Abstract
The endothelium is considered to be the gatekeeper of the vessel wall, maintaining and regulating vascular integrity. In patients with chronic kidney disease, protective endothelial cell functions are impaired due to the proinflammatory, prothrombotic and uremic environment caused by the decline in kidney function, adding to the increase in cardiovascular complications in this vulnerable patient population. In this review, we discuss endothelial cell functioning in healthy conditions and the contribution of endothelial cell dysfunction to cardiovascular disease. Further, we summarize the phenotypic changes of the endothelium in chronic kidney disease patients and the relation of endothelial cell dysfunction to cardiovascular risk in chronic kidney disease. We also review the mechanisms that underlie endothelial changes in chronic kidney disease and consider potential pharmacological interventions that can ameliorate endothelial health.
Keywords: atherosclerosis, cardiovascular diseases, chronic kidney diseases, endothelial cells, vascular stiffness
Increased Cardiovascular Risk in Chronic Kidney Disease
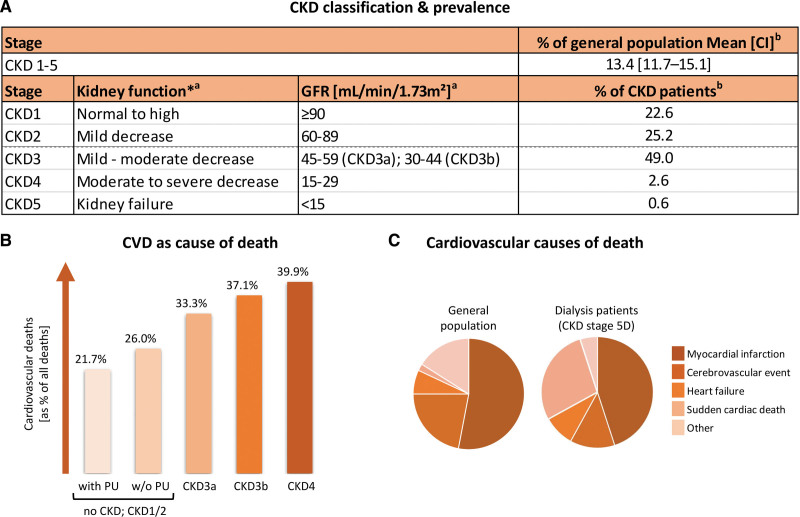
Chronic kidney disease (CKD) is defined by kidney damage or a reduced kidney filtration function (glomerular filtration rate <60 mL/minute per 1.73 m²) for a period beyond 3 months that affects health.1 With a global prevalence of ≈13.4%,2 CKD imposes a serious burden on our socioeconomic and health care system. CKD is a progressive disease, classified into stages 1 to 5 based on the reduction in kidney function (Figure 1A).1 In end-stage kidney disease (CKD stage 5: glomerular filtration rate <15 mL/minute per 1.73 m²), patients require kidney support therapy as dialysis or kidney transplantation to replace the failing kidney function.
Of note, patients with moderate to advanced CKD are at increased cardiovascular risk compared with the general population and patients with mild CKD, with lower estimated glomerular filtration rate and higher albuminuria identified as risk factors for all-cause and cardiovascular mortality, independent of traditional cardiovascular risk factors.5,6 Overall, 33.3% to 37.1% of CKD3(a/b) patients and 39.9% of CKD4 patients die from cardiovascular diseases (CVD) compared with 21.7% to 26% of the general population (Figure 1B).3 For patients with end-stage kidney disease aged 25 to 34, annual mortality is even increased 500 to 1000 times compared with similarly aged controls with healthy kidney function and is comparable to 85-year olds in the general population, underscoring the high cardiovascular burden in CKD.6
Figure 1.
Chronic kidney disease (CKD) prevalence and cardiovascular risk in CKD. A, CKD classification and prevalence. *Compared with young adult level. a/bFigure based on data by aInker et al1 and bHill et al.2 B, Percentage of cardiovascular deaths according to CKD stage (age and sex adjusted). Figure based on data by Thompson et al.3 C, Cardiovascular causes of death in the general population compared with dialysis patients (CKD stage 5D). Figure based on data by Roberts et al.4 CVD indicates cardiovascular disease; GFR, glomerular filtration rate; and PU, proteinuria.
In the general population, myocardial infarction and cerebrovascular events are the most important cardiovascular causes of death, underlying ≈75% of all CVD-related deaths (Figure 1C).4 Also, in CKD5 patients on dialysis (CKD5D), these CVD types remain important as they account for 45% (myocardial infarction) and 13% (cerebrovascular events) of deaths by cardiovascular causes, though with a joint responsibility of ≈58% of all CVD-associated deaths, they are reduced in relative importance compared with the general population. Instead, CKD5D patients show a relative increase in sudden cardiac death and heart failure, being responsible for 28% and 9% of all CVD-associated deaths compared with 2% and 7% as observed in the general population (Figure 1C).4 This reveals a high increase especially in the risk of sudden cardiac death in advanced CKD.4
Atherosclerosis and Myocardial Infarction
The main underlying cause of both myocardial infarction and stroke is atherosclerosis, a lipid-driven inflammatory disease of medium to large-sized arteries triggering the development of atherosclerotic lesions.7 These lesions gradually grow over time and can ultimately restrict blood flow or trigger thrombosis through plaque rupture or erosion.7,8 Patients with CKD3-5D show an increased prevalence of subclinical atherosclerotic lesions compared with the general population, with a larger increase in more advanced CKD stages after adjustment for sex, age and diabetes.9,10 Furthermore, compared with patients without CKD progression, patients with CKD progression over 24 months displayed more frequently also a progression of atherosclerotic lesions as detected by ultrasound.10,11 After acute myocardial infarction, patients with CKD show a reduced survival over time compared with non-CKD patients, with an increased risk of death as well as non-fatal cardiac events with increasing CKD stage.12
Sudden Cardiac Death and Uremic Cardiomyopathy
In the general population, coronary heart disease is responsible for 80% of sudden cardiac deaths.13 The disproportional increase in sudden cardiac death in patients with advanced CKD suggests differences in its pathophysiology and causes as kidney function declines. Left ventricular hypertrophy is significantly associated with increased risk of sudden cardiac death in the general population14 and can be caused by cardiac preload (intravascular volume overload), cardiac afterload (pressure overload), or afterload/preload-independent factors.15 Patients with CKD present more frequently with left ventricular hypertrophy, with a prevalence of up to 40% and even 75% in patients in CKD5D.16 Together with cardiac fibrosis, left ventricular hypertrophy is one of the hallmarks of uremic cardiomyopathy and may trigger cardiac electrical disturbances and lethal arrhythmias.15
Endothelial (Cell) Dysfunction as Contributor to Cardiovascular Risk
A main contributor to increased cardiovascular risk is endothelial cell dysfunction, which encompasses a whole array of maladaptive alterations in the endothelial cell functional phenotype associated with increased cardiovascular risk. This term was suggested in an excellent review by Gimbrone et al17 to provide a distinction from the more narrow term “endothelial dysfunction,” which typically has been used to refer to endothelial abnormalities triggering a reduction in nitric oxide bioavailability and associated vascular relaxation. Significant endothelial heterogeneity exists across the vascular tree, for example, when comparing arteries versus veins, as well as the macrovasculature (including the large elastic as well as muscular conduit arteries) versus the microvasculature (including the capillaries, arterioles, and venules) and both endothelial cell dysfunction at the macro- and microvascular level contributes to increased cardiovascular risk. In this review, we discuss the contribution of endothelial cell dysfunction to CVD with a special focus on patients with CKD. We review findings on molecular mechanisms underlying endothelial cell dysfunction in CKD as well as discuss the impact of pharmacological interventions.
Endothelial Cell Dysfunction as Contributor to Cardiovascular Risk
Endothelial Cell Dysfunction and Atherosclerotic Risk
The endothelial cell layer of the vasculature provides a semipermeable barrier enabling a regulated exchange of fluids, molecules, and cells and plays an important role in maintaining vascular health (Figure 2). Macrovascular endothelial cell dysfunction is an early event in the development of atherosclerotic lesions. On the one hand, it is influenced by hemodynamic factors: in atherosclerosis-resistant areas of the arteries, a laminar blood flow contributes to a protective endothelial cell phenotype. However, atherosclerosis-prone regions of the arterial vasculature are exposed to a disturbed, oscillatory blood flow and associated low time-averaged shear stress, which induce oxidative stress, endothelial phenotypic changes, and cell junction alterations as well as endothelial cell turnover (as discussed in more detail in the review by Gimbrone et al17). Furthermore, inflammatory triggers as proinflammatory cytokines, oxLDL (oxidized low-density protein) as well as different cardiovascular risk factors as metabolic disturbances and smoking, contribute to endothelial cell dysfunction. Also, excessive stretch on blood vessels can trigger endothelial permeability, inflammatory responses and oxidative stress.18 Combined, this triggers proinflammatory signaling in endothelial cells with an upregulation of proinflammatory cytokines (eg, IL [interleukin]-1, IL-8), chemokines (eg, C-C motif chemokine ligand 2), and endothelial-leukocyte adhesion molecules (VCAM-1 [vascular cell adhesion molecule 1], ICAM-1 [intercellular adhesion molecule 1], P-selectin), reduces endothelial production of atheroprotective nitric oxide and increases endothelial permeability. As a result, inflammatory leukocytes are recruited, adhere to the inflamed endothelium and infiltrate into the vascular wall, where they together with accumulated lipids contribute to the development and progression of atherosclerotic lesions.7 The atheroprotective phenotype of the endothelium is regulated by master transcription factors as KLF (Kruppel-like factor)-2, KLF-4, and NRF (nuclear factor erythroid 2-related factor)-2, whereas NF-κB (nuclear factor-κB) is a key transcription factor driving endothelial inflammation.17 Furthermore, endothelial cells demonstrate a de-differentiation and increased heterogeneity during atherosclerosis progression, with also signs of endothelial-to-mesenchymal transition. Endothelial-to-mesenchymal transition is characterized by the acquirance of mesenchymal cell functions as ECM (extracellular matrix) production and is mainly driven by the transcription factors Snail, Slug, and Twist1. Its extent has been associated with the severity of atherosclerosis plaques in human arteries,19,20 and animal studies investigating key regulators of endothelial-to-mesenchymal transition suggested an important role in plaque progression20 and calcification,21 as discussed in detail in an excellent review by Souilhol et al.22
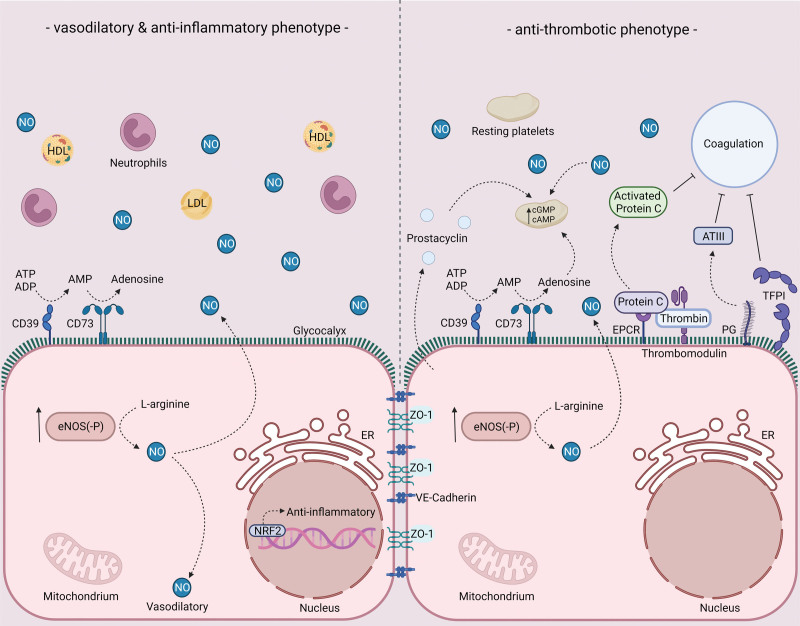
Figure 2.
Endothelial phenotype in healthy conditions in relation to thrombosis, vasoreactivity and inflammation. Vasodilatory, anti-inflammatory, and antithrombotic characteristics of healthy endothelium. For more information, see text. ADP indicates adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; ATIII, antithrombin III; cAMP, cyclic adenosine monophosphate; CD39, ectonucleoside triphosphate diphosphohydrolase-1; CD73, ecto-5’-nucleotidase; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; EPCR, endothelial protein C receptor; ER, endoplasmic reticulum; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NO, nitric oxide; NRF-2, nuclear factor erythroid 2-related factor 2; PG, proteoglycans; TFPI, tissue factor pathway inhibitor; and ZO, zonula occludens.
