Abstract
Lymph node swelling is a side effect of the mRNA COVID-19 vaccines, a distressing side effect for women treated for breast cancer. The purpose of this study is to present side effects reported by a cohort of patients treated for breast cancer. A survey link was sent to 4945 women who received breast cancer treatment and were prospectively screened for breast cancer-related lymphedema. In total, 621 patients who received an mRNA vaccine and responded to the survey were included in analysis. We assessed the frequency and predictors of side effects. The most frequent side effects reported were injection site soreness, fatigue, generalized muscle soreness, headache, and chills, with median duration ≤ 48 h. Lymph node swelling occurred most often in the axilla ipsilateral to the vaccine. The median duration was 1 week or less after all doses. These data will inform patient education regarding future vaccine doses, including reassurances about which side effects to expect, particularly lymph node swelling which may impact mammograms after vaccination. Type and duration of side effects were similar to that reported by the general population in Phase 3 testing trials of the mRNA vaccines. Clinical Trial Registration NCT04872738 posted May 4, 2021.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-023-01050-z.
Keywords: COVID-19, Vaccines, Breast cancer, Lymphedema, Lymphoedema
Background
Since the introduction of the COVID-19 vaccines, some patients have voiced concerns regarding vaccination, especially vaccine-associated side effects. Side effects, specifically lymph node (LN) swelling associated with the mRNA vaccines, are of particular concern for patients with a history of breast cancer (BC), as lymphadenopathy is also a sign of BC recurrence [1–3]. Research focused specifically on COVID-19 vaccination in women treated for BC is limited but warranted to assuage their unique concerns [4]. Both the Moderna and Pfizer vaccines elicit a strong immune response [5, 6]. In the lymph nodes, white blood cells recognize the antigen from the vaccine and create antibodies to fight the virus. It is this cellular activity that can lead to inflammation and swelling of the lymph nodes, specifically the lymph nodes closest to the injection site [7, 8]. Since vaccines are typically given in the deltoid muscle [3], the lymph nodes that would be most impacted are the axillary and supraclavicular lymph nodes which are also the lymph nodes most closely associated with BC. When an injection is given, this swelling is a sign that the immune system is acting as it should.
For patients with BC and breast cancer-related lymphedema (BCRL), a dysfunction of the lymphatic system, LN swelling can be a concerning side effect. BCRL is a devastating sequelae of BC treatment that affects over 20% of treated women and is caused by fluid buildup after damage (surgery or radiation) to the lymph nodes [10]. BCRL is highly feared in this population [9]. Risk factors for BCRL include axillary lymph node dissection, regional lymph node radiation, and high body mass index (BMI) at time of breast cancer diagnosis [10–16]. Although sentinel lymph node biopsy significantly reduces the risk of BCRL compared to axillary lymph node dissection, the risk is not negligible [10, 14, 16–18]. Patients with BCRL may experience a range of symptoms, including heaviness, pain, tightness, changes in skin quality, and decreased range of motion, and they are at a higher risk for infection in the affected area(s) [19–22]. The morbidity resulting from BCRL is significant and negatively impacts almost all aspects of patients’ lives, contributing to anxiety and depression [23–26].
The Lymphedema Research Program at Massachusetts General Hospital (MGH) prospectively screens patients with BC for BCRL and has screened >6,500 women since 2005 [27] (ClinicalTrials.gov identifier: NCT01521741; institutional review board identifiers: 2008P000540 and 2005P001038). Upon the advent of the COVID-19 vaccines, the program received many calls of concern from patients at MGH and across the country. Patients were concerned about the newly announced side effect of the COVID-19 vaccine and worried that LN swelling may incite or worsen BCRL. We initiated the LymphVAX study to better understand vaccine side effects experienced by patients treated for BC and to provide patient education, increase knowledge, and empower patient self-advocacy. In addition to LN swelling, other side effects of COVID-19 vaccines that may also be experienced secondary to BC treatment include fatigue, generalized muscle soreness (GMS), nausea, and vomiting [28–30]. Due to the inherent activity of mRNA vaccines and the immune system, as well as the role of the lymph nodes in BC and in BCRL, this study analyzed the side effect profile in patients treated for BC and who received up to three doses of the COVID-19 vaccinations [Moderna, Pfizer]. A separate analysis is presented on results from participants who received the Johnson & Johnson (J&J) vaccine as lymph node swelling is not a listed side effect of this non-mRNA vaccine and only a small proportion of the population received this vaccine (n = 49).
Older participants and participants who had received more intense cancer treatment (i.e., radiation, chemotherapy) were hypothesized to experience fewer side effects than younger participants and those with less intense treatment. The former group was anticipated to experience a lower immune response to a vaccine than the latter group due to the natural weakening of the immune system as adults age, and certain cancer treatments which may weaken the immune system temporarily [31–35]. Additionally, more participants were expected to experience side effects with their second dose than after their first dose, as the immune system should recognize the vaccine and initiate a more intense immune response to subsequent doses.
This study aims to assess side effect profiles for patients treated for BC across vaccine doses and whether age and BMI, BC surgery type, axillary surgery, chemotherapy, or radiation therapy influenced side effects experienced after the COVID-19 mRNA vaccinations (Moderna and Pfizer).
