Abstract
Background: Pulmonary lung involvement is the most common extra-glandular manifestation in patients with primary Sjögren’s syndrome (pSS), leading to a worsening of the patient’s prognosis. To date, different studies have assessed the prevalence of pulmonary involvement and interstitial lung disease (ILD) in pSS patients with different results. Methods: We performed a systematic literature review and meta-analysis on ILD pooled prevalence in pSS according to the PRISMA and MOOSE guidelines. Furthermore, we explored the pooled prevalence of the two main presentations of pSS-ILD, nonspecific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP). Results: We analysed the pSS-ILD prevalence in 30 studies including 8255 pSS patients. The pSS-ILD pooled prevalence was 23% (95% CI: 16–30). For NSIP, we found a pooled prevalence of 52% (CI 41–64), and for UIP we found a pooled prevalence of 44% (CI: 32–55). Regarding the meta-regression analysis, male gender, DLco value, country, and HRCT seem to contribute to the ILD presence. Conclusions: At least 20% of pSS patients have a comorbid ILD, usually NSIP. Male gender and alteration in DLco value may be considered the most important independent factors supporting an active search of lung complications during the clinical history of pSS patients.
Keywords: Sjogren’s syndrome, pulmonary involvement, interstitial lung disease, UIP, NSIP, meta-analysis, HRCT
1. Introduction
Sjögren’s syndrome (pSS) is a systemic autoimmune disease primarily affecting the exocrine glands with lymphocytic infiltrations, leading to their loss of function and the dryness of major mucosal surfaces, and eventually involving several internal organs [1]. When present, the internal organs’ involvement dramatically influences the clinical course of the disease and the prognosis of pSS patients [2]. Pulmonary manifestations are the most prevalent extra-glandular complications, often subclinical and difficult to assess, that must be suspected when impaired respiratory function or dry cough appears [3]. Some controversial data may be found in the available literature concerning the incidence of lung involvement, its histopathologic features, and factors associated with the development of severe complications [4,5,6]. These concerns may partially explain why lung involvement in pSS patients still represents a major challenge leading to poor survival and an increase in mortality [4,5,6].
Interstitial lung disease (ILD) is the most serious pulmonary complication in pSS patients, and some reports showed that the ILD cumulative incidence in pSS was 10% 1 year after diagnosis, increasing to 20% after 5 years. ILD may be diagnosed based on clinical presentations, high-resolution computed tomography (HRCT), pulmonary function tests (PFTs) and, eventually, lung biopsy [1,5,7]. HRCT is a sensitive diagnostic tool for ILD and strongly correlates with pulmonary histology and PFTs, the most common HRCT patterns found in pSS patients being nonspecific interstitial pneumonia (NSIP), the usual interstitial pneumonia (UIP), lymphoid interstitial pneumonia, organizing pneumonia (OP), and finally bronchiolitis. A minority of patients may show an indeterminate radiologic pattern [6]. In pSS patients, different studies suggest that NSIP is the most frequent radiologic pattern observed in 41–45% of patients, followed by UIP in about 10% and OP in 4% of the patients. A combination of these patterns can be seen in up to 40% of pSS patients [7]. Many risk factors have been associated with the development of pulmonary involvement in pSS, some depending on lifestyle, others related to co-morbidities, and finally some linked to specific biologic activities of the disease, mainly related to B-cells activation, such as hypergammaglobulinemia and the presence of autoantibodies [6].
To better define the pSS-ILD pooled prevalence, to assess the HRCT’s more frequent patterns, and to identify the risk factors associated with ILD, we performed a systematic literature review (SRL) and consequently analysed all the available data deriving from many studies from the beginning of the 1980s until now.
Our results concerning pSS-ILD, deriving from all the available literature retrieved from three scientific sources, allow us to give a clearer picture of this systemic manifestation, better defining the subgroups of patients affected by ILD and, finally, filling the gaps deriving from the single studies results.
2. Materials and Methods
2.1. Protocol
This study was carried out in accordance with Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocols (PRISMA-P) statement [8]. It also complies with the guidelines of Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) [9]. The PRISMA-P and MOOSE checklists have been presented as Supplementary Tables S1 and S2, respectively.
2.2. Search Strategy
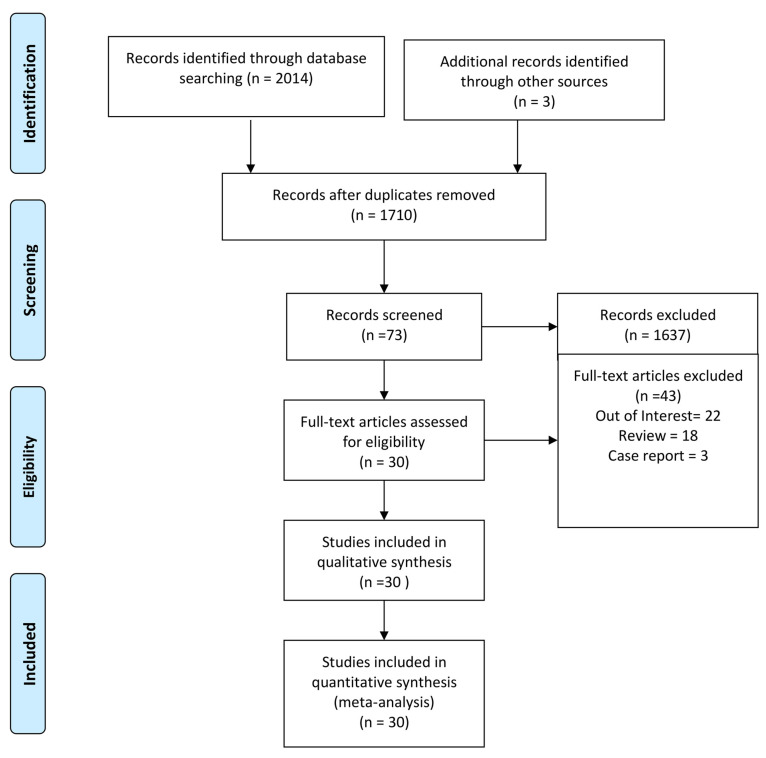
In the review, we incorporated all the peer-reviewed published papers reporting data on lung involvement in pSS patients. We included all the works conducted in pSS patients (Population) with lung involvement evaluation (Intervention and Control) that reported ILD prevalence (Outcome). No time limit on study publications was set during research. We conducted a systematic search in MedLine (via PubMed), Embase and Cochrane databases up to 7 December 2022. The main search was conducted using the string (“interstitial lung disease” OR “ILD pattern” OR “ILD” OR “pulmonary involvement” OR “progressive fibrosis” OR “pulmonary fibrosis” OR “active pulmonary involvement” OR “fibrosis” OR “lung involvement”) AND (“Sjogren’s” OR “Sjogren’s syndrome” OR “sjogren syndrome” OR “Sjogren” OR “primary sjogren’s syndrome” OR “pss” OR “sjs”). In addition, relevant keywords were used in different combinations for freehand search, and the bibliography of the selected articles was revised to improve the search strategy’s sensitivity, as shown in Figure 1.
