Abstract
Preterm birth is a principal cause of neurological disability later in life, including cognitive and behavioral deficits. Notably, cognitive impairment has greater impact on quality of life than physical disability. Survivors of preterm birth commonly have deficits of executive function. Difficulties with tasks and planning complexity correlate positively with increasing disability. To overcome these barriers for children born preterm, preclinical and clinical studies have emphasized the importance of neurorestoration. Erythropoietin (EPO) is a endogenous cytokine with multiple beneficial mechanisms of action following perinatal brain injury. While most preclinical investigations have focused on pathology and molecular mechanisms, translational studies of repair using clinically viable biobehavioral biomarkers are still lacking. Here, using an established model of encephalopathy of prematurity secondary to placental insufficiency, we tested the hypothesis that administration of EPO in the neonatal period would attenuate deficits in recognition memory and cognitive flexibility in adult rats of both sexes. We assessed cognition and executive function in two ways. First, using the classic test of novel object recognition and second, using a touchscreen platform. Touchscreen testing allows for rigorous testing of cognition and executive function in preclinical and clinical scenarios. Data show that adult rats exhibit deficits in recognition memory and cognitive flexibility following in utero placental insufficiency. Notably, neonatal treatment of EPO attenuates these deficits in adulthood and facilitates functional repair. Together, these data validate EPO neurorestoration using a clinically relevant outcome measure and support the concept that postnatal treatment following in utero injury can improve cognition and executive function through adulthood.
Keywords: novel object recognition, prematurity, preterm, reversal, touchscreen operant chambers, visual discrimination
1 ∣. INTRODUCTION
Rates of preterm birth remain unacceptably high in the United States despite multiple prevention efforts and sophisticated medical care (Martin et al., 2019). Survivors of preterm birth are prone to chronic neurological deficits, including cerebral palsy, epilepsy and most commonly, behavioral and cognitive deficits (Anderson, 2014). Cognitive deficits affect up to 50% of children born very preterm (<32 weeks) (Anderson, 2014), and these deficits persist into adolescence and adulthood, thus increasing the cumulative burden of disability throughout the lifespan (Eves et al., 2020; Suikkanen et al., 2020; Vollmer & Stalnacke, 2019). Indeed, cognitive deficits have perhaps the greatest impact on quality of life, relative to other neurological deficits (Vos et al., 2013). Executive function, broadly defined as one's ability to plan, adjust, and respond to actions and changes in environmental stimuli, is impacted in very preterm survivors. For example, former very preterm infants exhibit difficulty in cognitive tasks as tests become more complex and challenging (Wehrle et al., 2016). Cumulatively, these executive function deficits contribute to the challenges which former very preterm infants face in becoming independent adults.
Over the past two decades, more preterm infants have survived with chronic deficits, and thus driven the impetus to accelerate neurorestoration. Initially, the focus for this patient population was on the prevention and repair of the motor deficits that lead to cerebral palsy. More recently, however, the need to address the broader array of deficits associated with preterm birth has been widely recognized in clinical trials, as well as in preclinical models (L. Jantzie et al., 2018; Mazur et al., 2010). Notably, the importance of testing repair of cognition in preclinical models of perinatal brain injury using highly translatable outcomes measures in mature animals has become emphasized (Jantzie et al., 2018, 2020; Maxwell et al., 2020; Mazur et al., 2010). To successfully translate neuroreparative strategies from the bench to the bedside, rigorous techniques to test executive function in rodents that accurately predict successful improvement in humans are required (Bussey et al., ,2008, 2012). Touchscreen operant chambers with paradigms and platforms that parallel the Cambridge Neuropsychological Test Automated Battery (CANTAB) permit rigorous testing of multiple pillars of cognition, including cognitive flexibility (Bussey et al., 2008, 2012; Mar et al., 2013; Nithianantharajah et al., 2015). For example, dxl mutations in both humans and mice yields similar outcomes on touchscreen testing (Nithianantharajah et al., 2015). Notably, the tasks assess the same cognitive dimensions in both species because contingencies can be changed, requiring shifts in strategies to gain reward. Despite this promising avenue for translation, few studies thus far have tested neuroreparative strategies in perinatal brain injury using these techniques (Jantzie et al., 2018, 2020; Maxwell et al., 2020; Olguin et al., 2020).
Erythropoietin (EPO) is well known for its role in hematopoiesis in response to hypoxia. Over the past two decades, EPO has also emerged as a neurorestorative agent (Brines et al., 2000) for multiple types of central nervous system (CNS) insults, including for perinatal brain injury (Jantzie et al., 2013, 2014, 2015, 2016; Juul et al., 2004; Mazur et al., 2010; Robinson et al., 2017; Robinson, Conteh, et al., 2018). Multiple overlapping, beneficial mechanisms of action that catalyze neurorestoration and facilitate EPO’s beneficial effects have been identified (Jantzie, Corbett, et al., 2015; Mazur et al., 2010; Robinson et al., 2017; Wu et al., 2012). These include attenuation of neuroinflammation, mitigation of calpain activity, increased support for oligodendrogenesis, and mitigation of oxidative stress (Jantzie et al., 2013, 2014; Jantzie, Corbett, et al., 2015; Mazur et al., 2010). EPO signaling through EPO receptors (EPOR) is essential for CNS development, and for repair after stroke in the mature brain (Tsai et al., 2006). EPOR are expressed on most neural cell types including neurons, oligodendroglial lineage cells, astrocytes, and microglia (Iwai et al., 2007; Jantzie et al., 2013; Mazur et al., 2010). In the developing brain after injury, EPOR are upregulated on most neural cell types, while the EPO ligand is not (Mazur et al., 2010). However, if the ligand deficit is replaced with exogenous EPO over an extended neonatal course, then biochemical, cellular, and most importantly, functional neurorepair occurs after injury (Mazur et al., 2010; Robinson, Conteh, et al., 2018; Robinson, Winer, et al., 2018).
EPO has been shown to be safe in clinical trials involving neonates with perinatal brain injury (Fauchere et al., 2015; Juul et al., 2008, 2020; Leuchter et al., 2014; Ohls et al., 2014, 2016; Wu et al., 2016). Here, we assessed cognitive outcomes in adult rats using an established model of prenatal injury and neonatal treatment with EPO. We assessed cognition and executive function in adult animals using a traditional novel object recognition assay (NOR), in addition to a touchscreen test of visual discrimination and reversal learning. We hypothesized that a neonatal regimen of systemic EPO treatment would effectively repair deficits of cognition as assessed by both NOR and reversal learning paradigms. Herein, we demonstrate that a neonatal treatment of EPO facilitates functional repair and attenuates deficits in cognitive flexibility in adulthood. We also demonstrate that touchscreen testing provides a rigorous, reproducible, and validated technique to test cognition and assess biobehavioral biomarkers of executive function in preclinical models of perinatal brain injury.
2 ∣. METHODS AND MATERIALS
2.1 ∣. Ethical approval
The Institutional Care and Use Committees at Johns Hopkins University and Boston Children's Hospital approved all experimental procedures. ARRIVE guidelines were followed (Kilkenny et al., 2010).
2.2 ∣. Animals
Sprague Dawley rats of both sexes were obtained from Charles River (Wilmington, MA). Pregnant dams were housed in individual cages under a 12 hr light–dark cycle (Lights on 06:00) with a room temperature of 18–25°C (mean 22°C) and a humidity of 30%–70% (mean 44.6%). Food and water were provided ad libitum. Cages were changed twice per week with minimal handling. Rat pups were weaned on postnatal day (P) 21, and were housed in same sex dyads or triads. Physical enrichment included a small plastic chew toy, squiggles, and nestlets. All rats were euthanized by decapitation consistent with American Veterinary Medical Association (AVMA) guidelines.
For all experiments, animals from more than one litter were used for each group and sex was balanced. For litters with prenatal injury, half of the pups from each litter were randomized to each treatment group (EPO or saline vehicle). Investigators were blinded to injury and treatment group. Importantly, for both the NOR and the touchscreen, the parameters for testing, as well as the pass/fail criteria, were set a priori prior to the experiments. Three cohorts were used for NOR (total: sham n = 29, TSHI-veh n = 31 and TSHI-EPO n = 23) and three separate cohorts were used for touchscreen (total: sham n = 27, TSHI-veh n = 21 and TSHI-EPO n = 29).
