the co-culture of muscle and fat cells to obtain a good texture and juiciness of the final product. Marbling, which refers to the intramuscular fat between muscle fibers, is the main characteristic responsible for meat juiciness and is an important parameter used to grade meat products [32]. Thus to obtain quality in vitro meat products, several studies relied on the co-culturing of adipose and muscle cells [1,6,11]. However, co-culturing these different cell types has faced challenges because of their mutual influence [33]. Similarly to the in vivo conditions where adipose and muscle tissues are in a physical and functional relationship [34,35], in an in vitro co-culture system, such a cross talk can strongly influence the outcome of the culture itself [26]. Thus, several authors have underlined the influence adipocytes growing near muscle fibers can exert in vitro, mainly impairing myogenesis [36,37,38,39,40,41,42].
2.2. Adipose and Muscle Cells—The Cross Talk
Research on the molecular mechanisms at the base of this cross talk is mainly performed using rodent or human lineage cells. This step is necessary for obtaining basic information to develop a model for in vitro meat using farm animal cells. Table 1 below schematically summarizes the reported literature.
Table 1.
The literature on the in vitro cross talk between adipose and muscle cells.
| Authors | Cell Types | Culture System | Outcome | Doi | Year |
|---|---|---|---|---|---|
| Seo et al. [36] | 3T3-L1 | Indirect co-culture | Inhibition of muscle cells differentiation | 10.1111/ asj.13145 |
2019 |
| C2C12 | |||||
| Artaza et al. [39] | 10T(1/2) | Monoculture: | 10.1210/en.2005-0362 | 2005 | |
| +Recombinant myostatin | Adipogenic differentiation | ||||
| +Recombinant anti-myostatin | Myogenic differentiation | ||||
| Takegahara et al. [40] | Rat muscle progenitors | Indirect co-culture: | 10.1016/j.yexcr.2014.03.021 | 2014 | |
| 2G11 rat preadipocytes | Preadipocytes + muscle progenitors | No inhibition of muscle differentiation | |||
| 2G11 rat mature adipocytes | Mature adipocytes + muscle progenitors | Inhibition of muscle differentiation | |||
| Choi et al. [41] | Bovine satellite cells | Indirect co-culture | Inhibition of muscle differentiation | 10.1016/j.jnutbio.2012.01.015 | 2013 |
| Bovine preadipocytes | |||||
| Guo et al. [42] | Chicken satellite cells | Indirect co-culture | Inhibition of muscle differentiation | 10.1186/s12864-018-5209-5 | 2018 |
| Chicken intramuscular adipocytes |
The existence of such a cross talk could lean on the common embryological mesodermal origin of both skeletal muscles and adipose tissue [42,43]. They both result from mesenchymal precursors, which may explain their strong relationship and level of mutual influence [26], detectable in both in vivo and in vitro conditions.
Seo et al. [36] investigated this cross talk by co-culturing preadipocytes with murine muscle cells. Murine preadipocytes 3T3-L1 were cultured and differentiated on inserts for up to 10 days; then, inserts were placed in 24-well cell culture companion plates where murine myoblasts were seeded the day before starting co-culture. The co-culture was carried out for 5 days prior to analyses. The authors observed a paracrine cross talk between the two cell types, with downregulating effects of adipocytes on the differentiation of myoblasts mainly related to a downregulation in myogenin and upregulation in myostatin, atrophy-related ubiquitin E3 ligases (Atrogin1) and muscle RING-finger protein-1 (MurF-1) in muscle cells. Myogenin is a basic helix–loop–helix transcription factor that belongs to the myogenesis regulatory factors (MRFs) family. It is activated by the myoblast determination protein 1 (MyoD), and it plays a role in myocyte fusion and contractile protein synthesis during myogenesis [44,45,46]. Myostatin, also known as growth differentiation factor 8 (GDF8), is an adipomyokine produced by muscle cells to counteract muscle growth by indirectly activating the protein mothers against decapentaplegic homolog 3 (Smad3), a key element in the transforming growth factor beta pathway which inhibits myogenesis [37,47,48]. Smad3 binds to the basic helix–loop–helix (bHLH) region of MyoD so that MyoD cannot bind the DNA response element enhancer box (E-Box) CAXXTG and consequently cannot activate myogenin expression [49]. The myostatin pathway also leads to an overexpression of the ubiquitin–proteasome system [50], in particular of atrophy-related ubiquitin E3 ligases (Atrogin1) and muscle RING-finger protein-1 (MurF-1) by binding the Activin type II receptor (ActRIIB) [51]. Atrogin1 and MuRF-1 are tissue-specific proteins, typically expressed and upregulated in the proteasome machinery during muscle wasting [51,52,53]. They are responsible for the proteolysis that leads to muscle atrophy [53,54], a condition of skeletal muscle loss to which the main contributor is the ubiquitin–proteasome proteolytic system [55]. Moreover, Seo et al. detected an increased adipomyokine Interleukin-6 (IL-6) level in the murine C2C12 myoblasts. In the study, adipocyte-induced IL-6 played an inhibitory role in muscle cell differentiation [36].
A similar effect of IL-6 on muscle differentiation was also observed by Pelosi et al., who demonstrated that the regulation of muscle differentiation is dependent on the activation of signal transduction cascades with the complex involvement of several kinases [56].
The effects of myostatin on the faith of multipotent cell differentiation were also assessed by Artaza et al. [39], who demonstrated that 10T(1/2) murine mesenchymal multipotent cells stimulated by 5′-azacytidine for myogenic differentiation could undergo such lineage according to the presence or absence of myostatin [39]. They tested two different culture conditions, one characterized by the presence of recombinant myostatin and the other one by the presence of recombinant anti-myostatin. In the first situation, they observed that the levels of MyoD and myogenin were downregulated, with a consequently lower myoblasts fusion index. Interestingly, a higher level of the CCAAT/enhancer-binding proteins alpha (C/EBP alpha) and adiponectin were also recorded, which meant that cells did not undergo the myogenic differentiation pathway in favor of the adipogenic one [39]. C/EBP alpha and the peroxisome proliferator-activated receptor gamma (PPARγ), a key lipid metabolism and energy balance factor, are essential for activating the genes involved in the terminal phase of the adipogenic differentiation [57]. On the other hand, adiponectin is an adipokine that, when upregulated, interacts with PPARγ entering the adipogenesis pathway, as demonstrated on murine preadipocytes by Yang et al. [58]. In the second condition, with the recombinant anti-myostatin supplementation, cells underwent myogenic differentiation, with high levels of MRFs and the absence of adipogenic markers. The study proved that myostatin could interfere with myogenic differentiation, reverting it towards the adipogenic one [39].
