Abstract
Background: Many preclinical studies have demonstrated the effectiveness of genetically modified probiotics (gm probiotics) in animal models of inflammatory bowel disease (IBD). Objective: This systematic review was performed to investigate the role of gm probiotics in treating IBD and to clarify the involved mechanisms. Methods: PubMed, Web of Science, Cochrane Library, and Medline were searched from their inception to 18 September 2022 to identify preclinical and clinical studies exploring the efficacy of gm probiotics in IBD animal models or IBD patients. Two independent researchers extracted data from the included studies, and the data were pooled by the type of study; that is, preclinical or clinical. Results: Forty-five preclinical studies were included. In these studies, sodium dextran sulfate and trinitrobenzene sulfonic acid were used to induce colitis. Eleven probiotic species have been genetically modified to produce therapeutic substances, including IL-10, antimicrobial peptides, antioxidant enzymes, and short-chain fatty acids, with potential therapeutic properties against colitis. The results showed generally positive effects of gm probiotics in reducing disease activity and ameliorating intestinal damage in IBD models; however, the efficacy of gm probiotics compared to that of wild-type probiotics in many studies was unclear. The main mechanisms identified include modulation of the diversity and composition of the gut microbiota, production of regulatory metabolites by beneficial bacteria, reduction of the pro- to anti-inflammatory cytokine ratio in colonic tissue and plasma, modulation of oxidative stress activity in the colon, and improvement of intestinal barrier integrity. Moreover, only one clinical trial with 10 patients with Crohn’s disease was included, which showed that L. lactis producing IL-10 was safe, and a decrease in disease activity was observed in these patients. Conclusions: Gm probiotics have a certain efficacy in colitis models through several mechanisms. However, given the scarcity of clinical trials, it is important for researchers to pay more attention to gm probiotics that are more effective and safer than wild-type probiotics to facilitate further clinical translation.
Keywords: inflammatory bowel disease, colitis, genetically modified probiotics, efficacy
1. Introduction
Inflammatory bowel disease (IBD), largely classified as Crohn’s disease (CD) or ulcerative colitis (UC), is a chronic intestinal inflammatory disorder mediated by genetic, immune, microbial, and environmental factors [1]. However, its precise etiology has not yet been clarified [2]. Over the last few decades, the incidence of IBD has risen rapidly not only in Western countries [3] but also in Asian countries such as China and India, entailing a growing socioeconomic burden [4]. Considering that chronic inflammation in the gastrointestinal tract (GIT) is ultimately a dysregulated and overactive immune response to the destruction of the intestinal environment in the host, immune abnormalities, such as adaptive and innate immunity, have been mostly explored in the investigation of IBD pathogenesis [2]. Consequently, in addition to conventional therapies such as 5-ASAs, antibiotics, and steroids, immunosuppressive drugs, including immunomodulators and biologics, are widely used in an attempt to regulate compromised immune homeostasis and achieve positive efficacy in the treatment of many IBD patients [5]. However, the effectiveness of these drugs is individually specific and may be limited by their short half-life in vivo, instability in the upper GIT, and systemic side effects caused by intravenous and subcutaneous administration.
Probiotics are live microorganisms similar to beneficial bacteria that are naturally present in the human GIT and provide beneficial health effects when orally administered in adequate amounts [6]. To date, they have been widely used to prevent and treat various medical conditions, including IBD, irritable bowel syndrome, Helicobacter pylori infections, Clostridium difficile infections, antibiotic-associated diarrhea, Parkinson’s disease, and even cancer [7,8,9]. The probiotic cell-free supernatant has also attracted attention as a safe and targeted alternative therapy and has been reported to alleviate indomethacin-induced colitis resembling Crohn’s disease by downregulating the inflammatory response and oxidative stress [10]. In recent years, probiotics have been shown to be effective vehicles for the delivery of therapeutic substances to treat specific conditions [11], which has raised the opportunity for researchers to genetically modify them to develop more pragmatic probiotics that produce and deliver IBD therapeutic proteins to the GIT locally, with lower cost, greater effectiveness, and fewer side effects than conventional immunosuppressive drugs administered by injection [12].
Although many in vivo and in vitro experimental studies have suggested the possible effectiveness of gm probiotics in different animal models of IBD, there is still little evidence of their effects compared with those of wild-type probiotics. Hence, we conducted this systematic review to summarize the efficacy of different gm probiotics compared to wild-type probiotics in the treatment of IBD in animal models and patients and to investigate the specific effects and main mechanisms involved. Additionally, a critical assessment of preclinical and clinical experiments was conducted to identify their methodological weakness to serve as guidance for future research.
2. Methods
2.1. Literature Search
The PubMed, Web of Science, Cochrane Library, and Medline databases were searched systematically from their inception to 18 September 2022, by two independent researchers to identify related preclinical and clinical studies. The lists of references from the included studies were manually searched to identify other possible studies. The present study was designed and conducted in line with the recommendations included in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. This systematic review was registered in PROSPERO, and the registration ID is CRD42022351738.
2.2. Eligibility Criteria
In this systematic review, the PICOS strategy was used to identify studies that met the following inclusion criteria:
Population: rodents with colitis and patients with IBD;
Intervention: supplementation with gm probiotics;
Comparisons: placebo; wild-type probiotics, etc.;
Outcomes: weight loss, colon length, disease activity, intestinal damage, anti- and pro-inflammatory cytokines, oxidative stress-related indicators, mucosal barrier function, etc.
Study design: preclinical studies, randomized controlled trials, cohort studies, etc.
2.3. Exclusion Criteria
The exclusion criteria included the following:
Duplicated studies;
In vitro studies or studies not related to our research topic;
Papers published in a language other than English;
Publication type: reviews, meta-analyses, and consensus papers
Papers without the data we focused on or without full text.
2.4. Data Extraction and Risk of Bias
Two authors independently extracted the following variables from each preclinical study: first author, publication year, country, experimental model features (lineage, sex, age, and type of colitis), research method (number of groups, number of animals per group, wild-type probiotics, constructed plasmid, recombinant probiotics, therapeutic substances, administration method, dose, and duration), and the outcomes mentioned in Section 2.2. For clinical studies, variables including the first author, publication year, country, population features (sex, age, and number of participants), experimental design, intervention (gm probiotics, dose and frequency of administration, and duration), and main outcomes were collected.
The criteria set in Animal Research: Reporting In Vivo experiments (ARRIVE) guidelines [14] were used to evaluate the risk of bias in preclinical studies in vivo, and the criteria proposed by Downs and Black were used to assess the risk of bias in clinical studies [15]. The quality of the clinical studies was classified by total scores as poor (≤4 of 13 points), intermediate (5–8 of 13 points), or good (≥9 of 13 points).
If there were any inconsistencies in the process of data extraction and the risk of bias assessment, the two authors discussed these issues, or an independent expert in this field was consulted to reach a consensus.
3. Results
3.1. Study Selection
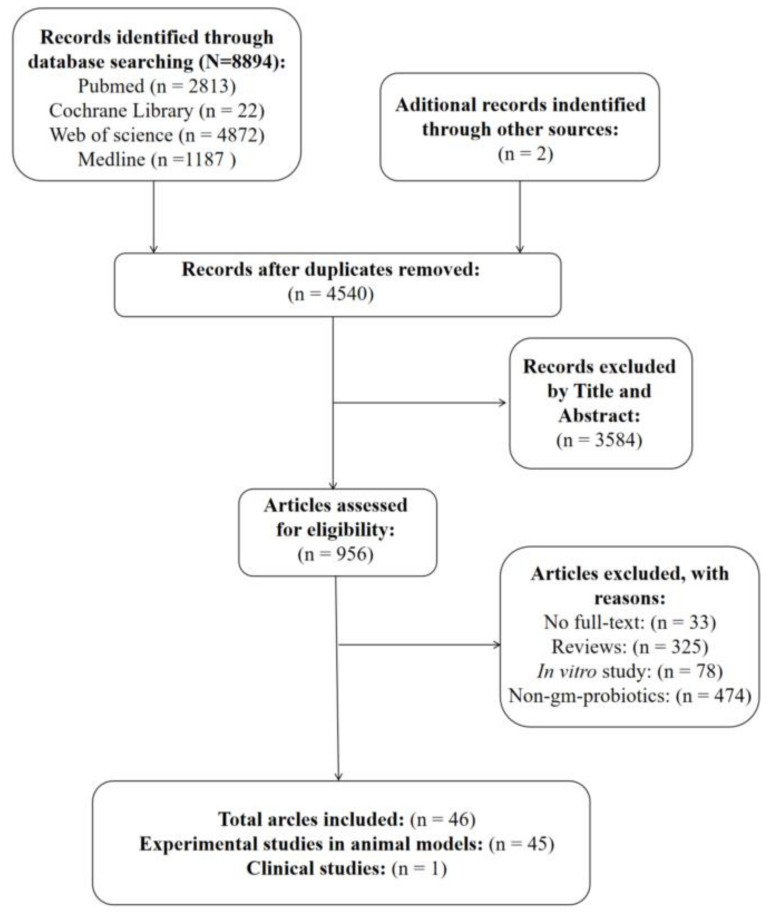
As shown in Figure 1, 8894 records were identified in the initial database search. A manual search of the reference lists yielded two additional studies. After removing duplicates, 4540 studies were reviewed by titles and abstracts, of which 3584 studies were excluded because they were not associated with our research topic. The full texts of the remaining 956 papers were further screened, and 910 articles were excluded; the reasons for this are shown in Figure 1. Finally, 45 preclinical studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] and 1 clinical study [61] fulfilled the inclusion criteria for this systematic review.
