Abstract
Purpose
To evaluate the 1-year efficacy, durability, and safety of faricimab versus aflibercept in patients with neovascular age-related macular degeneration (nAMD) enrolled in the Japan subgroup of the TENAYA trial.
Study design
TENAYA (NCT03823287) was a global, phase 3, multicenter, randomized, active comparator–controlled, double-masked, noninferiority, parallel-group, 112-week trial. After completion of global enrollment, additional patients were enrolled in the Japan extension of TENAYA.
Methods
Treatment-naïve patients aged ≥ 50 years with nAMD were randomized (1:1) to intravitreal faricimab 6 mg up to every 16 weeks (Q16W) after 4 initial Q4W doses based on disease activity at weeks 20 and 24 or aflibercept 2 mg Q8W after 3 initial Q4W doses. Primary endpoint was mean change in best-corrected visual acuity (BCVA) from baseline averaged over weeks 40, 44, and 48. Anatomical/durability outcomes were assessed.
Results
Overall, 133 patients were included in the TENAYA Japan subgroup analysis (faricimab, n = 66; aflibercept, n = 67). The adjusted mean (95% confidence interval) BCVA changes were + 7.1 (4.6‒9.7) and + 7.7 (5.2‒10.1) letters in the faricimab and aflibercept treatment groups, respectively. At week 48, 66.1%, 22.6%, and 11.3% of patients in the faricimab group were on Q16W, Q12W, Q8W and dosing intervals, respectively. Ocular adverse event rates were similar between treatment groups (faricimab, n = 14 [21.2%] versus aflibercept, n = 17 [25.4%]).
Conclusion
The TENAYA Japan subgroup analysis showed that faricimab up to Q16W had sustained efficacy with an acceptable safety profile. These findings are consistent with the global TENAYA and LUCERNE findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10384-023-00985-w.
Keywords: Angiopoietin-2, Anti-VEGF therapy, Choroidal neovascularization, Faricimab, Neovascular age-related macular degeneration
Introduction
Neovascular age-related macular degeneration (nAMD) is a common cause of vision impairment and blindness among the elderly in Japan [1], with a prevalence of approximately 0.5% [1]. The number of patients with nAMD in Japan has increased by more than 300% between 2005 and 2013 and is expected to continue to rise [2]. As a result, new treatment options are required.
Anti-vascular endothelial growth factor (VEGF) therapy, administered through regular intravitreal injections [3], is currently the first-line treatment for nAMD in Japan [4]. However, initial improvements in vision with anti-VEGF therapy are not always maintained long term in clinical practice [5–7]. This is likely, at least partially, the result of undertreatment and nonpersistence due to the burden of managing nAMD, such as requiring frequent injections and hospital visits for monitoring [6, 8–11].
Some currently used anti-VEGF treatments for nAMD have longer dosing intervals and, thus, the potential to reduce treatment burden. Although such treatments have a low risk for intraocular inflammation (IOI) [12, 13], there is evidence suggesting that Japanese patients may have an increased risk of IOI when treated with certain anti-VEGF agents [14]. New therapeutic approaches, with acceptable safety profiles and long dosing intervals, are therefore needed to further reduce the treatment burden and improve long-term efficacy in Japanese patients with nAMD. Novel agents that target other pathways involved in nAMD, in addition to VEGF, may be beneficial.
Faricimab is the first humanized, bispecific, immunoglobulin G monoclonal antibody designed for intraocular use via intravitreal injection [15, 16]. In contrast to the current standard of care anti-VEGF treatments, faricimab targets 2 pathways by independently binding and neutralizing both angiopoietin-2 (Ang-2) and VEGF-A. Anti–Ang-2 stabilizes vessels, reduces vascular leakage, and reduces inflammation, whereas anti–VEGF-A reduces vascular leakage and inhibits neovascularization [15]. Engineered by using 2 different antigen-binding domains with CrossMAb technology, faricimab has a reduced systemic half-life and, in turn, decreases the likelihood of inflammatory side effects [16].The efficacy of faricimab in nAMD was demonstrated in the pivotal phase 3 TENAYA (NCT0382328) and LUCERNE (NCT0382330) trials [17]. Data collected from phase 2 STAIRWAY (NCT03038880) and AVENUE (NCT02484690) trials show that after 4 consecutive doses of faricimab 6.0 mg every 4 weeks in the induction period, efficacy was similar to that of ranibizumab, despite longer dosing intervals [18, 19]. Based on these results, 4 consecutive initiation doses of 6.0 mg faricimab were administered every 4 weeks, and then every 8, 12, or 16 weeks in the maintenance phase at the most appropriate intervals based on individual nAMD symptoms. Data from the phase 3 global trials shows that best-corrected visual acuity (BCVA) changes from baseline with faricimab dosed up to every 16 weeks (Q16W) were non-inferior to aflibercept Q8W. Further, anatomical outcomes and rates of ocular adverse events were similar between faricimab up to Q16W and aflibercept Q8W. Based on these findings and those from the corresponding YOSEMITE/RHINE phase 3 trials in patients with diabetic macular edema [20], faricimab has been approved for the treatment of nAMD and diabetic macular edema in several countries in North America (including the United States), Europe, and Asia Pacific as of June 2022.
Here we present findings on the 1-year efficacy, durability, and safety of faricimab for the TENAYA Japan subgroup.