2.3 ∣. Transient systemic hypoxia-ischemia (TSHI) model
Pregnant rats underwent laparotomy on embryonic day 18 (E18) under isoflurane anesthesia, as previously described (Mazur et al., 2010; Robinson et al., 2005). Uterine arteries were transiently occluded for 60 min to mimic TSHI from placental insufficiency, while shams underwent only laparotomy and anesthesia for 60 min. Pups were born at term on E22. The prenatal TSHI model has been demonstrated to yield a reproducible insult with consistent fetal mortality and postnatal outcomes (Coq et al., 2016, 2018, 2019; Jantzie et al., 2013, 2016; Jantzie, Corbett, et al., 2015; Jantzie, Getsy, et al., 2015; Mazur et al., 2010; Robinson et al., 2017).
2.4 ∣. Neonatal erythropoietin treatment
On postnatal day 1 (P1), all pups were randomized to receive either EPO (2,000 U/kg, R&D Systems, Minneapolis, MN) or vehicle (sterile saline) intraperitoneally and were treated daily from P1 to P5 (Mazur et al., 2010), which is analogous to 30 to 35 weeks gestation in humans (Jantzie & Robinson, 2015). This established dosing regimen was previously shown to be effective and safe (Jantzie, Corbett, et al., 2015; Jantzie et al., 2013, 2014, 2016; Mazur et al., 2010), and is comparable to that used in human neonatal clinical trials.
2.5 ∣. Novel object recognition
For NOR, P28-29 young adult rats were first habituated to the testing arena (100 × 100 cm with 35 cm walls) housed in a quiet, dimly lit room (30 lumens). After 2 days of habituation, rats underwent a familiarization trial. Each rat was placed in the arena with two identical novel objects spaced 30 cm apart for 10 min. The amount of time each rat spent interacting with the novel objects, defined as having its nose within 2 cm of an object, was recorded using video-tracking software (Anymaze, Stoelting, Wood Dale, IL). The minimum threshold for interaction with either object during the familiarization stage was set a priori at 10 s, and those rats failing the a priori limits were excluded from the subsequent recognition trial. The proportion of each group that failed to interact with objects did not differ (sham: 52%,TSHI-veh: 48%, and TSHI-EPO: 43%), demonstrating that groups exhibited generally similar object engagement. After 1 hr, a recognition trial was performed. Each rat was placed in the arena with one familiar object (from familiarization trial) and one novel object (never encountered before) in the same location as during the familiarization trial for 10 min. The time interacting with each object was recorded. Novel object order of presentation was counterbalanced with equal placement on the left or right side of the arena to minimize bias. The arena was cleaned with 70% ethanol after each test. Magnitude of novelty reaction (discrimination index) was measured (ratio of NO duration minus familiar object duration/total exploration duration × 100%) (Bevins & Besheer, 2006; Leger et al., 2013).
2.6 ∣. Touchscreen visual discrimination and cognitive flexibility
Rats underwent touchscreen testing to assess specific areas of cognition and executive function. As previously described (Jantzie et al., 2018, 2020; Maxwell et al., 2020; Robinson, Winer, et al., 2018), light- and sound-attenuating chambers (Med Associates, St. Albans, VT, USA) with a touchscreen apparatus (Conclusive Solutions, Sawbridgeworth, UK) were used for operant behavior testing. The touchscreen was divided into two response windows with an overlying acrylic aperture plate. K-Limbic Software (Conclusive Solutions) was used for stimulus presentation and response recording. Dustless Precision 45 mg food pellets (Rodent Purified Diet, Bioserv, Frenchtown, NJ, USA) were delivered via a pellet dispenser as a reward for correct responses and were associated with a light and sound.
2.6.1 ∣. Pre-training
Rats began mild food restriction at P28 for 5 days. Their weight was limited to 85% of their free feeding weight. Rats were weighed daily and assessed for their general health. Each were given 25 pellets per day for 5 days in their home cage to acclimatize to the reward pellets. All rats tolerated the mild food restriction well. Beginning on P35, rats were habituated to the touchscreen boxes and underwent training, including pellet retrieval, auto-shaping, and three stages of visual discrimination training, with one 60 min session per day. Rats completing 48 correct trials out of 60 trials in under 60 min were advanced to the next training stage.
2.6.2 ∣. Visual discrimination and reversal learning
Once all pre-training stages were completed, rats moved on to visual discrimination (VD) testing. All rats were tested on a pairwise visual discrimination-reversal paradigm for up to 60 min daily. For discriminative learning, two novel equiluminescent stimuli were presented in a spatially pseudo-randomized manner over 60-trial sessions with a 5 s inter-trial interval (Bussey et al., 2012; Jantzie et al., 2018; Robinson, Winer, et al., 2018). Stimuli have previously been shown to be equivalent in capturing rodent attention (Horner et al., 2013). Stimuli remained on the screen until a response was made. Responses at one stimulus yielded a reward, whereas responses at the other stimulus led to a 5 s timeout period with extinguishing of the house light without correction trials. The designation of the stimulus associated with the reward was randomized across treatment groups. Passing criteria were set a priori as ≥80% correct responses for two consecutive days.
After VD passing criteria were attained, rats were assessed for reversal learning. Here, the designation of correct and incorrect stimuli was reversed. Similar to VD, rats were tested daily for up to 60 min with a 60-trial session, and passing criteria were set a priori as ≥80% correct responses for two consecutive days. When errors were made on first presentation reversal trials, correction trials followed until a correct response was made or the session ended. Failing criteria were set a priori at 21 sessions (days) for both VD and reversal.
Dependent measures were recorded during VD and reversal including total sessions, correct responses, errors (incorrect responses), correction errors (correction trials for reversal only), reaction time (time from stimulus presentation to touchscreen response) and magazine latency (time from touchscreen response to reward retrieval). Discrimination performance was analyzed across all sessions required to reach passing criteria. For reversal, errors and correction errors were also analyzed and were separated into distinct phases of early perseverative and late learning, defined as performance <50% correct and from 50% correct to passing criterion, respectively, as described previously (Brigman et al., 2015; Jantzie et al., 2018, 2020; Maxwell et al., 2020; Olguin et al., 2020; Robinson, Winer, et al., 2018).
2.7 ∣. Statistical analysis
All analyses were performed by blinded observers. We used a priori criteria to strengthen the rigor of the NOR and touchscreen analyses and planned a priori to analyze each sex separately in addition to both sexes combined. Statistical analyses were carried out using SPSS25 (IBM, Armonk, NY, USA). Data are reported as mean ± SEM. With respect to animal number, we used G* power to determine the number of samples assuming a power of 0.5 and an alpha of 0.05 for multiple group comparisons, consistent with our previous publications (Jantzie et al., 2018; Robinson, Winer, et al., 2018). Rats were randomized to sham, TSHI-veh, or TSHI-EPO groups. All parametric variables for the sham, TSHI-veh, and TSHI-EPO groups were tested for normal distribution with the Shapiro–Wilk test and confirmed with Levene's test for homogeneity of variances. For touchscreen, outliers beyond two standard deviations from the mean were excluded. For comparisons of multiple groups, a two-way ANOVA (injury × treatment) was performed with Bonferroni's post hoc correction. For NOR, a mixed model ANOVA (injury × treatment) and repeated measures (familiarization/recognition) with Bonferroni's post hoc correction was performed. For non-parametric variables, a Kruskal–Wallis test with Dunn's post hoc correction (KW with Dunn) was used. Both sexes were analyzed together, and then each sex was analyzed separately. Significant differences were designated as <0.05.
