Abstract
Respiratory viral infections (RVIs) are of major clinical importance in immunocompromised patients and represent a substantial cause of morbidity and mortality in patients with hematologic malignancies and those who have undergone hematopoietic cell transplantation. Similarly, patients receiving immunotherapy with CD19-targeted chimeric antigen receptor–modified T cells, natural killer cells, and genetically modified T-cell receptors are susceptible to RVIs and progression to lower respiratory tract infections. In adoptive cellular therapy recipients, this enhanced susceptibility to RVIs results from previous chemotherapy regimens such as lymphocyte-depleting chemotherapy conditioning regimens, underlying B-cell malignancies, immune-related toxicities, and secondary prolonged, profound hypogammaglobulinemia. The aggregated risk factors for RVIs have both immediate and long-term consequences. This review summarizes the current literature on the pathogenesis, epidemiology, and clinical aspects of RVIs that are unique to recipients of adoptive cellular therapy, the preventive and therapeutic options for common RVIs, and appropriate infection control and preventive strategies.
Keywords: adoptive cellular therapy, chimeric antigen receptor–modified CAR-T, infection control, respiratory viral infections, RVIs
This is a review of the pathogenesis, epidemiology, clinical characteristics, and management of respiratory viral infections after receipt of adoptive cell therapies. We also provide an outline of the corresponding appropriate infection control and preventive strategies for immunocompromised populations.
Respiratory viral infections (RVIs) remain a major cause of morbidity in patients with hematologic malignancies and those who have undergone hematopoietic cell transplantation (HCT). Similarly, recipients of CD19-targeted chimeric antigen receptor (CAR)–modified T cells, B-cell maturation antigen (BCMA)–targeted CAR T cells, and natural killer cells, among others, are also susceptible to severe RVIs, with subsequent impact on morbidity and mortality [1, 2].
Recipients of adoptive therapy undergo lymphocyte-depleting conditioning regimens and often suffer from prolonged, profound lymphopenia and hypogammaglobulinemia [1]. Potential complications from these cellular therapies include cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS); management often requires corticosteroids and interleukin blockers such as anakinra and tocilizumab. These therapies can derange the intricate balance of humoral and cell-mediated immunity. Currently, data are still limited regarding the risk factors and management strategies for RVIs in patients who have undergone cellular therapies.
This review summarizes the pathogenesis, epidemiology, and clinical aspects of RVIs that are unique to recipients of adoptive cellular therapy and introduces preventive and therapeutic options for common RVIs.
ADOPTIVE CELLULAR THERAPIES AND CELLULAR IMMUNOTHERAPIES
Adoptive cellular therapies are promising salvage treatments for patients with relapsed or refractory (r/r) hematological malignancies [3]. These therapies involve T cells and natural killer cells that have been genetically modified to express CARs. The CAR expression on these immune cells targets cancer cells by recognizing their tumor-associated antigens [4]. CAR T-cell therapies directed against CD19 are being used in the treatment of r/r lymphomas affecting B-cell lineages and acute and chronic lymphoblastic leukemia while BCMA-directed CAR T-cell therapies are used in the treatment of r/r multiple myeloma [5]. Before the CAR-modified immune cell infusion, patients undergo lymphodepletion chemotherapy to allow efficient cell engraftment.
INFECTIOUS RISK ASSOCIATED WITH CELLULAR IMMUNOTHERAPIES
The immune derangements detected in recipients of cellular immunotherapies are multifactorial. The different underlying malignancies lead to distinct inherent immune derangements related to the primary disease process. Lymphoblastic leukemia and multiple myeloma are associated with baseline cell-mediated immune defects, hypogammaglobulinemia, complement defects, and abnormal phagocytosis whereas lymphoma mainly causes qualitative and quantitative lymphocyte abnormalities [6]. These patients are often exposed to multiple lines of chemotherapy that result in prolonged cytopenia, B-cell depletion, and hypogammaglobulinemia, which is known to be associated with an increased risk of mortality due to community-acquired RVIs [7, 8]. In addition, 38%–55% of these patients undergo either autologous or allogeneic HCT preceding adoptive cellular therapy [2]. In this setting, the type of HCT-related lymphodepletion regimen impacts the duration of bone marrow suppression [1] and many patients experience delayed recovery of B- and T-lymphocyte subsets [9]. Further immunological dysregulations ensue when graft-versus-host disease occurs with subsequent use of steroids and other immunosuppressants. These cumulative immunodeficiencies predispose patients to significant clinical consequences and diminished responses to available vaccines.
In addition to all of the above factors, different types of CAR therapies are associated with infusion toxicities often requiring corticosteroids and interleukin blockers, such as anakinra and tocilizumab, and unique on-target–off-tumor toxicities leading to short-term and long-term immunodeficiencies [10]. The CD19 cell surface antigen is expressed on earlier-stage naive and memory B cells and lost on the terminal plasma cells affecting the development and immunoglobulin activation of B cells while BCMA expression remains present on long-lived plasma cells, which represent the long-lived antibody-producing plasma cells and hence are hypothesized to lead to a higher extent of immunoglobulins deficiencies with subsequent higher risks for viral infections than CD19-directed CAR T-cell therapy [4, 10].
