Abstract
Background
Uncertainty remains about the association of potassium (K) intake with depression and anxiety status. We explored their relationship using 24‐h urinary K, reflecting K intake, in general population.
Methods
We collected 24‐h urine and performed self‐rating depression and anxiety scales (SDS, SAS) cross‐sectionally in adults selected by random sampling in China. SDS and SAS standard score ≥50 defined depression and anxiety status. Participants were divided into three groups (T1, T2, and T3) by 24‐h urinary K tertile. Odds ratios (OR) and 95% confidence intervals were calculated. Sensitivity analysis was performed by excluding anti‐hypertensive agent takers.
Results
546 participants comprised current analytical sample. First, T1 and T2 groups showed higher SDS scores (40.0 vs 40.0 vs 36.0, p = .001), prevalence (19.8 vs 15.9 vs 7.1%, p = .002), whereas increased adjusted odds for depression status only in T1 group (OR = 2.71, p = .017), compared with T3 group. Second, T1 and T2 groups showed higher SAS scores (38.0 vs 40 vs 35.0, p < .001) and prevalence (14.8 vs 21.4 vs 8.8%, p = .003), whereas increased adjusted odds for anxiety status only in T2 group (OR = 2.07, p = .042), compared with T3 groups. Third, T1 and T2 groups showed higher prevalence (10.4% vs 11.5% vs 2.7%, p = .004) and adjusted odds (OR = 3.71, p = .013; OR = 3.66, p = .014) for co‐existent anxiety and depression status, compared with T3 group. Most results remained consistent in sensitivity analysis.
Conclusions
Lower K intake is implicated in presence of anxiety and depression status in general population; this may provide basis for programs to increase K intake and prevent disease.
Keywords: 24‐h urinary potassium excretion, anxiety status, depression status, potassium intake
We classified dietary potassium intake with 24‐h urine collection, found a independent association between potassium intake and depression and anxiety status.
1. INTRODUCTION
Mental disorders have become one of the serious public health problems in the world, with depression and anxiety as the two most common mental disorders in the general medical setting (Andrade et al., 2003; Kroenke et al., 2007). Studies show that the lifetime prevalence of depression and anxiety averaged 11.2% and 3.7%, respectively (Kessler et al., 2015; Ruscio et al., 2017). Since the 1990s, depressive disorders, second only to ischemic heart disease, have become the second greatest contributor to global disease burden, quantified as years of life lived in less than ideal health (Vos et al., 2012). In addition, disability‐adjusted life‐years of depression and anxiety increased by 14.3% and 12.8% during 2007−2017 (GBD 2017 Dalys & Hale Collaborators, 2018). Therefore, exploring modifiable risk factors is necessary to prevent depression and anxiety.
Although accumulating studies have been conducted in the past few decades, the etiologies for depression and anxiety are not understood clearly. Female gender, low socioeconomic status, less social support, stress, alcohol and drug abuse, genetic and epigenetic factors, dysregulation of gut microbiota, and several disease conditions contribute to increased risk for depression and anxiety (Hosseinzadeh et al., 2016). In addition, balanced diet or dietary patterns play important roles in human model of thinking and human behavior, as the intake of foods affects human cognition, memory capacity, and emotions (Huang et al., 2019); balanced dietary patterns such as the Mediterranean diet have been uniquely associated with a lower risk of depression or depressive symptoms (Parletta et al., 2019; Rienks et al., 2013). Besides a balanced diet, isolated nutrients are another element that might also be involved in mental disorders. Emerging evidence suggests that potassium (K) intake might be one of the modifiable risk factors for depression and anxiety, whereas inconclusive. In 1423 Japanese elderly population, K intake is significantly and negatively correlated with depressive symptoms among female participants (Thi Thu Nguyen et al., 2019). Furthermore, K intake is found lower in 59 patients with depression aged ≥18 years in another study (Kaner et al., 2015) and serum K is decreased slightly in 200 preoperative patients with anxiety (McCleane & Watters, 1990). Moreover, consumption of vegetables and fruits, well‐known as sources of K and recommended for the prevention of depression (Opie et al., 2017), is negatively correlated with severity of depressive symptoms (Mamplekou et al., 2010) and presence of anxiety (Sadeghi et al., 2021; Saghafian et al., 2018). Importantly, an intervention study reports that a low‐sodium and high‐K diet seems to have positive effects on general mood state including assessment of depression and anxiety (Mrug et al., 2019; Torres et al., 2008). Therefore, it is reasonable to speculate that K intake is associated with the development of depression and anxiety and a proper K intake may have positive effects on the prevention/reduction of the disease status, if the association between the two can be established.
Overall, the associations between K and depression and anxiety have been evaluated with premature status in previous studies and not without limitations (Faghih et al., 2020; Kaner et al., 2015; Karkishchenko & Khaĭtin, 1983; Mamplekou et al., 2010; McCleane & Watters, 1990; Mrug et al., 2019; Opie et al., 2017; Sadeghi et al., 2021; Saghafian et al., 2018; Shakya et al., 2021; Thi Thu Nguyen et al., 2019; Torres & Nowson, 2012; Torres et al., 2008; Yannakoulia et al., 2008). Some studies were conducted in selected population (patients (McCleane & Watters, 1990; Torres & Nowson, 2012), single gender (Torres & Nowson, 2012), the elderly (Thi Thu Nguyen et al., 2019), or the young (Faghih et al., 2020; Mrug et al., 2019), and in small sample (Kaner et al., 2015; Torres et al., 2008), and some failed to use suggested methods for estimation of K intake (questionnaires (Kaner et al., 2015; Karkishchenko & Khaĭtin, 1983; McCleane & Watters, 1990; Sadeghi et al., 2021; Shakya et al., 2021; Thi Thu Nguyen et al., 2019), or 12‐hour urinary K excretion (Mrug et al., 2019). It has been reported that 77% of K ingested is excreted through urine, which makes 24‐h urine sample collection as the gold standard to objectively measure K intake (World Health Organization, 2012). Therefore, we explored the association of K, using 24‐h urinary K (24‐h UK) excretion, a recommended method for K intake assessment, and depression and anxiety status in Chinese adults from general population.
2. MATERIALS AND METHODS
2.1. Study population
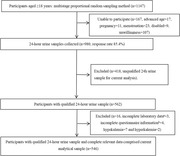
In this cross‐sectional study, we obtained study participants aged ≥18 years using multistage proportional random sampling method from Emin county, Xinjiang, China between March and June 2019. Eligible populations were asked to participate in 24‐h urine collection and post examination. Details were described in our previous study (Abudoureyimu et al., 2021; Li et al., 2022; Wang et al., 2021). In brief, the county was divided into 20 sites at the first stage. At the second stage, 10 sites were selected. At the third stage, participants were selected from locals, based on inclusion and exclusion criteria as in Figure 1.
