ABSTRACT
Aims
Baseline body mass index (BMI) and weight loss promoted by sodium–glucose cotransporter 2 inhibitors may impact outcomes in patients with heart failure with reduced ejection fraction (HFrEF). We assessed in the EMPEROR‐Reduced population treated with empagliflozin versus placebo the relationship between baseline BMI, weight loss and effects on the primary (time to first hospitalization for heart failure [HHF] or cardiovascular death) and key secondary outcomes.
Methods and results
We categorized patients according to their baseline BMI: <20 kg/m2 (n = 180); 20 to <25 kg/m2 (n = 1038); 25 to <30 kg/m2 (n = 1345); 30 to <35 kg/m2 (n = 774) and ≥35 kg/m2 (n = 393). The treatment effect of empagliflozin on the primary outcome was consistent across all BMI categories (hazard ratios in subgroups 0.66–0.88, interaction trend p = 0.32), as was the effect on total (first plus recurrent) HHF (interaction trend p = 0.31). Empagliflozin reduced the rate of estimated glomerular filtration rate decline consistently across the BMI categories (interaction trend p = 0.67). Overall, incidence rates of any or serious adverse events were comparable between the treatment groups across all BMI categories. A total of 313 (17.4%) patients treated with empagliflozin experienced a weight loss of more than 5% at week 52 versus 230 (12.8%) in placebo. When analysed separately within each treatment group, presence of weight loss was similarly associated with an increased risk of all‐cause mortality.
Conclusion
The benefits of empagliflozin versus placebo were consistently present across all BMI categories in HFrEF patients. Weight loss was associated with higher risk of all‐cause mortality, regardless of treatment group.
Keywords: Empagliflozin, Heart failure, Body mass index, Weight loss
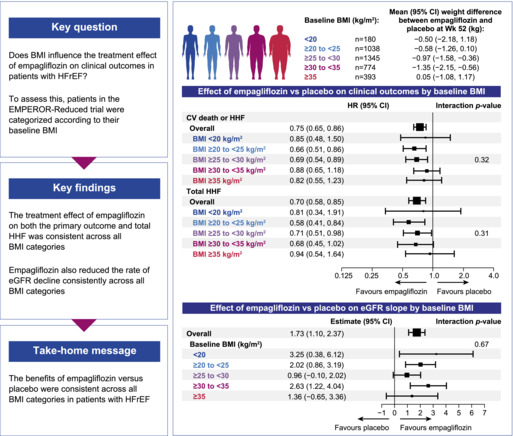
EMPEROR‐Reduced: effect of empagliflozin on heart failure outcomes, kidney function decline and weight according to body mass index categories. BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; HR, hazard ratio; Wk, week.
Introduction
The prevalence of obesity and heart failure (HF) continue to increase worldwide making them two of the most significant public health challenges. 1 , 2 While it is known that overweight and obesity independently increases the risk of incident HF, 3 , 4 , 5 and body mass index (BMI) appears causally linked to greater HF risks, 6 the association between BMI and outcomes in patients with established HF remains controversial. Previous studies have shown that relative to normal weight, overweight and obesity are associated with lower mortality in patients with both chronic compensated and acute decompensated HF. 7 , 8 , 9 This apparent protective association of elevated BMI in HF, also known as the ‘obesity paradox’, has been widely recognized in other cardiovascular (CV) and non‐CV conditions as well. 10 Moreover, cardiac cachexia, i.e. wasting with unintentional weight loss accompanied by inflammation and abnormal biochemistry, is also a predictor of adverse clinical outcomes in HF. 11 , 12
Sodium–glucose cotransporter‐2 (SGLT2) inhibitors have been shown to promote weight loss in several populations. Across clinical trials conducted in type 2 diabetes mellitus (T2DM) populations, a mean weight loss of around 2–3 kg has been observed with SGLT2 inhibitors. 13 , 14 , 15 In populations with HF with reduced ejection fraction (HFrEF), of which approximately 50% had T2DM, the weight loss was lower with a mean of around 1 kg as observed with empagliflozin and dapagliflozin in EMPEROR‐Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) and DAPA‐HF (Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure), respectively. 16 , 17 A recent post‐hoc analysis from DAPA‐HF showed that the beneficial effect of dapagliflozin was consistent across the wide range of BMI. 18 However, these results would benefit from validation.
The longitudinal effect of weight loss or weight gain with SGLT2 inhibitors versus placebo on clinical outcomes is still debated. Moreover, the degree of weight loss which can predict clinical outcomes remains uncertain, especially with regards to SGLT2 inhibitors. Therefore, in this post‐hoc analysis of EMPEROR‐Reduced, we aimed to assess the relationship between baseline BMI, weight loss and clinical outcomes in patients with HFrEF, and investigate the impact of weight loss and weight gain on clinical outcomes, and how SGLT2 inhibitors fit into this context.
Methods
Trial design
The EMPEROR‐Reduced trial was a randomized, double‐blind, parallel‐group, placebo‐controlled, event‐driven study. Patients were recruited into EMPEROR‐Reduced between 25 April 2017 and 8 November 2019 at 520 centres in 20 countries. The design and results of this trial have been published previously. 16 , 19 The trial was approved by the ethics committee at each study site, complies with the Declaration of Helsinki, and all patients provided written informed consent.
