Abstract
Objective:
To study whether delivering definitive radiotherapy to sites of oligoprogression in metastatic renal cell carcinoma enabled deferral of systemic therapy changes without compromising disease control or survival.
Materials/Methods:
We identified patients with metastatic renal cell carcinoma who received radiotherapy to ≤3 sites of extracranial progressive disease in 2014–2019 at a large tertiary cancer center. Inclusion criteria were (1) controlled disease for ≥3 months before oligoprogression, (2) all oligoprogression sites treated with a biologically effective dose of ≥100 Gy, and (3) availability of follow-up imaging. Time-to-event endpoints were calculated from the start of radiotherapy.
Results:
Seventy-two patients were identified (median follow-up time 22 months, 95% confidence interval [CI] 19–32 months), with oligoprogressive lesions in lung/mediastinum (n=35), spine (n=30), and non-spine bone (n=5). The most common systemic therapies before oligoprogression were none (n=33), tyrosine kinase inhibitor (n=23), and immunotherapy (n=13). At 1 year, the local control rate was 96% (95% CI 87%–99%); progression free survival, 52% (95% CI 40%–63%); and overall survival, 91% (95% CI 82%–96%). At oligoprogression, systemic therapy was escalated (n=16), maintained (n=49), or discontinued (n=7), with corresponding median progression free survival intervals of 19.7 months (95% CI 8.2–27.2 months), 10.1 months (95% CI 6.9–13.2 months), and 9.8 months (95% CI 2.4–28.9 months). Of the 49 patients maintained on the same systemic therapy at oligoprogression, 21 did not subsequently have systemic therapy escalation.
Conclusion:
Patients with oligoprogressive metastatic renal cell carcinoma treated with radiotherapy had comparable progression free survival regardless of systemic therapy strategy, suggesting that radiotherapy may be a viable approach for delaying systemic therapy escalation. Randomized controlled trials comparing treatment of oligoprogression with radiotherapy vs. systemic therapy alone are needed.
Keywords: metastatic renal cell carcinoma, oligoprogression, systemic therapy, stereotactic body radiotherapy
INTRODUCTION
Each year approximately 400,000 patients are diagnosed with renal cell carcinoma (RCC) worldwide, of whom approximately one-third will eventually develop metastatic disease.1,2 The mainstay of treatment for metastatic renal cell carcinoma (mRCC) is systemic therapy (ST),3 the options for which have expanded considerably over the past 15 years to include small-molecule tyrosine kinase inhibitors (TKIs) of vascular endothelial growth factor receptors (VEGFR; axitinib, sunitinib, pazopanib, cabozantinib and lenvatinib), small-molecule inhibitors of mammalian target of rapamycin (mTOR, everolimus and temsirolimus), and monoclonal antibodies of PD1 (nivolumab, pembrolizumab), PDL1 (avelumab), and CTLA4 (ipilimumab).4 Metastatic disease often responds well to these systemic therapies, resulting in improved disease control and outcomes.
Despite the growing availability of novel systemic agents for mRCC, complete and durable responses are uncommon, and one or more metastatic lesions inevitably develop resistance to therapy.5 Isolated sites of progression can develop in otherwise controlled disease, a phenomenon called oligoprogression.6,7 Oligoprogressive disease is of particular concern in mRCC because of its significant intra- and intertumoral heterogeneity, for which use of systemic therapies may lead to the selection of resistant clones.8 For disease progression, escalation of systemic therapy (STE) to the next line of treatment is a major strategy, but it can be associated with considerable decreases in quality of life and increases in financial costs.9,10
Interest in metastasis-directed therapy for progressive lesions is growing as a strategy to improve disease control and defer STE.11 Unlike targeted agents, local therapies such as radiotherapy (RT) can target resistant clonal populations without regard to mutation profile or burden. In this context, we sought to characterize outcomes and toxicities of mRCC patients who received metastasis-directed RT, including local control, distant control, and latency to STE.
MATERIALS AND METHODS
Patient selection
This review of medical records (protocol 2020–0065) was approved by the institutional review board, with the need for informed patient consent waived. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and its later amendments.12 We used the Oncora platform (Oncora Medical, Philadelphia, PA) to identify all patients aged ≥18 years with mRCC who received metastasis-directed RT at a single tertiary cancer care center from 2014 through 2019. All patients must have received RT to all oligoprogressive disease sites, defined as 1–3 progressing or new extracranial lesions; patients with progressing intracranial lesions at the time of oligoprogression were excluded given that they were likely to have management and outcomes distinct from those with extracranial progression alone. Lesion progression was evaluated with the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) as an increase of the sum of the longest diameters of the target lesions by ≥20%, an increase of ≥5 mm along any single dimension of the target lesions, or the development of a new lesion measuring ≥1 cm in long axis for non-nodes and ≥1.5 cm for lymph nodes as measured on computed tomography (CT) or magnetic resonance imaging (MRI).13 All images were reviewed retrospectively by a board-certified radiologist to identify oligoprogressive lesions according to RECIST v1.1. Other inclusion criteria were (1) disease control outside the oligoprogressive sites for ≥3 months before oligoprogression; (2) receipt of RT to the sites of oligoprogression to a biologically effective dose (BED) of ≥100 Gy; and (3) having follow-up imaging available for review. For calculations of BED, an α/β ratio of 2.63 was assumed for RCC.14
Data collection and analysis
Data regarding demographics, tumor histology, sites of progression, lesion dimensions, RT dose and fractionation, systemic therapies, disease control, toxicity, and survival were extracted and recorded. The International Metastatic RCC Database Consortium (IMDC) risk score was used as the basis for calculation of a modified IMDC score, in which 1 point is assigned for intervals of <1 year from diagnosis to ST or RT.15,16
Median follow-up time was calculated by using the reverse Kaplan-Meier method. Assessments of local control (LC), progression-free survival (PFS), overall survival (OS), and freedom from subsequent escalation (FFSE) were performed on a per-patient basis indexing to the first RT course for the oligoprogression. All endpoints were calculated from the start of RT. The Kaplan-Meier method and life tables were used for time-to-event analyses. The ST strategy was compared across three time points: before oligoprogression, just after RT for oligoprogression, and at the patient’s next progression event. STE was defined as beginning ST after observation or a change from one ST to another. Univariable analyses were conducted with Cox proportional hazards. Multivariable analysis was not performed owing to small sample sizes. Statistical analyses were done with Stata Version 13.0 (StataCorp, College Station, TX, USA) and JMP Version 14.0 (SAS Institute, Cary, NC, USA).
