Abstract
Cellular quiescence is an important physiological state both in unicellular and multicellular eukaryotes. Quiescent cells are halted for proliferation and stop the cell cycle at the G0 stage. Using fission yeast as a model organism, we have previously found that several subunits of a conserved chromatin remodeling complex, Ino80C (INOsitol requiring nucleosome remodeling factor), are required for survival in quiescence. Here, we demonstrate that Ino80C has a key function in the regulation of gene expression in G0 cells. We show that null mutants for two Ino80C subunits, Iec1 and Ies2, a putative subunit Arp42, a null mutant for the histone variant H2A.Z, and a null mutant for the Inositol kinase Asp1 have very similar phenotypes in quiescence. These mutants show reduced transcription genome-wide and specifically fail to activate 149 quiescence genes, of which many are localized to the subtelomeric regions. Using spike in normalized ChIP-seq experiments, we show that there is a global reduction of H2A.Z levels in quiescent wild-type cells but not in iec1∆ cells and that a subtelomeric chromosome boundary element is strongly affected by Ino80C. Based on these observations, we propose a model in which Ino80C is evicting H2A.Z from chromatin in quiescent cells, thereby inactivating the subtelomeric boundary element, leading to a reorganization of the chromosome structure and activation of genes required to survive in quiescence.
Supplementary information
The online version contains supplementary material available at 10.1007/s10577-023-09723-x.
Keywords: Cellular quiescence, fission yeast, chromatin remodelling, histone variant, Ino80, H2A.Z, eviction, telomere, boundary element
Introduction
Cellular quiescence is a reversible dormant state in which cells are changing their metabolism and cytology to adapt to an environment that does not permit proliferation. The ability to exit the cell cycle and enter quiescence is essential for tissue development and homeostasis in multicellular organisms. It is also an important survival strategy for unicellular organisms in harsh conditions. When fission yeast, Schizosacharomyces pombe, cells are starved for nitrogen in the absence of cells of the opposite mating type, they stop dividing and enter a quiescent state. In this state, the cell cycle is halted at the G0 stage before DNA replication. In G0, cells adapt the metabolism to survive until a nitrogen source becomes available so that the cells can re-enter the cell cycle and start proliferating. The survival in quiescence depends on a global change in gene expression (Marguerat et al. 2012). RNA transcription is generally reduced; however, some genes need to be activated to cope with the new physiological situation, for example, authophagy genes, proteasome-encoding genes, and genes for hexose and amino acid transporters (Takeda et al. 2010) (Oya et al. 2019). The nuclei of quiescent fission yeast cells are dramatically reorganized presumably to accommodate these profound changes in gene expression (Sajiki et al. 2009). To gain more insight into the role of chromatin structure changes in this process, we conducted a genetic screen in fission yeast for genes required to maintain viability during cellular quiescence (Zahedi et al. 2020). The screen identified the Ino80 complex (Ino80C). Ino80 is the catalytic subunit of this large chromatin remodeling complex consisting of approximately 10–14 protein subunits depending on the species. In fission yeast Ino80C consists of the following core subunits: Ino80, Arp5, Arp8, Rvb1, Rvb2, Alp5 (Arp4), Act1, Ies2, Ies4, Ies6, and Taf14 (Tfg3), and the accessory subunits: Iec1, Hap2, Iec3, Iec5, and Nht1 (Hogan et al. 2010) (Shevchenko et al. 2008) (Shan et al. 2020). The Ino80C subunit Iec1 (Ino Eighty Complex subunit 1) in fission yeast is similar to the Ying-Yang 1 (YY1) subunit of the human Ino80C and the function of Iec1 is to recruit Ino80C to target genes (Hogan et al. 2010). We have previously shown that mutations in nine tested subunits of Ino80C: Ies6, Nht1, Iec1, Iec3, Tfg3, Arp8, Ies2, Ies4, and the putative Ino80C subunit Arp42, lead to quiescence mortality phenotypes (Zahedi et al. 2020). Mutations in Hap2, Iec1, Arp8, Iec3, Nht1, Arp5, Ies4, and Ies2 were recently shown to be short-lived in stationary phase in fission yeast, implicating Ino80C in chronological ageing (Romila et al. 2021). Thus, at least six Ino80C subunits: Iec1, Arp8, Iec3, Nht1, Ies4, and Ies2 are implicated both in survival in quiescence and chronological ageing.
The molecular function of Ino80C is to remove the histone variant H2A.Z from nucleosomes by a histone exchange mechanism with H2.A in a process driven by ATP hydrolysis (Papamichos-Chronakis et al. 2011). This function is important for the repair of double-strand breaks, DNA replication, and the regulation of transcription (Poli et al. 2017). The H2A.Z exchange mechanism may also involve RNA polymerase II (Pol II) activity (Ranjan et al. 2020). Ino80 has recently been reported to be involved in gene regulation in several different species. Ino80 is required to activate the transcription of genes involved in thermomorphogenesis in plants (Xue et al. 2021). This mechanism of gene activation by Ino80 in plants involves H2A.Z eviction in response to elevated temperature. In Candida albicans, Ino80 is required for hyphal development by H2A.Z eviction at hyphal genes (Zhao et al. 2022). In mouse embryonic stem cells, Ino80 is required for regulation of cell cycle transitions by activating cell cycle genes (Yoo et al. 2022). Hence, it is likely that the requirement for Ino80 during quiescence involves some aspect of transcription regulation.
In fission yeast, Ino80C was shown to be important also for histone H3 exchange (Singh et al. 2020). We found that the genes for H2A.Z (pht1) and histone H3 (hht2) are both essential for surviving quiescence suggesting that H2A.Z deposition or removal and new histone H3 expression are required to maintain viability in G0 (Zahedi et al. 2020). H2A.Z is deposited by the Swr1C complex (Swi2/Snf2-related ATPase) in fission yeast (Buchanan et al. 2009). However, mutations affecting Swr1C do not affect survival in quiescence, indicating that H2A.Z deposition in G0 is less important than its removal by Ino80C. The activity of Ino80C in budding yeast, Saccharomyces cerevisiae, is modulated by Inositol polyphosphates (Shen et al. 2003). Curiously, the inositol kinase, Asp1, was recently reported to be important for the survival of quiescence in fission yeast (Sajiki et al. 2018). To advance the understanding of the role of Inositol polyphosphates and histone exchange by Ino80C in fission yeast quiescence, here, we investigate the functions of Asp1, Iec1, Arp42, Ies2, H2A.Z, and histone H3 in the regulation of gene expression in G0 cells.
Results
Time course analysis of viability and RNA expression in G0
We performed RNA sequencing (RNA-seq) analysis in wild-type cells and in three Ino80C-related mutants: arp42∆, iec1∆, and ies2∆, in pht1∆ cells carrying a gene deletion for H2A.Z, in asp1∆ cells carrying a gene deletion for an inositol kinase, and in hht2∆ cells with a gene deletion for a histone H3 encoding gene. Cells were grown to the logarithmic phase in minimal medium and shifted to nitrogen-free minimal medium. Samples were taken for RNA extractions at time zero (T0, before shift) and after 24 h (T1D), 1 week (T1W), and/or 2 weeks (T2W) of nitrogen starvation. We measured the viability of the cultures at each time point using flow cytometry (Fig. 1, Table 1). All the cultures entered efficiently into G0, as judged from the percentage of cells 1C DNA content, within 24 h (T1D) from the shift to nitrogen-free medium (Table 2). Consistent with our previous observations the Ino80C mutants iec1∆∆ arp42∆, ies2∆ and pht1∆ showed a reduced viability after 1 week in G0 (Table 1). We also found that asp1∆ cells lost their viability after 1 week in quiescence. This is also in agreement with a previous report (Sajiki et al. 2018). However, hht2∆ cells displayed a milder mortality phenotype in quiescence, only showing reduced viability after 2 weeks.
Fig. 1.
Measurements of viability and DNA content using flow cytometry. The gating strategy for measurement of viability and the proportion of G0 arrested cells during quiescence is illustrated with examples of FACS profiles for wild type (smt0) and the different mutant cells (as indicated)
Table 1.
Viability measurements by FACS
| Genotype | T0 | T1D | T1w | T2w |
|---|---|---|---|---|
| smt-0 (wt) | 99,9 0,6 | 99,7 0,2 | 98,8 1,3 | 98,30 0,3 |
| hht2∆ | 98,0 5,8 | 96,5 2,5 | 95,1 1,0 | 92,7 2,8 |
| pht1∆ | 96,0 3,7 | 93,7 0,3 | 85,0 1,7 | 70,9 11,5 |
| iec1∆ | 96,8 2,3 | 92,6 1,4 | 85,1 1,7 | 68,1 0,8 |
| asp1∆ | 99,5 0,6 | 98,7 0,1 | 95,1 0,1 | 62,8 3,7 |
| arp42∆ | 99,4 0,5 | 98,7 0,1 | 87,2 1,1 | 75,4 2,7 |
| ies2∆ | *99,0; 99,6 | *98,0; 98,2 | *78,0; 82,5 | *68,2; 65,1 |
The percentage of viable cells is indicated
Mean value standard deviation (n = 3)
*shows two measurements
Table 2.
