Abstract
Screening for drug-induced hyperprolactinaemia, a condition characterised by higher-than-normal levels of serum prolactin induced by drug treatments, requires a comprehensive understanding of the clinical presentations and long-term complications of the condition. Using two databases, Embase and MEDLINE, we summarised the available evidence on the clinical presentations and long-term complications of drug-induced hyperprolactinaemia. Clinical and observational studies reporting on drug treatments known or suspected to induce hyperprolactinaemia were included. Database searches were limited to the English language; no date or geographic restrictions were applied. Fifty studies were identified for inclusion, comprising a variety of study designs and patient populations. Most data were reported in patients treated with antipsychotics, but symptoms were also described among patients receiving other drugs, such as prokinetic drugs and antidepressants. Notably, the diagnosis of drug-induced hyperprolactinaemia varied across studies since a standard definition of elevated prolactin levels was not consistently applied. Frequent clinical presentations of hyperprolactinaemia were menstrual cycle bleeding, breast or lactation disorders, and sexual dysfunctions, described in 80% (40/50), 74% (37/50), and 42% (21/50) of the included studies, respectively. In the few studies reporting such symptoms, the prevalence of vaginal dryness impacted up to 53% of females, and infertility in both sexes ranged from 15 to 31%. Clinicians should be aware of these symptoms related to drug-induced hyperprolactinaemia when treating patients with drugs that can alter prolactin levels. Future research should explore the long-term complications of drug-induced hyperprolactinaemia and apply accepted thresholds of elevated prolactin levels (i.e., 20 ng/mL for males and 25 ng/mL for females) to diagnose hyperprolactinaemia as a drug-induced adverse event.
Trial Registration PROSPERO International Prospective Register Of Systematic Reviews (CRD42021245259).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40290-023-00462-2.
Key Points
| The 50 identified studies reporting clinical information about complications of drug-induced hyperprolactinaemia applied varying thresholds of prolactin levels to diagnose the condition. Future research should apply accepted consensus on elevated prolactin levels to allow better comparison across studies and likely more consistent results. |
| Most of the symptoms reported were menstrual cycle bleeding disorders, breast or lactation disorders, and sexual dysfunction. Patients presenting with these complications were receiving antipsychotics, prokinetic drugs, and antidepressants. |
| Limited clinical information exists about the complications of hyperprolactinaemia induced by drugs, especially in the long term, so additional research is needed to understand these adverse effects. |
Introduction
Hyperprolactinaemia (HPL), a condition characterised by higher-than-normal levels of serum prolactin (PRL), is estimated to occur in 13.8 people per 100,000 person-years and 3.5 times more females than males [1]. Under normal serum levels (i.e., PRL < 25 ng/mL in women, and PRL < 20 ng/mL in men) [2, 3], PRL is involved in several physiological processes—from lactation and regulation of sexual and reproduction functions, to newly described involvement in the proliferation of pancreatic β cells [4, 5]. Elevated serum levels of PRL—leading to HPL—may result in a range of symptoms and disorders, such as galactorrhoea, sexual dysfunction, and infertility in both sexes; females can be affected by irregular menstruation and amenorrhoea [6–9].
Elevated PRL levels have been reported under certain physiological conditions, such as pregnancy or breastfeeding, stress, exercise, and anxiety [2, 6]. Although PRL-secreting pituitary tumours (prolactinomas) are the most common cause of HPL [10, 11], other pituitary and hypothalamus disorders and tumours, systemic disorders (such as chronic kidney disease), and drugs are known to interfere with the neuroendocrine regulation of PRL [2, 12]. Drugs can induce HPL through different mechanisms. The inhibition of dopamine through drugs that are dopamine receptor antagonists (e.g., antipsychotics and metoclopramide), or drugs that inhibit dopamine synthesis (e.g., oestrogen) impact the hypothalamic suppression of prolactin synthesis, which in turns leads to increased PRL secretion [13]. Other drugs affect PRL levels by promoting the release of different factors that impact the tonic suppression of prolactin synthesis in the hypothalamus, such as serotonin and GABA [13].
The estimated prevalence of drug-induced HPL ranges from 15 to 45% [1, 10], and antipsychotics, tricyclic antidepressants, and prokinetic drugs (e.g., metoclopramide) can affect PRL levels and induce HPL as an adverse event (AE) [1, 10, 12]. Symptoms associated with drug-induced HPL can vary according to different drugs and treatment periods (i.e., short vs longer treatment periods); this poses a challenge for clinical identification and monitoring.
Screening for symptoms of drug-induced HPL requires a comprehensive understanding of the possible clinical presentations and long-term complications of the condition. However, current knowledge derives from unsystematic observations and clinical consensus, thus hindering diagnosis and treatment [14]. Our aim was to systematically review the evidence on the clinical presentations and long-term complications of drug-induced HPL and summarise the available data related to symptoms experienced by patients with drug-induced HPL.
Literature Search and Synthesis Methods
Following the review approach established in the review protocol (PROSPERO register CRD42021245259), we included studies based on the populations, interventions and comparators, outcomes, and study design (PICOS) framework (Table 1). The included studies comprised clinical trials (randomised, non-randomised, and single-arm) and observational studies (prospective, retrospective, and cross-sectional) that reported on the clinical presentations or long-term complications of drug-induced HPL in adults and children. Studies reporting on any drug treatment known or suspected to induce HPL were considered relevant for inclusion in the review.
Table 1.
PICOS selection criteria
| Criterium | Inclusion |
|---|---|
| Population | Children and adults with drug-induced HPL |
| Intervention | Drug treatment known or suspected to induce HPL |
| Comparator | Any or no comparator |
| Outcomes | Clinical presentation, including symptoms and signs of druginduced HPL, such as reproductive dysfunction, sexual impairment, breast dysfunctions, abnormalities associated with chronic hypogonadism, behavioural and mood alterations |
| Long-term complications of drug-induced HPL | |
| Study design |
Interventional and non-interventional studies: • Non-interventional/observational studies (prospective, retrospective, cross-sectional studies) • Interventional studies (randomised and non-randomised clinical trials) |
HPL hyperprolactinaemia, PICOS populations, interventions and comparators, outcomes, and study design
Studies involving patients who developed physiologic or pathological HPL not induced by drugs (e.g., Cushing syndrome, hypothalamus diseases, cirrhosis) were excluded. Studies assessing a population with a mixed aetiology of HPL were included only if data were reported separately for patients with drug-induced HPL. In addition, studies not reporting data on the outcomes of interest were excluded.
Information Sources and Search Terms
Electronic searches were conducted in Embase®, MEDLINE®, and MEDLINE® In-Process using the OvidSP® platform on 18 March 2021. The searches utilised a combination of controlled vocabulary and keywords specific to each database. Appropriate search strings filtering out study designs and publication types not of interest (e.g., case reports or opinion pieces) were employed, and the searches were limited to the English language. No date limit was applied. The search strategies are listed in Table S1.
As a search validation step, the protocol included checking the reference lists of systematic literature reviews published since 2018 for additional eligible publications. However, this step was not performed because no relevant systematic literature review was identified. Grey literature searches were not conducted.
Screening and Eligibility Assessment
Search results were imported into Endnote Version X9®, and duplicates were removed. Screening was conducted in DistillerSR® version 2.35.10 in two stages. First, the title and abstract of each retrieved publication were screened against the PICOS criteria by two independent reviewers. Second, the full texts of any records deemed potentially relevant were obtained and screened independently by two reviewers. In all stages, multiple reviewers worked independently as part of the review team; disagreements between reviewers were resolved by a third, senior researcher (DJ and CCC).
Data Extraction and Data Items
Data from the included publications were extracted into a Microsoft Excel® data extraction template by one reviewer, and independently validated by a second, senior reviewer. The data items extracted from the studies were pre-specified in the protocol, and included the following variables.
Study characteristics: study design, study duration/observation period, geographic location, study setting, study population, sample size, and patient inclusion/exclusion criteria.
