Abstract
The effects of resistant starch at high doses have been well-characterized, but the potential prebiotic effects of resistant starch at doses comparable to oligosaccharide prebiotics have not been evaluated. A three-arm randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the effect of 3.5 g and 7 g daily doses of Solnul™ resistant potato starch (RPS) on beneficial populations of gut bacteria and stool consistency after a 4-week period. The relative abundance of Bifidobacterium and Akkermansia was determined by employing 16Sv4 sequencing of stool samples. To assess the effect of RPS on laxation and bowel movements, stools were recorded and scored using the Bristol Stool Form Scale. Participants consuming 3.5 g/day of RPS experienced significantly greater changes in Bifidobacterium and Akkermansia compared to the placebo after 4 weeks. The number of diarrhea- and constipation-associated bowel movements were both significantly lower in the 3.5 g RPS arm compared to the placebo group. Participants consuming 7 g of RPS responded similarly to those in the 3.5 g arm. Our analyses demonstrate that Solnul™ RPS has a prebiotic effect when consumed for 4 weeks at the 3.5 g per day dose, stimulating increases in beneficial health-associated bacteria and reducing diarrhea- and constipation-associated bowel movements when compared to the placebo group.
Keywords: prebiotic, constipation, diarrhea, potato, resistant starch, Bifidobacterium, Akkermansia, microbiome
1. Introduction
Resistant starch (RS) is defined as ‘the sum of starch and starch-degradation products that, on average, reach the human large intestine’ [1]. Most RS is classified into five types based on the natural or manufactured form, with RS Type 1 (RS1) including starch physically inaccessible to amylase, RS Type 2 (RS2) including naturally occurring starch that resists amylase due to the shape of the granule, RS Type 3 (RS3) including heated-then-cooled starches that adopt a retrograded crystalline structure that resists amylase degradation, RS Type 4 (RS4) including chemically modified starches that resist amylase degradation, and RS Type 5 (RS5) including starch-lipid complexes that evade amylase degradation [2]. Uncooked potato, green banana, and high amylose maize are all naturally occurring sources of RS2, while cooked and cooled pasta and cooled baked potato are sources of RS3 [3]. RS2 and RS4 are recognized as forms of dietary fiber in Canada (Health Canada) and the United States (Food and Drug Administration), though the fermentability of RS4 and its effect on gut microbiota vary depending on the material it was derived from [4]. Resistant potato starch (RPS) is exceptional among the various types of dietary fiber in that it is insoluble but fully fermentable [5], the latter being a characteristic that is typically associated with soluble fibers.
While Australian dietary fiber levels approached those recommended by the National Health and Medical Research Council, bowel cancer rates remained high, and subsequent investigations revealed that RS levels in the Australian diet remained low (3–9 g per day; Commonwealth Scientific and Industrial Research Organization, Australia), suggesting that RS may play a valuable role among various forms of dietary fiber [6,7]. Americans consume approximately 4 g of RS per day, mostly RS2 and RS3 [8], with suggested dietary targets ranging from 15–20 g per day for optimal metabolic and bowel health [8,9]. Closing this gap through diet is challenging, given that most foods contain only small amounts of RS [3]. Furthermore, the food commonly consumed by Americans that contributes the most dietary RS is French fries [8], a food associated with negative health consequences, making it unreasonable to use such dietary sources to close the gap between current RS intake and suggested dietary targets.
Clinical trials evaluating the health benefits of RS fortification have typically investigated high doses in food formats, producing benefits including laxation [10,11,12,13], lower fasting blood glucose or HbA1c [14,15,16,17,18], and improved insulin metabolism [14,17,19,20,21,22,23,24,25,26]. Evidence from animal and human studies indicates that short-chain fatty acids derived from RS fermentation can attenuate inflammatory and oxidative stress pathways, which may mitigate the progression of diabetic kidney disease [27], an active area of investigation [28]. Other studies have investigated the effects of RS consumption on the composition of the gut microbiota, which is generally characterized by an increased abundance of Bifidobacterium for RPS and an increased abundance of Ruminococcus for high amylose maize starch [29]. Only a subset of RS2 clinical trials has measured both a health benefit and specific changes in the microbiome [14,30,31,32,33], which are required to demonstrate a prebiotic effect [34].
While current evidence suggests that higher doses of RS2 (i.e., 15–30 g/day) are needed to achieve metabolic improvements [8,14], we hypothesized that lower doses of RPS might provide a prebiotic effect by enhancing the abundance of beneficial bacteria while improving stool consistency. To test this, we designed and performed a study evaluating these effects in response to 3.5 g or 7 g RPS per day for four weeks in healthy individuals in a randomized, double-blind, placebo-controlled clinical trial.
2. Materials and Methods
2.1. Investigational Product
The resistant starch (RS) used in this study was Solnul™ (MSP Starch Products Inc., Carberry, MB, Canada), an unmodified resistant potato starch (RPS; a form of RS Type 2) produced via a unique processing method that preserves RS resulting in a stable RS content of 60% (AOAC 2002.02). The placebo used was fully digestible corn starch (Amioca; Ingredion, Brampton, ON, Canada) and contained no RS [30]. Investigational products were packaged in identical single-serving sachets and labeled in accordance with the randomization codes from Nutrasource Pharmaceutical and Nutraceutical Services (Guelph, ON, Canada) by personnel not involved in the collection of study data to ensure blinding of the study.
2.2. Study Design and Participant Selection
This study was conducted between 30 October 2019 and 6 January 2020 in Guelph, ON, Canada, and recruited participants from the general population in Guelph and the surrounding area. On-site monitoring was conducted according to the Clinical Monitoring Plan. The data management and statistical analyses for this study were conducted according to Standard Operating Procedures based on the International Council for Harmonization (ICH; www.ich.org, accessed on 26 October 2019), Health Canada Natural Health Product Regulations (https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/clinical-trials.html, accessed on 26 October 2019), and the Food and Drug Administration (FDA) regulations and guidance documents (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trials-guidance-documents, accessed on 26 October 2019).
The study protocol and related documents were approved by Canadian Shield Ethics Review Board (REB Tracking Number: 19-10-001; Burlington, ON, Canada) on 29 October 2019. A sample size calculation determined that a minimum total sample of n = 20 in each arm would have power = 0.80 to detect a Cohen’s f effect size of 0.33 between treatment arms and placebo with a nominal α = 0.05 for similar gastrointestinal and microbiome-related outcomes [14,30]. To allow for participant attrition, a total sample size of n = 25 was selected. This study was conducted in accordance with the protocol and with the consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable ICH Good Clinical Practice guidelines, and applicable local and federal laws and regulations. The trial is registered at ClinicalTrials.gov (NCT05242913).
The investigator or investigator’s representative explained the nature of the study to the participant or the participant’s legally authorized representative and answered all questions regarding the study. Participants were informed that their participation was voluntary and that any study report or publication of the study results would not disclose the participant’s identity without specific consent. Participants wishing to participate in the study, or their legally authorized representative, were required to sign a statement of informed consent that met the requirements of local regulations, ICH guidelines, and the research ethics board. The authorized person obtaining the informed consent also signed the informed consent form (ICF). A copy of the ICF was provided to the participant or the participant’s legally authorized representative. Written informed consent was obtained prior to any study-related procedures.