Subjects and methods
Study design
The study design for TENAYA and identically designed LUCERNE have been described in full previously [17]. TENAYA (NCT03823287) and LUCERNE (NCT03823300) are global, phase 3, multicenter, randomized, active comparator–controlled, double-masked, noninferiority, parallel-group, 112-week trials conducted at 271 clinical sites worldwide (TENAYA 149 sites in 15 countries, LUCERNE 122 sites in 20 countries). A total of 41 TENAYA sites in Japan enrolled patients.
After global enrollment was completed, additional patients were enrolled in a Japan extension of TENAYA to ensure that total enrollment was sufficient to support faricimab registration in Japan. The Japan extension began shortly after the conclusion of the TENAYA global enrollment. All patients in the Japan subgroup (including those in the Japan extension) were enrolled at registered TENAYA sites in Japan and all were Japan residents. All patients from Japan enrolled in the global enrollment phase were included in the TENAYA primary analyses. Per the study protocol, data from patients enrolled in the Japan extension were not included in the primary analyses.
Study protocols were approved by appropriate regulatory authorities, applicable.
institutional review boards, and ethics committees. Studies were conducted in accordance with the Declaration of Helsinki and principles of Good Clinical Practice.
Study population
The eligibility criteria for TENAYA and LUCERNE have been described in full previously [17]. In brief, key eligibility criteria were age ≥ 50 at randomization; presence of treatment-naïve choroidal neovascularization (CNV; also referred to as macular neovascularization [21]) secondary to nAMD; subfoveal CNV or juxtafoveal or extrafoveal CNV, with subfoveal component related to CNV activity, confirmed on fluorescein angiography, and CNV exudation confirmed on spectral-domain optical coherence tomography (SD-OCT); CNV lesion size of ≤ 9 disc areas and CNV component area ≥ 50% of total lesion area; and Early Treatment Diabetic Retinopathy Study BCVA 78 − 24 letters, inclusive (20/32–20/320 approximate Snellen equivalent). All participants provided written informed consent.
Randomization and masking
Patients were randomized in a 1:1 ratio to faricimab up to Q16W or aflibercept Q8W using identification numbers assigned through an interactive voice- or web-based response system. To preserve masking, all patients attended study visits every 4 weeks and received sham injections at non-active dosing visits. Further details about randomization and masking have been described previously [17].
Treatment protocol
The treatment protocols for TENAYA and LUCERNE have been described previously (Online Resource 1) [17]. From day 1, patients who were randomly assigned to the faricimab group initially received intravitreal faricimab 6.0 mg Q4W up to week 12 (4 injections) and patients in the aflibercept group received intravitreal aflibercept 2.0 mg Q4W up to week 8 (3 injections), followed by fixed Q8W dosing to study end, without allowing rescue or additional treatments during the study. After the first 4 monthly doses (day 1 and weeks 4, 8, and 12), patients in the faricimab group were assessed at weeks 20 and 24 for protocol-defined disease activity based on structural and functional criteria and treating physician clinical assessment. Patients with active disease at week 20 were treated with faricimab and subsequently continued to receive faricimab on fixed Q8W dosing until week 60. After a second disease activity assessment at week 24, patients with active disease (excluding those already on the Q8W regimen) received faricimab and subsequently continued on fixed Q12W dosing up to week 60. Patients in the faricimab group who did not have active disease at weeks 20 and 24 received faricimab at week 28 and continued a Q16W regimen up to week 60. In year 2 of the study, all patients in the faricimab group received an active dose of faricimab starting at week 60 and will be treated according to a protocol-driven treat-and-extend personalized treatment interval (PTI) regimen. In the PTI regimen, dosing intervals can be extended in 4-week increments or reduced in 4- or 8-week increments to a minimum of Q8W, a maximum of Q16W, or maintained based on disease activity assessments at study drug dosing visits. Study treatment will be administered up to week 108, with the final visit at week 112.
Outcome measures
Outcomes for this analysis were assessed for the TENAYA Japan subgroup and the pooled global TENAYA/LUCERNE trials. The primary efficacy endpoint was the mean change in BCVA from baseline averaged over weeks 40, 44, and 48. The outcome was averaged over the 3 time points to limit the impact of measurement variability and account for differences in time from the last dose received by patients across treatment groups on different dosing intervals. Secondary efficacy endpoints reported herein are the proportion of faricimab-treated patients on up to Q16W, Q12W, and Q8W schedules at week 48 and the change in BCVA over time. Anatomic outcomes reported herein are the change in SD-OCT–measured central subfield thickness (CST) from baseline at primary endpoint visits and over time. Safety endpoints reported include the incidence and severity of ocular adverse events (AEs) and the incidence and severity of non-ocular AEs. For indocyanine green angiography (ICGA) determined classifications for CNV lesions, study sites conducted arbitrary ICGA analyses. The ICGA data collection was performed at the discretion of each site.
Statistical analysis
Sample size calculations for TENAYA and LUCERNE have been described previously [17]. The pre-planned TENAYA Japan subgroup analyses included all patients randomized in the TENAYA trial at sites in Japan and in the TENAYA Japan extension cohort. The Japan subgroup safety analyses included all patients randomized in the TENAYA trial at sites in Japan and in the TENAYA Japan extension cohort who received at least 1 injection of study treatment (faricimab or aflibercept) in the study eye, grouped according to actual treatment received up to week 48.