Patient characteristics: age, sex, race/ethnicity, key diagnosis for which the drug is known or suspected to have induced HPL was administered, drug name/class of treatment known or suspected to have induced HPL, comorbidities, serum PRL levels.
Clinical presentations and long-term complications of HPL: symptoms and signs, time since start of drug treatment until the symptom or long-term complication of HPL presented.
When considering clinical trials (either randomised or non-randomised) allocating patients with drug-induced HPL to receive treatment for the condition, patient characteristics and clinical presentation data were extracted from the baseline characteristics. Additionally, only data on patients with confirmed drug-induced HPL were extracted.
Data Synthesis
The information assessed in this review was expected to be reported as occurrence (n, %) of symptoms and signs characterising drug-induced HPL. Consequently, the data synthesis comprised a descriptive summary of the results—i.e., an overview of the data according to the study design, patient characteristics, symptoms and signs reported as representative of the clinical presentations, and long-term complications of drug-induced HPL.
Summary of Findings
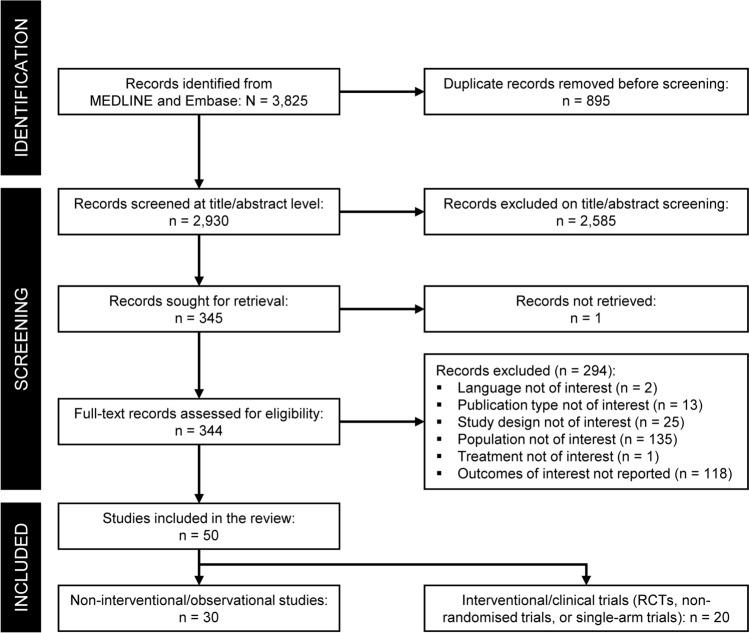
The electronic searches returned 3825 records; of those, 2930 unique records were screened after removing duplicates (Fig. 1). Following screening, 50 studies were included, comprising 20 clinical trials and 30 observational studies.
Fig. 1.
PRISMA diagram. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-analyses, RCT randomised controlled trial
Study Characteristics
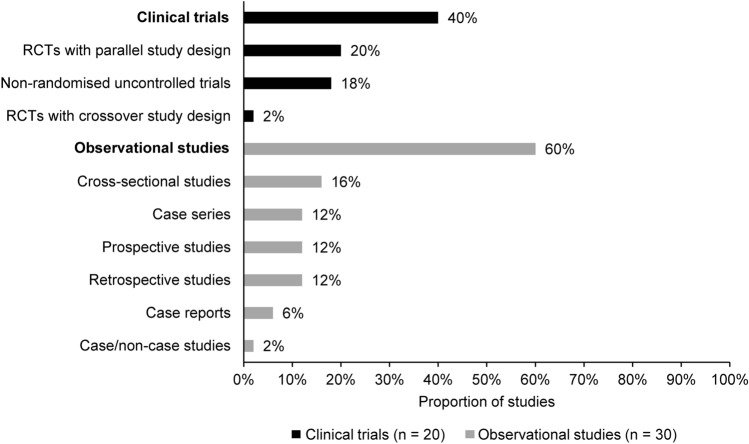
Figure 2 describes the study design of the included studies, and Table 2 summarises their characteristics. Table S2 provides additional details of study characteristics. Half of the clinical trials (10/20; 50%) applied a randomised parallel design, while nearly one-third of observational studies (9/30; 30%) were case series or case reports. Over half of the clinical trials were conducted in Asia (55%; 11/20) [15–25], while most observational studies took place in European (43%; 13/30) or Asian countries (37%; 11/30) [26–38]. Study settings at the time of patient enrolment were not reported in 36% (18/50) of the included studies. Most studies reporting this information recruited participants through outpatient settings, which accounted for 58% (7/12) of the clinical trials [17, 18, 22, 39–42] and 65% (13/20) of the observational studies [27, 29, 32, 34, 35, 43–50]. Information on the study period of enrolment was available from 54% (27/50) of the studies included in this review. Among the clinical trials that provided information about their enrolment period, most (9/10; 90%) enrolled patients from year 2000 onwards [15, 17, 20–22, 25, 39, 41, 51]. One clinical trial was conducted from 1987 to 1991 (totalling 4 years of enrolment) [16]. Among the observational studies that reported the enrolment period, 71% (12/17) enrolled patients from 2000 to 2018 [10, 28, 34, 38, 43, 46, 47, 49, 52–55]. The remaining studies (5/17; 29%) were conducted between 1976 and 1988 [27, 35, 36, 48, 50].
Fig. 2.
Study design of the included studies. RCT randomised controlled trial
Table 2.
Characteristics of included studies
| Characteristic | Clinical trials (n = 20) | Observational studies (n = 30) |
|---|---|---|
| Geographic location, n (%) | ||
| Asiaa | 11 (55%) | 11 (37%)b |
| Europe | 6 (30%) | 13 (44%) |
| North America | 2 (1%) | 3 (10%) |
| South America | 0 (0%) | 1 (3%) |
| Africa | 0 (0%) | 1 (3%) |
| Multiple countries | 1 (5%) | 0 (0%) |
| Not reported | 0 (0%) | 1 (3%) |
| Study setting, n (%) | ||
| Inpatient | 1 (5%) | 6 (20%) |
| Outpatient | 7 (35%) | 13 (44%) |
| Inpatient and outpatient | 4 (20%) | 1 (3%) |
| Not reported | 8 (40%) | 10 (33%) |
| Enrolment period, n (%) | ||
| After 2000 | 9 (45%) | 12 (40%) |
| Before 2000 | 1 (5%) | 5 (17%) |
| Not reported | 10 (50%) | 13 (43%) |
aIncluding Turkey as an Asian country (Turkey is situated in both Asia and Europe, with its larger territory lying on the Asian continent)
bIncluding Russia
The studies included in this review documented HPL among patients receiving various drug treatments. However, none were designed with the primary objective of assessing the causal relationship between the drug treatment, the development of HPL, and the symptoms experienced by patients. Instead, patients were identified with drug-induced HPL through different clinical approaches describing the drug treatment as the primary suspected cause of the HPL. Among the included studies, the definition of HPL varied substantially. In general, PRL levels used to define HPL ranged from ≥ 10 ng/mL to ≥ 30 ng/mL. Some studies defined specific PRL levels for males and females to diagnose HPL, while others applied different levels of PRL excess to classify mild and severe HPL cases. Study-specific definitions of HPL are provided in Table S2.
Finally, the duration of treatments suspected to have caused HPL differed among studies, and half of the included studies (25/50; 50%) did not report data on treatment duration. Fifteen clinical trials (of 20; 75%) reported a study duration ranging from 6 weeks to 2 years, and most trials (10/15; 67%) lasted between 2 and 4 months [17, 18, 21–24, 39, 42, 56, 57]. Ten observational studies (of 30; 10%) reported study durations varying from 2 months to 24 months. Most observational studies (7/10; 70%) lasted 6 months or less [26, 29, 44, 45, 47, 49, 58, 59].
