Introduction
Advances in melanoma management have introduced new treatment paradigms for patients with sentinel lymph node (SLN) metastases. Two randomized surgical trials, the German Cooperative Dermatologic Oncology Group study (DeCOG-SLT) published in 2016 and the Second Multicenter Selective Lymphadenectomy Trial (MSLT-II) published in 2017, demonstrated the survival equivalence of nodal observation to routine completion lymph node dissection (CLND), prompting surgeons to reconsider the necessity of regional surgery for SLN-positive disease.1–3 Simultaneous publication of positive adjuvant systemic therapy trials showed that anti-CTLA4, anti-PD1, and BRAF/MEK inhibitors are more effective and less toxic than historic alternatives, providing additional treatment options for surgically-resected melanoma patients at high risk of recurrence and death.4–7 Based on these findings, the FDA approved ipilimumab in 2015, nivolumab in 2017, dabrafenib/trametinib in 2018, and pembrolizumab in 2019 for adjuvant treatment of resected stage III melanoma, with subsequent approvals by the corresponding regulatory bodies in Europe, the United Kingdom, and Australia (Figure 1).8
Figure 1. Proportion of patients who underwent completion lymph node dissection and received adjuvant systemic therapy before and after DeCOG-SLT and MSLT-2 publication and region-specific regulatory approvals of adjuvant immunotherapies and BRAF/MEK inhibitor therapy.
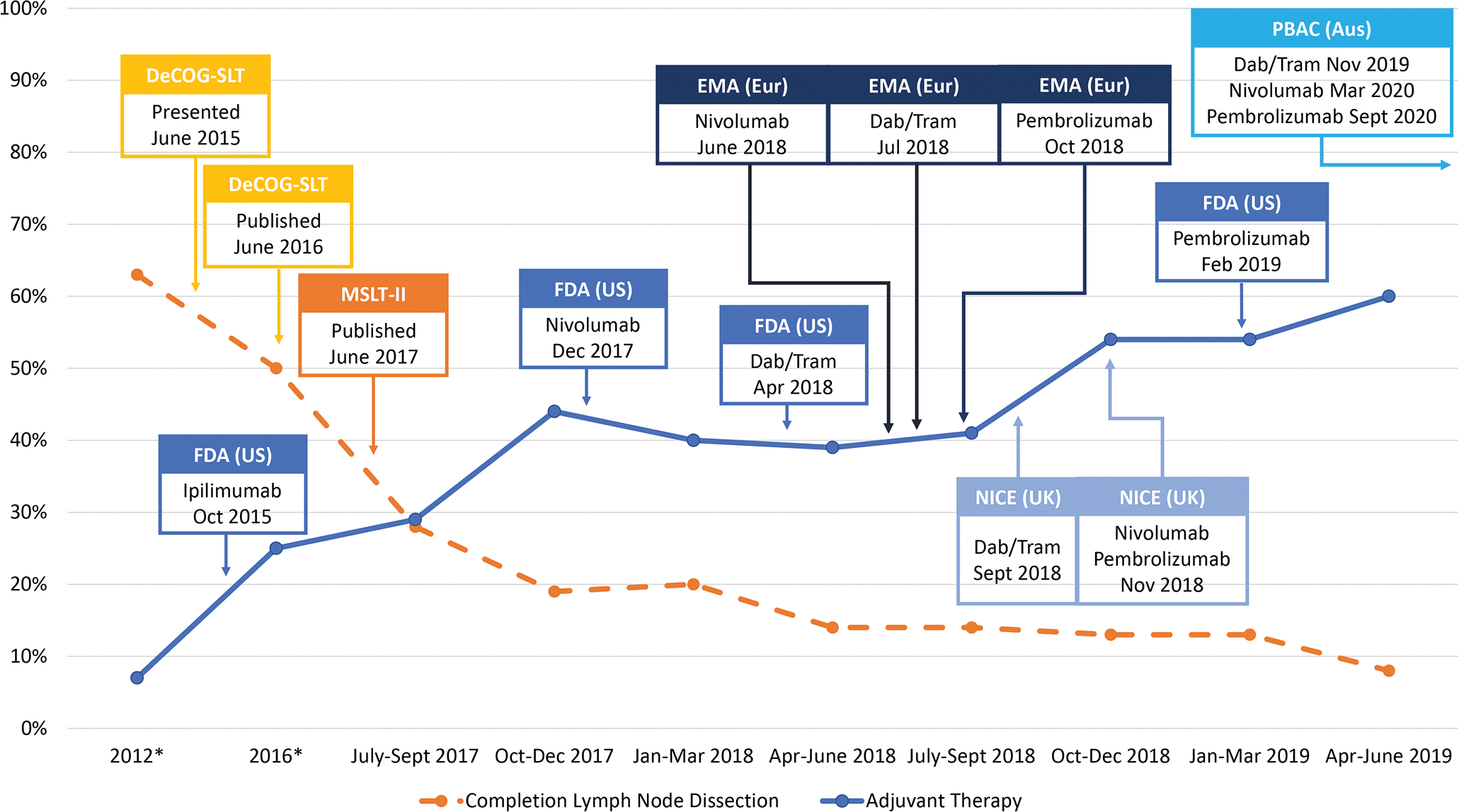
*Historical rates of CLND for years 2012 and 2016 and of adjuvant systemic therapy for 2016 for resected stage III melanoma were obtained from the National Cancer Database; historical rate of adjuvant systemic therapy from 2012 derived from MSLT-II and DeCOG-SLT publications
Abbreviations: DeCOG-SLT = German Dermatologic Oncology Group Trial; MSLT-II = Second Multicenter Selective Lymphadenectomy Trial; FDA = United States Food and Drug Administration; EMA = European Medicines Agency (Europe); NICE = National Institute for Healthcare Excellence (United Kingdom); PBAC = Pharmaceutical Benefits Advisory Committee (Australia); Dab/tram = dabrafenib/trametinib
These landmark trials provide evidence to omit (de-implement) regional surgery for SLN-positive patients while simultaneously administering (implementing) new medical therapies to high-risk patients in the adjuvant setting.9 While there is some evidence to suggest a long average time to implementation for most practices, less is known about how quickly practices are de-implemented or reasons for variation in de-implementation practices.10,11 Further, it is unknown how implementation of systemic therapies might influence de-implementation of local or regional treatments such as CLND. While nodal observation and adjuvant systemic therapy trials were performed in parallel, neither were studied in combination, leaving patients and physicians with four potential treatment strategies – nodal observation alone, nodal observation with adjuvant systemic therapy, CLND alone, or CLND with adjuvant systemic therapy – with widely ranging treatment intensity, morbidity, and cost. There are currently no comparative data to discern which option is optimal for each individual patient.