Participants enrolled in this study were healthy adults between 18–69 years of age with a body mass index (BMI) of 18.0 to 34.9 kg/m2 (Table 1). Eligible participants agreed to not any vitamins, minerals, or dietary supplements 14 days prior to the randomization visit until the completion of the final visit since consumption of these products could impact the results for the investigational product. Participants were counseled to follow their habitual diet throughout the study period. Individuals with a BMI over 34.9 kg/m2 were excluded as their health, and any related metabolic changes may impact the results of the study independent of the RPS or placebo. Additionally, individuals with a diagnosis of irritable bowel syndrome (IBS), dyspepsia, significant gastrointestinal disorders or other major diseases were excluded.
Table 1.
Baseline demographic data for the FAS population.
| 7 g RPS Dose | 3.5 g RPS Dose | Placebo | Total | |
|---|---|---|---|---|
| Number of Participants | 24 | 24 | 24 | 72 |
| Age (Year) # | ||||
| Mean (SD) | 36.6 (15.85) | 38.5 (14.63) | 39.1 (16.50) | 38.1 (15.49) |
| Median | 27.5 | 41.5 | 37.5 | 35.0 |
| Min, Max | 19, 64 | 20, 66 | 19, 66 | 19, 66 |
| Sex | ||||
| Male | 9 (37.5%) | 6 (25.0%) | 7 (29.2%) | 22 (30.6%) |
| Female | 15 (62.5%) | 18 (75.0%) | 17 (70.8%) | 50 (69.4%) |
| Race | ||||
| Indigenous | 0 (0.0%) | 0 (0.0%) | 1 (4.2%) | 1 (1.4%) |
| Asian | 3 (12.5%) | 3 (12.5%) | 0 (0.0%) | 6 (8.3%) |
| Black or African Canadian | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| White | 19 (79.2%) | 21 (87.5%) | 23 (95.8%) | 63 (87.5%) |
| Other | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| Ethnicity | ||||
| Hispanic or Latino | 0 (0.0%) | 0 (0.0%) | 2 (8.3%) | 2 (2.8%) |
| Not Hispanic or Latino | 24 (100%) | 24 (100%) | 22 (91.7%) | 70 (97.2%) |
| Height (cm) | ||||
| Mean (SD) | 168.73 (8.919) | 165.60 (7.351) | 167.87 (9.682) | 167.40 (8.684) |
| Median | 167.05 | 165.10 | 166.25 | 166.20 |
| Min, Max | 151.5, 186.9 | 153.5, 186.5 | 151.3, 184.5 | 151.3, 186.9 |
| Weight (kg) | ||||
| Mean (SD) | 69.12 (13.836) | 73.40 (12.281) | 71.20 (13.811) | 71.24 (13.257) |
| Median | 65.20 | 74.60 | 71.00 | 71.40 |
| Min, Max | 47.4, 102.8 | 54.1, 98.2 | 47.0, 105.8 | 47.0, 105.8 |
| BMI (kg/m2) | ||||
| Mean (SD) | 24.10 (3.420) | 26.78 (4.291) | 25.14 (3.735) | 25.34 (3.938) |
| Median | 23.25 | 26.90 | 24.30 | 24.80 |
| Min, Max | 18.8, 31.8 | 19.6, 33.8 | 19.8, 33.3 | 18.8, 33.8 |
Age, sex, race, ethnicity, height, weight, and body mass index (BMI) for full analysis set (FAS) participants at baseline. # Age calculated based on the date of informed consent signed and date of birth. Standard deviation (SD) is indicated.
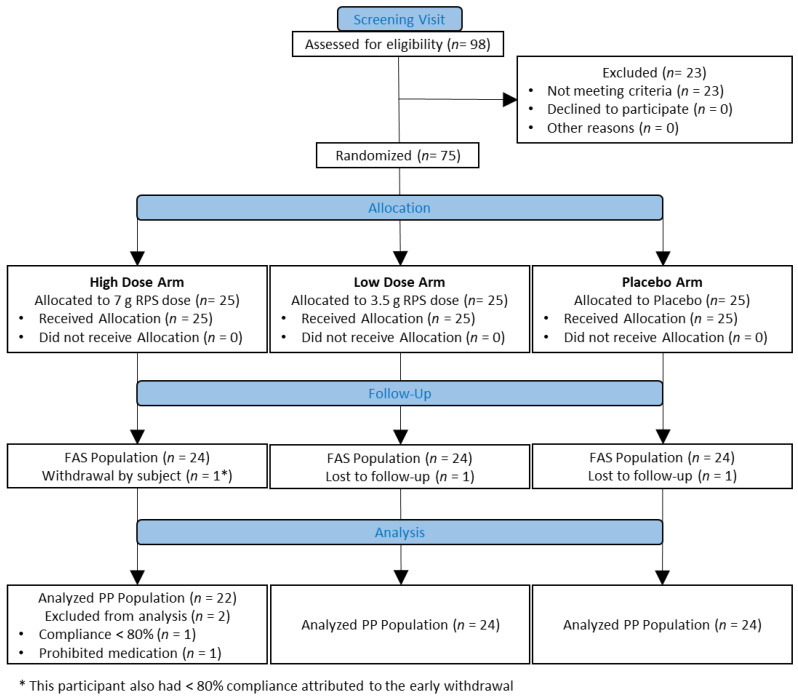
This was a randomized, double-blind, placebo-controlled three-arm parallel-group study (CONSORT Diagram; Figure 1). A total of 98 participants were screened for eligibility, of which 75 participants (25 participants per study arm) were eligible to be enrolled in the study. The study included a screening visit from 30 days up to 14 days prior to randomization, a run-in period of 14–17 days prior to randomization, a baseline visit (Day 0) during which the randomization was performed, and two subsequent study visits at Weeks 1 and 4, respectively (Table 2). During the screening visit, stool/bowel movement records and stool collection instructions and materials (including two fecal sample collection containers (DNA Genotek, Ottawa, ON, Canada)) were provided. During the run-in period, participants recorded their daily bowel habits for 14–17 days in a diary. As fecal sample collection was spontaneous, participants who produced a fecal sample that was collected prior to 72 h before the end of the study were documented, but this was not considered a protocol deviation.
Figure 1.
CONSORT flow diagram. Ninety-eight people were assessed for eligibility, of which 75 were randomized to one of three treatment arms: High dose (7 g Resistant potato starch (RPS) per day), low dose (3.5 g RPS per day), or placebo (Corn starch). Full analysis set (FAS) and per protocol (PP) populations are indicated, including reasons for participant attrition.
Table 2.
Study visits and timeline of events.
| Name | Events | Day | |
|---|---|---|---|
| Visit 1 | Screening | Inclusion/exclusion criteria reviewed, informed consent obtained, medical history and exam, vital signs and blood safety sample taken, fecal collection kits and diaries provided | −30 to −14 days |
| Visit 2 | Baseline | Inclusion/exclusion criteria, dietary supplements and medications, and medical history reviewed, pregnancy test (females only), fecal sample and diaries collected, randomization | Day 0 |
| Visit 3 | Week 1 | Fecal sample and diaries collected, unused study products collected, compliance calculated, and product re-dispensed, review medication and dietary supplement history, new fecal collection kits and diaries dispensed | ~Day 8 (+2 day window) |
| Visit 4 | Week 4 | Fecal sample and diaries collected, unused study products collected and compliance calculated, review medication and dietary supplement history | ~Day 29 (+3 day window) |
Visit number, corresponding event, and respective day number for the major activities during the trial.