Of note, endothelial cell dysfunction does not only contribute to plaque initiation, progression, and destabilization with subsequent plaque rupture but also to atherosclerotic plaque erosion, which is expected to be responsible for one-third of acute coronary syndromes. Here, chronic low-grade endothelial activation by for example TLR (toll-like receptor)-2 ligands combined with endothelial cell apoptosis and catabolism of basement membrane components can trigger endothelial cell detachment with subsequent thrombus formation on the denuded area.8
Endothelial Cell Dysfunction and Thrombotic Risk
A healthy, functional endothelial layer at macro- and microvascular levels is crucial in the regulation of hemostasis and interferes both at the level of primary as well as secondary hemostasis to prevent unwanted platelet activation and coagulation (Figure 2). At the level of primary hemostasis, the endothelium elicits strong platelet inhibition by the continuous secretion of nitric oxide (NO) and prostacyclin causing an increase in intraplatelet cGMP (cyclic guanosine monophosphate) and cAMP (cyclic adenosine monophosphate) levels, respectively.23 To prevent platelet activation by extracellular ATP and ADP, the endothelial layer expresses CD39 and CD73, ectonucleases that convert ATP and ADP to adenosine, a platelet inhibitor that by increasing platelet cAMP levels elevates the platelet’s activation threshold.24 In addition, the endothelial glycocalyx repels platelets by its negative charge and as such aids in the prevention of platelet adhesion.25,26 At the level of secondary hemostasis, within the glycocalyx, heparan sulfate proteoglycans bind and promote the activity of ATIII (antithrombin III), a potent inhibitor of multiple coagulation factors including thrombin, FIXa, FXa, FXIa, and FXIIa.26,27 Furthermore, endothelial cells express TFPI (tissue factor pathway inhibitor), a serine protease that—as its name suggests—interferes with TF (tissue factor)-induced coagulation and thereby limits the activity of the extrinsic pathway.27 Next to TFPI, endothelial cells constitutively express thrombomodulin, a membrane-bound protein that captures thrombin from the circulation and upon binding increases the affinity of thrombin for the anticoagulant protein C. Together with protein S, activated protein C disables FVa and FVIIIa.27
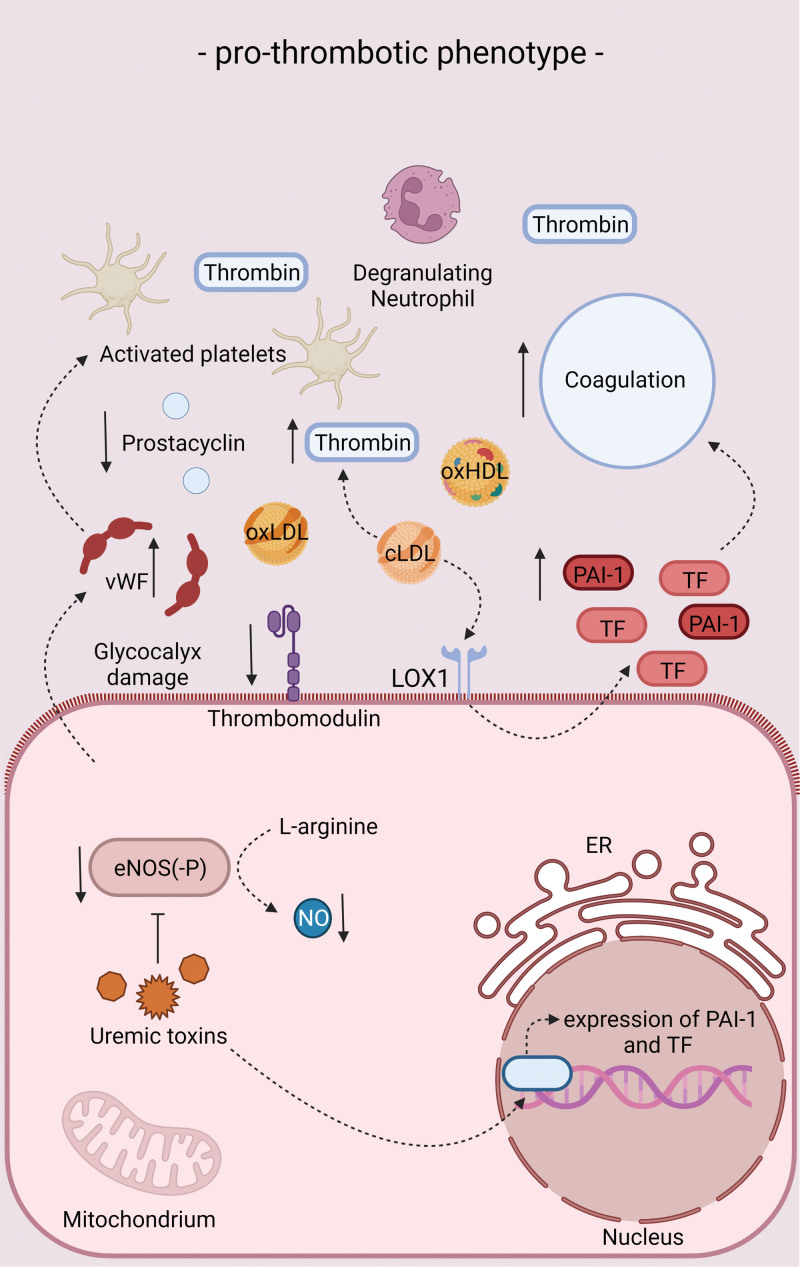
Upon disturbances in lipid metabolism, inflammation, oxidative stress, and pathophysiological shear stress, endothelial cell dysfunction develops, characterized by a diminishment of antithrombotic and anti-inflammatory properties and a degradation of the glycocalyx. Concomitantly, the dysfunctional endothelium takes on proinflammatory and prothrombotic characteristics (Figures 3 and 4).27 As a result, the production of NO and prostacyclin decreases, while the secretion of prothrombotic and proinflammatory molecules like vWF (von Willebrand factor) and C-C motif chemokine ligand 2 increases.27 Furthermore, the expression of thrombomodulin is strongly downregulated upon endothelial cell dysfunction resulting in a downregulation of protein C activation, whilst the expression of TF is upregulated favoring the activation of coagulation.27 Already during the early stages of atherogenesis, neutrophil extracellular traps are implicated in endothelial cell dysfunction and fuel the thromboinflammatory response.28
Figure 3.
Endothelial phenotype in chronic kidney disease (CKD) conditions in relation to thrombosis. Endothelial phenotype in CKD conditions in terms of thrombosis. For more information, see text. cLDL indicates carbamylated low-density lipoprotein; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; LOX-1, lectin-type oxidized low-density lipoprotein receptor 1; NO, nitric oxide; oxLDL, oxidized low-density lipoprotein; PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; and vWF, von Willebrand factor.
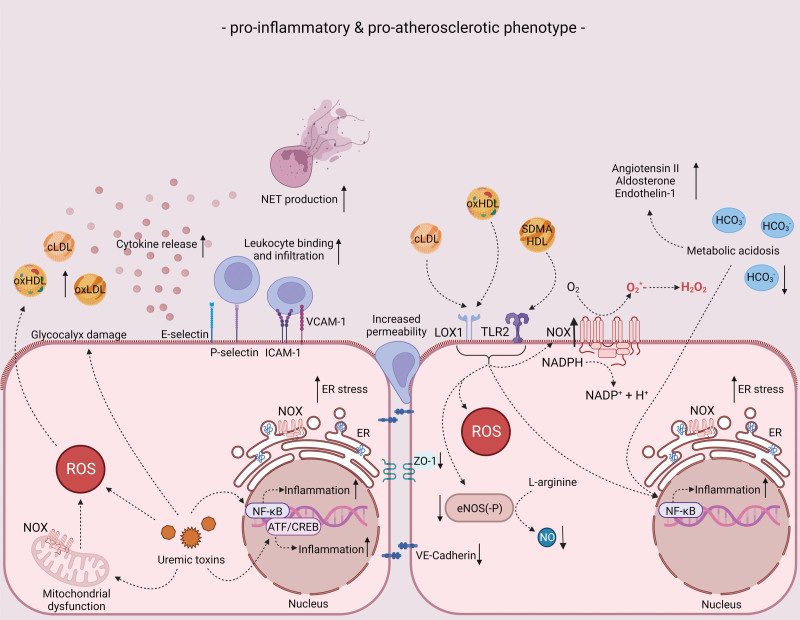
Figure 4.
Endothelial phenotype in chronic kidney disease (CKD) conditions in relation to inflammation and atherosclerosis. Endothelial phenotype in CKD conditions in terms of proinflammatory and proatherosclerotic characteristics. For more information, see text. ATF indicates activating transcription factor; cLDL, carbamylated low-density lipoprotein; CREB, cyclic adenosine monophosphate response element-binding protein; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; E-selectin, endothelial-leukocyte adhesion molecule 1; HCO3−, bicarbonate; H2O2, hydrogen peroxide; ICAM-1, intercellular adhesion molecule 1; LOX-1, lectin-type oxidized low-density lipoprotein receptor 1; NADPH, nicotinamide adenine dinucleotide phosphate; NET, neutrophil extracellular trap; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; O2, oxygen; O2*−, superoxide; oxHDL, oxidized high-density lipoprotein; oxLDL, oxidized low-density lipoprotein; P-selectin, granule membrane protein 140; ROS, reactive oxygen species; SDMA, symmetric dimethylarginine; TLR2, toll-like receptor 2; VCAM-1, vascular cell adhesion molecule 1; and ZO, zonula occludens.
All in all, these prothrombotic and proinflammatory responses of the dysfunctional endothelium spiral out of control, creating a vicious circle in which endothelial cell dysfunction progresses and vascular integrity is lost, resulting in a strongly increased thrombotic risk.
Endothelial Dysfunction, Reduced Vasorelaxation, Increased Vascular Stiffness, and Cardiovascular Risk
Aging as well as pathophysiological remodeling of the vascular wall by cardiovascular risk factors (eg, hypertension, diabetes, kidney disease) induce arterial stiffening, which reduces arterial compliance and increases pulsative shear and pressure on the vasculature. On structural level, arterial stiffness is characterized by collagen deposition and elastin degradation in the ECM.29 Furthermore, vascular smooth muscle cell (VSMC) tone and endothelial dysfunction impact vascular reactivity, with an impaired relaxation capacity of VSMCs as well as a reduced endothelium-dependent vasorelaxation contributing to arterial stiffness. Endothelial cells play an important role in vasorelaxation through NO production by eNOS (endothelial NO synthase; Figure 2), with reduced production and/or bioavailability of NO or other vasodilatory substances reducing the vascular relaxation capacity (Figure 5).
Figure 5.
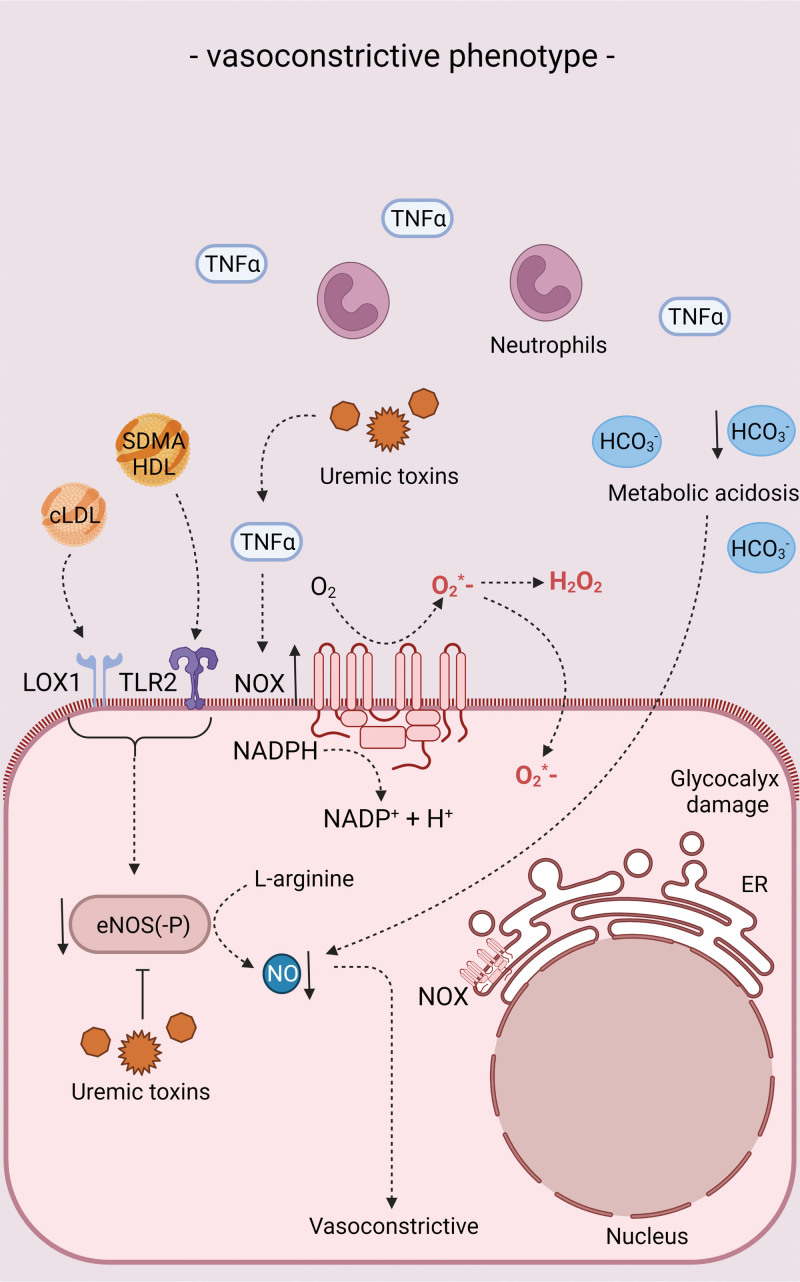
Endothelial phenotype in chronic kidney disease (CKD) conditions in relation to vasoreactivity. Endothelial phenotype in CKD conditions in terms of vasodilation/vasoconstriction. For more information, see text. eNOS indicates endothelial nitric oxide synthase; ER, endoplasmic reticulum; HCO3−, bicarbonate; HDL, high-density lipoprotein; H2O2, hydrogen peroxide; LOX-1, lectin-type oxidized low-density lipoprotein receptor 1; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; NOX, NADPH oxidase; O2, oxygen; O2*−, superoxide; SDMA, symmetric dimethylarginine; TLR2, toll-like receptor 2; and TNFɑ, tumor necrosis factor ɑ.
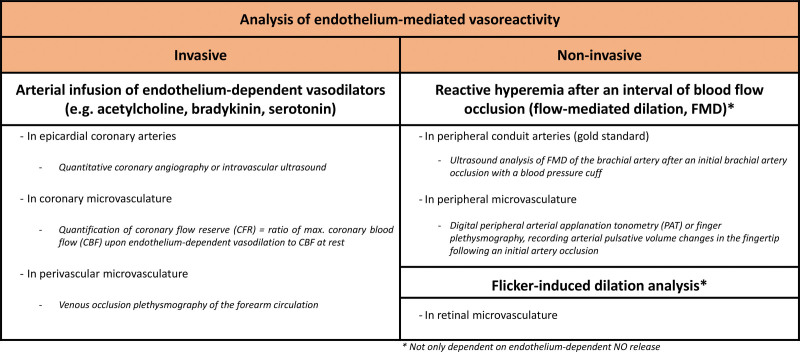
The gold standard for quantifying arterial stiffness is the analysis of blood flow rate, measured as pulse wave velocity (PWV, analyzed as carotid-femoral PWV or brachial-ankle PWV). Alternatively, the shape of the arterial pressure waveform (pulse wave analysis) provides insights into arterial stiffness through quantification of the augmentation index. Endothelial function in terms of vasodilatory responses can be measured either invasively or noninvasively within the epicardial coronary arteries, the peripheral conduit arteries (analyzing flow-mediated dilation [FMD] of the brachial artery as gold standard) or within the coronary or peripheral microvasculature (Figure 6). Peripheral and coronary endothelial dysfunction have been reported to correlate with each other,30,32 although others have reported on rather modest correlations between peripheral and coronary endothelial dysfunction and suggested a potential reflection of different pathologies or vascular beds.33 Furthermore, whereas PWV and the augmentation index were shown to correlate well, this was not always the case for the augmentation index and the “reactive hyperemia index” as noninvasive endothelial functional readout in the periphery, potentially due to the impact of other factors than endothelial function on vascular stiffness.34
Figure 6.
Overview of endothelium-dependent vasoreactivity analysis in patients. For a more in-depth discussion of these methods, we refer to 2 excellent reviews.30,31 CBF indicates coronary blood flow; FMD, flow-mediated dilation; and PAT, peripheral arterial tonometry.