For the pooled global TENAYA/LUCERNE trials, the efficacy analyses included all patients randomized in the TENAYA and LUCERNE trials. The pooled global safety analyses included all patients randomized in the TENAYA and LUCERNE trials in addition to the Japan extension cohort who received at least 1 injection of study treatment (faricimab or aflibercept) in the study eye, grouped according to actual treatment received up to week 48.
Efficacy endpoints measured on a continuous scale were analyzed using a mixed model for repeated measures. The model was adjusted for treatment group, visit, visit-by-treatment group interaction, baseline variable of interest (continuous), baseline BCVA (≥ 74, 73–55, and ≤ 54 letters), and baseline lower limit of detection (< 33 and ≥ 33 letters). In the pooled global TENAYA/LUCERNE trials, efficacy analyses were additionally adjusted/stratified by region (United States and Canada, Asia, and the rest of the world) and study (TENAYA and LUCERNE). An unstructured covariance structure was used.
Safety was assessed through descriptive summaries of ocular and systemic AEs, deaths, and ocular assessments up to week 52. AEs were coded per the Medical Dictionary for Regulatory Activities, version 24.0.
For the primary analysis, COVID-19-related intercurrent events (study treatment discontinuation, use of any prohibited systemic treatment or therapy in the study eye, missed dose or doses with potential impact on efficacy [i.e., immediately preceding and at primary endpoint visits], or death) were handled using a hypothetical strategy where all values were censored after the intercurrent event. For intercurrent events, such as study treatment discontinuation due to AEs or lack of efficacy or use of prohibited systemic treatment or prohibited therapy in study eye not due to COVID-19, a treatment policy strategy was applied, where all observed values were used regardless of occurrence of the intercurrent event.
No formal statistical testing of the comparison between faricimab and the active comparator, aflibercept, was performed for the Japan subgroup. Efficacy analyses in the TENAYA Japan subgroup and the TENAYA primary analyses were performed using 95.03% confidence intervals (CIs). Consequently, 95% CIs reflect rounding of 95.03%. Efficacy analyses in the pooled TENAYA/LUCERNE trials were performed using 95.00% CIs. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
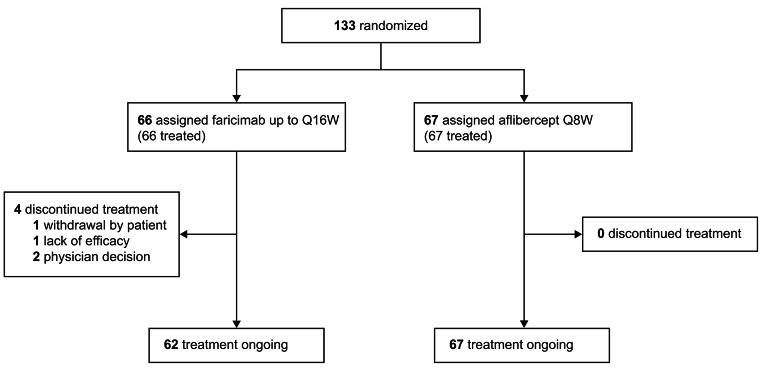
A total of 1329 patients were enrolled in the phase 3 global TENAYA and LUCERNE trials [17]. A total of 133 Japanese patients were enrolled in TENAYA (faricimab, n = 66; aflibercept, n = 67); 52 during global enrollment and 81 during the Japan extension (Fig. 1). In the Japan subgroup, 4 patients discontinued treatment; 1 withdrawal by patient (to prioritize treatment of gastric cancer), 1 lack of efficacy (patient had high CST and wanted to receive regular non-blinded treatment), 2 physician decision (one patient withdrawn due to hospitalization and one patient withdrawn due to treatment non-compliance).
Fig. 1.
Trial profile for the TENAYA Japan subgroup. Q8W every 8 weeks, Q16W every 16 weeks
Baseline demographic and ocular characteristics
Baseline characteristics were generally balanced between treatment groups in the TENAYA Japan subgroup (Table 1). There were lower proportions of women and patients with occult CNV in the faricimab group compared with the aflibercept group. ICGA analyses showed that a greater proportion of patients in the TENAYA Japan subgroup had PCV compared with patients in the pooled global TENAYA/LUCERNE trials.
Table 1.
