Abstract
Inflammatory bowel disease (IBD) pathogenesis is thought to be induced by a mix of genetic susceptibility, microbial populations, and immune triggers such as infections. Severe acute respiratory syndrome coronavirus 2 (SARS-nCoV2) may have increased capacity to generate autoimmune disease as evidenced by known spikes in diseases such as type 1 diabetes mellitus. Public health interventions like masking and closures additionally created remarkable drops in typical viral infections, with remarkable shifts in influenza-like illness reporting in 2020. This study aims to evaluate the impact of SARS-nCoV2 and associated interventions on pediatric IBD presentation in New York City using records of new diagnoses at a consortium of 4 institutions between 2016 and June 2022. We fit time series model (autoregressive integrated moving average model) to monthly and quarterly number of cases of each disease for January 2016–March 2020 and forecast the period between April 2020 and June 2022. We note no decrease in ulcerative colitis (UC) or Crohn disease (CD) in the aftermath of historic low levels of overall viral illness, and statistically significant increases in CD diagnoses and elevation in UC diagnoses creating a trend suggesting overall increase in IBD diagnoses exceeding the baseline rate of increase. These data suggest a possible linkage between SARS-nCoV2 infection rates and subsequent pediatric IBD presentation.
Keywords: COVID, Crohn disease, inflammatory bowel disease, SARS-nCoV2
What Is Known.
Infections may precipitate inflammatory bowel disease (IBD) pathogenesis.
SARS-nCoV2-related mitigation interventions substantially decreased viral infections in New York City (NYC).
SARS-nCoV2 has been associated with increases in some autoimmune diseases.
What Is New
Overall viral infections and non-SARS-nCoV2 infections in specific did not create a decline in IBD rates.
Crohn disease rates increased 4–6 months after SARS-nCoV2 infections spiked in NYC.
Rates of IBD diagnoses may be higher than expected.
The severe acute respiratory syndrome coronavirus 2 (SARS-nCoV2) pandemic has created multiple profound secondary effects in health care, but perhaps one of the most concerning is a possible capacity to generate increased rates of autoimmune disorders. Both pediatric and adult studies have demonstrated an increase in type 1 diabetes diagnoses, and case reports and series suggest possible impacts in other autoimmune disorders (1–5). Inflammatory bowel disease (IBD) is one of our greatest autoimmune disease concerns in the framework of pediatric gastroenterology. As currently understood, IBD pathogenesis appears to be a mix of genetic susceptibility, microbial interaction, and immune triggers such as infections. SARS-nCoV2 has a known predilection for intestinal tissue: gastrointestinal illness and protracted viral activity and shedding in stool accompany infection (6–9). With interventions such as face masks and social distancing adopted at the onset of the SARS-nCoV2 pandemic, influenza-like illness and other infectious disease incidence plummeted in the United States (10,11). Based on our theoretical understanding of IBD pathogenesis, reduced infectious disease during the pandemic would be expected to decrease IBD presentation rates, but decreased IBD incidence has not been observed clinically. As the location of many cases early in the SARS-nCoV2 pandemic, New York City (NYC) represents a valuable source of data to examine for any possible linkage with infections and IBD presentations, either positive or negative. Our study evaluated the association between the pandemic and new IBD diagnoses in NYC using data from a consortium of 4 NYC institutions: SUNY Downstate (Brooklyn), Maimonides Medical Center (Brooklyn), NYU Hassenfeld Children’s Hospital (Manhattan), and the Children’s Hospital at Montefiore (Bronx). We compared IBD rates after the SARS-nCoV2 pandemic with rates from the 5 years prior.
METHODS
Data
We extracted data for IBD patients presenting in pediatric gastrointestinal clinic at 4 institutions between January 2016 and June 2022 (n = 587) from electronic health records (EHRs) after institutional review board approval of respective institutions.
