Abstract
Context
The effects of androgen therapy on arterial function in transgender men (TM) are not fully understood, particularly concerning long-term androgen treatment.
Objective
To evaluate arterial stiffness in TM receiving long-term gender-affirming hormone therapy by carotid–femoral pulse wave velocity (cf-PWV).
Methods
A cross-sectional case–control study at the Gender Dysphoria Unit of the Division of Endocrinology, HC-FMUSP, Sao Paulo, Brazil. Thirty-three TM receiving intramuscular testosterone esters as regular treatment for an average time of 14 ± 8 years were compared with 111 healthy cisgender men and women controls matched for age and body mass index. Aortic stiffness was evaluated by cf-PWV measurements using Complior device post-testosterone therapy. The main outcome measure was aortic stiffness by cf-PWV as a cardiovascular risk marker in TM and control group.
Results
The cf-PWV after long-term testosterone therapy was significantly higher in TM (7.4 ± 0.9 m/s; range 5.8-8.9 m/s) than in cisgender men (6.6 ± 1.0 m/s; range 3.8-9.0 m/s, P < .01) and cisgender women controls (6.9 ± .9 m/s; range 4.8-9.1 m/s, P = .02). The cf-PWV was significantly and positively correlated with age. Analysis using blood pressure as a covariate showed a significant relationship between TM systolic blood pressure (SBP) and cf-PWV in relation to cisgender women but not to cisgender men. Age, SBP, and diagnosis of hypertension were independently associated with cf-PWV in the TM group.
Conclusion
The TM group on long-term treatment with testosterone had higher aging-related aortic stiffening than the control groups. These findings indicate that aortic stiffness might be accelerated in the TM group receiving gender-affirming hormone treatment, and suggest a potential deleterious effect of testosterone on arterial function. Preventive measures in TM individuals receiving testosterone treatment, who are at higher risk for cardiovascular events, are highly recommended.
Keywords: testosterone, female to male transsexual, transgender man, arterial stiffness, carotid–femoral pulse wave velocity
The goals of gender-affirming hormone therapy using testosterone in transgender men (TM) are to promote the interruption of menstrual cycles and to induce virilization by producing a male pattern of facial and body hair growth, clitoral enlargement, and increased muscle mass [1]. These effects promote the phenotypic gender transition.
Long-term androgen treatment effects on the cardiovascular systems of TM are not yet well established, because most of the literature data are based on small cohorts of younger TM, who have received gender-affirming hormone therapy for a short period of time [2‐4].
Some of the potential side effects of androgen treatment for TM, such as erythrocytosis, weight gain, hypertension, and lipid abnormalities, are known to be risk factors for cardiovascular diseases (CVDs) [5].
The exact role of male sex hormones in arterial vessels is not well recognized, but sex steroids seem to be able to modulate arterial properties. An indirect effect of testosterone, through estrogen originating from the peripheral aromatization of testosterone, has been demonstrated in the vasculature [6]. Additionally, direct testosterone action on arterial vessels has been suggested by evidence of androgen receptor expression in both the endothelial and smooth muscle cells of multiple vessels [7].
Increased cardiovascular risk and progression of subclinical arterial disease has been established in cisgender women with endogenous hyperandrogenism [8]. Furthermore, cardiovascular comorbidities, including systemic arterial hypertension and endothelial dysfunction, have been observed in young women with polycystic ovary syndrome who are chronically exposed to androgen excess [9, 10]. The current evidence for the role of testosterone therapy in the atherogenic profile and cardiovascular risk in cisgender women is controversial. Some studies that evaluated the chronic use of testosterone in climacteric patients demonstrated an increase in arterial vasodilation [11‐13], while others showed an increase in ischemic arterial disease and coronary disease [14].
Changes in the structural and functional properties of the large arteries are correlated with increased cardiovascular risk in different populations [15, 16]. Nonclassical cardiovascular risk markers, such as arterial stiffness estimated by carotid–femoral pulse wave velocity (cf-PWV) measurements and carotid structure evaluated by carotid intima–media thickness measurements, are recommended by different guidelines for the complementary assessment of subclinical atherosclerosis and as predictors of additional cardiovascular risk [17, 18].
Some studies have demonstrated the effects of testosterone on these markers in different conditions. A more androgenic profile of endogenous sex hormones was associated with worse endothelial function in a large cohort of postmenopausal women analyzed in the Multi-Ethnic Study of Atherosclerosis (MESA) [19]. Moreover, patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency, a disease of adrenal origin that is manifested by adrenal failure and hyperandrogenism, presented higher values of carotid intima–media thickness than control subjects [20].