Methods
Beginning in April 2021, a survey link was sent to 4,945 women over the age of 18 who had been treated for BC and prospectively screened for BCRL with perometry (Mitaka USA, Inc. Wheat Ridge, CO, USA) by the MGH Lymphedema Research Program. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at MGH, a secure, web-based software platform designed to support data capture for research studies. BC treatment data were collected through medical record review, and additional survey links and phone calls were made as reminders and to obtain booster dose information. The MGH Institutional Review Board approved this study in April 2021 (2021P000528), and data were collected prospectively through June 2022. Recall bias is a potential concern for this study; therefore, data collection ended roughly a year after survey distribution.
Participants were asked about each dose of the vaccine they received. This included the first two doses for the Moderna (mRNA-1273) and Pfizer (BNT162b2) vaccinations along with a third booster dose, if received, or one dose of J&J (Ad26.COV2.S) along with a booster dose, if received. First, participants were asked about vaccine type, education received regarding the vaccine, where on the body the vaccine was received and reasons behind that decision. Second, participants were asked which side effects they experienced and duration of side effects after vaccination. Solicited side effects included: injection site symptoms (soreness, swelling, or redness), symptoms in the arm on the side of injection (swelling, numbness, or heaviness), and generalized symptoms [muscle soreness, feeling tired (fatigue), headache, joint pain, chills, nausea, vomiting, fever, Bell’s palsy], other, or none of the above. Lastly, participants were asked specifically about LN swelling after each dose of the vaccine. LN swelling was defined as “discomfort or noticeable swelling when looking or touching” and questions specified whether this occurred in the axilla (underarm/armpit) or in the supraclavicular region (lower neck/above the collarbone) and laterality of the swelling. While it was not feasible for this study to have a professional examine participants’ lymph nodes, the definitions likely standardized any bias that would be a result of the patient-reported outcome. Survey questions were compiled based on clinician expertise from a certified lymphedema therapist and a breast radiation oncologist. The side effects list was derived from side effects published by Moderna and Pfizer. A patient advocate was consulted for ease of completion of the survey, and ensuring questions were appropriately worded. All survey questions are presented in the supplementary information (Additional File 1).
Statistical Methods
Side effect frequencies and median duration for each mRNA dose were analyzed. To investigate predictors of side effects, multivariable logistic regression models across all doses were fit separately for each side effect, with random effects for participants to account for clustered responses. We investigated age, BMI ( < 25, 25–30, ≥ 30 kg/m2), surgery type (lumpectomy, mastectomy, mastectomy with reconstruction), axillary surgery (none, sentinel lymph node biopsy, axillary lymph node dissection), radiation (yes/no), and chemotherapy (none, neoadjuvant, adjuvant only). Analysis was conducted across all three doses to determine any differences in side effect profiles. Coefficients with p < 0.05 were considered significant predictors of that side effect. Because lymph node swelling is not a listed side effect of the J&J vaccine, and because far fewer patients received that vaccine, we analyzed side effects among those receiving the J&J vaccine separately.
General Population
Data from the LymphVAX study were compared to two randomized, placebo-controlled, phase 3 trials conducted in adults who were healthy or with stable chronic medical conditions for the Pfizer and Moderna vaccines [5, 6]. The Pfizer study enrolled participants over the age of 16 to receive two doses of the BNT162b2 vaccine (n = 21,720) or a placebo (n = 21,728) 21 days apart at 152 sites worldwide, with 8183 participants reporting on reactogenicity up to 7 days after each injection. The Moderna study enrolled participants over the age of 18 to receive two doses of the mRNA-1273 vaccine (n = 15,210) or a placebo (n = 15,210) 28 days apart at 99 centers across the USA, monitoring reactogenicity for 7 days after each injection.
The solicited side effects listed in the general population studies were also solicited in the LymphVAX study, with the addition of swelling, heaviness, and arm numbness in the LymphVAX study as these are symptoms which have been found to be predictive of BCRL [21, 22]. The Pfizer study of the general population did not include LN swelling as a solicited event; however, it was reported as an adverse unsolicited event in 64 participants compared with 6 participants in the placebo group [6]. In contrast, the Moderna study of the general population included lymphadenopathy (LN swelling) as a solicited event, similar to the LymphVAX study. Therefore, LN swelling in patients in the LymphVAX study is compared to the Moderna group in the general population to maximize similarities between study designs.
In addition, an international, randomized, placebo-controlled, phase 3 trial tested the efficacy and safety of the J&J vaccine in the general population over the age of 18. The cohort included 3,356 participants who received the Ad26.COV2.S vaccine and reported on reactogenicity for 7 days after the injection [36].