RESULTS
Overview of clinical outcomes
Of 90 patients with clinically presumed oligoprogressive mRCC, 72 patients (80%) with 87 oligoprogressive lesions were retrospectively identified by a radiologist to meet selection criteria. All progressive lesions were treated definitive-intent RT between 2014 through 2019. Patient, disease, and treatment characteristics for the whole cohort and stratified by systemic therapy strategy are summarized in Table 1. Most of the patients were male, had clear cell histology, and had oligoprogression in the lung/mediastinum or vertebral spine at a median 41 months after initial diagnosis (interquartile range [IQR] 22–94 months). The modified IMDC score for 66 patients was 0–2, representing favorable- to intermediate-risk disease. At the time of oligoprogression, 33 patients had not had ST and the rest were receiving mostly VEGFR-TKI monotherapy or immune checkpoint therapy (Supplementary Table S1). The RT for oligoprogressive disease was delivered to 1 lesion in 59 patients, 2 lesions in 11 patients, and 3 lesions in 2 patients. The median dose was 50 Gy (IQR 24–53 Gy) delivered in a median 4 fractions (IQR 1–10 fractions), and the median BED2.63 was 243 Gy (IQR, 200–264 Gy).
Table 1.
Patient, tumor, and treatment characteristics of patients treated with metastasis-directed radiotherapy for oligoprogressive metastatic renal cell carcinoma
| Systemic therapy strategy | ||||
|---|---|---|---|---|
| Attribute | All patients | Discontinued | Maintained | Escalated |
| Number of patients treated | 72 | 7 | 49 | 16 |
| Number of lesions treated | 87 | 11 | 57 | 19 |
| Median age at treatment, years (quartiles) | 67 (60–73) | 73 (69–77) | 66 (61–73) | 65 (54–71) |
| Sex | ||||
| Male | 55 (76%) | 3 (43%) | 41 (84%) | 11 (69%) |
| Female | 17 (24%) | 4 (57%) | 8 (16%) | 5 (31%) |
| Histology | ||||
| Clear cell | 68 (94%) | 7 (100%) | 45 (92%) | 16 (100%) |
| Chromophobe | 2 (3%) | - | 2 (4%) | - |
| Papillary | 1 (1%) | - | 1 (2%) | - |
| Unclassified | 1 (1%) | - | 1 (2%) | - |
| Site of oligoprogression | ||||
| Lung/mediastinum | 35 (49%) | 6 (86%) | 28 (57%) | 1 (6%) |
| Spine | 30 (42%) | 1 (14%) | 16 (33%) | 13 (81%) |
| Non-spine bone | 5 (7%) | - | 4 (8%) | 1 (6%) |
| Other | 2 (3%) | - | 1 (2%) | 1 (6%) |
| Median interval from initial diagnosis to OP (quartiles), months | 41 (22–94) | 26 (6–61) | 45 (22–101) | 38 (26–58) |
| Median largest dimension of lesion at OP (quartiles), cm | 2.7 (1.7–3.7) | 2.5 (2.1–4.3) | 2.4 (1.6–3.3) | 3.6 (2.5–4.7) |
| Modified IMDC score at time of OP (n=71) | ||||
| 0 | 15 (21%) | 2 (29%) | 12 (24%) | 1 (7%) |
| 1 | 35 (49%) | 2 (29%) | 22 (45%) | 11 (73%) |
| 2 | 16 (23%) | 2 (29%) | 12 (24%) | 2 (13%) |
| 3 | 5 (7%) | 1 (14%) | 3 (6%) | 1 (7%) |
| Systemic therapy before OP | ||||
| None | 33 (46%) | - | 23 (47%) | 10 (63%) |
| TKI monotherapy | 23 (32%) | 6 (86%) | 13 (27%) | 4 (25%) |
| Immunotherapy | 13 (18%) | 1 (14%) | 10 (20%) | 2 (13%) |
| Immunotherapy/TKI combination | 2 (3%) | - | 2 (4%) | - |
| Other | 1 (1%) | - | 1 (2%) | - |
| Median dose, Gy (quartiles) | 50 (24–53) | 50 (50–54) | 50 (24–53) | 24 (24–27) |
| Median BED 2.63 , Gy (quartiles) | 243 (200–264) | 288 (122–288) | 243 (200–288) | 243 (222–243) |
| Median number of fractions (quartiles) | 4 (1–10) | 4 (1–10) | 4 (3–10) | 1 (1–3) |
| Systemic therapy strategy at OP | ||||
| Discontinuation | 7 (10%) | 7 (100%) | - | - |
| Maintenance | 49 (67%) | - | 49 (100%) | - |
| Escalation | 16 (22%) | - | - | 16 (100%) |
| Therapy switch patterns at OP (n=23) | ||||
| TKI → TKI | 2 (9%) | - | - | 2 (13%) |
| TKI → Immunotherapy | 2 (9%) | - | - | 2 (13%) |
| Immunotherapy → TKI | 2 (9%) | - | - | 2 (13%) |
| None → TKI | 10 (43%) | - | - | 10 (63%) |
| TKI → None | 6 (26%) | 6 (86%) | - | - |
| Immunotherapy → None | 1 (4%) | 1 (14%) | - | - |
| Median time to next therapy, months | 6.9 | 5.8 | 6.1 | 11.1 |
Abbreviations: OP, oligoprogression; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; TKI, tyrosine kinase inhibitor; BED, biologically effective dose; several percentages do not sum to 100% due to rounding
The median follow-up time for the full cohort was 21.7 months (95% confidence interval [CI] 18.7–32.3). The median follow-up time among the 56 patients who were alive at last follow up was 19.1 months (95% CI, 17–23.2). The median OS and PFS times after initiation of RT for oligoprogression were 60.8 months (95% CI 41.5–undefined) and 12.1 months (95% CI 9.0–15.8). The 1-year OS and PFS probabilities were 91% (95% CI 82%–96%) and 52% (95% CI 40%–63%). The median FFSE time after oligoprogression was 18.2 months (95% CI 11.1–40.7 months) and the 1-year FFSE probability was 62% (95% CI 49%–72%). Among patients who had a change in systemic therapy after oligoprogression, the median time to next therapy was 6.9 months. Seven patients had local failures (i.e., at the treated oligoprogression site) at a median of 14.7 months, of whom 4 had local failure as the first site of subsequent progression. The 1-year LC probability was 96% (95% CI 87%–99%).
