Abstract
Cancer is a hard-to-treat disease with a high reoccurrence rate that affects health and lives globally. The condition has a high occurrence rate and is the second leading cause of mortality after cardiovascular disorders. Increased research and more profound knowledge of the mechanisms contributing to the disease’s onset and progression have led to drug discovery and development. Various drugs are on the market against cancer; however, the drugs face challenges of chemoresistance. The other major problem is the side effects of these drugs. Therefore, using complementary and additional medicines from natural sources is the best strategy to overcome these issues. The naturally occurring phytochemicals are a vast source of novel drugs against various ailments. The modes of action by which phytochemicals show their anti-cancer effects can be the induction of apoptosis, the onset of cell cycle arrest, kinase inhibition, and the blocking of carcinogens. This review aims to describe different phytochemicals, their classification, the role of phytochemicals as anti-cancer agents, the mode of action of phytochemicals, and their role in various types of cancer.
Keywords: phytochemicals, phenolic compounds, cancer therapeutics, natural products, cell signaling
1. Introduction
Cancer has emerged as a major health issue and is known to be the most prevalent disease after cardiovascular diseases. In 2018, around 18 million cancer cases emerged globally; this number is estimated to increase to more than 23 million new cases annually by 2030 [1]. The disease is hard to treat and has a high chance of reversal after treatment. The presently accessible cancer treatment involves the removal by surgery and radiotherapy of the biomass accumulated by the cancer, and the procedure is followed by chemotherapy. The chemotherapeutic treatments include various antimetabolites, DNA-interacting agents, hormones, and molecular targeting agents [2]. Chemotherapy is effective, yet it faces major challenges such as chemoresistance by cancer cells, recurrence, and toxicity exerted on normal cells, ultimately impairing life quality. Thus, many rely on complementary and alternative medicine (CAM) [3]. The primary area of research in anti-cancer therapy is chemoprevention, focusing on numerous aspects ranging from nutritional to pharmacological factors. To tackle the problems associated with current therapies in cancer treatment, there is still a search for anticancer agents with enhanced efficiency and minimal side effects [4]. A major task in cancer management is overpowering chemoresistance and the failure of current chemotherapies. Resistance to chemotherapy is associated with modulated metabolism in cancer [5]. The compounds have also gained FDA approval for administration in regulated amounts [6]. Figure 1 depicts the use of natural compounds to treat various human ailments. Therefore, the metabolic modulations render the cancer cells more resistant to chemotherapy and increase at an enhanced rate [7]. Resistance to drugs in cancer is closely associated with an increase in glycolysis, even under sufficient oxygen conditions [8]. The colon cancer cells exhibiting chemoresistance show reduced production of ATP and increased aerobic glycolysis. Recent research focuses on identifying the genes responsible for providing chemoresistance and finding a safe and effective method to overcome cancer and drug resistance.
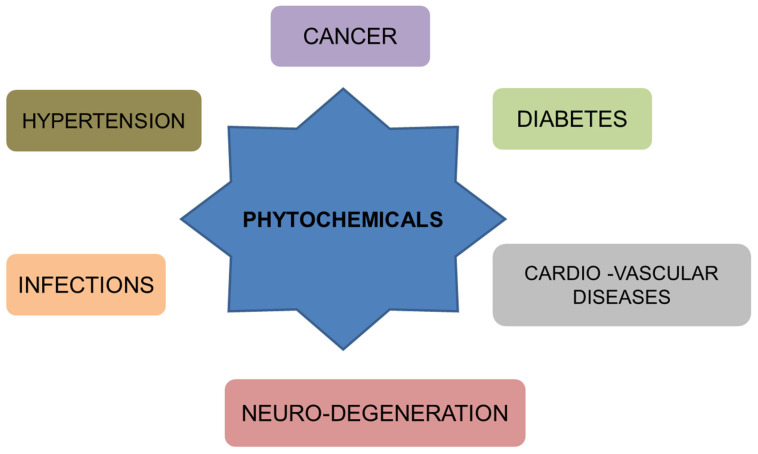
Figure 1.
Therapeutic potential of phytochemicals in different human ailments.
Phytochemicals are naturally occurring chemicals derived from plants, and various naturally occurring compounds have shown promising results in various human ailments with no adverse effects. Phytochemicals and their derivatives are biologically active compounds and have shown anti-cancer effects [9,10,11,12]. The development of phytochemical-based anti-cancer agents involves the extraction, separation, and purification of different compounds. The separated compounds are further tested on various cell lines in vitro and in vivo. The traditional knowledge that involved the selection of plants, collection methods, preparation of drugs, and their use was passed on from generation to generation. The drugs were used in various forms, such as teas, powders, formulations, decoctions, etc. [13,14], until the 18th century. The first breakthrough in drug discovery was the isolation of an analgesic from the plant Papaver somniferum, known as morphine. Afterward, many other drugs were derived from plants, including cocaine from Erythroxylum coca, aspirin from Salix sp, quinine from Cinchona officinalis, digitoxin from Digitalis purpurea, and many more having pharmacological activities [13,15,16]. Some of the most widely used anti-cancer agents derived from plant sources are Taxol, Demecolcine, Colcemid, Paclitaxel, etc. [17].
Plants are a rich source of phytochemicals and chemical entities and have many therapeutic applications [18]. Although modern and easy chemotherapeutic drugs offer first-line treatment, the problem associated with them is their various side effects. Therefore, researchers are interested in treatments with minimal side effects [19]. The phytochemicals effectively target different cancers and minimize various hallmarks of cancer, reducing its intensity. The chemo-protective roles of the phytochemicals are exerted by modulating the signaling pathways involved in cancer. This is found to have connections with the apoptosis induction and suppression of the epithelial to mesenchymal EMT, thereby resulting in the blockage of the metastatic behavior of cancer cells [19]. The phytochemicals interfere with various signaling cascades such as MAPK pathway, nuclear factor kappa B (NF-κB) signaling, PI3K-mTOR pathway, etc. [20,21]. The natural compounds also interfere with some of the protein kinases overexpressed in cancers, such as MARKs, AMPKs, PDKs, and SPHKs. Inhibition of these protein kinases with natural compounds provides a safe and effective approach in cancer therapeutics [11,20,22,23].
The phytochemicals also target the cancer stem cells, affecting the cells’ sensitivity toward chemotherapeutic drugs [24]. Phytochemicals have also shown modulatory metabolic properties in cancer cells by governing different steps in the cancer signaling pathway [25]. The chemicals can also modulate the membrane potential of the mitochondrial membrane and control the mitochondrial pathways [26]. The natural compounds have also shown immunoprotective effects. The phytochemicals modulate the immunosuppressive behavior of the cancer cells by modulating the T-regulatory (Treg) cells. Some of the natural products with immune-modulatory effects are [27]. This review elaborates on the classification of phytochemicals and the anti-cancer roles of phytochemicals.
2. Phytochemicals
Phytochemicals are very active constituents and are abundant in nature. As mentioned above, they are grouped and have significant roles in preventing various diseases. The use of these phytochemicals is done in a combination of multiple phytochemicals and other drugs as well [9,10,11,12,13,14]. Phytochemicals exhibit a wide range of therapeutic roles, including antioxidant, anti-inflammatory, anti-diabetic, analgesic, anti-cancer, neuroprotective, and anti-microbial activities [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Phytochemicals are an essential source for the development and discovery of new potent drugs [48]. The effects include apoptosis, alterations in signaling pathways, cell cycle blockage, DNA damage, etc. [28].
Various anti-cancer agents originating from plant sources have found their use and approval, such as vincristine, taxol, paclitaxel, camptothecin derivatives, chinconine, etc. [29]. Various studies have shown that the compound curcumin (originating from the roots of Curcuma longa L.) shows anticancer effects by inducing apoptosis, thereby inhibiting the proliferation of cancer cells and resulting in cell cycle arrest in various cancer cell lines [30]. Some organosulfur components obtained from the Allium sativum L. plant, such as S-allylcysteine, show retarding effects on the growth of the tumor in various in vivo models [31]. Epigallocatechin-3-gallate (EGCG) from green tea also shows anti-cancer and anti-microbial effects and is a very vital phytochemical [32,33]. The Catharanthus roseus (L.) plant is a rich source of alkaloids such as vinblastine and vincristine, which are used in the current treatment of various types of cancer such as breast cancer (BC), lung cancer, lymphomas, and leukemia [34]. Gymnemagenol is obtained from Gymnema sylvestre and shows promising anti-cancer potential against hepatic cancer cell lines. MTT assay to estimate the anti-proliferative activity of the phytochemical against HeLa cell lines was performed, and gymnemagenol showed an IC50 value of 37 μg/m [35]. In another study, baicalein, isolated from Oroxylumindicum, exhibited an antitumor effect on human cancer cell lines by inhibiting the HL-60 cell line proliferation [36]. Antitumor activity of various phytochemicals has been reported and is undergoing clinical trials. The compounds are at different stages of chemical trials for cancer. Table 1 lists the phytochemicals tested in clinical trials. Some of the phytochemicals showing therapeutic effects are shown in Figure 2a,b.
Table 1.
Some of the phytochemicals used against cancer, the methodology, and the final outcomes.