Central arterial stiffness, aortic stiffness, has emerged as a strong cardiovascular risk factor [21]. The cf-PWV, a methodology to measure aortic stiffness, has been shown to have good accuracy in estimating cardiovascular and all-cause mortality risk, and an excellent accuracy to estimate cardiovascular mortality [22]. The cf-PWV is the recommended arterial stiffness measurement method according to the American Heart Association scientific statement [23], the European expert consensus document [24], and European Society of Cardiology and the European Society of Hypertension guidelines for the management of arterial hypertension [25] due to the large preponderance of longitudinal data from cohort studies. The cf-PWV, a parameter with simple reproducibility and high reliability, has been considered the gold standard method for assessing aortic stiffness [24, 26].
Although not all studies are in concordance, transgender literature has suggested that TM receiving testosterone therapy may be at higher risk for myocardial infarction and that the vascular changes might be an underlying mechanisms [27, 28]. Testosterone therapeutic use in the TM represents a particular model for evaluating this relationship between an exogenous source of testosterone and vascular modifications in 46,XX subjects.
In this study, we evaluated arterial stiffness by cf-PWV measurements of TM after long-term testosterone therapy to investigate the presence of subclinical atherosclerosis and to establish a predictor of cardiovascular risk.
Patients and Methods
Subjects
This study protocol was approved by the Ethics Committee of the Hospital das Clínicas, Universidade de São Paulo (HCFMUSP: protocol number 43078815.4.0000.0068), and written informed consent was obtained from all participants.
Thirty-three TM followed at the Gender Dysphoria Unit of the Division of Endocrinology of the HC-FMUSP were evaluated in a cross-sectional case–control study. The criteria for a gender dysphoria/gender incongruence diagnosis were in accordance with DSM IV-V/ICD-11 guidelines. The mean age of the TM group was 44 ± 10 years (range 26-61 years). At the beginning of the study, all TM who were selected already had phenotypic male features (mean phallus length: 5.3 ± 1.0 cm; mean total Ferriman–Gallwey score: 22 ± 7; male pattern baldness and absence of menstrual cycles). Most of the TM had undergone sex reassignment surgery by the start of the study (n = 24; 73%), including hysterectomy plus bilateral oophorectomy. The mean duration of testosterone therapy was 14 ± 8 years (range 4-32 years).
The TM group received 200 mg of intramuscular testosterone cypionate every 2 weeks. The total testosterone levels of the TM group were 456 ± 250 ng/dL. The total testosterone levels were preferentially measured on the day preceding the next dose administration (nadir values).
The cisgender control group was composed of 111 healthy subjects, 55 cisgender women (aged 43 ± 10 years) and 56 cisgender men (aged 42 ± 10 years), who were age and body mass index (BMI) matched to the TM group. The following conditions were evaluated by clinical and laboratory assessments and subjects with these conditions were excluded from the cisgender control group: smoking; chronic kidney disease; hepatic failure; systemic arterial hypertension (blood pressure ≥ 140/90 mmHg or patient on antihypertensive medication); inflammatory diseases; neoplasia; endocrine disorders including diabetes mellitus and hypogonadism; hematological diseases; peripheral vascular diseases; dyslipidemia (or patient on lipid-lowering medication); cardiomyopathies; valvular heart diseases; and congenital diseases.
The participants of the control groups were recruited from research projects duly approved by the Ethics Committee of the Sao Paulo University Medical School as described elsewhere in detail [29]. For sample calculation, a minimum number of 30 patients per group was considered, based on the power to detect changes in cf-PWV measurements, according to the reproducibility and sensitivity of the test observed at our service.
Assessment of Arterial Parameters
Evaluations of arterial stiffness were performed for all participants by cf-PWV measurements using the Complior device (Artech, France) [30]. Briefly, 2 external sensors were positioned at 2 points of the arterial tree (carotid and femoral arteries), and the pulse curves were simultaneously registered. The cf-PWV was automatically calculated by inserting the distance between the 2 points. This method has been validated in several studies [31, 32].
Clinical and Biochemical Analyses
All subjects underwent the following laboratory tests after a 12-hour fast using standard methods in the clinical laboratory: hematocrit, fasting plasma glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, creatinine, and total testosterone and estradiol levels (immunofluorometric assay—AutoDELFIA, Wallac, Finland).
An automated and validated Microlife device was used to measure the systolic blood pressure (SDP) and diastolic blood pressure (DBP), and a mean of the 2 measurements was considered. The pulse pressure was calculated by the difference between the SDP and DBP [17]. Among the transgender group, 8 subjects were hypertensive, and all were on regular use of antihypertensive drugs.