Study patients
Participants included patients ≥18 years who had chronic HF (New York Heart Association functional class II, III, or IV) with a left ventricular ejection fraction ≤40% and BMI <45 kg/m2. To enroll patients at increased risk of events, the number of patients with an ejection fraction >30% was limited by requiring that they had been hospitalized for HF within 12 months or had exceptionally high levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), i.e. >1000 pg/ml or >2500 pg/ml in those with an ejection fraction of 31–35% or 36–40%, respectively; these thresholds were doubled in patients with atrial fibrillation. Exclusion criteria included symptomatic hypotension, systolic blood pressure of <100 mmHg or ≥180 mmHg, or an estimated glomerular filtration rate (eGFR) <20 ml/min/1.73 m2. For the current analyses patients were categorized according to their BMI at baseline in the following categories: <20, 20 to <25, 25 to <30, 30 to <35, and ≥35 kg/m2, according to the World Health Organization classification of obesity. 20 We chose BMI 20 kg/m2 as the lower cut‐off and 35 kg/m2 as the higher cut‐off due to low sample size below BMI 20 kg/m2 and above 35 kg/m2. In addition, to assess whether weight change was impacted by signs of congestion, we investigated change in weight in patients with versus without peripheral oedema at baseline.
Patients were randomized double‐blind (in a 1:1 ratio) to receive placebo or empagliflozin 10 mg daily, in addition to their usual therapy for HF. Following entry into the trial, all appropriate treatments for HF or other medical conditions could be initiated or altered at the clinical discretion of the investigator. Patients were periodically assessed at study visits for major outcomes, symptoms and functional capacity related to HF, vital signs and biomarkers reflecting changes in the course of HF, and adverse events. All randomized patients were followed for the occurrence of pre‐specified outcomes for the entire duration of the trial, regardless of whether the study participants were taking their study medications.
Trial endpoints
The primary endpoint of the EMPEROR‐Reduced trial was the time‐to‐first‐event analysis of the combined risk of CV death or hospitalization for heart failure (HHF). This analysis was based on adjudicated events, as assessed by a clinical events committee, which applied pre‐specified definitions and was blinded to treatment assignment. The key secondary endpoints of the study were (i) the total number of adjudicated hospitalizations for HF (including first and recurrent events); and (ii) the slope of the change in eGFR during double‐blind treatment. Further secondary endpoints included first HHF, CV death, all‐cause mortality, a composite renal endpoint that was defined as the need for chronic dialysis or renal transplant or a ≥40% sustained reduction in eGFR (creatinine‐based Chronic Kidney Disease Epidemiology Collaboration) or a sustained eGFR <15 ml/min/1.73 m2 (if baseline eGFR was ≥30 ml/min/1.73 m2) or <10 ml/min/1.73 m2 (if baseline eGFR was <30 ml/min/1.73 m2), as well as the difference in change from baseline to week 52 in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS).
Analyses of differences between treatment groups in change in body weight from baseline are displayed at weeks 12, 32 and 52. Safety analyses included serious adverse events, adverse events leading to discontinuation of study drug, adverse events of special interest and specific adverse events (e.g. hypotension, hypoglycaemia, acute renal failure and genital infections).
Statistical analyses
For time‐to‐first‐event analyses, differences between the placebo and empagliflozin groups for the primary endpoint across the various BMI categories were assessed for statistical significance using a Cox proportional hazards model, with pre‐specified covariates of age, gender, geographical region, diabetes status at baseline, left ventricular ejection fraction, and eGFR at baseline. These analyses were performed according to the intention‐to‐treat principle for all randomized patients and included data up to the end of the planned treatment period. Event rates per 100 patient‐years and adjusted hazard ratios (HR) are reported for each BMI category. For the analysis of total (first and repeated) events, between‐group differences were assessed using a joint frailty model, with CV death as a competing risk. The effect of empagliflozin versus placebo on the primary endpoint, and the components HHF and CV mortality were also evaluated using BMI as a continuous variable. For the analysis of changes in eGFR, KCCQ‐CSS and vital signs, treatment effects were assessed based on changes from baseline using a mixed model for repeated measures (MMRM). Between‐group difference in the slope of change during the treatment period in eGFR were analysed using a random intercept random model in the treated set. The MMRM, the slope model and the joint frailty model included the same covariates as the Cox model. To assess the consistency of effects across subgroups, subgroup‐by‐treatment interaction terms were added in the models. Analyses for safety were performed including all the patients who had received at least one dose of empagliflozin or placebo. A restricted cubic spline‐regression model with four knots was used to present the relationship between baseline BMI as continuous variable and all‐cause mortality and CV death. The model was conducted with and without NT‐proBNP on a logarithmic scale, added to the standard covariates as in the Cox model.
Additionally, the proportions of patients with >1% weight loss/gain from baseline to week 12, 32 and 52 were calculated. The impact of empagliflozin on body weight over follow‐up was assessed using the MMRM model using the same covariates as the Cox model. This analysis was also conducted by stratifying patients according to presence of peripheral oedema at baseline. The analysis of all‐cause mortality was repeated with weight loss and weight gain as separate time‐dependent covariates to assess the association of weight gain or loss and all‐cause mortality. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). All p‐values reported are 2‐sided, and p < 0.05 was considered as statistically significant in all cases. No adjustments for multiple testing were made.
