Abstract
Background
Taenia solium is a tapeworm that causes taeniosis in humans and cysticercosis in humans and pigs. Within Eastern and Southern Africa (ESA), information on the presence of human taeniosis and cysticercosis seems scarce. This systematic review aimed to describe the current information available and gaps in the epidemiology of human T. solium infections in ESA.
Methods/Principle findings
Scientific literature published between 1st January 2000 and 20th June 2022 in international databases [MEDLINE (Ovid), Embase (Ovid), Global Health (Ovid), Scopus (Elsevier), African Index Medicus (via WHO Global Index Medicus), and Open Grey] was systematically reviewed for ESA. The study area included 27 countries that make up the ESA region. Information on either taeniosis, cysticercosis or NCC was available for 16 of 27 countries within the region and a total of 113 reports were retained for the review. Most case reports for cysticercosis and NCC were from South Africa, while Tanzania had the most aggregated cysticercosis reports. Eleven countries reported on NCC with seven countries reporting data on NCC and epilepsy. Unconfirmed human T. solium taeniosis cases were reported in nine countries while two countries (Madagascar and Zambia) reported confirmed T. solium cases. The cysticercosis seroprevalence ranged between 0.7–40.8% on antigen (Ag) ELISA and between 13.1–45.3% on antibody (Ab) ELISA. Based on immunoblot tests the Ab seroprevalence was between 1.7–39.3%, while the proportion of NCC-suggestive lesions on brain CT scans was between 1.0–76% depending on the study population. The human taeniosis prevalence based on microscopy ranged between 0.1–14.7%. Based on Copro Ag-ELISA studies conducted in Kenya, Rwanda, Tanzania, and Zambia, the highest prevalence of 19.7% was reported in Kenya.
Conclusions
Despite the public health and economic impact of T. solium in ESA, there are still large gaps in knowledge about the occurrence of the parasite, and the resulting One Health disease complex, and monitoring of T. solium taeniosis and cysticercosis is mostly not in place.
Author summary
Taenia solium is a tapeworm that causes three diseases, taeniosis in humans and cysticercosis in humans and pigs. Neurocysticercosis, which occurs when the central nervous system is involved has been associated with up to 57% of epilepsy cases in sub–Saharan Africa. Diagnosing neurocysticercosis among people with epilepsy is vital to prevent further morbidity and mortality from the disease as well as to reduce the negative socio-cultural beliefs associated with epilepsy. Within Eastern and Southern Africa, there are many countries in which information on the presence of human taeniosis, cysticercosis and neurocysticercosis seems scarce. This systematic review aimed to describe the current information available and gaps in the epidemiology of human T. solium infections in Eastern and Southern Africa. We found that Information on either taeniosis, cysticercosis or NCC was available only for 16 of 27 countries within the region. We also found that most of the studies on T. solium taeniosis, cysticercosis and neurocysticercosis within the region have been done in Kenya, Madagascar, Mozambique, Rwanda, South Africa, Tanzania and Zambia. Understanding the epidemiology of T. solium infections is essential for monitoring, prevention and control of the disease complex in a One Health approach.
Introduction
Taenia solium is a tapeworm that causes taeniosis in humans and cysticercosis in humans and pigs. The life cycle of T. solium involves pigs as intermediate hosts (cysticercosis) while humans are definitive hosts (taeniosis). Humans may also act as accidental intermediate hosts when larvae of the parasite settle in muscles, subcutaneous or organ tissues causing human cysticercosis (HC). If they lodge in the central nervous system (CNS), including the brain and the spinal cord, the disease is called neurocysticercosis (NCC) [1,2]. An individual may also have cysticerci in the CNS as well as in other parts of the body, which is referred to as (neuro) cysticercosis. NCC may be asymptomatic, but it can also cause various neurological signs/symptoms such as epileptic seizures and epilepsy, chronic progressive headache, focal neurological deficits, signs and symptoms of increased intracranial pressure and in rare cases, death [3–5]. Globally, NCC is estimated to be responsible for 30% of cases of acquired epilepsy in endemic areas [6] and 22% in sub-Saharan Africa [7]. However, this proportion depends on the infection pressure of T. solium and thus may vary considerably.
T. solium is endemic in Africa, Asia and Latin America, especially in areas where pigs are reared under free-ranging conditions, where pork is eaten and where hygiene is limited [8–10]. Nevertheless, information on the endemicity of T. solium is limited and there are many countries from which no published information is available for either human or porcine cysticercosis. Within the Eastern and Southern African region, information on porcine cysticercosis was recently published [11]. However, not much is known about the epidemiology of T. solium infections in humans. The purpose of this review was to gather all published information on human T. solium infections (T. solium taeniosis/(neuro)cysticercosis (TSTC)) in Eastern and Southern Africa (ESA) within the period 1st January 2000 and 20th June 2022 and describe the gaps in the epidemiology of TSTC in this area.
Methods
The systematic review was conducted following a pre-registered protocol and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12] (S1 Table). The review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD 42022343072).
Search strategy
All published articles were searched using the electronic databases, MEDLINE (Ovid), Embase (Ovid), Global Health (Ovid), Scopus (Elsevier), African Index Medicus (via WHO Global Index Medicus), and Open Grey. The reference lists of included studies were also scanned to identify further eligible documents. The searches were conducted on 20th June 2022.
For all questions subject headings (where applicable) and text words describing T. solium taeniosis/cysticercosis/neurocysticercosis and prevalence, epidemiology, control, elimination, or eradication were searched. The search strategy was intended to obtain all available literature on human taeniosis, cysticercosis and neurocysticercosis in ESA conducted and published in the last 22 years. Grey literature was also searched for any relevant publications. The search terms for all databases can be found in (S1 File).
Procedures
Data management
Literature search results were uploaded to the bibliographic software Mendeley Desktop and Covidence. Duplicates were removed based on author names and titles. Screening questions based on the inclusion and exclusion criteria were developed and citation abstracts and full-text articles were uploaded.
Selection process
The selection process was conducted in two stages. Firstly, titles and abstracts were screened independently by two reviewers (GZ and CM) and then full-text reports were obtained by the same reviewers for a detailed assessment with respect to the inclusion criteria. For records that referred to the same study, only one was selected.
Data collection process
The data were collected using standardized data collection forms in Microsoft Excel (S2 File). Data extracted included demographic information, methodology, and all the relevant reported outcomes. GZ carried out the data extraction with verification by CM.
Data items
Predefined tables summarizing individual cases included the year of diagnosis, age, gender, the country where cases were detected, diagnostic method, and location of the lesion (for cysticercosis). Tables summarizing aggregated cases or prevalence data included country, level of data collection (e.g. national or regional), timeframe, number of cases (or prevalence proportion), number of people tested, and diagnostic method.
Eligibility criteria
Inclusion criteria
We included all studies describing human TSTC in ESA, which was defined as the area covered by the following countries/territories: Angola, Botswana, Burundi, Comoros, Djibouti, Eritrea, Ethiopia, Kenya, Lesotho, Madagascar, Malawi, Mauritius, Mayotte, Mozambique, Namibia, Reunion, Rwanda, Seychelles, Socotra, Somalia, Somaliland, South Africa, Eswatini (former Kingdom of Swaziland), Tanzania, Uganda, Zambia and Zimbabwe. Included in this review were studies that describe the epidemiology, disease occurrence, burden, prevalence, incidence, prevention and control of TSTC in any of the twenty-seven countries of ESA. All observational studies including case series and case reports and systematic reviews of such studies were included. Only studies conducted between 1st January 2000 and 20th June 2022 were considered. No language limits were imposed on the search, although studies in languages other than English were only included if they could be adequately translated by using Google Translate.
Exclusion criteria
We excluded studies that did not concern T. solium, did not concern humans, did not report data from within ESA and studies conducted before 1st January 2000.
Risk of bias assessment
The risk of bias in the studies was assessed using items recommended by the Agency for Health Care Research and Quality (AHRQ) [13]. This assessment covered baseline characteristics, inclusion-exclusion criteria, confounding and modifying variables, performance bias, attrition bias, detection bias and reporting bias. Each of these factors was rated and categorized as low, or high risk of bias. If the information available was insufficient, the risk of bias was considered ’unsure’.
Statistical analyses
No statistical pooling of results was conducted and the findings of the review are presented in a narrative synthesis with tables and figures to aid data presentation. Prevalence data if not already provided were calculated using the numerator and denominator of the study sample. Data were analyzed separately for taeniosis, cysticercosis, and neurocysticercosis. For the latter two, data were also presented separately for the general population and people with epilepsy.
Ethical considerations
Ethical recommendation was not required for this review.
Results
Study selection
Our search yielded 2114 records of which 1198 were duplicates. Through screening of titles and abstracts, 776 (85%) records were excluded and for a further 8, no full text was found. Full-text assessment with respect to the inclusion criteria was performed on 132 records of which 19 (14%) were excluded (17 reported data described in other included studies, one did not concern T. solium, and one reported results outside the scope of the study). One hundred and thirteen (113) studies were finally included in the review. Of those, 49 were concerned with only (neuro) cysticercosis, 57 with taeniosis, and 7 with both cysticercosis and taeniosis. Of the 113 reports reviewed, 25 were case reports, 82 were cross-sectional, 3 cohort and 3 case-control studies. The flow diagram for the search is shown in (Fig 1). The reference for included studies is shown in (S2 Table).
Fig 1. PRISMA flow diagram.
Risk of bias
The risk of bias assessment revealed that most studies had a low risk of reporting, detection, attrition and performance bias. However, 7 studies had a high risk of performance bias, 20 had a high risk of bias due to inadequate inclusion and exclusion criteria, and 45 articles had a high risk of bias due to confounding and modifying variables.
Results of individual studies
Human (neuro) cysticercosis
Information on human (neuro) cysticercosis was obtained from 62 sources. In total, 25 (40%) records provided individual information on NCC and CC. Twelve (48%) of these records were on NCC only, seven (28%) on CC and six (24%) on both NCC and CC. Thirty-seven (60%) records reported aggregated information on NCC and CC. Ten (27%) of these records were on NCC only, twenty-one (57%) on CC only, and six (16%) on both NCC and CC. Information on human neuro(cysticercosis) was available for 14 of 27 ESA countries. No reports were available for Angola, Botswana, Comoros, Djibouti, Eritrea, Ethiopia, Lesotho, Mayotte, Reunion, Seychelles, Socotra, Somaliland and Somalia.
Human cysticercosis case reports
Individual human CC cases during the period 2000 to 2022 were reported in 13 hospital case records from six of the ESA countries with, South Africa reporting four cases; Madagascar, Malawi, Rwanda and Tanzania reporting two cases each and Mauritius reporting one case (Table 1). Individual NCC cases were reported in 18 hospital records from eight of the ESA countries with South Africa reporting eight cases while Madagascar, Malawi and Zambia reported two cases each. Mozambique, Eswatini, Tanzania and Zimbabwe reported one case each (Table 2). All reports were based on individual patients presenting with various signs/symptoms, and investigations leading to their diagnosis of either CC or NCC. Twelve (48%) of the 25 case reports described only NCC diagnosed through the use of brain computed tomography (CT) scan [7] and magnetic resonance imaging (MRI) [5], while seven (28%) presented with CC affecting other organs i.e. bronchus, breast, abdomen, neck and subcutaneous tissue diagnosed through histopathology and other investigations. Six (24%) case reports were on patients who presented with both NCC and CC affecting other organs (disseminated CC). At a district hospital in Rwanda ten cases of cutaneous CC were reported with the youngest being only 2 months old [14]. The oldest reported case of NCC was from South Africa in an 86-year-old female with a pharyngeal cyst, cutaneous cysts and active brain cysts [15]. The median age in years was 41 (17–48 years). Twenty-three (92%) of the case reports had information on the affected gender with 54% of the reported cases being female and 46% being male.
Table 1. Individual human cysticercosis case reports as detected through histopathology of sample specimens from patients in Eastern and Southern Africa (2000–2022).
| Country | Age (yrs.) | Gender | Sample | Location of pathogen | Comment | Reference |
|---|---|---|---|---|---|---|
| Madagascar | 20 | Male | Bronchial specimen | Bronchus | A 20-year-old male who presented with hemoptysis following bronchial cysticercosis. | [16] |
| 15 | Female | Breast lamp | Breast | Breast cysticercosis in a 15-year-old girl. | [17] | |
| Malawi | 41 | Female | Skin nodule | Subcutaneous all over the body* | A 41-year-old patient on ARVs with disseminated cysticercosis and more than 50 cysts in the brain. | [18] |
| 56 | Female | Biopsy of neck mass | Left anterior and posterior triangles of the neck | A 56-year-old woman with a history of gradual but progressive growing left sided neck swelling for the past 25 years. | [19] | |
| Mauritius | 22 | Female | Excision biopsy | Upper part of right breast | A 22-year-old woman with history of painless mobile swelling in the right side of the breast. | [20] |
| Rwanda | 2 months—65 yrs. | - | Anatomical department files | Subcutaneous nodules | A case report of 10 cases of cutaneous cysticercosis confirmed by histopathology in the anatomical pathology department at a district hospital (Nyamasheke) in eastern Rwanda. | [14] |
| 46 | Female | Subcutaneous nodule | Subcutaneous nodules on trunk and extremities | A 46-year-old woman who presented with a two-week history of bilateral lower limb weakness, causing difficulty walking. | [21] | |
| South Africa | 42 | Male | Biopsy of subcutaneous nodule | Extensive calcifications in the muscle and subcutaneous tissue. Active cysts within myocardium# | A 42-year-old man with a rare case of myocardial cysticercosis with multiple calcifications in the muscle and subcutaneous tissues. | [22] |
| 86 | Female | Excised cyst from pharynx Subcutaneous nodule biopsy |
Left posterior oropharynx Subcutaneous nodules on the back |
An 86-year-old woman with cysts in the left posterior oropharynx, brain and subcutaneous tissues on the neck and back. | [15] | |
| 49 | Male | Excisional nodule biopsy | Subcutaneous nodules on the trunk and upper extremities | A 49-year-old man with generalized cysticercosis. | [23] | |
| 8 | Nr | Cyst from pleural effusion | Right lung | An 8-year-old child with pulmonary cysticercosis who presented with a large right pleural effusion. | [24] | |
| Tanzania | 4 | Male | Subcutaneous sample biopsy | Sub mandibular and cervical region and abdomen | A 4-year-old HIV positive child with disseminated cysticercosis. | [25] |
| 48 | Male | Subcutaneous nodule | Neck | A 48-year-old male driver with disseminated cysticercosis treated as malaria. | [26] |
ARV, Antiretroviral medication; HIV, Human immunodeficiency virus; Yrs., Years.