Measures
Date of diagnosis was coded as year and month of diagnosis with the day removed for identifiability reasons. We measured the following demographic variables: age in years at diagnosis, EHR-recorded sex, race, and ethnicity. Age was categorized by Food and Drug Administration SARS-nCoV2 vaccination approval age groups: 0–4, 5–11, 12–15, and 16–21. Race and ethnicity were measured by a free-entry field in the medical record, and more than 1 race and ethnicity were possible; however, race was often not reported for Hispanic/Latino-identified participants.
Individual-level association between SARS-nCoV2 infection and subsequent IBD would give stronger inference than temporal changes in IBD infection. For that aim, we gathered data about past SARS-nCoV2 infection before diagnosis.
Analysis
Due to potentially different seasonal variation and time to presentation, we analyzed Crohn disease (CD) and ulcerative colitis (UC) separately and excluded 29 cases of indeterminate IBD. Using April 2020 as the first month of the SARS-nCoV2 pandemic, we compared pre-pandemic versus pandemic periods with the Pearson chi-square test. For each disease, we also fit simple Poisson regression models for quarterly counts of CD and UC with an indicator for the pandemic that accounted for overdispersion, greater variance than expected in a Poisson distribution.
The quarterly counts of CD and UC appeared to be normal using both visual inspection of a quantile-quantile plot and the Shapiro-Wilk test: CD (P = 0.06) and UC (P = 0.6). We therefore fit an autoregressive integrated moving average model (ARIMA) to the quarterly number of cases of each disease for January 2016–March 2020 and forecast the subsequent 9 quarters (April 2020–June 2022) with 80% and 95% prediction intervals using the forecast library (5). We repeated the ARIMA analysis with monthly data by fitting a model to pre-pandemic data and forecasting the subsequent 27 months (April 2020–June 2022).
RESULTS
Of patients reviewed in this study, 43.1% were female. Median age at diagnosis was 14.0 years (range 2–21 years): 4.3% were ages 0–4 years, 25.6% were ages 5–11, 35.9% were ages 12–15, and 36.3% were 16–21. Race and ethnicity were provided as free-entry fields: 47.4% were White, 9.5% Black, 5.3% Asian, and 8.7% not reported; 22.5% were Hispanic, and 11.8% had Hispanic ethnicity status not reported. Patients diagnosed with IBD during the pandemic were less likely to be White and more likely to be Hispanic; patients did not differ in diagnosis, gender, age, Black or Asian identity, or county of residence from patients diagnosed before the pandemic (Table 1). The distribution of quarterly counts of CD and UC before and during the pandemic did not differ according to a Wilcoxon test (Table 1) or Poisson regression with overdispersion [CD incidence rate ratio (IRR) 1.26 (0.94, 1.68) with dispersion factor of 2.0; UC IRR 1.18 (0.78, 1.76) with dispersion factor of 1.6].
TABLE 1.
