Abstract
Background:
Higher intake of ultraprocessed foods (UPFs) might be associated with increased risk of cardiovascular disease.
Objectives:
Our objective was to examine the association between usual percentage of calories (%kcal) from UPFs and the American Heart Association’s “Life’s Simple 7” cardiovascular health (CVH) metrics in US adults.
Methods:
We analyzed data from 11,246 adults aged ≥20 y from the NHANES 2011–2016 (a cross-sectional, nationally representative survey). UPF designation was assigned on the basis of the NOVA classification system, according to the extent and purpose of food processing. Each CVH metric was given a score of 0, 1, or 2 representing poor, intermediate, or ideal health, respectively. Scores of the 6 metrics (excluding diet) were summed, and CVH was categorized as inadequate (0–4), average (5–8), or optimum (9–12). We used the National Cancer Institute’s methods to estimate the usual %kcal from UPFs, and multivariable linear and multinomial logistic regression to assess the association between UPFs and CVH, adjusted for age, sex, race and Hispanic origin, education, and poverty.
Results:
The weighted prevalence of inadequate, average, and optimum CVH was 8.0%, 51.7%, and 40.3%, respectively. The mean usual %kcal from UPFs was 55.4%, and midpoint of quartiles of intake ranged from 40.4% (quartile 1) to 70.5% (quartile 4). Every 5% increase in calories from UPFs was associated with 0.14 points lower CVH score (P < 0.001). The adjusted ORs for inadequate CVH were 1.40 (95% CI: 1.23, 1.60), 1.82 (1.45, 2.29), and 2.57 (1.79, 3.70), respectively, comparing quartiles 2, 3, and 4 with quartile 1 of UPF intake. The pattern of association was largely consistent across subgroups.
Conclusions:
Usual %kcal from UPFs represented more than half of total calorie intake in US adults. A graded inverse association between %kcal from UPFs and CVH was observed.
Keywords: ultraprocessed foods, usual percentage of calories, cardiovascular health, NHANES, odds ratio
Introduction
Ultraprocessed foods (UPFs), as defined by the NOVA food classification system, are formulations of macronutrients (starches, sugars, fats, and protein isolates) with little, if any, whole food and often with added flavors, colors, emulsifiers, and other cosmetic additives (1). Examples of UPFs include soft drinks, packaged salty snacks, cookies and cakes, processed meats, chicken nuggets, and powdered and packaged instant soups. UPFs are typically energy-dense products, high in calories, added sugar, unhealthy fats, and salt, and low in dietary fiber, protein, vitamins, and minerals. The level of consumption is generally high in high-income countries and is increasing in low-and middle-income countries (2). Researchers have shown that high intake of UPFs is associated with overweight (3, 4), obesity (3–6), dyslipidemia (7), hypertension (8), metabolic syndrome (9, 10), type 2 diabetes (11), cancer (12), cardiovascular disease (CVD) incidence (13), and all-cause mortality (14–16).
The American Heart Association’s (AHA’s) “Life’s Simple 7” includes 7 cardiovascular health (CVH) metrics (i.e., BMI, smoking, physical activity, dietary intake, total cholesterol, blood pressure, and fasting glucose) (17, 18). The presence of a higher number of ideal CVH metrics is associated with a graded and significantly lower risk of CVD incidence and mortality (19–22). Although a few studies (3–11) have examined the association between UPFs and individual cardiovascular risk factors, none have assessed the association between UPFs and the AHA’s CVH metrics. Given the potential role that UPFs have on several individual cardiovascular risk factors, we hypothesized that intake of UPFs is associated with reduced CVH. In this study, we examined the association between usual percentage of total daily calories (%kcal) from UPFs and CVH by using data from nationally representative samples of US adults.
Methods
Data source and participants
NHANES is a large, multistage, complex survey of the civilian, noninstitutionalized US population conducted by the National Center for Health Statistics (NCHS), CDC. Detailed descriptions of NHANES methods are published elsewhere (23). Briefly, a stratified, multistage probability cluster sampling design was used to collect health and nutritional data from a representative sample of the US population. During the 2011–2016 NHANES cycles, 17,048 participants aged ≥20 y were interviewed; of those, 14,865 had a complete and reliable first 24-h dietary recall. We sequentially excluded 174 pregnant women, 2514 participants who had history of CVD (defined as having coronary heart disease, heart attack, angina, or stroke, n = 1406) or cancer (n = 1108), and 931 participants who had missing information on CVH, or covariates, leaving 11,246 adults for analysis (Supplemental Figure 1). Study protocols for NHANES were approved by the NCHS ethics review board. Signed informed consent was obtained from all adult participants.
Estimated UPF intake
Data on the intake of UPFs were assessed by using up to two 24-h dietary recalls. The first recall was administered in person, followed by a second recall administered via phone 3–10 d later. Trained interviewers administered the 24-h dietary recalls by using the automated multipass method (23). Nutrient values were assigned to foods by using the USDA Food and Nutrient Database for Diet Studies (FNDDS) (24). Briefly, USDA’s FNDDS converts foods and beverages consumed by participants into gram amounts and determines their nutrient values using 8-digit food codes. The NOVA system was applied to FNDDS data to classify all foods and beverages into 4 groups on the basis of nature, extent, and purpose of industrial food processing: 1) unprocessed or minimally processed foods; 2) processed culinary ingredients; 3) processed foods; and 4) ultraprocessed foods. For all food items (Food Codes) judged to be a handmade recipe, the classification was applied to the underlying ingredients Standard Reference (SR) Codes) obtained from the USDA’s FNDDS (USDA SR) (24). The detailed procedures to classify food items according to NOVA (25) and estimate NOVA calorie contributions have been described in detail elsewhere (5, 25). Our analyses focused on UPFs.
We used a method developed by the National Cancer Institute (NCI) to estimate the usual %kcal from UPFs, accounting for between-and within-person variation in intake (26). This method allowed us to estimate the distribution of the usual %kcal from UPFs in our study population (27) and to examine the nutrient disease association corrected for measurement error, also known as the regression calibration, a statistical method for adjusting point and interval estimates of effect from the regression models for bias from measurement error (28, 29).