Figure 1.
Flow diagram of the literature search and screening process.
3.2. Qualitative Data
The general characteristics of all the included preclinical studies are presented in Table 1. All eligible preclinical studies were published after 2000 and were conducted in 13 countries. The animal lineages used for modeling included BALB/c mice (n = 19), C57BL/6 mice (n = 22), Wistar rats (n = 2), and Sprague Dawley (SD) rats (n = 2). Most studies used male animals (n = 23), and only two studies used both male and female animals; four studies did not report sex. The age of the animals ranged from 2 to 20 weeks, although four studies did not report this information. Regarding the colitis models, dextran sulphate sodium (DSS) (n = 34) was most commonly used to induce colitis, followed by trinitrobenzene sulfonic acid (TNBS) (n = 10), dinitro-benzenesulfonic acid (DNBs) (n = 2), anti-CD3 antibody (n = 1), IL-10 knockout (IL-10−/−) (n = 4), and T-cell transfer (n = 1); interestingly, five studies used more than one type of colitis model.
Table 1.
Characteristics of preclinical studies.
| Author | Year | Country | Lineage | Sex * | Age (Week) | Number of Groups | Number of Animals | Model | Acute/Chronic Course |
|---|---|---|---|---|---|---|---|---|---|
| Del Carmen et al. [29] | 2015 | Argentina | BALB/C mice | Female | 5 | 4 | 32 | TNBS-induced colitis | Acute |
| Gardlik et al. [21] | 2012 | Slovak Republic | C57BL/6 mice | Male | 10 | 6 | 60 | DSS-induced colitis | Acute |
| Foligné et al. [17] | 2006 | France | BALB/C mice | Female | 7–8 | 4 | 32–48 | TNBS-induced colitis | Acute |
| Del Carmen et al. [26] | 2014 | Argentina | BALB/C mice | Female | 5 | 5 | 90 | TNBS induced colitis | Chronic |
| Martín et al. [27] | 2014 | France | C57BL/6 mice | Male | 6–8 | 4 | 64 | DNBs induced colitis | Chronic |
| Steidler et al. [16] | 2000 | Belgium | BALB/C mice | Female | N.A. | 13 | 130 | DSS induced colitis | Chronic |
| 129 SvIEv IL-10−/− mice | Female | 3–7 | 3 | 15 | IL-10−/− mice | Chronic | |||
| Hamady et al. [23] | 2013 | Britain | C57BL/6 mice | Male | 8 | 7 | 56 | DSS induced colitis | Acute |
| Bermúdez-Humarán et al. [28] | 2015 | France | C57BL/6 mice | N.A. | 6–8 | 10 | 60–80 | DSS induced colitis | Acute |
| Liu et al. [36] | 2020 | Taiwan | C57BL/6JNarl mice | Male | 7–8 | 7 | 38 | DSS induced colitis | Acute |
| Chiabai et al. [33] | 2019 | Brazil | C57BL/6 mice | Female | 10 | 4 | 16–20 | DSS induced colitis | Acute |
| Namai et al. [37] | 2020 | Japan | C57BL/6 mice | Female | 7 | 2 | 36 | DSS induced colitis | Acute |
| Zhang et al. [32] | 2018 | China | BALB/C mice | Male | 6–8 | 4 | 40 | DSS induced colitis | Acute |
| Wang et al. [35] | 2019 | China | C57BL/6 mice | Female | 6–8 | 6 | 30 | DSS induced colitis | Acute |
| Zhang et al. [24] | 2013 | China | BALB/C mice | Female | 7 | 5 | 40 | DSS induced colitis | Acute |
| Xie et al. [31] | 2017 | China | Wistar rats | Male | 9–10 | 4 | 48 | TNBS induced colitis | Acute |
| LeBlanc et al. [20] | 2011 | Argentina | BALB/C mice | Female | 5 | 5 | 90 | TNBS induced colitis | Acute |
| Del Carmen et al. [25] | 2014 | Argentina | BALB/C mice | Female | 5 | 6 | 36 | TNBS induced colitis | Chronic |
| Han et al. [18] | 2006 | France | Wistar rats | Male | N.A. | 15 | 110 | TNBS induced colitis | Acute |
| Wong et al. [22] | 2012 | China | BALB/C mice | Male | 6–8 | 10 | 94 | DSS induced colitis | Acute |
| Li et al. [40] | 2021 | China | C57BL/6 mice | Male | 7–8 | 6 | 30 | DSS induced colitis | Acute |
| Zeng et al. [38] | 2020 | China | C57BL/6 mice | Male | 6–8 | 4 | 28 | DSS induced colitis | Acute |
| Esposito et al. [39] | 2021 | Italy | C57BL/6J mice | Male | 6 | 7 | 70 | DSS induced colitis | Acute |
| Park et al. [41] | 2021 | Korea | C57BL/6J mice | Male | 8 | 6 | 60 | DSS induced colitis | Acute |
| Yan et al. [45] | 2021 | China | C57BL/6J mice | Male | 7 | 5 | 25 | DSS induced colitis | Acute |
| Yoon et al. [19] | 2008 | Korea | BALB/C mice | Female | 6 | 4 | 20 | DSS induced colitis | Acute |
| Shigemori et al. [30] | 2015 | Japan | C57BL/6 mice | Female | 7 | 4 | 39 | DSS induced colitis | Acute |
| Praveschotinunt et al. [34] | 2019 | USA | C57BL/6NCrl mice | Female | 8–9 | 8 | 38–49 | DSS induced colitis | Acute |
| Sun et al. [43] | 2021 | China | C57BL/6 mice | Male | 6–8 | 4 | 26 | DSS induced colitis | Acute |
| Scott at al [42] | 2021 | Canada | C57BL/6J mice | Male | 8–10 | 4 | 36 | TNBS induced colitis | Acute |
| Female | 8–10 | 4 | 41 | DSS induced colitis | Chronic | ||||
| Female | 8–10 | 5 | 29 | Anti-CD3 antibody-induced enteritis | Acute | ||||
| Wang et al. [44] | 2021 | China | BALB/C mice | Male | 6 | 9 | 37–53 | DSS-induced colitis | Acute |
| Wei et al. [54] | 2016 | China | SD rats | Male and Female | N.A. | 4 | 48 | DSS-induced colitis | Acute |
| Wei et al. [55] | 2016 | China | BALB/c mice | Male | 6–12 | 4 | 40 | DSS-induced colitis | Acute |
| Vandenbroucke et al. [46] | 2010 | Belgium | BALB/c mice | Female | 11 | 5 | 50 | DSS-induced colitis | Chronic |
| IL10 knockout mice | N.A. | 20 | 10 | 87 | IL10 knockout mice | Chronic | |||
| Liu et al. [53] | 2016 | China | BALB/c mice | Female | 8 | 5 | 40 | DSS-induced colitis | Acute |
| Zurita-Turk et al. [58] | 2020 | Brazil | IL-10−/− mice and wild-type mice | N.A. | 2 | 4 | About 36 | IL-10−/− | Chronic |
| Qiu et al. [49] | 2013 | China | BALB/c mice | Female | 4–6 | 8 | 64 | DSS-induced colitis | Acute |
| Yao et al. [48] | 2011 | China | BALB/c mice | Male | 6 | 5 | 50 | DSS-induced colitis | Acute |
| Hanson et al. [50] | 2014 | USA | C57BL/6 and Rag1−/− | Male | 7.5 | N.A. | N.A. | Transfer of CD4 + CD45RBhi T cells-induced colitis | Chronic |
| Whelan et al. [52] | 2014 | Germany | C57BL/6 mice | Male | 9–11 | 4 | 45 | DSS induced colitis | Acute |
| Breyner et al. [57] | 2019 | France | C57BL/6 mice | N.A. | 6–8 | 4 | N.A. | DNBS-induced colitis | Acute |
| 4 | N.A. | DSS-induced colitis | Acute | ||||||
| Liu et al. [56] | 2018 | China | SD rats | Male | N.A. | 4 | 48 | DSS-induced colitis | Acute |
| Hou et al. [51] | 2014 | China | BALB/c mice | Female | 6 | 4 | 60 | TNBS-induced colitis | Acute |
| Watterlot et al. [47] | 2010 | France | BALB/c mice | Male | 7 | 5 | 50 | DSS-induced colitis | Acute |
| Aubry et al. [60] | 2015 | France | C57BL/6 mice | N.A. | 6 | 6 | DSS-induced colitis | Acute | |
| Foligne et al. [59] | 2007 | France | BALB/c and C57BL/6 | Female | 7–9 | 4 | 40 | TNBS-induced colitis | Acute |
| 4 | 40 | IL-10−/− and TNBS-induced colitis | Acute | ||||||
| 8 | 80 | DSS-induced colitis | Acute |
* We only extracted information on the animals used to evaluate the efficacy and safety of gm probiotics.