*detected through musculoskeletal ultrasound of nodules
#detected through chest and soft tissue x-rays
Table 2. Individual human neurocysticercosis (NCC) case reports as detected by Brain CT in Eastern and Southern Africa (2000–2022).
| Country | Age (yrs.) | Gender | Location of pathogen | Comment | Reference |
|---|---|---|---|---|---|
| Eswatini | 40 | Male | Multiple intra-parenchymal cysts, mural scoleces, calcified granulomas, right sided and parafalcine septate subdural collections, pathognomonic of racemose, stage I and stage IV NCC | A 40-year-old male with a history of chronic headache, unsteady gait and progressive deterioration in vision. | [27] |
| Madagascar | 71 | Male | Not described* | A 71-year-old man with dysarthria and memory problems. | [28] |
| 31 | Male | Two ring enhancing lesions in the left capsulo-lenticular area¶ | A 31-year-old man presenting with dysarthria, blurred vision and monoparesis of the right upper limb 7 weeks after starting anti-tuberculosis medication. | [29] | |
| Malawi | 58 | Female | Multicystic lesion in the left temporal lobe¶# | A 58-year-old woman with coincidental racemose NCC who was being treated for HIV associated cryptococcal meningitis, clinically and microbiologically responding to antifungal therapy. | [30] |
| 41 | Female | Multiple intra-parenchymal cysts in the brain | A 41-year-old patient on ARVs with disseminated cysticercosis and more than 50 cysts in brain. | [18] | |
| Mozambique | 18 | Female | Multiple lesions with different stages ranging from cerebral ring-enhancing cysts with scolices to transitional or degenerative colloidal cysts to multiple parenchymal calcifications | An 18-year-old female admitted to Hospital Central de Quelimane, Zambezia province in Mozambique, 8 days after delivery with a misdiagnosis of eclampsia that turned out to be NCC. | [31] |
| South Africa | 52 | Female | Granuloma within bilateral frontal and temporal lobes; Multicystic racemose lesion in the right cerebellar hemisphere; active cyst within the left cerebellar hemisphere; hydrocephalus with blocked ventriculo- peritoneal shunt; Intraventricular cyst | A 52-year-old woman with multiple types of NCC pathology. | [32] |
| 42 | Male | Multiple active and calcified lesions in the brain. | A 42-year-old man with NCC and disseminated cysticercosis with myocardial involvement and with multiple calcifications in the muscle and subcutaneous regions. | [22] | |
| 86 | Female | Active brain cysts (not specific) | An 86-year-old woman with NCC and cysts in the left posterior oropharynx and subcutaneous tissues on the neck and back. | [15] | |
| 49 | Male | Bilateral multiple parenchymal non-enhancing cerebral cystic lesions | A 49-year-old man with generalized cysticercosis. | [23] | |
| 46 | Female | Spinal and vertebra region. A spinal lesion at T12, causing vertebral collapse#$† | A 46-year-old HIV+ patient with NCC affecting the spine with severe pain with spasms and weakness in lower limbs. | [33] | |
| 30 | Female | Cyst in the occipital horn of the right lateral ventricle | A 30-year-old woman with acute unilateral hydrocephalus. | [34] | |
| 8 | - | A non-specific solitary sub centimeter calcified granuloma abutting the tentorium cerebellum | Letter to the editor of a case report on an 8-year-old child with pulmonary cysticercosis who presented to Red Cross children’ s hospital, Cape Town, with a large right pleural effusion. | [24] | |
| 32 | Male | Brain with isolated cystic lesions with scolex and ring enhancing lesion in colloidal stage | A 32-year-old known PWE admitted with COVID-19 and developed recurrent epileptic seizures due to dying cysts after beginning of NCC treatment. | [35] | |
| 42 | Female | Not described | A 42-year-old known PWE admitted with COIVD-19 and NCC with poor response to treatment and in status epilepticus for a long time. | ||
| Tanzania | 48 | Male | Multiple cystic lesions with dot sign diffusely distributed in the brain, neck, chest, including the heart, abdomen, and pelvis⁋ | A 48-year-old driver with disseminated cysticercosis treated as malaria. | [26] |
| Zambia | 48 | Female | Numerous round points with T1 hypo intense, T2 hyper intense dot-form cores seen in all cerebral slices. # | A 48-year-old woman who presented with subcutaneous nodules on the face, recurrent headache and epileptic seizures in Chambishi Zambia. | [36] |
| 60 | Male | Thoracic intramedullary cystic lesions. Also, cystic lesions in subcutaneous tissue#† | A 60-year-old man with progressive lower extremity weakness and numbness at University Teaching Hospital in Zambia. | [37] | |
| Zimbabwe | 35 | Male | A Suprasellar cystic lesion compressing the optic nerve#$ | A 35-year-old man presenting with pain accompanied by mild vision loss in the right eye due to a cystic mass compressing the optic nerve. | [38] |
ARVs, Antiretroviral medication; HIV, Human immunodeficiency virus; NCC, Neurocysticercosis; Yrs., Years.
* Serology with Ag-ELISA and EITB also used for diagnosis
¶ CSF Ag-ELISA also used for diagnosis
# MRI employed as detection method
⁋ CT of head, neck chest, abdomen, pelvis employed as detection method
$ Histopathology employed as detection method
† NCC affecting the spine with no brain involvement
Aggregated human cysticercosis
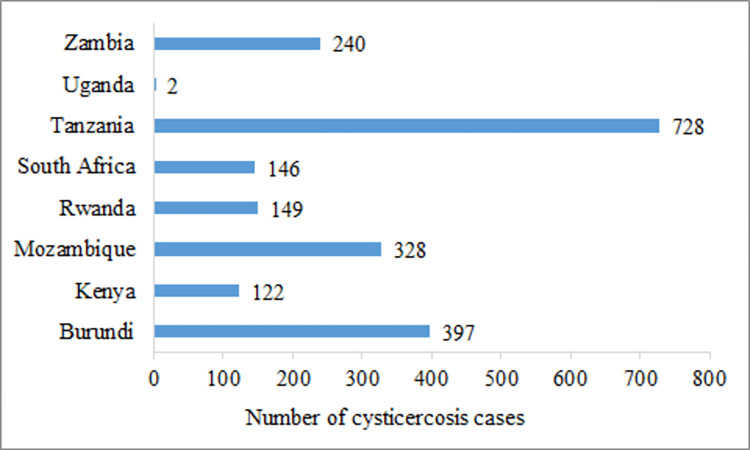
The number of aggregated human CC reports (community-based, cross-sectional studies) for the period 2000 to 2022 in ESA was 27 (Table 3). These were reports from nine countries, the majority of which were conducted in Tanzania (n = 7) followed by Madagascar (n = 6) and Zambia (n = 4). South Africa, Mozambique, Rwanda and Kenya had two reports each. For Burundi and Uganda one report each was available. There was great variation in the aggregated number of human CC cases across countries with over 2307 cases reported from Madagascar alone. The number of human CC cases identified in other ESA countries in documents published between 2000 and 2022 is shown in (Fig 2) below.
Table 3. Aggregated human cysticercosis cases identified in Eastern and Southern Africa (2000–2022).
| Country | No. Tested | Prevalence | Method | Comment | Reference |
|---|---|---|---|---|---|
| No. (%) | |||||
| Burundi | 972 | 397 (40.8) | Ag-ELISA | A prevalence matched case-control design used in the Kiremba area, Burundi to evaluate the role of cysticercosis in PWE. | [39] |
| Kenya | 2113 | 122 (5.8) | Ag-ELISA | A cross-sectional survey around the Lake Victoria region to evaluate human and animal zoonotic and non-zoonotic disease in a rural farming community. | [40] |
| 17 12 |
0 (0) ⁋ 1 (8.3) ⁋ |
Ag-ELISA | A cross-sectional study to determine the prevalence of cysticercosis and NCC among PWE (17) and PWOE (12) in a pig keeping community in Western Kenya. | [41] | |
| Madagascar | 73 | 6 (8.2) ⁋ | EITB | Screening of peace corps volunteers for cysticercosis and NCC before their return from Madagascar. | [42] |
| 1751 | 485 (27.7) | Ag-ELISA | A cross-sectional study to investigate the prevalence of active cysticercosis and associated risk factors in school children (3-16years) in seven cities in Madagascar. | [43] | |
| 2094 | 527 (25.2) | Ag-ELISA | An epidemiology study to evaluate cysticercosis seroprevalence in Madagascar in two groups. Group 1: All age groups (2–24) in Antananarivo, capital of Madagascar (n = 2094). | [44] | |
| 2048 | 269 (13.1) | Ab-ELISA* | |||
| 1760 | 489 (27.8) | Ag-ELISA | Group 2: Children aged between 3 to 16 years old from seven different cities (n = 1799). | ||
| 1799 | 242 (13.4) | Ab-ELISA* | |||
| 3619 | 76 (2.1) | Serology (not specified) | A retrospective study on 76 patients hospitalized for NCC in the pediatrics department of the Tamatave University Hospital Center to evaluate the epidemio-clinical aspect of NCC. | [45] | |
| 237 | 35 (14.8) | Immunoblot | A cross-sectional descriptive study at the Regional Referral Hospital in Antsirabe to determine the seroprevalence of cysticercosis as well as its associated risk factors in patients from the region of Vakinankaratra with clinical suspicion. | [46] | |
| 543 | 166 (30.6) | Ag-ELISA | A review of cysticercosis in Madagascar. Data for 2017 national survey in Infanadiana district. | [47] | |
| 12 (2.2) | EITB | ||||
| Mozambique Mozambique |
1723 | 267 (15.5) ⁋ | Ag-ELISA | A cross-sectional study to estimate the prevalence of NCC in Angónia district, Tete province, Mozambique based on prevalence of human T. solium cysticercosis. | [48] |
| 601 | 61 (10.2) | Immunoblot | A cross-sectional serological study among HIV infected people in Beira (Ponta Gea Health Centre (PGHC)), located in the central part of the Mozambique. | [49] | |
| Rwanda | 211 | 46 (21.8) ⁋ | Immunoblot | A health-facility based study in the southern province of Rwanda to determine the prevalence of NCC among PWE. | [50] |
| 572 | 76 (13.3) | EITB | A cross-sectional study conducted in 680 children from a rural primary school in Gakenke district, Northern province to assess the prevalence of T. solium taeniosis cysticercosis. | [51] | |
| 71 | 27 (38) | Ag-ELISA | |||
| South Africa | 113 | 42 (37.2) ⁋ | Immunoblot | A descriptive prospective study to survey the prevalence of NCC in PWE in Lusikisiki, Eastern Cape province. | [52] |
| 281 | 89 (32.6) ⁋ | Ab-ELISA# | A cross-sectional study of NCC prevalence among PWE at St Elizabeth hospital Eastern Cape province. | [53] | |
| 15 (7.9) | Ag-ELISA | ||||
| Tanzania | 20 | 6 (30) ⁋ | Ag-ELISA | A cross-sectional study to assess the prevalence of NCC among PWE in northern Tanzania. | [54] |
| 830 | 139 (16.7) ⁋ | Ag-ELISA | A cross-sectional study to determine prevalence and risk factors of human T. solium infections in Mbeya Region of Tanzania. | [55] | |
| 376 (45.3) | Ab-ELISA* | ||||
| 218 | 6 (2.8) ⁋ | Immunoblot | A cross-sectional study among PWE in a door-to-door study in an established demographic surveillance site in Hai district North East Tanzania. | [56] | |
| 404 | 10 (2.5) | EITB | A cross-sectional study to estimate the prevalence of and factors associated with T. solium infections among HIV+ and HIV—individuals in Mbulu district, northern Tanzania. | [57] | |
| 3 (0.7) | Ag-ELISA | ||||
| 302 | 3 (0.99) | Ag-ELISA | A cross-sectional study to investigate the presence and prevalence of TSTC in PWE in Dar es Salaam. | [58] | |
| 8 (2.65) | EITB | ||||
| 528 | 9 (1.7) | Immunoblot | Tanzania results from a multi-center case-control study using prevalent cases of ACE identified from HDSS in Tanzania, Kenya, Uganda and South Africa. | [59] | |
| 1051 | 171 (16.3) ⁋ | Immunoblot | A cross-sectional study conducted to determine the status and health burden of NCC in Mbulu district. | [60] | |
| Uganda | 84 | 2 (2.4) | Immunoblot | Uganda results from a multi centre case-control study using prevalent cases of ACE identified from HDSS in Tanzania, Kenya, Uganda and South Africa. | [61] |
| Zambia | 708 | 41 (5.8) | Ag-ELISA | A community-based cross-sectional survey to determine the prevalence of human taeniosis and cysticercosis in Petauke district in Eastern province. | [62] |
| 867 | (12.2–14.5) | Ag-ELISA | A prospective study conducted to ascertain the incidence of human cysticercosis in Katete district in Eastern province. | [63] | |
| (33.5–38.5) | EITB | ||||
| 56 | 22 (39.3) ⁋ | EITB | A cross-sectional study to assess NCC prevalence among PWE in Katete district in Eastern province. | [64] | |
| 13 (23.2) | Ag-ELISA | ||||
| 9 | 9 (-) | Histopathology Full body dissection postmortem | A descriptive study looking at postmortem examinations of the past 12 months for cardiac cysticercosis and NCC in sudden and unexpected community deaths in Lusaka. | [65] |
ACE, Active convulsive epilepsy; Ab-ELISA, Antibody based ELISA; Ag-ELISA, Antigen based ELISA; EITB, Enzyme linked electroimmunotransfer blot; ELISA, Enzyme linked immunosorbent Assay; HDSS, Health demographic surveillance systems; HIV, Human immunodeficiency virus; NCC, Neurocysticercosis; No., Number; PWE, People with epilepsy; PWOE, People without epilepsy; TSTC, T. solium taeniosis cysticercosis.
*Antibodies detected using the rT24-ELISA
#Antibodies detected using the IgG-ELISA
⁋ Employed imaging in addition to serology.
Fig 2. Aggregated human cysticercosis cases reported in community-based cross-sectional studies in Eastern and Southern Africa between the years 2000 and 2022.
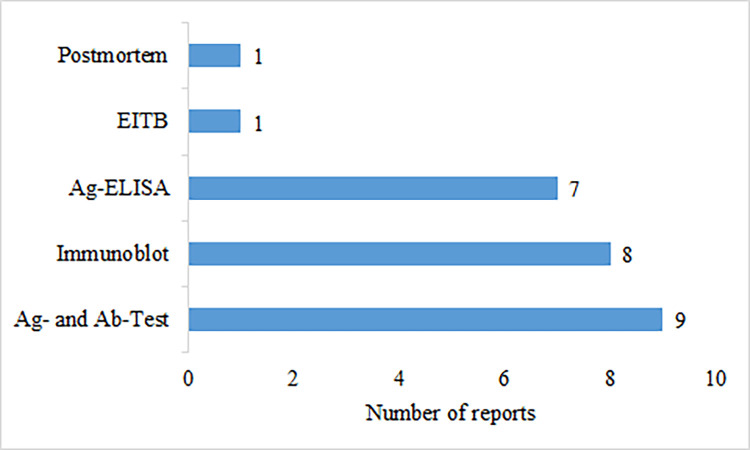
To ascertain cases of aggregated human CC, various diagnostic methods were utilized in the included reports with nine (33%) studies employing a combination of antibody and antigen based serologic tests. Seven (26%) studies employed only the Ag-ELISA, while eight (30%) utilized only the immunoblot methods. One report (4%) utilized only the electroimmunotransfer blot assay (EITB) and one report (4%) was based on postmortem findings of CC through full-body dissection [65]. One report did not specify the type of serologic test used. The methods used for CC diagnosis are shown in (Fig 3).
