Abstract
It has been assumed that connections between the postparotid terminal branches of the facial nerve are purely motor. However, the nature of their fibers remains unexplored. The aim of this study is to determine whether these connections comprise motor fibers exclusively. In total 17 connections between terminal facial nerve branches were obtained from 13 different facial nerves. Choline acetyltransferase antibody (ChAT) was used to stain the fibers in the connections and determine whether or not all of them were motor. All connections contained ChAT positive and negative fibers. The average number of fibers overall was 287 (84–587) and the average proportion of positive fibers was 63% (37.7%–91.5%). In 29% of the nerves, >75% of the fibers were ChAT+ (strongly positive); in 52.94%, 50%–75% were ChAT+ (intermediately positive); and in 17.65%, <50% were ChAT+ (weakly positive). Fibers traveling inside the postparotid terminal cranial nerve VII branch connections are not exclusively motor.
Keywords: choline acetyltransferase, facial nerve, facial nerve motor disorders, immunohistochemistry, nerve fibers
1. INTRODUCTION
Connections between the five terminal branches of the facial nerve (cranial nerve [cn] VII) have been described since the second half of the nineteenth century. They constitute a structure with multiple connected branches called the “subparotid plexus” (Hovelaque, 1927; Sappey, 1879) or “parastenon plexus” (Pons‐Tortella, 1947). Most authors have used this description and it has served as a basis for different proposed facial nerve classifications (Alomar, 2021; Bernstein & Nelson, 1984; Davis et al., 1956; Katz & Catalano, 1987; Kitamura & Yamazaki, 1958; Martínez Pascual et al., 2019; Myint et al., 1992; Tzafetta & Terzis, 2010).
Connections between facial nerve branches have been found more frequently in the temporofacial division (TF). This has been attributed to its greater number of branches and its plexiform nature; the cervicofacial division (CF) supplies fewer branches, so connections between them are less common (Davis et al., 1956; Diamond et al., 2011; Lineaweaver et al., 1997; Martínez Pascual et al., 2019; Pons‐Tortella, 1947; Salame et al., 2002; Tansatit et al., 2015).
It has been assumed that the fibers within these connections are motor because the five terminal branches of the facial nerve supply the mimic muscles, and the sensory innervation of the face depends on the trigeminal nerve (cn V) (Shoja et al., 2014). However, branches of cn VII also communicate with terminal ramifications of cn V in the face: the auriculotemporal (Kwak et al., 2004; Namking et al., 1994; Tansatit et al., 2015), supraorbital (Hwang et al., 2005; Li et al., 2009; Martínez Pascual et al., 2019), infraorbital (Hu et al., 2007; Hwang et al., 2004; Martínez Pascual et al., 2019; Tansatit et al., 2016), and mental (Hwang et al., 2007; Kim et al., 2009; Martínez Pascual et al., 2019; Touré et al., 2019) nerves, or the well‐known connection between the lingual nerve and the chorda tympani conveying the sense of taste (Dixon, 1899; Kwak et al., 2004; Hwang et al., 2007; Diamond et al., 2011; Shoja et al., 2014; Takezawa & Kageyama, 2015).
Different types of fibers have been proposed to constitute the VII‐V connections: autonomic (Bowden & Mahran, 1960; Lewy et al., 1938; Tansatit et al., 2015), motor (Conley, 1964; Martin & Helsper, 1957; Odobescu et al., 2012) or sensory (Baumel, 1974; Cobo, Abbate, et al., 2017; Cobo, Solé‐Magdalena, et al., 2017; Odobescu et al., 2012; Yang et al., 2013). Thus, non‐motor fibers of VII‐V connections could continue to travel through the postparotid facial connections, so not all the fibers inside those connections are necessarily motor type. However, all these studies of the VII‐VII and V‐VII connections are based on anatomical dissection, which does not reveal the real functions of the component fibers.
Therefore, the goal of our study is to determine, using specific immunohistochemical techniques, whether the connections between the terminal branches of the facial nerve are purely motor or whether they also carry other types of fiber.
2. MATERIALS AND METHODS
The study was carried out on 13 hemiheads from embalmed adult Caucasian bodies (seven men, five women) from the Body Donations and Dissecting Rooms Centre of the Complutense University of Madrid. The average age of the cadavers was 83 years (range 75–90 years) at the time of death.
The authors state that every effort was made to follow all local and international ethical guidelines and laws that pertain to the use of human cadaveric donors in anatomical research (Iwanaga et al., 2022).
2.1. Microdissection
The extrapetrous course of 13 facial nerves (seven right, six left) was dissected from proximal to distal using microsurgical forceps and scissors with the help of surgical loupes (2.5x) (Optimedic®). The facial nerve was dissected and the postparotid terminal facial‐facial connections were identified, sectioned, and extracted for processing.