Results
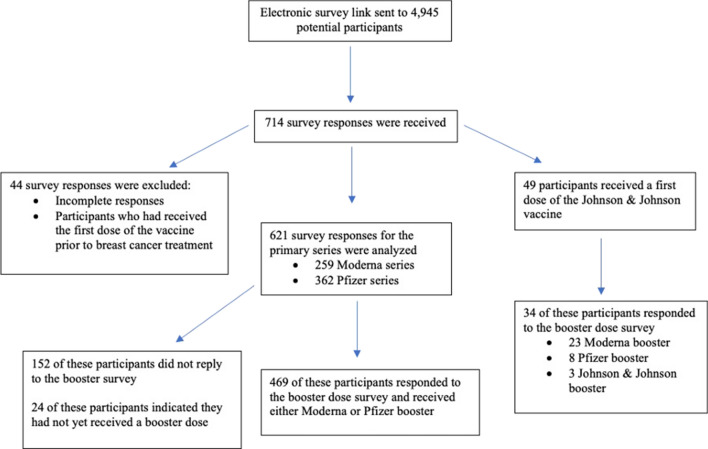
We received 714 survey responses regarding patients’ primary vaccination series (two doses of Pfizer or Moderna and one dose of J&J). We excluded 44 incomplete surveys or surveys from patients who received the first vaccine prior to BC treatment, resulting in data from 670 patients for analysis. For booster dose surveys (either a third dose of Moderna or Pfizer or a second dose of any vaccine after receiving J&J), 503 responses were received from patients who had completed the initial vaccination survey (75%). An additional 24 patients indicated over the phone that they had not yet received a booster dose when the survey was distributed.
Patient Population
In total, 621 patients who received an mRNA vaccine (259 Moderna, 362 Pfizer) and responded to the survey were included in the analysis, and 469 of those participants completed booster dose surveys. In total, 49 participants received the J&J vaccine and 34 of these participants completed booster surveys (23 received a Moderna booster, 8 received a Pfizer booster, 3 received a J&J booster). The primary analysis was restricted to the sample with mRNA primary doses, and we report results for the J&J recipients separately as previously discussed (Fig. 1).
Fig. 1.
Study Population
Demographic and breast cancer-related characteristics for the 621 patients receiving an mRNA primary vaccine series are presented in Table 1. The median age was 53.0 years with a median BMI of 26.0 kg/m2. 43.9% of participants underwent mastectomy, 28.3% underwent axillary lymph node dissection, 57.3% had BMI > 25 at time of BC diagnosis, and 30.3% of participants underwent regional lymph node radiation. The median follow-up time between breast surgery and date of first vaccine dose was 69.0 months.
Table 1.
Demographics and treatment-related characteristics
| Factor | Total Cohort |
|---|---|
| (n = 621) | |
| Number (%) | |
| Age* | 53.0 (26.2, 83.6) |
| BMI* | 26.0 (15.8, 50.4) |
| BMI > 25 | 356 (57.3%) |
| Missing Data | 2 |
| Surgery | |
| Mastectomy with Reconstruction | 222 (35.7%) |
| Mastectomy without Reconstruction | 51 (8.2%) |
| Lumpectomy | 348 (56.0%) |
| Nodal surgery | |
| Axillary Lymph Node Dissection | 176 (28.3%) |
| Sentinel Lymph Node Biopsy | 393 (63.3%) |
| No Nodal Surgery | 52 (8.4%) |
| Regional Lymph Node Radiation | 188 (30.3%) |
| Neoadjuvant ± adjuvant chemotherapy | 109 (17.6%) |
| Adjuvant chemotherapy, only | 196 (31.6%) |
| Time between breast surgery & first dose* | 69.0 months (0.13, 361.5) |
*median (range)
Most Frequently Reported Side Effects
The most frequently reported local side effects were injection site soreness, injection site swelling, injection site redness, LN swelling, and arm heaviness (Figure 2A). The most frequently reported systemic side effects were fatigue, GMS, headache, chills, and joint pain (Figure 2B). Aside from injection site soreness, all systemic side effects were more commonly reported than the other local side effects. After the first, second, and booster doses, 14.3%, 12.1%, and 15.4% of patients reported experiencing no side effects at all.
Fig 2.
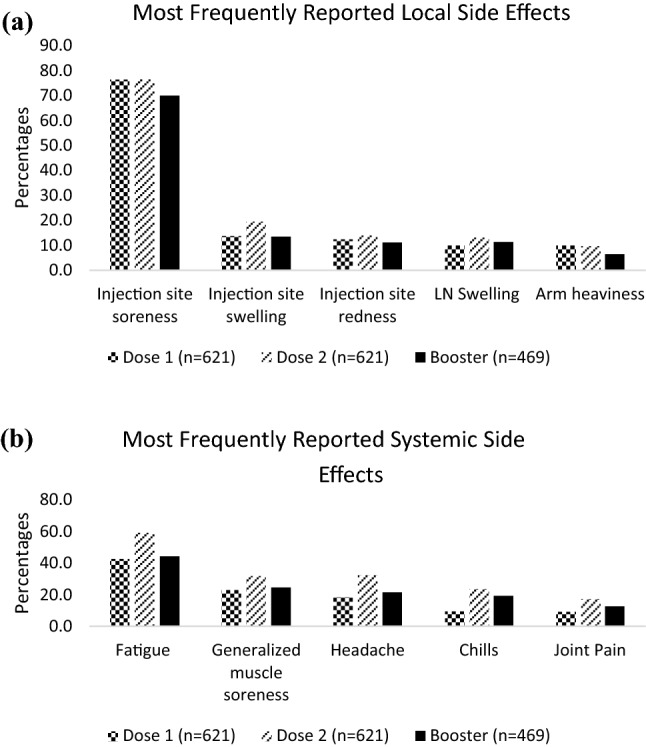
Most Frequently Reported Local (a) and Systemic (b) Side Effects among Participants receiving mRNA Vaccines as a Primary Series (n = 621 Dose 1, n = 621 Dose 2, n = 469 Booster)
Multivariable model results: Systemic side effects
In the multivariable model, fatigue, GMS, headache, chills, and joint pain were reported by a significantly lower percentage of older participants (set linearly with odds ratios at 10-year increments) than younger participants (p < 0.001). These side effects were also all reported significantly more after dose 2 compared with dose 1 (p < 0.001). Headache (p = 0.046), chills (p < 0.001), and joint pain (p = 0.014) were reported significantly more after dose 3 compared with dose 1. Complete results from the multivariable models are presented in the supplementary information (Additional File 2).