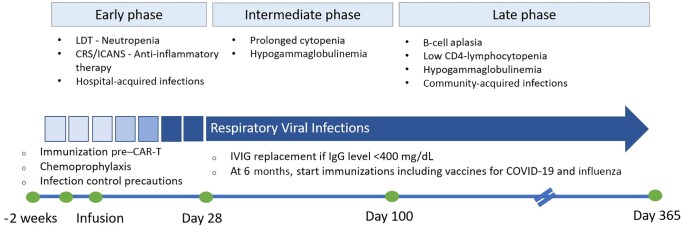
Like the HCT clinical categorization, the timeline from CAR T-cell infusion comprises multiple phases, with the early phase from day 0 to 30, the intermediate phase from day 31 to day 100, and the late phase beyond day 100 [8] (Figure 1).
Figure 1.
Timeline of host immune defects, predictable respiratory viral infection incidence, and corresponding preventive practices for recipients of cellular therapy. Abbreviations: CAR-T, chimeric antigen receptor–modified T-cell therapy; COVID-19, coronavirus disease; CRS/ICANS, cytokine release syndrome/immune effector cell–associated neurotoxicity syndrome; IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; LDT, lymphodepleting chemotherapy.
Early Phase
Cytopenias occur in up to 48% of patients during the early phase [11]. These are attributed to lymphodepleting regimens, bridging chemotherapy, or radiotherapy before CAR T-cell infusion, as well as to therapy-related toxicities, like CRS, macrophage activation syndrome, and ICANS [12]. CRS and ICANS occur in relatively high proportions and vary with the type and burden of the underlying malignancies, ranging from 37% to 93% and 23% to 67%, respectively [13]. Their treatment protocols include anti–interleukin 6 receptor antibodies such as tocilizumab in addition to corticosteroids when severe [13]. Bacteria are the most common pathogens during the early phase, accounting for 54%–61% of infections, followed by viral infections, of which 8%–38% are RVIs [2, 8, 14].
Intermediate and Late Phase
Following day 30, bone marrow toxicities with prolonged cytopenias, such as neutropenia, B-cell aplasia, and hypogammaglobulinemia, are increasingly noted as therapy-related adverse effects [12]. The likelihood of developing extended immunosuppression and off-target effects, such as B-cell aplasia leading to long-term hypogammaglobulinemia, increases with the number of previous lines of therapy [12]. This has been observed within 9 weeks following receipt of CAR T-cell infusion and may persist through 4 years [3]. In a pooled analysis of 3 clinical trials (NCT01029366 and NCT02640209 for tisagenlecleucel, and NCT01747486 for CART-19/4-1 BB) that included 67 patients who received CD19-directed CAR T-cell therapy [15], 44% of patients had hypogammaglobulinemia before therapy, and 81% developed a new or persistent decline in their immunoglobulin levels following infusion [15].
Persistent cytopenias were noted in 27% of patients at 1 year and in 11% of patients at 2 years in the ZUMA-1 and ZUMA-9 trials [11]. Similarly, in the TRANSCEND NHL-001 trial that evaluated lisocabtagene maraleucel therapy in patients with relapsed or refractory large B-cell lymphoma, 37% of the patients (N = 100/269) developed cytopenias at day 29 [16]. Other retrospective analyses reported similar results; the rate of persistent neutropenia, defined as neutropenia beyond day 28 in some studies and beyond day 42 in others, ranged from 9% to 70% in these analyses [17]. The intermediate and late phases after CAR T-cell infusion are mostly complicated by RVIs [8, 14].
INCIDENCE AND PATTERNS OF RVIs AFTER ADOPTIVE CELL THERAPY
To date, there are scarce data on the incidence and outcomes of RVIs after CAR T-cell therapy. Most of the literature was generated from post hoc evaluation of the main CAR T-cell therapy trials. These studies’ rates of RVIs ranged from 8.3% to 19.5% in the early phase (within 30 days of CAR T-cell infusion) [14, 18–20] and in some studies reached 58% from days 30 to 90 and 53% within 1 year during monitoring (late phase) [1, 8, 19, 21, 22]. The high overall incidence of these RVIs after CAR T-cell therapy during the late phase is likely attributable to patients reengaging with their communities while experiencing the long-term immunodeficiency described above [23].