2.2. Immunohistochemistry
Immunohistochemistry was performed in the Section of Anatomy from Department of Neuroscience at the University of Padova and the Department of Immunology, Ophthalmology and ENT at the Complutense University School of Medicine in Madrid. The connections were processed and embedded in paraffin blocks. Transverse sections (6 μm thickness) were cut with a microtome and mounted on slides, deparaffinized, and rehydrated before staining. Antigen retrieval was performed with sodium citrate (pH 6.1) for 20 min at 95°C. The sections were then washed in phosphate‐buffered saline (PBS) and placed in 1% hydrogen peroxide (H2O2) in PBS for 10 min at room temperature, and then put into blocking buffer (0.2% bovine serum albumin in PBS) for 1 h at room temperature. After washing with PBS, they were incubated for 24 h at 4°C with the primary antibody anti‐choline acetyl transferase (ChAT) (Gene Tex® [N1N3]; 1:800). A negative control was performed for each different sample. The slides were washed with PBS and incubated with secondary antibody (goat anti‐rabbit, Jackson Immunoreserch®; 1:300) in blocking buffer for 1 h at room temperature. After further washing, color was developed with DAKO chromogen for 30 s. The samples were washed with distilled water and finally counterstained with hematoxylin, dehydrated and mounted.
2.3. Images analysis
The immunohistochemical images were photographed under a microscope (Nikon E800M). Motor axons (ChAT positive) (Figure 1) were counted in every sample using ImageJ Fiji 1.52p software (National Institutes of Health®) with 20× magnification. The connections were classified on the basis of number of motor fibers: strongly positive (>75% ChAT+ fibers), intermediately positive (50%–75% ChAT+ fibers) and weakly positive (< 50% ChAT+ fibers) (Figures 2A–C).
FIGURE 1.
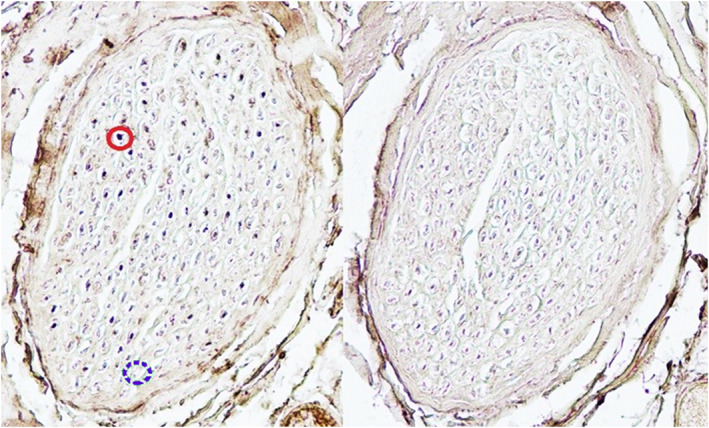
Example of images used for counting axons. The continuous circle shows a ChAT+ motor fiber. The discontinuous circle shows a ChAT− fiber.
FIGURE 2.
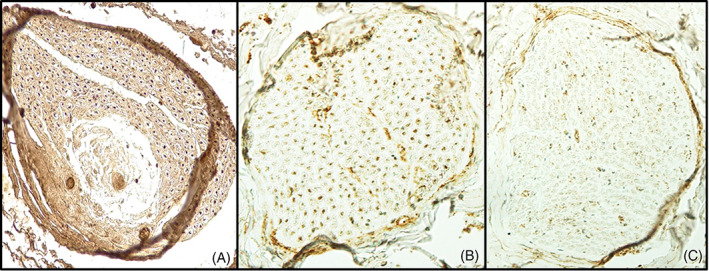
Classification of facial nerve connections based on number of motor fibers. (A) Strongly positive connection (>75% of ChAT+); (B) Intermediately positive (50%–75% of ChAT+); (C) Weakly positive (<50% of ChAT+).
2.4. Statistical analysis
Both descriptive and analytical statistics were used; percentages, means, ranges, and standard deviations were collected and compared. A one‐factor experimental design was used to detect significant differences in the average number of fibers with respect to side. The Kolmogorov–Smirnov Test was used to determine the normality of the underlying data distribution. A significance level alpha = 0.05 was used for all tests. SPSS software version 22 (IBM Corporation, Armonk) was used for the analyses.
3. RESULTS
A total of 17 VII‐VII connections were analyzed. These connections were more frequent on the left side (10 connections) than on the right (seven). Nine of the connections were in male specimens while the other eight were in female cadavers. Tables 1 and 2 show the distribution of connections by side and sex respectively.
TABLE 1.