Results
Patient characteristics
Of the 3730 randomized patients, 180 had a baseline BMI <20 kg/m2, 1038 had BMI 20 to <25 kg/m2, 1345 had BMI 25 to <30 kg/m2, 774 had BMI 30 to <35 kg/m2, and 393 had BMI ≥35 kg/m2. Baseline demographic and clinical characteristics according to BMI categories are shown in Table 1 . Compared with patients with a lower BMI, those with a higher BMI were younger, more often white, had more often concomitant diabetes and atrial fibrillation, and had higher blood pressure and hemoglobin levels. Left ventricular ejection fraction was similar across the different BMI categories. Patients with higher BMI more frequently had poorer functional capacity with New York Heart Association class III–IV, and signs of volume overload with peripheral oedema/congestion. KCCQ scores were lower in patients with the higher BMI. There was no significant difference between the different BMI categories for serum low‐density lipoprotein cholesterol and total cholesterol levels. Proportion of patients who had history of chronic obstructive pulmonary disease or malignancies were largely similar across the different categories of BMI (Table 1 ).
Table 1.
Baseline demographic and clinical characteristics according to body mass index categories
| Baseline characteristics | <20 kg/m2 (n = 180) | 20 to <25 kg/m2 (n = 1038) | 25 to <30 kg/m2 (n = 1345) | 30 to <35 kg/m2 (n = 774) | ≥35 kg/m2 (n = 393) | p‐value for trend |
|---|---|---|---|---|---|---|
| Age, years | 67.5 ± 13.2 | 67.9 ± 11.1 | 67.6 ± 10.6 | 65.4 ± 10.5 | 64.0 ± 11.7 | <0.0001 |
| Male sex, n (%) | 118 (65.6) | 798 (76.9) | 1064 (79.1) | 592 (76.5) | 265 (67.4) | 0.2824 |
| Race, n (%) | <0.0001 | |||||
| White | 68 (37.8) | 570 (54.9) | 1018 (75.7) | 643 (83.1) | 330 (84.0) | |
| Black‐African‐American | 8 (4.4) | 72 (6.9) | 96 (7.1) | 48 (6.2) | 33 (8.4) | |
| Asian | 94 (52.2) | 348 (33.5) | 173 (12.9) | 45 (5.8) | 12 (3.1) | |
| Region, n (%) | <0.0001 | |||||
| North America | 14 (7.8) | 77 (7.4) | 159 (11.8) | 104 (13.4) | 71 (18.1) | |
| Latin America | 47 (26.1) | 343 (33.0) | 472 (35.1) | 279 (36.0) | 145 (36.9) | |
| Europe | 28 (15.6) | 278 (26.8) | 539 (40.1) | 343 (44.3) | 165 (42.0) | |
| Asia | 65 (36.1) | 266 (25.6) | 121 (9.0) | 32 (4.1) | 9 (2.3) | |
| eGFR, ml/min/1.73 m2 | 65.3 ± 23.8 | 62.5 ± 21.4 | 61.2 ± 21.1 | 62.0 ± 21.5 | 62.2 ± 22.9 | 0.2205 |
| HbA1c, % | 6.15 ± 1.09 | 6.43 ± 1.41 | 6.48 ± 1.30 | 6.82 ± 1.56 | 6.83 ± 1.49 | <0.0001 |
| BMI, kg/m2 | 18.6 ± 1.2 | 22.9 ± 1.4 | 27.4 ± 1.4 | 32.2 ± 1.4 | 38.3 ± 2.9 | |
| Haemoglobin, g/dl | 127.8 ± 17.5 | 134.7 ± 15.6 | 137.9 ± 15.7 | 139.4 ± 16.6 | 139.1 ± 17.4 | <0.0001 |
| KCCQ‐CSS | 75.19 ± 20.05 | 73.82 ± 21.21 | 71.57 ± 21.60 | 67.59 ± 22.43 | 63.75 ± 22.57 | <0.0001 |
| Lipid levels, mg/dl | ||||||
| Total cholesterol | 161.8 ± 40.9 | 157.4 ± 42.3 | 157.7 ± 41.0 | 156.9 ± 42.6 | 154.6 ± 42.8 | 0.1670 |
| LDL | 83.8 ± 31.7 | 83.7 ± 34.9 | 84.4 ± 34.3 | 83.1 ± 35.0 | 81.3 ± 34.7 | 0.3543 |
| HDL | 55.7 ± 18.0 | 49.3 ± 14.4 | 46.4 ± 13.3 | 44.5 ± 12.8 | 42.8 ± 12.0 | <0.001 |
| Triglycerides | 110.9 ± 63.1 | 123.5 ± 71.9 | 139.3 ± 110.8 | 150.2 ± 93.7 | 157.3 ± 106.8 | <0.0001 |
| Medical history | ||||||
| HHF last 12 months, n (%) | 58 (32.2) | 329 (31.7) | 394 (29.3) | 234 (30.2) | 136 (34.6) | 0.8824 |
| DM at baseline, n (%) | 56 (31.1) | 447 (43.1) | 661 (49.1) | 452 (58.4) | 240 (61.1) | <0.0001 |
| Atrial fibrillation a , n (%) | 58 (32.2) | 345 (33.2) | 488 (36.3) | 308 (39.8) | 170 (43.3) | <0.0001 |
| Systolic BP, mmHg | 119.1 ± 15.0 | 119.0 ± 15.1 | 122.2 ± 15.7 | 124.2 ± 15.6 | 126.2 ± 15.5 | <0.0001 |
| Diastolic BP, mmHg | 71.6 ± 10.9 | 72.0 ± 10.7 | 73.9 ± 10.6 | 75.1 ± 10.6 | 77.4 ± 10.7 | <0.0001 |
| LVEF, % | 27.5 ± 6.3 | 27.4 ± 6.2 | 27.5 ± 6.0 | 27.6 ± 5.8 | 27.3 ± 6.2 | 0.9405 |