Participants collected a fecal sample within 72 h prior to the Day 0 visit and transferred it to the clinic site within 24 h of collection. Stool samples for microbiome analysis were collected using the OMNIgene-Gut kits as per the manufacturer’s instructions. During the baseline visit (Day 0), the participants were randomized to receive one of three study interventions as indicated by the randomization scheme: High dose (7 g resistant potato starch (RPS)), low dose (3.5 g RPS combined with 3.5 g digestible corn starch), or placebo (7 g digestible corn starch). The randomization scheme was generated by Nutrasource Pharmaceutical and Nutraceutical Services using SAS 9.4 PROC PLAN with Seed Number: 1887363180. Seventy-five participants were randomized into 25 blocks, with each block containing 3 participants. The first dose of study intervention was demonstrated by study staff and administered by mixing the product in approximately 125 mL of cool or room temperature water and having the participant drink it immediately before the investigational product settled. Participants were instructed to consume the investigational product in the morning. Bowel habit/daily diaries, stool collection supplies, and a 31-day supply of the study intervention were provided to the study participants during the baseline visit.
At Visit 3 (Week 1), previous bowel habit diaries, unused study interventions, and empty product packaging were collected, and compliance was calculated. New bowel habit diaries and stool collection supplies were provided to the participants. At Visit 4 (Week 4, the final study visit), previous bowel habit diaries, unused study products, and empty packaging were collected, and compliance was calculated. Participants collected fecal samples within 72 h prior to Visit 3 (Week 1) and Visit 4 (Week 4) and transferred them to the clinic site within 24 h of each collection.
Participants returned all sachets, including any open or unopened sachets, during both the 3rd and 4th visits. Compliance was calculated based on the amount of study product consumed compared to the total amount of study product expected to have been consumed for the given duration. Compliance for this study was considered acceptable if participants consumed an average of ≥80% of the study product for the given duration. Safety profiles were based on the safety analysis set (SAF; n = 75), while the full analysis set (FAS; n = 72) included participants who received at least one dose of the study product and had at least one outcome assessment after dosing and the per-protocol population (PP; n = 70) included only those who completed the study with overall compliance with study parameters.
2.3. Safety
The safety population (SAF) consisted of all participants who received at least 1 dose of the study product. Treatment-emergent adverse events (TEAEs) were defined as adverse events (AEs) with the onset time on or after the first dose of the study product. The number and percentage of participants and TEAEs are summarized based on Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC), Preferred Term (PT), severity, and relationship to the study product by study arm. The relationship between the study intervention and each AE was assessed by the study clinician using their best judgment by examining and evaluating the participant based on the temporal relationship to the AE.
2.4. Microbiome Analysis
16Sv4 amplicons generated from fecal samples collected in OMNIgene-Gut kits (DNA Genotek) were sequenced on a MiSeq platform (Illumina, San Diego, CA, USA) at Microbiome Insights (Vancouver, BC, Canada). MiSeq-generated Fastq files were quality-filtered and clustered into 97% similarity operational taxonomic units (OTUs) using the mothur software package and the Greengenes v13.8 database [35]. The resulting dataset had 59,086 OTUs (including those occurring once with a count of 1 or singletons). An average of 30,860 quality-filtered reads were generated per sample. Sequencing quality for read one (R1) and read 2 (R2) was determined using FastQC 0.11.5. Bifidobacterium and Akkermansia were identified a priori as candidate genera that would increase in response to RPS consumption [29,30,36,37].
2.5. Self-Reported Bristol Stool Chart Scoring
Participants scored their bowel movements using the Bristol Stool Form Scale, also known as the Bristol Stool Chart (BSC), where Type 1 = constipation with hard, round stools, Type 2 = lumpy and sausage-like stools, Types 3 and 4 are soft, easily passed ‘normal’ stools, Type 5 = soft blobs of stool with clear-cut edges, Type 6 = stools with mushy consistency and ragged edges, and Type 7 = watery diarrhea [38]. In addition to quantifying extreme scores (i.e., Type 1 or Type 7), scores were grouped into constipation- (i.e., Type 1 or 2) or diarrhea-related (i.e., Type 6 or 7; Types 5, 6 or 7) bowel movement groups. Effects on either end of the scale can be muted when BSC scores are averaged. For example, a participant with equal numbers of Type 1 (constipation) and Type 7 (diarrhea) bowel movements would produce an average score of 4, falsely indicating that this participant experienced normal bowel movements. For this reason, BSC Type scores were not averaged and were kept discrete to evaluate the effects on both ends of the scale. The number of bowel movements meeting the categories above was quantified during the last seven recorded bowel movements to align with the microbiome analysis at 4 weeks, which was based on a stool sample collected 72 h before Visit 4.
2.6. Statistical Analysis
Change in relative abundance was determined by subtracting the baseline relative abundance from the relative abundance at week 4 for both Bifidobacterium and Akkermansia. Outliers were identified using the inter-quartile range (IQR) method, whereby changes in relative abundance greater and less than 1.5 times the IQR are considered outliers ([39,40,41,42]; Excel, Redmond, WA, USA). After removing outliers, RPS-dependent increases in bacterium levels were discretely compared to those in the placebo via Student’s t-tests (Excel). Bristol Stool Chart scores of bowel movements at 4 weeks were compared discretely between the placebo group and 3.5 g or 7 g treatment arms using 2-tailed Fisher’s exact tests (QuickCalcs, GraphPad Software, San Diego, CA, USA). In all instances, p < 0.05 was considered statistically significant.
3. Results
3.1. Participant Characteristics
One participant per treatment arm discontinued the study before Visit 4, and 2 participants in RPS high-dose arm were excluded due to non-compliance (<80% product consumption) and use of study-prohibited medication (Figure 1). Participants enrolled in the study were excluded if they had various gastrointestinal complaints, including a diagnosis of irritable bowel syndrome (IBS). Despite being healthy and excluded for these conditions, participants in each arm experienced a variety of bowel movement types during the run-in period (Table 3).
Table 3.
Baseline Bristol Stool Chart (BSC) scores.
| Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 | Total | |
|---|---|---|---|---|---|---|---|---|
| Placebo | 12 | 34 | 50 | 190 | 92 | 32 | 15 | 429 |
| 3.5 g RPS | 9 | 27 | 100 | 209 | 53 | 46 | 11 | 455 |
| 7 g RPS | 34 | 49 | 50 | 136 | 109 | 40 | 5 | 423 |
Baseline (BSC) scores for all bowel movements recorded during the run-in period (15–17 days, depending on the participant).