FIGURE 1.
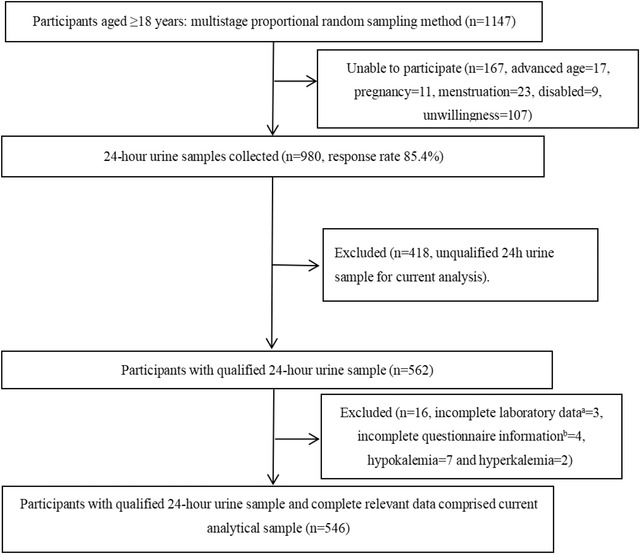
Flowchart for study participants. (a) Laboratory data include serum potassium test value, 24‐h urinary potassium test value, and 24‐h urinary sodium test value. (b) Questionnaire information included general information, SAS data, SDS data, and PSQI data.
2.2. Measures
2.2.1. 24‐h urine sample collection and measurement
As described previously (Abudoureyimu et al., 2021; Wang et al., 2021), a complete scheme was applied to the collection and detection process of 24‐h urine samples. Urine samples meeting any of the following exclusion criteria were not included in this study: the urine volume < 500 ml; the duration of specimen collection < 20 h; reported more than 100 ml of urine lost during collection; the 24‐h urinary creatinine (24‐h UCr) per kilogram of body weight was not up to standard (Mohammadifard et al., 2019; Nerbass et al., 2014). The detection of electrolytes (i.e., K, sodium, etc.) and creatinine in urine samples were carried out uniformly.
2.2.2. Data and blood sample collection
Data were collected on participants’ demographic characteristics (age, gender), socioeconomic status (occupation, education), lifestyles (cigarette and alcohol consumption), sleep quality using Pittsburgh sleep quality index (PSQI), depression and anxiety status using self‐rating depression scale (SDS) and self‐rating anxiety scale (SAS), and medical histories (hypertension, diabetes, dyslipidemia, and relevant therapeutic agents). Trained investigators also measured body weight, height, and blood pressure (BP), according to protocol.
Body weight was accurate to 0.1 kg. Height was measured to the nearest 0.1 cm. BP was expressed as the average value of three measurements of the electronic sphygmomanometer. Body mass index (BMI) was calculated by dividing body weight by the square of height (kg/m2).
Venous blood samples were obtained after an overnight fasting and serum creatinine, lipid profiles, and glucose were measured at People's hospital of Tacheng on the same day of collection, to where driving takes 40 min.
2.2.3. SDS and SAS scales, depression, and anxiety status
The SDS is a self‐report scale, which contains 20 items reflecting subjective feelings of depression, of which 10 are positive and 10 are negative. Each item was rated with respect to how participants felt using a four‐point Likert‐type scale. Options include: 1 = little or no time, 2 = a small part of the time, 3 = quite a lot of time, 4 = most or all of the time. Forward scoring questions were scored as 1, 2, 3, and 4; reverse scoring questions were scored as 4, 3, 2, and 1. Reverse scoring question number: 2, 5, 6, 11, 12, 14, 16, 17, 18, and 20. The original total score of SDS is between 20 and 80, and the result is usually expressed as the standard total score of SDS, which is obtained by multiplying the original score by 1.25 and taking the integer part (Zung, 1965).
The SAS is also a self‐report scale with 20 items covering a series of anxiety symptoms (Zung, 1971). In the current study, participants were instructed to choose how often they experienced each symptom over the past week given on a four‐point Likert‐type scale, ranging from 1 (little or no time) to 4 (most or all of the time). Items include positive and negative experiences. Forward scoring questions were scored as 1, 2, 3, and 4; reverse scoring questions were scored as 4, 3, 2, and 1. Reverse scoring question number: 5, 9, 13, 17, and 19. The standard total score of the SAS ranges from 25 to 100. The higher the standard score, the more serious the symptom.
Depression status was defined as SDS standard score ≥50, anxiety status as SAS standard score ≥50, and coexistence of the two as SDS standard score ≥50 and SAS standard score ≥50 (Zung et al., 1990).
2.2.4. Definitions of other covariates
Hypertension: systolic BP ≥140 mmHg and or diastolic BP ≥90 mmHg and or antihypertensive agent intake within the previous 2 weeks of survey (Joint committee for guideline revision, 2019). Diabetes mellitus: fasting blood glucose (FBG) ≥ 7.0 mmol/L and or self‐reported previous diagnosis by clinicians and or intake of hypoglycemic agents within the past 2 weeks of survey (Alberti & Zimmet, 1998). Dyslipidemia: triglyceride (TG) ≥2.3 mmol/L and or total cholesterol (TC) ≥6.2 mmol/L and or high density lipoprotein cholesterol < 1.0 mmol/L and or low density lipoprotein cholesterol ≥4.1 mmol/L and or having received lipid‐lowering treatment during the past 2 weeks (Joint committee for guideline revision, 2018). Poor sleep quality was defined as a PSQI score > 6 (Antza et al., 2018; Buysse et al., 1989; Zheng et al., 2013). Education attainment status was categorized as middle school and lower and high school and higher. Occupations were divided into two types as mental and manual work. Alcohol intake was defined as drinking at least once a week within 1 month at the survey time point (Wang et al., 2018). Cigarette consumption was defined as smoking more than 20 packs of cigarettes and smoking currently (Wang et al., 2018).
2.3. Statistical analysis
Participants were divided into three groups by the tertile of 24‐h UK as T1, T2, and T3 groups. Continuous variables including age, BMI, BP, 24‐h UNa, and 24‐h UK were presented as means ± standard deviations and were analyzed using ANOVA test if normally distributed; otherwise, presented as median and 25th–75th percentiles and analyzed by Mann–Whitney U test or Kruskal–Wallis H test. Categorical variables were displayed in the form of frequency (n) and proportion (%) and examined through Chi‐square test.