Figure 1.
PRISMA 2009 flow diagram [8].
Excluded from this study were review papers, case studies, correspondences, concise reports, non-English language publications, and those with missing data. The list of all excluded papers after the evaluation of the full text is provided in Supplementary Table S3.
2.3. Eligibility Criteria
For the primary search, based on preliminary scouting, we included all the clinical studies reporting results regarding the ILD prevalence in pSS patients.
2.4. Data Extraction and Quality Assessment
Data from the chosen articles were extracted, collected and summarized by three independent reviewers (AM, SDA, IG), and verified by two senior reviewers (OB, LN). From each selected article, the following features have been collected: first author; year of publication; origin; study design; total number of participants; age of participants; gender; methodology used to assess ILD; serological markers (anti-nuclear antibodies (ANA), anti-Ro (SSA), anti-La (SSB), C-reactive protein (CRP)), and carbon dioxide diffusing capacity corrected for haemoglobin concentration (DLco). The mean values of CRP, SSA, SSB, age of the participants, and DLco values were also extracted, when available. When summary statistics were not fully reported, these data were calculated whenever possible [10]. Where data were missing, incomplete or inconsistent, the authors were contacted to obtain necessary information.
2.5. Assessment of Methodological Quality
The quality of the studies was assessed using an adapted Assessment Tool for Prevalence Studies [11]. This tool evaluates the risk of bias in prevalence studies. It considers ten different items, such as the representativeness and the selection of the study population, the likelihood of non-response bias, the process of data collection, the appropriateness of the definition of cases (subjects with ILD), and the measurement of the parameter of interest (prevalence of ILD). The quality of the studies included in the quantitative analysis was assessed using the “star system” of the Newcastle–Ottawa Quality Assessment Scale (NOS) [12]. The score ranges from 0 to 9 stars (Supplementary Table S4). Studies that scored ≥7 stars were considered high quality. For case series studies, we evaluated the quality using the Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group proposed by the National Heart, Lung, and Blood Institute—US Department of Health and Human Services (https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after, accessed on 1 December 2022). After scoring each item, an overall rate (good, fair, or poor) was assigned by each reviewer (Supplementary Table S5). Quality evaluation was performed independently by two reviewers (AM and IG). If there was any disagreement in the scores, a third reviewer (SDA) was involved to re-evaluate the original study.
2.6. Statistical Analysis
Analyses of data and graphs were performed using the package ‘metafor’ of the R statistical software (version 4.1.2, 2021; The R Foundation for Statistical Computing, Vienna, Austria). The pooled prevalence of ILD was estimated using a random-effects model. This model assumes that the included studies have varying effect sizes, thus providing a conservative estimate of the overall effect. The 95% confidence intervals (CIs) of the prevalence reported for each study were estimated from the proportion of cases and the specific sample size, using the binomial Clopper–Pearson exact method. Freeman–Tukey double arcsine transformation was applied to the primary study data to approximate normality. The final pooled results and 95% CIs were transformed and expressed as percentages for a simpler interpretation. An inverse variance method was used for weighting each study in the pooled estimates. We used Cochran’s Chi-square (Cochran’s Q) and I2 test to analyse the statistical heterogeneity between the results of different studies: I2 > 50% and/or p < 0.05 showed substantial heterogeneity [13]. An additional subgroup analysis was conducted, according to the diagnosis of ILD performing HRCT, to detect the possible source of the between-study heterogeneity. Sensitivity analyses were performed with the leave-one-out cross-validation test, by the sequential omission of individual studies to determine the contribution of each study to the pooled estimates, thus evaluating the stability and reliability of the results. Publication bias was explored through funnel plots [14] and the Begg adjusted rank correlation test [15]. To correct for publication bias, Duval and Tweedie’s ‘trim-and-fill’ analysis was carried out [16]. In the presence of an asymmetric funnel shape, this test detects putative missing studies to rebalance the distribution and provides an adjusted pooled estimate taking the additional studies into account, thus correcting the analysis for publication bias. Available covariates that could affect the estimates, such as publication year, study design, geographic region, and mean values of CRP, anti-SSA antibodies, anti-SSB antibodies, and DLco values of the study populations, were included in linear meta-regression models.
3. Results
3.1. Study Selection and Characteristics
Using the search strategy, 2017 peer-reviewed articles were retrieved. After the first scrutiny checking titles and abstracts, 73 articles were selected for full-text assessment. After review, 30 studies were included in the qualitative and quantitative analysis. Eleven studies had a prospective design, and among them four were conducted in Greece [17,18,19,20], three in Sweden [21,22,23], one in Spain [24], one in the Netherlands [25], two in Italy [26,27], and one in China [28]. Nineteen studies had a retrospective design, and among them ten were conducted in China [28,29,30,31,32,33,34,35,36,37], three in Turkey [38,39,40], two in France [41,42], one in Italy [26], one in the Netherlands [43], one in Japan [44], one in Spain [45], and one in Germany [46]. Many of them referred to pSS patients fulfilling the revised criteria proposed by the American–European Consensus Group [47]. In 23 studies out of the 30 included HRCT was performed to investigate lung involvement [19,20,25,26,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45,46]. The main characteristics of the selected studies are reported in Table 1. The overall quality of the selected studies is high, although studies with a control group have a lower quality (Tables S4 and S5).
Table 1.
Main characteristics of included studies.