Distribution of facial nerve connections by side.
| Name of connection (n = 17) | Average N° fibers | Right (7) | Left (10) | Total % | |||
|---|---|---|---|---|---|---|---|
| ChAT+ | ChAT− | ChAT+ | ChAT− | ChAT+ | ChAT− | ||
|
Temporo‐temporal (n = 1; 1 R) |
84 |
76.2% (64/84) |
23.8% (20/84) |
— | — |
76.2% (64/84) |
23.8% (20/84) |
|
Temporo‐zygomatic (n = 2; 2 L) |
234 (134–355) |
— | — |
46.1% (216/469) |
53.9% (253/469) |
46.1% (216/469) |
53.9% (253/469) |
|
Zygomatico‐zygomatic (n = 2; 1 R, 1 L) |
343 (259–406) |
56.6% (241/426) |
43.4% (185/426) |
91.5% (237/259) |
8.5% (22/259) |
69.8% (478/685) |
30.2% (207/685) |
|
Zygomatico‐buccal (n = 6; 2 R, 4 L) |
312 (144–587) |
60.1% (345/574) |
39.9% (229/574) |
51.5% (664/1271) |
48.5% (633/1271) |
53.9% (1009/1871) |
46.1% (862/1871) |
|
Bucco‐buccal (n = 1; 1 R) |
115 |
80% (92/115) |
20% (23/115) |
— | — |
80% (92/115) |
20% (23/115) |
|
Bucco‐mandibular (n = 3; 1 R, 2 L) |
286 (259–322) |
65.2% (167/256) |
34.8% (89/256) |
69.3% (417/602) |
30.7% (185/602) |
68.1% (584/858) |
31.9% (274/858) |
|
Mandibulo‐cervical (n = 2; 1 R, 1 L) |
399 (232–566) |
75% (174/232) |
25% (58/232) |
64.1% (363/566) |
35.9% (203/566) |
67.3% (537/798) |
32.7% (261/798) |
Abbreviations: L, left; n, number of samples; R, right.
TABLE 2.
Distribution of facial nerve connections by sex.
| Name of connection (n = 17) | Average N° fibers | Male (9) | Female (8) | Total % | |||
|---|---|---|---|---|---|---|---|
| ChAT+ | ChAT− | ChAT+ | ChAT− | ChAT+ | ChAT− | ||
|
Temporo‐temporal (n = 1; 1 M) |
84 |
76.2% (64/84) |
23.8% (20/84) |
— | — |
76.2% (64/84) |
23.8% (20/84) |
|
Temporo‐zygomatic (n = 2; 2 M) |
234 (134–355) |
46.1% (216/469) |
53.9% (253/469) |
— | — |
46.1% (216/469) |
53.9% (253/469) |
|
Zygomatico‐zygomatic (n = 2; 1 M, 1 F) |
343 (259–406). |
56.6% (241/426) |
43.4% (185/426) |
91.5% (237/259) |
8.5% (22/259) |
69.8% (478/685) |
30.2% (207/685) |
|
Zygomatico‐buccal (n = 6 3 M, 3 F) |
312 (144–587) |
55% (639/1161) |
45% (522/1161) |
52.1% (370/710) |
47.9% (340/710) |
53.9% (1009/1871) |
46.1% (862/1871) |
|
Bucco‐buccal (n = 1; 1 F) |
115 | — | — |
80% (92/115) |
20% (23/115) |
80% (92/115) |
20% (23/115) |
|
Bucco‐mandibular (n = 3; 2 M, 1 F) |
286 (259–322) |
75.3% (435/578) |
24.7% (143/578) |
53.2% (149/280) |
46.8% (131/280) |
68.1% (584/858) |
31.9% (274/858) |
|
Mandibulo‐cervical (n = 2; 2 F) |
399 (232–566) |
— | — |
67.3% (537/798) |
31.7% (261/798) |
67.3% (537/798) |
31.7% (261/798) |
Abbreviations: F, female; L, left; M, male; n, number of samples; R, right.
The different types of connections are reported first, followed by a global overview.
3.1. Temporo‐temporal
A temporo‐temporal (tt) connection was found in one case. It had 84 fibers, 76.2% (64/84) of them ChAT positive (ChAT+) and the other 23.8% (20/84) ChAT negative (ChAT−). Therefore, this connection was strongly positive (Figure 3A).
FIGURE 3.
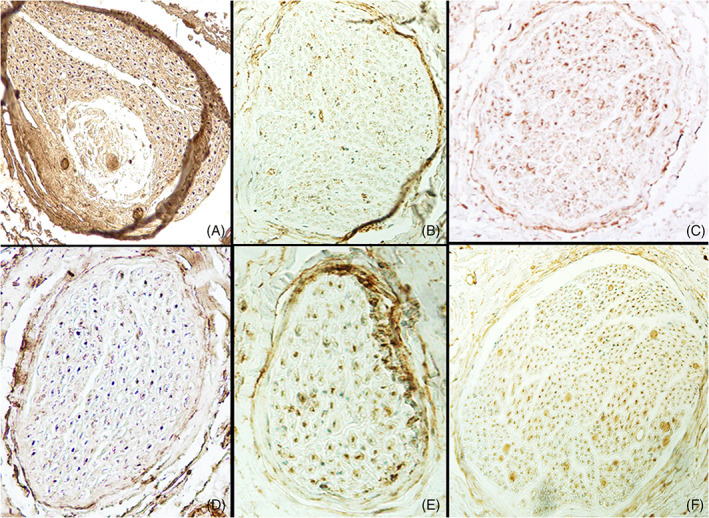
Different types of terminal facial nerve connections. (A) Temporo‐temporal (strongly +); (B) Temporo‐zygomatic (weakly +); (C) Zygomatic‐zygomatic (intermediately +); (D) Bucco‐buccal (strongly +); (E) Bucco‐mandibular (intermediately +); (F) Mandibulo‐cervical (strongly +).