Logistic regression analysis was used to assess the association of the tertiles of 24‐h UK (T3 group of 24‐h UK as the reference) and the presence of depression status, anxiety status, and co‐existence of the two, and results were presented as unadjusted and adjusted odds ratios (ORs) and the 95% confidence intervals (CI).
Independent variables significantly relevant to depression and or anxiety status (p < .1) in univariate Logistic regression analysis were adjusted in multivariate logistic analysis. Tolerance and the variance inflation factor were examined to identify multicollinearity, which could be concerned if the variance inflation factor was > 10 and the tolerance was < 0.10.
Sensitivity analysis was conducted to compare SDS, SAS score, and logistic regression analysis by excluding participants with hypertension under anti‐hypertensive treatment.
Results were considered statistically significant if two‐tailed p value was less than .05. All analyses were performed with SPSS statistical software, version 20.0 (Chicago, IL, USA).
3. RESULTS
3.1. Participant characteristics at baseline
As in Figure 1, in total, 1147 adults aged ≥18 years were randomly selected and asked to participate, of whom 980 agreed to participate with a response rate of 85.4%. Among 980 participants, 562 participants provided complete 24‐h urine sample. Finally, 546 participants with complete data on 24‐h urine and relevant parameters comprised current analytical sample.
3.2. General information of participants
In current analytical participants, median age was 48.2 (36.2, 55.7) years and 57.9% were women (Table 1). Participants were tertiled into three grous as T1 (< 970.9 mg), T2 (970.9–1337.8 mg), and T3 (≥1337.8 mg) groups by 24‐h UK.
TABLE 1.
Characteristics of total study participants and by tertile of 24‐h urinary potassium excretion
| Characteristics | Total | T1 (< 970.9 mg) | T2 (970.9 to 1337.8 mg) | T3 (≥1337.8 mg) | X2, Z/P |
|---|---|---|---|---|---|
| N | 546 | 182 | 182 | 182 | |
| Age (years) | 48.2 (36.2, 55.7) | 45.8 (32.2, 54.0) | 48.2 (34.8, 55.4) | 49.8 (42.9, 56.6) | 13.001/0.002 |
| Gender (women, n, %) | 316 (57.9) | 109 (59.9) | 120 (65.9) | 87 (47.8) | 12.703/0.002 |
| Education (≥high school, n, %) | 244 (44.7) | 77 (42.3) | 91 (50.0) | 76 (41.8) | 3.121/0.210 |
| Occupation (mental worker, n, %) | 202 (37.0) | 65 (35.7) | 76 (41.8) | 61 (33.5) | 2.839/0.242 |
| Cigarette consumption (n, %) | 101 (18.5) | 40 (22.0) | 35 (19.2) | 26 (14.3) | 3.662/0.160 |
| Alcohol intake (n, %) | 130 (23.8) | 32 (17.6) | 39 (21.4) | 59 (32.4) | 11.871/0.003 |
| Hypertension (n, %) | 197 (36.1) | 61 (33.5) | 64 (35.2) | 72 (39.6) | 1.538/0.464 |
| Anti‐hypertensive agents (n, %) | 105 (19.2) | 29 (15.9) | 43 (23.6) | 33 (18.1) | 3.672/0.159 |
| Systolic blood pressure (mmHg) | 125.2 (115.3, 138.8) | 122.3 (114.5, 136.8) | 125.5 (115.3, 138.7) | 126.8 (116.6, 141.0) | 3.491/0.175 |
| Diastolic blood pressure (mmHg) | 79.5 (73.0, 88.0) | 79.9 ± 11.9 | 80.6 ± 11.2 | 80.5 (74.3, 88.2) | 1.808/0.405 |
| Diabetes (n, %) | 52 (9.5) | 10 (5.5) | 17 (9.3) | 25 (13.7) | 7.171/0.028 |
| Fasting blood glucose (mmol/L) | 5.11 (4.60, 5.82) | 5.0 (4.5, 5.6) | 5.1 (4.7, 5.8) | 5.2 (4.7, 6.1) | 5.433/0.066 |
| Dyslipidemia (n, %) | 181 (33.2) | 51 (28.0) | 58 (31.9) | 72 (39.6) | 5.659/0.059 |
| Serum total cholesterol (mmol/L) | 4.53 (3.81, 5.22) | 4.5 ± 1.1 | 4.5 (3.8, 5.2) | 4.6 (3.9, 5.4) | 1.738/0.419 |
| Serum triglyceride (mmol/L) | 1.10 (0.74, 1.68) | 1.0 (0.7, 1.5) | 1.1 (0.7, 1.5) | 1.3 (0.9, 2.0) | 19.731/ < 0.001 |
| Poor sleep quality (n, %) | 240 (44.0) | 83 (45.6) | 87 (47.8) | 70 (38.5) | 3.518/0.172 |
| PSQI score | 5.0 (3.0, 8.0) | 5.0 (3.0, 8.0) | 5.0 (3.0, 9.0) | 4.0 (3.0, 7.0) | 4.446/0.108 |
| Body mass index (kg/m2) | 26.6 (23.6, 29.2) | 25.6 (22.3, 28.5) | 25.9 (23.4, 29.3) | 27.4 (25.0, 29.8) | 20.253/ < 0.001 |
| Serum potassium (mmol/L) | 4.3 (4.1, 4.6) | 4.3 (4.1, 4.6) | 4.3 (4.1, 4.6) | 4.3 (4.1, 4.6) | 1.285/0.526 |
| 24‐h urinary potassium (mg) | 1133.5 (888.5, 1476.1) | 772.6 (635.3, 890.0) | 1133.5 (1036.2, 1224.6) | 1640.9 (1475.0, 1978.3) | 484.446/ < 0.001 |
| 24‐h urinary sodium (mg) | 3287.7 (2351.3, 4461.5) | 2602.7 (1837.0, 3780.8) | 2313.7 (3312.8, 4266.7) | 4238.1 (3071.2, 5341.6) | 79.772/ < 0.001 |
| 24‐h urine creatinine (mmol) | 8.1 (6.5, 10.2) | 6.9 (5.3, 8.5) | 7.9 (6.7, 9.5) | 9.8 (7.8, 12.8) | 102.386/ < 0.001 |
| 24‐h urine volume (ml) | 1241.5 (895.8, 1571.3) | 941.5 (670.0, 1313.5) | 1234.0 (903.3, 1623.5) | 1452.0 (1202.5, 1851.3) | 94.546/ < 0.001 |
Abbreviations: PSQI, Pittsburgh sleep quality index; SAS, self‐rating anxiety scale.; SDS, self‐rating depression scale.
