Background:
Biologic medications are recommended for treatment of moderately-to-severely active Crohn disease (CD) or ulcerative colitis (UC) in children. However, many patients require sequential biologic treatment because of nonresponse or loss of response to the initial biologic.
Methods:
We analyzed pediatric inflammatory bowel disease (IBD) data from the ImproveCareNow Network registry between May 2006 and September 2016, including time to biologic initiation, choice of first subsequent biologics, biologic durability, and reasons for discontinuation.
Results:
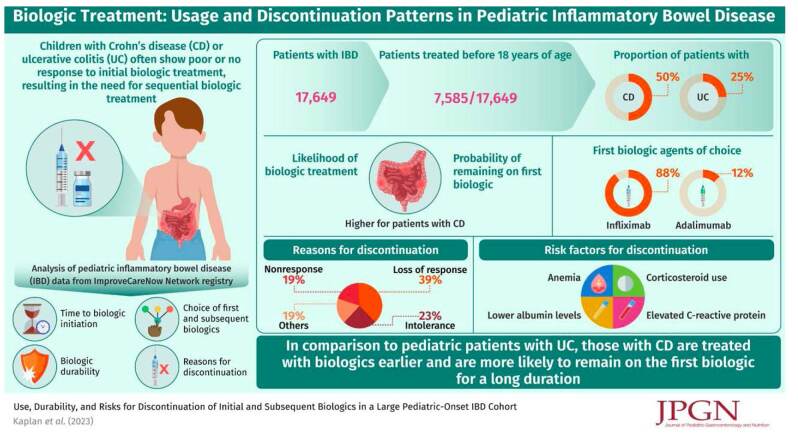
Of 17,649 patients with IBD [CD: 12,410 (70%); UC: 5239 (30%)], 7585 (43%) were treated with a biologic agent before age 18 (CD: 50%; UC: 25%). Biologic treatment was more likely for CD than UC (odds ratio, 3.0; 95% CI: 2.8–3.2; P < 0.0001). First biologic agents for all patients were anti-tumor necrosis factor agents (88% infliximab, 12% adalimumab). Probability of remaining on the first biologic was significantly higher in CD than UC (P < 0.0001). First biologics were discontinued because of loss of response (39%), intolerance (23%), and nonresponse (19%). In univariate analysis, factors associated with discontinuation of first and/or second biologics in CD include colonic-only disease, corticosteroid use, upper gastrointestinal tract involvement, and clinical and biochemical markers of severe disease. Biologic durability improved with later induction date.
Conclusions:
Treatment with biologic medications is common in pediatric IBD. Patients with CD are more likely to receive biologics, receive biologics earlier in disease course, and remain on the first biologic longer than patients with UC. Multiple factors may predict biologic durability in children with IBD.
Keywords: Crohn disease, persistence, real-world evidence, ulcerative colitis
What Is Known.
Biologic agents are safe and effective treatments for pediatric patients with inflammatory bowel disease.
Some patients do not respond or lose response to the initial biologic and require sequential biologic treatment.
What Is New
Pediatric patients with Crohn disease (CD) are treated with biologic therapies more frequently and earlier in the disease course than those with ulcerative colitis (UC).
Primary nonresponse to the first biologic agent is more common in UC than in CD.
Risk factors for biologic discontinuation in CD may include colonic-only disease location, corticosteroid use, and markers of more severe disease.
Biologic treatment is recommended for pediatric patients with moderately-to-severely active Crohn disease (CD) or ulcerative colitis (UC) (1,2). Several biologic agents are indicated for treatment of children with CD or UC (1,3–9) and biologic therapies indicated for adults are also used off-label to treat pediatric patients (1,3,10), including children aged <6 years (11).
While biologic therapies are effective in inducing and maintaining remission in children with inflammatory bowel disease (IBD) (12,13), some children do not respond or lose response (10) and require sequential biologic treatment. In pediatric CD trials, 12%–18% of children did not respond to anti-tumor necrosis factor (anti-TNF) agents following induction (12,13); of those who did, 19%–49% lost response in the first year of therapy (12). However, in small retrospective single-center studies, the durability of an anti-TNF agent in pediatric IBD was >50% after 3 years (14). Patterns of initial and subsequent biologic use and durability in real-world pediatric IBD cohorts are not well known.
ImproveCareNow (ICN) is an international pediatric IBD quality improvement and research network with >100 participating centers and approximately 30,000 patients (15,16). ICN centers range from the largest children’s hospitals to small office-based clinics. ICN center patients and their guardians have the option to consent to use their ICN registry data for research. We examined data from this large pediatric IBD cohort to determine frequency and patterns of biologic medication use, durability, and risk factors for discontinuation.
METHODS
Study Design
In this retrospective, multicenter, observational cohort study, we analyzed ICN pediatric IBD registry data from clinical care visits between May 2006 and September 2016 (study period). Diagnoses were based on clinical, radiographic, endoscopic, and pathologic abnormalities. Disease severity was determined by Physician Global Assessment and short Pediatric Crohn’s Disease Activity Index (sPCDAI) or Pediatric Ulcerative Colitis Activity Index (17,18).
We examined 3 patient cohorts: the Full Cohort, the Biologic Inception Cohort, and the Discontinuation Cohort. The Full Cohort included patients diagnosed with CD or UC (not IBD-unclassified) before age 18 who enrolled in ICN and consented to their data being used for research purposes. Data for this cohort were obtained from the ICN registry database. The follow-up period for the Full Cohort was from enrollment to last visit, or September 9, 2016, whichever was earlier. The Biologic Inception Cohort was a subgroup of the Full Cohort who received their first biologic dose after enrollment into ICN and before age 18. The Discontinuation Cohort was a subgroup of the Biologic Inception Cohort whose biologic discontinuation status was known and who had a clinic visit ≤60 days before biologic initiation. For the Biologic Inception and Discontinuation Cohorts, follow-up was from first biologic initiation to last visit, or September 9, 2016, whichever was earlier. Additional data for the Biologic Inception and Discontinuation Cohorts were obtained by patient medical record review at ICN sites. To assess potential differences in biologic discontinuation over the duration of the study, patients were divided into 2 groups by first biologic initiation date (Group 1: on or before June 2013; Group 2: July 2013–February 2015) for some analyses, allowing at least 18 months of follow-up.