This unique scenario provides an opportunity to understand how new results are incorporated into practice for a single disease site and to study the dynamics of concurrent de-implementation of surgical treatment and implementation of adjuvant systemic therapy. As results from large oncologic databases are not yet mature, we used the database from the International High-Risk Melanoma Consortium consisting of 21 melanoma referral centers.12 Our objectives were to evaluate overall trends and center-level variation in de-implementation of CLND and implementation of adjuvant systemic therapy for SLN-positive melanoma.
Methods
The International High-Risk Melanoma Consortium was established in 2017 and includes a geographically diverse network of 21 melanoma referral centers from Australia, Europe (including the United Kingdom), and the United States (US).12 In this retrospective cohort study, each participating center provided data on adult patients with SLN-positive cutaneous melanoma who were treated from July 1, 2017-June 30, 2019. Requirements for center participation included having a nodal surveillance protocol in place before study initiation, attainment of institutional ethics/review board approval, negotiation of a data use agreement with the coordinating center, Moffitt Cancer Center, and provision of de-identified patient data by established deadlines. There was no designated funding source for this study. Reporting is in accordance with EQUATOR guidelines (Supplemental File).
Data was collected during routine care of patients with clinically node negative melanoma who had metastatic melanoma in at least one SLN. Included patients were required to have margin-negative resection of the primary tumor and no evidence of distant metastases on staging studies performed either before or after positive SLN biopsy but prior to further treatment planning. Performance of nodal observation versus CLND and use of adjuvant systemic therapy (i.e., anti-PD1 or anti-CTLA4 immunotherapy, BRAF/MEK inhibitor) were determined by treating physicians and patients. Unlike prior adjuvant systemic therapy studies, patients were not required to undergo CLND before receiving adjuvant systemic treatment.
We determined center-level rates of de-implementation of CLND and implementation of adjuvant systemic therapy for each 3-month period (quarter) over the two years of study to describe change over time. We also described variation in comprehensive management for SLN-positive patients treated at each center including the four possible treatment strategies – nodal observation alone, nodal observation with adjuvant systemic therapy, CLND alone, or CLND with adjuvant systemic therapy. We performed a comparative analysis of treatment strategies by the following center-level characteristics: geographic region (Australia, Europe, or US), whether the center previously participated in the MLST-II trial (no DeCOG-SLT sites were included in this study), designation as a cancer center by the National Cancer Institute, the European Society of Medical Oncology, or self-designated for Australian centers, and number of SLN-positive patients treated (volume reported by tertile). Center-level adoption rates were compared using Wilcoxon rank-sum tests for variables with two categories and Kruskal Wallis tests for variables with more than two categories. For findings of significant association by Kruskal Wallis tests (alpha <0.05), Dunn’s tests with Bonferroni correction for multiple comparisons were used to determine which specific elements of each variable were associated with the treatment outcome.
To adjust for patient- and disease-specific characteristics, generalized linear mixed models with random intercepts for each center were used to assess variation in de-implementation of CLND and implementation of adjuvant systemic treatment. Separate models were created for each outcome (CLND and adjuvant systemic treatment). Models were adjusted for the following factors: primary site (head/neck, trunk, extremity), tumor ulceration, presence of microsatellitosis, American Joint Committee on Cancer (AJCC) 8th edition stage, size of largest nodal metastasis (<1 mm (millimeter) or ≥1 mm), and extranodal tumor extension. Values are reported as odds ratios with 95% Confidence Intervals (CI). To demonstrate center-level variation not explained by disease-specific factors, the models were used to determine the adjusted probability of CLND and adjuvant systemic treatment, respectively, by treating center. We evaluated the relative importance of each covariate by computing the Nagelkerke pseudo r-squares for each covariate alone and for all covariates except the covariate of focus. This enabled us to evaluate the contribution of the covariate by itself and its incremental effect (eTable 1).13
We also performed clinically relevant sensitivity analyses based on eligibility criteria for adjuvant therapy trials and pertinent treatment guidelines. As some guidelines do not recommend adjuvant systemic therapy for stage IIIA patients, we separately evaluated center-level variation in use of adjuvant systemic therapy for this group.14 Likewise, we examined differences in provision of adjuvant systemic therapy for patients with nodal tumor deposits <1 mm because eligibility criteria for clinical trials of adjuvant systemic therapy required a minimum nodal tumor deposit of 1 mm.4–7
Results
Temporal trends in de-implementation of CLND and implementation of adjuvant systemic therapy
Participating centers collectively treated 1,109 SLN-positive patients (Table 1). In the earliest quarter of study, which was concurrent with MSLT-II publication, 28% of patients underwent CLND. This was lower than previously published rates and decreased to 8% by the last quarter of the two-year study period. At the same time adjuvant systemic therapy use increased from 29% to 60% over the two-year period (Figure 1).
Table 1.