| Phytochemical | Cancer | Interventions | Effect | References |
|---|---|---|---|---|
| Allium sativum | Colorectal, liver, and pancreatic cancer patients Colorectal ademas |
500 mg of aged garlic extract (GE) in 4 capsules for 12 weeks 2.4 mL GE in 3 capsules twice a day for 1 year |
Natural killer (NK) cells increased in number and activity. Reduced size and number of colon adenomas. |
[40] [41] |
| Camptothecin (Ct) | Patients with refractory cancer Primary/metastatic lung cancer patients |
Ct: 3 weeks drug-1-week rest; Nitro-Ct: 5 day drug- 2 days rest 6.7–26.6 µg/kg of Ct in the form of aerosolized liposomes were given 5 days a week for 6 weeks, followed by a gap of 2 weeks. |
Both the compounds showed tumor regression in patients with breast cancer, prostate cancer, and melanomas. 3 lung patients stabilized upon dosage. |
[42] [43] |
| Curcumin | Urinary bladder cancer, uterine cervical neoplasm, and intestinal metaplasia Advanced pancreatic cancer |
500 mg/day, orally, for 3 months Dosage was 8 g/day for one month |
Improvement in 1 out of every 2 patients with bladder cancer and 1 out of 6 patients with intestinal Metaplasia, and 1 out of 4 patients with uterine cervical neoplasm. Study was conducted on 21 patients, of whom 1 had stable disease for >18 months and 1 had tumor reversion. |
[44] [45] |
| Green tea | Patients with high-grade prostate intraepithelial neoplasia Patients with adenocarcinoma of the prostate Esophageal cancer Patients with colon, rectum and pancreas cancer |
Green tea catechins (600 mg) were given daily, orally, for one year Tea consumption as a daily routine Usual green tea consumption Non-regular tea consumption |
Improved quality of life Risk declination of prostate cancer with increased consumption of green tea. Reduced risk of Esophageal cancer. Inverse relation was associated with cancer and green tea consumption. |
[46] [47] [48] [49] |
| Panax ginseng | Patients with cancer of uterine, ovary, rectum, stomach, etc | 3000 mg/day of the heat-processed ginseng for 12 weeks | Improvement of mental and physical functioning, and hence improved quality of life. | [50] |
| Isoflavones | Prostate cancer | (60 mg) daily for 12 months | Reducing prostate cancer incidence for patients aged 65 or more. | [51] |
| Synthetic genistein | Prostate cancer | 54 patients with localized prostate cancer. (30 mg) daily for 3–6 weeks | Decreasing level of serum prostate specific antigen (PSA). | [52] |
| Soy isoflavone | Prostate cancer | 86 patients with localized prostate cancer. (80 mg total isoflavones, 51 mg aglucon units) daily for 6 weeks | No significant change in serum hormone levels, total cholesterol, or PSA. | [53] |
| Flavonoid mixture | Colorectal cancer | (20 mg apigenin and 20 mg EGCG) for 3–4 years. 87 patients with resected colorectal cancer or polypectomy | Reducing the recurrence rate of colon neoplasia in patients with resected colon cancer. | [54] |
| Isoflavones and curcumin | Prostate cancer | Isoflavones (40 mg) and curcumin (100 mg) daily for 6 months | decreasing level of serum PSA. | [55] |
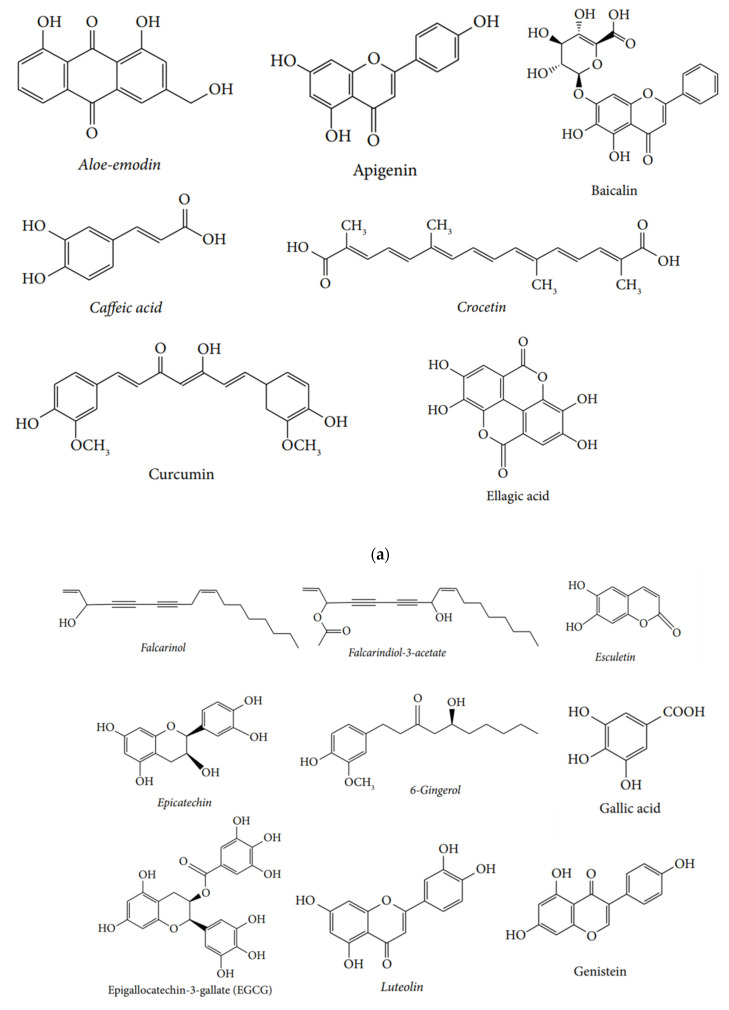
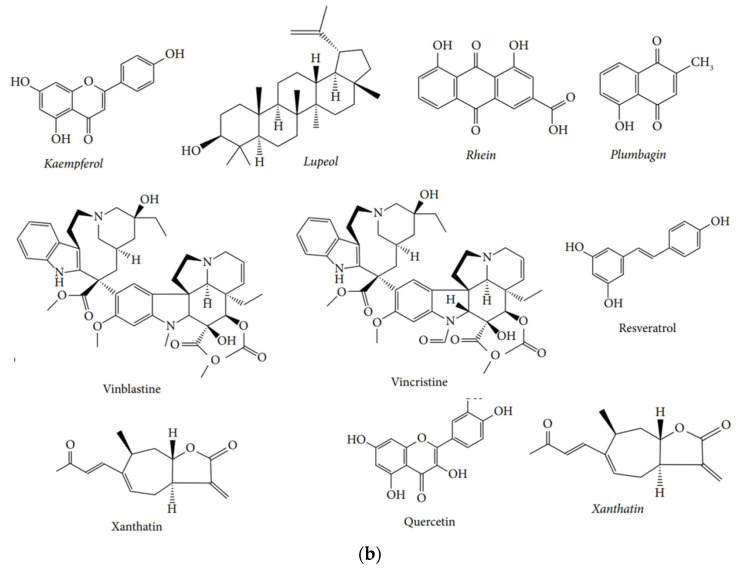
Figure 2.
(a) Different phytochemicals used as therapeutic agents: aloe-emodlin, apigenin, baicalin, caffeic acid, crocetin, curcumin, and ellagic acid; (b) Different phytochemicals used as therapeutic agents: epigallocatechin-3-gallate (EGCG). luteolin, genistein, kaempferol, lupeol, Rhein, plumbagin, vinblastine, vincristeine, resveratrol, xanthatin, quercetin, and xanthin.
Polyphenols have no exact classification, yet as they have diverse structures and are abundant in nature. The phytochemicals are generally classified as primary and secondary metabolites, as per their roles in plant metabolisms. The class of primary metabolites includes common sugars, nucleic acids, and proteins, all of which play an essential role in the basic survival of the plant. The secondary metabolites are plant chemicals that provide extra advantages over basic survival strategies such as flowering, defense mechanisms, anti-microbial agents, etc. [37,38].
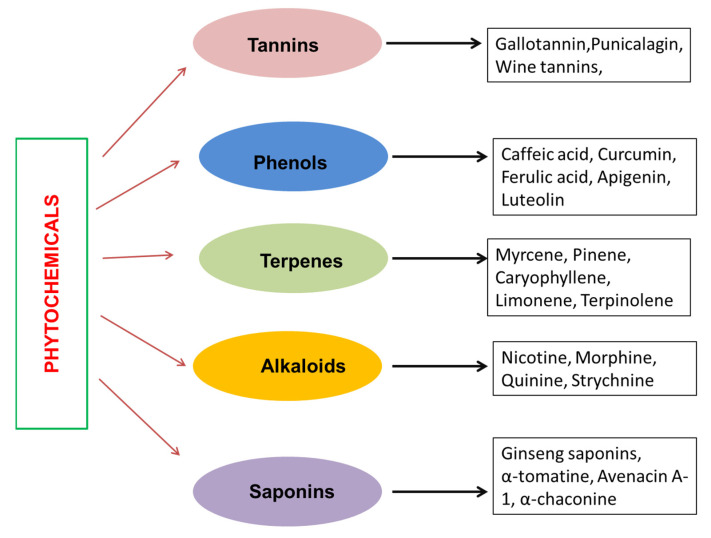
Secondary metabolites mainly consist of lignans, alkaloids, terpenes, phytoalexins, triterpenes, steroids, stilbenoids, bibenzyls, phenols, flavonoids, etc. [39]. Phenolics are known to be the most prevalent and structurally diverse phytochemicals. Figure 3 depicts the classification of phytochemicals.
Figure 3.
The diverse phytochemicals originating from plant sources are distinguished into five major classes based on their chemical structure and properties. The figure illustrates the different classes and gives examples of each type.
3. Phenolic Compounds and Their Role in Cancer Management
Phenolic compounds are the major components of phytochemicals that are widely distributed in the plant kingdom [56]. They aid in defense mechanisms as secondary metabolites. Additionally, phenolic compounds benefit humans in multiple ways; their antioxidant properties are widely considered a significant benefit for humans in this disease era. Flavonoids, phenolic acids, and polyphenols are three major groups of dietary phenolics. Flavonoids are a large group of phenols that occur ubiquitously as aglycones, glucosides, and methylated derivatives [57]. Many thousands of flavonoids, such as those found in fruits, vegetables, tea, and coffee, have been known to occur abundantly as a part of our diet [58]. Flavonoids have been used successfully in treating ailments since ancient times and have found their uses to date. Flavonoids usually occur in conjugation with sugars and are classified further as mono-, di-, and oligo-glycosides. Flavonoids are gaining attention due to their effect on various biological and pharmacological functions. Some effects exerted on biological functions include cytotoxic effects against cancer cell lines, anti-tumor effects, anti-inflammatory effects, and anti-microbial effects. Apart from therapeutic effects, the group of phytochemicals is known for its potent antioxidant activity, which plays a vital role in protection from the harmful effects of free radicals and reactive oxygen species (ROS). The phenolic acids form diverse groups and are abundantly distributed, such as hydroxylbenzoic acid (HBA) and hydroxycinnamic acid (HCA), and have one carboxylic acid functional group. HCAs are simple esters with an attached glucose or hydroxycarboxylic acid group. The phenolic compounds produced by plants have a different molecular structure, well known by the presence of hydroxylated aromatic rings [59]. The compounds are known for their antioxidant properties that prevent oxidative damage against ROS, thereby playing a vital role in neurodegeneration, cardiovascular diseases (CVDs), cancers, and many more. Tumor cells have a higher generation of ROS than normal cells and, therefore, are targeted by these compounds [60].
The importance of phenolic compounds is attributed to their effectiveness against the proliferation of various human cancer cell lines (HCCL) [61,62]. Cinnamic acid (CA) is a monocarboxylic acid derived from acrylic acid with a phenyl substituent. According to published literature, CA reduced the cell proliferation of the melanoma cell line (HT-44) with an IC50 value of 2.4 mM and inhibited the growth of the HT-44 cells by inhibiting the cells in the S phase [63]. Another study showed the arrest of the G2-M phase of the cell cycle in MDA-MB-231 and MCF-7 breast CCL when exposed to 4-Methyl-3-nitro-benzoic acid [64,65]. The efficacy of phenolics against cancer cell proliferation, migration, and invasion is well documented in many literatures [66,67]. P-coumaric acid decreased the viability of HCT15 and HT29 colorectal cancer cell lines [68,69]. Caffeic acid and its derivatives are also found to reduce the cell viability of cancer cell lines; caffeic and 5-caffeoylquinic acids reduced the cell proliferation of colorectal (HT-29) and fibrosarcoma cell lines (HT-1080) by modulating the cell cycles at various stages [70,71,72]. The phenol, di-caffeoylquinic acid, also reduced the proliferation of human colon CCC (DLD-1) [73]. Ferulic acid showed inhibition of pancreatic CCL MIA-Pa-Ca-2, and gallic acid inhibited CCL HeLa and HTB-35 [74,75]. Cinnamic acid derivatives with phenyl groups showed cytotoxicity in CCCs HT-29 (human colorectal CCL), A-549 (human lung CCL), MDA-MB-231, and HeLa (cervical CCL). The phenyl-substituted acids showed better efficacy in inhibiting cancer cell proliferation. At 0.1 mM concentration, the phenyl substitutes inhibited 84–92% of the cancer cells compared to non-substituted compounds, showing maximum inhibition of up to 63% [76].
The phenolics are studied for their toxicity in normal human cell lines. Compared to synthetic ones, naturally occurring phenolics showed less toxicity even at higher doses [77].The phytochemical protocatechuic acid was tested for safety and toxicity. Protocatechuic acid showed an LD50 value of 800 mg per kg by inter-peritoneal and 3.5 g/kg by intravenous routes [78]. A toxicity assessment of gallic acid (GA) in rats was conducted in which the rats were fed a GA-rich diet (up to 5%) for 13 weeks and no symptoms of toxicity were observed [79]. Similarly, p-coumaric acid also exhibited low toxicity, with an LD50 ~ 2850 mg/kg body weight [80]. In conclusion, phenolics and their derivatives are safe and have anti-proliferative effects on cancer cell lines. The compounds’ toxicity profile may vary depending on the structure, administration route, and dosage.
3.1. Curcumin
Curcumin has shown great potency in chemoprevention, isolated from Curcuma longa. The potent compound shows chemopreventive effects through ROS scavenging, signaling pathway modulations, apoptosis induction, and tumor microenvironment regulation. Curcumin is a safe and effective chemopreventive agent with low toxicity to normal cells. One of the essential aspects of curcumin is that it is budget-friendly, yet effective. In Asian countries, curcumin is used deliberately, and the plant’s root is used as a coloring and flavoring agent for food. The compound has many other benefits, such as being anti-inflammatory and having potent antioxidants [81]. The phytochemical is abundant in the spice turmeric and has a mixture of many bioactive compounds. The curcumin derivative in turmeric, tetrahydrocurcumin, has been a great attraction for research due to its anti-cancer effects and excellent solubility in water [82].