3.2. Temporo‐zygomatic
Two temporo‐zygomatic (tz) connections were found. The number of fibers in them totalled 469, and the average was 234 (range 134–335); 46.1% (216/469) were ChAT+ while 53.9% (253/469) were ChAT−. Both tz connections were weakly positive (Figure 3B).
3.3. Zygomatic‐zygomatic
There were two zygomatic‐zygomatic (zz) connections. The number of fibers in them totalled 685, and the average was 343 (259–426); 69.8% (478/685) were ChAT+ and 30.2% (207/685) were ChAT−. One connection was classed as strongly positive while the other was intermediately positive (Figure 3C).
3.4. Zygomatic‐buccal
The zygomatic‐bucal (zb) connection was the most frequent, being found in six cases. Five of the buccal branches (b) arose from the TF division and one from the CF division. The total number of fibers was 1871 and the average was 312 (144–587); 53.9% (1009/1871) were ChAT+ and 46.1% (862/1871) were ChAT−. Five were intermediately positive and the other was weakly positive (Figure 2B).
3.5. Bucco‐buccal
We found just one bucco‐buccal (bb) connection. Both b branches belonged to the TF division. It had 115 fibers, 20% (23/115) of them ChAT− and 80% (92/115) ChAT+, so it was a strongly positive connection (Figure 3D).
3.6. Bucco‐mandibular
There was a bucco‐mandibular (bm) connection in three facial nerves. Two b branches came from the TF division and the other from the CF. The total number of fibers was 858 and the average was 286 (259–322), 68.1% (584/858) of them ChAT+ and 31.9% (274/858) ChAT−. Two connections were intermediately positive while the other was strongly positive (Figure 3E).
3.7. Mandibulo‐cervical
There was a mandibulo‐cervical (mc) connection in two facial nerves. These had the highest number of fibers, averaging 399 (232–566) with a total number of 798, 32.7% (261/798) being ChAT− and 67.3% (537/798) ChAT+. One connection was strongly positive and the other intermediately positive (Figure 3F).
3.8. Global view
The average number of fibers in the VII‐VII connections overall was 287 with a standard deviation of 145.46 (range 84–587), and the average proportion of positive fibers was 63% with a standard deviation of 15% (range 37.7%–91.5%).
The average number of fibers on the left side was 319 with a standard deviation of 146.49 (range 134–587), and the average proportion of positive fibers was 58.9% with a standard deviation of 17.2% (range 37.6%–91.5%). In contrast, the average number of fibers in the right side was 241 with a standard deviation of 141.5 (range 84–430), and the average proportion of positive fibers was 68.9% with a standard deviation of 9.82% (range 55.6%–80%) (Figure 4).
FIGURE 4.
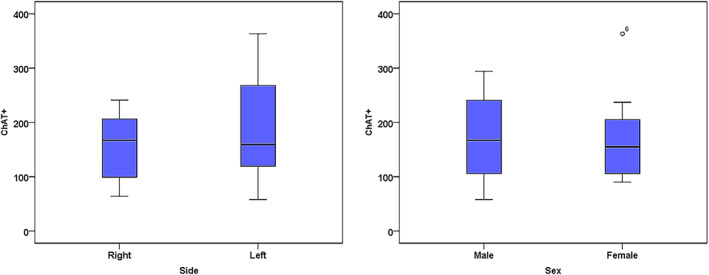
Box plot diagram of ChAT+ fibers by side and sex. The number of ChAT+ fibers was more variable on the left side than the right, and more variable in males than females, but these differences were not statistically significant.
The average number of fibers in males was 302 with a standard deviation of 164.81 (range 84–587) and the average proportion of positive fibers was 61.21% with a standard deviation of 14.03% (range 43.3%–83.2%). The average number of fibers in females was 270 with a standard deviation of 129.3 (range 115–566) and the average proportion of positive fibers was 64.9% with a standard deviation of 17.1% (range 37.6%–91.5%) (Figure 4).
The distributions of both ChAT+ and ChAT− fibers was normal (Kolmogorov–Smirnov Test p‐values = 0.821 and 0.871, respectively) and the ANOVA table associated with the one factor experimental design corroborated the hypothesis of equity between the average numbers of positive fibers by side (p‐value = 0.429) and of negative fibers by side (p‐value = 0.273).
Similar results were obtained for sex (p‐values = 0.926 for ChAT+ fibers and 0.483 for ChAT− fibers). Therefore, there were no statistically significant side or sex differences.