In these experimental studies, 11 different probiotic species, including Lactococcus lactis (n = 20), Escherichia coli (n = 7), Streptococcus thermophilus (n = 2), Lactobacillus casei (n = 4), Lactobacillus paracasei (n = 1), Bacteroides ovatus (n = 1), Saccharomyces boulardii (n = 1), Lactobacillus fermentum (n = 2), Bifidobacterium longum (n = 5), Lactobacillus plantarum (n = 1), and Saccharomyces cerevisiae (n = 2) were used as chassis to be genetically modified to secrete different therapeutic substances with potential properties against colitis. In all studies except one, the animals received gm probiotics via gastric gavage at doses ranging from 105 to 4 × 1012 colony-forming units (CFUs)/day, although one study did not report the dose. Finally, the duration of the intervention ranged from 3–6 weeks (Table 2).
Table 2.
Methods used in preclinical studies.
| Author | Year | Wild-Type Probiotic Strain | Constructed Plasmid with Function | Recombinant Probiotic Name | Secretions | Administration | Dose/Day | Length |
|---|---|---|---|---|---|---|---|---|
| del Carmen et al. [29] | 2015 | S. thermophilus CRL807 | pValac::il-10 | S. thermophilus CRL 807pValac::il-10 | IL-10 | Gastric gavage | 108 CFU | 12 days |
| Gardlik et al. [21] | 2012 | E. coli Nissle 1917 | pMEC-IL10 | Nissle 1917/pMEC-IL10 | IL-10 | Gastric gavage | 109 bacteria | 7 days |
| L. lactis | pMEC-IL10 | Lactococcus lactis/pMEC-IL10 | IL-10 | Gastric gavage | 109 bacteria | 7 days | ||
| Foligné et al. [17] | 2006 | L. lactis MG1363 | N.A. | LL-mIL-10 | mIL-10 | Gastric gavage | 105 to 109 CFU | 14 days |
| del Carmen et al. [26] | 2014(a) | L. lactis MG1363 | pValac:il-10 | LL-pValac:IL-10 | mIL-10 | Gastric gavage | 109 CFU | 14 days |
| L. lactis MG1363 | pGroeESL:il-10 | LL-pGroESL:IL-10 | mIL-10 | Gastric gavage | 109 CFU | 14 days | ||
| Martín et al. [27] | 2014 | L. lactis MG1363 | pLB350 | LL-IL10 | mIL-10 | Gastric gavage | 109 CFU | 10 days |
| Steidle et al. [16] | 2000 | L. lactis | N.A. | LL-mIL-10 | mIL-10 | Gastric gavage | 2 × 107 CFU or 2 × 109 CFU | 2 weeks or 4 weeks |
| Hamady et al. [23] | 2013 | B. ovatus | N.A. | BO-KGF | KGF-2 | Gastric gavage | 2 × 108 CFU | 5 days |
| B. ovatus | N.A. | BO-TGF | TGF-β1 | Gastric gavage | 2 × 108 CFU | 5 days | ||
| Bermúdez-Humarán et al. [28] | 2015 | L. lactis MG1363 | pSEC:mIL-10 | LL-IL-10 | mIL-10 | Gastric gavage | 5 × 109 CFU | 7 days |
| L. lactis MG1363 | pSEC:mTGF-β | LL-TGF-β | TGF-β | Gastric gavage | 5 × 109 CFU | 7 days | ||
| L. lactis MG1363 | pSEC:elafin | L. lactis Elafin | Elafin | Gastric gavage | 5 × 109 CFU | 7 days | ||
| L. lactis MG1363 | pSEC: mSLPI | L. lactis SLPI | SLPI | Gastric gavage | 5 × 109 CFU | 7 days | ||
| Liu et al. [36] | 2020 | S. boulardii | N.A. | N.A. | IL-10 | Gastric gavage | 109 CFU | 5 days |
| S. boulardii | N.A. | N.A. | TNFR1-ECD | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | AP | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | ANP | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | ANPm | Gastric gavage | 109 CFU | 5 days | ||
| Chiabai et al. [33] | 2019 | L. lactis MG1363 FnBPA + (LL-F) | pValac::anti-TNFα | LL-FT | scFv of anti-TNFα antibody | Gastric gavage | 2.0–2.5 × 109 CFU | 4 days |
| Namai et al. [37] | 2020 | L. lactis NZ9000 | pNZ8148#2:SEC-IL1Ra | NZ-IL1Ra | mIL-1Ra | Gastric gavage | 1010 CFU | 12 days |
| Zhang et al. [32] | 2018 | E. coli BL21(DE3) | pET-28a(+)-IL35 | E. coli/IL-35 | IL-35 | Gastric gavage | 1010 CFU | 5 days |
| Wang et al. [35] | 2019 | L. lactis NZ9000 | pNZ8148+IL-35 | NZ9000/IL-35 | mIL-35 | Gastric gavage | 109 CFU | 14 days (3 times weekly) |
| Zhang et al. [24] | 2013 | L. fermentum I5007 | pLK126 | L. fermentum P126 | CAT | Gastric gavage | 109 CFU | 7 days |
| Xie et al. [31] | 2017 | B. longum HB15 | pBsSOD | B. longum-rhMnSOD | MnSOD | Gastric gavage | 2 × 109 CFU | 7 days |
| LeBlanc et al. [20] | 2011 | L. casei BL23 | pLEM415-mnkat | Lb. casei BL23 pLEM415-mnkat | CAT | Gastric gavage | 109 CFU | 24 days |
| L. casei BL23 | pLEM415-sodA | Lb. casei BL23 pLEM415-sodA | SOD | Gastric gavage | 109 CFU | 24 days | ||
| del Carmen et al. [25] | 2014(b) | S. thermophilus CRL807 | pIL253-sodA | S. thermophilus CRL 807:SOD | SOD | Gastric gavage | 109 CFU or 3 × 1010 CFU | 14 days |
| S. thermophilus CRL807 | pIL253-mnkat | S. thermophilus CRL 807:CAT | CAT | Gastric gavage | 109 CFU or 3 × 1010 CFU | 14 days | ||
| Han et al. [18] | 2006 | L. lactis NZ9800 | pNZ8048sodA | L. lactis SOD+ | SOD | Gastric gavage | 109 CFU | 8 days |
| L. plantarum NCIMB8826 | pNZ8048sodA | L. plantarum SOD+ | SOD | Gastric gavage | 109 CFU | 8 days | ||
| Wong et al. [22] | 2012 | L. lactis NZ3900 (N0) | N.A. | N4 | mCRAMP | Gastric gavage | 108 or 1010 CFU | 7 days |
| Li et al. [40] | 2021 | L. lactis NZ9000 | pMG36e-Usp45-CRAMP | L.L-pMU45CR | CRAMP | Gastric gavage | 1010 CFU | 4 days |
| L. lactis NZ9000 | pNZ8148-Usp45-CRAMP | L.L- pNU45CR | CRAMP | Gastric gavage | 1010 CFU | 4 days | ||
| Zeng et al. [38] | 2020 | L. lactis NZ9000 | pN8148-SHD-5 | NZ9000SHD-5 | HD-5 | Gastric gavage | N.A. | 7 days |
| Esposito et al. [39] | 2021(a) | L. paracasei F19 | pTRKH3-slp-NAPE-PLD | pNAPE-LP | NAPE-PLD | Gastric gavage | 0.8–1.2 × 108 CFU | 5 days |
| Park et al. [41] | 2021 | E. coli MG1655 | pACYC184-BCD-BUT | MG1655-BCD-BUT | BCD and BUT | Gastric gavage | 0.2 × 109 CFU | 9 days |
| E. coli Nissle 1917 | pACYC184-BCD-BUT | EcN-BCD-BUT | BCD and BUT | Gastric gavage | 0.2 × 109 CFU | 9 days | ||
| Yan et al. [45] | 2021 | E. coli Nissle 1917 | pYX50 | EcNL4 (EcNΔldhA) | 3HB | Gastric gavage | 5 × 1010 cells | 7 days |
| Yoon et al. [19] | 2008 | L. casei BLS | pLUAT-ssMSH | L. casei-alpha-MSH | alpha-MSH | Gastric gavage | 1010 CFU | 7 days |
| Shigemori et al. [30] | 2015 | L. lactis NZ9000 | pNZ8148#2:SEC-mHO-1 | NZ-HO | rmHO-1 | Gastric gavage | 5 × 109 CFU | 7 days |
| Praveschotinunt et al. [34] | 2019 | E. coli Nissle 1917 | pBbB8k-CsgA-TFF3 | PBP8 CsgA-TFF3 | Trefoil factors 3 | Rectal administration | 108 CFU | 14 days |
| Sun et al. [43] | 2021 | S. cerevisiae BY4741 | N.A. | S. cerevisiae 39# | Lactic acid | Gastric gavage | 2 × 108 CFU | 7 days |
| Scott at al [42] | 2021 | S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | 11 days |
| S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | 21 days | ||
| S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | N.A. | ||
| Wang et al. [44] | 2021 | E. coli Nissle 1917 | pGEX-4T-1-Sj16-AsBD and pGEX-4T-1-Sj16-GFP-AsBD | EcN-Sj16 | Sj16 | Gastric gavage | 1 × 109 CFU | 3 days (Days 0, 4, and 8) |
| Wei et al. [54] | 2016 | B. longum HB15 | pDGMSH | B. longum-a-MSH | alpha-MSH | Gastric gavage | 2 × 1010 CFU | 7 days |
| Wei et al. [55] | 2016 | B. longum HB15 | pBDMSH | B. longum-a-MSH | alpha-MSH | Gastric gavage | 1 × 1010 CFU | 9 days |
| Vandenbroucke et al. [46] | 2010 | L. lactis MG1363 | N.A. | LL–MT1–MT1 | MT1–MT1 Nanobody | Gastric gavage | 2 × 109 CFU | 14 or 21 days |
| L. lactis MG1363 | N.A. | LL–MT1 | MT1 Nanobody | Gastric gavage | 3 × 109 CFU | 21 days | ||
| L. lactis MG1363 | pT1mIL10 | LL–Mil10 | IL-10 | Gastric gavage | 4 × 109 CFU | 14 or 21 days | ||
| Liu et al. [53] | 2016 | L. lactis NZ9000 | pNZ8148-pIGF-I3 | L. lactis NZ9000 (pNZ8148-pIGF-I3) | IGF-I | Gastric gavage | 4 × 1012 CFU | 10 days |
| Zurita-Turk et al. [58] | 2020 | L. lactis MG1363 | pValac:il-10 | L. lactis MG1363 FnBPA+ (pValac:il-10) | IL-10 | Gastric gavage | 2 × 109 CFU | 6 weeks |
| Qiu et al. [49] | 2013 | L. casei CECT 5276 | pIlac-sp-IL10 | N.A. | IL-10 | Gastric gavage | 0.6 × 107 or 0.6 × 108 or 0.6 × 109 CFU | 10 days |
| Yao et al. [48] | 2011 | B. longum NCC 2705 | pBBADs-hIL-10 | BL-hIL-10 | IL-10 | Gastric gavage | 1.2 × 108 CFU | 7 days |
| Hanson et al. [50] | 2014 | L. lactis | N.A. | LL-IL-27 | IL-27 | Gastric gavage | 2 × 108 CFU | 14 days |