3 ∣. RESULTS
3.1 ∣. Recognition memory recovers with post-injury EPO treatment
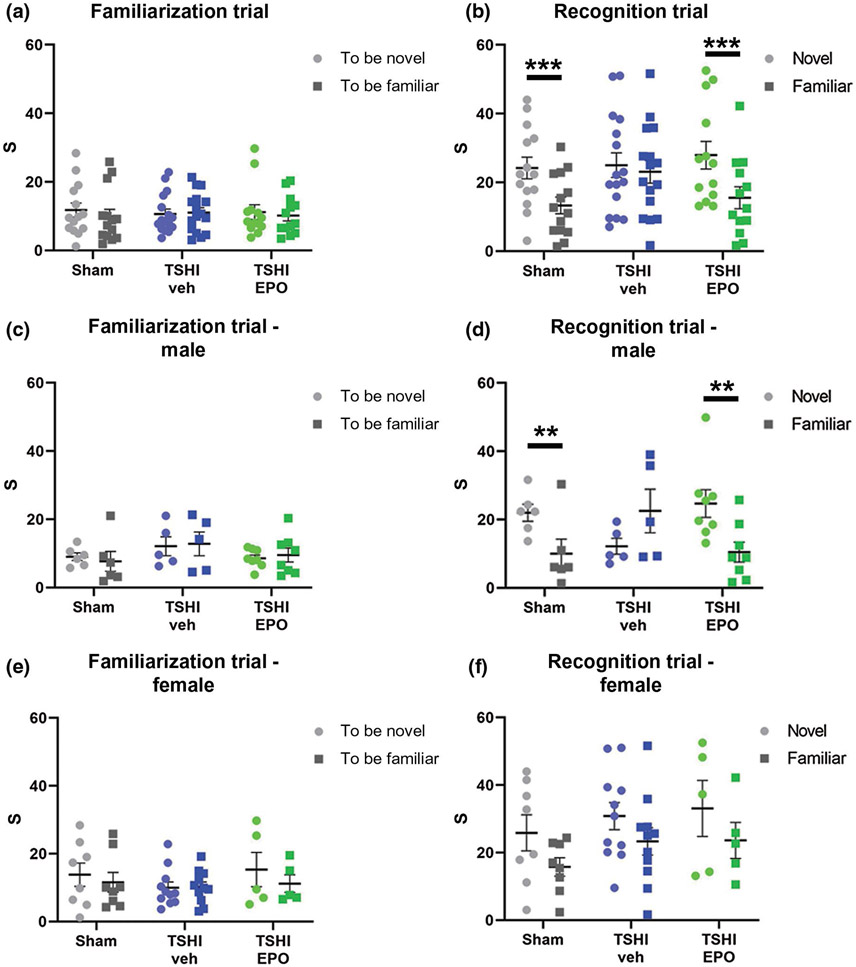
Rats show inherent curiosity with novel objects, and their ability to remember previously presented objects tests recognition memory. First, we tested whether adult rats that experienced placental insufficiency in utero had deficits in recognition memory, and whether these deficits could be prevented with a neonatal regimen of EPO. After habituation to the testing arena, rats from all three groups exhibited similar levels of interaction with the novel and familiar objects in the test. Specifically, the proportion of rats from each treatment group that interacted with the objects (white or black block and silver sphere) per a priori criterion did not differ [sham: 48% (14/29), TSHI-veh (16/31): 52%, and TSHI-EPO: 57% (13/23)]. Notably, Sham, TSHI-veh and TSHI-EPO rats did not demonstrate a preference for one object over the other during the familiarization stage (Figure 1a). However, when presented with one familiar object and one novel objected during the recognition trial, sham and TSHI+EPO rats exhibited the expected behavior and spent more time interacting with the novel, newly introduced object. Specifically, TSHI-veh rats (n = 16) showed no preference or difference in their interest between the novel and familiar objects, while sham rats (n = 14) and TSHI-EPO rats (n = 13) spent significantly more time with a novel object compared to a familiar object (mixed model ANOVA, (F(1, 12) = 8.063, p = 0.015, , Figure 1b). Together, these results demonstrate that prenatal TSHI causes a recognition memory deficit in young adult rats that is prevented by neonatal EPO treatment.
FIGURE 1.
Novel object recognition was tested after prenatal placental insufficiency, and postnatal erythropoietin (EPO) treatment. (a) During the familiarization trial, sham, vehicle-treated transient systemic hypoxia-ischemia (TSHI), and EPO-treated TSHI rats of both sexes exhibited a similar amount of interest in both objects. (b) During the recognition trial, TSHI-veh rats showed no difference in interest between the novel and familiar object, while both shams and EPO-treated TSHI rats showed significant interest in the novel object (mixed model ANOVA with Bonferroni's correction). (c, e) Neither male nor female rats in any group showed a preference during the familiarization trial. (d) Male TSHI-veh rats showed no difference in interest between the two objects, while male shams and male EPO-treated TSHI rats were significantly more interested in the novel object. (f) Female rats exhibited a similar pattern, but it failed to reach statistical significance. (**p < 0.01, ***p < 0.001)
When analyzed separately by sex, male rats and female rats from sham, TSHI-veh and TSHI-EPO groups showed no object preference during the familiarization stage (Figure 1c,e). In the discrimination stage, male sham rats (n = 6) and male TSHI-EPO rats (n = 8) spent more time with a novel object, whereas male TSHI-veh rats (n = 5) showed no preference (F(1, 7) = 13.435, p = 0.008, , Figure 1d). Similarly, female sham rats (n = 8) and female TSHI-EPO rats (n = 5) spent more time with the novel object, whereas the female TSHI-veh rats (n = 11) did not show a preference (p = 0.06, Figure 1f). Overall, both sexes showed improved recognition memory with EPO on a NOR test.
3.2 ∣. Touchscreen assessment of visual discrimination and reversal learning
To extend our assessment of executive function and cognition, we next performed assessments of visual discrimination and cognitive flexibility using a touchscreen platform. To begin, rats were first trained on the touchscreen platform. With the exception of two female TSHI-veh animals, all rats passed the five stages of necessary training. The two animals that did not advance through the training stages were excluded from further study and analysis. Indeed, similar proportions of rats passed touchscreen training from each of the three groups [Sham 100% (27/27); TSHI-veh 90.5% (19/21); TSHI-EPO 100% (29/29)]. After successful completion of the training sessions, rats performed a visual discrimination task. Rats in all three groups (sham, TSHI-veh, and TSHI-EPO) required a similar number of sessions to achieve a priori passing criteria (Figure 2a). Rats from all three groups also committed a similar number of errors prior to passing in completion of the VD task (Figure 2b). The overall proportion of rats from each group that met passing criteria and successfully completed the VD assessment did not differ. Likewise, rats from all three groups showed similar reaction time (Figure 2c) and latency to retrieve a reward (magazine latency, Figure 2d), consistent equivalent sensorimotor ability and motivation to perform during testing sessions. Analyses by sex revealed that both male (Figure 3a-d) and female rats (Figure 3e-h) performed similarly during VD. Thus, sham, TSHI-veh, and TSHI-EPO rats of both sexes successfully completed VD testing, demonstrating that they possessed adequate visual, motor, motivation, discrimination, and cognitive skills to perform the touchscreen testing.
FIGURE 2.
Visual discrimination (VD) testing with touchscreen chambers demonstrated no difference between shams, TSHI-veh and TSHI-EPO rats, indicating that all three groups have adequate visual, motor, and cognitive abilities to complete touchscreen testing. (a) Rats required a similar number of sessions to achieve a priori passing criteria in VD. (b) Similarly, rats committed a similar number of errors (incorrect responses) while achieving passing criteria. (c) The reaction time between stimulus presentation and response did not differ between the three groups. (d) Motivation (magazine latency, time to retrieve reward) also did not differ between groups
FIGURE 3.
Rats of both sexes successfully completed visual discrimination (VD) testing, indicating that the rats possess the motor, visual, and cognitive abilities to complete a discrimination task on a touchscreen platform. (a) Male rats from all three groups required a similar number of sessions to pass the a priori criterion for VD. (b) Male rats also committed a similar number of errors during VD. (c, d) The reaction latency and magazine latency did not differ between groups of male rats. (e) Female rats from all groups required a similar number of sessions to pass the a priori criteria for VD. (f) Female rats from all three groups also committed the same number of errors during VD testing. (g, h) Female rats from all groups had similar reaction latency and magazine latency
We next assessed cognitive flexibility using a reversal learning paradigm. In this test, the stimuli for a correct and incorrect response are reversed from the prior assessment of VD. Each rat is tested on how quickly and accurately it learns the new association, adjusts to the new rule of the task and shifts its cognitive set. During reversal, all three groups required a similar number of sessions to achieve a priori reversal learning passing criteria (Figure 4a), and committed a similar number of errors throughout the duration of the assessment (Figure 4b). Cumulatively, 59.3% (16/27) of sham rats passed the reversal paradigm, compared to 36.8% (7 of 19) of TSHI-veh rats, while 55.2% (16/29) of TSHI-EPO rats passed (Kruskal–Wallis (KW), NS). Subsequently, we assessed differences in the number of correction trials for rats in each treatment group. Following an incorrect response, rats receive a “second chance” or correction trial until the correct response is registered. Notably, TSHI-veh rats required significantly more correction trials than TSHI-EPO rats (KW with Dunn's: H(2) = 8.883, p = 0.019), or shams (p = 0.03, Figure 4c) in attempting to achieve the passing criterion. Similar to during VD, there was no difference in reaction time or magazine latency during reversal, indicating similar motivation to respond to visual stimuli or work for food reward and motor performance in the touchscreen chambers (Figure 4d,e).
FIGURE 4.