Statistical Analysis
Statistical analysis was performed using GraphPad Prisma 8.4.2. The D’Agostino–Pearson normality test was used to evaluate data distribution (Gaussian or non-Gaussian). For variables with normal distribution, the comparison among the 3 groups was done through analysis of variance, and for comparative subanalyses of 2 groups, the Tukey test was used. Nonparametric Kruskal–Wallis test was used for variables, and Dunn's multiple comparison test was used for comparative subanalyses of 2 groups. Pearson's correlation coefficient was used to measure the statistical relationship between continuous variables. Analyses of covariance (ANCOVA) covarying for SBP/DBP and cf-PWV values were employed to detect mean between-group differences. Multiple linear regression analysis was performed to determine the effect of different independent variables (age, SBP/DBP, hematocrit, creatinine, testosterone level, testosterone treatment duration, and systemic arterial hypertension) on the arterial stiffness parameter (dependent variable). Statistical significance was set at P < .05.
Results
The TM group presented significantly higher SDP and DBP and hematocrit levels than both cisgender women and men (Table 1). Testosterone levels and creatinine levels were similar between TM and cisgender men and were significantly lower in cisgender women than in TM and cisgender men. There were no significant differences in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, or estradiol levels between the TM and control groups (Table 1).
Table 1.
Comparison of the clinical characteristics and laboratory data among the transgender men group and cisgender control groups, matched for age and body mass index
| Variable | Groups | P value | |||||
|---|---|---|---|---|---|---|---|
| Transgender men | Cisgender women | Cisgender men | |||||
| Mean ± SD | Median (variation) | Mean ± SD | Median (variation) | Mean ± SD | Median (variation) | ||
| Age (yrs)a | 43.6 ± 9.5 | 44 (26-61) |
43 ± 10 | 43.5 (25-61) |
42 ± 10 | 43 (25-61) |
NS |
| BMI (kg/m²)a | 27 ± 5 | 25.9 (19.1-39.2) |
27 ± 5 | 25.4 (19.9-37.1) |
27 ± 4 | — | NS |
| SBP (mmHg)a | 129 ± 17 | 130 (90-180) |
118 ± 15 | 115 (90-160) |
121 ± 17 | 120 (85-180) |
.01* |
| DBP (mmHg) | 81 ± 11 | — | 74 ± 11 | — | 75 ± 14 | — | .02* |
| PP (mmHg) | 47 ± 11 | — | 44 ± 9 | — | 46 ± 11 | — | NS |
| Hematocrit (%) | 47 ± 3 | — | 40 ± 3 | — | 45 ± 2 | — | <.01* |
| TC (mg/dL) | 205 ± 41 | — | 200 ± 36 | — | 190 ± 36 | — | NS |
| HDL-c (mg/dL)a | 47 ± 15 | 46 (29-112) |
50 ± 11 | 48 (29-150) |
43 ± 9 | 41 (27-70) |
.04* |
| LDL-c (mg/dL) | 131 ± 32 | — | 129 ± 30 | — | 119 ± 31 | — | NS |
| Triglycerides (mg/dL)a | 145 ± 92 | 147 (41-540) |
115 ± 74 | 86 (42-346) |
135 ± 75 | 111 (40-425) |
NS |
| FPG (mg/dL)a | 89 ± 14 | 87 (59-128) |
94 ± 9 | 92 (77-113) |
94 ± 9 | 92 (77-113) |
NS |
| Creatinine (mg/dL) | 0.94 ± 0.16 | — | 0.79 ± 0.12 | — | 0.97 ± 0.12 | — | <.01* |
| Testosterone (ng/dL) | 456 ± 250 | — | 36 ± 12 | — | 514 ± 300 | — | <.01* |
| Estradiol (pg/mL) | 38 ± 34 | — | 40 ± 30 | — | 34 ± 26 | — | NS |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NS, nonsignificant; PP, pulse pressure; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; Yrs, years. Unit conversion: testosterone—1 ng/mL = 2.76 nmol/L.
P < .05 was considered to be statistically relevant.
Nonparametric Kruskal–Wallis test.
The mean values of cf-PWV were significantly higher in the TM group (7.4 ± 0.9 m/s; range 5.8-8.9 m/s) than in the groups of cisgender men (6.6 ± 1.0 m/s; range 3.8-9.0 m/s, P < .01) and cisgender women (6.9 ± 0.9 m/s; range 4.8-9.1 m/s, P = .02). Cisgender men and women controls presented similar cf-PWV values (P = .4) (Fig. 1).
Figure 1.