Patient Characteristics
Table 3 summarises the demographic characteristics of patients in the included studies. Among the clinical trials and observational studies, samples comprised mostly adults (42/50; 84%). One clinical trial [41] and two observational studies included children [28, 49], and one clinical trial [51] and four observational studies enrolled both children and adults [26, 34, 52, 53]. Most studies recruited both females and males; however, females were slightly more prevalent among patients with drug-induced HPL. Among the clinical trials, half of the studies (10/20; 50%) assessed at least one female-only treatment group [15–22, 25, 60], while the proportion of females ranged from 0 to 90% in the remaining trials [23, 24, 39–42, 51, 56, 57, 61]. Similarly, 77% (23/30) of the observational studies reported a proportion of females as ≥ 50%, with 37% (11/30) reporting data on females only for at least one of the treatment groups assessed.
Table 3.
Patient characteristics in included studies
| Patient characteristic | Top-level overview | N studies | Reported in studies |
|---|---|---|---|
| Clinical trials (n = 20) | |||
| Patient age (years) |
Mean: 16–47 Range: 21–62 |
14 | Andersen, 1982 [60]; Atmaca, 2002 [15]; Cavallaro, 2004 [39]; Düring, 2019 [61]; Karaman, 1993 [16]; Lee, 2006 [17]; Lee, 2010 [18]; Lu, 2008 [19]; Man, 2016 [20]; Mir, 2008 [40]; Qiao, 2016 [21]; Trives, 2013 [42]; Yoon, 2016 [24]; Yuan, 2008 [25] |
| Sex (female) | 0–49% | 5 | Düring, 2019 [61]; Kelly, 2006 [56]; Mir, 2008 [40]; Perez-Iglesias, 2012 [51]; Savitz, 2015 [41] |
| 50–90% | 5 | Cavallaro, 2004 [39]; Kinon, 2006 [57]; Shim, 2007 [23]; Trives, 2013 [42]; Yoon, 2016 [24] | |
| 100% | 10 | Andersen, 1982 [60]; Atmaca, 2002 [15]; Karaman, 1993 [16]; Lee, 2006 [17]; Lee, 2010 [18]; Lu, 2008 [19]; Man, 2016 [20]; Qiao, 2016 [21]; Ranjbar, 2015 [22]; Yuan, 2008 [25] | |
| Race/ethnicity |
48–100% White 26–67% Black/African American 1–100% other (Asian, Hispanic Chinese, American Indian or Alaskan native, other/mixed) |
6 | Kelly, 2006 [56]; Kinon, 2006 [57]; Mir, 2008 [40]; Qiao, 2016 [21]; Savitz, 2015 [41]; Trives, 2013 [42] |
| Serum PRL levelsa |
Mean: 32–168 ng/mL Range: 19–99 ng/mL |
11 | Andersen, 1982 [60]; Cavallaro, 2004 [39]; Lee, 2006 [17]; Lee, 2010 [18]; Lu, 2008 [19]; Man, 2016 [20]; Mir, 2008 [40]; Qiao, 2016 [21]; Trives, 2013 [42]; Yoon, 2016 [24]; Yuan, 2008 [25] |
| Observational studies (n = 30) | |||
| Patient age (years) |
Mean: 15–47 Median: 39–41 Range: 18–71 |
17 | Ahuja, 2008 [63]; Atluri, 2018 [43]; Barszcz, 2007 [26]; Chen, 2010 [44]; Emsley, 2008 [53]; Furuhjelm, 1980 [27]; Holzer, 2006 [28]; Kalkavoura, 2013 [29]; Kopecek, 2006 [30]; Kotan, 2011 [45]; Kulshreshtha, 2017 [46]; Matsuoka, 1986 [59]; Melkersson, 1999 [31]; Trenque, 2011 [36]; Vilar, 2008 [10]; Yunilainen, 2018 [38]; Zhang, 2018 [55] |
| Sex (female) | 0–49% | 7 | Alosaimi, 2018 [52]; Emsley, 2008 [53]; Holzer, 2006 [28]; Kotan, 2011 [45]; Leonard, 1989 [48]; Roke, 2012 [34]; Venetikou, 2008 [37] |
| 50–90% | 12 | Kalkavoura, 2013 [29]; Kopecek, 2006 [30]; Lee, 2006 [58]; Margari, 2015 [49]; Matsuoka, 1986 [59]; Melkersson, 1999 [31]; Melkersson, 2005 [32]; Park, 2016 [54]; Trenque, 2011 [36]; Vilar, 2008 [10]; Yunilainen, 2018 [38]; Zhang, 2018 [55] | |
| 100% | 11 | Ahuja, 2008 [63]; Atluri, 2018 [43]; Barszcz, 2007 [26]; Chen, 2010 [44]; Furuhjelm, 1980 [27]; Kulshreshtha, 2017 [46]; Lankford, 1981 [65]; Lee, 2005 [47]; Pollock, 1998 [33]; Seppala, 1977 [35]; Smith, 1992 [50] | |
| Race/ethnicity |
13–100% White/Caucasian 100% Taiwanese 78% Mixed |
4 | Chen, 2010 [44]; Emsley, 2008 [53]; Margari, 2015 [49]; Roke, 2012 [34] |
| Serum PRL levelsa |
Mean: 24–145 ng/mL Media: 59b Range: 25 to ≥ 200 ng/mL |
19 | Atluri, 2018 [43]; Barszcz, 2007 [26]; Chen, 2010 [44]; Furuhjelm, 1980 [27]; Holzer, 2006 [28]; Kopecek, 2006 [30]; Kulshreshtha, 2017 [46]; Lankford, 1981 [65]; Lee, 2005 [47]; Lee, 2006 [58]; Leonard, 1989 [48]; Margari, 2015 [49]; Melkersson, 2005 [32]; Park, 2016 [54]; Pollock, 1998 [33]; Roke, 2012 [34]; Smith, 1992 [50]; Vilar, 2008 [10]; Zhang, 2018 [55] |
HPL hyperprolactinaemia, PRL prolactin
aIn patients diagnosed with drug-induced HPL, regardless of treatment status
bReported in only one study
Two-thirds (33/50; 66%) of the included studies reported on the serum PRL level of patients with drug-induced HPL. Mean PRL levels of patients with drug-induced HPL varied from 32 to 168 ng/mL [17, 24]; one trial reported median and range values (84 ng/mL; 44–99 ng/mL) [42], and two studies reported only ranges [40, 60]. Over two-thirds of studies reported serum PRL levels (19/30; 63%); of those, 21% (4/19) described data among the overall set of participants enrolled in the study, regardless of the drug-induced HPL status [32, 34, 54, 55]. Fifteen studies (of 19; 79%) reported data on patients diagnosed with drug-induced HPL and assessed in at least one treatment group. Mean PRL levels of patients with drug-induced HPL ranged from 24 to 145 ng/mL [44, 46, 48]. Two studies reported only ranges [27, 33], and one study reported data separately for females and males (males: 505–1318 mIU/mL; females: 887–4250 mIU/mL) [30]. Additional details of patient characteristics are provided in Table S3.