Statistical Analysis
Demographics, basic disease characteristics, and exposure to biologics (≥1 dose of infliximab, adalimumab, certolizumab pegol, golimumab, natalizumab, ustekinumab, or vedolizumab) were summarized for all 3 cohorts using descriptive statistics. For the Biologic Inception Cohort, we analyzed time from IBD diagnosis to first biologic dose, treatment duration (durability) of first biologic agent, choice of first and subsequent biologics, and discontinuation reasons.
Associations between categorical variables and disease diagnosis were evaluated using an exact Chi-square test. Biologic agent continuation was estimated using Kaplan-Meier analysis. Log-rank testing was used to compare time to biologic discontinuation between disease groups. Factors associated with discontinuation of initial and subsequent biologics and time-to-discontinuation were assessed by Cox regression analysis (univariate and multivariable models). Sample sizes for each analysis varied because of missing data. We limited the analysis in the Discontinuation Cohort to patients treated with adalimumab or infliximab because only 0.2% were treated with other biologics. Proportional hazards assumptions were checked using the supremum test. When a significant interaction between a predictor and biologic agent was identified, hazard ratios (HRs) and P values for the predictor for each biologic were reported.
The ICN registry does not collect data on medications discontinued before enrollment. Therefore, some patients in the Full Cohort categorized as never exposed to biologics may have initiated and discontinued biologics prior to ICN registration. The rate of false-negative biologic exposures was estimated using the Clopper-Pearson exact method (19) by chart review of a random sample of Full Cohort patients.
Ethics
The study was approved by the institutional review boards of all participating sites and the ICN Research Committee.
RESULTS
Model Assumptions
No violations in the proportional hazards assumptions of the model were found using the supremum test.
Patient Demographics and Disease Characteristics
The Full Cohort included 17,649 patients (Figure, Supplemental Digital Content 1, http://links.lww.com/MPG/D78), 12,410 (70%) with CD and 5239 (30%) with UC. Over half the patients (53%) were diagnosed with IBD between ages 12 and 18; 9% were diagnosed before age 6 (Table, Supplemental Digital Content 2, http://links.lww.com/MPG/D79). Median follow-up for the Full Cohort was 1.68 years (IQR 0.67–3.15).
Of 1029 patients in the Biologic Inception Cohort, 809 (79%) had CD and 220 (21%) had UC. Of 846 patients in the Discontinuation Cohort, 678 (80%) had CD and 168 (20%) had UC. Median follow-up after first biologic initiation was 1.65 years (IQR 0.99–2.66) and 1.69 years (IQR 1.02–2.72) for Biologic Inception and Discontinuation Cohorts, respectively.
Biologic Use
In the Full Cohort, 43% of all IBD patients were treated with a biologic before age 18 (Table, Supplemental Digital Content 2, http://links.lww.com/MPG/D79) including 50% of CD and 25% of UC patients. CD patients were more likely to receive biologic treatment than UC patients (odds ratio 3.0; 95% CI: 2.8–3.2; P < 0.0001).
Use and Durability of Biologic Treatment
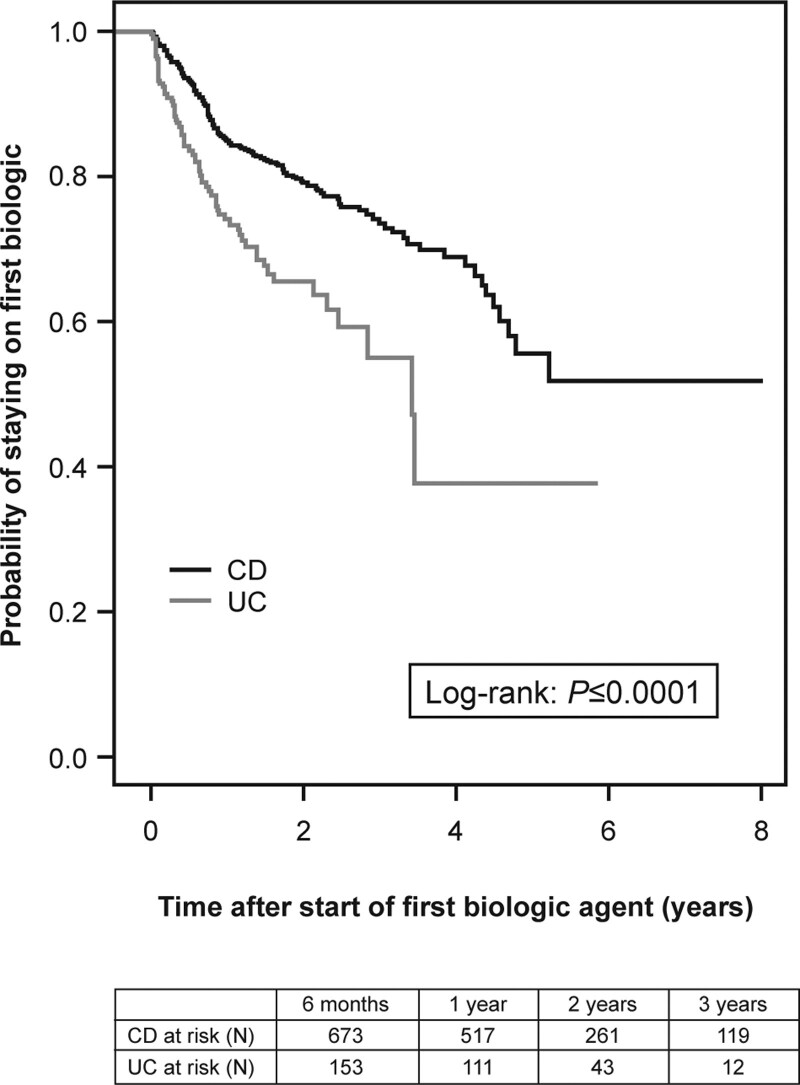
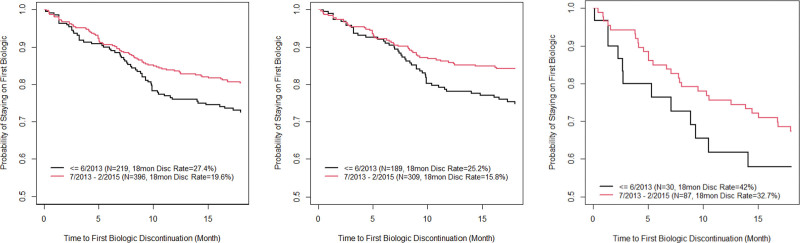