Fig 3. Diagnostic methods used for aggregated human cysticercosis cases in the included studies for Eastern and Southern Africa.
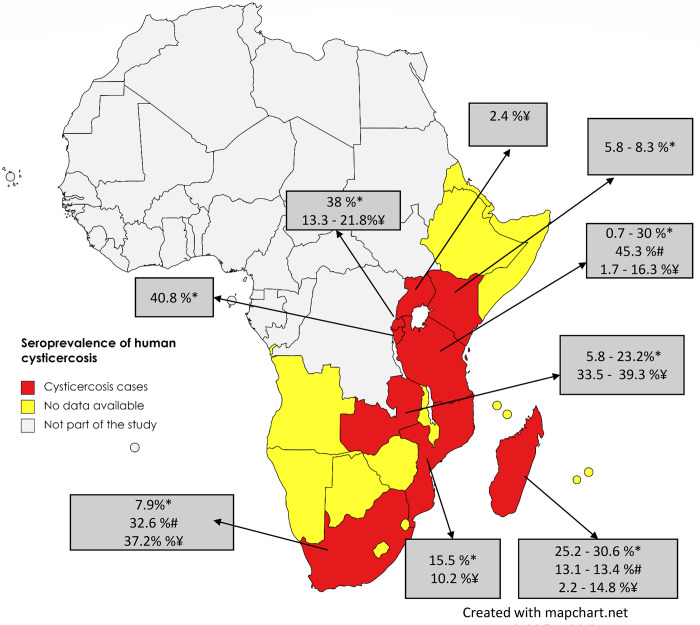
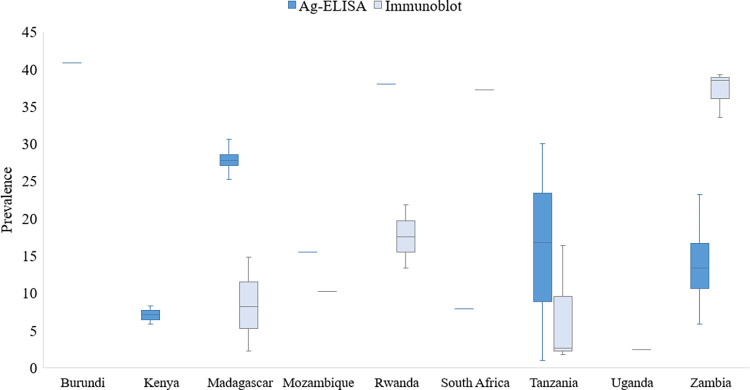
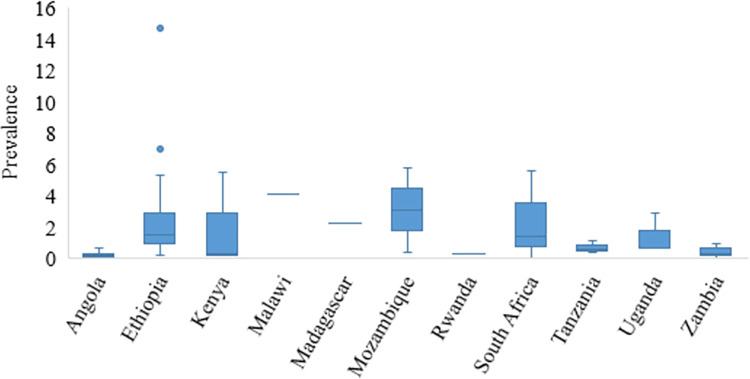
The prevalence of human CC in ESA in different study populations showed wide variation within and between countries (Fig 4 and Table 3). The CC seroprevalence ranged between 0.7–40.8% on Ag-ELISA and between 13.1–45.3% on Ab-ELISA tests. Based on Ab-tests using the immunoblot the CC seroprevalence ranged between 1.7–39.3%. The highest point prevalence based on serum Ab-ELISA was reported in Tanzania (45.3%) from a study conducted in the Mbozi area of Mbeya District [55].
Fig 4. The distribution of human cysticercosis seroprevalence in the included studies for Eastern and Southern Africa (*Ag-ELISA, #Ab-ELISA, ¥Immunoblot).
The maps were obtained from an openly available source, Mapchart.net for free. Link: https://www.mapchart.net/terms.html#licensing-maps. Permission has been obtained from the owner.)
For the studies conducted within Tanzania, this was an outlier value. High prevalence variation was also observed within countries as seen in Madagascar, Rwanda and Zambia (Fig 5). The lowest point prevalence (0.7%) was also reported from Tanzania. For details on sources refer to Table 3.
Fig 5. Prevalence of human cysticercosis in Eastern and Southern Africa based on serology data in reviewed studies with high variation observed in Madagascar, Rwanda, Tanzania and Zambia.
Aggregated human neurocysticercosis
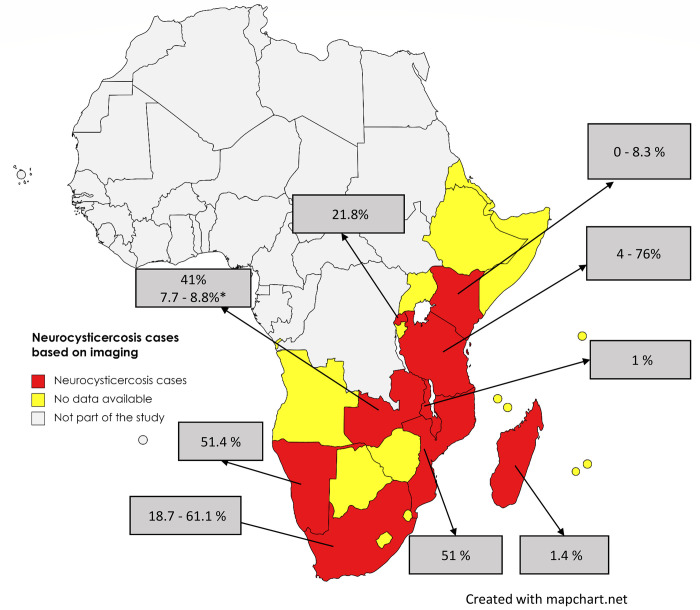
For reports presenting aggregated NCC cases (Table 4), 16 records were available. Four (25%) employed imaging (brain CT or MRI) only, whereas 11 (69%) incorporated both imaging and serological tests, and one (6%) was based on postmortem full-body dissection. The NCC-suggestive lesions on brain CT scans showed a proportion ranging between 1.0–76% in different study populations in ESA (Fig 6 and Table 4).
Table 4. Aggregated human neurocysticercosis cases as detected by Brain CT scan in eastern and southern Africa (2000–2022).
| Country | No. Tested | Proportion | Comment | Reference |
|---|---|---|---|---|
| No. (%) | ||||
| Kenya | 17 | 0 (0) ⁋ | A cross-sectional study to determine the prevalence of cysticercosis and NCC among PWE (17) and PWOE (12) in a pig keeping community in Western Kenya. | [41] |
| 12 | 1 (8.3) ⁋ | |||
| Madagascar | 73 | 1 (1.4) ⁋ | Screening of peace corps volunteers for cysticercosis and NCC before their return from Madagascar. | [42] |
| Malawi | 98 | 1 (1) | Hospital study on patients presenting with a stroke in Blantyre hospital. | [67] |
| Mozambique | 151 | 77 (51) ⁋ | A cross-sectional study to estimate the prevalence of NCC in Angónia district, Tete province, Mozambique. | [48] |
| Namibia | 117 | 96 (51.4) | An analytical cross-sectional study conducted among patients with a first seizure presenting to the casualty and medical in-patient wards of Oshakati hospital in northern Namibia. | [68] |
| Rwanda | 211 | 46 (21.8) ⁋ | A health-facility based study in the southern province of Rwanda to determine the prevalence of NCC among PWE. | [50] |
| South Africa | 113 | 69 (61.1) ⁋ | A descriptive prospective study to survey the prevalence of NCC in PWE in Lusikisiki, Eastern Cape province. | [52] |
| 92 | 34 (37) ⁋ | A cross-sectional study of NCC prevalence among PWE at St Elizabeth hospital Eastern Cape province. | [53] | |
| 75 | 14 (18.7) | A retrospective review of the brain CT findings in adults presenting with new-onset seizures in a tertiary hospital in Gauteng province. | [69] | |
| Tanzania | 212 | 68 (32.1) ⁋ | A cross-sectional study to assess the prevalence of NCC among PWE in northern Tanzania. | [54] |
| 55 | 30 (54.6) ⁋ | A cross-sectional study to determine prevalence and risk factors of human T. solium infections in Mbeya Region of Tanzania. | [55] | |
| 200 | 8 (4) ⁋ | A cross-sectional study among PWE in a door-to-door study in an established demographic surveillance site in Hai district North East Tanzania. | [56] | |
| 25 | 19 (76) ⁋ | A cross-sectional study conducted to determine the status and health burden of NCC in Mbulu district. | [60] | |
| Zambia | 49 | 20 (41) ⁋ | A cross-sectional study to assess NCC prevalence among PWE in Katete district in Eastern province. | [64] |
| 34 | 3 (8.8) * | Observational study of cognition and HIV in children’s hospital Lusaka. Three of 34 HIV+ participants and one of 13 controls were found to have neurocysticercosis. | [66] | |
| 13 | 1 (7.7) * | |||
| 9 | 9 (-) # | A descriptive study looking at postmortem examinations for the past 12 months for cardiac cysticercosis and NCC in sudden and unexpected community deaths in Lusaka. | [65] |
CT, Computed tomography; HIV, Human immunodeficiency virus; NCC, Neurocysticercosis; No., Number; PWE, People with epilepsy; PWOE, People without epilepsy.
* NCC detected through use of MRI
# NCC detected through full body dissection postmortem histopathology
⁋ Serology employed in addition to imaging
Fig 6. The distribution of human neurocysticercosis based on imaging data in reviewed studies in Eastern and Southern Africa.
*Proportion based on MRI. (The maps were obtained from an openly available source, Mapchart.net for free. Link: https://www.mapchart.net/terms.html#licensing-maps. Permission has been obtained from the owner).
One report utilized MRI to identify lesions suggestive of NCC in HIV+ children. In this report, four children (8.5% of the total) were found to have NCC, with imaging suggesting the vesicular (active) stage of NCC in all of them [66].
Neurocysticercosis and epilepsy
Among the 16 records reporting aggregated NCC, eight (50%) reported data linking NCC and epilepsy (Table 5). Three of these records were reported from South Africa whereas one record each was reported from Kenya, Mozambique, Rwanda, Tanzania and Zambia. Three (33%) of these NCC epilepsy studies were community-based studies and six (67%) were conducted in hospital/health facility settings. The prevalence of NCC among people with epilepsy ranged between 0–61%. One study in Kenya in a T. solium endemic area found none of the participants with epilepsy had serological evidence of cysticercosis and none had radiographic findings consistent with NCC [41]. Whereas at St Elizabeth’s Hospital, Lusikisiki, Eastern Cape, 61% of the patients presenting with epilepsy had NCC associated epilepsy, the prevalence being highest in the 10–19-year-old age group [52]. For details on sources refer to Table 5.
Table 5. Human neurocysticercosis with epilepsy as detected by Brain CT scan in eastern and southern Africa (2000–2022).
| Country | No Tested | Proportion | Comment | Reference |
|---|---|---|---|---|
| No (%) | ||||
| Kenya | 17 | 0 (0) ⁋ | A cross-sectional study to determine the prevalence of cysticercosis and NCC among PWE in a pig keeping community in Western Kenya | [41] |
| Mozambique | 151 | 77 (51) ⁋ | A cross-sectional study to estimate the prevalence of NCC in Angónia district, Tete province, Mozambique based on prevalence of human T. solium cysticercosis. | [48] |
| Rwanda | 211 | 46 (21.8) ⁋ | A health-facility based study in the southern province of Rwanda to determine the prevalence of NCC among PWE. | [50] |
| South Africa | 113 | 69 (61.1) ⁋ | A descriptive prospective study to survey the prevalence of NCC in PWE in Lusikisiki, Eastern Cape province. | [52] |
| 92 | 34 (37) ⁋ | A cross-sectional study of NCC prevalence among PWE at St Elizabeth hospital Eastern Cape province. | [53] | |
| 75 | 14 (18.7) | A retrospective review of the brain CT findings in adults presenting with new-onset seizures in a tertiary hospital in Gauteng province. | [69] | |
| Tanzania | 212 | 68 (32.1) ⁋ | A cross-sectional study to assess the prevalence of NCC among PWE in northern Tanzania. | [54] |
| Zambia | 49 | 20 (41) ⁋ | A cross-sectional study to assess NCC prevalence among PWE in Katete district in Eastern province. | [64] |
CT, Computed tomography; NCC, Neurocysticercosis; PWE, People with epilepsy.
⁋ Serology employed in addition to imaging
Human taeniosis
A total of 64 records were identified providing information on taeniosis cases in ESA for the period 2000 to 2022 (Table 6). These records were reported in 11 out of 27 ESA countries (Fig 7) with Ethiopia reporting the most records (n = 38) followed by Tanzania (n = 6) and Zambia (n = 5). Kenya and Uganda had three records each while Angola, Mozambique, and South Africa had two records each. Malawi, Madagascar and Rwanda had one record each. There were no cases reported from Botswana, Burundi, Comoros, Djibouti, Eritrea, Lesotho, Mauritius, Mayotte, Namibia, Reunion, Seychelles, Socotra, Somalia, Somaliland, Swaziland, and Zimbabwe.
