Abstract
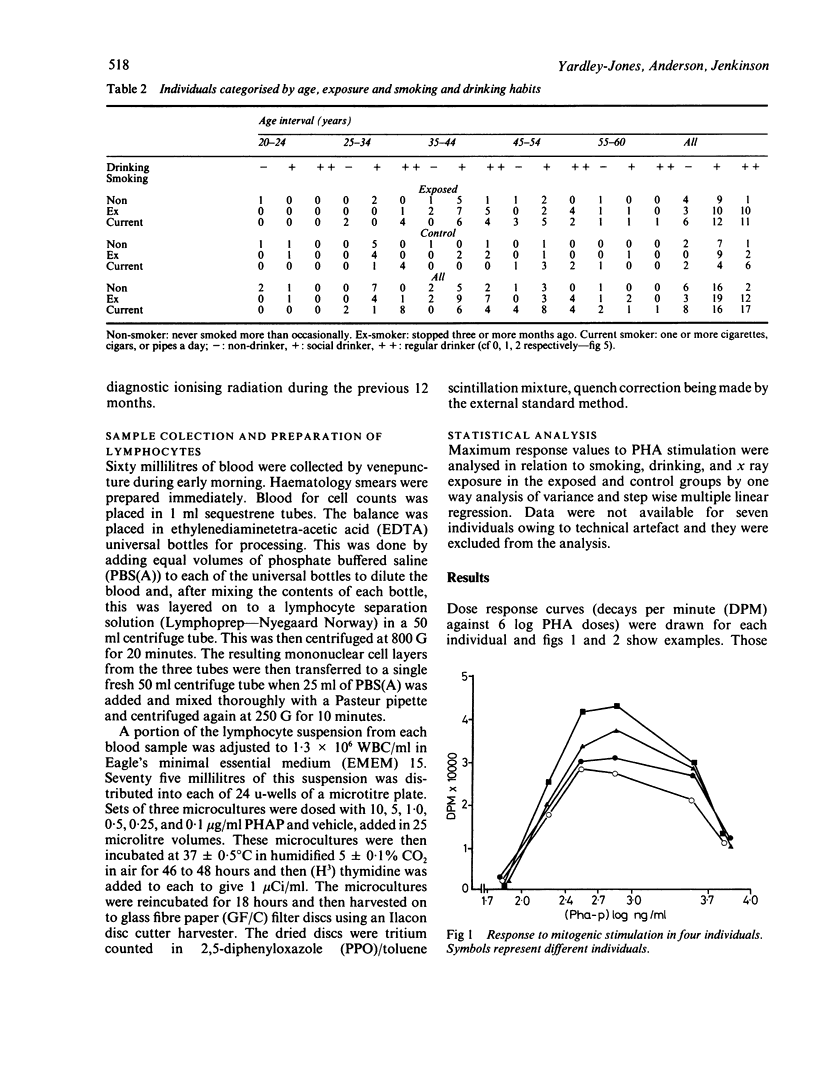
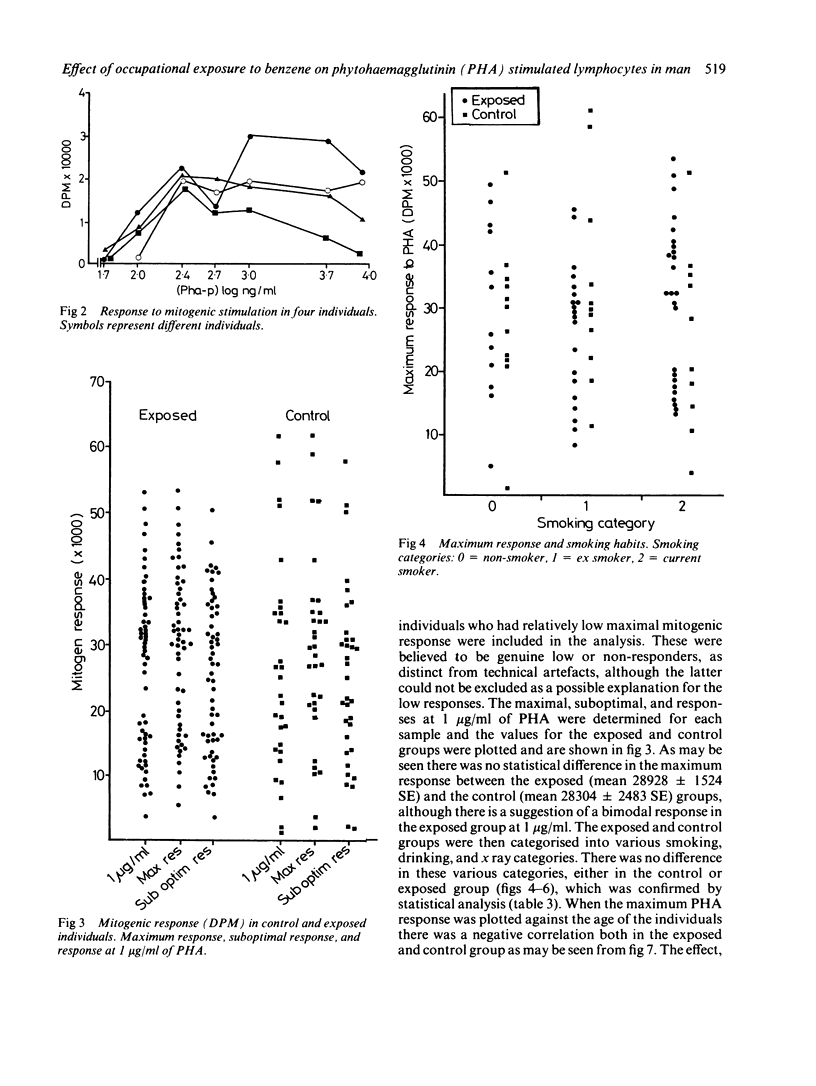
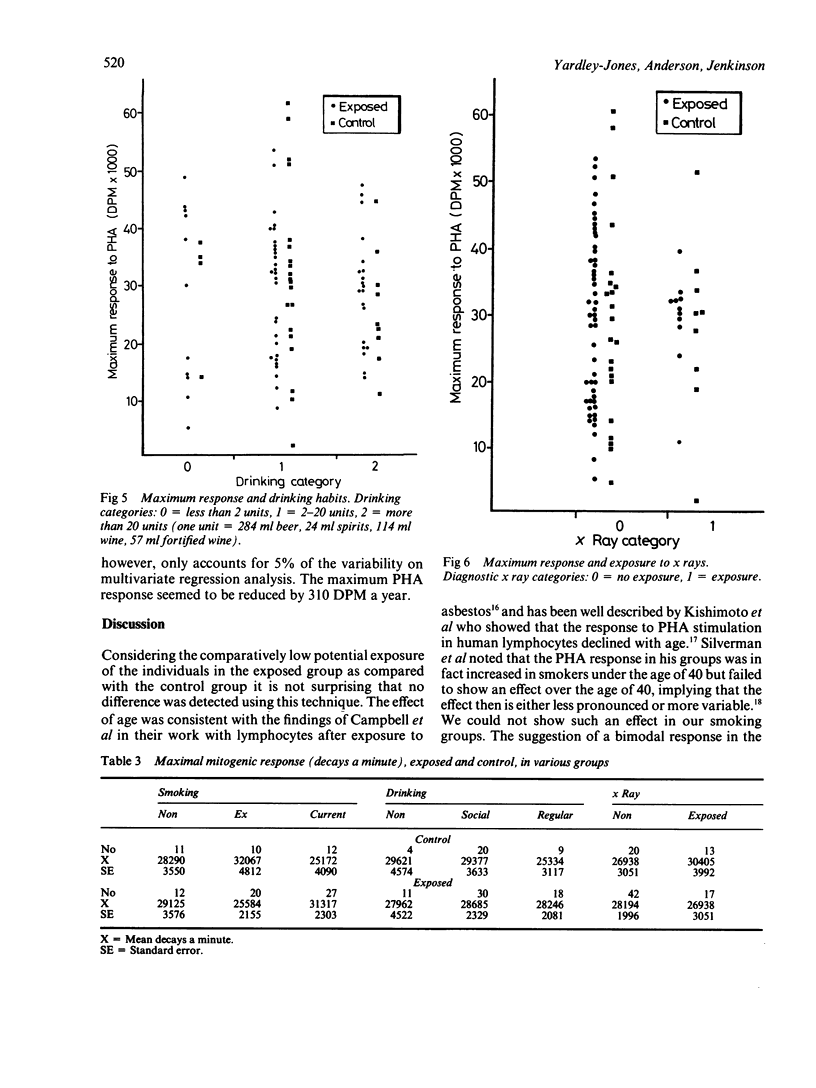
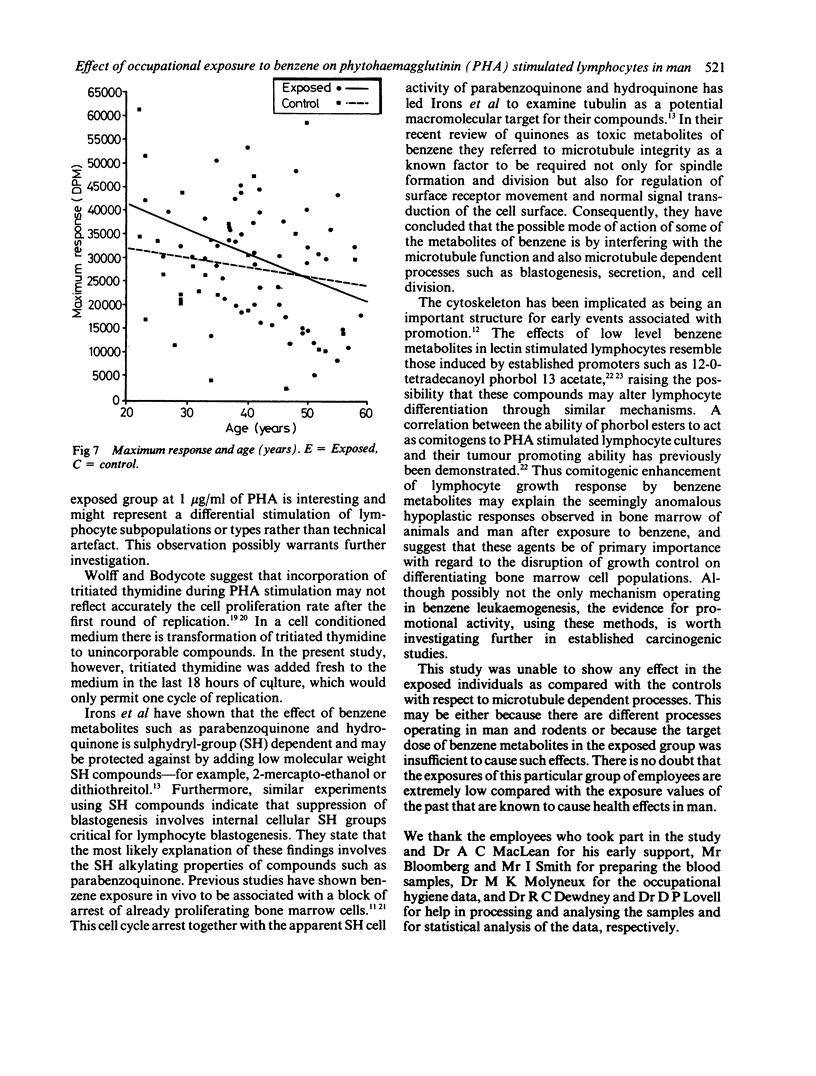
This study was carried out to investigate the effects of potential low level exposure to benzene on phytohaemagglutinin (PHA) stimulated lymphocytes. Sixty six male workers of a refinery population were studied and compared with 33 control workers in the same refinery who were not known to have been exposed to benzene. The responsiveness of the lymphocyte to PHA as a measure of blastogenesis was measured by the incorporation of radio labelled thymidine by the stimulated lymphocytes in vitro. Questionnaires were used to determine various lifestyle factors such as smoking, drinking, and exposure to ionising radiation. The results showed that there was no difference between the exposed group (mean 28928 + 1524 SE (decays per minute (DPM] as compared with the control group (mean 28304 + 2483 SE DPM). Furthermore, it was not possible to determine any effects attributable to various social factors. There was, however, a suggestion of a decrease in mitogenic response with age in both exposed and control workers that was consistent with other studies. It has been shown that products of benzene metabolism may