In the Biologic Inception Cohort (n = 1029), the first biologic used was an anti-TNF in all cases (88% infliximab, 12% adalimumab). Median time from diagnosis to biologic initiation was shorter in CD than in UC (325 vs 423 days, P = 0.004) (Table, Supplemental Digital Content 3, http://links.lww.com/MPG/D80). The probability of remaining on the first biologic was significantly higher in CD patients than in UC patients: 0.93 versus 0.84 at 6 months, 0.85 versus 0.75 at 12 months, 0.79 versus 0.66 at 24 months, and 0.74 versus 0.55 at 36 months; P < 0.0001 (Fig. 1). IBD and CD patients who started their first biologic during or after July 2013 had significantly lower rates of biologic discontinuation at 18 months than those who started biologics before July 2013 (IBD: 19.6% vs 27.4%, P = 0.03; CD: 15.8% vs 25.2%, P = 0.016) (Figure 2 and Table, Supplemental Digital Content 4, http://links.lww.com/MPG/D81). This discontinuation difference between early versus later induction date was not seen in the UC cohort. For the IBD cohort, the most common reasons for discontinuation of the first biologic were secondary loss of response (39%), intolerance (23%), and primary nonresponse (19%). Primary nonresponse was a more common reason for discontinuation in UC patients than CD patients (29% vs 15%) In the Biologic Inception Cohort, 17%, 2%, and 0.6% of patients were treated with at least 2, 3, or 4 biologics, respectively (Table, Supplemental Digital Content 3, http://links.lww.com/MPG/D80), over median follow-up of 1.65 years (IQR 0.99–2.66). The second biologic was often a second anti-TNF (95%); vedolizumab was the second or third biologic in 5% and 38% of patients, respectively (Table, Supplemental Digital Content 3, http://links.lww.com/MPG/D80). When used as the second or third biologic, anti-TNFs were discontinued by 26% (44/167) or 40% (6/15) of patients, respectively, versus 13% (1/8; P = 0.54) or 11% (1/9; P = 0.17) of vedolizumab patients.
FIGURE 1.
Kaplan-Meier analysis of continuation of first biologic agent over time in the Biologic Inception Cohort by IBD diagnosis (CD, n = 772; UC, n = 206); 51 patients were excluded because of missing time or discontinuation status. CD = Crohn disease; IBD = inflammatory bowel disease; UC = ulcerative colitis.
FIGURE 2.
Kaplan-Meier curves of discontinuation of first biologic by 18 months in 2 induction periods by diagnosis. Left: IBD cohort (log-rank test P = 0.03); middle: CD cohort (log-rank test P = 0.016); right: UC cohort (log-rank test P = 0.25). CD = Crohn disease; IBD = inflammatory bowel disease; UC = ulcerative colitis.
Primary nonresponse to the first anti-TNF occurred in 4.3% (44/1029) of all IBD patients (3.0% of CD patients; 9.1% of UC patients). Primary nonresponse to the second anti-TNF was 5.1% in IBD patients (5.3% of CD patients; 4.6% of UC patients), regardless of reason for discontinuation of the first biologic.
Risks for First Biologic Discontinuation
Among the Discontinuation Cohort (CD and UC; n = 846), the first biologic was infliximab in 757 (89%) patients and adalimumab in 89 patients (11%). Patients not receiving systemic corticosteroids when the first biologic was initiated were less likely to discontinue the first biologic versus those receiving corticosteroids [HR 0.73 (95% CI: 0.55–0.96; P = 0.025)] (Table 1). The durability of the first biologic was not affected by time from diagnosis to biologic initiation, C-reactive protein (CRP), body mass index, or concomitant immunomodulator use within 6 months after biologic initiation (Table 1).
TABLE 1.