Characteristics of treating centers and SLN-positive melanoma patients
| TREATING CENTERS | PATIENTS | ||
|---|---|---|---|
| Number of centers | 21 | Number of patients | 1,109 |
| Region | Male gender, N (%) | 672 (61%) | |
| Australia | 3 (14%) | Age, years, median (25th–75th %ile) | 61 (49–71) |
| Europe | 5 (24%) | Tumor location, N (%) | |
| United States | 13 (62%) | Head and Neck | 144 (13%) |
| Volume tertile (# SLN+ pts/yr) | Trunk | 428 (39%) | |
| Low (6–15) | 8 (38%) | Extremity | 537 (48%) |
| Middle (16–27) | 6 (29%) | Breslow depth, mm, median (25th–75th %ile) | 2.5 (1.5–4.2) |
| High (28–90) | 7 (33%) | Tumor ulceration, N (%) | 453 (41%) |
| Cancer center a | Microsatellites, N (%) | 95 (9%) | |
| No | 5 (24%) | Number of positive SLN, N (%) | |
| Yes | 16 (76%) | 1 | 842 (76%) |
| MSLT-2 trial participant | 2–3 | 247 (22%) | |
| No | 14 (67%) | 4 or more | 20 (2%) |
| Yes | 7 (33%) | Size of largest nodal tumor deposit, N (%) | |
| <1 mm | 508 (46%) | ||
| ≥1 mm | 475 (43%) | ||
| Unknown | 126 (11%) | ||
| Extranodal extension N (%) | 71 (6%) | ||
| AJCC 8th edition stage, N (%) | |||
| IIIA | 333 (30%) | ||
| IIIB | 242 (22%) | ||
| IIIC | 490 (44%) | ||
| IIID | 21 (2%) | ||
| III, not specified | 23 (2%) |
National Cancer Institute-designated, European Society of Medical Oncology-designated, or self-designated cancer centers for those outside NCI or ESMO jurisdiction
SLN=sentinel lymph node; pts=patients; yr=year; MSLT-2=Second Multicenter Selective Lymphadenectomy Trial; mm=millimeters; AJCC=American Joint Committee on Cancer
Center-level variation
Combining nodal management and adjuvant systemic treatment strategies, patients were managed with nodal observation alone (n=519, 47%), nodal observation with adjuvant systemic therapy (n=411, 37%), CLND alone (n=102, 9%), or CLND with adjuvant systemic therapy (n=77, 7%) (Figure 2). US centers treated more patients with adjuvant therapy than European centers during the period of study, whether doing nodal observation (p=0.01) or CLND (p=0.04), while adjuvant systemic therapy use at Australian centers was not significantly different from US or European centers (Table 2). At the center level there were no significant associations between performance of CLND or use of adjuvant systemic therapy and melanoma patient volume, region, cancer center designation, or prior participation in MSLT-II (Table 2).
Figure 2. Nodal management with observation versus completion lymph node dissection (CLND) and adjuvant systemic therapy use for patients with melanoma treated at twenty-one participating institutions in Australia, Europe, and the United States.
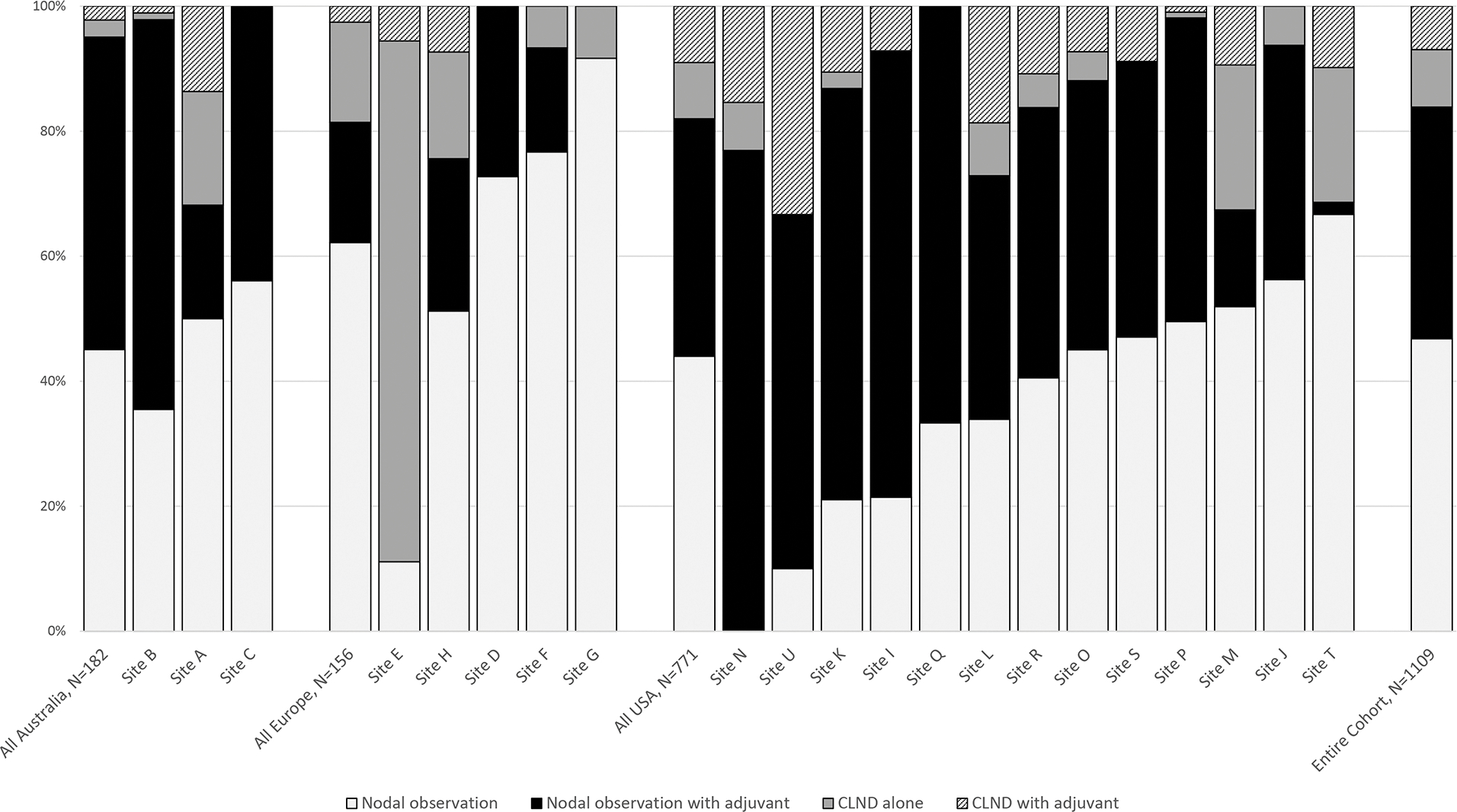
Table 2.