Interestingly, Takegahara et al. [40] observed a different influence on myogenesis when using mouse preadipocytes and mature adipocytes. Using an indirect co-culture system based on insert, they cultured together either freshly isolated rat 2G11 mature adipocytes or rat skeletal muscle progenitors. When compared, the two conditions gave different results; using mature adipocytes, a lower fusion index in myoblasts was recorded, with a lower positivity to myosin heavy chains, while using preadipocytes, both the fusion index and the positivity to myosin heavy chains were higher [40]. Moreover, considering the lack of direct contact between the two cell types, the cross talk between them appeared to be mediated by soluble factors. To prove this hypothesis, freshly isolated skeletal muscle progenitors from rat were cultured in two different conditioned media (CMs), one from a 2G11 rat mature adipocytes culture and the other from 2G11 rat preadipocytes [40]. Similar to the co-culture conditions, myogenesis was impaired or slowed when using the mature adipocytes conditioned medium (CM). These results may suggest an effect of myogenesis impairment only when using already differentiated adipose cells.
Accordingly, Choi et al. [41] demonstrated the effects of differentiated bovine adipocytes on bovine muscle cells. Starting from freshly isolated bovine skeletal muscle satellite cells and subcutaneous preadipocytes, cells were separately cultured and differentiated into myoblasts and adipocytes, respectively. Cells were then indirectly co-cultured, seeding adipocytes on an insert and myoblasts on a multi-well plate. Significant differences were found comparing the co-cultured myoblasts with the control monoculture of myoblasts. The co-cultured cells reported a higher expression of both C/EBP beta and PPARγ, both involved in the activation of adipogenesis, as observed by Jin et al. [57]. Choi et al. also observed some differences between adipocytes in the co-culture and the ones used as monoculture controls. Indeed, it showed that the level of G-protein-coupled receptor [48] (GPR43) in the co-cultured group was notably increased [41]. GPR43 is a cell surface receptor widely expressed in adipocytes that regulate metabolic processes and homeostasis [59]. It is responsible for the activation of AMP-activated protein kinase alpha (AMPK alpha), which blocks energy-consuming processes, such as fatty acid biosynthesis, to promote those that produce energy, such as fatty acid oxidation [59,60]. It could be stated that adipocytes co-cultured with myoblasts may lead to ineffective lipid synthesis and increased oxidation [41].
The effects of mature adipocytes co-cultured with satellite cells were also assessed in poultry by Guo et al. [42]. Muscle satellite cells and adipocytes were isolated from chicken Pectoralis major muscle. An indirect co-culture using a Transwell insert was set up, with adipocytes seeded on the insert and muscle satellite cells on the lower wells. Analyses revealed that satellite cells in the co-culture showed downregulation in myosin heavy chains (MHC) expression and an accumulation of lipid depots when compared with the control. Moreover, consistent with the reduction in MHC, their expression of myogenin, MyoD, and paired box protein 7 (PAX7), main factors in the myogenic pathway [46,61,62,63], was also reduced [42]. On the other hand, the notable deposition of lipid drops in the co-cultured satellite cells could be explained by the assessed over-expression of genes involved in the PPAR-gamma signaling pathway involved in late adipogenesis [38,56].
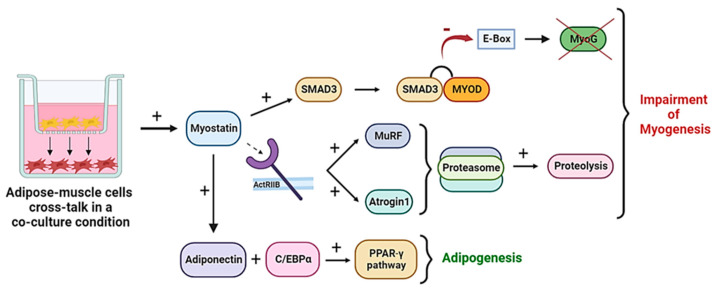
In light of these studies, the main effects exerted by adipose cells on muscle cells in a co-culture condition are myogenesis impairment and adipogenesis triggering, mainly due to the overexpression of myostatin, as shown in Figure 1. In summary, myostatin indirectly activates Smad 3 which binds MyoD. This way, MyoD cannot bind E-Box CAXXTG, resulting in a lack of activation of myogenin with consequent impairment of myogenesis. At the same time, the produced myostatin binds the cellular receptor ActRIIB, leading to the production of Atrogin1 and MurF-1, key elements of the proteasome machinery. The consequently triggered proteolysis takes part in myogenesis inhibition by degrading essential muscle proteins. Moreover, the increased level of myostatin is associated with increased adiponectin and C/EBP α, which are factors of the (PPARγ) pathway, which leads to the activation of adipogenic processes. The final effect of this cross talk is an impairment of myogenesis and a triggered adipogenesis.
Figure 1.
The effects of the cross talk between adipose and muscle cells. In an adipose-muscle cells co-culture, myostatin increases and activates Smad3, which blocks the activity of MyoD and the activation of myogenin (MyoG). Simultaneously, myostatin activates Atrogin1 and MurF-1, triggering proteolysis of muscle proteins. Moreover, adiponectin and C/EBP α, which are important factors of the (PPARγ) pathway, get activated and start adipogenic processes. The final effect of this cross talk is the impairment of myogenesis and a triggered adipogenesis.
The downregulation of myogenesis and muscular development was also observed in in vivo conditions, both in the physiological event of aging and under some pathological conditions, such as sarcopenia and obesity [64]. Though such cross talk between adipose and muscular tissue is still to be clarified, it is known that aging and pathologies lead to an inflammatory status for the tissues that, in response, become dysfunctional [65]. This results in altered production of cytokines, adipokines, myokines, and adipomyokines that cross talk, interfering and leading to reciprocal effects [66].