Risk factors for discontinuation of biologic agents in the overall discontinuation cohort (univariate analysis)
| First biologic agent | Second biologic agent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Measurement | N | Hazard ratio | 95% CI | P value | N | Hazard ratio | 95% CI | P value |
| Age at biologic initiation | Each additional year of age at biologic initiation | 844 | 1.003 | (0.957–1.052) | 0.8994 | 113 | 0.950 | (0.847–1.065) | 0.3805 |
| Age at diagnosis | Each additional year of age at diagnosis | 841 | 0.988 | (0.947–1.030) | 0.5670 | 113 | 0.953 | (0.859–1.056) | 0.3571 |
| Anemia at biologic initiation | No vs yes | 629 | 0.869 | (0.635–1.188) | 0.3785 | 73 | 0.470 | (0.194–1.143) | 0.0960 |
| Time from diagnosis to start of first biologic | 0 to 3 mo vs 3 to 12 mo | 841 | 0.872 | (0.582–1.305) | 0.7685 | 100 | 0.694 | (0.226–2.125) | 0.7155 |
| 0 to 3 mo vs >12 mo | 0.951 | (0.647–1.400) | 0.919 | (0.285–2.961) | |||||
| 3 to 12 mo vs >12 mo | 1.092 | (0.799–1.491) | 1.325 | (0.588–2.989) | |||||
| BMI z score at biologic initiation | Each additional BMI z score unit | 834 | 1.074 | (0.958–1.203) | 0.2192 | 108 | 1.067 | (0.769–1.481) | 0.6965 |
| CRP at biologic initiation | Normal vs elevated | 619 | 1.108 | (0.785–1.563) | 0.5595 | 62 | 0.252 | (0.082–0.772) | 0.0158 |
| CRP at biologic initiation relative to ULN range | Each additional unit relative to CRP ULN | 519 | 1.021 | (0.982–1.061) | 0.2993 | 54 | 1.098 | (1.009–1.195) | 0.0293 |
| Erythrocyte sedimentation rate at biologic initiation | Normal vs elevated | 572 | 0.840 | (0.589–1.198) | 0.3367 | 61 | 0.463 | (0.129–1.662) | 0.2375 |
| Ethnicity | Hispanic vs non-Hispanic | 585 | 1.721 | (0.871–3.400) | 0.1184 | 69 | 2.652 | (0.597–11.779) | 0.1998 |
| Hypoalbuminemia at biologic initiation | No vs yes | 602 | 1.114 | (0.696–1.785) | 0.6528 | 72 | 0.412 | (0.163–1.039) | 0.0603 |
| Immunomodulator use within the first 6 months post baseline biologic | No vs yes | 828 | 1.232 | (0.925–1.641) | 0.1532 | 107 | 0.619 | (0.292–1.312) | 0.2110 |
| Perianal disease at biologic initiation | No vs yes | 627 | 1.280 | (0.855–1.918) | 0.2307 | 83 | 0.737 | (0.309–1.758) | 0.4917 |
| Physician Global Assessment at biologic initiation | Mild vs moderate | 821 | 0.659 | (0.475–0.913) | 0.0568 | 106 | 0.624 | (0.246–1.586) | 0.0508 |
| Mild vs severe | 0.872 | (0.418–1.819) | 0.262 | (0.089–0.770) | |||||
| Moderate vs severe | 1.323 | (0.638–2.743) | 0.419 | (0.136–1.287) | |||||
| Quiescent vs mild | 1.527 | (1.017–2.294) | 0.802 | (0.274–2.348) | |||||
| Quiescent vs moderate | 1.006 | (0.678–1.492) | 0.501 | (0.163–1.534) | |||||
| Quiescent vs severe | 1.331 | (0.618–2.868) | 0.210 | (0.060–0.729) | |||||
| Prednisone or methyl-prednisolone usage at biologic initiation | No vs yes | 844 | 0.725 | (0.547–0.960) | 0.0248 | 113 | 1.251 | (0.551–2.843) | 0.5922 |
| Race | Black vs non-Black | 761 | 1.019 | (0.633–1.640) | 0.9374 | 102 | 1.588 | (0.594–4.246) | 0.3566 |
| Albumin at biologic initiation | Each additional albumin unit | 602 | 0.979 | (0.736–1.302) | 0.8837 | 72 | 0.416 | (0.220–0.786) | 0.0069 |
| ESR at biologic initiation | Each additional erythrocyte sedimentation rate unit | 572 | 1.006 | (0.999–1.013) | 0.1191 | 61 | 1.011 | (0.986–1.036) | 0.3842 |
| Hematocrit at biologic initiation | Each additional hematocrit unit | 629 | 0.982 | (0.947–1.019) | 0.3329 | 73 | 0.870 | (0.765–0.990) | 0.0351 |
Bold text indicates statistically significant P values.
BMI = body mass index; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; ULN = upper limit of normal.
Of 678 Discontinuation Cohort patients with CD, the first biologic was infliximab for 600 (88%) patients and adalimumab for 78 (12%) patients; 85%, 79%, and 74% remained on their first biologic for 12, 24, and 36 months, respectively. In univariate analysis, risk of first biologic discontinuation was significantly higher with colonic-only disease at diagnosis than ileocolonic disease [HR 1.94 (95% CI: 1.28–2.94, P = 0.004)] (Table 2). Similar to the entire IBD Discontinuation Cohort, CD patients receiving corticosteroids upon first biologic initiation were more likely to discontinue than patients not receiving corticosteroids (P = 0.017) (Table 2). Higher sPCDAI scores at biologic initiation were also a risk factor for discontinuation [HR 1.011 for each additional sPCDAI unit (95% CI: 1.001–1.022, P = 0.032)]. None of these variables remained statistically significant in multivariable analysis. Durability of the first biologic in CD was not influenced by the presence of perianal disease or disease phenotype (inflammatory vs penetrating and/or stricturing disease).
TABLE 2.