| Study | Design | Country | n | pSS Classification Criteria |
F, % | ILD, n (%) | HRCT | NSIP, n (%) | UIP, n (%) | PFTs | DLCO (mmol/min kPa) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Constantopoulos et al., 1985 [17] |
Prospective | Greece | 36 | * | 91.7% | 9 (25%) | - | - | - | - | |
| Papathanasiou et al., 1986 [18] |
Prospective | Greece | 40 | * | 100% | 15 (37.5%) | - | - | - | yes | 91.23 ± 18.86 |
| Papiris et al., 1999 [19] |
Prospective | Greece | 61 | 1993 ECCC | 95% | 19 (31%) | yes | - | - | yes | 85 ± 17.7 |
| Cervera et al., 2000 [24] |
Prospective | Spain | 223 | 1993 ECCC | 91.5% | 19 (8.5%) | - | - | - | - | |
| Taouli et al., 2002 [41] |
Retrospective | France | 35 | 1993 ECCC | 80% | 12 (34.3%) | yes | yes | 71.9 ± 15.7 | ||
| Lin et al., 2010 [29] |
Retrospective | China | 522 | 2002 AECG | 91% | 116 (22.2%) | yes | - | - | - | - |
| Botsios et al., 2011 [27] |
Retrospective | Italy | 336 | 2002 AECG | 96% | 5 (1.5%) | yes | - | - | - | - |
| Ter Borg et al., 2014 [25] |
Prospective | Netherlands | 83 | 2002 AECG | 89% | 6 (7.2%) | yes | 4 (4.8%) | - | - | |
| Kvarnstrom et al., 2015 [21] |
Prospective | Sweden | 199 | 2002 AECG | 93% | 2 (1%) | - | - | - | - | - |
| Li et al., 2015 [30] |
Retrospective | China | 315 | 2002 AECG | 96% | 66 (21%) | yes | yes | - | ||
| Zhao et al., 2015 [31] |
Retrospective | China | 483 | 2002 AECG | 94% | 59 (12.2%) | yes | - | - | - | - |
| Manfredi et al., 2017 [26] |
Prospective | Italy | 77 | 2002 AECG | 88% | 13 (16.9%) | yes | - | 7 (9%) | yes | - |
| Ramirez Sepulveda et al., 2017 [22] |
Prospective | Sweden/Norway | 967 | 2002 AECG | 93% | 48 (5%) | - | - | - | - | - |
| Roca et al., 2017 [42] |
Retrospective | France | 263 | 2002 AECG | - | 21 (8%) | yes | 7 (33.3%) | 5 (23.8%) | yes | |
| Strevens Bolmgren et al., 2017 [23] |
Prospective | Sweden | 51 | 2002 AECG | 96% | 9 (17.6%) | - | 6 (11.7%) | 0 | 6.5 ± 1.9 | |
| Ter Borg et al., 2017 [43] |
Retrospective | Netherlands | 140 | 2002 AECG | 89% | 17 (12.1%) | yes | - | - | - | - |
| Dong et al., 2018 [32] |
Retrospective | China | 527 | 2002 AECG/2016 ACR EULAR | 88% | 206 (39%) | yes | 86 (41.7%) | 22 (10.7%) | yes | 54.54 ± 21.25 |
| Gao et al., 2018 [33] |
Retrospective | China | 853 | 2002 AECG | - | 165 (31.7%) | yes | 27 (39.1%) | 11 (15.9%) | yes | 57.5 ± 21.2 |
| Kakugawa et al., 2018 [44] |
Retrospective | Japan | 101 | 2002 AECG | 94% | 32 (1.8%) | yes | 28 (27.7%) | 2 (2.0) | - | - |
| Wang et al., 2018 [28] |
Prospective | China | 201 | 2002 AECG | 88% | 158 (78.6%) | yes | 72 (45.5%) | 16 (10.1%) | yes | 42.9 ± 19.4 |
| Kampolis et al., 2018 [20] |
Prospective | Greece | 384 | 2002 AECG | 94.5% | 7 (1.8%) | yes | - | - | yes | 81.74 ± 17.38 |
| Guisado-Vasco et al., 2019 [45] |
Retrospective | Spain | 102 | 2016 ACR/EULAR | 93% | 36 (35.3%) | yes | 27 (26%) | 6 (5.9%) | yes | - |
| Sogkas et al., 2020 [46] |
Retrospective | Germany | 31 | 2016 ACR/EULAR | 71% | 19 (61%) | yes | 9 (29%) | 13 (42%) | yes | - |
| Shi et al., 2020 [34] |
Retrospective | China | 706 | 2002 AECG | 90.5% | 168 (23.8%) | - | - | - | - | - |
| Ufuk et al., 2020 [38] |
Retrospective | Turkey | 28 | 2016 ACR/EULAR | 86% | 34 (75%) | yes | 21 (75%) | 6 (21.4%) | yes | |
| Gao et al., 2021 [35] |
Retrospective | China | 934 | 2002 AECG | - | 178 (19%) | yes | 57 (44.9%) | 9 (15.0%) | yes | 72.4 ± 20.9 |
| Sahin Ozdemirel et al., 2021 [39] |
Retrospective | Turkey | 35 | 2016 ACR/EULAR | 94% | 1 (3%) | yes | - | - | yes | 91.28 ± 19.70 |
| Lin et al., 2022 [36] |
Retrospective | China | 333 | 2002 AECG | 93.1% | 66 (19.8%) | yes | 42 (63.6%) | 20 (30.3%) | yes | 58.82 ± 21.04 |
| Weng et al., 2022 [37] |
Retrospective | China | 69 | 2016 ACR/EULAR | 90% | 35 (50%) | yes | - | - | yes | - |
| Işik et al., 2022 [40] |
Retrospective | Turkey | 120 | 2016 ACR/EULAR | - | 16 (13.3%) | yes | 10 (62.5%) | 2 (12.5%) | yes | 60.1 ± 20.4 |
* Other criteria before 2002 AECG: xerostomia, sicca syndrome, focal lymphocyte infiltrate on minor salivary gland biopsy. n, number of patients; ILD, interstitial lung disease, UIP, usual interstitial pneumonia, NSIP, non-specific interstitial pneumonia; PFTs, pulmonary function tests. DLco results are presented as mean ± SD or as a percentage.