Demographics of participants (2016–June 2022)
| All participants | Jan. 2016–Mar. 2020 | April 2020–Jun. 2022 | P value | |
|---|---|---|---|---|
| N | 587 | 351 | 236 | |
| Diagnosis | 0.6 | |||
| Crohn disease (CD) | 399 (68.0%) | 238 (67.8%) | 161 (68.2%) | |
| Ulcerative colitis (UC) | 159 (27.1%) | 98 (27.9%) | 61 (25.8%) | |
| Undetermined | 29 (4.9%) | 15 (4.3%) | 14 (5.9%) | |
| Quarterly CD cases | Mean 15.3 (variance 34.3) | Mean 14.0 (variance 21.4) | Mean 17.7 (variance 54.5) | 0.2 |
| Median 14.5, IQR (11, 17) | Median 13, IQR (11, 16) | Median 16, IQR (14, 21) | ||
| Quarterly UC cases | Mean 6.1 (variance 9.5) | Mean 5.8 (variance 11.3) | Mean 6.8 (variance 6.2) | 0.3 |
| Median 6, IQR (4, 8) | Median 4, IQR (4, 8) | Median 7, IQR (6, 8) | ||
| Documented SARS-nCoV2 infection | 8 (1.4%) | 0 | 8 (3.4%) | n.a. |
| Gender | 0.7 | |||
| Male | 333 (56.7%) | 197 (56.1%) | 136 (57.9%) | |
| Female | 253 (43.1%) | 154 (43.9%) | 99 (41.9%) | |
| Missing | 1 (0.2%) | 0 | 1 (0.4%) | |
| Age at diagnosis | 0.8 | |||
| 0–4 | 15 (4.3%) | 9 (2.6%) | 4 (1.7%) | |
| 5–11 | 150 (25.6%) | 92 (26.2%) | 58 (25.6%) | |
| 12–15 | 211 (35.9%) | 121 (34.5%) | 90 (38.1%) | |
| 16–21 | 213 (36.3%) | 129 (36.8%) | 84 (35.6%) | |
| Institution | 0.01 | |||
| Downstate | 36 (6.1%) | 15 (4.3%) | 21 (8.9%) | |
| Maimonides | 91 (15.5%) | 63 (17.9%) | 28 (11.9%) | |
| Montefiore | 274 (46.7%) | 155 (44.2%) | 119 (50.4%) | |
| NYU | 186 (31.7%) | 118 (33.6%) | 68 (28.8%) | |
| Race and ethnicity | ||||
| White | 278 (47.4%) | 183 (52.1%) | 95 (40.3%) | 0.03 |
| Black | 56 (9.5%) | 30 (8.5%) | 26 (11.0%) | 0.2 |
| Asian | 31 (5.3%) | 14 (4.0%) | 17 (7.2%) | 0.06 |
| Hispanic | 132 (22.5%) | 70 (19.9%) | 62 (26.3%) | 0.02 |
| Race missing | 51 (8.7%) | 22 (6.3%) | 29 (12.3%) | |
| Ethnicity missing | 69 (11.8%) | 31 (8.8%) | 38 (16.1%) | |
| Year of diagnosis | n.a. | |||
| 2016 | 67 (11.4%) | 67 (19.1%) | 0 (0%) | |
| 2017 | 85 (14.5%) | 85 (24.2%) | 0 (0%) | |
| 2018 | 80 (13.6%) | 80 (22.8%) | 0 (0%) | |
| 2019 | 90 (15.3%) | 90 (25.6%) | 0 (0%) | |
| 2020 | 97 (16.5%) | 29 (8.3%) | 68 (28.8%) | |
| 2021 | 102 (17.4%) | 0 (0%) | 102 (43.2%) | |
| 2022 | 66 (11.2%) | 0 (0%) | 66 (11.2%) | |
| Location | 0.3 | |||
| Manhattan | 35 (6.0%) | 20 (5.7%) | 15 (6.4%) | |
| Brooklyn | 230 (39.2%) | 150 (42.7%) | 80 (33.9%) | |
| Queens | 21 (3.6%) | 12 (3.4%) | 9 (3.8%) | |
| Bronx | 172 (29.3%) | 100 (28.5%) | 72 (30.5%) | |
| Staten Island | 12 (2.0%) | 6 (1.7%) | 6 (2.5%) | |
| Westchester | 54 (9.2%) | 28 (8.0%) | 26 (11.0%) | |
| Rockland | 36 (6.1%) | 18 (5.1%) | 18 (7.6%) | |
| Long Island | 12 (2.0%) | 10 (2.8%) | 2 (0.8%) | |
| Other NY or PA or CT or NJ | 5 (0.9%) | 2 (0.6%) | 3 (1.3%) | |
| Not reported | 10 (1.7%) | 5 (1.4%) | 5 (2.1%) |
All percentages are column percentages. Race and ethnicity were reported in a free-entry field; they do not add to 100% because patients could be identified with multiple racial and ethnic categories. P value from chi-square test of association between time period (pre-pandemic vs pandemic) and each variable. The distribution of quarterly counts were compared with a Wilcoxon test; we present the means and variances for reference, but the Wilcoxon test does not compare these.