Stratified outcomes analyses
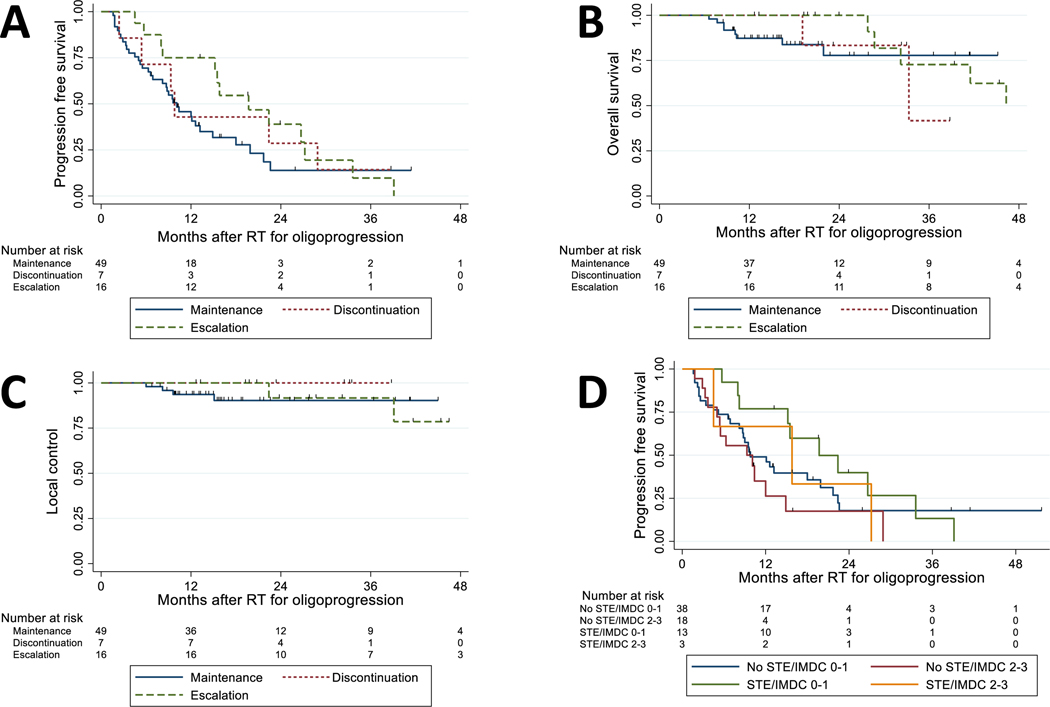
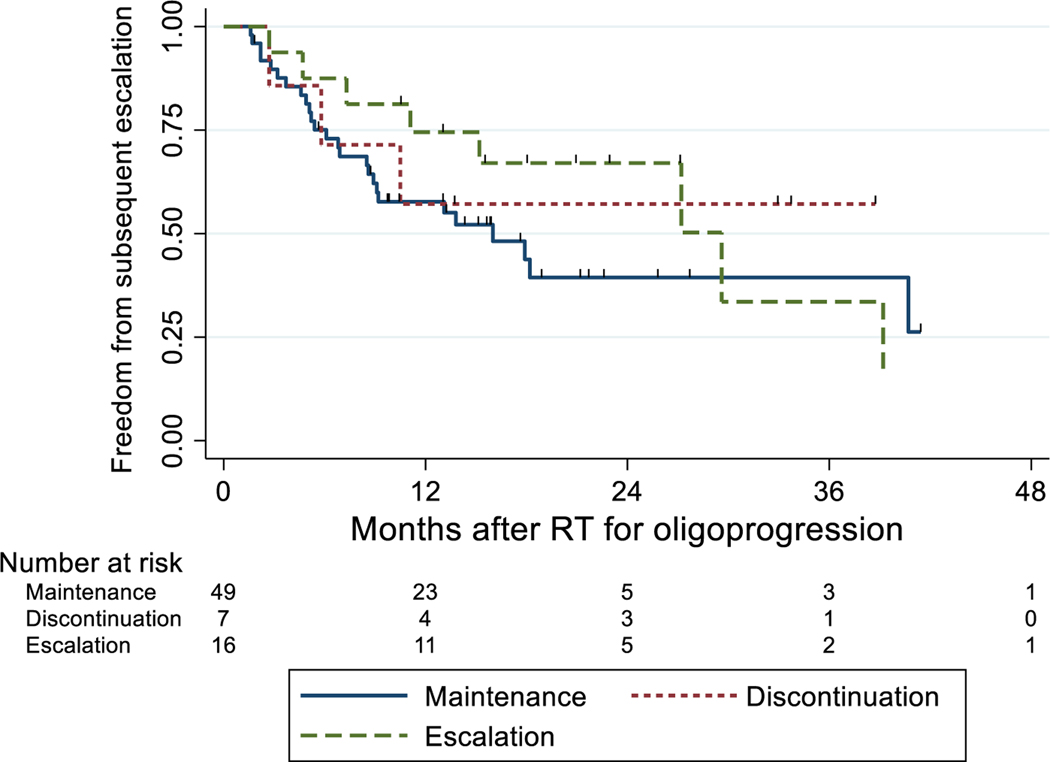
At the time of oligoprogression, 7 patients discontinued ST altogether, 49 patients maintained the same ST, and 16 patients escalated ST to another agent. Among 23 patients who did not maintain the same ST after oligoprogression, the most common switch patterns were from no ST to TKI in 10 patients and from TKI to no ST in 6 patients. On survival analysis, median PFS intervals after RT for the discontinued ST, maintained ST, and escalated ST groups were 9.8 months (95% CI 2.4–28.9 months), 10.1 months (95% CI 6.9–13.2 months), and 19.7 months (95% CI 8.2–27.2 months), respectively. Median OS intervals after RT for ST discontinuation and ST escalation were 33.3 months (95% CI 19.1 months–could not be estimated) and 46.3 months (95% CI 28.7 months–could not be estimated), whereas the median survival time for those who were on ST maintenance had not been reached at last follow-up. Survival probability curves for PFS, OS, and LC are displayed in Figure 1A–C, and PFS probability curves stratified by STE (yes vs. no) and by IMDC score (0–1 vs. 2–3) is shown in Figure 1D. The survival probability curves for FFSE are shown in Figure 2. At 1 year, the estimated probability of FFSE was 57% (95% CI 17%–84%) for ST discontinuation, 58% (95% CI 43%–71%) for ST maintenance, and 75% (95% CI 46%–90%) for ST escalation at the time of oligoprogression.