Table 6. Aggregated human taeniosis cases detected by stool microscopy and reported as Taenia spp. in eastern and southern Africa (2000–2022).
| Country | No. Tested | Prevalence | Comment | Reference |
|---|---|---|---|---|
| No (%) | ||||
| Angola | 1237 | 1 (0.1) | A cross-sectional study of 1,237 preschool children (0–5 year olds), 1,142 school-aged children (6–15 year olds) and 960 women (15 year olds) conducted to understand the distribution of malnutrition, anemia, malaria, schistosomiasis (intestinal and urinary) and geohelminths in north-western province. | [74] |
| 1142 | 2 (0.2) | |||
| 960 | 1 (0.1) | |||
| 328 | 2. (0.6) | A cross-sectional study conducted among 328 children attending a primary school in Lubango. | [75] | |
| Ethiopia | 93 | 4 (4.3) | A cross-sectional study among food handlers in Awassa town. | [76] |
| 372 | 2 (0.5) | A cross-sectional study among HIV infected and non-infected patients in Jimma zone. | [77] | |
| 1021 | 14 (1.4) | A cross-sectional community based parasitological survey conducted among population living around the Gilgel Gibe dam catchment areas. | [78] | |
| 248 | 2 (0.8) | A cross-sectional study among HIV negative subjects in Bahir Dar city. | [79] | |
| 459 | 13 (2.8) | A cross-sectional study to assess the magnitude and pattern of intestinal parasitism in lowland (2.8%) and highland (1.2%) dwellers in Gamo area, south Ethiopia. | [80] | |
| 399 | 5 (1.2) | |||
| 399 | 28 (7) | A cross-sectional study to assess prevalence of intestinal parasites infection and associated risk factors among school children in Dagi primary school. | [81] | |
| 343 | 14 (4.1) | A cross-sectional study to determine the prevalence of intestinal parasitic infections in patients living with HIV/AIDS at Hawassa University referral hospital. | [82] | |
| 778 | 10 (1.3) | A cross-sectional study among 12 primary schools in Bahir Dar region. | [83] | |
| 121 | 5 (4.1) | A cross-sectional study among prison inmates (4.1%) and tobacco farm workers (0.9%) in Northcentral Ethiopia. | [84] | |
| 115 | 1 (0.9) | |||
| 385 | 6 (1.6) | A cross-sectional study among elementary school children in Sanja, northwest Ethiopia. | [85] | |
| 415 | 1 (0.2) | A cross-sectional study among TB patients in Gondar hospital. | [86] | |
| 272 | 1 (0.4) | A cross-sectional study among food handlers in food establishments at Hassawa University. | [87] | |
| 260 | 1 (0.4) | A cross-sectional study among diarrhea children at Jimma health centre. | [88] | |
| Ethiopia | 400 | 3 (0.8) | A cross-sectional study among children of selected primary schools in Chencha town south Ethiopia. | [89] |
| 307 | 4 (1.3) | A cross-sectional study among university students at Mekelle University. | [90] | |
| 32,191 | 322 (1.0) | A retrospective observational study of all stool samples examined for parasites from patients presenting with diarrhea in the period 2007–2012 at Gambo Rural hospital. | [91] | |
| 464 | 4 (0.9) | A cross-sectional study among patients attending at Anbesame health center, South Gondar. | [92] | |
| 374 | 20 (5.3) | A cross-sectional study among school children in Dawro Zone, Southern Ethiopia. | [93] | |
| 95 | 4 (4.2) | A cross-sectional study to determine the prevalence of intestinal parasite among HIV/AIDS patients attending ART clinic at Arba Minch hospital southern Ethiopia. | [94] | |
| 345 | 14 (4.1) | A cross-sectional study among food handlers at Arba Minch University student’s cafeteria, South Ethiopia. | [95] | |
| 401 | 2 (0.5) | A cross-sectional study among preschool-aged children in Chuahit, Dembia district, Northwest Ethiopia | [96] | |
| 173 | 4 (2.3) | A cross-sectional study among HIV infected individuals in Wolaita Sodo hospital. | [97] | |
| 503 | 13 (2.6) | A cross-sectional parasitological and malacological survey among school children in Wolaita zone south Ethiopia. | [98] | |
| 94 | 2 (2.1) | A cross-sectional study among food handlers at cafeteria of Jimma University specialized hospital. | [99] | |
| 427 | 2 (0.5) | A cross-sectional study among patients seen at Adwa health centre North Ethiopia. | [100] | |
| 279 | 2 (2.4) | A cross-sectional study among primary school children of Haike primary school Northeast Ethiopia. | [101] | |
| 256 | 3 (1.2) | A cross-sectional study conducted at University of Gondar hospital to assess the prevalence of intestinal parasites among pulmonary tuberculosis suspected patients. | [102] | |
| 213 | 5 (2.3) | A cross-sectional study among TB patients in Arba Minch facility. | [103] | |
| 108 204 |
5 (4.6) 4 (2.0) |
A cross-sectional study to compare prevalence of intestinal parasites and associated factors among adult pre-ART (4.6%) and on ART (2.0%) patients in Goncha Siso Enesie Woreda, East Gojjam, Northwest Ethiopia. | [104] | |
| Ethiopia | 280 | 8 (2.9) | A cross-sectional study to determine the prevalence of schistosomiasis and other intestinal helminthiasis among school children and free ranging vervet monkeys in Bochessa area, Ziway. Results for school children. | [105] |
| 247 | 1 (0.4) | A cross-sectional study to assess intestinal parasitic infections among under-five children attending in Debre Birhan referral hospital, Northeast Ethiopia. | [106] | |
| 391 | 4 (1.0) | A cross-sectional study conducted among primary school children in Arbaminch Zuria district. | [107] | |
| 273 | 6 (2.2) | A cross-sectional study among rural school children in Northwest Ethiopia. | [108] | |
| 170 | 25 (14.7) | A cross-sectional study among food handlers at Wolkite University. | [71] | |
| 406 | 12 (3) | A cross-sectional study among primary school children in Jawi town, northwest Ethiopia. | [109] | |
| 200 | 3 (1.5) | A cross-sectional study among food handlers at Wollo University Northeastern Ethiopia. | [110] | |
| 383 | 5 (1.3) | A cross-sectional study conducted among Gara Riketa primary school children at Hawassa Tula Sub-City, Southern Ethiopia. | [111] | |
| 525 | 11 (2.1) | A cross-sectional study in three primary schools in Sululta after deworming. | [112] | |
| Kenya | 797 | 1 (0.13) | A cross-sectional study to determine a baseline prevalence of IPIs among children of five years and below at Webuye HDSS area in western Kenya. | [113] |
| 2113 | 416 (19.7) * 6 (0.3) |
A cross-sectional survey around the Lake Victoria region to evaluate human and animal zoonotic and non-zoonotic disease in a rural farming community. | [40] | |
| 310 | 17 (5.5) | A cross-sectional study among school children in lodwa municipality Turkana county. | [114] | |
| Madagascar | 459 | 10 (2.2) # | A cross-sectional study aimed at assessing the prevalence of T. Solium infections and associated risk factors in twelve remote villages surrounding Ranomafana national park, Ifanadiana district. | [70] |
| Malawi | 190 | 4 (2.1) | A cross-sectional survey among preschool children in Mangochi district. | [115] |
| Mozambique | 269 | 1 (0.4) | A cross-sectional study among children living in urban environs of Maputo and attending the health clinic at xipamanine. | [116] |
| 83,331 | 4833 (5.8) | A cross-sectional study among school children across the country. | [117] | |
| Rwanda | 144 | 2 (1.4) * | A cross-sectional study conducted in 680 children from a rural primary school in Gakenke district, Northern province to assess the prevalence of T. solium taeniosis cysticercosis. | [51] |
| South Africa | 510 | - (0–1.4) | A cross-sectional study conducted to estimate the prevalence of fecal helminths in a series of rural and urban African school pupils residing at a relatively high altitude above sea level. | [118] |
| 998 | 55 (5.6) | A cross-sectional study to determine the prevalence and risk factors of schistosomiasis and STH infection among preschool aged children aged 15 years in Ngwavuma area of Mkhanyakude district. | [119] | |
| Tanzania | 820 | 43 (5.2) * 34 (4.1) & 9 (1.1) |
A cross-sectional study conducted in Mbozi area of Mbeya district. Of the stool samples testing positive for taeniosis by copro-Ag-ELISA, 34 (4.1%) were Ab positive against adult T. solium. Taenia eggs were detected in 9 (1.1%) stool samples by routine coprology. | [55] |
| 404 | 5 (1.2) & | A cross-sectional study to estimate the prevalence of and factors associated with T. solium infections among HIV+ and HIV—individuals in Mbulu district, northern Tanzania | [57] | |
| 1517 1501 |
9 (0.6) * 7 (0.5) |
Taeniosis estimates after 3 rounds of MDA for schistosomiasis in the two regions Mbozi (0.6%) and Mbeya (0.5%). | [120] | |
| 1514 1500 |
12 (0.8) * 8 (0.5) |
Repeated MDA to school aged children in Mbozi (0.8%) and school aged children in Mbeya (0.5%), Tanzania. | [72] | |
| 302 | 5 (1.7) & | A cross-sectional study to investigate the presence and prevalence of TSTC in PWE in Dar es Salaam. | [58] | |
| Uganda | 162 | 1 (0.6) | A cross-sectional study among children attending Mwanamugimu nutrition unit of Mulago hospital, Kampala, Uganda. | [121] |
| 331 | 2 (0.6) | A cross-sectional study among slum dwellers without risk of flooding at least 2 km away from the Nakivubo wetland in Kampala. | [122] | |
| 476 | 14 (2.9) | A cross-sectional study aimed at determining the prevalence and the outcomes (including anaemia) of intestinal parasitic infections and co-infections among children in Kiryandongo refugee camp. | [123] | |
| Zambia | 297 | 1 (0.3) | Prospective study conducted among HIV infected patients in Lusaka. | [124] |
| 403 | 4 (0.9) | A cross-sectional study to determine the prevalence and intensity of STH infections, intestinal protozoa and the presence of multiple infections in children attending preschool centres in Kafue district Zambia. | [125] | |
| 718 712 |
2 (0.3) # 45 (6.3)* |
A community-based cross-sectional survey to determine the prevalence of human taeniosis and cysticercosis in Petauke district in Eastern province. | [62] | |
| 226 | 27 (11.9)* | A prospective study conducted to ascertain the incidence of human cysticercosis in Katete district in Eastern province. | [63] | |
| 54 | 8 (14.8)* | A cross-sectional study to assess NCC prevalence among PWE in Katete district in Eastern province. | [64] |
HDSS, Health and demographic surveillance system; HIV, Human immune deficiency virus; IPIs, Intestinal parasite infections; MDA, Mass drug administration; NCC, Neurocysticercosis; No., Number; PWE, People with epilepsy; STH, Soil transmitted helminths; TSTC, T. solium taeniosis cysticercosis.
*Detection by copro Ag-ELISA.
#T. solium species confirmed.
& Detection based on serology using EITB (rES33 immunoblot).
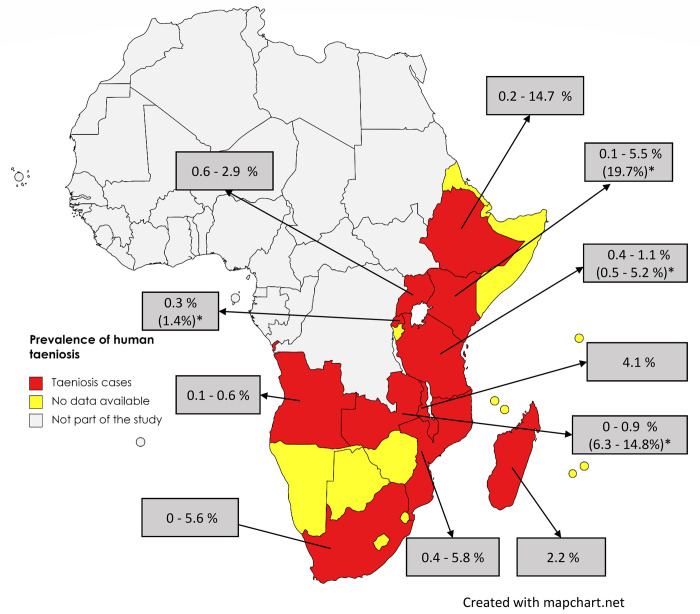
Fig 7. The distribution of human taeniosis in Eastern and Southern Africa showing prevalence data based on microscopy and *prevalence based on serology (rES33-immunoblot), and copro-Ag ELISA.
(The maps were obtained from an openly available source, Mapchart.net for free. Link: https://www.mapchart.net/terms.html#licensing-maps. Permission has been obtained from the owner).
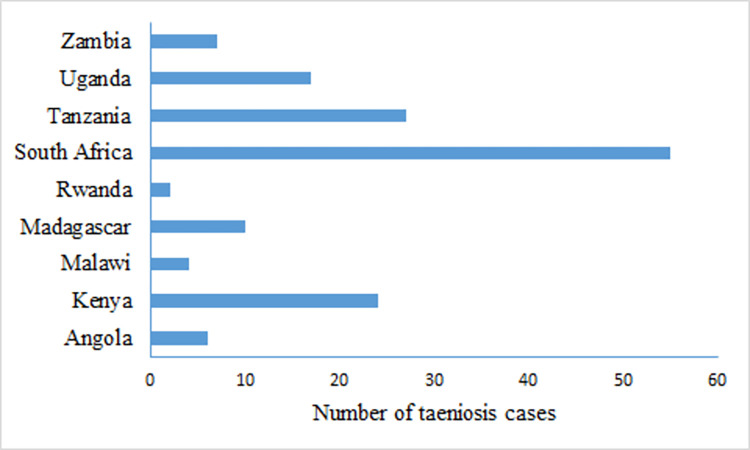
Several groups of people were studied for human taeniosis with 25 records reporting taeniosis cases in school children, 18 records on patients presenting at health facilities, 15 on consenting community volunteers, and six on food handlers. There was great variation in aggregated numbers of human taeniosis cases across countries with 595 cases reported from Ethiopia alone and even more (4834) reported from Mozambique. The number of human taeniosis cases identified for the other nine countries in ESA is shown in (Fig 8).
Fig 8. Taeniosis cases reported in Eastern and Southern Africa between 2000–2020.
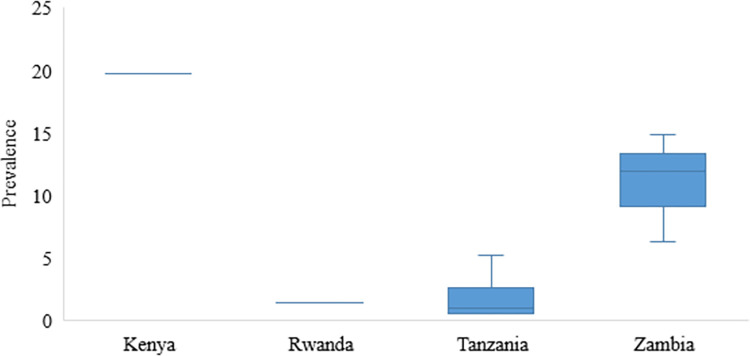
The largest number of cases were reported as Taenia spp. (Table 6) without specifying the type of species. One record from Madagascar and another from Zambia, reported confirmed T. solium using PCR; [62,70]. Microscopy alone was employed for 53 studies, and copro Ag-ELISA alone in three studies. Five studies used both copro Ag-ELISA and microscopy for taeniosis diagnosis. Three studies used the rES33 EITB for the presence of a tapeworm antibodies in the body. Immunological data (copro Ag-ELISA or rES33 EITB) on taeniosis was only available from Kenya, Rwanda, Tanzania, and Zambia.
Within the ESA region, the prevalence of human taeniosis based on microscopy ranged between 0.1–14.7% with the highest prevalence reported in a study conducted among food handlers at Wolkite University in Ethiopia [71] (Figs 7 and 9). Among studies based on copro Ag-ELISA conducted in Kenya, Rwanda, Tanzania, and Zambia, the highest prevalence was reported in Kenya at 19.7% [40]. Rwanda had a taeniosis prevalence of 1.4% [51], Tanzania ranged between 0.5 to 5.2% [55,72] [16], [22] and Zambia ranged between 0.3 to 13.8% [62,73] (Fig 10). For details on sources refer to Table 6.
Fig 9. Prevalence of taeniosis in Eastern and Southern Africa based on microscopy data in reviewed studies.
Fig 10. Prevalence of taeniosis based on copro-Ag ELISA in reviewed studies in Eastern and Southern Africa.