Vascular stiffness is an early prognostic marker of coronary heart disease.35 Vascular stiffness increases the load on the heart; it urges a greater energy demand for cardiac ejection and triggers cardiac hypertrophy.29 With increased arterial stiffness being associated with a reduced Windkessel function (ie, a reduction in the elastic buffering capacity required to dampen blood pressure fluctuations), arterial stiffness also imposes an increased pulsatility on the microcirculation and enhanced cyclical stretch on endothelial cells. Recently, it was shown that the latter triggers the secretion of protein GAS6 (growth arrest specific 6) by endothelial cells with subsequent proinflammatory GAS6/Axl signaling in monocytes and their transformation to macrophages and dendritic cells. As shown in a mouse model of Angiotensin II-induced chronic aortic remodeling, this then contributed to vascular and kidney inflammation,36,37 linking arterial stiffness to further end-organ damage.
As endothelial dysfunction is an early event in CVD that precedes macrovascular complications, also endothelial functional analyses in terms of vascular tone regulation have been intensively studied for a predictive capacity of cardiovascular risk beyond traditional cardiovascular risk factors. In multiple studies, macro- and microvascular endothelial dysfunction could independently predict cardiovascular events in patients at risk for coronary artery disease.31 For example, in relation to endothelial reactivity of the conduit arteries (macrovasculature), medium to low endothelial function recorded by brachial artery FMD was associated with increased risk of cardiac events in patients with peripheral artery disease undergoing vascular surgery.38 Also in patients with chronic heart failure, endothelial dysfunction analyzed by brachial and radial FMD was associated with future cardiac events or mortality.39–41 Others showed healthy endothelial function recorded by brachial FMD in apparently healthy individuals to result in a significantly higher survival over a 5-year follow-up, even after adjustment for traditional risk factors.42,43 Thus, endothelial cell dysfunction can add important information on cardiovascular risk beyond traditional risk factors.30 Of note, this value of endothelial dysfunction analysis for cardiovascular risk prediction seems to be influenced by the analyzed patient cohort, since others could not confirm a cardiovascular risk prediction value for FMD-based analysis of endothelial dysfunction in an elderly cohort, potentially due to reduced arterial compliance (i.e. the vascular ability to expand upon pressure increases) in elderly subjects.44 Nonetheless, this study did report an association of peripheral microvascular endothelial dysfunction with increased cardiovascular risk, as discussed in the next paragraph in more detail.
Microvascular Dysfunction and Cardiovascular Risk
Whereas larger arteries are predominantly affected by atherosclerotic changes, dysfunction of the microvasculature—the network of arterioles, capillaries, and venules enabling tissue perfusion—is of a different nature. Microvascular dysfunction (MVD) can develop at the level of the coronary microcirculation as well as in the periphery in the absence or presence of obstructive artery disease of the larger vessels. The development of MVD is multifactorial and can be the result of functional or structural changes or a combination thereof, depending on the underlying disease. In terms of functional changes, impaired vasodilatory responses—at least partially endothelium-dependent—underlie MVD. Furthermore, increases in the levels of vasoconstrictive substances in combination with an enhanced responsiveness toward these stimuli have been implicated in MVD, contributing to the occurrence of vascular spasm. Structural changes associated with MVD encompass luminal narrowing of the microvasculature due to adverse vascular remodeling and perivascular fibrosis; microvascular compression; microvascular rarefaction resulting in a loss of coherent microvascular trees (capillaries, small arterioles, and venules); and microembolization of atherosclerotic and thrombotic material.45–48
As for macrovascular endothelial dysfunction, also microvascular endothelial dysfunction has been associated with cardiovascular risk.49 This was for example the case in a population-based prospective study, in which peripheral microvascular endothelial dysfunction—but not FMD-mediated endothelial function analysis of conduit arteries—correlated with cardiovascular events (myocardial infarction, stroke or death) in elderly patients during 5 years of follow-up, even beyond major cardiovascular risk factors from the Framingham risk score.44 Along the same line, peripheral microvascular endothelial dysfunction predicted ischemic heart disease, and even performed better in future cardiovascular risk prediction in nonobstructive coronary artery disease compared with other risk scores.50 Also, retinal arteriolar endothelial dysfunction independently predicted MACE in patients with or at high risk of coronary artery disease.51
Also in relation to heart failure, both patients with heart failure with reduced left ventricular ejection fraction as well as patients with heart failure with preserved ejection fraction present with microvascular dysfunction. In failure with reduced left ventricular ejection fraction, peripheral microvascular endothelial function analysis was associated with an increased rate of HF-related events.52 Patients with heart failure with preserved ejection fraction present with both large vessel as well as microvascular dysfunction.53 Furthermore, they display a reduction in cardiac microvascular density54 as well as an increase in markers of endothelial cell dysfunction in myocardial biopsies, including the upregulation of endothelial adhesion molecules (E-selectin, ICAM-1), pro-oxidative regulators (NOX-2) and eNOS uncoupling.55 Coronary microvascular dysfunction has been proposed to contribute to cardiac wall stiffening and diastolic dysfunction in heart failure with preserved ejection fraction patients. However, whether this involves a real causal relation or rather a non-causal association between coronary microvascular dysfunction and heart failure with preserved ejection fraction remains debated.56,57 Of note, coronary microvascular dysfunction may also have a pathophysiological and prognostic role in other types of CVD, for which we refer to an excellent recent review by Del Buono et al.46
Endothelial Cell Dysfunction and Vascular Aging
Endothelial cell dysfunction with reduced endothelial vasodilation capacity, increased inflammation and permeability and enhanced prothrombotic properties, all described above, is an important hallmark of vascular aging. Endothelial cell dysfunction is not only observed in the macrocirculation but also contributes to microvascular dysfunction upon aging. Vascular aging is furthermore characterized by functional and structural changes of the vascular wall and adventitia by processes of e.g. inflammation, vascular calcification and ECM remodeling, which further contribute to increased vascular stiffness and cardiovascular risk.58
Increased Endothelial Cell Dysfunction in CKD
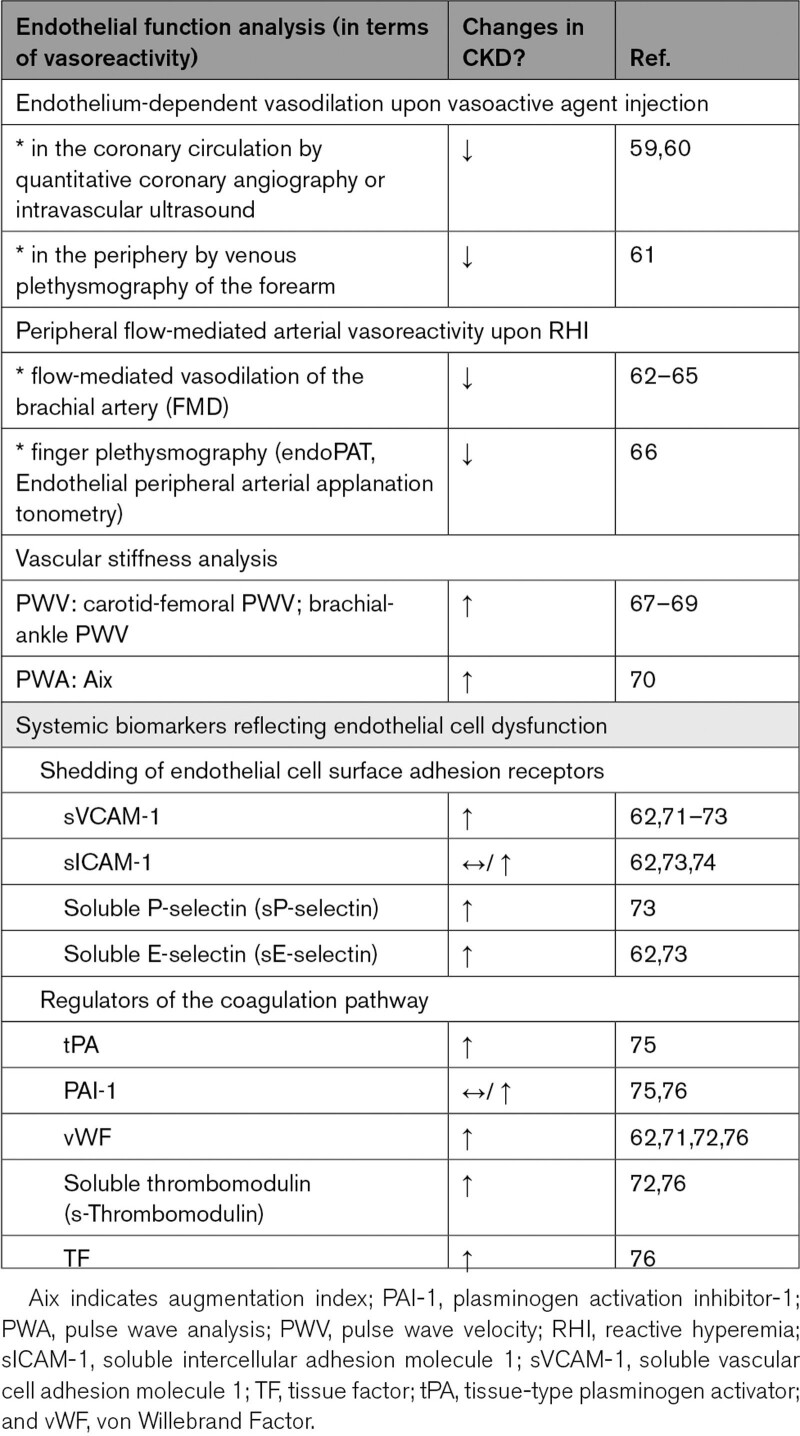
Endothelial cell dysfunction in the context of CKD has been extensively investigated both in animal studies as well as in patients (Table 1). Overall, these studies revealed a reduced endothelium-dependent vasodilation, endothelial glycocalyx damage, an increased endothelial permeability as well as increased proinflammatory and prothrombotic properties. Along with macrovascular endothelial cell dysfunction, CKD is associated with microvascular dysfunction and rarefaction. Also, CKD patients increasingly present with medial calcification, which has been associated with increased arterial stiffness.77,78 Combined with aging-associated characteristics of the vascular wall and the immune system,79 these pathophysiological changes underlie the vision of CKD as a process of increased vascular aging.80
Table 1.
Endothelial (Cell) Dysfunction Analyses in Patients With CKD
Insights From Animal Studies
Animal models of CKD have provided us with many valuable insights in CKD-induced endothelial cell dysfunction as they allow the in vivo assessment of endothelial (cell) function. Induction of CKD in rats has been shown to lead to a reduced endothelium-dependent relaxation,81 an increased endothelial permeability of the thoracic aorta,82 and an increase in microvascular permeability that was coupled to a loss of the endothelial surface layer in a rat model of proteinuric kidney disease.83 Furthermore, extensive damage to the glycocalyx was shown in 5/6-nephrectomized rats, indicated by a decreased thickness of the aortic glycocalyx and increased levels of the glycocalyx component syndecan-1 in plasma.84 Also, in mouse models of CKD, endothelial cell dysfunction has been demonstrated at macro- and microvascular level, illustrated by a significant reduction in glycocalyx thickness and density, an upregulated expression of glycocalyx components, decreased endothelium-dependent relaxation in response to acetylcholine of aortic rings and an increased endothelial expression of ICAM-1 and VCAM-1.85–87 Further, using microcomputed tomography imaging of the preglomerular arteries, Ehling et al47 showed that kidney dysfunction resulted in a reduction in vessel diameter and vessel branching as well as an increase in vessel tortuosity in 3 murine models of progressive kidney disease and kidney fibrosis. In combination with an Apolipoprotein E-deficient background, CKD induction furthermore resulted in a higher plaque burden, indicating a higher atherosclerotic risk in CKD.85
Insights From Patient Studies
Reduced Macrovascular Endothelium-Dependent Vasodilation in CKD
In terms of functional responses of the peripheral macrovasculature as assessed by FMD, multiple studies have shown that CKD patients have a significant impairment in endothelium-dependent vasodilation,62–64 which increases with increasing CKD severity,64,88 independently predicts cardiovascular outcome64 and correlates with left ventricular hypertrophy.65 Also, endothelium-independent vasodilation as measured by brachial FMD analysis upon nitroglycerin injection was significantly reduced in patients with advanced CKD—but not in patients with coronary artery disease and healthy kidney function—with vascular calcification suggested as potential underlying mechanism.89 Additionally, multiple studies showed that as CKD progresses arterial stiffness increases,67,68,70 although others could not confirm a correlation between PWV and kidney function after adjustment of covariates (including, but not limited to, age, sex, comorbidities, and medication use).69
MVD and Rarefaction in CKD
CKD has been associated with an increased dysfunction of both the peripheral as well as coronary microvasculature and as CKD advances the structural and functional capillary density declines.49,59,90 In human myocardial specimens of CKD patients, microvascular density was found to be reduced by on average 32%49; it could already be observed in CKD3-4 and was accompanied by increased endothelial-to-mesenchymal transition and myocardial fibrosis.49,91 Although CKD patients often present with comorbidities that strongly contribute to the development of MVD (eg, hypertension and diabetes), CKD-induced MVD—or uremic angiopathy—extends beyond these factors, with the resulting dysfunction of the microvasculature further adding to the progression of CKD. Evidence is derived from animal models of CKD as well as from CKD patient studies in which it was shown that CKD-induced microvascular rarefaction occurs in a heterogeneous pattern resulting in the development of large avascular areas.48,49
Venous and finger plethysmography measurements indicated a compromised endothelium-dependent vasodilatory response in nondialysis-dependent CKD,61,66 although Wang et al92 did not observe a correlation between estimated GFR and the reactive hyperemia index as measured by peripheral arterial tonometry. Focusing on coronary microvascular dysfunction, a recent systematic review with meta-analysis indicated CKD patients to have a significantly lower coronary flow reserve compared with non-CKD patients.59 As in the general population, reduced coronary flow reserve as readout of coronary microvascular dysfunction is predictive for cardiovascular risk in CKD patients, even after adjustment for traditional cardiovascular risk factors.60,93 Restoration of kidney function upon kidney transplantation partially ameliorated microvascular function as assessed by analysis of the sublingual microcirculation and the coronary flow reserve, suggesting that to some extent microvascular changes in CKD are reversible.94,95
Endothelial Glycocalyx Damage in CKD
In patients with CKD, increased levels of the glycocalyx components syndecan-1 and hyaluronan in combination with elevated hyaluronidase activity have been detected in serum, with highest levels observed in CKD patients on dialysis.71,84,96 Along the same line, treatment of human endothelial cells in vitro with uremic serum from pediatric CKD patients or adult hemodialysis patients led to a decrease in glycocalyx height and stiffening of the endothelial cells.97 Glycocalyx damage was confirmed in dermal biopsies of CKD patients, in which a severe reduction in staining with ulex europaeus agglutinin-1 (a lectin binding glycoproteins and glycolipids) was observed in biopsies obtained from nondialysis-dependent CKD patients as well as hemodialysis patients.98 Measurements of the perfused boundary region, as indicator of the thickness of the glycocalyx, are less consistent among studies. While Vlahu et al96 observed an increase in the perfused boundary region and perfused diameter, Liew et al71 detected no significant difference in the perfused boundary region. Three months after kidney transplantation, markers of the endothelial glycocalyx improved as the perfused boundary region decreased and serum syndecan-1, sVCAM-1, and vWF levels were reduced in comparison to pretransplant levels.99 Serum hyaluronan levels remained unchanged.99
Soluble Biomarkers of Endothelial Health in CKD
Systemic biomarkers have been used to provide insights into the extent of endothelial cell dysfunction in patients with increased cardiovascular risk (Table 1). Dysfunctional endothelium typically produces less NO through reduced production or activity of eNOS, but the short half-life of NO makes it a difficult readout for accurately assessing endothelial cell function. Alternative systemic biomarkers that have been used when studying endothelial cell dysfunction include shedded forms of inflammation-induced cell surface adhesion receptors (eg, soluble forms of VCAM-1, ICAM-1, P-selectin, and E-selectin, with the latter regarded as most selective for endothelial cells).100 With the endothelium playing an important regulating role in hemostasis, also molecules involved in platelet activation and coagulation are often quantified to assess the thrombotic responses of a potentially dysfunctional endothelium. This includes systemic levels of vWF, soluble thrombomodulin, tPA (tissue-type plasminogen activator), and PAI-1 (plasminogen activation inhibitor-1; a fibrinolysis inhibitor).100,101 However, it should be noted that most of these systemic biomarkers are not specific for inflamed or damaged endothelium and only provide indirect insights into potential endothelial cell dysfunction.