Curcumin fights cancer by its action on various essential signaling molecules such as CDKs, NF-kB, tumor necrosis factor-alpha (TNF-a), and cyclooxygenase-2 (COX-2) [83,84]. It shows considerable anti-inflammatory and anti-cancer effects in different clinical and preclinical studies. Many in vitro experiments also demonstrated diverse mechanisms by which curcumin inhibits cancer cells. CDK overexpression is associated with cancer, and breast and skin cancer treatment with curcumin decreases cancer progression by inhibiting CDK4 [85]. Curcumin downregulates gene expression in cancer onset and progression, such as VEGF, angiopoietin, MMP-9, and MMP-3 [86].
3.2. Resveratrol
Resveratrol is chemically 3, 5, 40-trihydroxy-trans-stilbenes and is abundant in grapes, berries, and many other plants. The compound has anti-ageing properties and has excellent roles in managing many diseases, including cancer, diabetes, neurodegeneration, arthritis, etc. [87]. Resveratrol modulates different signaling pathways in cancer onset, progression, and metastasis. It is also known to induce programmed cell death, reduce inflammatory responses, and aid in lowering the angiogenesis and conversion of a benign tumor into a malignant tumor [88,89]. The side effects of cancer treatment are significant complications in chemotherapy. Resveratrol has a major advantage: it eliminates the toxicity and side effects of cancer therapies and may be used as a combinatorial treatment [90,91,92]. The phytochemical reduces toxic heavy metals such as arsenic in renal cells. Using resveratrol inhibits the oxidative stress induced by arsenic trioxide, and a decline in arsenic concentration is observed in the hepatic cells [93,94]. The phytochemical is also beneficial in the treatment of acetaminophen-induced liver toxicity and cisplatin-induced kidney disorders [95]. External application of the phytochemical inhibits the effects of UV-B radiation on skin edema and reduces the production of hydrogen peroxide in mice. Extended application of resveratrol showed a tumor reduction and delayed the onset of cancer, whereas short-term application led to cytotoxicity against cancer growth [96,97]. Various research claims that resveratrol treatment modulates the signaling molecules associated with oncogenesis and shows inhibitory effects on cancer cells [98,99].
3.3. Apigenin
Apigenin is highly abundant in nature in the form of fruits and vegetables. The phytochemical is a flavone derivative and has anti-angiogenic properties. The properties are related to the modulation of signaling pathways associated with cancer induction, apoptosis, and cell cycle arrest [100]. Various research studies have shown the chemopreventive roles of apigenin in in vivo models. Different animal models were studied with variations in dosage, mode, and frequency of administration of the phytochemical. The major pathway modulated by apigenin is the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [101]. Apigenin reduces Her2/neu protein expression in mouse models of cancer [102]. The phytochemical shows chemopreventive effects by stimulating apoptotic cell death and cell cycle arrest. The natural phytochemical inhibits the progression of prostate cancer by inhibiting the NF-kB pathway [103]. Apigenin administration in the form of a parsley-rich diet improved antioxidant levels [104]. Other biological activities associated with phytochemicals include reduced plasma levels and platelet aggregation [105].
3.4. Gingerol
Gingerol, a phenolic compound, is a major bioactive compound present in ginger. According to a published study, mice treated with gingerol (5 mg/kg body weight) demonstrated inhibition of tumor growth and metastasis of breast cancer cells to other parts of the body by inhibiting caspase-3 expression [106]. Gingerol also inhibits metastatic lung cancer, breast cancer proliferation, metastasis, and invasion by suppressing the AKT and p38MAPK pathways [107].
3.5. Thymoquinone
Thymoquinone (TQ) is chemically 2-isopropyl-5-methyl-1,4-benzo-quinone and a bioactive constituent in black cumin seed oil. The compound has been extensively studied in in vivo models. When administered to BALB/c mice at 10 mg/kg, there is a decline in tumor size. TQ showed anti-cancer effects by inducing apoptosis and blocking STAT3 phosphorylation in gastric cancer cells; reduced STAT3 showed a reduction in JAK2 and c-Src activity [108]. Preclinical studies showed the potential role of TQ in combinatorial therapy with other chemotherapeutic agents [109]. BALB/c mice with transplanted breast cancer cells (EMT6/P cell line) were studied for inhibition by TQ along with melatonin. and it was found that it leads to decreased tumor size and cell death induction [110].
4. Tannins in Cancer Management
Tannins are high-molecular-weight (500–3000 Dalton), heterogeneous, and water-soluble compounds that are abundant in plants and common in food and beverages [91]. They are highly reactive, and owing to this, they form inter- and intra-molecular hydrogen bonds with other macromolecules such as proteins [111,112]. Tannins are classified into two classes: hydrolysable tannins and condensed tannins. Hydrolysable tannins are further classified into two groups. First are the gallotannins, which yield a sugar and gallic acid (GA) upon hydrolysis, and second are the ellagitannins, which yield an additional ellagic acid when hydrolyzed. The second class of tannins is the condensed tannins, the proanthocyanidins. The proanthocyanidins are highly abundant plant-derived polyphenols [112]. These compounds, unlike the hydrolysable tannins, do not hydrolyze in the presence of weak acid. However, under acidic and alcoholic conditions, they decompose and produce red pigments named phlobaphenes. The high structural complexity and the polymeric nature are responsible for less attention being paid to the tannins [113]. Proanthocyanidins and their monomers have drawn recent attention as they have various human health benefits, namely, antioxidant, anti-cancer, anti-inflammatory, anti-diabetic, etc. [114]. Table 2 lists the tannins and their roles against cancer proliferation.
4.1. Epigallocatechin Gallate
Green tea is a rich source of antioxidants and a proven preventive compound for numerous diseases. The major bioactive compound present in green tea is epigallocatechin gallate (EGCG), made up of three bound heterocyclic rings; delocalization of electrons leads to the scavenging of free electrons [115]. The tea catechins that contain the bioactive compound show redox properties with ROS. EGCG also acts as a metal chelating agent and prevents the production of ROS [116,117]. Although the compound is rich in health benefits, it has very low bioavailability, is indigestive, and has efflux properties [118,119,120]. Due to these reasons, EGCG shows a reduced effect in clinical trials. The major signaling pathways modulated by the compound are JAK/STAT, Janus kinases (JAK), signal transducer and activator of transcription proteins (STAT), NF-κB, MAPK, etc. [121,122]. The compounds have proven to exhibit tumor suppression and include genes such as p53, p21, p16, and Rb [123,124].
4.2. Gallic Acid
Tannin, or gallic acid (GA), shows anti-proliferative effects on multiple cancers such as lung, prostate, breast, colon, and esophageal cancer [125,126,127] by inducing cell death by apoptosis and other mechanisms. GA has shown antiproliferative effects on various human prostate cancer cell lines, such as LNCaP, PC-3, etc., by modulating multiple mechanisms [128,129]. In an in vivo study on BALB/C male nude mice, xenografts for DU145 and 22Rv1 were administered with GA in water for 6 weeks, and this resulted in reduced tumor size in the mouse models [114]. GA minimizes the proliferation of cancer cells by inducing apoptosis in H446, Calu-6, A549, etc., cell lines [130]. GA also stimulates mitogen-activated protein kinase (MAPK) inhibition, leading to apoptosis induction in lung cancer cells; GA reduced the number of viable NCI-H460 cells through induction of apoptosis and ultimately leading to G2/M phase arrest [131]. In another study, C57 black mice transplanted with LL-2 cells were administered GA (1 mg/mL) ad libitum, and it resulted in a reduction of tumor growth compared with the controls [132]. Nude NCI-H460 xenograft mice were administered GA orally, and it showed a reduction in tumor growth and induced caspases 3, 8, and 9 in the mouse model that induced apoptosis via the caspase-mediated mitochondrial pathway [133]. GA also induces apoptosis in human osteosarcoma cells by modulating the MAPK pathways. The compound shows inhibitory effects on the cancer cell lines U-2OS and HOS osteosarcoma cell lines. GA administration also inhibited the tumor growth in xenografts in a dose-dependent manner by downregulating PCNA and CD31 levels and thereby inducing apoptosis in the tumor cell lines [134].
Table 2.
Tannins and their roles against cancer proliferation.
| Tannins | Cancer | Effect on Cancer | Refs. |
|---|---|---|---|
| Tannic acid (TA) | Breast cancer cell lines (MCF-7, MDA-MB-231, BT474) Prostate Cancer Cells (PC-3 and LNCaP) Head and Neck Cancer (FaDu and YD-38) |
|
[135,136,137] [138] [139] |
| Ellagic acid (EA) | Human Bladder Cancer Cell Lines (T24, UM-UC-3, 5637, and HT-1376) Lung Cancer cell line A549 |
|
[140] [141,142,143] |
| EGCG | Breast cancer cell line 4T1 Human esophageal squamous carcinoma cells Eca109 Colorectal cancer (DLD-1 and SW480) Oral squamous cell carcinoma (HSC-3) |
|
[144] [145] [146] [147] |
| Gallic acid | Prostate cancer cell lines (DU145) Human lung cancer cells. Calu-6 and A549 Leukemia K562 cell line |
|
[127] [130] [148] |
| Procyanidins | Human breast cancer cell line MCF7 Non-small cell lung cancer (NSCLC) |
|
[149] [150] |
| Green tea catechins | Human lung cancer cell line PC-9 Human prostate cancer DU145 cell line |
|
[151] [152] |
| Epicatechin (flavon-3-ol monomer units) | Human bladder cancer TCCSUP cell line |
|
[153] |
5. Alkaloids in Cancer Treatment
Alkaloids are the phytochemicals that possess the most promising anti-cancer activities. The phytochemical class has diverse compounds derived from plants, animals, microbes, and many more [20]. The low molecular weight alkaloids are organic nitrogenous compounds. The compounds in this group are generally colorless and non-volatile and exhibit a low toxic effect on human cells. The action of alkaloids for cancer cell inhibition is to block the action of the topoisomerase enzyme, which further stalls DNA replication and promotes cell death [22]. For these reasons, alkaloids have been used as a parent molecule for designing and developing compounds possessing human health benefits [22]. Various alkaloids having anti-cancer effects include colchicine, vincristine, vinblastine, morphine, etc.
Colchicine is an anti-mitotic agent that prevents microtubule elongation by binding to tubulin and forming a tubulin-colchicine complex reversibly. However, at higher doses, the alkaloid causes significant damage to the normal tissues and limits its use in chemotherapy [96]. Vinblastine sulfate, USP, is obtained from the flowers of a common medicinal plant (Catharanthus roseus spp.). The compound shows its anti-cancer effect by halting cell growth at the metaphase [154]. The alkaloid vincristine is also used as an anti-cancer agent. The drug is administered intravenously due to its low bioavailability [150]. Vindesine, marketed as vindesine sulfate, gained FDA approval in 1994. Like other Vinca alkaloids, vindesine blocks the cells in metaphase during mitosis [154]. In vitro studies show that vindesine sulfate inhibits the malignancy and invasion of cancer cells. Vindesine sulfate has more potency than other alkaloid drugs. Vinorelbine is also a semi-synthetic vinca alkaloid sold under the brand name Navelbine [155]. It is a chemotherapeutic drug for treating non-small cell lung cancer that has spread metastatically (NSCLC) [155]. Table 3 lists alkaloids with their pharmacological mechanisms.
Table 3.