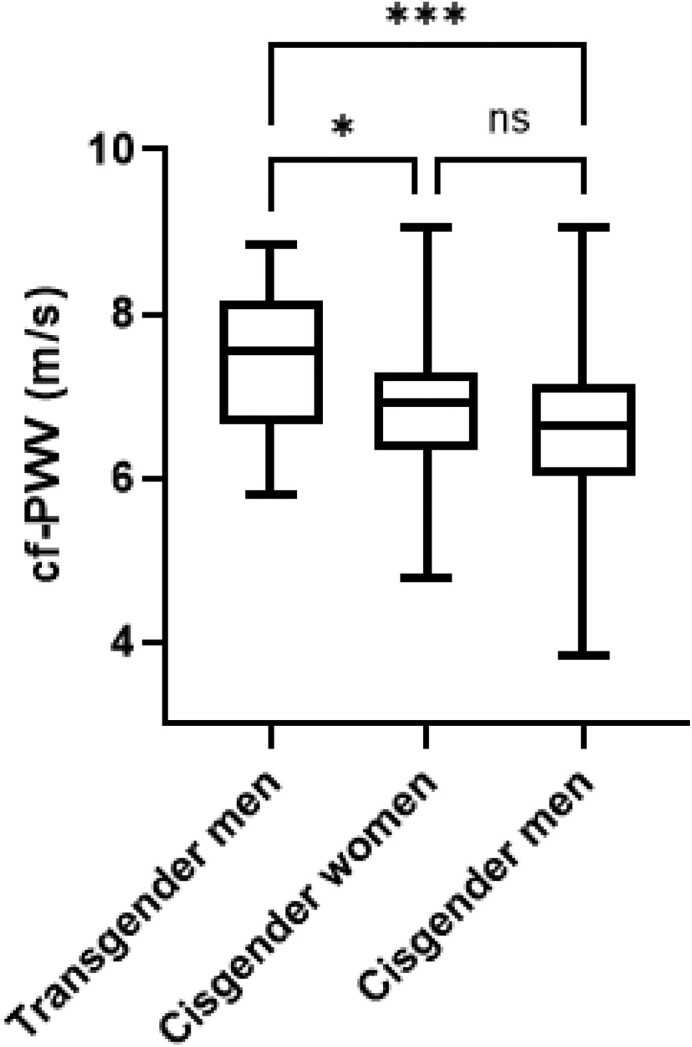
Comparative analysis of the carotid–femoral pulse wave velocity (cf-PWV) value in the transgender men (TM) group with long-term use of testosterone therapy, cisgender women health control group, and cisgender men healthy control group. The 3 groups were matched for age and body mass index. The mean duration of testosterone therapy in the TM group was 14 ± 8 years. A P < 0.05 was considered statistically relevant. *P = 0.02; ***P < 0.01; ns- non-significant.
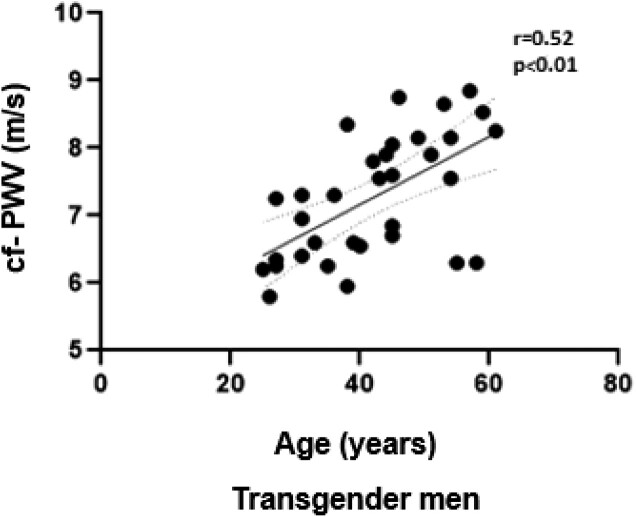
A significant positive correlation between cf-PWV values and age in the TM group was observed (r = 0.52; P < .01) (Fig. 2). In addition, a positive and significant correlation between cf-PWV and diagnosis of hypertension (r = 0.39; P = .01), cf-PWV and duration of testosterone treatment (r = 0.32; P = .04) were demonstrated in the TM group. The duration of testosterone treatment also showed a significant and positive correlation with the testosterone levels (r = 0.4; P = .004). The diagnosis of hypertension in the TM group was significantly and positively correlated with the age (r = 0.5; P = .01).
Figure 2.
Correlation between carotid–femoral pulse wave velocity and age in the transgender men group with long-term testosterone therapy. Dashed line = line of unity; solid line = regression line.