| NYHA class III/IV, n (%) | 43 (23.9) | 228 (22.0) | 297 (22.1) | 221 (28.6) | 141 (35.9) | <0.0001 |
| Volume overload with evidence of oedema or organ congestion, n (%) | 21 (11.7) | 124 (11.9) | 185 (13.8) | 127 (16.4) | 89 (22.6) | <0.0001 |
| COPD, n (%) | 23 (12.8) | 115 (11.1) | 150 (11.2) | 96 (12.4) | 59 (15.0) | 0.1183 |
| Malignancy, n (%) | 14 (7.8) | 85 (8.2) | 91 (6.8) | 53 (6.8) | 18 (4.6) | 0.0349 |
| Heart failure treatment, n (%) | ||||||
| ICD or CRT‐D b | 30 (16.7) | 255 (24.6) | 453 (33.7) | 288 (37.2) | 144 (36.6) | <0.0001 |
| CRT‐D or CRT‐P c | 12 (6.7) | 106 (10.2) | 173 (12.9) | 91 (11.8) | 56 (14.2) | 0.0111 |
| Use of ACEi or ARB d | 114 (63.3) | 695 (67.0) | 953 (70.9) | 565 (73.0) | 273 (69.5) | 0.0055 |
| Use of ARNI | 27 (15.0) | 186 (17.9) | 272 (20.2) | 154 (19.9) | 88 (22.4) | 0.0254 |
| MRA | 130 (72.2) | 721 (69.5) | 944 (70.2) | 561 (72.5) | 305 (77.6) | 0.0144 |
| Diuretics other than MRA | 153 (85.0) | 881 (84.9) | 1163 (86.5) | 693 (89.5) | 358 (91.1) | 0.0002 |
| Beta‐blocker | 156 (86.7) | 972 (93.6) | 1283 (95.4) | 744 (96.1) | 378 (96.2) | <0.0001 |
Values are given as mean ± standard deviation, unless otherwise indicated.
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacemaker; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; HHF, hospitalization for heart failure; ICD, implantable cardioverter defibrillator; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Atrial fibrillation reported in any ECG before treatment intake or history of atrial fibrillation reported as medical history.
ICD with or without cardiac resynchronization therapy.
Cardiac resynchronization therapy with or without a defibrillator.
Excluding valsartan when taken with sacubitril because sacubitril/valsartan is shown as ARNI.
Risk of clinical outcomes by baseline body mass index categories
The incidence rate of the primary outcome of CV death or HHF in the placebo group was highest in the BMI <20 kg/m2 category (24.6 events per 100 patient‐years) while the lowest incidence rate was in the BMI 25 to<30 kg/m2 category (19.3 events per 100 patient‐years). The association between BMI as a continuous variable and all‐cause mortality and CV death are shown in Figure 1 and online supplementary Figure Appendix S1 . When BMI was assessed as a continuous variable, we found that there was an association between BMI <25 kg/m2 and all‐cause mortality, without increased risk at higher BMI values after adjustment for potential confounders.
Figure 1.
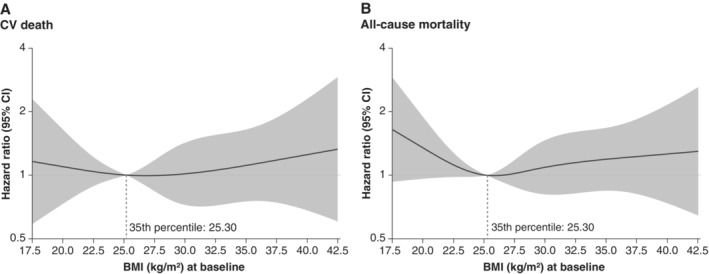
Risk of cardiovascular (CV) and all‐cause mortality according to baseline body mass index (BMI). The spline analyses are adjusted for age, sex, diabetes status, region, left ventricular ejection fraction, estimated glomerular filtration rate and N‐terminal pro‐B‐type natriuretic peptide. CI, confidence interval.
No trend in eGFR decline in the placebo group across BMI categories was observed (−1.96, −2.31, −1.71, −3.66, and −1.83, respectively).
Weight change
Overall, 313 (17.4%) of patients treated with empagliflozin had a weight loss of more than 5% at week 52, and 79 (4.4%) experienced a weight loss of more than 10%. The corresponding numbers in placebo were 230 (12.8%) and 69 (3.8%).
In the BMI <20 kg/m2 category, a slight increase in weight from baseline was seen in both treatment groups, however, weight increase was slightly less with empagliflozin (online supplementary Figure S2 ). In the BMI ≥35 kg/m2, in both treatment groups mean weight decreased from baseline, with early changes more pronounced in the empagliflozin group. In the three middle BMI categories, weight remained relatively stable in the placebo groups whereas modest weight reductions were observed in the empagliflozin group (mean change at week 52 ranging from 0.6 to 1.4 kg).