Alkaloids and their therapeutic effect and pharmacological mechanism.
| Alkaloid | Pharmacological Mechanism | Therapeutic Effect | Refs. |
|---|---|---|---|
| Vinblastine | -Binds to tubulin and prevents microtubules from binding. -Induce apoptosis and mitotic death. |
Cervical cancer Breast cancer Lung cancer Head and neck cancer Hodgkin’s lymphoma Testicular cancer |
[156,157] |
| Vincristine | -Binds tubulin dimer. -Prevents microtubule structure formation. |
Acute myeloid leukemia (AML, ANLL) Acute_lymphoblastic leukemia (ALL) Hodgkin’s_lymphoma Non-Hodgkin’s lymphoma |
[158,159] |
| Vindesine | Possess anti-mitotic activity | Melanoma Lung cancers Uterine malignancies |
[154] |
| Vinorelbine | Exhibits broad-spectrum antitumor activity. Antineoplastic activity |
Breast cancer Non-small cell lung cancer (NSCLC) |
[159,160] |
| Vinflunine | Decreases metaphase to anaphase transition, Prevents cancer cells from entering mitosis. Increases apoptosis |
Metastatic Urothelial carcinoma Transitional cell carcinoma Breast cancer |
[161] |
| Colchicine | Microtubule destabilizers perturb the assembly dynamics of microtubules. | Gastric cancer | [162,163] |
| Colcemid | Mitotic arrest Kinase inhibition |
Lung Cancer | [164] |
6. Terpenes in Cancer Treatment
Terpenes are highly abundant phytochemicals and are numerous. The terpenes are found in various sources, such as plants, flowers, and insects. The compounds are responsible for the taste and fragrance of the plants. We can classify terpenes based on the number of isoprene units and their organization [165]. Myrcene, a monoterpene, and the sesquiterpenes β-caryophyllene and α-humulene are the terpenes most common. Myrcene extracts have shown cytotoxic effects in cancer cell lines such as breast and colon cancer [166]. The terpene β-cp exhibits cytotoxic potential against lung and ovarian cancer cell lines by inducing cell cycle arrest and apoptosis [167,168]. The compound shows anti-proliferative effects in a glioblastoma model. β-cp at 20 µM induces proapoptotic and antiproliferative effects by modulating the JAK/STAT pathway in osteosarcoma cells [169]. Glycyrrhizin (Gy), a triterpene glycoside, is the active constituent found in the licorice root of Glycyrrhiza glabra. BALB/c nude mice xenografts of A549 cells (lung cancer) were transfected with TxA2 receptor (TPa), Gy with a dosage of 135 mg/kg, which reduced thromboxane synthase and PCNA expression by suppressing the TxA2 pathway [170]. It has anti-cancer and antioxidant activities. Gy also enhances NO production by stimulating with interferon-gamma (IFN-γ), and high NO concentrations are associated with cancer cell death [171]. Table 4 shows some of the terpenes and the anti-cancer potential of the compounds.
Table 4.
Anti-proliferative role of terpenes in cancer.
| Terpene | In Vitro Effects | In Vivo Effects | Clinical Trials | Refs. |
|---|---|---|---|---|
| Myrcene | Cytotoxic effects on cancer cell lines Reduced DNA damage |
Carcinogenic at higher doses | N/A | [172,173,174,175] |
| Limonene | Shown cytotoxic effects Mediates cell cycle arrest Decreased migration and invasion of cancer cells Apoptosis and autophagy induction Inhibition of the PI3K/Akt pathway |
Decreased tumor growth and metastasis, c-jun, and c-myc expression Induced apoptosis and latency period. |
Decreased the expression of proteins involved in tumor progression. | [176,177,178,179,180,181,182,183] |
| Pinene | Reduced cell viability. Induced apoptosis, ROS production, and cell cycle arrest |
Reduced the number and growth of tumors. | N/A | [184,185,186,187] |
| Elemene | Induced cell cycle arrest and apoptosis Inhibited MAPK pathway Reduced tumor migration and invasion Inhibited angiogenesis |
N/A | Effective agents in chemotherapy. Reduced toxicity of chemotherapy. |
[188,189,190,191,192,193,194] |
| Terpinene isomers | Reduced proliferation and induced apoptosis in cancer cells | N/A | N/A | [195,196,197,198] |
| Valencene | Reduced cellular proliferation and acted efficiently synergistically with doxorubicin | N/A | N/A | [199,200] |
| Nerolidol | Exhibited cytotoxic effects and induced apoptosis and cell cycle arrest. Acted synergistically with doxorubicin |
Inhibited cancer growth | N/A | [201,202,203,204,205] |
7. Mechanism of Action of Phytochemicals
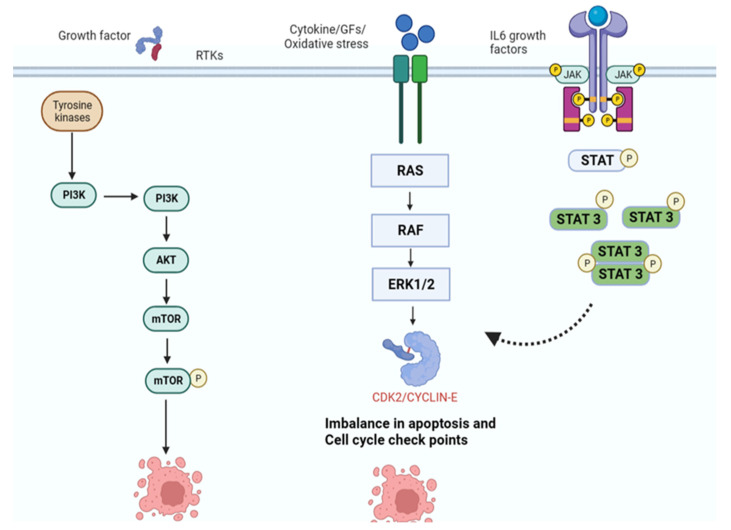
The phytochemicals exert deleterious effects on cancer cells through various mechanisms, including modulations in signaling pathways and the onset of apoptosis [206]. The anti-cancer agents show their effects by blocking the generation of carcinogenic species and obstructing the interaction between carcinogens and cells, thus delaying tumor formation [207]. The signaling pathways majorly associated with cancer are the mitogen-activated protein kinase (MAPK) pathway, nuclear factor kappa B (NF-Kb), and activator of transcription proteins (STAT) pathway. The signaling pathways are modulated so that they may be overactivated or blocked and govern the metabolic pathways in various cancers. The modulations further lead to cancer onset and proliferation, and they promote various hallmarks of cancer such as angiogenesis, increased glycolysis, and metastasis [9]. The signaling pathways and modulated enzymes and factors are a major target to inhibit or activate for the therapeutic treatment of cancers [23]. The MAPK pathway is associated with the onset of tumors such as melanomas and is a target for inhibiting the treatment of related cancers. Various phytochemicals such as quercetin, curcumin, ellagic acid, rosmarinic acid, etc. [208]. Refs. [209,210,211] are associated with halting the MAPK pathway, shown in Figure 4. Quercitin has demonstrated inhibitory effects on human hepatoma cell lines HepG2 by blocking the ERK pathway and phosphatidylinositol-3-kinase (PI3K)/Aurora kinase B (AKB) pathways [212]. Gallic acid showed a deleterious effect on the invasiveness of mouse brain endothelial cells and glioblastoma cells, U87 and U251, by blocking some pathways involved in cancer progression [213]. NF-kB pathway has major roles in cancer development and progression, promoting the proliferation of cancer cells, aiding metastasis, and skipping apoptosis. The phytochemicals have shown inhibitory effects against NF-kB; the phytochemicals involved are capsaicin, ursolic acid, gingerol, eugenol, etc. [214].
Figure 4.
Different signaling pathways targeted by the phytochemicals responsible for the onset of cancer and cancer proliferation, metastasis, and invasion.
Apoptosis is an essential process of programmed cell death and significantly eliminates tumor cells [22,215]. Phytochemicals have been shown to induce apoptotic effects on cells by upregulating caspase 3 and 9 expressions, decreasing the growth and development of colorectal cancer and lung cancer [216]. The phytochemicals involved in apoptosis are punicalagin and 5-methoxyangenylalkanni. Apigenin, a flavonoid derivative, is associated with the modulation of the kinase pathway and blocks the cells in the G2/M phase. Apigenin can inhibit the growth of HepG2 cells [217]. Esculetin induces apoptosis in various human cancer cell lines, including HSC4, HSC4 oral squamous cell carcinoma, the leukemia cell line U937, and melanoma cells G361 [218]. The phytochemical is also a potent inhibitor of the Wnt-β-catenin pathway. It blocks the formation of the β-catenin-Tcf complex, suppressing colon cancer cell proliferation [219]. In colon cancer, the phytochemical diosgenin s apoptosis by increasing caspase 3 activity, inhibiting Bcl-2 [220]. The phytochemical artabotryside A induces apoptosis in U87 cells by arresting the cell cycle at the G2/M phase of the cell cycle [221]. Caffeic acid-induced apoptosis was induced in the breast cancer cell T47D by activation of the Fas/FasL pathway [222]. Several other phytochemicals are known to induce anti-cancer effects by inducing apoptosis, such as lutein, capsaicin, rhein, etc.
Cell cycle progressions are associated with activating cyclin-dependent kinases (CDKs). The levels of CDKs are regulated by cyclin-dependent kinase inhibitors (CKIs), which maintain the level of CDKs [223]. Various phytochemicals, such as mangiferin [224], naringenin [225], berberine [226], fisetin [227], etc., have demonstrated inhibitory potentials for the progression of the cell cycle. Ferulic acid from Allium cepa has been studied to elevate the expression of genes associated with the association of centrosomes and arrest the cell cycle at the synthesis (S) phase, which results in the inhibition of colon cancer Caco-2 cells [228]. Withaferin A, isolated from Withaniasomnifera spp., arrests the cell cycle at the G2/M phase by lowering CDK levels in various cancer cell lines [229]. In addition, other phytochemicals such as capsaicin, kaempferol, and berberine induce arrest in the cell cycle. Cancer-related epigenetic variations are associated with chemical changes to histones and gene expression. DNA’s hyper- and hypomethylation leads to chromatin condensation and tumor inhibitory gene inhibition. Improper oncogene expression is also the result of methylated cytosines [230].
8. Conclusions and Future Perspectives
Phytochemicals have emerged as a major source for developing novel leads for drug discovery and development. An advanced approach to combining traditional knowledge with the drug discovery process can lead to the discovery of novel compounds that can aid in the management of various life-threatening diseases. The advancements in analytics and bioinformatics have also facilitated the entry of new leads from plants into the evaluation process. Cancer is a complex and hard-to-treat disease with various complications. The conventional methods of cancer therapeutics have a lot of drawbacks, such as side effects, chemoresistance, and reversal of cancer. The utmost need is to develop more potent therapeutic compounds with the least toxicity. Using phytochemicals in combination with current methods of cancer treatment can aid in reducing the effects of cancer. The phytochemicals work on cancer cells by modulating the cell signaling mechanism and inducing apoptosis in the cancer cells. Various phytochemicals have shown their anti-cancer effects in in vivo, in vitro, and clinical trials.
Detailed studies at preclinical and epidemiological levels are needed to identify more such beneficial compounds and their use against cancer alone as well as in combination with other drugs already available.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Program under grant number (RGP.2/137/1443). A.S. is also thankful to Ajman University for supporting the publication. M.A. is thankful to the Deanship of Scientific Research at Shaqra University for supporting this work.
Author Contributions
T.A.M.: writing—original draft, literature review and editing; S.A.A.: writing—original draft, literature review and editing; A.A. (Abdulrhman Alsayari): writing—original draft, literature review and editing; A.B.M.: writing—original draft, literature review and editing; M.A.: revisions and final editing; A.M.A.: literature review, revision, and final editing; A.S.: conceptualization, literature review, writing review and editing, project administration, and funding acquisition; A.A. (Akhtar Atiya): conceptualization, literature review, writing review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the associated Data is contained within the article.
Conflicts of Interest
The authors declare no competing financial interest.