DNA content measurements by FACS
| Genotype | T1D | T1w | T2w |
|---|---|---|---|
| smt-0 (wt) | 81,9 1,91 | 83,3 3,23 | 85,0 0,36 |
| hht2∆ | 83,8 1,07 | 86,4 2,36 | 87,1 2,51 |
| pht1∆ | 61,4 13,1 | 71,6 13,4 | 74,5 11,1 |
| iec1∆ | 40,8 11,5 | 40,8 13,6 | 15,2 0,42 |
| asp1∆ | 67,3 0,80 | 71,4 0,57 | 74,9 0,66 |
| arp42∆ | 67,3 0,85 | 71,9 4,13 | 80,4 1,51 |
| ies2∆ | *56,4; 56,9 | *64,5; 64,8 | *72,2; 70,6 |
The percentage of G0 cells is indicated (cells with a 1c DNA content)
Mean value standard deviation (n = 3)
*shows two measurements
A global repression of the transcriptome in G0 and an induction of subtelomeric genes
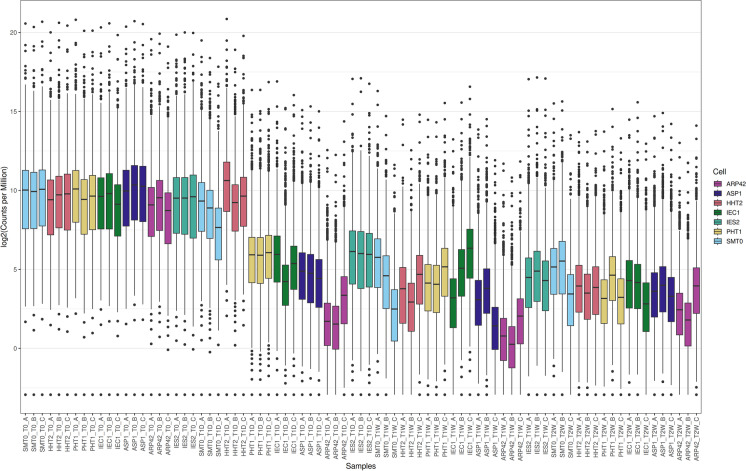
For RNA extractions, we used biological triplicates for wild type and each mutant. Because we expected a global change of transcription in quiescence, we could not normalize the RNA-seq data to the total number of reads, since it would give false negative and false positive results. Instead, we normalized the data to external RNA control consortium (ERCC) spike in controls that were added in proportion to the number of cells in each sample (Risso et al. 2014). It was previously shown that the fission yeast transcriptome is strongly downregulated in quiescence (Marguerat et al. 2012). Consistent with the earlier study, the ERCC normalization clearly showed that gene expression was globally repressed in wild-type cells after 1 and 2 weeks in G0 (Fig. 2). The hht2∆ cells showed a similar tendency as wild type. However, in pht1∆, iec1∆∆, arp42∆, ies2∆, and asp1∆ cells, the global repression had occurred already after 24 h (Fig. 2).
Fig. 2.
A representation of the ERCC spike in normalized RNA-seq data. The box plot shows triplicate RNA-seq samples as log2 values of numbers of normalized sequence reads (Y-axis) in wild-type cells (smt0) and the different mutants (as indicated in the X-axis)
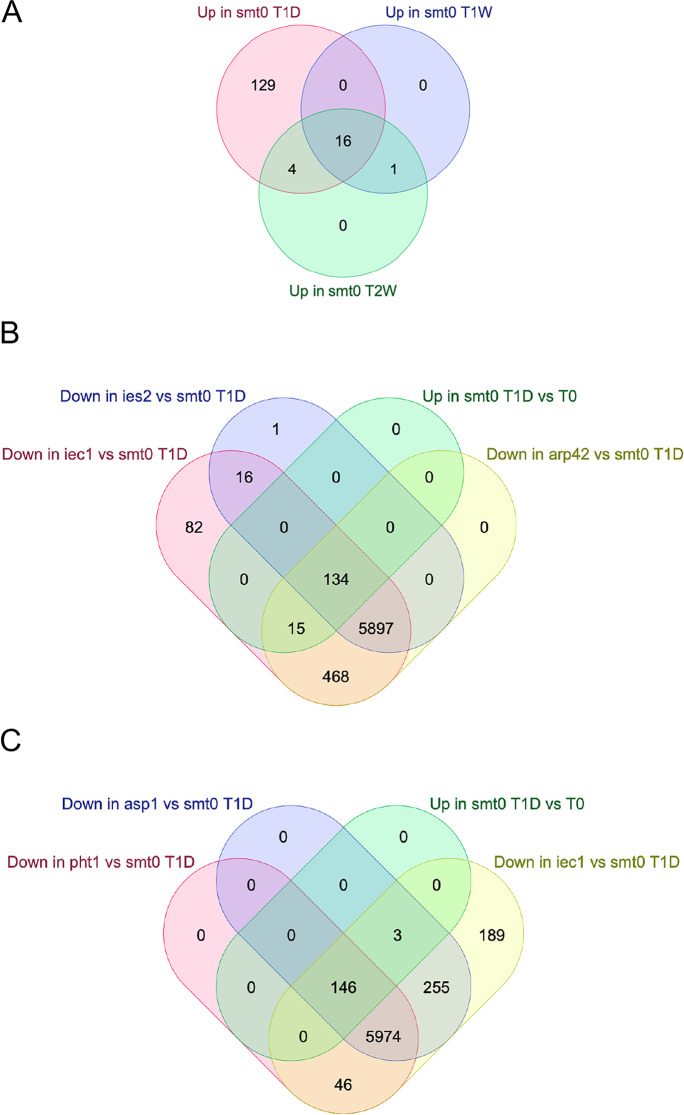
Next, a statistical threshold (FDR adjusted P < 0.05) was used to define up- and down-regulated genes. First, we defined genes affected by the shift to –nitrogen in wild type at T1D, T1W, and T2W compared to T0 (Table 3). These results are in nice agreement with earlier studies showing that most genes are downregulated and only a few genes are upregulated in G0. We compared the relatively few upregulated genes at the three time points in wild type (smt0) cells. There were 149 genes upregulated at T1D, 17 genes at T1W, and 21 genes at T2W. However, only 16 genes which were upregulated at all time points. Thus, we defined a set of 16 “core quiescence genes” that were found to be upregulated throughout the quiescence time course (Fig. 3A). Interestingly, 9 of these 16 genes (56.3%; CHI2 = 64; P < 0.001) reside in subtelomeric regions near tel1R and tel2L (Table 4). To validate the data, we compared with a previous quantitation of absolute numbers of mRNA molecules per cell (Marguerat et al. 2012). In all 16 cases, these measurements of mRNA molecules confirmed an induction of transcription in quiescent cells. Thus, our RNA-seq approach with ERCC normalization and a statistical cut off for affected genes was justified.
Table 3.
Number of genes differentially expressed in quiescent wild-type cells compared to vegetative cells
| Comparison | Up | Down | NS |
|---|---|---|---|
| T1D_T0_SMT0 | 149 | 1208 | 5255 |
| T1W_T0_SMT0 | 17 | 6436 | 159 |
| T2W_T0_SMT0 | 21 | 6360 | 231 |
The indicated gene lists were compared and the number of differentially expressed genes (up or down) are shown for each comparison (FDR adjusted P < 0.05)
RNA levels were normalized across all groups with ERCC spike-in controls
NS, not significant
Fig. 3.
Analysis of gene expression changes during quiescence in wild type and Ino80C mutants. A Venn diagram comparing lists of genes upregulated in wild-type cells (smt0) at 24 h (T1D) 1 week (T1W) and 2 weeks (T2W) after removal of nitrogen. B Venn diagram comparing lists of genes downregulated in Ino80C mutants iec1∆, ies2∆ and arp42∆ with a list of genes upregulated in wild-type cells (smt0) at 24 h (T1D) after removal of nitrogen. C Venn diagram paring lists of genes downregulated in the Inositol kinase null mutant asp1∆ with iec1∆ and a null mutant for the histone variant H2A.Z (pht1∆) as well as the list of genes upregulated in wild-type cells (smt0) at 24 h (T1D) after removal of nitrogen
Table 4.
Features of 16 core quiescence genes
| Geneid | Gene name | Quant. veg | Quant. G0 | Gene localization |
|---|---|---|---|---|
| SPNCRNA.821 | 0.038 | 0.17 | ||
| SPAC22H10.13 | zym1 | 2.7 | 17 | |
| SPAC3G9.11c | pdc201 | 1.7 | 6.4 | |
| SPAC1F7.06 | hsp3105 | 0.023 | 1.2 | |
| SPAC869.09 | 0.077 | 0.62 | subtel1R | |
| SPAC869.07c | mel1 | 0.069 | 0.66 | subtel1R |
| SPAC869.06c | hry1 | 0 | 0.19 | subtel1R |
| SPAC869.04 | 0.033 | 28 | subtel1R | |
| SPAC869.03c | 0.025 | 10 | subtel1R | |
| SPBPB21E7.01c | eno102 | 0.11 | 0.4 | subtel2L |
| SPBPB21E7.02c | 0 | 0.12 | subtel2L | |
| SPBPB21E7.10 | 0 | 0.11 | subtel2L | |
| SPBPB21E7.11 | 0.13 | 0.49 | subtel2L | |
| SPNCRNA.1364 | 0.079 | 0.94 | ||
| SPNCRNA.1573 | 0.078 | 0.68 | ||
| SPBC2G2.17c | 0.52 | 3.6 | ||
| SPNCRNA.577 | 0.016 | 4.6 |
Quantitative data (number of molecules per cell) from Marguerat, S.; Schmidt, A.; Codlin, S.; Chen, W.; Aebersold, R.; Bähler, J., Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell 2012, 151, 671–683
Next, we examined the 149 genes that were upregulated only at T1D, and a significant fraction of these genes are also localized close to telomeres. A total of 25 of the 149 genes (16.8%; CHI2 = 28,4; P < 0.001) are in 200 kb subtelomeric regions of chromosomes 1 and 2 (Table 5). Regarding the downregulated genes in G0, we found that as many as 1208 genes were significantly down after 24 h (T1D) and 6436 genes (including non-coding RNA genes) were down at T1W (Table 1). This represents 97.3% of the genome indicating that nearly the entire transcriptome is downregulated after 1 week in quiescence.
Table 5.