Risk factors for discontinuation of biologic agents in patients with Crohn disease in the discontinuation cohort (univariate analysis)
| First biologic | Second biologic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Measurement | N | Hazard ratio | 95% CI | P value | N | Hazard ratio | 95% CI | P value |
| Age at biologic initiation | Each additional year of age at biologic initiation | 678 | 0.985 | (0.929–1.043) | 0.5947 | 88 | 0.968 | (0.853–1.098) | 0.6137 |
| Age at diagnosis | Each additional year of age at diagnosis | 675 | 0.974 | (0.925–1.026) | 0.3199 | 88 | 0.977 | (0.864–1.104) | 0.7039 |
| Anemia at biologic initiation | No vs yes | 494 | 0.947 | (0.654–1.373) | 0.7750 | 56 | 0.393 | (0.146–1.056) | 0.0640 |
| Time from diagnosis to start of first biologic | 0 to 3 months vs 3 to 12 months | 675 | 0.986 | (0.627–1.552) | 0.9978 | 81 | 0.693 | (0.222–2.164) | 0.4945 |
| 0 to 3 months vs >12 months | 0.987 | (0.644–1.513) | 1.229 | (0.343–4.407) | |||||
| 3 to 12 months vs >12 months | 1.000 | (0.691–1.448) | 1.773 | (0.664–4.735) | |||||
| BMI z score at biologic initiation | Each additional unit of BMI z-score | 670 | 1.107 | (0.965–1.269) | 0.1459 | 84 | 0.968 | (0.682–1.374) | 0.8566 |
| CRP at biologic initiation | Normal vs elevated | 492 | 1.136 | (0.755–1.709) | 0.5421 | 49 | 0.301 | (0.095–0.953) | 0.0411 |
| Disease phenotype at diagnosis – CD | B1 vs B2/B3 | 644 | 1.468 | (0.811–2.658) | 0.2044 | – | – | – | – |
| ESR at biologic initiation | Normal vs elevated | 452 | 0.812 | (0.527–1.251) | 0.3454 | 46 | 0.574 | (0.148–2.225) | 0.4221 |
| Ethnicity | Hispanic vs non-Hispanic | 469 | 1.679 | (0.678–4.158) | 0.2626 | 54 | 2.405 | (0.532–10.884) | 0.2545 |
| Extent of disease at biologic initiation – macroscopic lower – CD | Colonic-only vs ileocolonic | 523 | 1.612 | (0.911–2.852) | 0.0958 | 71 | 2.145 | (0.744–6.189) | 0.4593 |
| Ileal only vs colonic-only | 0.663 | (0.324–1.359) | 0.265 | (0.031–2.287) | |||||
| Ileal only vs ileocolonic | 1.069 | (0.587–1.948) | 0.569 | (0.073–4.426) | |||||
| None vs colonic-only | 1.849 | (0.617–5.541) | – | – | |||||
| None vs ileal only | 2.788 | (0.916–8.482) | – | – | |||||
| None vs ileocolonic | 2.980 | (1.070–8.300) | – | – | |||||
| Extent of disease at biologic initiation – macroscopic upper distal – CD | No vs yes | 458 | 0.841 | (0.491–1.439) | 0.5264 | 68 | 1.962 | (0.546–7.055) | 0.3017 |
| Extent of disease at biologic initiation – macroscopic upper proximal – CD | No vs yes | 504 | 1.181 | (0.753–1.853) | 0.4687 | 72 | 0.308 | (0.107–0.888) | 0.0292 |
| Hypoalbuminemia at biologic initiation | No vs yes | 473 | 0.976 | (0.573–1.661) | 0.9276 | 55 | 0.387 | (0.137–1.094) | 0.0733 |
| Immunomodulator use within the first 6 months post baseline biologic | No vs yes | 663 | 1.230 | (0.880–1.720) | 0.2251 | 83 | 0.749 | (0.323–1.738) | 0.5010 |
| Extent of disease at diagnosis – macroscopic lower – CD | Colonic-only vs ileocolonic | 638 | 1.939 | (1.279–2.939) | 0.0040 | 77 | 1.297 | (0.461–3.645) | 0.7697 |
| Ileal only vs colonic-only | 0.734 | (0.433–1.246) | 0.536 | (0.108–2.673) | |||||
| Ileal only vs ileocolonic | 1.423 | (0.891–2.275) | 0.696 | (0.150–3.236) | |||||
| None vs colonic-only | 1.330 | (0.587–3.013) | 1.682 | (0.200–14.116) | |||||
| None vs ileal only | 1.811 | (0.780–4.204) | 3.135 | (0.283–34.681) | |||||
| None vs ileocolonic | 2.578 | (1.181–5.628) | 2.181 | (0.274–17.380) | |||||
| Perianal disease at biologic initiation | No vs yes | 623 | 1.286 | (0.859–1.927) | 0.2217 | 82 | 0.777 | (0.316–1.910) | 0.5828 |
| PGA at biologic initiation | Mild vs moderate | 659 | 0.702 | (0.483–1.020) | 0.1505 | 85 | 0.672 | (0.258–1.750) | 0.1818 |
| Mild vs severe | 1.201 | (0.434–3.319) | 0.406 | (0.087–1.898) | |||||
| Moderate vs severe | 1.711 | (0.619–4.727) | 0.604 | (0.128–2.859) | |||||
| Quiescent vs mild | 1.511 | (0.956–2.386) | 0.418 | (0.113–1.546) | |||||
| Quiescent vs moderate | 1.060 | (0.672–1.671) | 0.281 | (0.074–1.062) | |||||
| Quiescent vs severe | 1.814 | (0.636–5.173) | 0.170 | (0.028–1.024) | |||||
| Prednisone or methylprednisolone usage at biologic initiation | No vs yes | 678 | 0.670 | (0.482–0.931) | 0.0170 | 88 | 1.157 | (0.424–3.154) | 0.7757 |
| Race | Black vs non-Black | 611 | 1.193 | (0.697–2.042) | 0.5204 | 80 | 1.349 | (0.390–4.671) | 0.6362 |
| Short PCDAI | Each additional sPCDAI score unit | 472 | 1.011 | (1.001–1.022) | 0.0320 | 72 | 1.020 | (0.997–1.043) | 0.0854 |
| Albumin at biologic initiation | Each additional unit of albumin | 473 | 0.969 | (0.683–1.373) | 0.8578 | 55 | 0.368 | (0.181–0.748) | 0.0058 |
| ESR at biologic initiation | Each additional hematocrit unit | 452 | 1.005 | (0.996–1.014) | 0.2631 | 46 | 1.017 | (0.987–1.047) | 0.2627 |
| Hematocrit at biologic initiation | Each additional hematocrit unit | 494 | 0.982 | (0.937–1.030) | 0.4644 | 56 | 0.855 | (0.742–0.986) | 0.0316 |
| Phenotype at biologic initiation – CD | B1 vs B2/B3 | 649 | 1.285 | (0.751–2.198) | 0.3597 | – | – | ||
Bold text indicates statistically significant P values.
BMI = body mass index; CD = Crohn disease; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; PCDAI = Pediatric Crohn Disease Activity Index; PGA = Physician Global Assessment; sPCDAI = short PCDAI; ULN = upper limit of normal.
Among the 168 Discontinuation Cohort patients with UC, the first biologic was infliximab for 157 (93%) and adalimumab for 11 (7%) patients; 75%, 66%, and 55% remained on the first biologic for 12, 24, and 36 months, respectively. Corticosteroid treatment, disease duration, and extent of disease (pancolitis vs other) at biologic initiation did not affect first biologic discontinuation in patients with UC (Table, Supplemental Digital Content 3, http://links.lww.com/MPG/D80).