Figure 1.
(A) Progression free survival stratified by systemic therapy strategy at time of oligoprogression. (B) Overall survival stratified by systemic therapy strategy at time of oligoprogression. (C) Local control stratified by systemic therapy strategy at time of oligoprogression. (D) Progression free survival stratified by systemic therapy strategy and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score after oligoprogression for metastatic renal cell carcinoma. Abbreviations: RT, radiation therapy; STE, escalation of systemic therapy.
Figure 2.
Freedom from subsequent escalation stratified by systemic therapy strategy at time of oligoprogression. RT, radiation therapy.
The results of univariable Cox regression of factors associated with PFS and those associated with OS are shown in Table 2. One factor associated with a higher risk of death included being on a TKI at the time of oligoprogression (HR 1.48, 95% CI 1.16–11.82, P=0.028). One factor associated with a lower risk of death was not being on ST at the time of oligoprogression (HR 0.70, 95% CI 0.10–0.98, P=0.047).
Table 2.
Univariable analysis of factors associated with progression and overall survival after radiotherapy for oligoprogression.
| For Progression | For Death | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value |
| Age ≥ 65 at treatment | 0.85 | 0.50–1.47 | 0.563 | 2.64 | 0.83–8.34 | 0.099 |
| Male sex | 1.04 | 0.55–1.95 | 0.908 | 0.56 | 0.19–1.64 | 0.290 |
| Site of OP | ||||||
| Spine | (Reference) | (Reference) | ||||
| Lung/mediastinum | 0.78 | 0.44–1.38 | 0.393 | 0.76 | 0.25–2.31 | 0.627 |
| Bone | 0.96 | 0.29–3.18 | 0.944 | 1.48 | 0.18–12.52 | 0.717 |
| Other | 2.84 | 0.65–12.39 | 0.165 | 0.95 | 0.11–8.35 | 0.963 |
| Histology | ||||||
| Clear cell | (Reference) | (Reference) | ||||
| Other | 1.80 | 0.55–5.89 | 0.332 | Undefined | ||
| Time from initial diagnosis to OP | ||||||
| < 48 months | (Reference) | (Reference) | ||||
| ≥ 48 months | 0.70 | 0.37–1.30 | 0.255 | 0.53 | 0.15–1.88 | 0.327 |
| Number of sites treated | ||||||
| 1 | (Reference) | (Reference) | ||||
| >1 | 0.76 | 0.36–1.62 | 0.477 | 1.01 | 0.23–4.55 | 0.985 |
| Greatest dimension of lesion treated | ||||||
| ≤3 cm | (Reference) | (Reference) | ||||
| >3 cm | 0.98 | 0.57–1.69 | 0.938 | 0.87 | 0.32–2.41 | 0.791 |
| BED2.63 ≥ 250 | 0.62 | 0.35–1.12 | 0.114 | 0.72 | 0.25–2.09 | 0.543 |
| Systemic agent at time of OP | ||||||
| None | (Reference) | (Reference) | ||||
| TKI | 1.48 | 0.80–2.71 | 0.209 | 3.70 | 1.16–11.82 | 0.028* |
| Immunotherapy | 1.48 | 0.70–3.16 | 0.306 | 2.12 | 0.38–11.96 | 0.393 |
| TKI + immunotherapy | 1.50 | 0.35–6.46 | 0.583 | Undefined | ||
| No systemic therapy at time of OP | 0.70 | 0.40–1.20 | 0.195 | 0.31 | 0.10–0.98 | 0.047* |
| On immunotherapy at time of OP | 1.27 | 0.67–2.43 | 0.462 | 0.90 | 0.20–4.08 | 0.891 |
| Modified IMDC score at OP | ||||||
| 0–1 | (Reference) | (Reference) | ||||
| 2–3 | 1.50 | 0.83–2.69 | 0.176 | 1.10 | 0.38–3.16 | 0.866 |
| Therapy switch strategy | ||||||
| Maintenance | (Reference) | (Reference) | ||||
| Discontinuation | 0.73 | 0.30–1.77 | 0.486 | 1.22 | 0.25–5.93 | 0.802 |
| Escalation | 0.62 | 0.32–1.19 | 0.150 | 1.02 | 0.34–3.07 | 0.969 |
BED2.63, biologically effective dose assuming α/β ratio of 2.63; CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; OP, oligoprogression; TKI, tyrosine kinase inhibitor.