Baseline demographic and ocular characteristics in the TENAYA Japan subgroup and pooled global TENAYA/LUCERNE trials
| TENAYA Japan | Pooled global TENAYA/LUCERNE | |||
|---|---|---|---|---|
|
Faricimab
up to Q16W (n = 66) |
Aflibercept
Q8W (n = 67) |
Faricimab
up to Q16W (n = 665) |
Aflibercept
Q8W (n = 664) |
|
| Age, years, mean (SD) | 72.1 (7.5) | 71.8 (9.5) | 75.4 (8.5) | 76.4 (8.7) |
| Sex: Female, n (%) | 12 (18.2%) | 22 (32.8%) | 394 (59.2%) | 399 (60.1%) |
| Region, n (%) | ||||
| USA and Canada | 0 | 0 | 317 (47.7%) | 316 (47.6%) |
| Rest of the world | 0 | 0 | 287 (43.2%) | 289 (43.5%) |
| Asia | 66 (100%) | 67 (100%) | 61 (9.2%) | 59 (8.9%) |
| Race, n (%) | ||||
| White | 0 | 0 | 581 (87.4%) | 572 (86.1%) |
| Asian | 66 (100%) | 67 (100%) | 64 (9.6%) | 62 (9.3%) |
| American Indian or Alaska Native | 0 | 0 | 2 (0.3%) | 2 (0.3%) |
| Black or African American | 0 | 0 | 2 (0.3%) | 8 (1.2%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 0 | 0 | 61 (9.2%) | 72 (10.8%) |
| BCVA, ETDRS letters, mean (SD) | 59.1 (13.1) | 59.9 (13.2) | 60.0 (13.3) | 60.2 (13.1) |
| BCVA categories, n (%) | ||||
| ≥ 74 (20/32 or better) | 7 (10.6%) | 8 (11.9%) | 92 (13.8%) | 91 (13.7%) |
| 73–55 (between 20/80–20/40) | 38 (57.6%) | 38 (56.7%) | 381 (57.3%) | 384 (57.8%) |
| ≤ 54 (20/80 or worse) | 21 (31.8%) | 21 (31.3%) | 192 (28.9%) | 189 (28.5%) |
| CST (ILM-RPE), µm, mean (SD) | 354.1 (133.6) | 335.8 (142.6) | 356.8 (122.1) | 357.5 (119.4) |
| Presence of IRF, n (%) | 24 (36.4%) | 19 (28.4%) | 288 (43.3%) | 311 (46.8%) |
| Presence of SRF, n (%) | 51 (77.3%) | 50 (74.6%) | 437 (65.7%) | 447 (67.3%) |
| CNV location by FFA, n (%) | ||||
| Subfoveal | 36 (54.5%) | 30 (44.8%) | 410 (61.7%) | 377 (56.8%) |
| Juxtafoveal | 16 (24.2%) | 22 (32.8%) | 161 (24.2%) | 172 (25.9%) |
| Extrafoveal | 13 (19.7%) | 15 (22.4%) | 83 (12.5%) | 99 (14.9%) |
| Missing/Not Done | 1 (1.5%) | 0 | 11 (1.7%) | 16 (2.4%) |
| CNV lesion type by FFA, n (%) | ||||
| Predominantly classica | 13 (19.7%) | 10 (14.9%) | 205 (30.8%) | 217 (32.7%) |
| Minimally classic | 3 (4.5%) | 3 (4.5%) | 62 (9.3%) | 61 (9.2%) |
| Occult/ | 25 (37.9%) | 36 (53.7%) | 348 (52.3%) | 314 (47.3%) |
| Missing/Not done/Otherb | 25 (37.9%) | 18 (26.9%) | 50 (7.5%) | 72 (10.8%) |
| Total Area of CNV lesion by FFA, mm2, mean (SD) | 4.3 (4.4) | 5.6 (6.7) | 4.7 (4.8) | 4.4 (4.2) |
| PCV status per ICGA, n (%)c | 15 (31.3%) | 7 (18.9%) | 8 (4.8%) | 8 (5.6%) |
aIncludes classic CNV lesion types; bOther includes PCV and RAP; cFor the TENAYA Japan subgroup: faricimab, n = 48; aflibercept, n = 37; for the pooled global TENAYA/LUCERNE trials: faricimab, n = 167; aflibercept, n = 144
BCVA best-corrected visual acuity, CNV choroidal neovascularization, CST central subfield thickness, ETDRS Early Treatment Diabetic Retinopathy Study, FFA fundus fluorescein angiography, ICGA Indocyanine green angiography, ILM internal limiting membrane, IRF intraretinal fluid, PCV polypoidal choroidal vasculopathy, Q8W every 8 weeks, Q16W every 16 weeks, RAP retinal angiomatous proliferation, RPE retinal pigment epithelium, SD standard deviation, SRF subretinal fluid
Primary efficacy endpoint
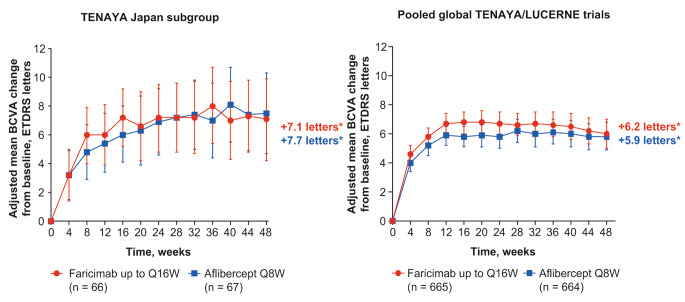
In the TENAYA Japan subgroup, the mean change from baseline in BCVA, averaged over weeks 40, 44, and 48 with faricimab up to Q16W, was similar to the mean change from baseline in BCVA with aflibercept. The adjusted mean (95% CI) BCVA change was + 7.1 letters (4.6 to 9.7) and + 7.7 letters (5.2 to 10.1) in the faricimab and aflibercept groups, respectively (Fig. 2a); the difference in adjusted means was − 0.5 letters (–4.1 to 3.0).
Fig. 2.