Figure 2 shows weight change in patients with (n = 504, 13.5%) and without (n = 3226, 86.5%) peripheral oedema at baseline. In patients with baseline peripheral oedema, body weight nominally decreased more than in patients without peripheral oedema, but there was no difference between the treatment groups – the adjusted mean change from baseline to week 12 in those with oedema and treated with empagliflozin was −0.90 kg (95% confidence interval [CI] −1.37 to −0.43) versus −1.17 kg (95% CI −1.63 to −0.72) in those on placebo. In patients without oedema at baseline, changes of body weight were +0.29 kg (95% CI 0.11 to 0.47) in those treated with empagliflozin and −0.68 kg (95% CI −0.86 to −0.50) in patients on placebo.
Figure 2.
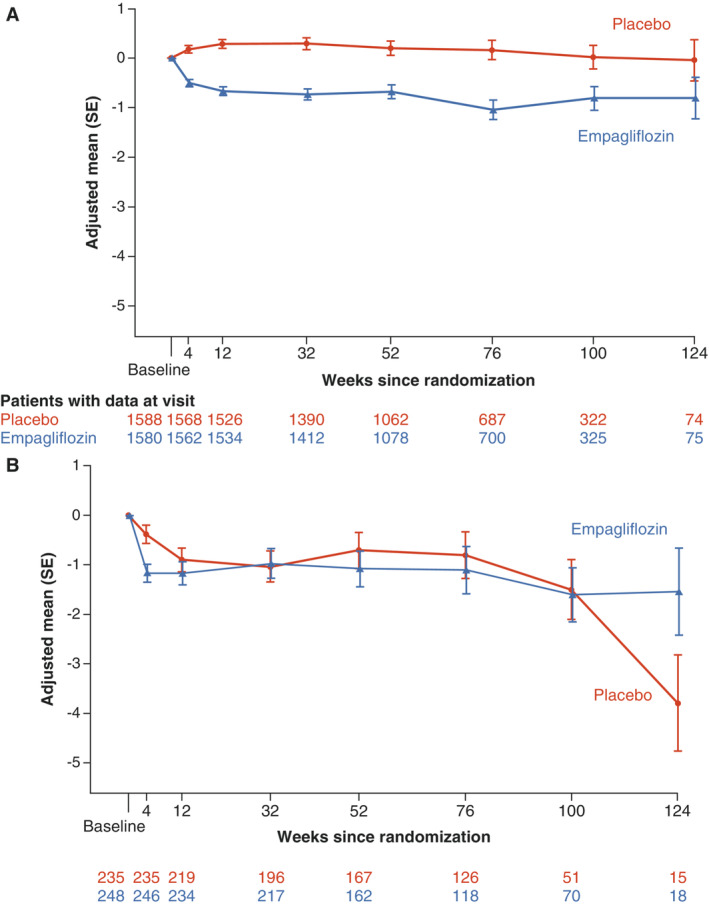
Change in weight by baseline peripheral oedema. Change from baseline in weight over time in patients without (A) and with (B) baseline peripheral oedema. Analysed by a mixed model of repeated measures. SE, standard error.
Weight change and clinical outcomes
In analyses with weight change as a time‐dependent covariate, weight loss was associated with a higher risk of all‐cause mortality in empagliflozin (HR per 1% weight loss: 1.0522 [95% CI 1.0300–1.0749], p < 0.01) and placebo arms (HR per 1% weight loss: 1.0599 [95% CI 1.0288–1.0919], p < 0.01), while weight gain was not associated with a higher risk of all‐cause mortality in placebo (HR per 1% weight gain: 0.9748 [95% CI 0.9337–1.0178], p = 0.25) or empagliflozin (HR per 1% weight gain: 0.9871 [95% CI 0.9437–1.0325]; p = 0.57) arm. A test for differences in these associations across treatment groups revealed no significant interaction between treatment and weight change on the risk of all‐cause mortality (p = 0.94), meaning that the treatment effect of empagliflozin versus placebo was not influenced by weight change when assessed as a time‐dependent covariate.
When analysing the primary endpoint, the pattern was somewhat different: a weight loss with empagliflozin was not associated with the primary endpoint (HR per 1% weight loss: 1.0115 [95% CI 0.9822–1.0417], p = 0.4443), whereas in placebo, weight loss was associated with a slightly higher risk (HR per 1% weight loss 1.0371 [95% CI 1.0067–1.0684], p = 0.0165). Weight gain was on the contrary not associated with the primary endpoint in placebo (HR per 1% weight gain: 1.0160 [95% CI 0.9865–1.0464], p = 0.2911), but associated with a slightly higher risk in empagliflozin (HR per 1% weight gain: 1.0345 [95% CI 1.0051–1.0647], p = 0.0210).
Effect of empagliflozin on clinical outcomes and quality of life by baseline body mass index
Figure 3 shows the effect of empagliflozin on HF endpoints and mortality across the BMI categories. Treatment effect of empagliflozin versus placebo on the primary outcome was consistent across all BMI categories (HR 0.85 [95% CI 0.48–1.50] for BMI < 20 kg/m2; HR 0.66 [95% CI 0.51–0.86] for BMI 20 to <25 kg/m2; HR 0.69 [95% CI 0.54–0.89] for BMI 25 to <30 kg/m2; HR 0.88 [95% CI 0.65–1.18] for BMI 30 to <35 kg/m2; and HR 0.82 [95% CI 0.55–1.23] for BMI ≥35 kg/m2 [p for interaction trend = 0.32]). Empagliflozin also significantly reduced the total number of HHF across all the BMI categories without evidence of treatment heterogeneity (HR 0.81 [95% CI 0.34–1.91] for BMI < 20 kg/m2; HR 0.58 [95% CI 0.41–0.84] for BMI 20 to <25 kg/m2; HR 0.71 [95% CI 0.51–0.98] for BMI 25 to <30 kg/m2; HR 0.68 [95% CI 0.45–1.02] for BMI 30 to <35 kg/m2; and HR 0.94 [95% CI 0.54–1.64] for BMI ≥35 kg/m2 [p for interaction trend = 0.31]). No evidence of treatment modification with respect to empagliflozin versus placebo was observed for all‐cause or CV mortality across all BMI subgroups. Empagliflozin had a favourable impact on the rate of decline of eGFR across the different BMI categories (p for interaction trend = 0.67) (Figure 4A ). Overall, the risk of the renal composite outcome was reduced with empagliflozin versus placebo with a HR 0.50 (95% CI 0.32–0.77), with no evidence of treatment heterogeneity across the different BMI categories (p for interaction trend = 0.76). The mean differences in KCCQ‐CSS change from baseline to week 52 were consistent across the BMI categories (p for interaction trend at week 52 >0.99) (Figure 4B ).