Funding Statement
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Program under grant number (RGP.2/137/1443). A.S. is also thankful to Ajman University for supporting the publication. M.A. is thankful to the Deanship of Scientific Research at Shaqra University for supporting this work.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaumer S., Bonnabry P., Veuthey J.-L., Fleury-Souverain S. Analysis of anticancer drugs: A review. Talanta. 2011;85:2265–2289. doi: 10.1016/j.talanta.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Judson P.L., Abdallah R., Xiong Y., Ebbert J., Lancaster J.M. Complementary and alternative medicine use in individuals presenting for care at a comprehensive cancer center. Integr. Cancer Ther. 2017;16:96–103. doi: 10.1177/1534735416660384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranjan A., Ramachandran S., Gupta N., Kaushik I., Wright S., Srivastava S., Das H., Srivastava S., Prasad S., Srivastava S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019;20:4981. doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo Y.M., Shin Y., Lee E.J., Lee S., Jeong S.H., Kong H.K., Park E.Y., Kim H.K., Han J., Chang M., et al. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PLoS ONE. 2015;10:e0132285. doi: 10.1371/journal.pone.0132285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Liberti M.V., Locasale J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y., Hou R., Yu J., Xing C., Zhuang C., Qu Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients. 2023;15:491. doi: 10.3390/nu15030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal M.K., Mohammad M., Parvin S.I., Islam M.M., Gazi H.A.R., Alberto A.K.M., da Costa M.J., Carvalho J.C.T. A Short Review on Anticancer Phytochemicals. Pharmacogn. Rev. 2023;17:11–23. doi: 10.5530/097627870236. [DOI] [Google Scholar]
- 11.Anwar S., Shamsi A., Shahbaaz M., Queen A., Khan P., Hasan G.M., Islam A., Alajmi M.F., Hussain A., Ahmad F., et al. Rosmarinic acid exhibits anticancer effects via MARK4 inhibition. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-65648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anwar S., Khan S., Anjum F., Shamsi A., Khan P., Fatima H., Shafie A., Islam A., Hassan M.I. Myricetin inhibits breast and lung cancer cells proliferation via inhibiting MARK4. J. Cell. Biochem. 2022;123:359–374. doi: 10.1002/jcb.30176. [DOI] [PubMed] [Google Scholar]
- 13.Ogbonna J., Kenechukwu F., Attama A., Chime S. Different approaches to formulation of herbal extracts/phytopharmaceuticals/bioactive phytochstituents-a review. Int. J. Pharm. Sci. Rev. Res. 2012;16:1–8. [Google Scholar]
- 14.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breinbauer R., Vetter I.R., Waldmann H. From protein domains to drug candidates—Natural products as guiding principles in the design and synthesis of compound libraries. Angew. Chem. Int. Ed. 2002;41:2878–2890. doi: 10.1002/1521-3773(20020816)41:16<2878::AID-ANIE2878>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Butler M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 17.Huang M., Lu J.-J., Ding J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021;11:5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamsi A., Anwar S., Mohammad T., Shahwan M., Hassan M.I., Islam A. Autism Spectrum Disorder and Alzheimer’s Disease: Advances in Research. Springer; Berlin/Heidelberg, Germany: 2022. Therapeutic potential of polyphenols in Alzheimer’s therapy: Broad-spectrum and minimal side effects as key aspects; pp. 111–133. [Google Scholar]
- 19.Panda A.K., Chakraborty D., Sarkar I., Khan T., Sa G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017;9:31–45. doi: 10.2147/JEP.S70568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choy K.W., Murugan D., Leong X.-F., Abas R., Alias A., Mustafa M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019;10:1295. doi: 10.3389/fphar.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tewari D., Patni P., Bishayee A., Sah A.N., Bishayee A. Proceedings of Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2022. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy; pp. 1–17. [DOI] [PubMed] [Google Scholar]
- 22.Anwar S., Mohammad T., Shamsi A., Queen A., Parveen S., Luqman S., Hasan G.M., Alamry K.A., Azum N., Asiri A.M., et al. Discovery of Hordenine as a potential inhibitor of pyruvate dehydrogenase kinase 3: Implication in lung Cancer therapy. Biomedicines. 2020;8:119. doi: 10.3390/biomedicines8050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anwar S., Shamsi A., Mohammad T., Islam A., Hassan M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2021;1876:188568. doi: 10.1016/j.bbcan.2021.188568. [DOI] [PubMed] [Google Scholar]
- 24.Sen G.S., Mohanty S., Hossain D.M.S., Bhattacharyya S., Banerjee S., Chakraborty J., Saha S., Ray P., Bhattacharjee P., Mandal D. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53-p300 in breast cancer. J. Biol. Chem. 2011;286:42232–42247. doi: 10.1074/jbc.M111.262295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui F.A., Prakasam G., Chattopadhyay S., Rehman A.U., Padder R.A., Ansari M.A., Irshad R., Mangalhara K., Bamezai R.N., Husain M. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018;8:8323. doi: 10.1038/s41598-018-25524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvaraji S., Poh L., Natarajan V., Mallilankaraman K., Arumugam T.V. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond. Med. 2019;2:30. [PMC free article] [PubMed] [Google Scholar]
- 27.Devasagayam T., Sainis K. Immune System and Antioxidants, Especially Those Derived from Indian Medicinal Plants. IJEB. 2002;40:639–655. [PubMed] [Google Scholar]
- 28.Akhtar M.F., Saleem A., Rasul A., Baig M.M.F.A., Bin-Jumah M., Daim M.M.A. Anticancer natural medicines: An overview of cell signaling and other targets of anticancer phytochemicals. Eur. J. Pharmacol. 2020;888:173488. doi: 10.1016/j.ejphar.2020.173488. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S., Mehta A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015;38:199–210. doi: 10.1007/s40415-015-0135-0. [DOI] [Google Scholar]
- 30.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Thomson M., Ali M. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- 32.Khan I., Abbas T., Anjum K., Abbas S.Q., Shagufta B.I., Ali Shah S.A., Akhter N. Antimicrobial potential of aqueous extract of Camellia sinensis against representative microbes. Pak. J. Pharm. Sci. 2019;32:631–636. [PubMed] [Google Scholar]
- 33.Sharif T., Alhosin M., Auger C., Minker C., Kim J.-H., Etienne-Selloum N., Bories P., Gronemeyer H., Lobstein A., Bronner C., et al. Aronia melanocarpa juice induces a redox-sensitive p73-related caspase 3-dependent apoptosis in human leukemia cells. PLoS ONE. 2012;7:e32526. doi: 10.1371/journal.pone.0032526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Tiwari P., Mishra B., Sangwan N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. BioMed Res. Int. 2014;2014:830285. doi: 10.1155/2014/830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar Roy M., Nakahara K., Na Thalang V., Trakoontivakorn G., Takenaka M., Isobe S., Tsushida T. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Die Pharm.-Int. J. Pharm. Sci. 2007;62:149–153. [PubMed] [Google Scholar]
- 37.Divekar P.A., Narayana S., Divekar B.A., Kumar R., Gadratagi B.G., Ray A., Singh A.K., Rani V., Singh V., Singh A.K., et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022;23:2690. doi: 10.3390/ijms23052690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeshi K., Crayn D., Ritmejerytė E., Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules. 2022;27:313. doi: 10.3390/molecules27010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392. [Google Scholar]
- 40.Ishikawa H., Saeki T., Otani T., Suzuki T., Shimozuma K., Nishino H., Fukuda S., Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006;136:816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S., Haruma K., Yoshihara M., Kajiyama G., Kira K., Amagase H., Chayama K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006;136:821S–826S. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- 42.Natelson E.A., Giovanella B.C., Verschraegen C.F., Fehir K.M., De Ipolyi P.D., Harris N., Stehlin J.S. Phase I clinical and pharmacological studies of 20-(S)-camptothecin and 20-(S)-9-nitrocamptothecin as anticancer agents. Ann. N. Y. Acad. Sci. 1996;803:224–230. doi: 10.1111/j.1749-6632.1996.tb26392.x. [DOI] [PubMed] [Google Scholar]
- 43.Verschraegen C.F., Gilbert B.E., Loyer E., Huaringa A., Walsh G., Newman R.A., Knight V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20 (s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.CCR-0929-3. [DOI] [PubMed] [Google Scholar]
- 44.Cheng A., Hsu C., Lin J., Hsu M., Ho Y., Shen T., Ko T., Lin J., Lin B., Ming-Shiang W., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high risk or pre-malignant lesion. Anti-Cancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 45.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 46.Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 47.Jian L., Xie L.P., Lee A.H., Binns C.W. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int. J. Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y.T., McLaughlin J.K., Blot W.J., Ji B.T., Dai Q., Fraumeni J.F. Reduced risk of esophageal cancer associated with green tea consumption. JNCI J. Natl. Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 49.Ji B.T., Chow W.H., Hsing A.W., McLaughlin J.K., Dai Q., Gao Y.T., Blot W.J., Fraumeni J.F., Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer. 1997;70:255–258. doi: 10.1002/(SICI)1097-0215(19970127)70:3<255::AID-IJC1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.H., Park C.Y., Lee S.J. Effects of sun ginseng on subjective quality of life in cancer patients: A double-blind, placebo-controlled pilot trial. J. Clin. Pharm. Ther. 2006;31:331–334. doi: 10.1111/j.1365-2710.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 51.Miyanaga N., Akaza H., Hinotsu S., Fujioka T., Naito S., Namiki M., Takahashi S., Hirao Y., Horie S., Tsukamoto T., et al. Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012;103:125–130. doi: 10.1111/j.1349-7006.2011.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarevic B., Boezelijn G., Diep L.M., Kvernrod K., Ogren O., Ramberg H., Moen A., Wessel N., Berg R.E., Egge-Jacobsen W., et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer. 2011;63:889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton-Reeves J.M., Banerjee S., Banerjee S.K., Holzbeierlein J.M., Thrasher J.B., Kambhampati S., Keighley J., Van Veldhuizen P. Short-term soy isoflavone intervention in patients with localized prostate cancer: A randomized, double-blind, placebo-controlled trial. PLoS ONE. 2013;8:e68331. doi: 10.1371/journal.pone.0068331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoensch H., Groh B., Edler L., Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. WJG. 2008;14:2187. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ide H., Tokiwa S., Sakamaki K., Nishio K., Isotani S., Muto S., Hama T., Masuda H., Horie S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70:1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 56.Walton N.J., Mayer M.J., Narbad A. Vanillin. Phytochemistry. 2003;63:505–515. doi: 10.1016/S0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 57.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pridham J.B. Proceedings of Phenolics in Plants in Health and Disease. Proceedings of a Plant Phenolics Group Symposium Held at Bristol, April 1959. Pergamon Press; Oxford, UK: London, UK: 1960. Phenolics in plants in health and disease. Proceedings of a Plant Phenolics Group Symposium held at Bristol, April 1959. [Google Scholar]
- 59.Teixeira J., Gaspar A., Garrido E.M., Garrido J., Borges F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/251754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandal S.M., Chakraborty D., Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010;5:359–368. doi: 10.4161/psb.5.4.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rahaiee S., Assadpour E., Esfanjani A.F., Silva A.S., Jafari S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020;279:102153. doi: 10.1016/j.cis.2020.102153. [DOI] [PubMed] [Google Scholar]