The existence of the cross talk between adipocytes and myocytes may represent a challenge when setting up a co-culture for in vitro meat production. However, thanks to advanced biotechnology and innovative cell culture strategies, research has moved towards effective solutions, such as the application of 3D scaffolds that can improve cultural conditions giving positive outcomes for the pursuit of a more realistic in vitro meat.
3. New Approaches and Strategies for In Vitro Meat Culturing
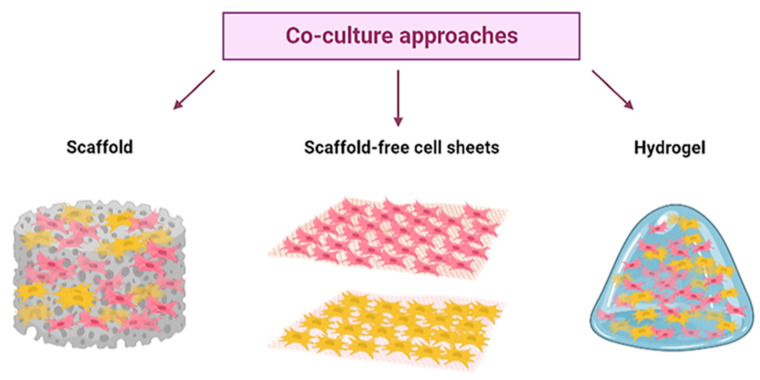
The organization of cells for cultured meat production can be achieved by either a scaffolding method or a cell assembly method. As shown in Figure 2, the most recent approaches to 3D meat culture rely on using three different structures: scaffolds, scaffold-free cellular sheets, and hydrogels, as reviewed by Singh et al. [67].
Figure 2.
The most recent three-dimensional co-culture approaches to meat agriculture.
Briefly, a scaffold is a 3D support, generally realized with biomaterials, able to promote cell adhesion, proliferation, and development in a tissue-like structure [68]. To be considered effective, scaffolds should be characterized by properties such as a wide surface and good porosity to promote cell adhesion, proliferation, and differentiation [69] and exhibit a biocompatible and noncytotoxic behavior to guarantee cell survival [70]. Scaffolds are widely used in tissue engineering and regenerative medicine [71], and their application is now finding its way into the field of cultured meat [72,73]. An ideal scaffold for in vitro meat production must be edible and nutritious and show mechanical properties in line with the desired texture for the final products to provide the optimal three-dimensional framework for obtaining reliable and realistic in vitro meat [67,68].
A scaffold-free cell sheet is an approach in which cells are cultured as sheets where the produced extracellular matrix keeps cells aggregated, forming a sort of veil. The cell sheets can be moved and stacked, forming 3D tissue-like structures useful for tissue engineering and meat culturing [74].
Finally, a hydrogel is a three-dimensional support for cell culture, characterized by a high water content, particularly appreciated for its resemblance to the extracellular matrix and for its ability to encourage cell adhesion [75]. A hydrogel shows tissue-like elasticity thanks to its hydration level; moreover, its structure and composition can be modified according to the desired chemical, physical, and biological characteristics [76]. For these reasons, hydrogels have found several practical applications in tissue engineering and are now employed in the meat culturing field. Table 2 schematically summarizes the relevant literature.
Table 2.
The most recent and innovative approaches to in vitro meat production.
| Support | Author | Species | Cell Types | Approach | Doi | Year |
|---|---|---|---|---|---|---|
| Scaffold - Edible scaffold |
Thyden et al. [77] | Bovine | Satellite cells | Decellularized broccoli floret + Rotating bioreactor | 10.3390/app12105155 | 2022 |
| Song et al. [78] | Pig | Adipose Mesenchymal Stromal Cells | Peanut Wire-drawing Protein scaffold | 10.1016/j.foodres.2022.111636 | 2022 | |
| Xiang et al. [79] | Mouse | C2C12 | Wheat gluten scaffold | 10.1016/j.biomaterials.2022.121543 | 2022 | |
| Bovine | Satellite cells | |||||
| Scaffold-free cell sheets | Shahin-Shamsabadi et al. [17] | Mouse | 3T3-L1 | ECM-free bio-fabricated cellular sheets | 10.1159/000511764 | 2022 |
| C2C12 | ||||||
| Tanaka et al. [80] | Bovine | Myoblasts | Scaffold-free cell-based sheets | 10.1038/s41538-022-00155-1 | 2022 | |
| Hydrogel sheets | Li et al. [81] | Mouse | 3T3-L1 | Soy milk gelatin sheets | 10.3389/fbioe.2022.875069 | 2022 |
| C2C12 | ||||||
| Fibrillar Hydrogel | Kang et al. [16] | Bovine | Adipose Mesenchymal Stromal Cells | Bath-assisted 3D printing + tendon gel integrated bioprinting | 10.1038/s41467-021-25236-9 | 2021 |
| Satellite cells | ||||||
| Hydrogel beads | Zagury et al. [82] | Bovine | Adipose Mesenchymal Stromal Cells | Alginate spherical Hydrogel | 10.1038/s42003-022-03852-5 | 2022 |
| Satellite cells | Alginate spherical Hydrogel | |||||
| Adipose Mesenchymal Stromal Cells | Alginate spherical Hydrogel | |||||
| Satellite cells | 3D-printed support | |||||
| Gelatin microcarrier | Liu et al. [15] | Mouse | 3T3-L1 | Microcarrier + spinner flasks Bioreactor + Mold | 10.1016/j.biomaterials.2022.121615 | 2022 |
| C2C12 | ||||||
| Pig | Satellite cells |
3.1. Scaffold: Edible Scaffolds
Scaffolds can be realized following several different approaches according to the desired architecture of the final product. Focusing specifically on meat culture, plant-based scaffolds have drawn attention as a possible edible solution for in vitro meat manufacturing [67]. There are several advantages to using vegetable scaffolds; they are biodegradable, cheap, and nutritious and provide a favorable environment for cell adhesion and growth [67]. Their application in cultured meat has already been explored; for example, Thyden et al. produced a scaffold using decellularized broccoli florets on which primary bovine satellite cells were seeded and successfully cultured in a suspension-style bioreactor [77].