Characteristics of Patients with Drug-Induced HPL
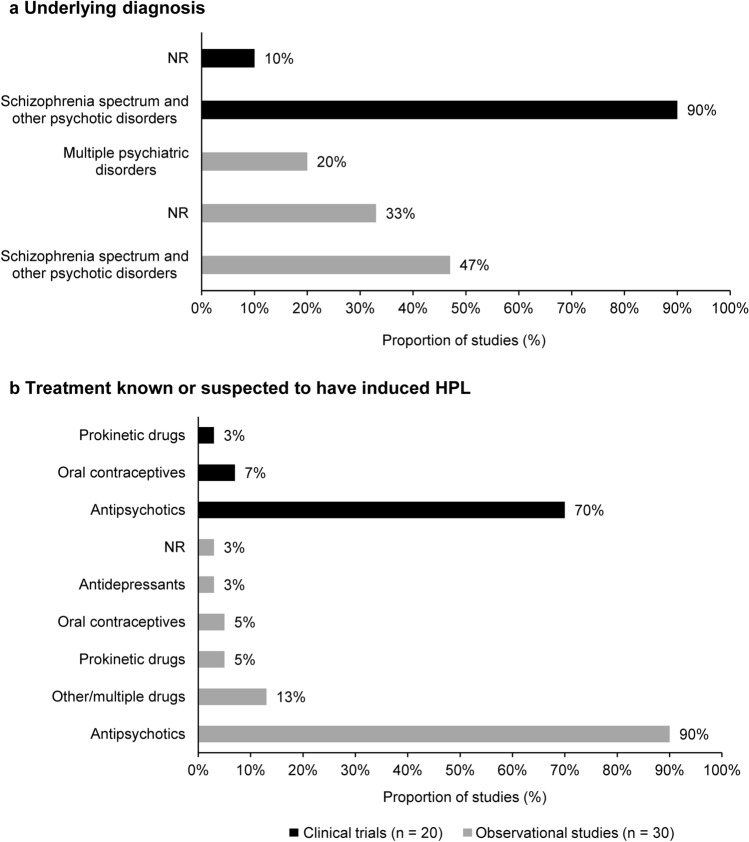
The number of patients with drug-induced HPL ranged from 2 (among 35 assessed patients) [15] to 220 (among 400 assessed patients) [41] among the clinical trials, and from 5 [28] to 442 [52] among the observational studies. The clinical presentations and long-term complications were reported in patients with a variety of diagnoses, but mostly (47/50; 94%) in patients with schizophrenia spectrum and other psychotic disorders. Consistent with the patient’s diagnosis, most drugs known or suspected to have induced HPL were antipsychotics, as reported in 76% (38/50) of the included studies. One clinical trial reported drug-induced HPL occurring in patients receiving treatment with prokinetic drugs [60], and another in patients taking oral contraceptives [16]. Oral contraceptives and prokinetic drugs were also reported as associated with drug-induced HPL in two observational studies [35, 43]; however, 20% (6/30) of the observational studies also reported a variety of other treatments as associated with drug-induced HPL, such as antihypertensives, antidepressants, and anxiolytic drugs [10, 36, 37, 46, 52]. Figure 3 summarises the underlying diagnosis of patients with drug-induced HPL and their related treatments.
Fig. 3.
Underlying diagnosis for which the drug known or suspected to have induced HPL was administered (a), and treatment known or suspected to have induced HPL (b). HPL hyperprolactinaemia, NR not reported. Note: Underlying diagnosis refers to the diagnosis for which the drug known or suspected to have induced HPL was administered. Some studies described patients with HPL, but the specific diagnoses and drugs related to the development of HPL were not reported. Importantly, only diagnoses that were clearly documented were extracted; no interpretations were made to avoid biasing the results
The reporting of treatment doses and durations varied significantly across studies. Treatment doses were reported according to the specific drugs and their established regimen. One-third (17/50; 34%) of the studies did not report treatment duration for the patients assessed. In those studies that did report treatment durations, these ranged from 18 days [57] to 12 years among the clinical trials [60], and from 15 days [30] to 8 years [32] among the observational studies. Table S4 provides details on the characteristics of patients with drug-induced HPL.
Clinical Presentations and Long-Term Complications of Patients with Drug-Induced HPL
The included studies described the clinical presentations of drug-induced HPL in various ways, using 79 unique terms to describe symptoms and signs experienced by this population. The reported clinical characteristics of drug-induced HPL were classified into seven symptom categories broadly based on the International Classification of Diseases 11th Revision (ICD-11) [62]: breast or lactation disorders, female genital system disorders, hair and skin abnormalities, infertility, menstrual cycle bleeding disorders, sexual dysfunctions, and other clinical presentations. The symptoms categorised among ‘other clinical presentations’ referred to presentations described as a combination of symptoms (e.g., amenorrhoea, decreased libido, and galactorrhoea), and symptoms that might be part of the range of AEs induced by the drug treatments assessed and not necessarily associated with drug-induced HPL (e.g., muscle rigidity and weight gain associated with antipsychotics). Table S5 provides further details on other clinical presentations reported by the studies.
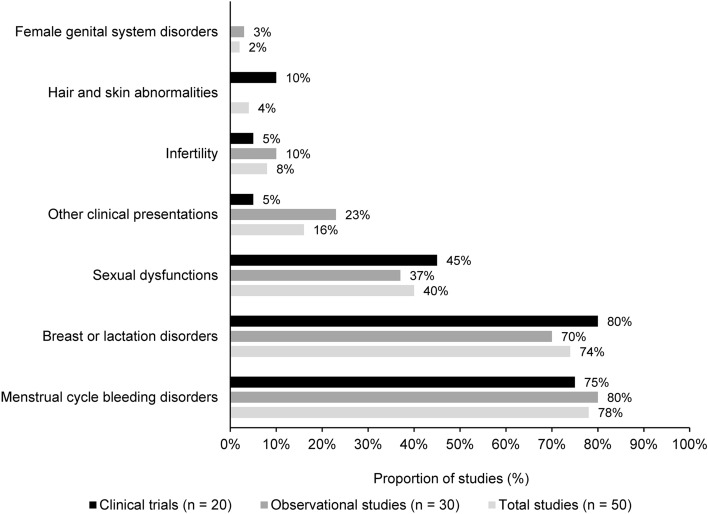
Across the main symptom categories, the clinical presentations most frequently reported in patients with drug-induced HPL were related to menstrual cycle bleeding disorders (40/50; 80%) and breast or lactation disorders (37/50; 74%), followed by sexual dysfunctions (21/50; 42%) (Fig. 4, Table 4). These clinical presentations were reported among patients treated with a range of drugs, but mostly antipsychotics. Other drugs suspected to have been associated with drug-induced HPL and associate symptoms are prokinetic agents (e.g., metoclopramide), oral contraceptives, antidepressants, mood stabilisers, and antihypertensives.
Fig. 4.
Clinical presentations according to symptom categories
Table 4.
Main clinical presentations of drug-induced HPL in clinical trials and observational studies
| Clinical presentation | Number of trials reporting data (n = 20) | Symptoms or long-term consequences in clinical trials (proportion of patients) | Number of observational studies reporting data (n = 30) | Symptom or long-term consequence in observational studies (proportion of patients) | Drug suspected |
|---|---|---|---|---|---|
| Breast and lactation disorders | 16 (80%) |
Galactorrhoea: 4–62% Gynaecomastia: 3–34%a |
21 (70%) |
Galactorrhoea: 6–85% Gynaecomastia: 5–60% |
Antipsychotics, oral contraceptives, and prokinetic drugs |
| Menstrual cycle bleeding disorders | 15 (75%) |
Amenorrhoea: 11–100% Oligomenorrhoea: 15–55% Other disturbances in the menstrual cycle: 3–100% |
24 (80%) |
Amenorrhoea: 6–100% Oligomenorrhoea: 8–50% Other disturbances in the menstrual cycle: 3–100% |
Dopamine antagonists, antipsychotics, antidepressants, oestrogen, anxiolytics, H2-antihistamines, antihypertensives, and prokinetic drugs |
| Sexual dysfunctions | 9 (45%) |
Ejaculatory or erectile dysfunctions: 1–32% Decreased libido: 1–77% Difficulty achieving orgasm: 9–22% |
11 (37%) |
Ejaculatory or erectile dysfunctions: 4–100% Decreased libido: 11–62% Difficulty achieving orgasm: 11–65% |
Antipsychotics |
| Female genital system disorders | 0 (0%) | NA | 1 (3%) | Vaginal dryness during intercourse: 53% (42/80) females | Antipsychotics |
| Hair and skin abnormalities | 2 (10%) | Facial acne and hirsutism: 3–5% | 0 (0%) | NA | Antipsychotics |
| Infertility | 1 (5%) | Anovulation: 34% (2/6) | 3 (10%) |
General infertility: 31–29% Oligospermia: 15% (1/7) of males |
Prokinetic drugs, antipsychotics |
HPL hyperprolactinaemia, NA not available
aOne trial reported a frequency of 100% based on one patient with drug-induced HPL
Only one study specified that the clinical presentation reported referred to a long-term complication of drug-induced HPL [27]; however, the definition of long-term was unclear. This study assessed infertility in patients experiencing amenorrhoea for at least 3 years—reported in 29% (2/7) of patients who developed HPL induced by oral contraceptives. Hereafter, clinical presentations refer to any symptom associated with drug-induced HPL, including the single study reporting on long-term complications.