- 62.Cosme P., Rodríguez A.B., Espino J., Garrido M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants. 2020;9:1263. doi: 10.3390/antiox9121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anantharaju P.G., Gowda P.C., Vimalambike M.G., Madhunapantula S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016;15:99. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casaburi I., Puoci F., Chimento A., Sirianni R., Ruggiero C., Avena P., Pezzi V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013;57:71–83. doi: 10.1002/mnfr.201200503. [DOI] [PubMed] [Google Scholar]
- 65.Jafari S., Saeidnia S., Abdollahi M. Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr. Pharm. Biotechnol. 2014;15:409–421. doi: 10.2174/1389201015666140813124832. [DOI] [PubMed] [Google Scholar]
- 66.Tsakiroglou P., VandenAkker N.E., Del Bo’ C., Riso P., Klimis-Zacas D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: An updated overview. Nutrients. 2019;11:1075. doi: 10.3390/nu11051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akl M.R., Ayoub N.M., Mohyeldin M.M., Busnena B.A., Foudah A.I., Liu Y.-Y., Sayed K.A.E. Olive phenolics as c-Met inhibitors:(-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE. 2014;9:e97622. doi: 10.1371/journal.pone.0097622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaganathan S.K., Supriyanto E., Mandal M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. WJG. 2013;19:7726. doi: 10.3748/wjg.v19.i43.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson L.R., Zhu S.t., Harris P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005;49:585–593. doi: 10.1002/mnfr.200500014. [DOI] [PubMed] [Google Scholar]
- 70.Murad L.D., Soares N.d.C.P., Brand C., Monteiro M.C., Teodoro A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer. 2015;67:532–542. doi: 10.1080/01635581.2015.1004736. [DOI] [PubMed] [Google Scholar]
- 71.Rezaei-Tavirani M., Tavirani M.R., Azodi M.Z. The bioinformatics aspects of gene screening of HT-29, human colon cell line treated with caffeic acid. Gastroenterol. Hepatol. Bed Bench. 2019;12:246. [PMC free article] [PubMed] [Google Scholar]
- 72.Rajendra Prasad N., Karthikeyan A., Karthikeyan S., Venkata Reddy B. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011;349:11–19. doi: 10.1007/s11010-010-0655-7. [DOI] [PubMed] [Google Scholar]
- 73.Kurata R., Adachi M., Yamakawa O., Yoshimoto M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007;55:185–190. doi: 10.1021/jf0620259. [DOI] [PubMed] [Google Scholar]
- 74.Sourani Z., Pourgheysari B., Rafieian-Kopaei M., Shirzad H., Shirzad M. The effect of gallic acid on Jurkat cell line. J. Herbmed Pharmacol. 2015;4:129–132. [Google Scholar]
- 75.Zhao B., Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013;6:1749–1755. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pontiki E., Hadjipavlou-Litina D., Litinas K., Geromichalos G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules. 2014;19:9655–9674. doi: 10.3390/molecules19079655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Habauzit V., Morand C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012;3:87–106. doi: 10.1177/2040622311430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kakkar S., Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niho N., Shibutani M., Tamura T., Toyoda K., Uneyama C., Takahashi N., Hirose M. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 2001;39:1063–1070. doi: 10.1016/S0278-6915(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 80.Pei K., Ou J., Huang J., Ou S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 81.Reddy B.S., Rao C.V. Cancer Chemoprevention: Promising Cancer Chemopreventive Agents. Humana Press Inc.; Totowa, NJ, USA: 2004. Chemoprevention of cancer by curcumin; pp. 169–175. [Google Scholar]
- 82.Lai C.-S., Ho C.-T., Pan M.-H. The cancer chemopreventive and therapeutic potential of tetrahydrocurcumin. Biomolecules. 2020;10:831. doi: 10.3390/biom10060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 84.Prakobwong S., Khoontawad J., Yongvanit P., Pairojkul C., Hiraku Y., Sithithaworn P., Pinlaor P., Aggarwal B.B., Pinlaor S. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int. J. Cancer. 2011;129:88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 85.Mukhopadhyay A., Banerjee S., Stafford L.J., Xia C., Liu M., Aggarwal B.B. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 86.Gururaj A.E., Belakavadi M., Venkatesh D.A., Marmé D., Salimath B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem. Biophys. Res. Commun. 2002;297:934–942. doi: 10.1016/S0006-291X(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 87.Ren B., Kwah M.X.-Y., Liu C., Ma Z., Shanmugam M.K., Ding L., Xiang X., Ho P.C.-L., Wang L., Ong P.S., et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63–72. doi: 10.1016/j.canlet.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 89.Saiko P., Szakmary A., Jaeger W., Szekeres T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Rev. Mutat. Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Harikumar K.B., Aggarwal B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 91.Kotecha R., Takami A., Espinoza J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget. 2016;7:52517. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berman A.Y., Motechin R.A., Wiesenfeld M.Y., Holz M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017;1:35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang W., Xue J., Ge M., Yu M., Liu L., Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol. 2013;51:87–92. doi: 10.1016/j.fct.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 94.Upadhyay G., Singh A.K., Kumar A., Prakash O., Singh M.P. Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur. J. Pharmacol. 2008;596:146–152. doi: 10.1016/j.ejphar.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Afaq F., Adhami V.M., Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003;186:28–37. doi: 10.1016/S0041-008X(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 96.Aziz M.H., Afaq F., Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in Survivin. Photochem. Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274.1. [DOI] [PubMed] [Google Scholar]
- 97.Aziz M.H., Reagan-Shaw S., Wu J., Longley B.J., Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 98.Adhami V.M., Afaq F., Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/S1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reagan-Shaw S., Afaq F., Aziz M.H., Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y., Mao Y., Chen H., Lin Y., Hu Z., Wu J., Xu X., Xu X., Qin J., Xie L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013;13:54. doi: 10.1186/1475-2867-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shukla S., Bhaskaran N., Babcook M.A., Fu P., MacLennan G.T., Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mafuvadze B., Liang Y., Besch-Williford C., Zhang X., Hyder S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm. Cancer. 2012;3:160–171. doi: 10.1007/s12672-012-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shukla S., Kanwal R., Shankar E., Datt M., Chance M.R., Fu P., MacLennan G.T., Gupta S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget. 2015;6:31216. doi: 10.18632/oncotarget.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nielsen S., Young J., Daneshvar B., Lauridsen S., Knuthsen P., Sandström B., Dragsted L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999;81:447–455. doi: 10.1017/S000711459900080X. [DOI] [PubMed] [Google Scholar]
- 105.Thiery-Vuillemin A., Nguyen T., Pivot X., Spano J., Dufresnne A., Soria J. Molecularly targeted agents: Their promise as cancer chemopreventive interventions. Eur. J. Cancer. 2005;41:2003–2015. doi: 10.1016/j.ejca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Martin A.C.B., Fuzer A.M., Becceneri A.B., da Silva J.A., Tomasin R., Denoyer D., Kim S.-H., McIntyre K.A., Pearson H.B., Yeo B., et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget. 2017;8:72260. doi: 10.18632/oncotarget.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joo J.-H., Hong S.-S., Cho Y.-R., Seo D.-W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016;35:779–784. doi: 10.3892/or.2015.4405. [DOI] [PubMed] [Google Scholar]
- 108.Zhu W.-Q., Wang J., Guo X.-F., Liu Z., Dong W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016;22:4149. doi: 10.3748/wjg.v22.i16.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mostofa A., Hossain M.K., Basak D., Bin Sayeed M.S. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017;8:295. doi: 10.3389/fphar.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Odeh L.H., Talib W.H., Basheti I.A. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J. Cancer Res. Ther. 2018;14:S324–S330. doi: 10.4103/0973-1482.235349. [DOI] [PubMed] [Google Scholar]
- 111.De Jesus N.Z.T., de Souza Falcão H., Gomes I.F., de Almeida Leite T.J., de Morais Lima G.R., Barbosa-Filho J.M., Tavares J.F., Silva M.S.d., de Athayde-Filho P.F., Batista L.M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012;13:3203–3228. doi: 10.3390/ijms13033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lamy E., Pinheiro C., Rodrigues L., Capela-Silva F., Lopes O., Tavares S., Gaspar R. Determinants of Tannin-Rich Food and Beverage Consumption: Oral Perception vs. Psychosocial Aspects. 2016. [(accessed on 15 March 2023)]. Available online: https://dspace.uevora.pt/rdpc/handle/10174/18018.
- 113.Serrano J., Puupponen-Pimiä R., Dauer A., Aura A.M., Saura-Calixto F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009;53:S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 114.Smeriglio A., Monteleone D., Trombetta D. Health effects of Vaccinium myrtillus L.: Evaluation of efficacy and technological strategies for preservation of active ingredients. Mini Rev. Med. Chem. 2014;14:567–584. doi: 10.2174/1389557514666140722083034. [DOI] [PubMed] [Google Scholar]
- 115.Bimonte S., Cascella M., Schiavone V., Mehrabi-Kermani F., Cuomo A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: An update on preclinical in vivo studies and future perspectives. Drug Des. Dev. Ther. 2017;11:2737–2742. doi: 10.2147/DDDT.S142475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupte A., Mumper R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Khan H.Y., Zubair H., Ullah M.F., Ahmad A., Hadi S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals. 2011;24:1169–1178. doi: 10.1007/s10534-011-9475-9. [DOI] [PubMed] [Google Scholar]
- 118.Auger C., Mullen W., Hara Y., Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J. Nutr. 2008;138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 119.Stalmach A., Troufflard S., Serafini M., Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009;53:S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 120.Mereles D., Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011;12:5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tedeschi E., Suzuki H., Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann. N. Y. Acad. Sci. 2002;973:435–437. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 122.Sen T., Dutta A., Chatterjee A. Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFκB and AP-1 in the human breast cancer cell line MDA-MB-231. Anti-Cancer Drugs. 2010;21:632–644. doi: 10.1097/CAD.0b013e32833a4385. [DOI] [PubMed] [Google Scholar]
- 123.Kang S.U., Lee B.-S., Lee S.-H., Baek S.J., Shin Y.S., Kim C.-H. Expression of NSAID-activated gene-1 by EGCG in head and neck cancer: Involvement of ATM-dependent p53 expression. J. Nutr. Biochem. 2013;24:986–999. doi: 10.1016/j.jnutbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Bhatia N., Agarwal C., Agarwal R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr. Cancer. 2001;39:292–299. doi: 10.1207/S15327914nc392_20. [DOI] [PubMed] [Google Scholar]
- 125.Liu K.-C., Huang A.-C., Wu P.-P., Lin H.-Y., Chueh F.-S., Yang J.-S., Lu C.-C., Chiang J.-H., Meng M., Chung J.-G. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and-9 signaling pathways. Oncol. Rep. 2011;26:177–184. doi: 10.3892/or.2011.1264. [DOI] [PubMed] [Google Scholar]