In the control group, the correlation between cf-PWV and age was significant and positive for cisgender women (r = 0.46; P < .01) and not significant for cisgender men (r = 0.12; P = .3). The cf-PWV was significantly and positively correlated with SBP (r = 0.35; P < .01) and DBP (r = 0.28; P = .03) in cisgender control women. The cf-PWV was not significantly correlated with SBP (r = .13; P = .3) and DPB (r = 0.12; P = .4) in cisgender control men.
ANCOVA, using SBP and DBP as covariates, showed a significant relationship between SBP and cf-PWV in TM in relation to cisgender women, but not to cisgender men.
Multiple linear regression analysis was performed to determine the effect of different variables on the arterial stiffness parameter, and age, SBP, and diagnosis of hypertension were independently associated with cf-PWV in TM (Table 2).
Table 2.
Multiple linear regression using carotid–femoral pulse wave velocity as a dependent variable
| Variable | B-coefficient | R2 | P value |
|---|---|---|---|
| Age (years) | .526 | 0.27 | .04* |
| Systolic blood pressure (mmHg) | −.710 | 0.01 | .04* |
| Diastolic blood pressure (mmHg) | .175 | 0.01 | NS |
| Hematocrit (%) | .203 | 0.05 | NS |
| Creatinine (mg/dL) | .109 | 0.01 | NS |
| Testosterone (ng/dL) | .102 | 0.01 | NS |
| Duration of testosterone therapy (years) | −.282 | 0.08 | NS |
| Hypertension | .102 | 0.01 | .03* |
*Statistical significance was set at P value < 0.05.
Discussion
To date, our study is the first to demonstrate that arterial stiffness measured by cf-PWV is elevated in TM after long-term testosterone use compared with age- and BMI-matched cisgender men and women. This result might indicate a pathological basis for the higher cardiovascular risk associated with testosterone therapy.
Epidemiological studies have shown a higher incidence of CVD in cisgender men than in cisgender premenopausal women, and these data suggest a role of sex hormones in this sex difference, indicating a protective effect of estrogen and a potential detrimental effect of testosterone [33]. Therefore, it was supposed that transgender individuals receiving testosterone were at higher risk, but the great majority of the studies evaluating cardiovascular endpoints in TM failed to show an increased cardiovascular mortality rate when compared with the general population [27, 34, 35]. The younger age and the relatively short period of androgen treatment in these TM may justify the negative results [36].
In contrast, the correlation between being transgender and the rate of myocardial infarction and CVD risk factors, based on 2017 Behavioural Risk Factor Surveillance System (BRFSS) data, has shown that TM are at higher risk for myocardial infarction than cisgender women after adjusting for CVD risk factors, including age, diabetes, hypertension, hypercholesterolemia, chronic kidney disease, smoking, and exercise [37].
Vascular changes in TM receiving gender-affirming hormone therapy have rarely been investigated, and few results have been reported. Emi et al evaluated 111 TM (48 treated with androgen for 45 ± 38 months and 63 untreated) using brachial–ankle PWV to measure arterial stiffness and showed that TM receiving testosterone presented higher brachial–ankle PWV than untreated subjects [2]. These results indicated a higher arterial stiffness, similarly to our findings. In contrast to the study of Emi et al, our methodology using cf-PWV measurements is considered the gold standard, and we also compared TM with cisgender men and women of similar ages. These data suggest that testosterone therapy in TM interferes with vascular function more than in age-matched cisgender men.
Nevertheless, Giltay et al showed no effect of testosterone treatment on carotid and femoral distensibility (an index of regional arterial stiffness) in a prospective evaluation of 18 TM (median age 23 years) during 12 months of androgen therapy [3]. In contrast to our results, the authors used a localized regional analysis of arterial stiffness, and the age of the patients and the time of testosterone use were lower than those in our population. These findings suggest the necessity of long-term androgen exposure to develop vascular stiffening. McCredie et al evaluated 12 TM, aged 33 ± 6 years, who had received testosterone for 38 ± 52 months, and encountered a significantly decreased sublingual nitroglycerin response when compared with 12 cisgender female healthy group, although the flow-mediated vasodilatation was not significantly decreased in the transgender group [4], suggesting a possible decreased response to vasodilators in TM receiving testosterone.
Several studies have demonstrated that age and blood pressure are the main determinants of cf-PWV [38‐41]. In our cohort, aortic stiffness was significantly and positively associated with the age of the TM, as observed in the general population. The mechanisms involved in acceleration of the aging-related aortic stiffening observed in TM receiving exogenous testosterone are still unknown.