Significant at 5% level
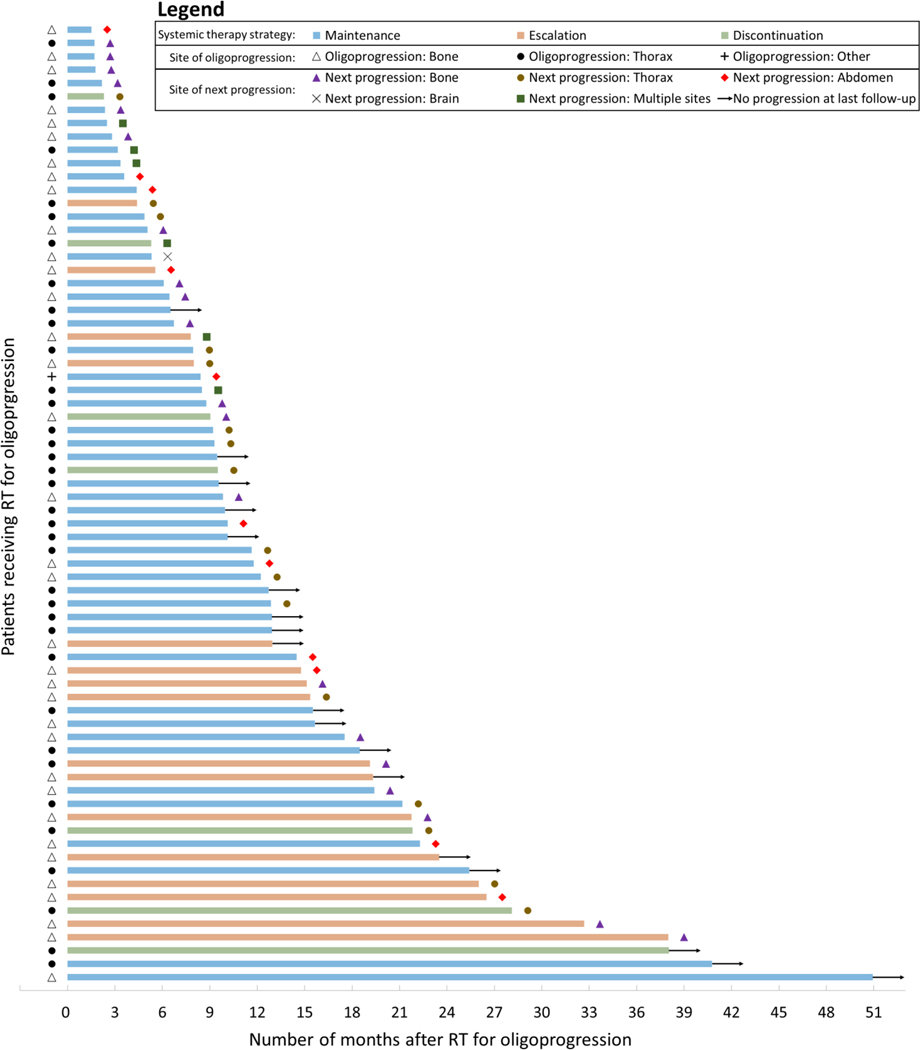
Subsequent progression and treatment
After oligoprogression, 41 patients had a change in ST (37 escalated and 4 discontinued), and 31 maintained ST. The reasons for a subsequent change in ST were progression (n=37), side effects (n=2), enrollment on a new clinical trial (n=1), and patient preference (n=1). At the most recent follow-up, a cumulative 45 patients had escalation of ST at oligoprogression or at any time thereafter. Of 49 patients who were maintained on the same or no systemic agent at the time of oligoprogression, 21 did not subsequently have escalation of ST at median follow-up time of 15.7 months (95% CI 9.6–41.3). Of the 21 patients who did not have any ST changes at oligoprogression or subsequent follow-up, the median OS time was not reached, and the 1-year OS probability was 95% (95% CI 71%–99%).
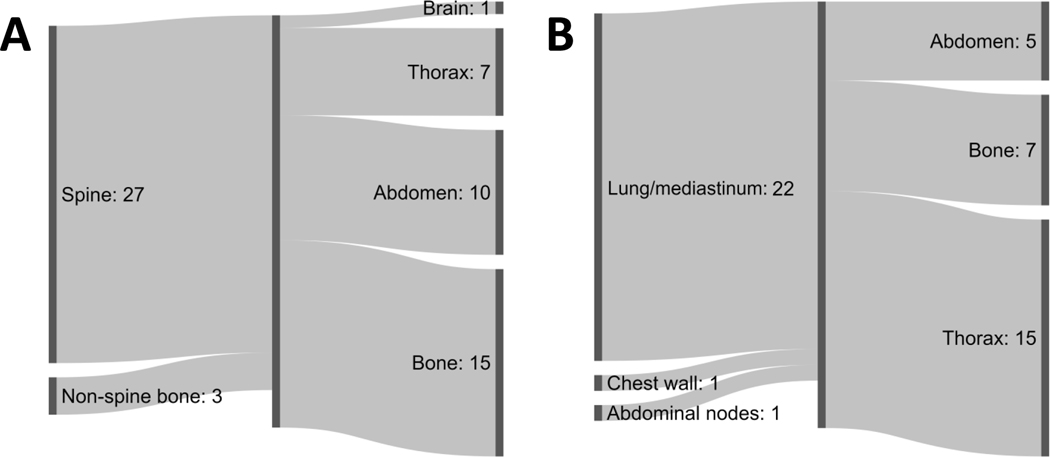
Descriptive analyses of the site of next progression after treatment of oligoprogression are shown in Figure 3. Of 30 patients treated for spine or non-spine bone oligoprogression sites who subsequently had disease progression, the most common site of next progression was at another osseous site (n=15, 45% of 33 total sites). Of 24 patients treated for non-bone OP lesions (22 lung/mediastinum, 1 chest wall, 1 abdominal nodes), the most common site of next progression was the thorax (n=15, 56% of 27 total sites). Only 1 patient treated for bone oligoprogressive lesions and no patients treated for non-bone oligoprogressive lesions developed brain lesion(s) as the next site of progression. Two patients treated for spine lesions and two patients treated for lung/mediastinum lesions developed local failure as the site of next progression. A swimmer plot of sites of oligoprogression and next progression stratified by ST strategy is shown in Figure 4.
Figure 3.
Sites of next progression stratified by primary site of treated oligoprogression: (A) Bone site or (B) non-bone site. Progression occurred at multiple sites in several cases, which accounts for the unbalanced sums when comparing totals from left to right.
Figure 4.
Patient-level outcomes for 72 patients receiving radiotherapy for oligoprogression of metastatic renal cell carcinoma. The latency between radiotherapy and outcome, either progression or no progression at last follow-up, is represented by the length of each bar shown.
Toxicity
Acute and chronic toxicities associated with radiation treatment are listed in Supplementary Table S2. No patients experienced grade 3 or higher acute toxicities. The most common acute toxicities were pain at the treatment site (n=11), pneumonitis (n=5), and nausea (n=5). The most common chronic toxicities were pain (n=7), insufficiency fracture (n=7), and pneumonitis (n=6). Grade 3 chronic toxicities were uncommon and included pain (n=2), brachial plexopathy (n=2), and fibrosis (n=1). No patients experienced grade 4 or 5 chronic toxicities at last follow up.