The NCI method requires that at least some respondents have multiple days of nutrient values to estimate the between-and within-person variations. The estimate of usual intake distribution was adjusted for age in years, sex, race and Hispanic origin, the first-or second-day dietary recalls (all participants had a first-day, and 87.7% had a second-day dietary recall), and the day of the week when 24-h recall was collected [weekday (Monday–Thursday) compared with weekend (Friday–Sunday)].
CVH metrics
CVH metrics were based on the “Life’s Simple 7” metrics developed by the AHA, which included smoking, physical activity, healthy dietary scores, BMI, total cholesterol, blood pressure, and fasting plasma glucose (17, 18). The definitions of ideal, intermediate, and poor CVH metrics for adults are presented in Supplemental Table 1. In the main analysis of the current study, the dietary score was not included. Fasting plasma glucose was available for 48.4% of participants because NHANES only collected fasting blood samples in half of the participants. Instead, glycated hemoglobin (HbA1c) was measured in almost all participants. To maximize the sample size, we used HbA1c values <5.7%, 5.7–6.4%, and ≥6.5% as a proxy for fasting plasma glucose concentrations <100 mg/dL, 100 to <126 mg/dL, and ≥126 mg/dL, respectively, as recommended by the American Diabetes Association (30). Backward calibration equation was used to adjust data of the NHANES 2015–2016 cycle to be consistent with earlier years, to account for changes in glucose measurement methods over time. Participants who reported having diabetes, or who were being treated with insulin or oral medication to lower blood glucose and who had an HbA1c concentration <6.5% and/or a fasting plasma glucose concentrations <126 mg/dL were categorized as intermediate health. Similarly, participants who reported taking antihypertensive medication and who were treated to goal (<140/90 mmHg), or who were taking cholesterol-lowering medication and treated to goal (<240 mg/dL) were categorized as “intermediate.” Participants who had either of these conditions who were untreated, or who were treated but not to goal, were categorized as “poor” for that health factor.
Use of antihypertensive, cholesterol-lowering, and glucoselowering medications was self-reported. Total cholesterol and plasma glucose were measured with the enzymatic method (23). BMI was calculated as weight in kilograms divided by height in meters squared. Mean blood pressure was estimated from ≤3 readings, obtained under standard conditions during a single physical examination. Each CVH component was given a point score of 0, 1, or 2 to represent poor, intermediate, or ideal health, respectively. On the basis of the sum of 6 components (diet component was not included because UPFs were derived from dietary data), an overall score ranging from 0 to 12 was categorized as inadequate (0–4), average (5–8), or optimum (9–12) CVH.
The primary outcome variables in our study are the summary of the 6-component CVH metrics scores (excluding diet component) as a continuous variable or categorized into 3 categories (inadequate, average, and optimum). The secondary outcome variables included the 4 CVH factors (BMI, total cholesterol, blood pressure, and fasting plasma glucose), 4 CVH factors plus dietary component, and all 7 CVH metrics.
Other covariates
Other sociodemographic data included age, sex, self-reported race and Hispanic origin (non-Hispanic white, non-Hispanic black, Hispanic, and other), educational attainment, and poverty-to-income ratio (PIR, the ratio of household income to the poverty threshold after accounting for inflation and family size). Educational attainment was categorized into less than high school, high-school graduate, and college or above. PIR was categorized into <1.30, ≥1.30, and missing (n = 886).
Statistical analyses
Data on characteristics were expressed as means and 95% CIs for continuous variables, or as percentages and 95% CIs for categorical variables, and data were compared across CVH metric categories (inadequate, average, and optimum). Trends across CVH metrics categories were assessed by t test.
We used the restricted cubic spline in the multivariable linear regression models with 4 knots (20th, 40th, 60th, and 80th percentiles) to examine departure from a linear relation between usual %kcal from UPFs and CVH scores (31); there was no evidence of departure from a linear relation (P = 0.10 for nonlinearity). We then calculated the adjusted differences in CVH scores by using the midpoint of the lowest quartile (quartile 1) of intake (40.4% of calories from UPFs) as the reference.
We used multinomial logistic regression to estimate the adjusted ORs for inadequate and average CVH compared with optimum CVH comparing quartiles 2, 3, and 4 with quartile 1 of usual %kcal from UPFs. The base model adjusted for age as a continuous variable, sex, and race and Hispanic origin; the second model was additionally adjusted for education and PIR. We presented the stratified analyses by age group (20–44, 45–64, and ≥65 y), sex, race and Hispanic origin, educational level, and PIR. We tested the interaction between UPFs and covariates by including the interaction terms in the multinomial logistic models based on the Wald F test.
We also estimated the association between UPFs and individual components of CVH metrics. When assessing the association with individual CVH components, we calculated ORs for poor and intermediate CVH compared with ideal CVH, comparing quartiles 2, 3, and 4 with quartile 1 of usual %kcal from UPFs and adjusted for the rest of CVH components in addition to covariates. False discovery rate (FDR)–adjusted P values were presented to take into account the multiple comparisons.
We used the first-day 24-h dietary recall sampling weights, and divided by 3 (data from 3 NHANES cycles) to represent the noninstitutionalized US population and account for sampling probability and nonresponse. Statistical analyses were performed using SUDAAN version 11 (RTI International) accounting for the complex sampling design. All tests of statistical significance were 2-tailed, and a P value <0.05 was considered significant.
Sensitivity analyses
The AHA Life’s Simple 7 include 7 CVD determinants of different nature regarding the link between UPFs and CVD. CVH health factors (BMI, total cholesterol, blood pressure, and fasting plasma glucose) and diet quality are potential mechanisms linking UPFs to CVD, whereas smoking and physical activity are not. Four sensitivity analyses were performed to test the robustness of the results.
The first sensitivity analysis was to assess the association between UPF intake and the 4 CVH health factors. We presented ORs of having 0–1 compared with 3–4, and 2 compared with 3–4 ideal CVH health factors by quartiles of UPF intake (Supplemental Table 2).