Multivariable model results: Local side effects
Injection site soreness was reported significantly less in older participants (p < 0.001) and after dose 3 compared with dose 1 (p = 0.004). Injection site swelling was reported significantly more after dose 2 compared with dose 1 (p < 0.001). Arm heaviness was reported significantly less after dose 3 compared with dose 1 (p < 0.033).
Lymph Node Swelling
After the first, second, and booster doses, 9.8% (61/621), 12.9% (80/621), and 11.3% (53/469) of participants reported LN swelling, respectively. Of note, 54.1% (33/61), 62.5% (50/80), and 71.7% (38/53) of participants who reported LN swelling reported it in the axilla ipsilateral to the vaccine after each dose. LN swelling was also reported in the axilla contralateral to the vaccine [45.9% (28/61) D1, 45.0% (36/80) D2, 24.5% (13/53) D3], supraclavicular region ipsilateral to [29.5% (18/61) D1, 26.3% (21/80) D2, 32.1% (17/53) D3], and contralateral to the vaccine [18.0% (11/61) D1, 20.0% (16/80) D2, 9.4% (5/53) D3] (Figure 3). Next, LN swelling was analyzed in relation to side of vaccine and side of BC and whether the swelling occurred on the affected side. 10.8% (67/621) of participants for the first 2 doses and 13.6% (64/469) of participants who received the booster were excluded from this analysis due to bilateral cancer or receiving a vaccine in the leg. For dose 1, dose 2, and dose 3, respectively, 78% (432/554), 78.5% (435/554), and 81.7% (331/405) of participants received the vaccine contralateral to side of BC treatment. LN swelling was reported on the side opposite to the vaccine on the side of BC treatment by 6.9%, 9.7%, and 3.6% of these participants, respectively. Conversely, 22.0% (122/554), 21.5% (119/554), and 18.3% (74/405) received the vaccine on the side of BC treatment. 11.5%, 14.3%, and 13.5% of these participants had LN swelling on the side of the vaccine and side of BC treatment (Figure 4). The median duration of LN swelling either in the supraclavicular region or the axilla was 1 week or less after all 3 doses. A higher percentage of participants reported longer duration of axillary LN swelling than supraclavicular swelling, although few participants experienced swelling after 1 week.
Fig. 3.
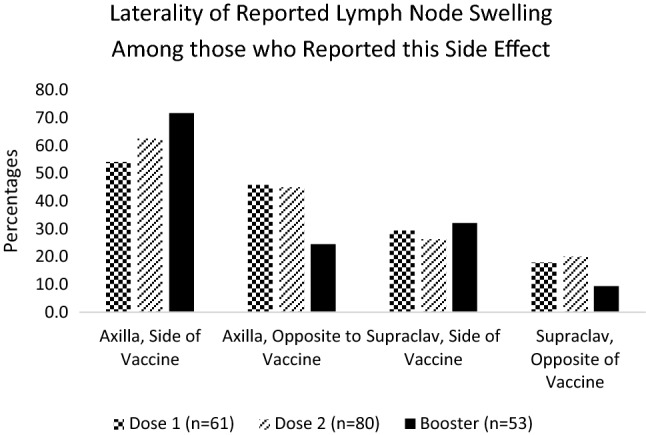
Laterality of Lymph Node Swelling among those who Reported this Side Effect (n = 61 Dose 1, n=80 Dose 2, n=53 Booster)
Fig. 4.
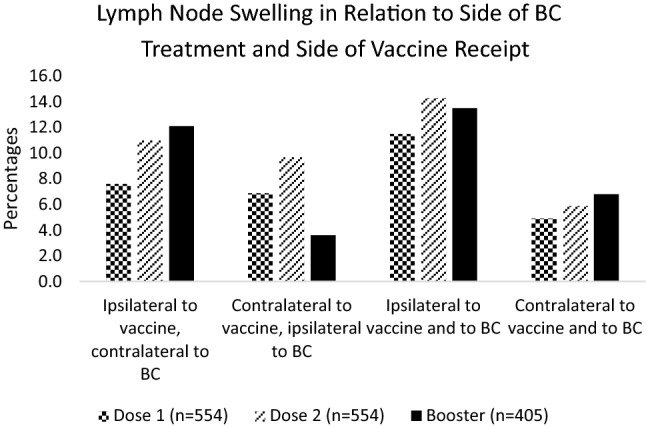
Lymph Node Swelling in Relation to Side of BC Treatment and Side of Vaccine Receipt (n = 554 Dose 1, n = 554 Dose 2, n = 405 Booster)
Side Effect Duration and Progression
The median duration of side effects for all three doses was 48 h or less, with the duration ranging from 24 h or less (41.0% D1, 38.7% D2, 44.1% D3) to more than 4 weeks (2.6% D1, 5.2% D2, 2.5% D3). Of the participants who reported side effects for multiple doses, 32.1% had side effects that lasted the same duration in each dose. 39.2% had side effects for a longer duration in at least one subsequent dose compared to an earlier dose, and 28.7% experienced their longest side effects after the first dose.