Participants in T3 and T2 groups were significantly older (49.8 vs 48.2 vs 45.8 years, p = .002), more alcohol takers (32.4 vs 21.4 vs 17.6%, p = .003), more diabetic (13.7 vs 9.3 vs 5.5%, p = .028), with higher TG (1.3 vs 1.1 vs 1.0 mmol/L), and higher BMI (27.4 vs 25.9 vs 25.6 kg/m2), compared with those in T1 group.
3.3. The SDS scores and depression status
As in Table 2, the median of SDS and SAS scores among the participants was 38.0 (32.0, 45.0) and 37.0 (32.0, 45.0), respectively.
TABLE 2.
Comparison of SDS and SAS standard score among different 24‐h UK tertile groups
| Overall | T1 (< 970.9 mg) | T2 (970.9 to 1337.8 mg) | T3 (≥1337.8 mg) | p | p 1 | p 2 | p 3 | |
|---|---|---|---|---|---|---|---|---|
| Total participants | n = 546 | n = 182 | n = 182 | n = 182 | ||||
| SDS standard score | 38.0 (32.0, 45.0) | 40.0 (33.0, 46.0) | 40.0 (33.0, 45.0) | 36.0 (31.0, 42.0) | .001 | 1.000 | .007 | .004 |
| SAS standard score | 37.0 (32.0, 45.0) | 38.0 (32.0, 45.0) | 40.0 (32.0, 46.2) | 35.0 (30.0, 41.0) | < .001 | .649 | .019 | < .001 |
| Not on antihypertensive agents | n = 441 | n = 147 | n = 147 | n = 147 | ||||
| SDS standard score | 38.0 (32.0, 43.0) | 40.0 (33.0, 46.0) | 40.0 (32.0, 45.0) | 36.0 (31.0, 41.0) | .001 | 1.000 | .001 | .009 |
| SAS standard score | 37.0 (31.0, 43.0) | 38.0 (32.0, 45.0) | 40.0 (32.0, 45.0) | 35.0 (30.0, 40.0) | .002 | 1.000 | .021 | .002 |
Abbreviations: SDS, Self‐rating depression scale; SAS, self‐rating anxiety scale.
p, among group comparison; p 1, T1 versus T2; p 2, T1 versus T3; p 3, T2 versus T3.
Participants in T1 (40.0 vs 36.0, p = .007) and T2 groups (40.0 vs 36.0, p = .004) showed significantly higher SDS scores than did those in T3 group, which remained consistent in sensitivity analysis by excluding hypertensives under anti‐hypertensive treatment.
Participants in T1 and T2 groups showed significantly higher prevalence of depression status in total participants (19.8 vs 15.9 vs 7.1%, p = .002), than did those in T3 groups, which is largely consistent in men (T1 vs T3: 16.9 vs 5.3%, p = .023) and in women participants (T1 vs T3: 21.9 vs 8.6%, p = .042) (Table 3).
TABLE 3.
Prevalence of depression and anxiety status in total participants and in stratified participants by tertile of 24‐h UK
| Overall | T1 (< 970.9 mg) | T2 (970.9 to 1337.8 mg) | T3 (≥1337.8 mg) | p | p 1 | p 2 | p 3 | |
|---|---|---|---|---|---|---|---|---|
| Total participants | 546 | 182 | 182 | 182 | ||||
| Depression (n, %) | 78 (14.3) | 36 (19.8) | 29 (15.9) | 13 (7.1) | .002 | .885 | .002 | .050 |
| Anxiety (n, %) | 82 (15.0) | 27 (14.8) | 39 (21.4) | 16 (8.8) | .003 | .236 | .321 | .002 |
| Depression and anxiety (n, %) | 45 (8.2) | 19 (10.4) | 21 (11.5) | 5 (2.7) | .004 | 1.000 | .023 | .007 |
| Men | 230 | 77 | 77 | 76 | ||||
| Depression (n, %) | 18 (7.8) | 13 (16.9) | 1 (0.1) | 4 (5.3) | .001 | .001 | .023 | 1.000 |
| Anxiety (n, %) | 12 (5.2) | 5 (6.5) | 4 (5.2) | 3 (3.9) | .779 | – | – | – |
| Depression and anxiety (n, %) | 6 (2.6) | 4 (5.2) | 1 (1.3) | 1 (1.3) | .219 | – | – | – |
| Women | 316 | 105 | 106 | 105 | ||||
| Depression (n, %) | 60 (19.0) | 23 (21.9) | 28 (26.4) | 9 (8.6) | .003 | 1.000 | .042 | .003 |
| Anxiety (n, %) | 70 (22.2) | 22 (21.0) | 35 (33.0) | 13 (12.4) | .001 | .105 | .406 | .001 |
| Depression and anxiety (n, %) | 39 (12.3) | 15 (14.3) | 20 (18.9) | 4 (3.8) | .003 | .937 | .064 | .003 |
p, among‐group comparison; p 1, T1 versus T2; p 2, T1 versus T3; p 3, T2 versus T3.
As in Table 4, compared with the participants in T3 group, participants in T2 (OR = 2.46, 95%CI: 1.24, 4.91, p = .010) and T1 (OR = 3.21, 95%CI: 1.64,6.28, p = .001) groups showed elevated ORs for presence of depression status, whereas the association remained significant only for T1 group in model adjusted for age, gender, education attainment status, occupation, body mass index, systolic and diastolic blood pressure, diabetes, dyslipidemia, sleep quality, serum K, and 24‐h UNa, which showed significant association with presence of depression status in univariate logistic regression (Table S1). Furthermore, the association was more augmented in sensitivity analysis by exclusion of patients with hypertension under anti‐hypertensive treatment (ORs of T3 vs T2 vs T1: 1 vs 2.65 vs 3.90, p = .066 and = .010, respectively).
TABLE 4.