Institutional factors associated with nodal management and adjuvant treatment
| Nodal observationa | P-Value | Nodal observation with adjuvanta | P-Value | CLND alonea | P-Value | CLND with adjuvanta | P-Value | |
|---|---|---|---|---|---|---|---|---|
| All Centers | 47% (0–92%) | 43% (0–77%) | 5% (0–83%) | 7% (0–33%) | N/A | |||
| Region | ||||||||
| Australia | 50% (35–56%) | 0.13 | 44% (18–62%) | 0.03c | 1% (0–18%) | 0.47 | 1% (0–14%) | 0.08d |
| Europe | 73% (11–92%) | 17% (0–27%) | 8% (0–83%) | 0% (0–7%) | ||||
| US | 41% (0–67%) | 44% (2–77%) | 5% (0–23%) | 9% (0–33%) | ||||
| Volume tertile (# SLN+ pts/yr) | ||||||||
| Low (6–15) | 27% (0–92%) | 0.42 | 38% (0–77%) | 0.95 | 8% (0–83%) | 0.77 | 7% (0–33%) | 0.65 |
| Mid (16–27) | 49% (21–67%) | 41% (2–66%) | 6% (0–22%) | 10% (0–11%) | ||||
| High (28–90) | 50% (34–73%) | 43% (15–62%) | 1% (0–23%) | 1% (0–19%) | ||||
| Cancer centerb | ||||||||
| Yes | 43% (0–73%) | 0.46 | 44% (0–77%) | 0.37 | 4% (0–83%) | 0.75 | 7% (0–19%) | 0.66 |
| No | 51% (10–92%) | 18% (0–57%) | 8% (0–18%) | 7% (0–33%) | ||||
| MSLT-2 trial participant | ||||||||
| Yes | 49% (21–56%) | 0.60 | 33% (0–77%) | 0.06 | 1% (0–83%) | 0.16 | 7% (0–33%) | 0.50 |
| No | 45% (0–92%) | 49% (24–71%) | 8% (0–17%) | 9% (0–11%) | ||||
Analysis by treating center reported as the proportion of patients at each treating center who received each treatment category with values representing center-level medians and ranges
National Cancer Institute-designated, European Society of Medical Oncology-designated, or self-designated cancer centers for those outside NCI or ESMO jurisdiction; CLND=completion lymph node dissection; US=United States; SLN+=sentinel lymph node positive; pts=patients; yr=year; MSLT-2=Second Multicenter Selective Lymphadenectomy Trial
p=0.01 for comparison of rates in US versus Europe, 1.00 for US versus Australia, 0.14 for Europe versus Australia
p=0.04 for comparison of rates in US versus Europe, 0.55 for US versus Australia, 0.64 for Europe versus Australia
Multi-level models
In the multilevel models, higher odds of CLND were associated with head and neck primary site (relative to extremity) and nodal tumor deposit of ≥1 mm (Table 3). Accounting for disease-specific factors, the adjusted probability of CLND based on treating center ranged from 1% to 83% (median 10%) (Figure 3). Odds of adjuvant systemic therapy increased for nodal tumor deposit of ≥1 mm and decreased for patients with stage IIIA disease relative to IIIC or IIID (Table 3). Adjusted probabilities of adjuvant systemic therapy ranged from 9% to 87% by treating center (median 46%) (Figure 3). For both CLND and adjuvant systemic therapy, the most influential covariates in explaining observed variation were treating center, tumor size, and stage.
Table 3.
Probability of completion lymph node dissection and adjuvant systemic therapy based on patient, disease, and treating center factorsa
| Completion Lymph Node Dissection | Adjuvant Systemic Therapy | |||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P-Value | Odds Ratio (95% Confidence Interval) | P-Value | |
| Primary site | ||||
| Head and neck | 2.19 (1.26, 3.80) | 0.006 | 1.04 (0.66, 1.61) | 0.877 |
| Trunk | 1.10 (0.73, 1.66) | 0.657 | 1.06 (0.77, 1.47) | 0.705 |
| Extremity | Reference | Reference | ||
| Tumor ulceration | 0.88 (0.54, 1.43) | 0.594 | 1.20 (0.82, 1.75) | 0.349 |
| Microsatellites | 0.84 (0.45, 1.56) | 0.582 | 0.92 (0.54, 1.55) | 0.746 |
| AJCC 8th edition stage | ||||
| IIIA | 0.70 (0.39, 1.26) | 0.234 | 0.37 (0.23, 0.59) | <0.001 |
| IIIB | 0.57 (0.32, 1.02) | 0.060 | 0.71 (0.47, 1.08) | 0.108 |
| IIIC/D | Reference | Reference | ||
| Nodal tumor ≥1 millimeter | 3.62 (2.33, 5.63) | <0.001 | 1.65 (1.20, 2.27) | 0.002 |
| Extranodal tumor extension | 1.70 (0.89, 3.24) | 0.106 | 1.52 (0.84, 2.76) | 0.165 |
Models contained random intercept to account for clustering of patients within facility
Figure 3: Probability of completion lymph node dissection (CLND) and adjuvant systemic treatment by treating centera.
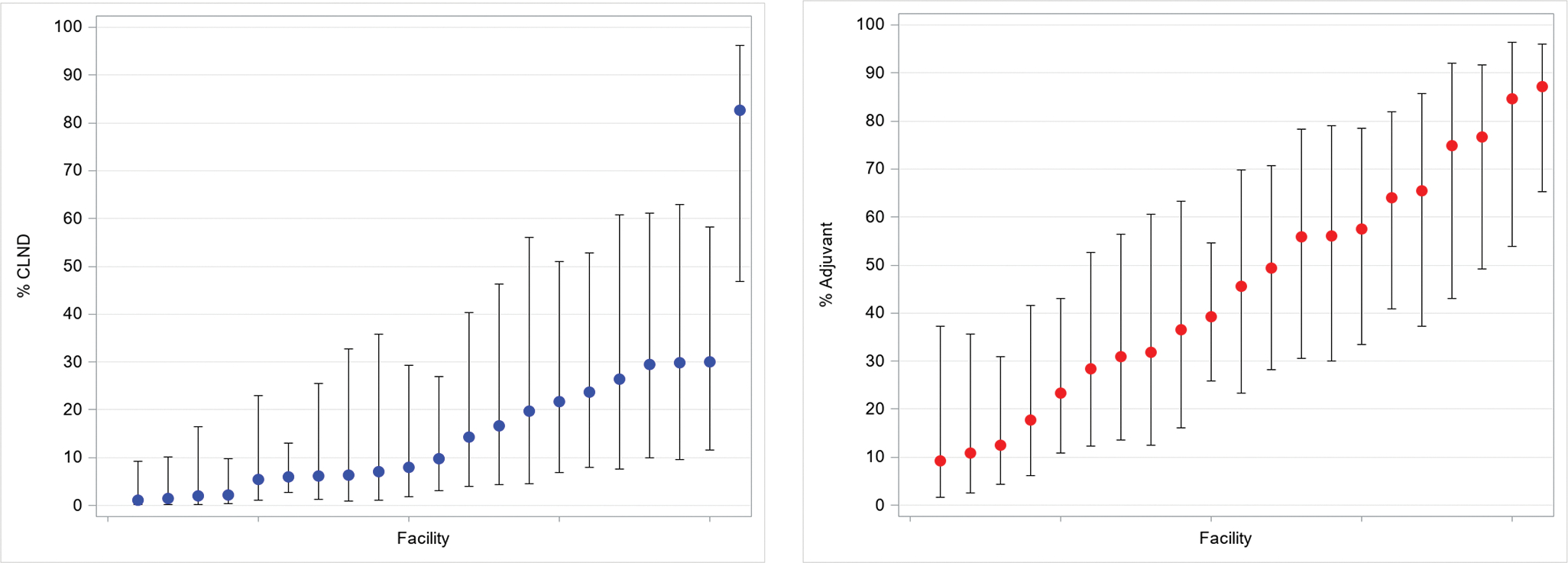
aAdjusted for primary tumor site, ulceration, AJCC 8th edition stage, size of largest nodal tumor deposit, microsatellitosis, and extranodal tumor extension; models contained random intercept to account for clustering of patients within facility.