Breast or Lactation Disorders
Sixteen clinical trials (of 20; 80%) [15–22, 25, 41, 42, 50, 56, 57, 60, 61] and 21 observational studies (of 30; 70%) reported data on breast and lactation disorders in people with drug-induced HPL [10, 26–28, 30, 31, 33–36, 38, 43, 46, 47, 49, 50, 52–54, 58, 59]. Among the clinical trials, galactorrhoea was the most frequently observed presentation of HPL, occurring in 4–62% of patients. High frequencies of galactorrhoea were observed among clinical trial patients with HPL induced by antipsychotics (43%) [17] and oral contraceptives (62%) [16]; an incidence of 17% was described among menstruating females exposed to prokinetic drugs [60]. One trial reported one patient with both galactorrhoea and gynaecomastia [56].
Among the observational studies, galactorrhoea was the most frequently observed clinical presentation of drug-induced HPL, with a prevalence ranging from 6 to 85%. High frequencies of galactorrhoea were reported among patients with HPL induced by antipsychotics (69%) [59] and prokinetic drugs (82%) [43]. Gynaecomastia occurred among 5–60% of patients with drug-induced HPL; one trial reported a frequency of 100% but this was based on only one patient with drug-induced HPL [56]. The higher frequencies of 34% and 60% were described in patients treated with antipsychotics [28, 36], and a prevalence of 29% was reported among patients receiving antidepressants. One study described gynaecomastia in 25% (10/40) of males with HPL induced by antipsychotics [38], and one study reported breast pain occurring in 12% of patients exposed to antidepressants [36].
Table S6 provides further details on the clinical presentations of breast and lactation disorders.
Female Genital System Disorders
One observational study (of 30; 3%) [38] reported data on female patients with drug-induced HPL presenting with genital system disorders. The symptom related to vaginal dryness during intercourse, assessed by the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ) scale, was reported in up to 53% (42/80) of females treated with antipsychotics [38]. Table S7 provides further details on clinical presentations of female genital system disorders.
Hair and Skin Abnormalities
Two clinical trials (of 20; 10%) [20, 25] reported data on patients with drug-induced HPL presenting with hair and skin abnormalities. The patients assessed in both trials developed HPL induced by antipsychotics, in frequencies ranging from 3 to 5%. Table S8 provides further details on clinical presentations of hair and skin abnormalities.
Infertility
One clinical trial (of 20; 5%) [60] and three observational studies (of 30; 10%) [27, 32, 43] reported data on infertility associated with drug-induced HPL. Anovulation was observed in 34% (2/6) of patients with drug-induced HPL evaluated in a clinical trial after a 1-week exposure to prokinetic drugs [60]. Among the observational studies, oligospermia was described among 15% (1/7) of males treated with antipsychotics [32], and general infertility was reported in two studies on females with a prevalence ranging from 31 to 29%. Table S9 summarises additional information on clinical presentations of infertility.
Menstrual Cycle Bleeding Disorders
Fifteen clinical trials (of 20; 75%) [16–20, 22–25, 40–42, 56, 57, 61] and 24 observational studies (of 30; 80%) [10, 26, 27, 29–33, 35, 36, 38, 43–47, 49, 50, 52–54, 58, 59, 63] reported on patients presenting with menstrual cycle disorders. Among the clinical trials, amenorrhoea was described with frequencies ranging from 11 to 80% of those with drug-induced HPL. Two trials described amenorrhoea presenting in 100% of patients exposed to oral contraceptives or antipsychotics [16, 17]. Oligomenorrhoea occurred in 15–55% of females treated with antipsychotics, and various other disturbances in the menstrual cycle (reported using generic terms such as abnormal menstrual duration, change in menstruation, or menstrual irregularities) were experienced by 3–100% of the population of these trials [20, 22, 25, 40, 41, 57, 61].
Among the observational studies, amenorrhoea was described with frequencies ranging from 6 to 96% in patients with drug-induced HPL. Three studies reported amenorrhoea among all patients assessed after receiving treatment with antipsychotics [47] and oral contraceptives [27, 35]. Two studies reported similar frequencies of amenorrhoea among patients taking different drugs, such as prokinetic drugs, antipsychotics and antidepressants, oestrogens, anxiolytics, H2-antihistamines, antihypertensives, prokinetic drugs, antidepressants, or antipsychotics [10, 46]. Oligomenorrhoea occurred in frequencies varying from 8 to 50%, and other disturbances in the menstrual cycle (described by generic terms such as abnormal menstruation, menstrual cycle impairments) were experienced by 3–100% of patients.
Premenstrual tension was reported only in the clinical trials, with a frequency of 8% among patients treated with antipsychotics [42]. Conversely, dysmenorrhoea was only described among patients in the observational studies. The prevalence of dysmenorrhoea ranged from 4 to 80%, and was reported among patients with HPL associated with the use of antipsychotics [26, 38].
Table S10 provides further details on clinical presentations of menstrual cycle bleeding disorders.
Sexual Dysfunctions
Nine clinical trials (of 20; 45%) [20, 22, 24, 39–42, 51, 61] and 11 observational studies (of 20; 55%) [32, 34, 36–38, 45, 48, 52, 55, 58, 59] reported on these data. Among the clinical trials, ejaculatory or erectile dysfunctions were described in frequencies ranging from 1 to 32%, and were associated with antipsychotic treatment [39–42, 61]. Decreased libido was also reported among patients with HPL associated with antipsychotic treatment, with incidences ranging from 1 to 77% [20, 40–42, 61].
Among the observational studies, ejaculatory or erectile dysfunctions were described in frequencies from 4 to 100% in patients exposed to antipsychotics [38], antidepressants [36], and other/multiple drugs [37]. Decreased libido was described with a prevalence of 11–62% with drug-induced HPL, and mostly in patients exposed to antipsychotics.
Difficulty achieving orgasm was reported in the observational studies (11–65%) slightly more frequently than in the clinical trials (9–22%). One observational study evaluated the quality of life in patients with drug-induced HPL and sexual dysfunctions [38]. Decreased quality of life owing to sexual impairments was reported among 51% (61/120) of patients who received treatment with antipsychotics. The proportion of patients with reduced quality of life owing to sexual impairments was higher among females, and estimated at 56% (45/80) of patients [38].
Table S11 provides further details on clinical presentations of sexual dysfunctions.
Discussion
In this review, we analysed the evidence landscape on the clinical presentations and long-term effects described among patients with drug-induced HPL. Our review depicted that menstrual cycle disturbances, breast and lactation disorders, and sexual dysfunctions are presentations commonly reported in studies reporting the clinical presentations of patients with drug-induced HPL. A range of other symptoms comprise female genital system disorders, hair and skin abnormalities, and infertility. In line with the literature, these symptoms were described mostly among patients treated with antipsychotics and patients treated with other drugs that can increase PRL levels, such as antidepressants, anti-contraceptives, and prokinetic drugs.