Some of the landmark CAR T-cell trials that have described RVIs among their participants include the phase 2, multicenter ZUMA-1 trial of axicabtagene ciloleucel [24], the JULIET trial of tisagenlecleucel [25], and the TRANSCEND trial of lisocabtagene maraleucel, among others [26–28] (Table 1). In the ZUMA-1 trial, 12 upper respiratory tract infections (URTIs) occurred among the 101 participants within 8 months of CAR T-cell infusion. Two recurrent URTIs were noted on follow-up at 16 months [24]. In the phase 1 trial for 19-28z CAR T-cell therapy, 11 RVIs were reported; 3 occurred in the first month after the infusion, and 8 occurred between 31 and 180 days after the infusion, with a median of 48 days [25]. In 2 cohorts of patients with r/r diffuse large-B-cell lymphoma receiving axicabtagene ciloleucel or tisagenlecleucel, RVIs occurred less frequently during the first 30 days (up to 11.8%) than during day 30 to 1 year after receipt of adoptive cellular therapy (up to 23%) [14, 20]. None of these infections were associated with death during the follow-up period. Among the studies that reported the incidence of specific viruses, rhinovirus infections were the most common (35/102 [34.3%]), followed by influenza (22/102 [21.6%]) and human parainfluenza virus (hPIV) (15/102 [14.7%]) infections (Table 1). The prevalence of coronavirus disease 2019 (COVID-19) was 4.8% among an observational cohort of 353 patients who received CAR T-cell therapy in 2020 prior to vaccine development [29], with half of the patients having received their therapy within 6 months of acquiring the virus [29].
Table 1.
Summary Table of Published Chimeric Antigen Receptor T-Cell Studies and Respiratory Viral Infections
| Study [Reference] | No. of Patients | Underlying Malignancy | Study Name, Identifier, Product | No. and Type of RVIs |
|---|---|---|---|---|
| Clinical trials | ||||
| Cappell et al, 2020 [25] | 53 | B-ALL | Phase 1 clinical trial, NCT01044069 19–28z+ CAR-T |
Total: 11 <30 d, n = 3 Parainfluenza, n = 2 Rhinovirus, n = 1 31–180 d, n = 8 Coronavirus, n = 1 Influenza A, n = 2 Rhinovirus/enterovirus, n = 4 Unknown, n = 1 |
| Hill et al, 2018 [2] | 133 | Relapsed refractory CD19+ ALL, CLL, NHL | Phase 1/2 clinical trial NCT01865617 Autologous anti-CD19-CAR-4-1BB-CD3zeta-EGFRt–expressing T lymphocytes |
Total: 20 Viral pneumonia, n = 3 <28 d, n = 10 Rhinovirus, n = 4 Parainfluenza 3, n = 2 (1 LRTI) Parainfluenza 4, n = 1 (LRTI) Influenza A, n = 1 hMPV, n = 1 Coronavirus, n = 1 29–90 d, n = 10 Rhinovirus, n = 4 Parainfluenza 3, n = 2 Influenza A, n = 1 Influenza B, n = 1 (LRTI) hMPV, n = 1 Coronavirus, n = 1 |
| Locke et al, 2019 [24] | 101 | DLBCL | ZUMA-1 trial, NCT02348216 (axicabtagene ciloleucel) |
Total: 12 Viral pneumonia, n = 5 Influenza A, n = 2 Influenza B, n = 6 Rhinovirus, n = 1 Parainfluenza, n = 1 |
| Schuster et al, 2019 [26] | 111 | DLBCL | JULIET trial, phase 2, NCT02445248 CTL019 (tisagenlecleucel-T) |
Total: 13 Unspecified, n = 13 |
| Frey et al, 2020 [27] | 42 | Relapsed or refractory B-ALL | Phase 2 trial, NCT02030847, NCT01029366 CTL019 (tisagenlecleucel) |
Total: 2 Influenza, n = 1 Unspecified, n = 1 |
| Cappell et al, 2020 [25] | 43 | Primary mediastinal BCL, DLBCL | Phase 1/2 trial, NCT00924326 (FMC63-28Z) |
Total: 1 Influenza, n = 1 |
| Abramson et al, 2020 [16] | 269 | LBCL | TRANSCEND trial, NCT02631044 (lisocabtagene maraleucel) |
Total: 3 Coronavirus, n = 1 Parainfluenza, n = 1 Unspecified, n = 1 |
| Retrospective studies | ||||
| Schuster et al, 2017 [28] | 28 | DLBCL, follicular lymphoma | Phase 2a trial, NCT02030834 CTL019 (tisagenlecleucel) |
Total: 8 Unspecifieda, n = 8 |
| Wudhikarn et al, 2020 [14] | 60 | DLBCL | Axicabtagene ciloleucel or tisagenlecleucel | Total: 27 ≤30 d, n = 5 Rhinovirus, n = 2 Parainfluenza, n = 1 Coronavirus, n = 1 hMPV, n = 1 >30 d, n = 22 Rhinovirus, n = 8 Parainfluenza, n = 1 Coronavirus, n = 2 hMPV, n = 3 Adenovirus, n = 2 Influenza, n = 2 Unidentifieda, n = 4 |
| Dayagi et al, 2021 [19] | 88 | ALL, NHL | CD28-based CD19 CAR T cells | Total: 5 <30 d, n = 5 Parainfluenza, n = 2 hMPV, n = 2 Influenza B, n = 1 >30 d, n = 0 |
| Logue et al, 2021 [18] | 85 | Relapsed or refractory LBCL | Axicabtagene ciloleucel | Total: 29 <30 d, n = 10 Rhinovirus, n = 7 Influenza, n = 2 RSV, n = 1 >30 d, n = 19 Rhinovirus, n = 4 Influenza, n = 2 Parainfluenza, n = 2 RSV, n = 1 Unspecifieda, n = 10 |
| Baird et al, 2021 [20] | 41 | Relapsed or refractory CD19+ LBCL | Axicabtagene ciloleucel | Total: 14 ≤28 d, n = 4 Unspecifieda, n = 4 >28 d, n = 10 Unspecifieda, n = 10 |
| Kambhampati et al, 2022 [22] |
55 | Relapsed refractory multiple myeloma | BCMA CAR-T (JCARH125, BB2121, BB21217, JNJ-4528) | Total: 25 Unspecified, n = 25 |
Abbreviations: ALL, acute lymphoblastic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; BCL, B-cell lymphoma; BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor–modified T-cell therapy; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; hMPV, human metapneumovirus; LBCL, large B-cell lymphoma; LRTI, lower respiratory tract infection; NHL, non-Hodgkin lymphoma; RSV, respiratory syncytial virus; RVI, respiratory viral infection.