IQR = interquartile range; CT = Connecticut; NJ = New Jersey; NY = New York; NYU = New York University.
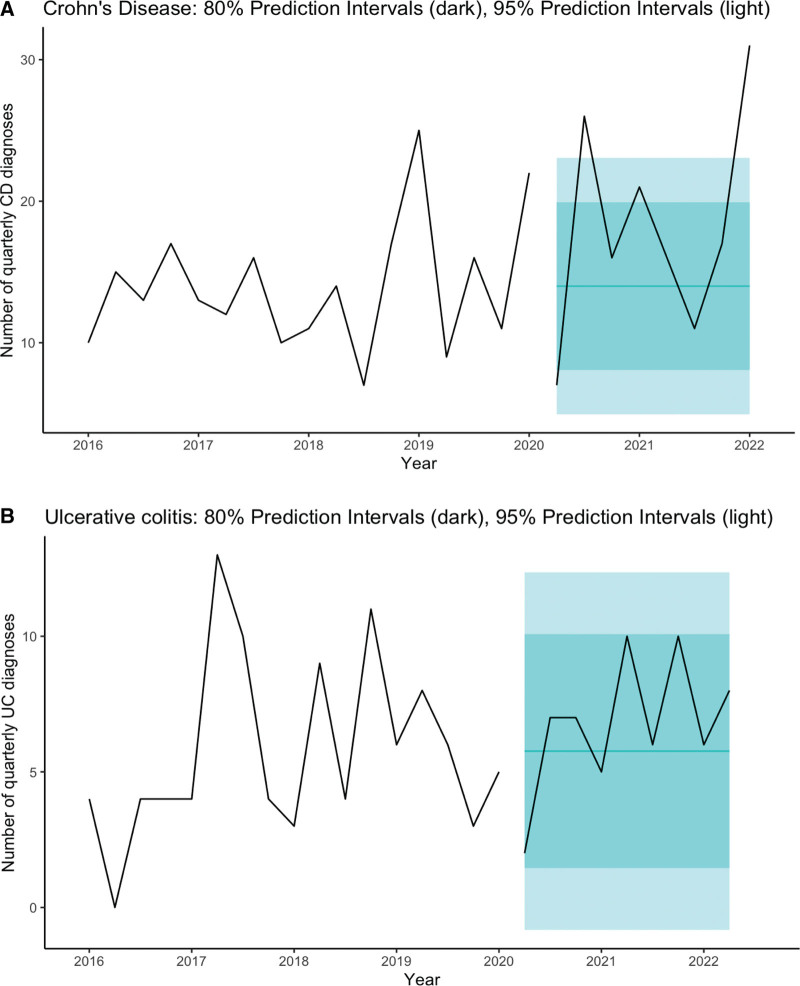
The quarterly pre-pandemic model for UC was ARIMA(0,0,0) (white noise) with a mean of 5.76 cases per quarter (SE = 0.79), residual standard deviation of 11.3; during the pandemic period, the forecast was an average of 5.76 cases per quarter with 80% prediction interval (1.45, 10.1) and 95% prediction interval (−0.83, 12.4) (Fig. 1 and Table 1, Supplemental Digital Content, http://links.lww.com/MPG/D83).
FIGURE 1.
Time series (ARIMA) of UC and CD. ARIMA time series analysis of (A) UC and (B) CD presentation by 3-month period from 2016 to June 2022 with 80% and 95% prediction intervals. CD = Crohn disease; UC = ulcerative colitis.
