Abstract
Background and aims
Mutations in FDXR gene, involved in mitochondrial pathway, cause a rare recessive neurological disorder with variable severity of phenotypes. The most common presentation includes optic and/or auditory neuropathy, variably associated to developmental delay or regression, global hypotonia, pyramidal, cerebellar signs, and seizures. The review of clinical findings in previously described cases from literature reveals also a significant incidence of sensorimotor peripheral polyneuropathy (22.72%) and ataxia (43.18%). To date, 44 patients with FDXR mutations have been reported. We describe here on two new patients, siblings, who presented with a quite different phenotype compared to previously described patients.
Methods
Clinical, neurophysiological, and genetic features of two siblings and a systematic literature review focused on the clinical spectrum of the disease are described.
Results
Both patients presented with an acute–sub-acute onset of peripheral neuropathy and only in later stages of the disease developed the typical features of FDXR-associated disease.
Interpretation
The peculiar clinical presentation at onset and the evolution of the disease in our patients and in some cases revised from the literature shed lights on a new possible phenotype of FDXR-associated disease: a peripheral neuropathy which can mimic an acute inflammatory disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-023-06790-0.
Keywords: Peripheral neuropathy, FDXR, Phenotype
Introduction
FDXR mutations have been identified as cause of an autosomal recessive neurological disorder with a large spectrum of neurological presentations. The gene encodes for ferredoxin reductase, an enzyme involved in the biosynthesis of iron sulfur clusters, essential to the mitochondrial pathway [1]. Recent studies revealed that FDXR mutations cause an abnormal iron accumulation in mitochondria of several tissue, namely, the brain, heart, and liver, causing their dysfunction and, in central nervous system (CNS), neurodegeneration [2, 3].
To date, 44 patients with FDXR-associated disease have been reported in the literature. The clinical presentation was characterized by a wide range of neurological signs and symptoms variably associated. Most of the patients had onset in the first or second decade of life with optic and acoustic neuropathy [1], either isolated or more rarely associated to ataxia and/or hypotonia. A few patients suffered from a more complex clinical phenotype with an earlier disease onset, developmental delay or regression, pyramidal signs, microcephaly, and seizures in addition to the typical features of the disease, namely, acoustic and optic neuropathy. Recently, a more severe clinical and neuroradiological presentations consistent with Leigh syndrome was described [4].
We report here on two previously unreported cases who presented with a quite different phenotype compared to so far described patients. We also provide a systematic literature review focused on the clinical spectrum of the disease.
Materials and methods
The study adheres to the principles of the Code of Ethics of the World Medical Association — Helsinki Declaration and concerns data gathered during routine diagnostic activity. The study complied with the institutional regulations for anonymized retrospective studies.
Genetic analysis methods
Genomic DNA was extracted from peripheral blood samples taken from the two probands and their parents using standard procedures. The exonic regions and flanking splice junctions of the genome were captured using the Clinical Research Exome v.2 kit (Agilent Technologies). Sequencing was done on a NextSeq500 Illumina system with 150 bp paired-end reads. Reads were aligned to human genome build GRCh37/UCSC hg19 and analyzed for sequence variants using a custom-developed analysis tool. Additional sequencing technology and the variant interpretation protocol have been previously described [5]. Coverage on target for the index was ≥ 10 × for 98.2% with a mean coverage of 210 × .