| Whelan et al. [52] | 2014 | E. coli Nissle 1917 | pMU13 -AvCys | EcN-AvCys | Nematode cystatin | Gastric gavage | 2 × 109 CFU | 4 days |
| Breyner et al. [57] | 2019 | L. lactis NZ9000 | pSEC:PAP | LL-PAP | PAP | Gastric gavage | 5 × 109 CFU | 9 or 17 days |
| Liu et al. [56] | 2018 | B. longum HB25 | pBDMnSOD | B. longum-PEP-1-rhMn-SOD | rhMn-SOD | Gastric gavage | 2 × 109 CFU | 7 days |
| Hou et al. [51] | 2014 | L. fermentum I5007 | pMF009 | L. fermentum (pMF009) | SOD | Gastric gavage | 5 × 109 CFU | 6 days |
| Watterlot et al. [47] | 2010 | L. casei | pILKSsodA | Lb. casei pILKSsodA | SOD | Gastric gavage | 5 × 109 CFU | 9 days |
| L. casei | pVE3874 | Lb. casei BL23 pVE3874 | CAT | Gastric gavage | 5 × 109 CFU | 9 days | ||
| Aubry et al. [60] | 2015 | L. lactis MG1363 | pGroESL-TSLP | LL-TSLP | TSLP | Gastric gavage | 1–5 × 109 CFU | 4 or 12 or 17 days |
| Foligne et al. [59] | 2007 | L. lactis MG1363 | pMEC237 | LL-LcrV | Immunomodulatory Yersinia LcrV Protein | Gastric gavage | 2 × 108 CFU | 5 days |
Only one human study [61] performed in the Netherlands was included. This study was a placebo-uncontrolled trial in 2006, with 10 CD patients receiving 10 capsules of 1 × 1010 CFU of genetically modified L. lactis producing IL-10 (LL-Thy12) twice daily for 7 days.
We focused on the efficacy of gm probiotics relative to wild-type probiotics in treating patients with IBD or animal models, unless these data were not accessible, in which case only the properties against colitis were described. The extracted data are presented in Supplementary Material S1.
3.3. The Efficacy of Gm Probiotics Secreting Immunoregulatory Cytokines on Colitis Models and IBD Patients
3.3.1. IL-10
Twelve preclinical studies [16,17,21,26,27,28,29,36,46,48,49,58] engineered probiotics to secrete IL-10. Of these studies, disease activity [48,49] and colon length [21,48,49] were assessed in only two and three studies, respectively, with a significant improvement in the gm probiotics group compared to the group receiving wild-type probiotics or the untreated group. Relative body weight presented inconsistent or even contradictory results, with either increases [26,29,58], decreases [21], or no changes [21,27,36] in the group using gm probiotics compared to the group using wild-type probiotics. As for intestinal damage, we were able to capture a relatively consistent trend that gm probiotics producing IL-10 can reduce intestinal damage observed macroscopically compared to wild-type probiotics in most studies.
Cytokine profile analysis generally showed that gm probiotics promoted higher IL-10 expression in the colon or serum than that of the wild-type probiotics. Although the levels of the other cytokines were reported without any consistent results, it is notable that B. longum producing IL-10 elicited higher suppression of the levels of IFN-γ, TNF-α, IL-1β, and IL-6 compared to the wild-type strain [48]. Three studies [27,28,46] reported the activity of colonic myeloperoxidase (MPO), a marker of neutrophil infiltration and oxidative stress, but only one study showed a significant improvement [28].
Intriguingly, a clinical study [61] with only ten CD patients showed that treatment with LL-Thy12 was safe, with minor adverse events, and a decrease in disease activity was observed in these patients, indicating that the use of gm probiotics for the mucosal delivery of IL-10 might be a feasible strategy for treating IBD. However, given the small sample size and limited outcome measures, these results should be interpreted with caution.
3.3.2. IL-27
IL-27, a pleiotropic cytokine belonging to the IL-12 family, has immunosuppressive and therapeutic effects in colitis [62]. Hanson et al. [50] developed a strategy for delivering IL-27 to the GIT by genetically modifying L. lactis to synthesize bioactive IL-27 (LL-IL-27) in situ. In this study, compared with L. lactis, oral administration of LL-IL-27 showed a stronger protective effect against CD4+CD45RBhi T-cell transfer-induced colitis by alleviating intestinal damage and promoting IL-10 expression in the colon.
3.3.3. IL-35
Two studies [32,35] constructed recombinant E. coli and L. lactis strains expressing IL-35. Both have a greater ability to alleviate intestinal damage and improve the disease activity index (DAI) score and colon length than wild-type probiotics. Additionally, they have been shown to modulate the expression of anti- and pro-inflammatory cytokines in the colon and plasma of colitis models [35] and have a higher ability to suppress IL-6 and increase IL-10 levels than wild-type probiotics [32].
3.3.4. Growth Factors
Growth factors play a key role in intestinal growth, regeneration, damage repair, and immunoregulation; however, it is difficult to achieve therapeutic functions with oral administration owing to their instability in the upper GIT. A study by Hamady et al. [23] explored the gm probiotics producing keratinocyte growth factor-2 (KGF-2) or transforming growth factor-beta (TGF-β), which showed a significant prophylactic effect on limiting the development of intestinal inflammation in comparison to wild-type probiotics. Additionally, oral administration of recombinant L. lactis expressing insulin-like growth factor I (IGF-I) improved intestinal damage and barrier integrity and reduced colonic MPO activity in DSS-induced colitis mice; however, when compared with wild-type probiotics, it only achieved better alleviation of the histological damage score [53].
3.3.5. Other Immunoregulatory Cytokines
Trefoil factors (TFFs), a family of human cytokines known to promote intestinal barrier function and epithelial restitution, do not yield therapeutic outcomes in IBD after oral delivery, as they adhere strongly to the mucus layer of the small bowel [63]. To overcome this limitation, Praveschotinunt et al. [34] used E. coli Nissle 1917 (EcN) as a vehicle to produce curli fibrous matrices displaying and tethering TFF3, which showed enhanced protective effects against acute colitis for rectal administration; however, these effects were not significant when compared to the parental strains.
The efficacy of gm probiotics in colitis differed not only in terms of the therapeutic substances it produced, but also the phases of administration; for instance, a short and early administration of recombinant L. lactis producing thymic stromal lymphopoietin (TSLP), a cytokine in mature dendritic cells with properties of inhibiting IL-12 secretion and inducing differentiation of anti-inflammatory FoxP3+ Treg, was more effective than a long-lasting treatment [60].
3.3.6. Antibodies or Receptor Antagonist for Pro-Inflammatory Cytokines
Interventions targeting pro-inflammatory cytokine signaling have also been demonstrated as an effective approach in several animal studies [46,64]. However, they have been reported to cause serious side effects when administered intravenously or subcutaneously in clinical patients; thus, local delivery of these therapeutic substances to the GIT is desirable to restrict these side effects [65].
A study by Chiabai et al. [33] using L. lactis as a vehicle for delivering anti-TNFα to GIT of acute colitis model showed a higher efficacy than L. lactis. Moreover, compared to L. lactis and L. lactis producing IL-10, it exerted a greater ability to alleviate chronic colitis induced by DSS and IL-10−/− [46]. Similarly, Namai et al. [37] used L. lactis as a chassis for the delivery of IL-1Ra to the intestinal mucosa, which showed a significantly higher efficacy in suppressing disease activity in mice with acute colitis than with wild-type probiotics. These results suggest that a novel, effective, and inexpensive IBD therapy that blocks pro-inflammatory cytokine signaling has been successfully developed.