affect the mitogenic response of lymphocytes in a similar way to known promoting agents. This study was unable to show these effects, probably as a result of the low exposures encountered by the individuals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell M. J., Wagner M. M., Scott M. P., Brown D. G. Sequential immunological studies in an asbestos-exposed population. II. Factors affecting lymphocyte function. Clin Exp Immunol. 1980 Jan;39(1):176–182. [PMC free article] [PubMed] [Google Scholar]
- Decouflé P., Blattner W. A., Blair A. Mortality among chemical workers exposed to benzene and other agents. Environ Res. 1983 Feb;30(1):16–25. doi: 10.1016/0013-9351(83)90161-5. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Dent J. G., Baker T. S., Rickert D. E. Benzene is metabolized and covalently bound in bone marrow in situ. Chem Biol Interact. 1980 May;30(2):241–245. doi: 10.1016/0009-2797(80)90130-1. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Neptun D. A. Effects of the principal hydroxy-metabolites of benzene on microtubule polymerization. Arch Toxicol. 1980 Oct;45(4):297–305. doi: 10.1007/BF00293810. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Neptun D. A., Pfeifer R. W. Inhibition of lymphocyte transformation and microtubule assembly by quinone metabolites of benzene: evidence for a common mechanism. J Reticuloendothel Soc. 1981 Nov;30(5):359–372. [PubMed] [Google Scholar]