Results
Patient 1
He is the fourth child of eight siblings from consanguineous parents native from Gambia. Family history was negative, and a normal psychomotor development was reported. At the age of 9 years, he presented with recurrent nocturnal enuresis, and since the age of 10 years, the parent noticed the onset of walking disturbances, for which he had not been investigated. At 11 years of age, a sub-acute clinical worsening was observed, since the boy developed marked difficulties in autonomous gait and frequent falls over a period of a few weeks, in association to lower limb pain. At that time, the neurological examination showed lower limbs proximal and mainly distal asymmetric muscle weakness and reduced tendon reflexes. Brain and spinal cord MRI, electroneurography, and cerebrospinal fluid (CSF) protein and cell count were tested normal. Sensory-evoked potentials (SEP) detected alterations of central conduction at lower limbs with impaired cortical responses. The possibility of a sub-acute inflammatory acquired condition was considered, and the boy was treated with intra-venous (IV) methylprednisolone up to 10 mg/kg/day followed by oral steroid with partial recover. Seven months later, he suffered from a further worsening of gait and painful hyperesthesia at distal upper and lower limbs. He repeated nerve conduction studies, which highlighted at first a reduction of compound muscle action potential (CMAP) amplitude of the lower limb motor nerves and a reduction of sensory nerve action potential (SNAP) amplitude of upper limb sensory nerves, which evolved, in the following assessment, in a secondary chronic axonal damage, with a more severe involvement of lower limbs. SEP confirmed marked dysfunction along the spinal ascending pathways, and motor-evoked potentials demonstrated an involvement of descending ones. An auditory impairment was clinically noted and brainstem auditory-evoked potentials (BAEP) detected dysfunction of the brainstem acoustic pathway of peripheral and retro-cochlear origin. Visual-evoked potentials (VEP), as well as brain and spinal cord control MR examination, were normal. The boy was treated again by IV methylprednisolone followed by 2 cycles of IV immunoglobulin at a dose of 2 g/kg, with benefit on sensitive symptoms and partial recovery of autonomous gait. He was discharged with oral prednisone 1 mg/kg/day.
During the following years, the disease showed a slowly progressive course with 1–2 episodes per year of exacerbation with pain, hyperesthesia, and gait worsening, without correlation with any triggering events except for the attempts of corticosteroid therapy lowering. Treatment with steroids and IV immunoglobulin where then repeated with only partial benefit; azathioprine was started at 14 years of age without effect in preventing the occurrence of further episodes of deterioration.
Optic neuropathy was also documented for the first time at 13 years: VEP and electroretinography showed reduced amplitude and increase of latency and time dispersion, associated with predominantly scotopic retinal alterations. Auditory impairment worsened during years, and at 14 years of age, he was fitted with hearing aid.
From a diagnostic point of a view, a wide spectrum of acquired and genetic conditions have been considered. CSF repeated analysis did not show significant changes; several metabolic, autoimmune, and infectious analyses have been performed (plasma folate, vitamin B12, vitamin E, free homocysteine, alanine, phytanic acid, ammonium, amino acids, very long fatty acids, arylsulfatase A, galactocerebroside beta-galactosidase; urinary organic acids; lactate and pyruvate on serum and CSF; serum anti-ganglioside GM1 and GM2 antibodies; Borrelia, mycoplasma, EBV, HSV1-2–6, CMV, HZV, Coxsackie, echovirus, West Nile, HIV, HTLV) showing normal results.
Patient 2
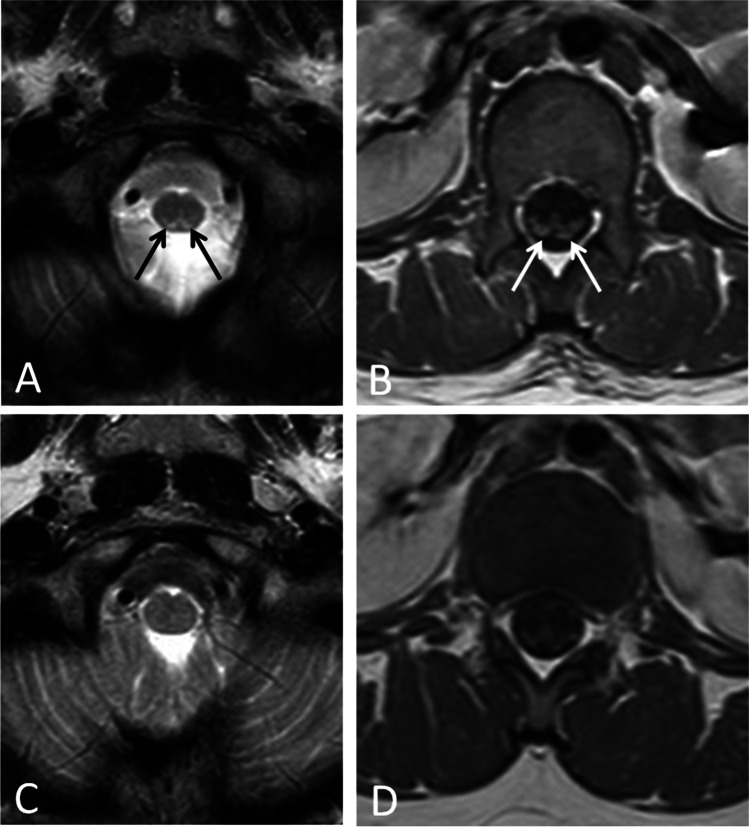