3.2. ILD Prevalence in Sjogren’s Syndrome
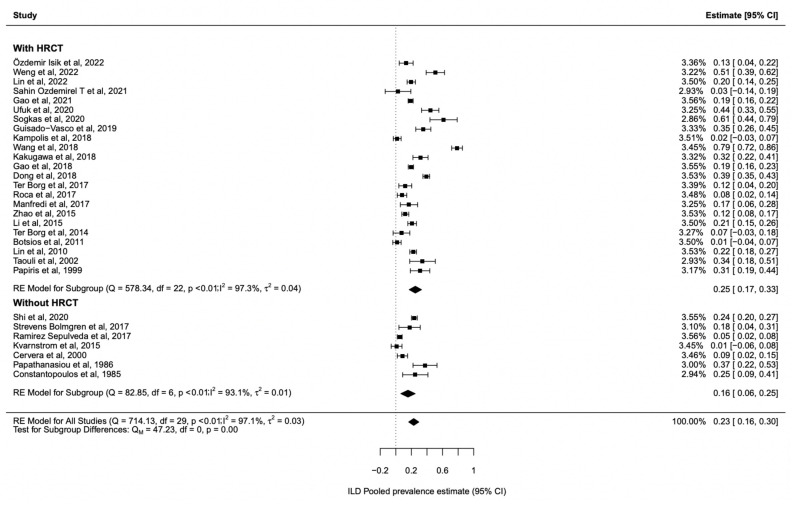
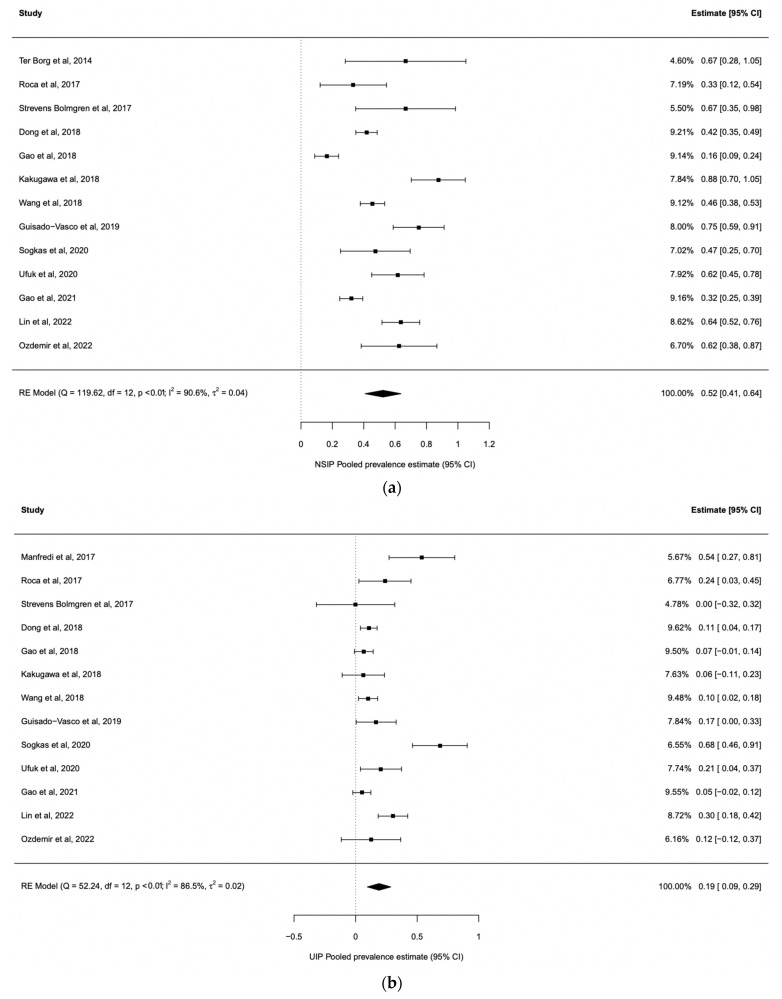
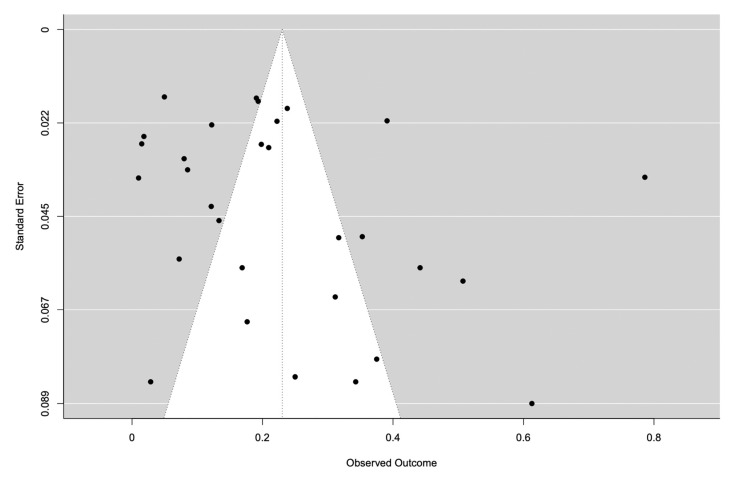
We analysed the pSS-ILD prevalence in 30 studies with 1557 pSS-ILD patients among 8255 pSS patients (Table 1). The prevalence of pSS-ILD in the selected studies ranged from 1% to 75%, and the pooled pSS-ILD prevalence was 23% (95% CI: 16–30) (Figure 2) with a prominent heterogeneity (I2 = 97%) (Table 1). We also explored the role of HRCT to better define the presence of ILD in these patients, performing a subgroup analysis. Our results show that the use of HRCT is associated with a significant higher prevalence of pSS-ILD (25%, 95% CI 17–33) when compared with the prevalence of pSS-ILD diagnosed by using PTFs (16%, 95% CI 6–25) (p < 0.001), as shown in Figure 2. Furthermore, we evaluated the pooled prevalence of the two main clinical radiological patterns, NSIP and UIP, in these patients. As far as the NSIP prevalence was concerned, we retrieved 13 papers [25,28,32,33,35,36,38,40,42,44,45,46] including 3599 patients with a pooled prevalence of 52% (95% CI: 41–64), with a high heterogeneity I2 = 90.6% (Figure 3a). On the other hand, UIP prevalence was reported in 13 studies [26,28,32,33,35,36,38,40,42,44,45,46] including 3621 patients with a pooled prevalence of 44% (95% CI: 32–55, I2 = 91%) (Figure 3b). Publication bias was evaluated with the funnel plot. The Egger’s test was used to test funnel plot asymmetry, and the analysis showed t = 1.7700, p = 0.077, not suggesting asymmetry (Figure 4). The “leave-one-out” test did not identify a single study which could influence the estimate overall effect-size (Supplementary Table S6). By this methodology, p-values were always <0.0001.
Figure 2.
Forest plots depicting the pooled prevalence estimate for ILD in Sjogren patients with subgroup analyses for HRCT. Diamonds indicate the overall summary estimates, and width of the diamonds represents the 95% confidence interval (CI); boxes indicate the weight of individual studies in the pooled results [17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Figure 3.
Forest plots depicting the pooled prevalence estimate for NSIP and UIP in ILD patients. (a) Forest plots depicting the pooled prevalence estimate for NSIP in ILD patients. Diamonds indicate the overall summary estimates, and width of the diamonds represents the 95% confidence interval (CI); boxes indicate the weight of individual studies in the pooled results. (b) Forest plots depicting the pooled prevalence estimate for UIP in ILD patients. Diamonds indicate the overall summary estimates, and width of the diamonds represents the 95% confidence interval (CI); boxes indicate the weight of individual studies in the pooled results [17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Figure 4.
Contour-enhanced funnel plot. The funnel is centred at 0. Various levels of statistical significance of the points, representative of the studies, are indicated in the plot.
3.3. Risk Factors Associated with pSS-ILD
A meta-regression analysis was conducted to test whether part of the heterogeneity might be due to the influence of moderators. ANA positivity, antiRo-SSA positivity, and antiLa-SSB positivity did not significantly contribute to the observed heterogeneity. On the other hand, male gender, DLco value, country, and HRCT seemed to significantly contribute to the observed heterogeneity, as listed in Table 2.