Genomic features of 149 quiescence genes. Subtelomeric genes on chromosomes I and II (< 200 kb from ends) are in bold letters
| Gene id | Chr | Start | End | Gene_name | T1_T0_SMT0_logFC | T1_T0_SMT0 P-value |
|---|---|---|---|---|---|---|
| SPNCRNA.602 | I | 50851 | 51545 | SPNCRNA.602 | 3,25973596 | 2,7274E-05 |
| SPAC1F8.04c | I | 92387 | 93931 | SPAC1F8.04c | 3,53511935 | 3,0867E-06 |
| SPNCRNA.607 | I | 99046 | 101140 | fta5-antisense-1 | 2,7031025 | 0,00024108 |
| SPAC11D3.19 | I | 106893 | 108361 | SPAC11D3.19 | 3,01020676 | 4,5012E-05 |
| SPAC11D3.03c | I;I;I | 110904;112098;112378 | 112046;112333;112499 | SPAC11D3.03c | 4,44873642 | 8,8882E-09 |
| SPAC11D3.16c | I | 140381 | 141653 | SPAC11D3.16c | 2,48746727 | 0,000418 |
| SPAC11D3.17 | I;I | 141199;141559 | 141510;144768 | SPAC11D3.17 | 2,1184002 | 0,00225435 |
| SPAC13G6.08 | I | 187701 | 190031 | SPAC13G6.08 | 2,11089891 | 0,00512897 |
| SPNCRNA.643 | I | 388352 | 389177 | trm112-antisense-1 | 2,47596303 | 0,00370115 |
| SPNCRNA.649 | I | 446175 | 446702 | lsd2-antisense-1 | 2,79371868 | 0,00369267 |
| SPAC23E2.03c | I | 450860 | 453603 | ste7 | 3,25111192 | 7,4801E-06 |
| SPNCRNA.673 | I | 664677 | 665246 | rad15-antisense-1 | 2,42711956 | 0,00934543 |
| SPNCRNA.690 | I | 829234 | 831663 | prh1-antisense-1 | 3,67766921 | 0,00038004 |
| SPNCRNA.220 | I | 1015475 | 1015830 | SPNCRNA.220 | 3,3952792 | 0,0028625 |
| SPAC56F8.15 | I | 1151431 | 1153638 | SPAC56F8.15 | 1,96273702 | 0,00753558 |
| SPNCRNA.737 | I | 1357621 | 1359568 | SPNCRNA.737 | 1,93983373 | 0,00875406 |
| SPAC1002.19 | I | 1835270 | 1837060 | urg1 | 3,37759044 | 3,9743E-06 |
| SPAC1399.03 | I | 1841908 | 1843995 | fur4 | 1,81787099 | 0,00812566 |
| SPNCRNA.178 | I | 1844130 | 1845339 | SPNCRNA.178 | 2,90780759 | 0,00018621 |
| SPAP11E10.02c | I | 1856833 | 1860727 | mam3 | 2,58249425 | 0,00026631 |
| SPAPB1A10.14 | I | 1890529 | 1892090 | pof15 | 2,58471259 | 0,00031153 |
| SPAC3C7.02c | I | 2064853 | 2066004 | pil2 | 2,01101362 | 0,00384745 |
| SPNCRNA.791 | I | 2066217 | 2067343 | rad55-antisense-1 | 3,26641538 | 7,9496E-05 |
| SPAC20H4.11c | I;I | 2131685;2132545 | 2132426;2133066 | rho5 | 2,23485497 | 0,00175906 |
| SPAC13F5.07c | I;I | 2184654;2185472 | 2185282;2185990 | hpz2 | 2,95890939 | 6,5014E-05 |
| SPAC13G7.13c | I | 2318492 | 2322664 | msa1 | 2,01610833 | 0,00357637 |
| SPNCRNA.821 | I | 2380416 | 2381234 | SPNCRNA.821 | 5,22655651 | 2,8497E-07 |
| SPAC22H10.13 | I | 2381690 | 2382200 | zym1 | 5,19708373 | 9,4432E-11 |
| SPNCRNA.194 | I | 2424735 | 2425515 | SPNCRNA.194 | 2,33292483 | 0,00869259 |
| SPNCRNA.43 | I | 2432340 | 2432921 | prl43 | 3,36282112 | 0,00071322 |
| SPAC4A8.04 | I | 2544815 | 2546981 | isp6 | 2,06781376 | 0,00291872 |
| SPNCRNA.835 | I | 2547093 | 2547449 | SPNCRNA.835 | 2,20795116 | 0,0071483 |
| SPSNRNA.06 | I;I | 2562276;2562374 | 2562323;2562427 | snu6 | 1,87091948 | 0,00697876 |
| SPNCRNA.198 | I | 2703965 | 2704030 | SPNCRNA.198 | 2,78396522 | 0,00553514 |
| SPNCRNA.853 | I | 2784605 | 2785492 | SPNCRNA.853 | 4,11372383 | 3,3265E-07 |
| SPNCRNA.860 | I | 2931622 | 2932800 | SPNCRNA.860 | 5,01730583 | 3,0063E-09 |
| SPAC2E1P3.04 | I | 2931763 | 2935115 | cao1 | 2,22542246 | 0,00144898 |
| SPAC31G5.09c | I | 2999546 | 3001687 | spk1 | 1,84222622 | 0,00732841 |
| SPNCRNA.71 | I | 3001761 | 3001930 | SPNCRNA.71 | 2,61362541 | 0,00116616 |
| SPNCRNA.877 | I | 3032579 | 3033397 | SPNCRNA.877 | 2,8566656 | 0,00067403 |
| SPAC24C9.16c | I;I;I;I | 3042749;3042872;3043096;3043225 | 3042817;3042909;3043157;3043380 | cox8 | 1,88137663 | 0,00786675 |
| SPAC3G9.11c | I | 3159753 | 3162245 | pdc201 | 4,74996563 | 1,3887E-09 |
| SPAC6G10.06 | I;I;I | 3226929;3227042;3227456 | 3226980;3227383;3228192 | SPAC6G10.06 | 2,51110956 | 0,0013486 |
| SPNCRNA.931 | I | 3731313 | 3732279 | grx2-antisense-1 | 2,91994619 | 0,0014984 |
| SPNCRNA.935 | I | 3745778 | 3748833 | SPNCRNA.935 | 3,9191636 | 1,7498E-07 |
| SPATRNAGLU.04 | I | 3776768 | 3776839 | SPATRNAGLU.04 | 1,95115116 | 0,01020029 |
| SPNCRNA.955 | I | 4004553 | 4005632 | SPAC27E2.02-antisense-1 | 2,33505779 | 0,00572603 |
| SPSNORNA.13 | I | 4154912 | 4155002 | snoR69b | 2,03181845 | 0,00326288 |
| SPAC25B8.13c | I | 4179961 | 4183074 | isp7 | 2,4050579 | 0,00062889 |
| SPAC1F7.06 | I | 4230615 | 4232557 | hsp3105 | 5,80756074 | 2,7966E-11 |
| SPNCRNA.243 | I | 4231812 | 4232976 | SPNCRNA.243 | 2,57917641 | 0,00141246 |
| SPAC9E9.17c | I;I;I;I | 4437311;4437606;4437732;4437860 | 4437401;4437648;4437813;4437862 | SPAC9E9.17c | 5,21541272 | 8,4446E-09 |
| SPNCRNA.987 | I | 4437466 | 4438921 | SPAC9E9.17c-antisense-1 | 2,17485116 | 0,00635584 |
| SPNCRNA.12 | I | 4439118 | 4439451 | prl12 | 3,78490716 | 0,00470787 |
| SPAC17C9.16c | I;I | 4472472;4473325 | 4473271;4474744 | mfs1 | 1,83115795 | 0,00796667 |
| SPAC27D7.03c | I | 4510983 | 4515015 | mei2 | 2,22978588 | 0,00141295 |
| SPNCRNA.993 | I | 4515182 | 4515993 | SPNCRNA.993 | 2,45668478 | 0,00117438 |
| SPAC11H11.04 | I | 4780224 | 4781922 | mam2 | 4,13282167 | 4,892E-08 |
| SPAC4F10.17 | I | 4868326 | 4869283 | SPAC4F10.17 | 3,91344782 | 1,839E-06 |
| SPAC4F10.22 | I | 4878241 | 4878453 | cmc4 | 2,0237062 | 0,00648228 |
| SPAPB8E5.04c | I | 4913971 | 4915513 | npc2 | 1,84500451 | 0,00745054 |
| SPAPB8E5.05 | I;I;I | 4916603;4916921;4917286 | 4916841;4917214;4917521 | mfm1 | 7,30766685 | 1,2375E-16 |
| SPAPJ691.02 | I | 5187647 | 5189187 | SPAPJ691.02 | 4,53526941 | 4,6374E-09 |
| SPAC19D5.07 | I | 5222447 | 5224795 | uga1 | 1,88556666 | 0,00622319 |
| SPAC2H10.01 | I | 5273012 | 5275983 | SPAC2H10.01 | 2,6493213 | 0,00027561 |
| SPNCRNA.1068 | I | 5309027 | 5311689 | SPNCRNA.1068 | 2,30679984 | 0,00316561 |
| SPNCRNA.1075 | I | 5353770 | 5354310 | new12-antisense-1 | 3,31258143 | 9,0267E-05 |
| SPAC1039.03 | I | 5452105 | 5453867 | SPAC1039.03 | 2,12458926 | 0,00219715 |
| SPAC1039.09 | I | 5465377 | 5468649 | isp5 | 2,15393594 | 0,00202143 |
| SPAC1039.10 | I;I;I | 5470432;5470574;5471284 | 5470525;5471227;5471520 | mmf2 | 1,86772985 | 0,00694076 |
| SPAC922.09 | I | 5482662 | 5482877 | SPAC922.09 | 5,12304127 | 4,3744E-05 |
| SPAC922.06 | I | 5485096 | 5486287 | SPAC922.06 | 2,20990467 | 0,00153079 |
| SPNCRNA.1092 | I | 5495270 | 5496597 | SPNCRNA.1092 | 2,16976747 | 0,00364619 |
| SPAC869.09 | I | 5496844 | 5497448 | SPAC869.09 | 4,67744479 | 8,7417E-06 |
| SPAC869.08 | I | 5497918 | 5499280 | pcm2 | 4,23373637 | 4,4033E-07 |
| SPAC869.07c | I | 5499768 | 5501236 | mel1 | 4,70824039 | 6,0466E-09 |
| SPAC869.06c | I | 5502560 | 5503291 | SPAC869.06c | 3,4837771 | 0,00042619 |
| SPAC869.04 | I | 5511137 | 5512369 | SPAC869.04 | 9,23276947 | 6,5934E-22 |
| SPAC869.03c | I | 5512757 | 5514865 | SPAC869.03c | 9,16835946 | 9,8841E-22 |
| SPAC869.01 | I | 5521275 | 5523181 | SPAC869.01 | 3,00278941 | 0,00011382 |
| SPBPB21E7.02c | II;II | 60553;61119 | 61107;61205 | SPBPB21E7.02c | 10,5415628 | 0,00031848 |
| SPBPB21E7.10 | II;II | 61362;61632 | 61526;62126 | SPBPB21E7.10 | 10,3509696 | 0,00048232 |