The studies contributing data to this review were diverse in study design (randomised control trials with or without crossover, non-randomised trials; prospective and retrospective study designs; cross-sectional studies; case series and reports) and patient characteristics (sex, age, race/ethnicity, serum PRL levels) and the overall data provided by the studies spanned from 1976 to 2018, a range of 42 years that may be a contributing factor to the heterogeneity of the studies. Importantly, diagnostic definitions, laboratory assays, medical treatments, and terminology have evolved over the years, which can affect the reported incidence and prevalence of disease cases and related clinical presentations. For instance, PRL levels up to 30 ng/mL were considered a normal range for males in 1986 [64], while the current consensus is that upper normal values of serum PRL are 20 ng/mL for males and 25 ng/mL for females [2, 3]. Additionally, case definitions can vary among prescribers, laboratories, and the scientific literature. In the studies included in this review, there was wide variance among the definitions provided for elevated PRL levels—and therefore drug-induced HPL. The variability in study duration—and consequently of patient follow-up duration—combined with the inconsistent case definitions of drug-induced HPL might have an impact on the robustness of the information summarised related to the clinical presentations. Moreover, the available evidence might not represent the symptoms experienced by patients presenting with elevated PRL levels according to contemporary standard ranges.
The clinical presentation profile of patients with drug-induced HPL identified in this review ranged across five symptom categories. Long-term complications of drug-induced HPL were specifically documented by only one study [27]; however, among observational studies with available data, reported study duration ranged up to 24 months. We also found that clinical symptoms related to drug-induced HPL were commonly reported in studies assessing patients receiving antipsychotics. Consistent with the profile of AEs induced by antipsychotics [9], female patients represented the majority of symptomatic cases reported in the studies summarised in this review. Overall, the clinical presentations of drug-induced HPL most frequently included menstrual cycle bleeding disorders. Elevated PRL levels can be associated with hypogonadism, but infertility was reported in only a limited number of studies with small sample sizes evaluated in this review. Other symptoms found to be associated with HPL-inducing antipsychotics (such as loss of bone mass, dyslipidaemia, and cardiovascular disease) [9] were not reported in the studies identified in this review.
Strengths and Limitations
We conducted a comprehensive literature review of evidence landscape on the clinical presentations and long-term effects of drug-induced HPL in both clinical trials and observational studies. To accomplish this, thorough searches with pre-defined eligibility criteria were undertaken across several databases, with articles subsequently screened for inclusion in this review. Study selection and data extraction were conducted by two independent reviewers. An additional strength of the review was the use of a pre-specified protocol and search criteria. The study protocol was registered with PROSPERO (CRD42021245259) to ensure transparency and allow for future replication or updates.
Our study has some limitations. Despite conducting comprehensive literature searches, the risks of missing relevant publications exist. There was considerable heterogeneity among publications regarding study designs, patient populations, definitions used for elevated PRL levels, and reporting the findings of interest. Across the studies, 79 unique terms were used to define the signs and symptoms of drug-induced HPL. Additionally, we summarised evidence from clinical trials (n = 20) and observational studies (n = 30) not designed to test the causal relationship between the drug treatment and HPL symptoms. Therefore, some of the clinical presentations collected from the included studies could be either a disease complication or an AE related to the drug by a mechanism other than HPL, instead of a clinical presentation of drug-induced HPL. Nevertheless, most drugs described among the patients with drug-induced HPL were anticipated to induce HPL, symptoms detected as potentially confounding AEs, such as muscle rigidity among patients treated with prokinetic drugs, were not included in the summary of the results despite being documented in this review.
Crucially, there was not a standard definition of elevated PRL levels; therefore, drug-induced HPL was diagnosed differently across the included studies. Overall, 25 different definitions of HPL were identified—11 were reported in the clinical trials, and 14 were reported in the observational studies. In addition, some studies used specific PRL thresholds for each sex, while other studies used various metrics to define mild, moderate, and severe HPL. As a result, patients may have been classified as having drug-induced HPL in one study but as not having it in another, thus impacting the evidence consistency across studies. Moreover, the studies included in this literature review were conducted over a broad time span, from 1976 to 2018; therefore, generalisations from older studies must be made with caution, as findings may not apply to patients diagnosed with drug-induced HPL according to contemporary standard ranges.
Finally, this review focused on the clinical features of HPL when this condition occurs as an AE of drug treatments. When making treatment decisions, clinicians should use the findings of this review alongside effectiveness data.
Evidence Gaps
The findings of this literature review are consistent with previous reports that focus on antipsychotics as the predominant treatment that causes drug-induced HPL. However, there is a lack of understanding of the clinical presentations of HPL when induced by other drugs. Additionally, future research should consistently apply the accepted consensus on elevated prolactin level thresholds to define HPL and, therefore, drug-induced HPL (i.e., upper normal values of serum PRL are 20 ng/mL for males and 25 ng/mL for females) [2, 3]. This would allow quantitative comparisons across studies and more consistent results.
Importantly, there is a lack of studies describing the long-term complications of drug-induced HPL. Only one was identified [27]; further, this study was published in 1980, making it challenging to apply the findings to the current treatment management landscape. Studies specifically exploring the long-term complications of drug-induced HPL are needed. Most of the studies identified in this review did not have the primary objective of describing the clinical presentations and long-term complications of drug-induced HPL. Instead, several studies reported clinical presentations of HPL as part of their findings when evaluating drug efficacy or effectiveness to treat HPL. Studies specifically aimed at the evaluation of clinical presentations and long-term complications are needed to obtain a richer, more comprehensive picture of this patient population.
Conclusion
In conclusion, this review is a comprehensive synthesis of the evidence of the clinical presentations of drug-induced HPL from both clinical trials and observational studies. Most of the symptoms reported in studies assessing patients with drug-induced HPL describe menstrual cycle bleeding disorders, breast or lactation disorders, and sexual dysfunction among frequent observed symptoms. However, the substantial heterogeneity in HPL definitions and the terms for various symptoms made it difficult to determine consistent trends across the included studies, which should be considered when interpreting the review findings. Future research should explore the long-term complications of drug-induced HPL, and establish a universal definition of elevated PRL levels in the diagnosis of HPL as a drug-induced AE.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr Andreas Freitag for his involvement in the early stages of designing this literature review, and Dr Van Thu Nguyen for support in the writing of the manuscript. We are also grateful for the team supporting the data collection from the included studies, Thea Drachen and Solange Gonzalez. Editorial assistance was provided by Oxford PharmaGenesis, Oxford, UK, and was supported by Takeda Development Center Americas, Inc.
Declarations
Funding
This study was sponsored by Takeda Development Center Americas, Inc.
Conflict of interest
DB is a current and SYH is a former employee of Takeda Development Center Americas, Inc. and received stock or stock options at the time of study. Currently SYH is employed by Ironwood Pharmaceuticals. DRJ and CCiC provide consultancy support to Takeda Development Center Americas, Inc. as employees of Evidera.
Ethics approval
Not applicable.
Consent to participate
Study analyzed anonymously using already published data: Not applicable.
Consent for publication
Study analysed anonymously using already published data: Not applicable.
Data availability
All data presented is available in manuscript and/or supplementary material.
Code availability
Not applicable.
Author contributions
DRJ, DB, SYH, and CCC led the research conceptualisation and methodology. DRJ led the data curation, formal analysis, investigation, project administration, and visualisation. She wrote the original draft and worked with all co-authors in reviewing and editing the final manuscript. All authors reviewed and approved the final manuscript.