The patient is the younger sister of patient 1. She is a 7-year-old previously healthy girl who was admitted at the hospital because of severe abdominal pain, acute onset (over 4 days) of flaccid paralysis with loss of trunk control, and autonomous deambulation. Neurological examination disclosed mild dysarthria, diffuse hypotonia, upper limb hyperreflexia and proximal weakness, lower limb areflexia and flaccid paralysis, dysesthesia, and pain in distal regions. Nerve conduction study revealed reduced CMAP amplitude of the upper limbs and reduced SNAP amplitude of the lower limb sensory nerves, without signs of active denervation at electromyographic assessment. VEP showed bilaterally increased latency, reduced amplitude, and a disrupted wave morphology. BAEP were normal. CSF cell count, glucose and protein were normal. Serum IgG against SARS-COV2 were found positive. Brain MRI detected only slight chiasm and optic nerves atrophy, while spinal cord MRI disclosed T2 hyperintensities in the posterior columns and posterior root contrast enhancement (Fig. 1A–B). EEG showed spread theta–delta activity. The acute onset of axonal neuropathy with MRI contrast enhancement of spinal root led to consider the possibility of an acquired inflammatory peripheral neuropathy. Treatment with IV IgG (400 mg/kg/day for 5 consecutive days) was administered corresponding to a slow improvement of strength gain in the upper and lower limbs.
Fig. 1.
MR examination performed at the onset of symptoms and at follow-up: A axial T2-weighted image at the level of craniocervical junction demonstrates subtle T2 signal hyperintensities in the posterior columns of spinal cord (arrows). B Axial post-contrast T1-weighted image at the level of cauda equina shows contrast enhancement of posterior roots (arrows). C–D Follow-up MR examination performed 1 year later (same sections and technique, respectively, of A and B). C Resolution of signal abnormalities in posterior columns. D No more evidence of posterior roots contrast enhancement
At the second assessment of nerve conduction studies, performed 15 days later, nerve conduction parameters returned to normal values. Considering some atypical clinical and neurophysiological features, and given the history of the older brother, the possibility of a genetic disorder was suspected, and in parallel, an extensive biochemical work-up excluding metabolic, autoimmune, and infectious diseases has been performed (urinary porphyrin, porphobilinogen, aminolevulinic acid (ALA), fecal porphyrin, erythrocyte porphyrin, zinc protoporphyrins, dosage of delta-aminolevulinic acid dehydratase enzyme activity, plasma vitamin B12, total plasma homocysteine, plasmatic copper and ceruloplasmin, plasmatic and urinary amino acid and urinary organic acids, plasma lactate and pyruvate, serum and CSF anti-ganglioside antibodies (anti-GM1 IgG, anti-GM1 IgM, anti-GQ1b IgG, anti-GM2 IgG, anti-GM2 IgM, anti-GD1a IgG, anti-GD1a IgM, anti-GD1b IgG, anti-GD1b IgM, and anti-sulfatidies), CSF PCR of EBV, HSV1-2, VZV, CMV, enterovirus). All these analyses were tested normal. A trio-based whole-exome sequencing (WES) was performed revealing an homozygous missense pathogenic variant on the FDXR gene (c.463C > T, p.Arg155Trp) in the proband, inherited from the healthy parents. This variant was already reported in literature [2], and the frequency in the general population is gnomAD MAF 4/282390. The same homozygous mutation was detected in the affected brother.
Given the likely mitochondrial dysfunction underlying the disease, we started a supplementation with Q10 coenzyme, riboflavin, thiamine, and lipoic acid. At the last evaluation, 2 years after the onset, dysesthesia and four limb strength were recovered, she regained head and trunk control, and she was able to walk independently with wide base gait. Moreover, follow-up MRI revealed no longer evidence of root contrast enhancement and no more signal abnormalities in posterior columns (Fig. 1C–D).
Literature review
A literature review, targeted to all published cases with FDXR mutation, was conducted in PubMed. Search criteria included “FDXR,” “ferredoxin reductase gene,” and “iron sulfur.”
At the time of this paper drafting, 44 patients, from 35 different families, affected with FDXR-associated disease (supplementary data Table 2) have been reported [1, 2, 4, 6–9].