| SPBPB21E7.11 | II;II;II | 61526;62598;62944 | 62449;62885;63086 | SPBPB21E7.11 | 4,55305877 | 2,1632E-06 |
| SPBC1683.05 | II | 147915 | 150574 | SPBC1683.05 | 1,81584673 | 0,00821874 |
| SPBC1271.09 | II;II | 350692;350787 | 350740;352369 | tgp1 | 2,42462905 | 0,00053769 |
| SPNCRNA.1346 | II | 359391 | 359866 | SPNCRNA.1346 | 3,2706138 | 0,00044942 |
| SPSNRNA.04 | II | 467233 | 467361 | snu4 | 3,23066699 | 0,00022388 |
| SPNCRNA.1364 | II | 503961 | 505097 | SPNCRNA.1364 | 4,8789878 | 5,6148E-10 |
| SPBC1685.05 | II | 504397 | 509056 | SPBC1685.05 | 2,22522485 | 0,0014316 |
| SPNCRNA.66 | II | 547325 | 547761 | prl66 | 2,67117747 | 0,0054103 |
| SPBC354.12 | II;II | 578063;578725 | 578181;580056 | gpd3 | 2,77080717 | 9,979E-05 |
| SPBPJ4664.03 | II;II | 700291;700544 | 700476;700700 | mfm3 | 7,90991958 | 3,2907E-18 |
| SPNCRNA.1405 | II | 1013315 | 1014054 | prp2-antisense-1 | 3,05365283 | 0,00040845 |
| SPNCRNA.1415 | II | 1150424 | 1150863 | SPNCRNA.1415 | 6,49255957 | 7,8126E-08 |
| SPSNORNA.21 | II | 1308634 | 1308745 | snoU14 | 3,06732048 | 1,982E-05 |
| SPNCRNA.15 | II | 1329046 | 1329760 | prl15 | 7,65836499 | 9,1712E-11 |
| SPBC83.19c | II | 1541452 | 1541903 | SPBC83.19c | 11,1826139 | 4,0742E-05 |
| SPNCRNA.352 | II | 1542196 | 1542364 | SPNCRNA.352 | 11,0312641 | 6,4796E-05 |
| SPBC29B5.02c | II | 1550293 | 1553467 | isp4 | 2,76293742 | 0,00010681 |
| SPNCRNA.1443 | II | 1554843 | 1557638 | SPNCRNA.1443 | 2,04831502 | 0,00323332 |
| SPBC1D7.02c | II | 1752100 | 1755619 | scr1 | 2,04685207 | 0,0032455 |
| SPBC9B6.03 | II | 1817454 | 1819848 | SPBC9B6.03 | 2,88027667 | 5,562E-05 |
| SPBC23G7.11 | II | 2120231 | 2121050 | mag2 | 2,67196421 | 0,00085483 |
| SPNCRNA.1512 | II | 2417501 | 2418682 | pvg3-antisense-1 | 3,40867882 | 0,00292084 |
| SPNCRNA.1525 | II | 2543249 | 2544500 | psm1-antisense-1 | 3,50755144 | 1,6382E-05 |
| SPNCRNA.1530 | II | 2580400 | 2581182 | SPNCRNA.1530 | 9,90488058 | 0,0021415 |
| SPBC25B2.08 | II | 2611366 | 2612917 | SPBC25B2.08 | 2,18518869 | 0,00197078 |
| SPBC19C7.04c | II | 2825251 | 2826700 | SPBC19C7.04c | 2,71034397 | 0,00015799 |
| SPNCRNA.1564 | II | 2934727 | 2935979 | SPBC1703.11-antisense-1 | 2,52684517 | 0,00180399 |
| SPNCRNA.1573 | II | 3020526 | 3022271 | SPBC15D4.05-antisense-1 | 6,22415483 | 4,9082E-12 |
| SPNCRNA.413 | II | 3150075 | 3150475 | SPNCRNA.413 | 2,82207927 | 0,00847413 |
| SPBC13A2.04c | II | 3405575 | 3408492 | ptr2 | 2,25820836 | 0,00121891 |
| SPBC2G2.17c | II | 3466270 | 3467797 | SPBC2G2.17c | 5,69625442 | 5,1176E-12 |
| SPBC887.16 | II | 3574869 | 3575250 | SPBC887.16 | 2,87571225 | 0,00131414 |
| SPSNORNA.27 | II | 3654333 | 3654416 | snoR47 | 2,08042095 | 0,0026702 |
| SPNCRNA.1660 | II | 4049029 | 4049473 | SPBC215.10-antisense-1 | 2,32255749 | 0,00367613 |
| SPBC1347.11 | II | 4081869 | 4083198 | sro1 | 2,14480969 | 0,00202154 |
| SPNCRNA.1670 | II | 4109027 | 4109600 | SPNCRNA.1670 | 5,26102836 | 1,1998E-06 |
| SPNCRNA.577 | II | 4255755 | 4259164 | SPBC1652.02-antisense-1 | 5,33538848 | 2,9985E-11 |
| SPNCRNA.451 | III | 49802 | 50142 | SPNCRNA.451 | 4,52899464 | 1,8957E-08 |
| SPCC757.13 | III | 77080 | 79816 | SPCC757.13 | 2,56643263 | 0,00028782 |
| SPNCRNA.1126 | III | 289992 | 293300 | SPCC553.08c-antisense-1 | 2,13247264 | 0,00505195 |
| SPNCRNA.1137 | III | 394357 | 396657 | ubp16-antisense-1 | 3,60875293 | 7,5933E-06 |
| SPCC1183.12 | III;III | 604366;604857 | 604805;604961 | spo13 | 2,01450177 | 0,00823742 |
| SPNCRNA.1160 | III | 815167 | 816476 | SPCC1393.09c-antisense-1 | 3,32393748 | 0,00134978 |
| SPCPB16A4.06c | III;III | 956012;957195 | 957014;957627 | SPCPB16A4.06c | 1,91007479 | 0,00587734 |
| SPCC550.07 | III | 1196729 | 1199999 | SPCC550.07 | 3,43129476 | 2,8671E-06 |
| SPCC550.10 | III | 1204315 | 1206695 | atd3 | 2,60470778 | 0,00022774 |
| SPCC338.18 | III | 1340628 | 1341847 | SPCC338.18 | 2,64277685 | 0,00033584 |
| SPCC1281.04 | III | 1386524 | 1387961 | SPCC1281.04 | 2,38484333 | 0,00223696 |
| SPCC188.12 | III;III | 1504862;1505062 | 1504999;1506851 | spn6 | 4,13670216 | 1,5614E-07 |
| SPNCRNA.51 | III | 1514062 | 1514343 | prl51 | 3,34790704 | 0,00037929 |
| SPCC584.13 | III | 1516197 | 1518230 | SPCC584.13 | 2,22673511 | 0,00137358 |
| SPNCRNA.1205 | III | 1559743 | 1561240 | SPNCRNA.1205 | 5,35400218 | 1,6137E-05 |
| SPCC417.02 | III | 1669944 | 1671551 | dad5 | 4,36062922 | 1,3103E-08 |
| SPNCRNA.1215 | III | 1671883 | 1674238 | SPCC417.03-antisense-1 | 2,29927601 | 0,00099408 |
| SPCC417.06c | III;III;III | 1677093;1678329;1678828 | 1678286;1678778;1679436 | mug27 | 3,07341949 | 0,00197289 |
| SPCC417.10 | III | 1694169 | 1697592 | dal51 | 1,98405831 | 0,00416259 |
| SPCC1450.07c | III;III | 1736500;1738213 | 1737778;1738318 | dao1 | 2,1507211 | 0,00212075 |
| SPCC1442.01 | III | 1765913 | 1768971 | ste6 | 2,32561278 | 0,00091354 |
| SPCC1223.09 | III | 1857150 | 1858621 | SPCC1223.09 | 2,18680094 | 0,00172594 |
| SPNCRNA.1236 | III | 1859963 | 1860577 | SPNCRNA.1236 | 1,78076961 | 0,00932741 |
| SPCC74.04 | III | 1935474 | 1938754 | SPCC74.04 | 2,86776786 | 6,048E-05 |
| SPCC576.01c | III | 2079421 | 2080662 | xan1 | 4,08792138 | 6,3569E-08 |
| SPCC830.04c | III | 2186723 | 2187154 | mug128 | 2,84134074 | 0,00746144 |
| SPCC965.13 | III | 2310096 | 2314400 | SPCC965.13 | 2,22721546 | 0,00139801 |
| SPCC70.04c | III;III;III | 2352568;2353746;2353974 | 2353701;2353913;2354572 | SPCC70.04c | 2,53908379 | 0,00031576 |
| SPNCRNA.519 | III | 2362179 | 2362725 | SPNCRNA.519 | 2,33386995 | 0,0013251 |
| SPCC569.09 | III;III | 2413,905;2414158 | 2414089;2414790 | SPCC569.09 | 274,408957 | 0,00012036 |
Transcription changes in G0 and reduced viability of Asp1, Ino80C, and H2A.Z mutants
Next, we analyzed the changes of the G0 transcriptome in the mutants. It was clear already by looking at the total number of reads after ERCC normalization that all mutants showed overall changes in the G0 transcriptome as compared to wild type (Fig. 2). The overall tendency was that the mutants showed a further reduction of global transcription as compared to the wild type. The genes affected in the different mutants at each time point as compared to the wild type were defined (Table 6). This analysis revealed substantial changes in gene expression in all the tested mutants. Again, hht2∆ cells showed a weaker phenotype at T1D and T1W compared to the other mutants, but 1286 genes were downregulated in hht2∆ cells at T2W. The hht2+ gene is constitutively expressed in contrast to the other two histone H3-encoding genes (hht1+ and hht3+) in fission yeast which are strictly expressed during the S phase (Takayama and Takahashi 2007). Thus, hht2+ is the sole histone H3 gene expressed in G0 cells. The viability of hht2∆ cells drops significantly compared to wild type after 2 weeks in quiescence (TTEST; P = 0.013) and is correlated with reduced transcription (Table 1, Fig. 2, Table 6). It is possible that this reduction of transcription, caused by reduced histone H3 levels, is contributing to the mortality of hht2∆ cells in G0.
