Abstract
Objectives:
This case control study aimed to investigate whether the routine hemogram and biochemical parameters of pediatric patients who have undergone surgery for inguinal hernia are associated with the etiopathogenesis of the disease.
Methods:
Eighty cases of inguinal hernia surgery performed between January 2019 and November 2022 were included in the study. A control group was also established using hospital records, consisting of eighty pediatric patients without any known history of hematological or metabolic disease or use of regular medication. Statistical analysis was conducted to compare the total hemoglobin (Hgb), hematocrit (Htc), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), erythrocyte distribution width (RDW) and thrombocyte (PLT) values in both groups.
Results:
The age range of the pediatric patients was 1-14 years. Of the eighty children, 47 (58.8%) were male and 33 (41.3%) were female, with a mean age of 5.79±3.26. The values of Hgb, Htc, MCH, MCHC, and MCV in the inguinal hernia patients were found to be statistically significantly lower than those in the control group (p<0.05). Additionally, the patient RDW values were found to be statistically significantly higher than those in the control group (p<0.05).
Conclusion:
Compared to the control group, the observed decrease in MCH, MCHC, MCV, Hgb, HTC values, as well as the increase in RDW in patient group, suggests a predisposing effect of iron deficiency. These specific changes suggested that iron deficiency may lead structural changes in the collagen construction and may contribute the etiopathogenesis of childhood inguinal hernia.
Keywords: Child, Erythrocyte Distribution Width (RDW), inguinal hernia, iron deficiency
Inguinal hernia repair is a common surgical procedure in pediatric populations worldwide. Extensive researches have been undertaken to identify the factors contributing to the etiopathogenesis of pediatric inguinal hernia. Despite advances in surgical techniques, issues such as relapse, surgical complications, persistent pain, and patient discomfort remain unresolved challenges for pediatric surgeons. Recent studies have demonstrated changes in the collagen structure of inguinal hernia that may contribute to its development.[1-3]
It has been demonstrated that an increase in the activity of matrix metalloproteinases (MMPs), which are responsible for the degradation of extracellular matrix proteins, is associated with variations in collagen ratios in inguinal hernia cases. Furthermore, there are additional findings that suggest a decrease in lysyl oxidase activity, which is responsible for collagen and elastin cross-linking, also causes changes in its effect on the strength and elasticity of the connective tissue.[4,5]
Collagen construction comprises three polypeptide chains arranged in a helical structure. Every single chain is made of approximately one thousand separate ribosomes. Prior to the construction and release of the triple-helical molecule into the interstitial space, the procollagen peptide chains undergo many posttranslational changes.[6] The hydroxylation of proline to hydroxyproline is a crucial posttranslational enzymatic process. Procollagen's helical structure is formed through two intracellular enzymatic processes: hydroxilation of lysine by lysl hydroxilase, followed by glycosylation. Iron, oxygen and α-ketogluturate are crucial for the Lysine hydroxylase activity.[7,8]
Possible alterations in type-1 and type-2 collagen synthesis, which may change the properties of collagen matrix and its functional contribution to chidhood inguinoscrotal pathologies have not been widely investigated. In this study we aimed to investigate any predictive parameters that may be associated with inguinal hernia. Based on the previous research results, it is hypothesized that microcytic anemia due to iron deficiency is a predisposing factor for developing inguinal hernia in children.
Methods
This retrospective study included a patient group of eighty children who were operated for inguinal hernia between January 2019 and November 2022 and a control group consisting of same number of cases established based on the hospital records. In both groups, children with a history of known hematological or metabolic disease or regular medication use were excluded from the study. Demographic and clinical data were recorded for all cases using a data list. Laboratory tests and pathology reports were obtained from the medical registry system of Malatya Turgut Özal University Training and Research Hospital. Biochemical tests and hemograms were obtained through Abbott Architect c16000 (Illinois, USA) and Sysmex Corporation XN-10 (Kobe, Japan), respectively. This retrospective study was approved by the Institutional Ethics Review Board for Clinical Research (2022/179).