Specifically in nondialysis-dependent patients in CKD stage 3-5, plasma and serum levels of asymmetric dimethylarginine (an eNOS inhibitor), L-arginine (the precursor of NO), sVCAM-1, sE-selectin, sP-selectin, s-thrombomodulin, tPA, uPA (urokinase-type plasminogen activator), TF and vWF were elevated (Table 1).71–73,75,76,102 These markers of endothelial activation associated significantly with albuminuria and a decreased estimated GFR as markers for CKD severity.62,71 Even a mild impairment in kidney function associated independently with plasma levels of vWF and sVCAM-1 as markers of endothelial activation.74 The effects of CKD on systemic levels of PAI-1 and sICAM-1 are less clear, varying from unchanged62,74,76 to increased levels in nondialysis CKD patients.73,75
Mechanistic Insights Into Endothelial Cell Dysfunction in CKD
A diversity of pathophysiological processes has been identified to contribute to endothelial cell dysfunction in CKD. These include chronic low-grade inflammation, increased oxidative stress, the accumulation of uremic toxins and associated posttranslational modifications of proteins and lipoprotein particles, as well as metabolic acidosis and high sympathetic activation (Figures 3 through 5). Furthermore, patients with CKD display signs of advanced vascular aging and present with vascular calcification. In the following paragraphs, the interaction of each of these different factors with endothelial cell dysfunction in CKD is being discussed. Effects of traditional cardiovascular risk factors such as hypertension and diabetes as frequent comorbidities and causes of CKD, are not discussed.
Chronic Low-Grade Inflammation
In the damaged kidney, unresolved low-grade inflammation triggers resident kidney cells to produce proinflammatory cytokines and chemokines and induce the deposition of ECM contributing to tubulointerstitial fibrosis. Thereby, chronic low-grade inflammation is an important driver of CKD progression.60 Furthermore, the reduced filtration function of the kidneys in CKD leads to a systemic uremic milieu, which triggers systemic, chronic low-grade inflammation by altering the expression and release of proinflammatory mediators, such as cytokines, in the blood.103 Hallmarks of low-grade inflammation in CKD are elevated systemic levels of the proinflammatory cytokines IL-1β, IL-6, TNF (tumor necrosis factor)-α and hsCRP (high-sensitivity C-reactive protein), which accumulate in blood due to an increased release as well as a reduced kidney clearance.104,105
Systemic proinflammatory biomarkers are associated with CKD progression106 as well as with cardiovascular risk in patients with CKD.107 For example, serum levels of hsCRP could predict cardiovascular events in CKD patients,108,109 with an adjusted hazard risk of 1.94 for cardiovascular mortality over a median follow-up of 10 years for CKD3-4 patients with high CRP (≥3 mg/L) compared with low CRP (<3 mg/L).109 Also, systemic levels of IL-6 correlated with a poor outcome in CKD, and both IL-6 and TNF-α could predict all-cause mortality in CKD5D patients after adjustment for age, sex, diabetes, smoking, baseline kidney function, protein energy wasting as well as other inflammation biomarkers as hsCRP (high-sensitivity C-reactive protein).104,107 On the other hand, also the anti-inflammatory IL-10 is upregulated in patients with CKD through increased secretion by uremic monocytes and reduced kidney clearance, with IL-10 counteracting the activated inflammatory response.104 Increased IL-10 levels were found to be positively correlated with cardiovascular events in CKD patients at follow-up, with increased IL-10 levels interpreted as an increased compensatory effect in an overall proinflammatory state in CKD patients.110
Antibody-mediated blockade of IL-1β in the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) reduced the occurrence of major adverse cardiovascular events in patients with moderate CKD (CKD3a) patients over a mean follow-up of 3.7 years.111 Blocking IL-6 for 24 weeks in patients with CKD3-5D and residual inflammatory risk in the RESCUE trial significantly reduced hsCRP as systemic inflammation marker, with the ZEUS study (Zlitivekimab Cardiovascular Outcomes Study) currently evaluating a potential benefit on cardiovascular outcome in CKD3-4 patients with high residual inflammatory risk.112
Chronic inflammation as well as the abovementioned cytokines are linked with endothelial inflammation and endothelial cell dysfunction. For example, IL-6 is known to trigger CRP production, with both IL-6113 and CRP114 decreasing endothelial production and/or activation of eNOS and thereby NO as crucial molecule in the maintenance of vascular health. IL-6 also actively damages the endothelial barrier and increases endothelial permeability by reducing endothelial expression of the adherens junction protein VE-Cadherin via the trans-signaling pathway115 as well as the junctional localization of the tight junction protein ZO-1 via STAT3 phosphorylation.116 TNF-α reduced endothelial-dependent vasodilation of mouse aortas by increasing superoxide production by activation of the NADPH oxidases,117 and both increased TNF-α and reduced NO could be causally linked to endothelial apoptosis in aged arteries of rats.118
In addition to elevated cytokine levels, patients with CKD display increased levels of danger-associated molecular pattern molecules such as HMGB1, S100 proteins and advanced glycation endproducts (AGEs), which trigger proinflammatory responses over RAGE (receptor for advanced glycation endproduct), TLRs, and NLRP3 inflammasome activation.119,120 Furthermore, neutrophil extracellular traps as produced by activated neutrophils induce endothelial inflammation and enhanced TF expression and activity, thereby promoting coagulation.121 Other contributors to a chronic inflammatory state in CKD are a gut dysbiosis79 as well as uremic toxins (discussed in more detail below).
A low-grade inflammatory state is not only observed in CKD, but also in other chronic diseases as well as upon aging, and is therefore referred to as inflammageing.79 Overall, low-grade inflammation induces endothelial cell dysfunction as well as oxidative stress, which in turn amplifies the inflammatory state, as further discussed below.
Oxidative Stress
Oxidative stress is defined as the imbalance between pro-oxidants and antioxidants. An increase in pro-oxidants and the generation of reactive oxygen species (ROS) and reactive nitrogen species affects the metabolism of cells and can trigger severe cell damage and apoptosis.122 It can be caused by mitochondrial dysfunction resulting in increased superoxide levels, increased activity of NOX (NADPH oxidase) leading to increased hydrogen peroxide levels as well as through eNOS uncoupling resulting in peroxynitrite production.122
In the vasculature, NOX is the major contributor to the generation of ROS.123 Of the 7 NOX isoforms, endothelial cells express 4: NOX-1 (NADPH oxidase 1), NOX-2, NOX-4, and NOX-5, with NOX-4 being the most prominently expressed subtype in endothelial cells.123,124 Especially NOX-2 and NOX-4 are often linked to the initiation and progression of cardiovascular complications.124 Extended NOX activation, as e.g. through stimulation by inflammatory mediators such as TNF-α, increases ROS production in endothelial cells and thereby mediates NF-κB signaling, enhancing vascular inflammation and inducing a vicious circle of inflammation and oxidative stress.125,126 Also, a range of uremic toxins has been described to trigger inflammation and by prolonged NOX activation to lead to oxidative stress in endothelial cells.127 For an extensive overview on the different NOX isoforms in CVD, we refer to a detailed review by Zhang et al.128
Lately, ROS-induced ROS release, a form of intracellular communication between ROS derived from NOX enzymes and mitochondria, has been presented as a feed-forward mechanism to maintain and amplify ROS signaling.123 In the context of CKD with increased systemic AGE levels, endothelial cells stimulated with AGEs showed increased levels of NOX-2, cytosolic and mitochondrial ROS but decreased levels of mitochondrial sirtuin-3, with analysis of sirtuin-3 blockade suggesting a role for mitochondrial ROS in cytoplasmic ROS production.129
In addition, uncoupling of eNOS is induced through CKD-mediated posttranslational modifications of LDL (low-density lipoprotein) and HDL (high-density lipoprotein), resulting in increased endothelial ROS production130–132 (posttranslational modifications described in more detail below). Finally, a reduction in antioxidative mechanisms could increase the overall oxidative stress level. For example, patients with advanced CKD display a reduction in NRF-2 (nuclear factor erythroid 2-related factor-2), a cellular protector from oxidative stress.133,134 Inducing the NRF-2 pathway in endothelial cells could therefore be a novel therapeutic approach treating inflammatory diseases such as atherosclerosis by protecting the endothelium for oxidative damage as shown in.135
Based on these disturbances in the pro-/antioxidative balance also in the context of CKD,122 oxidative stress is believed to be an important contributor to cardiovascular morbidity in the general population as well as in CKD patients.122,136
Uremic Toxins
Uremic toxins are broadly defined as substances of organic or inorganic origin that accumulate in the circulation due to kidney function decline and/or increased production, with harmful effects on the body. Currently >140 of such solutes have been identified.137 Overall, the uremic milieu triggers proinflammatory effects (eg, VCAM-1 and C-C motif chemokine ligand 2 expression), NOX expression and ROS production, as well as reduced antioxidant enzyme activity in endothelial cells, as demonstrated upon incubation of endothelial cells with uremic serum in vitro (Table 2).127,145 Furthermore, uremic serum collected from patients with CKD gradually reduces the endothelial glycocalyx height along with increasing CKD stage and increases the stiffness of the glycocalyx as well as of the actin-rich cortex beneath the plasma membrane in vitro. This, as well as uremic serum-induced reduction of eNOS and NO production in vitro, could be counteracted by blocking the mineralocorticoid receptors and the epithelial Na+ channel as its downstream target.146
Table 2.
Impact of Uremic Toxins on Signaling Pathways in Endothelial Cells with Negative Impact on Endothelial Function
The recent systematic review by Harlacher et al127 described in detail known uremic toxins in relation to detrimental effects on the endothelium. This revealed that uremic toxins as p-cresylsulfate, indoxylsulfate, cyanate, AGEs, asymmetric dimethylarginine and uric acid induce oxidative stress (ROS production, NOX activation) and inflammation and promote the adhesion of inflammatory leukocytes to the endothelium.127,145 Furthermore, a subset of these uremic toxins reduces the proliferative capacity of endothelial cells and is able to trigger cell death. Also, cyanate enhances the prothrombotic effects of the endothelium by triggering the expression of TF and PAI-1.127 As underlying mechanisms, these uremic toxins activate MAPK [mitogen-activated protein kinase]/NF-κB, RAGE, CREB (cAMP response element-binding protein)/ATF1 (AMP-dependent transcription factor 1) and AhR (aryl hydrocarbon receptor)-dependent pathways in endothelial cells (Table 2),127,145 which are known to induce among others oxidative stress and inflammation. Indoxylsulfate also upregulates the endothelial expression of solute carrier family 22 member 6 (OAT1 [organic anion transporter 1]), a membrane transporter molecule mediating cellular uptake of p-cresylsulfate and indoxylsulfate.145
Furthermore, AGEs were shown to enhance endothelial permeability and induce endothelial senescence as indicated by senescence-associated β-galactosidase staining and expression of the senescence-associated proteins p53, p21, and p16.152 p-Cresylsulfate, indoxylsulfate, cyanate, AGEs, and uric acid reduced the expression and activity of eNOS, thereby decreasing NO bioavailability and increasing vascular stiffness.127 Along the same line, the uremic toxin kynurenine triggered an increase of superoxide production in the vasculature at least partly via AhR-dependent signaling in endothelial cells, thereby reducing NO-mediated vasorelaxation.144 In patients with CKD, the plasma concentration of kynurenine positively correlated with sICAM-1, sVCAM-1, vWF, and thrombomodullin as markers of endothelial cell dysfunction.153
Combined, this suggests an important contribution of uremic toxins to cardiovascular risk in patients with CKD. Meta-analysis studies concluded on a significant association of the uremic toxin p-cresylsulfate with cardiovascular risk in patients with CKD,154 whereas indoxylsulfate and asymmetric dimethylarginine correlated with overall mortality but not cardiovascular mortality in CKD.154,155
Posttranslational Modifications
Uremic toxin accumulation and oxidative stress in CKD do not only trigger proinflammatory signaling but also induce posttranslational modifications, which may alter the function of the targeted proteins as well as of lipoprotein particles. For example, triggered by increased urea concentrations in patients with CKD, the intracellular sorting receptor sortilin is carbamylated in CKD. Carbamylated sortilin promotes VSMC calcification in vitro and is associated with increased coronary artery calcification in CKD patients.156 Also, PTMs have been identified to negatively affect lipoprotein particle function in CKD, with a negative impact on endothelial health. Both LDL and HDL particles are oxidized and carbamylated in patients with CKD,157 triggered by increased oxidative stress and plasma urea concentrations in CKD, respectively. oxLDL is well known for its proinflammatory effects in both CVD and CKD patients.157,158 Carbamylated LDL, but not native LDL, impaired endothelium-dependent vascular relaxation and enhanced ROS production through NADPH-oxidase activation and eNOS uncoupling through the LOX-1 receptor.130 Carbamylated LDL was also shown to induce autophagy, cell death, and DNA fragmentation in endothelial cells.159 Furthermore, it enhanced thrombin generation and injury-induced thrombus formation in a mouse model, as well as increased the production of TF and PAI-1 in endothelial cells through LOX-1.160
Whereas HDL exerts anti-inflammatory and proproliferative effects in endothelial cells,130,161 oxHDL (oxidized HDL) triggered NOX2-mediated ROS production as well as proinflammatory NF-κB signaling and cytokine expression in endothelial cells through LOX-1.131 Along this line, carbamylated HDL reduced endothelial migration and proliferation.161 Also, HDL from patients with CKD showed an enrichment in the proinflammatory protein SAA (serum amyloid A) as well as in the uremic toxin SDMA. SDMA-enriched HDL and CKD-HDL increased ROS through NADPH oxidase activation and reduced endothelial NO production via TLR2 in vitro. In contrast to HDL from healthy donors, SDMA-enriched HDL and CKD-HDL did not support endothelial repair after injury of the carotid artery in a mouse model.132
In summary, CKD-induced alterations of lipoprotein particles make LDL an even more noxious particle and convert HDL from a protective to a damaging lipoprotein particle. For more details and additional alterations in lipoprotein particles in CKD, we refer to a recent detailed review by Noels et al.157
Metabolic Acidosis
With a prevalence of 39% in predialysis patients with a glomerular filtration rate <20, chronic metabolic acidosis is a common complication in patients with advanced CKD,162 although it is consistently underdiagnosed and undertreated.163 It is caused by a reduced excretion of metabolically produced acids, leading to decreased systemic bicarbonate levels. Metabolic acidosis in CKD has been associated with CKD progression as well as with an increased risk of adverse cardiovascular events, including heart failure.164,165 On molecular level, chronic metabolic acidosis has been shown to induce ammoniagenesis and to increase the production of angiotensin II, aldosterone, and endothelin-1, in order to enhance net acid excretion.166 However, sustained upregulation of these mediators exerts proinflammatory and profibrotic effects on the kidney, thus again contributing to CKD progression. Furthermore, these mediators exert proinflammatory and vasoconstrictive effects on the endothelium.167,168 Also, in vitro studies revealed acidosis to trigger proinflammatory NF-κB signaling, endoplasmic reticulum stress, and the unfolded protein response in the endothelium via the proton-sensing receptor GPR4.169,170 Specifically extracellular acidification inhibited store-operated Ca2+ entry through divalent cation channels and thereby interfered with agonist-mediated production of the protective factors NO and prostaglandin I2 by endothelial cells.171 A pilot study identified an improvement of endothelial function in CKD stage 3b-4 patients upon sodium bicarbonate treatment for 6 weeks, as detected by a 1.8% increase in brachial artery flow-mediated dilation. Overall effects on cardiovascular outcome were not examined.172
Sympathetic Nerve Activity
Sympathetic nerve activity—for example, as measured by plasma levels of norepinephrine or catecholamines—increases along with kidney function decline, potentially triggered by increased renin-angiotensin-aldosterone system signaling, reduced NO bioavailability, and increased oxidative stress, among other factors.173,174 Increased sympathetic nerve activity contributes to hypertension, but also independent of blood pressure effects, high sympathetic nerve activity is associated with CKD progression as well as with increased cardiovascular risk in both predialysis and dialysis CKD patients.173,175,176 Sympathetic nerve activation reduces endothelial-dependent vasodilation and increases vascular stiffness, as discussed in detail by Kaur et al.173 In animal models, blocking sympathetic nerve activity reduced microvascular rarefaction.49 Furthermore, in vitro studies showed that high levels of catecholamine neurotransmitters trigger endothelial adrenergic receptors, leading to endothelial permeability and glycocalyx loss in endothelial cells.177 Combined, these findings suggest also a contribution of increased sympathetic nerve activity to endothelial dysfunction in CKD.