Strongly positive ChAT+ connections were found in 29% of the nerves in the sample (five cases in 17), intermediately positive in 52.94% (nine cases in 17) and weakly positive in 17.65% (three cases in 17).
4. DISCUSSION
Previous studies of VII‐VII connections were based on anatomical dissection, so the real functions of those fibers could not be established. No other immunohistochemical studies of the types of fibers in the postparotid facial connections in humans were found in the literature studied, so our results cannot be compared with others.
ChAT antibody has been proved specific/selective for motor axons in peripheral nerves (Castro et al., 2008; Courties et al., 2020; Kim et al., 2018; Lago et al., 2007; Lago & Navarro, 2006; Zhou et al., 2021), even after nerve injury (Castro et al., 2008; Kim et al., 2018; Lago et al., 2007; Lago & Navarro, 2006; Zhou et al., 2021). Therefore, it was chosen in this study to establish whether or not the VII‐VII connections are exclusively motor. The results showed that every connection had ChAT+ and ChAT– fibers, so they all contained both motor and non motor fibers.
The connection with the highest percentage of ChAT− fibers was the temporo‐zygomatic (53.9%), while the bucco‐buccal had the highest percentage of ChAT+ fibers (80%). This could be because the buccal branches innervate the middle and lower facial thirds, where there are more muscles (Le Louarn, 2009) than in the upper third (Abramo, 2018; Abramo et al., 2016); bucco‐buccal connections could therefore carry a greater motor axonal load.
Connections between the sensory terminal ramifications of cn V in the face and the terminal branches of the extrapetrous cn VII have already been described with the auriculotemporal (Kwak et al., 2004; Namking et al., 1994; Tansatit et al., 2015), supraorbital (Hwang et al., 2005; Li et al., 2009; Martínez Pascual et al., 2019), infraorbital (Hu et al., 2007; Hwang et al., 2004; Martínez Pascual et al., 2019; Tansatit et al., 2016), and mental (Hwang et al., 2007; Kim et al., 2009; Martínez Pascual et al., 2019; Touré et al., 2019) nerves. The nature of those connections has been discussed by many authors. Some believe they can be autonomic (Bowden & Mahran, 1960; Lewy et al., 1938; Tansatit et al., 2015), some motor (Conley, 1964; Martin & Helsper, 1957; Odobescu et al., 2012) and some sensory (Baumel, 1974; Cobo, Solé‐Magdalena, et al., 2017; Odobescu et al., 2012; Yang et al., 2013). Thus, assuming that these fibers continue to travel from the V‐VII connection through the rest of the extrapetrous facial nerve, it follows that the terminal branches of the facial nerve and their connections also have non‐motor fibers (Cattaneo & Pavesi, 2014; Cobo, Solé‐Magdalena, et al., 2017).
Facial muscles typically lack proprioceptors, and facial proprioceptive impulses travel via branches of the trigeminal nerve to the central nervous system (Cattaneo & Pavesi, 2014; Cobo, Solé‐Magdalena, et al., 2017). Those propioceptive fibers from the trigeminal nerve in facial‐trigeminal connections could also be conveyed in VII‐VII connections and could innervate sensory structures in facial muscles that substitute for typical muscle spindles in facial proprioception (Cattaneo & Pavesi, 2014; Cobo, Abbate, et al., 2017).
These findings could explain some of the feelings experienced by a patient with facial palsy after partial recovery, such as painful pressure at some points of the face (e.g. the zygomatic muscles, chin, forehead) (Valls‐Solé, 2013). Furthermore, muscle mass contraction or synkinesis, both observed in facial palsy patients, could also be related to this mixture of fibers traveling through the multiple connections in the face (Raslan et al., 2020; Valls‐Solé, 2013). Indeed, some authors explain synkinesis in terms of activation of latent nervous circuits pre‐existing in the healthy subject (Ton Van & Giot, 2021). We believe that these preexisting neural circuits can be conveyed by VII‐VII connections.
Therefore, we can affirm that the nerve fibers traveling inside the postparotid terminal facial branch connections are not exclusively motor. The nature of the fibers that do not stain for ChAT still needs to be studied using antibodies specific/selective for different types of sensory and autonomic nerves.
ACKNOWLEDGMENTS
The authors thank Martina Contran and Anna Agostinelli from Padova University and all the technicians from the Body Donations and Dissecting Rooms Centre of the Complutense University of Madrid for their invaluable help. The authors also sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind's overall knowledge and improve patient care. Therefore, these donors and their families deserve our highest gratitude (Iwanaga et al., 2021).