Statistical Analysis
All data analyses were performed using the SPSS for Windows v.25 software (IBM, New York, USA). The normality of the data was checked using the Kolmogorov-Smirnov test.[9] The significance level (p) for the comparison tests was set at 0.05. Since the data violated the normality assumption (p>0.05), the analyses were performed using the non-parametric Mann-Whitney U test. A chi-square (χ2) test was performed by creating cross-tables for the analysis of the categorical data. ROC analysis was employed to determine the cutoff point.[10]
Results
Demographic Characteristics of Patients with Inguinal hernia and the Healthy Control Group
Out of eighty children, 47 (58.8%) were male and 33 (41.3%) were female. No statistically significant difference was found between the groups according to the distribution of gender (p>0.05, Table 1). While the mean patient age was 5.79, it was 5.84 in the control group, and no statistically significant age-related difference was observed between the groups (p>0.05, Table 1).
Table 1.
Comparison of the Groups by Gender and Age Distribution
| Variable | Hernia | Control | Total | Test | p |
|---|---|---|---|---|---|
| Gender | |||||
| Girl | |||||
| n | 33 | 35 | 68 | 0.102 | 0.749 |
| % | 41.3 | 43.8 | 42.5 | ||
| Boy | |||||
| n | 47 | 45 | 92 | ||
| % | 58.8 | 56.3 | 57.5 | ||
| Total | |||||
| n | 80 | 80 | 160 | ||
| % | 100.0 | 100.0 | 100.0 | ||
| Age | |||||
| Mean±SD | 5.79±3.26 | 5.84±3.28 | 5.81±3.26 | 3106.500 | 0.749 |
| M (Min-Max) | 5 (1-14) | 6 (1-13) | 5.0 (1-14) |
n; frequency, %; percent, sd; standart deviation, M; Median, pa; Chi-Square Test value ((χ2), pb; Mann Whitney Test Value.
Laboratory Markers of Hernia Patients and Healthy Control Group
There was no statistically significant difference between the patient and control groups in terms of thrombocyte (PLT) (p>0.05, Table 2). A statistically significant difference was found between the groups according to total hemoglobin (Hgb), Hematocrit (HCT), mean corpuscular volume (MCV), mean red blood cell hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC), Erythrocyte Distribution Width (RDW) levels (p<0.05, Table 2) (Figs. 1-6).
Table 2.
Changes in inguina hernia patient and control groups laboratory results
| Variable | Hernia (n=80) | Control (n=80) | Test | p | ||
|---|---|---|---|---|---|---|
| Mean±SD | M (Min-Max) | Mean±SD | M (Min-Max) | |||
| HGB (g/dl) | 12.2±1.07 | 12.2 (9.4-14,4) | 13.54±1.06 | 13.5 (10.3-16.2) | 1235.500 | 0.001* |
| HCT (%) | 36.36±2.64 | 36.4 (31.1-43) | 39.48±2.98 | 39.3 (32.7-48.4) | 1415.000 | 0.001* |
| MCH (pg) | 25.32±2.69 | 25.8 (17-30.8) | 27.3±1.53 | 27.3 (23.6-31.2) | 1710.500 | 0.001* |
| MCHC (g/L) | 33.53±1.32 | 33.8 (30.2-36.7) | 34.29±0.96 | 34.25 (31.5-36.3) | 2159.000 | 0.001* |
| MCV (fl) | 75.39±6.36 | 76 (54.3-88.3) | 79.64±3.93 | 79.7 (70.5-95.5) | 1849.500 | 0.001* |
| RDW (%) | 14.19±1.76 | 13.7 (11.9-19.8) | 12.63±0.66 | 12.6 (11.4-14.6) | 1111.000 | 0.001* |
| PLT (%) | 0.32±0.09 | 0.31 (0.09-0.67) | 0.3±0.05 | 0.3 (0.18-0.44) | 2896.000 | 0.299 |
SD: standart deviation; M: Median; pa: Chi-Square Test value (χ2); p: Mann Whitney Test Value*p<0,05; referres a statistically significant difference between the groups. HGB: hemoglobin; HCT: Hematocrit; MCV: mean corpuscular volume; MCH: mean red blood cell hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW: Erythrocyte Distribution Width.
Figure 1.