To test cognitive flexibility the correct response was reversed, and TSHI-veh rats performed poorly compared to TSHI-EPO and sham rats. (a) Rats from all three groups required a similar number of sessions to achieve a priori passing criteria for reversal. (b) Rats from all three groups also committed a similar number of errors prior to passing reversal. (c) TSHI-veh rats required more correction trials than TSHI-EPO rats or sham rats. (d) Reaction time was similar across the groups. (e) Similarly, motivation did not differ across sham, TSHI-veh and TSHI-EPO rats. (*p < 0.05)
We analyzed cognitive performance across both stages of reversal learning (early perseverative (<50% correct) vs. later learning phases (>50% correct)). This was done to clarify whether the executive function deficit in TSHI-veh rats reflected poor cognitive flexibility and perseverative behavior or deficits in learning acquisition. Analysis of reversal performance by learning phase revealed that TSHI-veh rats were profoundly perseverative compared to sham and TSHI-EPO treated rats. Specifically, TSHI-veh rats required significantly more correction trials than shams (KW with Dunn's: H(2) = 11.92, p = 0.0152) or TSHI-EPO rats (p = 0.0030, Figure 5a) during the early perseverative phase (<50% correct). Similarly, TSHI-veh rats required more sessions (KW with Dunn's: H(2) = 19.59, p < 0.0001) and made more errors (KW with Dunn's: H(2) = 10.57, p = 0.0035), compared to TSHI-EPO rats during the early perseverative phase (Figure 5b,c). By contrast, during the choice relearning phase (>50% correct) performance was intact in all three groups with no differences in correction trials, sessions required to pass, or incorrect responses (Figure 5d-f). Importantly, the significant increase in correction errors, a measure of maladaptive perseveration, during early reversal was not due to motivation to respond or retrieve reward or sensorimotor impairment, as measured by reaction time or magazine latency on either phase of reversal learning. Thus, adult TSHI-veh exhibited maladaptive perseveration and poor cognitive flexibility, a behavioral phenotype that was attenuated by neonatal EPO treatment.
FIGURE 5.
Neonatal erythropoietin (EPO) treatment after prenatal injury in treated transient systemic hypoxia-ischemia (TSHI) rats effectively repaired deficits in perseveration, with correct less than chance (<50%). (a) TSHI-veh rats required more correction trials during perseveration than TSHI-EPO or sham rats. (b) TSHI-rats also required more sessions during perseveration than TSHI-EPO rats. (c) Similarly, TSHI-veh rats committed more errors during perseveration than TSHI-EPO rats. (d) By contrast, during learning, there was no difference in the number of correction trials between the three groups. (e) Likewise, there was no difference in the number of sessions during learning. (f) There was also no difference in the number of errors during learning. (**p < 0.01)
Because sex is an independent predictor of executive function in children born preterm, we next analyzed the performance of female and male rats separately during early perseverative and later learning stages of reversal. During perseveration, male TSHI-veh rats required more correction trials than male TSHI-EPO treated rats (KW with Dunn's: H(2) = 14.4, p = 0.0007), and male shams (p = 0.0159, Figure 6a). Male TSHI-veh rats also required significantly more sessions to pass (KW with Dunn's: H(2) = 17.86, p < 0.0001), and made more errors during the early perseverative phase than male TSHI-EPO rats (KW with Dunn's: H(2) = 11.5, p < 0.0024, Figure 6b,c). Interestingly, male TSHI-veh rats performed similar to male TSHI-EPO rats and male sham rats during the learning phase of reversal (Figure 6d). In contrast to male TSHI-veh rats, female TSHI-veh rats demonstrated a similar overall pattern of cognitive flexibility during perseveration and learning, compared to female TSHI-EPO rats and female shams (Figure 6e-h). In summary, male TSHI-veh rats were particularly challenged by the reversal paradigm and demonstrated the most significant maladaptive preservative behavior, a deficit that was prevented by neonatal EPO treatment.
FIGURE 6.
Males with prenatal treated transient systemic hypoxia-ischemia (TSHI) exhibit more difficulty during perseveration, while females are less affected. (a) Male TSHI-veh rats required more correction trials during perseveration than male TSHI-EPO rats or male shams. (b) Male TSHI-veh rats also required more sessions to pass than male TSHI-EPO rats. (c) Male TSHI-veh rats also committed more errors during perseveration than TSHI-EPO rats. (d) By contrast, male TSHI-veh rats did not require more correction trials during learning, compared to male TSHI-EPO rats or male shams. (e) Female TSHI-veh rats required more correction trials during perseveration than female TSHI-EPO rats or female sham rats, but the differences were not significant. (f) Female TSHI-veh rats also required more sessions than TSHI-EPO rats, but the trend was not significant. (g) Female TSHI-veh rats also committed slightly more errors during perseveration than female TSHI-EPO rats. (h) Female TSHI-veh rats did not require more correction trials during learning. (*p < 0.05, **p < 0.01, ***p < 0.001)
4 ∣. DISCUSSION
As more infants survive extreme prematurity, the need to prevent long-term neurological sequelae has become readily apparent. Indeed, survivors of very preterm birth exhibit a spectrum of deficits across multiple pillars of cognition (Anderson, 2014). While cognition comprises many domains including attention, memory and processing speed, executive function is a key determinant of independent living in adulthood. Thus, optimization of neurodevelopmental trajectory, long-term cognitive development and support of executive function is a critical clinical and research priority for survivors of preterm birth. Here, we assessed a viable therapeutic strategy for neurorepair in a translational preclinical model of encephalopathy of prematurity and the associated perinatal brain injury. Specifically, we assessed cognition and executive function in adult rats that were subject to an insult in utero. Significantly, we found that five doses of erythropoietin administered in the neonatal period after placental insufficiency not only attenuated deficits in recognition memory in adulthood, but also mitigated perseverative behavior and deficits in cognitive flexibility.
Erythropoietin is a 30.4 kDa endogenous glycoprotein secreted postnatally by the peritubular interstitial cells of the kidney (Fisher, 2010). In humans (and all mammals), fetal EPO is made in the liver (Zanjani et al., 1977). After birth, production switches from the liver to the kidneys, a transition that is completed by 40 days postpartum (Fisher, 2010; Zanjani et al., 1977). EPO is an FDA-approved medication routinely used clinically to treat anemia in patients of all ages. However, EPO and other erythropoiesis stimulating agents are presently being studied in clinical trials to ameliorate CNS injury from extremely preterm birth and hypoxic-ischemic encephalopathy (Fauchere et al., 2008, 2015; Juul, 2012; Juul et al., 2008, 2020; Ohls et al., 2014, 2016; Wu et al., 2016, 2019). Despite multiple confirmations of safety and acute efficacy, EPO’s clinical viability and utility in the long term and as a therapeutic for a neonatal patient population with evolving brain injury is unknown. The small, multi-center BRITE (Brain Imaging and Developmental Follow up of Infants Treated with Erythropoietin) trial (NCT01207778) of erythropoiesis-stimulating agents in very preterm infants has shown reduction of cerebral palsy (Ohls et al., 2014), and improvement in preschool age cognition and behavior (Ohls et al., 2016). The much larger EpoRepair trial (NCT02036073) found a significant reduction in death and moderate/severe neurological disability at 18 months after perinatal brain injury (Song et al., 2016). Results from the PENUT (Preterm Erythropoietin Neuroprotection Trial) Phase III trial (NCT01378273) that enrolled >940 extremely preterm infants are beginning to be reported. Despite no adverse events and an excellent safety profile, initial data are thus far inconclusive with respect to efficacy and this trial's outcome measures. To this end, we show that neonatal EPO treatment normalized executive function, specifically cognitive flexibility, and reversal learning in adult rats using a touchscreen platform. These data emphasize the need for precise trial design and the requirement for long-term cognitive outcomes in preclinical and clinical evaluations, including confirmed functional normalization in adulthood. Notably, significant cortical reorganization occurs during adolescence and myelination is ongoing through the second decade of life. The human cortex, particularly the prefrontal cortex, decreases in volume during adolescence and undergoes considerable pruning (Juraska & Drzewiecki, 2020). Changes in the GABAergic system, including parvalbumin interneurons and formation of perineuronal nets allow for efficient neural circuitry and fast spiking. Together with myelination, modulations to the neural circuity contribute extensively to executive function and cognitive performance and these changes occur in development far beyond the first few years of life commonly examined in primary outcome measures in clinical trials (i.e., Bayley Scales of Infant and Toddler Development). Given the significant cost of clinical trials, and relatively long time frame from trial initiation to results in cognition, it is essential that that emerging therapeutics be fully vetted using rigorous preclinical paradigms that encompass as much of the lifespan as possible.
