Abstract
The purpose of this work was to investigate, for the first time to our knowledge, the chemical composition and bioactivity of methanolic extracts (roots, stems, leaves, and flowers) from Cladanthus mixtus (L.) Chevall. that grows wild in northern Morocco (the Tangier-Tetouan-Al Hoceima region). The phenolic and flavonoid contents were determined by spectrophotometer methods, and the composition of derivatized methanolic extracts from C. mixtus using N-O-bis(trimethylsilyl) trifluoroacetamide (BSTFA) was analyzed by gas chromatography–mass spectrometry (GC-MS). The antioxidant activity was carried out by applying the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and DPPH (2,2-diphenyl-1-picrylhydrazyl) tests. The micro-dilution technique was chosen to investigate the antimicrobial activity of methanolic extracts against two bacterial strains and three fungal species. The results showed that the values of total phenolic and flavonoid contents were found to be higher in flower extracts (30.55 ± 0.85 mg of gallic acid equivalents (GAE)/g of dried weight (DW) and 26.00 ±1.34 mg of quercetin equivalents (QE)/g DW, respectively). Other groups of chemical compounds were revealed by GC-MS, such as carbohydrates (27.25–64.87%), fatty acids (1.58–9.08%), organic acids (11.81–18.82%), and amino acids (1.26–7.10%). Root and flower methanolic extracts showed the highest antioxidant activity using ABTS (39.49 mg of Trolox equivalents (TE)/g DW) and DPPH (36.23 mg TE/g DW), respectively. A positive correlation between antioxidant activity and polyphenol and flavonoid amounts was found. Antibacterial tests showed that the best activity was presented by the leaf extract against Staphylococcus aureus (minimum inhibitory concentration (MIC) = minimum bactericidal concentration (MBC) = 20 mg/mL) and Escherichia coli (MIC of 30 mg/mL and MBC of 35 mg/mL). S. aureus was more sensitive to the extracts compared to E. coli. All extracts showed antifungal activity against Trichophyton rubrum, with the best efficacy reported by the flower and leaf extracts (MIC = 1.25 mg/mL and minimum fungicidal concentration (MFC) = 2.5 mg/mL). In general, extracts of C. mixtus appeared less effective against Candida albicans and Aspergillus fumigatus.
Keywords: antioxidant activity, antibacterial activity, antifungal activity, Aspergillus fumigatus, Cladanthus mixtus, Candida albicans, Escherichia coli, medicinal-aromatic plants (MAPs), phytotherapy, Staphylococcus aureus, Trichophyton rubrum
1. Introduction
Plants are used in traditional medicine to treat a wide range of ailments. These medicinal-aromatic plants (MAPs) are well known for their biological activity. The World Health Organization estimates that over 80% of the global population still has confidence in conventional and folk medicine, mostly based on herbal remedies [1,2]. Herbs and plant-derived products have a long history of safe use as natural products in the treatment of various diseases [3].
Morocco is known for its beneficial geographical location as a country with Mediterranean and Atlantic coasts, which has contributed to an interesting plant diversity [4,5]. The Asteraceae family is the largest flowering herb family, with over 1700 genera and 34,000 species worldwide. It involves several plants with medicinal values, such as chamomile, wormwood, and dandelion, among others [6].
Studies have shown that some Asteraceae plants have many biological properties, such as antioxidant [7], antifungal [8], antibacterial [9], anti-inflammatory [10], and anticancer activities [11].
Cladanthus mixtus (L.) Chevall. (Moroccan chamomile or simple leaved chamomile, synonymous with C. mixtus (L.) Oberpr. and Vogt., Anthemis mixta L., Chamaemelum mixtum (L.) All., and Ormenis mixta subsp. mixta) belongs to the Asteraceae family [12]. This traditional medicinal plant is widespread in Morocco and in the northern and eastern zones of the Mediterranean basin. The flowers and leaves of C. mixtus are the most commonly used parts as an infusion to treat various diseases [13,14]. Furthermore, C. mixtus is used by therapists and herbalists as an antispasmodic, analgesic, antiallergic, anti-inflammatory, carminative, digestive, febrifuge, fungicide, vermifuge [15], antioxidant agent [16], and anticancer treatment [11].
In a previous study by El Mihyaoui et al. [11], the HPLC-MS analysis of methanolic extracts from C. mixtus revealed the presence of 23 phenolic compounds identified in the flowers and 24 compounds in the leaves, stems, and roots extracts; the GC-MS analysis of methanolic extracts without derivatization showed that C. mixtus is rich in biomolecules, including terpenoids, alcohols, esters, alkanes, fatty acids, organic acids, benzenes, phenols, ketones, sterols, carbonyls, amines, and other groups.
Plants can produce many diverse bioactive compounds, and several factors can affect the yield of these compounds, such as different extraction solvents and techniques as well as the particular isolation and purification of bioactive molecules [17].
The aim of this work is to investigate the chemical composition of methanolic extracts from different plant organs (flowers, leaves, stems, and roots) of wild Moroccan C. mixtus and to evaluate their biological activities, including antioxidant, antibacterial, and antifungal properties. To the best of our knowledge, this is the first comparative research study on the chemical characterizations and biological activities of different C. mixtus organs (flowers, leaves, stems, and roots) from northern Morocco (Tangier-Tetouan-Al Hoceima region).
2. Results
2.1. Extraction Yield and Total Polyphenol and Flavonoid Contents
The extraction yield was determined on 2 g of dry plant material and was expressed as a percentage. The results obtained are shown in Table 1. The flowers of C. mixtus gave the highest yield (25.86%). The roots also gave a good yield (20.65%), followed by leaves (19.40%), and stems (18.75%).
Table 1.
Extraction yield, polyphenol, and flavonoid contents in methanolic extracts of Cladanthus mixtus.
| Organs | Extraction Yield (%) | Polyphenols (mg GAE/g DW) | Flavonoids (mg QE/g DW) |
|---|---|---|---|
| Roots | 20.65 ± 2.86 | 18.83 ± 1.04 b,* | 8.74 ± 0.33 c |
| Stems | 18.75 ± 3.41 | 18.77 ± 0.39 b | 14.37 ± 0.55 b |
| Leaves | 19.40 ± 4.11 | 16.43 ± 0.32 b | 13.24 ± 0.05 b |
| Flowers | 25.86 ± 0.73 | 30.55 ± 0.85 a | 26.00 ± 1.34 a |
* Values are expressed as the mean ± SD of three replicates. Different letters indicate significant differences between the organs in the same column at p < 0.05.
The results in Table 1 also illustrated the polyphenol and flavonoid contents of methanolic extracts of the different organs of C. mixtus. The polyphenol contents ranged from 16.43 to 30.55 mg GAE/g DW. The content was significantly higher in flowers (p < 0.05). Roots and stems showed almost the same content (18.83 and 18.77 mg GAE/g DW, respectively), while the lowest content was observed in leaves (16.43 mg GAE/g DW). Concerning flavonoids, the contents ranged from 8.74 to 26.00 mg QE/g DW, following the order: flowers > stems > leaves > roots. Consequently, flowers showed the highest content of polyphenols and flavonoids.
2.2. Biochemical Constituents of Cladanthus mixtus Organs by GC-MS
GC-MS chromatograms of derivatized extracts from flowers, leaves, stems, and roots of C. mixtus at different retention times (Figure 1, Figure 2, Figure 3 and Figure 4) revealed the presence of 42, 74, 70, and 83 phytochemical compounds, respectively. These compounds can be mainly divided into six groups, including carbohydrates, lactones, organic acids, fatty acids, phenols, amino acids, and other biomolecule groups. In general, the extracts from flowers, leaves, stems, and roots were dominated by sugars (27.25, 54.8, 64.87, and 62.57%, respectively) (Table 2). Sucrose was observed to be the main biomolecule detected in roots (22.47%), flowers (17.01%), and stems (14.84%), while myo-inositol was the major compound identified in leaves (9.38%).
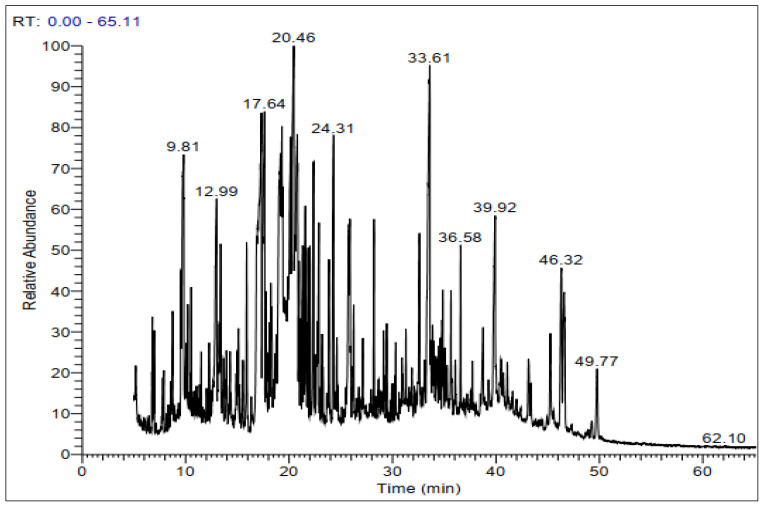
Figure 1.
Representative GC-MS chromatogram of derivatized methanolic extract of C. mixtus flowers.