Clinical diagnosis without microbiological confirmation.
Poor outcomes and higher mortality due to RVIs were reported in patients post–adoptive cellular therapy [8]. Influenza virus–associated lower respiratory tract infections (LRTIs) occurred in patients after receipt of FMC63-28Z and autologous anti-CD19 CAR T lymphocytes [4, 29]. Death attributed to influenza infection was also reported in a patient enrolled in the ZUMA-1 trial [24]. Furthermore, during the COVID-19 pandemic, patients with hematologic malignancies, including those who had received CAR T-cell products, were at higher risk of developing severe COVID-19 and had a higher death rate [4]. Two European cohorts of unvaccinated CD19 and BCMA-directed CAR T-cell recipients with concomitant COVID-19 were evaluated. In the first cohort with a total of 30 patients, 66.7% experienced severe disease, 43.3% required intensive care unit (ICU) admission, and 50% died [29]. In the second cohort of 56 patients, 80% required hospitalization, 42.9% received oxygen support, and 39.3% required ICU admission, and COVID-19–attributed mortality was 41.1% [30]. In addition, few studies highlighted the persistent and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral detection by reverse-transcription polymerase chain reaction (PCR) assays from nasopharyngeal swabs beyond 2 months in some patients [29, 31].
DIAGNOSIS OF RESPIRATORY ILLNESSES AND AVAILABLE THERAPIES FOR RECIPIENTS OF CELLULAR THERAPY
Adoptive cellular therapy recipients exhibiting upper or lower respiratory symptoms, with or without fever, should be evaluated for respiratory viruses in circulation. Because the available rapid detection techniques for influenza and respiratory syncytial virus (RSV) antigens have low sensitivity and specificity [23], comprehensive nasopharyngeal multiplex PCR testing for COVID-19 and other respiratory illnesses can improve the timeliness of care. As antiviral therapies are available for some pathogens, early, specific viral diagnostics can help prevent the progression to LRTI.
For patients with presumptive or confirmed RVI before CAR T-cell therapy, lymphodepleting chemotherapy should be delayed for 2 weeks or until symptoms resolve [23], especially for RSV, hPIV types 1–4, influenza virus A or B, and metapneumovirus [32]. Although delaying CAR T-cell therapy until the resolution of symptoms may be preferred, this must be balanced against the risk of malignancy progression [32]. Pulmonary radiography can help guide clinical decisions and risk stratification on infected patients.
To date, no prospective studies evaluate the specific use of antiviral therapy in recipients of adoptive cellular therapy. Instead, current practices are extrapolated from data and available guidelines for recipients of HCT [33]. This is one of the limitations to our review, which underscores the need for more related research.
Respiratory Syncytial Virus
An immunodeficiency scoring index was originally developed to risk-stratify RSV infections in recipients of allogeneic HCT and help define their risk of progressing to LRTI and mortality [34]. Although the index has not been validated in recipients of CAR T-cell therapy, this population would benefit from a similar tool to identify those at high risk of severe infection and to guide antiviral therapy. As in HCT recipients, patients with a low likelihood of progression to LRTI do not require therapy, but those with evidence of LRTI and those at moderate-to-high risk of progression to LRTI may benefit from oral ribavirin therapy [34].
Intravenous immunoglobulin (IVIG) is a recommended adjunct therapy in HCT recipients with RSV infections that have progressed to LRTI [34]. Further studies are needed to determine its role in recipients of adoptive cellular therapy, but it may be considered in patients with low immunoglobulin G (IgG) titers. Newer agents, including EDP-938 (NCT04196101) and sisunatovir (RV521; NCT04267822), are being actively investigated as treatments for RSV infections in recipients of HCT.