Adjusted mean change in best-corrected visual acuity (BCVA; Early Treatment Diabetic Retinopathy Study [ETDRS] letters) from baseline up to week 48 in the a TENAYA Japan subgroup and b pooled global TENAYA/LUCERNE trials
aAdjusted mean BCVA change from baseline averaged over weeks 40, 44, and 48
Results are based on mixed model for repeated measures (MMRM) analysis; missing data were implicitly imputed by MMRM. Error bars represent 95.03% confidence intervals (CIs) for the TENAYA Japan subgroup and 95% CIs for the pooled global TENAYA/LUCERNE trials. ITT intention-to-treat, Q8W every 8 weeks, Q16W every 16 weeks
In the pooled global TENAYA/LUCERNE trials, the adjusted mean (95% CI) BCVA change was + 6.2 letters (5.3 to 7.1) and + 5.9 letters (5.0 to 6.7) in faricimab and aflibercept groups, respectively (Fig. 2b); the difference in adjusted means was + 0.4 letters (–0.9 to 1.6).
Secondary efficacy endpoints
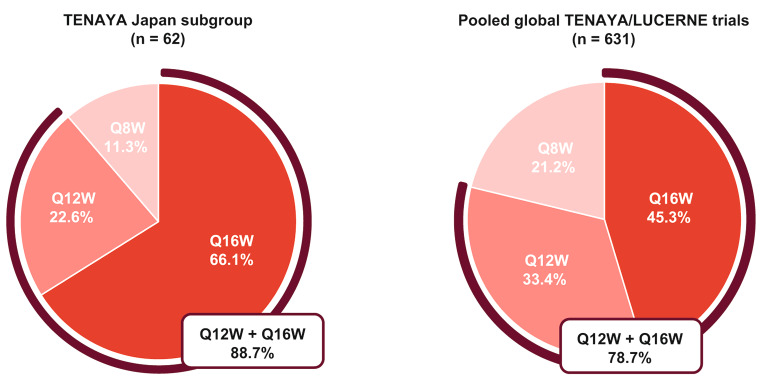
The proportions of patients in the faricimab group on Q16W, Q12W, and Q8W dosing intervals in the TENAYA Japan subgroup were 66.1% (41/62), 22.6% (14/62), and 11.3% (7/62), respectively. At week 48, 88.7% (55/62) of faricimab-treated patients were on Q12W or Q16W dosing intervals (Fig. 3a).
Fig. 3.
Proportions of patients in the faricimab group who completed week 48 on every-16-week (Q16W), every-12-week (Q12W), and every-8-week (Q8W) fixed-dosing intervals in the a TENAYA Japan subgroup and b pooled global TENAYA/LUCERNE trials
Percentages are based on number of patients randomly assigned to the faricimab group who had not discontinued the study at week 48. Treatment interval at week 48 is defined as the treatment interval decision followed at that visit. Red lines indicate the proportion of faricimab-treated patients on Q12W or Q16W dosing intervals at week 48. ITT intention-to-treat
The proportions of patients in the faricimab group on Q16W, Q12W, and Q8W dosing intervals in the pooled global TENAYA/LUCERNE trials were 45.3% (286/631), 33.4% (211/631), and 21.2% (134/631), respectively. At week 48, 78.8% (497/631) of faricimab-treated patients were on Q12W or Q16W dosing intervals (Fig. 3b).
Initial BCVA gains were sustained up to week 48 and were similar between treatment groups in the TENAYA Japan subgroup and the pooled global TENAYA/LUCERNE trials (Fig. 2).
Anatomic outcomes
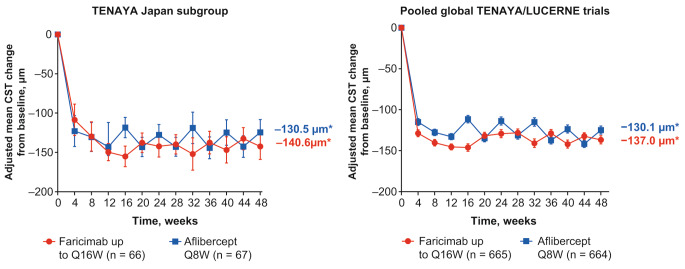
In the TENAYA Japan subgroup, the mean change from baseline in CST averaged over weeks 40, 44, and 48 with faricimab up to Q16W was similar to the mean change from baseline in CST with aflibercept. The adjusted mean (95% CI) CST change was − 140.6 μm (− 154.5 to − 126.7) and − 130.5 μm (− 144.1 to − 117.0) in the faricimab and aflibercept groups, respectively (Fig. 4a).
Fig. 4.
Adjusted mean change in central subfield thickness (CST) from baseline up to week 48 in the a TENAYA Japan subgroup and b pooled global TENAYA/LUCERNE trials. aAdjusted mean CST change from baseline averaged over weeks 40, 44, and 48. Results are based on mixed model for repeated measures (MMRM) analysis; missing data were implicitly imputed by MMRM. Error bars represent 95.03% confidence intervals (CIs) for the TENAYA Japan subgroup and 95% CIs for the pooled global TENAYA/LUCERNE trials. ITT intention-to-treat, Q8W every 8 weeks, Q16W every 16 weeks
In the pooled global TENAYA/LUCERNE trials, the adjusted mean (95% CI) CST change was − 137.0 μm (–141.2 to − 132.9) and − 130.1 μm (–134.2 to − 125.9) in the faricimab and aflibercept groups, respectively (Fig. 4b).
Initial CST reductions were sustained up to week 48 for patients in the faricimab up to Q16W group in both the TENAYA Japan subgroup and the pooled global TENAYA/LUCERNE trials (Fig. 4).