Higher blood pressure levels were observed in TM group than in cisgender groups (Table 1). Higher SBP levels, as a determining factor for the arterial stiffness among the groups, represented the influence variable evaluated in our study by ANCOVA analysis. The comparison between the arterial stiffness of TM and cisgender women group showed that the SBP levels contributed significantly to the negative impact of cf-PWV in TM group. A parallel can be drawn with anabolic androgenic steroids abusers. A case–control study showed that current and former anabolic androgenic steroids abusers displayed increased arterial stiffness compared with age-matched controls, related to high SBP and low plasmatic natriuretic peptides [42].
A study analyzing the flow-mediated vasodilation of the brachial artery in TM using physiological doses of testosterone showed that the magnitude of the vasodilator response was significantly lower in TM than in cisgender women matched for age and health conditions. This difference was independent of baseline arterial diameter or CV risk factors such as smoking, BMI, or serum lipid levels, suggesting that other factors such as inflammation or endothelial dysfunction could explain the differences that were observed [43]. Additionally, an increase in leukocyte–endothelium interactions, adhesion molecules, and proinflammatory cytokines was demonstrated in TM after 12 weeks of testosterone treatment [44].
The increased arterial stiffness caused by ageing involves mechanisms such as the degeneration of the elastic fibers in the vascular wall with partial replacement of elastin by collagen. In addition, high blood pressure, present in most elderly people, contributes to the fragmentation of the elastin in the vessel wall, promoting additional arterial stiffening. Receptors for steroid hormones have been identified in human arterial vessels such as the aorta, carotid arteries, and coronary arteries [45]. In the culture of aortic smooth muscle cells, testosterone reduces the elastin/collagen ratio, providing a structural basis for the increase in arterial stiffness, since collagen is considered a substance that can be up to 1000 times stiffer than elastin [46, 47]. Long-term testosterone exposure could enhance this effect.
The short-term effect of testosterone ester use on the patients studied resulted in a great variability of the total testosterone levels, based on the intervals of the injections and the personal ability to metabolize androgen. Therefore, we suggest that the duration of androgen treatment can represent a better parameter than the measurement of serum basal testosterone level in evaluating the chronic action of exogenous testosterone treatment in transgender subjects. We suppose that the chronic and cyclic supraphysiological levels of testosterone cypionate, achieved between 2 and 7 days after injection, are potentially associated with arterial stiffening. Reinforcing this idea, our study demonstrated that the duration of androgen treatment, but not the testosterone levels, was significantly and positively correlated with cf-PWV.
Furthermore, the differences in arterial stiffness observed in our study could also have been due to possible structural and functional vascular differences intrinsically linked to genetic sex or a combination of sex hormone effects. As described above, arterial stiffness increases with age in both sexes beginning in childhood [48]. However, this increase appears to be more pronounced in cisgender women, since in the postmenopausal phase, they present higher arterial stiffness than cisgender men of the same age [48]. At early ages, differences in arterial stiffness between cisgender men and women also seem to exist. Ahismastos et al evaluated the arterial stiffening pattern of 58 prepubertal and 52 postpubertal children of both sexes by measurement of PWV and arterial compliance. In the prepubertal phase, girls had higher arterial stiffness (higher cf-PWV and lower arterial compliance) than boys, but this difference was eliminated in the postpubertal phase. Both sexes demonstrated increased arterial capacitance after puberty, but girls developed vessels with higher distensibility, and boys developed stiffer vessels [46]. These results suggest that there are intrinsic sex differences in arterial stiffness that can be modulated by male and female sex hormones.
Although the present study showed interesting results, it has some limitations. First, the sample size was small. The absence of aortic stiffness measurements in transgender individuals prior to beginning testosterone therapy constituted another limitation of the study. Correlations of vascular indices with the waist–hip ratio and other parameters of body composition accessed by bioimpedance analysis or dual-energy x-ray absorptiometry were not performed, but would likely add information to the study, since the BMI may be an insufficient parameter to match the groups. Finally, we cannot affirm that our results can be extended to patients using other testosterone formulations which lead to fewer peaks and troughs, such as longer acting testosterone formulations or transdermal testosterone gel.
To the best of our knowledge, this report presents the first demonstration that TM receiving long-term treatment with testosterone had higher aortic stiffness correlated with age than cisgender controls matched for age and BMI. These findings indicate that aortic stiffness might be accelerated in the TM group receiving gender-affirming hormone treatment, and suggest a potential deleterious effect of testosterone on arterial function. Preventive measures in TM individuals receiving testosterone treatment, who are at higher risk for cardiovascular events, are highly recommended.