- 126.Wang R., Ma L., Weng D., Yao J., Liu X., Jin F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 2016;35:3075–3083. doi: 10.3892/or.2016.4690. [DOI] [PubMed] [Google Scholar]
- 127.Kaur M., Velmurugan B., Rajamanickam S., Agarwal R., Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sherin L., Sohail A., Shujaat S. Time-dependent AI-modeling of the anticancer efficacy of synthesized gallic acid analogues. Comput. Biol. Chem. 2019;79:137–146. doi: 10.1016/j.compbiolchem.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Y., Pei J., Zheng Y., Miao Y.-J., Duan B.-Z., Huang L.-F. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2021;28:1–11. doi: 10.1007/s11655-021-3345-2. [DOI] [PubMed] [Google Scholar]
- 130.Yeh R.-D., Chen J.-C., Lai T.-Y., Yang J.-S., Yu C.-S., Chiang J.-H., Lu C.-C., Yang S.-T., Yu C.-C., Chang S.-J. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer. Res. 2011;31:2821–2832. [PubMed] [Google Scholar]
- 131.Ji B.-C., Hsu W.-H., Yang J.-S., Hsia T.-C., Lu C.-C., Chiang J.-H., Yang J.-L., Lin C.-H., Lin J.-J., Suen L.-J.W., et al. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J. Agric. Food Chem. 2009;57:7596–7604. doi: 10.1021/jf901308p. [DOI] [PubMed] [Google Scholar]
- 132.Agarwal C., Tyagi A., Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 133.Kawada M., Ohno Y., Ri Y., Ikoma T., Yuugetu H., Asai T., Watanabe M., Yasuda N., Akao S., Takemura G., et al. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anti-Cancer Drugs. 2001;12:847–852. doi: 10.1097/00001813-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 134.Liang C.-Z., Zhang X., Li H., Tao Y.-Q., Tao L.-J., Yang Z.-R., Zhou X.-P., Shi Z.-L., Tao H.-M. Gallic acid induces the apoptosis of human osteosarcoma cells in vitro and in vivo via the regulation of mitogen-activated protein kinase pathways. Cancer Biother. Radiopharm. 2012;27:701–710. doi: 10.1089/cbr.2012.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Booth B.W., Inskeep B.D., Shah H., Park J.P., Hay E.J., Burg K.J. Tannic acid preferentially targets estrogen receptor-positive breast cancer. Int. J. Breast Cancer. 2013;2013:369609. doi: 10.1155/2013/369609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ngobili T.A., Shah H., Park J.P., Kwist K.W., Inskeep B., Burg K.J., Booth B.W. Remodeling of tannic acid crosslinked collagen type I induces apoptosis in ER+ breast cancer cells. Anticancer Res. 2015;35:1285–1290. [PubMed] [Google Scholar]
- 137.Jordan L.G., Booth B.W. HER2+ breast cancer cells undergo apoptosis upon exposure to tannic acid released from remodeled cross-linked collagen type I. J. Biomed. Mater. Res. Part A. 2018;106:26–32. doi: 10.1002/jbm.a.36205. [DOI] [PubMed] [Google Scholar]
- 138.Karakurt S., Adali O. Tannic acid inhibits proliferation, migration, invasion of prostate cancer and modulates drug metabolizing and antioxidant enzymes. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016;16:781–789. doi: 10.2174/1871520616666151111115809. [DOI] [PubMed] [Google Scholar]
- 139.Ta L.T., Nguyen T.T.K., Yoo H. Tannic acid-induced apoptosis in FaDu hypopharyngeal squamous cell carcinoma. Int. J. Oral Biol. 2019;44:43–49. doi: 10.11620/IJOB.2019.44.2.43. [DOI] [Google Scholar]
- 140.Ceci C., Tentori L., Atzori M.G., Lacal P.M., Bonanno E., Scimeca M., Cicconi R., Mattei M., De Martino M.G., Vespasiani G., et al. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients. 2016;8:744. doi: 10.3390/nu8110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dahiya R., Mohammad T., Gupta P., Haque A., Alajmi M.F., Hussain A., Hassan M.I. Molecular interaction studies on ellagic acid for its anticancer potential targeting pyruvate dehydrogenase kinase 3. RSC Adv. 2019;9:23302–23315. doi: 10.1039/C9RA02864A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gupta P., Mohammad T., Khan P., Alajmi M.F., Hussain A., Rehman M.T., Hassan M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019;118:109245. doi: 10.1016/j.biopha.2019.109245. [DOI] [PubMed] [Google Scholar]
- 143.Liu Q., Liang X., Niu C., Wang X. Ellagic acid promotes A549 cell apoptosis via regulating the phosphoinositide 3-kinase/protein kinase B pathway. Exp. Ther. Med. 2018;16:347–352. doi: 10.3892/etm.2018.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei R., Mao L., Xu P., Zheng X., Hackman R.M., Mackenzie G.G., Wang Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018;9:5682–5696. doi: 10.1039/C8FO01397G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu L., Ju Y., Wang J., Zhou R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. -Res. Pract. 2017;213:1242–1250. doi: 10.1016/j.prp.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 146.Chen Y., Wang X.-Q., Zhang Q., Zhu J.-Y., Li Y., Xie C.-F., Li X.-T., Wu J.-S., Geng S.-S., Zhong C.-Y., et al. (−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Nutrients. 2017;9:572. doi: 10.3390/nu9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshimura H., Yoshida H., Matsuda S., Ryoke T., Ohta K., Ohmori M., Yamamoto S., Kiyoshima T., Kobayashi M., Sano K. The therapeutic potential of epigallocatechin-3-gallate against human oral squamous cell carcinoma through inhibition of cell proliferation and induction of apoptosis: In vitro and in vivo murine xenograft study. Mol. Med. Rep. 2019;20:1139–1148. doi: 10.3892/mmr.2019.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reddy T.C., Reddy D.B., Aparna A., Arunasree K.M., Gupta G., Achari C., Reddy G., Lakshmipathi V., Subramanyam A., Reddanna P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: Downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol. In Vitro. 2012;26:396–405. doi: 10.1016/j.tiv.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 149.Kong F.-T., He C.-X., Kong F.-L., Han S.-F., Kong X.-S., Han W.-Q., Yang L.-X. Grape Seed Procyanidins Inhibit the Growth of Breast Cancer MCF-7 Cells by Down-Regulating the EGFR/VEGF/MMP9 Pathway. Nat. Prod. Commun. 2021;16:1934578X21991691. doi: 10.1177/1934578X21991691. [DOI] [Google Scholar]
- 150.Wu Y.-Y., Cao T.-T., Liu C.-L. Combined effect of vorinostat and grape seed proanthocyanidins on modulation of thymidine phosphorylase in non-small cell lung cancer. Trop. J. Pharm. Res. 2015;14:953–959. doi: 10.4314/tjpr.v14i6.3. [DOI] [Google Scholar]
- 151.Fujiki H., Sueoka E., Watanabe T., Suganuma M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015;141:1511–1522. doi: 10.1007/s00432-014-1899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pan M.-H., Chiou Y.-S., Wang Y.-J., Ho C.-T., Lin J.-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011;2:101–110. doi: 10.1039/c0fo00174k. [DOI] [PubMed] [Google Scholar]
- 153.Philips B.J., Coyle C.H., Morrisroe S.N., Chancellor M.B., Yoshimura N. Induction of apoptosis in human bladder cancer cells by green tea catechins. Biomed. Res. 2009;30:207–215. doi: 10.2220/biomedres.30.207. [DOI] [PubMed] [Google Scholar]
- 154.Moudi M., Go R., Yien C.Y.S., Nazre M. Vinca alkaloids. Int. J. Prev. Med. 2013;4:1231. [PMC free article] [PubMed] [Google Scholar]
- 155.Dhyani P., Quispe C., Sharma E., Bahukhandi A., Sati P., Attri D.C., Szopa A., Sharifi-Rad J., Docea A.O., Mardare I., et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22:206. doi: 10.1186/s12935-022-02624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trybus W., Trybus E., Król T. Emodin Sensitizes Cervical Cancer Cells to Vinblastine by Inducing Apoptosis and Mitotic Death. Int. J. Mol. Sci. 2022;23:8510. doi: 10.3390/ijms23158510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meyers M.A. Happy Accidents: Serendipity in Modern Medical Breakthroughs. Arcade Publishing; New York, NY, USA: 2007. [Google Scholar]
- 158.Arora R., Malhotra P., Mathur A.K., Mathur A., Govil C., Ahuja P. Anticancer alkaloids of Catharanthus roseus: Transition from traditional to modern medicine. Herbal Medicine: A Cancer Chemopreventive and Therapeutic Perspective. Jaypee Brothers Medical Publishers Pvt. Ltd.; New Delhi, India: 2010. pp. 292–310. [Google Scholar]
- 159.Škubník J., Pavlíčková V.S., Ruml T., Rimpelová S. Vincristine in combination therapy of cancer: Emerging trends in clinics. Biology. 2021;10:849. doi: 10.3390/biology10090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goa K.L., Faulds D. Vinorelbine: A review of its pharmacological properties and clinical use in cancer chemotherapy. Drugs Aging. 1994;5:200–234. doi: 10.2165/00002512-199405030-00006. [DOI] [PubMed] [Google Scholar]
- 161.Bennouna J., Delord J.-P., Campone M., Nguyen L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008;14:1625–1632. doi: 10.1158/1078-0432.CCR-07-2219. [DOI] [PubMed] [Google Scholar]
- 162.Bhattacharyya B., Panda D., Gupta S., Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008;28:155–183. doi: 10.1002/med.20097. [DOI] [PubMed] [Google Scholar]
- 163.Lin Z.-Y., Wu C.-C., Chuang Y.-H., Chuang W.-L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013;93:323–328. doi: 10.1016/j.lfs.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 164.Roy S., Khan S., Jairajpuri D.S., Hussain A., Alajmi M.F., Islam A., Luqman S., Parvez S., Hassan M.I. Investigation of sphingosine kinase 1 inhibitory potential of cinchonine and colcemid targeting anticancer therapy. J. Biomol. Struct. Dyn. 2022;40:6350–6362. doi: 10.1080/07391102.2021.1882341. [DOI] [PubMed] [Google Scholar]
- 165.Alim A., Goze I., Goze H.M., Tepe B., Serkedjieva J. In vitro antimicrobial and antiviral activities of the essential oil and various extracts of Salvia cedronella Boiss. J. Med. Plants Res. 2009;3:413–419. [Google Scholar]
- 166.Tomko A.M., Whynot E.G., Ellis L.D., Dupré D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12:1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chung K.-S., Hong J.Y., Lee J.-H., Lee H.-J., Park J.Y., Choi J.-H., Park H.-J., Hong J., Lee K.-T. β-caryophyllene in the essential oil from chrysanthemum boreale induces G1 phase cell cycle arrest in human lung cancer cells. Molecules. 2019;24:3754. doi: 10.3390/molecules24203754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arul S., Rajagopalan H., Ravi J., Dayalan H. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2020;20:1530–1537. doi: 10.2174/1871520620666200227093216. [DOI] [PubMed] [Google Scholar]
- 169.Annamalai V., Kotakonda M., Periyannan V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020;34:e22514. doi: 10.1002/jbt.22514. [DOI] [PubMed] [Google Scholar]
- 170.Deng Q.-P., Wang M.-J., Zeng X., Chen G.G., Huang R.-Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017;41:1383–1392. doi: 10.1159/000467897. [DOI] [PubMed] [Google Scholar]
- 171.Kato T., Horie N., Hashimoto K., Satoh K., Shimoyama T., Kaneko T., Kusama K., Sakagami H. Bimodal effect of glycyrrhizin on macrophage nitric oxide and prostaglandin E2 production. In Vivo. 2008;22:583–586. [PubMed] [Google Scholar]
- 172.Saleh M., Hashem F., Glombitza K. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var. filicinum. Pharm. Pharmacol. Lett. 1998;8:98. [Google Scholar]
- 173.Ferraz R.P., Bomfim D.S., Carvalho N.C., Soares M.B., da Silva T.B., Machado W.J., Prata A.P.N., Costa E.V., Moraes V.R.S., Nogueira P.C.L., et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae) Phytomedicine. 2013;20:615–621. doi: 10.1016/j.phymed.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 174.Silva S.L.D., Figueiredo P.M., Yano T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007;37:281–286. doi: 10.1590/S0044-59672007000200015. [DOI] [Google Scholar]
- 175.Sobral M.V., Xavier A.L., Lima T.C., de Sousa D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014;2014:953451. doi: 10.1155/2014/953451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye Z., Liang Z., Mi Q., Guo Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON./Off. J. Balk. Union. Oncol. 2020;25:280–285. [PubMed] [Google Scholar]
- 177.Jia S.-S., Xi G.-P., Zhang M., Chen Y.-B., Lei B., Dong X.-S., Yang Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013;29:349–354. doi: 10.3892/or.2012.2093. [DOI] [PubMed] [Google Scholar]
- 178.Hafidh R.R., Hussein S.Z., MalAllah M.Q., Abdulamir A.S., Abu Bakar F. A high-throughput quantitative expression analysis of cancer-related genes in human HepG2 cells in response to limonene, a potential anticancer agent. Curr. Cancer Drug Targets. 2018;18:807–815. doi: 10.2174/1568009617666171114144236. [DOI] [PubMed] [Google Scholar]