Table 2.
Meta-regression analysis.
| Moderator | Coefficient | SE | Z Value | p Value | 95% CI |
|---|---|---|---|---|---|
| Male gender | 0.24 | 0.044 | 5.54 | <0.0001 | 0.16–0.33 |
| ANA positivity | 0.19 | 0.25 | 0.77 | 0.44 | −0.30–0.68 |
| Anti-Ro/SSa positivity | 0.59 | 0.35 | 1.69 | 0.091 | −0.096–1.28 |
| Anti-La/SSb positivity | 0.42 | 0.29 | 1.45 | 0.15 | −0.15–0.98 |
| DLCO value | 0.47 | 0.17 | 2.68 | 0.0074 | 0.12–0.81 |
| Country | 0.18 | 0.045 | 4.02 | <0.0001 | 0.092–0.27 |
| HRCT | 0.16 | 0.069 | 2.35 | 0.019 | 0.027–0.30 |
SE, standard error; CI, interval of confidence.
4. Discussion
pSS is usually described as a disease with a low mortality risk, except for the development of lymphoma, but in recent years the presence of lung involvement and ILD has been described as organ complications associated with an increased risk of death, with RR 2.54 (95% CI:1.28, 5.04) [48]. These data account for a new interest concerning the ILD prevalence in pSS, and consequently for the better management of the lung involvement associated with this disease. In this study, we performed an SLR and meta-analysis of all the available studies for ILD evaluation in patients with pSS and, of note, it is the first paper evaluating the impact of HRCT in defining in a more accurate way the ILD prevalence in these patients. By this strategy, our results show a pooled pSS-ILD prevalence of 23% (95% CI: 16–30), which is higher than the prevalence reported in another study, which showed a pooled prevalence of 13% (95% CI: 9–19) [49]. Compared to this previous meta-analysis, performed more than 5 years ago, we found nine new papers published in recent years exploring the pSS-ILD prevalence [34,35,36,37,38,39,40,45,46]. These data confirm, on one hand, that pSS-ILD is an emerging hot topic, and on the other hand the need for “refreshing” previous metanalyses considering the relatively fast increase of knowledge in the field of autoimmune diseases. Among the papers published in recent years, only one study did not use HRCT for the ILD diagnosis [34]. This observation confirms the increased importance of HRCT to diagnose ILD, in contrast to what has been used in past years, in which many studies defined ILD only by PFTs, thus missing a substantial number of patients. In fact, the use of HRCT for ILD assessment is associated with a higher ILD prevalence, as shown in Figure 2, where studies with HRCT had a higher ILD prevalence when compared to the others (25%, 95% CI 17–33 vs. 16%, 95% CI: 6–25, p < 0.001). These data mirror what has already been described in systemic sclerosis (SSc): HRCT improves the sensitivity for ILD diagnosis, when compared to the other tests such as PFTs [50]. In fact, data on SSc showed that 90% of SSc patients had interstitial abnormalities detected by HRCT, while only a percentage ranging from 40 to 75% of them had changes detectable by PFTs [51]. Further, we explored the pooled prevalence of NSIP and UIP in our patients to define the most common ILD radiologic pattern associated with pSS. Our study shows that NSIP is the most frequent pattern, with a pooled prevalence of 52% (CI: 41–64), while UIP pattern was detected in 44% (CI: 32–55) of the patients enrolled in the studies (Figure 3). Data regarding other patterns, such as LIP, were scarce. Our results resemble what was already observed in other systemic autoimmune disease, such as SSc and systemic lupus erythematosus, and suggest that different pathogenic mechanisms associated with specific autoimmune diseases may finally lead to common radiologic changes [52,53]. When we explored the factors associated with pSS-ILD, male gender, DLco value, country, and HRCT were associated with a higher pSS-ILD prevalence. Unfortunately, data retrieved from the available literature did not allow us to evaluate whether any of these moderators may correlate with a specific radiologic pattern. According to our work, mirroring a previous meta-analysis, male gender is significantly associated with pSS-ILD prevalence [49]. A different clinical phenotype in male pSS patients when compared to female pSS patients has already been shown [54]. Male patients have a higher frequency of lymphoma and an increased prevalence of serum anti-La/SSB antibodies when compared to females [54,55]. A decrease in DLco, although not specific, is a suitable and reliable clinical biomarker associated with ILD progression in patients with connective tissue diseases, thus supporting its role in the follow-up of the patients [56]. As far as the association with the geographical localization of the patients is concerned, many data support the role of geographic and ethnic backgrounds in determining the clinical phenotype in pSS patients [57]. Furthermore, a previous meta-analysis showed a significantly higher pSS-ILD prevalence in Asia than in Europe [49]. To observe the role of ethnicity, Brito-Zeron et al. analysed data from 9352 pSS patients living in Europe but with different origins. They found a significant different distribution of ILD in pSS patients with different ethnicities, with a lower distribution in the Hispanic group when compared to White, Black/African American, and Asian patients [57].
We did not find any association between the serologic status in terms of autoantibodies presence and the ILD prevalence. We know that anti-Ro/SSA antibodies have a pivotal role in pSS classification and mirror what happens in the salivary glands, and their presence is associated with early diagnoses, parotidomegaly, and sicca symptoms [58,59,60]. Data regarding the association between anti-Ro/SSA antibodies presence and pSS-ILD are controversial, and their relation should be clarified [32,61,62].
We are aware of some weakness of our paper, deriving from the small number of prospective studies published in the available literature, the heterogeneity of the study design, the small number of patients enrolled in the studies, and the variability in ILD definition. These differences partially explain the large variability observed in the results. On the other hand, ILD is now considered one of the most common and severe morbidities in many immunologic diseases, both autoimmune and autoinflammatory, sometimes being the main cause of death in these patients [58,59,60,63,64,65]. More data are needed to better explore ILD prevalence, and to clarify whether the observed differences may be related to the studies’ characteristics. Alternatively, it may be possible that different clinical and biological phenotypes may be associated with a different pulmonary involvement.
To date, no drug is specifically approved for pSS treatment. Guidelines for ILD treatment during pSS should be related to the extension and progression of the pulmonary involvement and, borrowing from experiences in other immune mediated diseases, glucocorticoids or other immunosuppressive drugs should be used [66]. Data deriving from studies specifically designed in pSS-ILD are still a strong unmet need, especially with the advent of anti-fibrotic drugs, and for these reasons it is impossible to derive any generalization.