General Population
Compared with the general population, fatigue, GMS (new or worsening muscle pain/myalgia), and chills were reported by a similar but slightly higher percentage of the LymphVAX cohort after dose 1 (fatigue: 42.7% vs. 38.1%; GMS 23.0% vs. 21.7%; chills 9.5% vs. 8.8%) [5–8]. Additional comparisons are presented in Table 2. A very similar percentage of patients in the LymphVAX study reported LN swelling (9.8% and 12.9% after dose 1 and 2) to that in the general population (10.2% and 14.0% after dose 1 and 2), although due to study methodology, the data were not suitable for statistical analysis.
Table 2.
Most frequently reported side effects: Comparison of women treated for BC with GP
| Injection site soreness | Fatigue | Generalized muscle soreness (GMS) | Headache | Chills | |
|---|---|---|---|---|---|
| Dose 1 | 76.0% BC | 42.7% BC | 23.0% BC | 18.4% BC | 9.5% BC |
| 82.4% GP | 38.1% GP | 21.7% GP | 33.1% GP | 8.8% GP | |
| Dose 2 | 76.2% BC | 59.3% BC | 31.9% BC | 32.4% BC | 23.7% BC |
| 85.0% GP | 63.2% GP | 52.5% GP | 55.9% GP | 40.8% GP |
*BC = women treated for breast cancer (n = 621 Dose 1, n = 621 Dose 2)
*GP = general population reported by NEJM (n = 19256 Dose 1, n = 17702 Dose 2)
Johnson & Johnson (J&J) Cohort
Of the 49 patients who received the J&J vaccine, 69.4% (34/49) received a booster and responded to that survey. 67.6% (23/34) received Moderna, 23.5% (8/34) received Pfizer, and 8.8% (3/34) received a second dose of J&J. 73.5% reported experiencing any side effect for dose 1, and 70.6% reported experiencing any side effect for dose 2. The five most frequently reported side effects included injection site soreness (63.3% D1, 55.9% D2), fatigue (42.9% D1, 29.4% D2), headache (30.6% D1, 23.5% D2), chills (20.4% D1, 14.7% D2), and GMS (18.4% D1, 14.7% D2). For dose 2, 14.7% of participants experienced a fever. The median duration of side effects for both doses 1 and 2 was 48 h or less, with many participants reporting side effects lasting 24 h or less (44.4% D1, 50% D2). Although LN swelling is not a listed side effect of the J&J vaccine, 8.2% (4/49) of participants in this study reported LN swelling on the side of vaccine (three in the axilla and one in the supraclavicular lymph nodes). Side effects reported by those participants who received the J&J vaccine are presented in Table 3. Injection site soreness and fatigue were more common in women treated for BC who received the J&J vaccine in the LymphVAX study than the general population (63.3% vs. 48.6% injection site soreness; 42.9% vs. 38.2% fatigue); headache and GMS were less common in women who received J&J in the LymphVAX study (30.6% vs. 38.9% headache; 18.4% vs. 33.2% GMS). Due to study methodology, the data were not suitable for statistical analysis.
Table 3.
Johnson & Johnson Cohort – Reported Side Effects
| Side effect reported | Dose 1 (n = 49) (%) | Booster (n = 34) (%) |
|---|---|---|
| Injection site soreness | 63.3 | 55.9 |
| Fatigue | 42.9 | 29.4 |
| Headache | 30.6 | 23.5 |
| Chills | 20.4 | 14.7 |
| Generalized muscle soreness | 18.4 | 14.7 |
| Joint pain | 16.3 | 11.8 |
| Fever | 14.3 | 14.7 |
| Injection site swelling | 10.2 | 5.9 |
| Nausea | 8.2 | 11.8 |
| Injection site redness | 8.2 | 2.9 |
| Lymph node swelling | 8.2 | 2.9 |
| Arm heaviness | 4.1 | 5.9 |
| Arm swelling | 2.0 | 2.9 |
| Other | 2.0 | 0 |
| Vomiting | 0 | 2.9 |
| Arm numbness | 0 | 0 |
| Bell's Palsy | 0 | 0 |
Discussion
When a vaccine is given in the deltoid muscle, LN swelling in the axillary and supraclavicular lymph nodes is a sign that the immune system is responding to the vaccine as it should [37]. However, LN swelling can also raise concerns of cancer progression or recurrence, or that the swelling may incite or worsen lymphedema [1, 3, 38]. The CANVAX study was conducted in multiple cancer centers at MGH and found that patients with a history of or active cancer, including BC, had a lower immune response to the COVID-19 vaccines; however, most still had antigen concentrations and neutralization titers predicted to be protective against illness [39]. In addition, participants in the CANVAX study who reported more systemic symptoms after receiving the vaccines were more likely to have a higher immune response. While it is promising that the COVID-19 vaccine was found to be effective in patients with cancer, side effects such as LN swelling remain a serious concern for this population. The results of the LymphVAX study are therefore imperative to inform patient education regarding side effect profiles for women treated for BC.