3.2. RPS Supplementation Increases Relative Abundance of Bifidobacterium
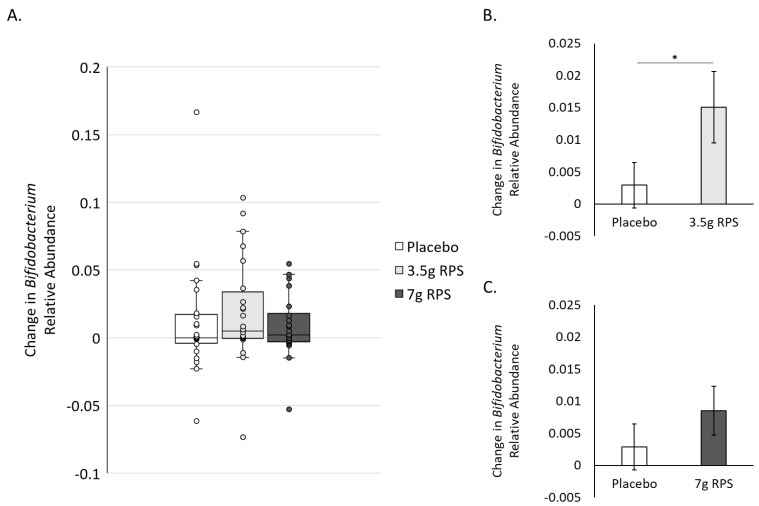
Bifidobacterium is known to be the primary degrader of RPS [43], and the relative abundance of Bifidobacterium increased in stool samples from participants consuming 30 g of RPS daily for three months [30]. We, therefore, asked whether supplementation of 3.5 g of RPS for four weeks increased Bifidobacterium levels greater than placebo. Bifidogenic responses to 3.5 g RPS and placebo were variable (Figure 2A), and we identified three outliers in the 3.5 g RPS group and 4 outliers in the placebo group [39]. After removing outliers [40,41,42], we compared the mean change in relative abundance in Bifidobacterium in the 3.5 g RPS group to the placebo and found that the RPS increase in the relative abundance of Bifidobacterium was significantly greater than placebo (p = 0.038; Figure 2B). The relative abundance of Bifidobacterium also varied in the 7 g RPS group (Figure 2A), with two outliers identified. Mean comparison of changes in the relative abundance of Bifidobacterium in response to 7 g RPS did not show a statistically significant increase compared to placebo (p = 0.14; Figure 2C), although there was a trend towards increasing Bifidobacterium in the 7 g RPS group.
Figure 2.
Increases in the relative abundance of Bifidobacterium after 4 weeks in response to placebo, 3.5 g RPS, or 7 g RPS. (A) Box and whisker plots revealing interquartile range (IQR; box), median (line inside box), and 1.5 times the IQR (error bars), as well as individual data points (circles). (B) The increase in the relative abundance of Bifidobacterium was significantly higher in the 3.5 g RPS group compared to the placebo arm (p = 0.038). (C) The increase in the relative abundance of Bifidobacterium was not significantly higher in the 7 g RPS group (p = 0.14) compared to the placebo arm (Student’s t-test, * p < 0.05).
3.3. RPS Supplementation Increases Relative Abundance of Akkermansia
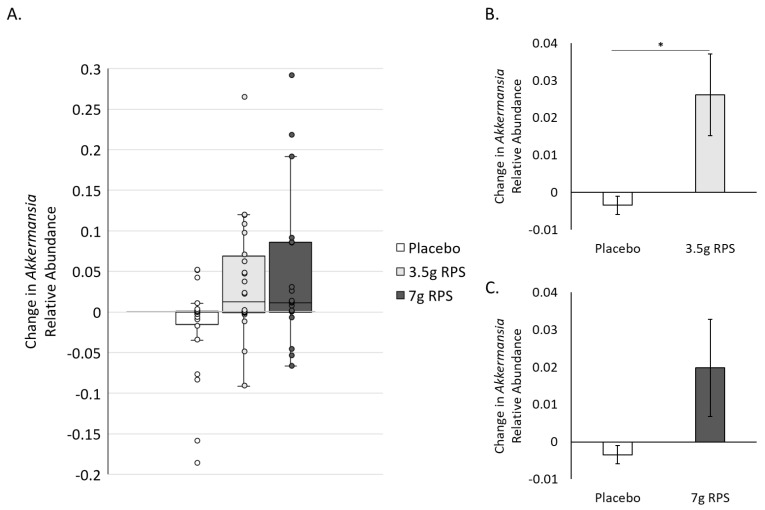
Probiotic supplementation with strains of Bifidobacterium [44,45,46,47,48], supplementation with high amylose maize starch [49], and a blend of RS [37] has been shown to increase levels of Akkermansia, a genus associated with numerous health benefits [50,51]. We asked whether RPS might simultaneously increase the relative abundance of Akkermansia, potentially as a function of Bifidobacterium increases. Changes in the relative abundance of Akkermansia were variable (Figure 3A), yielding 1 outlier in the 3.5 g RPS group and 6 outliers in the placebo group [39]. After removing outliers [40,41,42], we compared the mean change in Akkermansia in the 3.5 g RPS group to the placebo and found that the RPS increase in the relative abundance of Akkermansia was significant (p = 0.014; Figure 3B). Relative abundance of Akkermansia also varied in the 7 g RPS group (Figure 3A), with 2 outliers identified. The mean comparison of changes in Akkermansia in response to 7 g RPS did not show a statistically significant increase compared to placebo (p = 0.056; Figure 3C), although there was a trend towards increasing Akkermansia in the 7 g RPS group.
Figure 3.
Increases in the relative abundance of Akkermansia after 4 weeks in response to placebo, 3.5 g RPS, or 7 g RPS. (A) Box and whisker plots revealing interquartile range (IQR; box), median (line inside box), and 1.5 times the IQR (error bars), as well as individual data points (circles). (B) The increase in the relative abundance of Akkermansia was significantly higher in the 3.5 g RPS group compared to the placebo arm (p = 0.014). (C) The increase in the relative abundance of Akkermansia was not significantly higher in the 7 g RPS group (p = 0.056) compared to the placebo arm (Student’s t-test, * p < 0.05).
3.4. RPS Supplementation Improves Constipation- and Diarrhea-Associated Bowel Movements
The frequency of different Bristol Stool Chart (BSC) types during the last seven bowel movements, corresponding to the week 4 Bifidobacterium and Akkermansia analysis time point, in the 3.5 g RPS and placebo arms were analyzed. There was a significant difference between the number of Type 7 (liquid consistency with no solid pieces) bowel movements at 4 weeks between the 3.5 g RPS and placebo arms (Table 4). Notably, there were no reports of Type 7 bowel movements in the 3.5 g RPS group. There were no significant differences in the number of other diarrhea-associated bowel movement scores, including Type 6 or 7 or Types 5, 6 or 7 (Table 4). While there was no difference in the number of Type 1 scores (separate hard lumps), there was a significant difference in the number of Type 1 or 2 (separate hard lumps and/or lumpy and sausage-like) scores at 4 weeks between the 3.5 g RPS and placebo arms (Table 4). We similarly compared BSC scores at 4 weeks between the 7 g RPS and placebo arms and found that only bowel movements of Type 5, 6 or 7 (soft blobs with clear-cut edges and/or mushy consistency with ragged edges and/or liquid consistency with no solid pieces) were significantly different (Table 5).
Table 4.