Detailed description of clinical presentation is available in 36 patients, of whom one suffered from an early-onset severe multisystemic presentation with multiple malformations that lead to early death, and ten presented with a subtle onset in the first years of life, a slowly progressive course, with hypotonia, developmental delay, followed by signs of hearing and/or visual impairment in almost all cases. A sub-acute onset is described in 19 patients whose symptoms started in the first decade of life, with acoustic and/or visual defects, ataxia, and nystagmus sometimes associated to developmental regression. Six children had an abrupt onset during infections, two of them presenting with ataxia and two with signs of encephalopathy.
Across the various presentations of the disease spectrum, the common main features included optic neuropathy (93.2%) and acoustic neuropathy (50%). Sensorimotor peripheral polyneuropathy has been described in 20.5% of patients. Most of the patients developed, throughout the years, signs of neurological and ophthalmological involvement, namely, ataxia (40.9%), hypotonia (40.9%), pyramidal signs (22.7%), microcephaly (15.9%), movement disorders (13.6%), seizures (6.8%), retinal dystrophy (29.5%), nystagmus (18.2%), cataract (11.4%), and ophthalmoplegia (4.5%).
Clinical manifestations associated with FDXR variants are summarized in Table 1.
Table 1.
Clinical manifestations associate with FDXR variants (including reported cases from literature and patients reported in this paper)
| Clinical features | % |
|---|---|
| Optic neuropathy | 93.5 |
| Acoustic neuropathy | 50.0 |
| Ataxia | 43.9 |
| Hypotonia | 41.3 |
| Developmental delay | 39.1 |
| Developmental regression | 34.8 |
| Retinal dystrophy | 30.4 |
| Peripheral neuropathy | 23.9 |
| Pyramidal signs | 23.9 |
| Speech issues | 23.9 |
| Nystagmus | 17.4 |
| Loss of deambulation | 15.2 |
| Microcephaly | 15.2 |
| Cataract | 10.9 |
| Encephalopathic episodes | 10.9 |
| Dystonia | 8.7 |
| Seizures | 6.5 |
| Ophthalmoplegia | 4.3 |
| Tremor | 4.3 |
Table 1 summarizes all the clinical manifestations associated with FDXR variants, listed in order of frequency in all the reported cases both from literature and this paper
Brain MRI was reported to be normal in 38.6% of cases. The other patients developed optic nerve atrophy (27.27%); basal ganglia involvement with bilateral T2 hyperintensities in the globus pallidus, thalami, and substantia nigra (15.91%); cerebellar atrophy/hypoplasia (13.63%); cerebral atrophy (11.36%); thin corpus callosum (9.09%); and myelination delay (4.55%).
Discussion
We described two new unreported FDXR-mutated siblings presenting with acute onset of neurological disorder mimicking an inflammatory condition.
To date, 44 cases with FDXR mutations have been reported. The most common presentation includes optic and/or auditory neuropathy, with onset in the first two decades of life, in some cases associated to developmental delay or regression, global hypotonia, pyramidal, cerebellar signs, and seizures. Neuroimaging appeared normal or, in more complex cases, characterized by cerebral and cerebellar atrophy, basal ganglia abnormalities, delayed myelination, and thin corpus callosum. Spinal cord atrophy and posterior column T2 hyperintensity have been reported. A few cases presenting clinical and radiological features fulfilling criteria for Leigh disease have been described [4]. The clinical presentation is therefore wide, but available data are still too limited to define the presence of any genotype–phenotype correlations. Interestingly, one of the previously described patients shared the same pathogenic variants of our siblings [2]. He presented with acute onset of ataxia in a context of infection, with normal brain and spine MRI examinations, progressing with episodes of intermittent ataxia up to persistent gait abnormalities and visual impairment; some of these features are similar to those of our cases; however, neuroimaging was normal at the disease onset, and detailed neurophysiological examinations were not described.
Sensorimotor peripheral polyneuropathy was the most prominent signs in our patients. It has been reported only in 20.5% of the patients, even if it is of note that almost half of the cases presented with ataxia and/or gait impairment, and it cannot be excluded that, at least in part of them, these symptoms were caused by an undiagnosed peripheral neuropathy not fully investigated.
Primary axonal impairment is described as the most common cause of peripheral neuropathy in mitochondrial disorders [10, 11]. In both our patients, nerve conduction studies showed at first a reduction in amplitude of CMAP and SNAP which appears congruent with distal conduction block. In patient 2, nerve conduction data quickly returned to normal values, configuring a reversible conduction block, while in patient 1, who had a longer follow-up, nerve conduction data evolved progressively to a chronic axonal damage.