Risks for Second Biologic Discontinuation
In the Discontinuation Cohort (CD and UC), 119 patients received a second biologic [18 (15%) infliximab; 101 (85%) adalimumab]. In univariate analysis, elevated CRP concentrations at second biologic initiation were associated with an almost 4 times higher risk of discontinuation than CRP concentrations in the normal range [HR 3.97 (95% CI: 1.30–12.14, P = 0.016)] (Table 1). Each additional unit above the CRP upper limit of normal was associated with a significant increased risk of second biologic discontinuation [HR 1.10 (95% CI: 1.01−1.20, P = 0.029)]. Higher albumin and hematocrit at second biologic initiation were both associated with reduced risk of biologic discontinuation [HR 0.42 per additional unit of albumin (95% CI: 0.22–0.790, P = 0.007) and 0.87 per additional unit of hematocrit (95% CI: 0.77–0.99, P = 0.035), respectively] (Table 1).
Ninety-two (14%) Discontinuation Cohort CD patients received a second biologic; of these 13 (14%) received infliximab and 79 (86%) received adalimumab. Risk of second biologic discontinuation was greater for CD patients with elevated CRP concentrations at biologic initiation than with normal concentrations [HR 3.33 (95% CI: 1.05–10.55, P = 0.041)] (Table 2). CD patients without macroscopic proximal upper gastrointestinal (GI) tract involvement at biologic initiation were less likely to discontinue second biologic treatment [HR 0.31 (95% CI: 0.11–0.89, P = 0.029)], although proximal upper GI tract disease was not shown to be a risk factor for first biologic discontinuation. As with the full IBD Discontinuation Cohort, higher albumin and hematocrit at biologic initiation were both associated with reduced risk of a second biologic discontinuation in CD patients [HR 0.37 (95% CI: 0.18–0.75, P = 0.006) and 0.86 (95% CI: 0.74–0.99, P = 0.032)] (Table 2). None of these variables remained statistically significant in multivariable analysis.
Of 27 Discontinuation Cohort UC patients who received a second biologic, 5 (19%) received infliximab and 22 (81%) received adalimumab. For these patients, none of the examined factors were associated with an increased risk of second biologic discontinuation (Table, Supplemental Digital Content 5, http://links.lww.com/MPG/D82).
DISCUSSION
In this large real-world cohort study of more than 17,000 pediatric patients with IBD, 50% of children with CD and 25% with UC were treated with biologic therapy before age 18. Biologic therapy was used significantly earlier in the disease course and was more durable in CD patients than UC patients. Sequential use of biologics during a median follow-up of 1.65 years occurred in 17% of children with IBD who initiated biologic treatment.
Sequential anti-TNF therapy (infliximab followed by adalimumab or vice-versa) was the most common use pattern. Biologic therapies other than anti-TNFs were rarely used. However, during the study period, neither ustekinumab nor tofacitinib had been approved for use in adult or pediatric IBD, and vedolizumab was approved for use in adults with IBD toward the end of the study period.
In univariate analysis, we found several risk factors for biologic discontinuation. For CD, patients receiving corticosteroids at first biologic initiation were more likely to discontinue than those not treated with corticosteroids, possibly because corticosteroid use is a marker for more severe and/or refractory disease. Other markers of severe disease, such as higher sPCDAI scores, higher CRP, and low albumin and hematocrit, were also risk factors for discontinuation. However, none of these factors remained statistically significant in multivariable analysis. The first biologic was less durable in CD patients with colonic-only disease than patients with ileocolonic disease, contrary to findings in adults (20). In the Biologic Inception Cohort, the probabilities of remaining on the first biologic for CD (0.79 and 0.74 at 24 and 36 months, respectively) are consistent with results from a systematic review of real-world studies on pediatric luminal CD that reported 67%–91% and 61%–85% of patients remained on infliximab at 2 and 3 years, respectively, while 50%–79% and 79% remained on adalimumab at 2 and 3 years (14), respectively. Another review of pediatric CD reported that the likelihood of remaining on infliximab at 2 and 3 years was 78% and 67%, respectively (21).
In clinical practice, pediatric IBD patients discontinued biologics because of loss of response, poor initial response, immunogenicity, and personal choice (21–25). Pediatric UC treatment guidelines indicate that immunogenicity and lack of response are key reasons for switching biologic treatments (3). Similar reasons for discontinuation were observed in our analyses. Biologic response is associated with serum drug concentration (14). Lack of response to therapy among children with IBD often results from low serum drug concentrations or drug immunogenicity (26,27). Although we did not capture therapeutic drug monitoring (TDM) data, we did find that IBD and CD patients who started on first biologic therapy before July 2013 were more likely to discontinue 18 months after initiation than patients who started initial biologic therapy after July 2013. This observation may be due to changes in clinical practice over the course of the study, likely including increased clinician comfort with higher biologic dosing strategies and increasing use of TDM.
Concomitant immunomodulator use decreases immunogenicity of anti-TNFs in adults with IBD and it is recommended to allow patients who experience mild immunogenicity to continue their course of anti-TNF treatment (3). In 2 observational studies of anti-TNF use among pediatric patients with IBD and CD, 79% and 90%, respectively, of participants also received immunomodulator treatment (21,25). Immunomodulator use has been associated with increased durability of anti-TNFs in children (14). We did not find that concomitant immunomodulator use prolonged biologic durability, although we defined immunomodulator use broadly as documentation of ≥1 dose in the 6 months following biologic initiation; thus, we may have overestimated persistent use.