The presence of bowel movements scored according to the Bristol Stool Chart (BSC) at 4 weeks in the 3.5 g RPS and placebo arms.
| Type 1 | Not Type 1 | p Value | |
|---|---|---|---|
| Placebo | 3 | 165 | 0.99 |
| 3.5 g RPS | 2 | 166 | |
| Type 1 or 2 | Not Type 1 or 2 | ||
| Placebo | 23 | 145 | 0.0085 |
| 3.5 g RPS | 9 | 159 | |
| Type 7 | Not Type 7 | ||
| Placebo | 6 | 162 | 0.03 |
| 3.5 g RPS | 0 | 168 | |
| Type 6 or 7 | Not Type 6 or 7 | ||
| Placebo | 13 | 155 | 0.35 |
| 3.5 g RPS | 19 | 149 | |
| Type 5, 6 or 7 | Not Type 5, 6 or 7 | ||
| Placebo | 49 | 119 | 0.26 |
| 3.5 g RPS | 39 | 129 |
The last seven bowel movements recorded for each participant (corresponding with a 4-week timepoint) were scored according to the BSC and compared between the 3.5 g RPS and Placebo treatment groups using Fisher’s exact test.
Table 5.
Presence of bowel movements scored according to the Bristol Stool Chart (BSC) at 4 weeks in the 7 g RPS and placebo arms.
| Type 1 | Not Type 1 | p Value | |
|---|---|---|---|
| Placebo | 3 | 165 | 0.13 |
| 7 g RPS | 8 | 146 | |
| Type 1 or 2 | Not Type 1 or 2 | ||
| Placebo | 23 | 145 | 0.44 |
| 7 g RPS | 26 | 128 | |
| Type 7 | Not Type 7 | ||
| Placebo | 6 | 162 | 0.51 |
| 7 g RPS | 3 | 151 | |
| Type 6 or 7 | Not Type 6 or 7 | ||
| Placebo | 13 | 155 | 0.26 |
| 7 g RPS | 7 | 147 | |
| Type 5, 6 or 7 | Not Type 5, 6 or 7 | ||
| Placebo | 49 | 119 | 0.026 |
| 7 g RPS | 28 | 126 |
The last seven bowel movements recorded for each participant (corresponding with a 4-week timepoint) were scored according to the BSC and compared between the 7 g RPS and Placebo treatment groups using Fisher’s exact test.
3.5. Safety
Overall, there were similar incidences of treatment-emergent adverse events (TEAEs) in treatment and placebo arms. Thirty-three participants experienced 56 TEAEs during the study period (Table 6). Most AEs were gastrointestinal (GI) in nature, with 16 participants reporting 24 (42.9%) GI-related AEs, followed by infections, including cold and flu, upper respiratory tract infections and urinary tract infections, reported by 12 participants reporting 12 (21.4%) of these AEs. All TEAEs assessed by the PI as being possibly related to the study products were reported within the gastrointestinal disorders SOC (11 participants [14.7%] reporting 17 events). Of the 11 participants having AEs possibly related to the study product, two participants received a placebo, two received a low dose, and seven received a high dose. The two participants receiving a placebo reported increased flatulence (n = 2), increased bloating (n = 1) and nausea (n = 1). In the low-dose arm, flatulence (n = 1) and bloating (n = 1) were reported as possibly related AEs. In the high-dose arm, the possibly related AEs were increased belching (n = 2), increased flatulence (n = 6), increased bloating (n = 2), and abdominal pain (n = 1). The gastrointestinal events reported as possibly related were similar in type between all groups, and the number of participants reporting these events was similar between the placebo arm and the low-dose arm. There were no serious AEs or deaths during the study period.
Table 6.
Summary of TEAEs in the FAS population.
| 7 g RPS Dose | 3.5 g RPS Dose | Placebo | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Participants (n = 25) |
Events (n = 14) |
Participants (n = 25) |
Events (n = 22) |
Participants (n = 25) |
Events (n = 20) |
Participants (n = 72) |
Events (n = 56) |
|
| Overall | 10 (40.0%) | 14 (100%) | 11 (44.0%) | 22 (100%) | 12 (50.0%) | 20 (100%) | 33 (44.0%) | 56 (100%) |
| Serious | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severity | ||||||||
| Mild | 9 (36.0%) | 12 (85.7%) | 6 (24.0%) | 9 (40.9%) | 10 (40.0%) | 15 (75.0%) | 25 (33.3%) | 36 (64.3%) |
| Moderate | 1 (4.0%) | 1 (7.1%) | 8 (32.0%) | 12 (54.5%) | 4 (16.0%) | 5 (25.0%) | 13 (17.3%) | 18 (32.1%) |
| Severe | 1 (4.0%) | 1 (7.1%) | 1 (4.0%) | 1 (4.5%) | 0 | 0 | 2 (2.7%) | 2 (3.6%) |
| Relationship | ||||||||
| Related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Possibly related | 2 (8.0%) | 2 (14.3%) | 7 (28.0%) | 10 (45.5%) | 2 (8.0%) | 5 (25.0%) | 11 (14.7%) | 17 (30.4%) |
| Not related | 9 (36.0%) | 12 (85.7%) | 7 (28.0%) | 12 (54.5%) | 12 (48.0%) | 15 (75.0%) | 28 (37.3%) | 39 (69.6%) |
Treatment-emergent adverse events (TEAEs) in the full analysis set (FAS) population were neither serious nor fatal, and no TEAE led to study discontinuation. Participant numbers in the Severity and Relationship columns exceed the number in the Overall column because some participants experienced multiple events with discrete severity and/or relationship to the interventions. The majority of TEAEs were determined to be unrelated to the interventions, while less than a third were possibly related to the interventions, and no TEAEs were definitively related to the interventions.
4. Discussion
We tested the prebiotic properties of resistant potato starch (RPS) by administering 3.5 g or 7 g of RPS per day for 4 weeks and measuring the relative abundance of Bifidobacterium and scores of bowel movement consistency. Participants in the 3.5 g RPS arm demonstrated significantly higher increases in the relative abundance of Bifidobacterium compared to the placebo arm, along with fewer Type 7 (liquid consistency with no solid pieces) and Type 1 or 2 (separate hard lumps and/or lumpy and sausage-like) bowel movements compared to the placebo groups, thereby meeting prebiotic criteria [34]. Additionally, participants in the 3.5 g RPS arm displayed statistically significant increases in Akkermansia levels compared to the placebo group, further supporting the role that RPS can play in promoting the growth of health-associated microbes. These microbiome improvements are consistent with previous studies of RPS at high doses (i.e., 30 g/day), which increased Bifidobacterium [29,30,36] and promoted normal glucose metabolism [14]. Other sources of RS2, including high amylose maize starch [18,22] and green banana starch [52], have similarly been evaluated at high doses but studies evaluating low (i.e., <10 g/day) doses have not been reported.
Participants in the 7 g RPS arm had responses similar to those in the 3.5 g arm, but only improvements in diarrhea-associated bowel movement scores met statistical significance. Interestingly, while the relative abundance of Bifidobacterium increased in response to 7 g of RPS, the average increase was approximately 33% lower than what was observed in the 3.5 g arm. Curiously, this was also the case for Akkermansia. Several factors likely influenced this discrepancy, including the diets of participants, which were not controlled as part of the clinical trial. Dietary patterns improved predictions of gut microbiota species abundance in people undergoing daily monitoring of both diet and gut microbiome composition [53], suggesting that some of the variability in changes in Bifidobacterium and Akkermansia relative abundance could be due to participants consuming atypical foods. The median age of participants in the 7 g arm was 27.5 years, while the median ages in the placebo and 3.5 g arms were 37.5 and 41.5 years, respectively. Despite the mean ages being comparable, it is possible that age-related differences contribute to the lack of significant microbiome and bowel movement improvements in this group.