Discussion
This study aimed at collecting epidemiological data on T. solium infections in humans in the ESA region for the period 2000 to 2022. For both cysticercosis and taeniosis, there was no data from Comoros, Djibouti, Eritrea, Mayotte, Reunion, Seychelles, Socotra, Somalia and Somaliland that could be retrieved. Seventy per cent of the Somali human population subsists in pastoralism with sheep and goats being the dominant animals. Camels are primarily raised for milk production and small ruminants are for generating cash income for the family [126]. Somalia has for some time now experienced internal conflicts and this may have a bearing on the diagnosis and reporting of human cysticercosis and taeniosis cases. In addition, Somaliland and Somalia are predominantly Muslim communities where pig rearing and pork consumption is prohibited [127]. For the other six island states and territories, a lack of T. solium research in reporting cases has been cited [128]. The same can be said about Botswana and Lesotho from which data on cysticercosis and taeniosis was also lacking. Cysticercosis data was lacking in even more countries.
According to the World Bank IBRD-IDA report of 2022 [129] the population using safely managed sanitation services for both Comoros and Djibouti only stood at 37% with no data for other island states. People practicing open defecation were higher in Eritrea (67%) with Somalia reporting (23%) and Djibouti (16%). On the Réunion island, pork is said to be the most popular meat with a total annual pork consumption of about 20 000 tones. Half of this is produced on the island and the rest is imported frozen from Europe [130]. Comoros on the other hand has a small livestock sector with most of its meat including pork imported from Tanzania, Madagascar and France [131]. For Mayotte, Eritrea and Djibouti, information on pig rearing could not be found as these countries focus mostly on cattle, sheep, goats, and camels [132]. However, for Eritrea, pig breeding has recently begun to be promoted [133] and so is the case for Seychelles whose 2011 census of agriculture showed that 2% (483/24770) of the households were raising pigs in small holdings [134]. While it is not possible to ascertain the presence or absence of human T. solium infections in these countries, there is a possibility that these infections do exist as pork consumption seems to be practiced and risk factors are present. Therefore, the numbers collected for this review represent an underestimation of the T. solium epidemiology for human disease in ESA.
Regarding human cysticercosis cases, T. solium tapeworm carriers being the source of T. solium eggs are the sole cause of infection in both rural and urban areas of the ESA region [10,135]. T. solium tapeworm carriers, if not treated, pose a risk to themselves if they ingest infective eggs leading to cysticercosis but can also pose a risk to other people in contact [70,136]. A study in Madagascar found that the overall prevalence of cysticercosis at a household level was 46% and 34% detected by Ab-ELISA and EITB respectively, in the same household where a tapeworm carrier was found [70]. Individual human cysticercosis cases were identified from a third of the 27 ESA countries. South Africa reported the highest number of individual human cysticercosis cases followed by Madagascar. This could be due to increased awareness of cysticercosis as a possible diagnosis in, for example, people with epilepsy in these countries compared to other countries within the region. South Africa also has more resources to carry out testing compared to the other countries within the region. For example, as early as 2003 South Africa already had 214 CT scanners and 111 neurologists. This is an advantage in terms of diagnosis and management of particularly NCC that is not present in other countries within the region [137]. With poor pig management, poor meat inspection, and poor sanitation those who do not eat pork are equally at risk of infection with cysticercosis as those eating pork [138–140]. Additionally, certain cultural practices within the region have also been cited as contributing factors to cysticercosis. For example, in South Africa, it has been reported that some unqualified traditional healers "baloi" used Taenia segments and added them to medicinal mixtures as strengthening ingredients. There are also reports of T. solium segments being added to the beer of unfaithful husbands or lovers as punishment [137].
NCC has been associated with up to 57% of epilepsy cases in sub–Saharan Africa to which the region under consideration belongs [7,64]. This is higher than the global average of 30% of NCC among PWE [6]. For most countries within ESA, the diagnosis of NCC is problematic due to the scarcity of neuroimaging, serology and trained neurologists [141,142]. This diagnostic challenge is not only present in ESA but also in Europe where a lack of awareness of NCC leads to underdiagnoses [143]. Only a quarter of the countries within the ESA reported data on NCC and epilepsy. Epilepsy is one of the most common neurological disorders in many parts of sub-Saharan Africa and NCC seems to be a major cause of it in T. solium cysticercosis endemic areas. Diagnosing NCC among people with epilepsy is vital to prevent further morbidity and mortality from the disease as well as to reduce the social stigma associated with epilepsy in the region [141,144–146].
Regarding human T. solium taeniosis in ESA, only Kenya, Madagascar, Rwanda, Tanzania, and Zambia conducted community-based studies and employed serological tests that specifically aimed at determining the burden of infection. The other countries reported data on taeniosis as incidental findings following microscopic examinations of stool samples conducted for soil-transmitted helminths. The reported human taeniosis cases were also reported on an aggregated level without evidence of species determination. Thus, cases reported as either taeniosis or Taenia spp. could not for instance be differentiated from cases due to Taenia saginata which is also widely distributed in ESA [128]. Only two studies, one from Madagascar and another from Zambia were able to confirm the T. solium species after collecting tapeworm proglottids [62,70].
This lack of species differentiation creates uncertainty regarding the epidemiology of T. solium infections in ESA. Therefore, the prevalence of T. solium taeniosis in the region may be overestimated as most Taenia spp. diagnoses could be T. saginata as beef is consumed in most countries, while in some regions, the consumption of pork is prohibited for religious reasons. This is worsened by the fact that T. solium taeniosis is not even a notifiable disease in most of these countries. Application of molecular methods to differentiate T. solium species on stool examination is not widely practiced and it is not possible to differentiate Taenia eggs based on microscopy [135,147]. More sensitive and specific diagnostic tools need to be used to identify the true prevalence and outline the epidemiology of T. solium.
Human taeniosis in the region is due to the high prevalence of porcine cysticercosis in ESA countries which is ranked amongst the highest in the world [10]. The pooled prevalence for porcine cysticercosis within the region was recently estimated to be as high as 27% by carcass dissection which is a gold standard technique for porcine cysticercosis diagnosis [11]. Over the last decade, pig production as a risk factor for human taeniosis in the rural communities of ESA countries has increased mainly because pig raising is an attractive alternative to other animals as it does not require grazing land compared to ruminants. Free-range pig keeping is popular, especially in rural areas of the region as it is easy and cheap. This coupled with the general lack of slaughterhouses and inspection of pork with a lack of sanitation facilities has contributed to the risk for people to acquire taeniosis and cysticercosis [3,10,148].
Study limitation
Our study is limited by the fact that search platforms were used only for online publications. Thus, unpublished data which could have contributed significantly to a more nuanced picture of the epidemiology of human T. solium infections in ESA could have been missed. Another limitation of our study is the difficulty in comparing epidemiological data from different study populations using different diagnostic tests used and the different diagnostic approaches.
Conclusion
In this review on human T. solium taeniosis and (neuro) cysticercosis within ESA, we found wide variations in the prevailing prevalence estimates depending on the quality of the study and the diagnostic methods used. There remain large gaps with regards to T. solium infections within the ESA region with 11 countries without any information on human taeniosis and cysticercosis, and even more countries [18] without any information for cysticercosis.
Considering the public health and economic impact that T. solium infections have, it is important to understand its epidemiology in humans. Human taeniosis, cysticercosis and NCC cases need to be reported and notified to public health authorities. More community-based surveys as well as ante-mortem and postmortem porcine surveys must be conducted for control and surveillance purposes. Lack of awareness about the presence of T. solium among medical, community and government authorities including inadequate technology for diagnosis of taeniosis, cysticercosis and NCC, in both humans and pigs (cysticercosis) remains a challenge that needs to be addressed for proper surveillance, prevention and control.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the German Federal Ministry of Education and Research (BMBF) under CYSTINET-Africa grant number 81203604 (CSS) and 01KA1618 (ASW). The funder had no role in the design of the study, data collection, analysis and interpretation and in writing the manuscript.
References
- 1.Sotelo J, Del Brutto OH. Review of neurocysticercosis. Neurosurg Focus [Internet]. 2002. [cited 2018 Feb 22];12(6):e1. Available fro://www.juliosotelo.org/web/wp-content/uploads/2013/09/S240.pdf [DOI] [PubMed] [Google Scholar]
- 2.Murrell KD. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cysticercosis. Paris: World Health Organisation for Animal Health (OIE), 2005. [Internet]. 2005 [cited 2019 May 6]. Available from: http://www.oie.int [Google Scholar]
- 3.Carabin H, Millogo A, Cissé A, Gabriël S, Sahlu I, Dorny P, et al. Prevalence of and Factors Associated with Human Cysticercosis in 60 Villages in Three Provinces of Burkina Faso. PLoS Negl Trop Dis [Internet]. 2015. Nov [cited 2018 Feb 22];9(11):e0004248. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26588468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler A, Richter H. Landscape analysis : management of neurocysticercosis with an emphasis on low- and middle-income countries. World Heal Organ [Internet]. 2015. [cited 2018 Feb 22];65. Available from: www.neurokopfzentrum.med.tum.de/neurologie/42b.html [Google Scholar]
- 5.Stelzle D, Schmidt V, Keller L, Ngowi BJ, Matuja W, Escheu G, et al. Characteristics of people with epilepsy and Neurocysticercosis in three eastern African countries–A pooled analysis. PLoS Negl Trop Dis [Internet]. 2022. Nov 7 [cited 2022 Dec 8];16(11):e0010870. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. Preux P-M, editor. PLoS Negl Trop Dis [Internet]. 2010. Nov 2 [cited 2018 Feb 22];4(11):e870. Available from: doi: 10.1371/journal.pntd.0000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owolabi LF, Adamu B, Jibo AM, Owolabi SD, Imam AI, Alhaji ID. Neurocysticercosis in people with epilepsy in Sub-Saharan Africa: A systematic review and meta-analysis of the prevalence and strength of association [Internet]. Vol. 76, Seizure:European Journal of Epilepsy. Elsevier; 2020. [cited 2021 Jul 2]. p. 1–11. Available from: http://www.seizure-journal.com/article/S1059131119306399/fulltext [DOI] [PubMed] [Google Scholar]
- 8.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos J, et al. Epilepsy and Neurocysticercosis in Latin America: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis [Internet]. 2013. Oct 1 [cited 2022 Nov 11];7(10). Available from: https://pubmed.ncbi.nlm.nih.gov/24205415/ doi: 10.1371/journal.pntd.0002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng-Nguyen D, Traub RJ, Nguyen VAT, Breen K, Stevenson MA. Spatial distribution of Taenia solium exposure in humans and pigs in the Central Highlands of Vietnam. PLoS Negl Trop Dis [Internet]. 2018. Sep 1 [cited 2022 Nov 11];12(9). Available from: /pmc/articles/PMC6168177/ doi: 10.1371/journal.pntd.0006810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phiri IK, Ngowi H, Afonso S, Matenga E, Boa M, Mukaratirwa S, et al. The emergence of Taenia solium cysticercosis in Eastern and Southern Africa as a serious agricultural problem and public health risk. In: Acta Tropica [Internet]. Elsevier; 2003. [cited 2018 Feb 22]. p. 13–23. Available from: https://www.sciencedirect.com/science/article/pii/S0001706X03000512?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 11.Gulelat Y, Eguale T, Kebede N, Aleme H, Fèvre EM, Cook EAJ. Epidemiology of Porcine Cysticercosis in Eastern and Southern Africa: Systematic Review and Meta-Analysis. Front Public Heal. 2022. Mar 16;10:477. doi: 10.3389/fpubh.2022.836177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews [Internet]. Vol. 372, The BMJ. BMJ Publishing Group; 2021. [cited 2021 Jul 3]. Available from: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanathan M, MT A, Berkman N, Chang S, Hartling L, McPheeters M et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions—methods guide–chapter | AHRQ effective health care program [Internet]. 2016. [cited 2018 Sep 7]. Available from: www.effectivehealthcare.ahrq.gov. [Google Scholar]
- 14.Mugabe M, Ruhangaza D, Hakizimana E. Cysticercosis: Report of 10 cases diagnosed at a district hospital in Rwanda. Mod Pathol [Internet]. 2019;32(3). Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emexb&NEWS=N&AN=631814441 [Google Scholar]
- 15.Sobnach S, Khosa SA, Pather S, Longhurst S, Kahn D, Raubenheimer PJ. First case report of pharyngeal cysticercosis. Trans R Soc Trop Med Hyg [Internet]. 2009. Feb 1 [cited 2021 Oct 27];103(2):206–8. Available from: https://academic.oup.com/trstmh/article/103/2/206/1906630 doi: 10.1016/j.trstmh.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 16.Rabenjamina FR, Ranaivoravo J, Raholimina V, Ramialiharisoa A. A case report of bronchial cysticercosis. Arch Inst Pasteur Madagascar [Internet]. 2000. Jan 1 [cited 2021 Oct 23];66(1–2):43–5. Available from: https://europepmc.org/article/med/12463034 [PubMed] [Google Scholar]