Song et al. investigated the possibility of expanding porcine adipose-derived mesenchymal stromal cells (ADSCs) by inoculating them on peanut wire-drawing protein (PWP) scaffolds. They obtained differentiated mature fat, engineered tissue with an improved composition in terms of volatile products compared with the control scaffold-free culture [78].
In addition, wheat gluten was applied in manufacturing 3D scaffolds for cultured meat [79] because it lends itself to producing nontoxic scaffolds with different pore sizes and densities. Xiang et al. investigated the potential applicability of this biomaterial in the production of scaffolds for muscle cell culturing. By adjusting the pore size and the mechanical properties known to affect cell adhesion and proliferation, the authors obtained a wheat gluten scaffold to promote bovine satellite cell proliferation and migration inside the 3D structure [79].
Though the application of scaffolds in cultured meat is promising, to the best of our knowledge, no co-culture of adipose and muscle cells was performed using this kind of support. It is reasonable to state that a cross talk between these two cell populations results in practical issues when realizing a co-culture using scaffolds. Further improvements are required to bypass the inhibition exerted by adipose cells on muscle cell development and make the application of scaffolds, particularly the edible ones, feasible in meat agriculture.
3.2. Scaffold-Free Cell Sheets
In 2022, Shahin-Shamsabadi et al. [17] proposed, for the first time, an interesting approach involving the production of layered bio-fabricated cellular sheets. The authors used murine cells as a proof-of-concept model, specifying that this blueprint represents only the preliminary assessment for future farm animal cell development. Shahin-Shamsabadi’s technique was based on producing cellular sheets of muscle and adipose cells starting from partially differentiated murine 3T3-L1 adipocytes and murine C2C12 myoblasts that completed their differentiations co-cultured together in a bi-dimensional condition. The obtained sheets were removed through a delamination process promoted by consequent pH variation and were finally overlapped, forming a meat-like structure [17]. The main advantage of this approach was the lack of a scaffold since the sheets were made stable and robust by the extracellular matrix (ECM) produced by cells. As highlighted by the authors, this approach needs further realization, using, for instance, bioreactors and automated handling to make it suitable for large-scale in vitro meat production [17]. Although some improvements are needed, this sheet model represents a promising opportunity for meat culturing. Indeed, this approach has already been adapted and used for in vitro meat-like structures, using not only murine cells but also bovine ones [79,80]. To our knowledge, the only application of farm animal cells using this strategy was performed by Tanaka et al. [80], who applied the monoculture of freshly isolated bovine myocytes on a scaffold-free cell sheet. The authors used temperature-responsive culture dishes (TRCDs) to obtain cell sheets, and these cultural plates were characterized by a poly(N-isopropylacrylamide) coating, a thermo-responsive polymer, hydrophilic below 32 °C and hydrophobic at 37 °C [82,83,84]. At 37 °C, cells were attached and proliferated during culturing. Then, by lowering the temperature below 32 °C and using ultrasonic washing, the cell sheet detached without degenerating, thanks to the produced ECM. The layers were then layered and cultured to guarantee their attachment and staking. The final result was a meat-like structure composed of piled cellular sheets [80]. Though the authors did not perform a co-culture, their work represents the first employment of large animal cells to the scaffold-free cell sheet approach. Taken together, the works of Tanaka et al. and Shahin-Shamsabadi et al. represent a solid base for applying the cell sheet technique to meat cultured using two different cell types.
3.3. Hydrogel
3.3.1. Hydrogel Sheets
Unlike Shahin-Shamsabadi et al., who used no support [17], Li et al. fabricated soy milk gelatin sheets as a culturing matrix. Exploiting the staking-sheets approach, they demonstrated the possibility of generating a solid meat-like structure by consecutively piling three muscle–adipose layers and two adipose layers [81]. The choice of a support made of gelatin and soy milk was made according to the advantages these materials can guarantee. On the one hand, gelatin is rich in integrin-binding sites that promote cell adhesion and migration and favors cell settlement in the support [71,85]. On the other hand, soymilk was chosen for its bioactive isoflavones content that can promote myogenesis by activating myoblast determination protein 1 (MyoD) expression via protein kinase B (Akt or PKB) and p38 pathway [86], and bioactive compounds, such as 6-hydroxydaidzein and protein peptic hydrolysate, that stimulate adipogenesis by increasing the expression of PPAR gamma gene and so promoting lipid accumulation [87,88,89]
3.3.2. Fibrillar Hydrogel
Kang et al. implemented an innovative system based on the assembly of 3D-printed muscle, fat, and endothelial gelatin fibers [16]. Muscle satellite cells and adipose-derived stem cells were isolated from bovine masseter samples. After culturing, their respective differentiation into muscle and adipose and endothelial cells was performed in a 3D bio-printed fibrillar hydrogel of edible gelatin or gellan gum using a supporting bath-assisted 3D printer (SBP) [16]. Moreover, to avoid the collapsing of the fibers into a globular structure, Kang et al. introduced tendon gels to guarantee the linearity of cell fibers during culturing; they named this approach tendon-gel integrated bioprinting (TIP). Once cells were appropriately differentiated, the fibers were physically assembled into a meat-like structure, which was made even more resistant thanks to the addition of transglutaminase, an edible enzyme used as a cross-linker in the food industry [16].
3.3.3. Hydrogel Beads
Zagury et al. [82] also obtained a meat-like structure by engineering bovine adipose tissue; mesenchymal stromal cells were obtained from bovine fat tissue and cultured in alginate hydrogel beads for differentiation. Once the differentiation was reached, integrating adipose cells with muscle cells was performed following two different approaches [82]. On the one hand, differentiated adipose alginate beads were cut and bound through chelation with muscle cells differentiated on freeze-dried alginate scaffolds obtained as previously described by Ben-Arye et al. [73]. On the other hand, adipose cells were extracted from the alginate beads by dissolving the hydrogel and then loading through chelation on alginate-pea protein 3D-printed fibers, on which bovine satellite cells had previously been cultured and differentiated, according to the technique previously developed by Ianovici et al. [90]. Zagury et al. demonstrated the potentiality of chelation as a way to obtain a solid 3D meat-like structure [82]. Moreover, the authors evaluated the dimensions of the final construct and observed better cell maturation when using alginate. This result represents an interesting strategy for extensive cell expansion prior to differentiation to yield a high volume of cellular material starting from limited tissue samples [82].