Influenza Virus
Influenza virus infection can result in pneumonia and acute respiratory failure in recipients of adoptive cellular therapies. In these patients, antiviral therapy should be initiated immediately in all symptomatic patients with confirmed infection, even if symptom onset is beyond 48 hours, and the duration of therapy should be extended up to 10 days in patients with severe or prolonged illness [35]. Neuraminidase inhibitors are recommended treatments for influenza A and B; oseltamivir is the preferred agent except for neuraminidase-mutated influenza A(H1N1) [35]. Inhaled zanamivir is an alternative for both subtypes [35]. Intravenous peramivir has not been tested in immunocompromised patients. Still, it is an alternative when intestinal absorption is compromised by mucositis or diarrhea and inhaled zanamivir is not an option [23]. Baloxavir marboxil, a new antiviral agent with limited efficacy data [33, 36], is used in rare instances as salvage therapy. Combining baloxavir with standard-of-care neuraminidase inhibitors in hospitalized patients with severe influenza infection did not result in superior clinical outcomes but did show greater virologic efficacy [36]. However, the study did not report specifically on immunocompromised patients with underlying malignancies except noting the development of viral resistance in 2 patients with related immunosuppression due to their underlying malignancies [36]. In a subset analysis of immunocompromised patients who were not clearly defined, no significant difference in the median time to clinical recovery was observed (n = 17 and n = 13 in the baloxavir and the standard-of-care arms, respectively) [36]. A single-center clinical trial of baloxavir in combination with oseltamivir in HCT recipients is currently under way (NCT05170009).
Chemoprophylaxis is a strategy for preventing overt influenza infection in susceptible patients. Therapeutic dosing of oseltamivir within 48 hours postinfluenza exposure or during institutional outbreaks should be considered in unvaccinated patients or those with a poor vaccine response [32]. Available studies have shown significantly lower rates of cultured (0.4% oseltamivir vs 3.8% placebo) or PCR-confirmed (1.7% oseltamivir vs 8.4% placebo) influenza infections upon prophylaxis against influenza [33].
Human Parainfluenza Virus
Currently, there are no licensed anti-hPIV therapies. Therefore, supportive care and sometimes adjunctive IVIG are utilized [33]. Ribavirin therapy has been used despite the scarcity of data [33]. Sialidase DAS181, a novel inhaled host-active antiviral that cleaves the sialic acids from the surface of the respiratory epithelium preventing infection, demonstrated more rapid clinical stability and survival in the phase 2 trial [37]. A phase 3 trial of DAS181 in immunocompromised patients is under way (NCT03808922).
Rhinovirus
There are no approved antirhinovirus-specific therapies. Several agents have been studied, including capsid-binding proteins, protease inhibitors, and soluble intercellular adhesion molecules; however, these have failed to demonstrate clinical efficacy and may induce viral resistance [38].
COVID-19
The optimal approach to treating COVID-19 in immunocompromised patients is under investigation. Available therapies for patients with moderate to severe infection include antiviral and anti-inflammatory agents [39]. Therapeutic options should be guided by disease severity, as suggested by clinical parameters, including the presence of symptoms, age (>65 years), preexisting cardiopulmonary disease, severe obesity (body mass index >40 kg/m2), recent myeloablative chemotherapy, history of HCT, CAR T-cell therapy, severe lymphopenia (<300 cells/μL), hypogammaglobulinemia, radiographic evidence of LRTI, supplementation oxygen requirements, mechanical ventilation, and multiorgan dysfunction.
Early antiviral treatment with remdesivir showed a small effect against death and progression to ventilation in hospitalized patients, but no benefit was noted once mechanical ventilation was needed. The recommended duration of therapy spans from 3 to 10 days depending on illness severity [40]. Longer duration of therapy may be warranted for this population, unlike the general population. Persistent COVID-19 and clinical relapse have been reported in immunocompromised patients, and prolonging the duration of remdesivir course or repeating a course may be warranted [40, 41]. Oral antiviral agents—including the combination of the oral protease inhibitors nirmatrelvir and ritonavir, and molnupiravir, a nucleoside analogue that inhibits SARS-CoV-2 replication—are currently indicated for use in patients at risk of severe complications from COVID-19 to prevent hospitalization and progression to severe disease [42].
The arsenal of monoclonal antibodies in the treatment of COVID-19 has been jeopardized by rapidly emerging SARS-CoV-2 variants of concern [43], as these agents do not retain substantial efficacy against the currently dominant variant strains.
Regarding anti-inflammatory agents, dexamethasone reduces mortality in patients at least 7 days from COVID-19 symptom onset who require supplemental oxygen beyond their baseline; delaying treatment until day 7 may decrease the risk of corticosteroid-induced immunosuppression exacerbating viral replication. Tocilizumab may be used with dexamethasone and remdesivir for COVID-19 pneumonia in select patients requiring varying degrees of persistent supplemental oxygen [39]. In patients requiring supplemental oxygen beyond baseline who are not candidates for corticosteroid therapy, baricitinib may be considered if the benefits of the drug outweigh the risks (eg, further immunosuppression, superinfections, and thrombosis) [39].
INFECTION PREVENTION STRATEGIES
Infection prevention and control (IPC) strategies are integral to the care of patients with hematological malignancies, recipients of HCT, and adoptive cellular therapies. IPC precautions should be implemented for patients with suspected or documented RVIs. Standard precautions to prevent RVIs include hand hygiene, control of air quality, and transmission-based droplet precautions. Patients with RVIs who are hospitalized should be placed on isolation precautions to prevent transmission to healthcare workers (HCWs) and other vulnerable patients. The recommended personal protective equipment for HCWs caring for patients with RVIs includes gloves, gowns, masks, and sometimes eye protection. Visitors should also be asked about URTI symptoms and should delay visitation when symptoms are present. Similarly, HCWs with respiratory symptoms should refrain from direct patient care until symptom resolution to avoid nosocomial transmission (Table 2) [44].