Logistic regression analysis for the associations of tertiles of 24‐h UK excretion with the presence of depression and anxiety (OR, 95%CI, p)
| Depression status | Anxiety status | Depression and anxiety status | ||||
|---|---|---|---|---|---|---|
| Crude model | Adjusted model | Crude model | Adjusted Model | Crude model | Adjusted model | |
| Total participants | ||||||
| 24‐h UK tertiles | ||||||
| T3 | Ref | Ref | Ref | Ref | Ref | Ref |
| T1 | 3.21 (1.64, 6.28), 0.001 | 2.71 (1.20, 6.14), 0.017 | 1.81 (0.94, 3.48), 0.077 | 1.16 (0.54, 2.48), 0.710 | 4.13 (1.51, 11.31), 0.006 | 3.71 (1.31, 10.50), 0.013 |
| T2 | 2.46 (1.24, 4.91), 0.010 | 1.61 (0.72, 3.62), 0.249 | 2.83 (1.52, 5.28), 0.001 | 2.07 (1.03, 4.18), 0.042 | 4.62 (1.70, 12.53), 0.003 | 3.66 (1.30, 10.30), 0.014 |
| Sensitivity analyses by exclusion of antihypertensive agent takers | ||||||
| T3 | Ref | Ref | Ref | Ref | Ref | Ref |
| T1 | 5.36 (2.28, 12.59), < 0.001 | 3.90 (1.38, 11.06), 0.010 | 1.92 (0.91, 4.03), 0.086 | 1.18 (0.50, 2.77), 0.708 | 4.23 (1.38, 12.98), 0.012 | 3.99 (1.26, 12.62), 0.019 |
| T2 | 3.81 (1.57, 9.24), 0.003 | 2.65 (0.94, 7.51), 0.066 | 2.38 (1.14, 4.97), 0.021 | 1.87 (0.84, 4.19), 0.126 | 4.72 (1.54, 14.48), 0.007 | 3.74 (1.18, 11.86), 0.025 |
For depression status, adjusted model was adjusted for age, gender, education attainment status, occupation, body mass index, systolic and diastolic blood pressure, diabetes, dyslipidemia, sleep quality, serum K, and 24‐h urinary sodium excretion. For anxiety status, adjusted model was adjusted for gender, education attainment status, occupation, cigarette and alcohol use, systolic and diastolic blood pressure, dyslipidemia, sleep quality, and 24‐h urinary sodium excretion. For co‐existent depression and anxiety status, adjusted model was adjusted for gender, education attainment status, occupation, systolic and diastolic blood pressure, and sleep quality.
3.4. The SAS score and anxiety status
Participants in T1 (38.0 vs 35.0, p = .019) and T2 groups (40.0 vs 35.0, p < .001) showed significantly higher SDS scores than did those in T3 group, which remained consistent in sensitivity analysis by excluding hypertensives under anti‐hypertensive treatment (Table 2).
Accordingly, participants in T1 (14.8% vs 8.8%, p = .321) and T2 (21.4% vs 8.8%, p = .002) groups showed higher prevalence of anxiety status in total participants, compared with those in T3 groups, whereas with statistical significance only between T2 and T3 groups, which is largely consistent in women (T2 vs T3: 33.0 vs 12.4%, p = .001), but not men (Table 3).
In logistic regression analysis (Table 4), compared with the reference T3 group, participants in T1 (OR = 1.81, 95%CI: 0.94, 3.48, p = .077) and T2 (OR = 2.83, 95%CI: 1.52,5.28, p = .001) groups showed marginal and significant elevation in ORs for the presence of anxiety in crude model, which remained significant only for T2 group (OR = 2.07, 95%CI: 1.03, 4.18, p = .042) in adjusted model for variables selected using univariate logistic regression (Table S1). The association was diminished in adjusted model of sensitivity analysis by exclusion of hypertensives under treatment.
3.5. Co‐existence of anxiety and depression status
As in Table 3, compared with T3 groups, participants in T1 (10.4% vs 2.7%, p = .023) and T2 (11.5% vs 2.7%, p = .007) groups showed significantly higher presence of co‐existent anxiety and depression status in total participants, which is largely consistent in women (T1 vs T2 vs T3: 14.3% vs 18.9 vs 3.8%, p = .003) but not in men.
In logistic regression analysis (Table 4), compared with the participants in T3 group, participants in T1 (OR = 3.71, 95%CI: 1.31, 10.50, p = .013) and T2 (OR = 3.66, 95%CI: 1.30, 10.30, p = .014) groups showed significantly higher ORs for co‐existent anxiety and depression status in the model adjusted for gender, education attainment status, occupation, BP, and sleep quality that showed higher ORs for the outcome as Table S2. Furthermore, the association remained consistent in sensitivity analysis by exclusion of hypertensives under treatment (ORs of T3 vs T2 vs T1: 1 vs 3.74 vs 3.99, p = .025 and = .019, respectively).
4. DISCUSSION
To our knowledge, current study is the first to explore the relationship between 24‐h UK excretion and depression status, anxiety status, and co‐existence of the both in relatively large sample community‐based general population. Main results showed that SDS and SAS score and prevalence of depression status, anxiety status, and co‐existence of the two are significantly higher in participants with lower 24‐h UK excretion, compared to those with higher 24‐h UK excretion. In logistic regression analysis, compared with those in the highest tertile of 24‐h UK excretion, participants in the lower tertiles showed elevated odds for presence of depression status, anxiety status, and their co‐existence, which remained consistent in sensitivity analysis by excluding potential confounding of anti‐hypertensive agents for depression status and for co‐existence of the both. These results imply that lower K intake, indicated by lower UK excretion, may be involved in the development of depression status and or anxiety status in the general population.
Observations from the current study add evidence on the ongoing uncertainty of K and depression and anxiety status. Current findings are consistent with results of previous reports. For example, some studies based on DASH diet model have shown that low sodium and high potassium intake can significantly reduce depression and anxiety score and improve depression and anxiety status (Mrug et al., 2019; Torres et al., 2008). In addition, the current findings may extend some previous studies from the clinical environment to the general adult population. For example, consumption of foods high in sodium and low in potassium contributes to the development of depressive and anxiety symptoms in early adolescence (Mrug et al., 2019), and low‐sodium and high‐potassium diet seems to have an overall positive effect on depressive and anxiety mood state (Torres et al., 2008).
Urinary potassium excretion has been established as a marker of overall diet quality, with higher potassium excretion positively correlated with greater intake of vegetables, fruits, whole grains, fish, and poultry, and negatively correlated with the intake of fast food and red meat (Mente et al., 2009). Therefore, with regard to public health, prevention, control, or improvement of depression, anxiety, and the comorbidity by K supplements could have public health implications since it is feasible to increase K intake by fresh vegetables and fruits, and even salt substitute, which is a practical and cost‐effective approach to supplement K and has been shown to slow the incidence of hypertension (Bernabe‐Ortiz et al., 2020), another risk factor for depression and anxiety.