The second sensitivity analysis examined the association between UPF intake and the 4 CVH health factors plus the dietary component, which can also be a potential mechanism linking UPFs to CVD. We presented ORs of having 0–1 compared with 4–5, and 2–3 compared with 4–5 ideal CVH health components by quartiles of UPF intake (Supplemental Table 3). We used Healthy Eating Index 2010 (HEI-2010) score instead of AHA’s dietary score because the current recommendation of daily sodium intake in federal guidelines is <2300 mg/d sodium, which does not match the 1500 mg/d in the AHA dietary scores, and also because HEI-2010 is a continuous scale and therefore more sensitive and informative. HEI-2010 scores were based on a 12-component index: total fruit, whole fruit, total vegetables, grains and beans, whole grains, dairy, total protein foods, seafood and plant protein, fatty acid, refined grains, sodium, and empty calories, with total scores ranging from 0 to 100 and a higher score indicating a healthier diet (32). HEI-2010 scores were calculated using the first-day 24-h dietary recall. We used HEI-2010 scores after removing added sugars because they are part of empty calories, and studies have shown that 90% of added sugars come from UPFs (25). Before excluding added sugar, 51.5%, 46.0%, and 2.5% of participants had poor, intermediate, and ideal diet based on ≤50, 51–80, and ≥81 HEI-2010 scores, respectively. We used percentiles 51.5 and 97.5 to define poor, intermediate, and ideal diet in our sensitivity analyses after excluding added sugars.
In the third sensitivity analysis, we examined the association between %kcal from UPFs and the AHA 7 CVH metrics (including diet component HEI-2010). We presented ORs for inadequate (CVH scores 0–4) compared with optimum (CVH scores 10–14), and average (CVH scores 5–9) compared with optimum health by quartiles of UPF intake (Supplemental Table 4).
The fourth sensitivity analysis examined the association between the percentage of UPFs by weight and CVH because some studies have pointed out that assessing the proportion of UPFs out of the total weight of foods and beverages consumed can better account for UPFs that do not contribute to energy intake (e.g., artificially sweetened drinks), as well as properties directly related to food processing rather than those related to their nutritional characteristics (11, 12). We presented ORs for inadequate compared with optimum, and average compared with optimum health by quartiles of UPF intake (Supplemental Table 5).
Results
Of the 11,246 participants, the mean age was 44.6 y. About half (51%) of US adults were female, 63% were non-Hispanic white, 65% had higher education, and 72% had a PIR ≥130%. The weighted prevalence of inadequate, average, and optimum CVH was 8.0%, 51.7%, and 40.3%, respectively. Participants who were of non-Hispanic white descent, younger age, higher education level, or a higher PIR were more likely to have higher CVH scores; whereas non-Hispanic black adults were more likely to have lower CVH scores (Table 1).
TABLE 1.
Comparison of selected characteristics by cardiovascular health metric categories in US adults aged ≥20 y, NHANES 2011–20161
| Characteristics | Overall (n = 11,246) | CVH score 0–4 (n = 1064) | CVH score 5–8 (n = 6089) | CVH score 9–12 (n = 4093) | P value for trend2 |
|---|---|---|---|---|---|
| Age, y (mean ± SE) | 44.6 ± 0.38 | 53.7 ± 0.40 | 48.4 ± 0.38 | 38.0 ± 0.50 | <0.001 |
| Male, % (SE) | 49.3 (0.63) | 46.7 (2.33) | 50.0 (0.86) | 48.8 (1.03) | 0.425 |
| Race and Hispanic origin, % (SE) | |||||
| Non-Hispanic white | 63.3 (2.21) | 59.0 (3.34) | 63.0 (2.43) | 64.6 (2.14) | 0.040 |
| Non-Hispanic black | 11.3 (1.18) | 16.1 (2.16) | 12.5 (1.34) | 8.98 (1.00) | <0.001 |
| Hispanic | 16.3 (1.64) | 17.0 (2.38) | 16.7 (1.75) | 15.8 (1.55) | 0.463 |
| Other | 9.01 (0.68) | 7.96 (1.43) | 7.89 (0.67) | 10.7 (0.87) | 0.096 |
| Education, % (SE) | |||||
| <12 y | 14.4 (1.03) | 25.3 (2.24) | 17.0 (1.15) | 8.82 (0.93) | <0.001 |
| 12 y | 21.1 (0.83) | 26.8 (2.17) | 24.8 (1.11) | 15.1 (0.90) | <0.001 |
| >12 y | 64.6 (1.49) | 47.9 (2.56) | 58.2 (1.64) | 76.1 (1.46) | <0.001 |
| Poverty-to-income ratio,3 % (SE) | |||||
| 0–129% | 22.0 (1.18) | 32.4 (2.35) | 22.6 (1.36) | 19.1 (1.52) | <0.001 |
| ≥130% | 71.8 (1.31) | 60.1 (2.58) | 71.2 (1.48) | 74.9 (1.63) | <0.001 |
| Missing | 6.21 (0.46) | 7.51 (1.19) | 6.20 (0.53) | 5.96 (0.61) | 0.213 |
CVH: cardiovascular health (excluding diet component).
P value for trend across CVH metric categories was assessed by t test.
Poverty-to-income ratio is the ratio of family income to the Department of Health and Human Services poverty measure.
The mean usual %kcal from UPFs was 55.4%, and midpoint of quartiles of intake ranged from 40.4% (quartile 1) to 70.5% (quartile 4). Every 5% increase in calories from UPFs was associated with a 0.14 points lower CVH score (P < 0.001) (Figure 1). Comparing quartiles 2, 3, and 4 with the lowest quartile (quartile 1) of UPF intake, the fully adjusted ORs for inadequate CVH (compared with optimum) were 1.40 (95% CI: 1.23, 1.60), 1.82 (95% CI: 1.45, 2.29), and 2.57 (95% CI: 1.79, 3.70), respectively. The corresponding ORs for average CVH (compared with optimum) were 1.35 (95% CI: 1.25, 1.45), 1.70 (95% CI: 1.49, 1.94), and 2.30 (95% CI: 1.87, 2.84) (Table 2).