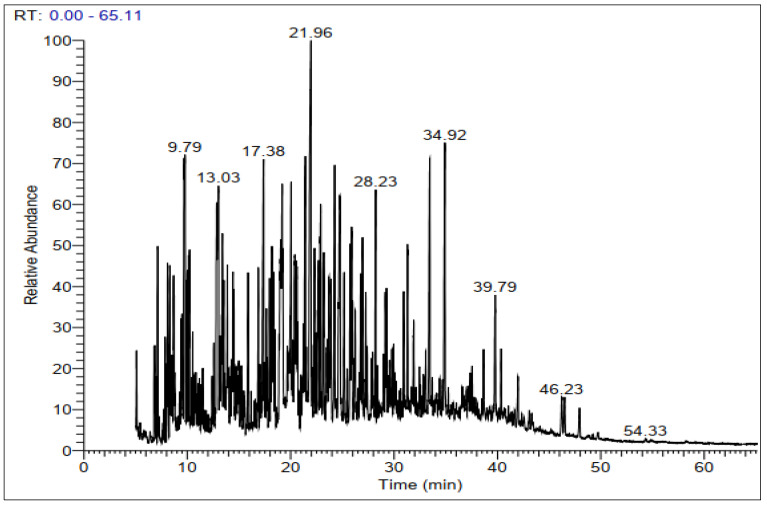
Figure 2.
Representative GC-MS chromatogram of derivatized methanolic extract of C. mixtus leaves.
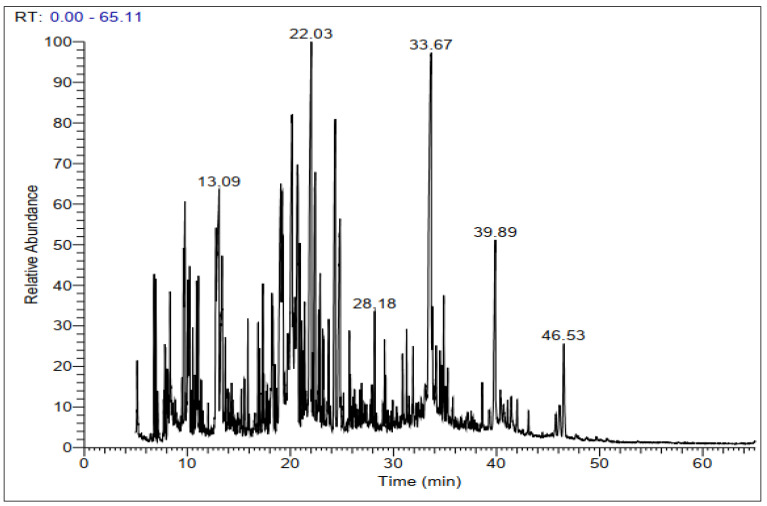
Figure 3.
Representative GC-MS chromatogram of derivatized methanolic extract of C. mixtus stems.
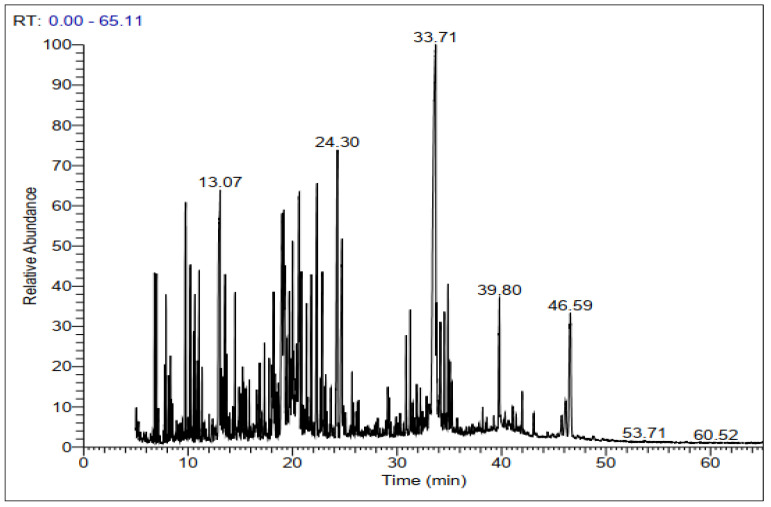
Figure 4.
Representative GC-MS chromatogram of derivatized methanolic extract of C. mixtus roots.
Table 2.
Biomolecule groups of derivatized methanolic extracts from Cladanthus mixtus analyzed by GC-MS.
| Area (%) | ||||
|---|---|---|---|---|
| Compound Groups | Flowers | Leaves | Stems | Roots |
| Carbohydrates | 27.25 | 54.80 | 64.87 | 62.57 |
| Lactones | 24.60 | -- | -- | -- |
| Organic acids | 11.81 | 18.82 | 18.68 | 13.00 |
| Fatty acids | 8.13 | 9.08 | 1.58 | 3.18 |
| Phenols | 7.08 | 1.50 | 8.46 | 6.66 |
| Amino acids | -- | 7.10 | 1.26 | 2.28 |
| Others | 21.13 | 8.69 | 5.15 | 12.31 |
| Total | 100 | 99.99 | 100 | 100 |
(--): Not detected.
As presented in Table 3, the results of derivatized methanolic extracts from C. mixtus showed that carbohydrates were important and varied. Flower extracts contained 10 compounds, representing 27.25% of the total biomolecules detected, and sucrose (17.01%) was the main constituent identified. In contrast, 20 biomolecules were identified in leaf extracts, representing 54.80% of leaf compounds, which were dominated by myo-inositol (9.38%), meso-erythritol (6.60%), and glucose (6.39%). In stem and root extracts, 28 and 27 compounds were detected, representing 64.87% and 62.57% of compounds, with sucrose (14.84% and 22.47%), myo-inositol (13.9% and 6.65%), and D-fructofuranose (7.37% and 8.46%) as the major carbohydrates in both organ extracts, respectively.
Table 3.
Carbohydrate composition of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| Sucrose | C12H22O11 | 342.2 | 17.01 | 0.13 | 14.84 | 22.47 |
| Myo-Inositol | C6H12O6 | 180.1 | -- | 9.38 | 13.9 | 6.65 |
| Xylitol | C5H12O5 | 152.1 | -- | 6.15 | 0.66 | -- |
| Trehalose | C12H22O11 | 342.3 | 1.22 | 4.61 | 0.98 | 3.93 |
| Glucose | C6H12O6 | 180.1 | -- | 6.39 | 0.25 | 0.23 |
| D-Fructofuranose | C6H12O6 | 180.1 | -- | 3.19 | 7.37 | 8.46 |
| α-Lactose | C12H22O11 | 342.3 | 0.63 | 2.04 | 0.38 | 0.23 |
| D-Allofuranose | C6H12O6 | 180.1 | -- | -- | 0.03 | 5.22 |
| Glyceric acid | C3H6O4 | 106.0 | 0.84 | 0.41 | 0.59 | 0.59 |
| α-Mannobiose | C12H22O11 | 342.1 | 2.00 | 0.33 | 0.79 | 0.21 |
| D-Galacturonic acid | C6H10O7 | 194.1 | 0.74 | 2.14 | 0.52 | -- |
| D-Ribofuranose | C5H10O5 | 150.1 | 1.40 | -- | 0.20 | 1.65 |
| Lactitol | C12H24O11 | 344.3 | 1.74 | -- | -- | -- |
| Turanose | C12H22O11 | 342.3 | 1.57 | 0.51 | 1.78 | 0.73 |
| Palatinose | C12H22O11 | 342.3 | 0.10 | -- | -- | -- |
| D-Glucosamine | C6H13NO5 | 179.1 | -- | 0.69 | -- | -- |
| meso-Erythritol | C4H10O4 | 122.1 | -- | 6.60 | -- | -- |
| Levoglucosan | C6H10O5 | 162.1 | -- | 1.53 | -- | -- |
| D-Glucitol | C6H14O6 | 182.1 | -- | 3.08 | 4.47 | -- |
| D-Gluconic acid | C6H12O7 | 196.1 | -- | 1.89 | 2.29 | 0.47 |
| Valproic acid glucuronide | C14H24O8 | 320.3 | -- | 1.34 | -- | -- |
| D-Glucopyranose | C6H12O6 | 180.1 | -- | 2.11 | -- | -- |
| Galactinol | C12H22O11 | 342.3 | -- | 1.65 | -- | 0.16 |
| α-D-Mannopyranose | C6H12O6 | 180.1 | -- | 0.63 | -- | -- |
| Glycerol | C3H8O3 | 92.0 | -- | -- | 3.14 | -- |
| Arabinonic acid, 1,4-lactone | C5H8O5 | 148.1 | -- | -- | 0.21 | -- |
| D-Glucuronic acid | C6H10O7 | 194.1 | -- | -- | 0.34 | |
| D-Glucuronic acid-γ-lactone | C6H8O6 | 176.1 | -- | -- | 0.61 | -- |
| 3-Deoxy-ribo-hexonic acid, 1,4-lactone | C6H10O5 | 162.1 | -- | -- | 2.14 | -- |
| D-Glucopyranose | C6H12O6 | 180.1 | -- | -- | 3.75 | 3.79 |
| D-Galactopyranoside (= D-galactose) | C6H12O6 | 180.1 | -- | -- | 0.56 | -- |
| Methyl galactoside | C7H14O6 | 194.1 | -- | -- | 0.33 | -- |
| 1,5-Anhydrohexitol | C6H12O5 | 164.1 | -- | -- | 1.01 | -- |
| Glyceryl-glycoside | C27H66O8Si6 | 687.3 | -- | -- | 0.64 | -- |
| Arabinofuranose | C5H10O5 | 150.1 | -- | -- | 0.66 | -- |
| D-Arabinopyranose | C5H10O5 | 150.1 | -- | -- | -- | 0.34 |
| D-Allopyranose | C6H12O6 | 180.1 | -- | -- | 0.62 | 1.22 |
| D-Tagatofuranose | C6H12O6 | 180.1 | -- | -- | 0.24 | -- |
| D-Arabitol | C5H12O5 | 152.1 | -- | -- | 1.91 | 1.10 |
| D-Ribofuranose | C5H10O5 | 150.1 | -- | -- | -- | 0.46 |
| 1,5-Anhydrohexitol | C6H12O5 | 164.1 | -- | -- | -- | 0.92 |
| D-Psicose | C6H12O6 | 180.1 | -- | -- | -- | 1.11 |
| Dulcitol | C6H14O6 | 182.1 | -- | -- | -- | 0.84 |
| α-Glucopyranose phosphate | C6H13O9P | 260.1 | -- | -- | -- | 0.22 |
| Beta-D-Lactose, (isomer 2) | C12H22O11 | 342.3 | -- | -- | -- | 0.11 |
| D-Psicofuranose | C6H12O6 | 180.1 | -- | -- | -- | 0.16 |
| Maltose | C12H22O11 | 342.3 | -- | -- | -- | 0.39 |
| Dihydroxyacetone | C3H6O3 | 90.0 | -- | -- | -- | 0.58 |
| Total | 27.25 | 54.8 | 64.87 | 62.57 | ||
(--): Not detected.