- 17.Rakoto-Ratsimba HN, Rabesalama SSEN, Razafimahandry HJC, Ranaivozanany A. Case report of solitary breast cysticercosis in Madagascar. Med Trop [Internet]. 2007. [cited 2021 Oct 18];67(2):179–80. Available from: https://pubmed.ncbi.nlm.nih.gov/17691439/ [PubMed] [Google Scholar]
- 18.Heller T, Wallrauch C, Kaminstein D, Phiri S. Case report: Cysticercosis: sonographic diagnosis of a treatable cause of epilepsy and skin nodules. Am J Trop Med Hyg [Internet]. 2017. Oct 2 [cited 2021 Oct 21];97(6):1827–9. Available from: https://www.ajtmh.org/view/journals/tpmd/97/6/article-p1827.xml doi: 10.4269/ajtmh.17-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uledi SJ. A rare gigantic solitary cysticercosis pseudotumour of the neck. J Surg Case Reports [Internet]. 2010. Nov [cited 2022 Jul 5];2010(9):5–5. Available from: https://pubmed.ncbi.nlm.nih.gov/24946357/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agnihotri S, Talwar OP, Pudasaini S, Baral R. Cysticercosis of breast—A case report. Polish J Pathol [Internet]. 2006. [cited 2021 Oct 18];57(1):53–4. Available from: https://www.researchgate.net/publication/7042840_Cysticercosis_of_breast_-_A_case_report [PubMed] [Google Scholar]
- 21.Tuan J, Kailani L, Ngabitsinze P, Umuganwa S, Munyaneza F, Musoni E, et al. Disseminated cysticercosis in rwanda—case report of a patient presenting with difficulty with walking and skin nodules [Internet]. Vol. 77, Rwanda Medical Journal. 2020. [cited 2022 Jul 5]. p. 1–4. Available from: https://www.google.com/search?q=Disseminated+cysticercosis+in+rwanda—case+report+of+a+patient+presenting+with+difficulty+with+walking+and+skin+nodules&oq=Disseminated+cysticercosis+in+rwanda—case+report+of+a+patient+presenting+with+difficulty+with+walking [Google Scholar]
- 22.Thomas MB, Thomas KM, Awotedu AA, Blanco-Blanco E, Anwary M. Cardiocysticercosis. SAMJ South African Med J [Internet]. 2007. Jul 1 [cited 2021 Oct 24];97(7):504–6. Available from: https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=02569574&v=2.1&it=r&id=GALE%7CA167881603&sid=googleScholar&linkaccess=fulltext [PubMed] [Google Scholar]
- 23.Agaba E, Modi D, Gunduz O, Modi Z. Subcutaneous nodules of cysticercosis as a sign of asymptomatic neurocysticercosis in an HIV positive patient. Rev Soc Bras Med Trop [Internet]. 2018. Nov 1 [cited 2021 Oct 18];51(6):861–3. Available from: http://www.scielo.br/j/rsbmt/a/CC3Bbf3Zj7ChrGrzmxXTPZK/?lang=en doi: 10.1590/0037-8682-0178-2018 [DOI] [PubMed] [Google Scholar]
- 24.Nowalaza Z, Zampoli M, Pillay K, Singh S, Zar HJ. Pulmonary cysticercosis in an urban South African child [Internet]. Vol. 56, Pediatric Pulmonology. Authorea; 2021. [cited 2022 Jul 6]. p. 4060–2. Available from: https://www.authorea.com/users/404410/articles/515592-isolated-pulmonary-cysticercosis-in-an-urban-south-african-child [DOI] [PubMed] [Google Scholar]
- 25.McCormick DW, Bacha JM, El-Mallawany NK, Kovarik CL, Slone JS, Campbell LR. Disseminated cysticercosis and Kaposi sarcoma in a child with HIV/AIDS: A case report. BMC Infect Dis [Internet]. 2020. Apr 25 [cited 2021 Oct 22];20(1):1–5. Available from: https://bmcinfectdis.biomedcentral.com/articles/doi: 10.1186/s12879-020-05039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyimo FR, Jusabani AM, Makungu H, Mtolera M, Surani S. Thinking Outside Malaria: A Rare Case of Disseminated Cysticercosis With Cardiopulmonary Involvement From Urban Tanzania. Cureus [Internet]. 2021. Jan 22 [cited 2022 Jul 5];13(1). Available from: https://www.cureus.com/articles/49925-thinking-outside-malaria-a-rare-case-of-disseminated-cysticercosis-with-cardiopulmonary-involvement-from-urban-tanzania doi: 10.7759/cureus.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ndlovu B. Combined surgical and medical treatment of racemose neurocysticercosis in Swaziland. Neuroradiology [Internet]. 2018;60(1):109–10. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L621458617&from=export29080918 [Google Scholar]
- 28.Rajaonarison P, Ralamboson S, Andriamamonjy C, Ramanampamonjy R, Ce R, Razafindratrimo F, et al. Diagnostic de la neurocysticercose : à propos d ‘ un cas. Arch Inst Pasteur Madagascar [Internet]. 2001. [cited 2021 Oct 23];67:53–6. Available from: https://www.researchgate.net/publication/237675160_Diagnostic_de_la_neurocysticercose_a_propos_d’un_cas [PubMed] [Google Scholar]
- 29.Rakotoarivelo RA, Andrianasolo RL, Ranoharison D, Rakoto ROS, Sendrasoa FA, de Dieu Randria MJ. Neurocysticercosis as an important differential of paradoxical response during antituberculosis therapy in HIV-negative patient. Asian Pacific J Trop Dis. 2011. Dec;1(4):333–4. [Google Scholar]
- 30.Kalata N, Ellis J, Benjamin L, Kampondeni S, Chiodini P, Harrison T, et al. Neurological deterioration in a patient with HIV-associated cryptococcal meningitis initially improving on antifungal treatment: a case report of coincidental racemose neurocysticercosis. BMC Infect Dis [Internet]. 2021. Dec 1 [cited 2022 Jul 5];21(1):1–5. Available from: https://bmcinfectdis.biomedcentral.com/articles/doi: 10.1186/s12879-021-06425-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saldanha V, Saldanha G, Reys RP, Benson CA, Noormahomed EV. Neurocysticercosis in Child Bearing Women: An Overlooked Condition in Mozambique and a Potentially Missed Diagnosis in Women Presenting with Eclampsia. EC Microbiol [Internet]. 2018. Nov [cited 2022 Jul 5];14(11):736–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31681909%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC6824723 [PMC free article] [PubMed] [Google Scholar]
- 32.Vitali AM, Jithoo R. Multiple forms of neurocysticercosis in a single patient. Br J Neurosurg [Internet]. 2004. Dec [cited 2021 Oct 22];18(6):650–1. Available from: https://www.tandfonline.com/doi/abs/10.1080/02688690400022847 [DOI] [PubMed] [Google Scholar]
- 33.Motsepe T, Ackerman D. Spinal and vertebral neurocysticercosis in an HIV-positive female patient. South African J Epidemiol Infect [Internet]. 2012. Jan [cited 2021 Oct 22];27(3):133–6. Available from: https://www.tandfonline.com/doi/abs/10.1080/10158782.2012.11441499 [Google Scholar]
- 34.Jivan K, Mochan A, Modi G. Intraventricular neurocysticercosis causing acute unilateral hydrocephalus. African J Psychiatry (South Africa) [Internet]. 2010. [cited 2021 Oct 22];13(4):315–7. Available from: https://www.researchgate.net/publication/47460261_Intraventricular_neurocysticercosis_causing_acute_unilateral_hydrocephalus [PubMed] [Google Scholar]
- 35.Sibat HF. Neurocysticercosis, Epilepsy, COVID-19 and a Novel Hypothesis: Cases Series and Systematic Review [Internet]. Vol. 15, Clinical Schizophrenia and Related Psychoses. 2021. [cited 2022 Jul 8]. Available from: https://www.clinicalschizophrenia.net/abstract/neurocysticercosis-epilepsy-covid19-and-a-novel-hypothesis-cases-series-and-systematic-review-84090.html [Google Scholar]
- 36.Abstracts Mukonde F. Epilepsia. 2014. Jun;55(Suppl.2):4–246. [DOI] [PubMed] [Google Scholar]
- 37.Manske J, McVey C, Chengazi H, Siddiqi O, Saylor D. Intramedullary spinal neurocysticercosis (P5.9–031). Neurology. 2019;92(15 Supplement). [Google Scholar]
- 38.Musara A, Soko N, Shamu S. Suprasellar cysticercosis cyst with optic nerve compression masquerading as an arachnoid cyst. Middle East Afr J Ophthalmol [Internet]. 2019. Apr 1 [cited 2021 Oct 23];26(2):114–6. Available from: http://www.meajo.org/article.asp?issn=0974-9233;year=2019;volume=26;issue=2;spage=114;epage=116;aulast=Musara doi: 10.4103/meajo.MEAJO_142_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nsengiyumva G, Druet-Cabanac M, Ramanankandrasana B, Bouteille B, Nsizabira L, Preux PM. Cysticercosis as a major risk factor for epilepsy in Burundi, East Africa. Epilepsia [Internet]. 2003. Jul 1 [cited 2021 Oct 22];44(7):950–5. Available from: https://pubmed.ncbi.nlm.nih.gov/12823579/ doi: 10.1046/j.1528-1157.2003.55302.x [DOI] [PubMed] [Google Scholar]
- 40.Fèvre EM, de Glanville WA, Thomas LF, Cook EAJ, Kariuki S, Wamae CN. An integrated study of human and animal infectious disease in the Lake Victoria crescent small-holder crop-livestock production system, Kenya. BMC Infect Dis [Internet]. 2017. Jun 30 [cited 2021 Oct 23];17(1):1–14. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz MM, Sokhi D, Noh J, Ngugi AK, Minja FJ, Reddi P, et al. Prevalence of Epilepsy, Human Cysticercosis, and Porcine Cysticercosis in Western Kenya. Am J Trop Med Hyg [Internet]. 2022. May 4 [cited 2022 Jul 5];106(5):1450–5. Available from: https://www.ajtmh.org/view/journals/tpmd/106/5/article-p1450.xml [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leutscher P, Andriantsimahavandy A. Cysticercosis in Peace Corps Volunteers in Madagascar. N Engl J Med [Internet]. 2004. Oct 8 [cited 2021 Oct 23];350(3):311–2. Available from: https://www.nejm.org/doi/full/10.1056/NEJM200401153500325 [DOI] [PubMed] [Google Scholar]
- 43.Carod J., Razafimahefa J, Randrianarison M, Ramahefarisoa R., Rakotondrazaka M, Andriantseheno M. The burden of neurocysticercosis: A multi-purpose survey in Madagascar [Internet]. Vol. 18, Clinical Microbiology and Infection. 2012. [cited 2022 Jul 8]. p. 52–3. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70822228 [Google Scholar]
- 44.Carod J., Rakotondrazaka M, Ramahefarisoa R., Menard D, Soares J., Dorny P, et al. Updated epidemiology of cysticercosis in Madagascar [Internet]. Vol. 16, Tropical Medicine and International Health. 2011. [cited 2022 Jul 9]. p. 270. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70589687 [Google Scholar]
- 45.Rakotomahefa Narison ML, Ratsimbazafy ABA, Randrianjafinimpanana H, Randrianaivo N, Andriatahina TN, Raobijaona HS. Aspect épidémio-clinique de la neurocysticercose dans le service de pédiatrie au CHU de Tamatave- Madagascar. Arch Pediatr. 2014. May 1;21(5):810. [Google Scholar]
- 46.Zafindraibe NJ, Ralalarinivo J, Rakotoniaina AI, Maeder MN, Andrianarivelo MR, Contamin B, et al. Séroprévalence de la cysticercose et facteurs de risque associés chez un groupe de patients vus au centre hospitalier régional de référence d’antsirabe, madagascar. Pan Afr Med J [Internet]. 2017. Nov 23 [cited 2021 Oct 27];28(260). Available from: https://www.panafrican-med-journal.com/content/article/28/260/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carod JF, Dorny P. Cysticercosis in Madagascar [Internet]. Vol. 14, Journal of Infection in Developing Countries. J Infect Dev Ctries; 2020. [cited 2022 Nov 17]. p. 1031–42. Available from: https://pubmed.ncbi.nlm.nih.gov/33031077/ [DOI] [PubMed] [Google Scholar]
- 48.Assane YA, Trevisan C, Schutte CM, Noormahomed EV, Johansen MV, Magnussen P. Neurocysticercosis in a rural population with extensive pig production in Angónia district, Tete Province, Mozambique. Acta Trop [Internet]. 2017. [cited 2021 Oct 23];165:155–60. Available from: 10.1016/j.actatropica.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noormahomed EV, Nhacupe N, Mascaró-Lazcano C, Mauaie MN, Buene T, Funzamo CA, et al. A Cross-sectional Serological Study of Cysticercosis, Schistosomiasis, Toxocariasis and Echinococcosis in HIV-1 Infected People in Beira, Mozambique. PLoS Negl Trop Dis [Internet]. 2014. Sep 1 [cited 2021 Oct 27];8(9):e3121. Available from: https://journals.plos.org/plosntds/article?id=doi:10.1371/journal.pntd.0003121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rottbeck R, Nshimiyimana JF, Tugirimana P, Düll UE, Sattler J, Hategekimana JC, et al. High Prevalence of Cysticercosis in People with Epilepsy in Southern Rwanda. PLoS Negl Trop Dis [Internet]. 2013. [cited 2021 Oct 22];7(11). Available from: /pmc/articles/PMC3828157/ doi: 10.1371/journal.pntd.0002558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acosta Soto L, Parker LA, Irisarri-Gutiérrez MJ, Bustos JA, Castillo Y, Perez E, et al. Evidence for Transmission of Taenia solium Taeniasis/Cysticercosis in a Rural Area of Northern Rwanda. Front Vet Sci. 2021. Apr 20;8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ocana GS, Sablon JCO, Tamayo IO, Arena LA, Ocana LMS, Govender S. Neurocysticercosis in patients presenting with epilepsy at St. Elizabeth’s Hospital, Lusikisiki. South African Med J [Internet]. 2009. Feb 9 [cited 2021 Oct 24];99(8):588–91. Available from: https://www.ajol.info/index.php/samj/article/view/50818 [PubMed] [Google Scholar]
- 53.Foyaca-Sibat H, Cowan LD, Carabin H, Targonska I, Anwary MA, Serrano-Ocaña G, et al. Accuracy of serological testing for the diagnosis of prevalent neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa. PLoS Negl Trop Dis [Internet]. 2009. Dec [cited 2021 Oct 23];3(12):e562. Available from: https://journals.plos.org/plosntds/article?id=doi:10.1371/journal.pntd.0000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Epilepsy and neurocysticercosis in rural Tanzania—An imaging study. Epilepsia. 2009. May;50(5):987–93. doi: 10.1111/j.1528-1167.2008.01867.x [DOI] [PubMed] [Google Scholar]
- 55.Mwanjali G, Kihamia C, Kakoko DVC, Lekule F, Ngowi H, Johansen MV, et al. Prevalence and Risk Factors Associated with Human Taenia Solium Infections in Mbozi District, Mbeya Region, Tanzania. Jones MK, editor. PLoS Negl Trop Dis [Internet]. 2013. Mar 14 [cited 2018 Feb 22];7(3):e2102. Available from: http://dx.plos.org/doi: 10.1371/journal.pntd.0002102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunter E, Burton K, Iqbal A, Birchall D, Jackson M, Rogathe J, et al. Cysticercosis and epilepsy in rural Tanzania: A community-based case-control and imaging study. Trop Med Int Heal [Internet]. 2015. Sep 1 [cited 2021 Oct 21];20(9):1171–9. Available from: https://onlinelibrary.wiley.com/doi/full/ doi: 10.1111/tmi.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt V, Kositz C, Herbinger KH, Carabin H, Ngowi B, Naman E, et al. Association between Taenia solium infection and HIV/AIDS in northern Tanzania: A matched cross sectional-study. Infect Dis Poverty. 2016. Dec 1;5(1). doi: 10.1186/s40249-016-0209-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt V, O’Hara MC, Ngowi B, Herbinger KH, Noh J, Wilkins PP, et al. Taenia solium cysticercosis and taeniasis in urban settings: Epidemiological evidence from a health-center based study among people with epilepsy in Dar es Salaam, Tanzania. PLoS Negl Trop Dis [Internet]. 2019. [cited 2021 Oct 24];13(12):e0007751. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0007751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mageto JO, Muleke CI, Newton CR, Samuel G. Multiple parasitic infections as risk factors of active convulsive epilepsy. Sch J Appl Med Sci [Internet]. 2015;3(1C):178–84. Available from: http://saspublisher.com/wp-content/uploads/2015/01/SJAMS-31C178-184.pdf [Google Scholar]