Vascular Aging
Although aging is a natural process, in case of CKD, it is accelerated. An indicator for cellular aging and subsequent decline in function is telomere length. A reduction in telomere length can drive endothelial cells into senescence, characterized by a stable arrest in cell growth and a proinflammatory phenotype.178 Although telomere shortening has been observed in CKD independent of age,179 a recent meta-analysis indicated a paradoxical association between CKD and telomere length. As such, the authors postulated that the shortening of telomeres associated with a declining kidney function is likely offset by cellular telomere reparative mechanisms in patients who survive longer with CKD,180 but more research is needed. Moreover, to which extent telomere shortening occurs in the endothelial layer as a consequence of a reduced kidney function is unclear.
Premature aging takes place partially due to systemic inflammation (“inflammageing”).79 The other way around, senescent cells can develop a senescence-associated secretory phenotype to release, among others, proinflammatory cytokines, growth factors and soluble receptors contributing to local as well as systemic inflammation, which accelerates tissue damage in patients with CKD.80
Furthermore, patients with CKD display a reduction in Klotho due to impaired kidney function. Klotho is a protective protein with antioxidant, antiapoptotic, and antisenescent effects, also toward endothelial cells,181 and its depletion is a crucial contributor to premature vascular aging in CKD.80,182,183 Animal studies by Shi et al linked a reduction in Klotho to a reduction in autophagy. Early αKlotho administration increased autophagic flux induction upon acute kidney injury and protected from kidney damage progression into CKD, suggesting the administration of Klotho as a potential therapeutic treatment after acute kidney injury to reverse kidney failure.184 A disturbed autophagic flux is additionally induced by uremic toxins such as indoxylsulfate, p-cresyl sulfate, and indole acetic acid, leading to the accumulation of oxidized proteins and organelles and increasing the sensitivity of endothelial cells toward oxidative stress.185
A depletion of Klotho in aortic endothelial and smooth muscle cells is accompanied by a significant reduction in SIRT1 (sirtuin-1). Similar to Klotho, SIRT1 is anti-inflammatory, antioxidative, antiapoptotic, and antisenescent; it inhibits the activation of NADPH oxidases and prohibits the production of ROS in endothelial cells.186 Blocking of SIRT1 triggered a proinflammatory phenotype illustrated by an increased ROS production in aortic endothelial cells and reduced endothelial-dependent vascular relaxation through impaired NO production.187,188 Beyond counteracting inflammation, oxidative stress and senescence, SIRT1 also protects from CKD-associated fibrosis and vascular calcification, suggesting SIRT1 as a potential future therapeutic target for CKD.186
Smooth Muscle—Endothelium Interaction and Vascular Calcification
Within the vasculature, endothelial cells and VSMCs can bidirectionally communicate.189 In relation to VSMC communication to endothelial cells, endothelial cells cocultured with VSMCs express increased levels of MMP-2 and MMP-9.190 Synthetic VSMCs produce proinflammatory IL-1β and IL-6, which induced NF-κB activation and E-selectin expression in cocultured endothelial cells.191 Also, mechanical stress-induced microparticle production by VSMCs induced proinflammatory responses in endothelial cells.192 Despite these findings, the overall contribution of VSMC changes to endothelial cell dysfunction in CVD remains unclear. This is also true in the context of CKD. CKD patients frequently display medial vascular calcification, as for example identified in 88% of dialysis patients aged 20 to 30 years old.193 Medial vascular calcification is associated with increased vascular stiffness as well as cardiovascular mortality in CKD patients.194,195 Whereas the impact of vascular calcification on endothelial function has not been studied to our knowledge, a dysfunctional endothelial cell layer does contribute to medial calcification. For example, NO produced by endothelial cells counteracts VSMC calcification.196 Also, inhibition of eNOS-mediated NO production by L-NAME increases warfarin-induced medial calcification in rats,197 with warfarin triggering vascular calcification by interfering with the activation of the calcification inhibitor matrix gla protein. As discussed above, in CKD, endothelial cells show a reduced eNOS expression and activation resulting in a reduced NO bioavailability, for example, triggered by uremic toxins as well as hyper- and hypophosphatemia.127 Further, uremic toxins can trigger endothelial inflammation,127 with inflammatory mediators such as TNF-α and IL-1β reported to be able to sensitize endothelial cells to BMP-induced osteogenic differentiation into osteoprogenitor cells, which could contribute to vascular calcification.198
Impact of Pharmacological Interventions on Endothelial Cell Dysfunction in CKD
Considering the function of the endothelium as gatekeeper of vascular health, maintaining its integrity by pharmacological intervention could contribute to alleviating the cardiovascular risk of patients with CKD. Drugs administered to patients with CKD to treat comorbidities as hypertension, hyperlipidemia, and diabetes have been extensively examined in light of endothelial cell function. Consequently, direct and indirect endothelial protective effects of antihypertensive, lipid-lowering (statins), and antihyperglycemic drugs have been well documented.199 For an extensive review of the mechanisms responsible for these beneficial effects on the endothelium, we refer to the recent review by Xu et al.199 Also in the context of CKD, endothelial protective effects of antihypertensive drugs with or without statin add-on therapy have been observed,200,201 although it needs to be noted that ACE inhibitors—but not angiotensin receptor blockers—increased proinflammatory asymmetric dimethylarginine levels in hemodialysis patients.202
In recent years, SGLT2 (sodium-glucose cotransporter-2) inhibitors received much attention due to their cardiovascular and kidney protective effects. Also, in patients with CKD, SGLT2 inhibitors were associated with an improvement in cardiovascular and kidney health, irrespective of diabetes status.203 In terms of endothelial protective effects, a recent meta-analysis showed that treatment with the SGLT2 inhibitor dapagliflozin resulted in an improved FMD in patients with type 2 diabetes.204 The clinical trial PROCEED is currently ongoing to determine whether SGLT2 inhibitors are also capable of improving endothelial function in patients with diabetes and CKD.205 Interestingly, treatment of human cardiac microvascular endothelial cells with the SGLT2 inhibitor empagliflozin could counteract the increase in oxidative stress and the reduction in endothelial nitric oxide bioavailability caused by uremic serum exposure, but the underlying mechanisms remain unclear.206
Mineralocorticoid receptor antagonists, being potassium-sparing diuretics, have been shown to have endothelial protective effects as well. In patients with stable mild to moderate chronic heart failure, spironolactone treatment improved endothelium-dependent vasodilation and increased NO bioactivity.207 Also in the context of CKD, endothelial protective effects of mineralocorticoid receptor antagonists have been observed. In a small cohort of stable chronic hemodialysis patients, spironolactone treatment for 4 months resulted in an improvement of endothelial function as assessed by venous occlusion plethysmography.208 In animal models of kidney dysfunction, spironolactone and finerenone ameliorated endothelial dysfunction by enhancing NO bioavailability and reducing oxidative stress.129,209,210
In terms of drugs targeting inflammation, blocking IL-1α/β with rilonacept for 12 weeks in patients with CKD3-4 improved FMD of the brachial artery and reduced systemic levels of hsCRP as well as endothelial expression of NADPH oxidase.211 Allopurinol, a xanthine oxidase inhibitor used to treat hyperuricemia, has contrasting results on its endothelial effects, with studies reporting no effect212,213 to studies showing an improvement in endothelial function in terms of vasodilatory responses in patients with CKD treated with allopurinol for 8 weeks or 9 months.214,215 Similarly, conflicting results have been published on the endothelial protective effects of Vitamin D. Whereas clinical trials have shown improvements in endothelial function upon Vitamin D supplementation,216,217 other trials observed no change.218–220
In recent years, many other clinical trials have been initiated investigating the effect of a wide range of pharmacological interventions or dietary supplements and/or adaptations (eg, endothelin receptor antagonism, antioxidant molecule mitoQ, low-AGE diet, plant-derived supplements or prebiotics) on endothelial function in CKD; however, outcomes were not always clear or were not yet reported (based on a search of the www.clinicaltrials.gov database for clinical trials in CKD measuring endothelial function). As the effects of CKD on the endothelium are multifactorial and the CKD patient population is very heterogeneous, protecting and maintaining endothelial integrity might require an early and multifactorial approach as well.
Conclusions
A healthy endothelial layer is a crucial gatekeeper counteracting CVD development. Patients with CKD display an impaired endothelial protective function due to the proinflammatory, prothrombotic, and uremic environment caused by their decline in kidney function, which contributes to the increased cardiovascular risk of these patients. Over the past decade, studies have started to reveal cellular and molecular mechanisms that underlie endothelial cell dysfunction in CKD, identifying a role for detrimental inflammatory and uremic mediators that are upregulated in CKD in contrast to a downregulation of protective factors. Clinical trials evaluating the effect of selected pharmacological interventions on endothelial function specifically in patients with CKD have been initiated and are ongoing, for example with a focus on targeting reduced endothelial nitric oxide bioavailability as well as increased inflammation and oxidative stress, and are expected to provide additional insights on patient level in the upcoming years. Additionally, preclinical and clinical studies should further support the development of new therapeutic options by unraveling novel disease mechanisms of increased cardiovascular risk specifically in this CKD population. This should also include a further focus on the level of immune-thrombosis interactions with the endothelium as gatekeeper of cardiovascular health. Overall, these efforts should support further cardiovascular risk reduction in this specific vulnerable patient population.
Article Information
Author Contributions
C.C.F.M.J. Baaten, S. Vondenhoff, and H. Noels performed literature research and wrote the article. S. Vondenhoff made the figures and tables.
Sources of Funding
This work was supported by the Dutch Heart Foundation (2020T020 to C.C.F.M.J. Baaten); the START-Program of the Faculty of Medicine of the RWTH Aachen University (105/20 to C.C.F.M.J. Baaten and H. Noels); a grant from the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University (PTD 1-12 to H. Noels); by the German Research Foundation (DFG) Project-ID 322900939–SFB/TRR219 (M-05 to H. Noels) and Project-ID 403224013—SFB 1382 (A-04 to H. Noels); and the German Center for Cardiovascular Research (DZHK-B23 to H. Noels). Further funding was provided by the “Else Kröner-Fresenius-Stiftung” (Project 2020_EKEA.60 to H. Noels).
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Nonstandard Abbreviations and Acronyms
- AhR
- aryl hydrocarbon receptor
- ATF1
- cyclic AMP-dependent transcription factor 1
- ATIII
- antithrombin III
- cAMP
- cyclic adenosine monophosphate
- CD73
- ecto-5’-nucleotidase
- cGMP
- cyclic guanosine monophosphate
- CKD
- chronic kidney disease
- CREB
- cAMP response element-binding protein
- CVD
- cardiovascular disease
- ECM
- extracellular matrix
- eNOS
- endothelial NO synthase
- FMD
- flow-mediated dilation
- GAS6
- growth arrest specific 6
- HDL
- high-density lipoprotein
- hsCRP
- high-sensitivity C-reactive protein
- ICAM-1
- intercellular adhesion molecule 1
- IL
- interleukin
- KLF
- Kruppel-like factor
- MAPK
- mitogen-activated protein kinase
- MVD
- microvascular dysfunction
- NADPH
- nicotinamide adenine dinucleotide phosphate
- NF-κB
- nuclear factor-κB
- NO
- nitric oxide
- NOX-1
- NADPH oxidase 1
- NRF-2
- nuclear factor erythroid 2-related factor-2
- OAT1
- organic anion transporter 1
- oxLDL
- oxidized low-density lipoprotein
- PAI-1
- plasminogen activation inhibitor-1
- PWV
- pulse wave velocity
- ROS
- reactive oxygen species
- SAA
- serum amyloid A
- SGLT2
- sodium-glucose cotransporter-2
- SIRT1
- sirtuin-1
- SLC22A6
- solute carrier family 22 member 6
- TF
- tissue factor
- TFPI
- tissue factor pathway inhibitor
- TLR
- toll-like receptor
- TNF-α
- tumor necrosis factor-α
- tPA
- tissue plasminogen activator
- uPA
- urokinase-type plasminogen activator
- VCAM-1
- vascular cell adhesion molecule 1
- VSMC
- vascular smooth muscle cell
- vWF
- von Willebrand factor
For Sources of Funding and Disclosures, see page 986.