Concerning lactones, the GC-MS analysis showed their presence only in flower extracts (Table 4). The extract contained 2 compounds: D-glucurono-γ-lactone (24.26%) and erythrono-1,4-lactone (0.34%).
Table 4.
Lactones of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| D-Glucurono-γ-lactone (isomer 1) | C6H8O6 | 176.12 | 24.26 | -- | -- | -- |
| Erythrono-1,4-lactone | C4H6O4 | 118.09 | 0.34 | -- | -- | -- |
| Total | 24.60 | -- | -- | -- | ||
(--): Not detected.
Following carbohydrates, organic acids represented an important percentage of biomolecules identified in C. mixtus derivatized extracts, whereas 11.81%, 18.82%, 17.66%, and 13.00% of organic acids were observed in flowers (5 compounds), leaves (10 compounds), stems (8 compounds), and roots (11 compounds), respectively (Table 5). Malic acid was the major organic acid detected in the four organ extracts.
Table 5.
Organic acids of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| Malic acid | C4H6O5 | 134.0 | 9.16 | 6.02 | 12.26 | 6.67 |
| Malonic acid | C3H4O4 | 104.0 | 1.06 | 1.45 | -- | -- |
| Lactic acid | C3H6O3 | 90.0 | 0.76 | 0.45 | 0.83 | 0.71 |
| Methyl malonate | C8H14O4 | 174.1 | 0.43 | -- | -- | -- |
| Glycolic acid | C2H4O3 | 76.0 | 0.40 | 0.30 | 0.57 | 0.67 |
| 3-Hydroxypropionic acid | C3H6O3 | 90.0 | -- | 0.38 | 0.61 | 0.6 |
| Succinic Acid | C4H6O4 | 118.0 | -- | 2.12 | 1.85 | -- |
| 3-Hydroxyisobutyric acid | C4H8O3 | 104.1 | -- | 1.46 | 0.52 | 0.38 |
| 3-Hydroxyadipic acid | C6H10O5 | 162.1 | -- | 0.51 | -- | -- |
| Citric acid | C6H8O7 | 192.1 | -- | 3.71 | -- | -- |
| Quininic acid | C11H9NO3 | 203.1 | -- | 2.42 | -- | 1.90 |
| Phosphoric acid, monomethyl ester | CH5O4P | 112.0 | -- | -- | -- | 0.16 |
| Propyl acetate | C5H10O2 | 102.1 | -- | -- | 0.93 | -- |
| Fumaric acid | C4H4O4 | 116.0 | -- | -- | 0.09 | -- |
| 2-Furoic acid | C5H4O3 | 112.0 | -- | -- | -- | 0.43 |
| Methyl 3-methoxyacrylate | C5H8O3 | 116.1 | -- | -- | -- | 0.44 |
| Pyruvic acid | C3H4O3 | 88.06 | -- | -- | -- | 0.64 |
| (R)-3-Hydroxybutyric acid | C4H8O3 | 104.1 | -- | -- | -- | 0.40 |
| Total | 11.81 | 18.82 | 17.66 | 13.00 | ||
(--): Not detected.
Regarding fatty acids (Table 6), 2 molecules were detected in the flower and stem extracts, with linoelaidic acid (6.73%) as the main compound in the flowers and dimethyl malate (1.05%) in the stems. Leaf and root extracts contained 7 and 6 compounds, respectively, with docosahexaenoic acid (2.71%) as a major component in leaves and palmitic acid (1.83%) in roots.
Table 6.
Fatty acids of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| Linoelaidic acid | C18H32O2 | 280.4 | 6.73 | 1.63 | -- | 0.38 |
| Palmitic Acid | C16H32O2 | 256.4 | -- | 2.45 | -- | 1.83 |
| Stearic acid | C18H36O2 | 284.4 | 1.40 | 0.73 | -- | 0.18 |
| Dimethyl malate | C6H10O5 | 162.1 | -- | -- | 1.05 | -- |
| Elaidic Acid | C18H34O2 | 282.5 | -- | -- | 0.22 | |
| Hexanoic acid (Caproic acid) | C6H12O2 | 116.1 | -- | -- | 0.18 | |
| Docosahexaenoic acid | C22H32O2 | 328.5 | -- | 2.71 | -- | -- |
| β-D-Glucopyranosiduronic acid, 3-(5-ethylhexahydro-2,4,6-trioxo-5-pyrimidinyl)-1,1-dimethylpropyl 2,3,4-tris-O-(trimethylsilyl)-, methyl ester | C27H52N2O10Si3 | 649.0 | -- | 0.16 | -- | -- |
| 2-Hydroxybut-2-enedioic acid | C4H4O5 | 132.0 | -- | 0.56 | -- | -- |
| 1-Monopalmitin | C19H38O4 | 330.5 | -- | 0.84 | 0.53 | 0.39 |
| Total | 8.13 | 9.08 | 1.58 | 3.18 | ||
(--): Not detected.
For phenolics, the GC-MS analysis illustrated the presence of 2 biomolecules in flowers: chlorogenic acid (5.92%) and naringenin (1.16%). However, chlorogenic acid (1.5%) was the only molecule detected in the leaves. Otherwise, chlorogenic acid and caffeic acid were the phenolics identified in the stem (4.03% and 4.43%) and root (2.26% and 4.01%) extracts, respectively (Table 7).
Table 7.
Phenolics of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| Chlorogenic acid | C16H18O9 | 354.3 | 5.92 | 1.50 | 4.03 | 2.26 |
| Caffeic acid | C9H8O4 | 180.1 | -- | -- | 4.43 | 4.01 |
| Naringenin | C15H12O5 | 272.2 | 1.16 | -- | -- | -- |
| Isovanillic acid | C8H8O4 | 168.1 | -- | -- | -- | 0.39 |
| Total | 7.08 | 1.50 | 8.46 | 6.66 | ||
(--): Not detected.
Concerning amino acids, the results differed widely between the studied organs (Table 8), whereas no amino acids were detected in flower extracts, unlike the 6 compounds identified in leaves and 2 in each of the stem and root extracts, representing a total of 7.10%, 1.26%, and 2.28%, respectively.
Table 8.
Amino acids of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| L-Homoserine | C4H9NO3 | 119.1 | -- | 1.28 | -- | -- |
| Proline | C5H9NO2 | 115.1 | -- | 1.52 | -- | -- |
| Serine | C3H7NO3 | 105.0 | -- | 1.37 | -- | -- |
| Threonine | C4H9NO3 | 119.1 | -- | 1.44 | 0.62 | -- |
| Leucine | C6H13NO2 | 131.1 | -- | 0.34 | -- | -- |
| Pidolic acid | C5H7NO3 | 129.1 | -- | 1.15 | -- | 1.91 |
| DL-Homophenylalanine | C10H13NO2 | 179.2 | -- | -- | -- | 0.37 |
| Sarcosine | C3H7NO2 | 89.0 | -- | -- | 0.64 | -- |
| Total | -- | 7.10 | 1.26 | 2.28 | ||
(--): Not detected.
For the rest of the biomolecules obtained by GC-MS (Table 9), many biological groups were detected, including terpenoids, alcohols, benzenoids, alkanes, and pyrimidines. In C. mixtus roots, 19 molecules (12.31%) were reported, while 14 molecules (21.13%) were reported in flowers, 14 molecules (8.69%) in leaves, and 11 molecules (5.15%) in stems.
Table 9.
Chemical composition of other molecules of derivatized methanolic extracts obtained by GC-MS.