3.3.4. Gelatin Microcarrier
Finally, among the newest approaches scientists are attempting in order to obtain in vitro meat, the edible 3D porous gelatin microcarrier (PoGelat-MCs) by Liu et al. [15] must be cited. Microcarriers are mini 3D scaffolds endowed with a high surface-to-volume ratio that can be modified for culturing meat purposes to become biodegradable, edible, cell-adhesive, and able to provide a large cultured surface [91], as in the case of the developed PoGelat microcarrier [15]. Freshly isolated pig skeletal muscle progenitor cells and murine 3T3L1 preadipocytes were separately differentiated in flasks; the obtained muscle and adipose cells were seeded and cultured on PoGelat microcarriers into the spinner–flask bioreactor to obtain muscle and adipose microtissues. Once gained, they were assembled in 3D-printed spherical molds where the addition of transglutaminase supported their adhesion into a meatball-like product [15]. Since this only represented a trial, further developments using large animal adipose cells are required to obtain a consumer-acceptable product.
Scaffold-free cell sheets and hydrogels are promising approaches to manufacturing in vitro meat; however, the limitations of such techniques stem from the fact that the co-culture could begin only when the two differentiation processes have already been carried out separately. Indeed, the two cultures are combined and cultured together only at the end of the process to guarantee a successful formation of a 3D meat-like structure thanks to the formation of ECM.
4. Conclusions
In vitro meat could represent an ethical and sustainable solution to the increasing demand for meat products. Different attempts to reproduce meat tenderness and juiciness in vitro have been performed by co-culturing those cell types that are typical of muscles. For this purpose, muscle and adipose cell co-culturing strategies have been followed, leading to preliminary studies on the cross talk between cell populations. It has been demonstrated that the main effects of muscle–adipose cell cross talk are the impairment of myogenesis and the triggering of adipogenic processes, which inevitably lead to practical difficulties in performing a successful co-culture. For this reason, it is essential to consider the molecular mechanisms at the base of such cross talk to appoint a co-culture strategy that overcomes myogenesis inhibition. It has been observed that the effects of adipose–muscle cross talk can be successfully overtaken when co-culturing already differentiated cells: it is likely that the strong influence exerted by adipose cells on muscle ones tends to increase or weaken according to the cell differentiation stage. Thus, the most successful approaches in in vitro meat production have involved the use of already differentiated cells and 3D supports in co-cultures, paving the way toward a more realistic product. Edible scaffolds are promising solutions since they are economical, natural, non-impactful, nutritious, and safe. However, to our knowledge, they have been applied only in monoculture, and further developments are likely required to make them functional to host a co-culture. On the other hand, scaffold-free cell sheets and hydrogels were successfully employed in co-culture strategies and could thus represent an optimal solution for meat culture. Moreover, employing large-animal-derived cells for such support makes obtaining reliable in vitro meat even more exciting and successful. Thus far, the results are promising, but further developments and improvements are still required to obtain a well-standardized protocol applicable to large-scale production. Moreover, some challenges still need to be overcome to improve cultured meat manufacturing; above all, there is a necessity to identify the most suitable species and the most appropriate age as optimal sources for cell sorting, since these can be crucial variables that are able to affect the outcome of the process.
Acknowledgments
A.F. thanks “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods” funded by the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.3—D.D. 341 15/03/2022, PE00000003).
Author Contributions
Conceptualization, A.D.G., S.C.M. and M.P.; methodology, M.P., A.F. and G.M.; writing—original draft preparation, A.D.G., S.C.M., L.A., A.F., M.P., G.M. and P.S.; writing—review and editing, A.D.G., S.C.M., M.P., A.F., L.A., G.M. and P.S.; supervision, A.D.G., S.C.M. and L.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Luis A.D., Hayman D.T.S., O’shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R.C., Mills J.N., Timonin M.E., Willis C.K.R., Cunningham A.A., et al. The Epic of In Vitro Meat Production—A Fiction into Reality. Foods. 2021;10:1395. doi: 10.3390/FOODS10061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton T., Bieg C., Harwatt H., Pudassaini R., Wellesley L. Food System Impacts on Biodiversity Loss Three Levers for Food. Chatham House; London, UK: 2021. [Google Scholar]
- 3.Bar-On Y.M., Phillips R., Milo R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FAO . FAO Publications Catalogue 2022. FAO; Rome, Italy: 2023. [DOI] [Google Scholar]
- 5.International Energy Agency Global Energy Review: CO2 Emissions in 2021. Global Emissions Rebound Sharply to Highest Ever Level. 2021. [(accessed on 13 March 2023)]. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2.
- 6.Mateo-Sagasta J., Zadeh S.M., Turral H. Water Pollution from Agriculture: A Global Review. Executive Summary. FAO; Rome, Italy: International Water Management Institute (IWMI); Colombo, Sri Lanka: 2017. [Google Scholar]
- 7.Takhar M. In Vitro Meat: An Ethical Solution to an Unsustainable Practice. UC Merced Undergrad. Res. J. 2018:10. doi: 10.5070/M4102038933. [DOI] [Google Scholar]
- 8.Chodkowska K.A., Wódz K., Wojciechowski J. Sustainable Future Protein Foods: The Challenges and the Future of Cultivated Meat. Foods. 2022;11:4008. doi: 10.3390/foods11244008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross R. The Smooth Muscle Cell. J. Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha A. First lab-grown hamburger gets full marks for ‘mouth feel’. The Guardian. Aug 6, 2013.