FIGURE 1.
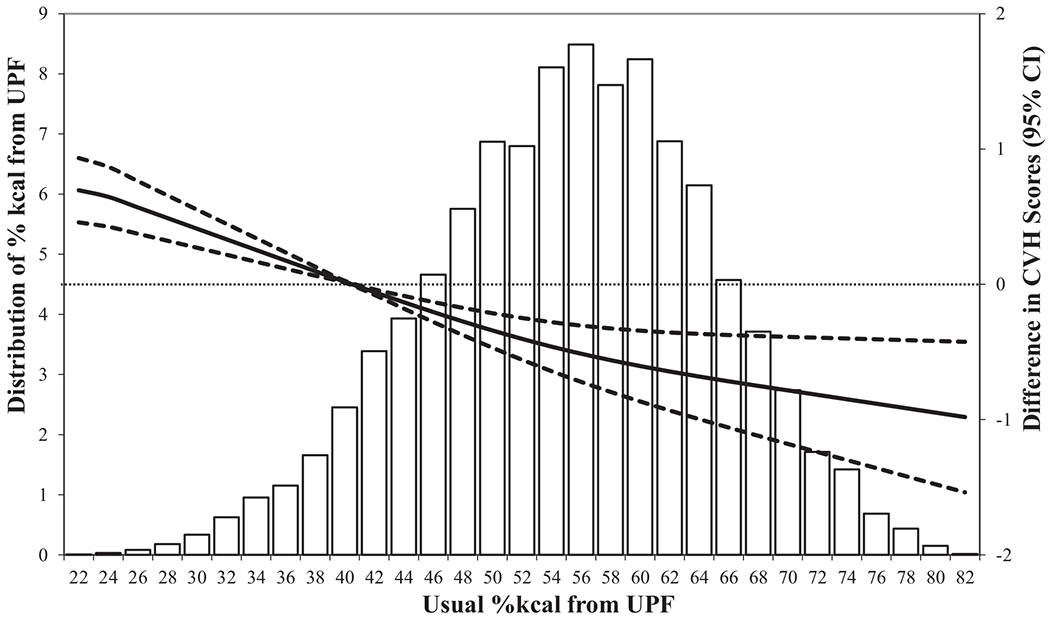
Distributions of usual %kcal from UPFs and adjusted differences in CVH scores (40.4% as reference), US adults (n = 11,246), NHANES 2011–2016. Multivariable linear regression models were used to estimate the adjusted differences in CVH scores and corresponding 95% CI, and were adjusted by age, sex, race and Hispanic origin, education, and poverty-to-income ratio. CVH, cardiovascular health (excluding diet component); UPF, ultraprocessed food; %kcal, percentage of calories.
TABLE 2.
ORs and 95% CIs for cardiovascular health metrics associated with UPFs: US adults, NHANES 2011–20161
| Quartiles of usual percentage of calories from UPFs (n = 11,246) |
|||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P value3 | |
| Midpoint and range of usual percentage of calories from UPFs | 40.4% (26.1–46.6%) | 51.2% (46.7–55.3%) | 59.5% (55.4–64.2%) | 70.5% (64.3–86.0%) | |
| Health metrics | |||||
| Age, sex, and race and Hispanic origin adjusted | |||||
| Average CVH vs. optimum | 1.00 | 1.43 (1.32, 1.55) | 1.89 (1.64, 2.17) | 2.72 (2.18, 3.39) | <0.001 |
| Inadequate CVH vs. optimum | 1.00 | 1.55 (1.36, 1.77) | 2.18 (1.73, 2.74) | 3.40 (2.37, 4.89) | <0.001 |
| Fully adjusted2 | |||||
| Average CVH vs. optimum | 1.00 | 1.35 (1.25, 1.45) | 1.70 (1.49, 1.94) | 2.30 (1.87, 2.84) | <0.001 |
| Inadequate CVH vs. optimum | 1.00 | 1.40 (1.23, 1.60) | 1.82 (1.45, 2.29) | 2.57 (1.79, 3.70) | <0.001 |
Optimum CVH: CVH metrics scores 9–12; average CVH: CVH metrics scores 5–8; inadequate CVH: CVH metrics scores 0–4. CVH, cardiovascular health (excluding diet component); Q, quartile; UPF: ultraprocessed food.
Multinomial logistic regression models were used to estimate ORs and corresponding 95% CIs, and were adjusted for age, sex, race and Hispanic origin, education, and poverty-to-income ratio (the ratio of family income to the Department of Health and Human Services poverty measure).
P value of β-coefficient for percentage of calories (continuous) from UPFs in the multinomial logistic regression models.
Figure 2 shows the fully adjusted ORs (95% CI) for poor or intermediate CVH (compared with ideal) comparing quartiles 2, 3, and 4 with quartile 1 of UPF intake for each individual CVH metric. Usual %kcal from UPFs was significantly associated with BMI, poor smoking status, physical inactivity, and diabetes, but not with blood pressure, and was inversely associated with total cholesterol.
FIGURE 2.