| Area (%) | ||||||
|---|---|---|---|---|---|---|
| Molecules | Chemical Formula | Molecular Weight | Flowers | Leaves | Stems | Roots |
| Ethaneperoxoic acid, 1-cyano-4,4-dimethyl-1-phenylpentyl ester | C16H21NO3 | 275.3 | 0.24 | -- | -- | -- |
| 4-Methyl-2-(2-nitro-5-piperidin-1-yl-phenyl)-2H-phthalazin-1-one | C20H20N4O3 | 364.4 | -- | 1.84 | -- | -- |
| Salbutamol | C13H21NO3 | 239.3 | -- | -- | 0.05 | -- |
| 2-(2-Methoxyphenyl)propan-2-ol | C10H14O2 | 166.2 | -- | -- | -- | 0.13 |
| Terephthalic acid, di(4,4-dimethylpent-3-yl) ester | C22H34O4 | 362.5 | -- | -- | -- | 1.98 |
| N-Isopropyl-2-[4-(1,2,3-thiadiazol-4-yl) phenyl]hydrazine-1-carboxamide | C12H15N5OS | 277.3 | -- | -- | -- | 1.31 |
| Propanedinitrile, 2-(5-phenylthio-2-thienylmethylene)- | C14H8N2S2 | 268.4 | -- | -- | -- | 0.48 |
| Phosphonic acid | H2O3P+ | 80.9 | -- | -- | 1.02 | -- |
| Phytol | C20H40O | 296.5 | -- | 1.24 | -- | |
| Stigmasterol | C29H48O | 412.7 | -- | 0.53 | 0.30 | 0.43 |
| β-Sitosterol | C29H50O | 414.7 | -- | 0.19 | 0.21 | 0.24 |
| Aucubin | C15H22O9 | 346.3 | -- | 0.54 | -- | -- |
| Lupenyl acetate | C32H52O2 | 468.8 | 0.37 | 0.05 | -- | -- |
| Ethyl cholate | C26H44O5 | 436.6 | 1.14 | -- | -- | -- |
| alpha-Amyrin | C30H50O | 426.7 | 0.54 | -- | -- | -- |
| Lanost-8-en-26-oic acid, 3α,12α-dihydroxy-24-methylene-, methyl ester | C34H54O5 | 542.7 | -- | -- | 0.02 | |
| 1,2,4,5-Cyclohexanetetrol | C6H12O4 | 148.1 | 0.66 | -- | -- | -- |
| 5-p-Coumaroylquinic acid | C16H18O8 | 338.1 | 0.80 | -- | -- | -- |
| 1,2-Ethenediol | C2H4O2 | 60.0 | 0.04 | -- | -- | -- |
| Tetracosane | C24H50 | 338.6 | 0.64 | -- | -- | -- |
| Octadecylcyclohexane | C24H48 | 336.6 | 0.04 | -- | -- | -- |
| 5-Methyluridine | C10H14N2O6 | 258.2 | 3.68 | 0.63 | -- | -- |
| 5-Methylcytosine | C5H7N3O | 125.1 | 0.53 | -- | -- | 0.81 |
| Deanol | C4H11NO | 89.1 | 1.17 | -- | 0.70 | -- |
| Copper phthalocyanine | C32H16CuN8 | 576.1 | 1.01 | -- | -- | -- |
| Silanol, trimethyl-, phosphate (3:1) | H3PO4 or H3O4P | 97.995 | 10.27 | -- | -- | 3.20 |
| 17-Pentatriacontene | C35H70 | 490.9 | -- | 0.06 | -- | -- |
| Tricyclo [20.8.0.0(7,16)]triacontane, 1(22),7(16)-diepoxy- | C30H52O2 | 444.7 | -- | 0.06 | -- | -- |
| 6-Ethoxy-4-methylquinoline-2-thiol | C12H13NOS | 219.3 | -- | 1.50 | -- | -- |
| Uridine | C9H12N2O6 | 244.2 | -- | 0.34 | -- | 0.15 |
| 2-Oxa-3-azabicyclo [4.4.0]dec-3-ene, 5-methyl-1-trimethylsilyloxy-, N-oxide | C12H23NO3Si | 257.0 | -- | 0.69 | -- | -- |
| 2-Methoxyethyl(trimethyl)silane | C6H16OSi | 132.2 | -- | 0.62 | -- | -- |
| Emulphor | C20H40O2 | 312.5 | -- | 0.40 | ||
| 1,3-Propanediol | C3H8O2 | 76.0 | -- | -- | 0.95 | -- |
| Ethylene glycol | C2H6O2 | 62.0 | -- | -- | 0.32 | -- |
| Dodecamethyl-pentasiloxane | C12H36O4Si5 | 384.8 | -- | -- | 1.60 | -- |
| 2,3-Dihydroxypropyl acetate | C5H10O4 | 134.1 | -- | -- | 0.20 | -- |
| Glycerol monostearate | C21H42O4 | 358.6 | -- | -- | 0.27 | -- |
| Cortisol | C21H30O5 | 362.5 | -- | -- | 0.53 | -- |
| Hexadecyl (E)-m-coumarate | 0.02 | 0.03 | ||||
| Isopropyl alcohol | C3H8O | 60.1 | -- | -- | -- | 0.23 |
| 1-Octanol | C8H18O | 130.2 | -- | -- | -- | 0.14 |
| 2-Butene-1,4-diol | C4H8O2 | 88.1 | -- | -- | -- | 1.09 |
| 2-(2-Chloroethyl)-1-methylpyrrolidine | C7H14ClN | 147.6 | -- | -- | -- | 0.23 |
| Acetamide | C2H5NO | 59.0 | -- | -- | -- | 0.06 |
| Silane, diethylheptyloxyisobutoxy- | C15H34O2Si | 274.5 | -- | -- | -- | 1.00 |
| α-Glycerophosphoric acid | C3H9O6P | 172.0 | -- | -- | -- | 0.78 |
| Total | 21.13 | 8.69 | 5.15 | 12.31 | ||
(--): Not detected.
2.3. Antioxidant Activity
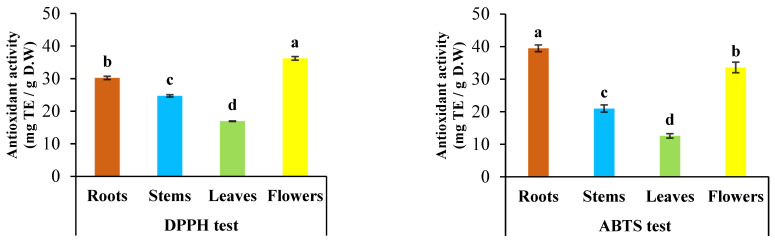
The antioxidant activity of methanolic extracts from different organs of C. mixtus was determined by DPPH and ABTS methods, and the results are shown in Figure 5. The DPPH results ranged from 16.96 to 36.23 mg TE/g DW, with a significant difference between all organs (p < 0.05). Flowers showed the highest activity (36.23 mg TE/g DW), followed by roots (30.24 mg TE/g DW), stems (24.72 mg TE/g DW), and leaves (16.96 mg TE/g DW). For the antioxidant activity of C. mixtus extracts determined by the ABTS method, the results were significantly different (p < 0.05) between the four organs, with values ranging from 12.58 to 39.49 mg TE/g DW. The strongest antioxidant activity was obtained from roots (39.49 mg TE/g DW), followed by flowers (33.6 mg TE/g DW), stems (20.99 mg TE/g DW), and leaves (12.58 mg TE/g DW).
Figure 5.
Antioxidant activity of different organ extracts of Cladanthus mixtus by the DPPH and ABTS tests. Different letters indicate significant differences between the organs of one plant at p < 0.05. Values are means ± S.D. for three replicates.
2.4. Antibacterial Activity
The extracts of the four organs showed antibacterial activity in vitro against the Gram-positive and Gram-negative bacterial strains S. aureus and E. coli (Table 10). S. aureus was most sensitive to the leaf and flower extracts (MIC = MBC = 20 and 32 mg/mL, respectively). Moreover, the stem and root extracts were also active against S. aureus, with MIC and MBC values of 40 mg/mL. For E. coli, the leaf extracts showed the best activity, with an MIC value of 30 mg/mL and MBC of 35 mg/mL. The other extracts from the roots, stems, and flowers showed activity against E. coli with MIC and MBC values of 40 mg/mL. Between the two strains tested, S. aureus was more sensitive to the extracts.
Table 10.
MIC and MBC values of methanolic extracts of Cladanthus mixtus and a reference antibiotic agent.
| Methanolic Extracts (mg/mL) | Bacterial Species | |||
|---|---|---|---|---|
| Gram-Positive Staphylococcus aureus |
Gram-Negative Escherichia coli |
|||
| MIC | MBC | MIC | MBC | |
| Roots | 40.00 ± 0.00 | 40.00 ± 0.00 | 40.00 ± 0.00 | 40.00 ± 0.00 |
| Stems | 40.00 ± 0.00 | 40.00 ± 0.00 | 40.00 ± 0.00 | >40.00 |
| Leaves | 20.00 ± 0.00 | 20.00 ± 0.00 | 30.00 ± 10.00 | 35.00 ± 8.66 |
| Flowers | 32.00 ± 9.80 | 32.00 ± 9.80 | 40.00 ± 0.00 | 40.00 ± 0.00 |
| Gentamicin (µg/mL) | 0.33 ± 0.11 | 32.00 ± 1.63 | 2.00 ± 0.00 | 64.00 ± 3.26 |
The results are presented as mean ± SD (n = 6).
When comparing the antibacterial activity of the extracts with that of a reference antibiotic (gentamicin), the difference in potency was found to be very significant, with gentamicin being more effective with lower MIC and MBC values ranging from 0.33 to 2 µg/mL and 32 to 64 µg/mL, respectively.
2.5. Antifungal Activity
Methanolic extracts of four C. mixtus organs were evaluated for their antifungal properties against three human pathogens, including one yeast (Candida albicans) and two filamentous fungi (Trichophyton rubrum and Aspergillus fumigatus). The MIC and MFC values were determined and summarized in Table 11. The three fungal pathogens were tested at concentrations ranging from 1.25 to 40 mg/mL. The results showed that C. mixtus extracts appeared to be less effective against C. albicans and A. fumigatus than against T. rubrum. The fungus A. fumigatus was noted to be the most resistant to all extracts tested, with MIC and MFC values higher than 40 mg/mL. In the activity against C. albicans, the best result was reported by the stem extracts, with a MIC value of 40 mg/mL and MFC higher than 40 mg/mL, unlike the other extracts, which showed MIC and MFC values higher than 40 mg/mL. Concerning the activity against the T. rubrum strain, all extracts showed important antifungal activity, with the highest efficacy reported by the flower and leaf extracts (MIC = 1.25 and MFC = 2.5 mg/mL). Roots and stems extracts also showed antifungal properties, with MIC = 2.5 mg/mL and MFC = 5 mg/mL for roots and MIC = 3.57 mg/mL and MFC = 6.67 mg/mL for stems.
Table 11.
MIC and MFC values of methanolic extracts of Cladanthus mixtus and a reference antifungal agent.
| Methanolic Extracts (mg/mL) | Fungal Species | |||||
|---|---|---|---|---|---|---|
| Candida albicans | Trichophyton rubrum | Aspergillus fumigatus | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| Roots | >40 | >40 | 2.5 ± 0.00 | 5.00 ± 0.00 | >40 | >40 |
| Stems | 40.00 ± 0.00 | >40 | 3.57 ± 1.24 | 6.67 ± 2.36 | >40 | >40 |
| Leaves | >40 | >40 | 1.25 ± 0.00 | 2.5 ± 0.00 | >40 | >40 |
| Flowers | >40 | >40 | 1.25 ± 0.00 | 2.5 ± 0.00 | >40 | >40 |
| Voriconazole (µg/mL) | 0.25 ± 0.00 | 4.00 ± 0.00 | 0.12 ± 0.00 | 1.66 ± 0.47 | 0.25 ± 0.00 | 0.66 ± 0.23 |
The results are presented as the mean (n = 6).