Limitations
Data collection occurred only at US sites, which limits generalizability of these findings to countries or regions where access to biologic therapy is limited or thresholds for biologic initiation are significantly different. This study evaluated biologic durability, not biologic efficacy. In real-world practice, patients may be continued on ineffective medications if no superior alternative exists. Thus, although there is correlation between durability and efficacy, we cannot draw full conclusions about biologic effectiveness from these data. Although patient data prior to ICN enrollment were not available for the Full Cohort, a random sample analysis estimated that approximately 9% of patients had previous biologic treatment not listed in the registry. Thus, biologic exposure in the Full Cohort was likely even higher than what we report. In addition, information on indications for biologic initiation and reasons for selection of specific first and subsequent agents was limited. Biologic dosing higher than FDA-approved doses is frequently used in clinical practice and can affect response and durability, but dosing was not known in most cases so it was not evaluated in this study. Serum concentrations of biologic drugs and anti-drug antibodies were not analyzed in this study, as TDM was not commonly performed during the study period. Drug concentrations and anti-drug antibodies play a role in biologic discontinuation and choice for subsequent treatment in the clinical setting, and evaluating their role would have provided important information. Finally, the treatment landscape has changed since conclusion of the data collection period. No new treatments have been approved for pediatric CD and UC apart from adalimumab (UC) and infliximab and adalimumab biosimilars, but ustekinumab, tofacitinib, and upadacitinib have been approved for adults with IBD. Vedolizumab was not available until later in the study period. More experience has now been gained with off-label use of biologic therapies in children, and use of advanced therapies beyond anti-TNFs for children has increased. Therefore, the frequency, duration, and patterns of biologic use reported here may not completely reflect current trends of biologic use.
CONCLUSIONS
Pediatric patients with CD are treated more frequently with biologics, are treated with biologics earlier in the disease course, and remain on their first biologic therapy longer than those with UC. While loss of response was the most common reason for discontinuation in both CD and UC, primary nonresponse was more common in UC. As of 2016, more than half of children with CD and more than one-quarter of children with UC in the ICN registry had been treated with 1 or more biologics before age 18. Likely, even more children with IBD are receiving treatment with biologics now, including both anti-TNF and non-anti-TNF therapies.
Factors associated with discontinuation of first and/or second biologics in CD included surrogate markers of more severe disease at biologic treatment initiation including corticosteroid use, higher sPCDAI score, elevated CRP, lower albumin levels, and anemia. Colonic-only CD was associated with first biologic discontinuation, and proximal upper GI tract involvement was associated with second biologic discontinuation in CD. While prospective studies are needed to confirm these findings, these clinical factors should be considered when starting biologic therapy in children with IBD.
Additional ImproveCareNow Network Contributing Authors: Each one has contributed data, critically reviewed the manuscript, and given approval of the final manuscript. Jeremy Adler, Howard I. Baron, José M. Cabrera, Jill M. Dorsey, Dana M.H. Dykes, Dawn R. Ebach, Monica P. Garin-Laflam, Benjamin D. Gold, John Grunow, Leslie M. Higuchi, Traci W. Jester, Sameer P. Lapsia, Ian Leibowitz, Tiffany M. Linvlle, Tina L. Morhardt, Jonathan Moses, Dedrick Moulton, Shaida Nasiri-Blomgren, B. Joanna Niklinska-Schirtz, Nicholas A. Ogunmola, Pablo J. Palomo, K.T. Park, Dinesh S. Pashankar, Brad A. Pasternak, Marcella C. Radano, Charles M. Samson, Kelly C. Sandberg, Marc E. Schaefer, Harohalli Shashidhar, Steven J. Steiner; Jillian S. Sullivan, Gitit Tomer, Sophia G. Verstraete.
Acknowledgments
The authors thank Kelly Olano, BA, Cincinnati Children’s Hospital, Cincinnati, OH, USA, for her contributions to the statistical programming for the study.
The authors also thank the staff and patients at the participating ImproveCareNow Centers: Advocate Children’s Medical Group, Boston Children’s Hospital, Carilion Children’s Hospital, Children’s Health Care of Atlanta at Egleston/Emory University, Children’s Healthcare of Atlanta at Scottish Rite-GI Care for Kids, Children’s Hospital of Alabama, Children’s Hospital at Dartmouth, Children’s Hospital of The King’s Daughters, Children’s Hospital of Wisconsin, Children’s Mercy Hospital, Cincinnati Children’s Hospital Medical Center, Cook Children’s Medical Center, Dayton Children’s Hospital, Floating Hospital for Children at Tufts Medical Center, Helen DeVos Children’s Hospital, Levine Children’s Hospital, Massachusetts General Hospital for Children, Mayo Clinic-Children’s Center, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nemours Children’s Health System/Orlando, Nemours Children’s Health System/Wilmington, Nemours Children’s Specialty Care/Jacksonville, New Hampshire’s Hospital for Children, Oklahoma University Medical Center, Pediatric Gastroenterology & Nutrition Associates, Pediatric Specialists of Virginia, Penn State Hershey Children’s Hospital, Phoenix Children’s Hospital, Rainbow Babies & Children’s Hospital, Riley Hospital for Children, St. Louis Children’s Hospital, Stanford Children’s Health, The Children’s Hospital at Montefiore, UCSF Benioff Children’s Hospital, University of Iowa Stead Family Children’s Hospital, University of Michigan-CS Mott Children’s Hospital, UT, Southwestern/Children’s Hospital, University of Vermont Children’s Hospital, and Yale-New Haven Children’s Hospital.
Supplementary Material
Footnotes
R.B.C. received financial support for research in Janssen Biotech and received consultancy fees from Janssen Biotech and Janssen Research and Development. A.S.P. received lecture fee(s) from Janssen Biotech, AbbVie, and Abbott Nutrition and received consultancy fees from QOL. N.C. is an employee of Takeda Pharmaceuticals U.S.A., Inc., and owns stock or stock options. T.L. was an employee of Takeda Pharmaceuticals U.S.A., Inc., at the time the study was conducted. S.S. is in the Speaker’s Bureau for AbbVie, Inc. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
Sources of Funding: This study was supported by funding provided by Takeda Pharmaceuticals U.S.A., Inc., to the ImproveCareNow Network. Medical writing assistance was provided by Reza Sayeed, MA, and Jennie G. Jacobson, PhD, CMPP, on behalf of Syneos Health Medical Communications, LLC. This assistance was funded by Takeda Pharmaceuticals U.S.A., Inc.
Role of the funder: Some of the authors are employees of Takeda Pharmaceuticals U.S.A., Inc. In addition, peer reviews of the manuscript were performed by other employees of Takeda Pharmaceuticals U.S.A., Inc. The funder had no other role.