Based on the results of this study, the vast majority of women treated for BC may expect to experience at least one side effect after any dose of the mRNA COVID-19 vaccines, most commonly injection site soreness, fatigue, GMS, headache, and chills. Side effects commonly last 24 h or less, with a median of 48 h or less; however, for some participants, the duration of side effects increased in a subsequent dose. Aside from injection site soreness, systemic side effects were much more common than local side effects, including LN swelling and arm swelling, which is promising for women at risk of BCRL.
Older participants reported significantly less fatigue, GMS, headache, chills, joint pain, and injection site soreness, consistent with the hypothesis that participants with weaker immune systems would experience fewer side effects [31–34]. Reports of fatigue, GMS, headache, chills, joint pain, and injection site swelling all significantly increased for the second dose of the vaccine compared to the first dose. This is consistent with the hypothesis that after repeated vaccine doses, the immune system already has the antigens ready to fight the virus; thus, the side effects may worsen as the immune response has increased [40].
General Population
We felt it important to comment on whether the side effect profile looks different in patients who have been treated for BC than in the general population. This information may assist patients in making informed decisions about the COVID-19 vaccine.
It should be noted that although numbers between the two studies are very close, due to study methodology, statistical analysis was not possible. The value that stands out most is that the percentage of patients reporting fatigue after the vaccination was higher in the LymphVAX cohort. Given these participants had endured cancer treatment in the past, it is possible that some could still be experiencing cancer-related fatigue, a known long-term side effect of treatment that can result in functional deficits [29]. This, in combination with the COVID-19 vaccine, may result in higher numbers of participants previously treated for BC reporting fatigue after vaccination. Otherwise, injection site soreness (pain) and headache after dose 1, and all most frequently reported LymphVAX side effects after dose 2 occurred more in the general population than in women treated for BC.
Perhaps the most distressing side effect of COVID-19 vaccination reported by patients who have been treated for BC is LN swelling, which is particularly prominent after COVID-19 vaccinations compared with others as these elicit higher immune responses [4–6]. As previously discussed, this mimics signs of BC recurrence or progression; therefore, this side effect has received great attention from patients treated for BC who have found it difficult to find information or advice to help guide their decisions around COVID-19 vaccination. The Society of Breast Imaging no longer suggests delaying screening mammograms; however, previous recommendation was to receive the first dose of the vaccine after screening mammogram or to delay screening until 4–6 weeks after second dose of the vaccine. Guidelines continue to be updated as information regarding further doses of the COVID-19 vaccines becomes available [41]. Additionally, MGH guidelines suggest that patients with BC receive the vaccine in the arm opposite to BC (i.e., without node removal), or in the case of bilateral cancer, in the thigh if possible, or alternatively in the arm where fewer lymph nodes were removed if thigh administration is not possible [42–46]. Further research is required to determine the long-term impact of LN swelling as a result of the vaccine and to draw conclusions regarding the impact of the COVID-19 vaccine on risk of BCRL after BC treatment. Although lymph node swelling is not a listed side effect of the J&J vaccine, side effects are still a concern to the study population. Women treated for BC should receive education about expected side effects, particularly LN swelling, in order to make informed decisions regarding their health.
Lymph Node Swelling and Patient Education
The proportion of patients in this study who reported LN swelling was qualitatively similar to those who reported LN swelling in the Phase 3 Moderna trial. Furthermore, it is reassuring that LN swelling was found to be more common ipsilateral to the vaccine, regardless of side of BC. Although less common, some patients reported swelling contralateral to the vaccine, and for the majority of these patients, the swelling did occur on the side of BC. It is important that patients are aware that LN swelling may occur contralateral to the site of vaccination. As doses progressed, fewer patients received the vaccine on the side of BC, which is expected due to more prominent patient education prior to booster doses that the vaccine should be administered on the contralateral side to BC [42–46].
As a precautionary measure, women treated for BC were historically told to avoid injections in the arm at risk of BCRL. However, ipsilateral injections were not associated with increased arm volume in a prospective study of 632 patients who were prospectively screened for BCRL with 3041 arm volume measurements [47]. However, this study did not analyze data from vaccines that specifically target the immune system and carry a known side effect of LN swelling. Therefore, the conclusions from this study cannot be applied to patients treated for BC receiving the COVID-19 vaccine. The thigh or the arm opposite to BC (i.e., without nodal removal) is recommended in the interim as the safest option as more research is required to make informed decisions. As previously stated, for women with bilateral cancer, the thigh is a recommended alternative vaccination site to the deltoid for COVID-19 vaccination [48, 49]. Further research is warranted and will be conducted with the results of the LymphVAX study, to determine the relationship between LN swelling from the vaccine and BCRL risk.
Limitations
It is difficult to determine the exact response rate for this study as the survey link was sent at a time when many patients had not yet received the vaccine and institutional barriers limited survey dissemination; therefore, recall bias may have played a role. Questions regarding LN swelling relied on self-report as it was not feasible to include imaging or clinical examination within the scope of this study. The surveys do not include questions regarding the severity of the side effects reported; therefore, conclusions about severity of side effects cannot be drawn. Methodological limitations prevent statistical comparisons with studies of the general population.