- 60.Mwang’Onde BJ, Chacha MJ, Nkwengulila G. The status and health burden of neurocysticercosis in Mbulu district, northern Tanzania. BMC Res Notes [Internet]. 2018. Dec 13 [cited 2021 Oct 21];11(1):1–5. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-018-3999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamuyu G, Bottomley C, Mageto J, Lowe B, Wilkins PP, Noh JC, et al. Exposure to Multiple Parasites Is Associated with the Prevalence of Active Convulsive Epilepsy in Sub-Saharan Africa. PLoS Negl Trop Dis [Internet]. 2014. [cited 2021 Oct 23];8(5):e2908. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0002908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mwape KE, Phiri IK, Praet N, Muma JB, Zulu G, van den Bossche P, et al. Taenia solium infections in a rural area of Eastern Zambia-A community based study. Jex AR, editor. PLoS Negl Trop Dis [Internet]. 2012. Mar 27 [cited 2018 Feb 22];6(3):1–9. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0001594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mwape KE, Phiri IK, Praet N, Speybroeck N, Muma JB, Dorny P, et al. The Incidence of Human Cysticercosis in a Rural Community of Eastern Zambia. Flisser A, editor. PLoS Negl Trop Dis [Internet]. 2013. Mar 21 [cited 2018 Feb 22];7(3):e2142. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mwape KE, Blocher J, Wiefek J, Schmidt K, Dorny P, Praet N, et al. Prevalence of neurocysticercosis in people with epilepsy in the Eastern province of Zambia. Flisser A, editor. PLoS Negl Trop Dis [Internet]. 2015. Aug 18 [cited 2018 Feb 22];9(8):e0003972. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Himwaze C, Mucheleng’anga LA, Telendiy V, Hamukale A, Tembo J, Kapata N, et al. Cardiac cysticercosis and neurocysticercosis in sudden and unexpected community deaths in Lusaka, Zambia: a descriptive medico-legal post-mortem examination study. Int J Infect Dis [Internet]. 2022. Feb 1 [cited 2022 Jul 5];115:195–200. Available from: http://www.ijidonline.com/article/S1201971221008894/fulltext doi: 10.1016/j.ijid.2021.11.042 [DOI] [PubMed] [Google Scholar]
- 66.Buda A, Dean O, Adams HR, Mwanza-Kabaghe S, Potchen MJ, Mbewe EG, et al. Neurocysticercosis Among Zambian Children and Adolescents With Human Immunodeficiency Virus: A Geographic Information Systems Approach. Pediatr Neurol [Internet]. 2020. Jan 1 [cited 2021 Nov 2];102:36–43. Available from: /pmc/articles/PMC7864625/ doi: 10.1016/j.pediatrneurol.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumwenda JJJ, Mateyu G, Kampondeni S, Van Dam AP, Van Lieshout L, Zijlstra EEE, et al. Differential diagnosis of stroke in a setting of high HIV prevalence in Blantyre, Malawi. Stroke [Internet]. 2005. Dec [cited 2021 Oct 23];17(4):960–4. Available from: /pmc/articles/PMC3345513/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Segamwenge IL, Kioko NP, Mukulu C, Jacob O, Humphrey W, Augustinus J. Neurocysticercosis among patients with first time seizure in Northern Namibia. Pan Afr Med J [Internet]. 2016. [cited 2021 Oct 24];24(127). Available from: https://www.panafrican-med-journal.com/content/article/24/127/full doi: 10.11604/pamj.2016.24.127.8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mabaso SH, Bhana-Nathoo D, Lucas S. An audit of CT brain findings in adults with new-onset seizures in a resource restricted setting in South Africa. South African J Radiol [Internet]. 2022. Jan 20 [cited 2022 Jul 5];26(1):7. Available from: https://sajr.org.za/index.php/sajr/article/view/2294/3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahantamalala A, Rakotoarison RL, Rakotomalala E, Rakotondrazaka M, Kiernan J, Castle PM, et al. Prevalence and factors associated with human Taenia solium taeniosis and cysticercosis in twelve remote villages of Ranomafana rainforest, Madagascar. PLoS Negl Trop Dis [Internet]. 2022. Apr 1 [cited 2022 Jul 5];16(4):e0010265. Available from: https://journals.plos.org/plosntds/article?id = doi: 10.1371/journal.pntd.0010265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bafa TA, Sherif EM, Hantalo AH, Woldeamanuel GG. Magnitude of enteropathogens and associated factors among apparently healthy food handlers at Wolkite University Student’s Cafeteria, Southern Ethiopia. BMC Res Notes [Internet]. 2019. Sep 11 [cited 2021 Oct 27];12(1):1–6. Available from: https://bmcresnotes.biomedcentral.com/articles/doi: 10.1186/s13104-019-4599-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braae UC, Magnussen P, Ndawi B, Harrison W, Lekule F, Johansen MV. Effect of repeated mass drug administration with praziquantel and track and treat of taeniosis cases on the prevalence of taeniosis in Taenia solium endemic rural communities of Tanzania. Acta Trop. 2017. Jan 1;165:246–51. doi: 10.1016/j.actatropica.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 73.Mwape KE, Phiri IK, Praet N, Dorny P, Muma JB, Zulu G, et al. Study and ranking of determinants of taenia solium infections by classification tree models. Am J Trop Med Hyg [Internet]. 2015. Jan 7 [cited 2018 Feb 22];92(1):56–63. Available from: http://www.ajtmh.org/docserver/fulltext/14761645/92/1/56.pdf?expires=1519301298&id=id&accname=guest&checksum=B0D8FC99A90C4EAAE9849F41B1D24ADA doi: 10.4269/ajtmh.13-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fançony C, Langa AJ, Magalhães RJS, et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One [Internet]. 2012. Apr 6 [cited 2021 Oct 27];7(4):e33189. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0033189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school-aged children from southern Angola. PLoS One [Internet]. 2015. Sep 15 [cited 2021 Oct 25];10(9):e0137327. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0137327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mariam ST, Roma B, Sorsa S, Worku S, Erosie L. Assessment of sanitary and hygienic status of catering establishments of Awassa Town. Ethiop J Heal Dev. 2000. Jan 1;14(1). [Google Scholar]
- 77.Awole M, Gebre-Selassie S, Kassa T, Kibru G. Prevalence of intestinal parasites in HIV-infected adult patients in Southwestern Ethiopia. Ethiop J Heal Dev. 2003. Jan 1;17(1). [Google Scholar]
- 78.Mekonnen Z, Suleman S, Biruksew A, Tefera T, Chelkeba L. Intestinal polyparasitism with special emphasis to soil-transmitted helminths among residents around Gilgel Gibe Dam, Southwest Ethiopia: a community based survey. BMC Public Health [Internet]. 2016. Nov 23 [cited 2021 Oct 23];16(1):1–7. Available from: /pmc/articles/PMC5121972/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alemu A, Shiferaw Y, Getnet G, Yalew A, Addis Z. Opportunistic and other intestinal parasites among HIV/AIDS patients attending Gambi higher clinic in Bahir Dar city, North West Ethiopia. Asian Pac J Trop Med [Internet]. 2011. Aug [cited 2021 Oct 18];4(8):661–5. Available from: https://pubmed.ncbi.nlm.nih.gov/21914548/ doi: 10.1016/S1995-7645(11)60168-5 [DOI] [PubMed] [Google Scholar]
- 80.Wegayehu T, Tsalla T, Seifu B, Teklu T. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health [Internet]. 2013. Feb 18 [cited 2021 Oct 24];13(1):1–7. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alamir M, Awoke W, Feleke A. Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Health (Irvine Calif) [Internet]. 2013. Sep 29 [cited 2021 Oct 18];05(10):1697–701. Available from: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=38007 [Google Scholar]
- 82.Fekadu S, Taye K, Teshome W, Asnake S. Prevalence of parasitic infections in HIV-positive patients in southern Ethiopia: A cross-sectional study. J Infect Dev Ctries [Internet]. 2013. Nov 15 [cited 2021 Oct 27];7(11):868–72. Available from: https://jidc.org/index.php/journal/article/view/2906 doi: 10.3855/jidc.2906 [DOI] [PubMed] [Google Scholar]
- 83.Abera B, Alem G, Yimer M, Herrador Z. Epidemiology of soil-transmitted helminths, Schistosoma mansoni, and haematocrit values among schoolchildren in Ethiopia. J Infect Dev Ctries [Internet]. 2013. Mar 14 [cited 2021 Oct 18];7(3):253–60. Available from: https://jidc.org/index.php/journal/article/view/2539 doi: 10.3855/jidc.2539 [DOI] [PubMed] [Google Scholar]
- 84.Mamo H. Intestinal parasitic infections among prison inmates and tobacco farm workers in Shewa Robit, north-central Ethiopia. PLoS One [Internet]. 2014. Jun 13 [cited 2021 Oct 21];9(6):e99559. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0099559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Worku L, Damte D, Endris M, Tesfa H, Aemero M. Schistosoma mansoni infection and associated determinant factors among school children in Sanja Town, northwest Ethiopia. J Parasitol Res. 2014;2014. doi: 10.1155/2014/792536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alemayehu M, Birhan W, Belyhun Y, Sahle M, Tessema B. Prevalence of smear positive tuberculosis, intestinal parasites and their co-infection among tuberculosis suspects in Gondar University Hospital and Gondar Poly Clinic, North West Ethiopia. J Microb Biochem Technol [Internet]. 2014. [cited 2021 Oct 22];6(4):179–84. Available from: https://www.longdom.org/abstract/prevalence-of-smear-positive-tuberculosis-intestinal-parasites-and-theircoinfection-among-tuberculosis-suspects-in-gonda-9392.html [Google Scholar]
- 87.Desta M, Asrat D, Yimtubezinash W, Demiss N. Prevalence of intestinal parasites and Salmonella and Shigella among food handlers at food service establishments in the main campus and Health Sciences College of Hawassa University, Hawassa, Ethiopia. Ethiop J Heal Dev [Internet]. 2014. [cited 2021 Oct 18];28(1):29–34. Available from: http://www.etpha.org/publications/the-ethiopian-journal-of-health-development.html%0Ahttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=cagh&AN=20153041959%0A http://wa4py6yj8t.search.serialssolutions.com/?url_ver=Z39.88-2004&rft_val_fmt= [Google Scholar]
- 88.Beyene G, Tasew H. Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health center, Jimma southwest Ethiopia: A cross sectional study. Ann Clin Microbiol Antimicrob [Internet]. 2014. Feb 5 [cited 2021 Oct 21];13(1):10. Available from: /pmc/articles/PMC3922032/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014. Feb 14;14(1). doi: 10.1186/1471-2458-14-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gebreyesus A, Adane K, Negash L, Asmelash T, Belay S, Alemu M, et al. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Mekelle University student cafeteria, Mekelle, Ethiopia. Food Control [Internet]. 2014. [cited 2021 Oct 23];44:45–8. Available from: 10.1016/j.foodcont.2014.03.040 [DOI] [Google Scholar]
- 91.Ramos JM, Rodríguez-Valero N, Tisiano G, Fano H, Yohannes T, Gosa A, et al. Different profile of intestinal protozoa and helminthic infections among patients with diarrhoea according to age attending a rural hospital in southern Ethiopia. Trop Biomed [Internet]. 2014. [cited 2021 Oct 27];31(2):392–7. Available from: https://pubmed.ncbi.nlm.nih.gov/25134911/ [PubMed] [Google Scholar]
- 92.Shiferaw MB, Mengistu AD. Helminthiasis: Hookworm infection remains a public health problem in Dera District, South Gondar, Ethiopia. PLoS One [Internet]. 2015. Dec 1 [cited 2021 Oct 18];10(12):e0144588. Available from: https://journals.plos.org/plosone/article?id = doi: 10.1371/journal.pone.0144588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bereket A, Zewdneh T. Prevalence of intestinal helminthiasis and associated risk factors among schoolchildren in Dawro Zone, Southern Ethiopia. J Biol Agric Healthc [Internet]. 2015. [cited 2021 Oct 27];5(11):76–82. Available from: https://www.iiste.org/Journals/index.php/JBAH/article/view/23238 [Google Scholar]
- 94.Tigist G, Fantahun E, Hailu E, Kibe H, Fesseha O, Godana W, et al. Prevalence of Intestinal Parasitosis Among HIV / AIDS Patients Attending ART Clinic of Arbaminch Hospital. researchgate.net [Internet]. 2015. [cited 2021 Oct 22];(November 2015):1–6. Available from: https://www.researchgate.net/profile/Wanzahun-Godana/publication/283351146_PREVALENCE_OF_INTESTINAL_PARASITOSIS_AMONG_HIVAIDS_PATIENTS_ATTENDING_ART_CLINIC_OF_ARBAMINCH_HOSPITAL/links/5636572908aeb786b703f0ae/PREVALENCE-OF-INTESTINAL-PARASITOSIS-AMONG-HIV [Google Scholar]
- 95.Mama M, Alemu G. Prevalence and factors associated with intestinal parasitic infections among food handlers of Southern Ethiopia: Cross sectional study Infectious Disease epidemiology. BMC Public Health [Internet]. 2016. Feb 1 [cited 2021 Oct 24];16(1):1–7. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-2790-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alemu A, Tegegne Y, Damte D, Melku M. Schistosoma mansoni and soil-transmitted helminths among preschool-aged children in Chuahit, Dembia district, Northwest Ethiopia: Prevalence, intensity of infection and associated risk factors. BMC Public Health [Internet]. 2016. May 23 [cited 2021 Oct 18];16(1). Available from: /pmc/articles/PMC4876558/ doi: 10.1186/s12889-016-2864-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arota Amado A, Wadilo Wada F, Solomon Bisetegn F, Abreham Leka Y. Non-opportunistic intestinal parasitic infections among HIV-infected individuals at Wolaita Sodo Hospital, South Ethiopia. J Coast Life Med. 2016. May;4(5):353–7. [Google Scholar]
- 98.Alemayehu B, Tomass Z, Wadilo F, Leja D, Liang S, Erko B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health. 2017. Jun 20;17(1). doi: 10.1186/s12889-017-4499-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Girma H, Beyene G, Mekonnen Z. Prevalence of intestinal parasites among food handlers at cafeteria of Jimma University Specialized Hospital, Southwest Ethiopia. Asian Pacific J Trop Dis. 2017;7(8):467–71. [Google Scholar]
- 100.Alemu M, Kinfe B, Tadesse D, Mulu W, Hailu T, Yizengaw E. Intestinal parasitosis and anaemia among patients in a Health Center, North Ethiopia. BMC Res Notes [Internet]. 2017. Nov 28 [cited 2021 Oct 22];10(1):1–7. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-017-2957-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feleke DG, Arega S, Tekleweini M, Kindie K, Gedefie A. Schistosoma mansoni and other helminthes infections at Haike primary school children, North-East, Ethiopia: A cross-sectional study. BMC Res Notes [Internet]. 2017. Nov 21 [cited 2021 Oct 18];10(1):1–6. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-017-2942-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tegegne Y, Wondmagegn T, Worku L, Jejaw Zeleke A. Prevalence of Intestinal Parasites and Associated Factors among Pulmonary Tuberculosis Suspected Patients Attending University of Gondar Hospital, Gondar, Northwest Ethiopia. J Parasitol Res [Internet]. 2018. [cited 2021 Oct 27];2018. Available from: /pmc/articles/PMC5832163/ doi: 10.1155/2018/9372145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alemu G, Mama M. Intestinal helminth co-infection and associated factors among tuberculosis patients in Arba Minch, Ethiopia. BMC Infect Dis [Internet]. 2017. Jan 13 [cited 2021 Oct 21];17(1):1–9. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asmare T, Awoke W, Alem G. Prevalence of Intestinal Parasites and Associated Factors among AdultPre-ART and ART Patients in Goncha Siso Enesie Woreda, East Gojjam,Northwest Ethiopia, 2014. J AIDS Clin Res. 2017;8(10). [Google Scholar]