Compared to the reference antifungal voriconazole, the difference in activity was very significant; the latter was much more potent than the extract, with MIC and MFC values between 0.12 and 0.25 µg/mL and 0.66 and 4 µg/mL, respectively.
3. Discussion
Plant extracts containing phytochemicals are increasingly marketed as products with a positive impact on the human health system. This work aimed to characterize the metabolites of four organs from C. mixtus and evaluate their antioxidant, antibacterial, and antifungal activities.
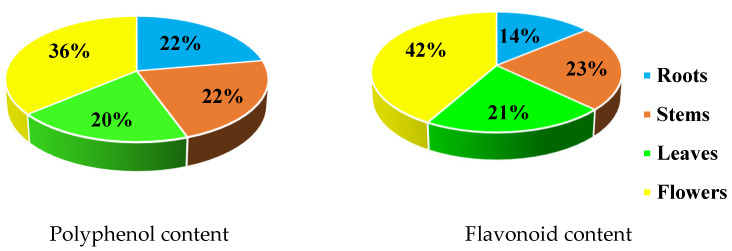
Methanolic extracts of C. mixtus flowers showed the highest content of polyphenols (36%), followed by flavonoids (42%) (Figure 6). Polyphenol contents were similar in roots and stems (22%), followed by leaves (20%). For flavonoids, the stems showed a content close to that of the leaves (23% and 21%, respectively), followed by the roots (14%).
Figure 6.
Percentage of polyphenols and flavonoids in methanolic extracts of Cladanthus mixtus.
The total phenolic content of the C. mixtus flower extract found in this work (30.55 mg GAE/g DW) is slightly higher than that found in the flower extract of a similar study with Italian Matricaria chamomilla (2689.2 mg GAE/100 g DW) [18] and much higher than a study with Egyptian M. chamomilla (3.7 mg GAE/g DW) [19] and a commercial M. chamomilla product from the United Arab Emirates (21.4 mg GAE/g DW) [20].
In a previous study, aerial part extracts of C. mixtus (obtained from Bouznika, Morocco) presented a total phenolic content of 19.5 mg GAE/g DW in a methanolic extract and 38.2 mg GAE/g DW in an aqueous extract [21], showing a lower content than that found in current research (65.75 mg GAE/g DW). Elouaddari et al. [21] also reported that in aerial part extracts of C. mixtus, the total flavonoid content was 2.7 and 3.2 mg QE/g DW in aqueous and methanolic extracts, respectively. In this investigation, our estimation showed a much greater amount of flavonoid content (53.61 mg QE/g DW) (Table 1) than in the aforementioned study by Elouaddari et al. [21]. The differences in extraction methods and the solvent and timing used may influence the composition of polyphenols, flavonoids, and other compounds and therefore also affect their biological activities.
In the present study, we applied the endpoint method developed by Arnao et al. [22]. This technique uses 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) as a chromogen to estimate total antioxidant activity. It is a robust method, that is widely used and applied to various biological samples [23]. Although this method was originally developed for the study of plant foodstuffs, it can also be used to characterize plant extracts [16]. The ABTS·+ chromogen used in our method was compared to another widely used radical chromogen, which is 2,2-diphenyl-1-picrylhydrazyl (DPPH·). Furthermore, the extraction method and the solvent used can influence the composition of polyphenols, flavonoids, and other compounds and therefore also affect the antioxidant activity [19,24].
Zeroual et al. [25] showed that methanolic extracts of C. mixtus flowers had the highest free radical scavenging activity (IC50 = 55.50 µg/mL), followed by the ethanolic extract (IC50 = 121.5 µg/mL), ethyl acetate (IC50 = 240.9 µg/mL), and n-hexane (IC50 = 259 µg/mL). Similarly, the DPPH test showed that methanolic extracts of chamomile flowers (Matricaria chamomilla) had the strongest antiradical power (IC50 = 0.0022 µMoles) compared to ethanolic, diethyl ether, and hexane extracts [19]. In contrast, methanolic extracts of the aerial part of C. mixtus showed lower antioxidant activity than the aqueous extract by DPPH and ABTS tests [21].
Furthermore, the choice of extraction organ plays an important role in antioxidant activity. In our study, we chose to separate the plant organs (flowers, leaves, stems, and roots) to compare their antioxidant activity. Methanolic extracts of the roots showed the best activity with ABTS, while the flowers showed a better result with DPPH. On the other hand, the leaves of C. mixtus showed the lowest activity in both tests.
A correlation analysis was carried out to study the relationship between the phenolic and flavonoid contents of the extracts and their antioxidant activities (Table 12). Methanolic extracts of stem, leaf, and flower showed a very significant positive correlation between the content of phenolics and the antioxidant activity by the ABTS (r² = 0.93, p < 0.05) and DPPH (r2 = 0.94, p < 0.05) tests. These organs also showed a strong correlation between their total flavonoid content and antioxidant activity by the ABTS (r² = 0.89, p < 0.05) and DPPH (r2 = 0.90, p < 0.05) tests.
Table 12.
Correlation between antioxidant activities and total phenol and flavonoid contents of Cladanthus mixtus extracts.
| Parameters Relationship | Organ | Equation | r2 * |
|---|---|---|---|
| DPPH Test | |||
| Polyphenol content vs. antioxidant activity | Stems, leaves, and flowers | y = 1.2486x − 1.5089 | 0.94 |
| Flavonoid content vs. antioxidant activity | Stems, leaves, and flowers | y = 1.3098x + 2.4508 | 0.90 |
| ABTS Test | |||
| Polyphenol content vs. antioxidant activity | Stems, leaves, and flowers | y = 1.3533x − 7.2704 | 0.93 |
| Flavonoid content vs. antioxidant activity | Stems, leaves, and flowers | y = 1.4176x − 2.9432 | 0.89 |
* Indicates a significant correlation at p < 0.05.
The ability of flavonoids to act as in vitro antioxidants has been the subject of several studies in recent years, and important structure-activity relationships for antioxidant activity have been established [26,27]. Almost all flavonoid groups can act as antioxidants, but it has been reported that flavones and catechins appear to be the most potent flavonoids in protecting the body against reactive oxygen species [28].
Moreover, many studies have reported the benefits of phenolic compounds, such as vanillin, that have antioxidant and antidepressant activities and neuroprotective, antimutagenic, and anticarcinogenic effects [29].
Based on the GC-MS analysis, the present research reveals that derivatized methanolic extracts from C. mixtus contain carbohydrates, lactones, fatty acids, organic acids, amino acids, terpenoids, alcohols, phenolics, alkanes, and other compounds, with carbohydrate compositions in abundance. Comparing these results with others obtained of methanolic extracts from the same plant without derivatization [11], we found that the flower extracts were dominated by fatty acids (27.86%), leaf extracts by terpenoids (46.20%), stem extracts by esters (30.11%), and root extracts by alcohols (24.49%) and esters (21.91%). The choice of the derivatization technique was made to improve and increase the volatility, sensitivity, thermal stability, greater selectivity, and separation behavior of the analytes [30]. Another study on aqueous extracts of two Moroccan chamomiles, C. mixtus and M. chamomilla, discovered the presence of alkaloids, terpenoids, saponins, flavonoids, and tannins, but not anthraquinones [31].
Elouaddari et al. (2019) [32] examined the chemical composition and biological activities of C. mixtus essential oils (EOs). According to the authors review, a total of 264 compounds constitute the EOs of C. mixtus, which vary greatly depending on diverse parameters, including geographies, plant parts, extraction methods, and ecological factors. The distribution of these chemicals is as follows: oxygenated monoterpenes (30–45.3%), sesquiterpene hydrocarbons (14–33.9%), monoterpene hydrocarbons (15–24.5%), sesquiterpenes (4–11.7%), and others (traces–4.5%). Many properties, including antimicrobial, anticorrosive, and cytotoxic activity against human cervical cancer cell lines, are caused by these biomolecules.
Our results of HPLC-MS analysis reported in the previous study [11] showed that the different methanolic extracts of the C. mixtus plant are very rich in glycosides and aglycones (luteolin, apigenin, luteolin-7-O-glucoside, apigenin-7-glucoside, quercetin, rutin, naringin, catechin, vanillin, kaempferol, and isorhamnetin) and phenolic acids (gallic, protocatechuic, chlorogenic, salicylic, p-hydroxybenzoic, caffeic, vanillic, syringic, methyl paraben, rosmarinic, p-coumaric, and ferulic acids). The presence of phenolic compounds and flavonoids may contribute to the activity of the extracts [33]. Flavonoids, such as epicatechin and rutin, have been reported to be powerful radical scavengers [28]. The scavenging ability of rutin may be due to its inhibitory activity on the xanthine oxidase enzyme [28]. We revealed the presence of rutin in all extracts of C. mixtus with interesting values, and the highest value was detected in C. mixtus flowers (673.12 µg/g DW) [11].
Additionally, the antimicrobial activities of natural products have attracted much attention due to the increasing incidence of pathogens that have become drug-resistant [34]. In this study, the antibacterial activity of methanolic extracts of the flowers, leaves, stems, and roots of C. mixtus was tested against S. aureus and E. coli strains isolated from clinical samples. C. mixtus extracts exhibited antibacterial activity against the two selected strains, with the best result obtained with the leaf extract. The other organs showed almost the same activity against E. coli.
However, extracts are more effective against Gram-positive bacteria than Gram-negative bacteria. This difference in sensitivity is due to membrane permeability. Gram-negative bacteria have a complex and rigid membrane rich in lipopolysaccharide, which can limit the passage of antimicrobial constituents [35].