The genus Akkermansia, including Akkermansia muciniphilia, primarily utilizes host mucus-derived glycans as an energy source [54], making our findings that low-dose RPS administration increased Akkermansia levels surprising. Akkermansia muciniphilia has gained attention for promoting healthy metabolism and has been developed into probiotic and postbiotic formats [50,51]. Clinical trials evaluating the effects of administering probiotic strains of Bifidobacterium also show increases in Akkermansia levels [44,45,46,47,48], suggesting that the RPS-dependent increases could be a consequence of Bifidobacterium activities or other RPS-dependent changes in the microbial environment. In the absence of Bifidobacterium increases, high doses of prebiotic inulin produced modest increases in Akkermansia in hemodialysis patients [55], as did high doses of high amylose maize starch in insulin-resistant individuals [49] and an RS blend containing RPS, green banana starch, and apple pectin [37], indicating that prebiotics may support Akkermansia independent of probiotic-associated bacterium increases. Intriguingly, the magnitude of the increase in the relative abundance of Akkermansia (0.026 +/− 0.011) was greater than the Bifidobacterium increase (0.015 +/− 0.006) for the 3.5 g RPS dose (Figure 2A and Figure 3A). Cross-feeding between Bifidobacterium and butyrate-producing bacteria has been hypothesized to enhance mucin secretion, thereby increasing Akkermansia, suggesting that the beneficial actions of Bifidobacterium are more important in promoting Akkermansia levels than relative Bifidobacterium abundance per se [46].
This novel study is the first to demonstrate increases in health-associated bacteria Bifidobacterium and Akkermansia in response to a low dose of RS, as were the improvements in stool form. Previous studies using RS from a variety of sources have found improvements in bowel movement characteristics at higher dosage levels (i.e., 17–30 g/day; [10,11,12,13]). Oligosaccharide prebiotics like lactulose reduce constipation by increasing stool bulk via microbial cell growth and increasing luminal osmotic pressure in the small intestines, which can lead to diarrhea when administered at too high a dose (i.e., 20–40 g/day) or for too long a duration [56]. Participants consuming a 3.5 g RPS dose had significantly fewer diarrhea- and constipation-associated bowel movements after 4 weeks compared to the placebo group (Table 5), indicating that supplementation with RPS relieves constipation without promoting excessively soft stools. Importantly, Type 7 (liquid diarrhea with no solid pieces) bowel movements were completely absent in the 3.5 g arm after 4 weeks, demonstrating that this dose of RPS effectively normalizes stool form. Reductions in both diarrhea- and constipation-associated bowel movements are uncommon among dietary fiber intervention studies, and poorly fermentable psyllium is the best example of a fiber that promotes improvements in participants who experience both overly firm and overly loose stools, effects attributed to the water-holding and fecal bulking properties of this fiber [57]. To our knowledge, this is the first report of a low-dose fermentable fiber normalizing stool form in a clinical study of healthy individuals.
There are limitations to this study, including the short duration. Bowel movement records were not kept beyond the 4-week timepoint, making it difficult to connect changes in bowel movement scores to changes in the composition of the gut microbiota derived from a stool sample collected at the end of the study. We compensated for this limitation by analyzing the final seven bowel movements recorded for each participant, which we reasoned was an appropriate approximation of the bowel movements consistent with the microbiota composition at 4 weeks. A longer study would also be useful to confirm the stability of these findings. It is possible that baseline levels of bacteria and stool consistency reflect cessation of vitamin and/or dietary supplement consumption, and future studies would benefit from recruiting participants earlier for a longer ‘wash-out’ period. The short duration also limited the investigation into the effects of RPS on the gut microbiota, which has pronounced beneficial effects at higher doses for longer durations [30]. Another limitation was the exclusion of individuals clinically diagnosed with digestive symptoms, which was necessary to evaluate the efficacy of formulation into dietary supplements. Our study indicates that RPS could be beneficial for individuals suffering from IBS, especially those who alternate between diarrheal and constipated bowel movements or experience a ‘mix’ of symptoms [58]. Future studies could benefit from a cross-over design, in which participants consume each dose with wash-out periods in between, facilitating dose-response comparisons within individuals. Such a study could better account for the influences of dietary preferences, host glycan production, and baseline microbiome-dependent effects.
5. Conclusions
We demonstrate that RPS has a prebiotic effect at a 3.5 g daily dose, which is comparable to prebiotic oligosaccharide doses (i.e., 3–7 g/day; [59]). RPS significantly increased Bifidobacterium and Akkermansia levels and decreased diarrhea- and constipation-associated bowel movements compared to the placebo, meeting the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus criteria for the definition of a prebiotic [34]. Bifidobacterium and Akkermansia increases in the 7 g RPS arm trended positively towards statistical significance compared to the placebo, consistent with the 3.5 g dose, and improvements in bowel movement scores were significantly different from the placebo group in both treatment arms. RPS was safe and well tolerated at both a 3.5 g daily dose and a 7 g daily dose. Future studies examining the other host benefits at low RPS doses and benefits in healthy people and patients with IBS are warranted.
Acknowledgments
We thank the clinical trial participants, as well as Saif Abdulwahhab, Stephanie Recker, Ana Samborski, Sandra Pacheco (Nutrasource, Guelph, ON, Canada), Karl Moran, Pedro Dimitriu, and Khoi Nguyen (Microbiome Insights, Vancouver, BC, Canada). We also thank Anna Bearpark, Diane Clayton, Belinda Gray, Natasha Haskey, Madhura Maiya, Ida Gisela Pantoja Feliciano, and Noelle Patno, for the helpful comments that greatly improved this manuscript.
Author Contributions
Conceptualization, J.R.B. and J.B.; methodology, J.R.B. and J.B.; trial supervision, J.B.; formal analysis, J.R.B.; investigation, J.R.B.; resources, J.R.B.; data curation, J.R.B. and J.B.; writing—original draft preparation, J.R.B.; writing—review and editing, J.R.B., J.B., S.V.H. and M.J.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Canadian Shield Ethics Review Board (Burlington, ON, Canada; REB Tracking Number: 19-10-001 on 29 October 2019).
Informed Consent Statement
Informed consent was obtained from all participants (or their legally authorized representative) involved in the study.
Data Availability Statement
The data that support the findings of this study are available from MSP Starch Products Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of MSP Starch Products Inc. Individual patient data is unavailable as per the informed consent form and the interventional study protocol registration.
Conflicts of Interest
Jason R. Bush is employed by and Michelle J. Alfa and Scott V. Harding previously provided consulting services for MSP Starch Products Inc., Carberry, MB, Canada. Joshua Baisley is employed by Nutrasource Pharmaceutical and Nutraceutical Services, Guelph, ON, the company that MSP Starch Products contracted to conduct the study. MSP Starch Products Inc.’s sister company McPharma Biotech holds relevant patents US11058711B2, CA3024201A1, and AU2017294806A1.