Because signaling by EPO through its receptor EPOR is essential for the development of most, if not all neural cell types (Mazur et al., 2010), exogenous EPO can likely mitigate deleterious effects from perinatal insults (Jantzie, Corbett, et al., 2015; Juul, 2004; Mazur et al., 2010; Robinson et al., 2017). This beneficial effect may be most salient to neuronal and oligodendroglial lineages in the third trimester (Iwai et al., 2010; Jantzie et al., 2013; Mazur et al., 2010). Indeed, the role of EPO/EPOR in neural cell health is approximately 10-fold greater prenatally, and gradually diminishes as the CNS matures, highlighting the importance of EPO/EPOR homeostasis and downstream signal transduction in the context of the developing brain (Sanchez et al., 2009). During development, the amount of EPO present dictates neural cell survival, including number and maturation (Knabe et al., 2004, 2005). If EPO ligand binds EPOR, the neural cell survives, whereas unbound EPORs trigger cell death through upregulation of GSK-3β, which facilitates the initiation of apoptosis through formation of the mitochondrial permeability transition pore (Knabe et al., 2005). Thus, the central principle of EPO repair is to supplement this existing endogenous signaling cascade with administration of exogenous EPO. In the present study, we hypothesized that EPO’s role in neurorepair, maintenance of neural homeostasis and circuit development would be beneficial to cognitive development in adult rats following perinatal brain injury. Using this same model of prenatal injury secondary to placental insufficiency that mimics multiple CNS deficits of children born very preterm, we have previously shown that neonatal EPO prevents deficits in gait, seizure threshold, social interaction, and motor inhibition (Jantzie, Corbett, et al., 2015; Mazur et al., 2010; Robinson et al., 2017). Here, NOR revealed a deficit in spatial recognition, particularly in male TSHI-veh rats. We have previously shown that TSHI rats also exhibit gait deficits (Coq et al., 2016, 2018; Jantzie, Corbett, et al., 2015; Mazur et al., 2010; Robinson et al., 2017). Interestingly, using a similar model of intrauterine hypoperfusion yielding a motor phenotype similar to cerebral palsy and evidence of developmental coordination disorder, Coq and colleagues did not detect functional abnormalities in Morris water maze performance. (Coq et al., 2019). However, our data here do corroborate other prior preclinical investigations of functional outcome, where post-injury EPO treatment effectively improved cognitive function in adult rats secondary to infant traumatic brain injury (Robinson, Winer, et al., 2018; Schober et al., 2014). Similarly, our data are consistent with prior reports demonstrating that neonatal EPO treatment attenuates hyperoxia-induced cognitive deficits in adult rats (Dewan et al., 2020), and cognitive development after intraventricular hemorrhage long term (Hierro-Bujalance et al., 2020; Robinson, Conteh, et al., 2018; Robinson, Winer, et al., 2018). They are also consistent with data demonstrating that EPO can improve performance beyond what is observed in sham animals (Mazur et al., 2010), and that females fair better than males after perinatal brain injury (Nagy et al., 2021; O'Driscoll et al., 2018). These data emphasize differences in neural substrates of cognition, specific brain regions associated with different types of cognitive assessments and the clinical picture associated with encephalopathy of prematurity.
In addition to NOR, a standard paradigm used in numerous preclinical investigations and the studies referenced above (Dewan et al., 2020; Hierro-Bujalance et al., 2020; Schober et al., 2014), we used a touchscreen platform to test visual discrimination and reversal learning to confirm deficits in multiple cognitive domains and to detect repair by neonatal EPO treatment. Reversal learning is a paradigm used to assess cognitive flexibility in the setting of changing stimulus-outcome or response-outcome (Izquierdo et al., 2017). Importantly, different stages of learning can be assessed by looking at specific times during the reversal paradigm, including early (<50% correct) and late (>50% correct). This is important as children born preterm have complex memory deficits, and often struggle when cognitive tasks gain complexity (Wehrle et al., 2016). Furthermore, it has been shown that adults who were born preterm continue to exhibit executive function deficits throughout the lifespan (Eves et al., 2020; Suikkanen et al., 2020; Vollmer & Stalnacke, 2019). Indeed, the touchscreen platform incorporates stimuli, learning rules and response actions over time and in adult animals. Assessment of discrimination and reversal learning is heavily dependent on orbitofrontal-striatal connections in both humans and rodents (Bergstrom et al., 2020; Brigman et al., 2013; Izquierdo et al., 2017). Placental insufficiency did not impact response learning, as animals in all treatment groups were able to complete a visual discrimination task. This in utero insult also did not affect secondary latency measures, such as reaction time and the time to retrieve a reward, indicating that placental insufficiency did not globally impair motor behavior, or motivation to work for food reward. Interestingly, TSHI-vehicle treated rats exhibited perseveration, a significant lack of cognitive flexibility. Reversal learning, and particularly the early, perseverative phase, has consistently been shown to be mediated by the orbitofrontal cortex across species (Hamilton & Brigman, 2015; Izquierdo et al., 2017; Kennerley et al., 2011; Rudebeck et al., 2013). In particular, the deficits observed in TSHI-rats were most prominent in correction trials during reversal. These data are consistent with significantly maladaptive perseveration and demonstrated lack of cognitive flexibility.
More importantly, EPO treatment prevented this deficit in mature rats, suggesting that cognitive flexibility can be modulated by neonatal therapeutic intervention. Lack of cognitive flexibility, particularly perseveration, may be related to deficient serotonin signaling in the orbitofrontal cortex (Alsio et al., ,2015, 2020). As such, elevating serotonin signaling can improve reversal learning (Brigman et al., 2010). Preterm survivors who have a complex, complicated, and unstable NICU course requiring multiple interventions for their prematurity have epigenetic loss of the serotonin transporter, and subsequent difficulties with mood regulation and social interaction (Fumagalli et al., 2018; Provenzi et al., 2020). Our rats with preterm brain injury demonstrate deficits similar to preterm infants with impaired cognitive control, including perseveration, who also exhibit structural, anatomical, and functional connectivity abnormalities in the orbitofrontal cortex and cortico-striatal pathways (Degnan et al., 2015; Kelly et al., 2020; Robinson et al., 2017; Thompson et al., 2020; Tokariev et al., 2019).
Many studies have used both NOR and reversal assessments together to dissect deficits in cognition and executive function. Carron and colleagues have used this approach in models of epilepsy (Carron et al., 2019). Berg and colleagues found that ubc3a mutant mice did not exhibit deficits on a NOR test, but displayed impaired reversal learning using touchscreens (Berg et al., 2020). Accordingly, the touchscreen platform has gained significant traction with respect to sensitivity and rigor (Dumont et al., 2020). Touchscreen platforms were originally developed to bring unbiased and computerized assessments to the translation of cognitive interventions for schizophrenia, bipolar disorder, and similar neuropsychiatric illnesses (Bussey et al., ,2008, 2012). More recently, touchscreen platforms have also been used to quantify executive function deficits after a wide variety of perinatal insults including hypoxic-ischemic encephalopathy, opioid, and ethanol exposure (Jantzie et al., 2020; Kenton, Castillo, et al., 2020; Kenton, Ontiveros, et al., 2020; Marquardt et al., 2014, 2020; Maxwell et al., 2020; Olguin et al., 2020), and to test interventions to repair early brain injury secondary to chorioamnionitis and infant brain trauma (Jantzie et al., 2018; Robinson, Conteh, et al., 2018). Touchscreen platforms have some advantages compared to other behavioral tests, such as the existence of similar protocols to test multiple aspects of cognition in humans (Bussey et al., 2008, 2012). Indeed, testing of specific components of memory, learning, cognitive flexibility, and executive function has been validated in rats (Horner et al., 2013; Mar et al., 2013; Oomen et al., 2013). Furthermore, because rodents are tested in a soundproof, environmentally controlled chamber, the potential influence of distractions is markedly reduced. Computerized delivery of the testing paradigms and collection of all data minimizes operator influence or error. To this end, in this study, approximately half of the rats from all groups met the a priori criteria for object engagement during NOR, while the vast majority of rats from all groups passed touchscreen training, suggesting that potentially smaller cohorts can be used for touchscreen testing. However unlike NOR, touchscreen assessments do not capitalize on a rodent's natural tendency to explore, but rather is contingent on tight stimulus control and two-dimensional stimuli. The relationship between performance, test difficulty, and frustration after unsuccessful trials is important considerations when choosing appropriate paradigms for behavioral testing after brain injury or neurological disease. Indeed, it is likely that fewer rats are lost to performance issues in touchscreen assays, as in the case with NOR, due to the motivation of food restriction. While the touchscreen platform utilizes reward-based stimuli rather than the aversive stimuli found in Morris Water Maze, food restriction is needed and is required to be maintained throughout the testing paradigm. Similarly, touchscreen platforms are sensitive to prefrontal cortex deficits at a younger age in mice with a background mimicking Alzheimer's disease (Van den Broeck et al., 2020), but the impact of two-dimensional stimuli, which limits direct animal interaction, is unknown.