Sensitivity analyses
By stage, the proportion of patients receiving adjuvant systemic therapy was IIIA 28%, IIIB 44%, IIIC/D 55%. There were differences in adjuvant treatment for Stage IIIA versus Stage IIIB-D disease at the regional and center levels (Figure 4). By center, the proportion of stage IIIA patients who received adjuvant systemic therapy ranged from 0% to 88% with five centers not treating any stage IIIA patient with adjuvant systemic therapy and ten centers using adjuvant therapy in more than one-quarter of patients with stage IIIA disease. Center-level variation was similarly observed when patients were stratified by size of largest nodal tumor deposit. Median rates of adjuvant systemic therapy use for patients with nodal tumor deposits <1 mm ranged by center from 0 to 100% with 10 of 21 centers using adjuvant systemic therapy for more than one-quarter of their patients with nodal tumor deposits <1 mm.
Figure 4. Nodal management and adjuvant systemic therapy for AJCC 8th Edition Stage IIIA (A) versus Stage IIIB-D (B) melanoma patients based on region of treating center.
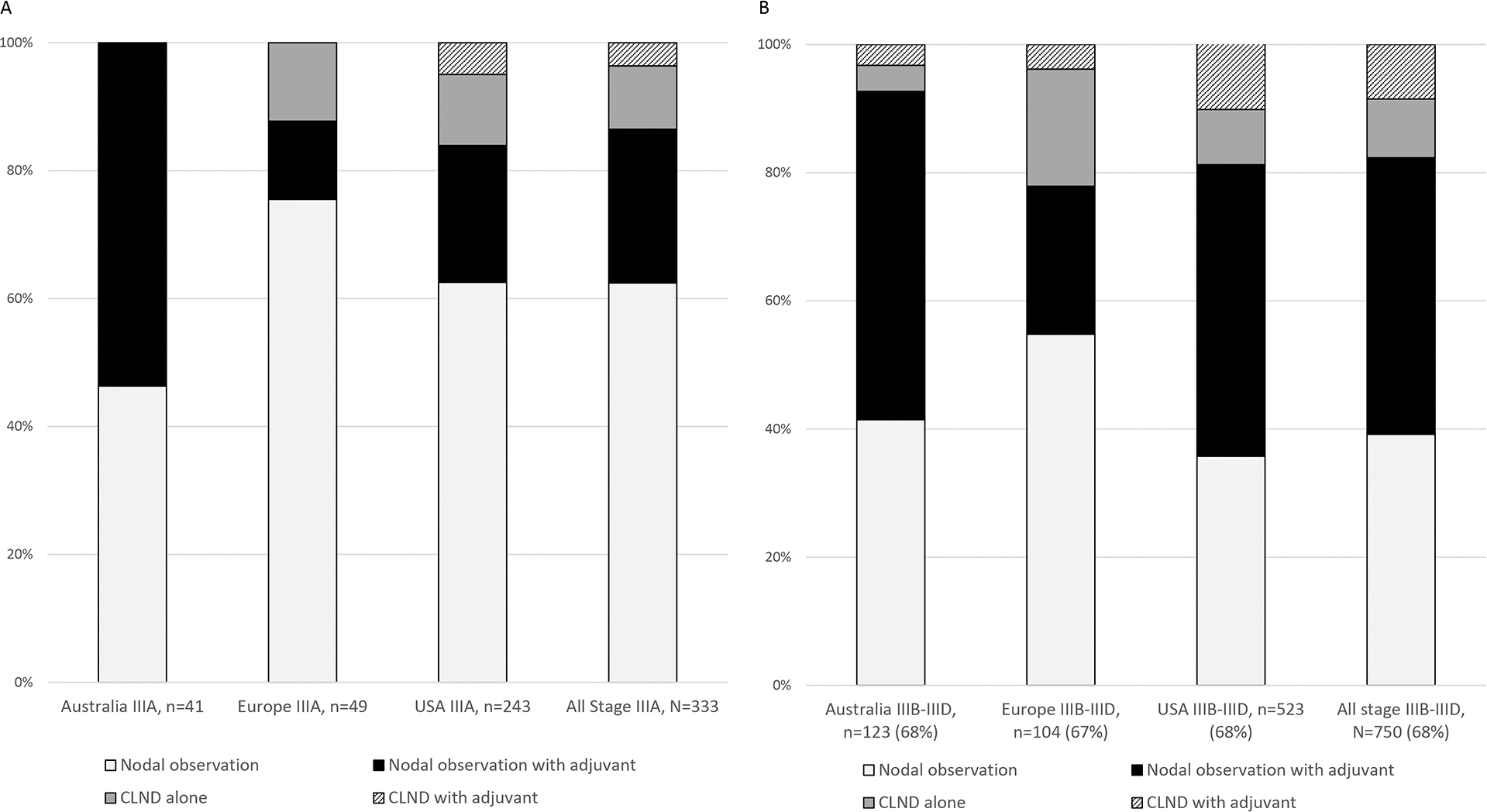
AJCC = American Joint Committee on Cancer; CLND=completion lymph node dissection; USA =United States of America
Discussion
This study has three main findings. First, at major melanoma centers world-wide there has been rapid but varied incorporation of surgical trial findings into routine care for SLN-positive patients. Second, there has been a simultaneous increase in use of adjuvant therapy in SLN-positive patients. Third, while performance of CLND and administration of adjuvant systemic treatment were associated with disease-specific factors including primary tumor features and burden of SLN-positive disease, there was also significant variation in CLND and adjuvant systemic treatment patterns based on the center where patients received care.