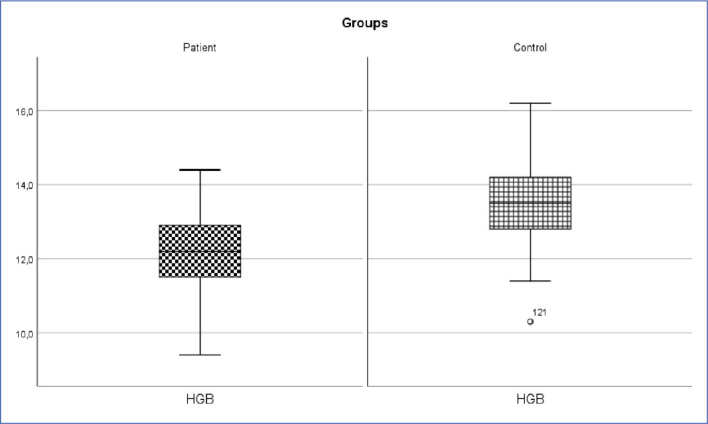
Inguinal hernia patient and control group Hgb change (p<0.05).
HGB: Hemoglobin.
Figure 6.
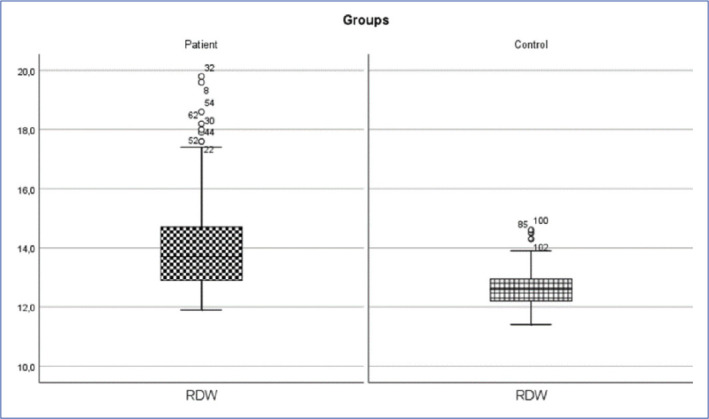
Inguinal hernia patient and control group RDW change (p<0.05).
RDW: Erythrocyte Distribution Width.
Figure 2.
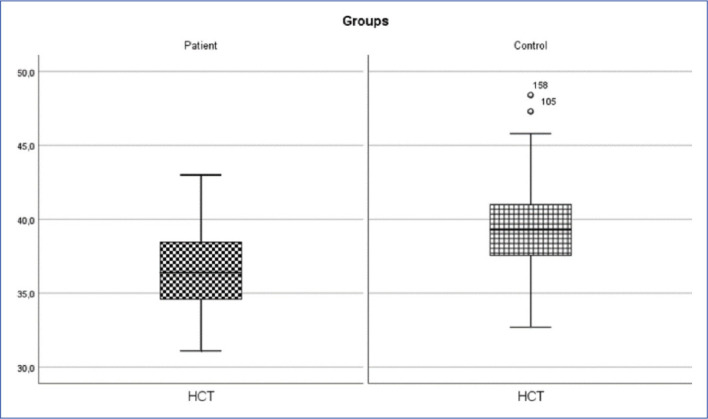
Inguinal hernia patient and control group HCT change (p<0.05).
HTC: Hematocrite.
Figure 3.
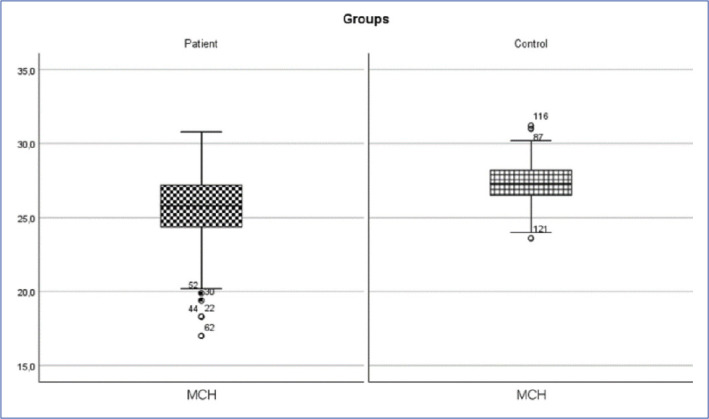
Inguinal hernia patient and control group MCH change (p<0.05).