Table 2.
Preventive and Therapeutic Recommendations for Recipients of Cellular Therapy With Respiratory Viral Infections
| Virus | Prophylaxis | Available Therapies | Infection Control Procedures and Prevention Strategies |
|---|---|---|---|
| Respiratory syncytial virus | Not indicated | − Oral or aerosolized ribavirin − RSV polyclonal or monoclonal antibodies, EDP-938 (sisunatovir; under investigation) |
− Transmission-based contact plus droplet precautions |
| Rhinovirus | No chemoprophylaxis available | Supportive care | − Transmission-based contact plus droplet precautions − Consider longer ICP measures for prolonged shedding |
| Parainfluenza virus | No chemoprophylaxis available | − IVIG − DAS181 (a sialidase; under investigation) |
Transmission-based contact plus droplet precautions |
| Influenza A and B | Postexposure: − Within 48 h of exposure in unvaccinated patients or those with poor vaccine response − Oral oseltamivir (preferred) or inhaled zanamivir |
− Oral oseltamivir − Inhaled zanamivir − Intravenous peramivir − Oral baloxavir marboxil in combination therapy |
− Transmission-based contact plus droplet precautions − Seasonal vaccination (lifelong): at least 2 wk prior to the initiation of lymphodepleting therapy and 3 mo after CAR T-cell infusion; then annually thereafter − Vaccination of close contacts |
| Human metapneumovirus | No chemoprophylaxis available | Supportive therapy | Transmission-based contact plus droplet precautions |
| Coronaviruses | No chemoprophylaxis available | Supportive care | Transmission-based contact plus droplet precautions |
| SARS-CoV-2 | … | − Oral ritonavir-boosted nirmatrelvir (Paxlovid) − Molnupiravir − Remdesivir − Dexamethasone − Tocilizumab − Baricitinib |
− Transmission-based contact plus airborne precautions − Serial vaccination series: 2 wk prior to the initiation of lymphodepleting therapy and 90 d after CAR T-cell infusion |
Abbreviations: CAR, chimeric antigen receptor; ICP, infection control and prevention; IVIG, intravenous immunoglobulin; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The COVID-19 pandemic demonstrated the utility of universal masking in patient care areas. The rates of non–COVID-19 RVIs decreased in institutions where universal masking was adopted for HCWs and patients [45]. Prepandemic, a prospective trial noted a significant decrease in the overall incidence of RVI rates from 10.3% to 4.4% in HCT recipients after a mandatory universal masking policy on all individuals in contact with this patient population [46].
Clinical surveillance of community-acquired respiratory viruses, especially during times of high viral circulation in the community or during hospital outbreaks, should include daily screening for signs and symptoms of RVIs. Furthermore, prolonged viral shedding should be considered in recipients of adoptive cellular therapy, including HCT and immunotherapy cell recipients, and corresponding infection control precautions should be taken. This is especially important for rhinovirus, as shedding time in infected patients may exceed 21 days (Table 2).
VACCINATION AND OTHER STRATEGIES FOR PREVENTING RVIs
The immunogenicity of vaccines following CAR T-cell therapy is not well established. Available studies show mixed vaccine responses to influenza and SARS-CoV-2.
Influenza A(H1N1)–specific titers were significantly lower in response to vaccination in CAR T-cell therapy recipients compared to a control group, despite similar vaccination frequency [47]. In contrast, a prospective study on inactivated influenza vaccine given before or after CD19-directed CAR T-cell therapy found evidence of preserved immunogenicity, even among patients with low immunoglobulin and CD19+ B-cell levels [48].
A pooled analysis of 11 studies evaluating the immune response to the SARS-CoV-2 vaccination series in which CAR T-cell recipients received either 2 or 3 vaccine doses found a cumulative humoral response of 35.9% [49], suggesting either a poor vaccine response or a rapidly waning responses.
The current practice of immunization of adoptive cell therapy recipients is not standardized. Experts recommend administering nonlive vaccines 2–4 weeks before CAR T-cell infusion [50]. Live vaccines should be avoided 6–8 weeks before CAR T-cell therapy [50]. After infusion, the patient population becomes heterogeneous. For patients in remission who do not require additional chemotherapy, vaccinations with killed or inactivated vaccines should be considered 6 months to 1 year after CAR T-cell therapy, as long as the patients have not received IVIG supplementation within 2 months [32]. For influenza, experts suggest administering the annual vaccine at least 2 weeks before lymphodepleting therapy or 6 months after CAR T-cell infusion, similar to the other inactivated vaccines, and then yearly thereafter [32]. It is reasonable however, during the influenza season and outbreaks, to administer the vaccine starting 3 months post–CAR T-cell therapy despite the possible inadequate response. European and United States transplant guidelines recommend COVID-19 vaccination as early as 3 months after transplantation or adoptive cellular therapy to prevent infection and severe disease [23, 34]. These recommendations were based on limited data from few small sample size studies on COVID-19 vaccines and on previous clinical experience with other vaccine-preventable infections [23, 51]. The Pfizer-BioNTech and Moderna messenger RNA COVID-19 vaccines are preferred for primary and booster vaccinations [51]. COVID-19 vaccination should not be delayed in patients receiving IVIG [51]. Interestingly, the COVID-19 vaccines developed against earlier strains during the pandemic provided clinical protection despite decreased humoral responses after the emergence of highly mutated SARS-CoV-2 variants. This potentially underscored the role of the spike-specific T-cell immunity induced by COVID-19 vaccinations [52, 53]. Oh et al showed that vaccination with BNT162b2 induced spike-specific T-cell immunity in a small cohort of 8 patients treated with anti-CD19 4-1BB-CD3z CAR T-cell therapy [53]. As numerous epitopes are targeted along the whole spike protein, the generated T-cell immunity remained intact against the mutated variants [53].