Martínez‐Pascual, P. , Pérez‐Lloret, P. , Alcaide, E. M. , Sanz‐García, C. , Simón de Blas, C. , Sanudo, J. , Konschake, M. , Porzionato, A. , De Caro, R. , & Macchi, V. (2023). Connections between postparotid terminal branches of the facial nerve: An immunohistochemistry study. Clinical Anatomy, 36(1), 28–35. 10.1002/ca.23972
Funding information Atracción de Talento de la Comunidad Autónoma de Madrid, Grant/Award Numbers: 2019‐T1/BMD‐13313, PID2020‐113299RA‐I00
REFERENCES
- Abramo, A. C. (2018). Muscle insertion and strength of the muscle contraction as guidelines to enhance duration of the botulinum toxin effect in the upper face. Aesthetic Plastic Surgery, 42(5), 1379–1387. [DOI] [PubMed] [Google Scholar]
- Abramo, A. C. , Amaral, T. P., Do , Lessio, B. P. , & Lima, G. A., De . (2016). Anatomy of forehead, glabellar, nasal and orbital muscles, and their correlation with distinctive patterns of skin lines on the upper third of the face: Reviewing concepts. Aesthetic Plastic Surgery, 40(6), 962–971. [DOI] [PubMed] [Google Scholar]
- Alomar, O. S. K. (2021). New classification of branching pattern of facial nerve during parotidectomy: A cross sectional study. Annals of Medicine and Surgery, 62, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel, J. (1974). Trigeminal‐facial nerve communications. Archives of Otolaryngology, 99(1), 34–44. [DOI] [PubMed] [Google Scholar]
- Bernstein, L. , & Nelson, R. H. (1984). Surgical anatomy of the extraparotid distribution of the facial nerve. Archives of Otolaryngology, 110(3), 177–183. [DOI] [PubMed] [Google Scholar]
- Bowden, R. E. M. , & Mahran, Z. Y. (1960). Experimental and histological studies of the extrapetrous portion of the facial nerve and its communications with the trigeminal nerve in the rabbit. Journal of Anatomy, 94(Pt 3), 375–386. [PMC free article] [PubMed] [Google Scholar]
- Castro, J. , Negredo, P. , & Avendaño, C. (2008). Fiber composition of the rat sciatic nerve and its modification during regeneration through a sieve electrode. Brain Research, 23(1190), 65–77. [DOI] [PubMed] [Google Scholar]
- Cattaneo, L. , & Pavesi, G. (2014). The facial motor system. Neuroscience and Biobehavioral Reviews, 38, 135–159. [DOI] [PubMed] [Google Scholar]
- Cobo, J. L. , Abbate, F. , de Vicente, J. C. , Cobo, J. , & Vega, J. A. (2017). Searching for proprioceptors in human facial muscles. Neuroscience Letters, 15(640), 1–5. [DOI] [PubMed] [Google Scholar]
- Cobo, J. L. , Solé‐Magdalena, A. , Menéndez, I. , de Vicente, J. C. , & Vega, J. A. (2017). Connections between the facial and trigeminal nerves: Anatomical basis for facial muscle proprioception. JPRAS Open, 12, 9–18. [Google Scholar]
- Conley, J. J. (1964). Accesory neuromotor pathways to the face. Transactions – American Academy of Ophthalmology and Otolaryngology, 68, 1064–1067. [PubMed] [Google Scholar]
- Courties, A. , Belle, M. , Senay, S. , Cambon‐Binder, A. , Sautet, A. , Chédotal, A. , Berenbaum, F. , & Sellam, J. (2020). Clearing method for 3‐dimensional immunofluorescence of osteoarthritic subchondral human bone reveals peripheral cholinergic nerves. Scientific Reports, 10(1), 8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. A. , Anson, B. J. , Budinger, J. M. , & Kurth, L. R. (1956). Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surgery, Gynecology & Obstetrics, 102(4), 385–412. [PubMed] [Google Scholar]
- Diamond, M. , Wartmann, C. T. , Tubbs, R. S. , Shoja, M. M. , Cohen‐Gadol, A. A. , & Loukas, M. (2011). Peripheral facial nerve communications and their clinical implications. Clinical Anatomy, 24(1), 10–18. [DOI] [PubMed] [Google Scholar]
- Dixon, A. F. (1899). The sensory distribution of the facial nerve in man. J Anat Physiol, 33(Pt 3), 471–92. [PMC free article] [PubMed] [Google Scholar]
- Hovelaque, A. (1927). Anatomie des nerfs craniens et rachidiens et du système grand sympathique chez l'homme. G. Doin et Cie. [Google Scholar]
- Hu, K. S. , Kwak, J. , Koh, K. S. , Abe, S. , Fontaine, C. , & Kim, H. J. (2007). Topographic distribution area of the infraorbital nerve. Surgical and Radiologic Anatomy, 29(5), 383–388. [DOI] [PubMed] [Google Scholar]