Table 6.
Number of genes differentially expressed in mutant cells compared to wild-type cells
| Comparison | Up | Down | NS |
|---|---|---|---|
| HHT2_SMT0_T0 | 2 | 6 | 6605 |
| PHT1_SMT0_T0 | 5 | 9 | 6599 |
| IEC1_SMT0_T0 | 20 | 11 | 6582 |
| ASP1_SMT0_T0 | 26 | 19 | 6569 |
| ARP42_SMT0_T0 | 9 | 114 | 6490 |
| IES2_SMT0_T0 | 9 | 29 | 6575 |
| HHT2_SMT0_T1D | 91 | 1 (hht2) | 6521 |
| PHT1_SMT0_T1D | 6 | 6143 | 464 |
| IEC1_SMT0_T1D | 8 | 6194 | 410 |
| ASP1_SMT0_T1D | 2 | 6368 | 243 |
| ARP42_SMT0_T1D | 0 | 6519 | 94 |
| IES2_SMT0_T1D | 7 | 6048 | 558 |
| HHT2_SMT0_T1W | 1 | 1 | 6611 |
| PHT1_SMT0_T1W | 29 | 1 | 6583 |
| IEC1_SMT0_T1W | 88 | 0 | 6525 |
| ASP1_SMT0_T1W | 230 | 3504 | 2879 |
| ARP42_SMT0_T1W | 5 | 6145 | 463 |
| IES2_SMT0_T1W | 24 | 6 | 6583 |
| HHT2_SMT0_T2W | 190 | 1129 | 5294 |
| PHT1_SMT0_T2W | 16 | 33 | 6564 |
| IEC1_SMT0_T2W | 43 | 78 | 6492 |
| ASP1_SMT0_T2W | 144 | 1898 | 4571 |
| ARP42_SMT0_T2W | 5 | 5238 | 1370 |
The indicated gene lists were compared and the number of differentially expressed genes (up or down) are shown for each comparison (FDR adjusted P < 0.05)
RNA levels were normalized across all groups with ERCC spike-in controls
NS, not significant
In contrast to hht2∆, pht1∆ cells show strong changes of the transcriptome already at 24 h, i.e., prior to the reduction in viability that occurs after 1 week (Fig. 1, Fig. 2, Table 6). This observation is consistent with a key role for the histone variant H2A.Z in gene regulation, being essential for survival in quiescence. Ino80C is required for the removal of H2A.Z by its chromatin remodeling activity driven by ATP hydrolysis (Papamichos-Chronakis et al. 2011). Our results show that null mutations in three Ino80C-related genes, arp42∆, iec1∆, and ies2∆ cause a massive reduction of the G0 transcriptome after 24 h as compared to the wild type, and arp42∆ shows a further reductions of gene expression after one and 2 weeks. Thus, a vast majority of genes are prematurely downregulated in Ino80C mutants compared to wild type (Fig. 2, Table 6). Although the observed transcription patterns are not strictly correlated to mortality, the generally reduced transcription is likely explaining the essential role of Ino80C in cellular quiescence that we previously observed (Zahedi et al. 2020).
Failure to induce quiescence genes in Ino80C mutants
Next, we studied the behavior of the larger set of 149 genes that are upregulated in the wild type after 24 h in nitrogen starvation (T1D). We found that none of these genes were upregulated in arp42∆, iec1∆, and ies2∆ cells (Fig. 3B). In fact, all the 149 genes were downregulated in these mutants compared to wild type (smt0) after 24 h in G0. This shows that Ino80C is essential for the activation of these genes in response to nitrogen starvation. Gene ontology analysis revealed that the list of 149 upregulated genes is significantly enriched for several GO terms including the fungal vacuole, amino acid, dipeptide, and nucleobase transmembrane transport (Table 7). The vacuole is required in quiescence for the autophagy process to recycle amino acids, and these transmembrane transport processes are crucial for survival during cellular quiescence. Thus, the upregulated expression of these genes is required for survival in G0 by adapting the cellular metabolism. For example, the SPAC11D3.16c gene located in near tel1L is annotated as being essential for viability in G0 (Harris et al. 2021). Two genes near tel1R encoding membrane transporters, isp5+ and SPAC869.03c fail to be activated in Ino80C mutants. Therefore, it is conceivable that the reason Ino80C mutants are dying in G0 is due to the inability to activate genes needed for the metabolic change and cellular uptake that normally occur during quiescence.
Table 7.
Gene ontology analysis of 149 genes induced in quiescent wild-type cells after 24 h
| GO | Term | N | Gene list T1D_T0_SMT0_up | P-value |
|---|---|---|---|---|
| GO:0,000,750 | pheromone-dependent signal transduction involved in conjugation with cellular fusion | 14 | 5 | 9,23456E-06 |
| GO:0,035,442 | dipeptide transmembrane transport | 3 | 3 | 1,12193E-05 |
| GO:0,071,916 | dipeptide transmembrane transporter activity | 3 | 3 | 1,12193E-05 |
| GO:0,043,864 | indoleacetamide hydrolase activity | 3 | 2 | 0,001,491,016 |
| GO:0,015,205 | nucleobase transmembrane transporter activity | 3 | 2 | 0,001,491,016 |
| GO:0,007,267 | cell–cell signaling | 4 | 2 | 0,002,937,898 |
| GO:0,000,772 | mating pheromone activity | 4 | 2 | 0,002,937,898 |
| GO:0,000,324 | fungal-type vacuole | 68 | 6 | 0,004,105,498 |
| GO:0,035,673 | oligopeptide transmembrane transporter activity | 5 | 2 | 0,004,824,158 |
| GO:0,003,333 | amino acid transmembrane transport | 17 | 3 | 0,006,047,446 |
| GO:0,031,520 | plasma membrane of cell tip | 20 | 3 | 0,009,650,323 |
| GO:0,006,878 | cellular copper ion homeostasis | 9 | 2 | 0,016,367,608 |
Gene Ontology (GO) analysis of GO terms obtained from PomBase
Analysis done on the upregulated genes of the wild type (SMT0) cells between T0 and T1D
N column, the number of genes in the GO
A role of Ino80 and H2A.Z in activation of quiescence genes
To get some insights into the role of H2A.Z in quiescence, we compared the list of downregulated genes in the pht1∆ mutant at T1D with the gene lists of Ino80C mutants, arp42∆ and iec1∆, and a list of genes induced in the wild type at T1D. This comparison revealed that all of the 149 genes that are upregulated in wild type (smt0) at T1D fail to be induced in pht1∆ cells lacking H2A.Z (Fig. 3C). Also, there is a very strong overlap genome-wide between genes downregulated in pht1∆ cells and those downregulated in arp42∆ and iec1∆ mutants. A total of 5951 genes were downregulated in all three mutants. Based on this and our observations, we concluded that H2A.Z and Ino80C are both somehow required for the activation of genes induced in G0, in particularly in subtelomeric regions.
Changes of H2A.Z localization in quiescent cells at subtelomeric LTR boundary elements
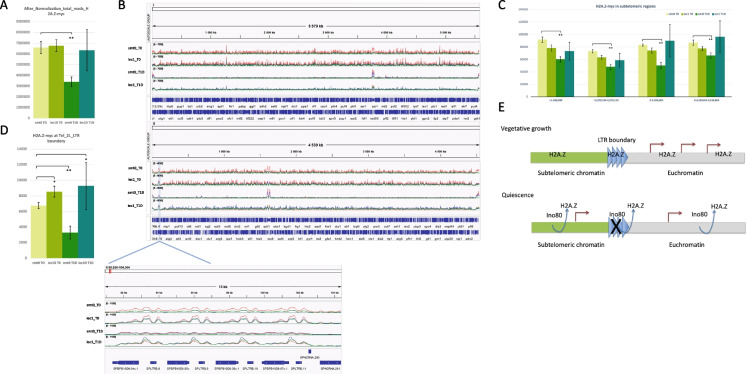
The molecular function of Ino80C is to remove H2A.Z in a nucleosome disassembly mechanism in which H2A.Z is exchanged with H2A (Papamichos-Chronakis et al. 2011). Furthermore, Ino80 has been shown to evict H2A.Z in diverse organisms leading to changes in gene expression. To test if the observed changes in gene expression in quiescent cells depend on H2A.Z, we performed ChIP-seq of epitope tagged H2A.Z (pht1-myc) in wild type and iec1∆ cells. We used the Drosophila spike in chromatin methodology to allow measurement of global changes of H2A.Z occupancy by ChIP-seq (see the “Materials and methods” section). The total number of matched reads after the spike in normalization revealed a strong and significant reduction of H2A.Z-myc occupancy in quiescent wild type (smt0) cells at T1D compared to vegetative wild-type cells (T0), but not redusction was observed in quiescent iec1∆ cells (Fig. 4A). The chromosomal browser view confirmed this observation showing very low signals of H2A.Z-myc along all three chromosomes in wild-type cells at T1D, whereas signals remained high in iec1∆ cells (Fig. 4B). Thus, we conclude that there is an eviction process of H2A.Z from chromsosomes in quiescent cells, which is dependent on Ino80C.