Figure 3.
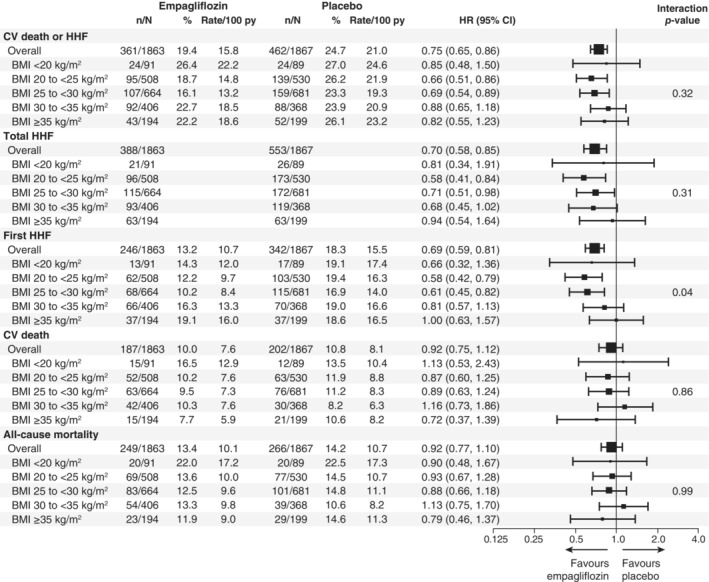
Effects of empagliflozin versus placebo on heart failure outcomes and mortality. BMI, body mass index; CI, confidence interval; CV, cardiovascular; HHF, hospitalization for heart failure; HR, hazard ratio; py, patient‐years.
Figure 4.
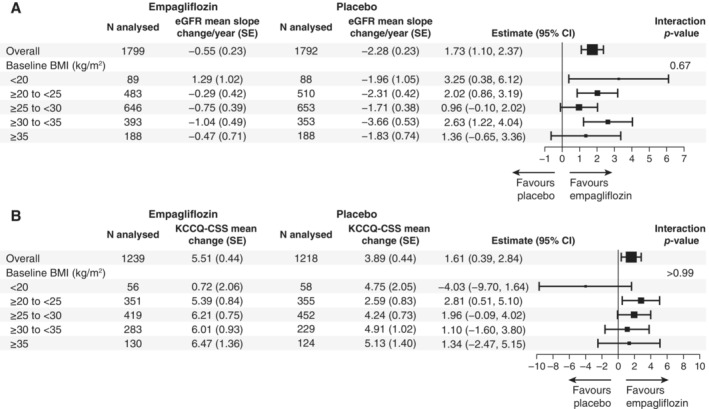
Treatment effect on estimated glomerular filtration rate (eGFR) slope (A) and Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS) (B) according to baseline body mass index (BMI) categories. Forest plot summarizing the treatment effects on eGFR slope estimates and difference in change in KCCQ‐CSS from baseline to week 52 across BMI categories. Between‐group difference in the slope of change during the treatment period in eGFR were analysed using a random intercept random model in the treated set. The model includes age, baseline eGFR, region, baseline diabetes status, sex, baseline left ventricular ejection fraction, treatment, baseline BMI categories, and treatment by baseline BMI categories interaction. CI, confidence interval; SE, standard error.
When analysing BMI as a continuous variable, the effect of empagliflozin on CV death or HHF was consistent across the BMI spectrum. Similar results were obtained for the effect of empagliflozin on the time to first HHF and CV mortality (Figure 5 ).
Figure 5.
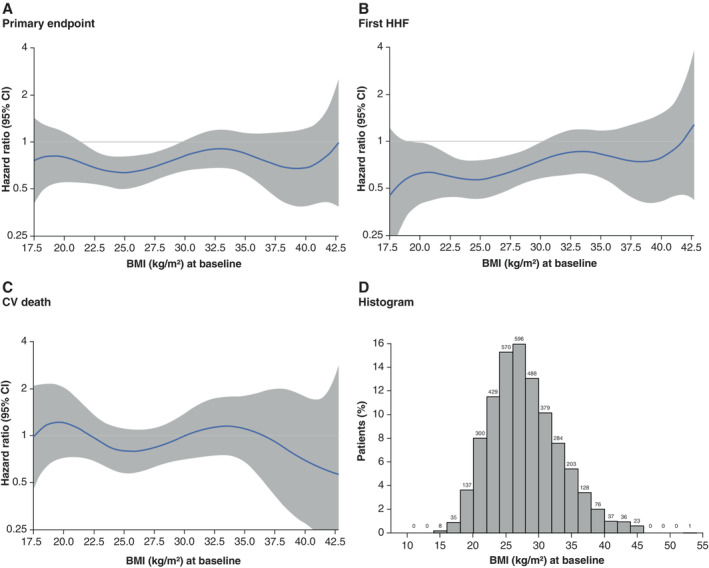
Effect of empagliflozin by body mass index (BMI) as a continuous variable. Spline regression models showing the treatment effect of empagliflozin versus placebo across BMI as a continuous variable for (A) the primary endpoint, (B) first hospitalization for heart failure (HHF), (C) cardiovascular (CV) death, and (D) a histogram with BMI distribution. CI, confidence interval.