MCH: mean red blood cell hemoglobin.
Figure 4.
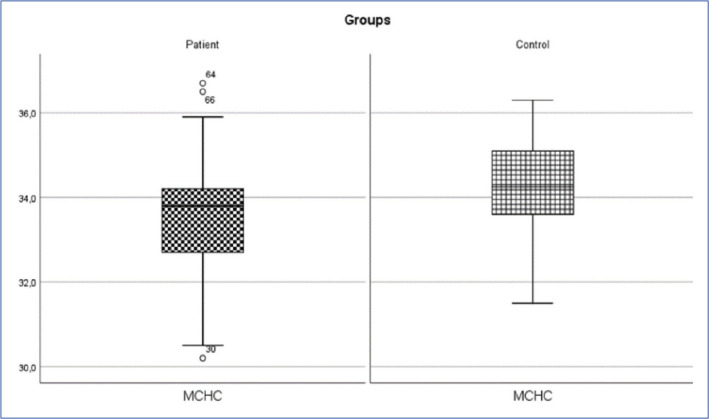
Inguinal hernia patient and control group MCHC change (p<0.05).
MCHC: Mean corpuscular hemoglobin concentration.
Figure 5.
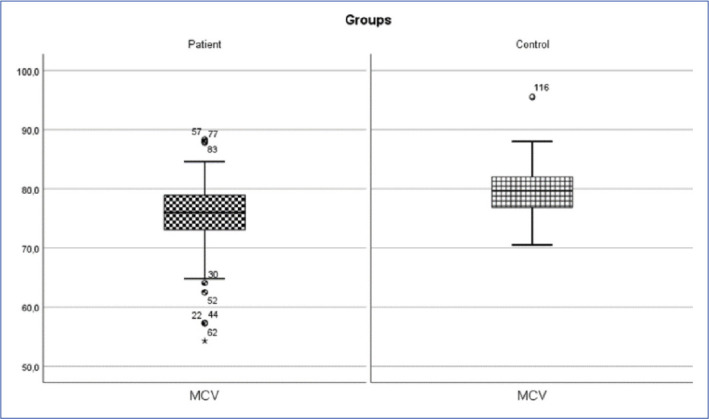
Inguinal hernia patient and control group MCV change (p<0.05).
MCV: mean corpuscular volume.
ROC Analysis
ROC analysis results are given in Table 3, and ROC curves of all analyzed parameters are presented in Figure 1. The areas under the curve calculated for the PLT variable were not found statistically significant (Table 3, Fig. 7).
Table 3.
ROC Analysis Results of inguinal hernia group biochemistry parameters
| Test Result Variable(s) | Cut Off | Sensitivity | Specificity | AUC | p | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| HGB (g/dl) | 12.500 | 0.550 | 0.450 | 0.192 | 0.001* | 0.126 | 0.259 |
| HCT (%) | 36.250 | 0.513 | 0.413 | 0.218 | 0.001* | 0.148 | 0.288 |
| _MCH (pg) | 24.350 | 0.775 | 0.738 | 0.267 | 0.001* | 0.189 | 0.345 |
| MCHC (g/dL) | 32.850 | 0.713 | 0.638 | 0.337 | 0.001* | 0.253 | 0.421 |
| MCV (fL) | 75.100 | 0.588 | 0.463 | 0.289 | 0.001* | 0.209 | 0.369 |
| RDW (%) | 12.150 | 0.963 | 0.750 | 0.826 | 0.001* | 0.764 | 0.889 |
| _PLT (109/L) | 0.295 | 0.563 | 0.075 | 0.548 | 0.301 | 0.457 | 0.638 |
p<0.05; referres a statistically significant difference between the groups., AUC; Area Under the Curve, HGB: hemoglobin, HCT: Hematocrit, MCV: mean corpuscular volume, MCH: mean red blood cell hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, RDW: Erythrocyte Distribution Width.
Figure 7.