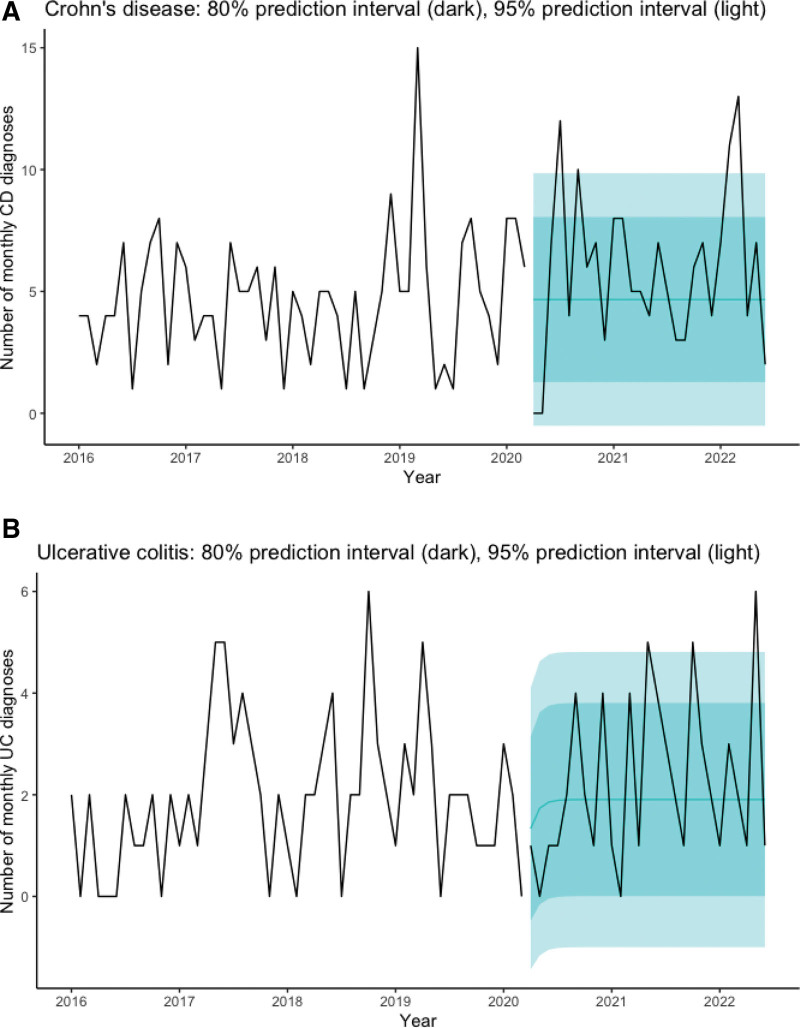
The monthly pre-pandemic model for UC was ARIMA(1,0,0) with a mean of 1.91 cases per month (SE = 0.28), lag-1 autocorrelation (AR1) of 0.30 (SE = 0.13), and residual standard deviation of 2.00. The forecast varied only slightly across the interval. For UC, beginning in August 2020 an average of 1.90 cases were forecast with 80% prediction interval (0.00, 3.80) and 95% prediction interval (−1.00, 4.81). We noted more UC cases than forecast in May 2021 (5 cases), October 2021 (5 cases), and May 2022 (6 cases), compared with upper limit of 95% prediction interval of 4.81 cases (Fig. 2 and Table 1, Supplemental Digital Content, http://links.lww.com/MPG/D83).
FIGURE 2.
Time series (ARIMA) of UC and CD. ARIMA time series analysis of (A) UC and (B) CD presentation by month from 2016 to June 2022 with 80% and 95% prediction intervals. CD = Crohn disease; UC = ulcerative colitis.
Overall, and trend for increased UC diagnoses was noted in the post-SARS-nCoV2 period, exceeding expected increases from the pre-pandemic trend. The pre-pandemic model for UC was ARIMA(1,0,0) with a mean of 1.91 cases per month (SE = 0.28) and lag-1 autocorrelation (AR1) of 0.30 (SE = 0.13). The residual standard deviation is 2.00.