The antibacterial activity of Matricaria chamomilla extract (Asteraceae family) from Djibouti was investigated [36]. Methanolic extracts of M. chamomilla leaves showed higher antibacterial activity against E. coli (MIC and MBC = 25 µg/mL) compared to S. aureus (MIC and MBC = 100 µg/mL).
Concerning the antifungal activity, against C. albicans and A. fumigatus, C. mixtus extracts appeared to be less effective, and A. fumigatus was the most resistant (MIC and MFC > 40 mg/mL). In another report, the antifungal activity of the aerial part (leaves and flowers) of M. chamomilla extracts (aqueous, methanol, and chloroform) was studied against C. albicans and Fusarium spp., and the results showed that the extracts had no effect on the fungal strains tested [37]. In reverse, methanolic extracts of Montanoa sp. and Schistocarpha sinforosi Cuatrec. from the Asteraceae family showed moderate activity against C. albicans (MICs = 0.62 and 2.50 mg/mL, respectively) [38].
In a study reported by Mekonnen et al. [39], the essential oil from M. chamomilla flowers had no inhibitory effect against all strains of Trichophyton and Aspergillus. Comparing these results with the present study, all the methanolic extracts of C. mixtus gave very good results against T. rubrum.
Accordingly, it can be inferred from our results that the extracts of both plants showed antibacterial and antifungal activity. This can be explained by their chemical composition, which is rich in phenolic acids and flavonoids. They are also very rich in terpenoids, fatty acids, organic acids, esters, and ketones [11].
Protocatechuic acid was isolated from the aerial parts of Centaurea spruneri of the Asteraceae family to test its antibacterial activity [40]. The MIC and MBC values against S. aureus, E. coli, Bacillus cereus, Micrococcus flavus, Listeria monocytogenes, Pseudomonas aeruginosa, Proteus mirabilis, and Salmonella typhimurium were between 100 and 400 mg/L. Several studies have revealed that other phenolic compounds have antimicrobial activities, such as ellagic acid [41], gallic acid [42,43], and p-hydroxybenzoic acid [44].
Overall, the findings of the present work demonstrated that all extracts of C. mixtus organs exhibited good antioxidant and antimicrobial activities, and results differed from one organ to another. Therefore, the extracts of the plant materials studied could be recommended as a source of pharmaceutical materials necessary for the preparation of new antioxidant and antimicrobial agents.
4. Materials and Methods
4.1. Standards and Chemical Reagents
Dimethyl sulfoxide (DMSO) was obtained from Sigma (Darmstadt, Germany). Mueller–Hinton Broth (MHB) and Mueller–Hinton Agar (MHA) media were purchased from Liofilchem (Teramo, Italy). RPMI-1640 broth medium was obtained from Biochrom AG (Berlin, Germany). Sabouraud dextrose agar (SDA) was purchased from BioMérieux (Marcy L’Étoile, France). 2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), horseradish peroxidase (HRP) type VI, and 2,2-diphenyl-1-picrylhydrazyl 95% (DPPH) were obtained from Sigma Chem. Co. (Madrid, Spain). H2O2 (30% v/v) was purchased from Aldrich Chem. Co. (Madrid, Spain). Methanol, N-O-bis(trimethylsilyl) trifluoroacetamide (BSTFA), and pyridine were obtained from Merck KGaA (Darmstadt, Germany).
4.2. Plant Material and Preparation of Methanolic Extracts
The C. mixtus plants were collected in May 2018 at full maturity from the Beni Hassane region, province of Tanger-Tetouan-Al Hoceima in northern Morocco (N 35°21′20.865″, W 5°22′12.677″) and brought to the laboratory. The identification of the plant was carried out by Prof. Ahmed Lamarti from the Faculty of Science in Tetouan (Morocco). The organs (roots, stems, leaves, and flowers) of the fresh plant were separated before the material was dried in an oven until it reached a stable dry weight at 50 °C. After that, it was ground at 8000 rpm in a Microtron MB 550 (Kinematica AG, Eschbach, Germany). The powder was made up of particles with a diameter of about 0.2 mm and was kept at room temperature in the dark.
Methanolic extraction was performed following the previous work by Barros et al. [45] with a slight modification. Two grams of fine dried powder from each plant part were extracted by stirring with 100 mL of methanol at 25 °C at 150 rpm for 24 h and were filtered afterward through Whatman No 4 paper. The filtration residue was extracted twice more using the same method. Methanolic extracts of each plant part were combined and dried using a rotary evaporator under vacuum (Rotavapor® R-210, BÜCHI, Flawil, Switzerland) at 45 °C. Then, the dried extracts of roots, stems, leaves, and flowers were weighed and stored at −80 °C for further use. For each organ, the extraction yield was calculated.
4.3. Estimation of the Total Phenolic Content from Cladathus mixtus
The Folin–Ciocalteu reagent was used with some modifications to determine the total phenolic content (TPC) in each organ as described by Singleton and Rossi [46]. In a glass test tube, 50 µL of the sample was placed, followed by 950 µL of distilled water, 50 µL of 1 M sodium carbonate, and 50 µL of Folin–Ciocalteu reagent. After 15 min of standing in a water bath at 30 °C, the absorbance at 715 nm was measured. The results were given in milligrams of gallic acid equivalents per gram of dry weight (mg GAE g−1 DW). A Perkin-Elmer Lambda-2S UV-VIS spectrophotometer (Loughborough, UK) was used to take photometric measurements. Experiments were carried out in triplicate.
4.4. Estimation of the Total Flavonoids Content from Cladathus mixtus
The total flavonoid content (TFC) was determined by using an aluminum chloride colorimetric method modified by Woisky and Salatino [47] with a slight modification. Briefly, 0.5 mL of methanolic extract was mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. The absorbance of the reaction mixture was measured at 415 nm after 30 min of incubation at room temperature. The findings were reported in mg of quercetin equivalents per gram of dry weight (mg QE/g DW). Moreover, the experiments were carried out in triplicate.
4.5. GC-MS Analysis of Methanolic Extracts from Cladathus mixtus
A chemical analysis of methanolic extracts from C. mixtus was performed using gas chromatography (GC) in a Trace 1300 gas chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) coupled to mass spectrometry (MS) (ISQ single quadrupole mass spectrometer; Thermo Fisher Scientific) and an automatic injector. The GC was outfitted with a capillary column DB-5 (30 µm, 0.25 mm i.d., film thickness 0.25 µm) with a non-polar stationary phase (5% phenyl, 95% dimethylpolysiloxane) from Thermo Fisher Scientific (Waltham, MA, USA). The temperature of the column was programmed to rise from 50 to 350 °C at a rate of 5 °C/min. At a flow rate of 0.75 mL/min, helium was used as the carrier gas [48]. To perform GC-MS analysis, the dried methanolic extracts were derivatized by mixing 10 mg of each sample with 100 µL of anhydrous pyridine and 100 µL BSTFA, then the mixture was heated at 65 °C for 30 min and diluted with 200 µL chloroform. Finally, the derivatized solution (50 µL) was analyzed using GC-MS.
4.6. Estimation of Antioxidant Activity of the Cladanthus mixtus Extracts
4.6.1. Free-Radical Scavenging Activity on 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
The DPPH* test is the oldest indirect method for determining antioxidant activity and was first used to determine the antioxidant potential of phenolic compounds [49]. DPPH* is a very stable radical chromogen that is acquired directly without preparation (ready to dissolve). It has a dark blue color and is a long-lived nitrogen radical species due to its inability to undergo dimerization [50]. A 0.1 mM DPPH solution was prepared in methanol. Of the DPPH stock solution, 900 μL were mixed with 100 μL of plant extract solution. Trolox was used as a reference standard. The reaction was performed in triplicate and allowed to stand at room temperature for 30 min. The decrease in absorbance at 517 nm, which is proportional to soaked (DPPH*), was determined in mg trolox equivalents per g dry weight (mg TE/g DW).
4.6.2. 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS)
The antioxidant activity was measured using the ABTS/H2O2/HRP decoloration methods according to the procedure employed by Arnao et al. [22] and previously described by El Mihyaoui et al. [16]. The reaction mixture contained 1 mM ABTS, 75 µM hydrogen peroxide (H2O2), and 6 µM horseradish peroxidase (HRP) type VI in acidified ethanol (pure ethanol with phosphoric acid, 0.7% w/v), in a total volume of 1 mL prepared at 25 °C. A volume of 40 µL of each methanolic extract was added to the reaction medium, and the decrease in absorbance at 730 nm was measured after 6 min. The absorbance decrease was measured from the difference between the absorbance at 730 nm values before 6 min and after sample addition. Antioxidant activity was calculated as moles of ABTS·+ quenched by 1 mole of trolox. The results were expressed as trolox equivalents per gram of dry weight (mg TE/g DW). Experiments were conducted in triplicate.
4.7. Estimation of Antimicrobial Activities of the Cladanthus mixtus Extracts
4.7.1. Microorganism Strains
Two strains of Gram-negative and Gram-positive bacteria were used to evaluate the antibacterial activity: Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922). Stock cultures were maintained on Mueller–Hinton broth (MHB) medium with 15% glycerol at −80 °C and subcultured on Mueller–Hinton agar (MHA) before each test.
The antifungal activity of methanolic extracts of C. mixtus was checked against three fungi: Candida albicans (ATCC 10231), Aspergillus fumigatus (ATCC 46645), and Trichophyton rubrum (clinical strain, FF 5). All microorganisms were stored in SDB (Sabouraud dextrose broth) with 20% glycerol at −80 °C and subcultured in SDA (Sabouraud dextrose agar) or potato dextrose agar (PDA) before each test to provide pure and ideal growing conditions. The strains were obtained from the Department of Microbiology, Faculty of Pharmacy, Porto University (Portugal), where all the susceptibility tests were completed.