DISCUSSION
The advent of several classes of systemic agents for the treatment of mRCC has led to partial or complete responses, as measured by RECIST v1.1, in most cases.17 Nonetheless, the deferral of initiation or escalation of ST for mRCC may confer benefits to patients with regard to cost, quality of life, and the ability to reserve potentially effective therapies for later stages of disease. Rising out-of-pocket costs for ST in mRCC have been shown to be a significant barrier to medication access and compliance with therapy.18 Recent studies have also shown that most patients receiving ST for mRCC experience bothersome side effects and immune checkpoint inhibitors in particular are associated with severe immune-related adverse events in up to 40% of patients.19,20 Active surveillance with deferred initiation of ST has been shown to confer superior quality of life without affecting mRCC-specific survival for patients with low-burden or indolent disease.21 However, it remains unclear whether using metastasis-directed therapy for oligoprogressive lesions is a conduit to deferral of ST or a change without compromise of durable disease control. In the current study, we found that patients who did not undergo STE had similar LC, PFS, and OS as did those who did have STE, suggesting that maintenance of ST may be an appropriate strategy for patients receiving metastasis-directed therapy to sites of oligoprogression.
Data regarding local therapy for oligoprogressive mRCC are limited and are summarized in Supplementary Table S3. A key conclusion of analyses to date, including the present study, is that the use of metastasis-directed RT may enable deferral of the introduction of ST or escalation to the next line of therapy. For instance, one study by the Groupe d’Etude des Tumeurs Uro-Génitales (GETUG) examined 188 patients with either oligometastatic or oligoprogressive disease, defined as ≤5 lesions. Among 101 patients with oligoprogressive mRCC (143 lesions treated with RT), median local recurrence-free survival time was 19.3 months, time to initiation of ST was 10.5 months, PFS time was 8.6 months, and OS time was 23.2 months. However, the RT doses used were considerably lower (mean BED3 = 78 Gy, range, 28–276 Gy) than in the present study, suggesting that a significant proportion of patients were treated with palliative doses. The influence of ST strategy also was not explicitly explored in that analysis.11 Another multi-institutional retrospective study analyzed 55 patients with oligoprogressive mRCC treated with ablative techniques (n=5), surgery (n=25), or RT (n=25). Among patients who maintained the same agent vs. changed agents, no difference was found in PFS time (15 vs. 7 months, P=0.207), but OS (39 vs. 11 months, P=0.014) favored patients who maintained a systemic agent. Another retrospective analysis of 51 patients receiving RT for sites of oligoprogressive mRCC found median PFS and OS to be 8.6 and 30.5 months, respectively, with 2-year PFS of 12%.22 Finally, findings from a single-arm phase II trial examining the role of stereotactic RT for oligoprogression in patients with mRCC receiving TKI therapy (NCT02019576) were recently published in abstract form. Patients were maintained on the same TKI, and had a median PFS time of 9.6 months, a 2-year LC rate of 96%, and a 2-year OS rate of 77%. The cumulative incidence of ST change was 47% at 1 year and 75% at 2 years.23,24 Current trials at Sunnybrook, UT Southwestern, and the Centre Francois Baclesse in conjunction with GETUG are ongoing.25–27
Metastasis-directed therapy for oligoprogressive disease, either with surgery, stereotactic RT, or radiofrequency ablation, has shown early promise for several types of primary cancer.28 One single-institution review of patients with solid tumors receiving immune checkpoint therapy found that oligoprogression was noted in 4% of all patients, and 16% of patients achieved at least stable disease on immune checkpoint therapy. Of patients who were maintained on immune-checkpoint therapy and received local therapy, the PFS interval was 15 months.29 In other studies of patients with non-small cell lung cancer with mutations in epidermal growth factor receptor (EGFR) and/or anaplastic lymphoma kinase (ALK) who were maintained on the same TKI at the time of oligoprogression, metastasis-directed therapy was shown in several retrospective series to confer LC rates ranging from 48%−86% at 12 months and median PFS intervals of 6–14 months.23,30–33 Several retrospective series of metastasis-directed therapy for oligoprogressive castrate-resistant prostate cancer treated with abiraterone or enzalutamide showed median PFS intervals ranging from 7 to 11 months.34–36 Other small series of oligoprogressive disease in head & neck, skin, colorectal, urothelial, pleural, soft tissue, and gynecologic malignancies have also shown the potential of metastasis-directed therapy for prolonging PFS and deferring changes in ST.37–44 Although existing evidence is largely limited to retrospective analyses at this time, several prospective randomized trials aimed at addressing the utility of metastasis-directed therapy for patients with lung, breast, and prostate cancers are ongoing.45–48 Interest has also been expressed in the interactions between RT and immune-checkpoint therapy for mRCC and other types of cancer.49
The location of the metastases in mRCC has implications for prognosis and differences in response to treatment.50,51 Molecular analyses in mRCC have suggested that metastatic spread has distinct, constrained routes.52 However, it remains unclear if the location of oligoprogressive disease can predict site of subsequent progression and influence the optimal selection of local or ST. In the current study, we found that bone metastases most commonly had subsequent progression at osseous sites, whereas non-bone metastases—predominantly in lung/mediastinal locations—most often had subsequent progression in the thorax. Information on patterns of progression in mRCC has been limited. Data from the current study suggest that for patients with treated oligoprogressive disease in bone, a bone scan and CT of the chest and abdomen may be more informative than MRI at routine follow-up visits. The influence of local therapy, ST, and sites of disease in determining patterns of subsequent progression and prognosis warrants further investigation.
This study had some limitations. We used an inclusion threshold of ≥ 3 months of systemic disease control, which may have overestimated progression in the absence of serial imaging studies to confirm lesion growth. However, it should be noted that a majority of patients were selected for inclusion by review of serial imaging over longer durations. Systemic therapy selection was non-random and may have reflected clinician assessment of disease status, individual clinician preference for or against specific agents, and/or anticipated tolerance of patients for side effects of therapy. It is possible that patients chosen for STE represent a healthier subgroup more likely to tolerate side effects, which would overestimate the true time to progression associated with STE. Additionally, subgroup sample sizes were small resulting in large confidence intervals for time-to-event endpoints and hazard ratios on Cox analysis. Also, although STE may be a clinically meaningful endpoint, it is subject to bias and heterogeneity between practitioners and patients. Furthermore, our findings also suggest that RT may have limited applicability for oligoprogressive mRCC given that the vast majority of the patients studied were referred for bone, lung, or nodal metastases and generally not for intra-abdominal (liver, kidney, or pancreatic) metastases. Taken together, these limitations limit our ability to perform a causal analysis aimed at directly guiding patient selection, treatment selection, and treatment sequencing in a clinical setting. Nonetheless, the current study demonstrates that treatment of oligoprogression lesions with metastasis-directed RT is associated with excellent LC, a median OS time of 61 months, and a favorable toxicity profile. Prospective trials examining ablative RT for sites of oligoprogression are warranted and may provide valuable insight into disease control, survival, and quality of life after such therapy.