3.3.7. Comparisons of Different Gm Probiotics
Interestingly, two studies [28,36] have compared the efficacy of different gm probiotics, with one study [28] using probiotics producing anti-inflammatory cytokines (IL-10 and TGF-β1) and serine protease inhibitors (Elafin and SLPI) and another using [36] probiotics secreting IL-10, TNFR1-ECD, alkaline phosphatase (AP), and atrial natriuretic peptide (ANP). Finally, the results demonstrated that L. lactis secreting serine protease inhibitors and S. boulardii secreting ANP may be the most effective probiotics for the treatment of colitis.
3.4. The Efficacy of Gm Probiotics Secreting Antioxidant Enzymes on Colitis Models
Eight studies [18,20,24,25,31,47,51,56] have successfully expressed catalase (CAT) or superoxide dismutase (SOD) in different probiotics, with prominent antioxidant activity.
L. fermentum P126 producing CAT showed superior effects in improving the intestinal damage, reducing the activity of lipid peroxidation and MPO, as well as activating NF-κB in colon tissue when compared to wild-type probiotics [24]. Consistently, the administration of SOD-secreting gm probiotics also revealed a significant improvement in intestinal damage, inflammation, and oxidative stress in colitis models [18,56], and compared to wild-type probiotics, B. longum [31] and L. fermentum [51] producing SOD exerted a higher efficacy.
Three publications [20,25,47] focused on both CAT- and SOD-producing probiotics. LeBlanc et al. [20] found that mice with acute colitis receiving CAT- or SOD-producing L. casei BL23 showed faster recovery from weight loss and increased SOD and CAT activities in the colon compared to mice receiving the wild-type strain. Interestingly, CAT- and SOD-producing S. thermophilus CRL 807 were administered to mice with chronic colitis as a suspension in saline solution or in fermented milk in a study conducted by del Carmen et al. [25], which revealed that these gm probiotics in fermented milk were more effective than in saline solution; for instance, the former significantly alleviated intestinal damage compared to the parental strain, but the latter showed almost no superiority. Furthermore, beneficial effects were improved in mice receiving a mixture of both CAT- and SOD-producing S. thermophilus CRL807; however, these effects were not obvious in a study performed by Watterlot et al. [47].
Heme oxygenase-1 (HO-1) is an antioxidant enzyme induced by inflammatory stimuli and oxidative stress [66]. Only one study [30] showed that oral administration of L. lactis NZ9000 secreting HO-1 significantly alleviated colitis-associated symptoms, histological damage, and immune disorders in mice compared to L. lactis NZ9000 with an empty vector.
3.5. The Efficacy of Gm probiotics Secreting Antimicrobial Peptide on Colitis Models
Antimicrobial peptides such as defensins and cathelin-related antimicrobial peptide (CRAMP) are protective factors that constitute complex chemical barriers on the layer of continuous epithelial cells in the GIT [67]. Increased expression of antimicrobial peptides in colonic mucosa has been reported in response to inflammation and infection [22], indicating an essential role in immune regulation and wound healing. However, their short half-lives and sensitivity to acidic environments in the GIT greatly limit their clinical application in IBD therapy [40].
Four studies engineered L. lactis NZ3900 [22,38,40,57] to produce antimicrobial peptides. Of these studies, two [22,40] focused on CRAMP-producing L. lactis, confirming its effectiveness in preventing and attenuating colitis, especially at 1010 CFU daily [22]. Likewise, the defensin-5-secreting L. lactis can also alleviate mucosal damage by suppressing NF-κB signaling pathway [38].
Pancreatitis-associated protein (PAP), a C-type lectin belonging to the regenerating islet-derived III protein family, plays a protective role in colitis. Breyner et al. [57] tested the efficacy of L. lactis secreting PAP (LL-PAP) in DNBS- and DSS-induced colitis, and protective effects were detected only in the DNBS colitis model. Moreover, compared to L. lactis, LL-PAP significantly improved the gut microbial composition, especially that of butyrate-producing bacteria such as Eubacterium plexicaudatum.
3.6. The Efficacy of Gm Probiotics Promoting Production of Short-Chain Fatty Acids (SCFAs) or Related Organic Acids in GIT
SCFAs, produced by bacteria that ferment fibers in the GIT, are carboxylic acids with aliphatic tails of 1–6 carbons, of which acetate, propionate, and butyrate are the most abundant [68]. Due to their anti-inflammatory properties, gut barrier protection, and immunomodulation, SCFAs are unfortunately reported to be typically reduced in the feces and gut mucosa of patients with IBD [68].
Park et al. [41] engineered E. coli MG1655 (MG1655-BCD-BUT) and EcN (EcN-BCD-BUT) to produce butyric acid and showed a significant amelioration of the DAI score and lower expression of IL-6 and MPO in the colonic tissue of DSS-induced colitis models. In particular, compared with MG1655-BCD-BUT, EcN-BCD-BUT alleviated intestinal damage and inflammation more significantly, proving its potential superiority.
These gm probiotics have shown great effects in improving gut microbiota homeostasis. For instance, S. cerevisiae secreting lactic acid appeared to improve α-diversity and decrease the Firmicutes to Bacteroides ratio in DSS-induced colitis mice [43]. Yan et al. [45] demonstrated that compared with EcN, oral application of EcN producing (R)-3-hydroxybutyrate (EcNL4) was more effective at improving mouse weight, colon length, histological damage, MPO activity, and SCFA levels in the colon, as well as serum cytokine levels. Furthermore, the abundance of Akkermansia and Prevotella significantly increased in the EcNL4 group.
Notably, the genetically engineered EcN highly expressed schistosome immunoregulatory protein Sj16, which was found to promote the growth of Ruminococcaceae in the GIT and therefore enhance the production of butyrate, mediating the attenuation of the disease activity of colitis [44]. This is an excellent example of a gm probiotic indirectly promoting SCFA production.
3.7. The Efficacy of Gm Probiotics Secreting Alpha-Melanocyte-Stimulating Hormone (α-MSH) on Colitis Models
α-MSH is a neuropeptide that elicits anti-inflammatory properties in various disease models, including IBD and arthritis. However, its clinical application is limited because of its extremely short duration in vivo; thus, probiotics might be effective carriers to facilitate efficient oral delivery of α-MSH to address this limitation [69].
α-MSH-secreting L. casei showed a significant effect on attenuating acute colitis as assessed by body weight loss, intestinal damage score, MPO activity, pro- and anti-inflammatory cytokines levels, and survival rate; however, its efficacy relative to wild-type probiotics is unknown [19]. Subsequently, two studies [54,55] utilizing α-MSH-producing B. longum against DSS-induced acute colitis revealed that B. longum-α-MSH was more effective than B. longum.
3.8. The Efficacy of Gm Probiotics Secreting Other Therapeutic Substances on Colitis Models
Palmitoylethanolamide (PEA), produced by the conjugation of palmitate and ethanolamine through N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) [70], harbors properties of modulating several physiological processes, including analgesia, neuroprotection, and inflammation [71]. By activating the peroxisome proliferator–activated receptor-α (PPARα), PEA exerts potent anti-inflammatory effects to improve intestinal inflammation [72]. However, even though it is safe without serious side effects, high doses are required to achieve therapeutic effects, which limits its use in current clinical practice. To overcome this limitation, a study [39] has genetically modified L. paracasei F19 to secrete NAPE-PLD (pNAPE-LP), which can release PEA from the GIT in the presence of ultra-low doses of exogenous palmitate. The results revealed significant amelioration of colitis in the group administered with pNAPE-LP.
Extracellular adenosine triphosphate (eATP), produced by commensal microbiota and immune cells in the host, activates purinergic signaling via purinergic receptors, boosting inflammation and pathological damage in the intestine. Thus, purinergic signaling is a potential therapeutic target in IBD [42]. Scott et al. [42] developed a self-tunable S. cerevisiae (APTM-3) which secreted the CD39-like eATP-degrading enzyme apyrase when engineered human P2Y2 receptors detected eATP. The researchers used three colitis models: TNBS-, DSS-, and anti-CD3 antibody-induced colitis, which showed significantly suppressed intestinal inflammation and damage, reduced fibrosis, and improved gut microbiota in the APTM-3 group compared to the wild-type strain group.
Secretions from pathogens such as parasitic nematodes and pathogenic yersiniae can modulate host immune responses to induce an anti-inflammatory environment that favors their persistence and reproduction in hosts. Thus, these molecules can be viewed as potential therapeutic agents for IBD. Whelan et al. [52] demonstrated stronger anti-inflammatory properties of recombinant EcN secreting cystatin from the rodent nematode Acanthocheilonema viteae (AvCys) than those from EcN. Consistently, oral administration of recombinant L. lactis producing a low-calcium response V (LcrV) protein from the enteropathogenic species Yersinia pseudotuberculosis also showed prominent efficacy in both TNBS- and DSS-induced models, in contrast with L. lactis [59]. Additionally, TNBS-induced colitis was not prevented in IL-10−/− mice, indicating that IL-10 is required for LcrV-mediated protection against IBD.