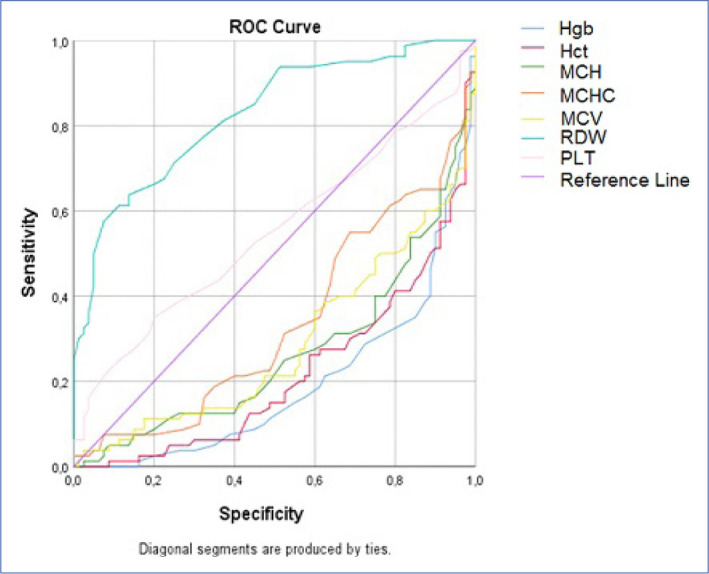
The cut-off points according to the ROC analysis.
According to ROC analysis results, Hgb, HCT, MCH, MCHC, MCV, RDW values were found to be distinguishing parameters for inguinal hernia. Among these parameters, RDW was observed to be the parameter with the highest AUC value. As a result of the ROC analysis for RDW, the cutoff point was 12.15, which corresponds with the highest sensitivity and specificity point. At this point, the sensitivity of the scale was found to be 0.963 and the specificity was determined as 0.75. The parameter with the highest AUC value after RDW is MCHC. At a cutoff value of 32.850, the sensitivity and specificity of the MCHC parameter was 0.713 and 0.638, respectively (Table 3, Fig. 7).
Discussion
Collagen is one of the main components of connective tissue. The total amount and the ratio of type-1 to type-3 collagen alterations affect the tensile strength and mechanical stability of the tissues. It is determined that patients with inguinal hernia have low collagen levels and the alterations in the ratio of collagen types weakens the abdominal fascia and decreases the enzymatic activities involved in connective tissue homeostasis.[11,12]
In a comprehensive investigation on children of various ages, IDA (Iron deficiency anemia) was reported 30-78%.[13] IDA mostly affects babies aged 4-to-24 months, school-aged children and teenage females.[14] In addition to malnutrition, intestinal parasites, malnutrition, celiac disease, and pica also contribute to IDA.[15,8]
During collagen synthesis, iron takes part as a cofactor involved in prolyl 4-hydroxylase[1-4] enzyme. This enzyme catalyzes the procollagen molecules during the synthesis steps of procollagen molecules. The hydroxilation step ensures the stability of the triple helix of procollagen.[6,16]
Currently, advanced technologies utilized in new hematological analyzers allow for the detection of erythrocyte problems prior to the development of anemia. These analyzers can alert the clinicians by displaying a histogram. For instance, in iron deficiency, an increase in RDW values is observed before other parameters, which can be detected by the clinician on a histogram.[17,18] However, when only small erythrocytes are present, the MCV falls below normal level. Patients with iron deficiency have low MCV and increased RDW values. Even in advanced iron deficiency, RDW continues to increase. MCV typically remains normal in the majority of cases with chronic anemia. In such cases, an accompanying cause other than chronic disease should be suspected if RDW is found to be high. In Thalassemia, if the anemia is not severe, RDW remains normal and, because most of these patients have mild anemia, MCV keeps decreasing and RDW values remain normal. In almost all anemic cases, MCW and RDW values remains normal as in healthy individuals due to the chronic nature of the disease. In autoimmune hemolytic anemia, both MCV and RDW are elevated in newborns; however, patients with aplastic anemia or preleukemia have a normal RDW despite macrocytosis.[19] In the present study, Hgb, Hct, MCV, MCH, and MCHC values were found to be statistically lower in hernia group in comparison with the control cases, while RDW levels were statistically significantly higher (p<0.05, see Table 2). RDW is an early predictor of oxidative stress, and it is suggested that RDW increases with the inflammation markers such as CRP, IL-6, and TNF- alpha in iron mobilization disorder and iron deficiency anemia.[20,21] According to results of our ROC analysis, RDW (AUC:0.826) was found to be the most powerful predictor in pediatric inguinal hernia, with a detected sensitivity of 96.3% (95% CI, 76.4%–88.9%) and specificity of 75.0% (95% CI, 76,4 %– 88.9%) (Table 3, Fig. 1). According to our study, MCV values (Mean±SD: 75,39±6,369) were lower than the control groups (Mean±SD: 79,64±3,93)
Iron deficiency (ID) is the most common dietary deficit and a significant cause of anemia. According to a large multinational research, almost 1,2 billion individuals suffer from iron deficiency anemia (IDA), while iron deficiency without anemia (IDWA) is predicted to be at least twice as prevalent.[22,23] Iron deficiency is a prevalent clinical problem[23], but the diagnosis can be difficult when blood count is normal. It is still unclear what non-hematologic consequences of iron deficiency emerge prior to the development of anemia.[24,25] Our result shown that Hgb level (Mean±SD: 12,2±1,07) is definitely not at the levels of anemia, but significantly lower than that in the control group (p<0.05).