- 11.Handral H.K., Tay S.H., Chan W.W., Choudhury D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2022;62:272–281. doi: 10.1080/10408398.2020.1815172. [DOI] [PubMed] [Google Scholar]
- 12.Pandurangan M., Kim D.H. A novel approach for in vitro meat production. Appl. Microbiol. Biotechnol. 2015;99:5391–5395. doi: 10.1007/s00253-015-6671-5. [DOI] [PubMed] [Google Scholar]
- 13.Bomkamp C., Skaalure S.C., Fernando G.F., Ben-Arye T., Swartz E.W., Specht E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022;9:2102908. doi: 10.1002/advs.202102908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domeneghini C., Di Giancamillo A., Corino C. Conjugated linoleic acids (CLAs) and white adipose tissue: How both in vitro and in vivo studies tell the story of a relationship. Histol. Histopathol. 2006;21:663–672. doi: 10.14670/HH-21.663. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Wang R., Ding S., Deng L., Zhang Y., Li J., Shi Z., Wu Z., Liang K., Yan X., et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials. 2022;287:121615. doi: 10.1016/j.biomaterials.2022.121615. [DOI] [PubMed] [Google Scholar]
- 16.Kang D.-H., Louis F., Liu H., Shimoda H., Nishiyama Y., Nozawa H., Kakitani M., Takagi D., Kasa D., Nagamori E., et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021;12:5059. doi: 10.1038/s41467-021-25236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahin-Shamsabadi A., Selvaganapathy P.R. Engineering Murine Adipocytes and Skeletal Muscle Cells in Meat-like Constructs Using Self-Assembled Layer-by-Layer Biofabrication: A Platform for Development of Cultivated Meat. Cells Tissues Organs. 2022;211:304–312. doi: 10.1159/000511764. [DOI] [PubMed] [Google Scholar]
- 18.Weaver A.D., Kauffman R.G., Greaser M.L., Guo W. Part II Meat Science. In: Hui Y.H., editor. Handbook of Meat and Meat Processing. CRC Press; Boca Raton, FL, USA: 2012. [Google Scholar]
- 19.Warner R. Meat: Conversion of Muscle into Meat. Encycl. Food Health. 2015:677–684. doi: 10.1016/B978-0-12-384947-2.00452-9. [DOI] [Google Scholar]
- 20.Purslow P.P. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front. Physiol. 2020;11:495. doi: 10.3389/fphys.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weston A.R., Rogers R.W., Althen T.G. The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002;18:107–111. doi: 10.15232/S1080-7446(15)31497-2. [DOI] [Google Scholar]
- 22.Listrat A., Gagaoua M., Normand J., Gruffat D., Andueza D., Mairesse G., Mourot B.P., Chesneau G., Gobert C., Picard B. Contribution of connective tissue components, muscle fibres and marbling to beef tenderness variability in longissimus thoracis, rectus abdominis, semimembranosus and semitendinosus muscles. J. Sci. Food Agric. 2020;100:2502–2511. doi: 10.1002/jsfa.10275. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe G., Motoyama M., Nakajima I., Sasaki K. Relationship between water-holding capacity and intramuscular fat content in Japanese commercial pork loin. Asian-Australas. J. Anim. Sci. 2018;31:914. doi: 10.5713/ajas.17.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council (US) Committee on Technological Options to Improve the Nutritional Attributes of Animal Products The Role of Fat in the Palatability of Beef, Pork, and Lamb. [(accessed on 2 January 2023)];1988 Available online: https://www.ncbi.nlm.nih.gov/books/NBK218173/
- 25.Maltin C.A., Warkup C.C., Matthews K.R., Grant C.M., Porter A.D., Delday M.I. Pig muscle fibre characteristics as a source of variation in eating quality. Meat Sci. 1997;47:237–248. doi: 10.1016/S0309-1740(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuppusamy P., Kim D., Soundharrajan I., Hwang I., Choi K.C. Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology. 2021;10:6. doi: 10.3390/biology10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goers L., Freemont P., Polizzi K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface. 2014;11:20140065. doi: 10.1098/rsif.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin W.S., Hemmi C., Toyo-oka T. Co-culture and crosstalk between endothelial cells and vascular smooth muscle cells mediated by intracellular calcium. Methods Mol. Biol. 2002;188:347–357. doi: 10.1385/1-59259-185-X:347. [DOI] [PubMed] [Google Scholar]
- 29.Renaud J., Martinoli M.-G. Development of an Insert Co-culture System of Two Cellular Types in the Absence of Cell-Cell Contact. J. Vis. Exp. 2016;113:e54356. doi: 10.3791/54356. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov D.P., Parker T.L., Walker D.A., Alexander C., Ashford M.B., Gellert P.R., Garnett M.C. In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J. Biotechnol. 2015;205:3–13. doi: 10.1016/j.jbiotec.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Dohle E., Scherrieble A., Doser M., Al-Maawi S., Hoss M., Dauner M., Sader R., Kirkpatrick C.J., Ghanaati S. Co-culture Model for Cutaneous Wound Healing to Assess a Porous Fiber-Based Drug Delivery System. Tissue Eng. Part C Methods. 2020;26:475–484. doi: 10.1089/ten.tec.2020.0145. [DOI] [PubMed] [Google Scholar]
- 32.Lonergan S.M., Topel D.G., Marple D.N. The Science of Animal Growth and Meat Technology. Elsevier; Amsterdam, The Netherlands: 2019. Fat and fat cells in domestic animals; pp. 51–69. [Google Scholar]
- 33.Pedersen B.K. Muscle-to-fat interaction: A two-way street? J. Physiol. 2010;588:21. doi: 10.1113/jphysiol.2009.184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamboni M., Mazzali G., Brunelli A., Saatchi T., Urbani S., Giani A., Rossi A.P., Zoico E., Fantin F. The Role of Crosstalk between Adipose Cells and Myocytes in the Pathogenesis of Sarcopenic Obesity in the Elderly. Cells. 2022;11:3361. doi: 10.3390/cells11213361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Seo K., Suzuki T., Kobayashi K., Nishimura T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 2019;90:423–434. doi: 10.1111/asj.13145. [DOI] [PubMed] [Google Scholar]