Our data demonstrate the pace of CLND de-implementation was swift over the ensuing two years of study, corroborating the findings of single institution studies and demonstrating a much shorter time to practice change than the average 17 years often cited in implementation research.15–17 One contributing factor may have been a pre-existing acknowledgement of the limitations of CLND. Prior to MSLT-II publication, several large retrospective cohort studies already suggested limited benefit of CLND, with most patients having no additional positive (non-sentinel) nodes in CLND specimens.18–20 At the time, several risk prediction tools for non-sentinel node positivity were available to support a decision not to perform CLND.18,21–24 Rates of CLND at this study initiation and in prior studies demonstrate that CLND was already being performed selectively prior to publication or presentation of DeCOG-SLT or MSLT-II results. At American College of Surgeons Commission on Cancer (CoC) participating centers in the US, the rate of CLND was 63% in 2012 and 50% in 2016.25 Even in the MSLT-II trial, more patients who were randomized to CLND did not undergo the prescribed intervention. Patients randomized to CLND who did not undergo node dissection had significantly lower nodal disease burdens, suggesting that the perceived risk of positive non-sentinel nodes influenced patients and/or physicians in their decision to accept the assigned treatment.1 Additional explanations for low baseline performance of CLND and swift de-implementation might include surgeons’ level of comfort with performing the procedure and the risk of potentially life-altering lymphedema.26
Further, MLST-II trial results were well-disseminated, with a recent survey of the Society of Surgical Oncology membership finding that 98% of respondents were aware of its findings.27 Research findings that are particularly impactful to a highly specialized provider group may disseminate more quickly due to the close-knit nature of subspecialty practitioners who routinely seek information and colleagues’ advice from outside their immediate practice environment. Finally, it is notable that the curves for de-implementation of CLND and implementation of adjuvant systemic therapy have an inverse relationship. While there is no available evidence to suggest that adjuvant systemic treatment is an effective replacement for CLND or that it confers additional regional control, patients and physicians may have been more comfortable forgoing additional surgery when alternative treatments were available to mitigate recurrence.9
Similar to trends in de-implementation of CLND, the implementation of adjuvant systemic therapy for SLN-positive melanoma began before the start of this study but rapidly escalated in a comparable timeframe. In our 21 melanoma referral centers, adjuvant systemic treatment increased from 29% in July 2017 to 60% in June 2019. Centers with high adoption used adjuvant systemic therapy in up to 92% of SLN-positive patients, including large proportions of patients with stage IIIA disease and nodal tumor deposits <1 mm. Other reports from single institution cohorts of SLN-positive patients not undergoing CLND have reported use of adjuvant systemic therapy in 69–75%.16,28
There are several potential reasons for the accelerated implementation of adjuvant systemic therapy in SLN-positive melanoma. Historically, regionally metastatic melanoma carried a poor prognosis, with only 28–44% of patients having recurrence-free survival at 5 years. Effective, well-tolerated adjuvant treatments represented a significant therapeutic advance.29 These agents had previously been tested in the setting of stage IV and unresectable stage III disease, demonstrating often dramatic response rates and significant improvements in progression-free survival.30–32 Finally, concurrent trials of immunotherapy in other solid tumors increased widespread knowledge within and outside the medical community, with drug companies broadly disseminating information about the medications, including direct to consumer advertising in the US.33
Despite overall adoption of these evidence-based practices, there remained variation both in de-implementation of CLND and implementation of adjuvant systemic treatment based on where patients were treated. Several possible reasons exist. First, unmeasured patient factors such as travel time to the treating center may have influenced patients’ preferences for both nodal observation and receiving a year of adjuvant systemic treatment. Secondly, while FDA approval came during the study period for several of the contemporary adjuvant systemic therapies, regulatory approvals were later in Europe and Australia. While the payer mix in US centers is quite heterogenous, all participating centers in Europe and Australia have some form of universal, government-run healthcare, which initially might delay or limit access to new, expensive adjuvant systemic treatments. Still, even within the studied US centers there was profound variation in both nodal observation and adjuvant systemic treatment rates for SLN-positive patients.
A final potential contributor to the observed variation in both CLND and adjuvant systemic treatment is physicians’ interpretation and application of available evidence. A recent survey demonstrated that most SLN-positive melanoma patients prefer to follow their physicians’ recommendations regarding CLND, highlighting the importance of the local context in which patients receive care and the constitution of patients’ treatment teams.9,34 Evidence from randomized trials of nodal observation and adjuvant systemic treatment have been informative, but several knowledge gaps remain. Adjuvant trials, for instance, mandated CLND prior to systemic treatment, while under 10% of nodal observation trial participants received adjuvant therapy. As a result, high-level evidence is lacking on outcomes of nodal observation in adjuvant systemic therapy recipients.1,2,5,6,35,36 Also, certain populations of SLN-positive patients were underrepresented in these trials. While adjuvant systemic therapy trials required a minimal nodal tumor deposit dimension of 1 mm, patients with low nodal tumor burden constituted the majority of participants in the randomized surgical trials of nodal observation.1,2,5,6,35,36 Despite these significant differences in the study populations, our data demonstrate that many treatment teams have readily integrated the two contemporary strategies, offering patients nodal observation with adjuvant systemic treatment despite a lack of randomized evidence and only limited survival data from observational cohorts, even in patients whose tumor and nodal burdens were not represented in the randomized trials.16,28,37,38
In certain cases and centers, interpretations of available evidence may have resulted in overuse of adjuvant systemic therapy or non-evidence-based de-implementation of CLND in patients who were not represented in the nodal management trials.12 For example, a sizeable proportion of stage IIIA patients with nodal tumor deposits of <1 mm received adjuvant systemic therapy despite the absence of efficacy data for patients with low nodal tumor burdens.14 For such patients, the risk of adjuvant systemic treatment-related adverse events may exceed potential benefits. The observed variation in treatment intensity for SLN-positive melanoma, from nodal observation alone to CLND with adjuvant treatment, is associated significant differences in patient morbidity, travel burden, anxiety, and cost. Hence, it is critical to develop indications and understand outcomes for each of these combined treatment strategies.