- 179.Berliocchi L., Chiappini C., Adornetto A., Gentile D., Cerri S., Russo R., Bagetta G., Corasaniti M.T. Early LC3 lipidation induced by d-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine. 2018;40:98–105. doi: 10.1016/j.phymed.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 180.Yu X., Lin H., Wang Y., Lv W., Zhang S., Qian Y., Deng X., Feng N., Yu H., Qian B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018;11:1833–1847. doi: 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Manuele M.G., Barreiro Arcos M.L., Davicino R., Ferraro G., Cremaschi G., Anesini C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2009;28:135–145. doi: 10.3109/07357900903179583. [DOI] [PubMed] [Google Scholar]
- 182.Uedo N., Tatsuta M., Iishi H., Baba M., Sakai N., Yano H., Otani T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999;137:131–136. doi: 10.1016/S0304-3835(98)00340-1. [DOI] [PubMed] [Google Scholar]
- 183.Miller J.A., Pappan K., Thompson P.A., Want E.J., Siskos A.P., Keun H.C., Wulff J., Hu C., Lang J.E., Chow H.-H.S. Plasma Metabolomic Profiles of Breast Cancer Patients after Short-term Limonene InterventionMetabolomics of Limonene Intervention. Cancer Prev. Res. 2015;8:86–93. doi: 10.1158/1940-6207.CAPR-14-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hou J., Zhang Y., Zhu Y., Zhou B., Ren C., Liang S., Guo Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:6631. doi: 10.12659/MSM.916419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu Q., Li M., Yang M., Yang J., Xie J., Lu X., Wang F., Chen W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018;38:BSR20180980. doi: 10.1042/BSR20180980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao Y., Chen R., Wang Y., Yang Y. α-Pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy. 2018;63:1–7. doi: 10.1159/000479863. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Z., Guo S., Liu X., Gao X. Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC) Drug Res. 2015;65:214–218. doi: 10.1055/s-0034-1377025. [DOI] [PubMed] [Google Scholar]
- 188.Yao Y.-Q., Ding X., Jia Y.-C., Huang C.-X., Wang Y.-Z., Xu Y.-H. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008;264:127–134. doi: 10.1016/j.canlet.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 189.Wang G., Li X., Huang F., Zhao J., Ding H., Cunningham C., Coad J., Flynn D., Reed E., Li Q. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. CMLS. 2005;62:881–893. doi: 10.1007/s00018-005-5017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li X., Wang G., Zhao J., Ding H., Cunningham C., Chen F., Flynn D., Reed E., Li Q. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell. Mol. Life Sci. CMLS. 2005;62:894–904. doi: 10.1007/s00018-005-5027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu Z., Wang R., Xu L., Xie S., Dong J., Jing Y. β-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS ONE. 2011;6:e15843. doi: 10.1371/journal.pone.0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li Q.Q., Wang G., Huang F., Banda M., Reed E. Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010;62:1018–1027. doi: 10.1111/j.2042-7158.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 193.Chen W., Lu Y., Wu J., Gao M., Wang A., Xu B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011;67:799–808. doi: 10.1007/s00280-010-1378-x. [DOI] [PubMed] [Google Scholar]
- 194.Jiang S., Ling C., Li W., Jiang H., Zhi Q., Jiang M. Molecular mechanisms of anti-cancer activities of β-elemene: Targeting hallmarks of cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016;16:1426–1434. doi: 10.2174/1871520616666160211123424. [DOI] [PubMed] [Google Scholar]
- 195.Assmann C.E., Cadoná F.C., Bonadiman B.d.S.R., Dornelles E.B., Trevisan G., da Cruz I.B.M. Tea tree oil presents in vitro antitumor activity on breast cancer cells without cytotoxic effects on fibroblasts and on peripheral blood mononuclear cells. Biomed. Pharmacother. 2018;103:1253–1261. doi: 10.1016/j.biopha.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 196.Jamali T., Kavoosi G., Ardestani S.K. In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J. Ethnopharmacol. 2020;248:112313. doi: 10.1016/j.jep.2019.112313. [DOI] [PubMed] [Google Scholar]
- 197.Döll-Boscardin P.M., Sartoratto A., Sales Maia B.H.L.d.N., Padilha de Paula J., Nakashima T., Farago P.V., Kanunfre C.C. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid.-Based Complement. Altern. Med. 2012;2012:342652. doi: 10.1155/2012/342652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marrelli M., Conforti F., Formisano C., Rigano D., Arnold N.A., Menichini F., Senatore F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016;30:735–739. doi: 10.1080/14786419.2015.1040993. [DOI] [PubMed] [Google Scholar]
- 199.Ambrož M., Matoušková P., Skarka A., Zajdlová M., Žáková K., Skálová L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules. 2017;22:1021. doi: 10.3390/molecules22061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ambrož M., Boušová I., Skarka A., Hanušová V., Králová V., Matoušková P., Szotáková B., Skálová L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules. 2015;20:15343–15358. doi: 10.3390/molecules200815343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ryabchenko B., Tulupova E., Schmidt E., Wlcek K., Buchbauer G., Jirovetz L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008;3:1934578X0800300710. doi: 10.1177/1934578X0800300710. [DOI] [Google Scholar]
- 202.Boris R., Elena T., Erich S., Walter J., Gerhard B., Leopold J. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil Bear. Plants. 2011;14:316–319. doi: 10.1080/0972060X.2011.10643940. [DOI] [Google Scholar]
- 203.Tatman D., Mo H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002;175:129–139. doi: 10.1016/S0304-3835(01)00723-6. [DOI] [PubMed] [Google Scholar]
- 204.Wang H.-L., Chang J.-C., Fang L.-W., Hsu H.-F., Lee L.-C., Yang J.-F., Liang M.-T., Hsiao P.-C., Wang C.-P., Wang S.-W., et al. Bulnesia sarmientoi supercritical fluid extract exhibits necroptotic effects and anti-metastatic activity on lung cancer cells. Molecules. 2018;23:3304. doi: 10.3390/molecules23123304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang Q., Wu J., Luo Y., Huang N., Zhen N., Zhou Y., Sun F., Li Z., Pan Q., Li Y. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget. 2016;7:62585. doi: 10.18632/oncotarget.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ovais M., Hoque M.Z., Khalil A.T., Ayaz M., Ahmad I. Biogenic Nanoparticles for Cancer Theranostics. Elsevier; Amsterdam, The Netherlands: 2021. Mechanisms underlying the anticancer applications of biosynthesized nanoparticles; pp. 229–248. [Google Scholar]
- 207.Ayaz M., Nawaz A., Ahmad S., Mosa O.F., Eisa Hamdoon A.A., Khalifa M.A., Sadiq A., Ullah F., Wadood A., Kabra A., et al. Underlying anticancer mechanisms and synergistic combinations of phytochemicals with cancer chemotherapeutics: Potential benefits and risks. J. Food Qual. 2022;2022:1–15. doi: 10.1155/2022/1189034. [DOI] [Google Scholar]
- 208.Kowshik J., Giri H., Kranthi Kiran Kishore T., Kesavan R., Naik Vankudavath R., Bhanuprakash Reddy G., Dixit M., Nagini S. Ellagic acid inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the hamster cheek pouch carcinogenesis model. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014;14:1249–1260. doi: 10.2174/1871520614666140723114217. [DOI] [PubMed] [Google Scholar]
- 209.Liu R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 210.Peluso I., Yarla N.S., Ambra R., Pastore G., Perry G. Proceedings of Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2019. MAPK signalling pathway in cancers: Olive products as cancer preventive and therapeutic agents; pp. 185–195. [DOI] [PubMed] [Google Scholar]
- 211.Liao X.Z., Gao Y., Sun L.L., Liu J.H., Chen H.R., Yu L., Chen Z.Z., Chen W.H., Lin L.Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytother. Res. 2020;34:1142–1153. doi: 10.1002/ptr.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Granado-Serrano A.B., Martín M.A., Bravo L., Goya L., Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J. Nutr. 2006;136:2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 213.Lu Y., Jiang F., Jiang H., Wu K., Zheng X., Cai Y., Katakowski M., Chopp M., To S.-S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010;641:102–107. doi: 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shukla V., Chandra V., Sankhwar P., Popli P., Kaushal J.B., Sirohi V.K., Dwivedi A. Phytoestrogen genistein inhibits EGFR/PI3K/NF-kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Adv. 2015;5:56075–56085. doi: 10.1039/C5RA06167A. [DOI] [Google Scholar]
- 215.Anwar S., Shahwan M., Hasan G.M., Islam A., Hassan M.I. Microtubule-affinity regulating kinase 4: A potential drug target for cancer therapy. Cell. Signal. 2022;99:110434. doi: 10.1016/j.cellsig.2022.110434. [DOI] [PubMed] [Google Scholar]
- 216.Tung N.H., Du G.-J., Yuan C.-S., Shoyama Y., Wang C.-Z. Isolation and chemopreventive evaluation of novel naphthoquinone compounds from Alkanna tinctoria. Anti-Cancer Drugs. 2013;24 doi: 10.1097/CAD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhong Y., Krisanapun C., Lee S.-H., Nualsanit T., Sams C., Peungvicha P., Baek S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer. 2010;46:3365–3374. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jeon Y.-J., Jang J.-Y., Shim J.-H., Myung P.K., Chae J.-I. Esculetin, a coumarin derivative, exhibits anti-proliferative and pro-apoptotic activity in G361 human malignant melanoma. J. Cancer Prev. 2015;20:106. doi: 10.15430/JCP.2015.20.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Anand J.R., Rijhwani H., Malapati K., Kumar P., Saikia K., Lakhar M. Anticancer activity of esculetin via-modulation of Bcl-2 and NF-κB expression in benzo [a] pyrene induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2013;3:107–112. doi: 10.1016/j.bionut.2012.11.004. [DOI] [Google Scholar]
- 220.Raju J., Patlolla J.M., Swamy M.V., Rao C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2004;13:1392–1398. doi: 10.1158/1055-9965.1392.13.8. [DOI] [PubMed] [Google Scholar]
- 221.Khan M., Xiao Y., Yu B., Wang N., Rasul A., Yi F., Yang L., Yang H., Ma T. Artabotryside A, a constituent from Descurainia sophia (L.) induces cell death in U87 glioma cells through apoptosis and cell cycle arrest at G2/M phase. J. Med. Plants Res. 2012;6:3754–3765. [Google Scholar]
- 222.Kampa M., Alexaki V.-I., Notas G., Nifli A.-P., Nistikaki A., Hatzoglou A., Bakogeorgou E., Kouimtzoglou E., Blekas G., Boskou D., et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004;6:R63. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ashraf M.A., Sayed S., Bello M., Hussain N., Chando R.K., Alam S., Hasan M.K. CDK4 as a phytochemical based anticancer drug target. Inform. Med. Unlocked. 2022;28:100826. doi: 10.1016/j.imu.2021.100826. [DOI] [Google Scholar]
- 225.Motallebi M., Bhia M., Rajani H.F., Bhia I., Tabarraei H., Mohammadkhani N., Pereira-Silva M., Kasaii M.S., Nouri-Majd S., Mueller A.-L., et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022;305:120752. doi: 10.1016/j.lfs.2022.120752. [DOI] [PubMed] [Google Scholar]
- 226.Vuddanda P.R., Chakraborty S., Singh S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs. 2010;19:1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- 227.Kashyap D., Sharma A., Sak K., Tuli H.S., Buttar H.S., Bishayee A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018;194:75–87. doi: 10.1016/j.lfs.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 228.Janicke B., Hegardt C., Krogh M., Önning G., Åkesson B., Cirenajwis H.M., Oredsson S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer. 2011;63:611–622. doi: 10.1080/01635581.2011.538486. [DOI] [PubMed] [Google Scholar]
- 229.Stan S.D., Zeng Y., Singh S.V. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer. 2008;60:51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bennett R.L., Licht J.D. Targeting epigenetics in cancer. Annu. Rev. Pharmacol. Toxicol. 2018;58:187–207. doi: 10.1146/annurev-pharmtox-010716-105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the associated Data is contained within the article.