- 37.Yamanouchi K., Nakamura K., Takeuchi S., Hosoyama T., Matsuwaki T., Nishihara M. Suppression of MyoD induces spontaneous adipogenesis in skeletal muscle progenitor cell culture. Anim. Sci. J. 2021;92:e13573. doi: 10.1111/asj.13573. [DOI] [PubMed] [Google Scholar]
- 38.Deng B., Zhang F., Wen J., Ye S., Wang L., Yang Y., Gong P., Jiang S. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. 2017;14:29. doi: 10.1186/s12986-017-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artaza J.N., Bhasin S., Magee T.R., Reisz-Porszasz S., Shen R., Groome N.P., Fareez M.M., Gonzalez-Cadavid N.F. Myostatin Inhibits Myogenesis and Promotes Adipogenesis in C3H 10T(1/2) Mesenchymal Multipotent Cells. Endocrinology. 2005;146:3547–3557. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 40.Takegahara Y., Yamanouchi K., Nakamura K., Nakano S.-I., Nishihara M. Myotube formation is affected by adipogenic lineage cells in a cell-to-cell contact-independent manner. Exp. Cell Res. 2014;324:105–114. doi: 10.1016/j.yexcr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Choi S.H., Chung K.Y., Johnson B.J., Go G.W., Kim K.H., Choi C.W., Smith S.B. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARγ and C/EBPβ gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J. Nutr. Biochem. 2013;24:539–543. doi: 10.1016/j.jnutbio.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Guo L., Cui H., Zhao G., Liu R., Li Q., Zheng M., Guo Y., Wen J. Intramuscular preadipocytes impede differentiation and promote lipid deposition of muscle satellite cells in chickens. BMC Genom. 2018;19:838. doi: 10.1186/s12864-018-5209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGeady T.A., Quinn P.J., Fitzpatrick E.S., Ryan M.T., Kilroy D., Lonergan P. Veterinary Embriology. 2nd ed. John Wiley & Sons; Hoboken, NJ, USA: 2017. [Google Scholar]
- 44.Essentials of Domestic Animal Embryology—9780702028991|Elsevier Health. [(accessed on 7 January 2023)]. Available online: https://www.eu.elsevierhealth.com/essentials-of-domestic-animal-embryology-9780702028991.html?gclid=Cj0KCQiAzeSdBhC4ARIsACj36uHEBb9v8cha9RIh4CudIiwb0zUYJUVi-bzz5fL-arMqFJSlTRBpZfEaAlpzEALw_wcB&gclsrc=aw.ds.
- 45.Sabourin L.A., Rudnicki M.A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 46.Zammit P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Asfour H.A., Allouh M.Z., Said R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018;243:118–128. doi: 10.1177/1535370217749494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D., Black B.L., Derynck R. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapscott S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 50.Wang D.-T., Yang Y.-J., Huang R.-H., Zhang Z.-H., Lin X. Myostatin Activates the Ubiquitin-Proteasome and Autophagy-Lysosome Systems Contributing to Muscle Wasting in Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2015;2015:684965. doi: 10.1155/2015/684965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gumucio J.P., Mendias C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomes M.D., Lecker S.H., Jagoe R.T., Navon A., Goldberg A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heras G., Namuduri A.V., Traini L., Shevchenko G., Falk A., Bergström Lind S., Jia M., Tian G., Gastaldello S. Muscle RING-finger protein-1 (MuRF1) functions and cellular localization are regulated by SUMO1 post-translational modification. J. Mol. Cell Biol. 2019;11:356–370. doi: 10.1093/jmcb/mjy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin L., Li N., Jia W., Wang N., Liang M., Yang X., Du G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021;172:105807. doi: 10.1016/j.phrs.2021.105807. [DOI] [PubMed] [Google Scholar]
- 55.Mitch W.E., Price S.R. Mechanisms activating proteolysis to cause muscle atrophy in catabolic conditions. J. Ren. Nutr. 2003;13:149–152. doi: 10.1053/jren.2003.50019. [DOI] [PubMed] [Google Scholar]
- 56.Pelosi M., De Rossi M., Barberi L., Musarò A. IL-6 Impairs Myogenic Differentiation by Downmodulation of p90RSK/eEF2 and mTOR/p70S6K Axes, without Affecting AKT Activity. BioMed Res. Int. 2014;2014:206026. doi: 10.1155/2014/206026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Q., Zhang F., Yan T., Liu Z., Wang C., Ge X., Zhai Q. C/EBPα regulates SIRT1 expression during adipogenesis. Cell Res. 2010;20:470–479. doi: 10.1038/cr.2010.24. [DOI] [PubMed] [Google Scholar]
- 58.Yang W., Yang C., Luo J., Wei Y., Wang W., Zhong Y. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol. Med. Rep. 2017;17:428–435. doi: 10.3892/mmr.2017.7881. [DOI] [PubMed] [Google Scholar]
- 59.Nakajima A., Nakatani A., Hasegawa S., Irie J., Ozawa K., Tsujimoto G., Suganami T., Itoh H., Kimura I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE. 2017;12:e0179696. doi: 10.1371/journal.pone.0179696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardie D.G. Balancing Cellular Energy. Science. 2007;315:1671–1672. doi: 10.1126/science.1140737. [DOI] [PubMed] [Google Scholar]
- 61.Daval M., Foufelle F., Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sodhi S.S. Differential Expression Profiling of Myogenic Regulatory Factor Genes in Postnatal Longissimus dorsi Muscle of Indigenous and Large White Yorkshire Breeds of Pigs. J. Anim. Res. 2021;11:11–17. doi: 10.30954/2277-940X.01.2021.2. [DOI] [Google Scholar]