In this study Hgb, Hct, MCV, MCH and MCHC values were found to be statistically lower in hernia group, while RDW levels were also statistically significantly higher in comparison with the control cases (p<0.05, see Table 2). RDW is considered an early predictor of oxidative stress and has been suggested to increase in conjunction with the inflammation markers such as CRP, IL-6, and TNF- alpha in iron mobilization disorder and iron deficiency anemia.[23,24] Results of our ROC analysis revealed that the RDW (AUC:0.826) was the most powerful predictor for pediatric inguinal hernia, with a detected sensitivity of 96.3% (95% CI, 76.4%–88.9%) and specificity of 75.0% (95% CI, 76,4 %– 88.9%) (Table 3, Fig. 1).
Conclusion
In conclusion according to the results of this study, decreased Hgb, HTC, MCV, MCH, MCHC and elevated RDW levels in pediatric inguinal hernia patients compared to healthy controls, suggests an iron deficiency in children. With the cofactor effect of iron, the hydroxylation of procollagen and effective collagen synthesis and secretion is regulated. In iron deficiency, it may cause a lack of stability in cross-linking of collagen and elastin polypeptides. Therefore, it is suggested that defects in cross-linking due to iron deficiency may be a risk factor for hernia formation.
One of the limitations of this study is the lack of parameters affecting iron level, such as ferritin or transferrin, since they are not included in routine preoperative tests. Therefore, based on the obtained results, further detailed prospective studies may assist in recognizing the etiopathogenesis of pediatric inguinal hernia.
Footnotes
Please cite this article as ”Harma B, Kiran TR, Inceoglu F. Iron Deficiency may be a Risk Factor for Inguinal Hernia Development in Children. Med Bull Sisli Etfal Hosp 2023;57(1):105–110”.
Disclosures
Ethics Committee Approval
This study received approval from the institutional noninvasive human research ethics commitee (2022/179) and was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. Informed consent of the subjects was weived by the ethics commitee because the sample consisted of medical records.
Peer-review
Externally peer-reviewed.
Conflict of Interest
None declared.
Authorship Contributions
Concept – B.H.; Design – B.H.; Supervision – B.H.; Materials – B.H., T.R.K.; Data collection &/or processing – B.H., F.I.; Analysis and/or interpretation – B.H., F.I.; Literature search – B.H., T.R.K.; Writing – B.H.; Critical review – B.H., T.R.K., F.I.