These features are strongly suggestive of the presence of paranodopathy, a condition well described both in inflammatory and non-inflammatory peripheral neuropathies [12]. Some data suggest that paranodopathies can represent an alternative mechanism of peripheral neuropathy in some mitochondrial disorders, probably due to ischemic-like depolarization of the axonal membrane consistent with disruption of energy-dependent process [13, 14]. Therefore, taking into account the role in the mitochondrial pathway of FDXR gene, we can suggest that a paranodopathy can be the underlying mechanism in sensorimotor peripheral polyneuropathy in FDXR-associated disease.
In most of the cases described in literature, peripheral neuropathy appeared in the last stages of the disease, while in our patients, this was present since clinical onset. Of note, in patient 2, this was the presenting feature with limb flaccid paralysis, anterior and posterior root contrast enhancement on spinal MRI, and neurophysiological signs of peripheral nerve involvement, leading to consider the possibility of an inflammatory causative mechanism, similarly to two other cases from literature [6]. For this reason, an immunomodulatory treatment was started, and a concomitant improvement of the clinical picture was observed in both siblings. The reason of this improvement is not clear: it is possible that it was only a chance association to treatment, as previously observed in other genetic diseases [15] or that it was related to the partial restoring of energetic demand after the resolution of the infectious events that may have triggered the relapses of the disease; it is to note that no positive response to IV immunoglobulin was found in the case reported from the literature [6]. Moreover, at least 15/44 published cases (34%) displayed episodes of clinical deterioration triggered by infections, and this was followed by spontaneous partial recovery after the resolution of the intercurrent illness.
FDXR is a mitochondrial membrane protein implicated in the biosynthesis of iron sulfur clusters, essential components for the mitochondrial machinery function. It would not therefore be surprising that the clinical picture has features commonly observed in mitochondrial disorders and in other disorders of Fe‐S protein metabolism, such as Friedreich ataxia. In most of the patients, a progressive course with recurrence of relapses and remissions were observed, mostly triggered by infections. This is frequently observed in the course of mitochondrial disorders, but in the first stages of the disease, it can be misdiagnosed with the acute onset of an acquired inflammatory disorder. While mitochondrial disorders are already well-known as possible differential diagnosis of acquired inflammatory conditions affecting CNS, our two cases in addition to the other two previously published [6] underline the importance of including FDXR mutation in the differential diagnosis of inflammatory peripheral neuropathies, especially when an acquired origin is ruled out or not totally convincing.
In conclusion, FDXR biallelic mutations can be associated to a wide spectrum of clinical presentation; our cases suggest that peripheral neuropathy can be the predominant manifestation and neurophysiological data suggest that it could be related to a primary nodal impairment. In some case, peripheral neuropathy can be the presenting symptoms, and it can be misdiagnosed as an acquired inflammatory condition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Some authors of this publication are members of the European Reference Network for Rare Neurological Disease and European Reference Network for Neuromuscular Disease (IM, LC).
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S.M., R.P., D.T., M.O., I.M., and C.D. The first draft of the manuscript was written by S.M., R.P., D.T., M.O., and I.M., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Anonymized data that support the findings of this study are available from the corresponding author (S.M.) on reasonable request. Not all of the data are publicly available because they contain information that could compromise children’s privacy and their family consent.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study adheres to the principles of the Code of Ethics of the World Medical Association — Helsinki Declaration, and concerns data gathered during routine diagnostic activity. The study complied with our institutional regulations for anonymized retrospective studies.
Informed consent
The parents of the patients gave written consent for the analysis and publication of the data.
Statement on liability
The authors confirm the correctness of data and statement made in the manuscript.
Copyright
The authors confirm that this submitted manuscript represent original research not previously published and not considered for publication elsewhere.
Footnotes
Highlights
• FDXR disease spectrum includes a high incidence of cases presented with ataxia and peripheral neuropathy, which can be the predominant manifestation.
• Neurophysiological data suggest that peripheral polyneuropathy in FDXR disease could be related to a primary nodal impairment.
• FDXR can present at disease onset with an acute/sub-acute flaccid paralysis mimicking acquired inflammatory peripheral neuropathies.
• FDXR symptoms can be triggered by infections.