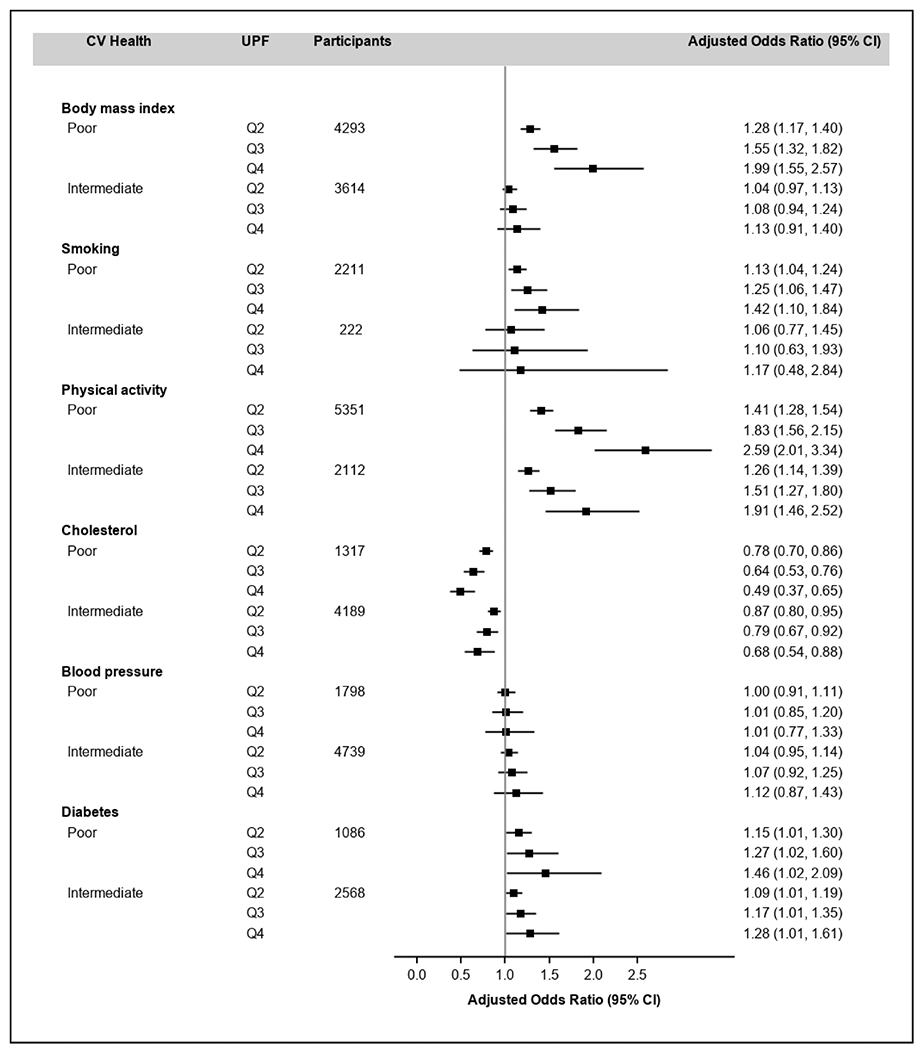
Adjusted ORs (95% CI) for poor or intermediate CVH of each individual CVH metric associated with usual %kcal from UPFs, US adults (n = 11,246), NHANES 2011–2016. Multinomial logistic regression models were used to estimate ORs and corresponding 95% CIs, and were adjusted by age, sex, race and Hispanic origin, education, and poverty-to-income ratio. The midpoints (range) of usual %kcal from UPFs were 40.4% (26.1–46.6%), 51.2% (46.7–55.3%), 59.5% (55.4–64.2%), and 70.5% (64.3–86.0%) for quartiles 1–4, respectively. CVH, cardiovascular health (excluding diet component); Q, quartile; UPF, ultraprocessed food; %kcal, percentage of calories.
The associations between %kcal from UPFs and CVH were largely consistent by age, sex, education years, and PIR subgroups (Table 3, FDR-adjusted P values >0.05 for all interactions). The FDR-adjusted P value for the interaction between UPFs and race and Hispanic origin was 0.049, and the association appeared to be stronger in the other race group.
TABLE 3.
Adjusted ORs and 95% CIs for CVH metrics associated with UPFs by selected subgroups: US adults, NHANES 2011–20161
| Quartiles of usual percentage of calories from UPFs2 (n = 11,246) |
FDR-adjusted P value3 | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Midpoint and range of usual percentage of calories from UPFs | 40.4% (26.1–46.6%) | 51.2% (46.7–55.3%) | 59.5% (55.4–64.2%) | 70.5% (64.3–86.0%) | |
| Age 20–44 y (n = 5591) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.28 (1.02, 1.60) | 1.54 (1.03, 2.30) | 1.97 (1.05, 3.70) | 0.054 |
| Average CVH vs. optimum | 1.00 | 1.32 (1.19, 1.47) | 1.64 (1.36, 1.96) | 2.17 (1.63, 2.89) | <0.001 |
| Age 45–64 y (n = 3952) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.57 (1.27, 1.94) | 2.22 (1.52, 3.24) | 3.50 (1.93, 6.36) | <0.001 |
| Average CVH vs. optimum | 1.00 | 1.44 (1.27, 1.64) | 1.91 (1.53, 2.39) | 2.78 (1.96, 3.94) | <0.001 |
| Age ≥65 y (n = 1703) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.40 (1.09, 1.80) | 1.83 (1.17, 2.86) | 2.61 (1.28, 5.31) | 0.010 |
| Average CVH vs. optimum | 1.00 | 1.31 (1.06, 1.62) | 1.62 (1.11, 2.36) | 2.14 (1.18, 3.91) | 0.023 |
| Sex | |||||
| Male (n = 5480) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.19 (0.96, 1.48) | 1.37 (0.94, 2.00) | 1.64 (0.90, 3.00) | 0.126 |
| Average CVH vs. optimum | 1.00 | 1.27 (1.15, 1.40) | 1.52 (1.28, 1.81) | 1.94 (1.47, 2.57) | <0.001 |
| Female (n = 5766) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.64 (1.40, 1.93) | 2.40 (1.81, 3.20) | 3.97 (2.53, 6.26) | <0.001 |
| Average CVH vs. optimum | 1.00 | 1.43 (1.26, 1.62) | 1.89 (1.52, 2.36) | 2.72 (1.92, 3.88) | <0.001 |
| Race and Hispanic origin | |||||
| NHW (n = 4047) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.26 (1.06, 1.50) | 1.50 (1.11, 2.04) | 1.90 (1.18, 3.06) | 0.017 |
| Average CVH vs. optimum | 1.00 | 1.34 (1.20, 1.49) | 1.67 (1.39, 2.01) | 2.23 (1.67, 2.98) | <0.001 |
| NHB (n = 2510) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.43 (1.14, 1.79) | 1.87 (1.26, 2.79) | 2.67 (1.43, 4.98) | 0.006 |
| Average CVH vs. optimum | 1.00 | 1.22 (1.05, 1.41) | 1.42 (1.10, 1.83) | 1.72 (1.16, 2.57) | 0.017 |
| Hispanic (n = 2935) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.21 (1.01, 1.47) | 1.42 (1.01, 1.99) | 1.74 (1.02, 2.97) | 0.060 |
| Average CVH vs. optimum | 1.00 | 1.24 (1.13, 1.37) | 1.47 (1.24, 1.75) | 1.85 (1.40, 2.43) | <0.001 |
| Other (n = 1754) | |||||
| Inadequate CVH vs. optimum | 1.00 | 2.47 (1.58, 3.85) | 5.29 (2.34, 11.98) | 15.59 (4.05, 59.97) | <0.001 |
| Average CVH vs. optimum | 1.00 | 1.51 (1.26, 1.80) | 2.13 (1.54, 2.96) | 3.49 (2.04, 5.98) | <0.001 |
| Education years | |||||
| <12 (n = 2371) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.26 (0.90, 1.77) | 1.52 (0.84, 2.75) | 1.93 (0.75, 4.93) | 0.187 |
| Average CVH vs. optimum | 1.00 | 1.20 (0.98, 1.46) | 1.38 (0.97, 1.97) | 1.66 (0.95, 2.91) | 0.099 |
| 12 (n = 2440) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.09 (0.85, 1.40) | 1.17 (0.75, 1.82) | 1.28 (0.64, 2.55) | 0.503 |
| Average CVH vs. optimum | 1.00 | 1.09 (0.90, 1.33) | 1.17 (0.83, 1.65) | 1.28 (0.75, 2.19) | 0.390 |
| >12 (n = 6435) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.51 (1.26, 1.80) | 2.08 (1.51, 2.85) | 3.16 (1.92, 5.21) | <0.001 |
| Average CVH vs. optimum | 1.00 | 1.44 (1.29, 1.60) | 1.91 (1.58, 2.32) | 2.77 (2.05, 3.76) | <0.001 |
| PIR | |||||
| 0–129% (n = 3383) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.17 (0.95, 1.44) | 1.32 (0.92, 1.90) | 1.55 (0.88, 2.75) | 0.150 |