- Irons R. D., Pfeifer R. W., Aune T. M., Pierce C. W. Soluble immune response suppressor (SIRS) inhibits microtubule function in vivo and microtubule assembly in vitro. J Immunol. 1984 Oct;133(4):2032–2036. [PubMed] [Google Scholar]
- Irons R. D. Quinones as toxic metabolites of benzene. J Toxicol Environ Health. 1985;16(5):673–678. doi: 10.1080/15287398509530777. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Mueller G. C. Retinoic acid inhibition of the comitogenic action of mezerein and phorbol esters in bovine lymphocytes. Cancer Res. 1978 Mar;38(3):771–775. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Inomata K., Kotegawa S., Saito T., Kuroki M., Mitsuya H., Hisamitsu S. Age-related changes in the subsets and functions of human T lymphocytes. J Immunol. 1978 Nov;121(5):1773–1780. [PubMed] [Google Scholar]
- Maltoni C., Conti B., Cotti G. Benzene: a multipotential carcinogen. Results of long-term bioassays performed at the Bologna Institute of Oncology. Am J Ind Med. 1983;4(5):589–630. doi: 10.1002/ajim.4700040503. [DOI] [PubMed] [Google Scholar]
- Pfeifer R. W., Irons R. D. Alteration of lymphocyte function by quinones through a sulfhydryl-dependent disruption of microtubule assembly. Int J Immunopharmacol. 1983;5(5):463–470. doi: 10.1016/0192-0561(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Pfeifer R. W., Irons R. D. Effect of benzene metabolites on phytohemagglutinin-stimulated lymphopoiesis in rat bone marrow. J Reticuloendothel Soc. 1982 Feb;31(2):155–170. [PubMed] [Google Scholar]
- Silverman N. A., Potvin C., Alexander J. C., Jr, Chretien P. B. In vitro lymphocyte reactivity and T-cell levels in chronic cigarette smokers. Clin Exp Immunol. 1975 Nov;22(2):285–292. [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L., Hadden J. W., Touraine F., Hadden E. M., Estensen R., Good R. A. Phorbol myristate acetate: a mitogen selective for a T-lymphocyte subpopulation. J Exp Med. 1977 Feb 1;145(2):460–465. doi: 10.1084/jem.145.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunek A., Platt K. L., Przybylski M., Oesch F. Multi-step metabolic activation of benzene. Effect of superoxide dismutase on covalent binding to microsomal macromolecules, and identification of glutathione conjugates using high pressure liquid chromatography and field desorption mass spectrometry. Chem Biol Interact. 1980 Dec;33(1):1–17. doi: 10.1016/0009-2797(80)90040-x. [DOI] [PubMed] [Google Scholar]