• Genetic conditions can mimic acquired inflammatory peripheral neuropathies.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paul A, Drecourt A, Petit F, Deguine DD, et al. FDXR mutations cause sensorial neuropathies and expand the spectrum of mitochondrial Fe-S-synthesis diseases. Am J Hum Genet. 2017;101(4):630–637. doi: 10.1016/j.ajhg.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slone J, Peng Y, Chamberlin A, Harris B, et al. Biallelic mutations in FDXR cause neurodegeneration associated with inflammation. J Hum Genet. 2018;63(12):1211–1222. doi: 10.1038/s10038-018-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slone JD, Yang L, Peng Y, Queme LF, et al. Integrated analysis of the molecular pathogenesis of FDXR-associated disease. Cell Death Dis. 2020;11(6):423. doi: 10.1038/s41419-020-2637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenton SL, Piekutowska-Abramczuk D, Kulterer L, Kopajtich R et al (2021) Expanding the clinical and genetic spectrum of FDXR deficiency by functional validation of variants of uncertain significance. Hum Mutat 42(3):310–319. 10.1002/humu.24160 [DOI] [PubMed]
- 5.Pezzani L, Marchetti D, Cereda A, Caffi LG et al (2018) Atypical presentation of pediatric BRAF RASopathy with acute encephalopathy. Am J Med Genet A 176(12):2867–2871. 10.1002/ajmg.a.40635 [DOI] [PubMed]
- 6.Jurkute N, Shanmugarajah PD, Hadjivassiliou M, Higgs J, et al. Expanding the FDXR-associated disease phenotype: retinal dystrophy is a recurrent ocular feature. Invest Ophthalmol Vis Sci. 2021;62(6):2. doi: 10.1167/iovs.62.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y, Shinde DN, Valencia CA, Mo JS, et al. Biallelic mutations in the ferredoxin reductase gene cause novel mitochondriopathy with optic atrophy. Hum Mol Genet. 2017;26(24):4937–4950. doi: 10.1093/hmg/ddx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SJ, Hong Y, Xu K, Zhang C (2021) Novel biallelic compound heterozygous mutations in FDXR cause optic atrophy in a young female patient: a case report. Int J Ophthalmol 14(11):1796–1798. 10.18240/ijo.2021.11.22 [DOI] [PMC free article] [PubMed]
- 9.Yang C, Zhang Y, Li J, Song Z, et al. Report of a case with ferredoxin reductase (FDXR) gene variants in a Chinese boy exhibiting hearing loss, visual impairment, and motor retardation. Int J Dev Neurosci. 2021;81(4):364–369. doi: 10.1002/jdn.10104. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso M, Orsucci D, Angelini C et al (2016) “Mitochondrial neuropathies”: a survey from the large cohort of the Italian Network Neuromuscul Disord. 26(4–5):272–6. 10.1016/j.nmd.2016.02.008 [DOI] [PubMed]
- 11.Luigetti M, Sauchelli D, Primiano G, Cuccagna C et al (2016) Peripheral neuropathy is a common manifestation of mitochondrial diseases: a single-centre experience. Eur J Neurol 23(6):1020–1027. 10.1111/ene.12954 [DOI] [PubMed]
- 12.Uncini A, Kuwabara SJ (2015) Nodopathies of the peripheral nerve: an emerging concept. Neurol Neurosurg Psychiatry 86(11):1186–95. 10.1136/jnnp-2014-310097 [DOI] [PubMed]
- 13.Uncini A, Santoro L (2020) The electrophysiology of axonal neuropathies: more than just evidence of axonal loss. Clin Neurophysiol 131(10):2367–2374. 10.1016/j.clinph.2020.07.014 [DOI] [PubMed]
- 14.Farrar MA, Lin CS, Krishnan AV, Park SB et al (2010) Acute, reversible axonal energy failure during stroke-like episodes in MELAS. Pediatrics 126(3):e734–e739. 10.1542/peds.2009-2930 [DOI] [PubMed]
- 15.Fernandez-Garcia MA, Stettner GM, Kinali M, Clarke A, et al. Genetic neuropathies presenting with CIDP-like features in childhood. Neuromuscul Disord. 2021;31(2):113–122. doi: 10.1016/j.nmd.2020.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data that support the findings of this study are available from the corresponding author (S.M.) on reasonable request. Not all of the data are publicly available because they contain information that could compromise children’s privacy and their family consent.