| Average CVH vs. optimum | 1.00 | 1.14 (0.99, 1.31) | 1.26 (0.97, 1.62) | 1.43 (0.96, 2.14) | 0.100 |
| ≥130% (n = 6977) | |||||
| Inadequate CVH vs. optimum | 1.00 | 1.53 (1.28, 1.83) | 2.11 (1.54, 2.90) | 3.24 (1.97, 5.33) | <0.001 |
| Average CVH vs. optimum | 1.00 | 1.40 (1.26, 1.54) | 1.80 (1.51, 2.15) | 2.53 (1.92, 3.34) | <0.001 |
| Missing PIR (n = 886) | |||||
| Inadequate CVH vs. optimum | 1.00 | 0.97 (0.66, 1.41) | 0.94 (0.48, 1.85) | 0.91 (0.31, 2.65) | 0.855 |
| Average CVH vs. optimum | 1.00 | 1.38 (1.06, 1.80) | 1.78 (1.11, 2.85) | 2.49 (1.18, 5.26) | 0.028 |
CVH, cardiovascular health (excluding diet component); FDR, false discovery rate; NHW, non-Hispanic whites; NHB, non-Hispanic blacks; PIR, poverty-to-income ratio; UPF, ultraprocessed food.
Multinomial logistic regression models were used to estimate ORs and the corresponding 95% CIs, and were adjusted for age, sex, race and Hispanic origin, education, and PIR (the ratio of family income to the Department of Health and Human Services poverty measure). Optimum CVH: CVH metrics scores 9–12; average CVH: CVH metrics scores 5–8; inadequate CVH: CVH metrics scores 0–4.
FDR-adjusted P value of β-coefficient for percentage of calories (continuous) from UPFs in the multinomial logistic regression models. The FDR-adjusted P values for the interaction between UPFs and age group, sex, race and Hispanic origin, education, and PIR were 0.491, 0.171, 0.049, 0.217, and 0.171, respectively.
In sensitivity analyses, we examined the association between %kcal from UPFs and 4 health factors, 4 health factors plus HEI-2010 excluding added sugar metric as well as all 7 CVH components, and the association between proportion of UPFs by weight and 6 CVH metrics. The pattern of association remained largely consistent, though the association was stronger for the 7 CVH component metric (Supplemental Tables 2–5).
Discussion
In this nationally representative study, we found that UPFs account for slightly more than half of US adults’ daily total calories, and measures of CVH decrease as UPF consumption rises. The pattern of the association was largely consistent across age, sex, education, and PIR subgroups. Sensitivity analyses suggested that the association remained significant and that the strength of association increased with the number of factors taken into consideration. To the best of our knowledge, this is the first study to assess consumption of UPFs and its relation with CVH.
There has been rapid growth in consumption of UPFs in both high-income and middle-income countries (2). UPFs have displaced unprocessed or minimally processed foods, as well as freshly prepared dishes and meals made from these foods together with processed culinary ingredients (1). In several countries, public health authorities have recently started to promote unprocessed or minimally processed foods and to recommend limiting the consumption of UPFs (33, 34). Many observational studies have indicated that high consumption of UPFs is associated with several major CVD risk factors, such as overweight and obesity in children and adults (3–6), incidence of hypertension in middle-aged adults (8), increased total cholesterol and LDLs in preschool to school-age children (7), metabolic syndrome in adolescents and adults (9, 10), and type 2 diabetes in adults (11). Studies have also suggested that high consumption of UPFs is associated with increased CVD incidence and early death from all-cause mortality (13–16). One recent randomized controlled crossover trial observed that participants had higher energy intake and weight gain during a UPF diet, whereas they lost weight when consuming a nonultraprocessed diet (35). Our analyses showed a graded inverse association between %kcal from UPFs and CVH. Many high-cholesterol foods (eggs, cheese, beef, shellfish, etc.) are not classified as UPFs, which could explain the negative association between UPFs and cholesterol. Further, people consuming a high-UPF diet can have lower HDLs and higher triglycerides, because both are associated with greater consumption of added sugar and UPF consumption has been shown to be associated with added sugar (25), but we would not have captured this association because our measure only captured total cholesterol and self-reported high cholesterol. In addition, reverse causality might explain the negative relation between UPF consumption and total cholesterol. People who develop hyperlipidemia might improve their diet and reduce UPF consumption.