3.9. Risk of Bias
All the included studies had sufficient scientific contextualization and objectives. Eight studies did not provide ethical statements. Twenty-six studies provided information regarding whether the experiments were performed in a blind-controlled manner. None of the studies reported the time of day chosen for treatment administration, the rationale for choice of a specific route of administration, an explanation regarding the decision of animal numbers and details of sample size calculation, or the order in which the animals in the different experimental groups were treated and assessed. Only one study has described the rationale for choosing a specific dosage; that is, 2 × 109 CFU, which represents the technically maximum reliable dose for freshly cultured strains. Sixteen studies reported the animals’ weight ranges before the intervention. Thirty articles did not describe how animals were allocated to the experimental groups. Only 12 studies reported mortality rates.
Based on the criteria proposed by Downs and Black, the clinical study included in this systematic review was classified as intermediate quality. Therefore, further high-quality studies are required (Supplementary Material S1).
4. Discussion
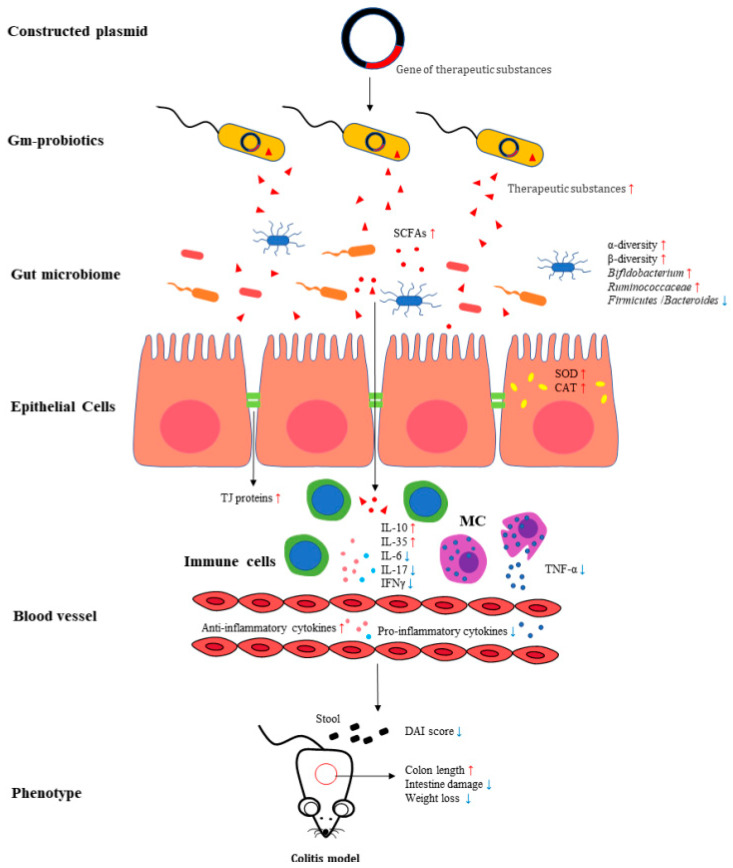
To the best of our knowledge, this is the first systematic review to summarize the efficacy of different gm probiotics in the treatment of IBD in animal models and patients, providing reference values for conducting and improving subsequent clinical research, and thus further improving the quality of life of patients with IBD. Despite the heterogeneity of these studies, our findings showed a certain effect of gm probiotics against colitis. Several protective mechanisms have been identified: reduction of the pro- to anti-inflammatory cytokine ratio in colonic tissue and plasma, modulation of the activity of oxidative stress in the colon, improvement of intestinal barrier integrity, modulation of the diversity and composition of gut microbiota, and production of favorable metabolites, including SCFAs, by beneficial bacteria. These mechanisms may contribute to the alleviation of phenotypes such as weight loss, colon length, disease activity, and intestinal damage in colitis models (Figure 2). Furthermore, it is notable that many studies have not reported the outcomes of the efficacy of gm probiotics compared to that of wild-type probiotics, making it difficult to further evaluate the properties of these gm probiotics. Only gm probiotics that are superior to wild-type probiotics can be regarded as successful.
Figure 2.
The mechanism of gm probiotics involved in treating colitis models. Constructed plasmids containing genes for therapeutic substances are integrated into wild-type probiotics to treat colitis animal models by gavage or rectal administration. The gm probiotics ameliorated the clinical phenotypes of colitis, such as DAI score, body weight loss, and intestinal damages, via the mechanisms involved in improving gut microbiota, increasing the level of short-chain fatty acids, regulating immune cells, reducing expression of the pro-inflammatory cytokines, increasing anti-inflammatory cytokines levels, and increasing the expression of tight junction proteins. (SCFAs: short-chain fatty acids; MC: mast cells; TJ proteins: tight junction proteins; DAI: disease activity index).
Most studies were preclinical experiments, and only one phase I clinical trial [61] performed by Braat et al. in 2006, was included in this systematic review, which indicated that the barriers to using gm probiotics to treat IBD still exist in human studies. Several factors may contribute to the limited number of clinical studies: IBD patients are susceptible to intestinal microbiota translocation, leading to systemic sepsis. Thus, although the probiotics are generally regarded as safe, there still may be risks in the IBD population [73]. The colonization of the gm probiotics in the intestine was not sufficient to maintain the long-term efficacy; hence, the probiotics must be taken frequently, which may in turn increase the risk of side effects. The preclinical studies of some gm probiotics were scarce, and the laboratory data obtained were insufficient to support their clinical use and the limitations of IBD models. Thus, more well-designed preclinical studies and large-scale multicenter randomized controlled trials are needed to evaluate the beneficial effects and safety of gm probiotics in IBD.
There are many candidate therapeutic substances that have shown potential efficacy against colitis in previous studies; however, their further development to clinical applications have been limited by several shortages. For instance, as summarized in the Section 3, the growth factors were not stable in upper GIT, TFFs were reported to adhere strongly to the mucus layer of the small bowel, antibodies or receptor antagonist for pro-inflammatory cytokines were reported to cause serious side effects through intravenous or subcutaneous administration in clinical patients, and CRAMP and α-MSH showed a short half-life in vivo. Orally administered SCFAs have low bioavailability because they are efficiently absorbed by the upper GIT, reducing their therapeutic function in the lower GIT or colon of patients [74]. Additionally, SCFAs have a certain sour odor and poor palatability, which makes it particularly important to optimize the delivery methods of SCFAs, such as coating technology. The effectiveness of these administration methods for patients still needs to be confirmed by further research. Thus, using probiotics as a chassis to deliver therapeutic substances into the GIT can not only improve drug utilization, but also allows it to fully exert its therapeutic effect in local lesions while reducing systemic adverse reactions, which is beneficial for clinical application.
A wide variety of probiotics were used as chassis to deliver therapeutic substances into GIT, and given the properties of immunomodulation and surviving passage through the GIT, the species L. lactis, including L. lactis MG1363 and L. lactis NZ9000, was used most often [75]. The first evidence of this application in a colitis mouse model was found in an experiment performed by Steidler et al. in 2000 [16]; thereafter in 2006, L. lactis secreting IL-10 was used in clinical trials to treat Crohn’s disease and showed potential efficacy [61]. In addition to L. lactis and E. coli, the development of a new chassis in more dominant microorganisms from the gut microbiome is essential because these species may be more effective depending on their multiple properties, such as better adaptation to the human intestine, better colonization to achieve high cell numbers in the GIT, and safe interaction with the immune system [7]. However, there is still no consensus in the literature as to which is the most effective and active species or strain to be used as a chassis for delivering therapeutic molecules; thus, experiments comparing the biological activities of these probiotics are also needed. Moreover, the evaluation of the properties of gm probiotics or the colonization of wild-type strains in the intestine is important and has been ignored in many studies.
The dose of gm probiotics is still undefined and varies across studies, ranging from 105 to 4 × 1012 CFU per day per mouse or rat in this systematic review, making it impracticable to suggest a specific dose. Few studies have simultaneously compared the efficacy of different concentrations of gm probiotics in the treatment of colitis. Qiu et al. [49] tested the effectiveness of three doses (2 × 107, 2 × 108, and 2 × 109 CFU/mL) of recombinant L. casei CECT 5276 secreting IL-10, with the highest concentration (2 × 109 CFU/mL) being the most effective. However, the dose-dependent effect of gm probiotics requires further validation in more studies. The time effect of gm probiotics on colitis models is undefined and worthy of exploration.
The survival rate of gm probiotics in GIT could improve when multiple complementary species are administered simultaneously. This may be because different parts of a genetic circuit in plasmids could be distributed among different species, increasing their co-dependence and relieving the metabolic strain [7]. Additionally, mixing several grams of probiotics that secrete different proteins may produce additional effects. In the study performed by del Carmen et al. [25], the greatest anti-inflammatory activity was observed in the group that received a mixture of both CAT- and SOD-producing Streptococci. In contrast, Watterlot et al. [47] observed that the combination of Lb. casei MnKat and Lb. casei BL23MnSOD showed no such effects. Despite the conflicting results of these two studies, this method still deserves to be used as a reference for subsequent studies as well as a promising treatment for IBD.
Many IBD models are available for use, and according to a review by Mizoguchi et al. [76], they can be classified into five major groups: the chemically induced model, the cell-transfer model, the spontaneous model, the congenital model, and the genetically engineered model. Chemically induced models such as DSS-induced- and TNBS-induced colitis were most frequently used to evaluate the efficacy of gm probiotics. Each model has specific advantages over others; for example, the DSS model was mainly used for exploring epithelial homeostasis/regeneration and wound healing processes, and IL10−/− mice, which were also used in some studies, contributed to understanding the mechanisms of probiotics, Helicobacter, and NSAIDs in IBD. Thus, it is a general trend to utilize more than one colitis model to verify the function of gm probiotics in one study.