Supplementary Material
Acknowledgments:
The authors would like to thank Christine Wogan from the Department of Radiation Oncology Research for reviewing the manuscript.
Funding statement:
This work was supported in part by Cancer Center Support (Core) grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center. Chad Tang is a Sabin Scholar and is supported by a Cancer Prevention & Research Institute of Texas early clinical investigator award and individual investigator award. Pavlos Msaouel is supported by a Young Investigator Award by the Kidney Cancer Association, a Career Development Award by the American Society of Clinical Oncology, by a Concept Award by the United States Department of Defense, and by the MD Anderson Khalifa Scholar award.
Footnotes
Conflicts of interest: BD reports honoraria from Sermo, Inc. PM reports research funding from Mirati Therapeutics, Bristol-Myers Squibb, Takeda Pharmaceutical Company, UT MD Anderson Cancer Center; consultancy/advisory board participation with Mirati Therapeutics, Bristol-Myers Squibb, Exelixis, Axiom Healthcare Strategies; non-branded educational programs with Exelixis, Pfizer. JL reports research funding from Bristol Myers Squibb, Medtronic. NMT reports consulting/advisory relationships with Bristol-Myers-Squibb, Pfizer, Nektar Therapeutics, Exelisis, Inc, Eisai Medical Research, Eli Lilly, Oncorena, Ono Pharmaceutical, Calithera Bioscience, Surface Oncology, Novartis, Ipsen; research funding from Bristol-Myers-Squibb, Pfizer, Nektar Therapeutics, Exelisis, Inc, Calithera Bioscience, Mirati Therapeutics, Epizyme Inc., Arrowhead Pharmaceuticals, Inc.; serving on the scientific advisory committees of Nektar Therapeutics, Pfizer, Oncorena, Eli Lilly, and Eisai Medical Research. AJZ reports consulting/advisory roles for AstraZeneca, Bayer; research funding to his institution from Infinity Pharma; honoraria from Pfizer, Janssen. JAK reports consulting/advisory board/honoraria from Pfizer, Merck, Roche/Genentech; institutional research funding from Roche/Genentech, Mirati, Merck, Elypta; stock ownership in Allogene, MedTek, ROM Technologies. CT reports consulting fees from Reflexion. All other authors have no disclosures. All reported conflicts are unrelated to the current work.
Data availability statement:
The data that support the findings of this study are available from the corresponding author, CT, upon reasonable request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Campbell SC, Cho HY, et al. The Epidemiology of Renal Cell Carcinoma. European Urology 2011;60:615–21. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Cancer. NCCN Clinical Practice Guidelines in Oncology 2020. [Google Scholar]
- 4.Adashek JJ, Genovese G, Tannir NM, Msaouel P. Recent advancements in the treatment of metastatic clear cell renal cell carcinoma: A review of the evidence using second-generation p-values. Cancer treatment and research communications 2020;23:100166. [DOI] [PubMed] [Google Scholar]
- 5.Barata PC, Mendiratta P, Kotecha R, et al. Effect of Switching Systemic Treatment After Stereotactic Radiosurgery for Oligoprogressive, Metastatic Renal Cell Carcinoma. Clinical Genitourinary Cancer 2018;16:413–9.e1. [DOI] [PubMed] [Google Scholar]
- 6.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weichselbaum RR, Hellman S. Oligometastases revisited. Nature reviews Clinical oncology 2011;8:378–82. [DOI] [PubMed] [Google Scholar]
- 8.Fisher R, Larkin J, Swanton C. Inter and intratumour heterogeneity: a barrier to individualized medical therapy in renal cell carcinoma? Frontiers in oncology 2012;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien CR, Geynisman DM, Kim B, Xu Y, Shih YT. Economic Burden of Renal Cell Carcinoma-Part I: An Updated Review. PharmacoEconomics 2019;37:301–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK, Gandhi S, George S. Second-line systemic therapy in metastatic renal-cell carcinoma: A review. Urologic oncology 2017;35:640–6. [DOI] [PubMed] [Google Scholar]
- 11.Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. European Journal of Cancer 2018;98:38–47. [DOI] [PubMed] [Google Scholar]
- 12.The World Medical Association: Ferney-Voltaire F. Declaration of Helsinki: Ethical principles for medical research involving human subjects. 2013. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990) 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14.Ning S, Trisler K, Wessels BW, Knox SJ. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997;80:2519–28. [DOI] [PubMed] [Google Scholar]
- 15.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The Lancet Oncology 2013;14:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 17.Jonasch E, Pagliaro LC, Tannir NM. Long-term management of patients with metastatic renal cell carcinoma on targeted agents. Expert review of anticancer therapy 2010;10:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Wong Y-N, Jahnke J, Pettit AR, Doshi JA. Association of high cost sharing and targeted therapy initiation among elderly Medicare patients with metastatic renal cell carcinoma. 2018;7:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battle D, Rathmell WK, Jonasch E, et al. Patient-reported outcomes on treatment-related side effects in renal cell carcinoma. 2020;38:654-. [Google Scholar]
- 20.Rini BI, Battle D, Figlin RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019;7:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. The Lancet Oncology 2016;17:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenhals J, Mohamad O, Christie A, et al. Stereotactic Ablative Radiotherapy for Oligoprogressive Metastatic Renal Cell Carcinoma. International journal of radiation oncology, biology, physics 2019;105:E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung PJTBJoR. Stereotactic body radiotherapy for oligoprogressive cancer. 2016;89:20160251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung P, Patel S, North SA, et al. A phase II multicenter study of stereotactic radiotherapy (SRT) for oligoprogression in metastatic renal cell cancer (mRCC) patients receiving tyrosine kinase inhibitor (TKI) therapy. 2020;38:5065-. [DOI] [PubMed] [Google Scholar]
- 25.Centre SHS, Pfizer. Stereotactic Radiotherapy for Metastatic Kidney Cancer Being Treated With Sunitinib. https://ClinicalTrials.gov/show/NCT02019576; 2014.