4.7.2. Broth Microdilution Method
Broth microdilution method, based on the Clinical and Laboratory Standards Institute (CLSI) (M07-A8, bacteria; M27-A3, yeasts; and M38-A2, filamentous fungi) and previously described by Erbiai et al. [51], was used to determine the antimicrobial activities of C. mixtus extracts. In brief, the extracts were dissolved in 25% dimethyl sulfoxide (DMSO), and serial dilutions were prepared in MHB for bacteria and in RPMI-1640 for fungi. Of each concentration medium, 100 µL was then distributed into sterile 96-well plates, followed by 100 µL of the final cell suspension (1–2 × 105 CFU/mL for bacteria, 1–5 × 103 CFU/mL for yeasts, 0.4–5 × 104 CFU/mL for Aspergillus, and 1–3 × 103 CFU/mL for dermatophytes), which was diluted in fresh MHB for bacteria and RPMI 1640 for fungi. Then, the plates were incubated without agitation at 37 °C for 24 h for bacteria, 48 h for C. albicans and A. fumigatus, and for seven days at 25 °C for T. rubrum. The same organisms were also tested against the reference antibacterial drug, gentamicin, and the reference antifungal drug, voriconazole, for comparison of results and quality control.
The concentration that induced no visible growth was referred to as the minimal inhibitory concentration (MIC). To estimate the minimum bactericidal (MBC) and fungicidal concentrations (MFC), 10 µL from the wells with no turbidity were inoculated into a Petri dish containing MHA medium for bacteria and SDA medium for fungi. Under the previously specified incubation conditions, the MBC and MFC were determined as the lowest concentrations that completely inhibited the development of the tested strains.
4.8. Statistical Analysis
The SPSS program (Chicago, IL, USA) was used, applying a one-way ANOVA to evaluate the statistical differences among the group and the Tukey multiple range test to establish significant differences between the evaluated parameters. The results of three independent experiments are represented as the mean ± SD for extraction yield, phenolic and flavonoid contents, and antioxidant and antimicrobial activities.
5. Conclusions
This is the first comparative study on the four organs of the C. mixtus plant from the Beni Hassane region, province of Tanger-Tetouan-Al Hoceima, in northern Morocco. The total content of phenolic and flavonoid compounds from methanolic extracts was highly significant. Therefore, it can be concluded that C. mixtus is rich in phenolics and flavonoids, mainly in the flowers. Furthermore, many biomolecules were identified in derivatized methanolic extracts using GC-MS analysis. In addition, the extracts showed strong antioxidant activity, which was correlated with their phenolic and flavonoid contents. The extracts also showed antimicrobial activity, with S. aureus being more sensitive than E. coli. Moreover, the extracts were less effective against C. albicans and A. fumigatus than against T. rubrum. All extracts showed good antifungal properties against the T. rubrum strain, whereas flower and leaf extracts were the most effective. Overall, the important biological properties of C. mixtus were due to its richness in many bioactive compounds, which differed from one organ to another. Herein, this research suggests that C. mixtus exhibits interesting health-related bioactivities, but more specific research should be performed to determine the phytotherapeutic and dietary applications of interest.
Acknowledgments
Lamarti Ahmed, Laboratory of Plant Biotechnology, Department of Biology, Faculty of Sciences, Abdelmalek Essaadi University, Tetouan.
Abbreviations
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| BSTFA | N-O-bis(trimethylsilyl) trifluoroacetamide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | dry weight |
| EOs | essential oils |
| GAE | gallic acid equivalents |
| GC-MS | gas chromatography–mass spectrometry |
| HPLC-MS | high-performance liquid chromatography-mass spectrometry |
| MAPs | medicinal-aromatic plants |
| MBC | minimum bactericidal concentration |
| MFC | minimum fungicidal concentration |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| QE | quercetin equivalent |
| TE | trolox equivalent |
Author Contributions
Conceptualization, A.E.M., J.C.G.E.d.S. and M.B.A.; methodology, A.E.M., J.C.G.E.d.S., M.B.A., E.P. and E.H.E.; software, A.E.M. and E.H.E.; validation, A.E.M., E.P., J.C.G.E.d.S. and E.P.; formal analysis, A.E.M., E.H.E. and E.P.; writing—original draft preparation, A.E.M.; Writing—review and editing, A.E.M., S.C., J.C.G.E.d.S., E.P., E.H.E., M.B.A., A.C., J.H.-R., A.L. and A.B.; visualization, A.E.M., J.C.G.E.d.S., A.L., M.E.C.C. and M.B.A.; supervision, J.C.G.E.d.S., M.B.A., A.L. and M.E.C.C.; project administration, A.E.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all the compounds are not available from the authors.
Funding Statement
Joaquim C.G. Esteves da Silva, the funder of R&D Units CIQUP (UIDB/000081/2020) and the Associated Laboratory IMS (LA/P/0056/2020), and UIDB/04423/2020, UIDP/04423/2020 (CIIMAR). El Hadi Erbiai the FCT for funding his postdoctoral position (under project PTDC/QQUI-QUI-QFI/2870/2020).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tourchi Roudsari M., Arslan A., Iranshai M. Biological Effects of Arctiin from Some Medicinal Plants of Asteraceae Family. Am. J. Biol. Life Sci. 2016;4:41–47. [Google Scholar]
- 2.Eruygur N., Koçyiğit U.M., Taslimi P., Ataş M., Tekin M., Gülçin İ. Screening the In Vitro Antioxidant, Antimicrobial, Anticholinesterase, Antidiabetic Activities of Endemic Achillea cucullata (Asteraceae) Ethanol Extract. S. Afr. J. Bot. 2019;120:141–145. doi: 10.1016/j.sajb.2018.04.001. [DOI] [Google Scholar]
- 3.Rizwana H., Alwhibi M.S., Khan F., Soliman D.A. Chemical Composition and Antimicrobial Activity of Eruca sativa Seeds against Pathogenic Bacteria and Fungi. J. Anim. Plant Sci. 2016;26:1859–1871. [Google Scholar]
- 4.Ghanmi M., Strani B., Aberchane M., Ismaili M.R., Aafi A., El Abid A. Plantes Aromatiques et Médicinales Du Maroc: Les Mille et Une Vertus. Cent. Natl. La Rech. For. Rabat. 2011;130:46–180. [Google Scholar]
- 5.Scherrer A.M., Motti R., Weckerle C.S. Traditional Plant Use in the Areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy) J. Ethnopharmacol. 2005;97:129–143. doi: 10.1016/j.jep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Nikolić M., Stevović S. Family Asteraceae as a Sustainable Planning Tool in Phytoremediation and Its Relevance in Urban Areas. Urban For. Urban Green. 2015;14:782–789. doi: 10.1016/j.ufug.2015.08.002. [DOI] [Google Scholar]
- 7.Aghraz A., Gonçalves S., Rodríguez-Solana R., Dra L.A., Di Stefano V., Dugo G., Cicero N., Larhsini M., Markouk M., Romano A. Antioxidant Activity and Enzymes Inhibitory Properties of Several Extracts from Two Moroccan Asteraceae Species. S. Afr. J. Bot. 2018;118:58–64. doi: 10.1016/j.sajb.2018.06.017. [DOI] [Google Scholar]
- 8.Bammou M., Tariq Bouhlali E.D., Sellam K., Derouich M., El-Rhaffari L., Ibijbijen J., Nassiri L. Chemical Profile and Antimicrobial Properties of Liquid and Vapor Phases of The Essential Oil of Cladanthus eriolepis: An Endemic Asteraceae Growing in The Moroccan Oases. J. Essent. Oil Bear. Plants. 2020;23:1042–1053. doi: 10.1080/0972060X.2020.1822758. [DOI] [Google Scholar]
- 9.Mohamed Abdoul-Latif F., Ainane A., Oumaskour K., Boujaber N., Mohamed J., Ainane T. Chemical Composition and Antimicrobial Activity of the Essential Oil of Chamaemelum nobile (L.) All. Pharmacologyonline. 2021;2:449–457. [Google Scholar]
- 10.Hajjaj G., Bounihi A., Tajani M., Cherrah Y., Zellou A. Anti-Inflammatory Avaluation of Aqueous Extract of Matricaria chamomilla L. (Asteraceae) in Experimental Animal Models from Morocco. World J. Pharm. Res. 2013;2:1218–1228. [Google Scholar]
- 11.El Mihyaoui A., Charfi S., Erbiai E.H., Pereira M., Duarte D., Vale N., Candela Castillo M.E., Badoc A., Lamarti A., Esteves da Silva J.C.G., et al. Phytochemical Compounds and Anticancer Activity of Cladanthus mixtus Extracts from Northern Morocco. Cancers. 2022;15:152. doi: 10.3390/cancers15010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conservatoire et Jardin Botaniques de La Ville de Genève and South African National Biodiversity Institute African Plants Database. [(accessed on 29 March 2023)]. Available online: http://www.ville-ge.ch/musinfo/bd/cjb/chg/index.php?lang=fr.