Future Directions
Patients are prospectively screened for BCRL as standard of care at MGH [27]. As patients receive further COVID-19 vaccinations, additional analysis will be conducted to analyze the effect of mRNA COVID-19 vaccinations on BCRL risk, comparing reported side effects with immunogenicity data, and investigating patterns in side effects among patients infected with COVID-19 prior to vaccination. These data, collected specifically for patients with BC, provide an evidence base to enhance guidelines for more structured and universal education regarding future booster doses of the vaccine. This will allow patients to have a better understanding of COVID-19 vaccine side effect profiles after BC treatment, empowering women treated for BC to make informed decisions prior to future doses.
Conclusions
In summary, patients treated for BC experience injection site soreness, fatigue, GMS, headache, and chills most commonly after COVID-19 vaccination. These symptoms resolve in less than 1 week in most cases. The percentage of patients experiencing each symptom is similar to that in the general population (including LN swelling), although statistical conclusions cannot be made. These side effect profiles for a cohort of women treated for BC can be used to provide evidence-based patient education regarding future COVID-19 vaccine administration. Finally, the effect of the COVID-19 vaccines on BCRL risk is currently unknown and more research is required. In the interim, we would recommend vaccination away from the side of LN removal, either in the thigh or the contralateral arm. We believe that more patients would take advantage of having the vaccination in the thigh if it were available; however, this is proving difficult for patients as many locations where vaccines are administered do not offer the thigh as an option. It would be beneficial for patients treated for BC if national organizations would make this option more widely available. The goal of this study was to address concerns surrounding side effects, and the informed education that can be produced based on these results will hopefully ease the fears of women treated for BC and empower them to make informed decisions regarding future vaccine doses.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work has been presented as a poster (Side Effects of the mRNA Covid-19 Vaccines in Women Treated for Breast Cancer; San Antonio Breast Cancer Symposium; December 6–10, 2022) and in part during a symposium (Covid-19 and Breast Cancer-Related Lymphedema: Patient Experiences and the LymphVAX Study; Presented by Cheryl Brunelle; Lymphatic Education & Research Network; March 9, 2022).
Authors’ contributions
AGT, CLB, GEN, MCB, LKB, and BCJ designed the concept of the study and manuscript. LHS and BCJ performed data analysis for the paper. AWJ, HSA, EKH, MCB, LKB, TB, and BCJ conducted the study. TB and BCJ performed literature review. All authors edited and approved the manuscript.
Funding
The project was supported by award no. R01CA139118 (A. G. Taghian) and award no. P50CA08393 (A.G. Taghian) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This program was supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema, the Heinz Family Foundation, and the Olayan-Xefos Family Fund for Breast Cancer Research.
Availability of data and materials
Data are not available to share. Please reach out to the corresponding author with questions.
Declarations
Conflict of interest
Alphonse G. Taghian has been loaned equipment from ImpediMed for use in an investigator-initiated clinical trial. Alphonse G. Taghian is on the Scientific Advisory Board of Puretech Health (not paid) and is a previous consultant for VisionRT. Both involvements are unrelated to this study. Cheryl L. Brunelle is on the Scientific Advisory Board of Puretech Health. The remaining authors have no conflict of interest.
Ethics approval and consent to participate
The Massachusetts General Hospital Institutional Review Board approved protocols 2021P000528 (COVID-19 vaccine survey) and 2021P003414 (secondary use protocol to analyze the vaccine survey in conjunction with breast cancer-related demographic and treatment factors). Consent was obtained electronically from participants prior to beginning the survey. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung HL, Whitman GJ, Leung JWT, Sun J, Middleton LP, Le-Petross HT. Ultrasound features to differentiate COVID-19 vaccine-induced benign adenopathy from breast cancer related malignant adenopathy. Acad Radiol. 2022;29(7):1004–1012. doi: 10.1016/j.acra.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recurrent Breast Cancer. Mayo Clin. 2022; Available from: https://www.mayoclinic.org/diseases-conditions/recurrent-breast-cancer/symptoms-causes/syc-20377135
- 3.Lehman CD, Lamb LR, D’Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. Am J Roentgenol. 2021;217(3):584586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 4.Tu W, Gierada DS, Joe BN. Covid-19 vaccination–related lymphadenopathy: What to be aware of. Radiol Imaging Cancer. 2021;3(3). [DOI] [PMC free article] [PubMed]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Website. Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events. Last reviewed 06/21/2022. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
- 8.Center for Disease Control and Prevention. Website. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
- 9.Foldi E, Foldi M. Foldi’s textbook of lymphology. In: Foldi M, editor. Foldi’s textbook of lymphology. Munich, Germany: Elsevier; 2006. pp. 417–427. [Google Scholar]
- 10.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–72. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 12.Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19(8):2580–9. doi: 10.1245/s10434-012-2290-x. [DOI] [PubMed] [Google Scholar]