References
- 1.Klinge U, Binnebösel M, Mertens PR. Are collagens the culprits in the development of incisional and inguinal hernia disease? Hernia. 2006;10:472–7. doi: 10.1007/s10029-006-0145-8. [DOI] [PubMed] [Google Scholar]
- 2.Pans A, Albert A, Lapière CM, Nusgens B. Biochemical study of collagen in adult groin hernias. J Surg Res. 2001;95:107–13. doi: 10.1006/jsre.2000.6024. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DW, Boyd CD, Norton P, Greco RS, Boyarsky AH, Mackenzie JW, et al. Increases in type III collagen gene expression and protein synthesis in patients with inguinal hernias. Ann Surg. 1993;218:754–60. doi: 10.1097/00000658-199312000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascual G, Rodríguez M, Mecham RP, Sommer P, Buján J, Bellón JM. Lysyl oxidase like-1 dysregulation and its contribution to direct inguinal hernia. Eur J Clin Invest. 2009;39:328–37. doi: 10.1111/j.1365-2362.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 5.Aren A, Gökçe AH, Gökçe FS, Dursun N. Roles of matrix metalloproteinases in the etiology of inguinal hernia. Hernia. 2011;15:667–71. doi: 10.1007/s10029-011-0846-5. [DOI] [PubMed] [Google Scholar]
- 6.Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders (first of two parts) N Engl J Med. 1979;301:13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- 7.Kivirikko KI, Prockop DJ. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc Natl Acad Sci U S A. 1967;57:782–9. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedler-Nagy C, Bruckner P, Hayashi T, Prockop DJ. Isolation of unhydroxylated type I procollagen folding of the protein in vitro. Arch Biochem Biophys. 1981;212:668–77. doi: 10.1016/0003-9861(81)90411-2. [DOI] [PubMed] [Google Scholar]
- 9.Alpar R. 6ncı baskı. Ankara: Detay Yayıncılık; 2020. Uygulamalı İstatistik ve Geçerlik-Güvenirlik. [Google Scholar]
- 10.Dirican A. Evaluation of the diagnostic test’s performance and their comparisons. [Article in Turkish] Cerrahpaşa J Med. 2001;32:25–30. [Google Scholar]
- 11.Peeters E, De Hertogh G, Junge K, Klinge U, Miserez M. Skin as marker for collagen type I/III ratio in abdominal wall fascia. Hernia. 2014;18:519–25. doi: 10.1007/s10029-013-1128-1. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen NA, Yadete DH, Sorensen LT, Agren MS, Jorgensen LN. Connective tissue alteration in abdominal wall hernia. Br J Surg. 2011;98:210–9. doi: 10.1002/bjs.7339. [DOI] [PubMed] [Google Scholar]
- 13.Türk Hematoloji Derneği Yetişkinde demir eksikliği tanı ve tedavi kılavuzu. Eritrosit Hastalıkları ve Hemoglobin Bozuklukları Tanı ve Tedavi Kılavuzu 1.1. İstanbul. 2019:23–33. [Google Scholar]
- 14.World Health Organization The prevalence of anaemia in women: a tabulation of available information. 2nd ed. Available at: https://apps.who.int/iris/handle/10665/58994 Accessed Feb 8, 2023.
- 15.Dilek İ, Altun S, Tuncer İ, Uygan İ, Topal C, Aksoy H. Demir eksikliği anemisinde hemoglobin, hematokrit değerleri, eritrosit indeksleri ve etyolojik nedenlerin değerlendirilmesi. [Article in Turkish] Van Tıp Derg. 2000;7:51–6. [Google Scholar]
- 16.Calim A, Kanat E, Mazi EE, Oygen S, Karabay U, Borlu F. Evaluation of in-patients with iron deficiency anemia in terms of etiology. Sisli Etfal Hastan Tip Bul. 2020;54:428–32. doi: 10.14744/SEMB.2018.47354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bessman JD. Heterogeneity of red cell volume: quantitation, clinical correlations, and possible mechanisms. Johns Hopkins Med J. 1980;146:226–30. [PubMed] [Google Scholar]
- 18.Kaye FJ, Alter BP. Red-cell size distribution analysis: an evaluation of microcytic anemia in chronically ill patients. Mt Sinai J Med. 1985;52:319–23. [PubMed] [Google Scholar]
- 19.Bessman JD, Gilmer PR, Jr, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol. 1983;80:322–6. doi: 10.1093/ajcp/80.3.322. [DOI] [PubMed] [Google Scholar]
- 20.Karabulut A, Uzunlar B. Correlation between red cell distribution width and coronary ectasia in the acute myocardial infarction. Clin Appl Thromb Hemost. 2012;18:551–2. doi: 10.1177/1076029611436198. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012;64:380–7. doi: 10.1016/j.ihj.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 24.Camaschella C. Iron deficiency. Blood. 2019;133:30–39. doi: 10.1182/blood-2018-05-815944. [DOI] [PubMed] [Google Scholar]
- 25.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]