References
- 1.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 2.Hill NR, Fatoba ST, Oke JL, Hirst JA, O-Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M; Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58:64–72. doi: 10.1053/j.ajkd.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 5.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, Matsushita K, Coresh J, et al. ; Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 6.Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 8.Quillard T, Franck G, Mawson T, Folco E, Libby P. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol. 2017;28:434–441. doi: 10.1097/MOL.0000000000000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betrui A, Martinez-Alonso M, Arcidiacono MV, Cannata-Andia J, Pascual J, Valdivielso JM, Fernández E; Investigators from the NEFRONA Study. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: the NEFRONA study. Nephrol Dial Transplant. 2014;29:1415–1422. doi: 10.1093/ndt/gfu038 [DOI] [PubMed] [Google Scholar]
- 10.Valdivielso JM, Rodríguez-Puyol D, Pascual J, Barrios C, Bermúdez-López M, Sánchez-Niño MD, Pérez-Fernández M, Ortiz A. Atherosclerosis in chronic kidney disease: more, less or just different?. Arterioscler Thromb Vasc Biol. 2019;39:1938–1966. doi: 10.1161/ATVBAHA.119.312705 [DOI] [PubMed] [Google Scholar]
- 11.Gracia M, Betrui A, Martinez-Alonso M, Arroyo D, Abajo M, Fernández E, Valdivielso JM; NEFRONA Investigators. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin J Am Soc Nephrol. 2016;11:287–296. doi: 10.2215/CJN.01240215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anavekar NS, McMurray JJV, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365 [DOI] [PubMed] [Google Scholar]
- 13.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846 [DOI] [PubMed] [Google Scholar]
- 14.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0 [DOI] [PubMed] [Google Scholar]
- 15.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4:S79–S91. doi: 10.2215/CJN.04860709 [DOI] [PubMed] [Google Scholar]
- 16.Middleton R, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. doi: 10.1681/ASN.V1251079 [DOI] [PubMed] [Google Scholar]
- 17.Gimbrone MA, Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meza D, Musmacker B, Steadman E, Stransky T, Rubenstein DA, Yin W. Endothelial cell biomechanical responses are dependent on both fluid shear stress and tensile strain. Cell Mol Bioeng. 2019;12:311–325. doi: 10.1007/s12195-019-00585-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853. doi: 10.1038/ncomms11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–4528. doi: 10.1172/JCI82719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom KI, Yao J, Guihard PJ, Blazquez-Medela AM, Yao Y. Endothelial-mesenchymal transition in atherosclerotic lesion calcification. Atherosclerosis. 2016;253:124–127. doi: 10.1016/j.atherosclerosis.2016.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souilhol C, Harmsen MC, Evans PC, Krenning G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res. 2018;114:565–577. doi: 10.1093/cvr/cvx253 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588 [DOI] [PubMed] [Google Scholar]
- 24.Johnston-Cox HA, Ravid K. Adenosine and blood platelets. Purinergic Signal. 2011;7:357–365. doi: 10.1007/s11302-011-9220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Born GV, Palinski W. Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br J Exp Pathol. 1985;66:543–549. [PMC free article] [PubMed] [Google Scholar]
- 26.Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pfugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–743. doi: 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- 29.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29 [DOI] [PubMed] [Google Scholar]
- 30.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander Y, Osto E, Schmidt-Trucksass A, Shechter M, Trifunovic D, Duncker DJ, Aboyans V, Back M, Badimon L, Cosentino F, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 2021;117:29–42. doi: 10.1093/cvr/cvaa085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14 [DOI] [PubMed] [Google Scholar]
- 33.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, et al. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardovasc Imaging. 2011;4:371–380. doi: 10.1161/CIRCIMAGING.110.961557 [DOI] [PubMed] [Google Scholar]
- 34.Perrault R, Omelchenko A, Taylor CG, Zahradka P. Establishing the interchangeability of arterial stiffness but not endothelial function parameters in healthy individuals. BMC Cardiovasc Disord. 2019;19:190. doi: 10.1186/s12872-019-1167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonarjee VVS. Arterial stiffness: a prognostic marker in coronary heart disease. Available methods and clinical application. Front Cardiovasc Med. 2018;5:64. doi: 10.3389/fcvm.2018.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Beusecum JP, Barbaro NR, Smart CD, Patrick DM, Loperena R, Zhao S, de la Visitacion N, Ao M, Xiao L, Shibao CA, et al. Growth arrest specific-6 and Axl coordinate inflammation and hypertension. Circ Res. 2021;129:975–991. doi: 10.1161/CIRCRESAHA.121.319643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Van Beusecum JP, Xiao L, Patrick DM, Ao M, Zhao S, Lopez MG, Billings FT, Cavinato C, Caulk AW, et al. Role of Axl in target organ inflammation and damage due to hypertensive aortic remodeling. Am J Physiol Heart Circ Physiol. 2022;323:H917–H933. doi: 10.1152/ajpheart.00253.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral artery disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001 [DOI] [PubMed] [Google Scholar]
- 40.Meyer B, Mörtl D, Strecker K, Hülsmann M, Kulemann V, Neunteufl T, Pacher R, Berger R. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol. 2005;46:1011–1018. doi: 10.1016/j.jacc.2005.04.060 [DOI] [PubMed] [Google Scholar]
- 41.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patient with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF [DOI] [PubMed] [Google Scholar]
- 42.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Heath Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276 [DOI] [PubMed] [Google Scholar]
- 43.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047 [DOI] [PubMed] [Google Scholar]
- 45.Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med. 2020;9:2880. doi: 10.3390/jcm9092880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Buono MG, Montone RA, Camili M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC State-of-the-Art review. J Am Coll Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehling J, Babickova J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, Kiessling F, Floege J, Lammers T, Boor P. Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol. 2016;27:520–532. doi: 10.1681/ASN.2015020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prommer HU, Maurer J, von Websky K, Freise C, Sommer K, Nasser H, Samapati R, Reglin B, Guimaraes P, Pries AR, et al. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci Rep. 2018;8:5317. doi: 10.1038/s41598-018-23663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Querfeld U, Mak RH, Pries AR. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond). 2020;134:1333–1356. doi: 10.1042/CS20200279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. doi: 10.1016/j.jacc.2009.10.073 [DOI] [PubMed] [Google Scholar]
- 51.Theuerle JD, Al-Fiadh AH, Amirul Islam FM, Patel SK, Burrell LM, Wong TY, Farouque O. Impaired retinal microvascular function predicts long-term adverse events in patients with cardiovascular disease. Cardiovasc Res. 2021;117:1949–1957. doi: 10.1093/cvr/cvaa245 [DOI] [PubMed] [Google Scholar]
- 52.Fujisue K, Sugiyama S, Matsuzawa Y, Akiyama E, Sugamura K, Matsubara J, Kurokawa H, Maeda H, Hirata Y, Kusaka H, et al. Prognostic significance of peripheral microvascular endothelial dysfunction in heart failure with reduced left ventricular ejection fraction. Circ J. 2015;79:2623–2631. doi: 10.1253/circj.CJ-15-0671 [DOI] [PubMed] [Google Scholar]
- 53.Maréchaux S, Samson R, van Belle E, Breyne J, de Monte J, Dédrie C, Chebai N, Menet A, Banfi C, Bouabdallaoui N, et al. Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. J Card Fail. 2016;22:3–11. doi: 10.1016/j.cardfail.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 54.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 56.D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1347. doi: 10.3389/fphys.2019.01347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornuault L, Rouault P, Duplàa C, Couffinhal T, Renault MA. Endothelial dysfunction in heart failure with preserved ejection fraction: what are the experimental proofs?. Front Physiol. 2022;13:906272. doi: 10.3389/fphys.2022.906272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harvey A, Montezano AC, Alves Lopes R, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–668. doi: 10.1016/j.cjca.2016.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain V, Gupta K, Bhatia K, Rajapreyar I, Singh A, Zhou W, Klein A, Nanda NC, Prabhu SD, Bajaj NS. Coronary flow abnormalities in chronic kidney disease: a systematic review and meta-analysis. Echocardiogr. 2022;39:1382–1390. doi: 10.1111/echo.15445 [DOI] [PubMed] [Google Scholar]
- 60.Charytan DM, Skali H, Shah NR, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, et al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 2018;93:501–509. doi: 10.1016/j.kint.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Annuk M, Lind L, Linde T, Fellström B. Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant. 2001;16:302–306. doi: 10.1093/ndt/16.2.302 [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Hamm LL, Mohler ER, Hudaihed A, Arora R, Chen CS, Liu Y, Browne G, Mills KT, Kleinpeter MA, et al. Interrelationship of multiple endothelial dysfunction biomarkers with chronic kidney disease. PLoS One. 2015;10:e0132047. doi: 10.1371/journal.pone.0132047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kruger A, Stewart J, Sahityani R, O-Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157–164. doi: 10.1038/sj.ki.5001511 [DOI] [PubMed] [Google Scholar]
- 64.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26:3537–3543. doi: 10.1093/ndt/gfr081 [DOI] [PubMed] [Google Scholar]
- 65.Poulikakos D, Ross L, Recio-Mayoral A, Cole D, Andoh J, Chitalia N, Sharma R, Kaski JC, Banerjee D. Left ventricular hypertrophy and endothelial dysfunction in chronic kidney disease. Eur Heart J Cardiovasc Imaging. 2014;15:56–61. doi: 10.1093/ehjci/jet120 [DOI] [PubMed] [Google Scholar]
- 66.Hirata Y, Sugiyama S, Yamamoto E, Matsuzawa Y, Akiyama E, Kusaka H, Fujisue K, Kurokawa H, Matsubara J, Sugamura K, et al. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol. 2014;173:481–486. doi: 10.1016/j.ijcard.2014.03.085 [DOI] [PubMed] [Google Scholar]
- 67.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–357. doi: 10.1038/sj.ki.5000047 [DOI] [PubMed] [Google Scholar]
- 68.Yoon HE, Shin DI, J KD, Koh ES, Hwang HS, Chung S, Shin SJ. Brachial-ankle pulse wave velocity predicts decline in renal function and cardiovascular events in early stages of chronic kidney disease. Int J Med Sci. 2013;10:1430–1436. doi: 10.7150/ijms.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW. Association of pulse wave velocity and pulse pressure with decline in kidney function. J Clin Hypertens (Greenwich). 2014;16:372–377. doi: 10.1111/jch.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber T, Ammer M, Gündüz D, Bruckenberger P, Eber B, Wallner M. Association of increased arterial wave reflections with decline in renal function in chronic kidney disease stages 3 and 4. Am J Hypertens. 2011;24:762–769. doi: 10.1038/ajh.2011.45 [DOI] [PubMed] [Google Scholar]
- 71.Liew H, Roberts MA, Pope A, McMahon LP. Endothelial glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol. 2021;22:21. doi: 10.1186/s12882-020-02219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson SH, Egberg N, Hylander B, Lundahl J. Correlation between soluble markers of endothelial dysfunction in patients with renal failure. Am J Nephrol. 2002;22:42–47. doi: 10.1159/000046673 [DOI] [PubMed] [Google Scholar]
- 73.Bonomini M, Reale M, Santarelli P, Stuard S, Settefrati N, Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79:399–407. doi: 10.1159/000045084 [DOI] [PubMed] [Google Scholar]
- 74.Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CDA. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537–545. doi: 10.1681/ASN.2005080834 [DOI] [PubMed] [Google Scholar]
- 75.Kaminski TW, Pawlak K, Karbowska M, Mysliwiec M, Grzegorzewski W, Kuna J, Pawlak D. Association between uremic toxin-anthranilic acid and fibrinolytic system activity in predialysis patients at different stages of chronic kidney disease. Int Urol Nephrol. 2018;50:127–135. doi: 10.1007/s11255-017-1729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mezzano D, Tagle R, Panes O, Pérez M, Downey P, Muñoz B, Aranda E, Barja P, Thambo S, González F, et al. Endothelial cell markers in chronic uremia: relationship with hemostatic defects and severity of renal failure. Thromb Res. 1997;88:6. doi: 10.1016/s0049-3848(97)00280-6 [DOI] [PubMed] [Google Scholar]
- 77.Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014 [DOI] [PubMed] [Google Scholar]
- 78.Holmar J, de la Puente-Secades S, Floege J, Noels H, Jankowski J, Orth-Alampour S. Uremic toxins affecting cardiovascular calcification: a systematic review. Cells. 2020;9:2428. doi: 10.3390/cells9112428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande FM, Schalkwijk CG, Shiels PG, Stenvinkel P. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol. 2017;313:F938–F950. doi: 10.1152/ajprenal.00256.2017 [DOI] [PubMed] [Google Scholar]
- 80.Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J. 2019;17:721–729. doi: 10.1016/j.csbj.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu-Wong JR, Kawai M, Chen Y, Wessale JL, Huang CJ, Wu MT, Nakane M. Two novel vitamin D receptor modulators with similar structures exhibit different hypercalcemic effects in 5/6 nephrectomized uremic rats. Am J Nephrol. 2013;37:310–319. doi: 10.1159/000348755 [DOI] [PubMed] [Google Scholar]