The quarterly pre-pandemic model for CD was ARIMA(0,0,0) (white noise) with a mean of 14.0 cases per quarter (SE = 1.09), residual standard deviation of 21.4; during the pandemic period, the forecast was an average of 14.0 cases per quarter with 80% prediction interval (8.07, 19.93) and 95% prediction interval (4.94, 23.06). We noted more CD cases than forecast in the third quarter of 2020 and first quarter of 2022, which had respectively 26 cases and 31 cases compared with upper limit of 95% prediction interval of 23.0 (Fig. 1 and Table 1, Supplemental Digital Content, http://links.lww.com/MPG/D83).
The monthly pre-pandemic model for CD was ARIMA(0,0,0) (white noise) with mean of 4.67 cases per month (SE = 0.37), and residual standard deviation of 6.99. The forecast was uniform across the interval because the number of CD cases per month were white noise. For CD, an average of 4.67 cases was forecast with 80% prediction interval (1.28, 8.05) and 95% prediction interval (−0.51, 9.85). We noted more CD cases than forecast in July 2020 (12 cases), September 2020 (10 cases), February 2022 (11 cases), and March 2022 (13 cases), compared with upper limit of 95% prediction interval of 9.85 cases (Fig. 2 and Table 1, Supplemental Digital Content, http://links.lww.com/MPG/D83).
One patient had a positive antibody test before diagnosis and 7 patients had positive polymerase chain reactions test before diagnosis. No records indicated negative tests.
DISCUSSION
We observe 2 key findings: first, the substantial decrease in overall rates of viral infection did not decrease CD and UC presentation rates, suggesting either diminished importance of viral infection as a pathogenic trigger of IBD or a strong capacity for SARS-nCoV2 to drive IBD pathogenesis. Second, we observe a significant increase in CD diagnoses with a possible temporal linkage of 4–6 months after pre-Omicron peak SARS-nCoV2 infection in NYC in March/April 2020, as well as peaks in diagnoses at approximately the same timing occurring in the aftermath of the Omicron wave starting November 2021. Notably, the demographic shifts in IBD diagnosis corresponds with SARS-nCoV2 infection demographics: more common in Hispanic populations and less likely in White populations (12). Our finding of a decreased proportion of Caucasian patients and increased proportions of Hispanic patients aligns well with documented SARS-nCoV2 prevalence in the time frame of our study, suggesting a possible linkage. These temporal and demographic associations suggest an effect, but they do not demonstrate a linkage of CD with SARS-nCoV2 due to lack of testing data in the EHRs and multiple confounding effects which could generate an elevation in IBD: delays in care during lockdowns initially, recurrence of other viral infections, and overall delays in presentation for CD diagnosis.
Our study also shows the importance of incorporating SARS-nCoV2 test data and vaccination data into EHRs. We anticipate that SARS-nCoV2 infections were underestimated because children were under-tested during the early pandemic (13), and because children may have been tested through programs that did not submit to the EHR, such as the NYC school-based surveillance testing program, NYC’s testing program run by the public hospital system (NYC Health and Hospital Corporation), urgent care clinics, other health systems, or by home rapid tests. The results of school testing programs are reported to public health authorities, but these results do not come to the EHR except through ad hoc self-report. These problems will be compounded by at-home rapid tests that are not even reported to public health authorities.
Our study provides unique data from a city that experienced a substantial SARS-nCoV2 burden at the start of the pandemic from a population of patients attending pediatric gastroenterology clinics, as well as some of the most profound non-pharmaceutical interventions and decreases in viral illness in the United States. However, our study shows temporal and demographic associations with the pandemic but not individual associations due to lack of testing data in the EHRs. The 4–6 month lag between the city’s peak infection rate and CD could be consistent with a SARS-nCoV2 infection pathogenesis or with delayed presentation from cases during the lockdown period, although later trends suggesting elevation in CD diagnoses at other periods suggest a need for more detailed evaluation at a larger scale. IBD does not present with consistent patterns, resulting in wide variability in data. We also do not have documentation of the timing of SARS-nCoV2 infections, but specific variant lineage may drive different levels of autoimmunity; Multisystem Inflammatory Syndrome in Children incidence was lower when Omicron was the dominant variant compared with earlier variants of concern.