Acknowledgments
We thank Patrícia Braga, Rogério Prado, and Divaldo Martins for their assistance with the statistical analysis.
Abbreviations
- ANCOVA
analyses of covariance
- BMI
body mass index
- cf-PWV
carotid–femoral pulse wave velocity
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- TM
transgender men
Contributor Information
Flávia Siqueira Cunha, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Tania Aparecida Sartori Sanchez Bachega, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Elaine Maria Frade Costa, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Vinicius Nahime Brito, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Leonardo Azevedo Alvares, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil; Centro Universitário São Camilo, Curso de Medicina, Departamento de Endocrinologia, 04264-030 São Paulo, SP, Brazil.
Valéria Aparecida Costa-Hong, Unidade de Hipertensão Arterial do Instituto do Coração (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Renata Gomes Sanches Verardino, Unidade de Hipertensão Arterial do Instituto do Coração (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Maria Helena Palma Sircili, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Berenice Bilharinho de Mendonça, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Luiz Aparecido Bortolotto, Unidade de Hipertensão Arterial do Instituto do Coração (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Sorahia Domenice, Email: sorahiad@gmail.com, Disciplina de Endocrinologia e Metabologia, Laboratório de Hormônios e Genética Molecular/LIM42, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, 05403-900 São Paulo, SP, Brazil.
Funding
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grants 303002/2016-6 and 457978/2014 (to B.B.M.) and 308873/2018-1 (to S.D.).
Author Contributions
Study conception and design: S.D., B.B.M., T.A.S.S.B. Acquisition and analysis of the data: F.S.C., M.H.P.S., L.A.B., V.A.C.H., R.G.S.V., L.A.A. Drafting of the manuscript: F.S.C., S.D. Critical revision: S.D., B.B.M., T.A.S.S.B., E.M.F.C., L.A.B.
Disclosures
The authors have nothing to disclose.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869‐3903. [DOI] [PubMed] [Google Scholar]
- 2. Emi Y, Adachi M, Sasaki A, Nakamura Y, Nakatsuka M. Increased arterial stiffness in female-to-male transsexuals treated with androgen. J Obstet Gynaecol Res. 2008;34(5):890‐897. [DOI] [PubMed] [Google Scholar]
- 3. Giltay EJ, Lambert J, Gooren LJ, Elbers JM, Steyn M, Stehouwer CD. Sex steroids, insulin, and arterial stiffness in women and men. Hypertension. 1999;34(4):590‐597. [DOI] [PubMed] [Google Scholar]
- 4. McCredie RJ, McCrohon JA, Turner L, Griffiths KA, Handelsman DJ, Celermajer DS. Vascular reactivity is impaired in genetic females taking high-dose androgens. J Am Coll Cardiol. 1998;32(5):1331‐1335. [DOI] [PubMed] [Google Scholar]
- 5. Dutra E, Lee J, Torbati T, Garcia M, Merz CNB, Shufelt C. Cardiovascular implications of gender-affirming hormone treatment in the transgender population. Maturitas. 2019;129:45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98(6):3589‐3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatakeyama H, Nishizawa M, Nakagawa A, Nakano S, Kigoshi T, Uchida K. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002;530(1-3):129‐132. [DOI] [PubMed] [Google Scholar]
- 8. Mesch VR, Siseles NO, Maidana PN, et al. Androgens in relationship to cardiovascular risk factors in the menopausal transition. Climacteric. 2008;11(6):509‐517. [DOI] [PubMed] [Google Scholar]
- 9. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30(7):399‐404 [DOI] [PubMed] [Google Scholar]
- 10. Usselman CW, Yarovinsky TO, Steele FE, et al. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: role of the endothelin B receptor. J Physiol. 2019;597(11):2853‐2865. [DOI] [PubMed] [Google Scholar]
- 11. Davis SR, Wahlin-Jacobsen S. Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980‐992. [DOI] [PubMed] [Google Scholar]
- 12. Britto R, Araújo L, Barbosa I, Silva L. Improvement of the lipid profile in post menopausal women who use estradiol and testosterone implants. Gynecol Endocrinol. 2012;28(10):767‐769. [DOI] [PubMed] [Google Scholar]
- 13. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847‐3853. [DOI] [PubMed] [Google Scholar]
- 14. Spoletini I, Vitale C, Pelliccia F, Fossati C, Rosano GM. Androgens and cardiovascular disease in postmenopausal women: a systematic review. Climacteric. 2014;17(6):625‐634. [DOI] [PubMed] [Google Scholar]
- 15. Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384‐3390. [DOI] [PubMed] [Google Scholar]
- 16. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236‐1241. [DOI] [PubMed] [Google Scholar]