Passive immunization against SARS-CoV-2 was utilized during the pandemic to prevent disease progression in immunosuppressed patients; however, its efficacy did not last with the subsequent new variants and subvariants.
Vaccination of HCWs and household contacts of immunocompromised individuals remains paramount in protecting high-risk patients from vaccine-preventable diseases.
IMMUNOGLOBULIN REPLACEMENT
The prophylactic use of immunoglobulin replacement in patients with secondary hypogammaglobulinemia is limited to recommendations based on the consensus of experts who adapted guidelines for managing primary humoral immune deficiencies for patients with hematologic malignancies who had received HCT. For CAR T-cell recipients, current practices differ among institutions. Some offer IVIG replacement to patients with recurrent or severe infections, while others offer it to patients with IgG titers <400 mg/dL [54].
FUTURE DIRECTIONS
The current gap in knowledge of the underlying immunological and cellular consequences of adoptive cellular therapies will continue to exist with the ongoing development of new-generation CAR T cells. Each new product and construct may carry a novel and unique infectious risk profile. In the realm of RVIs, the focus remains on developing the optimal preventive and prophylactic strategies while new antiviral agents are being developed.
Risk stratification using patient and treatment factors may prove to be useful in guiding the intensity of supportive care following CAR T-cell therapies. Validating risk scores may assist in management as is the case of immunodeficiency scoring index for RSV and other respiratory viruses in allogeneic HCT recipients [55]. The CAR-HEMATOTOX score evaluates the bone marrow reserves and inflammatory status to predict hematotoxicity and, although it is not validated for viral infections, may play a role in stratifying patients at risk of developing severe infections and poor outcomes [56].
The duration of immunity defects, the timing of vaccinations, whether revaccination or boosting is required, and if this is contingent on the different antigens that the CAR cells are designed to target are areas of research that remain to be determined in future studies. On the other hand, mitigating therapy-related toxicities will be significant. Strategies to improve the on-target–off-tumor effects are being investigated such as the use of selective antigens, deleting the target on normal hematopoietic stem cells, and optimizing the CAR with dual and affinity-tuned CARs [57].
CONCLUSIONS
Patients receiving cellular therapies are particularly vulnerable to RVIs, placing them at risk of higher morbidity and mortality. Understanding the acute and delayed infectious complications after CAR T-cell therapy and treatment of CAR T-cell toxicities plays a substantial role in determining the optimal RVI management strategies and highlights the need for further research to promote the development of novel antiviral compounds.
Contributor Information
Rita Wilson Dib, Department of Internal Medicine, Division of Infectious Diseases, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, Texas, USA; Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ella Ariza-Heredia, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Amy Spallone, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Roy F Chemaly, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Notes
Acknowledgments . The authors thank Laura L. Russell of the Department of Scientific Publications at The MD Anderson Cancer Center for editorial assistance.
Patient consent . This review does not include factors necessitating patient consent.
Financial support . No financial support was used for this review.