In the current study, we included several confounding factors for parameters of interest. For example, we had data on anthropometric (BMI) and socioeconomic indicators (education attainment status and occupation), disease history (diabetes, hypertension), and lifestyle factors (cigarette and alcohol use and sleep quality), which have effects on interest of outcome. We also collected data on anti‐hypertensive agent use, most of which exerts effects on K and Na. Furthermore, we performed univariate and multi‐variate logistic regression and sensitivity analysis to obtain objective association of 24‐h UK and depression and/or anxiety status, although some of the data showed significant differences among groups. For example, BMI increased significantly from T1 to T3 group of UK tertile, whereas its effects on the results were ruled out using regression analysis.
The main strength of this study is the objective measurement of K excretion using 24‐h urine samples. Second, the current study was conducted in general population with wide age ranges from both genders. Therefore, results may be generalizable, although limited to a certain county. Third, we adjusted for the sleep quality, an independent risk factor for depression and anxiety (Huang & Zhu, 2020), in logistic regression analysis, and the association still remained significant. Nonetheless, the current study also contains some limitations. A key limitation is that we failed to obtain causality between K with depression and or anxiety due to the cross‐sectional nature of the study. Second, due to seasonal or daily changes in urinary potassium excretion, a single 24‐h urine sample may not reflect the usual dietary intake or pattern of participants. Nevertheless, we collected urine samples on weekdays and weekends, and from spring to summer, which may have reduced the relevant variability. In addition, we used SDS and SAS to evaluate the depressive and anxiety mood of the study population and roughly screen out possible depression and anxiety status patients. Nevertheless, SDS and SAS have been widely used including scientific researches with higher diagnostic sensitivity (SDS: 92%; SAS: 89.0%) and specificity (SDS: 77%; SAS: 69.0%) (Dunstan et al., 2017; Gabrys & Peters, 1985). In addition, both questionnaires are confirmed to be reliable and valid in Chinese population (Liu et al., 2018). Moreover, we failed to assess overall dietary intake pattern in this study population, which may provide more information on the association of K intake and depression and or anxiety. However, the main objective of the study was to assess the association of K and depression and or anxiety status, and K in 24‐h urine sample may provide more accurate assessment of 24‐h K intake, since studies show that 77% of K ingested is excreted through urine (World Health Organization, 2012).
5. CONCLUSIONS
Lower 24‐h UK excretion shows independent association with higher SDS and SAS scores and with higher prevalent depression and or anxiety status in general adults from China, suggesting lower K intake may be involved in depression and or anxiety, and this could lead to optimization programs focused on increasing potassium intake at the population level, which has been shown to be feasible and result in disease prevention.
AUTHOR CONTRIBUTIONS
Study design was performed by Nanfang Li1, Mulalibieke Heizhati, and Zihao Wu. Data collection was performed by Mulalibieke Heizhati, Junli Hu, Mengyue Lin, Mei Li, Wenbo Yang, Ling Yao, Jing Hong, Le Sun, Jing Li, and Wei Li; and data analysis/interpretation was performed by Zihao Wu. Manuscript drafting or manuscript revision for important intellectual content was performed by Zihao Wu, Nanfang Li, Junli Hu, and Mulalibieke Heizhati. All authors contributed to the writing, review, and editing of the article. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2842.
Supporting information
Table S1 Variable selection to be adjusted using univariate logistic regression analysis and multicollinearity assessment.
Table S2 Variable selection to be adjusted using univariate logistic regression analysis and multicollinearity assessment.
ACKNOWLEDGMENTS
We gratefully acknowledge all of the study participants and staffs in local hospitals for their assistance during the study. This study was supported by the Xinjiang Uygur Autonomous Region Health Young Medical Science and Technology Talents Special Research Project [grant number WJWY‐202124].
Wu, Z. , Heizhati, M. , Hu, J. , Lin, M. , Gan, L. , Li, M. , Yang, W. , Yao, L. , Hong, J. , Sun, L. , Li, J. , Li, W. , & Li, N. (2023). Lower 24‐h urinary potassium excretion is associated with higher prevalent depression and anxiety status in general population. Brain and Behavior, 13, e2842. 10.1002/brb3.2842
Contributor Information
Mulalibieke Heizhati, Email: morale118@126.com.
Nanfang Li, Email: Lnanfang2016@sina.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abudoureyimu, R. , Heizhati, M. , Wang, L. , Li, M. , Zhang, D. , Wang, Z. , Yang, Z. , Hong, J. , & Li, N. (2021). Lower 24‐h urinary potassium excretion is negatively associated with excessive daytime sleepiness in the general population. Sleep & Breathing = Schlaf & Atmung, 26(2), 733–741. Advance online publication. 10.1007/s11325-021-02444-7 [DOI] [PubMed] [Google Scholar]
- Alberti, K. G. , & Zimmet, P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine: A Journal of the British Diabetic Association, 15(7), 539–553. [DOI] [PubMed] [Google Scholar]
- Andrade, L. , Caraveo‐Anduaga, J. J. , Berglund, P. , Bijl, R. V. , De Graaf, R. , Vollebergh, W. , Dragomirecka, E. , Kohn, R. , Keller, M. , Kessler, R. C. , Kawakami, N. , Kiliç, C. , Offord, D. , Ustun, T. B. , & Wittchen, H. U. (2003). The epidemiology of major depressive episodes: Results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. International Journal of Methods in Psychiatric Research, 12(1), 3–21. 10.1002/mpr.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antza, C. , Stabouli, S. , & Kotsis, V. (2018). Gut microbiota in kidney disease and hypertension. Pharmacological Research, 130, 198–203. 10.1016/j.phrs.2018.02.028 [DOI] [PubMed] [Google Scholar]