This study is not without limitations and is constrained by scope. While cognitive flexibility and visual discrimination was assessed, and compelling data on long-term neurological repair ascertained, further study is needed to assess attention, learning inhibition, processing speed, cognitive control, and performance using more complex tasks such as five-choice serial reaction. Similarly, longitudinal neuroimaging of specific pathways underlying complex circuitry and functional neuroimaging or electroencephalogram would extend these data with secondary histopathological analyses. Despite this and the inherent limitations of working with rodents over large animals, the results of this study confirm long-term brain repair with EPO. Taken together with a growing body of preclinical and clinical data, these emphasize the absolute essentiality of inclusion of long-term outcome measures in future clinical trials for preterm infants and the need for long-term follow-up in patients with brain injury regardless of their treatment status in the NICU.
5 ∣. CONCLUSION
Postnatal administration of EPO following an in utero insult improves cognitive flexibility and attenuates perseverative learning deficits in adult animals using a clinically relevant touchscreen platform. These data support EPO’s use as a neurorestorative therapy, highlight long-term repair through adulthood, and emphasize the use of touchscreen technology in translational investigations of encephalopathy of prematurity. Sophisticated testing to evaluate emerging therapies prior to expensive and long clinical trials is essential.
Supplementary Material
Significance.
Preterm birth is associated with cognitive and executive function deficits. The endogenous cytokine erythropoietin (EPO) mediates brain repair following developmental brain injury. While behavior is often tested in preclinical studies, translational touchscreen assessments that mimic human cognitive assessments enhance rigor and translatability to clinical realms. Here, we demonstrate that postnatal administration of EPO following an in utero insult improves cognitive flexibility and attenuates perseverative learning deficits in adult animals using a clinically relevant touchscreen platform. These data highlight the endurance of EPO-driven long-term repair through adulthood and emphasize the use of touchscreen technology in translational investigations of encephalopathy of prematurity.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (NIH). Specifically, this work was supported by R01HL139492 to LLJ and R56NS060765 to SR. The authors are grateful for the technical assistance of Christine Schrock, MD, Akosua Oppong, BA, Tracylyn R Yellowhair, BS, Jessie Newville, BS, Riley Sevensky, BS, Nagat El Demerdash, MD, PhD, and Nicholas Andrews, PhD.
Funding information
National Institute of Neurological Disorders and Stroke; National Heart, Lung, and Blood Institute
Footnotes
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
The authors disclose no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
Transparent Peer Review Report
Transparent Science Questionnaire for Authors
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alsiö J, Lehmann O, McKenzie C, Theobald DE, Searle L, Xia J, Dalley JW, & Robbins TW (2020). Serotonergic innervations of the orbitofrontal and medial-prefrontal cortices are differentially involved in visual discrimination and reversal learning in rats. Cerebral Cortex, 31(2), 1090–1105. 10.1093/cercor/bhaa277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsiö J, Nilsson SRO, Gastambide F, Wang RAH, Dam SA, Mar AC, Tricklebank M, & Robbins TW (2015). The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: A cross-site study. Psychopharmacology, 232(21–22), 4017–4031. 10.1007/s00213-015-3963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PJ (2014). Neuropsychological outcomes of children born very preterm. Seminars in Fetal & Neonatal Medicine, 19(2), 90–96. 10.1016/j.siny.2013.11.012 [DOI] [PubMed] [Google Scholar]
- Berg EL, Pride MC, Petkova SP, Lee RD, Copping NA, Shen Y, Adhikari A, Fenton TA, Pedersen LR, Noakes LS, Nieman BJ, Lerch JP, Harris S, Born HA, Peters MM, Deng P, Cameron DL, Fink KD, Beitnere U, … Silverman JL (2020). Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Translational Psychiatry, 10(1), 39. 10.1038/s41398-020-0720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Lieberman AG, Graybeal C, Lipkin AM, & Holmes A (2020). Dorsolateral striatum engagement during reversal learning. Learning & Memory, 27(10), 418–422. 10.1101/lm.051714.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, & Besheer J (2006). Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study 'recognition memory'. Nature Protocols, 1(3), 1306–1311. 10.1038/nprot.2006.205 [DOI] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Saksida L, Bussey TJ, Nakazawa K, & Holmes A (2015). Impaired discrimination learning in interneuronal NMDAR-GluN2B mutant mice. NeuroReport, 26(9), 489–494. 10.1097/WNR.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, & Holmes A (2013). GluN2B in cortico-striatal circuits governs choice learning and choice shifting. Nature Neuroscience, 16(8), 1101–1110. 10.1038/nn.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Graybeal C, & Holmes A (2010). Predictably irrational: Assaying cognitive inflexibility in mouse models of schizophrenia. Frontiers in Neuroscience, 4, 13. 10.3389/neuro.01.013.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, & Cerami A (2000). Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proceedings of the National Academy of Sciences of the United States of America, 97, 10526–10531. 10.1073/pnas.97.19.10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister K, Nithianantharajah J, Oomen CA, & Saksida LM (2012). New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology, 62(3), 1191–1203. 10.1016/j.neuropharm.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, & Saksida LM (2008). The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learn Mem, 15(7), 516–523. 10.1101/lm.987808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron S, Dezsi G, Ozturk E, Nithianantharajah J, & Jones NC (2019). Cognitive deficits in a rat model of temporal lobe epilepsy using touchscreen-based translational tools. Epilepsia, 60(8), 1650–1660. 10.1111/epi.16291 [DOI] [PubMed] [Google Scholar]
- Coq JO, Delcour M, Massicotte VS, Baud O, & Barbe MF (2016). Prenatal ischemia deteriorates white matter, brain organization, and function: Implications for prematurity and cerebral palsy. Developmental Medicine and Child Neurology, 58(Suppl. 4), 7–11. 10.1111/dmcn.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq J-O, Delcour M, Ogawa Y, Peyronnet J, Castets F, Turle-Lorenzo N, Montel V, Bodineau L, Cardot P, Brocard C, Liabeuf S, Bastide B, Canu M-H, Tsuji M, & Cayetanot F (2018). Mild intrauterine hypoperfusion leads to lumbar and cortical hyperexcitability, spasticity, and muscle dysfunctions in rats: implications for prematurity. Frontiers in Neurology, 9, 423. 10.3389/fneur.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq J-O, Kochmann M, Lacerda DC, Khalki H, Delcour M, Toscano AE, Cayetanot F, Canu M-H, Barbe MF, & Tsuji M (2019). From cerebral palsy to developmental coordination disorder: Development of preclinical rat models corresponding to recent epidemiological changes. Annals of Physical and Rehabilitation Medicine, 63(5), 422–430. 10.1016/j.rehab.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Degnan LA, Milstone AM, Diener-West M, & Lee CK (2015). Extended-spectrum beta-lactamase bacteria from urine isolates in children. Journal of Pediatric Pharmacology and Therapeutics, 20(5), 373–377. 10.5863/1551-6776-20.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MV, Serdar M, van de Looij Y, Kowallick M, Hadamitzky M, Endesfelder S, Fandrey J, Sizonenko SV, Herz J, Felderhoff-Müser U, & Bendix I (2020). Repetitive erythropoietin treatment improves long-term neurocognitive outcome by attenuating hyperoxia-induced hypomyelination in the developing brain. Frontiers in Neurology, 11, 804. 10.3389/fneur.2020.00804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Salewski R, & Beraldo F (2020). Critical mass: The rise of a touchscreen technology community for rodent cognitive testing. Genes, Brain, and Behavior, 20(1), e12650. 10.1111/gbb.12650 [DOI] [PubMed] [Google Scholar]
- Eves R, von Mühlenen A, Mendonça M, Johnson S, O'Reilly H, Bartmann P, Marlow N, & Wolke D (2020). The role of executive and general cognitive functioning in the attention problems of very and extremely preterm adults. Journal of Developmental and Behavioral Pediatrics, 41(6), 461–469. 10.1097/DBP.0000000000000806 [DOI] [PubMed] [Google Scholar]