Our data does not include all pediatric gastroenterology groups in NYC, which may be impacted by variations in choice of institution for gastroenterology care. Vaccines were available for over a year for two-thirds of the patients in the cohort with new diagnoses since April 2020, and vaccines were widely available to NYC children and adolescents through mass vaccination sites and widespread vaccine bus visits including all public schools. However, our study is not able to assess the impact of vaccinations due to significant heterogeneity in vaccine documentation. It is challenging to assess the potential impact on data beyond 2020 due to a partial recurrence of viral infections; however, data derived from the New York State Department of Health Influenza-Like-Illness tracker demonstrates a substantial drop over regional baseline which continued through the 2021–2022 season (14).
CONCLUSIONS
Our study suggests two possible interpretations, both with substantial potential impact on our understanding of IBD. One possible interpretation is that viral illness is not substantially related to pathogenesis of IBD, and the data we have captured here reflects solely the historical trend for continued growth in IBD rates. Our analysis strongly demonstrates that the absence of non-SARS-nCoV2 infections did not create any decline in IBD diagnostic rates. However, these findings are also congruent with an alternative interpretation, that viral illness remains a risk factor in IBD pathogenesis, and SARS-nCoV2 is particularly capable of engendering the autoimmune response leading to IBD, and in particular CD. This interpretation is consistent with prior findings that SARS-nCoV2 increases risk of type 1 diabetes. Further study is needed with improved data infrastructure to incorporate SARS-nCoV2 test results and vaccine records, enabling monitoring of ongoing changes in IBD incidence as new viral lineages emerge and during future pandemics.
Acknowledgments
We would like to acknowledge the contributions of the following individuals, Dalia Arostegui MD, Chika Oragui MD, Agata Mann MD, Jon Dooley MD, and Dr Melanie Greifer MD who assisted in data collection, and Steven Schwarz MD who assisted in building our data gathering collaboration.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Gottesman BL, Yu J, Tanaka C, et al. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr 2022;176:414–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saad MA, Alfishawy M, Nassar M, Mohamed M, Esene IN, Elbendary A. COVID-19 and autoimmune diseases: a systematic review of reported cases. Curr Rheumatol Rev 2021;17:193–204. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years – United States, March 1, 2020–June 28, 2021. MMWR Morb Mortal Wkly Rep 2022;71:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev 2020;19:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydin MF, Taşdemir H. Ulcerative Colitis in a COVID-19 Patient: A Case Report. Turk J Gastroenterol 2021;32:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arostegui D, Castro K, Schwarz S, et al. Persistent SARS-CoV-2 nucleocapsid protein presence in the intestinal epithelium of a pediatric patient 3 months after acute infection. JPGN Rep 2022;3:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs JJL. Persistent SARS-2 infections contribute to long COVID-19. Med Hypotheses 2021;149:110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalkeri R, Goebel S, Sharma GD. SARS-CoV-2 shedding from asymptomatic patients: contribution of potential extrapulmonary tissue reservoirs. Am J Trop Med Hyg 2020;103:18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yusuf F, Fahriani M, Mamada SS, et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Res 2021;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opin Gastroenterol 2011;27:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill 2020;25:2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Yuan F. Historical redlining and resident exposure to COVID-19: a study of New York City. Race Soc Probl 2022;14:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peaper DR, Murdzek C, Oliveira CR, Murray TS. Severe acute respiratory syndrome coronavirus 2 testing in children in a large regional US health system during the coronavirus disease 2019 pandemic. Pediatr Infect Dis J 2021;40:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Health New York State. Weekly Influenza Surveillance Report – June 25th, 2022. 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.