- 105.Teklemariam D, Legesse M, Degarege A, Liang S, Erko B. Schistosoma mansoni and other intestinal parasitic infections in schoolchildren and vervet monkeys in Lake Ziway area, Ethiopia. BMC Res Notes [Internet]. 2018. Feb 20 [cited 2021 Oct 24];11(1):1–6. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-018-3248-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zemene T, Shiferaw MB. Prevalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes [Internet]. 2018. Jan 22 [cited 2021 Oct 24];11(1):1–6. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-018-3166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alemu G, Aschalew Z, Zerihun E. Burden of intestinal helminths and associated factors three years after initiation of mass drug administration in Arbaminch Zuria district, Southern Ethiopia. BMC Infect Dis [Internet]. 2018. Aug 29 [cited 2021 Oct 21];18(1). Available from: /pmc/articles/PMC6114701/ doi: 10.1186/s12879-018-3330-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alemu M, Anley A, Tedla K. Magnitude of Intestinal Parasitosis and Associated Factors in Rural School Children, Northwest Ethiopia. Ethiop J Health Sci [Internet]. 2019. Jun 4 [cited 2021 Oct 25];29(1):923–8. Available from: https://www.ajol.info/index.php/ejhs/article/view/187204 doi: 10.4314/ejhs.v29i1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sitotaw B, Mekuriaw H, Damtie D. Prevalence of intestinal parasitic infections and associated risk factors among Jawi primary school children, Jawi town, north-west Ethiopia. BMC Infect Dis [Internet]. 2019. [cited 2021 Oct 27];19(1). Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3971-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kebede E, Seid A, Akele S. Prevalence and associated risk factors of intestinal parasitic infections among asymptomatic food handlers in Wollo University student’s cafeteria, Northeastern Ethiopia. BMC Res Notes [Internet]. 2019. Mar 14 [cited 2021 Oct 21];12(1):1–6. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-019-4182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eyamo T, Girma M, Alemayehu T, Bedewi Z. Soil-Transmitted Helminths And Other Intestinal Parasites Among Schoolchildren In Southern Ethiopia. Res Rep Trop Med [Internet]. 2019. Oct 24 [cited 2021 Oct 22];Volume 10:137–43. Available from: https://www.dovepress.com/soil-transmitted-helminths-and-other-intestinal-parasites-among-school-peer-reviewed-fulltext-article-RRTM doi: 10.2147/RRTM.S210200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohamed N, Muse A, Wordofa M, Abera D, Mesfin A, Wolde M, et al. Increased prevalence of cestode infection associated with history of deworming among primary school children in Ethiopia. Am J Trop Med Hyg [Internet]. 2019. [cited 2021 Oct 25];101(3):641–9. Available from: /pmc/articles/PMC6726949/ doi: 10.4269/ajtmh.19-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Obala AA, Simiyu CJ, Odhiambo DO, Nanyu V, Chege P, Downing R, et al. Webuye health and demographic surveillance systems baseline survey of soil-transmitted helminths and intestinal protozoa among children up to five years. J Trop Med [Internet]. 2013. [cited 2021 Oct 23];2013. Available from: /pmc/articles/PMC3600298/ doi: 10.1155/2013/734562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lowoko BN, Ngugi B, Malai A, Mutai JK. Factors associated with intestinal parasites among school going children in Lodwar Municipality, Turkana County, Kenya. East Afr Med J [Internet]. 2018. May 29 [cited 2021 Oct 23];94(8):649–63. Available from: https://www.ajol.info/index.php/eamj/article/view/172053 [Google Scholar]
- 115.Jones TPW, Hart JD, Kalua K, Bailey RL. A prevalence survey of enteral parasites in preschool children in the Mangochi District of Malawi. Vol. 19, BMC Infectious Diseases. BioMed Central Ltd.; 2019. doi: 10.1186/s12879-019-4439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noormahomed E V., Pividal JG, Azzouz S, Mascaró C, Delgado-Rodríguez M, Osuna A. Seroprevalence of anti-cysticercus antibodies among the children living in the urban environs of Maputo, Mozambique. Ann Trop Med Parasitol [Internet]. 2003. Jan [cited 2021 Oct 22];97(1):31–5. Available from: https://pubmed.ncbi.nlm.nih.gov/12662420/ doi: 10.1179/000349803125002742 [DOI] [PubMed] [Google Scholar]
- 117.Augusto G, Nalá R, Casmo V, Sabonete A, Mapaco L, Monteiro J. Geographic distribution and prevalence of schistosomiasis and soil-transmitted helminths among schoolchildren in mozambique. Am J Trop Med Hyg [Internet]. 2009. Nov 1 [cited 2021 Oct 23];81(5):799–803. Available from: https://www.ajtmh.org/view/journals/tpmd/81/5/article-p799.xml doi: 10.4269/ajtmh.2009.08-0344 [DOI] [PubMed] [Google Scholar]
- 118.Walker ARP, Dini LA, Walker BF, Frean JA. Helminthiasis in African children in a relatively low risk region in south Africa: implications for treatment? S Afr J Epidemiol Infect South [Internet]. 2000. [cited 2021 Oct 22];15. Available from: https://www.cabdirect.org/cabdirect/abstract/20013078709 [Google Scholar]
- 119.Sacolo-Gwebu H, Chimbari M, Kalinda C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-Natal, South Africa: A cross-sectional study. Infect Dis Poverty [Internet]. 2019. Jun 16 [cited 2021 Oct 22];8(1):1–12. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-019-0561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Braae UC, Magnussen P, Harrison W, Ndawi B, Lekule F, Johansen MV. Effect of National Schistosomiasis Control Programme on Taenia solium taeniosis and porcine cysticercosis in rural communities of Tanzania. Parasite Epidemiol Control. 2016. Sep 1;1(3):245–51. doi: 10.1016/j.parepi.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buzigi E. Prevalence of Intestinal Parasites, and its Association with Severe Acute Malnutrition Related Diarrhoea. J Biol Agric Healthc [Internet]. 2015. [cited 2021 Oct 18];5(2). Available from: https://www.researchgate.net/publication/271763982_Prevalence_of_Intestinal_Parasites_and_its_Association_with_Severe_Acute_Malnutrition_Related_Diarrhoea [Google Scholar]
- 122.Fuhrimann S, Winkler MS, Kabatereine NB, Tukahebwa EM, Halage AA, Rutebemberwa E, et al. Risk of Intestinal Parasitic Infections in People with Different Exposures to Wastewater and Fecal Sludge in Kampala, Uganda: A Cross-Sectional Study. PLoS Negl Trop Dis [Internet]. 2016. Mar 3 [cited 2021 Nov 3];10(3):e0004469. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0004469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oboth P, Gavamukulya Y, Barugahare BJ. Prevalence and clinical outcomes of Plasmodium falciparum and intestinal parasitic infections among children in Kiryandongo refugee camp, mid-Western Uganda: A cross sectional study. BMC Infect Dis [Internet]. 2019. Apr 1 [cited 2021 Oct 24];19(1):1–8. Available from: https://bmcinfectdis.biomedcentral.com/articles/doi: 10.1186/s12879-019-3939-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Modjarrad K, Zulu I, Redden DT, Njobvu L, Freedman DO, Vermund SH. Prevalence and predictors of intestinal helminth infections among human immunodeficiency virus type 1-infected adults in an urban African setting. Am J Trop Med Hyg [Internet]. 2005. [cited 2021 Oct 18];73(4):777–82. Available from: /pmc/articles/PMC2749260/ [PMC free article] [PubMed] [Google Scholar]
- 125.Siwila J, Phiri IGK, Enemark HL, Nchito M, Olsen A. Intestinal helminths and protozoa in children in pre-schools in Kafue district, Zambia. Trans R Soc Trop Med Hyg [Internet]. 2010. Feb 1 [cited 2021 Oct 27];104(2):122–8. Available from: https://academic.oup.com/trstmh/article/104/2/122/1923270 doi: 10.1016/j.trstmh.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 126.Elmi AA. Livestock production in Somalia with special emphasis on camels [Internet]. Vol. 29, Nomadic Peoples. 1991. [cited 2022 Nov 17]. p. 87–103. Available from: https://www.jstor.org/stable/43123342 [Google Scholar]
- 127.Bonne K, Verbeke W. Muslim consumer’s motivations towards meat consumption in Belgium : qualitative exploratory insights from means-end chain analysis. Anthropol food [Internet]. 2006. May 1 [cited 2023 Jan 18];(5). Available from: http://journals.openedition.org/aof/90 [Google Scholar]
- 128.Dermauw V, Dorny P, Braae UC, Devleesschauwer B, Robertson LJ, Saratsis A, et al. Epidemiology of Taenia saginata taeniosis/cysticercosis: A systematic review of the distribution in southern and eastern Africa [Internet]. Vol. 11, Parasites and Vectors. BioMed Central; 2018. [cited 2019 Jul 22]. p. 578. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30400948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.The World Bank IBRD-IDA. Countries | Data [Internet]. 2022. [cited 2022 Nov 17]. Available from: https://data.worldbank.org/country [Google Scholar]
- 130.Phillips L. Successful meat production on Réunion Island [Internet]. Farmer’s Weekly. 2014. [cited 2022 Sep 27]. Available from: https://www.farmersweekly.co.za/animals/cattle/successful-meat-production-on-reunion-island/ [Google Scholar]
- 131.Metz HC. Comoros: A Country Study [Internet]. Washington: GPO for the Library of Congress. 1994 [cited 2022 Sep 27]. Available from: http://countrystudies.us/comoros/
- 132.EAFF 2022. East Africa Farmers Federation [Internet]. [cited 2022 Sep 23]. Available from: https://www.eaffu.org/djibouti/ [Google Scholar]
- 133.Eritrea Ministry of Information. Livestock Population and Types [Internet]. 2012. [cited 2022 Sep 27]. Available from: https://shabait.com/2012/12/24/livestock-population-and-types-of-eritrea/ [Google Scholar]
- 134.Naiken Mr Marc; Ah Time Ms. Laura. Seychelles Census of Agriculture. 2011. [cited 2022 Nov 17]; Available from: https://wedocs.unep.org/xmlui/handle/20.500.11822/9520 [Google Scholar]
- 135.Mwape KE, Gabriël S. The Parasitological, Immunological, and Molecular Diagnosis of Human Taeniasis with Special Emphasis on Taenia solium Taeniasis. Vol. 1, Current Tropical Medicine Reports. 2014. p. 173–80. [Google Scholar]
- 136.Garcia HH, Del Brutto OH. Neurocysticercosis: Updated concepts about an old disease [Internet]. Vol. 4, Lancet Neurology. 2005. p. 653–61. Available from: http://neurology.thelancet.com [DOI] [PubMed] [Google Scholar]
- 137.Mafojane NA, Appleton CC, Krecek RC, Michael LM, Willingham AL. The current status of neurocysticercosis in Eastern and Southern Africa. In: Acta Tropica. Elsevier; 2003. p. 25–33. doi: 10.1016/s0001-706x(03)00052-4 [DOI] [PubMed] [Google Scholar]
- 138.Heinz HJ, Macnab GM. Cysticercosis in the Bantu of southern Africa. S Afr J Med Sci [Internet]. 1965. [cited 2022 May 15];30(1):19–31. Available from: https://pubmed.ncbi.nlm.nih.gov/5854976/ [PubMed] [Google Scholar]
- 139.Phiri IK, Dorny P, Gabriel S, Willingham AL, Speybroeck N, Vercruysse J. The prevalence of porcine cysticercosis in Eastern and Southern provinces of Zambia. Vet Parasitol. 2002;108(1):31–9. doi: 10.1016/s0304-4017(02)00165-6 [DOI] [PubMed] [Google Scholar]
- 140.Jansen F, Dorny P, Gabriël S, Dermauw V, Johansen MV, Trevisan C. The survival and dispersal of Taenia eggs in the environment: what are the implications for transmission? A systematic review [Internet]. Vol. 14, Parasites and Vectors. BioMed Central Ltd; 2021. [cited 2022 Nov 16]. p. 1–16. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-021-04589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winkler Sylvia A. Epilepsy and Neurocysticercosis in Sub-Saharan Africa. Nov Asp Cysticercosis Neurocysticercosis [Internet]. 2013. Jan 30 [cited 2018 Feb 22]; Available from: http://www.intechopen.com/books/novel-aspects-on-cysticercosis-and-neurocysticercosis/epilepsy-and-neurocysticercosis-in-sub-saharan-africa [Google Scholar]
- 142.Millogo A, Kongnyu Njamnshi A, Kabwa-PierreLuabeya M. Neurocysticercosis and epilepsy in sub-Saharan Africa. Vol. 145, Brain Research Bulletin. Elsevier Inc.; 2019. p. 30–8. [DOI] [PubMed] [Google Scholar]
- 143.Laranjo-González M, Devleesschauwer B, Trevisan C, Allepuz A, Sotiraki S, Abraham A, et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Western Europe [Internet]. Vol. 10, Parasites and Vectors. BioMed Central Ltd.; 2017. [cited 2022 May 15]. p. 1–14. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2280-828049510 [Google Scholar]
- 144.Baskind R, Birbeck GL. Epilepsy-associated stigma in sub-Saharan Africa: The social landscape of a disease. Epilepsy Behav [Internet]. 2005. Aug 1 [cited 2018 Feb 22];7(1):68–73. Available from: https://www.sciencedirect.com/science/article/pii/S152550500500140X?via%3Dihub doi: 10.1016/j.yebeh.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 145.Atadzhanov M, Haworth A, Chomba EN, Mbewe EK, Birbeck GL. Epilepsy-associated stigma in Zambia: what factors predict greater felt stigma in a highly stigmatized population? Epilepsy Behav [Internet]. 2010. Nov [cited 2018 Feb 20];19(3):414–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20851056 doi: 10.1016/j.yebeh.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Medina MT, Aguilar-Estrada RL, Alvarez A, Durõn RM, Martínez L, Dubõn S, et al. Reduction in rate of epilepsy from neurocysticercosis by community interventions: The Salamá, Honduras study. Epilepsia [Internet]. 2011. Jun [cited 2022 Jul 1];52(6):1177–85. Available from: https://pubmed.ncbi.nlm.nih.gov/21275975/ [DOI] [PubMed] [Google Scholar]
- 147.McCarthy JS, Lustigman S, Yang GJ, Barakat RM, García HH, Sripa B, et al. A research agenda for helminth diseases of humans: Diagnostics for control and elimination programmes. Vol. 6, PLoS Neglected Tropical Diseases. 2012. doi: 10.1371/journal.pntd.0001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thys S, Mwape KE, Lefèvre P, Dorny P, Marcotty T, Phiri AM, et al. Why Latrines Are Not Used: Communities’ Perceptions and Practices Regarding Latrines in a Taenia solium Endemic Rural Area in Eastern Zambia. Gyapong M, editor. PLoS Negl Trop Dis [Internet]. 2015. Mar 4 [cited 2018 Feb 22];9(3):e0003570. Available from: http://dx.plos.org/10.1371/journal.pntd.0003570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.