The risk of bias assessments in our systematic review demonstrated that much information related to study design and results was neglected or not reported by many studies; thus, improvements in the methodology and reporting of animal experiments are needed to ensure the quality and persuasiveness of studies in the future.
5. Conclusions
These findings indicate that several factors may affect the efficacy of gm probiotics, such as the species or strains of wild-type probiotics, different gm probiotic combinations, therapeutic substances, the dose, and the IBD model. Overall, gm probiotics have a certain effect on colitis models, which might be attributed to the following mechanisms: reduction of the pro- to anti-inflammatory cytokine ratio in the colonic tissue and plasma, modulation of the activity of oxidative stress in the colon, improvement of intestinal barrier integrity, modulation of the diversity and composition of the gut microbiota, and production of regulatory metabolites by beneficial bacteria. It is also important for researchers to pay more attention to gm probiotics, which are more effective and safer than wild-type probiotics, to facilitate clinical translation. Additionally, the methodology and reporting of animal experiments and clinical trials should be improved to ensure the quality of studies.
Acknowledgments
We thank all authors who provided data for this systematic review.
Abbreviations
| CFU | Colony-forming unit |
| DAI | Disease activity index |
| GIT | Gastrointestinal tract |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| DSS | Dextran sulphate sodium |
| TNBS | Trinitrobenzene sulfonic acid |
| DNBS | Dinitro-benzenesulfonic-acid |
| Gm probiotics | Genetically modified probiotics |
| IBD | Inflammatory bowel disease |
| MPO | Myeloperoxidase |
| SCFAs | Short-chain fatty acids |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15071566/s1.
Author Contributions
Guarantor of the article: L.D. is the guarantor. T.Z., J.Z. and L.D. conceived and drafted the manuscript. J.Z. screened the abstracts and T.Z. collected all data. T.Z. and J.Z. analyzed and interpreted the data. T.Z. drafted the manuscript. L.D. acquired the funding and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
This study was funded by the National Natural Science Foundation of China (82170557) and National Key R&D Program of China (2019YFA0905604).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.De Souza H.S., Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Zenlea T., Peppercorn M.A. Immunosuppressive therapies for inflammatory bowel disease. World J. Gastroenterol. 2014;20:3146–3152. doi: 10.3748/wjg.v20.i12.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 7.Barra M., Danino T., Garrido D. Engineered Probiotics for Detection and Treatment of Inflammatory Intestinal Diseases. Front. Bioeng. Biotechnol. 2020;8:265. doi: 10.3389/fbioe.2020.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu K., Dong S., Wu X., Jin R., Chen H. Probiotics in Cancer. Front. Oncol. 2021;11:638148. doi: 10.3389/fonc.2021.638148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazerani P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019;20:4121. doi: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samer A., Toumi R., Soufli I., Touil-Boukoffa C. Cell-free probiotic supernatant (CFS) treatment alleviates indomethacin-induced enterocolopathy in BALB/c mice by down-modulating inflammatory response and oxidative stress: Potential alternative targeted treatment. Inflammopharmacology. 2022;30:1685–1703. doi: 10.1007/s10787-022-00996-y. [DOI] [PubMed] [Google Scholar]
- 11.Wyszyńska A., Kobierecka P., Bardowski J., Jagusztyn-Krynicka E.K. Lactic acid bacteria—20 years exploring their potential as live vectors for mucosal vaccination. Appl. Microbiol. Biotechnol. 2015;99:2967–2977. doi: 10.1007/s00253-015-6498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigemori S., Shimosato T. Applications of Genetically Modified Immunobiotics with High Immunoregulatory Capacity for Treatment of Inflammatory Bowel Diseases. Front. Immunol. 2017;8:22. doi: 10.3389/fimmu.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steidler L., Hans W., Schotte L., Neirynck S., Obermeier F., Falk W., Fiers W., Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 17.Foligné B., Nutten S., Steidler L., Dennin V., Goudercourt D., Mercenier A., Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: Technical and microbiological aspects. Dig. Dis. Sci. 2006;51:390–400. doi: 10.1007/s10620-006-3143-x. [DOI] [PubMed] [Google Scholar]
- 18.Han W., Mercenier A., Ait-Belgnaoui A., Pavan S., Lamine F., van Swam I.I., Kleerebezem M., Salvador-Cartier C., Hisbergues M., Bueno L., et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm. Bowel Dis. 2006;12:1044–1052. doi: 10.1097/01.mib.0000235101.09231.9e. [DOI] [PubMed] [Google Scholar]
- 19.Yoon S.W., Lee C.H., Kim J.Y., Kim J.Y., Sung M.H., Poo H. Lactobacillus casei secreting alpha-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c Mice. J. Microbiol. Biotechnol. 2008;18:1975–1983. [PubMed] [Google Scholar]
- 20.LeBlanc J.G., del Carmen S., Miyoshi A., Azevedo V., Sesma F., Langella P., Bermúdez-Humarán L.G., Watterlot L., Perdigon G., de Moreno de LeBlanc A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011;151:287–293. doi: 10.1016/j.jbiotec.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Gardlik R., Palffy R., Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol. 2012;58:238–245. [PubMed] [Google Scholar]
- 22.Wong C.C., Zhang L., Li Z.J., Wu W.K., Ren S.X., Chen Y.C., Ng T.B., Cho C.H. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J. Gastroenterol. Hepatol. 2012;27:1205–1212. doi: 10.1111/j.1440-1746.2012.07158.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamady Z.Z. Novel xylan-controlled delivery of therapeutic proteins to inflamed colon by the human anaerobic commensal bacterium. Ann. R. Coll. Surg. Engl. 2013;95:235–240. doi: 10.1308/003588413X13511609958217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Liu H., Wang Q., Hou C., Thacker P., Qiao S. Expression of catalase in Lactobacillus fermentum and evaluation of its anti-oxidative properties in a dextran sodium sulfate induced mouse colitis model. World J. Microbiol. Biotechnol. 2013;29:2293–2301. doi: 10.1007/s11274-013-1395-0. [DOI] [PubMed] [Google Scholar]
- 25.Del Carmen S., de Moreno de LeBlanc A., Martin R., Chain F., Langella P., Bermúdez-Humarán L.G., LeBlanc J.G. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl. Environ. Microbiol. 2014;80:869–877. doi: 10.1128/AEM.03296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Carmen S., Martín Rosique R., Saraiva T., Zurita-Turk M., Miyoshi A., Azevedo V., de Moreno de LeBlanc A., Langella P., Bermúdez-Humarán L.G., LeBlanc J.G. Protective effects of lactococci strains delivering either IL-10 protein or cDNA in a TNBS-induced chronic colitis model. J. Clin. Gastroenterol. 2014;48((Suppl. 1)):S12–S17. doi: 10.1097/MCG.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 27.Martín R., Chain F., Miquel S., Natividad J.M., Sokol H., Verdu E.F., Langella P., Bermúdez-Humarán L.G. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum. Vaccines Immunother. 2014;10:1611–1621. doi: 10.4161/hv.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bermúdez-Humarán L.G., Motta J.P., Aubry C., Kharrat P., Rous-Martin L., Sallenave J.M., Deraison C., Vergnolle N., Langella P. Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb. Cell Factories. 2015;14:26. doi: 10.1186/s12934-015-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Carmen S., Miyoshi A., Azevedo V., de Moreno de LeBlanc A., LeBlanc J.G. Evaluation of a Streptococcus thermophilus strain with innate anti-inflammatory properties as a vehicle for IL-10 cDNA delivery in an acute colitis model. Cytokine. 2015;73:177–183. doi: 10.1016/j.cyto.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Shigemori S., Watanabe T., Kudoh K., Ihara M., Nigar S., Yamamoto Y., Suda Y., Sato T., Kitazawa H., Shimosato T. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb. Cell Factories. 2015;14:189. doi: 10.1186/s12934-015-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y., Guo Q., Li S., Liu M., Zhang Q., Xu Z., Sun H. Anti-inflammatory properties of Bifidobacterium longum expressing human manganese superoxide dismutase using the TNBS-induced rats model of colitis. J. Microbiol. Biotechnol. 2017 doi: 10.4014/jmb.1703.03044. ahead of print . [DOI] [PubMed] [Google Scholar]
- 32.Zhang B., Liu Y., Lan X., Xu X., Zhang X., Li X., Zhao Y., Li G., Du C., Lu S., et al. Oral Escherichia coli expressing IL-35 meliorates experimental colitis in mice. J. Transl. Med. 2018;16:71. doi: 10.1186/s12967-018-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiabai M.J., Almeida J.F., de Azevedo M.G.D., Fernandes S.S., Pereira V.B., de Castro R.J.A., Jerônimo M.S., Sousa I.G., de Souza Vianna L.M., Miyoshi A., et al. Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice. BMC Biotechnol. 2019;19:38. doi: 10.1186/s12896-019-0518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Praveschotinunt P., Duraj-Thatte A.M., Gelfat I., Bahl F., Chou D.B., Joshi N.S. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019;10:5580. doi: 10.1038/s41467-019-13336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Tian M., Li W., Hao F. Preventative delivery of IL-35 by Lactococcus lactis ameliorates DSS-induced colitis in mice. Appl. Microbiol. Biotechnol. 2019;103:7931–7941. doi: 10.1007/s00253-019-10094-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu C.H., Chang J.H., Chang Y.C., Mou K.Y. Treatment of murine colitis by Saccharomyces boulardii secreting atrial natriuretic peptide. J. Mol. Med. 2020;98:1675–1687. doi: 10.1007/s00109-020-01987-8. [DOI] [PubMed] [Google Scholar]