- Fauchere J-C, Dame C, Vonthein R, Koller B, Arri S, Wolf M, & Bucher H (2008). An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics, 122(2), 375–382. 10.1542/peds.2007-2591 [DOI] [PubMed] [Google Scholar]
- Fauchere JC, Koller BM, Tschopp A, Dame C, Ruegger C, Bucher HU & Swiss Erythropoietin Neuroprotection Trial Group. (2015). Safety of early high-dose recombinant erythropoietin for neuroprotection in very preterm infants. Journal of Pediatrics, 167(1), 52–57.e3. 10.1016/j.jpeds.2015.02.052 [DOI] [PubMed] [Google Scholar]
- Fisher JW (2010). Landmark advances in the development of erythropoietin. Experimental Biology and Medicine, 235(12), 1398–1411. 10.1258/ebm.2010.010137 [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Provenzi L, De Carli P, Dessimone F, Sirgiovanni I, Giorda R, Cinnante C, Squarcina L, Pozzoli U, Triulzi F, Brambilla P, Borgatti R, Mosca F, & Montirosso R (2018). From early stress to 12-month development in very preterm infants: Preliminary findings on epigenetic mechanisms and brain growth. PLoS ONE, 13(1), e0190602. 10.1371/journal.pone.0190602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, & Brigman JL (2015). Behavioral flexibility in rats and mice: Contributions of distinct frontocortical regions. Genes, Brain, and Behavior, 14(1), 4–21. 10.1111/gbb.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierro-Bujalance C, Infante-Garcia C, Sanchez-Sotano D, del Marco A, Casado-Revuelta A, Mengual-Gonzalez CM, Lucena-Porras C, Bernal-Martin M, Benavente-Fernandez I, Lubian-Lopez S, & Garcia-Alloza M (2020). Erythropoietin improves atrophy, bleeding and cognition in the newborn intraventricular hemorrhage. Frontiers in Cell and Developmental Biology, 8, 571258. 10.3389/fcell.2020.571258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SRO, Alsiö J, Oomen CA, Holmes A, Saksida LM, & Bussey TJ (2013). The touchscreen operant platform for testing learning and memory in rats and mice. Nature Protocols, 8(10), 1961–1984. 10.1038/nprot.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, & Chen J (2007). Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke, 38(10), 2795–2803. 10.1161/STROKEAHA.107.483008 [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, & Cao G (2010). Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke, 41(5), 1032–1037. 10.1161/STROKEAHA.109.570325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, & Holmes A (2017). The neural basis of reversal learning: An updated perspective. Neuroscience, 345, 12–26. 10.1016/j.neuroscience.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Corbett CJ, Firl DJ, & Robinson S (2015). Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cerebral Cortex, 25(9), 2683–2695. 10.1093/cercor/bhu066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Getsy PM, Denson JL, Firl DJ, Maxwell JR, Rogers DA, Wilson CG, & Robinson S (2015). Prenatal hypoxia-ischemia induces abnormalities in CA3 microstructure, potassium chloride co-transporter 2 expression and inhibitory tone. Frontiers in Cellular Neuroscience, 9, 347. 10.3389/fncel.2015.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, & Robinson S (2014). Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Molecular and Cellular Neurosciences, 61, 152–162. 10.1016/j.mcn.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, Ramachandra S, Bakhireva LN, Northington FJ, Gerner G, Tekes A, Milio LA, Brigman JL, Robinson S, & Allan A (2020). Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain, Behavior, and Immunity, 84, 45–58. 10.1016/j.bbi.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Miller RH, & Robinson S (2013). Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatric Research, 74(6), 658–667. 10.1038/pr.2013.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Oppong AY, Conteh FS, Yellowhair TR, Kim J, Fink G, Wolin AR, Northington FJ, & Robinson S (2018). Repetitive neonatal erythropoietin and melatonin combinatorial treatment provides sustained repair of functional deficits in a rat model of cerebral palsy. Frontiers in Neurology, 9, 233, 10.3389/fneur.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, & Robinson S (2015). Preclinical models of encephalopathy of prematurity. Developmental Neuroscience, 37(4–5), 277–288. 10.1159/000371721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Winer JL, Corbett CJ, & Robinson S (2016). Erythropoietin modulates cerebral and serum degradation products from excess calpain activation following prenatal hypoxia-ischemia. Developmental Neuroscience, 38(1), 15–26. 10.1159/000441024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, & Drzewiecki CM (2020). Cortical reorganization during adolescence: What the rat can tell us about the cellular basis. Developmental Cognitive Neuroscience, 45, 100857. 10.1016/j.dcn.2020.100857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul S (2004). Recombinant erythropoietin as a neuroprotective treatment: In vitro and in vivo models. Clinics in Perinatology, 31(1), 129–142. 10.1016/j.clp.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Juul S (2012). Neuroprotective role of erythropoietin in neonates. Journal of Maternal-Fetal & Neonatal Medicine, 25(Suppl. 4), 105–107. 10.3109/14767058.2012.715025 [DOI] [PubMed] [Google Scholar]
- Juul S, McPherson R, Bauer L, Ledbetter K, Gleason C, & Mayock D (2008). A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: Pharmacokinetics and safety. Pediatrics, 122(2), 383–391. 10.1542/peds.2007-2711 [DOI] [PubMed] [Google Scholar]
- Juul S, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, & Gleason CA (2004). Erythropoieitin concentrations in cerebrospinal fluid of nonhuman primates adn fetal sheep following high-dose recombinant erythropoietin. Biology of the Neonate, 85, 138–144. [DOI] [PubMed] [Google Scholar]
- Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, Ahmad KA, Bendel-Stenzel E, Baserga M, LaGamma EF, Downey LC, Rao R, Fahim N, Lampland A, Frantz ID III, Khan JY, Weiss M, Gilmore MM, Ohls RK, … PENUT Trial Consortium. (2020). A randomized trial of erythropoietin for neuroprotection in preterm infants. New England Journal of Medicine, 382(3), 233–243. 10.1056/NEJMoa1907423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CE, Thompson DK, Cooper M, Pham J, Nguyen TD, Yang JYM, Ball G, Adamson C, Murray AL, Chen J, Inder TE, Cheong JLY, Doyle LW, & Anderson PJ (2020). White matter tracts related to memory and emotion in very preterm children. Pediatric Research. 10.1038/s41390-020-01134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Behrens TE, & Wallis JD (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nature Neuroscience, 14(12), 1581–1589. 10.1038/nn.2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton JA, Castillo VK, Kehrer PE, & Brigman JL (2020). Moderate prenatal alcohol exposure impairs visual-spatial discrimination in a sex-specific manner: Effects of testing order and difficulty on learning performance. Alcoholism, Clinical and Experimental Research, 44(10), 2008–2018. 10.1111/acer.14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton JA, Ontiveros T, Bird CW, Valenzuela CF, & Brigman JL (2020). Moderate prenatal alcohol exposure alters the number and function of GABAergic interneurons in the murine orbitofrontal cortex. Alcohol, 88, 33–41. 10.1016/j.alcohol.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, & Altman DG (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8(6), e1000412. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabe W, Knerlich F, Washausen S, Kietzmann T, Sirén AL, Brunnett G, Kuhn HJ, & Ehrenreich H (2004). Expression patterns of erythropoietin and its receptor in the developing midbrain. Anatomy and Embryology, 207, 503–512. 10.1007/s00429-003-0365-y [DOI] [PubMed] [Google Scholar]
- Knabe W, Siren A-L, Ehrenreigh H, & Kuhn H-J (2005). Expression patterns of erythropoietin and its receptor in the developing spinal cord and dorsal root ganglia. Anatomy and Embryology, 210, 209–219. 10.1007/s00429-005-0019-3 [DOI] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, & Freret T (2013). Object recognition test in mice. Nature Protocols, 8(12), 2531–2537. 10.1038/nprot.2013.155 [DOI] [PubMed] [Google Scholar]
- Leuchter R-V, Gui L, Poncet A, Hagmann C, Lodygensky GA, Martin E, Koller B, Darqué A, Bucher HU, & Hüppi PS (2014). Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA, 312(8), 817–824. 10.1001/jama.2014.9645 [DOI] [PubMed] [Google Scholar]
- Mar AC, Horner AE, Nilsson SRO, Alsiö J, Kent BA, Kim CH, Holmes A, Saksida LM, & Bussey TJ (2013). The touchscreen operant platform for assessing executive function in rats and mice. Nature Protocols, 8(10), 1985–2005. 10.1038/nprot.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Cavanagh JF, & Brigman JL (2020). Alcohol exposure in utero disrupts cortico-striatal coordination required for behavioral flexibility. Neuropharmacology, 162, 107832. 10.1016/j.neuropharm.2019.107832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Sigdel R, Caldwell K, & Brigman JL (2014). Prenatal ethanol exposure impairs executive function in mice into adulthood. Alcoholism, Clinical and Experimental Research, 38(12), 2962–2968. 10.1111/acer.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, & Driscoll AK (2019). Births: Final data for 2018. National Vital Statistics Reports, 68(13), 1–47. [PubMed] [Google Scholar]