- 17. Malachias MVB, Ferreira Filho S, Souza WKSB, Ribeiro JM, Miranda RD, Jardim TSV.. 7th Brazilian guideline of arterial hypertension: Chapter 11 - Arterial hypertension in the elderly. Arq Bras Cardiol. 2016;107(3 Suppl 3):64‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams B, Mancia G, Spiering W, et al. 2018 European society of hypertension-European society of cardiology guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 19. Mathews L, Subramanya V, Zhao D, et al. Endogenous sex hormone and endothelial function in postmenopausal women and men: the multi-ethnic study of atherosclerosis. J Women's Health. 2019;28(7):900‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sartorato P, Zulian E, Benedini S, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(3):1015‐1018. [DOI] [PubMed] [Google Scholar]
- 21. Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64(2):210‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sequí-Domínguez I, Cavero-Redondo I, Alvarez-Bueno C, Pozuelo-Carrascosa DP, De Arenas-Arroyo SN, Martínez-Vizcaíno V. Accuracy of pulse wave velocity predicting cardiovascular and all-cause mortality. A systematic review and metaanalysis. J Clin Med. 2020; 9(7):2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American heart association. Hypertension. 2015;66(3):698‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588‐2605 [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spierinnng W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology (ESC) and the European society of hypertension (ESH). Eur. Heart J. 2018;33(33):3021‐3104. [Google Scholar]
- 26. Lehmann ED. Terminology for the definition of arterial elastic properties. Pathol Biol (Paris). 1999;47(6):656‐664. [PubMed] [Google Scholar]
- 27. Getahun D, Nash R, Flanders WD, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med. 2018;169(4):205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. 2019;139(11):1461‐1462. [DOI] [PubMed] [Google Scholar]
- 29. Tolezani EC, Costa-Hong V, Correia G, Mansur AJ, Drager LF, Bortolotto LA. Determinants of functional and structural properties of large arteries in healthy individuals. Arq Bras Cardiol. 2014;103(5):426‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guimarães GV, Ciolac EG, Carvalho VO, D'Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. Interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33(6):627‐632. [DOI] [PubMed] [Google Scholar]
- 32. Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379‐1386. [DOI] [PubMed] [Google Scholar]
- 33. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24(3):313‐340. [DOI] [PubMed] [Google Scholar]
- 34. Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102(11):3914‐3923. [DOI] [PubMed] [Google Scholar]
- 35. Asscheman H, Giltay EJ, Megens JAJ, de Ronde W, van Trotsenburg MAA, Gooren LJG. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635‐642. [DOI] [PubMed] [Google Scholar]
- 36. Streed CG, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy. Ann Intern Med. 2017;167(4):256‐267. [DOI] [PubMed] [Google Scholar]
- 37. Alzahrani T, Nguyen T, Ryan A, et al. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ Cardiovasc Qual Outcomes. 2019;12(4):e005597. [DOI] [PubMed] [Google Scholar]
- 38. Smulyan H, Mookherjee S, Safar ME. The two faces of hypertension: role of aortic stiffness. J Am Soc Hypertens. 2016;10(2):175‐183. [DOI] [PubMed] [Google Scholar]
- 39. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore longitudinal study of aging. Hypertension. 2013;62(5):934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54(6):1328‐1336. [DOI] [PubMed] [Google Scholar]
- 42. Rasmussen JJ, Schou M, Madsen PL, et al. Increased blood pressure and aortic stiffness among abusers of anabolic androgenic steroids: potential effect of suppressed natriuretic peptides in plasma? J Hypertens. 2018;36(2):277‐285. [DOI] [PubMed] [Google Scholar]
- 43. Gulanski BI, Flannery CA, Peter PR, Leone CA, Stachenfeld NS. Compromised endothelial function in transgender men taking testosterone. Clin Endocrinol. 2020;92(2):138‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iannantuoni F, Salazar JD, Marañon AM, et al. Testosterone administration increases leukocyte-endothelium interactions and inflammation in transgender men. Fertil Steril. 2021;115(2):483‐489. [DOI] [PubMed] [Google Scholar]
- 45. Torres-Estay V, Carreño DV, San Francisco IF, Sotomayor P, Godoy AS, Smith GJ. Androgen receptor in human endothelial cells. J Endocrinol. 2015;224(3):131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab. 2003;88(11):5375‐5380. [DOI] [PubMed] [Google Scholar]
- 47. Natoli AK, Medley TL, Ahimastos AA, et al. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension. 2005;46(5):1129‐1134. [DOI] [PubMed] [Google Scholar]
- 48. Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19(12):2205‐2212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.