- 26.SAbR For Oligo-Progressive Renal Cell Cancer. https://ClinicalTrials.gov/show/NCT03696277. [Google Scholar]
- 27.Baclesse CF, National Cancer Institute F, GETUG. Study Assessing Stereotactic Radiotherapy in Therapeutic Strategy of Oligoprogressive Renal Cell Carcinoma Metastases. https://ClinicalTrials.gov/show/NCT04299646; 2020.
- 28.Patel PH, Palma D, McDonald F, Tree AC. The Dandelion Dilemma Revisited for Oligoprogression: Treat the Whole Lawn or Weed Selectively? Clinical oncology (Royal College of Radiologists (Great Britain)) 2019;31:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sindhu KK, Leiter A, Moshier E, et al. Durable disease control with local treatment for oligoprogression of metastatic solid tumors treated with immune checkpoint blockade. Cancer treatment and research communications 2020;25:100216. [DOI] [PubMed] [Google Scholar]
- 30.Campo M, Al-Halabi H, Khandekar M, Shaw AT, Sequist LV, Willers H. Integration of Stereotactic Body Radiation Therapy With Tyrosine Kinase Inhibitors in Stage IV Oncogene-Driven Lung Cancer. 2016;21:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elamin YY, Gomez DR, Antonoff MB, et al. Local Consolidation Therapy (LCT) After First Line Tyrosine Kinase Inhibitor (TKI) for Patients With EGFR Mutant Metastatic Non–small-cell Lung Cancer (NSCLC). Clinical Lung Cancer 2019;20:43–7. [DOI] [PubMed] [Google Scholar]
- 32.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 2018;29:iv192-iv237. [DOI] [PubMed] [Google Scholar]
- 33.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. International journal of radiation oncology, biology, physics 2014;88:892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berghen C, Joniau S, Ost P, et al. Progression-directed Therapy for Oligoprogression in Castration-refractory Prostate Cancer. European urology oncology 2019. [DOI] [PubMed] [Google Scholar]
- 35.Triggiani L, Mazzola R, Magrini SM, et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World journal of urology 2019;37:2631–7. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida S, Takahara T, Arita Y, et al. Progressive Site-Directed Therapy for Castration-Resistant Prostate Cancer: Localization of the Progressive Site as a Prognostic Factor. International journal of radiation oncology, biology, physics 2019;105:376–81. [DOI] [PubMed] [Google Scholar]
- 37.Onal C, Gultekin M, Oymak E, et al. Stereotactic radiotherapy in patients with oligometastatic or oligoprogressive gynecological malignancies: a multi-institutional analysis. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2020;30:865–72. [DOI] [PubMed] [Google Scholar]
- 38.Schröder C, Opitz I, Guckenberger M, et al. Stereotactic Body Radiation Therapy (SBRT) as Salvage Therapy for Oligorecurrent Pleural Mesothelioma After Multi-Modality Therapy. Frontiers in oncology 2019;9:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guida M, Bartolomeo N, De Risi I, et al. The Management of Oligoprogression in the Landscape of New Therapies for Metastatic Melanoma. Cancers 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson R, Cheung P, Chu W, et al. Outcomes of extra-cranial stereotactic body radiotherapy for metastatic colorectal cancer: Dose and site of metastases matter. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2020;142:236–45. [DOI] [PubMed] [Google Scholar]
- 41.Franzese C, Francolini G, Nicosia L, Alongi F, Livi L, Scorsetti M. Stereotactic Body Radiation Therapy in the Management of Oligometastatic and Oligoprogressive Bladder Cancer and Other Urothelial Malignancies. Clinical oncology (Royal College of Radiologists (Great Britain)) 2020. [DOI] [PubMed] [Google Scholar]
- 42.Bonomo P, Greto D, Desideri I, et al. Clinical outcome of stereotactic body radiotherapy for lung-only oligometastatic head and neck squamous cell carcinoma: Is the deferral of systemic therapy a potential goal? Oral oncology 2019;93:1–7. [DOI] [PubMed] [Google Scholar]
- 43.Farooqi A, Mitra D, Guadagnolo BA, Bishop AJ. The Evolving Role of Radiation Therapy in Patients with Metastatic Soft Tissue Sarcoma. Current oncology reports 2020;22:79. [DOI] [PubMed] [Google Scholar]
- 44.Comito F, Leslie I, Boos L, et al. Oligoprogression After Checkpoint Inhibition in Metastatic Melanoma Treated With Locoregional Therapy: A Single-center Retrospective Analysis. Journal of immunotherapy (Hagerstown, Md : 1997) 2020;43:250–5. [DOI] [PubMed] [Google Scholar]
- 45.Institute LHR. Stereotactic Radiotherapy for Oligo-Progressive Metastatic Cancer (The STOP Trial). https://ClinicalTrials.gov/show/NCT02756793; 2016.
- 46.Center MSKC. Randomized Study of Stereotactic Body Radiation Therapy (SBRT) in Patients With Oligoprogressive Metastatic Cancers of the Breast and Lung. https://ClinicalTrials.gov/show/NCT03808662; 2019.
- 47.Institute of Cancer Research UK. Stereotactic Body Radiotherapy for the Treatment of OPD. https://ClinicalTrials.gov/show/NCT03256981; 2017.
- 48.Center UoMRC. FOcal Radiation for Oligometastatic Castration-rEsistant Prostate Cancer (FORCE). https://ClinicalTrials.gov/show/NCT03556904; 2018.
- 49.Levitin M, Ofori J, Shin WJ, et al. Radiation and Checkpoint Inhibitor Immunotherapy Lead to Long Term Disease Control in a Metastatic RCC patient With Brain Metastases. Frontiers in oncology 2020;10:566070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. Metastasis in renal cell carcinoma: Biology and implications for therapy. Asian journal of urology 2016;3:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Annals of oncology : official journal of the European Society for Medical Oncology 2012;23:973–80. [DOI] [PubMed] [Google Scholar]
- 52.Turajlic S, Xu H, Litchfield K, et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell 2018;173:581–94.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, CT, upon reasonable request.