Safety and tolerability measures
Faricimab up to Q16W was well tolerated through week 52 (Table 2). No patients in the TENAYA Japan subgroup withdrew from the study due to an AE. The mean/median number of study drug administrations was 6.2/6.0 injections in the faricimab group, and 7.9/8.0 injections in the aflibercept group. These are consistent with the mean/median faricimab and aflibercept groups in the pooled global TENAYA/LUCERNE (6.6/6.0 injections and 7.6/8.0 injections), respectively. The most common ocular AEs were conjunctivitis allergic and nAMD (3.0% in both treatment groups). There was a low incidence of serious ocular AEs and IOI in both treatment groups, and no cases of endophthalmitis, retinal vasculitis, retinal occlusive events, or retinal pigment epithelial tear were reported. There were 65.2% (43/66) non-ocular AEs reported in the faricimab group and 52.2% (35/67) reported in the aflibercept group. There were no adjudicated Anti-Platelet Trialists’ Collaboration events or deaths.
Table 2.
Summary of AEs in the TENAYA Japan subgroup and pooled global TENAYA/LUCERNE trials
| TENAYA Japan | Pooled global TENAYA/LUCERNE | |||
|---|---|---|---|---|
| Faricimab up to Q16W (n = 66) |
Aflibercept Q8W (n = 67) |
Faricimab up to Q16W (n = 704) |
Aflibercept Q8W (n = 703) |
|
| Total number of AEsa | 158 | 102 | 1897 | 1863 |
| Total number of SAEsa | 14 | 8 | 134 | 207 |
| Patients with any ocular AE, n (%)b | 14 (21.2%) | 17 (25.4%) | 272 (38.6%) | 265 (37.7%) |
| Patients with any ocular SAE, n (%)b | 2 (3.0%) | 2 (3.0%) | 16 (2.3%) | 16 (2.3%) |
| Patients with any treatment-related ocular AE, n (%)b | 2 (3.0%) | 2 (3.0%) | 20 (2.8%) | 19 (2.7%) |
| Patients with any treatment-related ocular SAE, n (%)b | 0 | 1 (1.5%) | 8 (1.1%) | 3 (0.4%) |
| Patients with any AEs of IOI, n (%)c | 2 (3.0%) | 0 | 15 (2.1%) | 11 (1.6%) |
| Iritis | 1 (1.5%) | 0 | 4 (0.6%) | 2 (0.3%) |
| Uveitis | 1 (1.5%) | 0 | 4 (0.6%) | 2 (0.3%) |
| Keratic precipitates | 0 | 0 | 1 (0.1%) | 1 (0.1%) |
| Vitritis | 0 | 0 | 3 (0.4%) | 1 (0.1%) |
| Iridocyclitis | 0 | 0 | 3 (0.4%) | 1 (0.1%) |
| Chorioretinitis | 0 | 0 | 1 (0.1%) | 0 |
| Postprocedural inflammation | 0 | 0 | 0 | 3 (0.4%) |
| Noninfectious endophthalmitis | 0 | 0 | 0 | 1 (0.1%) |
| Patients with ocular SAE known to be associated with anti-VEGF | ||||
| Endophthalmitis, n (%) | 0 | 0 | 0 | 1 (0.1%) |
| Rhegmatogenous retinal detachment | 1 (1.5%) | 0 | 2 (0.3%) | 0 |
| Retinal tear | 0 | 0 | 0 | 0 |
| Retinal pigment epithelial tear | 0 | 0 | 4 (0.6%) | 0 |
| Intraocular pressure increased | 0 | 0 | 1 (0.1%) | 0 |
| Traumatic cataract | 0 | 0 | 0 | 0 |
| Patients with retinal vasculitis event, n (%) | 0 | 0 | 0 | 0 |
| Patients with retinal occlusive event, n (%) | 0 | 0 | 1 (0.1) | 0 |
| Retinal vein occlusion | 0 | 0 | 0 | 0 |
| Retinal artery occlusion | 0 | 0 | 0 | 0 |
| Retinal artery embolism | 0 | 0 | 1 (0.1%) | 0 |
| Patients with any APTC events, n (%)d | 0 | 0 | 13 (1.8%) | 11 (1.6%) |
aTotal number of AEs and SAEs includes nonocular and ocular events in the study or fellow eye
bOcular AEs and SAEs in the study eye only are presented
cExcluding endophthalmitis
dAPTC events were adjudicated by an external independent committee; all other events were investigator reported
Percentages are based on n in the column headings. Multiple occurrences of the same AE in an individual are counted only once, except for the “Total number of AEs” and “Total number of SAEs” rows, in which multiple occurrences of the same AE are counted separately. Includes AEs with onset up to day 377 (last day of week 52 analysis visit window)
The data aggregation period for TENAYA Japan and pooled global trials is 52 weeks
AE adverse event, APTC Anti-Platelet Trialists’ Collaboration, IOI intraocular inflammation, Q8W every 8 weeks, Q16W every 16 weeks, SAE serious adverse event, VEGF vascular endothelial growth factor
Discussion
This is the first report on the efficacy, durability, and safety of faricimab in Japan subgroup patients with treatment-naïve nAMD. The TENAYA Japan subgroup analysis showed that faricimab had sustained efficacy up to Q16W with an acceptable safety profile. The efficacy of faricimab was similar to that of the aflibercept Q8W fixed regimen, and in keeping with the previously reported finding of the overall TENAYA and LUCERNE analyses that faricimab up to Q16W was non-inferior to aflibercept Q8W for the change from baseline in BCVA [17].