- Bernabe‐Ortiz, A. , Sal Y Rosas, V. G. , Ponce‐Lucero, V. , Cárdenas, M. K. , Carrillo‐Larco, R. M. , Diez‐Canseco, F. , Pesantes, M. A. , Sacksteder, K. A. , Gilman, R. H. , & Miranda, J. J. (2020). Effect of salt substitution on community‐wide blood pressure and hypertension incidence. Nature Medicine, 26(3), 374–378. 10.1038/s41591-020-0754-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D. J. , Reynolds, C. F. 3rd , Monk, T. H. , Berman, S. R. , & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Dunstan, D. A. , Scott, N. , & Todd, A. K. (2017). Screening for anxiety and depression: Reassessing the utility of the Zung scales. BMC Psychiatry [Electronic Resource], 17(1), 329. 10.1186/s12888-017-1489-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghih, S. , Babajafari, S. , Mirzaei, A. , & Akhlaghi, M. (2020). Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and mental health in Iranian university students. European Journal of Nutrition, 59(3), 1001–1011. 10.1007/s00394-019-01961-2 [DOI] [PubMed] [Google Scholar]
- Gabrys, J. B. , & Peters, K. (1985). Reliability, discriminant and predictive validity of the Zung Self‐rating Depression Scale. Psychological Reports, 57(3 Pt 2), 1091–1096. 10.2466/pr0.1985.57.3f.1091 [DOI] [PubMed] [Google Scholar]
- GBD 2017 DALYs and HALE Collaborators . (2018). Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England), 392(10159), 1859–1922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh, M. , Vafa, M. , Esmaillzadeh, A. , Feizi, A. , Majdzadeh, R. , Afshar, H. , Keshteli, A. H. , & Adibi, P. (2016). Empirically derived dietary patterns in relation to psychological disorders. Public Health Nutrition, 19(2), 204–217. 10.1017/S136898001500172X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , & Zhu, M. (2020). Increased global PSQI score is associated with depressive symptoms in an adult population from the United States. Nature and Science of Sleep, 12, 487–495. 10.2147/NSS.S256625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Q. , Liu, H. , Suzuki, K. , Ma, S. , & Liu, C. (2019). Linking what we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants (Basel), 8(9), 376. 10.3390/antiox8090376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint committee for guideline revision . (2018). 2016 Chinese guidelines for the management of dyslipidemia in adults. Journal of Geriatric Cardiology: JGC, 15(1), 1–29. 10.11909/j.issn.1671-5411.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Committee for Guideline Revision . (2019). 2018 Chinese Guidelines for Prevention and Treatment of Hypertension–A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. Journal of Geriatric Cardiology: JGC, 16(3), 182–241. 10.11909/j.issn.1671-5411.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner, G. , Soylu, M. , Yüksel, N. , Inanç, N. , Ongan, D. , & Başmısırlı, E. (2015). Evaluation of nutritional status of patients with depression. BioMed Research International, 2015, 521481. 10.1155/2015/521481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkishchenko, N. N. , & Khaĭtin, M. I. (1983). Anksioliticheskiĭ éffekt kaliia orotata [Anxiolytic effect of potassium orotate]. Farmakologiia i Toksikologiia, 46(4), 68–71. [PubMed] [Google Scholar]
- Kessler, R. C. , Sampson, N. A. , Berglund, P. , Gruber, M. J. , Al‐Hamzawi, A. , Andrade, L. , Bunting, B. , Demyttenaere, K. , Florescu, S. , de Girolamo, G. , Gureje, O. , He, Y. , Hu, C. , Huang, Y. , Karam, E. , Kovess‐Masfety, V. , Lee, S. , Levinson, D. , Medina Mora, M. E. , … Wilcox, M. A. (2015). Anxious and non‐anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiology and Psychiatric Sciences, 24(3), 210–226. 10.1017/S2045796015000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. L. , Williams, J. B. , Monahan, P. O. , & Löwe, B. (2007). Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Annals of Internal Medicine, 146(5), 317–325. 10.7326/0003-4819-146-5-200703060-00004 [DOI] [PubMed] [Google Scholar]
- Li, M. , Heizhati, M. , Wang, L. , Wang, Z. , Abudoureyimu, R. , Yang, Z. , Pan, F. , Sun, L. , Li, W. , Li, J. , Lin, M. , Gan, L. , Lu, S. , & Li, N. (2022). 24‐hour urinary potassium excretion is negatively associated with subjective sleep quality in the general population, independent of sleep‐disordered breathing. Journal of Clinical Sleep Medicine, 18(11), 2589–2596. 10.5664/jcsm.10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. , Tang, A. , Wang, X. , & Shen, S. (2018). Assessment of quality of life in chinese patients with inflammatory bowel disease and their caregivers. Inflammatory Bowel Diseases, 24(9), 2039–2047. 10.1093/ibd/izy099 [DOI] [PubMed] [Google Scholar]
- Mamplekou, E. , Bountziouka, V. , Psaltopoulou, T. , Zeimbekis, A. , Tsakoundakis, N. , Papaerakleous, N. , Gotsis, E. , Metallinos, G. , Pounis, G. , Polychronopoulos, E. , Lionis, C. , & Panagiotakos, D. (2010). Urban environment, physical inactivity and unhealthy dietary habits correlate to depression among elderly living in eastern Mediterranean islands: The MEDIS (MEDiterranean ISlands Elderly) study. The Journal of Nutrition, Health & Aging, 14(6), 449–455. 10.1007/s12603-010-0091-0 [DOI] [PubMed] [Google Scholar]
- McCleane, G. J. , & Watters, C. H. (1990). Pre‐operative anxiety and serum potassium. Anaesthesia, 45(7), 583–585. 10.1111/j.1365-2044.1990.tb14837.x [DOI] [PubMed] [Google Scholar]
- Mohammadifard, N. , Khosravi, A. , Salas‐Salvadó, J. , Becerra‐Tomás, N. , Nouri, F. , Abdollahi, Z. , Jozan, M. , Bahonar, A. , & Sarrafzadegan, N. (2019). Trend of salt intake measured by 24‐hour urine collection samples among Iranian adults population between 1998 and 2013: The Isfahan salt study. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD, 29(12), 1323–1329. 10.1016/j.numecd.2019.07.019 [DOI] [PubMed] [Google Scholar]
- Mrug, S. , Orihuela, C. , Mrug, M. , & Sanders, P. W. (2019). Sodium and potassium excretion predict increased depression in urban adolescents. Physiological Reports, 7(16), e14213. 10.14814/phy2.14213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbass, F. B. , Pecoits‐Filho, R. , McIntyre, N. J. , McIntyre, C. W. , & Taal, M. W. (2014). Development of a formula for estimation of sodium intake from spot urine in people with chronic kidney disease. Nephron Clinical Practice, 128(1‐2), 61–66. 10.1159/000363297 [DOI] [PubMed] [Google Scholar]