- 37.Namai F., Shigemori S., Ogita T., Sato T., Shimosato T. Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Exp. Mol. Med. 2020;52:1627–1636. doi: 10.1038/s12276-020-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng L., Tan J., Xue M., Liu L., Wang M., Liang L., Deng J., Chen W., Chen Y. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-kB pathway. J. Transl. Med. 2020;18:107. doi: 10.1186/s12967-020-02272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esposito G., Pesce M., Seguella L., Lu J., Corpetti C., Del Re A., De Palma F.D.E., Esposito G., Sanseverino W., Sarnelli G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. Int. J. Mol. Sci. 2021;22:2945. doi: 10.3390/ijms22062945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Yu S., Pan X., Zhang M., Lv Z., Pan L.L., Sun J. Recombinant CRAMP-producing Lactococcus lactis attenuates dextran sulfate sodium-induced colitis by colonic colonization and inhibiting p38/NF-κB signaling. Food Nutr. Res. 2021;65 doi: 10.29219/fnr.v65.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y.T., Kim T., Ham J., Choi J., Lee H.S., Yeon Y.J., Choi S.I., Kim N., Kim Y.R., Seok Y.J. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 2022;15:832–843. doi: 10.1111/1751-7915.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott B.M., Gutiérrez-Vázquez C., Sanmarco L.M., da Silva Pereira J.A., Li Z., Plasencia A., Hewson P., Cox L.M., O’Brien M., Chen S.K., et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021;27:1212–1222. doi: 10.1038/s41591-021-01390-x. [DOI] [PubMed] [Google Scholar]
- 43.Sun S., Xu X., Liang L., Wang X., Bai X., Zhu L., He Q., Liang H., Xin X., Wang L., et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021;12:777665. doi: 10.3389/fimmu.2021.777665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Liao Y., Yang R., Zhu Z., Zhang L., Wu Z., Sun X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng. Transl. Med. 2021;6:e10219. doi: 10.1002/btm2.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X., Liu X.Y., Zhang D., Zhang Y.D., Li Z.H., Liu X., Wu F., Chen G.Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell. Mol. Immunol. 2021;18:2344–2357. doi: 10.1038/s41423-021-00760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenbroucke K., de Haard H., Beirnaert E., Dreier T., Lauwereys M., Huyck L., Van Huysse J., Demetter P., Steidler L., Remaut E., et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 47.Watterlot L., Rochat T., Sokol H., Cherbuy C., Bouloufa I., Lefèvre F., Gratadoux J.J., Honvo-Hueto E., Chilmonczyk S., Blugeon S., et al. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int. J. Food Microbiol. 2010;144:35–41. doi: 10.1016/j.ijfoodmicro.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Yao J., Wang J.Y., Lai M.G., Li Y.X., Zhu H.M., Shi R.Y., Mo J., Xun A.Y., Jia C.H., Feng J.L., et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol. Pharm. 2011;8:488–497. doi: 10.1021/mp100331r. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Z.B., Chen J., Chen J.J., Rong L., Ding W.Q., Yang H.J., Zhong L. Effect of recombinant Lactobacillus casei expressing interleukin-10 in dextran sulfate sodium-induced colitis mice. J. Dig. Dis. 2013;14:76–83. doi: 10.1111/1751-2980.12006. [DOI] [PubMed] [Google Scholar]
- 50.Hanson M.L., Hixon J.A., Li W., Felber B.K., Anver M.R., Stewart C.A., Janelsins B.M., Datta S.K., Shen W., McLean M.H., et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146:210–221.e213. doi: 10.1053/j.gastro.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou C.L., Zhang J., Liu X.T., Liu H., Zeng X.F., Qiao S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014;116:1621–1631. doi: 10.1111/jam.12461. [DOI] [PubMed] [Google Scholar]
- 52.Whelan R.A., Rausch S., Ebner F., Günzel D., Richter J.F., Hering N.A., Schulzke J.D., Kühl A.A., Keles A., Janczyk P., et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol. Ther. J. Am. Soc. Gene Ther. 2014;22:1730–1740. doi: 10.1038/mt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S., Li Y., Deng B., Xu Z. Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice. BMC Biotechnol. 2016;16:25. doi: 10.1186/s12896-016-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei P., Yang Y., Ding Q., Li X., Sun H., Liu Z., Huang J., Gong Y. Oral delivery of Bifidobacterium longum expressing α-melanocyte-stimulating hormone to combat ulcerative colitis. J. Med. Microbiol. 2016;65:160–168. doi: 10.1099/jmm.0.000197. [DOI] [PubMed] [Google Scholar]
- 55.Wei P., Yang Y., Liu Z., Huang J., Gong Y., Sun H. Oral Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to fight experimental colitis. Drug Deliv. 2016;23:2058–2064. doi: 10.3109/10717544.2015.1122672. [DOI] [PubMed] [Google Scholar]
- 56.Liu M., Li S., Zhang Q., Xu Z., Wang J., Sun H. Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int. Immunopharmacol. 2018;57:25–32. doi: 10.1016/j.intimp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Breyner N.M., Vilas Boas P.B., Fernandes G., de Carvalho R.D., Rochat T., Michel M.L., Chain F., Sokol H., de Azevedo M., Myioshi A., et al. Oral delivery of pancreatitis-associated protein by Lactococcus lactis displays protective effects in dinitro-benzenesulfonic-acid-induced colitis model and is able to modulate the composition of the microbiota. Environ. Microbiol. 2019;21:4020–4031. doi: 10.1111/1462-2920.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zurita-Turk M., Mendes Souza B., Prósperi de Castro C., Bastos Pereira V., Pecini da Cunha V., Melo Preisser T., Caetano de Faria A.M., Carmona Cara Machado D., Miyoshi A. Attenuation of intestinal inflammation in IL-10 deficient mice by a plasmid carrying Lactococcus lactis strain. BMC Biotechnol. 2020;20:38. doi: 10.1186/s12896-020-00631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foligne B., Dessein R., Marceau M., Poiret S., Chamaillard M., Pot B., Simonet M., Daniel C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology. 2007;133:862–874. doi: 10.1053/j.gastro.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Aubry C., Michon C., Chain F., Chvatchenko Y., Goffin L., Zimmerli S.C., Leguin S., Langella P., Bermudez-Humaran L., Chatel J.M. Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS-induced colitis mouse model. Microb. Cell Factories. 2015;14:176. doi: 10.1186/s12934-015-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braat H., Rottiers P., Hommes D.W., Huyghebaert N., Remaut E., Remon J.P., van Deventer S.J., Neirynck S., Peppelenbosch M.P., Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Sasaoka T., Ito M., Yamashita J., Nakajima K., Tanaka I., Narita M., Hara Y., Hada K., Takahashi M., Ohno Y., et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiology. Gastrointest. Liver Physiol. 2011;300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 63.Poulsen S.S., Thulesen J., Christensen L., Nexo E., Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516–522. doi: 10.1136/gut.45.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coccia M., Harrison O.J., Schiering C., Asquith M.J., Becher B., Powrie F., Maloy K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao J., Cheng J., Xi S., Qi X., Shen S., Ge Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V. 2019;137:112–121. doi: 10.1016/j.ejpb.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y., Sun Y., Wang G., Ge S., Liu H. Dendrobium Officinale Polysaccharides Protect against MNNG-Induced PLGC in Rats via Activating the NRF2 and Antioxidant Enzymes HO-1 and NQO-1. Oxidative Med. Cell. Longev. 2019;2019:9310245. doi: 10.1155/2019/9310245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wehkamp J., Schmid M., Stange E.F. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2007;23:370–378. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- 68.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajora N., Boccoli G., Catania A., Lipton J.M. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–385. doi: 10.1016/S0196-9781(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 70.Pesce M., D’Alessandro A., Borrelli O., Gigli S., Seguella L., Cuomo R., Esposito G., Sarnelli G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell. Mol. Med. 2018;22:706–715. doi: 10.1111/jcmm.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrosino S., Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017;174:1349–1365. doi: 10.1111/bph.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borrelli F., Romano B., Petrosino S., Pagano E., Capasso R., Coppola D., Battista G., Orlando P., Di Marzo V., Izzo A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015;172:142–158. doi: 10.1111/bph.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abraham B.P., Quigley E.M.M. Probiotics in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017;46:769–782. doi: 10.1016/j.gtc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Bai L., Gao M., Cheng X., Kang G., Cao X., Huang H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Factories. 2020;19:94. doi: 10.1186/s12934-020-01350-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song A.A., In L.L.A., Lim S.H.E., Rahim R.A. A review on Lactococcus lactis: From food to factory. Microb. Cell Factories. 2017;16:55. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizoguchi A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012;105:263–320. doi: 10.1016/b978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.