- Maxwell JR, Zimmerman AJ, Pavlik N, Newville JC, Carlin K, Robinson S, Brigman JL, Northington FJ, & Jantzie LL (2020). Neonatal hypoxic-ischemic encephalopathy yields permanent deficits in learning acquisition: A preclinical touchscreen assessment. Frontiers in Pediatrics, 8, 289. 10.3389/fped.2020.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur M, Miller R, & Robinson S (2010). Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. Journal of Neurosurgery: Pediatrics, 6, 206–221. 10.3171/2010.5.PEDS1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Kalmar M, Beke AM, Graf R,& Horvath E (2021). Intelligence and executive function of school-age preterm children in function of birth weight and perinatal complication. Appl Neuropsychol Child, 1–12. 10.1080/21622965.2020.1866571 [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, McKechanie AG, Stewart TJ, Johnstone M, Blackwood DH, St Clair D, Grant SGN, Bussey TJ, & Saksida LM (2015). Bridging the translational divide: Identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Scientific Reports, 5, 14613. 10.1038/srep14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll DN, McGovern M, Greene CM, & Molloy EJ (2018). Gender disparities in preterm neonatal outcomes. Acta Paediatrica, 107(9), 1494–1499. 10.1111/apa.14390 [DOI] [PubMed] [Google Scholar]
- Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, Steffen M, Yeo RA, Campbell R, Wiedmeier S, Baker S, Gonzales S, & Lowe J (2016). Preschool assessment of preterm infants treated with darbepoetin and erythropoietin. Pediatrics, 137(3), e20153859. 10.1542/peds.2015-3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, Lacy CB, Roohi M, Lambert DK, Burnett JJ, Pruckler B, Peceny H, Cannon DC, & Lowe JR (2014). Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics, 133(6), 1023–1030. 10.1542/peds.2013-4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin SL, Thompson SM, Young JW, & Brigman JL (2020). Moderate prenatal alcohol exposure impairs cognitive control, but not attention, on a rodent touchscreen continuous performance task. Genes, Brain, and Behavior, 20(1), e12652. 10.1111/gbb.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, & Saksida LM (2013). The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nature Protocols, 8(10), 2006–2021. 10.1038/nprot.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzi L, Fumagalli M, Scotto di Minico G, Giorda R, Morandi F, Sirgiovanni I, Schiavolin P, Mosca F, Borgatti R, & Montirosso R (2020). Pain-related increase in serotonin transporter gene methylation associates with emotional regulation in 4.5-year-old preterm-born children. Acta Paediatrica, 109(6), 1166–1174. 10.1111/apa.15077 [DOI] [PubMed] [Google Scholar]
- Robinson S, Conteh FS, Oppong AY, Yellowhair TR, Newville JC, Demerdash NE, Shrock CL, Maxwell JR, Jett S, Northington FJ, & Jantzie LL (2018). Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Frontiers in Cellular Neuroscience, 12, 322. 10.3389/fncel.2018.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Corbett CJ, Winer JL, Chan LAS, Maxwell JR, Anstine CV, Yellowhair TR, Andrews NA, Yang Y, Sillerud LO, & Jantzie LL (2017). Neonatal erythropoietin mitigates impaired gait, social interaction and diffusion tensor imaging abnormalities in a rat model of prenatal brain injury. Experimental Neurology, 302, 1–13. 10.1016/j.expneurol.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Petelenz K, Li Q, Cohen M, Buczek M, Lust D, & Miller R (2005). Developmental changes induced by prenatal hypoxia-ischemia insult in rats models human perinatal brain injury. Neurobiology of Diseases, 18, 568–581. [DOI] [PubMed] [Google Scholar]
- Robinson S, Winer JL, Chan LAS, Oppong AY, Yellowhair TR, Maxwell JR, Andrews N, Yang Y, Sillerud LO, Meehan WP, Mannix R, Brigman JL, & Jantzie LL (2018). Extended erythropoietin treatment prevents chronic executive functional and microstructural deficits following early severe traumatic brain injury in rats. Frontiers in Neurology, 9, 451. 10.3389/fneur.2018.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, & Murray EA (2013). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience, 16(8), 1140–1145. 10.1038/nn.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Navarro FP, Fares RP, Nadam J, Georges B, Moulin C, Le Cavorsin M, Bonnet C, Ryvlin P, Belmeguenai A, Bodennec J, Morales A, & Bezin L (2009). Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. Journal of Comparative Neurology, 514(4), 403–414. 10.1002/cne.22020 [DOI] [PubMed] [Google Scholar]
- Schober ME, Requena DF, Block B, Davis LJ, Rodesch C, Casper TC, Juul SE, Kesner RP, & Lane RH (2014). Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury. Journal of Neurotrauma, 31(4), 358–369. 10.1089/neu.2013.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Sun H, Xu F, Kang W, Gao L, Guo J, Zhang Y, Xia L, Wang X, & Zhu C (2016). Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Annals of Neurology, 80(1), 24–34. 10.1002/ana.24677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suikkanen J, Miettola S, Heinonen K, Vääräsmäki M, Tikanmäki M, Sipola M, Matinolli H-M, Järvelin M-R, Räikkönen K, Hovi P, & Kajantie E (2020). Reaction times, learning, and executive functioning in adults born preterm. Pediatric Research. 10.1038/s41390-020-0851-4 [DOI] [PubMed] [Google Scholar]
- Thompson DK, Loh WY, Connelly A, Cheong JLY, Spittle AJ, Chen J, Kelly CE, Inder TE, Doyle LW, & Anderson PJ (2020). Basal ganglia and thalamic tract connectivity in very preterm and full-term children; associations with 7-year neurodevelopment. Pediatric Research, 87(1), 48–56. 10.1038/s41390-019-0546-x [DOI] [PubMed] [Google Scholar]
- Tokariev A, Stjerna S, Lano A, Metsaranta M, Palva JM, & Vanhatalo S (2019). Preterm birth changes networks of newborn cortical activity. Cerebral Cortex, 29(2), 814–826. 10.1093/cercor/bhy012 [DOI] [PubMed] [Google Scholar]
- Tsai P, Ohab J, Kertesz N, Groszer M, Matter C, Gao J, & Carmichael S (2006). A critical role of erythropoietin receptor in neurogenesis and post-stroke-recovery. Journal of Neuroscience, 26, 1269–1274. 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck L, Sierksma A, Hansquine P, Thonnard D, Callaerts-Vegh Z, & D'Hooge R (2020). Comparison between touchscreen operant chambers and water maze to detect early prefrontal dysfunction in mice. Genes, Brain, and Behavior, 20(1), e12695. 10.1111/gbb.12695 [DOI] [PubMed] [Google Scholar]
- Vollmer B, & Stalnacke J (2019). Young adult motor, sensory, and cognitive outcomes and longitudinal development after very and extremely preterm birth. Neuropediatrics, 50(4), 219–227. 10.1055/s-0039-1688955 [DOI] [PubMed] [Google Scholar]
- Vos RC, Becher JG, Ketelaar M, Smits DW, Voorman JM, Tan SS, Reinders-Messelink HA, Dallmeijer AJ, & PERRIN+ Study Group. (2013). Developmental trajectories of daily activities in children and adolescents with cerebral palsy. Pediatrics, 132(4), e915–e923. 10.1542/peds.2013-0499 [DOI] [PubMed] [Google Scholar]
- Wehrle FM, Kaufmann L, Benz LD, Huber R, O'Gorman RL, Latal B, & Hagmann CF (2016). Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Human Development, 92, 37–43. 10.1016/j.earlhumdev.2015.10.021 [DOI] [PubMed] [Google Scholar]
- Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC, & Juul SE (2012). Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics, 130, 683–691. 10.1542/peds.2012-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Goodman AM, Chang T, Mulkey SB, Gonzalez FF, Mayock DE, Juul SE, Mathur AM, Van Meurs K, McKinstry RC, & Redline RW (2019). Placental pathology and neonatal brain MRI in a randomized trial of erythropoietin for hypoxic-ischemic encephalopathy. Pediatric Research, 87(5), 879–884. 10.1038/s41390-019-0493-6 [DOI] [PubMed] [Google Scholar]
- Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, Van Meurs KP, Rogers EE, Gonzalez FF, Comstock BA, Juul SE, Msall ME, Bonifacio SL, Glass HC, Massaro AN, Dong L, Tan KW, Heagerty PJ, & Ballard RA (2016). High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: A phase II trial. Pediatrics, 137(6), e20160191. 10.1542/peds.2016-0191 [DOI] [PubMed] [Google Scholar]
- Zanjani ED, Poster J, Burlington H, Mann LI, & Wasserman LR (1977). Liver as the primary site of erythropoietin formation in the fetus. Journal of Laboratory and Clinical Medicine, 89(3), 640–644. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.