Several mechanisms have been suggested for the association between UPFs and CVD risk factors. UPFs are typically high in sugar, sodium, and trans and saturated fats, and are energy dense (1). First, studies have shown that increased intakes of high-sugar foods—sugar-sweetened beverages and foods having high-energy density—were associated with obesity, metabolic syndrome, and diabetes (36–38). Consumption of a diet high in sodium is associated with high blood pressure (39, 40), and trans and saturated fat consumption has been associated with increased risk of CVD (41–43). Second, high-intensity flavoring makes ultraprocessed products extremely palatable, which can supersede natural satiety mechanisms (44), so people might eat more of these foods, even when they are no longer hungry. In addition, because UPFs often lack fiber (1), these foods might not make people feel as full as less processed foods would (45). In these ways, ultraprocessed products can facilitate overeating and high glycemic loads. Furthermore, adverse effects of chemical additives on CVD have been suggested in experimental studies on animal or cellular models. For example, high oral doses of sulfites can cause damage to rat hearts (46); doses of monosodium glutamate in mice can initiate atherosclerosis and other coronary heart diseases (47). Emulsifiers, particularly carboxymethylcellulose and polysorbate-80, have shown potential roles in inducing low-grade inflammation and obesity or metabolic syndrome in mice (48). Last, UPF consumption might increase exposure to currently used phthalates, environmental chemicals that are present in food packaging, which have been shown to be associated with adverse health outcomes, such as obesity, diabetes, hypertension, and coronary artery disease (49, 50).
Our study is the first to assess the association between UPFs and CVH in a large, nationally representative sample of US adults. Our analyses were based on individual consumption data, and we used a measurement error model to estimate usual %kcal from UPFs from two 24-h dietary recalls accounting for within-individual variation in intake.
Our study has several limitations. First, although NHANES collected some information indicating food processing, such as place of meals and product brands, these data are not consistently determined for all food items, which could lead to potential misclassification errors. Second, whereas some authors have suggested that the association between UPFs and outcomes might be explained by their nutrient content rather than the processing itself (51), some longitudinal studies have observed that the association between UPFs and outcomes remained significant after adjustment for markers of the nutritional quality of the diet (52, 53). Third, a previous validation study using 24-h dietary recalls suggested that energy intake can be underestimated by as much as 11% (54), which could affect the estimate of usual absolute intake but not necessarily the dietary contributions used in this study. Fourth, social desirability bias can lead to an underestimation of the dietary contribution of unhealthy foods such as UPFs, which might not have affected our results if it occurred nondifferentially, or could have biased our results toward the null if this occurred differentially in people who consumed the most UPFs. Furthermore, because common lifestyle risk factors tend to cluster, higher UPF consumption could be a proxy of an overall unhealthy diet or lifestyle, and the subsequent residual confounding could overestimate the strength of the association (55). In addition, reverse causality could underestimate the association between UPF consumption and CVH. People who develop any of the poor CVH health conditions (overweight/obesity, high total cholesterol and blood pressure, or diabetes), might change their diet and reduce UPF consumption. Finally, because our analysis was cross-sectional, causal associations between UPF and CVH could not be determined.
In conclusion, our study indicated that US adults consume >50% of their daily total calories from UPFs, and higher consumption of UPFs was associated with inadequate CVH. Raising awareness of the negative health effects of UPFs might help inform the public about healthier eating patterns, which are recommended to improve CVH (37).
Supplementary Material
Acknowledgments
Data collection was sponsored by the CDC.
The authors reported no funding received for this study.
The findings and conclusions expressed in this article are those of the authors and do not necessarily represent the official position of the CDC or the US Department of Health and Human Services. No private sponsor had any role in the study design, data collection, analysis, or interpretation of data, writing the report, or the decision to submit the manuscript.
Abbreviations used:
- AHA
American Heart Association
- CVD
cardiovascular disease
- CVH
cardiovascular health
- FDR
false discovery rate
- FNDDS
Food and Nutrient Database for Dietary Studies
- HbA1c
glycated hemoglobin
- HEI-2010
Healthy Eating Index 2010
- NCHS
National Center for Health Statistics
- NCI
National Cancer Institute
- PIR
poverty-to-income ratio
- UPF
ultraprocessed food
- %kcal
percentage of calories
Footnotes
The authors’ responsibilities were as follows—ZZ: planned data analyses and drafted the manuscript; SLJ, EM, CG, QY: contributed to study planning, analysis, and manuscript development; ZZ, QY: conducted data analysis; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Data described in the manuscript, code book, and analytic code will be made available pending e-mail request to the corresponding author.