Safety
Online supplementary Table Appendix S1 shows adverse events according to baseline BMI categories. Overall, incidence rates of any or serious adverse events were comparable between the treatment groups across all BMI categories. The incidence of symptomatic hypotension, acute renal failure and confirmed hypoglycaemia were similar in both treatment groups across the BMI categories.
Adverse events leading to discontinuation of therapy were highest in the BMI <20 kg/m2 category. There were few cases of genital infections with higher incidences in empagliflozin versus placebo independent of BMI categories.
Discussion
In this post‐hoc analysis of the EMPEROR‐Reduced trial, we report several key findings. First, the incidence of the primary outcome of time to first HHF or CV death was highest among low and high BMI categories. Second, a tendency of slightly greater weight loss with empagliflozin versus placebo was noted in higher BMI categories, and there was no significant weight loss in those with low baseline BMI. Third, we observed that weight loss was associated with a higher risk of all‐cause mortality in both empagliflozin and placebo arms, but despite this, the effect of empagliflozin versus placebo on HF and kidney outcomes was consistent across baseline BMI categories. Lastly, the benefits of empagliflozin on quality of life were consistent across the BMI categories. Overall, these results have important clinical implications for guiding clinical management for patients with HFrEF. They suggest empagliflozin should not be withheld in patients with HFrEF on the basis of their baseline BMI or weight loss.
We observed that the risk of all‐cause mortality in the high BMI category (BMI 30 to <35 kg/m2) was less than half that in the lowest BMI (BMI <20 kg/m2) category. When BMI was assessed as a continuous variable, we found that there was a U‐shaped association between BMI and mortality, with the lowest risk at around BMI 25 kg/m2 after adjusting for NT‐proBNP. In addition, weight loss but not weight gain in empagliflozin and placebo arms was associated with a higher risk of all‐cause mortality. Our findings are consistent with previous studies, which have demonstrated an inverse relation of body weight and weight change with mortality among patients with HF. 21 , 22 , 23 Therefore, distinction between unintentional weight loss versus intentional weight loss with treatment is important. We believe that the weight loss with empagliflozin is mainly driven by loss of calories and sodium in urine, and is not related to the mechanism of unintentional weight loss, which is generally linked to higher mortality. This is supported by the notion that patients without peripheral oedema lost weight with empagliflozin but not with placebo. It is also important to emphasize that weight loss in HFrEF patients may be due to a multitude of concurrent conditions that may increase all‐cause mortality risk, such as incident malignancies and inflammatory diseases. It is also important to highlight that for non‐fatal outcomes, in contrast, the obesity paradox is not well established. Instead, a higher BMI has been previously associated with an increased risk of hospitalizations and the development of various cardiometabolic diseases, including atrial fibrillation and diabetes. 24 , 25 , 26 Our finding of the U‐shaped relationship between the incidence of the primary outcome and BMI categories is in line with this.
In our analyses, we did not observe a consistent association between weight loss or gain and the primary endpoint in the two treatment groups. Moreover, our results demonstrate that despite the association between weight loss and mortality, the beneficial effect of empagliflozin on the primary outcome of time to first HHF or CV death and on the key secondary outcome total HHF was consistent across BMI categories. This is in line with and validates the findings from DAPA‐HF, which showed that the effects of dapagliflozin on all outcomes including HHF were similar across a wide range of BMI categories, 18 i.e. SGLT2 inhibitors have favourable effects in HFrEF despite the obesity paradox. 27 We extend the findings from DAPA‐HF by also reporting consistency of renal benefits according to baseline BMI. We observed a consistent reduction in renal composite events, and slower annualized decline in eGFR with empagliflozin across all categories of BMI. This shows that the beneficial cardiorenal effect of empagliflozin is not modified by baseline BMI. Moreover, the known safety profile of empagliflozin in HF with preserved ejection fraction was confirmed. These aspects are important as some doctors ask whether their leaner patients with HF should receive SGLT2 inhibitors. Our results provide reassurance in this respect.
There are some limitations to this analysis. First, given the retrospective nature of the analysis and despite our adjustment between the two groups, it is possible that several unknown confounders might have biased the results. Second, BMI as a measure of adiposity does not differentiate between lean and fat mass. Moreover, BMI does not measure fat distribution; other estimates of fat distribution such as waist circumference and skinfold thickness may be more accurate but were not captured in this trial. Lastly, regional and racial variability in the diagnostic thresholds and care‐seeking patterns may have influenced the observed associations between BMI and outcomes analysed.
In conclusion, in both treatment arms, weight loss was associated with higher risk of all‐cause mortality in patients with HFrEF. However, the benefits of empagliflozin versus placebo were consistent across all BMI groups. No new safety signals were observed.