- 82.Vila Cuenca M, Ferrantelli E, Meinster E, Pouw SM, Kovacevic I, de Menezes R, Niessen HW, Beelen RJ, Hordijk PL, Vervloet MG. Vitamin D attenuates endothelial dysfunction in uremic rats and maintains human endothelial stability. J Am Heart Assoc. 2018;7:e008776. doi: 10.1161/JAHA.118.008776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salmon AHJ, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, Lukasz A, Oberleithner H, Pavenstadt H, Brand M, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234:335–343. doi: 10.1016/j.atherosclerosis.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 85.Six I, Gross P, Rémond MC, Chillon JM, Poirot S, Drueke TB, Massy ZA. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis. 2015;243:248–256. doi: 10.1016/j.atherosclerosis.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 86.Verkaik M, Juni RP, van Loon EPM, van Poelgeest EM, Kwekkeboom RFJ, Gam Z, Richards WG, Ter Wee PM, Hoenderop JG, Eringa EC, et al. ; NIGRAM Consortium. FGF23 impairs peripheral microvascular function in renal failure. Am J Physiol Heart Circ Physiol. 2018;315:H1414–H1424. doi: 10.1152/ajpheart.00272.2018 [DOI] [PubMed] [Google Scholar]
- 87.Ermert K, Buhl EM, Klinkhammer BM, Floege J, Boor P. Reduction of endothelial glycocalyx on peritubular capillaries in chronic kidney disease. Am J Pathol. 2023;193:138–147. doi: 10.1016/j.ajpath.2022.11.003 [DOI] [PubMed] [Google Scholar]
- 88.Theodorakopoulou MP, Schoina M, Sarafidis P. Assessment of endothelial and microvascular function in CKD: older and newer techniques, associated risk factors, and relations with outcomes. Am J Nephrol. 2020;51:931–949. doi: 10.1159/000512263 [DOI] [PubMed] [Google Scholar]
- 89.Kopel T, Kaufman JS, Hamburg N, Sampalis JS, Vita JA, Dember LM. Endothelium-dependent and -independent vascular function in advanced chronic kidney disease. Clin J Am Soc Nephrol. 2017;12:1588–1594. doi: 10.2215/CJN.12811216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schoina M, Loutradis C, Memmos E, Dimitroulas T, Pagkopoulou E, Doumas M, Karagiannis A, Garyfallos A, Papagianni A, Sarafidis P. Microcirculatory function deteriorates with advancing stages of chronic kidney disease independently of arterial stiffness and atherosclerosis. Hypertens Res. 2021;44:179–187. doi: 10.1038/s41440-020-0525-y [DOI] [PubMed] [Google Scholar]
- 91.Charytan DM, Padera R, Helfand AM, Zeisberg M, Xu X, Liu X, Himmelfarb J, Cinelli A, Kalluri R, Zeisberg EM. Increased concentration of circulating angiogenesis and nitic oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol. 2014;176:99–109. doi: 10.1016/j.ijcard.2014.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L, Huang X, He W, Liu W, Yang J. Digital microvascular reactivitiy does not decline with impaired renal function in chronic kidney disease. BMC Nephrol. 2019;20:288. doi: 10.1186/s12882-019-1484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakanishi K, Fukuda S, Shimada K, Miyazaki C, Otsuka K, Kawarabayashi T, Watanabe H, Yoshikawa J, Yoshiyama M. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013;112:928–932. doi: 10.1016/j.amjcard.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 94.Caliskan Y, Oflaz H, Demirturk M, Yazici H, Turkmen A, Cimen A, Elitok A, Yildiz A. Coronary flow reserve dysfunction in hemodialysis and kidney transplant patients. Clin Transplant. 2008;22:785–793. doi: 10.1111/j.1399-0012.2008.00879.x [DOI] [PubMed] [Google Scholar]
- 95.Yeh YC, Chao A, Lee CY, Lee CT, Yeh CC, Liu CM, Tsai MK. An observational study of microcirculation in dialysis patients and kidney transplant recipients. Eur J Clin Invest. 2017;47:630–637. doi: 10.1111/eci.12784 [DOI] [PubMed] [Google Scholar]
- 96.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900–1908. doi: 10.1681/ASN.2011121181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fels B, Beyer A, Cazana-Perez V, Giraldez T, Navarro-Gonzalez JF, Alvarez de la Rosa D, Schaefer F, Bayazit AK, Obrycki L, Ranchin B, et al. Effects of chronic kidney disease on nanomechanics of the endothelial glycocalyx are mediated by the mineralocorticoid receptor. Int J Mol Sci. 2022;23:10659. doi: 10.3390/ijms231810659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koch J, Hijmans RS, Ossa Builes M, Dam WA, Pol RA, Bakker SJL, Pas HH, Franssen CFM, van den Born J. Direct evidence of endothelial dysfunction and glycocalyx loss in dermal biopsies of patients with chronic kidney disease and their association with markers of volume overload. Front Cell Dev Biol. 2021;9:733015. doi: 10.3389/fcell.2021.733015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liew H, Roberts MA, McMahon LP. Markers of the endothelial glycocalyx are improved following kidney transplantation. Kidney Blood Press Res. 2021;46:581–587. doi: 10.1159/000517317 [DOI] [PubMed] [Google Scholar]
- 100.Deanfield J, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859 [DOI] [PubMed] [Google Scholar]
- 101.Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304:H1585–H1597. doi: 10.1152/ajpheart.00096.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Hamm LL, Mohler ER, Hudaihed A, Arora R, Chen CS, Liu Y, Browne G, Mills KT, Kleinpeter MA, et al. Interrelationship of multiple endothelial dysfunction biomarkers with chronic kidney disease. PLoS One. 2015;10:e0132047. doi: 10.1371/journal.pone.0132047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, Mambet C, Anton G, Tanase C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373. doi: 10.1155/2018/2180373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M. Il-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia -- the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x [DOI] [PubMed] [Google Scholar]
- 105.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, et al. Inflammation and progression in CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun J, Axelsson J, Machowska A, Heimbürger O, Bárány P, Lindholm B, Lindström K, Stenvinkel P, Qureshi AR. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol. 2016;11:1163–1172. doi: 10.2215/CJN.10441015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jalal D, Chonchol M, Etgen T, Sander D. C-reactive protein as a predictor of cardiovascular events in eldery patients with chronic kidney disease. J Nephrol. 2012;25:719–725. doi: 10.5301/jn.5000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x [DOI] [PubMed] [Google Scholar]
- 110.Yilmaz MI, Solak Y, Saglam M, Cayci T, Acikel C, Unal HU, Eyileten T, Oguz Y, Sari S, Carrero JJ, et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin J Am Soc Nephrol. 2014;9:1207–1216. doi: 10.2215/CJN.08660813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin-1b by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71:2405–2414. doi: 10.1016/j.jacc.2018.03.490 [DOI] [PubMed] [Google Scholar]
- 112.Ridker PM. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction?. Cardiovasc Res. 2021;117:e138–e140. doi: 10.1093/cvr/cvab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JHS. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens. 2010;28:940–951. doi: 10.1097/HJH.0b013e32833992ef [DOI] [PubMed] [Google Scholar]
- 114.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9 [DOI] [PubMed] [Google Scholar]
- 115.Yang YC, Fu H, Zhang B, Wu YB. Interleukin-6 downregulates the expression of vascular endothelial-cadherin and increases permeability in renal glomerular endothelial cells via the trans-signaling pathway. Inflammation. 2022;45:2544–2558. doi: 10.1007/s10753-022-01711-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alsaffar H, Martino N, Garrett JP, Adam AP. Interleukin-6 promotes a sustained loss of endothelial barrier function via janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol. 2018;314:C589–C602. doi: 10.1152/ajpcell.00235.2017 [DOI] [PubMed] [Google Scholar]
- 117.Huang Y, Yan L, Rong S, Haller H, Kirch T. TNF-a induces endothelial dysfunction via PKC-z-dependent NADPH oxidase activation. J Huazhong Univ Sci Technolog Med Sci. 2012;32:642–647. doi: 10.1007/s11596-012-1011-9 [DOI] [PubMed] [Google Scholar]
- 118.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003 [DOI] [PubMed] [Google Scholar]
- 119.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–425. doi: 10.1681/ASN.2010040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hara M, Ando M, Morito T, Nokiba H, Iwasa Y, Tsuchiya K, Nitta K. S100A12 gene expression is increased in peripheral leukocytes in chronic kidney disease stage 4-5 patients with cardiovascular disease. Nephron Clin Pract. 2013;123:202–208. doi: 10.1159/000353808 [DOI] [PubMed] [Google Scholar]
- 121.Folco EJ, Mawson T, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1a and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34:975–991. doi: 10.1007/s00467-018-4005-4 [DOI] [PubMed] [Google Scholar]
- 123.Fukai T, Ushio-Fukai M. Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis. Cells. 2020;9:1849. doi: 10.3390/cells9081849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease?. Trends Endocrinol Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 125.Vaziri ND. Causal link between oxidative stress, inflammation and hypertension. Iran J Kidney Dis. 2008;2:1–10. [PubMed] [Google Scholar]
- 126.Zhao W, Feng H, Guo S, Han Y, Chen X. Danshenol A inhibits TNF-alpha-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep. 2017;7:12953. doi: 10.1038/s41598-017-13072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harlacher E, Wollenhaupt J, Baaten C, Noels H. Impact of uremic toxins on endothelial dysfunction in chronic kidney disease: a systematic review. Int J Mol Sci. 2022;23:531. doi: 10.3390/ijms23010531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. 2020;17:170–194. doi: 10.1038/s41569-019-0260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang CC, Lee AS, Liu SH, Chang KC, Shen MY, Chang CT. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019;20:351. doi: 10.1186/s12882-019-1534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Speer T, Owala FO, Holy EW, Zewinger S, Frenzel FL, Stähli BE, Razavi M, Triem S, Cvija H, Rohrer L, et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur Heart J. 2014;35:3021–3032. doi: 10.1093/eurheartj/ehu111 [DOI] [PubMed] [Google Scholar]
- 131.Pérez L, Vallejos A, Echeverria C, Varela D, Cabello-Verrugio C, Simon F. OxHDL controls LOX-1 expression and plasma membrane localization through a mechanism dependent on NOX/ROS/NF-κB pathway on endothelial cells. Lab Invest. 2019;99:421–437. doi: 10.1038/s41374-018-0151-3 [DOI] [PubMed] [Google Scholar]
- 132.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38:754–768. doi: 10.1016/j.immuni.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 133.Aranda-Rivera AK, Cruz-Gregorio A, Pedraza-Chaverri J, Scholze A. Nrf2 activation in chronic kidney disease: promises and pitfalls. Antioxidants (Basel). 2022;11:1112. doi: 10.3390/antiox11061112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Juul-Nielsen C, Shen J, Stenvinkel P, Scholze A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: alterations, interventions and relation to morbidity. Nephrol Dial Transplant. 2022;37:904–916. doi: 10.1093/ndt/gfab031 [DOI] [PubMed] [Google Scholar]
- 135.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005 [DOI] [PubMed] [Google Scholar]
- 136.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x [DOI] [PubMed] [Google Scholar]
- 137.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Group. Normal and pathological concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol. 2010;31:435–441. doi: 10.1159/000299798 [DOI] [PubMed] [Google Scholar]
- 139.Masai N, Tatebe J, Yoshino G, Morita T. Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor kB pathway. Circ J. 2010;74:2216–2224. doi: 10.1253/circj.cj-10-0117 [DOI] [PubMed] [Google Scholar]
- 140.El-Gamal D, Holzer M, Gauster M, Schicho R, Binder V, Konya V, Wadsack C, Schuligoi R, Heinemann A, Marsche G. Cyanate is a novel inducer of endothelial icam-1 expression. Antioxid Redox Signal. 2012;16:129–137. doi: 10.1089/ars.2011.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saum K, Campos B, Celdran-Bonafonte D, Nayak L, Sangwung P, Thakar C, Roy-Chaudhury P, Owens Iii AP. Uremic advanced glycation end products and protein-bound solutes induce endothelial dysfunction through suppression of Krüppel-like factor 2. J Am Heart Assoc. 2018;7:e007566. doi: 10.1161/JAHA.117.007566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, Lu X, Chen BH, Shan YX, Tian JW, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10:1699–1712. doi: 10.1089/ars.2007.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ito S, Osaka M, Edamatsu T, Itoh Y, Yoshida M. Crucial role of the aryl hydrocarbon receptor (AhR) in indoxyl sulfate-induced vascular inflammation. J Atheroscler Thromb. 2016;23:960–975. doi: 10.5551/jat.34462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakagawa K, Kobayashi F, Kamei Y, Tawa M, Ohkita M. Acute kynurenine exposure of rat thoracic aorta induces vascular dysfunction via superoxide anion production. Biol Pharm Bull. 2022;45:522–527. doi: 10.1248/bpb.b21-01079 [DOI] [PubMed] [Google Scholar]
- 145.Stafim da Cunha R, Gregório PC, Maciel RAP, Favretto G, Franco CRC, Gonçalves JP, de Azevedo MLV, Pecoits-Filho R, Stinghen AEM. Uremic toxins activate CREB/ATF1 in endothelial cells related to chronic kidney disease. Biochem Pharmacol. 2022;198:114984. doi: 10.1016/j.bcp.2022.114984 [DOI] [PubMed] [Google Scholar]
- 146.Fels B, Beyer A, Cazaña-Pérez V, Giraldez T, Navarro-González JF, Alvarez de la Rosa D, Schaefer F, Bayazit AK, Obrycki L, Ranchin B, et al. Effects of chronic kidney disease on nanomechanics of the endothelial glycocalyx are mediated by the mineralocorticoid receptor. Int J Mol Sci. 2022;23:10659. doi: 10.3390/ijms231810659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davel AP, Anwar IJ, Jaffe IZ. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens. 2017;26:97–104. doi: 10.1097/MNH.0000000000000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang K, Nie L, Huang Y, Zhang J, Xiao T, Guan X, Zhao J. Amelioration of uremic toxin indoxyl sulfate-induced endothelial cell dysfunction by Klotho protein. Toxicol Lett. 2012;215:77–83. doi: 10.1016/j.toxlet.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 149.El-Gamal D, Rao SP, Holzer M, Hallström S, Haybaeck J, Gauster M, Wadsack C, Kozina A, Frank S, Schicho R, et al. The urea decomposition product cyanate promotes endothelial dysfunction. Kidney Int. 2014;86:923–931. doi: 10.1038/ki.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol. 2008;3:691–698. doi: 10.2215/CJN.04291007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li P, Zhang L, Zhang M, Zhou C, Lin N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int J Mol Med. 2016;37:989–997. doi: 10.3892/ijmm.2016.2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cheng M, Yang Z, Qiao L, Yang Y, Deng Y, Zhang C, Mi T. AGEs induce endothelial cells senescence and endothelial barrier dysfunction via miR-1-3-p/MLCK signaling pathways. Gene. 2023;851:147030. doi: 10.1016/j.gene.2022.147030 [DOI] [PubMed] [Google Scholar]