Contributor Information
Collaborators: Jeremy Adler, Howard I. Baron, José M. Cabrera, Jill M. Dorsey, Dana M.H. Dykes, Dawn R. Ebach, Monica P. Garin-Laflam, Benjamin D. Gold, John Grunow, Leslie M. Higuchi, Traci W. Jester, Sameer P. Lapsia, Ian Leibowitz, Tiffany M. Linvlle, Tina L. Morhardt, Jonathan Moses, Dedrick Moulton, Shaida Nasiri-Blomgren, B. Joanna Niklinska-Schirtz, Nicholas A. Ogunmola, Pablo J. Palomo, K.T. Park, Dinesh S. Pashankar, Brad A. Pasternak, Marcella C. Radano, Charles M. Samson, Kelly C. Sandberg, Marc E. Schaefer, Harohalli Shashidhar, Steven J. Steiner, Jillian S. Sullivan, Gitit Tomer, and Sophia G. Verstraete
REFERENCES
- 1.Zimmerman L, Bousvaros A. The pharmacotherapeutic management of pediatric Crohn’s disease. Expert Opin Pharmacother 2019;20:2161–8. [DOI] [PubMed] [Google Scholar]
- 2.Bolia R, Rajanayagam J, Hardikar W, Alex G. Impact of changing treatment strategies on outcomes in pediatric ulcerative colitis. Inflamm Bowel Dis 2019;25:1838–44. [DOI] [PubMed] [Google Scholar]
- 3.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care—an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257–91. [DOI] [PubMed] [Google Scholar]
- 4.AVSOLA (infliximab-axxq). Package insert. Amgen; 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761086s000lbl.pdf. Accessed October 21, 2020. [Google Scholar]
- 5.Remicade (infliximab). Package insert. Janssen Biotech, Inc; 2013. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf. Accessed October 21, 2020. [Google Scholar]
- 6.Humira (adalimumab). Package insert. AbbVie; 2021. Available at: https://www.rxabbvie.com/pdf/humira.pdf. Accessed May 27, 2021. [Google Scholar]
- 7.Inflectra (infliximab-dyyb). Package insert. Celltrion; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125544s000lbl.pdf. Accessed October 21, 2020. [Google Scholar]
- 8.Renflexis (infliximab-abda). Package insert. Merck Sharp & Dohme Corp; 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761054orig1s000lbl.pdf. Accessed October 21, 2020. [Google Scholar]
- 9.IXIFI (infliximab-qbtx). Package insert. Pfizer; 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761072s000lbl.pdf. Accessed October 21, 2020. [Google Scholar]
- 10.Breton J, Kastl A, Conrad M, et al. Positioning biologic therapies in the management of pediatric inflammatory bowel disease. Gastroenterol Hepatol 2020;16:400–14. [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsen JR, Sullivan KE, Rabizadeh S, et al. NASPGHAN position paper on the evaluation and management for patients with Very Early-Onset Inflammatory Bowel Disease (VEO-IBD). J Pediatr Gastroenterol Nutr 2020;70:389–403. [DOI] [PubMed] [Google Scholar]
- 12.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007;132:863–73; quiz 1165. [DOI] [PubMed] [Google Scholar]
- 13.Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology 2012;143:365–74.e2. [DOI] [PubMed] [Google Scholar]
- 14.van Rheenen H, van Rheenen PF. Long-term efficacy of anti-tumor necrosis factor agents in pediatric luminal Crohn’s disease: a systematic review of real-world evidence studies. Pediatr Gastroenterol Hepatol Nutr 2020;23:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crandall W, Kappelman MD, Colletti RB, et al. ImproveCareNow: the development of a pediatric inflammatory bowel disease improvement network. Inflamm Bowel Dis 2011;17:450–7. [DOI] [PubMed] [Google Scholar]
- 16.ImproveCareNow. Purpose and success. 2016. Available at: https://www.improvecarenow.org/purpose-success. Accessed March 31, 2020.
- 17.Kappelman MD, Crandall WV, Colletti RB, et al. Short pediatric Crohn’s disease activity index for quality improvement and observational research. Inflamm Bowel Dis 2011;17:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 19.Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–16. [Google Scholar]
- 20.Juillerat P, Sokol H, Froehlich F, et al. Factors associated with durable response to infliximab in Crohn’s disease 5 years and beyond: a multicenter international cohort. Inflamm Bowel Dis 2014;21:60–70. [DOI] [PubMed] [Google Scholar]
- 21.Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis 2009;15:816–22. [DOI] [PubMed] [Google Scholar]
- 22.Aloi M, Bramuzzo M, Arrigo S, et al. Efficacy and safety of adalimumab in pediatric ulcerative colitis: a real-life experience from the SIGENP-IBD Registry. J Pediatr Gastroenterol Nutr 2018;66:920–5. [DOI] [PubMed] [Google Scholar]
- 23.Rosh JR, Lerer T, Markowitz J, et al. Retrospective Evaluation of the Safety and Effect of Adalimumab Therapy (RESEAT) in pediatric Crohn’s disease. Am J Gastroenterol 2009;104:3042–9. [DOI] [PubMed] [Google Scholar]
- 24.Dayan JR, Dolinger M, Benkov K, et al. Real world experience with ustekinumab in children and young adults at a tertiary care pediatric inflammatory bowel disease center. J Pediatr Gastroenterol Nutr 2019;69:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrick VM, Mortier K, Williams LJ, et al. Real-life anti-tumour necrosis factor experience in >500 paediatric United Kingdom inflammatory bowel disease patients. J Pediatr Gastroenterol Nutr 2018;66:274–80. [DOI] [PubMed] [Google Scholar]
- 26.Naviglio S, Lacorte D, Lucafo M, et al. Causes of treatment failure in children with inflammatory bowel disease treated with infliximab: a pharmacokinetic study. J Pediatr Gastroenterol Nutr 2019;68:37–44. [DOI] [PubMed] [Google Scholar]
- 27.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015;64:1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.