References
- 1.Soto-Pedre E, Newey PJ, Bevan JS, Greig N, Leese GP. The epidemiology of hyperprolactinaemia over 20 years in the Tayside region of Scotland: the Prolactin Epidemiology, Audit and Research Study (PROLEARS) Clin Endocrinol (Oxf) 2017;86(1):60–67. doi: 10.1111/cen.13156. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–288. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 3.Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 2006;65(2):265–273. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11(5):265–275. doi: 10.1038/nrendo.2015.36. [DOI] [PubMed] [Google Scholar]
- 5.Galdiero M, Pivonello R, Grasso LFS, Cozzolino A, Colao A. Growth hormone, prolactin, and sexuality. J Endocrinol Investig. 2012;35(8):782–794. doi: 10.1007/BF03345805. [DOI] [PubMed] [Google Scholar]
- 6.Horseman ND, Gregerson KA. Prolactin actions. J Mol Endocrinol. 2014;52(1):R95–106. doi: 10.1530/JME-13-0220. [DOI] [PubMed] [Google Scholar]
- 7.Klibanski A. Clinical practice Prolactinomas. N Engl J Med. 2010;362(13):1219–1226. doi: 10.1056/NEJMcp0912025. [DOI] [PubMed] [Google Scholar]
- 8.Eren E, TorelErgur A, Isguven SP, CelebiBitkin E, Berberoglu M, Siklar Z, et al. Clinical and laboratory characteristics of hyperprolactinemia in children and adolescents: national survey. J Clin Res Pediatr Endocrinol. 2019;11(2):149–156. doi: 10.4274/jcrpe.galenos.2018.2018.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young SL, Taylor M, Lawrie SM. "First do no harm." A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353–362. doi: 10.1177/0269881114562090. [DOI] [PubMed] [Google Scholar]
- 10.Vilar L, Freitas MC, Naves LA, Casulari LA, Azevedo M, Montenegro R, Jr, et al. Diagnosis and management of hyperprolactinemia: results of a Brazilian multicenter study with 1234 patients. J Endocrinol Investig. 2008;31(5):436–444. doi: 10.1007/BF03346388. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos LE, Gutierrez C, Smith T, Laws ER, Iorgulescu JB. Epidemiology of common and uncommon adult pituitary tumors in the U.S. according to the 2017 World Health Organisation classification. Pituitary. 2022;25:201–209. doi: 10.1007/s11102-021-01189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molitch ME. Medication-induced hyperprolactinemia. Mayo Clinic Proceedings. Amsterdam: Elsevier; 2005. pp. 1050–1057. [DOI] [PubMed] [Google Scholar]
- 13.Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281–297. doi: 10.1002/hup.1116. [DOI] [PubMed] [Google Scholar]
- 14.Vilar L, Vilar CF, Lyra R, da Conceicao FM. Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology. 2019;109(1):7–19. doi: 10.1159/000499694. [DOI] [PubMed] [Google Scholar]
- 15.Atmaca M, Kuloglu M, Tezcan E, Canatan H, Gecici O. Quetiapine is not associated with increase in prolactin secretion in contrast to haloperidol. Arch Med Res. 2002;33(6):562–565. doi: 10.1016/S0188-4409(02)00403-4. [DOI] [PubMed] [Google Scholar]
- 16.Karaman AS, Uran B, Erler A. Serum prolactin levels in postpill amenorrheic patients. Int J Gynaecol Obstet. 1993;43(2):177–180. doi: 10.1016/0020-7292(93)90326-R. [DOI] [PubMed] [Google Scholar]
- 17.Lee BH, Kim YK, Park SH. Using aripiprazole to resolve antipsychotic-induced symptomatic hyperprolactinemia: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):714–717. doi: 10.1016/j.pnpbp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Lee MS, Song HC, An H, Yang J, Ko YH, Jung IK, et al. Effect of bromocriptine on antipsychotic drug-induced hyperprolactinemia: eight-week randomised, single-blind, placebo-controlled, multicenter study. Psychiatry Clin Neurosci. 2010;64(1):19–27. doi: 10.1111/j.1440-1819.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu ML, Shen WW, Chen CH. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1978–1981. doi: 10.1016/j.pnpbp.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Man SC, Li XB, Wang HH, Yuan HN, Wang HN, Zhang RG, et al. Peony-glycyrrhiza decoction for antipsychotic-related hyperprolactinemia in women with schizophrenia: a randomised controlled trial. J Clin Psychopharmacol. 2016;36(6):572–579. doi: 10.1097/JCP.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 21.Qiao Y, Yang F, Li C, Guo Q, Wen H, Zhu S, et al. Add-on effects of a low-dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone. Psychiatry Res. 2016;237:83–89. doi: 10.1016/j.psychres.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Ranjbar F, Sadeghi-Bazargani H, Khams PN, Arfaie A, Salari A, Farahbakhsh M. Adjunctive treatment with aripiprazole for risperidone-induced hyperprolactinemia. Neuropsychiatr Dis Treat. 2015;11:549–555. doi: 10.2147/NDT.S69088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim JC, Shin JGK, Kelly DL, Jung DU, Seo YS, Liu KH, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404–1410. doi: 10.1176/appi.ajp.2007.06071075. [DOI] [PubMed] [Google Scholar]
- 24.Yoon HW, Lee JS, Park SJ, Lee SK, Choi WJ, Kim TY, et al. Comparing the effectiveness and safety of the addition of and switching to aripiprazole for resolving antipsychotic-induced hyperprolactinemia: a multicenter, open-label, prospective study. Clin Neuropharmacol. 2016;39(6):288–294. doi: 10.1097/WNF.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 25.Yuan HN, Wang CY, Sze CW, Tong Y, Tan QR, Feng XJ, et al. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J Clin Psychopharmacol. 2008;28(3):264–270. doi: 10.1097/JCP.0b013e318172473c. [DOI] [PubMed] [Google Scholar]
- 26.Barszcz Z, Mucha S, Rabe-Jablonska J. Co-medication with psychotropic drugs, including antipsychotic drugs, and bromocriptine: changes in clinical signs of hyperprolactinaemia and prolactin level. Psychiatria i Psychologia Kliniczna. 2007;7(3):154–164. [Google Scholar]
- 27.Furuhjelm M, Rydner T, Carlstrom K. Hyperprolactinemia in cases of infertility and amenorrhea. Acta Obstet Gynecol Scand. 1980;59(2):137–141. doi: 10.3109/00016348009154630. [DOI] [PubMed] [Google Scholar]
- 28.Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinemia in adolescents. J Clin Psychopharmacol. 2006;26(2):167–171. doi: 10.1097/01.jcp.0000203194.58087.9a. [DOI] [PubMed] [Google Scholar]
- 29.Kalkavoura CS, Michopoulos I, Arvanitakis P, Theodoropoulou P, Dimopoulou K, Tzebelikos E, et al. Effects of cabergoline on hyperprolactinemia, psychopathology, and sexual functioning in schizophrenic patients. Exp Clin Psychopharmacol. 2013;21(4):332–341. doi: 10.1037/a0033448. [DOI] [PubMed] [Google Scholar]
- 30.Kopecek M, Bares M, Horacek J, Mohr P. Low-dose risperidone augmentation of antidepressants or anxiolytics is associated with hyperprolactinemia. Neuroendocrinol Lett. 2006;27(6):803–806. [PubMed] [Google Scholar]
- 31.Melkersson K, Hulting AL, Hall K. Hormonal evaluation in schizophrenic patients treated with neuroleptics. Neuroendocrinol Lett. 1999;20(3–4):199–204. [PubMed] [Google Scholar]
- 32.Melkersson K. Differences in prolactin elevation and related symptoms of atypical antipsychotics in schizophrenic patients. J Clin Psychiatry. 2005;66(6):761–767. doi: 10.4088/JCP.v66n0614. [DOI] [PubMed] [Google Scholar]
- 33.Pollock A, McLaren EH. Serum prolactin concentration in patients taking neuroleptic drugs. Clin Endocrinol. 1998;49(4):513–516. doi: 10.1046/j.1365-2265.1998.00569.x. [DOI] [PubMed] [Google Scholar]
- 34.Roke Y, Buitelaar JK, Boot AM, Tenback D, Van Harten PN. Risk of hyperprolactinemia and sexual side effects in males 10–20 years old diagnosed with autism spectrum disorders or disruptive behavior disorder and treated with risperidone. J Child Adolesc Psychopharmacol. 2012;22(6):432–439. doi: 10.1089/cap.2011.0109. [DOI] [PubMed] [Google Scholar]