Funding Statement
This research and the APC were funded by MSP Starch Products Inc.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Englyst H.N., Kingman S.M., Hudson G.J., Cummings J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996;75:749–755. doi: 10.1079/BJN19960178. [DOI] [PubMed] [Google Scholar]
- 2.Sajilata M., Singhal R.S., Kulkarni P.R. Resistant Starch? A Review. Compr. Rev. Food Sci. Food Saf. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 3.Patterson M.A., Maiya M., Stewart M.L. Resistant Starch Content in Foods Commonly Consumed in the United States: A Narrative Review. J. Acad. Nutr. Diet. 2020;120:230–244. doi: 10.1016/j.jand.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Deehan E.C., Yang C., Perez-Muñoz M.E., Nguyen N.K., Cheng C.C., Triador L., Zhang Z., Bakal J.A., Walter J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe. 2020;27:389–404.e6. doi: 10.1016/j.chom.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Cummings J.H., Englyst H.N. Measurement of starch fermentation in the human large intestine. Can. J. Physiol. Pharmacol. 1991;69:121–129. doi: 10.1139/y91-018. [DOI] [PubMed] [Google Scholar]
- 6.Landon S., Colyer C.G.B., Salman H. The Resistant Starch Report. Food Australia Supplement. 2012. [(accessed on 27 February 2023)]. Available online: https://www.ingredion.com/content/dam/ingredion/pdf-downloads/apac/APAC_2013_Hi-maize_The_Resistant_Starch_Report.pdf.
- 7.Bird A.R., Conlon M.A., Christophersen C.T., Topping D.L. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef. Microbes. 2010;1:423–431. doi: 10.3920/BM2010.0041. [DOI] [PubMed] [Google Scholar]
- 8.Miketinas D.C., Shankar K., Maiya M., Patterson M.A. Usual Dietary Intake of Resistant Starch in US Adults from NHANES 2015–2016. J. Nutr. 2020;150:2738–2747. doi: 10.1093/jn/nxaa232. [DOI] [PubMed] [Google Scholar]
- 9.Baghurst P.A., Baghurst K.I., Record S.J. Dietary fibre, non-starch polysaccharides and resistant starch: A review. Food Aust. 1996;48:S3–S35. [Google Scholar]
- 10.Cummings J.H., Beatty E.R., Kingman S.M., Bingham S.A., Englyst H.N. Digestion and physiological properties of resistant starch in the human large bowel. Br. J. Nutr. 1996;75:733–747. doi: 10.1079/BJN19960177. [DOI] [PubMed] [Google Scholar]
- 11.Maki K.C., Sanders L., Reeves M.S., Kaden V.N., Rains T.M., Cartwright Y. Beneficial effects of resistant starch on laxation in healthy adults. Int. J. Food Sci. Nutr. 2009;60:296–305. doi: 10.1080/09637480903130538. [DOI] [PubMed] [Google Scholar]
- 12.Khosroshahi H.T., Vaziri N.D., Abedi B., Asl B.H., Ghojazadeh M., Jing W., Vatankhah A.M. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 2018;22:492–500. doi: 10.1111/hdi.12653. [DOI] [PubMed] [Google Scholar]
- 13.Cassettari V.M.G., Machado N.C., Lourenção P.L.T.D.A., Carvalho M.A., Ortolan E.V.P. Combinations of laxatives and green banana biomass on the treatment of functional constipation in children and adolescents: A randomized study. J. Pediatr. (Rio J.) 2019;95:27–33. doi: 10.1016/j.jped.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Alfa M.J., Strang D., Tappia P.S., Olson N., DeGagne P., Bray D., Murray B.-L., Hiebert B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018;4:260. doi: 10.3389/fmed.2017.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodinham C.L., Smith L., Wright J., Frost G.S., Robertson M.D. Dietary Fibre Improves First-phase Insulin Secretion in Overweight Individuals. PLoS ONE. 2012;7:e40834. doi: 10.1371/journal.pone.0040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargari B.P., Namazi N., Khalili M., Sarmadi B., Jafarabadi M.A., Dehghan P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement. Ther. Med. 2015;23:810–815. doi: 10.1016/j.ctim.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Karimi P., Farhangi M.A., Sarmadi B., Gargari B.P., Javid A.Z., Pouraghaei M., Dehghan P. The Therapeutic Potential of Resistant Starch in Modulation of Insulin Resistance, Endotoxemia, Oxidative Stress and Antioxidant Biomarkers in Women with Type 2 Diabetes: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2015;68:85–93. doi: 10.1159/000441683. [DOI] [PubMed] [Google Scholar]
- 18.Peterson C.M., Beyl R.A., Marlatt K.L., Martin C.K., Aryana K.J., Marco M.L., Martin R.J., Keenan M.J., Ravussin E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018;108:492–501. doi: 10.1093/ajcn/nqy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson M.D., Bickerton A.S., Dennis A.L., Vidal H., Frayn K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005;82:559–567. doi: 10.1093/ajcn/82.3.559. [DOI] [PubMed] [Google Scholar]
- 20.Maki K.C., Pelkman C.L., Finocchiaro E.T., Kelley K.M., Lawless A.L., Schild A.L., Rains T.M. Resistant Starch from High-Amylose Maize Increases Insulin Sensitivity in Overweight and Obese Men. J. Nutr. 2012;142:717–723. doi: 10.3945/jn.111.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston K.L., Thomas E., Bell J.D., Frost G.S., Robertson M.D. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet. Med. 2010;27:391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 22.Dainty S.A., Klingel S.L., E Pilkey S., McDonald E., McKeown B., Emes M.J., Duncan A.M. Resistant Starch Bagels Reduce Fasting and Postprandial Insulin in Adults at Risk of Type 2 Diabetes. J. Nutr. 2016;146:2252–2259. doi: 10.3945/jn.116.239418. [DOI] [PubMed] [Google Scholar]
- 23.Gower B.A., Bergman R., Stefanovski D., Darnell B., Ovalle F., Fisher G., Sweatt S.K., Resuehr H.S., Pelkman C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. 2016;13:2. doi: 10.1186/s12986-016-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardá F.A.H., Giuntini E.B., Gomez M.L.P., Lui M.C.Y., Negrini J.A., Tadini C.C., Lajolo F.M., Menezes E.W. Impact of resistant starch from unripe banana flour on hunger, satiety, and glucose homeostasis in healthy volunteers. J. Funct. Foods. 2016;24:63–74. doi: 10.1016/j.jff.2016.04.001. [DOI] [Google Scholar]
- 25.Menezes E.W., Dan M.C.T., Cardenette G.H.L., Goñi I., Bello-Pérez L.A., Lajolo F.M. In Vitro Colonic Fermentation and Glycemic Response of Different Kinds of Unripe Banana Flour. Plant Foods Hum. Nutr. 2010;65:379–385. doi: 10.1007/s11130-010-0190-4. [DOI] [PubMed] [Google Scholar]
- 26.Blé-Castillo J.L., Aparicio-Trápala M.A., Francisco-Luria M.U., Córdova-Uscanga R., Rodríguez-Hernández A., Méndez J.D., Díaz-Zagoya J.C. Effects of Native Banana Starch Supplementation on Body Weight and Insulin Sensitivity in Obese Type 2 Diabetics. Int. J. Environ. Res. Public Health. 2010;7:1953–1962. doi: 10.3390/ijerph7051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake A.M., Coughlan M.T., Christophersen C.T., Snelson M. Resistant Starch as a Dietary Intervention to Limit the Progression of Diabetic Kidney Disease. Nutrients. 2022;14:4547. doi: 10.3390/nu14214547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamloo M., Mollard R., Wang H., Kingra K., Tangri N., MacKay D. A randomized double-blind cross-over trial to study the effects of resistant starch prebiotic in chronic kidney disease (ReSPECKD) Trials. 2022;23:72. doi: 10.1186/s13063-022-06009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter N.T., Schmidt A.W., Venkataraman A., Kim K.S., Waldron C., Schmidt T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. Mbio. 2019;10:e02566-18. doi: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfa M.J., Strang D., Tappia P.S., Graham M., Van Domselaar G., Forbes J.D., Laminman V., Olson N., DeGagne P., Bray D., et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2017;37:797–807. doi: 10.1016/j.clnu.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Kemp J.A., Dos Santos H.F., de Jesus H.E., Esgalhado M., de Paiva B.R., Azevedo R., Stenvinkel P., Bergman P., Lindholm B., Ribeiro-Alves M., et al. Resistant Starch Type-2 Supplementation Does Not Decrease Trimethylamine N-Oxide (TMAO) Plasma Level in Hemodialysis Patients. J. Am. Nutr. Assoc. 2022;41:788–795. doi: 10.1080/07315724.2021.1967814. [DOI] [PubMed] [Google Scholar]