- Hwang, K. , Han, J. Y. , Battuvshin, D. , Kim, D. J. , & Chung, I. H. (2004). Communication of infraorbital nerve and facial nerve: Anatomic and histologic study. The Journal of Craniofacial Surgery, 15(1), 88–91. [DOI] [PubMed] [Google Scholar]
- Hwang, K. , Hwang, J. H. , Cho, H. J. , Kim, D. J. , & Chung, I. H. (2005). Horizontal branch of the supraorbital nerve and temporal branch of the facial nerve. The Journal of Craniofacial Surgery, 16(4), 647–649. [DOI] [PubMed] [Google Scholar]
- Hwang, K. , Jin, S. , Park, J. H. , Kim, D. J. , & Chung, I. H. (2007). Relation of mental nerve with mandibular branch of the facial nerve. The Journal of Craniofacial Surgery, 18(1), 165–168. [DOI] [PubMed] [Google Scholar]
- Iwanaga, J. , Singh, V. , Ohtsuka, A. , Hwang, Y. , Kim, H. J. , Moryś, J. , Ravi, K. S. , Ribatti, D. , Trainor, P. A. , Sañudo, J. R. , Apaydin, N. , Şengül, G. , Albertine, K. H. , Walocha, J. A. , Loukas, M. , Duparc, F. , Paulsen, F. , Sol, M., Del , Adds, P. , … Tubbs, R. S. (2021). Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clinical Anatomy, 34(1), 2–4. [DOI] [PubMed] [Google Scholar]
- Iwanaga, J. , Singh, V. , Takeda, S. , Ogeng'o, J. , Kim, H. J. , Moryś, J. , Ravi, K. S. , Ribatti, D. , Trainor, P. A. , Sañudo, J. R. , Apaydin, N. , Sharma, A. , Smith, H. F. , Walocha, J. A. , Hegazy, A. M. S. , Duparc, F. , Paulsen, F. , Sol, M., Del , Adds, P. , … Tubbs, R. S. (2022). Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: Recommendations from Anatomical Journal Editors‐in‐Chief. Clinical Anatomy, 35(4), 526–528. [DOI] [PubMed] [Google Scholar]
- Katz, A. , & Catalano, P. (1987). The clinical significance of the various anastomotic branches of the facial nerve. Report of 100 patients. Archives of Otolaryngology–Head & Neck Surgery, 113(9), 959–962. [DOI] [PubMed] [Google Scholar]
- Kim, D. I. , Nam, S. H. , Nam, Y. S. , Lee, K. S. , Chung, R. H. , & Han, S. H. (2009). The marginal mandibular branch of the facial nerve in Koreans. Clinical Anatomy, 22(2), 207–214. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kobayashi, S. , Shimizu‐Okabe, C. , Okabe, A. , Moon, C. , Shin, T. , & Takayama, C. (2018). Changes in the expression and localization of signaling molecules in mouse facial motor neurons during regeneration of facial nerves. Journal of Chemical Neuroanatomy, 88, 13–21. [DOI] [PubMed] [Google Scholar]
- Kitamura, T. , & Yamazaki, H. (1958). Distribution of facial nerve in the parotid gland. Japan Journal of Otolaryngology, 61, 141–144. [Google Scholar]
- Kwak, H. H. , Park, H. D. , Youn, K. H. , Hu, K. S. , Koh, K. S. , Han, S. H. , & Kim, H. J. (2004). Branching patterns of the facial nerve and its communication with the auriculotemporal nerve. Surgical and Radiologic Anatomy, 26(6), 494–500. [DOI] [PubMed] [Google Scholar]
- Lago, N. , & Navarro, X. (2006). Correlation between target reinnervation and distribution of motor axons in the injured rat sciatic nerve. Journal of Neurotrauma, 23(2), 227–240. [DOI] [PubMed] [Google Scholar]
- Lago, N. , Rodríguez, F. J. , Guzmán, M. S. , Jaramillo, J. , & Navarro, X. (2007). Effects of motor and sensory nerve transplants on amount and specificity of sciatic nerve regeneration. Journal of Neuroscience Research, 85(12), 2800–2812. [DOI] [PubMed] [Google Scholar]
- Louarn, C., Le . (2009). Midface region: Functional anatomy, ageing process, indications and concentric malar lift. Annales de Chirurgie Plastique et Esthétique, 54(5), 411–420. [DOI] [PubMed] [Google Scholar]
- Lewy, F. H. , Groff, R. A. , & Grant, F. C. (1938). Autonomic innervation of the face II. An experimental study. Archives of Neurology & Psychiatry, 39, 1238–1249. [Google Scholar]
- Li, C. , Jiang, X. Z. , & Zhao, Y. F. (2009). Connection of trigeminal nerve and facial nerve branches and its clinical significance. Shanghai Kou Qiang Yi Xue, 18(5), 545–550. [PubMed] [Google Scholar]
- Lineaweaver, W. , Rhoton, A. , & Habal, M. B. (1997). Microsurgical anatomy of the facial nerve. The Journal of Craniofacial Surgery, 8(1), 6–10. [PubMed] [Google Scholar]
- Martin, H. , & Helsper, J. (1957). Spontaneous return of function following surgical section or excision of the seventh cranial nerve in the surgery of parotid tumours. Annals of Surgery, 146(5), 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Pascual, P. , Maranillo, E. , Vázquez, T. , Simon de Blas, C. , Lasso, J. M. , & Sañudo, J. R. (2019). Extracranial course of the facial nerve revisited. The Anatomical Record, 302(4), 599–608. [DOI] [PubMed] [Google Scholar]