In our paper, as far as the pSS-ILD prevalence is concerned, a large variability was observed and a previous metanalysis, published five years ago, may be considered old due to the velocity of progress in medical science. The methodology applied to our SLR highlights the differences among all the studies exploring the pulmonary involvement in pSS, such as the study design, the patients’ characteristics and 4eselection, the different geographical areas, and the different diagnostic methods. This heterogeneity confirms the need for specifically designed studies to assess the lung involvement in pSS patients, using well-defined and homogeneous criteria of inclusion, aimed at providing firm conclusions.
In conclusion, our data show that at least 20% of pSS patients have a comorbid ILD, usually the NSIP radiologic pattern. Male gender and the decrease in DLco value may be considered the most important independent factors supporting an active search of lung complications during the clinical history of pSS patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12072586/s1, Table S1: PRISMA checklist; Table S2: MOOSE (Meta-analyses Of Observational Studies in Epidemiology) Checklist; Table S3: Papers excluded from the analysis with the main reason; Table S4: Newcastle-Ottawa Assessment Scale for case-control studies; Table S5: Quality assessment of the included studies without the control group; Table S6: Leave-one-out test.
Author Contributions
O.B., L.N. and R.G. contributed to the conception and design of the study. S.D.’A. performed the statistical analysis. O.B. wrote the first draft of the manuscript. O.B., S.D.’A., A.M., I.G. and D.C. wrote sections of the manuscript and participated in the literature review. A.R., L.A. and M.V. participated in the literature review. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study does not include data directly derived from patients.
Data Availability Statement
All the data that have been used are reported in the article and Supplementary Materials.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ramos-Casals M., Brito-Zerón P., Sisó-Almirall A., Bosch X. Primary Sjogren syndrome. BMJ. 2012;344:e3821. doi: 10.1136/bmj.e3821. [DOI] [PubMed] [Google Scholar]
- 2.Fox R.I. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 3.Ito I., Nagai S., Kitaichi M., Nicholson A.G., Johkoh T., Noma S., Kim D.S., Handa T., Izumi T., Mishima M. Pulmonary manifestations of primary Sjogren’s syndrome: A clinical, radiologic, and pathologic study. Am. J. Respir. Crit. Care Med. 2005;171:632–638. doi: 10.1164/rccm.200403-417OC. [DOI] [PubMed] [Google Scholar]
- 4.Cafaro G., Bursi R., Chatzis L.G., Fulvio G., Ferro F., Bartoloni E., Baldini C. One year in review 2021, Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021;133:3–13. doi: 10.55563/clinexprheumatol/eojaol. [DOI] [PubMed] [Google Scholar]
- 5.Nannini C., Jebakumar A.J., Crowson C.S., Ryu J.H., Matteson E.L. Primary Sjogren’s syndrome 1976-2005 and associated interstitial lung disease: A population-based study of incidence and mortality. BMJ Open. 2013;3:e003569. doi: 10.1136/bmjopen-2013-003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambataro G., Ferro F., Orlandi M., Sambataro D., Torrisi S.E., Quartuccio L., Vancheri C., Baldini C., Cerinic M.M. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjӧgren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun. Rev. 2020;19:102447. doi: 10.1016/j.autrev.2019.102447. [DOI] [PubMed] [Google Scholar]
- 7.Fischer A., Strek M.E., Cottin V., Dellaripa P.F., Bernstein E.J., Brown K.K., Danoff S.K., Distler O., Hirani N., Jones K.D., et al. Proceedings of the American College of Rheumatology/Association of Physicians of Great Britain and Ireland Connective Tissue Disease-Associated Interstitial Lung Disease Summit: A Multidisciplinary Approach to Address Challenges and Opportunities. Arthritis Rheumatol. 2019;71:182–195. doi: 10.1002/art.40769. [DOI] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooke B.S., Schwartz T.A., Pawlik T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156:787–788. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 10.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., Buchbinder R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. [(accessed on 1 December 2022)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.html.
- 13.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A., Egger M. Funnel plots for detecting bias in metaanalysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 15.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 16.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in metaanalysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Constantopoulos S.H., Papadimitriou C.S., Moutsopoulos H.M. Respiratory manifestations in primary Sjogren’s syndrome. A clinical, functional, and histologic study. Chest. 1985;88:226–229. doi: 10.1378/chest.88.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Papathanasiou M.P., Constantopoulos S.H., Tsampoulas C. Reappraisal of respiratory abnormalities in primary and secondary Sjogren’s syndrome. A Control. Study Chest. 1986;90:370–374. doi: 10.1378/chest.90.3.370. [DOI] [PubMed] [Google Scholar]
- 19.Papiris S.A., Maniati M., Constantopoulos S.H., Roussos C., Moutsopoulos H.M., Skopouli F.N. Lung involvement in primary Sjogren’s syndrome is mainly related to the small airway disease. Ann. Rheum. Dis. 1999;58:61–64. doi: 10.1136/ard.58.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampolis C.F., Fragkioudaki S., Mavragani C.P., Zormpala A., Samakovli A., Moutsopoulos H.M. Prevalence and spectrum of symptomatic pulmonary involvement in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2018;36:94–101. [PubMed] [Google Scholar]
- 21.Kvarnström M., Ottosson V., Nordmark B., Wahren-Herlenius M. Incident cases of primary Sjögren’s syndrome during a 5-year period in Stockholm County: A descriptive study of the patients and their characteristics. Scand. J. Rheumatol. 2015;44:135–142. doi: 10.3109/03009742.2014.931457. [DOI] [PubMed] [Google Scholar]