- 32.Fong J.V.N., Miketinas D., Moore L.W., Nguyen D.T., Graviss E.A., Ajami N., Patterson M.A. Precision Nutrition Model Predicts Glucose Control of Overweight Females Following the Consumption of Potatoes High in Resistant Starch. Nutrients. 2022;14:268. doi: 10.3390/nu14020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laffin M.R., Tayebi Khosroshahi H., Park H., Laffin L.J., Madsen K., Kafil H.S., Abedi B., Shiralizadeh S., Vaziri N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019;23:343–347. doi: 10.1111/hdi.12753. [DOI] [PubMed] [Google Scholar]
- 34.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 35.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkataraman A., Sieber J.R., Schmidt A.W., Waldron C., Theis K.R., Schmidt T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4:33. doi: 10.1186/s40168-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanes D., Nowinski B., Lamb J.J., Larson I.A., McDonald D., Knight R., Song S.J., Patno N. The gastrointestinal and microbiome impact of a resistant starch blend from potato, banana, and apple fibers: A randomized clinical trial using smart caps. Front. Nutr. 2022;9:987216. doi: 10.3389/fnut.2022.987216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis S.J., Heaton K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 39.Tukey J. Exploratory Data Analysis. 1st ed. Addison-Wesley Pub Co.; Reading, MA, USA: 1977. pp. 29–38. [Google Scholar]
- 40.Timmer C., Davids M., Nieuwdorp M., Levels J., Langendonk J., Breederveld M., Mozafari N.A., Langeveld M. Differences in faecal microbiome composition between adult patients with UCD and PKU and healthy control subjects. Mol. Genet. Metab. Rep. 2021;29:100794. doi: 10.1016/j.ymgmr.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caverly L.J., Lu J., Carmody L.A., Kalikin L.M., Shedden K., Opron K., Azar M., Cahalan S., Foster B., VanDevanter D.R., et al. Measures of Cystic Fibrosis Airway Microbiota during Periods of Clinical Stability. Ann. Am. Thorac. Soc. 2019;16:1534–1542. doi: 10.1513/AnnalsATS.201903-270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castaño-Rodríguez N., Underwood A., Merif J., Riordan S.M., Rawlinson W.D., Mitchell H.M., Kaakoush N.O. Gut Microbiome Analysis Identifies Potential Etiological Factors in Acute Gastroenteritis. Infect. Immun. 2018;86:e00060-18. doi: 10.1128/IAI.00060-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMartino P., Cockburn D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2019;61:66–71. doi: 10.1016/j.copbio.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Toscano M., De Grandi R., Stronati L., De Vecchi E., Drago L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017;23:2696–2704. doi: 10.3748/wjg.v23.i15.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hibberd A., Yde C., Ziegler M., Honoré A., Saarinen M., Lahtinen S., Stahl B., Jensen H., Stenman L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes. 2019;10:121–135. doi: 10.3920/BM2018.0028. [DOI] [PubMed] [Google Scholar]
- 46.Ryan J.J., Patno N. Short-Term Tolerability, Safety, and Gut Microbial Composition Responses to a Multi-Strain Probiotic Supplement: An Open-Label Study in Healthy Adults. Integr. Med. 2021;20:24–34. [PMC free article] [PubMed] [Google Scholar]
- 47.Dizman N., Hsu J., Bergerot P.G., Gillece J.D., Folkerts M., Reining L., Trent J., Highlander S.K., Pal S.K. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 2020;10:79–86. doi: 10.1002/cam4.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C.-H., Yen H.-R., Lu W.-L., Ho H.-H., Lin W.-Y., Kuo Y.-W., Huang Y.-Y., Tsai S.-Y., Lin H.-C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients with Type 1 Diabetes Mellitus. Front. Endocrinol. 2022;13:754401. doi: 10.3389/fendo.2022.754401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maier T.V., Lucio M., Lee L.H., VerBerkmoes N.C., Brislawn C.J., Bernhardt J., Lamendella R., McDermott J.E., Bergeron N., Heinzmann S.S., et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio. 2017;8:e01343-17. doi: 10.1128/mBio.01343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 51.Cani P.D., Depommier C., Derrien M., Everard A., de Vos W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022;19:625–637. doi: 10.1038/s41575-022-00631-9. [DOI] [PubMed] [Google Scholar]
- 52.Costa E.S., França C.N., Fonseca F.A.H., Kato J.T., Bianco H.T., Freitas T.T., Fonseca H.A.R., Neto A.M.F., Izar M.C. Beneficial effects of green banana biomass consumption in patients with pre-diabetes and type 2 diabetes: A randomised controlled trial. Br. J. Nutr. 2019;121:1365–1375. doi: 10.1017/S0007114519000576. [DOI] [PubMed] [Google Scholar]
- 53.Johnson A.J., Vangay P., Al-Ghalith G.A., Hillmann B.M., Ward T.L., Shields-Cutler R.R., Kim A.D., Shmagel A.K., Syed A.N., Walter J., et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe. 2019;25:789–802.e5. doi: 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Derrien M., Vaughan E.E., Plugge C.M., de Vos W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 55.Biruete A., Cross T.-W.L., Allen J.M., Kistler B.M., de Loor H., Evenepoel P., Fahey G.C., Bauer L., Swanson K.S., Wilund K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021;31:512–522. doi: 10.1053/j.jrn.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karakan T., Tuohy K.M., Solingen G.J.-V. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021;8:672925. doi: 10.3389/fnut.2021.672925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McRorie J.W., Jr., McKeown N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017;117:251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Oka P., Parr H., Barberio B., Black C.J., Savarino E.V., Ford A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5:908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 59.Reimer R.A., Soto-Vaca A., Nicolucci A.C., Mayengbam S., Park H., Madsen K.L., Menon R., Vaughan E.E. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020;111:1286–1296. doi: 10.1093/ajcn/nqaa074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from MSP Starch Products Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of MSP Starch Products Inc. Individual patient data is unavailable as per the informed consent form and the interventional study protocol registration.