We found that initial BCVA gains in the TENAYA Japan subgroup were sustained in the faricimab treatment group, averaged over weeks 40, 44, and 48. Mean vision gains were similar between the faricimab up to Q16W and aflibercept Q8W treatment groups. Notably, faricimab showed extended durability in the TENAYA Japan subgroup, with more than two-thirds of the faricimab-treated patients on extended fixed treatment intervals of Q16W. Initial CST reductions in the TENAYA Japan subgroup were also sustained for patients in the faricimab up to Q16W arm, averaged over weeks 40, 44, and 48. Reductions in CST from baseline were similar between the faricimab up to Q16W and aflibercept Q8W treatment groups. The vision, durability, and anatomic findings in the TENAYA Japan subgroup were generally consistent with the pooled global TENAYA/LUCERNE trial findings. Interestingly, a higher proportion of patients in the Japan subgroup (66%) were on extended fixed treatment intervals of Q16W compared with patients in the pooled global TENAYA/LUCERNE trials (45%). Several studies report that subtypes of nAMD can affect disease management [22–24] with findings from one study suggesting that there may be a relationship between Ang-2 and PCV phenotype [25]. Indeed, one possible explanation for this difference is the higher proportion of patients with polypoidal choroidal vasculopathy (PCV) in the Japan subgroup compared with the pooled global TENAYA/LUCERNE trials (15/48 [31.3%] versus 8/167 [4.8%]). There was however, a relatively low overall number of patients with PCV at baseline; hence, further investigation is warranted to evaluate this possibility and other potential underlying factor(s). To this end, in-depth analyses on PCV will be conducted in the SALWEEN study, which will evaluate the efficacy, durability, and safety of faricimab in patients with PCV. The results of this study will be important given that baseline characteristics and nAMD subtypes may play a role in understanding how patients’ respond to treatment [26].
The ALTAIR clinical trial examined the effects of a treat-and-extend treatment regimen using aflibercept [27]. The results from the trial show that approximately 60% of patients received injections at least every 12 weeks by the end of the study. In the TENAYA Japan subgroup, the percentage of patients on Q12W or Q16W dosing at week 48 was 88.7%. This suggests that faricimab is a good candidate for long-term durability in the Japanese population. However, we are unable to directly compare the results of TENAYA and ALTAIR studies due to the differences in treatment criteria.
Faricimab was well tolerated in the TENAYA Japan subgroup. Rates of ocular AEs and ocular inflammation were low, and no cases of retinal vasculitis or occlusive retinal vasculitis were reported. Overall, safety findings in the TENAYA Japan subgroup were consistent with the pooled global TENAYA/LUCERNE trial findings.
The TENAYA Japan subgroup analysis has several limitations, including sample size. Also, the effects of choroid thickness were not measured or described in this study and require further evaluation as they may be involved in the development of nAMD [28]. Another limitation was that the short follow-up period at the time of the primary analysis and the long-term efficacy, safety, and durability of faricimab when compared with the current treatments is yet to be assessed. As previously outlined [17], we could not directly compare the durability of faricimab and aflibercept because aflibercept was administered according to a fixed 8-week dosing regimen without the possibility to extend treatment intervals, as in the faricimab group. Consequently, these findings warrant confirmation in real-world clinical settings.
In conclusion, the results from the TENAYA Japan subgroup analysis evaluating the efficacy, durability, and safety of faricimab, dosed up to Q16W, showed that faricimab had vision and anatomical benefits similar to those of aflibercept Q8W, extended durability, and an acceptable safety profile. The TENAYA Japan subgroup findings were also generally consistent with the global TENAYA and LUCERNE findings. Faricimab offers the potential for extended treatment intervals with sustained efficacy in patients with nAMD, as well as associated reductions in treatment burden and improved long-term outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
F. Hoffmann-La Roche Ltd. (Basel, Switzerland), Genentech, Inc. (South San Francisco, California, USA), and Chugai Pharmaceutical Co. (Tokyo, Japan) provided financial support for the study. Funding was provided by Chugai Pharmaceutical Co. for third-party writing assistance, which was provided by Elizabeth Daniel, PhD, of Envision Pharma Group.
Data Sharing
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing this request platform is Vivli. https://vivli.org/ourmember/roche/. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Declarations
Conflict of Interest
R. Mori, Consulting fees (Novartis, Chugai, Boehringer Ingelheim), Payment or honoraria for Writing, speakers bureau (Novartis, Nikon), Payment or honoraria for speakers bureau (Bayer, Santen, Senju, JFC Sales Plan, Chugai); S. Honda, None; F. Gomi, Grants to the author’s institution (Senju, Santen), Consulting fees (Senju), Payment or honoraria for speakers bureau, lectures (Chugai), Payment or honoraria for Speakers bureau (Novartis, Bayer, Santen, Senju), Advisory Board (Senju, Boehringer Ingelheim) ; A. Tsujikawa, Grants or contracts (Santen, Senju, Novartis), Consulting fees (Santen, Senju), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Senju, Novartis, Bayer, Chugai), Payment for expert testimony (Senju, Chugai); H. Koizumi, Payment for lectures (Chugai, Roche); H. Ochi, Employee (Chugai); S. Ohsawa, Employee (Chugai); A. A. Okada, Consulting fees (Chugai, Bayer, Novartis, Apellis, Biocon), Payment or honoraria for Speakers bureau (Chugai, Santen), Payment or honoraria for Writing, speakers bureau (Novartis, Bayer, Senju), Stock or stock options (Hemera Biosciences).