Until national datasets mature, the experience of this multi-institutional international collaborative represents the best available data on de-implementation of CLND and implementation of adjuvant systemic therapy for SLN-positive melanoma. One limitation of the study is reliance on data from melanoma referral centers which may not reflect management in other patient populations. As location of care and specifically treatment at a cancer center has been found to significantly impact implementation of evidence-based care, trends in implementation of nodal observation and adjuvant systemic treatment at non-referral centers may differ.39–44 In addition, while our international collaborative represents countries with some of the highest worldwide incidences of melanoma, it was limited to higher income countries with populations of predominantly European ancestry, limiting generalizability to other populations.45 With this retrospective study using clinical data, we were not positioned to study the specific reasons for CLND or adjuvant systemic therapy use at each center, nor could we evaluate potentially time-variant changes in barriers to or facilitators of implementation such as availability of adjuvant systemic treatments or high-quality ultrasound to perform nodal basin surveillance.
Conclusions
In an evolving treatment landscape for SLN-positive melanoma, fewer patients are undergoing CLND and more are receiving adjuvant systemic therapy. These changes in practice began prior to the publication of landmark trials of nodal observation and adjuvant immunotherapy and targeted therapy but accelerated dramatically at the included melanoma referral centers over a two-year time period post-publication. Location of care contributed significantly to the observed variation in de-implementation of CLND and implementation of adjuvant systemic treatment and was not explained by differences in patient mix. As there are significant differences in potential morbidity and cost of available treatment strategies, future work should explore how the context of care delivery and interprofessional interactions impact the incorporation of evidence-based findings into clinical care.
Supplementary Material
Acknowledgements and Disclosures
Dr. Beasley is a consultant for Regeneron and receives clinical trial funding from Istari Oncology.
Dr. Bredbeck is supported by the Ruth L. Kirschstein Research Service Award from the National Cancer Institute (NCI; T32 CA009672).
Dr. Farma is a speaker for Novartis and serves on the Data Safety Monitoring Board for Delcath.
Dr. Gyorki has received honoraria from Amgen, Bayer, and Qbiotics. He has served on the advisory board for Amgen.
Dr. Hieken has received research funding from Genentech and the Breast Cancer Research Foundation.
Ms. Kottschade was received research funding from Bristol-Meyers Squibb and serves on the advisory board for Novartis.
Dr. Olofsson Bagge has received institutional research grants from Astra Zeneca, Bristol-Myers Squibb (BMS) and SkyLineDx, speaker honorarium from Roche and Pfizer and has served on advisory boards for Amgen, BD/BARD, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, Roche and Sanofi Genzyme.
Dr. Sarnaik is a consultant to Iovance Biotherapeutics, Guidepoint, Defined Health, and Gerson Lehman group. His institution has received research funding from Iovance Biotherapeutics and Provectus Inc. He has received speaker fees from Physicians’ Education Resource and Medscape.
Dr. Sondak is a paid consultant to Merck, Bristol Myers Squibb, Novartis, Regeneron, Array, Replimune, Pfizer, Genentech/Roche, Eisai, Aduro, Amgen, TRM Oncology, and Polynoma.
Dr. Thompson is supported by the National Health and Medical Research Council (Grant #APP1093017). He has received honoraria for advisory board participation from Bristol Meyers Squibb Australia, Glaxo Smith Kline, Merck Sharp & Dohme Australia, Provectus. He has received travel support from Glaxo Smith Kline and Provectus.
Dr. van Akkooi had received honoraria and/or served on advisory boards for Amgen, Bristol Myers Squibb, Novartis, MSD-Merck, Merck-Pfizer, Sanofi, and 4SC. He has received research grants from Amgen and Merck-Pfizer.
Dr. Vetto is a consultant for Castle Biosciences.
Dr. Zager has received research funding from Novartis, Philogen, Delcath Systems, Amgen, Provectus, Novartis and Castle Biosciences. He has served on Advisory Boards for Merck, Novartis and Pfizer and on the Speakers Bureau for Pfizer, Sun Pharma, and Castle Biosciences. He provides expert testimony to McGowan Hood and Bubalo Law.
All other authors reported no disclosures.
References
- 1.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8 [DOI] [PubMed] [Google Scholar]
- 3.Leiter U, Stadler R, Mauch C, et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients With Melanoma With Positive Sentinel Node. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(32):3000–3008. doi: 10.1200/JCO.18.02306 [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 6.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 8.Kwak M, Farrow NE, Salama AKS, et al. Updates in adjuvant systemic therapy for melanoma. J Surg Oncol. 2019;119(2):222–231. doi: 10.1002/jso.25298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci IS. 2020;15(1):2. doi: 10.1186/s13012-019-0960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen. Theories, models, and frameworks for de-implementation of low value care: A scoping review of the literature. Implement Res Pract. 2020;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Bredbeck BC, Sinco B, et al. Variations in Persistent Use of Low-Value Breast Cancer Surgery. JAMA Surg. Published online February 3, 2021. doi: 10.1001/jamasurg.2020.6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broman KK, Hughes TM, Dossett LA, et al. Surveillance of Sentinel Node-Positive Melanoma Patients with Reasons for Exclusion from MLST-II: Multi-Institutional Propensity Score Matched Analysis. J Am Coll Surg. Published online December 13, 2020. doi: 10.1016/j.jamcollsurg.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–692. [Google Scholar]