Fig. 4.
ChIP-seq analysis of H2A.Z localisation at a subtelomeric boundary element in vegetative (T0) and quiescent cells (T1D). A Quantitation of total number oif ChIP-seq reads for H2A.Z-myc. The bar diagram shows the total number of reads (after spike-in normalization) in wild-type cells (smt0) and the iec1∆ mutant at the vegetative stage (T0) and 24 h after removal of nitrogen (T1D). The error bars represent Standard deviation (SD) values from triplicate samples. Unpaired t-test was used to determine data significance. *P < 0.05, **P < 0.01. B Browser images of chromosomes I, II and the LTR boundary element at tel2L. The IGV genome browser tracks present chromosome 1, chromosome 2 and LTR boundary at chromosome II subtelomeric region (chrII: 93,039–102,657). C Quantitation of ChIP-seq reads for H2A.Z-myc in subtelomeric regions. The bar diagram shows the reads in 4 subtelomeric region, chrI:1–200,000, chrI:5,379,134–5,579,133, chrII:1–200,000 and chrII:4,339,805–4,539,804. The error bars represent SD values from triplicate samples. Unpaired t-test was used to determine data significance. **P < 0.01. D Quantitation of ChIP-seq reads for H2A.Z-myc the LTR boundary element at tel2L. The bar diagram shown the reads in LTR boundary element at chromosome II subtelomeric region (chrII: 93,039–102,657). The error bars represent SD values from triplicate samples. Unpaired t-test was used to determine data significance. *P < 0.05, **P < 0.01. E Model for regulation of the activity of the tel2L boundary element. For details see Discussion
Next, we examined the subtelomeric regions for changes of H2A.Z localization in quiescent cells (Fig. 4C). As illustrated by the bar diagrams subtelomeric regions (0–200 kb) of chromosomes I and II had a significant reduction (TTEST; P < 0.01) of H2A.Z in wild-type cells at T1D. Again this reduction was not observed in iec1∆ cells at T1D. Furthermore, it was clear that H2A.Z is localized in four peaks to the long terminal repaet (LTR) containing subtelomeric boundary element near tel2L (Fig. 4B; bottom). The activity of this boundary element has previously been shown to be maintained via Fft3 (Fun thirty homolog 3) in vegetative cells (Strålfors et al. 2011) (Steglich et al. 2015). In vegetative cells (T0), we observed a small but significant increase of H2A.Z at the tel2L boundary in iec1∆ cells compared to wild type (Fig. 4D). However, in quiescent wild-type cells, the H2A.Z peaks were strongly reduced (TTEST; P < 0.01) whereas the peaks could still be detected in quiescent iec1∆ cells (Fig. 4D). It was not possible to investigate if Ino80C also plays a role at the he other subtelomeric boundary elements since they are not cleary defined (Steglich et al. 2015). Taken together, this suggests that Ino80 is involved in the eviction or relocalization of H2A.Z genomewide, in subtelomeric regions of chromosomes I and II, and at a subtelomeric LTR boundary element (tel2L) especially during quiescence.
Discussion
A role for Ino80 in quiescence and ageing?
We have shown that Ino80C is required for the expression of genes in quiescence, including the hsp3105+ gene, encoding a ThiJ domain protein implicated in autophagy and oxidative stress resistance (Table 4). This gene was shown to be required for survival in the stationary phase (Su et al. 2015). Assuming that Ino80C drives expression of hsp3105+ in the stationary phase, then it could explain the short chronological lifespan phenotype that was reported for Ino80C mutants (Romila et al. 2021). It would also suggest that there is some commonality between chronological ageing in stationary phase and quiescence.
A possible role for Inositol polyphosphates restricting Ino80C activity in fission yeast?
Asp1 encodes an inositol kinase that may affect the activity of Ino80C. Inositol polyphosphates are synthesized by a series of enzymes including Asp1. The Asp1 kinase generates one specific inositol polyphosphate, IP8, and in fission yeast asp1∆ mutants, IP8 levels are strongly reduced whereas IP6 and IP7 levels are increased (Pascual-Ortiz et al. 2018). In budding yeast IP6 directly inhibits Ino80C (Shen et al. 2003). Thus, our results showing a similar effect on the G0 transcriptome and G0 mortality phenotypes between asp1∆ and Ino80C mutants and the strong overlap of downregulated genes (Fig. 3B) suggest that inositol polyphosphates IP6 and IP7, which accumulate in the asp1∆ mutant, may inhibit Ino80C activity also in fission yeast.
A change in nuclear organization in quiescence mediated by Ino80C?
In fission yeast, all four telomeres of chromosomes I and II, i.e., tel1L, tel1R, tel2L, and tel2R, form a peripheral cluster near the nucleolus in quiescent cells (Maestroni et al. 2020). It was previously shown that genes induced during meiosis and sporulation are enriched in subtelomeric regions (Mata et al. 2002). It is conceivable that Ino80C is involved in the formation of a transcriptionally active nuclear compartment comprising subtelomeres in response to nitrogen starvation or cellular quiescence when a mating partner is absent. Expression of this active compartment may support for mating and sporulation if cells of the opposite mating type are present. In agreement with this notion, the iec1∆ mutant was reported to show a decreased mating efficiency (Hogan et al. 2010). In budding yeast, Ino80C and its ATPase activity are required for chromosomal movements within the nucleus (Neumann et al. 2012). Hence, it is possible that the formation of this actively transcribed subtelomeric nuclear compartment, in response to nitrogen starvation, involves chromatin movements facilitated by Ino80C within the nucleus.
Based on our new results, we propose a model in which Ino80C activity, possibly modulated by inositol kinase Asp1, is required to remove H2A.Z from chromatin by a nucleosome disassembly mechanism in quiescent cells (Fig. 4E). This includes H2A.Z eviction at a subtelomeric boundary element leading to inactivation of the boundary and gene expression of subtelomeric genes, including transmembrane transporter genes, required to survive in quiescence. We hypothesize that this process may involve a drastic reorganization of chromosome structures in quiescent cells and clustering of telomeres to maintain an active nuclear compartment. Interestingly, it is known that fft3∆ cells have a reduced efficiency of to enter and exit quiescence (Sajiki et al. 2018; Zahedi et al. 2020). This is probably due to a failure in maintaining and restoring the subtelomeric boundary elements during these cellular transitions. To speculate further, the activation of quiescence genes by Ino80C is likely linked to the observed reduction of subtelomeric heterochromatin regions that occurs in quiescent cells (Oya et al. 2019). Consistent with this notion, it is known from studies in budding yeast that H2A.Z incorporation into acetylated chromatin by the SWR1-C complex is maintains heterochromatin boundary activity at silenced HMR loci and near telomeres (Zhou et al. 2010). Hence, in two distinct yeast species, H2A.Z is involved in maintaining heterochromatin boundaries.
In Drosophila, insulator boundary elements bound by the CTCF protein play important roles during development by partitioning the genome into distinct topologically associating domains (TADs), for example, in the Antennopedia gene complex where they prevent inappropriate enhancer promoter interactions between TADs (reviewed by (Batut et al. 2022)). The fission yeast genome is also organized into cohesion dependent TAD-like structures in vegetative cells (Mizuguchi et al. 2014). It is therefore tempting to speculate that the subtelomeric TAD structures are drastically reorganized in quiescent fission yeast cells is response to H2A.Z removal by Ino80. Finally, H2A.Z was recently implicated in regulating CTCF binding to chromatin by modulating the unwrapping of nucleosomes in mouse ES cells (Wen et al. 2020). Yeast cells do not have a CTCF protein; however, it is plausible that H2A.Z has a conserved function at boundary elements related to nucleosome disassembly both in unicellular and multicellular eukaryotes.
Materials and methods
Yeast strains and media
All five null mutants, hht2∆, asp1∆, iec1∆∆, arp42∆, pht1∆, and ies2∆ were derived from the version 5 Bioneer library, i.e., a large collection of gene deletion mutants carrying the kanMX4 cassette marking the gene deletion and leu1-32 ade6-M216/M210 ura4-D18 auxotrophic markers. The Bioneer strains cannot survive under the absence of nitrogen. Therefore, to produce prototrophic null mutant strains, the Bioneer strains were crossed with the Hu2843 mat1-M smt0 wild-type strain using standard methods (Ekwall and Thon 2017). The prototrophic mutants produced from each cross were selected using Edinburgh Minimal Medium (EMM) minus leucine, adenine, and uracil and subsequently YES medium containing G418 (150 ug/mL). The resulting mutant strains were named Hu3103 smt0 hht2∆ kanMX4, Hu3101 smt0 asp1∆ kanMX4, Hu3104 smt0 iec1∆ kanMX4, Hu3100 smt0 arp42∆ kanMX4, Hu3102 smt0 pht1∆ kanMX4, and Hu3113 smt0 ies2∆ kanMX4. The epitope tagged H2A.Z (pht1-myc) strains were produced from a cross using parental strains from (Buchanan et al. 2009) and Hu2843 resulting in the Hu3110 smt0 iec1D::ura4 pht1-myc and the Hu3112 smt0 pht1-myc strains.