- 22.Ramírez Sepúlveda J.I., Kvarnström M., Eriksson P., Mandl T., Norheim K.B., Johnsen S.J., Hammenfors D., Jonsson M.V., Skarstein K., Brun J.G., et al. Long-term follow-up in primary Sjogren’s syndrome reveals differences in clinical presentation between female and male patients. Biol. Sex. Differ. 2017;8:25. doi: 10.1186/s13293-017-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strevens Bolmgren V., Olsson P., Wollmer P., Hesselstrand R., Mandl T. Respiratory symptoms are poor predictors of concomitant chronic obstructive pulmonary disease in patients with primary Sjogren’s syndrome. Rheumatol. Int. 2017;37:813–818. doi: 10.1007/s00296-017-3678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervera R., Font J., Ramos-Casals M., García-Carrasco M., Rosas J., Morlà R.M., Muñoz F.J., Artigues A., Pallarés L., Ingelmo M. Primary Sjogren’s syndrome in men: Clinical and immunological characteristics. LUPUS. 2000;9:61–64. doi: 10.1177/096120330000900111. [DOI] [PubMed] [Google Scholar]
- 25.Ter Borg E.J., Kelder J.C. Lower prevalence of extra-glandular manifestations and anti-SSB antibodies in patients with primary Sjogren’s syndrome and widespread pain: Evidence for a relatively benign subset. Clin. Exp. Rheumatol. 2014;32:349–353. [PubMed] [Google Scholar]
- 26.Manfredi A., Sebastiani M., Cerri S., Cassone G., Bellini P., Casa G.D., Luppi F., Ferri C. Prevalence and characterization of non-sicca onset primary Sjogren syndrome with interstitial lung involvement. Clin. Rheumatol. 2017;36:1261–1268. doi: 10.1007/s10067-017-3601-1. [DOI] [PubMed] [Google Scholar]
- 27.Botsios C., Furlan A., Ostuni P., Sfriso P., Andretta M., Ometto F., Raffeiner B., Todesco S., Punzi L. Elderly onset of primary Sjögren’s syndrome: Clinical manifestations, serological features and oral/ocular diagnostic tests. Comparison with adult and young onset of the disease in a cohort of 336 Italian patients. Joint Bone Spine. 2011;78:171–174. doi: 10.1016/j.jbspin.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Hou Z., Qiu M., Ye Q. Risk factors for primary Sjogren syndrome-associated interstitial lung disease. J. Thorac. Dis. 2018;10:2108–2117. doi: 10.21037/jtd.2018.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin D.F., Yan S.M., Zhao Y., Zhang W., Li M.T., Zeng X.F., Zhang F.-C., Dong Y. Clinical and prognostic characteristics of 573 cases of primary Sjogren’s syndrome. Chin. Med. J. 2010;123:3252–3257. [PubMed] [Google Scholar]
- 30.Li X., Xu B., Ma Y., Li X., Cheng Q., Wang X., Wang G., Qian L., Wei L. Clinical and laboratory profiles of primary Sjogren’s syndrome in a Chinese population: A retrospective analysis of 315 patients. Int. J. Rheum. Dis. 2015;18:439–446. doi: 10.1111/1756-185X.12583. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y., Li Y., Wang L., Li X.F., Huang C.B., Wang G.C., Zhang X.-W., Zhang Z.-L., Zhang X., Xiao W.-G., et al. Primary Sjögren syndrome in Han Chinese: Clinical and immunological characteristics of 483 patients. Medicine. 2015;94:e667. doi: 10.1097/MD.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong X., Zhou J., Guo X., Li Y., Xu Y., Fu Q., Lu Y., Zheng Y. A retrospective analysis of distinguishing features of chest HRCT and clinical manifestation in primary Sjogren’s syndrome-related interstitial lung disease in a Chinese population. Clin. Rheumatol. 2018;37:2981–2988. doi: 10.1007/s10067-018-4289-6. [DOI] [PubMed] [Google Scholar]
- 33.Gao H., Zhang X.W., He J., Zhang J., An Y., Sun Y., Jia R.-L., Li S.-G., Zhang L.-J., Li Z.-G. Prevalence, risk factors, and prognosis of interstitial lung disease in a large cohort of Chinese primary Sjogren syndrome patients: A case-control study. Medicine. 2018;97:e11003. doi: 10.1097/MD.0000000000011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi L., Han X.L., Guo H.X., Wang J., Tang Y.P., Gao C., Li X.F. Increases in tumor markers are associated with primary Sjögren’s syndrome-associated interstitial lung disease. Ther. Adv. Chronic Dis. 2020;11:2040622320944802. doi: 10.1177/2040622320944802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao H., Sun Y., Zhang X.Y., Xie L., Zhang X.W., Zhong Y.C., Zhang J., Hou Y.-K., Li Z.-G. Characteristics and mortality in primary Sjögren syndrome-related interstitial lung disease. Medicine. 2021;100:e26777. doi: 10.1097/MD.0000000000026777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin W., Xin Z., Zhang J., Liu N., Ren X., Liu M., Su Y., Liu Y., Yang L., Guo S., et al. Interstitial lung disease in Primary Sjögren’s syndrome. BMC Pulm. Med. 2022;22:73. doi: 10.1186/s12890-022-01868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng L., Chen Y., Liang T., Lin Y., Liu D., Yu C., Hu Y., Lui W., Liu Y., Chen X., et al. Biomarkers of interstitial lung disease associated with primary Sjögren’s syndrome. Eur. J. Med. Res. 2022;27:199. doi: 10.1186/s40001-022-00828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ufuk F., Demirci M., Altinisik G., Karasu U. Quantitative analysis of Sjogren’s syndrome related interstitial lung disease with different methods. Eur. J. Radiol. 2020;128:109030. doi: 10.1016/j.ejrad.2020.109030. [DOI] [PubMed] [Google Scholar]
- 39.Sahin Ozdemirel T., Ozdemirel A.E., Akinci Ozyurek B., Yenibertiz D., Erdogan Y. The evaluation of lung involvement and functional capacities in patients diagnosed with primary Sjogren’s syndrome. Int. J. Clin. Pract. 2021;75:e14635. doi: 10.1111/ijcp.14635. [DOI] [PubMed] [Google Scholar]
- 40.Işık Ö.Ö., Yazıcı A., Çefle A. The respiratory manifestations in patients with primary Sjögren’s syndrome: Is. interstitial lung disease related to disease activity? Turk. J. Med. Sci. 2022;52:1737–1743. doi: 10.55730/1300-0144.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taouli B., Brauner M.W., Mourey I., Lemouchi D., Grenier P.A. Thinsection chest CT findings of primary Sjogren’s syndrome: Correlation with pulmonary function. Eur. Radiol. 2002;12:1504–1511. doi: 10.1007/s00330-001-1236-7. [DOI] [PubMed] [Google Scholar]
- 42.Roca F., Dominique S., Schmidt J., Smail A., Duhaut P., Lévesque H., Marie I. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun. Rev. 2017;16:48–54. doi: 10.1016/j.autrev.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Ter Borg E.J., Kelder J.C. Development of new extra-glandular manifestations or associated auto-immune diseases after establishing the diagnosis of primary Sjogren’s syndrome: A long-term study of the Antonius Nieuwegein Sjogren (ANS) cohort. Rheumatol. Int. 2017;37:1153–1158. doi: 10.1007/s00296-017-3715-4. [DOI] [PubMed] [Google Scholar]