- 14.Michielin O, van Akkooi A, Lorigan P, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(11):1449–1461. doi: 10.1016/j.annonc.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Bredbeck BC, Mubarak E, Zubieta DG, et al. Management of the positive sentinel lymph node in the post-MSLT-II era. J Surg Oncol. Published online September 6, 2020. doi: 10.1002/jso.26200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrow NE, Raman V, Williams TP, Nguyen KY, Tyler DS, Beasley GM. Adjuvant Therapy is Effective for Melanoma Patients with a Positive Sentinel Lymph Node Biopsy Who Forego Completion Lymphadenectomy. Ann Surg Oncol. Published online April 20, 2020. doi: 10.1245/s10434-020-08478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green LW. Closing the chasm between research and practice: evidence of and for change. Health Promot J Aust Off J Aust Assoc Health Promot Prof. 2014;25(1):25–29. doi: 10.1071/HE13101 [DOI] [PubMed] [Google Scholar]
- 18.Cochran AJ, Wen D-R, Huang R-R, Wang H-J, Elashoff R, Morton DL. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol Off J U S Can Acad Pathol Inc. 2004;17(7):747–755. doi: 10.1038/modpathol.3800117 [DOI] [PubMed] [Google Scholar]
- 19.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992 [DOI] [PubMed] [Google Scholar]
- 20.Bamboat ZM, Konstantinidis IT, Kuk D, Ariyan CE, Brady MS, Coit DG. Observation after a positive sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol. 2014;21(9):3117–3123. doi: 10.1245/s10434-014-3758-7 [DOI] [PubMed] [Google Scholar]
- 21.Bertolli E, Franke V, Calsavara VF, et al. Validation of a Nomogram for Non-sentinel Node Positivity in Melanoma Patients, and Its Clinical Implications: A Brazilian-Dutch Study. Ann Surg Oncol. 2019;26(2):395–405. doi: 10.1245/s10434-018-7038-9 [DOI] [PubMed] [Google Scholar]
- 22.Rossi CR, Mocellin S, Campana LG, et al. Prediction of Non-sentinel Node Status in Patients with Melanoma and Positive Sentinel Node Biopsy: An Italian Melanoma Intergroup (IMI) Study. Ann Surg Oncol. 2018;25(1):271–279. doi: 10.1245/s10434-017-6143-5 [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Essner R, Torisu-Itakura H, Wanek L, Wang H, Morton DL. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(18):3677–3684. doi: 10.1200/JCO.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 24.MacDonald S, Siever J, Baliski C. Performance of models predicting residual lymph node disease in melanoma patients following sentinel lymph node biopsy. Am J Surg. 2020;219(5):750–755. doi: 10.1016/j.amjsurg.2020.02.059 [DOI] [PubMed] [Google Scholar]
- 25.Herb JN, Dunham LN, Ollila DW, Stitzenberg KB, Meyers MO. Use of Completion Lymph Node Dissection for Sentinel Lymph Node-Positive Melanoma. J Am Coll Surg. 2020;230(4):515–524. doi: 10.1016/j.jamcollsurg.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 26.Faries MB, Thompson JF, Cochran A, et al. The impact on morbidity and length of stay of early versus delayed complete lymphadenectomy in melanoma: results of the Multicenter Selective Lymphadenectomy Trial (I). Ann Surg Oncol. 2010;17(12):3324–3329. doi: 10.1245/s10434-010-1203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui J, E Burke, K Broman, S Marmo, E Jensen, T Tuttle, Zager J. Surgeon Decision-Making for Management of Positive Sentinel Lymph Nodes in the Post-Multicenter Selective Lymphadenectomy Trial II Era: A Survey Study. J Surg Oncol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauwerdink DJW, Molina G, Frederick DT, Sharova T, Carmichael H, Boland GM. Adjuvant Therapy Failure Patterns in the Modern Era of Melanoma Management. Ann Surg Oncol. Published online May 23, 2020. doi: 10.1245/s10434-020-08631-2 [DOI] [PubMed] [Google Scholar]
- 29.Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe - a systematic review of the literature. Clin Epidemiol. 2016;8:109–122. doi: 10.2147/CLEP.S99021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626–636. doi: 10.1056/NEJMoa1904059 [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 33.Applequist J, Ball JG. An Updated Analysis of Direct-to-Consumer Television Advertisements for Prescription Drugs. Ann Fam Med. 2018;16(3):211–216. doi: 10.1370/afm.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mott N, Wang T, Miller J, et al. Medical Maximizing-Minimizing Preferences in Relation to Low-Value Services for Older Women with Hormone Receptor-Positive Breast Cancer: A Qualitative Study. Ann Surg Oncol. 2021;28(2):941–949. doi: 10.1245/s10434-020-08924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(11):1848. doi: 10.1093/annonc/mdz221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broman K, Hughes T, Dossett L, et al. Active Surveillance of Melanoma Patients with Sentinel Node Metastasis: An International Multi-Institution Evaluation of Post-MSLT-2 Adoption and Early Outcomes. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen CN, Shoushtari AN, Chauhan D, et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy☆. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(8):1075–1082. doi: 10.1016/j.annonc.2020.04.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard DH, Soulos PR, Chagpar AB, Mougalian S, Killelea B, Gross CP. Contrary To Conventional Wisdom, Physicians Abandoned A Breast Cancer Treatment After A Trial Concluded It Was Ineffective. Health Aff Proj Hope. 2016;35(7):1309–1315. doi: 10.1377/hlthaff.2015.1490 [DOI] [PubMed] [Google Scholar]
- 40.Hennigs A, Köpke M, Feißt M, et al. Which patients with sentinel node-positive breast cancer after breast conservation still receive completion axillary lymph node dissection in routine clinical practice? Breast Cancer Res Treat. 2019;173(2):429–438. doi: 10.1007/s10549-018-5009-2 [DOI] [PubMed] [Google Scholar]
- 41.Bilimoria KY, Balch CM, Wayne JD, et al. Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(11):1857–1863. doi: 10.1200/JCO.2008.18.7567 [DOI] [PubMed] [Google Scholar]
- 42.Minami CA, Wayne JD, Yang AD, et al. National Evaluation of Hospital Performance on the New Commission on Cancer Melanoma Quality Measures. Ann Surg Oncol. 2016;23(11):3548–3557. doi: 10.1245/s10434-016-5302-4 [DOI] [PubMed] [Google Scholar]
- 43.Tucker TC, Charlton ME, Schroeder MC, et al. Improving the Quality of Cancer Care in Community Hospitals. Ann Surg Oncol. 2021;28(2):632–638. doi: 10.1245/s10434-020-08867-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shulman LN, Browner AE, Palis BE, et al. Compliance with Cancer Quality Measures Over Time and Their Association with Survival Outcomes: The Commission on Cancer’s Experience with the Quality Measure Requiring at Least 12 Regional Lymph Nodes to be Removed and Analyzed with Colon Cancer Resections. Ann Surg Oncol. 2019;26(6):1613–1621. doi: 10.1245/s10434-019-07323-w [DOI] [PubMed] [Google Scholar]
- 45.Matthews NH, Li W-Q, Qureshi AA, Weinstock MA, Cho E. Epidemiology of Melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017. Accessed March 10, 2021. http://www.ncbi.nlm.nih.gov/books/NBK481862/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