All strains were grown in semi-solid YES complete media for 2 days 30 ℃ for 48 h and were regrown in liquid Pombe minimal glutamate medium (PMG) + nitrogen in a 200 ml flask to reach 106 cells /ml using a shaking incubator at 200 rpm at 30 °C. Before washing the cells, take 50 ml of culture as time 0 investigation and the rest of culture washed with 200 ml pre-warmed PMG-N and incubated them in 200 ml of PMG-N media then incubated (shaking incubator at 200 rpm at 30 °C).
Flow cytometry and viability measurements
For flow cytometry analysis (FACS) four time points were considered in this study, T0 (before shift to -nitrogen), T1D (24 h after shift), T1W (1 week after shift) and T2W (2 weeks after shift). For T0 and T1D 50 ml of culture and for T1D and T2W, 100 ml of culture was used. For each time point cells were pelleted and transferred into a 96-round bottom well plate and washed with 200 ∆l of PBS (centrifuged at 400 g, 5 min, at room temperature) and stained with 150 ∆l of Live-or-Dye™-Fixable Viability Stain (Biotium, Fremont, CA, USA). This stain was used at 1/1000 dilution in PBS, in the dark, and incubated for 30 min on ice with mild shaking. Then, cells were washed with 200 ∆l PBS and centrifuged (400 g, 5 min, at room temperature) followed by a fixation step using 200 ∆l of 70% ethanol and incubated for 30 min on ice in the dark. After washing with 200 ∆l PBS, cells were incubated for 15 min in 200 ∆l sodium citrate buffer (50 mM sodium citrate, pH 7.0), washed once with 200 ∆l sodium citrate buffer, pelleted and resuspended in 200 ∆l sodium citrate buffer containing 0.2 mg/ml DNAse-free RNase A (Roche diagnostics Scandinavia, Solna, Sweden, 10,109,169,001) and incubated for 3 h at 37 °C. Then, cells were stained with 100 ∆l of PBS containing 12.5 mg/ml propidium iodide (PI) (Invitrogen AB, Stockholm, Sweden, P4864) by incubating for 30 min at room temperature in the dark. Before FACS analysis, 100 μl of PBS was added into each well and the 96-well plate was immediately analyzed using the multiplex flow-cytometer CytoflexS (Beckman Coulter) and the CytExpert software (www.mybeckman.se). Slow mode running was used to collect and run the samples and the data was recorded based on 20,000 events of live cells in each sample. The total number of cells was selected via forward (FSC) and side (SSC) scattering, and single cells were sorted via FSC vs FSH (height) to exclude doublets. Then, the live cell population was selected via negative signal of Live-or-Dye™ Fixable Viability Staining λEx/λEm 642/662 nm through the FSC-A vs FL3A (R660) channels (FL3A::660A). The DNA content histogram analysis and cell cycle population analysis were performed on live cells population using the signals of PI staining using the gating strategy described in (Zahedi et al. 2020). At the T0 time point, the mononuclear G2 cell population was selected through the total area of DNA signal (DNA-A) vs. the width of the DNA signal (DNA-W), and in quiescence, G0 cells with 1C DNA content were selected via same gating (DNA-A negative, DNA-W negative). A minimal cut-off of 1000 single cells was considered for each sample measurement. The selected data was analyzed via FlowJo software version 9 (https://www.flowjo.com/solutions/flowjo/downloads).
RNA isolation.
Wild type and mutant strains were grown in a 200 ml liquid PMG + N medium using a shaking incubator (200 rpm at 30 °C) to reach between 1.0 × 106 and 10 × 106 cells/ml. For each culture, 100 ml was removed for the T0 timepoint, and the rest of the culture was washed with pre-warmed PMG-N and incubated for 24 h in 500 ml of pre-warmed PMG-N using a shaking incubator (200 rpm at 30 °C). For RNA extraction, cells were washed with ice-cold PBS and resuspended in 500 ∆l of ice-cold RNA extraction buffer (10 mM Tris–HCl pH 8.0, 1 mM EDTA, 2% Triton X-100, 1% SDS, 100 mM NaCl). Then we added 500 µl of Phenol (acidic phenol pH 4.5, Sigma) and 500 µl of glass beads (acid washed, Sigma). The tubes were vortexed vigorously and incubated at 65 °C for 45–60 min. Next, the tube was placed on ice for 5 min and centrifuged (1300 g, 5 min, 4 °C). The upper aqueous part was collected and transferred to a tube with 500 µl of chloroform (Sigma Aldrich), vortexed and centrifuged (1300 g, 5 min, 4 °C). The upper phase was collected and subjected to RNA precipitation at − 20 °C overnight. The precipitated RNA was washed once with 70% ethanol and dissolved in 30 ∆l H2O.
Chromatin immunoprecipitation sequencing (ChIP-Seq)
Log phase cells grown in PMG or PMG-N media were harvested and cross-linked by 1% formaldehyde for 30 min, and then 125 mM Glycine was added to quench the crosslinking for 5 min. After three time washing with cold PBS, the cells pellet was resuspended in ChIP lysis buffer with 0.5 mm Zirconia/Silica Beads, and then lysed in FastPrep machine for 7 times at max power 6.5. Sonication was done by using Bioruptor® Pico for 10 cycles, and then chromatin concentration was measured with Qubit dsDNA HS assay kit. Immunoprecipitation was performed with 20 µg sheared chromatin, 40 ng spike-in chromatin (activemotif 53,083), 1.6 µl spike-in antibody (activemotif 61,686) and 6 µl anti-c-Myc antibody (Sigma-Aldrich, M4439). After three times washing with low salt wash buffer, high salt wash buffer and LiCl wash buffer successively, ChIP-DNA was extracted by ChIP DNA Clean & Concentrator kit (ZYMO RESEARCH, D5205) and DNA concentration measured by Qubit dsDNA HS assay kit. Sequence library prepared by ThruPLEX DNA-Seq kit (TaKaRa, R400676) with DNA HT Dual Index Kit – 96N Set A (TaKaRa, R400660). Before sequencing, we performed quality control with bioanalyzer high sensitivity DNA analysis, and then the sequencing was performed using the Illumina Nextseq 2000 platform with P3 v3 50 kit (36 + 8 + 8 + 36 cycles, single-end sequencing) at the BEA facility (Huddinge, Sweden).
Raw sequencing data from Nextseq 2000 (Bcl files) were converted and demultiplexed to fastq files using the bcl2fastq v2.20.0.422 program. The STAR 2.7.9a program (Dobin et al. 2013) was used for alignment with Schizosaccharomyces pombe reference genome (ASM294v2) and Drosophila melanogaster reference genome (dm6). We used Drosophila spike in normalization strategy for ChIP-seq data normalization described in (Egan et al. 2016). Samtools was used to count the reads in specific regions, and then we normalized the reads by following spike-in normalization strategy. Data were visualized with the Integrated Genomics Viewer (IGV). For bar diagrams, Microsoft Excel was used to create bar diagrams with unpaired T-test statistics.
RNA-seq and bioinformatics
To remove rRNA, 3 µg of purified total RNA was treated with Ribominus Eukaryote System v.2 kit (Ambion, Thermo Fisher Scientific). To generate sequencing libraries, a total of 100 ng of rRNA-depleted stocks and Illumina Stranded mRNA Prep Ligation kit (Illumina) were used. To quantify the samples, Qubit (HS dsDNA) was used, and samples were sequenced using an Illumina Nextseq 2000 platform (P3 100 cycle kit, 58 + 58 cycles, paired-end sequencing) at the BEA facility (Huddinge, Sweden) following the manufacturer’s instruction. To normalize samples, ERCC RNA Spike-In Mix 1, dilution 1:100 (Invitrogen, Thermo Fisher Scientific) was added in proportion on the number of vegetative cells in each culture that was used for RNA isolation.
Raw sequencing data from Nextseq 2000 (Bcl files) were converted and demultiplexed to fastq files using the bcl2fastq v2.20.0.422 program. The STAR 2.7.9a program (Dobin et al. 2013) was used to index the Schizosaccharomyces_pombe reference genome (ASM294v2) and the ERCC spike in sequences, and then the resulting fastq files were aligned. The mapped reads were then counted in annotated exons using featureCounts v1.5.1 (Liao et al. 2014). The genome fasta file and annotations (Schizosaccharomyces_pombe.ASM294v2.35.gff3) were obtained from ensembl. The count table from ‘featureCounts’ was imported into the R/Bioconductor program and differential gene expression analysis was performed using the EdgeR package (Robinson et al. 2010). The linear models pipeline of EdgeR was used. For the gene expression analysis, genes that had > 1 count per million in 3 or more samples were used and normalized based only on the ERCC spike in counts using the TMM normalization. To correct for batch effects the second batch with ies2 samples were normalized at T0 with the average ERCC factor from the first batch.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the BEA core facility at Karolinska Institutet, particularly A. Damdimopoulos, for help in processing and analysis of gene expression data. We are grateful for services at the FACS core facility, Department of Haematology and Regenerative Medicine, Karolinska Institutet.
Author contribution
KE and YZ conceived and designed the research project. YZ, SZ, and KE performed the experiments. YZ, SZ, and KE analyzed that data. KE and YZ wrote the manuscript with help from SZ. All authors read and approved the manuscript.
Funding
Open access funding provided by Karolinska Institute. The Swedish Research Council, grant number 2021–02238, and Cancerfonden, grant number CAN 2021/1895, funded this research with grants to KE.