References
- 1. Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect Dis 2020; 7:ofaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018; 131:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buitrago J, Adkins S, Hawkins M, Iyamu K, Oort T. Adult survivorship: considerations following CAR T-cell therapy. Clin J Oncol Nurs 2019; 23:42–8. [DOI] [PubMed] [Google Scholar]
- 4. Meir J, Abid MA, Abid MB. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transplant Cell Ther 2021; 27:973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albinger N, Hartmann J, Ullrich E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther 2021; 28:513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison VA. Infections in patients with leukemia and lymphoma. Cancer Treat Res 2014; 161:319–49. [DOI] [PubMed] [Google Scholar]
- 7. Khanna N, Steffen I, Studt JD, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2009; 11:100–5. [DOI] [PubMed] [Google Scholar]
- 8. Stewart AG, Henden AS. Infectious complications of CAR T-cell therapy: a clinical update. Ther Adv Infect Dis 2021; 8:20499361211036773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol 2021; 21:277–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol 2022; 15:305–20. [DOI] [PubMed] [Google Scholar]
- 11. Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica 2021; 106:2667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma N, Reagan PM, Liesveld JL. Cytopenia after CAR-T cell therapy—a brief review of a complex problem. Cancers (Basel) 2022; 14:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol 2020; 11:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J 2020; 10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uy NF, Pequignot E, Frey NV, et al. Hypogammaglobulinemia and infection risk in chronic lymphocytic leukemia (CLL) patients treated with CD19-directed chimeric antigen receptor T (CAR-T) cells. Blood 2020; 136:30–2. [Google Scholar]
- 16. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020; 396:839–52. [DOI] [PubMed] [Google Scholar]
- 17. Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020; 26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 2021; 106:978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dayagi TW, Sherman G, Bielorai B, et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma 2021; 62:1692–701. [DOI] [PubMed] [Google Scholar]
- 20. Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv 2021; 5:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021; 384:705–16. [DOI] [PubMed] [Google Scholar]
- 22. Kambhampati S, Sheng Y, Huang CY, et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv 2022; 6:2045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Los-Arcos I, Iacoboni G, Aguilar-Guisado M, et al. Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: a position paper. Infection 2021; 49:215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cappell KM, Sherry RM, Yang JC, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol 2020; 38:3805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380:45–56. [DOI] [PubMed] [Google Scholar]
- 27. Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol 2020; 38:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377:2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Busca A, Salmanton-Garcia J, Corradini P, et al. COVID-19 and CAR T cells: a report on current challenges and future directions from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv 2022; 6:2427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spanjaart AM, Ljungman P, de La Camara R, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia 2021; 35:3585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hensley MK, Bain WG, Jacobs J, et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis 2021; 73:e815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 2020; 136:925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ison MG. Respiratory viral infections in the immunocompromised. Curr Opin Pulm Med 2022; 28:205–10. [DOI] [PubMed] [Google Scholar]
- 34. Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica 2019; 104:1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Principi N, Camilloni B, Alunno A, Polinori I, Argentiero A, Esposito S. Drugs for influenza treatment: is there significant news? Front Med (Lausanne) 2019; 6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar D, Ison MG, Mira JP, et al. Combining baloxavir marboxil with standard-of-care neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. Lancet Infect Dis 2022; 22:718–30. [DOI] [PubMed] [Google Scholar]
- 37. Chemaly RF, Marty FM, Wolfe CR, et al. DAS181 treatment of severe lower respiratory tract parainfluenza virus infection in immunocompromised patients: a phase 2 randomized, placebo-controlled study. Clin Infect Dis 2021; 73:e773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coultas JA, Cafferkey J, Mallia P, Johnston SL. Experimental antiviral therapeutic studies for human rhinovirus infections. J Exp Pharmacol 2021; 13:645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaer F E, Auletta JJ, Chemaly RF. How I treat and prevent COVID-19 in patients with hematologic malignancies and recipients of cellular therapies. Blood 2022; 140:673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dioverti V, Boghdadly ZE, Shahid Z, et al. Revised guidelines for coronavirus disease 19 management in hematopoietic cell transplantation and cellular therapy recipients (August 2022). Transplant Cell Ther 2022; 28:810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020; 222:1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petty LA, Malani PN. Oral antiviral medications for COVID-19. JAMA 2022; 327:2022–464. [DOI] [PubMed] [Google Scholar]
- 43. Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 2021; 27:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ariza-Heredia EJ, Chemaly RF. Update on infection control practices in cancer hospitals. CA Cancer J Clin 2018; 68:340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong SC, Lam GK, AuYeung CH, et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: implication of universal masking in hospitals. Infect Control Hosp Epidemiol 2021; 42:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sung AD, Sung JAM, Thomas S, et al. Universal mask usage for reduction of respiratory viral infections after stem cell transplant: a prospective trial. Clin Infect Dis 2016; 63:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walti CS, Loes AN, Shuey K, et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy: a prospective observational study. J Immunother Cancer 2021; 9:e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016; 128:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu X, Wang L, Shen L, He L, Tang K. Immune response to vaccination against SARS-CoV-2 in hematopoietic stem cell transplantation and CAR T-cell therapy recipients. J Hematol Oncol 2022; 15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamboj M, Shah MK. Vaccination of the stem cell transplant recipient and the hematologic malignancy patient. Infect Dis Clin North Am 2019; 33:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khawaja F, Dadwal S, Pergam SA, et al. ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients: frequently asked questions. Available at:https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients#:∼:text=We%20recommend%20that%20all%20close,(primary%20series%20and%20boosters. Accessed 20 August 2022.
- 52. Hurme A, Jalkanen P, Heroum J, et al. Long-lasting T cell responses in BNT162b2 COVID-19 mRNA vaccinees and COVID-19 convalescent patients. Front Immunol 2022; 13:869990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oh BLZ, Tan N, de Alwis R, et al. Enhanced BNT162b2 vaccine-induced cellular immunity in anti-CD19 CAR T cell-treated patients. Blood 2022; 140:156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T—and a side order of IgG, to go? Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019; 38:100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014; 123:3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rejeski K, Perez A, Iacoboni G, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer 2022; 10:e004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yanez L, Sanchez-Escamilla M, Perales MA. CAR T cell toxicity: current management and future directions. Hemasphere 2019; 3:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]