- Opie, R. S. , Itsiopoulos, C. , Parletta, N. , Sanchez‐Villegas, A. , Akbaraly, T. N. , Ruusunen, A. , & Jacka, F. N. (2017). Dietary recommendations for the prevention of depression. Nutritional Neuroscience, 20(3), 161–171. 10.1179/1476830515Y.0000000043 [DOI] [PubMed] [Google Scholar]
- Parletta, N. , Zarnowiecki, D. , Cho, J. , Wilson, A. , Bogomolova, S. , Villani, A. , Itsiopoulos, C. , Niyonsanga, T. , Blunden, S. , & Segal, L. (2019). A Mediterranean‐style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutritional Neuroscience, 22, 474–487. 10.1080/1028415X.2017 [DOI] [PubMed] [Google Scholar]
- Rienks, J. , Dobson, A. J. , & Mishra, G. D. (2013). Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid‐aged women: Results from a large community‐based prospective study. European Journal of Clinical Nutrition, 67, 75. 10.1038/ejcn.2012.193 [DOI] [PubMed] [Google Scholar]
- Ruscio, A. M. , Hallion, L. S. , Lim, C. , Aguilar‐Gaxiola, S. , Al‐Hamzawi, A. , Alonso, J. , Andrade, L. H. , Borges, G. , Bromet, E. J. , Bunting, B. , Caldas de Almeida, J. M. , Demyttenaere, K. , Florescu, S. , de Girolamo, G. , Gureje, O. , Haro, J. M. , He, Y. , Hinkov, H. , Hu, C. , … Scott, K. M. (2017). Cross‐sectional comparison of the epidemiology of DSM‐5 generalized anxiety disorder across the globe. JAMA Psychiatry, 74(5), 465–475. 10.1001/jamapsychiatry.2017.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi, O. , Keshteli, A. H. , Afshar, H. , Esmaillzadeh, A. , & Adibi, P. (2021). Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutritional Neuroscience, 24(4), 248–259. 10.1080/1028415X.2019.1620425 [DOI] [PubMed] [Google Scholar]
- Saghafian, F. , Malmir, H. , Saneei, P. , Keshteli, A. H. , Hosseinzadeh‐Attar, M. J. , Afshar, H. , Siassi, F. , Esmaillzadeh, A. , & Adibi, P. (2018). Consumption of fruit and vegetables in relation with psychological disorders in Iranian adults. European Journal of Nutrition, 57(6), 2295–2306. 10.1007/s00394-018-1652-y [DOI] [PubMed] [Google Scholar]
- Shakya, P. R. , Melaku, Y. A. , Page, A. J. , & Gill, T. K. (2021). Nutrient patterns and depressive symptoms among Australian adults. European Journal of Nutrition, 60(1), 329–343. 10.1007/s00394-020-02243-y [DOI] [PubMed] [Google Scholar]
- Thi Thu Nguyen, T. , Miyagi, S. , Tsujiguchi, H. , Kambayashi, Y. , Hara, A. , Nakamura, H. , Suzuki, K. , Yamada, Y. , Shimizu, Y. , & Nakamura, H. (2019). Association between lower intake of minerals and depressive symptoms among elderly Japanese women but not men: Findings from Shika Study. Nutrients, 11(2), 389. 10.3390/nu11020389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, S. J. , & Nowson, C. A. (2012). A moderate‐sodium DASH‐type diet improves mood in postmenopausal women. Nutrition (Burbank, Los Angeles County, Calif.), 28(9), 896–900. 10.1016/j.nut.2011.11.029 [DOI] [PubMed] [Google Scholar]
- Torres, S. J. , Nowson, C. A. , & Worsley, A. (2008). Dietary electrolytes are related to mood. The British Journal of Nutrition, 100(5), 1038–1045. 10.1017/S0007114508959201 [DOI] [PubMed] [Google Scholar]
- Vos, T. , Flaxman, A. D. , Naghavi, M. , Lozano, R. , Michaud, C. , Ezzati, M. , Shibuya, K. , Salomon, J. A. , Abdalla, S. , Aboyans, V. , Abraham, J. , Ackerman, I. , Aggarwal, R. , Ahn, S. Y. , Ali, M. K. , Alvarado, M. , Anderson, H. R. , Anderson, L. M. , Andrews, K. G. , … Memish, Z. A. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England), 380(9859), 2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Chen, Z. , Zhang, L. , Wang, X. , Hao, G. , Zhang, Z. , Shao, L. , Tian, Y. , Dong, Y. , Zheng, C. , Wang, J. , Zhu, M. , Weintraub, W. S. , Gao, R. , & China Hypertension Survey Investigators . (2018). Status of hypertension in China: Results from the China Hypertension Survey, 2012–2015. Circulation, 137(22), 2344–2356. 10.1161/CIRCULATIONAHA.117.032380 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Li, N. , Heizhati, M. , Wang, L. , Li, M. , Pan, F. , Yang, Z. , Abudureyimu, R. , Hong, J. , Sun, L. , Li, J. , & Li, W. (2021). Association between 24‐h urinary sodium to potassium ratio and mild cognitive impairment in community‐based general population. Public Health Nutrition, 24, 5795–5804. 10.1017/S1368980021001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2012). Guideline: Potassium Intake for Adults and Children. [PubMed]
- Yannakoulia, M. , Panagiotakos, D. B. , Pitsavos, C. , Tsetsekou, E. , Fappa, E. , Papageorgiou, C. , & Stefanadis, C. (2008). Eating habits in relations to anxiety symptoms among apparently healthy adults. A pattern analysis from the ATTICA Study. Appetite, 51(3), 519–525. 10.1016/j.appet.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. , Yu, B. , Alexander, D. , Manolio, T. A. , Aguilar, D. , Coresh, J. , Heiss, G. , Boerwinkle, E. , & Nettleton, J. A. (2013). Associations between metabolomic compounds and incident heart failure among African Americans: The ARIC Study. American Journal of Epidemiology, 178(4), 534–542. 10.1093/aje/kwt004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung, W. W. (1965). A self‐rating depression scale. Archives of General Psychiatry, 12, 63–70. 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- Zung, W. W. (1971). A rating instrument for anxiety disorders. Psychosomatics, 12(6), 371–379. 10.1016/S0033-3182(71)71479-0 [DOI] [PubMed] [Google Scholar]
- Zung, W. W. , Magruder‐Habib, K. , Velez, R. , & Alling, W. (1990). The comorbidity of anxiety and depression in general medical patients: A longitudinal study. The Journal of Clinical Psychiatry, 51, Suppl, 77–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Variable selection to be adjusted using univariate logistic regression analysis and multicollinearity assessment.
Table S2 Variable selection to be adjusted using univariate logistic regression analysis and multicollinearity assessment.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