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Reference
- 1.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 2018;21:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro CA, Moubarac J-C, Cannon G, Ng SW, Popkin B. Ultraprocessed products are becoming dominant in the global food system. Obes Rev 2013;14(Suppl. 2):21–8. [DOI] [PubMed] [Google Scholar]
- 3.Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, Bes-Rastrollo M. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 2016;104:1433–40. [DOI] [PubMed] [Google Scholar]
- 4.Canhada SL, Luft VC, Giatti L, Duncan BB, Chor D, Fonseca M, Matos SMA, Molina MDCB, Barreto SM, Levy RB, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr 2020;23:1076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr 2018;120:90–100. [DOI] [PubMed] [Google Scholar]
- 6.Nardocci M, Leclerc BS, Louzada ML, Monteiro CA, Batal M, Moubarac JC. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health 2019;110:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauber F, Campagnolo PD, Hoffman DJ, Vitolo MR. Consumption of ultra-processed food products and its effects on children’s lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis 2015;25:116–22. [DOI] [PubMed] [Google Scholar]
- 8.Mendonca RD, Lopes AC, Pimenta AM, Gea A, Martinez-Gonzalez MA, Bes-Rastrollo M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: the Seguimiento Universidad de Navarra Project. Am J Hypertens 2017;30:358–66. [DOI] [PubMed] [Google Scholar]
- 9.Martinez Steele E, Juul F, Neri D, Rauber F, Monteiro CA. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med 2019;125:40–8. [DOI] [PubMed] [Google Scholar]
- 10.Tavares LF, Fonseca SC, Garcia Rosa ML, Yokoo EM. Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian family doctor program. Public Health Nutr 2012;15:82–7. [DOI] [PubMed] [Google Scholar]
- 11.Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, Chazelas E, Deschasaux M, Hercberg S, Galan P, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern Med 2020;180:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Alles B, Mejean C, Deschasaux M, Fassier P, Latino-Martel P, Beslay M, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 2018;360:k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srour B, Fezeu LK, Kesse-Guyot E, Alles B, Mejean C, Andrianasolo RM, Chazelas E, Deschasaux M, Hercberg S, Galan P, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 2019;365:11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabel L, Kesse-Guyot E, Alles B, Touvier M, Srour B, Hercberg S, Buscail C, Julia C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med 2019;179:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rico-Campa A, Martinez-Gonzalez MA, Alvarez-Alvarez I, Mendonca RD, de la Fuente-Arrillaga C, Gomez-Donoso C, Bes-Rastrollo M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019;365:11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco-Rojo R, Sandoval-Insausti H, Lopez-Garcia E, Graciani A, Ordovas JM, Banegas JR, Rodríguez-Artalejo F, Guallar-Castillón P. Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc 2019;94:2178–88. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:948–54. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, Atherosclerosis Risk in Communities (ARIC) Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012;125:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012;307:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artero EG, Espana-Romero V, Lee DC, Sui X, Church TS, Lavie CJ, Blair SN. Ideal cardiovascular health and mortality: Aerobics Center Longitudinal Study. Mayo Clin Proc 2012;87:944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. National Health and Nutrition Examination Survey [Internet]. [cited January 31, 2020]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm.
- 24.USDA Agricultural Research Service. Food and Nutrient Database for Dietary Studies [Internet]. [cited January 31, 2020]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/.
- 25.Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 2016;6:e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr 1997;127:1106–12. [DOI] [PubMed] [Google Scholar]
- 27.Tooze JA, Midthune D, Dodd KW, Freedman LS, Krebs-Smith SM, Subar AF, Guenther PM, Carroll RJ, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc 2006;106:1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kipnis V, Midthune D, Buckman DW, Dodd KW, Guenther PM, Krebs-Smith SM, Subar AF, Tooze JA, Carroll RJ, Freedman LS. Modeling data with excess zeros and measurement error: application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics 2009;65:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol 1992;136:1400–13. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl 1):S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of Health of Brazil. Dietary guidelines for the Brazilian population, 2nd ed. [Internet]. 2014. [cited Sept. 25, 2020]. Available from: http://189.28.128.100/dab/docs/portaldab/publicacoes/guia_alimentar_populacao_ingles.pdf.
- 34.Haut Conseil de la santé publique. Avis relatif à la révision des repères alimentaires pour les adultes du futur Programme National Nutrition Sante 2017-2021. 2017. [Internet]. [cited Sept. 25, 2020]. Available from: www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspa20170216_reperesalimentairesactua2017.pdf.
- 35.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V,et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 2019;30:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scientific Report of the 2015 Dietary Guidelines Advisory Committee. [Internet]. [cited Sept. 25, 2020]. Available from: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [Google Scholar]
- 38.Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007;30:974–9. [DOI] [PubMed] [Google Scholar]
- 39.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Cogswell ME, Gillespie C, Fang J, Loustalot F, Dai S, Carriquiry AL, Kuklina EV, Hong Y, Merritt R, et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005–2010. PLoS One 2013;8(10):e75289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–44. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Gillespie C, Yang Q. Plasma trans-fatty acid concentrations continue to be associated with metabolic syndrome among US adults after reductions in trans-fatty acid intake. Nutr Res 2017;43:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nettleton JA, Brouwer IA, Geleijnse JM, Hornstra G. Saturated fat consumption and risk of coronary heart disease and ischemic stroke: a science update. Ann Nutr Metab 2017;70:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig DS. Technology, diet, and the burden of chronic disease. JAMA 2011;305:1352–3. [DOI] [PubMed] [Google Scholar]
- 45.Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr 1995;49:675–90. [PubMed] [Google Scholar]
- 46.Zhang Q, Bai Y, Yang Z, Tian J, Meng Z. The molecular mechanisms of sodium metabisulfite on the expression of K ATP and L-Ca2+ channels in rat hearts. Regul Toxicol Pharmacol 2015;72:440–6. [DOI] [PubMed] [Google Scholar]
- 47.Singh K, Ahluwalia P. Effect of monosodium glutamate on lipid peroxidation and certain antioxidant enzymes in cardiac tissue of alcoholic adult male mice. J Cardiovasc Dis Res 2012;3:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley JP, KIM H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int 2019;131:105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rancière F, Lyons JG, Loh VHY, Button J, Galloway T, Wang T, Shaw JE, Magliano DJ. Bisphenol A and the risk of cardiometabolic disorders: asystematicreview with meta-analysis of the epidemiological evidence. Environ Health 2015;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health—processing or nutrient content? Curr Obes Rep 2017;6:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Mejéan C, Andrianasolo RM, Chazelas E, Deschasaux M, Hercberg S, Galan P. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 2019;365:11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srour B, Fezeu LK, Kesse-Guyot E, Alles B, Debras C, Druesne-Pecollo N, Chazelas E, Deschasaux M, Hercberg S, Galan P, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern Med 2019;180:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, Robertson J, Brown WM, McFarlane M; TONE Cooperative Research Group. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol 2001;153:996–1006. [DOI] [PubMed] [Google Scholar]
- 55.Schuit AJ, van Loon AJ, Tijhuis M, Ocké M. Clustering of lifestyle risk factors in a general adult population. Prev Med 2002;35(3):219–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