- 44.Kakugawa T., Sakamoto N., Ishimoto H., Shimizu T., Nakamura H., Nawata A., Ito C., Sato S., Hanaka T., Oda K., et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjogren’s syndrome. Respir. Med. 2018;137:95–102. doi: 10.1016/j.rmed.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Guisado-Vasco P., Silva M., Duarte-Millán M.A., Sambataro G., Bertolazzi C., Pavone M.O., Luque-Pinill J., Santilli D., Sambataro D., Torr S.E., et al. Quantitative assessment of interstitial lung disease in Sjögren’s syndrome. PLoS ONE. 2019;14:e0224772. doi: 10.1371/journal.pone.0224772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sogkas G., Hirsch S., Olsson K.M., Hinrichs J.B., Thiele T., Seeliger T., Skripuletz T., Schmidt R.E., Witte T., Jablonka A., et al. Lung Involvement in Primary Sjögren’s Syndrome-An Under-Diagnosed Entity. Front. Med. 2020;7:332. doi: 10.3389/fmed.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H.M., Alexander E.L., Carsons S.E., Daniels T.E., Fox P.C., Fox R.I., Kassan S.S., et al. Classification Criteria for Sjögren’s Syndrome: A Revised Version of the European Criteria Proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H., Xie W., Geng Y., Fan Y., Zhang Z. Mortality in patients with primary Sjögren’s syndrome: A systematic review and meta-analysis. Rheumatology. 2021;60:4029–4038. doi: 10.1093/rheumatology/keab364. [DOI] [PubMed] [Google Scholar]
- 49.He C., Chen Z., Liu S., Chen H., Zhang F. Prevalence and risk factors of interstitial lung disease in patients with primary Sjögren’s syndrome: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2020;23:1009–1018. doi: 10.1111/1756-185X.13881. [DOI] [PubMed] [Google Scholar]
- 50.Ruaro B., Baratella E., Confalonieri P., Wade B., Marrocchio C., Geri P., Busca A., Pozzan R., Andrisano A.G., Cova M.A., et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics. 2021;11:1960. doi: 10.3390/diagnostics11111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkmann E.R., Tashkin D.P., Sim M., Li N., Goldmuntz E., Keyes-Elstein L., Pinckney A., Furst D.E., Clements P.J., Khanna D., et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann. Rheum. Dis. 2019;78:122–130. doi: 10.1136/annrheumdis-2018-213708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao T., Shi X., Yang S., Zhang W., Li X., Shu J., Alqalyoobi S., Zeki A.A., Leung P.S., Shuai Z. Interstitial Lung Disease in Connective Tissue Disease: A Common Lesion with Heterogeneous Mechanisms and Treatment Considerations. Front. Immunol. 2021;12:684699. doi: 10.3389/fimmu.2021.684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutsche M., Rosen G.D., Swigris J.J. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr. Respir. Care Rep. 2012;1:224–232. doi: 10.1007/s13665-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatzis L., Pezoulas V.C., Ferro F., Gandolfo S., Donati V., Binutti M., Callegher S.Z., Venetsanopoulou A., Zampeli E., Mavrommati M., et al. Sjögren’s Syndrome: The Clinical Spectrum of Male Patients. J. Clin. Med. 2020;9:2620. doi: 10.3390/jcm9082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandt J.E., Priori R., Valesini G., Fairweather D. Sex differences in Sjögren’s syndrome: A comprehensive review of immune mechanisms. Biol. Sex. Differ. 2015;6:19. doi: 10.1186/s13293-015-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh S.L., Sverzellati N., Devaraj A., Keir G.J., Wells A.U., Hansell D.M. Connective tissue disease related fibrotic lung disease: High resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216–222. doi: 10.1136/thoraxjnl-2013-203843. [DOI] [PubMed] [Google Scholar]
- 57.Brito-Zerón P., Acar-Denizli N., Ng W.F., Horváth I.F., Rasmussen A., Seror R., Li X., Baldini C., Gottenberg J.-E., Danda D., et al. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjögren’s syndrome. Rheumatology. 2020;59:2350–2359. doi: 10.1093/rheumatology/kez578. [DOI] [PubMed] [Google Scholar]
- 58.Berardicurti O., Ruscitti P., Di Benedetto P., D’Andrea S., Navarini L., Marino A., Cipriani P., Giacomelli R. Association Between Minor Salivary Gland Biopsy During Sjӧgren’s Syndrome and Serologic Biomarkers: A Systematic Review and Meta-Analysis. Front. Immunol. 2021;12:686457. doi: 10.3389/fimmu.2021.686457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzioufas A.G., Wassmuth R., Dafni U.G., Guialis A., Haga H.J., Isenberg D.A., Jonsson R., Kalden J.R., Kiener H., Sakarellos C., et al. Clinical, immunological, and immunogenetic aspects of autoantibody production against Ro/SSA, La/SSB and their linear epitopes in primary sjögren’s syndrome (pSS): A European multicentre study. Ann. Rheum. Dis. 2002;61:398–404. doi: 10.1136/ard.61.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzioufas A.G., Tatouli I.P., Moutsopoulos H.M. Autoantibodies in sjögren’s syndrome: Clinical presentation and regulatory mechanisms. Presse Med. 2012;41:e451–60. doi: 10.1016/j.lpm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Retamozo S., Akasbi M., Brito-Zerón P., Bosch X., Bove A., Perez-de-Lis M., Jimenez I., Soto-Cardenas M.-J., Gandia M., Diaz-Lagares C., et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2012;30:686–692. [PubMed] [Google Scholar]
- 62.Song J.S., Do J.H., Lee S.W. The prevalence and the clinical relevance of anti-Ro52 in Korean patients with primary Sjögren’s syndrome. Rheumatol. Int. 2012;32:491–495. doi: 10.1007/s00296-010-1790-x. [DOI] [PubMed] [Google Scholar]
- 63.Ruscitti P., Berardicurti O., Iacono D., Pantano I., Liakouli V., Caso F., Emmi G., Grembiale R.D., Cantatore F.P., Atzeni F., et al. Parenchymal lung disease in adult onset Still’s disease: An emergent marker of disease severity-characterisation and predictive factors from Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale (GIRRCS) cohort of patients. Arthritis Res. Ther. 2020;22:151. doi: 10.1186/s13075-020-02245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruscitti P., Bruno F., Berardicurti O., Acanfora C., Pavlych V., Palumbo P., Conforti A., Carubbi F., Di Cola I., Di Benedetto P., et al. Lung involvement in macrophage activation syndrome and severe COVID-19, results from a cross-sectional study to assess clinical, laboratory and artificial intelligence-radiological differences. Ann. Rheum. Dis. 2020;79:1152–1155. doi: 10.1136/annrheumdis-2020-218048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saper V.E., Chen G., Deutsch G.H., Guillerman R.P., Birgmeier J., Jagadeesh K., Canna S., Schulert G., Deterding R., Xu J., et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann. Rheum. Dis. 2019;78:1722–173. doi: 10.1136/annrheumdis-2019-216040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramos-Casals M., Brito-Zerón P., Bombardieri S., Bootsma H., De Vita S., Dörner T., Fisher B.A., Gottenberg J.-E., Hernandez-Molina G., Kocher A., et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020;79:3–18. doi: 10.1136/annrheumdis-2019-216114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data that have been used are reported in the article and Supplementary Materials.