- Myint, K. , Azian, A. L. , & Khairul, F. A. (1992). The clinical significance of the branching pattern of the facial nerve in Malaysian subjects. The Medical Journal of Malaysia, 47(2), 114–121. [PubMed] [Google Scholar]
- Namking, M. , Boonruangsri, P. , Woraputtaporn, W. , & Guldner, F. H. (1994). Communication between the facial and auriculotemporal nerves. Journal of Anatomy, 185(Pt 2), 421–426. [PMC free article] [PubMed] [Google Scholar]
- Odobescu, A. , Williams, H. B. , & Gilardino, M. S. (2012). Description of a communication between the facial and zygomaticotemporal nerves. Journal of Plastic, Reconstructive & Aesthetic Surgery, 65(9), 1188–1192. [DOI] [PubMed] [Google Scholar]
- Pons‐Tortella, E. (1947). The parotid plexus of the facial nerve and the peripheral connections between the facial and trigeminal nerve. Its significance in parotid gland surgery. Medicina Clínica, 9, 32–39. [Google Scholar]
- Raslan, A. , Guntinas‐Lichius, O. , & Volk, G. F. (2020). Altered facial muscle innervation pattern in patients with postparetic facial synkinesis. The Laryngoscope, 130(5), 320–326. [DOI] [PubMed] [Google Scholar]
- Salame, K. , Ouaknine, G. E. , Arensburg, B. , & Rochkind, S. (2002). Microsurgical anatomy of the facial nerve trunk. Clinical Anatomy, 15(2), 93–99. [DOI] [PubMed] [Google Scholar]
- Sappey, P. (1879). Traite d'anatomie. Adrien Delahaye et Cie. [Google Scholar]
- Shoja, M. M. , Oyesiku, N. M. , Griessenauer, C. J. , Radcliff, V. , Loukas, M. , Chern, J. J. , Benninger, B. , Rozzelle, C. J. , Shokouhi, G. , & Tubbs, R. S. (2014). Anastomoses between lower cranial and upper cervical nerves: A comprehensive review with potential significance during skull base and neck operations, part I: Trigeminal, facial, and vestibulocochlear nerves. Clinical Anatomy, 27(1), 118–130. [DOI] [PubMed] [Google Scholar]
- Takezawa, K. , & Kageyama, I. (2015). Nerve fiber analysis on the morphology of the lingual nerve. Anatomical Science International, 90(4), 298–302. [DOI] [PubMed] [Google Scholar]
- Tansatit, T. , Apinuntrum, P. , & Phetudom, T. (2015). Evidence suggesting that the buccal and zygomatic branches of the facial nerve may contain parasympathetic secretomotor fibers to the parotid gland by means of communications from the Auriculotemporal nerve. Aesthetic Plastic Surgery, 39(6), 1010–1017. [DOI] [PubMed] [Google Scholar]
- Tansatit, T. , Phanchart, P. , Chinnawong, D. , Apinuntrum, P. , Phetudom, T. , & Sahraoui, Y. M. (2016). A cadaveric study of the communication patterns between the buccal trunks of the facial nerve and the infraorbital nerve in the midface. The Journal of Craniofacial Surgery, 27(1), 214–218. [DOI] [PubMed] [Google Scholar]
- Ton Van, C. , & Giot, J. P. (2021). Synkinesis in facial palsy: What do we know about the physiopathology? Annales de Chirurgie Plastique et Esthétique, 66(5), 371–378. [DOI] [PubMed] [Google Scholar]
- Touré, G. , Tran de Fremicourt, M. K. , Randriamanantena, T. , Vlavonou, S. , Priano, V. , & Vacher, C. (2019). Vascular and nerve relations of the marginal mandibular nerve of the face: Anatomy and clinical relevance. Plastic and Reconstructive Surgery, 143(3), 888–899. [DOI] [PubMed] [Google Scholar]
- Tzafetta, K. , & Terzis, J. K. (2010). Essays on the facial nerve: Part I. Microanatomy. Plastic and Reconstructive Surgery, 125(3), 879–889. [DOI] [PubMed] [Google Scholar]
- Valls‐Solé, J. (2013). Facial nerve palsy and hemifacial spasm. Handbook of Clinical Neurology, 115, 367–380. [DOI] [PubMed] [Google Scholar]
- Yang, H. M. , Won, S. Y. , Kim, H. J. , & Hu, K. S. (2013). Sihler staining study of anastomosis between the facial and trigeminal nerves in the ocular area and its clinical implications. Muscle & Nerve, 48(4), 545–550. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Du, J. , Qing, L. , Mee, T. , Xu, X. , Wang, Z. , Xu, C. , & Jia, X. (2021). Identification of sensory and motor nerve fascicles by immunofluorescence staining after peripheral nerve injury. Journal of Translational Medicine, 19(1), 207. [DOI] [PMC free article] [PubMed] [Google Scholar]