Funding
The EMPEROR‐Reduced trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Conflict of interest: Dr Anker reports grants and personal fees from Vifor Int. and Abbott Vascular, and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Brahms, Cardiac Dimensions, Medtronic, Novartis, Occlutech, Servier, V‐Wave, and Vifor Int. Dr Khan reports no conflict. Dr Butler reports consulting fees from BI, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V‐Wave Ltd., and Vifor. Dr Ofstad, Dr Peil and Dr Pfarr are employees of Boehringer Ingelheim. Dr Doehner reports consulting fees from Boehringer Ingelheim related to work on clinical events committee during the conduct of the study and personal fees from Aimediq, Bayer, Boehringer Ingelheim (BI), Medtronic, Pfizer, Sanofi‐Aventis, Sphingotec, Vifor Pharma and research support from EU (Horizon2020), German ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, and ZS Pharma. Dr Sattar reports personal fees from Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi; and grant funding paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics outside the submitted work. Professor Coats declares having received honoraria and/or lecture fees from Astra Zeneca, Boehringer Ingelheim, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia, and Viatris. Dr Filippatos reports lecture fees and/or committee member contributions in clinical trials sponsored by Bayer, Medtronic, Vifor, Servier, Novartis, Amgen, and Boehringer Ingelheim, and research support from the European Union. Dr Ferreira reports consultancy fees from Boehringer Ingelheim, and receives research support from Bayer, Novartis, and AstraZeneca through his institution, the University of Porto. Dr Zannad has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius. Dr Pocock reports consultancy fees from Boehringer Ingelheim. Dr Packer reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Abbvie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, Salamandra, outside the submitted work.
Supporting information
Appendix S1. Supporting Information.
Acknowledgements
Graphical assistance, supported financially by Boehringer Ingelheim, was provided by 7.4 Limited and Elevate Scientific Solutions. Open Access funding by Boehringer Ingelheim.
Data availability statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer‐reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
References
- 1. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 4. Powell‐Wiley TM, Poirier P, Burke LE, Despres JP, Gordon‐Larsen P, Lavie CJ, et al.; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council . Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–43. [DOI] [PubMed] [Google Scholar]
- 6. Shah S, Henry A, Roselli C, Lin H, Sveinbjornsson G, Fatemifar G, et al. Genome‐wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet. 2003;361:1077–83. [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. [DOI] [PubMed] [Google Scholar]
- 9. Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co‐morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162:20–6. [DOI] [PubMed] [Google Scholar]
- 10. Doehner W, von Haehling S, Anker SD. Protective overweight in cardiovascular disease: moving from ‘paradox’ to ‘paradigm’. Eur Heart J. 2015;36:3729–2732. [DOI] [PubMed] [Google Scholar]
- 11. Lena A, Ebner N, Anker MS. Cardiac cachexia. Eur Heart J Suppl. 2019;21(Suppl L):L24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Springer J, Anker SD. Publication trends in cachexia and sarcopenia in elderly heart failure patients. Wien Klin Wochenschr. 2016;128:446–54. [DOI] [PubMed] [Google Scholar]
- 13. Cai X, Yang W, Gao X, Chen Y, Zhou L, Zhang S, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta‐analysis. Obesity. 2018;26:70–80. [DOI] [PubMed] [Google Scholar]
- 14. Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, et al. Long‐term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active‐controlled, parallel‐group, randomized, 78‐week open‐label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshida A, Kusakabe H, Takamura T, Kaku K, Suganami H. The SGLT2 inhibitor tofogliflozin reduces weight in people with type 2 diabetes due to fluid loss initially and to lipolysis in late. Diabetes. 2018;67:1152. [Google Scholar]
- 16. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 18. Adamson C, Jhund PS, Docherty KF, Bělohlávek J, Chiang CE, Diez M, et al.; EMPEROR‐Reduced Trail Committees and Investigators. Efficacy of dapagliflozin in heart failure with reduced ejection fraction according to body mass index. Eur J Heart Fail. 2021;23:1662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. Evaluation of the effect of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail. 2019;21:1270–8. [DOI] [PubMed] [Google Scholar]
- 20. World Heath Organisation . Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894); 2000. [PubMed]
- 21. Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–79. [DOI] [PubMed] [Google Scholar]
- 22. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M; ADHERE Scientific Advisory Committee and Investigators . An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. [DOI] [PubMed] [Google Scholar]
- 23. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Waist‐to‐hip ratio and mortality in heart failure. Eur J Heart Fail. 2018;20:1269–77. [DOI] [PubMed] [Google Scholar]
- 24. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Ccardiovascular and Stroke Nursing; Council on Hypertension, and Council on Quality and Outcomes Research . Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–e78. [DOI] [PubMed] [Google Scholar]
- 25. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, et al.; American Heart Association Electrocardiography and Arrhythmias Committee and Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Lifestyle and Cardiometabolic Health . Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e72. [DOI] [PubMed] [Google Scholar]
- 26. Javed S, Gupta D, Lip GYH. Obesity and atrial fibrillation: making inroads through fat. Eur Heart J Cardiovasc Pharmacother. 2021;7:59–67. [DOI] [PubMed] [Google Scholar]
- 27. Carbone S, daSilva‐deAbreu A, Lavie CJ. The sodium‐glucose co‐transporter 2 inhibitor dapagliflozin improves prognosis in systolic heart failure independent of the obesity paradox. Eur J Heart Fail. 2021;23:1673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer‐reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.