- 63.Ropka-Molik K., Eckert R., Piórkowska K. The expression pattern of myogenic regulatory factors MyoD, Myf6 and Pax7 in postnatal porcine skeletal muscles. Gene Expr. Patterns. 2011;11:79–83. doi: 10.1016/j.gep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Addison O., Drummond M.J., Lastayo P.C., Dibble L.E., Wende A.R., McClain D.A., Marcus R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging. 2014;18:532–538. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Leary M.F., Wallace G.R., Davis E.T., Murphy D.P., Nicholson T., Bennett A.J., Tsintzas K., Jones S.W. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin-mediated activation of NFκB. Sci. Rep. 2018;8:15360. doi: 10.1038/s41598-018-33840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahemi H., Nigam N., Wakeling J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface. 2015;12:20150365. doi: 10.1098/rsif.2015.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh A., Kumar V., Singh S.K., Gupta J., Kumar M., Sarma D.K., Verma V. Recent advances in bioengineered scaffold for in vitro meat production. Cell Tissue Res. 2022;391:235–247. doi: 10.1007/s00441-022-03718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Díaz Lantada A., Curras D., Mousa J., Hengsbach S. Studies in Mechanobiology, Tissue Engineering and Biomaterials. Volume 18. Springer; Cham, Switzerland: 2016. Tissue engineering scaffolds for 3D cell culture; pp. 249–268. [DOI] [Google Scholar]
- 69.Ghasemi-Mobarakeh L. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells. 2015;7:728. doi: 10.4252/wjsc.v7.i4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomillion C.T., Burg K.J.L. Adipose Tissue Engineering. Compr. Biomater. 2011;5:529–539. doi: 10.1016/B978-0-08-055294-1.00189-6. [DOI] [Google Scholar]
- 71.Chan B.P., Leong K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008;17:467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seah J.S.H., Singh S., Tan L.P., Choudhury D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022;42:311–323. doi: 10.1080/07388551.2021.1931803. [DOI] [PubMed] [Google Scholar]
- 73.Ben-Arye T., Shandalov Y., Ben-Shaul S., Landau S., Zagury Y., Ianovici I., Lavon N., Levenberg S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food. 2020;1:210–220. doi: 10.1038/s43016-020-0046-5. [DOI] [Google Scholar]
- 74.Chen G., Qi Y., Niu L., Di T., Zhong J., Fang T., Yan W. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015;3:749–757. doi: 10.3892/br.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cushing M.C., Anseth K.S. Hydrogel Cell Cultures. Science. 2007;316:1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 77.Thyden R., Perreault L.R., Jones J.D., Notman H., Varieur B.M., Patmanidis A.A., Dominko T., Gaudette G.R. An Edible, Decellularized Plant Derived Cell Carrier for Lab Grown Meat. Appl. Sci. 2022;12:5155. doi: 10.3390/app12105155. [DOI] [Google Scholar]
- 78.Song W.J., Liu P.P., Zheng Y.Y., Meng Z.Q., Zhu H.Z., Tang C.B., Li H.X., Ding S.J., Zhou G.H. Production of cultured fat with peanut wire-drawing protein scaffold and quality evaluation based on texture and volatile compounds analysis. Food Res. Int. 2022;160:111636. doi: 10.1016/j.foodres.2022.111636. [DOI] [PubMed] [Google Scholar]
- 79.Xiang N., Yuen J.S.K., Stout A.J., Rubio N.R., Chen Y., Kaplan D.L. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials. 2022;285:121543. doi: 10.1016/j.biomaterials.2022.121543. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka R., Sakaguchi K., Yoshida A., Takahashi H., Haraguchi Y., Shimizu T. Production of scaffold-free cell-based meat using cell sheet technology. npj Sci. Food. 2022;6:41. doi: 10.1038/s41538-022-00155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C.-H., Yang I.-H., Ke C.-J., Chi C.-Y., Matahum J., Kuan C.-Y., Celikkin N., Swieszkowski W., Lin F.-H. The Production of Fat-Containing Cultured Meat by Stacking Aligned Muscle Layers and Adipose Layers Formed From Gelatin-Soymilk Scaffold. Front. Bioeng. Biotechnol. 2022;10:875069. doi: 10.3389/fbioe.2022.875069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zagury Y., Ianovici I., Landau S., Lavon N., Levenberg S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 2022;5:927. doi: 10.1038/s42003-022-03852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamada N., Okano T., Sakai H., Karikusa F., Sawasaki Y., Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Chem. Rapid Commun. 1990;11:571–576. doi: 10.1002/marc.1990.030111109. [DOI] [Google Scholar]
- 84.Fukumori K., Akiyama Y., Kumashiro Y., Kobayashi J., Yamato M., Sakai K., Okano T. Characterization of Ultra-Thin Temperature-Responsive Polymer Layer and Its Polymer Thickness Dependency on Cell Attachment/Detachment Properties. Macromol. Biosci. 2010;10:1117–1129. doi: 10.1002/mabi.201000043. [DOI] [PubMed] [Google Scholar]
- 85.Shi J., Chen S., Wang L., Zhang X., Gao J., Jiang L., Tang D., Zhang L., Midgley A., Kong D., et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019;107:2040–2049. doi: 10.1002/jbm.b.34295. [DOI] [PubMed] [Google Scholar]
- 86.Lee S.-J., Vuong T.A., Go G.-Y., Song Y.-J., Lee S., Lee S.-Y., Kim S.-W., Lee J., Kim Y.K., Seo D.-W., et al. An isoflavone compound daidzein elicits myoblast differentiation and myotube growth. J. Funct. Foods. 2017;38:438–446. doi: 10.1016/j.jff.2017.09.016. [DOI] [Google Scholar]
- 87.Chien K.B., Shah R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012;8:694–703. doi: 10.1016/j.actbio.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 88.Chien K.B., Aguado B.A., Bryce P.J., Shah R.N. In vivo acute and humoral response to three-dimensional porous soy protein scaffolds. Acta Biomater. 2013;9:8983–8990. doi: 10.1016/j.actbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Goto T., Mori A., Nagaoka S. Soluble soy protein peptic hydrolysate stimulates adipocyte differentiation in 3T3-L1 cells. Mol. Nutr. Food Res. 2013;57:1435–1445. doi: 10.1002/mnfr.201200573. [DOI] [PubMed] [Google Scholar]
- 90.Ianovici I., Zagury Y., Redenski I., Lavon N., Levenberg S. 3D-printable plant protein-enriched scaffolds for cultivated meat development. Biomaterials. 2022;284:121487. doi: 10.1016/j.biomaterials.2022.121487. [DOI] [PubMed] [Google Scholar]
- 91.Bodiou V., Moutsatsou P., Post M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020;7:10. doi: 10.3389/fnut.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.