- 35.Seppala M, Lehtovirta P, Ranta T. Discordant patterns of hyperprolactinaemia and galactorrhoea in secondary amenorrhoea. Acta Endocrinol. 1977;86(3):457–463. doi: 10.1530/acta.0.0860457. [DOI] [PubMed] [Google Scholar]
- 36.Trenque T, Herlem E, Auriche P, Dram M. Serotonin reuptake inhibitors and hyperprolactinaemia: a case/non-case study in the French pharmacovigilance database. Drug Saf. 2011;34(12):1161–1166. doi: 10.2165/11595660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Venetikou MS, Lambou T, Gisani D. Hyperprolactinaemia due to hypothalamic-pituitary disease or drug-induced in patients with erectile dysfunction. Andrologia. 2008;40(4):240–244. doi: 10.1111/j.1439-0272.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 38.Yunilainen OA, Starostina EG, Dzeranova LK, Kudryashkina GN, Kessel'man LG, Baranov PA, et al. Neuroleptic-associated hyperprolactinemia: clinical manifestations and effects on sexual function. Neurosci Behav Physiol. 2018;48(3):358–366. doi: 10.1007/s11055-018-0571-y. [DOI] [Google Scholar]
- 39.Cavallaro R, Cocchi F, Angelone SM, Lattuada E, Smeraldi E. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry. 2004;65(2):187–190. doi: 10.4088/JCP.v65n0207. [DOI] [PubMed] [Google Scholar]
- 40.Mir A, Shivakumar K, Williamson RJ, McAllister V, O'Keane V, Aitchison KJ. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22(3):244–253. doi: 10.1177/0269881107082901. [DOI] [PubMed] [Google Scholar]
- 41.Savitz A, Lane R, Nuamah I, Singh J, Hough D, Gopal S. Long-term safety of paliperidone extended release in adolescents with schizophrenia: an open-label, flexible dose study. J Child Adolesc Psychopharmacol. 2015;25(7):548–557. doi: 10.1089/cap.2014.0130. [DOI] [PubMed] [Google Scholar]
- 42.Trives MZ, Llacer JMB, Escudero MAG, Pastor CJM. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol. 2013;33(4):538–541. doi: 10.1097/JCP.0b013e3182970431. [DOI] [PubMed] [Google Scholar]
- 43.Atluri S, Sarathi V, Goel A, Boppana R, Shivaprasad C. Etiological profile of galactorrhoea. Indian J Endocrinol Metab. 2018;22(4):489–493. doi: 10.4103/ijem.IJEM_89_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CK, Huang YS, Ree SC, Hsiao CC. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495–1499. doi: 10.1016/j.pnpbp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Kotan Z, Ertepe B, Akkaya C, Sarandol E, Ozkaya G, Kirli S. Metabolic, endocrinologic and cardiac effects of amisulpride: a 24-week follow-up study. Ther Adv Psychopharmacol. 2011;1(6):189–196. doi: 10.1177/2045125311426896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulshreshtha B, Pahuja I, Kothari D, Chawla I, Sharma N, Gupta S, et al. Menstrual cycle abnormalities in patients with prolactinoma and drug-induced hyperprolactinemia. Indian J Endocrinol Metab. 2017;21(4):545–550. doi: 10.4103/ijem.IJEM_515_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee BH, Han CS, Kim KH, Kim YK. Treatment in risperidone-induced amenorrhoea. Int J Psychiatry Clin Pract. 2005;9(1):29–34. doi: 10.1080/13651500510014747. [DOI] [PubMed] [Google Scholar]
- 48.Leonard MP, Nickel CJ, Morales A. Hyperprolactinemia and impotence: why, when and how to investigate. J Urol. 1989;142(4):992–994. doi: 10.1016/S0022-5347(17)38964-4. [DOI] [PubMed] [Google Scholar]
- 49.Margari L, Matera E, Petruzzelli MG, Simone M, Lamanna AL, Pastore A, et al. Prolactin variations during risperidone therapy in a sample of drug-naive children and adolescents. Int Clin Psychopharmacol. 2015;30(2):103–108. doi: 10.1097/YIC.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith S. Neuroleptic-associated hyperprolactinemia: Can it be treated with bromocriptine? J Reprod Med Obstet Gynecol. 1992;37(8):737–740. [PubMed] [Google Scholar]
- 51.Perez-Iglesias R, Mata I, Martinez-Garcia O, Garcia-Unzueta MT, Amado JA, Valdisan EM, et al. Long-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosis. J Clin Psychopharmacol. 2012;32(6):804–808. doi: 10.1097/JCP.0b013e318272688b. [DOI] [PubMed] [Google Scholar]
- 52.Alosaimi FD, Fallata EO, Abalhassan M, Alhabbad A, Alzain N, Alhaddad B, et al. Prevalence and risk factors of hyperprolactinemia among patients with various psychiatric diagnoses and medications. Int J Psychiatry Clin Pract. 2018;22(4):274–281. doi: 10.1080/13651501.2018.1425459. [DOI] [PubMed] [Google Scholar]
- 53.Emsley R, Medori R, Koen L, Oosthuisen PP, Niehaus DJH, Rabinowitz J. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210–213. doi: 10.1097/JCP.0b013e318167269d. [DOI] [PubMed] [Google Scholar]
- 54.Park YM, Lee SH, Lee BH, Lee KY, Lee KS, Kang SG, et al. Prolactin and macroprolactin levels in psychiatric patients receiving atypical antipsychotics: a preliminary study. Psychiatry Res. 2016;239:184–189. doi: 10.1016/j.psychres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Tang Z, Ruan Y, Huang C, Wu J, Lu Z, et al. Prolactin and thyroid stimulating hormone (TSH) levels and sexual dysfunction in patients with schizophrenia treated with conventional antipsychotic medication: a cross-sectional study. Med Sci Monit. 2018;24:9136–9143. doi: 10.12659/MSM.913759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly DL, Conley RR. A randomised double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology. 2006;31(3):340–346. doi: 10.1016/j.psyneuen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31(5):577–588. doi: 10.1016/j.psyneuen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Lee BH, Kim YK. The relationship between prolactin response and clinical efficacy of risperidone in acute psychotic inpatients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):658–662. doi: 10.1016/j.pnpbp.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 59.Matsuoka I, Nakai T, Miyake M, Hirai M, Ikawa G. Effects of bromocriptine on neuroleptic-induced amenorrhea, galactorrhea and impotence. Jpn J Psychiatry Neurol. 1986;40(4):639–646. doi: 10.1111/j.1440-1819.1986.tb03179.x. [DOI] [PubMed] [Google Scholar]
- 60.Andersen AN, Schioler V, Hertz J, Bennett P. Effect of metoclopramide induced hyperprolactinaemia on the gonadotrophic response to oestradiol and LRH. Acta Endocrinol. 1982;100(1):1–9. doi: 10.1530/acta.0.1000001. [DOI] [PubMed] [Google Scholar]
- 61.During SW, Nielsen MO, Bak N, Glenthoj BY, Ebdrup BH. Sexual dysfunction and hyperprolactinemia in schizophrenia before and after six weeks of D2/3 receptor blockade—an exploratory study. Psychiatry Res. 2019;274:58–65. doi: 10.1016/j.psychres.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organisation. International Classification of Diseases 11th Revision (ICD-11). https://icd.who.int/en [cited 2022 Feb 7].
- 63.Ahuja N, Moorhead S, Lloyd AJ, Cole AJ. Antipsychotic-induced hyperprolactinemia and delusion of pregnancy. Psychosomatics. 2008;49(2):163–167. doi: 10.1176/appi.psy.49.2.163. [DOI] [PubMed] [Google Scholar]
- 64.Jeffcoate SL, Bacon RR, Beastall GH, Diver MJ, Franks S, Seth J. Assays for prolactin: guidelines for the provision of a clinical biochemistry service. Ann Clin Biochem. 1986;23(Pt 6):638–651. doi: 10.1177/000456328602300603. [DOI] [PubMed] [Google Scholar]
- 65.Lankford HV, Blackard WG, Gardner DF, Tucker HS. Effects of thyrotropin-releasing hormone and metoclopramide in patients with phenothiazine-induced hyperprolactinemia. J Clin Endocrinol Metab. 1981;53(1):109–112. doi: 10.1210/jcem-53-1-109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented is available in manuscript and/or supplementary material.