Footnotes
Study group investigators The TENAYA and LUCERNE investigators and study sites are listed at the end of this manuscript.
Corresponding Author: Ryusaburo Mori.
The original publication has been revised due to replacement of Supplementary Material 2.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/8/2023
A Correction to this paper has been published: 10.1007/s10384-023-00996-7
References
- 1.Honda S, Yanagi Y, Koizumi H, Chen Y, Tanaka S, Arimoto M, et al. Impact of neovascular age-related macular degeneration: burden of patients receiving therapies in Japan. Sci Rep. 2021;11:13152. doi: 10.1038/s41598-021-92567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kume A, Ohshiro T, Sakurada Y, Kikushima W, Yoneyama S, Kashiwagi K. Treatment patterns and health care costs for age-related macular degeneration in Japan: an analysis of national insurance claims data. Ophthalmology. 2016;123:1263–8. doi: 10.1016/j.ophtha.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol. 2019;4:e000398. doi: 10.1136/bmjophth-2019-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Iida T, Ishida S, Crawford B, Sakai Y, Mochizuki A, et al. Effectiveness of current treatments for wet age-related macular degeneration in Japan: a systematic review and pooled data analysis. Clin Ophthalmol. 2022;16:531–40. doi: 10.2147/OPTH.S345403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4:19–30. doi: 10.1016/j.oret.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–6. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4:122–33. doi: 10.1016/j.oret.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Mitchell P, Finger RP, Eldem B, Talks SJ, Hirst C, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128:234–47. doi: 10.1016/j.ophtha.2020.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160:725–31e1. doi: 10.1016/j.ajo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Gomi F, Toyoda R, Yoon AH, Imai K. Factors of anti-vascular endothelial growth factor therapy withdrawal in patients with neovascular age-related macular degeneration: implications for improving patient adherence. J Clin Med. 2021;10:3106. doi: 10.3390/jcm10143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanemoto T, Hikichi Y, Kikuchi N, Kozawa T. The impact of different anti-vascular endothelial growth factor treatment regimens on reducing burden for caregivers and patients with wet age-related macular degeneration in a single-center real-world japanese setting. PLoS ONE. 2017;12:e0189035. doi: 10.1371/journal.pone.0189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monés J, Srivastava SK, Jaffe GJ, Tadayoni R, Albini TA, Kaiser PK, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050–9. doi: 10.1016/j.ophtha.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Motevasseli T, Mohammadi S, Abdi F, Freeman WR. Side effects of brolucizumab. J Ophthalmic Vis Res. 2021;16:670–5. doi: 10.18502/jovr.v16i4.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruko I, Okada AA, Iida T, Hasegawa T, Izumi T, Kawai M, et al. Brolucizumab-related intraocular inflammation in japanese patients with age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol. 2021;259:2857–9. doi: 10.1007/s00417-021-05136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regula JT, von Lundh P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8:1265–88. doi: 10.15252/emmm.201505889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108:11187–92. doi: 10.1073/pnas.1019002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 18.Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:964–72. doi: 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahni J, Dugel PU, Patel SS, Chittum ME, Berger B, Del Valle Rubido M, et al. Safety and Efficacy of different doses and regimens of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: the AVENUE phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020;138:955–63. doi: 10.1001/jamaophthalmol.2020.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741–55. doi: 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]
- 21.Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–36. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina. 2020;4:767–76. doi: 10.1016/j.oret.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5:16204. doi: 10.1038/srep16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozawa S, Ishikawa K, Ito Y, Nishihara H, Yamakoshi T, Hatta Y, et al. Differences in macular morphology between polypoidal choroidal vasculopathy and exudative age-related macular degeneration detected by optical coherence tomography. Retina. 2009;29:793–802. doi: 10.1097/IAE.0b013e3181a3b7d9. [DOI] [PubMed] [Google Scholar]
- 25.Inoda S, Takahashi H, Inoue Y, Tan X, Tampo H, Arai Y, et al. Cytokine profiles of macular neovascularization in the elderly based on a classification from a pachychoroid/drusen perspective. Graefes Arch Clin Exp Ophthalmol. 2022;260:747–58. doi: 10.1007/s00417-021-05445-0. [DOI] [PubMed] [Google Scholar]
- 26.Fenner BJ, Cheung CMG, Sim SS, Lee WK, Staurenghi G, Lai TYY, et al. Evolving treatment paradigms for PCV. Eye (Lond) 2022;36:257–65. doi: 10.1038/s41433-021-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37:1173–87. doi: 10.1007/s12325-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Invernizzi A, Benatti E, Cozzi M, Erba S, Vaishnavi S, Vupparaboina KK, et al. Choroidal structural changes correlate with neovascular activity in neovascular age related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:3836–41. doi: 10.1167/iovs.18-23960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing this request platform is Vivli. https://vivli.org/ourmember/roche/. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.