Data availability
Yeast strains can be requested by writing to KE. The RNA-seq data and the ChIP-seq data have been submitted to the NCBI Gene Expression Omnibus (GEO) under the accession number GSE200378. The processed ERCC normalized RNA-seq data is provided in Supplementary data excel file 1.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethics approval is not required for yeast research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Batut PJ, Bing XY, Sisco Z, Raimundo J, Levo M, Levine MS. Genome organization controls transcriptional dynamics during development. Science. 2022;375(6580):566–570. doi: 10.1126/science.abi7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan L, Durand-Dubief M, Roguev A, Sakalar C, Wilhelm B, Strålfors A, Shevchenko A, Aasland R, Shevchenko A, Ekwall K, Francis Stewart A. The Schizosaccharomyces pombe JmjC-protein, Msc1, prevents H2A.Z localization in centromeric and subtelomeric chromatin domains. PLoS Genet. 2009;5(11):e1000726. doi: 10.1371/journal.pgen.1000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Yuan C-C, Craske ML, Labhart P, Guler GD, Arnott D, et al. An alternative approach to ChIP-Seq normalization enables detection of genome-wide changes in histone H3 lysine 27 trimethylation upon EZH2 inhibition. PLoS ONE. 2016;11(11):e0166438. doi: 10.1371/journal.pone.0166438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall, K, Thon G (2017) Setting up Schizosaccharomyces pombe Crosses/Matings. Cold Spring Harb Protoc (7). 10.1101/pdbprot091694 [DOI] [PubMed]
- Harris MA, Rutherford KM, Hayles J, Lock A, Bähler J, Oliver SG, Mata J, Wood V (2022) Fission stories: using PomBase to understand Schizosaccharomyces pombe biology. Genetics 220(4). 10.1093/genetics/iyab222 [DOI] [PMC free article] [PubMed]
- Hogan CJ, Aligianni S, Durand-Dubief M, Persson J, Will WR, Webster J, Wheeler L, Mathews CK, Elderkin S, Oxley D, Ekwall K, Varga-Weisz PD. Fission yeast Iec1-ino80-mediated nucleosome eviction regulates nucleotide and phosphate metabolism. Mol Cell Biol. 2010;30(3):657–674. doi: 10.1128/MCB.01117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Maestroni L, Reyes C, Vaurs M, Gachet Y, Tournier S, Géli V, Coulon S. Nuclear envelope attachment of telomeres limits TERRA and telomeric rearrangements in quiescent fission yeast cells. Nucleic Acids Res. 2020;48(6):3029–3041. doi: 10.1093/nar/gkaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerat S, Schmidt A, Codlin S, Chen W, Aebersold R, Bähler J. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell. 2012;151(3):671–683. doi: 10.1016/j.cell.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Lyne R, Burns G, Bähler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet. 2002;32(1):143–147. doi: 10.1038/ng951. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Fudenberg G, Mehta S, Belton JM, Taneja N, Folco HD, FitzGerald P, Dekker J, Mirny L, Barrowman J, Grewal SIS. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature. 2014;516(7531):432–435. doi: 10.1038/nature13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann FR, Dion V, Gehlen LR, Tsai-Pflugfelder M, Schmid R, Taddei A, Gasser SM. Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev. 2012;26(4):369–383. doi: 10.1101/gad.176156.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya E, Durand-Dubief M, Cohen A, Maksimov V, Schurra C, Nakayama JI, Weisman R, Arcangioli B, Ekwall K. Leo1 is essential for the dynamic regulation of heterochromatin and gene expression during cellular quiescence. Epigenetics Chromatin. 2019;12(1):45. doi: 10.1186/s13072-019-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144(2):200–213. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Ortiz M, Saiardi A, Walla E, Jakopec V, Künzel NA, Span I, Vangala A, Fleig U. Asp1 bifunctional activity modulates spindle function via controlling cellular Inositol pyrophosphate levels in Schizosaccharomyces pombe. Mol Cell Biol. 2018;38(9):e00047–18. doi: 10.1128/MCB.00047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli J, Gasser SM, Papamichos-Chronakis M (2017) The INO80 remodeller in transcription, replication and repair. Philos Trans R Soc Lond B Biol Sci 372(1731). 10.1098/rstb.2016.0290 [DOI] [PMC free article] [PubMed]
- Ranjan A, Nguyen VQ, Liu S, Wisniewski J, Kim JM, Tang X, Mizuguchi G, Elalaoui E, Nickels TJ, Jou V, English BP, Zheng Q, Luk E, Lavis LD, Lionnet T, Wu C (2020) Live-cell single particle imaging reveals the role of RNA polymerase II in histone H2A.Z eviction. Elife (9):e5567. 10.7554/eLife.55667 [DOI] [PMC free article] [PubMed]
- Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32(9):896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romila CA, Townsend S, Malecki M, Kamrad S, Rodríguez-López M, Hillson O, Cotobal C, Ralser M, Bähler J. Barcode sequencing and a high-throughput assay for chronological lifespan uncover ageing-associated genes in fission yeast. Microb Cell. 2021;8(7):146–160. doi: 10.15698/mic2021.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajiki K, Hatanaka M, Nakamura T, Takeda K, Shimanuki M, Yoshida T, Hanyu Y, Hayashi T, Nakaseko Y, Yanagida M. Genetic control of cellular quiescence in S. pombe. J Cell Sci. 2009;122(Pt 9):1418–1429. doi: 10.1242/jcs.046466. [DOI] [PubMed] [Google Scholar]
- Sajiki K, Tahara Y, Uehara L, Sasaki T, Pluskal T, Yanagida M. Genetic regulation of mitotic competence in G(0) quiescent cells. Sci Adv. 2018;4(8):eaat5685. doi: 10.1126/sciadv.aat5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan CM, Bao K, Diedrich J, Chen X, Lu C, Yates JR, 3rd, Jia S. The INO80 complex regulates epigenetic inheritance of heterochromatin. Cell Rep. 2020;33(13):108561. doi: 10.1016/j.celrep.2020.108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Xiao H, Ranallo R, Wu WH, Wu C. Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science. 2003;299(5603):112–114. doi: 10.1126/science.1078068. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Roguev A, Schaft D, Buchanan L, Habermann B, Sakalar C, Thomas H, Krogan NJ, Shevchenko A, Stewart AF. Chromatin central: towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome Biol. 2008;9(11):R167. doi: 10.1186/gb-2008-9-11-r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Shukla M, White SA, Lafos M, Tong P, Auchynnikava T, Spanos C, Rappsilber J, Pidoux AL, Allshire RC. Hap2-Ino80-facilitated transcription promotes de novo establishment of CENP-A chromatin. Genes Dev. 2020;34(3–4):226–238. doi: 10.1101/gad.332536.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich B, Strålfors A, Khorosjutina O, Persson J, Smialowska A, Javerzat JP, Ekwall K. The Fun30 chromatin remodeler Fft3 controls nuclear organization and chromatin structure of insulators and subtelomeres in fission yeast. PLoS Genet. 2015;11(3):e1005101. doi: 10.1371/journal.pgen.1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strålfors A, Walfridsson J, Bhuiyan H, Ekwall K. The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet. 2011;7(3):e1001334. doi: 10.1371/journal.pgen.1001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Chen C, Huang L, Yan J, Huang Y. Schizosaccharomyces pombe homologs of human DJ-1 are stationary phase-associated proteins that are involved in autophagy and oxidative stress resistance. PLoS ONE. 2015;10(12):e0143888. doi: 10.1371/journal.pone.0143888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, Takahashi K. Differential regulation of repeated histone genes during the fission yeast cell cycle. Nucleic Acids Res. 2007;35(10):3223–3237. doi: 10.1093/nar/gkm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Yoshida T, Kikuchi S, Nagao K, Kokubu A, Pluskal T, Villar-Briones A, Nakamura T, Yanagida M. Synergistic roles of the proteasome and autophagy for mitochondrial maintenance and chronological lifespan in fission yeast. Proc Natl Acad Sci USA. 2010;107(8):3540–3545. doi: 10.1073/pnas.0911055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhang L, Ruan H, Li G. Histone variant H2A.Z regulates nucleosome unwrapping and CTCF binding in mouse ES cells. Nucleic Acids Res. 2020;48(11):5939–5952. doi: 10.1093/nar/gkaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Zhang H, Zhao F, Zhao T, Li H, Jiang D. The INO80 chromatin remodeling complex promotes thermomorphogenesis by connecting H2A.Z eviction and active transcription in Arabidopsis. Mol Plant. 2021;14(11):1799–1813. doi: 10.1016/j.molp.2021.07.001. [DOI] [PubMed] [Google Scholar]
- Yoo S, Lee EJ, Thang NX, La H, Lee H, Park C, Han DW, Uhm SJ, Song H, Do JT, Choi Y, Hong K. INO80 is required for the cell cycle control, survival, and differentiation of mouse ESCs by transcriptional regulation. Int J Mol Sci. 2022;23(23):15402. doi: 10.3390/ijms232315402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi Y, Durand-Dubief M, Ekwall K. High-Throughput FlowCytometry Combined with Genetic Analysis Brings New Insights into the Understanding of Chromatin Regulation of Cellular Quiescence. Int J Mol Sci. 2020;21(23):9022. doi: 10.3390/ijms21239022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Dai B, Wu H, Zhu W, Chen J. Ino80 is required for H2A.Z eviction from hypha-specific promoters and hyphal development of Candida albicans. Mol Microbiol. 2022;118(1–2):92–104. doi: 10.1111/mmi.14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Wang SS, Xu LX, Meng FL, Xuan YJ, Duan YM, Wang JY, Hu H, Dong X, Ding J, Zhou JQ. SWR1 complex poises heterochromatin boundaries for antisilencing activity propagation. Mol Cell Biol. 2010;30(10):2391–2400. doi: 10.1128/MCB.01106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Yeast strains can be requested by writing to KE. The RNA-seq data and the ChIP-seq data have been submitted to the NCBI Gene Expression Omnibus (GEO) under the accession number GSE200378. The processed ERCC normalized RNA-seq data is provided in Supplementary data excel file 1.