- 13.Merghoub N., Benbacer L., Amzazi S., Morjani H., El-Mzibri M. Cytotoxic Effect of Some Moroccan Medicinal Plant Extracts on Human Cervical Cell Lines. J. Med. Plants Res. 2009;3:1045–1050. doi: 10.3390/20103012489. [DOI] [Google Scholar]
- 14.Bellakhdar J. La Pharmacopée Marocaine Traditionnelle. Edition Ibis Press; Paris, France: 1997. p. 766. Médecine Arabe Ancienne et Savoirs Populaires. [Google Scholar]
- 15.Lahsissene H., Kahouadji A., Tijane M., Hseini S. Catalogue Des Plantes Médicinales Utilisées Dans La Région de Zaër (Maroc Occidental) Rev. Bot. 2009;186:1–26. [Google Scholar]
- 16.El Mihyaoui A., Candela Castillo E.M., Cano A., Hernández-Ruiz J., Lamarti A., Arnao M.B. Comparative study of wild chamomile plants from the north-west of Morocco: Bioactive Components and Total Antioxidant Activity. J. Med. Plants Res. 2021;5:431–441. doi: 10.5897/JMPR2021.7159. [DOI] [Google Scholar]
- 17.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formisano C., Delfine S., Oliviero F., Tenore G.C., Rigano D., Senatore F. Correlation among Environmental Factors, Chemical Composition and Antioxidative Properties of Essential Oil and Extracts of Chamomile (Matricaria chamomilla L.) Collected in Molise (South-Central Italy) Ind. Crops Prod. 2015;63:256–263. doi: 10.1016/j.indcrop.2014.09.042. [DOI] [Google Scholar]
- 19.Roby M.H.H., Sarhan M.A., Selim K.A.-H., Khalel K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare L.) and Chamomile (Matricaria chamomilla L.) Ind. Crops Prod. 2013;44:437–445. doi: 10.1016/j.indcrop.2012.10.012. [DOI] [Google Scholar]
- 20.Al-Dabbagh B., Elhaty I.A., Elhaw M., Murali C., Al Mansoori A., Awad B., Amin A. Antioxidant and Anticancer Activities of Chamomile (Matricaria recutita L.) BMC Res. Notes. 2019;12:3. doi: 10.1186/s13104-018-3960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elouaddari A., Elamrani A., Moutia M., Oubrim N., Habti N., JamalEddine J. Chemical Composition and Evaluation of Antioxidant, Antimicrobial and Cytotoxic Activities of Moroccan Cladanthus mixtus Essential Oil and Extracts. J. Essent. Oil Bear. Plants. 2019;22:1450–1466. doi: 10.1080/0972060X.2019.1691059. [DOI] [Google Scholar]
- 22.Arnao M.B., Cano A., Alcolea J.F., Acosta M. Estimation of Free Radical-Quenching Activity of Leaf Pigment Extracts. Phytochem. Anal. 2001;12:138–143. doi: 10.1002/pca.571. [DOI] [PubMed] [Google Scholar]
- 23.Cano A., Arnao M.B. Recent Trends and Applications. Wiley; Chichester, UK: 2018. ABTS/TEAC (2, 2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid)/Trolox®-Equivalent Antioxidant Capacity) Radical Scavenging Mixed-Mode Assay. Measurement of Antioxidant Activity & Capacity; pp. 117–139. Chapter 7. [Google Scholar]
- 24.Cvetanović A., Švarc-Gajić J., Mašković P., Savić S., Nikolić L. Antioxidant and Biological Activity of Chamomile Extracts Obtained by Different Techniques: Perspective of Using Superheated Water for Isolation of Biologically Active Compounds. Ind. Crops Prod. 2015;65:582–591. doi: 10.1016/j.indcrop.2014.09.044. [DOI] [Google Scholar]
- 25.Zeroual A., Sakar E.H., Eloutassi N., Mahjoubi F., Chaouch M., Chaqroune A. Wild Chamomile [Cladanthus mixtus (L.) Chevall.] Collected from Central-Northern Morocco: Phytochemical Profiling, Antioxidant, and Antimicrobial Activities. Biointerface Res. Appl. Chem. 2020;11:11440–11457. doi: 10.33263/BRIAC114.1144011457. [DOI] [Google Scholar]
- 26.Ren W., Qiao Z., Wang H., Zhu L., Zhang L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava N., Bezwada R. Flavonoids: The Health Boosters. INDOFINE Chemical Company; Hillsbrgh, NJ, USA: 2015. White Paper. [Google Scholar]
- 28.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016;5:15. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand A., Khurana R., Wahal N., Mahajan S., Mehta M., Satija S., Sharma N., Vyas M., Khurana N. Vanillin: A Comprehensive Review of Pharmacological Activities. Plant Arch. 2019;19:1000–1004. [Google Scholar]
- 30.Fodor B., Molnár-Perl I. The Role of Derivatization Techniques in the Analysis of Plant Cannabinoids by Gas Chromatography Mass Spectrometry. TrAC Trends Anal. Chem. 2017;95:149–158. doi: 10.1016/j.trac.2017.07.022. [DOI] [Google Scholar]
- 31.Hajjaj G., Bahlouli A., Sayah K., Tajani M., Cherrah Y., Zellou A. Phytochemical Screening and In Vivo Antipyretic Activity of the Aqueous Extracts of Three Moroccan Medicinal Plants. Pharm. Biol. Eval. 2017;4:188–192. doi: 10.26510/2394-0859.pbe.2017.30. [DOI] [Google Scholar]
- 32.Elouaddari A., Amrani A.E., Cayuela Sánchez J.A., Bellahcen T.O., Zouiten A., Eddine J.J. Chemical Composition and Biological Activities of the Cladanthus mixtus Essential Oil: A Review. Anal. Chem. Lett. 2019;9:649–663. doi: 10.1080/22297928.2019.1682665. [DOI] [Google Scholar]
- 33.Benammar C., Hichami A., Yessoufou A., Simonin A.M., Belarbi M., Allali H., Khan N.A. Zizyphus Lotus L. (Desf.) Modulates Antioxidant Activity and Human T-Cell Proliferation. BMC Complement. Altern. Med. 2010;10:1–9. doi: 10.1186/1472-6882-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyedjavadi S.S., Khani S., Zare-Zardini H., Halabian R., Goudarzi M., Khatami S., Imani Fooladi A.A., Amani J., Razzaghi-Abyaneh M. Isolation, Functional Characterization, and Biological Properties of MCh-AMP1, a Novel Antifungal Peptide from Matricaria chamomilla L. Chem. Biol. Drug Des. 2019;93:949–959. doi: 10.1111/cbdd.13500. [DOI] [PubMed] [Google Scholar]
- 35.Abdalla R.M., Abdelgadir A.E. Antibacterial Activity and Phytochemical Constituents of Cinnamomum verum and Matricaria chamomilla from Sudan. Bio Bull. 2016;2:8–12. [Google Scholar]
- 36.Abdoul-Latif F.M., Mohamed N., Edou P., Ali A.A., Djama S.O., Obame L.C., Bassolé I.H.N., Dicko M.H. Antimicrobial and Antioxidant Activities of Essential Oil and Methanol Extract of Matricaria chamomilla L. from Djibouti. J. Med. Plants Res. 2011;5:1512–1517. [Google Scholar]
- 37.Boudıeb K., Ait Slimane-Ait Kaki S., Oulebsir-Mohandkaci H., Bennacer A. Phytochemical Characterization and Antimicrobial Potentialities of Two Medicinal Plants, Chamaemelum nobile (L.) All and Matricaria chamomilla (L.) Int. J. Innov. Approaches Sci. Res. 2018;2:126–139. doi: 10.29329/ijiasr.2018.173.2. [DOI] [Google Scholar]
- 38.Niño J., Narváez D.M., Mosquera O.M., Correa Y.M. Antibacterial, Antifungal and Cytotoxic Activities of Eight Asteraceae and Two Rubiaceae Plants from Colombian Biodiversity. Brazilian J. Microbiol. 2006;37:566–570. doi: 10.1590/S1517-83822006000400030. [DOI] [Google Scholar]
- 39.Mekonnen A., Yitayew B., Tesema A., Taddese S. In Vitro Antimicrobial Activity of Essential Oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016;2016:9060649. doi: 10.1155/2016/9545693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ćirić A., Karioti A., Glamoĉlija J., Soković M., Skaltsa H. Antimicrobial Activity of Secondary Metabolites Isolated from Centaurea Spruneri Boiss. & Heldr. J. Serbian Chem. Soc. 2011;76:27–34. [Google Scholar]
- 41.Shakeri A., Zirak M.R., Sahebkar A. Ellagic Acid: A Logical Lead for Drug Development? Curr. Pharm. Des. 2018;24:106–122. doi: 10.2174/1381612823666171115094557. [DOI] [PubMed] [Google Scholar]
- 42.Naz S., Siddiqi R., Ahmad S., Rasool S.A., Sayeed S.A. Antibacterial Activity Directed Isolation of Compounds from Punica granatum. J. Food Sci. 2007;72:M341–M345. doi: 10.1111/j.1750-3841.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 43.Silva J.R.A., Rezende C.M., Pinto A.C., Amaral A.C.F. Cytotoxicity and Antibacterial Studies of Iridoids and Phenolic Compounds Isolated from the Latex of Himatanthus sucuuba. African J. Biotechnol. 2010;9:7357–7360. doi: 10.5897/AJB10.345. [DOI] [Google Scholar]
- 44.Elegir G., Kindl A., Sadocco P., Orlandi M. Development of Antimicrobial Cellulose Packaging through Laccase-Mediated Grafting of Phenolic Compounds. Enzyme Microb. Technol. 2008;43:84–92. doi: 10.1016/j.enzmictec.2007.10.003. [DOI] [Google Scholar]
- 45.Barros L., Calhelha R.C., Vaz J.A., Ferreira I.C.F.R., Baptista P., Estevinho L.M. Antimicrobial Activity and Bioactive Compounds of Portuguese Wild Edible Mushrooms Methanolic Extracts. Eur. Food Res. Technol. 2007;225:151–156. doi: 10.1007/s00217-006-0394-x. [DOI] [Google Scholar]
- 46.Singleton V.L., Rossi J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 47.Woisky R.G., Salatino A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998;37:99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- 48.Erbiai E.H., Maouni A., Pinto da Silva L., Saidi R., Legssyer M., Lamrani Z., Esteves da Silva J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules. 2023;28:1123. doi: 10.3390/molecules28031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roginsky V., Lissi E.A. Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food. Food Chem. 2005;92:235–254. doi: 10.1016/j.foodchem.2004.08.004. [DOI] [Google Scholar]
- 50.Gulcin İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020;94:651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 51.Erbiai E.H., Bouchra B., da Silva L.P., Lamrani Z., Pinto E., da Silva J.C.G.E., Maouni A. Chemical Composition and Antioxidant and Antimicrobial Activities of Lactarius Sanguifluus, a Wild Edible Mushroom from Northern Morocco. Euro-Mediterr. J. Environ. Integr. 2021;6:43. doi: 10.1007/s41207-021-00247-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.