- 13.Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88(3):565–71. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142(1):59–67. doi: 10.1007/s10549-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naoum GE, Roberts S, Brunelle CL, et al. Quantifying the impact of axillary surgery and nodal irradiation on breast cancer-related lymphedema and local tumor control: long-term results from a prospective screening trial. J Clin Oncol. 2020;38(29):3430–8. doi: 10.1200/JCO.20.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 18.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Fu MR. Breast cancer-related lymphedema: symptoms, diagnosis, risk reduction, and management. World J Clin Oncol. 2014;5(3):241–7. doi: 10.5306/wjco.v5.i3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asdourian MS, Skolny MN, Brunelle C, Seward CE, Salama L, Taghian AG. Precautions for breast cancer-related lymphoedema: risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016;17(9):e392–405. doi: 10.1016/S1470-2045(16)30204-2. [DOI] [PubMed] [Google Scholar]
- 21.Brunelle CL, Roberts SA, Horick NK, Gillespie TC, Jacobs JM, Daniell KM, Naoum GE, Taghian AG. Integrating symptoms into the diagnostic criteria for breast cancer–related lymphedema: applying results from a prospective surveillance program. Phys Therapy. 2020;100(12):2186–2197. doi: 10.1093/ptj/pzaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–9. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Jammallo LS, Miller CL, Horick NK, et al. Factors associated with fear of lymphedema after treatment for breast cancer. Oncol Nurs Forum. 2014;41(5):473–83. doi: 10.1188/14.ONF.473-483. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin SA, Staley AC, Vicini F, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: recommendations from a multidisciplinary expert Asbrs panel: part 1: definitions, assessments, education, and future directions. Ann Surg Oncol. 2017;24(10):2818–26. doi: 10.1245/s10434-017-5982-4. [DOI] [PubMed] [Google Scholar]
- 25.Eaton LH, Narkthong N, Hulett JM. Psychosocial issues associated with breast cancer-related lymphedema: a literature review. Curr Breast Cancer Rep. 2020;12(4):216–24. doi: 10.1007/s12609-020-00376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Zhang H, Zhong Q et al. Predictors of quality of life in patients with breast cancer-related lymphedema: effect of age, lymphedema severity, and anxiety. Lymphat Res Biol. 2021 [DOI] [PubMed]
- 27.Brunelle C, Skolny M, Ferguson C, Swaroop M, O’Toole J, Taghian AG. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: Lessons Learned. J Pers Med. 2015;5(2):153–64. doi: 10.3390/jpm5020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–79. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler S, Kneiss J, Adams B, Wood Magee LJ. Persistent cancer-related fatigue after breast cancer treatment predicts postural sway and postexertional changes in sit-to-stand strategy. Rehabil Oncol. 2022;40(4):162–171. doi: 10.1097/01.REO.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Website. Side Effects of Cancer Treatments. Last reviewed 06/09/2022. Available from: https://www.cdc.gov/cancer/survivors/patients/side-effects-of-treatment.htm
- 31.Montecino-rodriguez E, Berent-maoz B, Dorshkind K, Montecino-rodriguez E, Berent-maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–65. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElhaney JE, Dutz JP. Better influenza vaccines for older people: What will it take? J Infect Dis. 2008;198(5):632–4. doi: 10.1086/590435. [DOI] [PubMed] [Google Scholar]
- 33.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 34.Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005;23(1):S1–S9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Wyant T, Alteri R, Kalidas M, Ogoro C, Lubejko B, Eidsmoe K. Why people with cancer are more likely to get infections. American Cancer Society. 2021. pp. 1–41.
- 36.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021 doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cynthia V-G, Vaca-Cartagena BF, Becerril-Gaitan A. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7:1242. doi: 10.1001/jamaoncol.2021.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naranbhai V, Pernat CA, Gavralidis A, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX Cohort study. J Clin Oncol. 2022;40(1):12–23. doi: 10.1200/JCO.21.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Da Silva FT. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019 doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimm L, Srini A, Dontchos B, et al. Revised SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination Society of Breast Imaging Patient Care and Delivery Committee. 2022;(February):2020–3. Available from: https://www.sbi-online.org/Portals/0/PositionStatements/2021/SBI-recommendations-for-
- 42.Mortimer PS, Gordon K, Macallan D, et al. Consensus document on COVID-19 vaccination for patients with lymphoedema. UK: British Lymphology Society; 2021. [Google Scholar]
- 43.NLN Highlight on COVID-19 Vaccination Injection Site. National Lymphedema Network.
- 44.Caron C. Do I have to get the COVID vaccine in my arm? New York Times. 2021.
- 45.Wells J. Lymph node swelling and the COVID-19 vaccine. Mass General Giving. 2021.
- 46.Receiving the COVID-19 Vaccine After Cancer Surgery Requiring Lymph Node Removal Under the Arm. Mass General Cancer Center. 2021. Available from: https://www.massgeneral.org/cancer-center/news/covid-19-vaccine-after-cancer-surgery-requiring-lymph-node-removal#:~:text=Lymph node swelling is a,to lymphedema after cancer treatment.
- 47.Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol. 2016;34(7):691–8. doi: 10.1200/JCO.2015.61.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moderna COVID-19 Vaccine: Standing Orders for Administering Vaccine. 2022;10–3. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/downloads/standing-orders.pdf
- 49.Pfizer-BioNTech COVID-19 Vaccine: Standing Orders for Administering Vaccine. 2022;3–5. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/standing-orders.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available to share. Please reach out to the corresponding author with questions.