- 153.Pawlak K, Mysliwiec M, Pawlak D. Kynurenine pathway - a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci. 2010;55:196–203. doi: 10.2478/v10039-010-0015-6 [DOI] [PubMed] [Google Scholar]
- 154.Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. 2015;10:e0132589. doi: 10.1371/journal.pone.0132589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H, Xiang S, Dai Z, Fan Y. Asymmetric dimethylarginine level as biomarkers of cardiovascular or all-cause mortality in patients with chronic kidney disease: a meta-analysis. Biomarkers. 2021;26:579–585. doi: 10.1080/1354750X.2021.1954694 [DOI] [PubMed] [Google Scholar]
- 156.Jankowski V, Saritas T, Kjolby M, Hermann J, Speer T, Himmelsbach A, Mahr K, Augusto Heuschkel M, Schunk SJ, Thirup S, et al. Carbamylated sortilin associates with cardiovascular calcification in patients with chronic kidney disease. Kidney Int. 2022;101:574–584. doi: 10.1016/j.kint.2021.10.018 [DOI] [PubMed] [Google Scholar]
- 157.Noels H, Lehrke M, Vanholder R, Jankowski J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol. 2021;17:528–542. doi: 10.1038/s41581-021-00423-5 [DOI] [PubMed] [Google Scholar]
- 158.Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4–33. doi: 10.1016/j.addr.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 159.Bose C, Shah SV, Karaduta OK, Kaushal GP. Carbamylated Low-Density Lipoprotein (cLDL)-mediated induction of autophagy and its role in endothelial cell injury. PLoS One. 2016;11:e0165576. doi: 10.1371/journal.pone.0165576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Holy EW, Akhmedov A, Speer T, Camici GG, Zewinger S, Bonetti N, Beer JH, Lüscher TF, Tanner FC. Carbamylated low-density lipoproteins induce a prothrombotic state via LOX-1: impact on arterial thrombus formation in vivo. J Am Coll Cardiol. 2016;68:1664–1676. doi: 10.1016/j.jacc.2016.07.755 [DOI] [PubMed] [Google Scholar]
- 161.Sun JT, Yang K, Lu L, Zhu ZB, Zhu JZ, Ni JW, Han H, Chen N, Zhang RY. Increased carbamylation level of HDL in end-stage renal disease: carbamylated-HDL attenuated endothelial cell function. Am J Physiol Renal Physiol. 2016;310:F511–F517. doi: 10.1152/ajprenal.00508.2015 [DOI] [PubMed] [Google Scholar]
- 162.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M’Rad MB, Jacquot C, Houillier P, Stengel B, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whitlock RH, Ferguson TW, Komenda P, Rigatto C, Collister D, Bohm C, Reaven NL, Funk SE, Tangri N. Metabolic acidosis is undertreated and underdiagnosed: a retrospective cohort study. Nephrol Dial Transplant. 2022:gfac099. doi: 10.1093/ndt/gfac299 [DOI] [PubMed] [Google Scholar]
- 164.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kid Dis. 2013;62:670–678. doi: 10.1053/j.ajkd.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Collister D, Ferguson TW, Funk SE, Reaven NL, Mathur V, Tangri N. Metabolic acidosis and cardiovascular disease in CKD. Kid Med. 2021;3:753–761.e1. doi: 10.1016/j.xkme.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wesson DE, Buysse JM, Bushinsky DA. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol. 2020;31:469–482. doi: 10.1681/ASN.2019070677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006 [DOI] [PubMed] [Google Scholar]
- 168.Chen ZW, Tsai CH, Pan CT, Chou CH, Liao CW, Hung CS, Wu VC, Lin YH. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci. 2019;20:5214. doi: 10.3390/ijms20205214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One. 2013;8:e61991. doi: 10.1371/journal.pone.0061991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dong L, Krewson EA, Yang LV. Acidosis activates endoplasmic reticulum stress pathways through GPR4 in human vascular endothelial cells. Int J Mol Sci. 2017;18:278. doi: 10.3390/ijms18020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Asai M, Takeuchi K, Saotome M, Urushida T, Katoh H, Satoh H, Hayashi H, Watanabe H. Extracellular acidosis suppresses endothelial function by inhibiting store-operated Ca2+ entry via non-selective cation channels. Cardiovasc Res. 2009;83:97–105. doi: 10.1093/cvr/cvp105 [DOI] [PubMed] [Google Scholar]
- 172.Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, Chonchol M. Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study. Clin J Am Soc Nephrol. 2018;13:1463–1470. doi: 10.2215/CJN.00380118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci. 2017;18:1682. doi: 10.3390/ijms18081682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402 [DOI] [PubMed] [Google Scholar]
- 175.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261 [DOI] [PubMed] [Google Scholar]
- 176.Penne EL, Neumann J, Klein IH, Oey PL, Bots ML, Blankestijn PJ. Sympathetic hyperactivity and clinical outcome in chronic kidney disease patients during standard treatment. J Nephrol. 2009;22:208–215. [PubMed] [Google Scholar]
- 177.Lopez Garcia de Lomana A, Vilhjalmsson AI, McGarrity S, Sigurethardottir R, Anuforo O, Viktorsdottir AR, Kotronoulas A, Bergmann A, Franzson L, Halldorsson H, et al. Metabolic response in endothelial cells to catecholamine stimulation associated with increased vascular permeability. Int J Mol Sci. 2022;23:3162. doi: 10.3390/ijms23063162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park S, Lee S, Kim Y, Cho S, Kim K, Kim YC, Han SS, Lee H, Lee JP, Joo KW, et al. A mendelian randomization study found causal linkage between telomere attrition and chronic kidney disease. Kidney Int. 2021;100:1063–1070. doi: 10.1016/j.kint.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 180.Ameh OI, Okpechi IG, Dandara C, Kengne AP. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS. 2017;21:143–155. doi: 10.1089/omi.2016.0180 [DOI] [PubMed] [Google Scholar]
- 181.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094 [DOI] [PubMed] [Google Scholar]
- 182.Buendía P, Carracedo J, Soriano S, Madueño JA, Ortiz A, Martín-Malo A, Aljama P, Ramírez R. Klotho prevents NFkB translocation and protects endothelial cell from senescence induced by uremia. J Gerontol A Biol Sci Med Sci. 2015;70:1198–1209. doi: 10.1093/gerona/glu170 [DOI] [PubMed] [Google Scholar]
- 183.Izquierdo MC, Perez-Gomez M, Sanchez-Niño MD, Sanz AB, Ruiz-Andres O, Poveda J, Moreno JA, Egido J, Ortiz A. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant. 2012;27:iv6–iv10. doi: 10.1093/ndt/gfs426 [DOI] [PubMed] [Google Scholar]
- 184.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. aKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27:2331–2345. doi: 10.1681/ASN.2015060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rodrigues SD, Santos SS, Meireles T, Romero N, Glorieux G, Pecoits-Filho R, Zhang DD, Nakao LS. Uremic toxins promote accumulation of oxidized protein and increased sensitivity to hydrogen peroxide in endothelial cells by impairing the autophagic flux. Biochem Biophys Res Commun. 2020;523:123–129. doi: 10.1016/j.bbrc.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 186.Yan J, Wang J, He JC, Zhong Y. Sirtuin 1 in chronic kidney disease and therapeutic potential of targeting sirtuin 1. Front Endocrinol (Lausanne). 2022;13:917773. doi: 10.3389/fendo.2022.917773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez_Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 189.Zhang YX, Tang RN, Wang LT, Liu BC. Role of crosstalk between endothelial cells and smooth muscle cells in vascular calcification in chronic kidney disease. Cell Prolif. 2021;54:e12980. doi: 10.1111/cpr.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meng F, Zhao Y, Wang B, Li B, Sheng Y, Liu M, Li H, Xiu R. Endothelial cells promote calcification in aortic smooth muscle cells from spontaneously hypertensive rats. Cell Physiol Biochem. 2018;49:2371–2381. doi: 10.1159/000493837 [DOI] [PubMed] [Google Scholar]
- 191.Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jia LX, Zhang WM, Li TT, Liu Y, Piao CM, Ma YC, Lu Y, Wang Y, Liu TT, Qi YF, et al. ER stress dependent microparticles derived from smooth muscle cells promote endothelial dysfunction during thoracic aortic aneurysm and dissection. Clin Sci (Lond). 2017;131:1287–1299. doi: 10.1042/CS20170252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003 [DOI] [PubMed] [Google Scholar]
- 194.Chen Y, Zhao X, Wu H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscler Thromb Vasc Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Himmelsbach A, Ciliox C, Goettsch C. Cardiovascular calcification in chronic kidney disease - therapeutic opportunities. Toxins (Basel). 2020;12:181. doi: 10.3390/toxins12030181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kanno Y, Into T, Lowenstein CJ, Matsushita K. Nitric oxide regulates vascular calcification by interfering with TGF- signalling. Cardiovasc Res. 2008;77:221–230. doi: 10.1093/cvr/cvm049 [DOI] [PubMed] [Google Scholar]
- 197.Van den Bergh G, Van den Branden A, Opdebeeck B, Fransen P, Neven E, De Meyer GRY, D’Haese PC, Verhulst A. Endothelial dysfunction aggravates arterial media calcification in warfarin administered rats. FASEB J. 2022;36:e22315. doi: 10.1096/fj.202101919R [DOI] [PubMed] [Google Scholar]
- 198.Zhang L, Yao J, Yao Y, Boström KI. Contributions of the endothelium to vascular calcification. Front Cell Dev Biol. 2021;9:620882. doi: 10.3389/fcell.2021.620882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73:924–967. doi: 10.1124/pharmrev.120.000096 [DOI] [PubMed] [Google Scholar]
- 200.Yilmaz MI, Saglam M, Sonmez A, Caglar K, Cakir E, Kurt Y, Eyileten T, Tasar M, Acikel C, Oguz Y, et al. Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: ramipril versus valsartan. Blood Purif. 2007;25:327–335. doi: 10.1159/000107410 [DOI] [PubMed] [Google Scholar]
- 201.Han SH, Kang EW, Yoon HS, Lee HC, Yoo TH, Choi KH, Han DS, Kang SW. Combined vascular effects of HMG-CoA reductase inhibitor and angiotensin receptor blocker in non-diabetic patients undergoing peritoneal dialysis. Nephrol Dial Transplant. 2011;26:3722–3728. doi: 10.1093/ndt/gfr108 [DOI] [PubMed] [Google Scholar]
- 202.Gamboa JL, Pretorius M, Sprinkel KC, Brown NJ, Ikizler TA. Angiotensin converting enzyme inhibition increases ADMA concentration in patients on maintenance hemodialysis -- a randomized cross-over study. BMC Nephrol. 2015;16:167. doi: 10.1186/s12882-015-0162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nuffield Department of Population Health Renal Studies G and Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. doi: 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Patoulias D, Papadopoulos C, Kassimis G, Vassilikos V, Karagiannis A, Doumas M. Meta-analysis addressing the effect of sodium-glucose cotransporter 2 inhibitors on flow-mediated dilation in patients with type 2 diabetes mellitus. Am J Cardiol. 2022;165:133–135. doi: 10.1016/j.amjcard.2021.11.003 [DOI] [PubMed] [Google Scholar]
- 205.Tanaka A, Shimabukuro M, Okada Y, Sugimoto K, Kurozumi A, Torimoto K, Hirai H, Node K; PROCEED trial investigators. Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial. Cardiovasc Diabetol. 2020;19:85. doi: 10.1186/s12933-020-01065-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Juni RP, Al-Shama R, Kuster DWD, van der Velden J, Hamer HM, Vervloet MG, Eringa EC, Koolwijk P, van Hinsbergh VWM. Empagliflozin restores chronic kidney disease-induced impairment of endothelial regulation of cardiomyocyte relaxation and contraction. Kidney Int. 2021;99:1088–1101. doi: 10.1016/j.kint.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 207.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594 [DOI] [PubMed] [Google Scholar]
- 208.Flevari P, Kalogeropoulou S, Drakou A, Leftheriotis D, Panou F, Lekakis J, Kremastinos D, Vlahakos DV. Spironolactone improves endothelial and cardiac autonomic function in non heart failure hemodialysis patients. J Hypertens. 2013;31:1239–1244. doi: 10.1097/HJH.0b013e32835f955c [DOI] [PubMed] [Google Scholar]
- 209.Gil-Ortega M, Vega-Martin E, Martin-Ramos M, Gonzalez-Blazquez R, Pulido-Olmo H, Ruiz-Hurtado G, Schulz A, Ruilope LM, Kolkhof P, Somoza B, et al. Finerenone reduces intrinsic arterial stiffness in munich wistar fromter rats, a genetic model of chronic kidney disease. Am J Nephrol. 2020;51:294–303. doi: 10.1159/000506275 [DOI] [PubMed] [Google Scholar]
- 210.Gonzalez-Blazquez R, Somoza B, Gil-Ortega M, Martin Ramos M, Ramiro-Cortijo D, Vega-Martin E, Schulz A, Ruilope LM, Kolkhof P, Kreutz R, et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol. 2018;9:1131. doi: 10.3389/fphar.2018.01131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, et al. IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol. 2017;28:971–980. doi: 10.1681/ASN.2016040453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jalal DI, Decker E, Perrenoud L, Nowak KL, Bispham N, Mehta T, Smits G, You Z, Seals D, Chonchol M, et al. Vascular function and uric acid-lowering in stage 3 CKD. J Am Soc Nephrol. 2017;28:943–952. doi: 10.1681/ASN.2016050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rutherford E, Ireland S, Mangion K, Stewart GA, MacGregor MS, Roditi G, Woodward R, Gandy SJ, Houston JG, Jardine AG, et al. A randomized, controlled trial of the effect of allopurinol on left ventricular mass index in hemodialysis patients. Kidney Int Rep. 2020;6:146–155. doi: 10.1016/j.ekir.2020.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, Struthers AD. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yelken B, Caliskan Y, Gorgulu N, Altun I, Yilmaz A, Yazici H, Oflaz H, Yildiz A. Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin Nephrol. 2012;77:275–282. doi: 10.5414/cn107352 [DOI] [PubMed] [Google Scholar]
- 216.Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, Bolignano D, Cutrupi S, Politi R, Tripepi G, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64:1005–1011. doi: 10.1161/HYPERTENSIONAHA.114.03748 [DOI] [PubMed] [Google Scholar]
- 217.Chitalia N, Ismail T, Tooth L, Boa F, Hampson G, Goldsmith D, Kaski JC, Banerjee D. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One. 2014;9:e91363. doi: 10.1371/journal.pone.0091363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thethi TK, Bajwa MA, Ghanim H, Jo C, Weir M, Goldfine AB, Umpierrez G, Desouza C, Dandona P, Fang-Hollingsworth Y, et al. Effect of paricalcitol on endothelial function and inflammation in type 2 diabetes and chronic kidney disease. J Diabetes Complications. 2015;29:433–437. doi: 10.1016/j.jdiacomp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kendrick J, Andrews E, You Z, Moreau K, Nowak KL, Farmer-Bailey H, Seals DR, Chonchol M. Cholecalciferol, calcitriol, and vascular function in CKD: a randomized, double-blind trial. Clin J Am Soc Nephrol. 2017;12:1438–1446. doi: 10.2215/CJN.01870217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159 [DOI] [PubMed] [Google Scholar]